Introduction
Recent epidemiological studies have suggested that
colorectal cancer (CRC) is one of the most common malignancies, and
millions of new CRC cases are diagnosed worldwide every year
(1). Incidence and mortality
rates have been decreasing for several years because of historical
changes in risk factors, including reduced smoking and red meat
consumption and increased use of aspirin (2). Furthermore, knowledge regarding the
initiation, promotion and progression of CRC has increased over
recent years (2). However, no
effective therapeutic strategy has been identified to provide a
long-term cure. Notably, several factors have been identified to
increase the risk of CRC, including duration and extent of colitis,
family history of CRC and severity of histologic inflammation
(3). Chronic inflammation has
been increasingly demonstrated to contribute to all steps of tumor
development (4).
Astragalus saponins (AST), extracted from the
medicinal plant Astragalus membranaceus, are the main active
constituent in Radix Astragali, and its anti-tumor effects have
been investigated in various studies (5,6).
In China, AST has been commonly used as an immunomodulating agent
in mixed herbal decoctions to treat the common cold, diarrhea,
fatigue and anorexia, and has also been prescribed to patients with
cardiac diseases (7). Several
studies have reported that AST possesses anti-tumor and
apoptosis-inducing effects on various types of human cancer in
vitro and in vivo (6,8-10).
Furthermore, clinical studies have reported that AST
polysaccharides could increase the effectiveness of platinum-based
chemotherapy when combined with chemotherapy and improve the
quality of life in patients with advanced non-small cell lung
cancer (7,11,12). However, few studies have
demonstrated the impact of AST treatment on glucose metabolism and
growth conditions in CRC cells.
Considering this, the present study investigated the
effect of AST treatment on cell viability and apoptosis in
vitro. Additionally, the present study explored the effect of
AST treatment on glycolysis metabolism, including glucose uptake,
lactate production and expression of glycolytic enzymes. The
findings of the present study may provide a new therapeutic
strategy in CRC treatment.
Materials and methods
Cell culture
Human CRC cell lines HT-29 and SW620 were obtained
from American Type Culture Collection (Manassas, VA, USA). Cells
were respectively maintained in McCoy’s 5A medium or L-15 medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented
with 10% fetal bovine serum at 37°C in humidified tissue culture
incubator containing 5% CO2.
Preparation of AST
Radix Astragali [Astragalus membranaceus
(Fisch.) Bunge var. mongolicus] was obtained from Shaanxi
University of Chinese Medicine (Shanxi, China). The authenticity
and quality of the crude herb were confirmed in the Quality
Assurance Laboratory of the School of Chinese Medicine, Hong Kong
Baptist University (Hong Kong, China) by microscopic and
chromatographic analyses, as well as DNA finger-printing. To ensure
consistency between batches, voucher specimens were kept at the
herbarium center (Shanghai, China) for future reference. AST was
extracted as described previously (13). In brief, 500 g of crude herb was
refluxed in methanol for 1 h at room temperature. Following this,
n-butanol was added to the reconstituted residue for phase
separation to obtain the total AST. Butanol was removed in the
rotary evaporator. The resulting residue was reconstituted with
distilled water and lyophilized into dry powder. The dried and
lyophilized powder was reconstituted in ultrapure water to form a
10 mg/ml stock and stored at −20°C.
MTT assay
HT-29 and SW620 cells were seeded in 96-well plates
at a density of 1×104 cells/well and treated with AST at
the indicated concentrations (0, 0.02, 0.04, 0.08, 0.16 and 0.20
mg/ml) and time intervals (0, 12, 24, 48 and 72 h). Control cells
received McCoy’s 5A or L-15 treatment only. A sterile MTT solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to each
well, and the cells were incubated for an additional 4 h at 37°C.
The medium was removed and 150 µl dimethyl sulfoxide was
added to dissolve the formazan crystals formed in the viable cells.
Plates were examined at 570 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). At least three independent
experiments were performed.
Colony formation assay
HT-29 and SW620 cells were seeded in 6-well plates
at a density of 500 cells/well and treated with 50 µg/ml
AST. Cells treated with 25 µg/ml 5-fluorouracil (5-Fu,
Sigma-Aldrich; Merck KGaA) were used as a positive control group.
The medium (McCoy’s 5A or L-15) was refreshed every 2 days.
Following two weeks of incubation at 37°C, colonies were fixed with
methanol in 15 min at room temperature and then stained with Giemsa
solution (Sigma-Aldrich; Merck KGaA) for 10-15 min at room
temperature. Plates were washed with PBS, and colony formation was
photographed and analyzed using ImageJ 1.48 (National Institutes of
Health, Bethesda, MD, USA). Cloning efficiency is the number of
colonies divided by the number of cells plated.
Apoptosis assay
To investigate the effect of AST treatment on cell
apoptosis, cells treated with 50 µg/ml AST served as the
experimental group. Untreated cells were used as a control group.
Cell apoptosis was analyzed using an Annexin V/propidium iodide
apoptosis detection kit (BD Biosciences, San Jose, CA, USA)
according to the instructions of the manufacturer. Following 48 h
of AST treatment, the cells were harvested, washed with cold PBS
(pH 7.4), centrifuged at 400 × g at 4°C for 5 min, and
double-stained with Annexin V-Fluorescein isothiocyanate and
propidium iodide in binding buffer [10 mM HEPES (pH 7.4), 140 mM
NaCl and 2.5 mM CaCl2] for 15 min in the dark. The
samples were analyzed with Attune NxT Software version 2.5 and a
flow cytometer (Invitrogen Attune NxT; Thermo Fisher Scientific,
Inc.).
Reverse transcription-quantitative
polymerase change reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer’s instructions. Subsequenetly, cDNA was synthesized
with an iScript Advanced cDNA Synthesis kit (Bio-Rad Laboratories,
Inc.). PCR was performed in duplicate with a CFX96 PCR system and
iTaq Universal SYBR-Green Supermix according to the manufacturer’s
instructions (Bio-Rad Laboratories, Inc.). PCR amplification
conditions were as follows: 1 cycle of 95°C for 3 min, followed by
40 cycles of 95°C for 10 sec and 60°C for 22 sec. Primers used were
as follows: Human (h)β-actin forward, 5′-CCTGGGCATGGAGTCCTGTG-3′
and reverse, 5′-TCTTCATTGTGCTGGGTGCC-3′; (h)c-Myc forward,
5′-GGAGGAACAAGAAGATGAGGAAG-3′ and reverse,
5′-AGGACCAGTGGGCTGTGAGG-3′; (h) lactate dehydrogenase A (LDH-A),
forward, 5′-CCCCAGAATAAGATTACAGTTATTG-3′ and reverse,
5′-GAGCAAGTTCATCTGCCAAGT-3′; (h) hexokinase 2 (HK2) forward,
5′-GATTGTCCGTAACATTCTCATCGA-3′ and reverse,
5′-TGTCTTGAGCCGCTCTGAGAT-3′; (h)Glut-1 forward,
5′-TCTGGGCATGTGCTTCCAGTA-3′ and reverse,
5′-ATCGAAGGTCCGGCCTTTAGTC-3′; mouse (m) β-actin forward,
5′-ATGCCATCCTGCGTCTGGACCTGGC-3′ and reverse,
5′-AGCATTTGCGGTGCACGATGGAGGG-3′; (m)c-Myc forward,
5′-TCTCCATCCTATGTTGCGGTC-3′ and reverse,
5′-TCCAAGTAACTCGGTCATCATCT-3′; (m)LDH-A forward,
5′-GCTCCCCAGAACAAGATTACAG-3′ and reverse,
5′-TCGCCCTTGAGTTTGTCTTC-3′; and (m)phosphofructokinase (PFK)U
forward, 5′-CCGAGGAGCGTACAAAGT-3′ and reverse,
5′-CTGAGCGGTGGTGGTGAT-3′.
Western blot
HT-29 and SW620 cells were lysed in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Following this, total protein
concentration was determined using the BCA assay Kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amounts of protein (20
µg per lane) were separated using SDS-PAGE (on 10% gels),
transferred to nitrocellulose membranes (Amersham; GE Healthcare,
Chicago, IL, USA). Following blocking in 5% non-fatty milk at room
temperature for 60 min, membranes were incubated overnight at 4°C
with primary antibodies directed against β-actin (1:500; BM5422,
Wuhan Boster Biological Technology, Ltd., Wuhan, China), B-cell
lymphoma 2 (Bcl-2; 1:1,000; ab692), Bcl-2 associated X protein
(Bax; 1:1,000; ab32503; both Abcam), Bcl-2 homologous
antagonist/killer (Bak; 1:1,000; 12105, CST Biological Reagents
Co., Ltd., Shanghai, China), Bcl-2-associated agonist of cell death
(Bad; 1:1,000; 9268, CST Biological Reagents Co., Ltd.),
pro-caspase3 and poly (ADP-ribose) polymerase (PARP; 1:1,000; 9532,
CST Biological Reagents Co., Ltd.), respectively. The membranes
were then washed three times with phosphate buffered saline with
0.05% Tween-20 buffer and incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000; 14709, CST
Biological Reagents Co., Ltd.) for 1 h at room temperature. The
blots were detected using chemiluminescence (Pierce; Thermo Fisher
Scientific, Inc.) and an Odyssey Imaging System (Li-Cor
Biosciences, Lincoln, NE, USA).
Glucose uptake assay
Basal glucose uptake was measured as previously
reported for human cells in vitro (14). Briefly, HT-29 and SW620 cells were
plated at 1×104 cells/well in 96-well cell culture
plates and treated with 50 µg/ml AST at 37°C for 0, 12, 24
and 48 h. At every point in time, cells were treated with 100
µl of glucose-free DMEM culture medium containing 100
µg/ml 2-NBDG for 20 min. The medium was subsequently
replaced with 200 µl Hank’s Balanced Salt Solution and the
plate was centrifuged for 5 min at 30 × g (at room temperature).
The fluorescence intensity was measured at 530 nm using a plate
reader from Tecan Group Ltd. (Männedorf, Switzerland).
Glycolytic enzymes activity and lactate
assay
HK and LDH activities were measured according to the
manufacturer’s instructions (Nanjing Jiancheng Bioengineering
Institute (HK cat. no. A077-1 and LDH cat. no. A020-2; Nanjing,
China). Cell lactate production was determined as previously
reported in human cells (15). In
brief, HT-29 and SW620 cells were collected, lysed via
centrifugation at 6,000 × g for 10 min (at 4°C). The supernatant
was collected and total protein was examined using a BCA kit
(Biyuntian, Shanghai, China).
Animals and treatment
A total of 32 male 9-week-old C57BL/6J mice (mean
body weight of 21 g; range, 18-25 g) were purchased from Shanghai
SLAC Laboratory Animal Co., Ltd (Shanghai, China) and housed in
individually ventilated cages (IVC; Tianhuan, Shanghai, China)
supplied with filtered air and access to food and water. The mice
were maintained under the following conditions: Temperature,
20-22°C; relative humidity, 50-60%; and a 12-h light/dark cycle.
All mice were acclimatized for 1 week prior to starting dextran
sulfate sodium (DSS; molecular weight, 36-50 kDa; MP Biomedicals,
Solon, OH, USA) administration. Mice were randomly divided into the
following three groups: Mice were exposed to 2% DSS (n=8, positive
control), 0.1 mg/g AST (n=8, negative control) and 0.1 mg/g AST
treatment combined with 2% DSS in drinking water (n=8),
respectively. The body weight of all mice was monitored daily. All
mice were sacrificed 8 days following the administration of DSS.
Colitis was induced by administration of 2% DSS dissolved in
distilled water for 8 days. Once the colon length was measured, the
distal colon was extracted and fixed in 4% paraformaldehyde for 24
h at room temperature. Paraffin-embedded colon tissues (5 µm
thick) were prepared for hematoxylin and eosin staining. The
resulting tissue sections were stained with hematoxylin for 5 min
and eosin for 3 min at room temperature. The sections were observed
using a confocal microscope (LSM 5 PASCAL; Carl Zeiss AG,
Oberkochen, Germany). The remaining colon tissues were embedded in
paraffin or subjected to RNA and protein extraction. The procedures
employed for the handing and care of animals were approved by the
Renji Hospital Ethics Committee, Shanghai Jiao Tong University
School of Medicine (Shanghai, China), and conformed to the national
guidelines for animal use in research.
Statistical analysis
All analyses were performed using SPSS 17.0
statistical package (SPSS, Inc., Chicago, IL, USA). Data were
expression as the mean ± standard error of the mean. Data were
compared using one-way analysis of variance followed by an LSD
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
AST inhibits growth and proliferation in
CRC cells
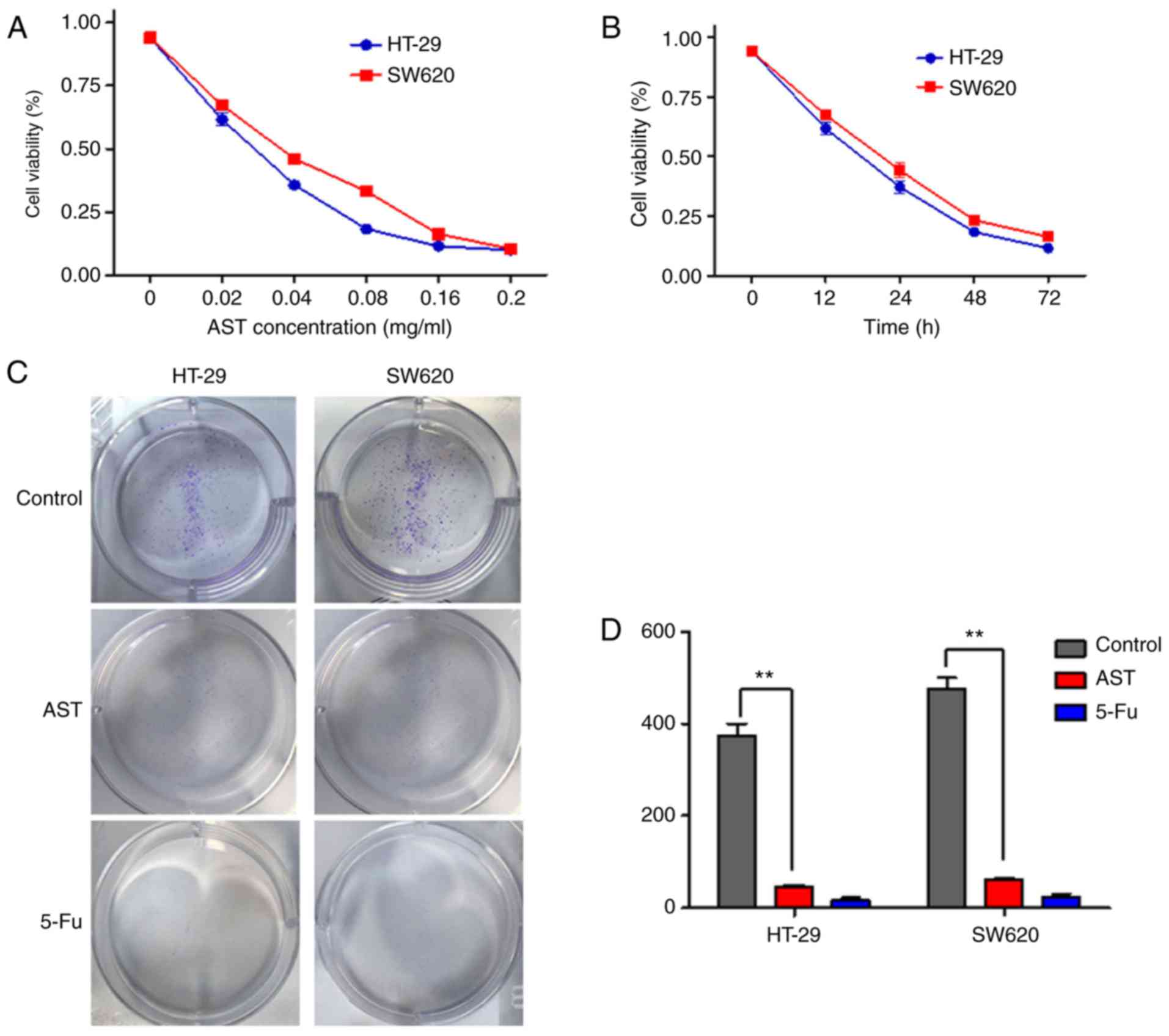
The MTT assay was performed to determine the growth
inhibitory effect of AST on CRC cells. It was revealed that AST
could inhibit the growth of CRC cells in a dose- and time-dependent
manner (Fig. 1A and B). The
IC50 values at 24 h in HT-29 and SW620 cells were ~35
and 46 µg/ml, respectively. Thus, a final concentration of
50 µg/ml AST was used in all subsequent experiments unless
otherwise specified. Following this, the inhibitory impact of AST
on HT-29 and SW620 cell proliferation over an extended time
interval was determined. To assess colony formation, cells were
seeded in 6-well plates and treated with 50 µg/ml AST (cells
treated with 25 µg/ml 5-Fu served as a positive control) for
2 weeks. Statistical analysis indicated that AST significantly
reduced the number of colonies formed by CRC cells compared with
the vehicle group (Fig. 1C and
D). The results illustrated that AST has a directly inhibitory
effect on CRC cell growth and viability over short- and long-term
time points.
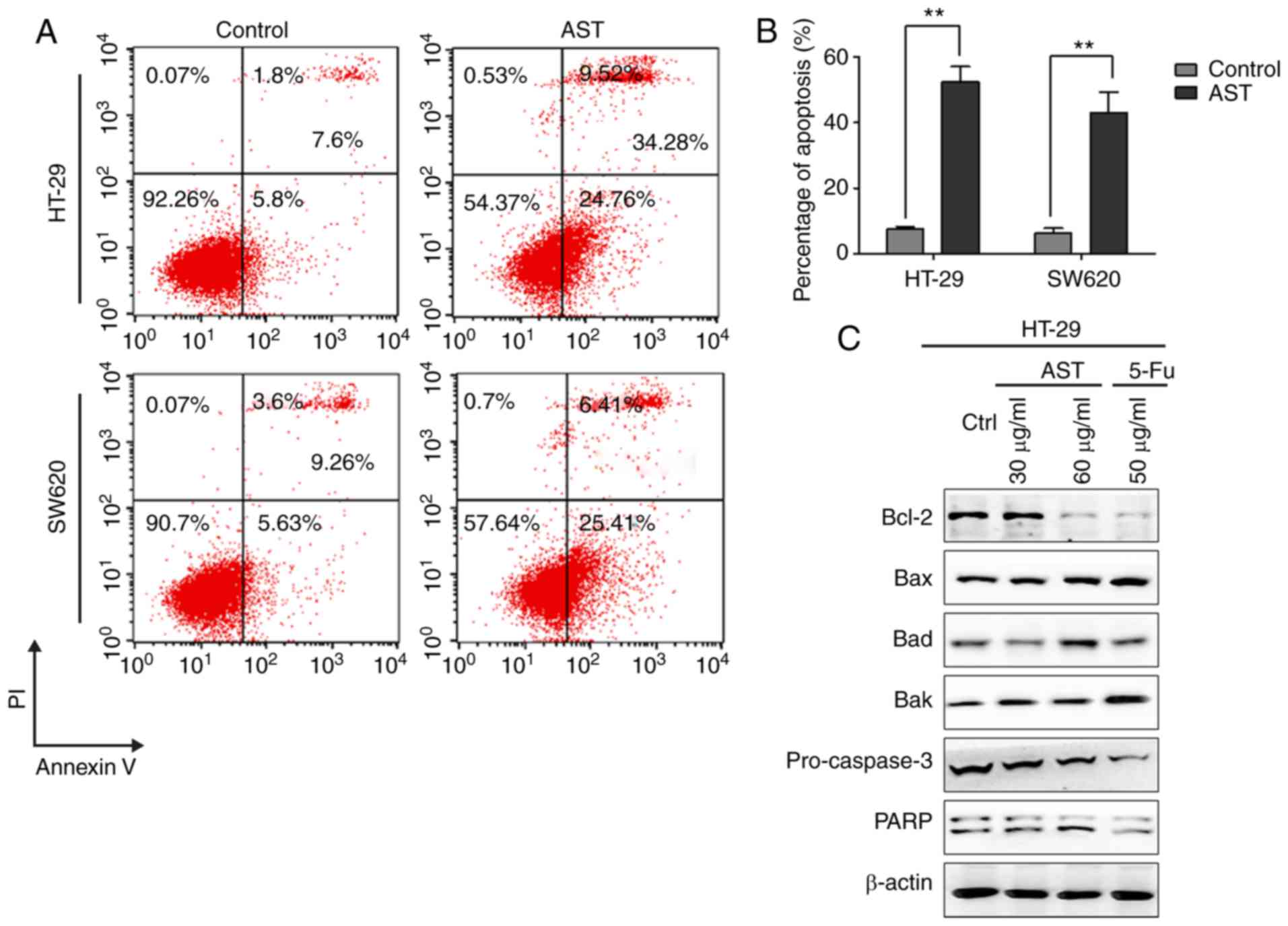
AST directly promotes apoptosis and the
expression of apoptosis-associated genes in vitro
To determine whether the cytotoxic effects of AST
are associated with the induction of apoptosis, flow cytometry was
performed when AST was applied to cells. AST treatment
significantly induced apoptosis in CRC cells (Fig. 2A and B). Furthermore, ~34% of
HT-29 cells and ~31% of SW620 cells were apoptotic in response to
AST. Notably, the expression levels of apoptosis-associated
proteins confirmed the induction of apoptosis by AST, including the
upregulation of Bax, Bad and Bak, and the downregulation of Bcl-2,
pro-caspase3, PARP (Fig. 2C).
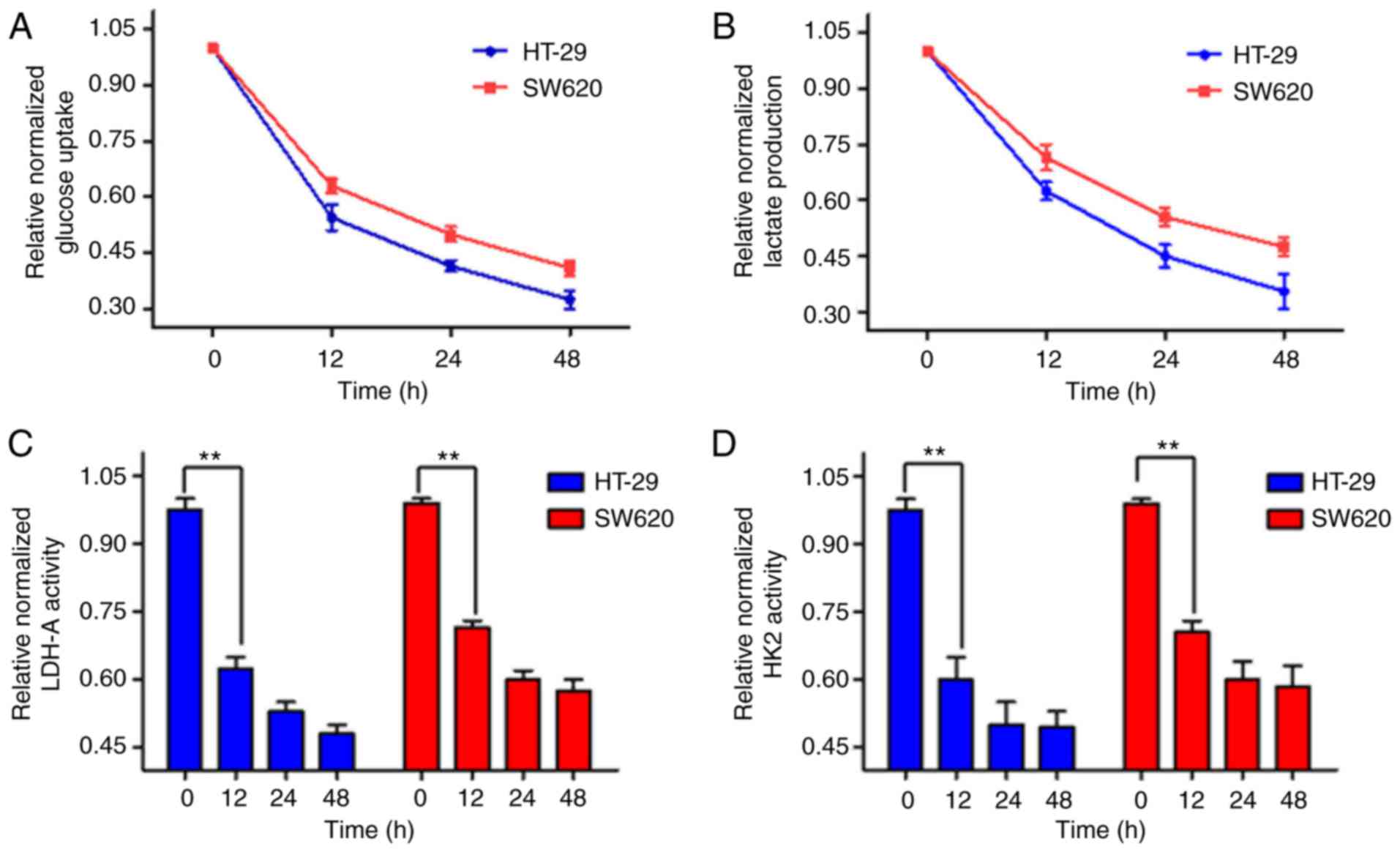
Glucose uptake and lactate production are
decreased by AST treatment in CRC cells
To explore the impact of AST on glycolysis
metabolism in CRC cells, glucose uptake and lactate levels were
measured. As indicated in Fig.
3A, the glucose uptake of CRC cells decreased with AST
treatment. Furthermore, lactate production was also reduced
(Fig. 3B). In addition, the
enzymes activities of HK2 and LDH-A in CRC cells were assessed. AST
significantly reduced the enzymes activities of HK2 and LDH-A
(Fig. 3C and D). The results
illustrated that AST could inhibit aerobic glycolysis and the
activities of glycolytic enzymes.
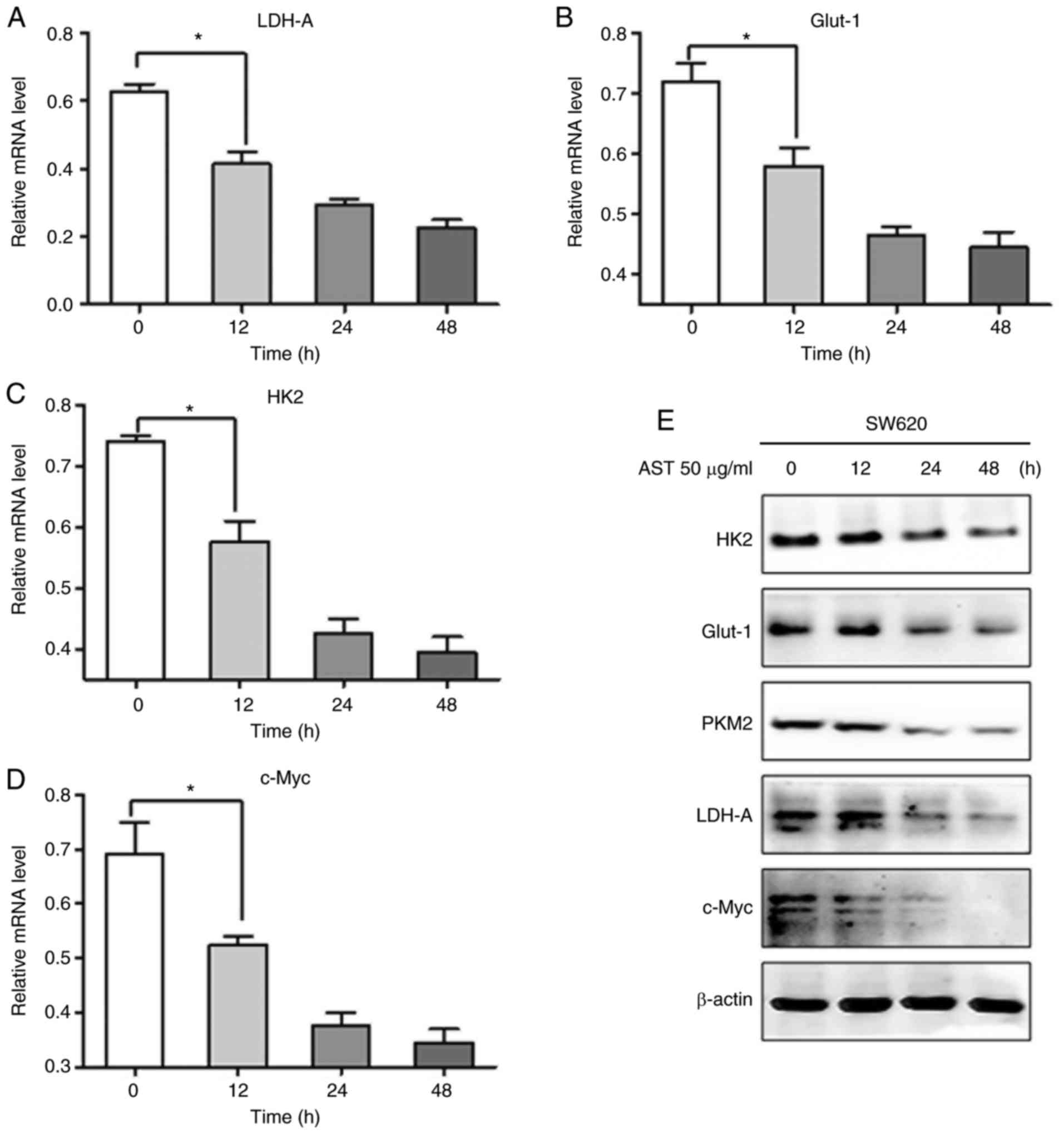
AST inhibits the expression levels of
glycolytic enzymes in CRC cells
To verify whether the expression levels of
glycolytic enzymes were also decreased by AST, western blot
analysis and RT-qPCR were performed. The relative mRNA expression
levels of LDH-A, Glut-1 and HK2 were significantly reduced by AST
in SW620 cells (Fig. 4A, B and
C). Interestingly, the expression of c-Myc, a primary oncogene
of carcinogenesis that is overexpressed in various cancer types
(16,17), was significantly decreased in CRC
cells treated with AST (Fig. 4D).
In addition, western blot analysis revealed similar trends to
RT-qPCR data (Fig. 4E).
AST attenuates the inflammatory response
and tumor-like aerobic glycolysis in colitis mice model
To uncover the possible impact of AST on
inflammation and glucose metabolism in vivo, a DSS-induced
colitis mouse model was established. DSS is a well-known agent for
inducing inflammation in order to identify the impact of
inflammation on the pathogenesis and the clinical course of
inflammation (18-20). In the DSS-induced colitis mouse
model of the present study, compared with the combined treatment of
AST and DSS, mice administered DSS alone were used as a positive
control, and those with AST treatment alone served as a negative
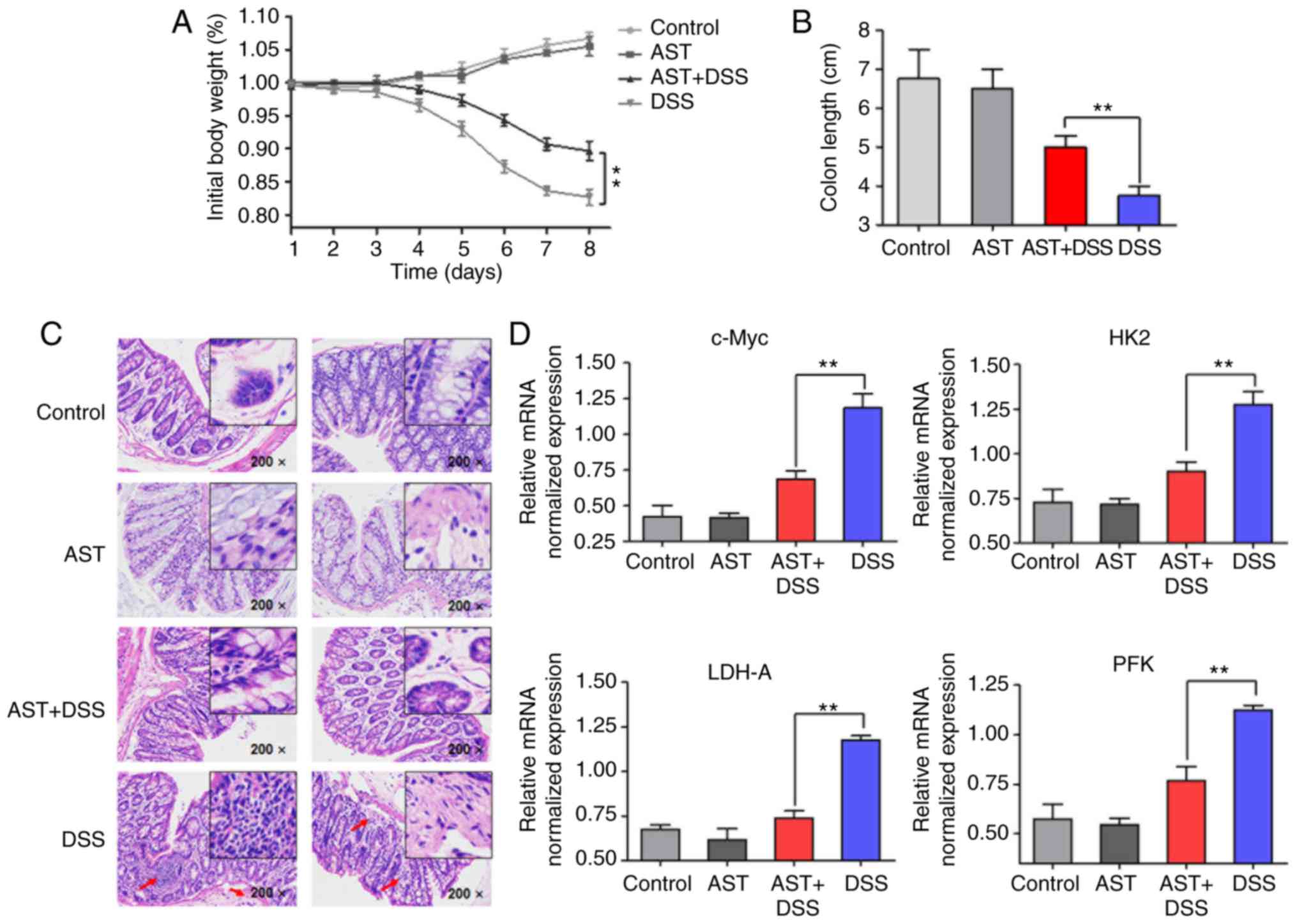
control. Body weight loss was observed over 8 days
post-administration of DSS. In addition, colon length and
histological analysis were observed 8 days post-administration of
DSS. Following DSS treatment, the body weight and colon length of
mice treated with DSS only were significantly reduced. Notably,
there was only a slight decrease in the combined treatment group
compared with control group and AST groups (Fig. 5A and B). In addition, the
inflammatory response of the colonic mucosa was assessed using
hematoxylin and eosin staining. As indicated in Fig. 5C, a large number of lobular
necro-inflammatory cells were observed in the DSS group; however,
this was reduced by AST treatment. The result suggests that AST
treatment could attenuate the inflammatory response induced by DSS.
Considering the decrease of body weight and the acute inflammatory
reactions associated with DSS, it was proposed that glucose
metabolism may be altered following inflammation enhancement. mRNA
expression levels of c-Myc and glycolytic enzymes were increased in
colon epithelial cells following 8-day DSS administration. However,
AST treatment significantly inhibited the tumor-like aerobic
glycolysis (the high level of glycose uptake and lactate
production) in mice with DSS-induced colitis (Fig. 5D), revealing almost the same
trends that were indicated in the in vitro cell
experiments.
Discussion
Astragalus is considered to be useful in traditional
Chinese medicine and has been used as a treatment for almost all
diseases believed to be caused by chi deficiency (21,22). Previous studies have suggested
that AST, a traditional Chinese medicine, has anti-tumor and
apoptosis-inducing effects on various types of human cancer in
vitro and in tumor xenografts (23-25). However, few studies have reported
the impact of AST on metabolic alterations in CRC cells. To address
this issue, the present study investigated a series of glycolysis
metabolism and growth condition alterations in CRC cells treated
with AST.
In the present study, the cytotoxic activity of AST
was determined in CRC cells. The results illustrated that AST could
inhibit growth and proliferation of CRC cells, and that AST could
directly induce apoptosis in vitro. Western blot analysis of
apoptosis-associated proteins further confirmed the induction of
apoptosis by AST. For instance, AST decreased the expression of
anti-apoptotic protein Bcl-2, PARP and pro-caspase 3, and increased
the expression of the pro-apoptotic proteins Bax, Bak and Bad in
CRC cells. In the 1920s, Warburg hypothesized that there was a
trend toward an increased rate of glycolysis in a variety of cancer
cells, triggering the excessive production of lactic acid from
glucose, which was termed the ‘Warburg effect’ (26,27). Accordingly, the impact of AST on
glycolysis metabolism was investigated in the present study. A
series of indexes of aerobic glycolysis, including glucose uptake,
lactate production and the activity of glycolytic enzymes, were all
decreased. Western blot analysis and RT-qPCR data revealed similar
trends. In addition, the present study established a DSS-induced
colitis mouse model, with the objective of exploring the possible
impact of AST on the inflammatory response and glucose metabolism.
The results suggested that AST could effectively attenuate the
inflammatory response and inhibit tumor-like aerobic glycolysis,
indicating that AST may have the capacity to resist inflammation
and maintain normal glucose homeostasis.
Furthermore, evidence has suggested the multifaceted
oncogene, c-Myc, exerts important regulatory impacts on multiple
aspects of cell transformation (16,28,29). It was reported that c-Myc could
activate the expression of LDH-A and increase lactate production
(30). The present study
indicated that AST decreased c-Myc expression in CRC cells. The
results suggested that AST-induced inhibition of growth and aerobic
glycolysis of CRC cells may be associated with the suppression of
c-Myc; specifically, AST may inhibit the Warburg effect through
c-Myc suppression.
In conclusion, the present findings indicated that
AST may be able to inhibit CRC development and attenuate aerobic
glycolysis, which may be associated with c-Myc downregulation. The
present study may provide a new therapeutic strategy for clinical
treatment of CRC.
Funding
The present study was supported by a grant from the
Shanghai Medical Key Specialist Construction plans (grant no.
ZK2015B10).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
ZS conceived and designed the study. HG, BW and JW
performed the drugs preparation and treatments, western blot
analysis and mice model, and were major contributors in writing the
manuscript. JZ and WY performed the reverse transcription
quantitative polymerase chain reaction and analyzed the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Renji Hospital
Ethics Committee, Shanghai Jiaotong University School of Medicine
(Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Tenesa A and Dunlop MG: New insights into
the aetiology of colorectal cancer from genome-wide association
studies. Nat Rev Genet. 10:353–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar
|
|
3
|
Potack J and Itzkowitz SH: Colorectal
cancer in inflammatory bowel disease. Gut Liver. 2:61–73. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ullman TA and Itzkowitz SH: Intestinal
inflammation and cancer. Gastroenterology. 140:1807–1816. 2011.
View Article : Google Scholar
|
|
5
|
Wei H, Sun R, Xiao W, Feng J, Zhen C, Xu X
and Tian Z: Traditional Chinese medicine Astragalus reverses
predominance of Th2 cytokines and their up-stream transcript
factors in lung cancer patients. Oncol Rep. 10:1507–1512. 2003.
|
|
6
|
McCulloch M, See C, Shu XJ, Broffman M,
Kramer A, Fan WY, Gao J, Lieb W, Shieh K and Colford JM Jr:
Astragalus-based Chinese herbs and platinum-based chemotherapy for
advanced non-small-cell lung cancer: Meta-analysis of randomized
trials. J Clin Oncol. 24:419–430. 2006. View Article : Google Scholar
|
|
7
|
Wu CY, Ke Y, Zeng YF, Zhang YW and Yu HJ:
Anticancer activity of Astragalus polysaccharide in human non-small
cell lung cancer cells. Cancer Cell Int. 17:1152017. View Article : Google Scholar
|
|
8
|
Ye MN and Chen HF: Effects of Astragalus
injection on proliferation of basal-like breast cancer cell line
MDA-MB-468. Zhong Xi Yi Jie He Xue Bao. 6:399–404. 2008.In Chinese.
View Article : Google Scholar
|
|
9
|
Deng Y and Chen HF: Effects of Astragalus
injection and its ingredients on proliferation and Akt
phosphorylation of breast cancer cell lines. Zhong Xi Yi Jie He Xue
Bao. 7:1174–1180. 2009.In Chinese. View Article : Google Scholar
|
|
10
|
Wang T, Xuan X, Li M, Gao P, Zheng Y, Zang
W and Zhao G: Astragalus saponins affect proliferation, invasion
and apoptosis of gastric cancer BGC-823 cells. Diagn Pathol.
8:1792013. View Article : Google Scholar :
|
|
11
|
Cassileth BR, Rizvi N, Deng G, Yeung KS,
Vickers A, Guillen S, Woo D, Coleton M and Kris MG: Safety and
pharmacokinetic trial of docetaxel plus an Astragalus-based herbal
formula for non-small cell lung cancer patients. Cancer Chemother
Pharmacol. 65:67–71. 2009. View Article : Google Scholar
|
|
12
|
Dugoua JJ, Wu P, Seely D, Eyawo O and
Mills E: Astragalus-containing Chinese herbal combinations for
advanced non-small-cell lung cancer: A meta-analysis of 65 clinical
trials enrolling 4751 patients. Lung Cancer (Auckl). 1:85–100.
2010.
|
|
13
|
Ma XQ, Shi Q, Duan JA, Dong TT and Tsim
KW: Chemical analysis of Radix Astragali (Huangqi) in China: A
comparison with its adulterants and seasonal variations. J Agric
Food Chem. 50:4861–4866. 2002. View Article : Google Scholar
|
|
14
|
Schlaepfer IR, Hitz CA, Gijón MA, Bergman
BC, Eckel RH and Jacobsen BM: Progestin modulates the lipid profile
and sensitivity of breast cancer cells to docetaxel. Mol Cell
Endocrinol. 363:111–121. 2012. View Article : Google Scholar
|
|
15
|
Zhan P, Wang Y, Zhao S, Liu C, Wang Y, Wen
M, Mao JH, Wei G and Zhang P: FBXW7 negatively regulates ENO1
expression and function in colorectal cancer. Lab Invest.
95:995–1004. 2015. View Article : Google Scholar
|
|
16
|
Stine ZE, Walton ZE, Altman BJ, Hsieh AL
and Dang CV: MYC, metabolism, and cancer. Cancer Discov.
5:1024–1039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller DM, Thomas SD, Islam A, Muench D
and Sedoris K: c-Myc and cancer metabolism. Clin Cancer Res.
18:5546–5553. 2012. View Article : Google Scholar
|
|
18
|
Parang B, Barrett CW and Williams CS:
AOM/DSS model of colitis-associated cancer. Methods Mol Biol.
1422:297–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antoniou E, Margonis GA, Angelou A,
Pikouli A, Argiri P, Karavokyros I, Papalois A and Pikoulis E: The
TNBS-induced colitis animal model: An overview. Ann Med Surg
(Lond). 11:9–15. 2016. View Article : Google Scholar
|
|
20
|
Qu D, Shen L, Liu S, Li H, Ma Y, Zhang R,
Wu K, Yao L, Li J and Zhang J: Chronic inflammation confers to the
metabolic reprogramming associated with tumorigenesis of colorectal
cancer. Cancer Biol Ther. 18:237–244. 2017. View Article : Google Scholar
|
|
21
|
Wang XM, Yu RC and Wang YT: Study on
advanced non-small cell lung cancer patients with Qi deficiency and
blood stasis syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi.
14:724–726. 1994.In Chinese. PubMed/NCBI
|
|
22
|
Yuan F, Zhou Y, Jiang Y, Liu R, Li JZ, Xie
YK, Li XM and Dai F: Therapeutic effect and apoptosis mechanism of
lung-tonifying and expectorant decoction on lung cancer rats with
Qi deficiency and blood stasis. Asian Pac J Trop Med. 8:983–988.
2015. View Article : Google Scholar
|
|
23
|
Dong JC, Dong XH and Zhao FD: Therapeutic
effects of Astragalus injection on lewis lung cancer in mice.
Zhonghua Zhong Liu Za Zhi. 28:272–273. 2006.In Chinese. PubMed/NCBI
|
|
24
|
Zou YH and Liu XM: Effect of astragalus
injection combined with chemotherapy on quality of life in patients
with advanced non-small cell lung cancer. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 23:733–735. 2003.In Chinese.
|
|
25
|
Na D, Liu FN, Miao ZF, Du ZM and Xu HM:
Astragalus extract inhibits destruction of gastric cancer cells to
mesothelial cells by anti-apoptosis. World J Gastroenterol.
15:570–577. 2009. View Article : Google Scholar
|
|
26
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Warburg O: On respiratory impairment in
cancer cells. Science. 124:269–270. 1956.
|
|
28
|
Dang CV: MYC on the path to cancer. Cell.
149:22–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh AL, Walton ZE, Altman BJ, Stine ZE
and Dang CV: MYC and metabolism on the path to cancer. Semin Cell
Dev Biol. 43:11–21. 2015. View Article : Google Scholar
|
|
30
|
Shim H, Dolde C, Lewis BC, Wu CS, Dang G,
Jungmann RA, Dalla-Favera R and Dang CV: c-Myc transactivation of
LDH-A: Implications for tumor metabolism and growth. Proc Natl Acad
Sci USA. 94:6658–6663. 1997. View Article : Google Scholar
|