Introduction
Acute lymphoblastic leukemia (ALL) is an aggressive
hematological malignancy that is mainly diagnosed in children. ALL
arises from the malignant transformation of T or B progenitor cells
in the bone marrow (BM) (1,2).
Current multi-agent chemotherapy regimens are highly effective in
patients with newly-diagnosed ALL (3); however, a significant number of
patients relapsed due to chemotherapy failure. Resistance to
chemotherapeutic agents is one of the major obstacles for the
successful treatment of ALL (3,4).
Previous studies showed that the mechanism of
resistance to chemotherapeutics agents is a complex network
involving multiple cellular and molecular mechanisms. In ALL, the
BM microenvironment provides growth and survival signals that may
confer resistance to chemotherapy (5-7).
Increasing evidence suggest that soluble factors in BM, including
extracellular matrix molecules, cytokines, and chemokines such as
osteopontin, CXCL12, and interleukin-6 (8,9),
provide a permissive environment for leukemogenesis and contribute
to drug resistance (10,11). Thus, studies on soluble factors in
BM provide a better understanding of the drug resistance of ALL and
facilitate the design of new treatments.
Cyr61/CCN1 is a secreted extracellular matrix (ECM)
protein, which is important for cell proliferation, survival,
adhesion, migration, and differentiation (12). As a secreted protein, the role of
Cyr61 has been extensively investigated in solid tumors, with
multiple studies showing that Cyr61 positively regulates tumor cell
growth and metastasis (13-16). More and more studies have shown
that Cyr61 confers on malignant cells resistance to
chemotherapeutic drugs in breast cancer, ovarian cancer, prostate
carcinoma and pancreatic ductal adenocarcinoma (17-20). Notably, Cyr61 is also involved in
stroma-induced chemo-resistance in acute myeloid leukemia (AML)
(21). In 2016, it was found that
the levels of Cyr61 are elevated in the plasma and BM supernatants
from patients with ALL compared with in samples from healthy
donors. It was also shown that increased Cyr61 promotes ALL cell
survival (22). However, whether
Cyr61 is involved in ALL cell resistance to chemotherapeutic drugs
has not yet been explored.
In the present study, it was revealed that Cyr61 is
highly expressed in BM mononuclear cells (BMMNCs) from patients
with ALL compared with those from healthy donors. Furthermore, the
role of Cyr61 in the chemotherapeutic sensitivity of ALL cells was
determined. Given that Cytosine arabinoside (Ara-C) is one of the
most important chemotherapeutic agents used to treat both children
and adults with acute leukemia (3), our study used Ara-C to evaluate the
role of Cyr61 in the chemotherapy resistance of ALL cells. The
present study found that Cyr61 could protect ALL cells from
Ara-C-induced apoptosis and that its effectiveness might be
partially due to the activation of the NF-κB pathway. Furthermore,
it was found that blocking the bioactivity of Cyr61 with an
anti-Cyr61 antibody 093G9 could improve the ALL cell response to
Ara-C. Therefore, the results indicated that Cyr61 may act as a
chemoprotective factor for ALL cells, and that targeting Cyr61
directly, or its relevant effector pathways, might improve the
clinical responses of patients with ALL.
Materials and methods
Reagents and chemicals
Recombinant human (rh) Cyr61 was obtained from
PeproTech, Inc., (Rocky Hill, NJ, USA), dissolved in PBS to a stock
concentration of 1 mg/ml and stored at 80°C until used. Ara-C was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany),
dissolved in DMSO to a stock concentration of 0.5 mM and stored at
−20°C until used. A mouse anti-human Cyr61 monoclonal antibody
(093G9) and a PEGFP-Cyr61 plasmid were kindly gifted by Dr Ningli
Li (Shanghai Jiao Tong University School of Medicine, Shanghai,
China). Rabbit anti-human monoclonal antibodies (mAb) against GAPDH
(1:1,000; cat. no. 8884S), Bcl-2 (1:1,000; cat. no. 4223S), NF-κB
p65 (1:1,000; cat. no. 4764S) and p-NF-κB p-p65 (1:1,000; cat. no.
3033S) used were all purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). An HRP-linked anti-rabbit IgG and HRP-linked
anti-mouse IgG were purchased from Cell Signaling Technology, Inc.
Pyrrolidine dithiocarbamate (PDTC; special inhibitor of NF-κB
activation) was purchased from Sigma-Aldrich (Merck KGaA).
Patients and specimens
All bone marrow (BM) aspirate samples were obtained
from Fujian Medical University Union Hospital (Fuzhou, China) from
July 2015 to October 2017. To analyze the level of Cyr61 in the BM
mononuclear cells (BMMNCs) from patients with ALL, BM samples from
newly diagnosed, non-treated patients with ALL (range, 6-37 years,
n=8) and healthy donors (range, 16-34, n=6) were collected
(Table I) and enriched for BMMNCs
using histopaque gradient centrifugation (density 1.077 g/ml;
Sigma-Aldrich; Merck KGaA) according to the manufacturer’s
instructions. The BM supernatant samples were obtained after
centrifugation of the total BM aspirates of consecutive patients
with ALL and stored at −80°C until used. These studies were
performed in accordance with the ethical guidelines under the
protocols approved by the Institutional Medical Ethics Review Board
of the Fujian Medical University Union Hospital. Informed consent
was obtained from all individual participants included in the
study.
 | Table IClinical characteristics of patients
with ALL included in the present study. |
Table I
Clinical characteristics of patients
with ALL included in the present study.
|
Characteristics | ALL (n=8) | Control (n=6) |
|---|
| Sex | | |
| Male | 3 | 3 |
| Female | 5 | 3 |
| Age (years) | 20.15±12.78 | 21.63±7.25 |
| Diagnosis | | |
| T-ALL | 2 | |
| B-ALL | 6 | |
Cell lines and culture conditions
Leukemia cell lines Jurkat and Nalm-6 cells were
kindly provided by Dr Qiang Chen (Shanghai Jiao Tong University
School of Medicine) and were maintained in RPMI-1640 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C and 5% CO2.
Evaluation of Cyr61’s effect on ALL cell
chemosensitivity and antibody neutralization assays
To address the effect of BM-derived Cyr61 on the
chemotherapeutic sensitivity to Ara-C of ALL cells, ALL cells were
cultured using BM supernatants from patients with ALL in which
overexpressed Cyr61 had been blocked with 1,000 pg/ml 093G9. A
murine isotype-matched antibody served as the control. Cells were
then treated with 1 µM Ara-C for 24 h and apoptotic cells were
quantified by Annexin V-FITC and PI double-staining kit (BD
Biosciences, San Jose, CA, USA).
Next, to explore rhCyr61’s effect on
chemosensitivity in ALL, Jurkat (5×106 cells/ml) and
Nalm-6 (1×107 cells/ml) cells were seeded into 24-well
plates (CoStar, Cambridge, MA, USA) and maintained in RPMI-1640
medium with 5% FBS. Jurkat and Nalm-6 were pre-incubated with
rhCyr61 at different concentrations for 24 h followed by treatment
with 1 µM Ara-C. After incubation for 24 h, cell apoptosis was
analyzed by Annexin V-FITC and PI double-staining kit (BD
Biosciences).
For the antibody blocking assay, rhCyr61 was
pre-incubated with a mouse anti-Cyr61 mAb (093G9) for 2 h prior to
adding to cell culture. A murine isotype-matched antibody served as
a control. After incubation for 24 h, Jurkat and Nalm-6 cells were
treated with 1 µM Ara-C for another 24 h. Cell apoptosis was
analyzed using an Annexin V-FITC and PI Double-Staining kit (BD
Biosciences).
Apoptosis assay
Cell apoptosis was measured according to the
manufacturer’s instruciton (BD Biosciences). The percentages of
cell apoptosis (FITC-positive) were analyzed by flow cytometry
using BD FACS Canto II flow cytometer and BD FACSDiva 6.0 software
(BD Biosciences).
Transfection and Ara-C induced apoptosis
assay
To construct Cyr61-overexpressing ALL cell models,
Jurkat and Nalm-6 cells were transfected with PEGFP-Cyr61 (the
plasmid carrying Cyr61 cDNA) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) in serum-free RPMI-1640 according
to the manufacturer’s instructions. ALL cells were transfected with
PEGFP-N3 (vector only) to generate matched control cells. After
transfection, cells were washed and then incubated in RPMI-1640
medium with 10% FBS for 48 h. For the apoptosis assay, the
transfected cells were treated with 1 µM Ara-C for 24 h. The
cells were then washed in 1X PBS and stained with Annexin V-PE (BD
Biosciences) according to the manufacturer’s instructions. The
percentages of apoptotic Jurkat or Nalm-6 cells (PE-positive) were
subsequently analyzed by flow cytometry using a BD FACS Canto II
flow cytometer and BD FACSDiva software 6.0 (BD Biosciences).
Real-time PCR
Jurkat and Nalm-6 were treated with Ara-C (1
µM) with or without rhCyr61 (100 ng/ml for Jurkat and 1,000
ng/ml for Nalm-6) for 8 h before RNA extraction. Total RNA was
extracted from specimens using a TriPure Isolation Reagent (Roche
Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s
instructions. Total RNA (1 µg) was reverse transcribed into
first strand cDNA using the RevertAid™ First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Inc.). Briefly, 1 µl of 50
µM oligo (dT)20 and 1 µl of 10 mM dNTPs mix were
added to the RNA, and the volume was adjusted to 11 µl using
RNase-free water. mRNA was converted to cDNA according to the kit
manufacturer’s instructions. Real-time PCR was performed using
SYBR®-Green Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer’s
instructions. The primers used in this study were as follows:
Bcl-2, forward, CTGGTGGGAGCTTGCATCAC; Bcl-2, reverse,
ACAGCCTGCAGCTTTGTTTC; BCL-xL, forward, TCAGGCTGCTTGGGATAAAGAT;
BCL-xL, reverse, AGAGGCTTCTGGAGGACATTTG; Bax, forward,
TGGAGCTGCAGAGGATGATTG; Bax, reverse, CCAGTTGAAGTTGCCGTCAGA; XIAP,
forward, TTGAGGAGTGTCTGGTAAG; XIAP, reverse, CCATTCGTATAGCTTCTTGT;
GAPDH, forward, CACATGGCCTCCAAGGAGTA; GAPDH, reverse,
TGAGGGTCTCTCTCTTCCTCTTGT. GAPDH was used as an internal control,
and the relative expression of each mRNA was analyzed using the
2−ΔΔCt method (23).
Probing of signaling pathways involved in
Cyr61-enhanced resistance to Ara-C in ALL cells
Approximately 2.5×105 cells/well Jurkat,
5×106 cells/well Nalm-6 were plated in 24-well plates
with 500 µl RPMI-1640 containing 5% FBS. Next, 4 µM
PDTC (an inhibitor of the NF-κB pathway) was added to the plates
along with rhCyr61 (100 ng/ml for Jurkat and 1,000 ng/ml for
Nalm-6). After pre-incubation for 24 h the plates were treated with
1 µM Ara-C. After incubation for 24 h, cell apoptosis was
measured using an Annexin V-FITC and PI double-staining kit (BD
Biosciences).
Western blot analysis
Protein immune blotting was performed as described
previously (24). Briefly, cells
were lysed with cell lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer’s
instructions and protein concentration was detected using a Bio-Rad
Protein Assay kit (Bio-Rad, Hercules, CA, USA). Proteins were
separated using a pre-cast 10% SDS-PAGE gel and subsequently
transferred onto a PVDF membrane (EMD Millipore, Billerica, MA,
USA) at 90 V for 90 min. The blots were blocked with 5% BSA for 1 h
at room temperature with gentle shaking, and then, respectively,
probed with rabbit anti-human mAbs against GAPDH and Bcl-2
(1:1,000) or mouse anti-human mAbs against Cyr61, with gentle
shaking overnight at 4°C overnight and subsequently rinsed with
PBS. The blots were then incubated with an HRP-linked anti-rabbit
IgG (1:2,000) or HRP-linked anti-mouse IgG (1:2,000) for 1 h at
room temperature with gentle shaking. After three rounds of washing
with PBS, the target proteins were examined with an ECL system (EMD
Millipore) and visualized with autoradiography film. The
housekeeping protein GAPDH was selected as an internal control for
equal protein loading.
Statistical analysis
Unless otherwise indicated, the results are
expressed as the mean ± standard error of the mean (SEM).
Statistical analyses were performed with SPSS software version 18.0
(SPSS Inc., Chicago, IL, USA). The difference in Cyr61 expression
between patients with ALL and healthy donors was analyzed by the
non-parametric Mann-Whitney U test. Statistical comparisons of
means between two groups were analyzed using the Student’s t-test.
Comparisons among multiple groups were analyzed using one-way ANOVA
with a post hoc SNK test for comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Level of Cyr61 expression in ALL cell
lines and ALL bone marrow samples
It was previously observed that Cyr61 is elevated in
both the plasma and BM supernatants from patients with ALL. In this
study, the levels of Cyr61 protein were examined in the BMMNCs
derived from eight patients with ALL and six healthy donors via
western blotting, and the results showed that Cyr61 levels in the
BMMNCs derived from patients increased to varying levels compared
with those derived from healthy donors (Fig. 1A). Further analysis showed that
Cyr61 protein was expressed in both Jurkat and Nalm-6 cells, and
that the level of Cyr61 protein in Jurkat cells was approximately
1.5-fold higher than in Nalm-6 cells (P<0.01, Student’s t-test;
Fig. 1B). Taken together, these
data indicate that the level of Cyr61 is upregulated in ALL bone
marrow samples, and that ALL cell lines (Jurkat and Nalm-6) also
express the Cyr61 protein.
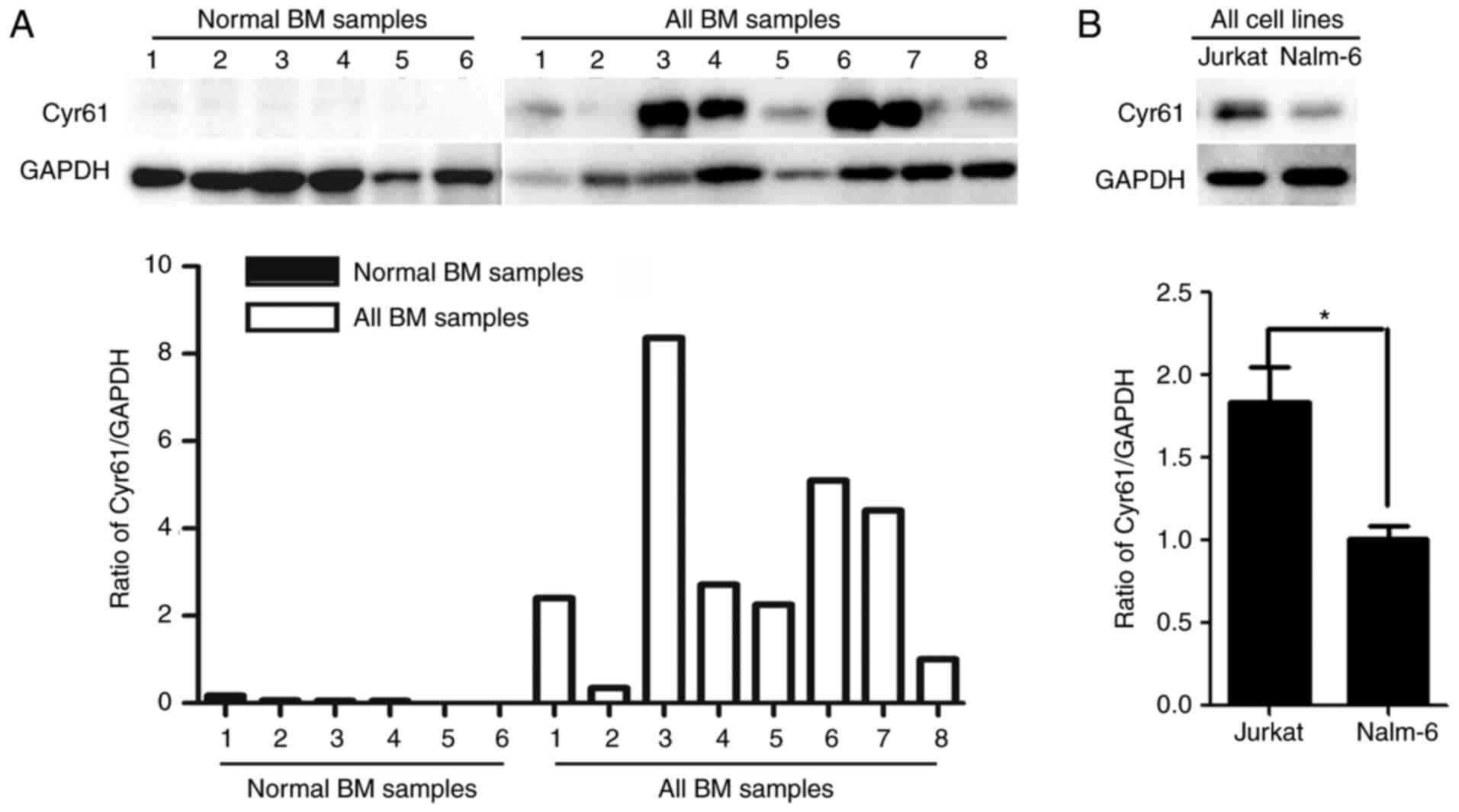 | Figure 1Level of Cyr61 expression in ALL cell
lines and ALL bone marrow samples. (A) Cyr61 protein levels were
determined in the BMMNCs derived from healthy donors and ALL
samples by western blotting, and the ratio of Cyr61/GAPDH in the
last ALL BM sample was taken as the control, and the ratio of
Cyr61/GAPDH was set as 1, to calculate the relative expression of
Cyr61 in other samples. (B) The level of Cyr61 protein was
determined in two ALL cell lines (Jurkat and Nalm-6) by western
blotting, and the ratio of Cyr61/GAPDH in Nalm-6 cells was taken as
the control, in which the ratio of Cyr61/GAPDH was set as 1, to
calculate the relative expression of Cyr61 in Jurkat cells.
*P<0.05. Cyr61, cysteine-rich 61; ALL, acute
lymphoblastic leukemia; BMMNCs, bone marrow mononuclear cells; BM,
bone marrow; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
Cyr61 effectively decreases Ara-C-induced
apoptosis in ALL cells
Previous studies have shown that Cyr61 is involved
in drug resistance by decreasing chemotherapeutic drug-induced
apoptosis in ovarian cancer, breast cancer and acute myeloid
leukemia cells (21,25,26). To explore the role of Cyr61 in the
drug resistance of ALL, Jurkat (T-ALL cell lines) and Nalm-6 (B-ALL
cell lines) cells were incubated with BM supernatants from newly
diagnosed patients with ALL with no treatment. Cell cultures were
subjected to Ara-C treatment in the presence or absence of an
anti-Cyr61 monoclonal antibody (mAb) 093G9. As shown in Fig. 2A, anti-Cyr61 monoclonal antibody
(mAb) 093G9 could increase the Ara-C-induced Jurkat and Nalm-6 cell
apoptosis, suggesting that the endogenous Cyr61 from ALL patient BM
could decrease ALL cell apoptosis induced by Ara-C. Next, Jurkat
and Nalm-6 cells were transiently transfected with a
Cyr61-expressing plasmid (PEGFP-Cyr61) to overexpress Cyr61 in ALL
cells. Transfection with PEGFP-Cyr61 significantly increased the
level of Cyr61 protein in both Jurkat and Nalm-6 cells (data not
shown). As expected, Ara-C-induced apoptosis was significantly
decreased in Cyr61-overexpressing (PEGFP-Cyr61) ALL cells (Fig. 2B), suggesting that autocrine
secretion of Cyr61 could confer resistance to Ara-C-induced
apoptosis. To further address Cyr61’s effect on the
chemosensitivity of ALL cells, Jurkat and Nalm-6 cells were treated
with exogenous Cyr61 (rhCyr61) at different concentrations, and
cellular sensitivity to Ara-C-induced apoptosis was subsequently
examined. As shown in Fig. 2C,
exogenous Cyr61 also significantly downregulated the level of
Ara-C-induced apoptosis in Jurkat and Nalm-6 cells in a
dose-dependent manner. Then, Jurkat and Nalm-6 cells were treated
with an anti-Cyr61 monoclonal antibody 093G9 to block rhCyr61
function, and the apoptosis induced by Ara-C were analyzed. The
results showed that exposure to 093G9 significantly sensitized both
Jurkat and Nalm-6 cells to Ara-C treatment, resulting in increased
apoptosis (Fig. 2D). Taken
together, these results suggest that Cyr61 has an important role in
the resistance of ALL cells to Ara-C.
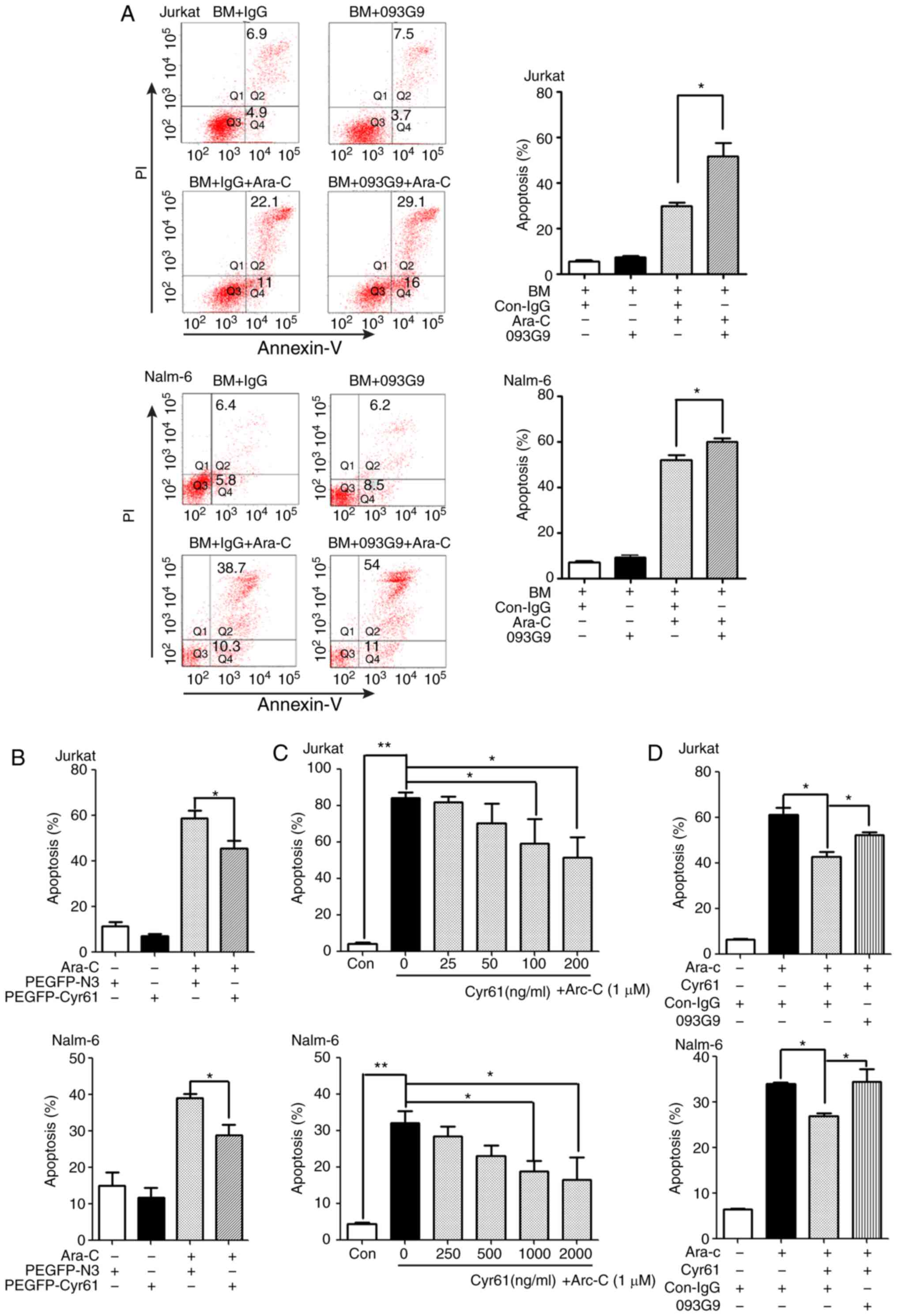 | Figure 2Cyr61 effectively decreases
Ara-C-induced apoptosis in ALL cells. (A) Jurkat and Nalm-6 cells
were incubated in BM supernatants (Cyr61 concentration is 185
pg/ml) from patients with ALL with or without 1,000 pg/ml
anti-human Cyr61 monoclonal antibody (093G9) for 24 h, followed by
exposure to 1 µM Ara-C. After incubation for 24 h, cells
were collected and stained with Annexin V-FITC/PI and the
percentages of apoptotic cells (FITC-positive) were measured using
flow cytometric analysis. A murine isotype-matched antibody
(con-IgG) served as a control. (B) Jurkat and Nalm-6 cells were
transfected with a Cyr61 expression plasmid (PEGFP-Cyr61) and an
empty plasmid (PEGFP-N3) for 48 h followed by exposure to 1
µM Ara-C for 24 h. Cells were then collected and stained
with Annexin V-PE. The percentages of apoptotic cells (PE-positive)
were measured with e flow cytometric analysis. (C) Jurkat and
Nalm-6 cells were pre-incubated with increasing concentrations of
rhCyr61 for 24 h, followed by exposure to 1 µM Ara-C for 24
h. Cell apoptosis was determined by flow cytometric analysis using
Annexin V-FITC /PI double staining. (D) Cyr61-decreased apoptosis
of Ara-C-induced ALL cells was restored by 093G9. rhCyr61 (100
ng/ml Cyr61 in Jurkat; 1,000 ng/ml Cyr61 in Nalm-6) were
pre-incubated with a mouse anti-Cyr61 mAb (093G9) (500 ng/ml in
Jurkat; 5,000 ng/ml in Nalm-6) for 2 h prior to adding to cell
culture. A murine isotype-matched antibody (con-IgG) served as a
control. After incubation for 24 h, Jurkat and Nalm-6 cells were
treated with 1 µM Ara-C for another 24 h. Cell apoptosis was
analyzed using an Annexin V-FITC/PI double-staining kit. Data are
expressed as the mean percentage of apoptotic cells ± SEM of at
least three independent experiments in triplicate.
*P<0.05, **P<0.01. Cyr61, cysteine-rich
61; ALL, acute lymphoblastic leukemia; Ara-C, cytosine arabinoside;
PI, propidium iodide; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
Exogenous Cyr61 upregulates Bcl-2 in
Ara-C-treated ALL cells
Since apoptosis is one of the key mechanisms of
cytotoxicity mediated by chemotherapeutic drugs, the proteins of
the Bcl-2 family have important roles in the regulation of
apop-tosis (27,28). Thus, to investigate the underlying
mechanism of Cyr61 induced drug resistance, real-time PCR and
western blotting were used to analyze whether Cyr61 has an effect
on Bcl-2 family molecules (Bcl-2, BCL-xL, Bax and XIAP) in
Ara-C-treated ALL cells. The result showed that exogenous Cyr61
up-regulated the level of Bcl-2 mRNA in Ara-C-treated ALL cells
(Fig. 3A, Jurkat cells,
P<0.05, Nalm-6 cells, P<0.05, Student’s t-test), but had
little effect on BCL-xL, Bax, and XIAP mRNA expression.
Additionally, it was found that exogenous Cyr61 could increase the
level of Bcl-2 protein in Ara-C-treated ALL cells (Fig. 3B, Jurkat cells, P<0.05, Nalm-6
cells, P<0.05, calculated by Student’s t-test). Together, these
results suggest that the existence of exogenous Cyr61 can
desensitize Ara-C treatment of ALL cells, likely through
upregulating Bcl-2 levels.
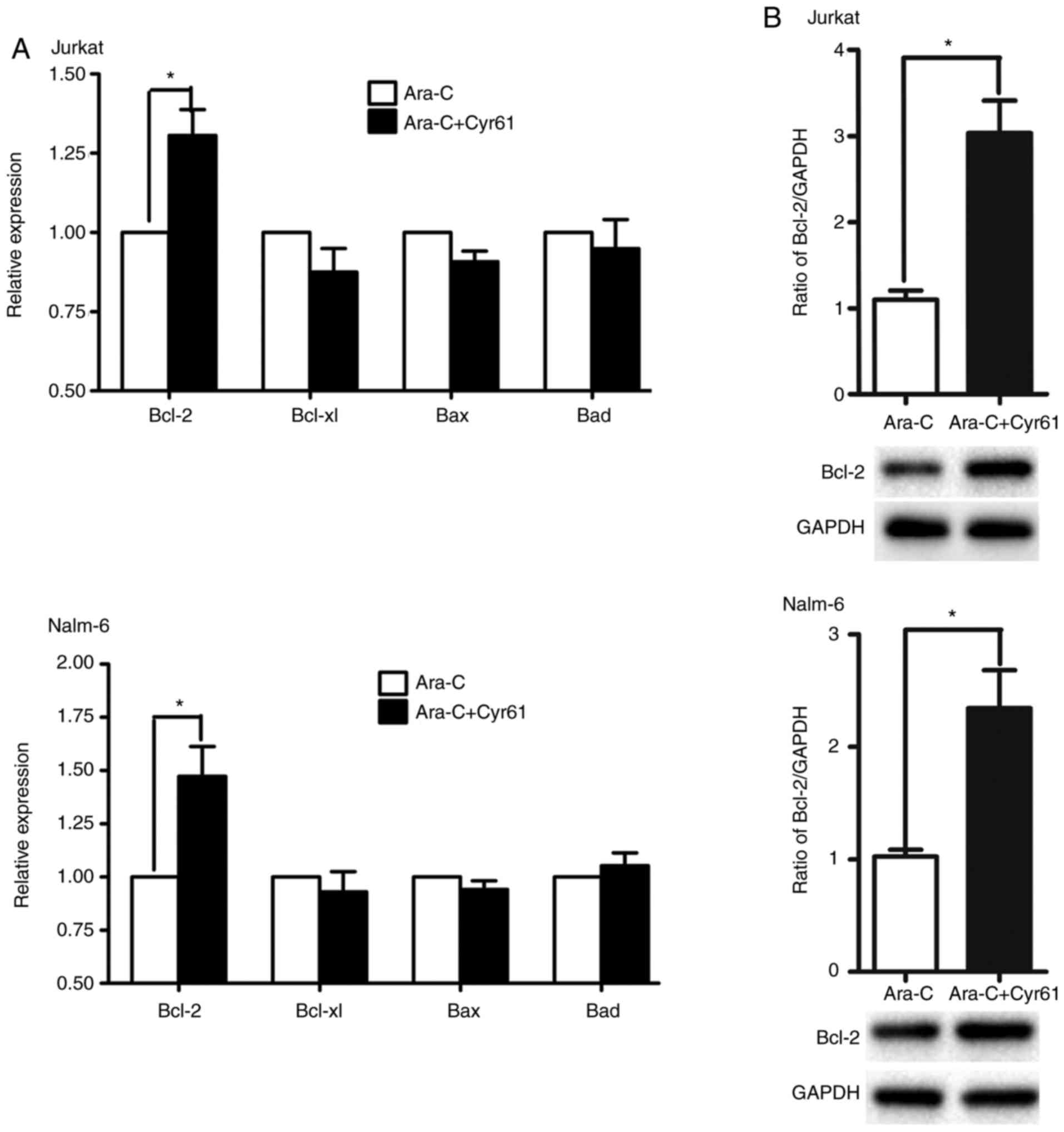 | Figure 3Exogenous Cyr61 upregulates Bcl-2 in
Ara-C-treated ALL cells. (A) Jurkat and Nalm-6 cells were co-treat
with Cyr61 (100 ng/ml for Jurkat cells; 1,000 ng/ml for Nalm-6
cells) and 1 µM Ara-C for 8 h, and Bcl-2, BCL-xL, Bax, and
XIAP mRNA expression were examined by real-time PCR. The expression
levels of target genes were calculated with the 2−ΔΔCt
method, employing GAPDH as an internal control. (B) Jurkat and
Nalm-6 cells were pre-incubated (24 h) with Cyr61 (100 ng/ml for
Jurkat cells; 1,000 ng/ml for Nalm-6 cells) followed by addition
with 1 µM Ara-C for 24 h. The relative level of Bcl-2
protein was examined by western blotting with the indicated
antibodies, and the Ara-C-treated group was taken as the control
sample, in which the ratio of Bcl-2/GAPDH was set as 1, to
calculate the relative expression of Bcl-2. Data are expressed as
the mean ± SEM of at least three independent experiments in
triplicate. *P<0.05. Cyr61, cysteine-rich 61; ALL,
acute lymphoblastic leukemia; Ara-C, cytosine arabinoside; Bcl-2,
B-cell lymphoma-2; BCL-xL, B-cell lymphoma-extra large; Bax,
Bcl-2-associated X protein; XIAP, X-linked inhibitor of apoptosis
protein; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
Exogenous Cyr61 decreases Ara-C-induced
apoptosis through the NF-κB signaling pathway
Several reports have observed constitutive NF-κB
activation in ALL cells (29-31); this pathway could be activated by
Cyr61, resulting in cellular proliferation and chemotherapy
resistance in ovarian and breast cancer cells. To determine whether
the NF-κB pathway is involved in the anti-apoptotic function of
Cyr61 in Ara-C-treated ALL cells, we evaluated the profile of the
NF-κB pathway using known inhibitors of this pathway, including
PDTC (an inhibitor of NF-κB activation). The results showed that
PDTC could significantly increase Ara-C-induced apoptosis in
Cyr61-treated ALL cells and PDTC alone have no effect on the
apoptosis of ALL cells (Fig. 4A).
Moreover, Cyr61 treatment led to a dramatic increase in the
phosphorylation of NF-κB but not its overall expression in both
Jurkat and Nalm-6 cells (Fig.
4B), indicating Cyr61 could activate NF-κB pathway in Jurkat
and Nalm-6 cells. Taken together, these results suggest that Cyr61
decreases Ara-C-induced apoptosis via the NF-κB pathway.
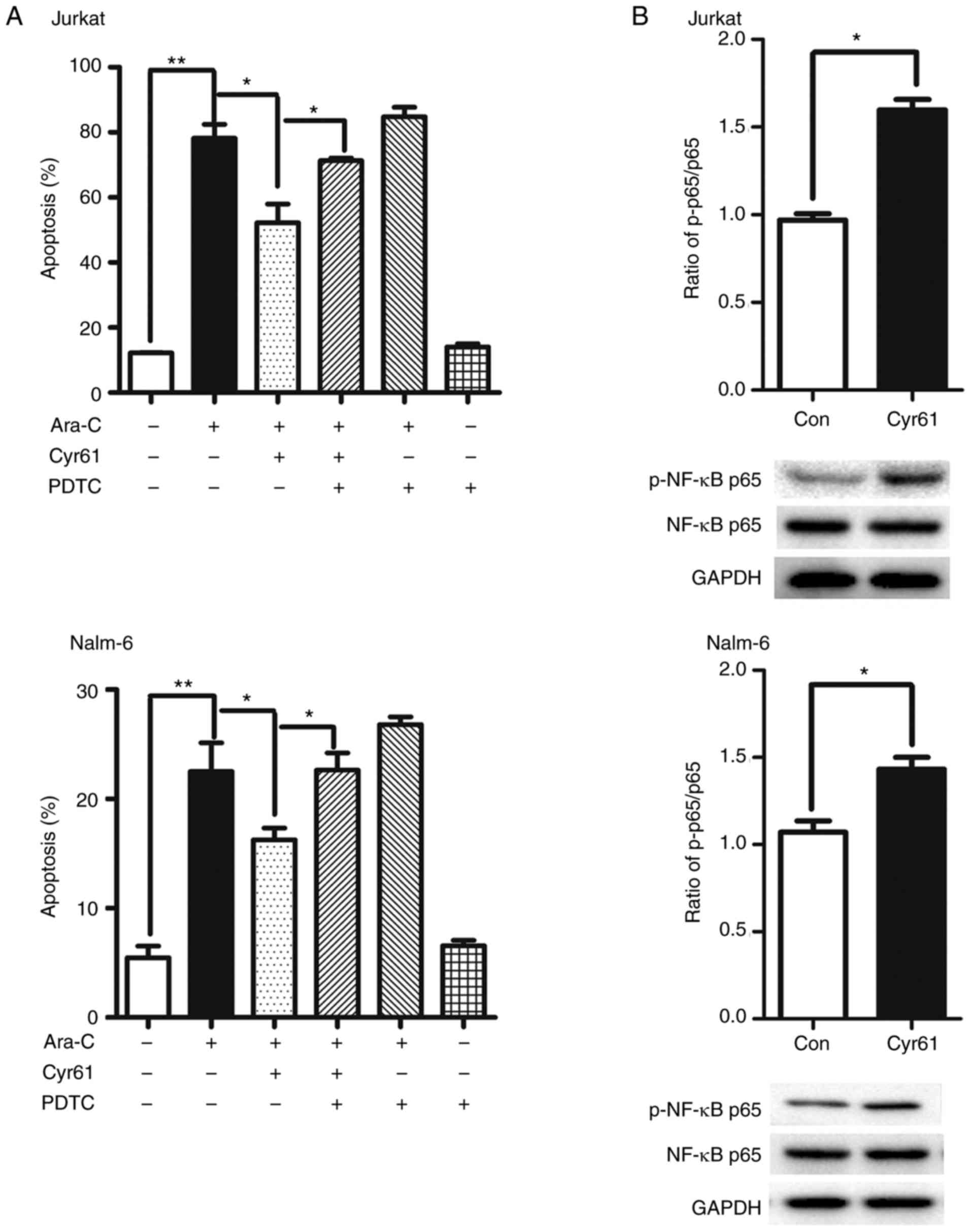 | Figure 4Exogenous Cyr61 decreases
Ara-C-induced apoptosis through the NF-κB signaling pathway. (A)
Jurkat and Nalm-6 cells were pre-incubated with 4 µM PDTC in
association with Cyr61 (100 ng/ml for Jurkat cells; 1,000 ng/ml for
Nalm-6 cells) for 24 h and then exposure to 1 µM Ara-C for
24 h. Cell apoptosis was analyzed using an Annexin V-FITC/PI
double-staining kit. (B) Jurkat and Nalm-6 cells were incubated
with Cyr61 (100 ng/ml for Jurkat cells; 1,000 ng/ml for Nalm-6
cells) for 10 min, and overall expression and phosphorylation of
NF-κB were examined by western blotting. The untreated group was
taken as the control sample, in which the ratio of Cyr61/GAPDH was
set as 1, to calculate the relative phosphorylation of NF-κB. Data
are expressed as the mean ± SEM of at least three independent
experiments in triplicate. *P<0.05,
**P<0.01. Cyr61, cysteine-rich 61; ALL, acute
lymphoblastic leukemia; Ara-C, cytosine arabinoside; PDTC, ammonium
pyrrolidinedithiocarbamate; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
Ara-C treatment increases the production
of Cyr61 in ALL cells
To explore whether Ara-C can affect the production
of Cyr61 in ALL cells, Jurkat and Nalm-6 cells were exposed to
Ara-C for 24 h. Western blotting was used to analyze the expression
profiles of Cyr61 in ALL cells. As shown in Fig. Ara-C could
increase the production of Cyr61 in ALL cells.
Discussion
In this study, we found that Cyr61 was overexpressed
in ALL BMMNCs. Furthermore, we observed that Cyr61 effectively
decreased Ara-C-induced apoptosis in ALL cells, indicating
chemoprotective effects of Cyr61 in ALL treatment.
Cyr61 is a secreted ECM protein, which is not only
important for cell proliferation, survival, and migration, but also
drug resistance in various tumors (12). It was recently reported that the
level of Cyr61 is increased in BM supernatants from patients with
ALL, and this change could promote ALL cell survival (22). Previous studies showed that BM
stromal cells are the major source of Cyr61 (21,32). Our current study showed that Cyr61
was overexpressed not only in the BMMNCs from patients with ALL,
but also two ALL cell lines. It is speculated that, in addition to
stromal cells, ALL cells could also be one of the sources
generating Cyr61 in the bone marrow in an autocrine manner.
Although multi-agent chemotherapy regimens are
highly effective for patients with ALL, some responding patients
eventually became refractory to initial therapy (3). Resistance to chemotherapeutic agents
is a significant clinical problem for the successful treatment of
leukemia. More studies have shown that the BM microenvironment
contributes to leukemia cell resistance to chemotherapeutic agents.
However, no study has yet explored the role of Cyr61 in ALL drug
resistance, to the best of our knowledge.
Induction of apoptosis is a critical mechanism of
cytotoxicity mediated by chemotherapeutic drugs, and resistance to
apoptosis is a major obstacle in chemotherapy treatment (33,34). In the present study, it was found
that BM-derived Cyr61 could decrease Ara-C-induced apoptosis in
Jurkat and Nalm-6 cells, and forced overexpression of Cyr61 in ALL
cells enhanced their resistance to Ara-C-induced apoptosis,
possibly through the autocrine secretion of Cyr61 into the
microenvironment. In addition, it was observed that recombinant
human Cyr61 increased the resistance of ALL cells to Ara-C, and
that this effect was antagonized by the anti-Cyr61 antibody 093G9.
Furthermore, it was observed that Jurkat cells (T-ALL cell lines)
were more sensitive to the chemoprotective effects of Cyr61 than
Nalm-6 cells (B-ALL cell lines), indicating that Cyr61 has
differential chemoprotective effects on diverse cell types. These
findings demonstrate, for the first time, that Cyr61 decreases the
sensitivity of ALL cells to Ara-C, and that inhibition of the
bioactivity of Cyr61 restores ALL cell response to Ara-C. The
findings reported herein are consistent with previous results that
Cyr61 decreases the apoptosis of tumor cells, leading to
chemotherapy resistance in breast cancer, ovarian cancer and acute
myeloid leukemia (21,25,26). Therefore, Cyr61 may be one of the
factors leading to drug resistance of ALL, and blocking pathways
involved with Cyr61 function could be used for treating relapsed
ALL. These results provide further evidence that BM
microenvironment-derived soluble factors have important roles in
the development and therapeutic response of leukemia cells.
It is well known that the Bcl-2 family of proteins
are important apoptosis regulators that have essential roles in the
apoptosis induced by chemotherapeutic drugs (27,28). To investigate the mechanism
underlying Cyr61-induced drug resistance, the influence of
exogenous Cyr61 on the expression of Bcl-2, BCL-xL, Bax, and XIAP
was evaluated as a possible mechanism for Cyr61-induced Ara-C
resistance. The results showed that Cyr61 could increase Bcl-2
production without affecting the expression levels of BCL-xL, Bax,
and XIAP in Ara-C-treated ALL cells. Considering that Bcl-2 is an
anti-apoptotic protein able to inhibit apoptosis, these findings
suggest that the Bcl-2 pathway is involved in Cyr61-induced Ara-C
resistance of ALL cells. Furthermore, it was found that
Cyr61-induced Bcl-2 production is higher in Jurkat cells than in
Nalm-6 cells, which may be one of the reasons why Jurkat cells were
more sensitive to Cyr61 chemoprotective effects than Nalm-6
cells.
Previous studies have shown that NF-κB is activated
downstream of Cyr61, conferring malignant cell resistance to
chemotherapy (17,18). As expected, the NF-κB pathway also
contributed to Cyr61-mediated ALL cell resistance to Ara-C. The
findings reported herein are consistent with those of several
previous reports in which Cyr61 activates the NF-κB signaling
pathway, and subsequently confers resistance to certain
chemotherapeutic drugs in breast cancer and ovarian cancer
(17,18). Numerous studies have demonstrated
that the Bcl-2 family and NF-κB proteins are closely associated
with cell apoptosis (35-37). Notably, our previous studies found
that the NF-κB signaling pathway is involved in Cyr61-induced Bcl-2
production in ALL cells (22). On
the basis of these results, NF-κB proteins may be upstream
controllers of Bcl-2 production in Cyr61-induced ALL cell
resistance to Ara-C.
The ability of cells to counteract stressful
conditions usually elicits the activation of pro-survival pathways
and the production of molecules with antioxidant and anti-apoptotic
activities to sustain cell survival. For example, Cyr61 is found to
be markedly increased in prostate carcinoma PC-3 cells in response
to N-acetylcysteine induced cytotoxicity, and are beneficial for
cell survival and anti-apoptosis under a cytotoxic microenvironment
(19). In the present study, the
results showed that Ara-C treatment markedly increased the levels
of Cyr61 in Jurkat and Nalm-6 cells. Therefore, it is speculated
that this is a part of the mechanism of ALL cell resistance to
Ara-C. Thus, the significance and mechanism of Ara-C-induced Cyr61
expression need to be further studied.
There are several limitations to this study. First,
the results rely solely on one chemotherapy drug (Ara-C). However,
there are many alternative chemotherapeutic drugs commonly used for
ALL treatment, such as vincristine, daunorubicin, and
dexamethasone. The role of Cyr61 in the chemosensitivity of ALL
cells to other drugs remains unknown and needs to be elucidated.
Second, the study on the Cyr61/NF-κB signaling pathway was
performed in vitro and thus lacks certain components of the
BM microenvironment; further study should be conducted to elucidate
the mechanism underlying Cyr61-mediated ALL cell resistance to
Ara-C in vivo.
In the present study, the results showed that Cyr61
was highly expressed in BMMNCs from patients with ALL, and elevated
Cyr61 levels conferred ALL cells with resistance to Ara-C-induced
apoptosis, partially via the activation of the NF-κB pathway. The
present study indicates, for the first time, that Cyr61 may act as
a chemoprotective factor for ALL cells, and that targeting Cyr61
directly or its relevant effector pathways might improve the
clinical responses of patients undergoing treatment for ALL.
Funding
This study was supported by the Fujian Medical
Innovation Project (grant no. 2017-CX-20), Fujian Province Joint
Funds for the Innovation of Science and Technology (grant no.
2017Y9051), the Natural Science Foundation of Fujian Province
(grant no. 2016J01569), the Training Project for Young and
Middle-aged Core Talents in Health System of Fujian Province (grant
nos. 2016-ZQN-31 and 2018-ZQN-69), the National Natural Science
Foundation of China (grant no. 81700098), the Fujian Provincial Key
Special Projects (grant nos. 2016Y9032 and 2016B041), the
Construction Project of Fujian Medical Center of Hematology (grant
no. Min201704), the National Key R&D Program of China (grant
no. 2016YFC0902800), the National Natural Science Foundation of
China (grant no. 81470326), Fujian Provincial Public Health Project
(grant no. WKJ2016-2-06), National and Fujian Provincial Key
Clinical Specialty Discipline Construction Program, P. R.C (grant
no. 2016-682) and the Fujian Provincial National Health and Family
Planning Commission Project for Young (grant no. 2016-1-45).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
XZ and JH conceived the research and performed
overall supervision in the study. YC, CW, XZ, YS, ZL, YK, PL, CZ,
QH and TH performed the experiments. XZ, CW, YS, YC and JH
performed data analysis. XZ, JH, YC and CW wrote the manuscript.
XZ, JH, YC and CW contributed to the discussion of results and to
the review of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
These studies were performed in accordance with the
ethical guidelines under the protocols approved by the
Institutional Medical Ethics Review Board of the Affiliated Union
Hospital of Fujian Medical University, Fuzhou, China.
Patient consent for publication
Not applicable.
Competing financial interests
The authors declare no competing financial
interests.
References
|
1
|
Pui CH: Childhood leukemias. N Engl J Med.
332:1618–1630. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pui CH and Evans WE: Acute lymphoblastic
leukemia. N Engl J Med. 339:605–615. 1998. View Article : Google Scholar
|
|
3
|
Pui CH and Jeha S: New therapeutic
strategies for the treatment of acute lymphoblastic leukaemia. Nat
Rev Drug Discov. 6:149–165. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rowe JM: Prognostic factors in adult acute
lymphoblastic leukaemia. Br J Haematol. 150:389–405. 2010.
|
|
5
|
Mudry RE, Fortney JE, York T, Hall BM and
Gibson LF: Stromal cells regulate survival of B-lineage leukemic
cells during chemotherapy. Blood. 96:1926–1932. 2000.
|
|
6
|
Gibson LF: Survival of B lineage leukemic
cells: Signals from the bone marrow microenvironment. Leuk
Lymphoma. 43:19–27. 2002. View Article : Google Scholar
|
|
7
|
Konopleva M and Andreeff M: Targeting the
leukemia microenvironment. Curr Drug Targets. 8:685–701. 2007.
View Article : Google Scholar
|
|
8
|
Boyerinas B, Zafrir M, Yesilkanal AE,
Price TT, Hyjek EM and Sipkins DA: Adhesion to osteopontin in the
bone marrow niche regulates lymphoblastic leukemia cell dormancy.
Blood. 121:4821–4831. 2013. View Article : Google Scholar
|
|
9
|
Meads MB, Hazlehurst LA and Dalton WS: The
bone marrow microenvironment as a tumor sanctuary and contributor
to drug resistance. Clin Cancer Res. 14:2519–2526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Masurekar A, Johnson S, Chakraborty
S, Griffiths J, Smith D, Alexander S, Dempsey C, Parker C, Harrison
S, et al: Stromal cell-mediated mitochondrial redox adaptation
regulates drug resistance in childhood acute lymphoblastic
leukemia. Oncotarget. 6:43048–43064. 2015.PubMed/NCBI
|
|
11
|
Dosen-Dahl G, Munthe E, Nygren MK,
Stubberud H, Hystad ME and Rian E: Bone marrow stroma cells
regulate TIEG1 expression in acute lymphoblastic leukemia cells:
Role of TGFbeta/BMP-6 and TIEG1 in chemotherapy escape. Int J
Cancer. 123:2759–2766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lau LF: CCN1/CYR61: The very model of a
modern matricellular protein. Cell Mol Life Sci. 68:3149–3163.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chuang JY, Yu NY, Chiang IP, Lai CH, Lin
CD and Tang CH: Cyr61 increases matrix metalloproteinase-3
expression and cell motility in human oral squamous cell carcinoma
cells. J Cell Biochem. 113:1977–1986. 2012. View Article : Google Scholar
|
|
14
|
Lin BR, Chang CC, Chen LR, Wu MH, Wang MY,
Kuo IH, Chu CY, Chang KJ, Lee PH, Chen WJ, et al: Cysteine-rich 61
(CCN1) enhances chemotactic migration, transendothelial cell
migration, and intravasation by concomitantly up-regulating
chemokine receptor 1 and 2. Mol Cancer Res. 5:1111–1123. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fromigue O, Hamidouche Z, Vaudin P,
Lecanda F, Patino A, Barbry P, Mari B and Marie PJ: CYR61
downregulation reduces osteosarcoma cell invasion, migration, and
metastasis. J Bone Miner Res. 26:1533–1542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leask A: A sticky situation: CCN1 promotes
both proliferation and apoptosis of cancer cells. J Cell Commun
Signal. 4:71–72. 2010. View Article : Google Scholar
|
|
17
|
Lee KB, Byun HJ, Park SH, Park CY, Lee SH
and Rho SB: CYR61 controls p53 and NF-κB expression through
PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells.
Cancer Lett. 315:86–95. 2012. View Article : Google Scholar
|
|
18
|
Lin MT, Chang CC, Chen ST, Chang HL, Su
JL, Chau YP and Kuo ML: Cyr61 expression confers resistance to
apoptosis in breast cancer MCF-7 cells by a mechanism of
NF-kappaB-dependent XIAP up-regulation. J Biol Chem.
279:24015–24023. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YJ, Lee DM and Lee SH: Production of
Cyr61 protein is modulated by extracellular acidification and
PI3K/Akt signaling in prostate carcinoma PC-3 cells. Food Chem
Toxicol. 58:169–176. 2013. View Article : Google Scholar
|
|
20
|
Hesler RA, Huang JJ, Starr MD, Treboschi
VM, Bernanke AG, Nixon AB, McCall SJ, White RR and Blobe GC:
TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in
pancreatic ductal adenocarcinoma through downregulation of the
nucleoside transporters hENT1 and hCNT3. Carcinogenesis.
37:1041–1051. 2016. View Article : Google Scholar
|
|
21
|
Long X, Yu Y, Perlaky L, Man TK and Redell
MS: Stromal CYR61 confers resistance to mitoxantrone via spleen
tyrosine kinase activation in human acute myeloid leukaemia. Br J
Haematol. 170:704–718. 2015. View Article : Google Scholar
|
|
22
|
Zhu X, Song Y, Wu C, Pan C, Lu P, Wang M,
Zheng P, Huo R, Zhang C, Li W, et al: Cyr61 participates in the
pathogenesis of acute lymphoblastic leukemia by enhancing cellular
survival via the AKT/NF-κB signaling pathway. Sci Rep. 6:340182016.
View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Zhang Q, Wu J, Cao Q, Xiao L, Wang L, He
D, Ouyang G, Lin J, Shen B, Shi Y, et al: A critical role of Cyr61
in interleukin-17-dependent proliferation of fibroblast-like
synoviocytes in rheumatoid arthritis. Arthritis Rheum.
60:3602–3612. 2009. View Article : Google Scholar
|
|
25
|
Rho SB, Woo JS, Chun T and Park SY:
Cysteine-rich 61 (CYR61) inhibits cisplatin-induced apoptosis in
ovarian carcinoma cells. Biotechnol Lett. 31:23–28. 2009.
View Article : Google Scholar
|
|
26
|
Lai D, Ho KC, Hao Y and Yang X: Taxol
resistance in breast cancer cells is mediated by the hippo pathway
component TAZ and its downstream transcriptional targets Cyr61 and
CTGF. Cancer Res. 71:2728–2738. 2011. View Article : Google Scholar
|
|
27
|
Youle RJ and Strasser A: The BCL-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar
|
|
28
|
Mirjolet JF, Barberi-Heyob M, Didelot C,
Peyrat JP, Abecassis J, Millon R and Merlin JL: Bcl-2/Bax protein
ratio predicts 5-fluorouracil sensitivity independently of p53
status. Br J Cancer. 83:1380–1386. 2000. View Article : Google Scholar
|
|
29
|
Kordes U, Krappmann D, Heissmeyer V,
Ludwig WD and Scheidereit C: Transcription factor NF-kappaB is
constitutively activated in acute lymphoblastic leukemia cells.
Leukemia. 14:399–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xue TY, Xu W, An Q, Wu Y, Xu CP and Zhang
XY: Expression of nuclear transcription factor kappaB in childhood
acute lymphoblastic leukemia and its significance. Zhongguo Shi Yan
Xue Ye Xue Za Zhi. 15:767–771. 2007.In Chinese. PubMed/NCBI
|
|
31
|
Dos Santos NR, Ghezzo MN, da Silva RC and
Fernandes MT: NF-κB in T-cell acute lymphoblastic leukemia:
Oncogenic functions in leukemic and in microenvironmental cells.
Cancers (Basel). 2:1838–1860. 2010. View Article : Google Scholar
|
|
32
|
Niu CC, Zhao C, Yang Z, Zhang XL, Pan J,
Zhao C and Si WK: Inhibiting CCN1 blocks AML cell growth by
disrupting the MEK/ERK pathway. Cancer Cell Int. 14:742014.
View Article : Google Scholar
|
|
33
|
Anand S, Penrhyn-Lowe S and Venkitaraman
AR: AURORA-A amplification overrides the mitotic spindle assembly
checkpoint, inducing resistance to Taxol. Cancer Cell. 3:51–62.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen G, Wang Y, Li M, Xu T, Wang X, Hong B
and Niu Y: Curcumol induces HSC-T6 cell death through suppression
of Bcl-2: Involvement of PI3K and NF-κB pathways. Eur J Pharm Sci.
65:21–28. 2014. View Article : Google Scholar
|
|
36
|
González-Ramos R, Defrère S and Devoto L:
Nuclear factor-kappaB: A main regulator of inflammation and cell
survival in endometriosis pathophysiology. Fertil Steril.
98:520–528. 2012. View Article : Google Scholar
|
|
37
|
Cao JP, Niu HY, Wang HJ, Huang XG and Gao
DS: NF-κB p65/p52 plays a role in GDNF up-regulating Bcl-2 and
Bcl-w expression in 6-OHDA-induced apoptosis of MN9D cell. Int J
Neurosci. 123:705–710. 2013. View Article : Google Scholar
|


















