Introduction
Peripheral nerve injury can be caused by accidents,
idiopathic damages, iatrogenic injuries or systemic diseases and is
typically characterized by loss of sensory and motoric functions
downstream of the defect. Approximately 300,000 cases of peripheral
nerve injury are reported every year in Europe alone. However,
unlike the central nervous system, the peripheral nervous system is
characterized by a much higher intrinsic potential to regenerate
(1). The current clinical gold
standard for the treatment of peripheral nerve injury is the
end-to-end suturing of nerves. In cases where tension-free suture
is not possible due to substantial loss of nerve tissue, autologous
nerve grafts are used (2). This,
however, shifts the problem of nerve defect to the donor site.
Furthermore, it has been demonstrated that, in peripheral nerve
surgery, microsurgery of the severed nerve alone fails to address
extensive cell death in the dorsal root ganglia (3). As a result, different research
approaches to enhance regeneration of peripheral nerve injuries are
being explored.
One such potential approach to enhance nerve
regeneration is to administer exogenous growth factors, either
directly or entrapped into biodegradable polymeric nano- and
microparticles for localized delivery (including silicone, PLGA,
PLA or polyphosphoesters of ethylene terephthalate), which may
promote axonal regeneration (4).
However, the success in terms of functional nerve regeneration of
the described systems and related treatments has been limited. One
reason for this failure might be the limitations of the delivery
systems, such as inadequate release kinetics, loss of bioactivity,
single (rather than multiple) factor delivery and lack of cellular
ingrowth. Cell-based delivery systems could be an alternative,
since the cells can be engineered to overexpress specific growth
factors and deliver these agents locally (2).
Another potential approach that is being
investigated is to transplant other cell types into the nerve
grafts to aid the process of regeneration, including Schwann cells
(SCs) (5), olfactory ensheathing
cells (OECs) (6), bone
marrow-derived stem cells (BMSCs) (7), adipose-derived stem cells (ASCs)
(8,9) and induced pluripotent stem cells
(iPSCs) (10). Stem cells have
the potential to increase the number of SCs and prolong their
ability to support regeneration. Furthermore, stem cells can
promote peripheral nerve regeneration by their ability to
differentiate into a SC phenotype, secrete neurotrophic factors,
essential for regeneration, and their potential for myelin
formation (11). Though SCs are
the most fundamental cell type for peripheral nerve regeneration,
their clinical application is limited due to the inability to
isolate a sufficient amount of these cells in a short period of
time (12). Tohill and Terenghi
(7), reported that, under
appropriate conditions, BMSCs can differentiate into non-mesodermal
lineages, such as neurons, astrocytes and SC-like cells (7). Multiple studies have demonstrated
that iPSCs have a pro-regenerative effect in small animal models of
central and peripheral nervous system injury (13-16).
The use of ASCs, in specific, may have practical and
clinical advantages, since they are characterized by a high
proliferation rate and can be derived by liposuction from
subcutaneous fat tissue in large quantities with limited donor site
morbidity and discomfort (17).
Approximately 400,000 liposuction procedures are conducted in the
US every year. Each procedure yields 100-3,000 ml of lipoaspirate
tissue (18), which is a
heterogeneous mixture of cells with a high number of ASCs (19). Several protocols have been
established to induce neuronal differentiation of undifferentiated
ASCs, such as treatment with epidermal growth factor and basic
fibroblast growth factor (bFGF) in a neural progenitor basal medium
resulting in floating neurosphere induction (20); additionally, differentiated cells
have been used in cell therapeutic approaches (3,13-26).
Although ASCs have the clear ability to myelinate
dorsal root ganglion (DRG) neurons in co-culture (27,28), long-term survival of the
transplanted cells has been questioned in a study directly
addressing this problem (29).
Thus it has been hypothesized that the beneficial effect of ASCs in
neuroregeneration depends to a large extent on secretion of
neurotrophic factors, reduction of inflammation and neuronal loss
(30). For instance, the
incorporation of differentiated ASCs into dorsal root ganglia
significantly increased anti-apoptotic Bcl-2 mRNA expression in DRG
neurons (23). Angiogenesis is
also an important factor (31).
Nevertheless, the intrinsic properties of cells in contact with
neurons need to be explored in further detail as a prerequisite for
optimal application of ASCs to enhance and support peripheral nerve
regeneration.
Different strategies have been developed to exploit
the therapeutic benefit of ASCs applied to neural damage. While
lipoaspirated adipose tissue filled in a segment of epigastric vein
impaired functional recovery of a 10 mm gap in a rat sciatic nerve,
processing of the adipose tissue to obtain either the stromal
vascular fraction or even ASCs appears important in the context of
nerve regeneration (22).
Branching of neurons at the site of injury is known
to be a dynamic process, generally decreasing over time, as
branches that fail to establish functional contact with the
periphery are believed to be eliminated. In the present study, the
immediate effect of undifferentiated ASC-DRG neuron interaction was
examined by a detailed analysis of DRG neurite outgrowth over 24
and 48 h in comparison to single-culture cells and cells stimulated
with nerve growth factor (NGF).
Materials and methods
ASC harvest and cell culture
All animals were treated according to the legal and
ethical requirements of the German Animal Welfare Act and were
approved by the Animal Ethics Committee of the Hannover Medical
School Central Animal Laboratory (approval no. 2014/52). Adipose
tissue was obtained from inguinal fat depots of adult male Lewis
rats, 8 weeks old, weighing 350-400 g (n=12) supplied by Charles
River Laboratories (Sulzfeld, Germany). The fat pads were carefully
dissected from the rats under inhalation isoflurane anesthesia,
subsequently the animals were sacrificed. After rinsing with Hank’s
balanced salt solution (HBSS; PAA Laboratories; GE Healthcare GmbH,
Solingen, Germany) and mincing, the fat tissue was digested with
collagenase type I, CLS I (2 mg/ml; Biochrom GmbH, Berlin, Germany)
for 60 min at 37°C under shaking. Following centrifugation for 10
min at 620 × g, at room temperature, the cell pellet was
resuspended in ASC culture medium: DMEM/F12 (Biochrom GmbH) with
100 U/ml penicillin, 100 mg/m streptomycin, 0.2 mM L-ascorbic
acid-2-phosphate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
and 10% fetal bovine serum (FBS; Biochrom GmbH). Cells were plated
on two 150 cm2 cell culture flasks under standard
conditions at 37°C, 100% atmosphere humidity and 5%
CO2.
Phenotypic characterization via surface
marker expression and flow cytometry
Cultured cells (passage 1-3, n=6 per passage) were
examined for ASC-specific surface markers using flow cytometry. The
following anti-rat antibodies conjugated to fluorochromes or
unconjugated were used: CD11b/c PerCP-eFluor 710 (cat. no.
MA1-81606; eBioscience; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), CD44H-fluorescein isothiocyanate (FITC; cat. no. 550974)
and CD105-FITC (cat. no. 562762; BD Biosciences, San Jose, CA,
USA), CD45-FITC (cat. no. 202205) and CD90-PE/CY7 (cat. no. 328123;
BioLegend, Inc., Fell, Germany), CD34 (cat. no. sc-7324; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and CD73 (cat. no. 551123; BD
Pharmingen; BD Biosciences). For immunolabelling, cells were
detached from culture flasks with 0.2% EDTA solution and washed
with PBS containing 10% FBS. The cells were blocked with 1% BSA in
PBS for 30 min at 4°C, centrifuged for 5 min at 200 × g, at room
temperature, resuspended in PBS and incubated with the primary
antibodies (final dilution 1:10) for 50 min at 4°C. After washing
with PBS, the probes with unconjugated primary antibodies were
incubated with 1:10 diluted fluorochrome-labeled secondary
antibodies bovine anti-goat IgG-PerCP-Cy5.5 or
goat-anti-mouse-IgG-PE (both Santa Cruz Biotechnology, Inc.) for 45
min at 4°C, washed and analyzed. The probes with conjugated
antibodies were analyzed directly after washing with PBS. A FC500
flow cytometer with CXP-software v2.2 (Beckman Coulter, Inc.,
Krefeld, Germany) was used for evaluation. The means and standard
deviations were calculated and statistical significance was
evaluated by analysis of variance (ANOVA) and Bonferroni’s post hoc
test.
Induction of adipogenic, osteogenic,
chondrogenic and SC differentiation
In preparation of adipogenic and osteogenic
differentiation, ASCs in passage 2 were plated at a cell density of
3×104 cells/cm2 into 40 mm dishes and allowed
to adhere for 20 h. To induce adipogenic differentiation, the
samples were cultured in standard ASC medium supplemented with 1
μM dexamethasone, 0.5 mM 1-methyl-3-isobutylxanthine, 1
ng/ml insulin and 100 μM indomethacin (all from
Sigma-Aldrich; Merck KGaA) for 3 weeks. To stimulate osteogenic
differentiation, 100 nM dexamethasone and 3 mM β-glycerophosphate
(Sigma-Aldrich; Merck KGaA) were added to standard medium over a
period of 4 weeks. After fixation with 4% (w/v) phosphate buffered
paraformaldehyde (PFA; Carl Roth Gmbh & Co. Kg, Karlsruhe,
Germany), for 15 min at room temperature, differentiation to fat
cells was visualized by staining lipid droplets with Oil Red O
(Serva, Mannheim, Germany), for 10 min at room temperature, while
osteogenic cells were detected by staining of calcium depositions
with Alizarin red (Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature.
To induce chondrogenic differentiation,
3×106 cells were incubated as pellets in an upright
centrifugation tube at 37°C, 5% CO2 and 100% humidity
for three weeks. The differentiated cell pellets were washed with
PBS and fixed with 4% paraformaldehyde in PBS, dehydrated, embedded
in paraffin, sectioned and stained with Alcian blue.
Differentiation to SCs was induced by supplementing
standard medium with 1% ITS+ (BD Biosciences), 1 mM sodium pyruvate
(Biochrom GmbH), 40 μg/ml proline, 40 ng/ml dexamethasone
(both from Sigma-Aldrich; Merck KGaA), 10 ng/mg TGFβ1 (PeproTech,
Ltd., Rocky Hill, NJ, USA). Successful differentiation was
evaluated by immunocytochemistry, as described by Kingham et
al(32). For this purpose
differentiated cells were trypsinised and replated on
μ-slides (ibidi GmbH, Martinsried, Germany) for 2 days.
Subsequently, cells were fixed with 4% PFA for 15 min at room
temperature and immunostained based on a standard protocol using
the primary antibody rabbit anti-S100 (1:500; Dako; Agilent
Technologies GmbH, Waldbronn, Germany) and the secondary antibody
goat-anti-rabbit Alexa Fluor 555 (1:400; Invitrogen; Thermo Fisher
Scientific, Inc.).
Cell size analysis and proliferation
assay
Cultured cells (passages 1-3, n=4 per passage) were
analyzed morphometrically, after confluence of 50-70% was reached.
A total of five random digital images (2,080×1,544 pixels, 1.15
pixels/μm) at magnification, ×4 were captured with an
Olympus CKX41 microscope with Color View Soft imaging system
(Olympus Corporation, Tokyo, Japan). Within these images, the
surface area occupied by 200 cells was measured using the public
domain Java-based image processing and analysis program
ImageJ_v1.15g (National Institutes of Health, Bethesda, MD,
USA).
At 80-90% confluence, cells were detached with
trypsin-EDTA solution (0.25%/0.02%; Biochrom GmbH), washed, seeded
in 6-well plates at 3,000 cells/cm2 and incubated under
standard conditions. After 24 h, the cells of three wells were
detached and counted using a Neubauer haemocytometer. The cells in
the remaining three wells were detached and counted following 48 h
of incubation. Dead cells were identified by trypan blue and
subtracted from the cell count. The proliferation factor was
calculated as the quotient of the cell count at 48 h and the cell
count at 24 h for each passage. Means and standard deviations were
calculated and statistical significance was evaluated by ANOVA
followed by Bonferroni’s post hoc test.
DRG harvest and culture
DRG neurons were obtained from 10-week old male
Wistar rats weighing 400-500 g (n=8) supplied by Charles River
Laboratories (Wilmington, MA, USA). Animals were sacrificed under
isoflurane anesthesia, ganglia were extracted, washed and incubated
in HBSS containing 1.7 mg/ml collagenase A (Roche Diagnostics GmbH,
Mannheim, Germany). The digested ganglia were gently dissociated in
DMEM/F12 + 6% D-glucose (Merck KGaA), centrifuged and washed.
Afterwards, a previously prepared 20% BSA solution in DMEM/F12 was
overlaid with the resuspended cells and centrifuged. After the
liquid layers containing debris were removed and the neurons at the
base were resuspended in a modified Bottenstein and Sato medium
[DRG medium: DMEM/F12 + 6% D-glucose supplemented with 100
μg/ml BSA, 100 μg/ml transferrin, 100 μM
putrescine, 30 nM sodium selenite, 20 nM progesterone, 10 nM
insulin (all purchased from Sigma-Aldrich; Merck KGaA), 100 U/ml
penicillin and 100 mg/ml streptomycin (PAA)], the neurons were
counted and plated onto laminin-coated 12 mm glass coverslips in
24-well plates at 1,000 neurons/coverslip.
DRG neuron and ASC co-culture
The DRG neurons were cultured with ASCs of passage
2. The cultures were maintained in an incubator at 37°C with
humidified atmosphere and 5% CO2. As a negative control,
neurons were cultured separately without ASCs in normal DRG medium
and, as a positive control, neurons were cultured without ASCs in
DRG medium supplemented with 10 ng/ml NGF (Sigma-Aldrich; Merck
KGaA). After 24 and 48 h, the cells were fixed with 4% PFA for 15
min, at room temperature. For immunofluorescence, anti-tubulin β
III (MMS-435P; Covance, Inc., Princeton, NJ, USA) was used as
primary antibody and Alexa Fluor 555 anti-rabbit IgG (cat. no.
A32732; Molecular Probes; Thermo Fisher Scientific, Inc.) as
secondary antibody. The sections were counterstained with DAPI
(Sigma-Aldrich; Merck KGaA). Phase contrast images (2,080×1,544
pixels, 2.9 pixels/μm) of all neurons were taken at ×10
objective magnification using the CKX41 imaging system (Olympus
Corporation). Neurons were analyzed morphometrically using ImageJ
software, with the number and length of outgrowing neurites were
measured. Means and standard deviations were calculated and tested
for statistical significance by ANOVA and Bonferroni’s post hoc
test (soma diameter), or Kruskal-Wallis-ANOVA followed by
Bonferroni corrected Mann-Whitney U-Test (number and length of
neurites).
Statistical analysis
Data are presented as means + standard deviation.
Differences between groups were evaluated by ANOVA and Bonferroni’s
post hoc test (soma diameter), or Kruskal-Wallis-ANOVA followed by
Bonferroni corrected Mann-Whitney U-Test (number and length of
neurites). Statistics based on the IBM SPSS v.20. (IBM, Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
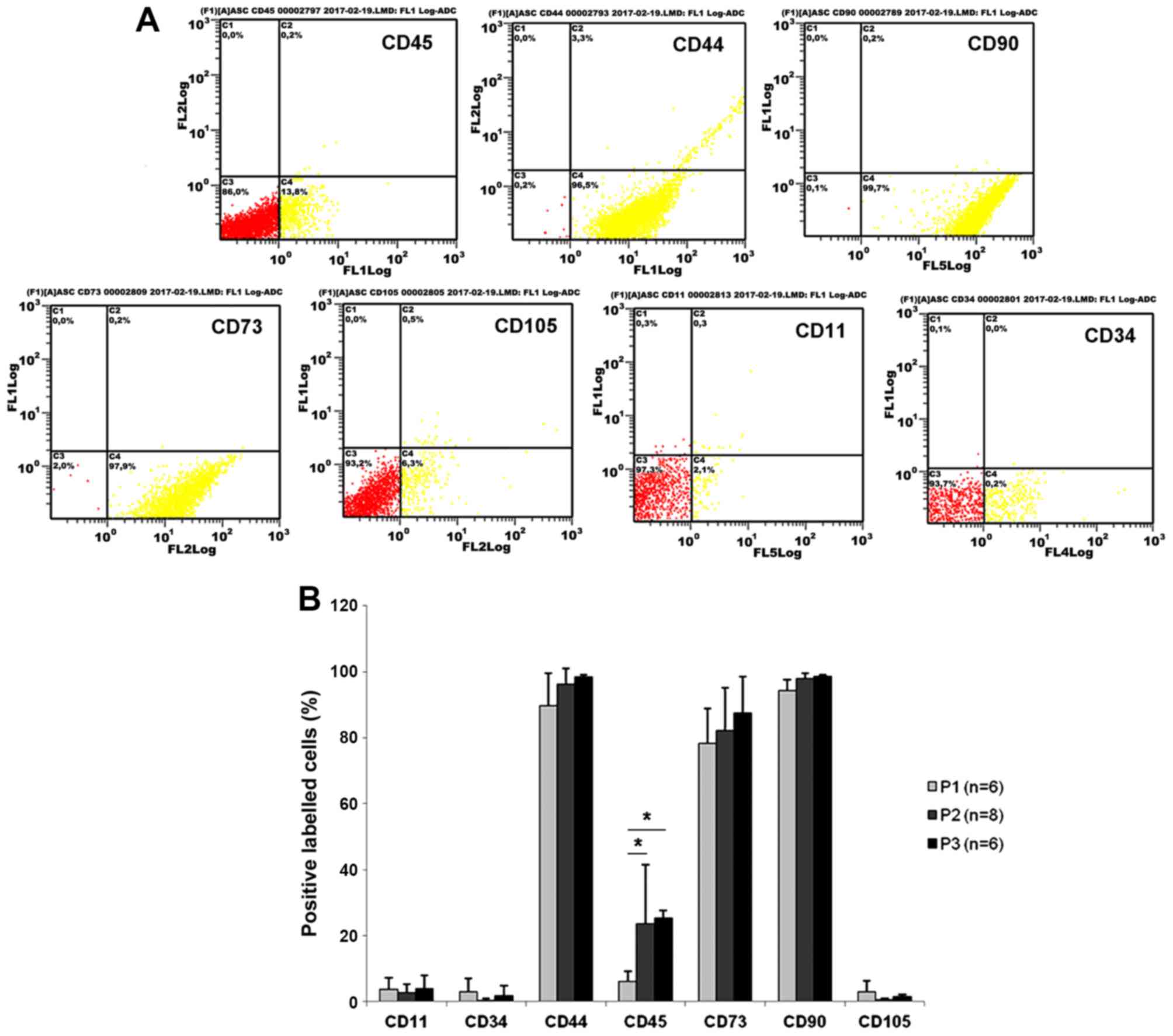
Phenotypic characterization of ASCs by
flow cytometry and changes over time with passaging
ASCs were harvested from inguinal fat pads of adult
rats as plastic adherent cells through removal of nonadherent cells
24 h after cell harvesting and primary cell seeding. For
characterization of the ASCs, cell surface protein expression was
detected by flow cytometry (Fig.
1A). Through all three investigated passages, the majority of
cells expressed the characteristic mesenchymal stem cell surface
markers CD44 (95.83±2.87%), CD73 (83.92±9.23%), and CD90
(98.23±1.61%), while <5% of the cells were positive for CD11,
CD105 and CD34 (Fig. 1B). Of
note, the percentage of CD45-positive cells was 6.15±2.99% at
passage 1 and increased significantly to 22.87±21.18% at passage 2
and 25.36±2.18% at passage 3 (Fig.
1B).
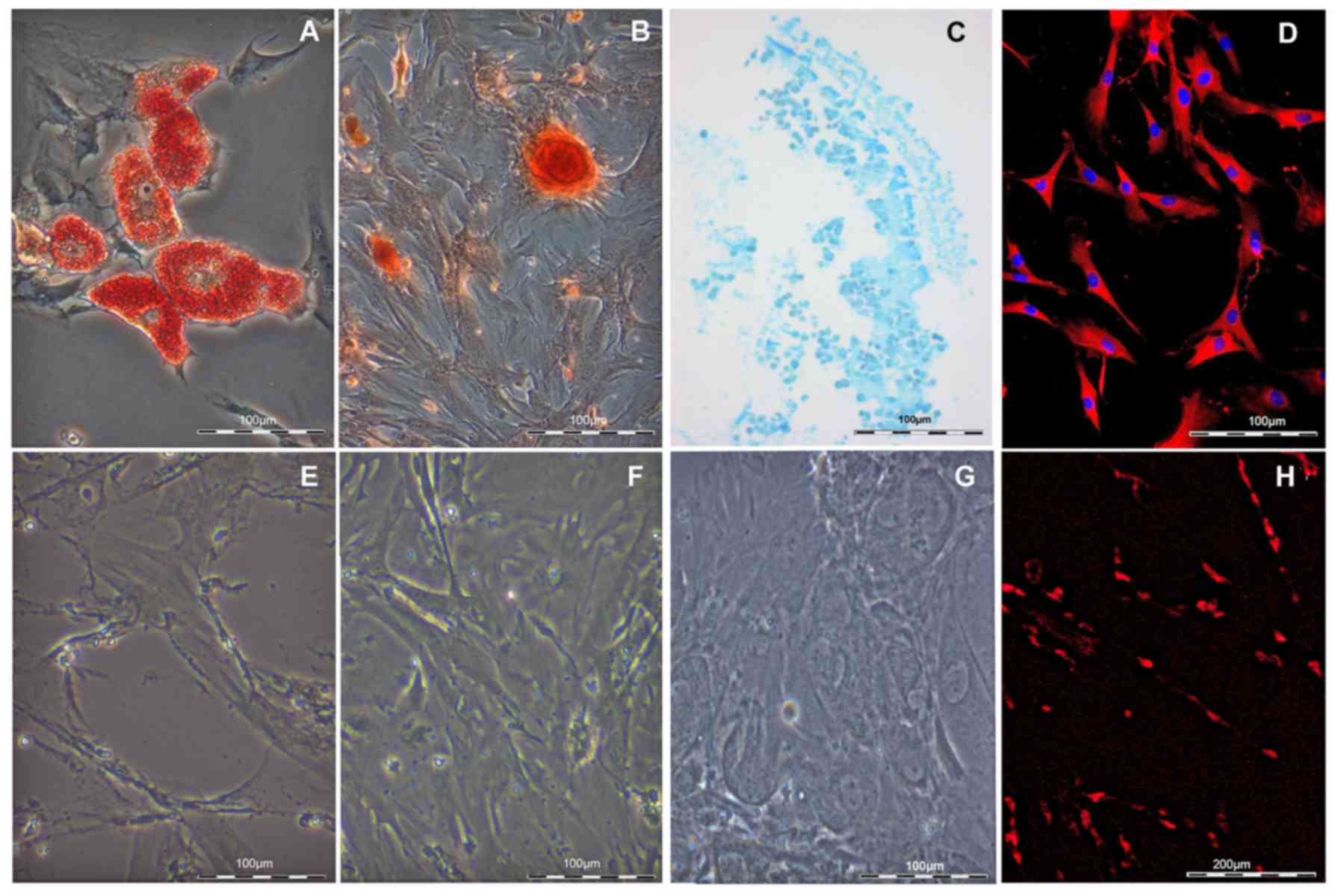
Differentiation capacity of ASCs
As mesenchymal stem cells are able to differentiate
into adipogenic and osteogenic lineages, differentiation of
isolated cell populations was induced into both directions. After
10 days in adipogenic differentiation medium, the existence of
lipid vesicles could be verified by positive staining with Oil Red
O (Fig. 2A). No staining was
observed in the non-induced control samples (Fig. 2E). The osteogenic potential of
ASCs was also evaluated. Four weeks after osteogenic induction,
typical calcified nodules were identified, visualized by Alizarin
red staining (Fig. 2B), in
contrast to the non-induced ASC control population (Fig. 2F). Thirdly, chrondrogenic cells
were identified by alcian blue staining in chondrogenicly-induced
ASCs (Fig. 2C), while none were
detected in the control non-induced samples (Fig. 2G).
To demonstrate neuronal differentiation capacity,
ASCs were successfully differentiated into a SC-like phenotype.
After 2 weeks of induction, cell shape alteration was observed from
a flat, fibroblast-like structure into a spindle-shaped morphology,
similar to SCs used as control (Fig.
2H). Successful differentiation to SC-like phenotype was
further confirmed by detection of S100 expression (Fig. 2D).
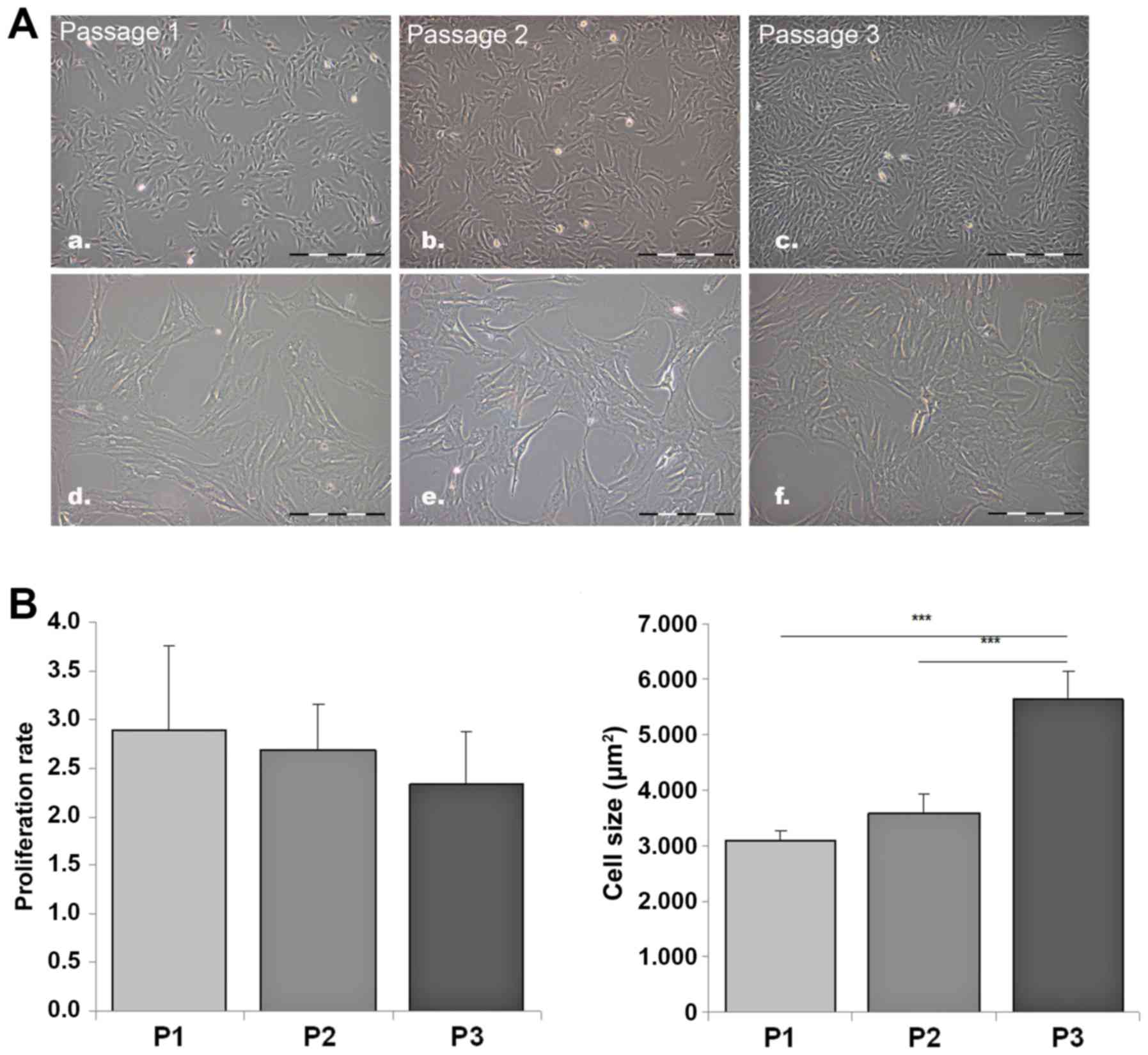
Morphologic characterization of ASCs and
effect of cell passaging
ASCs appeared as large and flat cells with a
fibroblast-like, irregular shape. The cells continuously
proliferated without significant changes in their fibroblast-like
morphology until passage 3 (Fig.
3A). The proliferation rate was stable; the observed minimal
decrease in proliferation rate with increasing passage numbers,
from a 2.9-fold at 24 h at passage 1 to a 2.3-fold at 24 h at
passage 3, was not statistically significant (Fig. 3B). The cell size of subconfluent
ASCs in progressive stages of cell culturing was increased by 82%
in passage 3 compared with passage 1 (Fig. 3B).
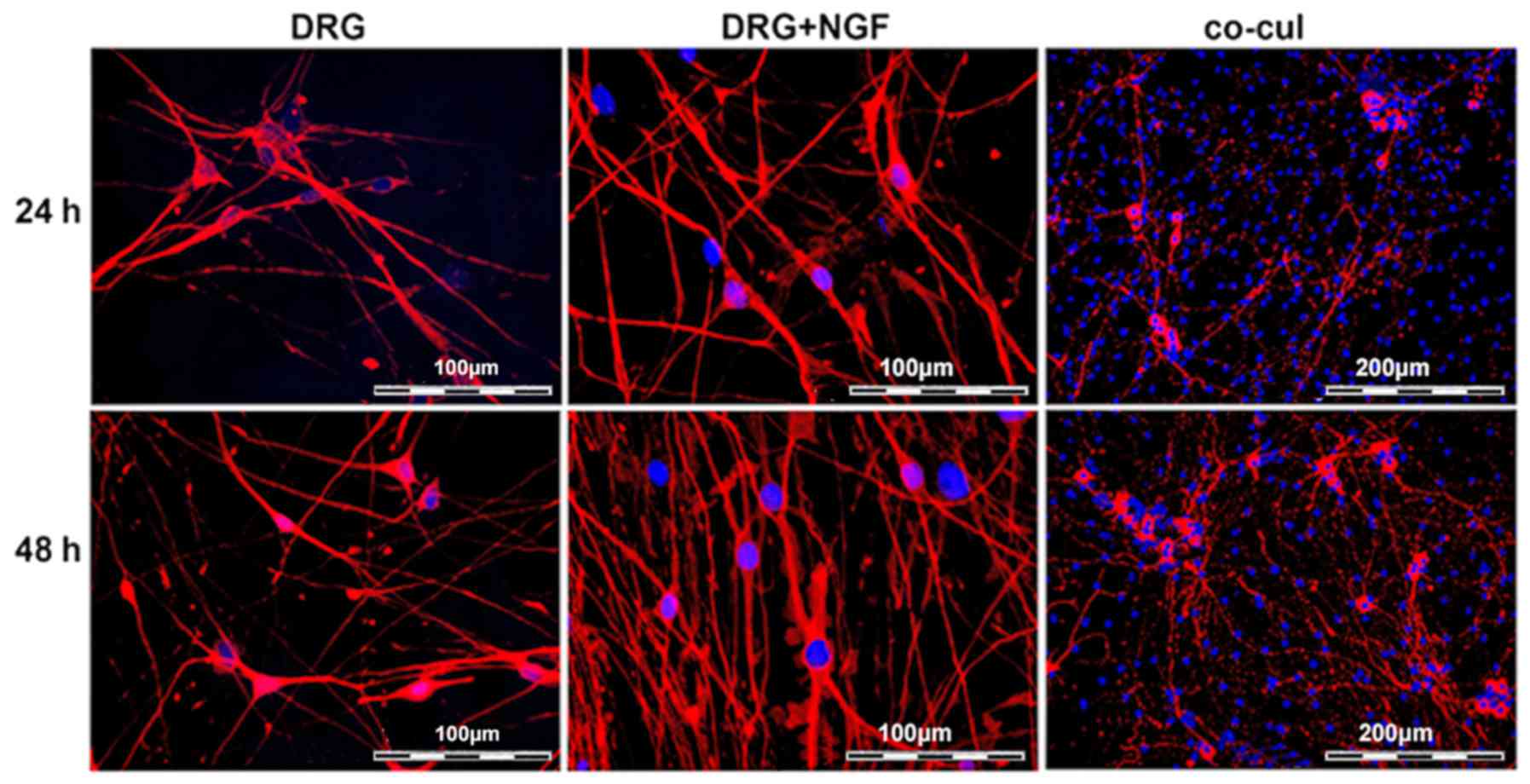
Directed neurite outgrowth in co-culture
with ASCs
Undifferentiated ASCs of passage 2 were co-cultured
with freshly dissociated DRG neurons as an in vitro model of
neurite outgrowth. Apart from the experimental group consisting of
the co-cultured ASCs and DRG neurons, two control groups were used.
The first contained neurons in single culture (DRG group) as a
negative control, while the second contained neurons in DRG medium
supplemented with NGF (NGF group) as a positive control. Under the
culture conditions, neurites protruded from the seeded cells within
24 h in culture verified by immunostaining of β-III-tubulin
(Fig. 4). After 48 h, the number
of neurites increased in all approaches, although the highest
number of neurites was observed in the cultures supplemented with
NGF (Fig. 4). In co-cultures with
undifferentiated ASCs, an alignment of neurites along the ASCs
could be observed, while ASCs themselves did not express
β-III-tubulin (Fig. 4). To
quantify the effect of ASCs on DRG neurons, several parameters were
evaluated: percentage of neurons that formed neurites, number of
neurites per neuron, mean and total length of neurites per neuron
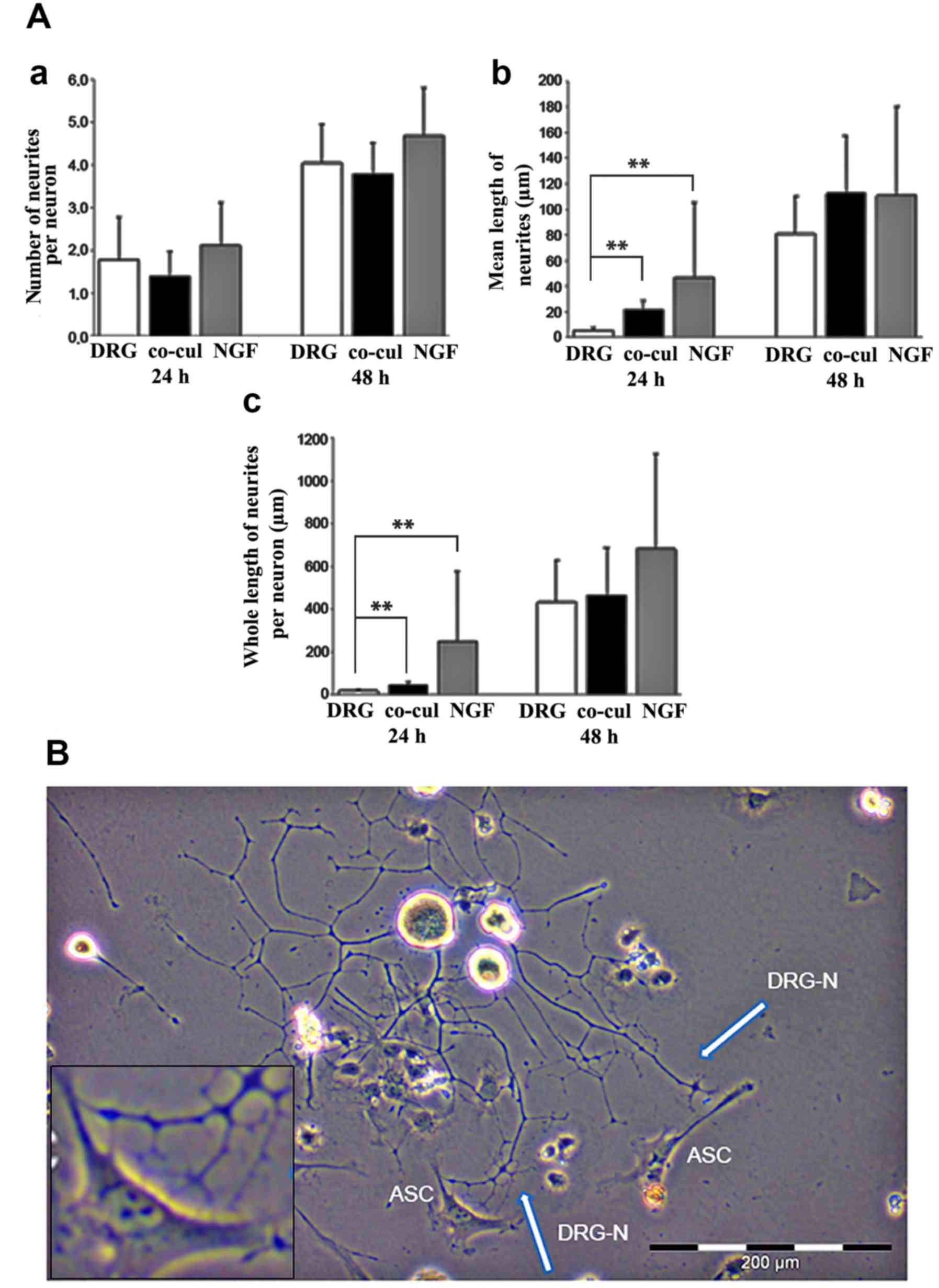
(Fig. 5A).
After 24 and 48 h of culture, the neurite outgrowth
was compared between the three groups. Within few hours numerous
neurons started to form neurites in all culture conditions. After
24 h, 46±16% of neurons in single culture and 56±13% of neurons in
co-cultures exhibited neurites. At 48 h, 82±8% and 92±6%,
respectively, formed neurites.
Both after 24 and 48 h, no significant difference
was observed regarding the number of neurites per neuron formed in
the three groups (Fig. 5A-a). ASC
co-culture, however, appeared to have an influence on the neurite
length at early time points. After 24 h, the mean length of
neurites was significantly increased in the co-cultures compared
with the DRG group (P=0.003; Fig.
5A-b). After 48 h, a statistically significant difference
between the three groups could not be detected any more (Fig. 5A-b). Similar results were observed
when the whole length of neurites per neuron was analyzed (Fig. 5A-c).
According to the stimulating influence on neurite
elongation, light microscopic analyses of the co-culture systems
between ASCs and DRG neurons revealed a directed neurite elongation
towards the ASCs. The neurites established direct contact with the
ASC (Fig. 5B).
Discussion
The present study examined the feasibility of using
ASCs as a source of stem cells for the differentiation of DRG
neurons by an in vitro co-culture approach. Previous studies
have demonstrated that rat ASCs can be differentiated toward a
SC-like phenotype, which promotes neurite outgrowth and myelination
(33,34). In addition, ASCs secrete a
plethora of growth factors that can mediate angiogenesis, wound
healing, tissue regeneration and immune cell reactions (35,36). In the regeneration of the nervous
system, ASCs produce a wide variety of neurotrophic factors which
can enhance neurite outgrowth and provide neuroprotection (37-40). It has been demonstrated that
ASC-derived soluble factors in ASC-conditioned medium supported
survival and proliferation of SCs and promoted neurite outgrowth in
DRG neurons (41). However, the
early effects of cell contact between undifferentiated ASC and DRG
neurons have not been investigated to date. The present study
demonstrated that neurite outgrowth of DRG neurons was enhanced by
co-culture with ASCs, as evidenced by increased neurite lengths
even after short periods of co-culture at 24 h. Acceleration of
neurite length is of particular interest in peripheral nerve
regeneration because the time period for successful regeneration is
limited. Axonal regeneration and irreversible loss of neurovascular
junction integrity are simultaneous ongoing processes, as long as
the regenerating nerve has not reached its effector. Additionally,
neurite branching was not increased in the present study,
indicating that axonal sprouting is controlled by decreasing
collateral axonal branching and possible reduced risk of neuroma
formation (42).
The ASC populations were expanded over three
passages without losing their proliferative capacity, which is in
line with the findings of Mantovani et al(43), who described ASCs isolated from
young adult rats with a significant reduction in their
proliferation rate only starting with passage 20. Nevertheless,
there was a significant increase in cell size at passage 3,
indicating cellular senescence (44). As an influence of replicative
senescence on secretion of neurotrophic factors cannot be excluded
(43), high passaging of ASCs was
avoided in the present study and only cells from passage 2 were
used for co-culture experiments.
The isolated ASCs were successfully characterized,
as required by the International Society for Cell Therapy, for
their expression of MSC-specific surface markers and their
differentiation capacity into adipocytes, chondrocytes and
osteoblasts. As expected, the primary cell cultures displayed a
great percentage of CD44, CD73 and CD90-positive cells. High
expression rates of CD44 have also been regarded as important for
stemness of different adult stem cells (45), which might compensate for low
levels of CD105 expression. The impact of CD105 expression on
aspects of nerve regeneration is so far unknown and has to be
characterized in further studies. In the present study, analysis of
CD45 expression, a marker for hematopoietic lineage, revealed that
its expression in the ASCs continuously increased from passage 1 to
passage 3. In the present study, analysis of CD45 expression, a
marker for hematopoietic lineage, revealed that its expression in
the ASCs continuously increased from passage 1 to passage 3. An
increase in CD45 expression has also been described for late ASC
passages in a previous study, which may indicate the ability of ASC
long-term cultures to activate the immune response or an
inclination towards hematopoietic differentiation (46). Proliferation rates of ASCs
isolated from animals of different ages showed no significant
difference and only small numbers of CD45-positive cells were
contained in the cultures (41).
The results of the present flow cytometry analyses confirmed the
presence of MSC surface markers (CD73, CD90, CD44 >80%; CD11,
CD105, CD34 <5%; CD45 >6% and <25%). Furthermore, the
differentiation capacity of the isolated ASCs into adipocytes,
chondrocytes and osteoblasts was confirmed. The present panel of
surface markers does not fully meet the criteria introduced by
Dominici et al(47).
However, based on the present surface phenotype analysis, in
conjunction with the differentiation functional criteria, the
isolated ASC cultures were confirmed based on the current state of
knowledge. Strict observation of the cultures concerning their
proper phenotype, differentiation and senescence is an important
factor for optimized results concerning stimulation of neurite
growth. These findings were taken into account in the experimental
design of the present study, avoiding high passages of the cells.
These strict criteria will also be important for future studies
in vivo.
Furthermore, the ASCs in passage 2 could
successfully be induced for chondrogenic, osteogenic, adipose and
glial cell lineage differentiation. Additionally, it was
demonstrated that ASCs could be differentiated into a functional
SC-like phenotype, expressing markers S100 and enhancing neurite
outgrowth in vitro. The ASC neurotrophic potential has also
been confirmed by in vivo peripheral nerve repair studies
(48,49), indicating that adult stem cells
may be of benefit for treatment of peripheral nerve injuries.
A direct contact of severed nerves with growth
factors, such as NGF, can lead to extreme pain and
hyperexcitability (6). Therefore,
the use of ASCs as a source of growth factors in the clinic may be
advantageous, because an uncontrollable growth factor
supplementation might lead to severe side effects. This is in
contrast to differentiated ASCs where secretion in high amounts of
neurothrophins and growth factors was demonstrated (41). This does not exclude the ability
of undifferentitated ASCs to produce growth factors, but their
production and secretion of growth factors might be more targeted
than an unspecific, uncontrollable exogenous supplementation. As
cell-to-cell contact in co-culture with direct neuritic contact on
the cell surface of the undifferentiated ASCs was observed in the
present study, the positive neuronal influence might be based on
other cell signaling mechanisms or direct crosstalk between the
cells.
In the present study, undifferentiated ASCs were
used due to their advantages in regard to possible clinical
applications. The majority of studies have demonstrated the
efficacy of ASCs following glial differentiation (50). In the case of a potential clinical
transfer, the application of unaltered cells is preferable. This
underlines the need for a more detailed knowledge regarding the
interaction and efficacy of undifferentiated ASCs on injured
neurons, in order to optimize the healing results and to reduce
undesired side effects.
The present results demonstrated that
undifferentiated ASCs significantly enhanced the speed of DRG
neuron neurite growth in a co-culture system. This effect is
important for nerve injury treatment, as a quick growth of neurites
is crucial for success in peripheral nerve regeneration. These
findings indicate that undifferentiated ASCs may have promise as a
method of direct transplantation in peripheral nerve
regeneration.
Acknowledgments
The authors would like to thank Miss Jieli Liu for
helping with the measurements of the neuronal parameters. The
authors would also thank Dr Stefanie Michael for writing
assistance.
Funding
The present study has been supported by the
Boehringer Ingelheim Foundation (grant no. 19470076).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors’ contributions
RS, SS and AF performed experiments and analyzed the
data. VB, DV and CTP analyzed the data and participated in the
interpretation of the data. PMV, KR and MF participated in the
interpretation of the data. KR, SS and MF revised the manuscript
critically. CR designed, analyzed and interpreted the study. VB
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animals were treated according to the legal and
ethical requirements of the German Animal Welfare Act and were
approved by the Animal Ethics Committee of the Hannover Medical
School Central Animal Laboratory (approval no. 2014/52).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Madduri S and Gander B: Schwann cell
delivery of neurotrophic factors for peripheral nerve regeneration.
J Peripher Nerv Syst. 15:93–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chimutengwende-Gordon M and Khan W: Recent
advances and developments in neural repair and regeneration for
hand surgery. Open Orthop J. 6:103–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reid AJ, Sun M, Wiberg M, Downes S,
Terenghi G and Kingham PJ: Nerve repair with adipose-derived stem
cells protects dorsal root ganglia neurons from apoptosis.
Neuroscience. 199:515–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong FS, Chan BP and Lo AC: Carriers in
cell-based therapies for neurological disorders. Int J Mol Sci.
15:10669–10723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ide C: Peripheral nerve regeneration.
Neurosci Res. 25:101–121. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Radtke C, Wewetzer K, Reimers K and Vogt
PM: Transplantation of olfactory ensheathing cells as adjunct cell
therapy for peripheral nerve injury. Cell Transplant. 20:145–152.
2011. View Article : Google Scholar
|
|
7
|
Tohill M and Terenghi G: Stem-cell
plasticity and therapy for injuries of the peripheral nervous
system. Biotechnol Appl Biochem. 40:17–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muschler GF, Nitto H, Boehm CA and Easley
KA: Age- and gender-related changes in the cellularity of human
bone marrow and the prevalence of osteoblastic progenitors. J
Orthop Res. 19:117–125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strem BM, Hicok KC, Zhu M, Wulur I,
Alfonso Z, Schreiber RE, Fraser JK and Hedrick MH: Multipotential
differentiation of adipose tissue-derived stem cells. Keio J Med.
54:132–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robinton DA and Daley GQ: The promise of
induced pluripotent stem cells in research and therapy. Nature.
481:295–305. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walsh S and Midha R: Use of stem cells to
augment nerve injury repair. Neurosurgery. 65(Suppl): A80–A86.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weinstein DE: The role of Schwann cells in
neural regeneration. Neuroscientist. 5:208–216. 1999. View Article : Google Scholar
|
|
13
|
Ikeda M, Uemura T, Takamatsu K, Okada M,
Kazuki K, Tabata Y, Ikada Y and Nakamura H: Acceleration of
peripheral nerve regeneration using nerve conduits in combination
with induced pluripotent stem cell technology and a basic
fibroblast growth factor drug delivery system. J Biomed Mater Res
A. 102:1370–1378. 2014. View Article : Google Scholar
|
|
14
|
Satarian L, Javan M, Kiani S, Hajikaram M,
Mirnajafi-Zadeh J and Baharvand H: Engrafted human induced
pluripotent stem cell-derived anterior specified neural progenitors
protect the rat crushed optic nerve. PLoS One. 8:e718552013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uemura T, Takamatsu K, Ikeda M, Okada M,
Kazuki K, Ikada Y and Nakamura H: Transplantation of induced
pluripotent stem cell-derived neurospheres for peripheral nerve
repair. Biochem Biophys Res Commun. 419:130–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang A, Tang Z, Park IH, Zhu Y, Patel S,
Daley GQ and Li S: Induced pluripotent stem cells for neural tissue
engineering. Biomaterials. 32:5023–5032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez AM, Elabd C, Amri EZ, Ailhaud G
and Dani C: The human adipose tissue is a source of multipotent
stem cells. Biochimie. 87:125–128. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gimble JM, Katz AJ and Bunnell BA:
Adipose-derived stem cells for regenerative medicine. Circ Res.
100:1249–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carvalho PP, Wu X, Yu G, Dias IR, Gomes
ME, Reis RL and Gimble JM: The effect of storage time on
adipose-derived stem cell recovery from human lipoaspirates. Cells
Tissues Organs. 194:494–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radtke C, Schmitz B, Spies M, Kocsis JD
and Vogt PM: Peripheral glial cell differentiation from
neurospheres derived from adipose mesenchymal stem cells. Int J Dev
Neurosci. 27:817–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren Z, Wang Y, Peng J, Zhao Q and Lu S:
Role of stem cells in the regeneration and repair of peripheral
nerves. Rev Neurosci. 23:135–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Papalia I, Raimondo S, Ronchi G, Magaudda
L, Giacobini-Robecchi MG and Geuna S: Repairing nerve gaps by vein
conduits filled with lipoaspirate-derived entire adipose tissue
hinders nerve regeneration. Ann Anat. 195:225–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Georgiou M, Golding JP, Loughlin AJ,
Kingham PJ and Phillips JB: Engineered neural tissue with aligned,
differentiated adipose-derived stem cells promotes peripheral nerve
regeneration across a critical sized defect in rat sciatic nerve.
Biomaterials. 37:242–251. 2015. View Article : Google Scholar
|
|
24
|
Hsueh YY, Chang YJ, Huang TC, Fan SC, Wang
DH, Chen JJ, Wu CC and Lin SC: Functional recoveries of sciatic
nerve regeneration by combining chitosan-coated conduit and
neurosphere cells induced from adipose-derived stem cells.
Biomaterials. 35:2234–2244. 2014. View Article : Google Scholar
|
|
25
|
Orbay H, Uysal AC, Hyakusoku H and Mizuno
H: Differentiated and undifferentiated adipose-derived stem cells
improve function in rats with peripheral nerve gaps. J Plast
Reconstr Aesthet Surg. 65:657–664. 2012. View Article : Google Scholar
|
|
26
|
Sun F, Zhou K, Mi WJ and Qiu JH: Combined
use of decellularized allogeneic artery conduits with autologous
transdifferentiated adipose-derived stem cells for facial nerve
regeneration in rats. Biomaterials. 32:8118–8128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ravasi M, Scuteri A, Pasini S, Bossi M,
Menendez VR, Maggioni D and Tredici G: Undifferentiated MSCs are
able to myelinate DRG neuron processes through p75. Exp Cell Res.
319:2989–2999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Razavi S, Mardani M, Kazemi M, Esfandiari
E, Narimani M, Esmaeili A and Ahmadi N: Effect of leukemia
inhibitory factor on the myelinogenic ability of Schwann-like cells
induced from human adipose-derived stem cells. Cell Mol Neurobiol.
33:283–289. 2013. View Article : Google Scholar
|
|
29
|
Erba P, Mantovani C, Kalbermatten DF,
Pierer G, Terenghi G and Kingham PJ: Regeneration potential and
survival of transplanted undifferentiated adipose tissue-derived
stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet
Surg. 63:e811–e817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Faroni A, Smith RJ and Reid AJ: Adipose
derived stem cells and nerve regeneration. Neural Regen Res.
9:1341–1346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kolar MK and Kingham PJ: Regenerative
effects of adipose-tissue-derived stem cells for treatment of
peripheral nerve injuries. Biochem Soc Trans. 42:697–701. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kingham PJ, Kalbermatten DF, Mahay D,
Armstrong SJ, Wiberg M and Terenghi G: Adipose-derived stem cells
differentiate into a Schwann cell phenotype and promote neurite
outgrowth in vitro. Exp Neurol. 207:267–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
di Summa PG, Kalbermatten DF, Raffoul W,
Terenghi G and Kingham PJ: Extracellular matrix molecules enhance
the neuro-trophic effect of Schwann cell-like differentiated
adipose-derived stem cells and increase cell survival under stress
conditions. Tissue Eng Part A. 19:368–379. 2013. View Article : Google Scholar
|
|
34
|
Xu Y, Liu L, Li Y, Zhou C, Xiong F, Liu Z,
Gu R, Hou X and Zhang C: Myelin-forming ability of Schwann
cell-like cells induced from rat adipose-derived stem cells in
vitro. Brain Res. 1239:49–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kapur SK and Katz AJ: Review of the
adipose derived stem cell secretome. Biochimie 9. 5:2222–2228.
2013. View Article : Google Scholar
|
|
36
|
Salgado AJ, Reis RL, Sousa NJ and Gimble
JM: Adipose tissue derived stem cells secretome: Soluble factors
and their roles in regenerative medicine. Curr Stem Cell Res Ther.
5:103–110. 2010. View Article : Google Scholar
|
|
37
|
Chung JY, Kim W, Im W, Yoo DY, Choi JH,
Hwang IK, Won MH, Chang IB, Cho BM, Hwang HS, et al:
Neuroprotective effects of adipose-derived stem cells against
ischemic neuronal damage in the rabbit spinal cord. J Neurol Sci.
317:40–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kalbermatten DF, Schaakxs D, Kingham PJ
and Wiberg M: Neurotrophic activity of human adipose stem cells
isolated from deep and superficial layers of abdominal fat. Cell
Tissue Res. 344:251–260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lattanzi W, Geloso MC, Saulnier N,
Giannetti S, Puglisi MA, Corvino V, Gasbarrini A and Michetti F:
Neurotrophic features of human adipose tissue-derived stromal
cells: In vitro and in vivo studies. J Biomed Biotechnol.
2011:4687052011. View Article : Google Scholar
|
|
40
|
Wei X, Zhao L, Zhong J, Gu H, Feng D,
Johnstone BH, March KL, Farlow MR and Du Y: Adipose stromal
cells-secreted neuroprotective media against neuronal apoptosis.
Neurosci Lett. 462:76–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sowa Y, Imura T, Numajiri T, Nishino K and
Fushiki S: Adipose-derived stem cells produce factors enhancing
peripheral nerve regeneration: Influence of age and anatomic site
of origin. Stem Cells Dev. 21:1852–1862. 2012. View Article : Google Scholar
|
|
42
|
Skouras E, Ozsoy U, Sarikcioglu L and
Angelov DN: Intrinsic and therapeutic factors determining the
recovery of motor function after peripheral nerve transection. Ann
Anat. 193:286–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mantovani C, Raimondo S, Haneef MS, Geuna
S, Terenghi G, Shawcross SG and Wiberg M: Morphological, molecular
and functional differences of adult bone marrow- and
adipose-derived stem cells isolated from rats of different ages.
Exp Cell Res. 318:2034–2048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simons JW: The use of frequency
distributions of cell diameters to characterize cell populations in
tissue culture. Exp Cell Res. 45:336–350. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maleki M, Ghanbarvand F, Reza Behvarz M,
Ejtemaei M and Ghadirkhomi E: Comparison of mesenchymal stem cell
markers in multiple human adult stem cells. Int J Stem Cells.
7:118–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wan Safwani WK, Makpol S, Sathapan S and
Chua KH: The changes of stemness biomarkers expression in human
adipose-derived stem cells during long-term manipulation.
Biotechnol Appl Biochem. 58:261–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
di Summa PG, Kalbermatten DF, Pralong E,
Raffoul W, Kingham PJ and Terenghi G: Long-term in vivo
regeneration of peripheral nerves through bioengineered nerve
grafts. Neuroscience. 181:278–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
di Summa PG, Kingham PJ, Raffoul W, Wiberg
M, Terenghi G and Kalbermatten DF: Adipose-derived stem cells
enhance peripheral nerve regeneration. J Plast Reconstr Aesthet
Surg. 63:1544–1552. 2010. View Article : Google Scholar
|
|
50
|
Zuck P: Adipose-derived stem cells in
tissue regeneration. ISRN Stem Cells. 2013:e7139592013.
|