Introduction
The concept of exosomes was first proposed by Trams
et al (1) in 1981, while
soon after, exosomes were identified in a study of reticulocyte
differentiation as a consequence of multivesicular endosome fusion
with the plasma membrane (2,3).
Recent studies have revealed that exosomes are vesicles of 30-100
nm in size (4), which are
secreted by cells into the extracellular space and composed of a
lipid bilayer containing several specific proteins and RNAs
(5,6). These small intraluminal vesicles
fuse with the plasma membrane of target cells when released into
the extracellular environment (7). Exosomes can be detected in all
bodily fluids, such as urine (8),
seminal fluid (9), blood
(10) and amniotic fluids
(11). In the transmission of
information and regulation of cell signal transduction between
different cells, exosomes serve a significant role by selectively
delivering biologically active substances to the target cells
(12). Exosomes also participate
in numerous important physiological and pathological processes,
including intercellular communication, cell motility, angiogenesis
(13), immune response (14), tumor development and metastasis
(15,16). Owing to their bioactive cargo,
exosomes may serve as messengers and may offer valuable information
for the diagnosis and prognosis of disease (17).
Differing from biopsy, the ‘liquid biopsy’ of
exosomes has various advantages, such as being less invasive, and
providing easy handling and sampling (18). As one of the body fluids, urine
also contains exosomes. Urinary exosomes are not susceptible to
external interference due to their natural lipid bilayers. They
also contain a large amount of biological information, which can be
communicated to target cells (19,20). Therefore, urinary exosomes can
serve as a sensitive biomarker of tumors in diagnosis and screening
(21).
A large number of strategies have been applied for
the isolation of urinary exosomes. However, the high cost of these
methods and the low purity of the obtained exosomes remain
important challenges, limiting further development of exosomes
(22). Among the available
strategies, ultra-centrifugation (UC) is the most widely used
method, which exhibits a low throughput and impure isolated
exosomes due to high-molecular-mass proteins, sample heterogeneity
and low stability, given the intensiveness of sequential UC steps
(23). Thus, there is an urgent
need to establish a simple and rapid method of isolating urinary
exosomes with high purity, production and biological activity for
further applications in research and clinical practice (24,25). Furthermore, a uniform standard
regarding the urine collection time in the isolation of urinary
exosomes is currently lacking.
The present study reports an optimized
ultrafiltration (OUF) method that can effectively isolate urinary
exosomes with high purity and quality in a much more simplified way
compared with UC. The results suggest that OUF may be useful for
further application in the field of exosome study for liquid
biopsy, clinical screening and early disease diagnosis.
Materials and methods
Ethics statement and sample
collection
Our previous research has mainly focused on the
study of microRNA (miRNA) and urine miRNA in ovarian cancer
(26-28). In order to determine the clinical
value of urinary exosome miRNA for ovarian cancer and other
female-specific conditions, therefore, urine samples were collected
from young women in the current study. Urine was collected from
Chinese female volunteers, aged between 22 and 27 years, subsequent
to obtaining written informed consent. Volunteers were requested to
collect samples of morning (first urination), afternoon and night
urine of one day during four consecutive days. All subjects were
relatively young and healthy, without kidney disease, diabetes or
other chronic medical history. Approximately 50 ml urine was
collected from each subject at each time, and the urine samples
from different individuals were not mixed. Specimens were stored at
−80°C immediately. The present study was approved by the Ethics
Committee of Central South University (Changsha, China) and
conducted in adherence with the Declaration of Helsinki.
Exosome purification methods
Urine samples of approximately 50 ml were collected
from each individual in the morning (first urination of the day),
afternoon (14:00-18:00) and evening (18:00-22:00). The workflows of
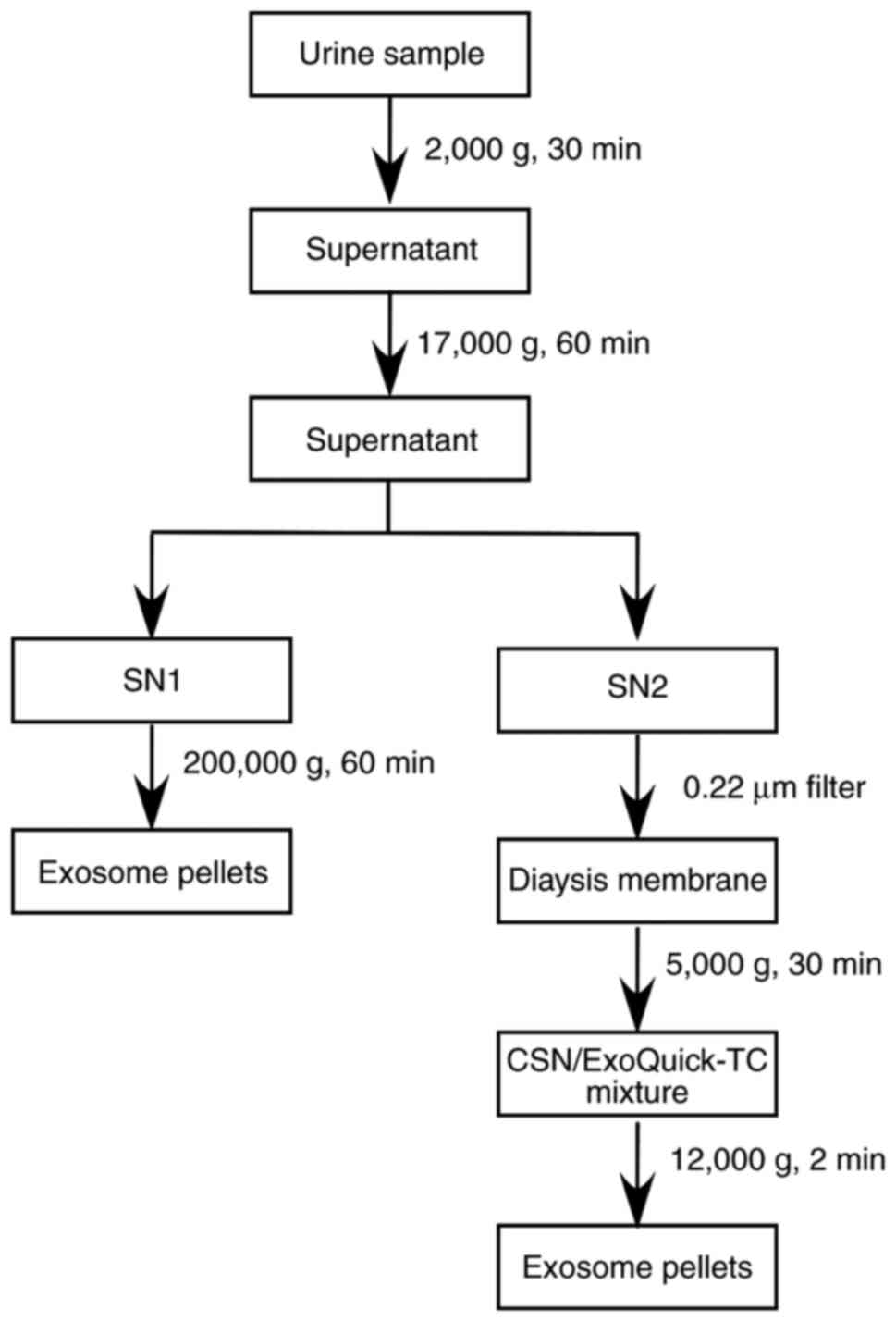
the two methods for isolating exosomes from urine samples can be
briefly summarized as follows: First, urine samples were
centrifuged at 2,000 x g for 30 min at 4°C to remove the cells,
cell debris, bacteria and the majority of Tamm-Horsfall protein
(THP) (29-33). Next, the remaining macropolymers
and THP were removed by further centrifugation for 60 min at 17,000
x g and 4°C. The supernatant was split into two fractions with the
same volume, namely supernatant 1 (SN1) and SN2. In the UC method,
SN1 was directly ultracentrifuged (Beckman L-80XP 70 Ti; Beckman
Coulter, Inc. Brea, CA, USA) at 200,000 x g for 60 min at 4°C in
order to collect exosome pellets. In comparison, in the OUF method,
SN2 was passed through a 0.22-µm filter to remove proteins
with diameters of >0.22 µm. The filtered solution was
then centrifuged at 3,000 x g for 30 min at 4°C in the dialysis
tube with a molecular weight cut-off (MWCO) membrane (Merck KGaA,
Darmstadt, Germany). In these two steps, SN2 was passed through two
types of filter to excess interference from soluble protein and
then concentrated to 1/50 of the original volume. Next, the
concentrated supernatant (CSN) was incubated with ExoQuick-TC™
exosome precipitation solution (cat. no. EXOTC50A-1; System
Biosciences, Palo Alto, CA, USA) for 30 min at 4°C. Subsequently,
the mixture was spun at 15,279 x g for 2 min at 4°C to harvest the
yellow pellets of exosomes (Figs.
1 and 2A).
Nanoparticle tracking analysis (NTA)
A NanoSight LM10 (Malvern Panalytical Ltd., Malvern,
UK) was used to detect the size of the exosomes. Briefly, 10
µl exosomes samples were diluted to 1:100 in 1X PBS. Next,
the mixture was placed into the 1 ml injector and injected into the
nanoparticle tracking analyzer in order to analyze the size of the
exosomes.
Transmission electron microscopy
(TEM)
Exosome samples were fixed with 1% glutaraldehyde in
PBS at an optimal concentration. The mixture was then spotted onto
a 300 mesh carbon/formvar-coated grids and dried at room
temperature. Next, the grids were washed with PBS and stained for
contrast using uranyl acetate in water for 10 min. Subsequent to
staining, samples were imaged by TEM (FEI, Hillsboro, OR, USA), and
images were captured with an AMT CCD Camera (Advanced Microscopy
Techniques, Danvers, MA, USA).
Protein assay, colloidal
Coomassie-stained gel and western blotting
Exosomes were lysed in radioimmunoprecipitation
assay lysis buffer (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Protein concentrations were determined with a bicinchoninic
acid protein assay. Denaturation of protein was obtained by
appropriately mixing with 5 mM β-mercaptoethanol (Thermo Fisher
Scientific, Inc.) in a water bath at 98°C for 10 min. A total of 20
µg protein (for each sample) was separated by 8% SDS-PAGE,
and then stained at room temperature for 1 h in 0.01% (w/v)
Coomassie Brilliant Blue G-250 (Thermo Fisher Scientific, Inc.),
4.7% (w/v) ethanol and 8.5% (w/v) phosphoric acid as previously
described (34), or transferred
onto 0.45 µm polyvinylidene fluoride membranes (Merck KGaA)
by wet electrophoretic transfer. For western blotting, the protein
blot was blocked with 5% skimmed milk for 2 h at room temperature
and incubated overnight at 4°C with the following primary
antibodies, according to manufacturer's protocol: Anti-CD63 (cat.
no. Ab134045; 1:500; Abcam, Cambridge, MA, USA) and anti-heat shock
protein 70 (anti-Hsp70; cat. no. Ab2787; 1:500; Abcam). Following
washing in 0.1% PBS-Tween 20, the protein blot was incubated with
horseradish peroxidase-conjugated goat anti-mouse IgG1 secondary
antibody (cat. no. SA00001-1; 1:5,000; ProteinTech, Chicago, IL,
USA). Subsequently, the protein blot was washed four times in 0.1%
PBS-Tween 20 on an orbital shaker. Enhanced chemiluminescence
mixture (Sigma-Aldrich; Merck KGaA) was then added to the blot, and
images were captured using a gel imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
RNA isolation and reverse
transcription-quantitative poly- merase chain reaction (RT-qPCR)
analysis
Total RNA was isolated using TRIzol Plus RNA
purification kit (Thermo Fisher Scientific, Inc.). RNA
concentration was measured with a NanoDrop® ND-1000
(Thermo Fisher Scientific, Inc.). Subsequently, reverse
transcription was performed using All-in-One™ miRNA First-Strand
cDNA Synthesis Kit (cat. no. QP013; GeneCopoeia Biosciences,
Guangzhou, China) according to manufacturer's protocol. Next, qPCR
was conducted according to the protocol described in the
All-in-One™ miRNA qPCR kit (cat. no. QP016; GeneCopoeia
Biosciences) in a 20 µl reaction tube. The thermocycling
conditions of qPCR were as follows: Initial denaturation at 95°C
for 10 min; followed by 40 cycles of denaturation at 95°C for 10
sec, annealing at 60°C for 30 sec and extension at 72°C for 38 sec.
The primers of miR-205, miR-7-5p and RNU6 were purchased as
ready-to-order specific primer pairs (sequences unavailable) from
GeneCopoeia, Inc. (Rockville, MD, USA). Amplification was performed
with an ABI-7500 machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The data of RT-qPCR were analyzed with the SDS
relative quantification software, version 2.2.2 (Thermo Fisher
Scientific, Inc.). Relative fold-changes in expression were
calculated using the 2−ΔΔCq method (35).
Statistical analysis
Values are presented as the mean ± standard
deviation. Statistical analysis was performed using IBM SPSS
software, version 20.0 (IBM Corp., Armonk, NY, USA). An unpaired
Student's t-test was used to compare the miRNA expression in
different groups. P<0.05 indicated that a difference was
statistically significant. All results have been verified three
times.
Results
Exosome purity and abundance
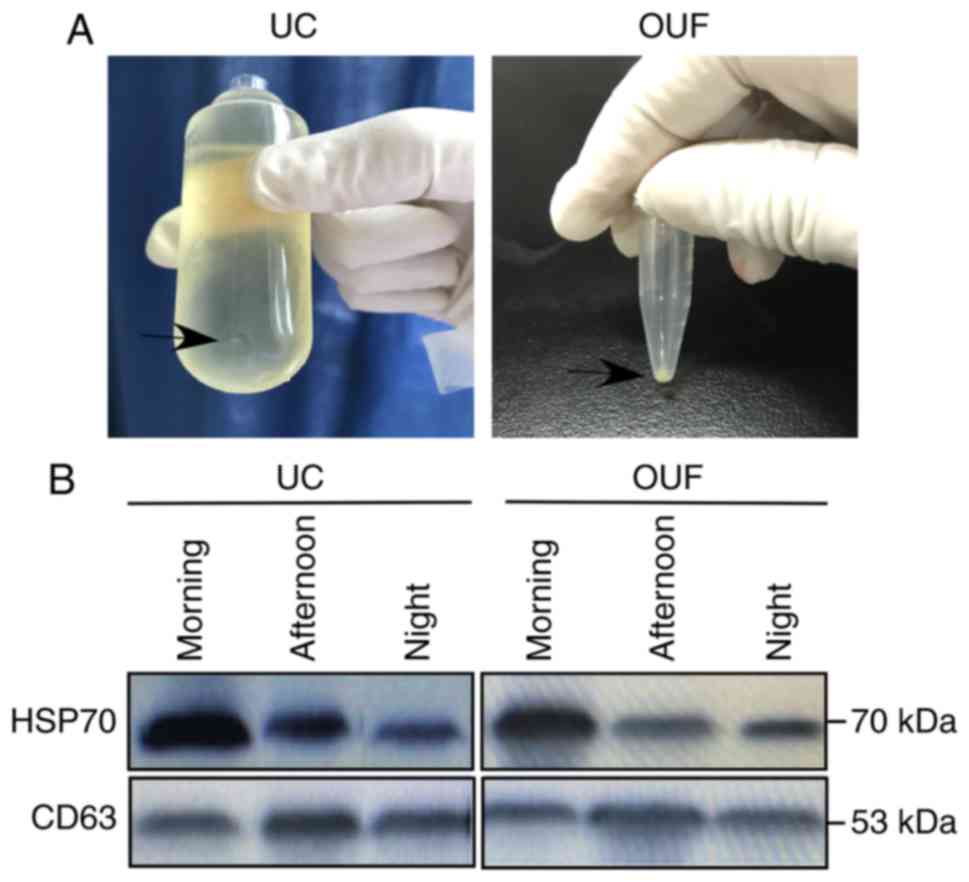
UC and OUF were used to isolate exosomes from the
same volume of urine. The results demonstrated that pellets
(denoted by the black arrow in Fig.
2A) isolated from the OUF group appeared to be larger in
comparison with those from the UC group. To ensure that the pellets
isolated by OUF and UC were indeed exosomes, they were identified
by western blotting, TEM and NTA. The results of western blotting
demonstrated the presence of canonical exosome proteins CD63 and
Hsp70 in the isolated sediments, which confirmed that OUF and UC
successfully isolated exosomes from the urine samples (Fig. 2B).
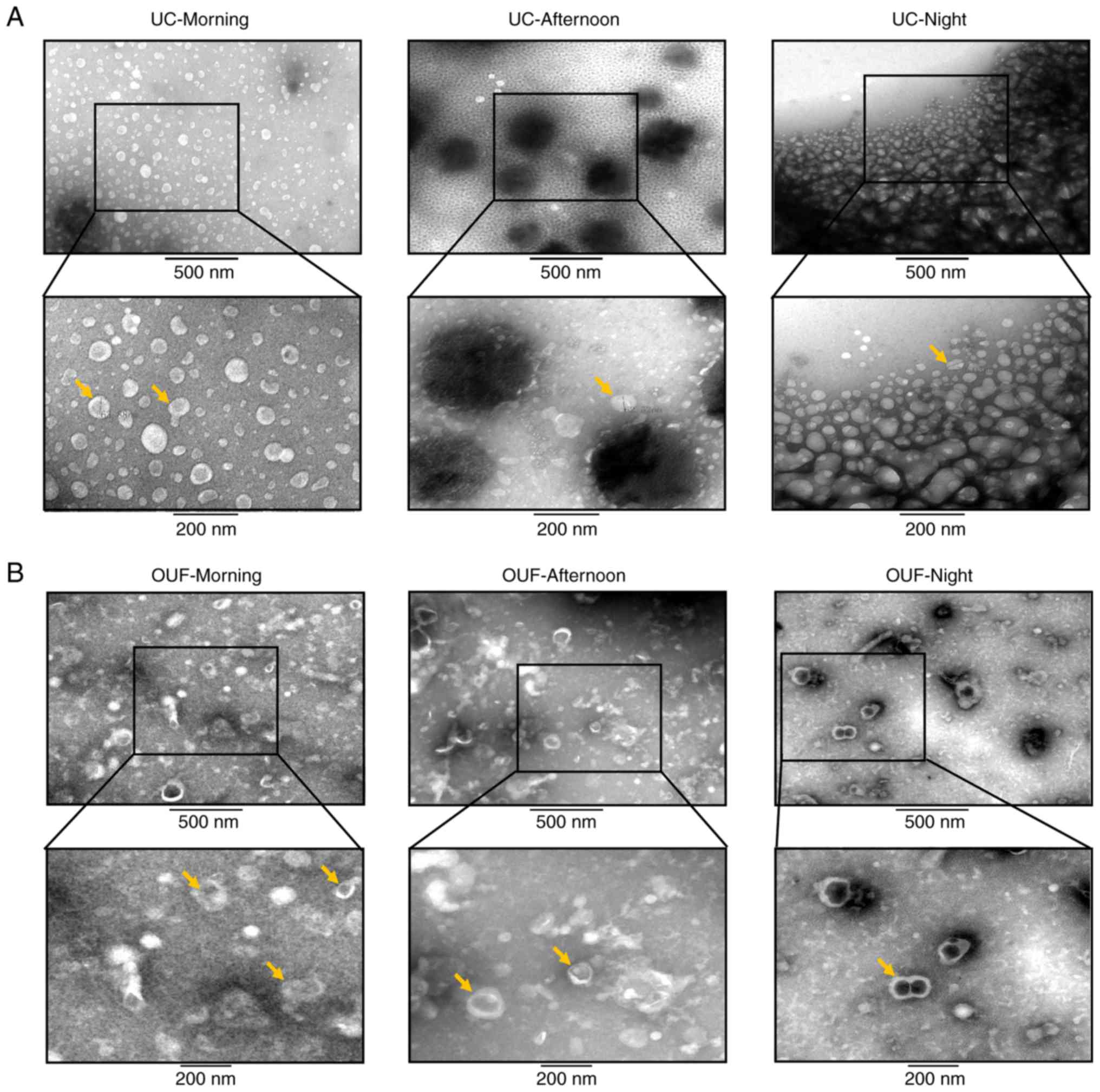
TEM analysis was used to observe the morphology and
size of exosomes. Notably, the TEM images of the OUF group revealed
that exosomes displayed a cup-shaped morphology, with relative
sizes ranging between 30 and 100 nm (Fig. 3A and B). However, fewer exosomes
were detected in the field of vision in the UC group when compared
with the OUF group. The majority of the vesicles isolated from the
UC group presented as larger particles that were white, without the
classical exosome morphology and with a diameter of 50-200 nm
(Fig. 3A). In addition, the TEM
images of exosomes isolated in the three different collection
periods were compared. The results indicated that the exosomes in
the morning group exhibited better morphology in both UC and OUF,
and it is thus proposed that morning would be the best time for
collecting urine samples for exosome isolation.
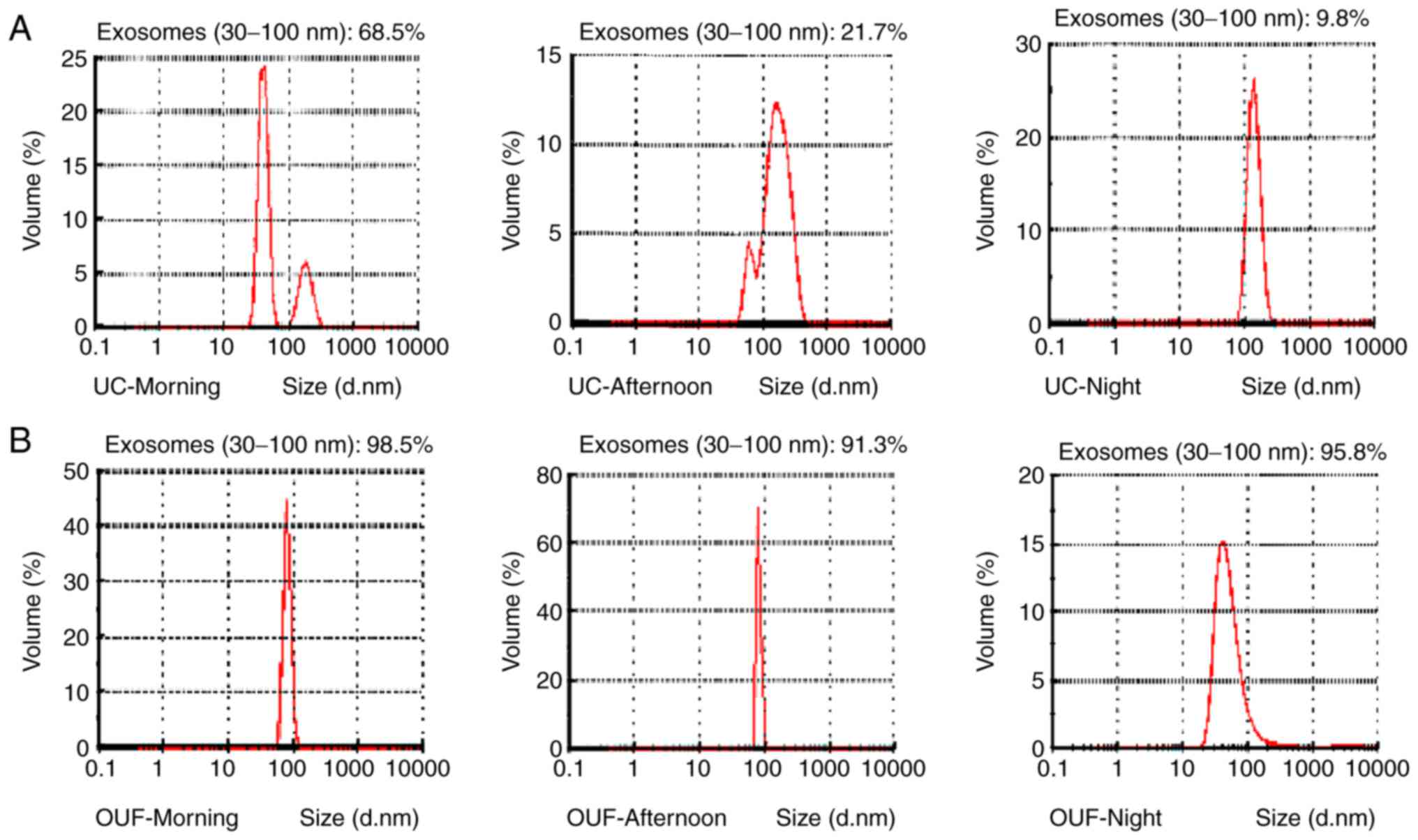
Next, NTA was used to measure the size distribution
of particles consistent with the size range of exosomes. The
results revealed that these vesicles ranged in size between 50 and
250 nm. NTA also demonstrated that the exosomes derived from the
morning group constituted a higher proportion of the total vesicles
as compared with those in the afternoon and evening groups in both
UC and OUF, which again proved that morning is the best time for
collecting urine samples for the isolation of exosomes (Fig. 4). Furthermore, as seen in Fig. 4, the exosomes (30-100 nm) isolated
by OUF constituted a significantly higher proportion of the total
vesicles as compared with those obtained from UC in all three time
periods (morning, 68.5 vs. 98.5%; afternoon, 21.7 vs. 91.3%;
evening, 9.8 vs. 95.8%; Fig. 4).
These results clearly suggested the existence of exosomes in urine
and the superiority of OUF over UC for isolating these
exosomes.
Stability of the biological function and
structural integrity of exosomes
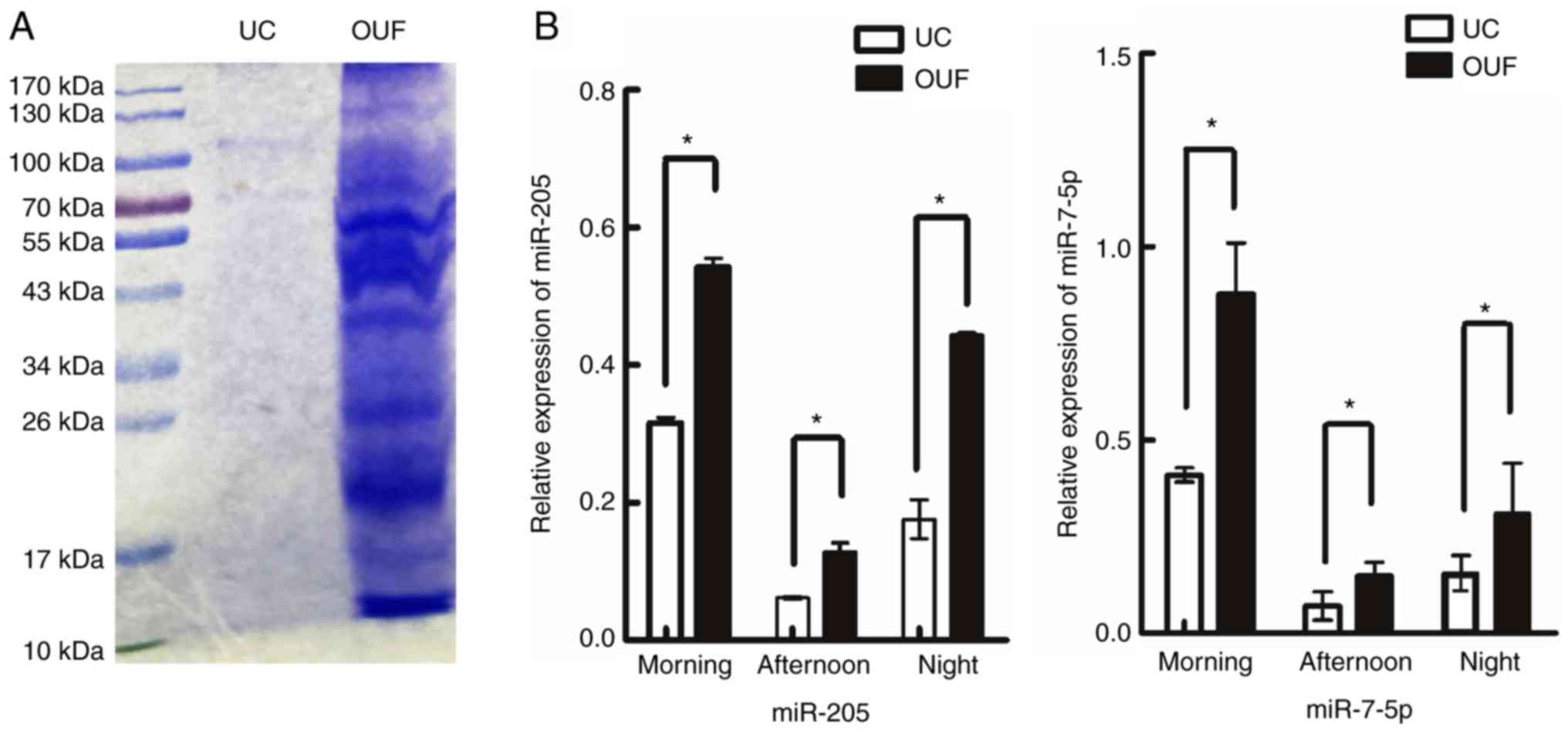
To visualize the difference in protein levels
between UC and OUF fractions, the same volumes of urine samples
isolated by these two protocols were treated under the same
conditions. A protein sample of the exosomes of approximately 20
µl was loaded onto a colloidal Coomassie-stained gel
(Fig. 5A). In the OUF group,
there was increased protein expression as compared with that in the
UC group under identical culture conditions (Fig. 5A).
Recent studies have demonstrated that
exosome-derived miRNAs mediate the communication between a tumor
and the surrounding microenvironment, and suggested that they are
potential biomarkers of cancer (26,36). In addition, Li et al
(27) indicated that the increase
of miR-205 was significantly correlated with poor survival outcome
in ovarian cancer. Furthermore, miR-7-5p has been found to be
downregulated in breast cancer and may function as a potential
prognostic biomarker of breast cancer (37). Therefore, in the present study,
exosomal miRNAs were purified, and RT-qPCR was performed to detect
the expression of these two miRNAs, hsa-miR-205 and hsa-miR-7-5p.
The results revealed that the expression levels of miR-205 and
miR-7-5p in urinary exosomes isolated by OUF were significantly
higher compared with those isolated by UC at all time points
(P<0.05; Fig. 5B).
Discussion
In recent decades, numerous studies illustrating the
structure and function of exosomes have been conducted. Early
studies favored the notion that exosomes may function as cellular
garbage bags storing excess material and have no physiological
functions (2-4). At present, increasing research
suggests that exosomes serve a significant role in cell-to-cell
interaction and have great potential as biomarkers for a number of
diseases. However, severe issues associated with the inefficiency
of nonstandardized exosome isolation protocols remain unresolved,
limiting associated research and applications. UC, the most widely
used exosome isolation strategy, has long been considered the gold
standard for this purpose (38,39). However, this method has various
procedural issues, including a long process, the need for costly
ultracentrifuges and continuous UC steps, which inevitably damage
the isolated exosomes. As a popular alternative protocol, the
synthetic polymer-based precipitation method is relatively simple
and produces a large amount of exosomes (40); however, the cost of this method is
high, and it usually provides exosomes with low purity and
stability. In the present study, we propose a simple and efficient
method for addressing these problems.
Urine samples were first centrifuged at 2,000 and
17,000 x g to remove cellular debris, bacteria, urinary casts and
the majority of the THP. The exosomal vesicles can be obtained by
direct UC at 200,000 x g in the UC group, however, the intense
centrifugal force may destroy the integrity of exosomes. To
minimize the damage to exosomes produced by the centrifugal force
as much as possible, a filtration step with a 0.22-µm filter
was used to purify SN2. Next, a dialysis membrane with MWCO of
10,000 kDa was introduced to concentrate the filtered fluids up to
1/50 of the initial volume. This concentration step was aimed to
reduce the consumption of ExoQuick-TC and the incubation time of
CSN and ExoQuick-TC mixture. Subsequently, ExoQuick-TC was
incubated with CSN to gently precipitate the exosomes from the
mixture (Fig. 1). The
aforementioned improvements included in the OUF method resulted in
the production of more exosomes, which were of higher purity and
biological stability, as demonstrated through various
characterization procedures.
The exosome marker proteins CD63 and Hsp70 were
detectable in the exosome pellets, indicating that UC and OUF were
both able to isolate a considerable amount of exosomes (Fig. 2B). However, according to the
results shown in Fig. 2, the
exosome pellets harvested from the OUF group were clearly larger in
comparison with those from the UC group (Fig. 2B). These results indicated that
use of the OUF method extracted a higher number of exosomes. For
further analysis of these two methods regarding the purity and
quantity of isolated exosomes, these exosomes were observed by TEM
and NTA, respectively. The results of TEM revealed that exosomes
had a clear cup-shaped morphology with a diameter of 100-130 nm
(Fig. 3A and B). Few typical
exosome-like vesicles were identified in the UC group, and the
majority of these particles were heterogeneous (50-200 nm), without
a classical exosome structure (Fig.
3A). By contrast, TEM images of the OUF group demonstrated a
high concentration of exosomes, which were relatively homogeneous
with a size of 100-130 nm (Fig.
3B). These results indicated that the purity of the exosomes
isolated by OUF was higher in comparison with that of UC-isolated
exosomes. Subsequently, NTA was applied to measure the proportion
of vesicles consistent with the size range of exosomes. This
revealed that the mean proportion of exosomes in the OUF group
(95.2%) was higher compared with that in the UC group (33.33%;
Fig. 4). This suggested that the
abundance of exosomes isolated by OUF was higher as compared with
that obtained by UC.
It is well known that exosomes containing specific
proteins and miRNA are likely to be carriers of information between
cells, and thus the stability of biological functions and
structural integrity of exosomes are very important for their
further study and clinical application. Therefore, in the current
study, the protein and miRNA expression levels in exosomes were
measured. The SDS-PAGE protein pattern in the OUF group exhibited
increased protein levels compared with those in the UC group
(Fig. 5A), while the quantities
of miR-205 and miR-7-5p in urinary exosomes isolated by OUF were
all significantly higher compared with those in UC (P<0.05;
Fig. 5B). These findings indicate
that the OUF method may prevent damage to the stability and
integrity of exosomes. Finally, the time and cost between UC and
OUF in the entire process of isolating exosomes were compared
(Table I). The results implied
that high-quality exosomes were obtained by OUF in a similar time
and lower cost than when UC was performed (Table I).
 | Table IComparative performance of UC and
OUF. |
Table I
Comparative performance of UC and
OUF.
| Method | UC | OUF |
|---|
| Detectable CD63 and
Hsp70 levels | Yes | Yes |
| Pellet size | Small | Large |
| TEM analysis | Less exosomes,
contains numerous non-exosome particles | More exosomes,
contains few non-exosome particles |
| NTA analysis | ~33.33% exosomes in
all vesicles | ~95.2% exosomes in
all vesicles |
| Protein expression
pattern | Low protein
levels | Increased protein
levels |
| Expression of
miR-205 and miR-7-5p | Low | High |
| Time consumed | 150 min | 152 min |
| Cost | Approximately ¥ 400
(60-ml sample)
(matched tube x2 = ¥ 100; UC time:1 h = ¥ 300) |
Approximately ¥ 240 (60-ml sample)
(ExoQuick-TC volume: 200 µl = ¥ 50; MWCO tube x2 = ¥ 180;
0.22 µm filter = ¥ 10) |
In conclusion, the present study successfully
developed a highly efficient method to isolate urinary exosomes
with high purity, production and biological stability. Compared
with conventional UC, OUF offers a simple alternative for
harvesting high-quality urinary exosomes using a simpler method.
Furthermore, the miRNA expression in OUF exosomes was high, which
may be suitable for the large-scale extraction of clinical urine
samples and subsequent experiments, such as small RNA sequencing
and RT-qPCR. It is noteworthy that the OUF approach is more
suitable for the isolation of exosomes from samples with a large
amount of liquid, such as urine or supernatant of cells, whereas it
may not suitable for blood or other specimens with less fluid,
which may inevitable have some sample lost in the procedure of
isolation. The development of a functional filter that can
concentrate and reduce the amount of sample lost is ongoing in our
laboratory.
Acknowledgments
We thank Liwen Bianji of Edanz Group China for
language editing the manuscript draft.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81172469) and
Technology Program of Changsha City Science Technology Bureau
(grant no. K1403050-31).
Availability of data and materials
The analyzed and/or datasets generated during the
study are available from the corresponding author on reasonable
request.
Authors' contributions
LH conceived and designed the study, performed the
experiments, processed the data and wrote the paper. DZ and JW
performed the experiments and processed the data. XW contributed to
the study design, processed the data, and reviewed and edited the
drafts of the paper. All authors provided help during the research,
including providing subject design, data acquisition and analysis,
article drafting and writing assistance. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Central South University (Changsha, China) and
conducted in adherence with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of
interest regarding the publication of this manuscript.
References
|
1
|
Trams EG, Lauter CJ, Salem N Jr and Heine
U: Exfoliation of membrane ecto-enzymes in the form of
micro-vesicles. Biochim Biophys Acta. 645:63–70. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harding C, Heuser J and Stahl P:
Endocytosis and intracellular processing of transferrin and
colloidal gold-transferrin in rat reticulocytes: Demonstration of a
pathway for receptor shedding. Eur J Cell Biol. 35:256–263.
1984.PubMed/NCBI
|
|
3
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
4
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao L, You B, Shi S, Shan Y, Zhang Q, Yue
H, Zhang J, Zhang W, Shi Y, Liu Y, et al: Metastasis-associated
miR-23a from nasopharyngeal carcinoma-derived exosomes mediates
angiogenesis by repressing a novel target gene TSGA10. Oncogene.
37:2873–2889. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Lu FB, Chen DZ, Wu JL, Hu ED, Xu
LM, Zheng MH, Li H, Huang Y, Jin XY, et al: BMSCs-derived
miR-223-containing exosomes contribute to liver protection in
experimental autoimmune hepatitis. Mol Immunol. 93:38–46. 2018.
View Article : Google Scholar
|
|
7
|
Rajendran L, Bali J, Barr MM, Court FA,
Krämer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G,
Wang J and Breakefield XO: Emerging roles of extracellular vesicles
in the nervous system. J Neurosci. 34:15482–15489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pathare G, Dhayat N, Mohebbi N, Wagner CA,
Cheval L, Neuhaus TJ and Fuster DG: Acute regulated expression of
pendrin in human urinary exosomes. Pflugers Arch. 470:427–438.
2018. View Article : Google Scholar
|
|
9
|
Bai R, Latifi Z, Kusama K, Nakamura K,
Shimada M and Imakawa K: Induction of immune-related gene
expression by seminal exosomes in the porcine endometrium. Biochem
Biophys Res Commun. 495:1094–1101. 2018. View Article : Google Scholar
|
|
10
|
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo
J, Li HM, Zhang WS, Chen CY and Xie H: Exosomes from human
umbilical cord blood accelerate cutaneous wound healing through
miR-21 3p-mediated promotion of angiogenesis and fibroblast
function. Theranostics. 8:169–184. 2018. View Article : Google Scholar :
|
|
11
|
Asea A, Jean-Pierre C, Kaur P, Rao P,
Linhares IM, Skupski D and Witkin SS: Heat shock protein-containing
exosomes in mid-trimester amniotic fluids. J Reprod Immunol.
79:12–17. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zomer A, Maynard C, Verweij FJ, Kamermans
A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J,
Ellenbroek SI, et al: In Vivo imaging reveals extracellular
vesicle-mediated phenocopying of metastatic behavior. Cell.
161:1046–1057. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Wang Y, Bai M, Wang J, Zhu K, Liu
R, Ge S, Li J, Ning T, Deng T, et al: Exosomes serve as
nanoparticles to suppress tumor growth and angiogenesis in gastric
cancer by delivering hepatocyte growth factor siRNA. Cancer Sci.
109:629–641. 2018. View Article : Google Scholar :
|
|
14
|
Ferguson Bennit HR, Gonda A, McMullen JRW,
Kabagwira J and Wall NR: Peripheral blood cell interactions of
cancer-derived exosomes affect immune function. Cancer
Microenviron. Mar 30–2018.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung BH, Ketova T, Hoshino D, Zijlstra A
and Weaver AM: Directional cell movement through tissues is
controlled by exosome secretion. Nat Commun. 6:71642015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armstrong EA, Beal EW, Chakedis J, Paredes
AZ, Moris D, Pawlik TM, Schmidt CR and Dillhoff ME: Exosomes in
pancreatic cancer: From early detection to treatment. J
Gastrointest Surg. 22:737–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui S, Cheng Z, Qin W and Jiang L:
Exosomes as a liquid biopsy for lung cancer. Lung Cancer.
116:46–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murakami T, Oakes M, Ogura M, Tovar V,
Yamamoto C and Mitsuhashi M: Development of glomerulus-, tubule-,
and collecting duct-specific mRNA assay in human urinary exosomes
and microvesicles. PLoS One. 9:e1090742014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fernández-Llama P, Khositseth S, Gonzales
PA, Star RA, Pisitkun T and Knepper MA: Tamm-Horsfall protein and
urinary exosome isolation. Kidney Int. 77:736–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pathare G, Dhayat NA, Mohebbi N, Wagner
CA, Bobulescu IA, Moe OW and Fuster DG: Changes in V-ATPase
subunits of human urinary exosomes reflect the renal response to
acute acid/alkali loading and the defects in distal renal tubular
acidosis. Kidney Int. 93:871–880. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kesimer M and Gupta R: Physical
characterization and profiling of airway epithelial derived
exosomes using light scattering. Methods. 87:59–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez ML, Khosroheidari M, Kanchi Ravi R
and DiStefano JK: Comparison of protein, microRNA, and mRNA yields
using different methods of urinary exosome isolation for the
discovery of kidney disease biomarkers. Kidney Int. 82:1024–1032.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao W, Zheng XL and Zhao SP: Exosome and
its roles in cardiovascular diseases. Heart Fail Rev. 20:337–348.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tran TH, Mattheolabakis G, Aldawsari H and
Amiji M: Exosomes as nanocarriers for immunotherapy of cancer and
inflammatory diseases. Clin Immunol. 160:46–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou J, Gong G, Tan H, Dai F, Zhu X, Chen
Y, Wang J, Liu Y, Chen P, Wu X and Wen J: Urinary microRNA-30a-5p
is a potential biomarker for ovarian serous adenocarcinoma. Oncol
Rep. 33:2915–2923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Hu K, Gong G, Zhu D, Wang Y, Liu H
and Wu X: Upregulation of MiR-205 transcriptionally suppresses
SMAD4 and PTEN and contributes to human ovarian cancer progression.
Sci Rep. 7:413302017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Li L, Li Z, Gong G, Chen P, Liu H,
Wang J, Liu Y and Wu X: The role of miR-205 in the VEGF-mediated
promotion of human ovarian cancer cell invasion. Gynecol Oncol.
137:125–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Musante L, Tataruch D, Gu D, Benito-Martin
A, Calzaferri G, Aherne S and Holthofer H: A simplified method to
recover urinary vesicles for clinical applications, and sample
banking. Sci Rep. 4:75322014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Colombo M, Moita C, van Niel G, Kowal J,
Vigneron J, Benaroch P, Manel N, Moita LF, Théry C and Raposo G:
Analysis of ESCRT functions in exosome biogenesis, composition and
secretion highlights the heterogeneity of extracellular vesicles. J
Cell Sci. 126:5553–5565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lv LL, Cao Y, Liu D, Xu M, Liu H, Tang RN,
Ma KL and Liu BC: Isolation and quantification of microRNAs from
urinary exosomes/microvesicles for biomarker discovery. Int J Biol
Sci. 9:1021–1031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tkach M, Kowal J, Zucchetti AE, Enserink
L, Jouve M, Lankar D, Saitakis M, Martin-Jaular L and Théry C:
Qualitative differences in T-cell activation by dendritic
cell-derived extracellular vesicle subtypes. EMBO J. 36:3012–3028.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Candiano G, Bruschi M, Musante L, Santucci
L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L and Righetti PG:
Blue silver: A very sensitive colloidal Coomassie G-250 staining
for proteome analysis. Electrophoresis. 25:1327–1333. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
36
|
Casadei L, Calore F, Creighton CJ,
Guescini M, Batte K, Iwenofu OH, Zewdu A, Braggio DA, Bill KL,
Fadda P, et al: Exosome-derived miR-25-3p and miR-92a-3p stimulate
liposar-coma progression. Cancer Res. 77:3846–3856. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Block I, Burton M, Sørensen KP, Andersen
L, Larsen MJ, Bak M, Cold S, Thomassen M, Tan Q and Kruse TA:
Association of miR-548c-5p miR-7-5p miR-210-3p miR-128-3p with
recurrence in systemically untreated breast cancer. Oncotarget.
9:9030–9042. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lai RC, Yeo RW, Tan KH and Lim SK:
Mesenchymal stem cell exosome ameliorates reperfusion injury
through proteomic complementation. Regen Med. 8:197–209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gould SJ and Raposo G: As we wait: Coping
with an imperfect nomenclature for extracellular vesicles. J
Extracell Vesicles. 2:2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu R, Greening DW, Zhu HJ, Takahashi N and
Simpson RJ: Extracellular vesicle isolation and characterization:
Toward clinical application. J Clin Invest. 126:1152–1162. 2016.
View Article : Google Scholar : PubMed/NCBI
|