Introduction
As a dynamic metabolic system, bone modeling or
remodeling is modulated by two major bone cells: Osteoblasts, which
are able to secrete bone matrix and accelerate calcium (Ca)
deposition, and osteoclasts, which are responsible for resolving
mineralized bone matrix (1-3).
In the process of bone maintenance and repair, extracellular
signaling transduction between osteoblasts and osteoclasts serves a
crucial role in bone homeo-stasis (4). For example, osteoblast-secreted
receptor activator of nuclear factor-κB (NF-κB) (RANK) ligand
(RANKL) binds to the RANK receptor on osteoclasts, thus promoting
osteoclast survival and osteoclastogenesis (5). In addition, the binding of
macrophage colony-stimulating factor (M-CSF) to its receptor,
colony stimulating factor receptor, has been reported to be
essential for the generation of osteoclast precursor cells that are
present prior to RANKL stimulation (2). Furthermore, tumor necrosis factor
receptor-associated factor-6-activated NF-κB signaling induces the
initialization of nuclear factor of activated T cells, cytoplasmic
1 (NFATc1), which is a key transcription factor for
osteoclastogenesis and stimulates the expression of various
osteoclast-specific genes, including tartrate-resistant acid
phosphatase (TRAP), cathepsin K (Ctsk) and calcitonin receptor
(2,5).
MicroRNAs (miRNAs/miRs) are a class of small,
single-stranded, non-coding RNA molecules, 18-24 nucleotides in
length, which can regulate gene expression or translation by
targeting 3'-untranslated regions (3'-UTRs). miRNAs serve as a
novel class of post-transcriptional regulators that are involved in
diverse biological functions (6-8).
Previous studies have reported that miRNAs serve important roles in
regulating skeletal development (9,10).
For example, overexpression of miR-34a, miR-125a and miR-503
inhibits osteoclastogenesis to rescue bone loss (9,11,12). Conversely, miR-214 promotes
osteoclastogenesis, and inhibits osteoblast differentiation and
bone formation (10,13). miR-100 is a member of the miR-99
family (14) and participates in
various biological processes, including chemotherapy resistance,
apoptosis, osteogenic differentiation and osteoporotic fractures
(15-18). However, the role of miR-100 in
ovariectomy (OVX)-induced osteoporosis and osteoclastogenesis
remains unclear.
Fibroblast growth factor (FGF)21 is a member of the
FGF family, which is mainly expressed in the liver, and has
numerous metabolic functions (19). FGF21 has been demonstrated to
improve obesity-associated glucose and lipid metabolic disorders
(20), renal dysfunction
(21), cardiac hypertrophy
(22) and ethanol-associated
liver injury (23). Conversely,
upregulation of FGF21 is an independent risk factor associated with
the severity of non-alcoholic fatty liver disease (24). In a diet-induced mouse model of
obesity, a reduction in plasma FGF21 levels and hepatic FGF21
resistance by exenatide protects against high-fat diet-induced
non-alcoholic fatty liver disease (25). Emerging evidence has highlighted
that FGF21 may serve as a potent regulator of skeletal homeostasis
(26). In a Han Chinese
population, a significant inverse correlation is observed between
plasma FGF21 levels and bone mineral density (BMD) of the femoral
neck and Ward's triangle (27).
An increase in serum FGF21 levels is also negatively associated
with BMD and bone mineral content in human immunodeficiency
virus-1-infected patients (28).
However, fasting plasma FGF21 levels in healthy women exhibit a
strong positive association with total BMD and spine BMD (29). Notably, FGF21-knockout in mice
results in a high-bone-mass phenotype, whereas FGF21
gain-of-function decreases bone mass and promotes
osteoclastogenesis (26,30). However, the underlying molecular
mechanisms of FGF21-enhanced osteoclastogenesis and bone resorption
have not been completely clarified.
The present study screened the expression profile of
miRNAs in the process of M-CSF- and RANKL-induced
osteoclastogenesis using a miRNA microarray. The results
demonstrated that miR-100-5p was downregulated and functioned as a
negative regulator of osteoclastogenesis. miR-100-5p
gain-of-function significantly suppressed osteoclast activity in
vitro. In vivo, treatment with the agomir-miR-100-5p
inhibited FGF-21 expression and osteoclast activity, and prevented
bone loss in mice following OVX.
Materials and methods
Cell culture
The present study was approved by the Animal Ethical
Committee of Shengjing Hospital of China Medical University
(Shenyang, China; approval no. 2016PS361K). Murine bone
marrow-derived macrophages (BMMs) were obtained form female
C57BL/6J mice, as described previously (31). BMMs were maintained in α-Minimum
Essential Medium (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 1% penicillin-streptomycin
at 37˚C in an atmosphere containing 5% CO2. Non-adherent
cells were cultured in M-CSF (50 ng/ml; R&D Systems China Co.,
Ltd., Shanghai, China) for 3 days to induce osteoclast precursors.
For osteoclast differentiation, osteoclast precursors were
incubated with M-CSF (50 ng/ml) and RANKL (100 ng/ml; R&D
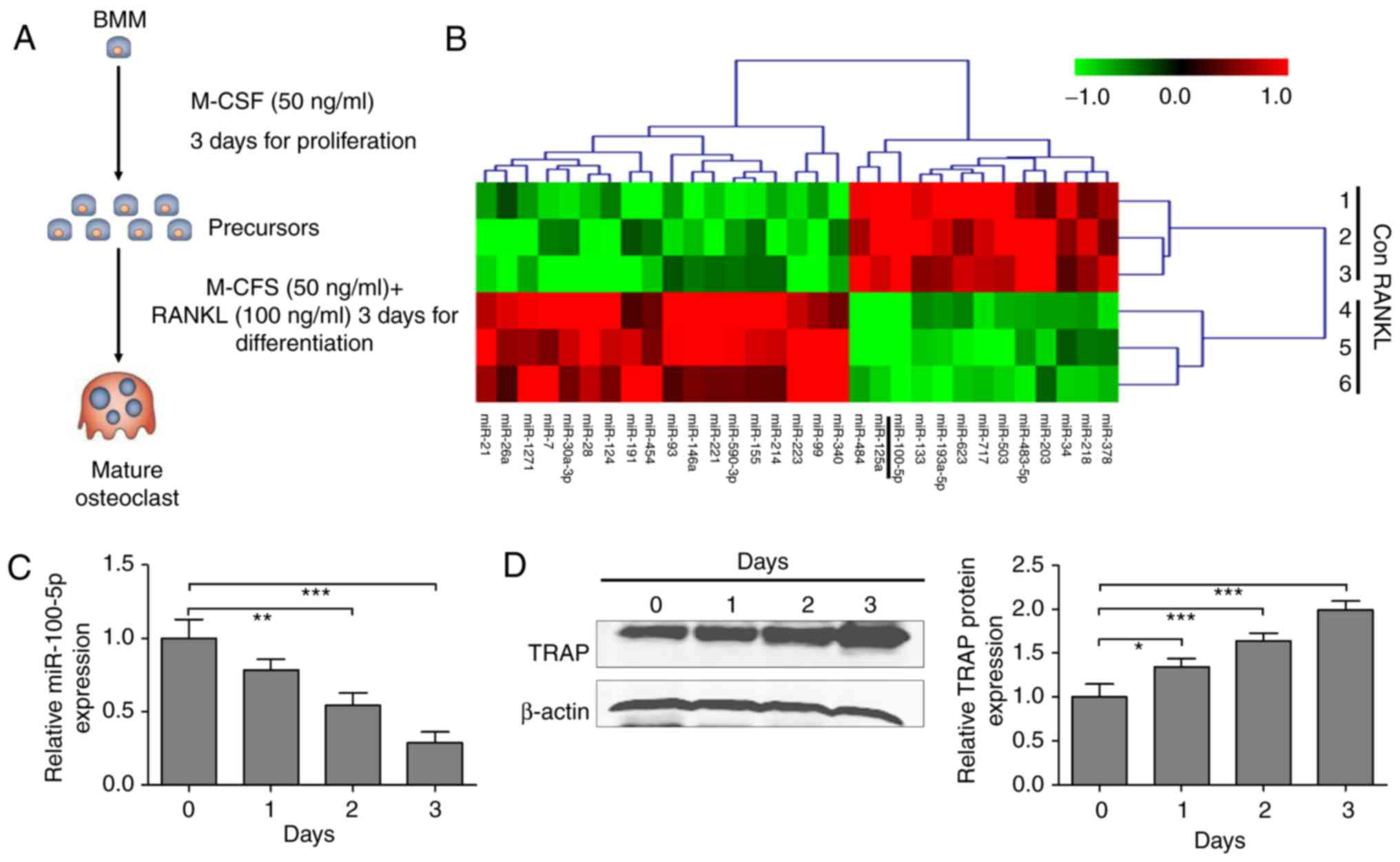
Systems China Co., Ltd.) for another 3 days (Fig. 1A). Osteoclasts with ≥3 nuclei/cell
were identified as TRAP-positive cells. The NCTC-1469 mouse normal
liver cell line (serial number: 3111C0001CCC000290) was obtained
from the Institute of Biochemistry and Cell Biology of the Chinese
Academy of Sciences & National Infrastructure of Cell Line
Resource (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 5% FBS, in a humidified incubator containing 5%
CO2 and 95% air (Thermo Fisher Scientific, Inc.).
Differentially expressed miRNAs in
RANKL-induced osteoclast differentiation
Osteoclast differentiation was induced as shown in
Fig. 1A. Subsequently, miRNA
expression profiling of osteoclasts was conducted using the mouse
miRNA expression profiling array V.2 (Illumina Inc., San Diego, CA,
USA), according to the manufacturer's protocol. Hierarchical
clustering was performed to determine similarity using complete
linkage and Euclidean distance using MeV software (version 4.2.6;
http://mev.tm4.org/#/welcome) (32). The miRNAs with a fold-change of
>2 in response to RANKL-stimulated osteoclast differentiation
compared with precursor cells were selected to determine the
expression profile.
Animal model
Female C57BL/6J mice (age, 8 weeks; body weight,
20±2 g) were obtained from the Animal Center of Shengjing Hospital
of China Medical University, and were allowed to acclimate for 1
week in a temperature-controlled environment (25±2˚C; humidity,
55±5%) under an artificial 12-h light/dark cycle with free access
to food and tap water. Mice were randomly divided into four groups
(n=6/group) and underwent either sham operation or bilateral OVX.
Agomir-miR-100-5p (5'-AAC CCG UAG AUC CGA ACU UGU G-3') and control
agomir (agomir-Con; 5'-AGU UUA GCU AAC GGU GAG CCG A-3') were
purchased from Guangzhou RiboBio Co. Ltd. Surgical operations were
performed under sodium pentobarbital anesthesia (50 mg/kg;
Sigma-Aldrich; Merck KGaA) by intraperitoneal injection. The sham
group received the same surgical operation as the OVX group without
ovariectomy, whereas mice in the OVX group the ovaries were
excised. The groups were as follows: i) Sham group, sham-operated
mice were treated with normal saline; ii) OVX group, OVX-operated
mice were treated with normal saline; iii) OVX + agomir-Con group,
OVX-operated mice were treated with agomir-Con; iv) OVX +
agomir-miR group, OVX-operated mice were treated with
agomir-miR-100-5p (100 mg/kg) via a tail vein injection. After a
4-week treatment with the agomirs (twice/week), mice were
euthanized and blood was harvested from the heart. Serum, liver and
tibia samples were immediately collected and maintained at −80°C
for further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from RANKL-stimulated
precursor cells, NCTC-1469 cells, and liver and tibia samples using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. RT was performed using
TaqMan® reverse transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. miR-100-5p was detected using TaqMan® MicroRNA
assay (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The thermocycling
conditions were as follows: 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec and 60°C for 60 sec. U6 small nuclear RNA was
used as an endogenous control. Relative miR-100-5p expression
levels were calculated using the 2−ΔΔCq method (33).
In addition, 2 µg total RNA was used to synthesize
cDNA with moloney murine leukemia virus reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. RT-qPCR was performed using the Applied
Biosystems 7300 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the TaqMan® Universal PCR
Master Mix (Thermo Fisher Scientific, Inc.). The cycling conditions
were as follows: 95°C for 10 min, followed by 40 cycles at 95°C for
15 sec, 60°C for 30 sec and 72°C for 30 sec, and a final extension
step at 72°C for 3 min. The relative mRNA expression levels were
calculated using the 2−ΔΔCq method (33) and were normalized to GAPDH. The
primers were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc. and sequences are presented in Table I.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| NFATc1 |
GCGAAGCCCAAGTCTCTTTCC |
GTATGGACCAGAATGTGA |
| Ctsk |
AGGCGGAGGTCGATGCCCCG |
CACGATGATGTCACCCTCGATGT |
| MMP-9 |
ACGCAGACATCGTCATCCAG |
CAGGGACCACAACTCGTCAT |
| TRAP |
GCTACTTGCGGTTTCACTATGGA |
TGGTCATTTCTTTGGGGCTTATCT |
| Runx2 |
GGCAGGTGCTTCAGAACTGG |
GTGGTGGCAGGTAGGTATGG |
| Osterix |
TGAGCTGGAACGTCACGTGC |
AAGAGGAGGCCAGCCAGACA |
| Osteocalcin |
CTCAGGGTTTCAGTGGTT |
TTTCCACGAGCACCCATC |
| ALP | CCCTCTCCAAGA
CATATAACAC |
TTGCCCTGAGTGGTGTTG |
| OPG |
CACACACACTGGGGACTCTG |
CAGCTGTGAGGAGAGGAAGG |
| RANKL |
CACAGCCCTCTCTCTTGAGC |
GACTGTGACCCCCTTCCATA |
| CA2 |
CATTACTGTCAGCAGCGAGCA |
GACGCCAGTTGTCCACCATC |
| FGF21 |
CAGGGAGGATGGAACAGTGGTA |
TGACACCCAGGATTTGAATGAC |
| GAPDH |
CACCATGGAGAAGGCCGGGG |
GACGGACACATTGGGGGTAG |
Cell transfection and plasmid
constructs
Pre-miR-control (Pre-miR-Con) and pre-miR-100-5p
were synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Osteoclast precursors (1×105) were seeded in 6-well
plates and trans-fected with pre-miR-Con (5'-AGU UUA GCU AAC GGU
GAG C CG A-3') or pre-miR-100-5p (5'-AAC CCG UAG AUC CGA ACU UGU
G-3') using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientifc, Inc., Waltham, MA, USA) for 48 h at 37°C at final
concentrations of 100 nM, according to the manufacturer's
protocol.
TRAP staining
RANKL-stimulated precursor cells and tibia samples
were fixed in 4% paraformaldehyde for 20 min and 24 h,
respectively, at room temperature. Tibia histological sections (5
µm) were obtained following decalcification in 0.5 M EDTA (pH 8.0)
and were embedded in paraffin, according to standard histological
procedures. RANKL-stimulated precursor cells and tibia histological
sections were stained for TRAP activity using a 0.1 M acetate
solution (pH 5.0) containing 6.76 mM sodium tartrate, 0.1 mg/ml
naphthol ASMX phosphate and 0.5 mg/ml Fast Red Violet. The TRAP
staining kit was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). TRAP-positive multinucleated cells with three
or more nuclei were scored and visualized under a microscope (Leica
DM 2500; Leica Microsystems GmbH, Wetzlar, Germany).
Hematoxylin and eosin (H&E)
staining
Tibias were fixed with 4% formalin at room
temperature for 24 h, decalcified in 0.5 M EDTA (pH 8.0) and were
then embedded in paraffin, according to standard histological
procedures. Tibia tissues were cut into 5 µm sections, which were
stained with H&E (Beyotime Institute of Biotechnology, Haimen,
China), according to the manufacturer's protocol. Stained slides
were visualized under an optical microscope (Leica DM 2500; Leica
Microsystems GmbH) and were analyzed using the OsteoMeasure system
(OsteoMetrics Inc., Decatur, GA, USA).
Bone Ca content and BMD
Ca content in the tibia was determined following
incineration of the samples using a muffle furnace (Thermo Fisher
Scientific, Inc.) at 800°C for 12 h. Subsequently, 10 mg bone ash
was dissolved in 1 ml 37% HCl diluted with Milli-Q®
water. The Ca content was determined using a kit (cat. no. C004-3;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China),
according to the manufacturer's protocol. The BMD of the tibia was
measured by dual-energy X-ray absorptiometry (Lunar DPXIQ; GE
Healthcare, Chicago, IL, USA), as described previously (34).
Physiological and biochemical markers in
serum and urine samples
The levels of FGF21 in mouse serum were detected
using a mouse bioactive ELISA kit (cat. no. E-EL-M0029c;
Elabscience Biotechnology, Wuhan, China), according to the
manufacturer's protocol. The levels of Ca (cat. no. C004-3),
phosphorus (P; cat. no. C006) and creatinine (cat. no. C011-1) in
serum and urine samples were measured using kits (Nanjing Jiancheng
Bioengineering Institute), according to the manufacturer's
protocol. Parathyroid hormone (cat. no. 60-2305; Immutopics, Inc.,
San Clemente, CA, USA), alkaline phosphatase (cat. no. E-EL-M2720c;
ALP; Elabscience Biotechnology), TRAP-5b (cat. no. SB-TR103;
Immunodiagnostic Systems, Inc., Scottsdale, AZ, USA), osteocalcin
(cat. no. 60-1305; Immutopics, Inc.) and C-terminal telopeptide of
type 1 collagen (CTX; E-EL-M0366c; Elabscience Biotechnology) were
detected using mouse bioactive ELISA kits, according to the
manufacturers' protocols.
Western blotting
Proteins were extracted from RANKL-stimulated
precursor cells, NCTC-1469 cells, and liver and tibia samples using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Protein concentration was measured using the
Bicinchoninic Acid kit for protein determination (cat. no.
BCA1-1KT; Sigma-Aldrich; Merck KGaA). Western blotting was
conducted as previously described (35). The membranes were incubated with
the following primary antibodies at room temperature for 2 h: TRAP
(cat. no. ab126775; 1:2,000; Abcam, Cambridge, UK), RANK (cat. no.
sc-59981; 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), RANKL (cat. no. ab45039; 1:1,000; Abcam), NFATc1 (cat. no.
sc-7294; 1:1,000; Santa Cruz Biotechnology, Inc.) and FGF21 (cat.
no. ab64857; 1:1,000; Abcam). Following incubation with primary
antibodies, the membranes were incubated at room temperature for 1
h with the appropriate horseradish peroxidase-conjugated anti-mouse
secondary antibody (cat. no. sc-516102; 1:10,000; Santa Cruz
Biotechnology, Inc.) and anti-rabbit secondary antibody (cat. no.
sc-2357; 1:10,000; Santa Cruz Biotechnology, Inc.) and were
visualized using chemiluminescence (Thermo Fisher Scientific,
Inc.). β-actin (1:2,000; cat. no. sc-130065; Santa Cruz
Biotechnology, Inc.) was used as the control antibody. Signals were
analyzed with Quantity One® software version 4.5 (Bio
Rad Laboratories, Inc., Hercules, CA, USA).
Luciferase reporter assay
The wild type (WT) or mutant-type (MUT) 3'-UTRs of
FGF21 were synthesized by Guangzhou RiboBio Co., Ltd. and were
inserted into multiple cloning sites of the luciferase expressing
pMIR-REPORT vector (Ambion; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. For the luciferase assay,
BMMs (1×105) were seeded into 24-wells and
co-transfected with luciferase reporter vectors containing WT or
MUT 3'-UTR (0.5 µg) of FGF21, and pre-miR-Con or pre-miR-100-5p
(100 nM) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) at 37°C for 48 h. Luciferase activity was
measured using a dual luciferase reporter assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol; and the WT vector group was used to normalize luciferase
activity.
Statistical analysis
Data are presented as the means ± standard error of
the mean. Statistical analysis was performed using SPSS Statistics
version 19.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism
version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Student's
t-test was used to analyze differences between two groups.
Inter-group differences were analyzed by one-way analysis of
variance, followed by a post hoc Tukey test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-100-5p is downregulated during RANKL-
and M-CSF-induced osteoclastogenesis
BMMs were initially cultured in M-CSF (50 ng/ml) for
3 days to induce formation of osteoclast precursors, which were
stimulated by M-CSF (50 ng/ml) and RANKL (100 ng/ml) for a further
3 days to induce osteoclastogenesis (Fig. 1A). A miRNA microarray analysis was
conducted to determine the molecular mechanism involved in RANKL-
and M-CSF-induced osteoclastogenesis. The results demonstrated that
31 miRNAs were differentially expressed in osteoclast precursors
with RANKL stimulation, compared with in those without RANKL
stimulation, among which 13 and 18 miRNAs were downregulated and
upregulated, respectively (Fig.
1B). The expression levels of miR-100-5p were most
significantly decreased in RANKL-stimulated cells. In addition, the
expression of miR-100-5p was validated by RT-qPCR; the results were
consistent with the microarray analysis and indicated that
expression was decreased in a time-dependent manner in response to
RANKL- and M-CSF-induced osteoclastogenesis (Fig. 1C). Furthermore, the protein
expression levels of TRAP were significantly augmented during the
process of osteoclastogenesis (Fig.
1D).
Overexpression of miR-100-5p suppresses
RANKL- and M-CSF-induced osteoclastogenesis
To investigate the function of miR-100-5p in
vitro, pre-miR-100-5p was transfected into osteoclast
precursors, in order to amplify the expression of miR-100-5p; the
results revealed a significant upregulation (~120-fold) of
miR-100-5p in osteoclast precursors (Fig. 2A). Post-transfection with
pre-miR-100-5p, the key transcription factor for
osteoclastogenesis, NFATc1 (Fig.
2B), and osteoclast-specific genes, Ctsk (Fig. 2C), matrix metalloproteinase-9
(MMP-9) (Fig. 2D) and TRAP
(Fig. 2E), were significantly
decreased compared with in the pre-miR-Con group. Furthermore,
osteoclast differentiation (TRAP-positive multinucleated cells) was
blocked by pre-miR-100-5p (Fig.
2F). These findings indicated that miR-100-5p, as a suppressor,
may neutralize RANKL- and M-CSF-induced osteoclastogenesis.
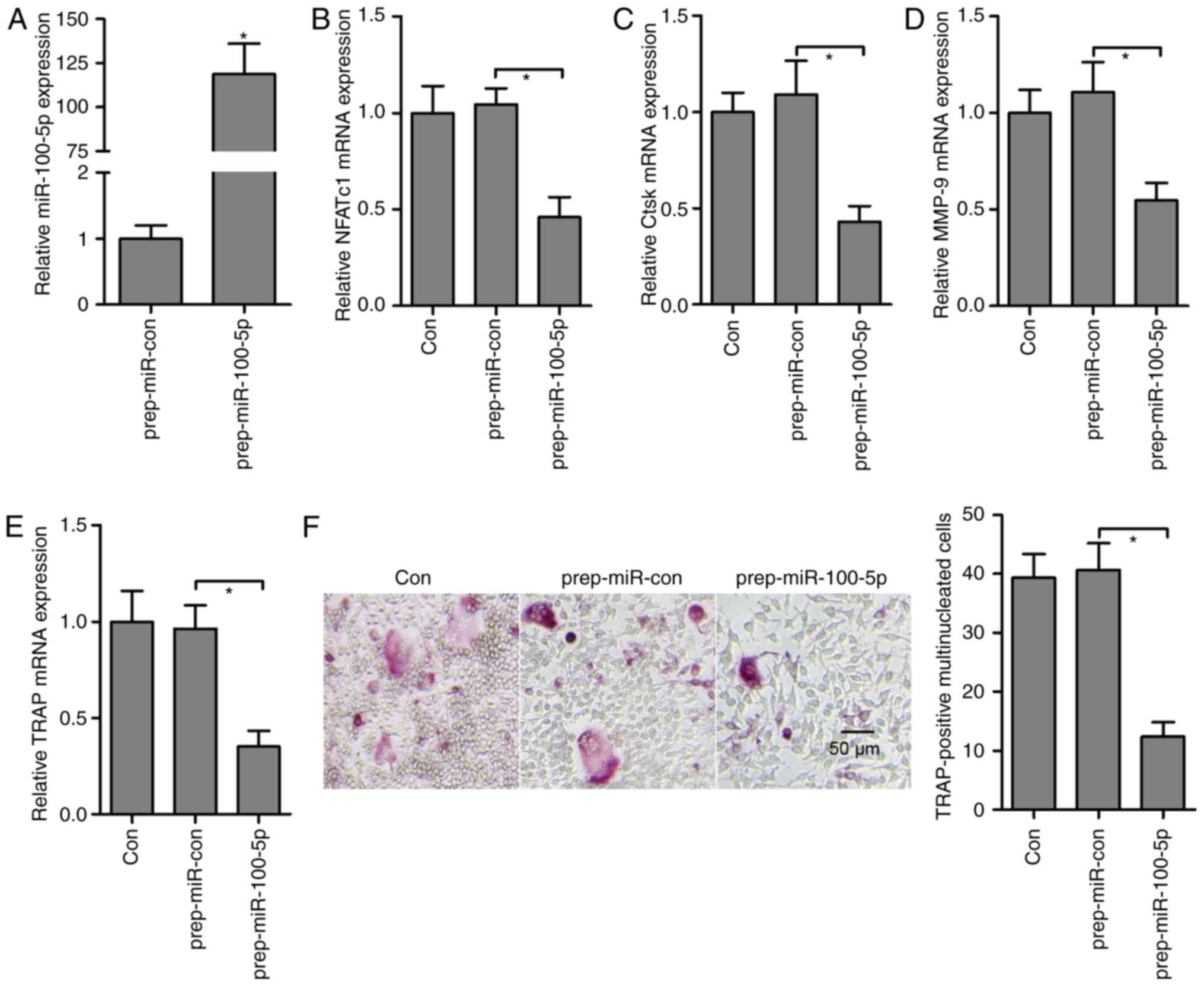 | Figure 2miR-100-5p gain-of-function inhibits
osteoclast differentiation. (A) Expression levels of miR-100-5p
were measured by RT-qPCR in osteoclast precursors post-transfection
with pre-miR-100-5p prior to RANKL stimulation. Expression of
osteoclast-specific genes, (B) NFATc1, (C) Ctsk, (D) MMP-9 and (E)
TRAP, was detected by RT-qPCR in response to M-CSF- and
RANKL-induced osteoclastogenesis and pre-miR-100-5p transfection.
(F) Osteoclast precursor cells transfected with pre-miR-100-5p were
cultured for an additional 3 days with M-CSF and RANKL, after
which, cultured cells were fixed and stained for TRAP, and the
number of osteoclasts was counted. Triplicate experiments were
performed in each group. *P<0.05. Ctsk, cathepsin K;
M-CSF, macrophage colony-stimulating factor; miRNA/miR, microRNA;
MMP-9, matrix metalloproteinase-9; NFATc1, nuclear factor of
activated T cells, cytoplasmic 1; pre-miR-Con, pre-miR-control;
RANKL, receptor activator of nuclear factor-κB ligand; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; TRAP,
tartrate-resistant acid phosphatase. |
Agomir-miR-100-5p blocks OVX-induced bone
resorption
The present study also examined whether
overexpression of miR-100-5p regulates bone metabolism in
vivo. Osteoporotic status was induced by OVX in mice, and
OVX-operated mice were administered agomir-miR-100-5p or agomir-Con
via a tail vein injection. ELISA analyses revealed that bone
formation markers, ALP and osteocalcin, and bone resorption
markers, TRAP-5b and CTX, were downregulated and upregulated,
respectively, in OVX-operated mice. Conversely, agomir-miR-100-5p
treatment reversed OVX-induced marker alterations, with the
exception of serum ALP (Table
II).
 | Table IIBiochemical parameters in
OVX-operated mice treated with agomir-miR. |
Table II
Biochemical parameters in
OVX-operated mice treated with agomir-miR.
| Parameters | Sham | OVX | OVX +
agomir-con | OVX +
agomir-miR |
|---|
| Serum Ca
(mg/dl) | 10.67±0.43 | 9.12±0.40a | 9.37±0.32 | 10.93±0.45b |
| Serum P
(mg/dl) | 6.06±0.31 | 6.12±0.35 | 6.25±0.36 | 6.17±0.32 |
| Urine Ca/Cre
(mg/mg) | 0.114±0.012 |
0.223±0.027a | 0.207±0.021 | 0.133±0.016b |
| Urine P/Cre
(mg/mg) | 1.41±0.20 | 1.39±0.26 | 1.55±0.33 | 1.53±0.31 |
| ALP (ng/ml) | 3.05±0.23 | 1.90±0.21a | 1.75±0.26 | 3.82±0.30 |
| Osteocalcin
(ng/ml) | 129.3±15.3 | 76.7±16.1a | 68.7±12.3 | 110.3±9.5b |
| TRAP-5b (U/l) | 8.94±0.42 | 12.75±0.54a | 12.70±0.58 | 10.09±0.45b |
| CTX (ng/ml) | 15.37±1.66 | 27.57±2.87a | 26.90±2.22 | 12.43±0.83b |
| Serum PTH
(pg/ml) | 123.3±24.5 | 256.3±35.6a | 271.3±33.2 | 171.3±26.6b |
RT-qPCR analysis revealed that miR-100-5p levels
were significantly downregulated in the tibia from OVX-operated
mice compared with in the tibia from sham-operated mice, whereas in
OVX-operated mice injected with agomir-miR-100-5p OVX-inhibited
miR-100-5p levels were markedly rescued in the tibia (Fig. 3A). Subsequently, the mRNA
expression levels of osteoblast-specific genes [Runt-related
transcription factor 2 (Runx2), osterix, osteocalcin and ALP] were
detected, and it was confirmed that these genes were reduced in the
tibia from OVX-operated mice, whereas agomir-miR-100-5p
administration in OVX-operated mice significantly upregulated the
mRNA expression levels of Runx2, osterix, osteocalcin and ALP
(Fig. 3B). Conversely, the mRNA
ratio of RANKL/osteoprotegrin (OPG) was elevated in the tibia from
OVX-operated mice, suggesting that an increase in osteoclast
activity in OVX-operated mice may be associated with overactivation
of RANKL availability; however, upregulation of the RANKL/OPG ratio
was reversed by agomir-miR-100-5p administration in OVX-operated
mice (Fig. 3C). To further
investigate whether miR-100-5p inhibited OVX-induced bone
resorption, osteoclast-specific genes, NFATc1, Ctsk, MMP-9,
carbonic anhydrase 2 (CA2) and TRAP, were detected by RT-qPCR; the
results demonstrated that agomir-miR-100-5p treatment markedly
abolished OVX-induced upregulation of osteoclast-specific genes in
the tibia (Fig. 3D). In addition,
as determined by western blotting, OVX-operated mice exhibited a
significant increase in RANK, RANKL and NFATc1, whereas
agomir-miR-100-5p administration decreased the protein expression
levels of RANK, RANKL and NFATc1 in the tibia from OVX-operated
mice(Fig. 3E and 3F).
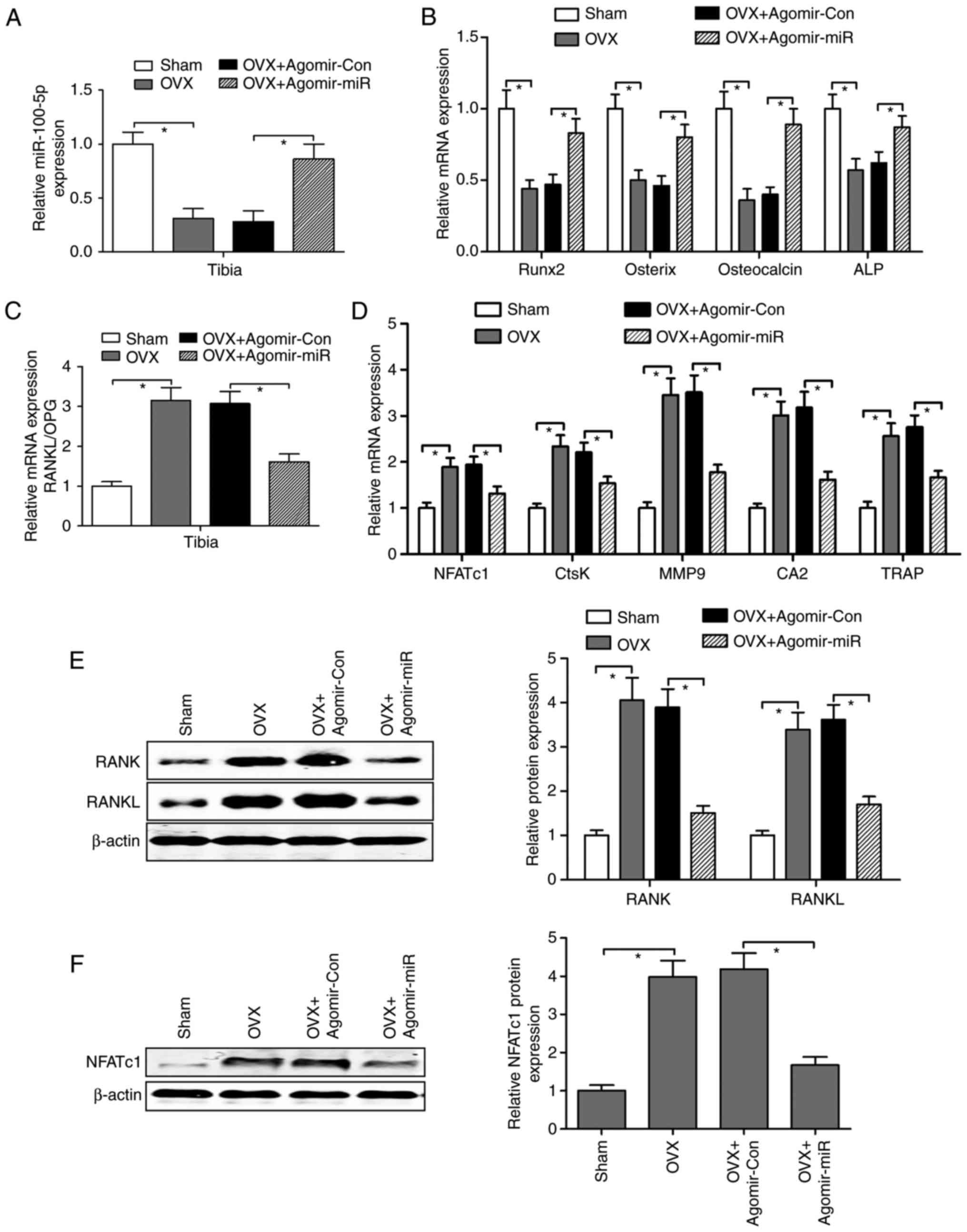 | Figure 3Agomir-miR regulates osteoblast- and
osteoclast-specific gene expression in OVX-operated mice. (A) Mice
in the OVX group were injected intravenously with agomir-miR-100-5p
or agomir-Con, and the expression levels of miR-100-5p were
measured by RT-qPCR in the tibia. (B) Osteoblast-specific genes,
Runx2, osterix, osteocalcin and ALP, were detected in the tibia by
RT-qPCR. (C) Ratio of RANKL mRNA to OPG mRNA in the tibia was
calculated as a marker of osteoclast activity. (D) RT-qPCR was
performed to analyze the mRNA expression levels of
osteoclast-specific genes in the tibia. (E and F) Protein
expression levels of RANK, RANKL and NFATc1 in the tibia were
detected using western blotting. Triplicate experiments were
performed in each group. *P<0.05. agomir-miR,
miR-100-5p agomir; agomir-Con, control agomir; CA2, carbonic
anhydrase 2; Ctsk, cathepsin K; miR, microRNA; MMP-9, matrix
metalloproteinase-9; NFATc1, nuclear factor of activated T cells,
cytoplasmic 1; OPG, osteoprotegrin; OVX, ovariectomy; RANK,
receptor activator of nuclear factor-κB RANKL, RANK ligand;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; TRAP, tartrate-resistant acid phosphatase. |
Ca and P metabolism were also analyzed in
OVX-operated mice in response to agomir-miR-100-5p administration.
Compared with in agomir-Con treated OVX-operated mice,
agomir-miR-100-5p administration significantly increased serum Ca
concentration and blocked urinary Ca secretion; however, the serum
and urine levels of P were not markedly altered in response to OVX
operation or agomir-miR-100-5p administration (Table II). Bone Ca content and tibia BMD
were decreased by OVX and were increased by agomir-miR-100-5p
administration in OVX-operated mice (Fig. 4A and B). Histomorphological
observation by H&E staining revealed that the integrity of the
trabecular bone microstructure network was destroyed in the
proximal epiphysis of the tibia by OVX. However, agomir-miR-100-5p
administration markedly improved trabecular bone structure and
accelerated bone remodeling (Fig. 4C
and D). Furthermore, the function of agomir-miR-100-5p on
osteoclast differentiation was determined in vivo. TRAP
staining revealed that the number of osteoclasts was increased by
OVX, whereas osteoclast numbers were significantly decreased by
agomir-miR-100-5p treatment (Fig. 4E
and F). Collectively, these findings suggested that increased
bone mass in agomir-miR-100-5p-administered OVX-operated mice may
be caused by a combination of increased bone formation and
decreased bone resorption.
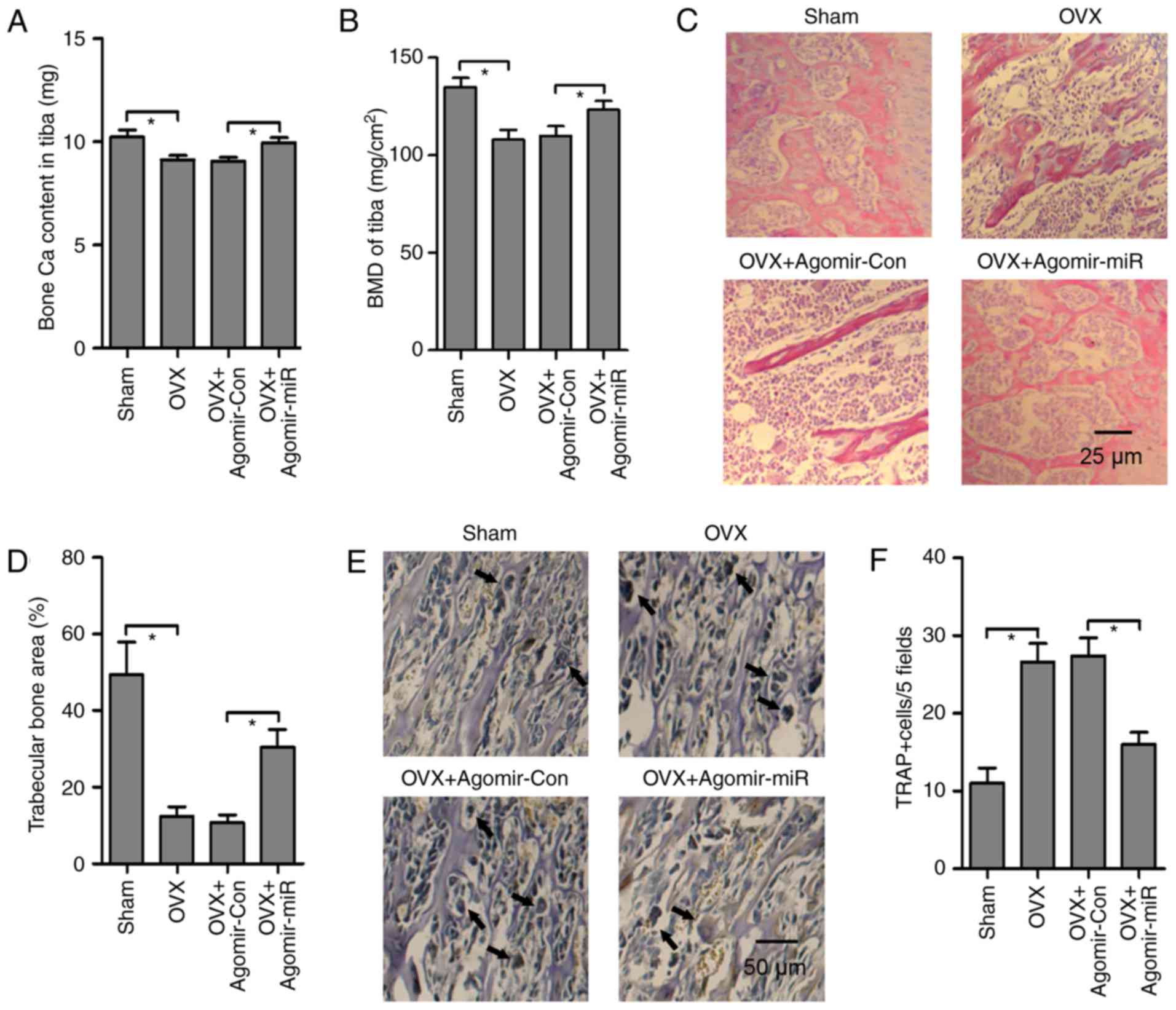 | Figure 4Agomir-miR inhibits bone loss in the
tibia from OVX-operated mice. (A) Bone Ca content and (B) tibia BMD
were measured in OVX-operated mice treated with agomir-miR or
agomir-Con injection. (C) Hematoxylin and eosin staining was
performed to assess the effects of agomir-miR on trabecular bone
microstructure in the proximal tibia from OVX-operated mice
(magnification, x200; scale bar, 25 µm). (D) Ratio of trabecular
bone area to total area in the proximal tibia was calculated to
estimate trabecular bone metabolism. (E) TRAP staining
(magnification, x100; scale bar, 50 µm) and (F) number of
osteoclasts in the tibia were measured to assess the effects of
agomir-miR on osteoclast activity (black arrows represent mature
osteoclasts). Triplicate experiments were performed in each group.
*P<0.05. Agomir-Con, control agomir; agomir-miR,
miR-100-5p agomir; BMD, bone mineral density; Ca, calcium; miR,
microRNA; OVX, ovariectomy; TRAP, tartrate-resistant acid
phosphatase. |
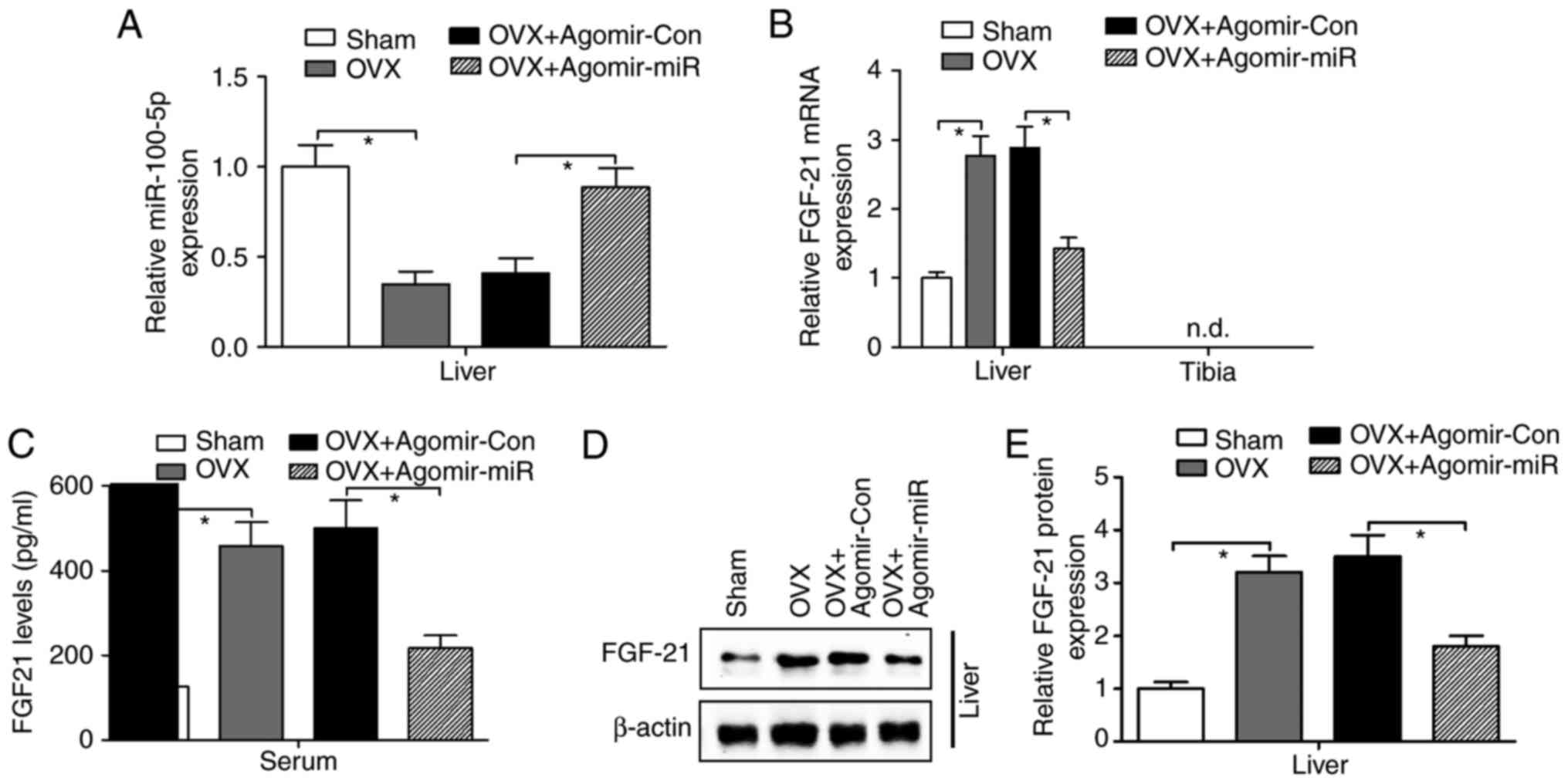
Agomir-miR-100-5p abolishes OVX-induced
FGF21 in the liver
Previous studies have reported that FGF21 is
inducible in the liver, but absent in the bone, and indirectly
promotes osteoclastogenesis and bone resorption via simultaneously
decreasing bone formation and increasing bone resorption (26,30). To determine the association
between miR-100-5p and FGF21, agomir-miR-100-5p or agomir-Con were
injected into OVX-operated mice via the tail vein; the results
revealed that miR-100-5p was significantly decreased in the liver
of OVX-operated mice compared with in the sham-operated group,
whereas agomir-miR-100-5p injection rescued OVX-induced
downregulation of miR-100-5p expression in the liver (Fig. 5A). Consistent with previous
findings (26,30), the present results indicated a
significant increase in the mRNA expression (Fig. 5B), serum levels (Fig. 5C) and protein expression (Fig. 5D and E) of FGF21 in the liver of
OVX-operated mice. Notably, both liver and serum FGF21 levels were
suppressed by agomir-miR-100-5p injection in OVX-operated mice
(Fig. 5B-E).
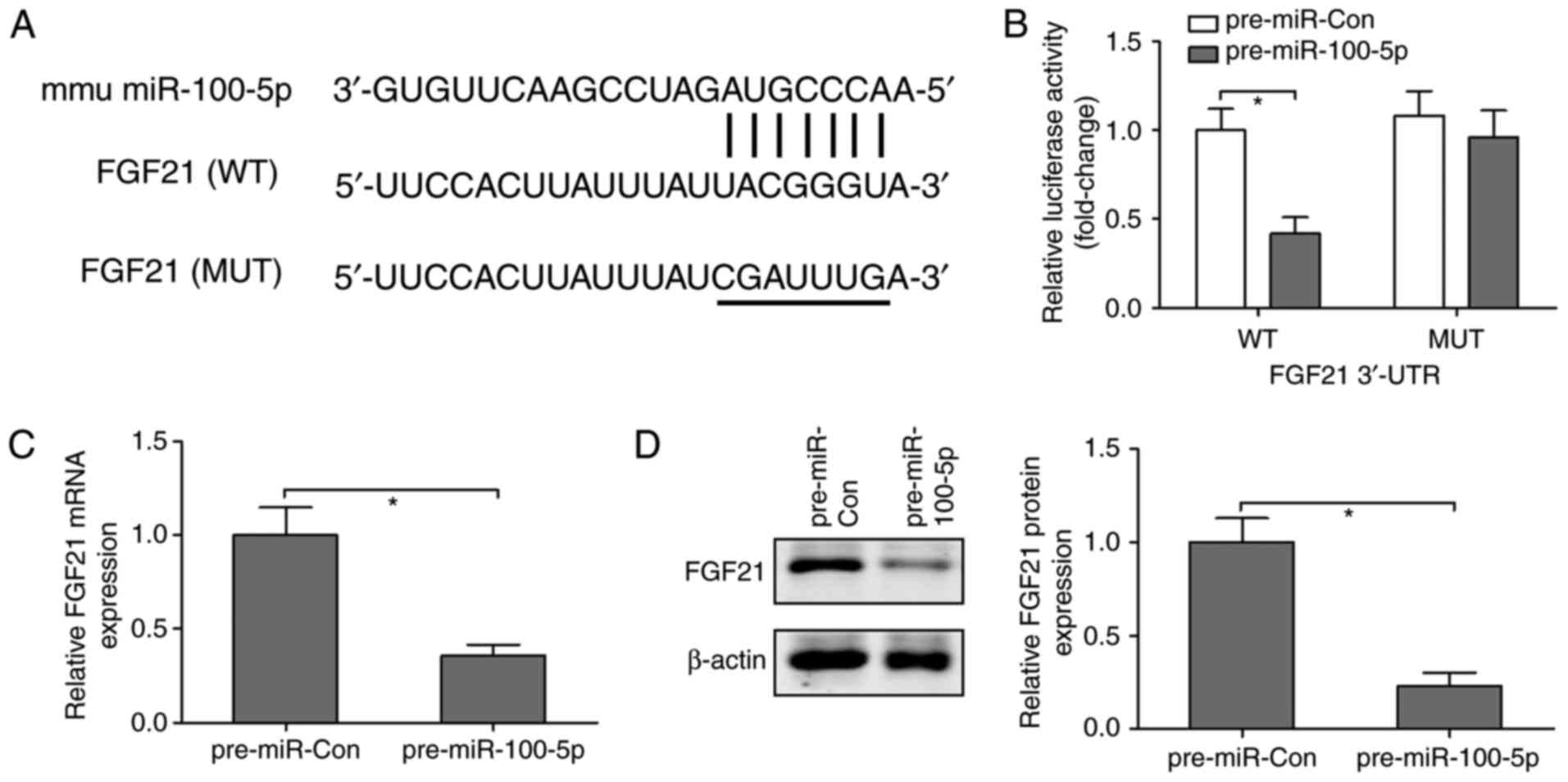
To further investigate whether FGF21 is a direct
target of miR-100-5p, the online prediction software TargetScan
(http://www.targetscan.org/vert_72/)
was used to predict the association between miR-100-5p and FGF21.
It was revealed that the 3'-UTR of FGF21 contained one conserved
binding site for miR-100-5p, which was presented as a complementary
pairing (Fig. 6A). To confirm
this finding, luciferase reporter plasmids containing WT or MUT
3'-UTR of FGF21 were co-transfected with pre-miR-Con and
pre-miR-100-5p into the NCTC-1469 mouse normal liver cell line. The
results revealed that the luciferase activity was reduced by ~60%
in cells transfected with plasmids containing WT 3'-UTR of FGF21
and pre-miR-100-5p; however, pre-miR-100-5p had no effect on
luciferase activity in cells transfected with plasmids containing
MUT 3'-UTR of FGF21 (Fig. 6B).
Furthermore, to identify the action of miR-100-5p on FGF21,
NCTC-1469 cells were transfected with pre-miR-Con or pre-miR-100-5p
and the mRNA and protein expression levels of FGF21 were measured.
Compared with in the pre-miR-Con group, overexpression of
miR-100-5p significantly downregulated the mRNA and protein
expression levels of FGF21 (Fig. 6C
and D). These findings suggested that FGF21 may be a direct
target of miR-100-5p.
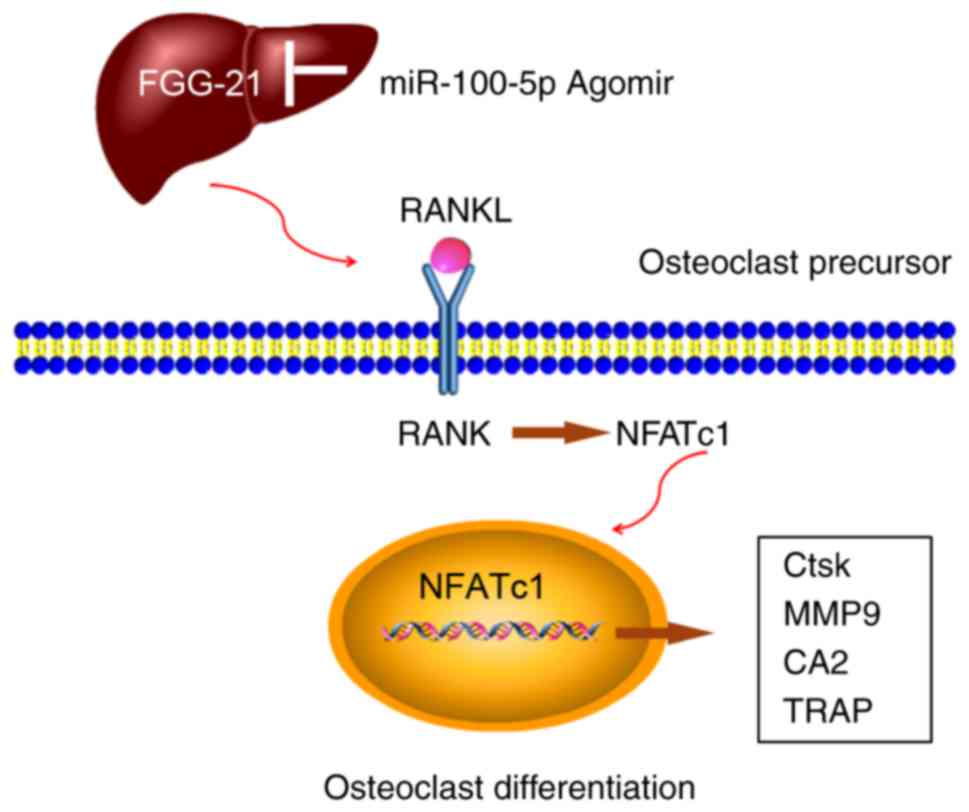
Based on the aforementioned findings, it was
revealed that miR-100-5p was inhibited during the process of
osteoclastogenesis in vitro and in OVX-induced bone loss
in vivo. FGF21, as a potential pro-osteoclastogenic liver
hormone, may be involved in RANKL/RANK/NFATc1 cascade
signaling-mediated osteoclast differentiation, whereas
agomir-miR-100-5p, as a post-transcriptional regulatory factor
targeted to the FGF21/RANKL/RANK/NFATc1 pathway, may block
osteoclastogenesis and OVX-induced bone loss (Fig. 7).
Discussion
The present study confirmed that the expression of
miR-100-5p was downregulated during RANKL-induced
osteoclastogenesis, as determined by microarray and RT-qPCR
analyses. miR-100-5p gain-of-function markedly inhibited the
process of osteoclast differentiation and negatively regulated
osteoclast function by suppressing the mRNA expression levels of
osteoclast-specific genes in vitro. The present results also
suggested that Ca metabolism and trabecular bone remodeling were
improved, and osteoclast differentiation and activity were
decreased by agomir-miR-100-5p in OVX-operated mice. Furthermore,
as a crucial liver hormone, FGF21 was upregulated and involved in
OVX-induced bone resorption. Notably, agomir-miR-100-5p abrogated
OVX-induced upregulation of FGF21 in the liver and serum; FGF21 was
validated as a direct target of miR-100-5p by bioinformatics
analysis and luciferase activity assay. Overexpression of
miR-100-5p resulted in the downregulation of FGF21 mRNA and protein
expression in NCTC-1469 cells. Based on these findings, it was
suggested that agomir-miR-100-5p alleviated osteoclast
differentiation and bone loss by blocking liver-secreted FGF-21;
FGF-21 may lead to overactivation of the RANKL/RANK/NFATc1
signaling pathway, thus mediating osteoclastic bone resorption.
miRNAs are post-transcriptional regulators that
mediate the expression of bone metabolism-associated genes at
transcriptional and translational levels (9-11,13). For example, overexpression of
miR-503 prevents bone resorption and osteoclast activity in
vitro and in vivo by inhibiting RANK expression
(11). miR-34a, as a key
osteoclast suppressor, blocks osteoporosis by suppressing
transforming growth factor-β-induced factor 2 in mice (9). Furthermore, miR-214 leads to a
decline in bone formation by inhibiting activating transcription
factor 4 and phosphatase and tensin homolog (10,13). These results suggested that a
regulatory mechanism including numerous miRNAs, or one miRNA, may
target various genes involved in the process of osteoclastogenesis
and bone loss (9-11,13). A previous study reported that
miR-100 modulates human mesenchymal stem cell (MSC) differentiation
into osteoblasts, and promotes osteogenesis in response to
mechanical stress (36).
Conversely, miR-100 serves a negative role in osteogenic
differentiation derived from human adipose-derived MSCs by
targeting bone morphogenetic protein receptor type II (18). The present study revealed that
downregulation of miR-100-5p was accompanied by an increase in the
expression levels of FGF21 in the liver samples of OVX-operated
mice. Further investigations indicated that FGF21 was a direct
target of miR-100-5p, which could inhibit FGF21 levels in the serum
and liver; osteoclast activity and bone loss were suppressed by
agomir-miR-100-5p injection in OVX-operated mice. These
observations provided an insight into liver-bone endocrine
signaling and suggested that miR-100-5p targeted FGF21 to inhibit
its hepatic expression and contributed to the regeneration of bone
under pathophysiological conditions.
FGF21 is a hepatokine that is mainly expressed in
the liver (26,30). FGF21 is considered a potential
novel drug for the treatment of obesity and diabetes by
potentiating the effect of peroxisome proliferator-activated
receptor γ (PPAR-γ) (37).
Furthermore, FGF21 inhibits osteoblastogenesis from MSCs and
stimulates osteoclastogenesis from hematopoietic stem cells by
activating PPAR-γ, whereas bone mass is markedly restored by FGF21
deletion (26,37). A previous study (36), and the present findings, revealed
that miR-100-5p exerted an opposite effect to FGF21 on osteoclast
differentiation. A functional study revealed that FGF21 was a
direct target of miR-100-5p, and agomir-miR-100-5p effectively
blocked FGF21 secretion from the liver, inhibited
osteoclastogenesis and osteoclast-specific gene expression, and
stimulated bone formation and osteoblast-specific gene expression.
FGF21 was inducible in the liver but absent in the bone, thus
suggesting that FGF21-stimulated osteoclast differentiation and
bone loss may be mediated via a liver-bone endocrine metabolic
mechanism in OVX-operated mice.
In conclusion, the present results demonstrated that
alterations in the expression levels of miR-100-5p may lead to an
alteration in bone homeostasis and osteoclast differentiation.
These phenomena may be associated with the hepatokine FGF-21. The
present experimental evidence from in vitro and in
vivo studies strongly indicated that miR-100-5p could prevent
bone resorption and osteoclastogenesis, at least partially, through
inhibition of FGF-21 secretion from the liver. This finding may
provide a novel insight into the management of osteoporosis and
osteoclast-associated bone diseases.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370981).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
The study was designed by LZ, HYS, LLG, LYY, SM and
QF. Literature research, data acquisition and data analysis were
performed by LZ, HYS and QF. Histological examination was conducted
by LLG, LYY and SM. Western blotting and RT-qPCR were performed by
LZ, HYS and QF. The manuscript was prepared and edited by LZ, HYS,
LLG, LYY, SM and QF. The manuscript was reviewed by LZ, HYS, LLG,
LYY, SM and QF. QF approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethical
Committee of Shengjing Hospital of China Medical University
(approval no. 2016PS361K).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Yuan FL, Wu QY, Miao ZN, Xu MH, Xu RS,
Jiang DL, Ye JX, Chen FH, Zhao MD, Wang HJ and Li X:
Osteoclast-derived extracellular vesicles: Novel regulators of
osteoclastogenesis and osteoclast-osteoblasts communication in bone
remodeling. Front Physiol. 9:6282018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neman J, Hambrecht A, Cadry C and Jandial
R: Stem cell-mediated osteogenesis: Therapeutic potential for bone
tissue engineering. Biologics. 6:47–57. 2012.PubMed/NCBI
|
|
3
|
Xiao W, Wang Y, Pacios S, Li S and Graves
DT: Cellular and molecular aspects of bone remodeling. Front Oral
Biol. 18:9–16. 2016. View Article : Google Scholar
|
|
4
|
Deng L, Wang Y, Peng Y, Wu Y, Ding Y,
Jiang Y, Shen Z and Fu Q: Osteoblast-derived microvesicles: A novel
mechanism for communication between osteoblasts and osteoclasts.
Bone. 79:37–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siddiqi MH, Siddiqi MZ, Ahn S, Kang S, Kim
YJ, Sathishkumar N, Yang DU and Yang DC: Ginseng saponins and the
treatment of osteoporosis: Mini literature review. J Ginseng Res.
37:261–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collison J: Bone: miR-106b promotes
osteoporosis in mice. Nat Rev Rheumatol. 13:1302017.
|
|
7
|
Szabo G and Bala S: MicroRNAs in liver
disease. Nat Rev Gastroenterol Hepatol. 10:542–552. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rottiers V and Naar AM: MicroRNAs in
metabolism and metabolic disorders. Nat Rev Mol Cell Biol.
13:239–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krzeszinski JY, Wei W, Huynh H, Jin Z,
Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, et
al: miR-34a blocks osteoporosis and bone metastasis by inhibiting
osteoclastogenesis and Tgif2. Nature. 512:431–435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Guo B, Li Q, Peng J, Yang Z, Wang
A, Li D, Hou Z, Lv K, Kan G, et al: miR-214 targets ATF4 to inhibit
bone formation. Nat Med. 19:93–100. 2013. View Article : Google Scholar
|
|
11
|
Chen C, Cheng P, Xie H, Zhou HD, Wu XP,
Liao EY and Luo XH: MiR-503 regulates osteoclastogenesis via
targeting RANK. J Bone Miner Res. 29:338–347. 2014. View Article : Google Scholar
|
|
12
|
Guo LJ, Liao L, Yang L, Li Y and Jiang TJ:
MiR-125a TNF receptor-associated factor 6 to inhibit
osteoclastogenesis. Exp Cell Res. 321:142–152. 2014. View Article : Google Scholar
|
|
13
|
Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao
D, Peng J, Wang A, Li Q, Song J, et al: miR-214 promotes
osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol.
12:343–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin Y, Tymen SD, Chen D, Fang ZJ, Zhao Y,
Dragas D, Dai Y, Marucha PT and Zhou X: MicroRNA-99 family targets
AKT/mTOR signaling pathway in dermal wound healing. PLoS One.
8:e644342013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Zhao X, Liu Q, Li C, Graves-Deal R,
Cao Z, Singh B, Franklin JL, Wang J, Hu H, et al: lncRNA
MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance
via Wnt/β-catenin signaling. Nat Med. 23:1331–1341. 2017.PubMed/NCBI
|
|
16
|
Liu M, Han T, Shi S and Chen E: Long
noncoding RNA HAGLROS regulates cell apoptosis and autophagy in
lipopolysaccharides-induced WI-38 cells via modulating
miR-100/NF-κB axis. Biochem Biophys Res Commun. 500:589–596. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seeliger C, Karpinski K, Haug AT, Vester
H, Schmitt A, Bauer JS and van Griensven M: Five freely circulating
miRNAs and bone tissue miRNAs are associated with osteoporotic
fractures. J Bone Miner Res. 29:1718–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng Y, Qu X, Li H, Huang S, Wang S, Xu Q,
Lin R, Han Q, Li J and Zhao RC: MicroRNA-100 regulates osteogenic
differentiation of human adipose-derived mesenchymal stem cells by
targeting BMPR2. FEBS Lett. 586:2375–2381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Xu Y, Hu Y and Wang G: The role of
fibroblast growth factor 21 in the pathogenesis of non-alcoholic
fatty liver disease and implications for therapy. Metabolism.
64:380–390. 2015. View Article : Google Scholar
|
|
20
|
Charoenphandhu N, Suntornsaratoon P,
Krishnamra N, Sa-Nguanmoo P, Tanajak P, Wang X, Liang G, Li X,
Jiang C, Chattipakorn N and Chattipakorn S: Fibroblast growth
factor-21 restores insulin sensitivity but induces aberrant bone
micro-structure in obese insulin-resistant rats. J Bone Miner
Metab. 35:142–149. 2017. View Article : Google Scholar
|
|
21
|
Anuwatmatee S, Tang S, Wu BJ, Rye KA and
Ong KL: Fibroblast growth factor 21 in chronic kidney disease. Clin
Chim Acta. S0009–8981. 30432–1. 2017.PubMed/NCBI
|
|
22
|
Planavila A, Redondo I, Hondares E,
Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt
M, van Bilsen M and Villarroya F: Fibroblast growth factor 21
protects against cardiac hypertrophy in mice. Nat Commun.
4:20192013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Desai BN, Singhal G, Watanabe M,
Stevanovic D, Lundasen T, Fisher FM, Mather ML, Vardeh HG, Douris
N, Adams AC, et al: Fibroblast growth factor 21 (FGF21) is robustly
induced by ethanol and has a protective role in ethanol associated
liver injury. Mol Metab. 6:1395–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Dong K, Fang Q, Hou X, Zhou M, Bao
Y, Xiang K, Xu A and Jia W: High serum level of fibroblast growth
factor 21 is an independent predictor of non-alcoholic fatty liver
disease: A 3-year prospective study in China. J Hepatol.
58:557–563. 2013. View Article : Google Scholar
|
|
25
|
Samson SL, Sathyanarayana P, Jogi M,
Gonzalez EV, Gutierrez A, Krishnamurthy R, Muthupillai R, Chan L
and Bajaj M: Exenatide decreases hepatic fibroblast growth factor
21 resistance in non-alcoholic fatty liver disease in a mouse model
of obesity and in a randomised controlled trial. Diabetologia.
54:3093–3100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei W, Dutchak PA, Wang X, Ding X, Wang X,
Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, et al:
Fibroblast growth factor 21 promotes bone loss by potentiating the
effects of peroxisome proliferator-activated receptor γ. Proc Natl
Acad Sci USA. 109:3143–3148. 2012. View Article : Google Scholar
|
|
27
|
Hao RH, Gao JL, Li M, Huang W, Zhu DL,
Thynn HN, Dong SS and Guo Y: Association between fibroblast growth
factor 21 and bone mineral density in adults. Endocrine.
59:296–303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gallego-Escuredo JM, Lamarca MK,
Villarroya J, Domingo JC, Mateo MG, Gutierrez MDM, Vidal F,
Villarroya F, Domingo P and Giralt M: High FGF21 levels are
associated with altered bone homeostasis in HIV-1-infected
patients. Metabolism. 71:163–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee P, Linderman J, Smith S, Brychta RJ,
Perron R, Idelson C, Werner CD, Chen KY and Celi FS: Fibroblast
growth factor 21 (FGF21) and bone: Is there a relationship in
humans. Osteoporos Int. 24:3053–3057. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Wei W, Krzeszinski JY, Wang Y and
Wan Y: A liver-bone endocrine relay by IGFBP1 promotes
osteoclasto-genesis and mediates FGF21-induced bone resorption.
Cell Metab. 22:811–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao H, Zhang J, Shao H, Liu J, Jin M,
Chen J and Huang Y: Transforming growth factor β1/Smad4 signaling
affects osteoclast differentiation via regulation of miR-155
expression. Mol Cells. 40:211–221. 2017.PubMed/NCBI
|
|
32
|
Saeed AI, Sharov V, White J, Li J, Liang
W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et
al: TM4: A free, open-source system for microarray data management
and analysis. Biotechniques. 34:374–378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
34
|
Gargiulo S, Gramanzini M, Megna R, Greco
A, Albanese S, Manfredi C and Brunetti A: Evaluation of growth
patterns and body composition in C57Bl/6J mice using dual energy
X-ray absorptiometry. Biomed Res Int. 2014:2530672014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu FY, Xie CQ, Sun JT, Peng W and Huang
XW: Overexpressed miR-145 inhibits osteoclastogenesis in
RANKL-induced bone marrow-derived macrophages and ovariectomized
mice by regulation of Smad3. Life Sci. 202:11–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frith JE, Kusuma GD, Carthew J, Li F,
Cloonan N, Gomez GA and Cooper-White JJ: Mechanically-sensitive
miRNAs bias human mesenchymal stem cell fate via mTOR signalling.
Nat Commun. 9:2572018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wan Y: Bone marrow mesenchymal stem cells:
Fat on and blast off by FGF21. Int J Biochem Cell Biol. 45:546–549.
2013. View Article : Google Scholar :
|