Introduction
The removal of afferents from adjacent dorsal root
ganglia (DRG) may initiate the sprouting of novel sensory neurons
to the denervated territory within the dorsal horn, which is known
as neuroplasticity of the spinal cord. The compensatory sprouting
of spared axons within the spinal cord following de-afferentation
has been confirmed previously (1-4).
Acupuncture, a well-known tool of traditional
Chinese medicine, has been reported to have therapeutic effects in
patients suffering from spinal cord injury (SCI).
Electro-acupuncture (EA), an effective acupuncture method, has been
demonstrated to be a promising therapeutic method for functional
recovery in patients with SCI (5). Previous studies have suggested that
EA may promote functional repair (5-8)
and enhance the intraspinal sprouting of spared afferents following
SCI (9). Accumulating evidence
suggested that alterations in the expression of neurotrophic
factors (NTFs) may be associated with EA therapy-induced
strengthening of spinal neuroplasticity following SCI (10,11). However, the underlying mechanisms
remain to be fully elucidated.
NTFs are important factors that influence neuronal
plasticity following nerve injury (12). Insulin-like growth factors (IGFs),
a sub-family of NTFs, are associated with neuronal growth. They
maintain neuronal survival and promote the proliferation,
differentiation and regeneration of injured neurons. In
vitro and in vivo studies have demonstrated that the IGF
system promotes the differentiation and proliferation of neurons
and sustains their survival, thus being key in brain development
(13-15). Insulin-like growth factor 1
(IGF-1), an isoform of the IGF family, is an NTF in the central
nervous system (CNS) that functions via autocrine and paracrine
signaling pathways (14). It has
been demonstrated that IGF-1 exerts neuroprotective and
proliferative effects through the promotion of cell survival
(15), prevention of apoptosis
(16) and stimulation of
neurogenesis within the injured CNS (17). However, the underlying mechanisms
are currently unclear.
Previous studies demonstrated that treadmill
exercise promotes the recovery of motor function by suppressing
apoptosis in the injured spinal cord. The beneficial effect of
exercise may be attributed to an increase in expression of NTFs,
including IGF-1, via activation of the phosphatidylinositol
3-kinase (PI3K)/Akt pathway (18). Impairment of insulin signaling
induction by IGF-1 has been demonstrated to be involved in
neurodegenerative diseases, including Alzheimer's disease (19-21) and Parkinson's disease (22-25). Our previous study investigated the
effects of EA on the endogenous expression of IGF-1 in the spared
DRG and the associated dorsal horns following adjacent dorsal root
ganglionectomies in cats, in order to understand the role of IGF-1
in the EA-mediated promotion of spinal cord plasticity. The results
demonstrated that the EA-induced increased expression of IGF-1 in
the spared L6 DRG and the associated dorsal horns may be associated
with the intra-spinal sprouting of the DRG neurons in the cats
subjected to adjacent dorsal root ganglionectomies (26). The aim of the present study was to
investigate the putative role of the IGF-1/PI3K/Akt signaling
pathway in the EA treatment induced-neuroplasticity of rats
receiving partial dorsal root ganglionectomies.
Materials and methods
Ethical aspects
The use and care of animals were in accordance with
animal care guidelines; they conformed to the Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health (Bethesda, MD, USA; publication no. 85-23,
revised 1996) and the Care and Use Guidelines of Experimental
Animals established by the Research Ethics Committee of Kunming
University of China (Kunming, China; permit no.
kmu-eac-2016047).
Construction of herpes simplex virus
(HSV)-IGF-1 and HSV-small interfering (si)RNA-IGF-1 vectors
The HSV CL-1 sequence (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), which does not contain ICP27,
ICP4 or ICP34.5 genes, was prepared for the framework construction
of the HSV vector. To generate the HSV-IGF-1 vector,
cytomegalovirus (CMV), Woodchuck hepatitis virus
post-transcriptional regulatory elements and poly A elements were
ligated into a pNX plasmid (pNX-CMV; Takara Biotechnology, Co.,
Ltd., Dalian, China). Full-length open reading frame (ORF) cDNA
clones of the IGF-1 gene (Rattus norvegicus; National Centre
for Biotechnology Information gene ID: 24482), were purchased from
Origene Technologies, Inc. (Rockville, MD, USA). A polymerase chain
reaction (PCR) was conducted to amplify the cDNA clones of IGF-1
ORF concluding restriction sites for HindIII and XhoI
enzymes, using the PCR Master Mix kit (Invitrogen; Thermo Fisher
Scientific, Inc.) for 30 cycles, consisting of denaturation at 94°C
for 1 min, annealing at 55°C for 30 sec, and extension at 72°C for
30 sec. The primers sequences were as follows: Sense, 5'-GAC TGG
ACT TGC TAT TGG GAC C-3' and anti-sense 5'-TAG GCT ATC TTG AGT CGG
ATT-3'. Subsequently, the ORF sequences were digested with
HindIII and XhoI restriction enzymes, and ligated to
pNX-CMV plasmid (pNX-CMV-IGF-1). The vector was subsequently
sequenced by Sanger sequencing. For HSV-siRNA-IGF-1 construction,
the recombinant pNX plasmid containing the U6 promoter and multiple
cloning sites (pNX-U6) was ligated with siRNA-IGF-1
(pNX-siRNA-IGF-1). The transformants were screened and identified
by PCR and restriction analyses. The pNX-CMV plasmid without cDNA
clones of the IGF-1 gene ORF was used as blank control for
pNX-CMV-IGF-1, and the pNX-U6 plasmid served as a control for
siRNA-IGF-1. Subsequently, DNA of the framework construction of the
HSV vector, pNX-CMV-IGF-1, pNX-CMV, pNX-siRNA-IGF-1 and pNX-U6 were
transfected into the OG01 cell line (Takara Biotechnology, Co.,
Ltd.) by calcium phosphate coprecipitation. The OG01 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum (FBS), 800 µg/ml neomycin and
700 µg/ml zeozin (all purchased from Invitrogen; Thermo
Fisher Scientific, Inc.). The successfully transfected cell clones
were selected for further PCR analysis, purification and
hyperfiltration to obtain the target HSV vectors, HSV-IGF-1,
HSV-pNX-CMV, HSV-siRNA-IGF-1 and HSV-pNX-U6, respectively. The
HSV-pNX-CMV and HSV-pNX-U6 vectors served as blank controls for
HSV-IGF-1 and HSV-siRNA-IGF-1, respectively.
Animal model
A total of 49 adult male Sprague-Dawley rats (weight
180-200 g, age 3-4 months), provided by the Laboratory Animal
Center of Kunming Medical University, were used in the present
study. The rats were housed with free access to food and water and
exposed to a 12-h light/dark cycle in a restricted access, in a
temperature-controlled (at 22°C) animal centre with 55-60%
humidity. All experimental procedures complied with the guidelines
for the care and use of animals stipulated by the National
Institutes of Health.
Details of the animal grouping are presented in
Table I. Group I rats served as
sham-operated controls that did not undergo DRG. The rats in Groups
II-VII were subjected to surgical removal of the bilateral four
lumbar (L1-L4) and sixth lumbar (L6) DRGs, sparing the L5 DRG. The
rats in Group II received dorsal root ganglionectomy only. The rats
in groups III, IV, V, VI and VII were subjected to bilateral dorsal
root ganglionectomies followed immediately by electrical
stimulation of the bilateral site at the zusanli (ST 36)-xuanzhong
(GB 39) and futu (ST 32)-sanyinjiao (SP 6) acupoints (7). The rats in groups IV, V, VI and VII
were injected with various HSV vectors at the site of the spared
DRG. The rats were then subjected to further analysis. They were
allowed to survive for 28 days post-operatively (dpo) as described
in Table I.
 | Table IAnimal grouping and treatments. |
Table I
Animal grouping and treatments.
| Group | Rats (n) | Treatment | Experimental
methods |
|---|
| I | 7 | Sham-operated |
RT-qPCR/WB/IHC/BBB/MWT/TWL |
| II | 7 | Model (operation
only) |
RT-qPCR/WB/IHC/BBB/MWT/TWL |
| III | 7 | EA Model (operation
+ EA) |
RT-qPCR/WB/IHC/BBB/MWT/TWL |
| IV | 7 | EA Model +
HSV-IGF-1 |
RT-qPCR/WB/IHC/BBB/MWT/TWL |
| V | 7 | EA Model +
HSV-pNX-CMV |
RT-qPCR/WB/IHC/BBB/MWT/TWL |
| VI | 7 | EA Model +
HSV-siRNA-IGF-1 |
RT-qPCR/WB/IHC/BBB/MWT/TWL |
| VII | 7 | EA Model +
HSV-pNX-U6 |
RT-qPCR/WB/IHC/BBB/MWT/TWL |
Prior to the surgical procedure, the animals were
deeply anesthetized with an intraperitoneal injection of
pentobarbital sodium solution (2%; 40 mg/kg body weight). A
bilateral hemilaminectomy was performed at lumbosacral levels as
described previously (7). The
articular processes of the respective vertebrae were removed with a
pair of rongeurs and the dura was cut with a small pair of
scissors. The L1-L4 and L6 DRGs were exposed and removed at their
respective intervertebral foramina, leaving the L5 DRG intact.
Following surgery, the overlaying skin and muscle were sutured, and
suitable post-operative care was provided regularly.
All injections into the DRG were performed using a
microprocessor-controlled injection system employing direct piston
displacement (Nanoliter 2000; World Precision Instruments,
Sarasota, FL, USA) mounted on a micromanipulator as described
previously (27). For direct
injection into the spared L5 DRG, the foramen was enlarged in a
cephalad direction using the rongeurs, in order to remove a
1-mm-deep crescent of laminar bone, exposing the distal third of
the DRG. A total of 2 µl HSV-IGF-1, HSV-siRNA-IGF-1, or the
associated control HSV-pNX-CMV and HSV-pNX-U6 vectors,
respectively, were injected into the DRG at a rate of 20 nl/sec.
Following completion of the injection, the pipette was left in
place for 5 min prior to removal to allow the fluid to distribute
and the pressure within the tissue to equalize. The wound was
closed in layers.
EA treatment
The rats in Groups III-VII were subjected to EA
treatment at two paired acupoints, zusanli (ST 36)-xuanzhong (GB
39) and futu (ST 32)-sanyinjiao (SP 6) (7,28).
Their locations were as follows: ST 36 is located 0.5 cm below the
front of the fibula head; GB 39, 1.0 cm above the front of the
lateral malleolus; ST 32, 1.5 cm above the lower end of the
patella; and SP 6, 0.5 cm above the posterior end of the medial
malleolus. These acupoints are known to lie in the dermatome of L5
as described in previous report (7). The rats were placed in plastic
holders to prevent movement of their body and hindlimbs when
treated with EA, with the animals remaining awake without
anesthesia in a convenient and safe manner. When the rats had been
acclimated to the holders, EA stimulation was performed. During EA
treatment, the conditions of the rats were monitored. If any signs
of discomfort were observed in the rat induced by EA, the EA
treatment was immediately terminated. Every day, these acupoints
were stimulated alternately at a frequency of 98-HZ pulses/min at
5V (98 HZ/5V), at 1.0 mA for 30 min delivered by an EA apparatus
(Hans electro-stimulator; Nanjing Jisheng Medical Technology Co.,
Ltd., Nanjing, China) with the electrodes connected to two
acupuncture needles. The electrodes were replaced every 15 min
during each acupuncture. A preliminary experiment was performed to
evaluate the effects of different stimulation intensities of EA on
rats and select the optimal intensity and duration. An EA intensity
at 98 HZ/5V was selected for the present study (data not
shown).
Evaluation of hindlimb locomotor
function
The hindlimb locomotor function of the rats in each
group was assessed using the Basso, Beattie, Bresnahan (BBB) rating
scale (29) at 7, 14, 21 and 28
dpo. The BBB scores following transfection ranged between 0 and 21.
The animals were allowed to walk around freely in an open field for
4 min, during which hindlimb movements were closely observed. Three
double-blinded individuals conducted the evaluations, and their
average scores were calculated. All behavioral evaluations were
performed daily at 8:00-9:00 a.m. following evacuation of the
bladder.
Mechanical withdrawal threshold (MWT)
test
MWT was determined for each hind-paw using von Frey
filaments (0.4-15.0 g; Stoelting, Co., Wood Dale, IL, USA) and an
‘up and down’ procedure between 10:00 a.m. and 12:00 p.m. every day
post-surgery, as previously described (30-34). If a withdrawal response to a
particular hair was observed at least five times, the value of that
hair in grams was considered as the withdrawal threshold. If a
withdrawal response did not occur with the 15.0 g von Frey
filament, it was considered a painless response. Paw withdrawal due
to animal movement was not considered a positive response. The data
were analyzed using the Dixon non-parametric test (31,35). Details of the treatment groups are
presented in Table I. A total of
seven animals were included in each treatment group.
Thermal withdrawal latency (TWL)
detection
TWL was assessed to determine the thermal
sensitivity of rats using a Hargreave's heat source (3A) with a
Halogen Photo Optic lamp (15V, 150W) (36). The average temperature at the
animal's hind-paw surface was 36.2°C at 10 sec, 39.2°C at 14 sec
and 41.3°C at 16 sec. The animals were placed in a clear Plexiglass
box on an elevated platform and allowed to acclimatize for 10 min.
A radiant heat source with constant intensity was aimed at the
mid-planter area of the hind-paw.
The paw TWL was recorded using a timer three times
with 10-min intervals between each trial, and the mean of these
three trials was then calculated. A cut-off time of 30 sec was used
to prevent potential tissue damage. If no paw withdrawal occurred
by 30 sec, the radiant heat was removed and TWL was recorded as 30
sec (34).
DRG culture
DRGs were isolated from the neonatal Sprague-Dawley
rats as described previously (37) with minor modifications. The rats
were sacrificed by exposure to increasing concentrations of
CO2 followed by cervical dislocation. Ganglia from all
spinal levels were dissected in chilled PBS (Invitrogen; Thermo
Fisher Scientific, Inc.). The DRG tissues were dissociated
following enzymatic digestion in 0.25% trypsin-EDTA (Gibco; Thermo
Fisher Scientific, Inc.). The tissue was triturated using a 1-ml
Gilson pipette. The DRG neurons were separated from myelin and
cellular debris by centrifugation for 10 min at 145 × g and 20-25°C
in 2 ml DMEM/Ham's F12 (F12; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS. Pellets containing the DRG neurons were
resuspended in DMEM/F12 media, containing 100 U/ml penicillin G,
100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific,
Inc.) and 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.). The
cells were maintained at 37°C, 95% O2 and 5%
CO2 prior to analysis.
DRG neuron transfection
The HSV-IGF-1, HSV-siRNA-IGF-1, HSV-pNX-CMV and
HSV-pNX-U6 vectors were each transfected into DRG neurons using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. In
total, ~1×105 DRG neurons were seeded in 35-mm plates
(24-well) and maintained in a conditioned medium consisting of
DMEM/F12 (1:1) supplemented with 100 U/ml penicillin G, 100
µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
and 10% FBS. The cells were incubated at 37°C, 5% CO2
overnight and the fresh DMEM/F12 medium was replaced every 2 days.
When the cultured cells reached 60% confluence, they were
transfected with the HSV-IGF-1, HSV-siRNA-IGF-1, HSV-pNX-CMV or
HSV-pNX-U6 plasmids. At 3 days post-transfection, the cells were
digested with 0.25% trypsin for 8 min, followed by the addition of
FBS containing medium to inhibit trypsin. The samples were
subsequently centrifuged at 500 × g and 4°C for 30 min. To confirm
successful transfection of the HSV-IGF-1 vector constructs, the
cells were subsequently subjected to reverse
transcription-quantitative PCR (RT-qPCR) and western blot analyses.
The effects of HSV-IGF-1 and HSV-siRNA-IGF-1 on DRG neural growth
were additionally evaluated by measuring the axon length, areas of
neurons and cell numbers using a LEICA DMI6000B microscope (LAS AF
system; Leica Microsystems GmbH, Wetzlar, Germany; magnification,
×200).
The PI3K/Akt inhibitor and Akt siRNA were used to
investigate the role of the PI3K/Akt signaling pathway in
neuroprotection induced by IGF-1. A specific synthetic inhibitor of
PI3K, LY294002 (cat. no. sc391584; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) was used to pre-treat the DRG neurons for 30 min
at a final concentration of 20 µmol/l (38). The cells were subsequently
transfected with HSV-IGF-1 using the aforementioned procedures. The
cells in the control group were treated with dimethyl
sulfoxide.
Specific siRNA duplexes targeting Akt were used to
inhibit the expression of Akt. The siRNA sequences were as follows:
5'-UUC AGG UAC UCA AAC UCG UUC AUG G-3' and 5'-CCA UGA ACG AGU UUG
AGU ACC UGA A-3' (39), and were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. Scrambled
siRNA duplexes served as a control. The Akt or scrambled siRNAs
were transfected into DRG neurons using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following
HSV-IGF-1 transfection, according to the manufacturer's
protocol.
RT-qPCR analysis
RT-qPCR was used to quantify the mRNA expression
levels of IGF-1. All the primers employed in the present study were
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. The
cultured DRG neurons and spared L5 DRG samples in the rats were
additionally obtained for IGF-1 detection, as previously described
(40). TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract
the total RNA, according to the manufacturer's protocol. Total RNA
was eluted in 20 µl RNase-free DNase I to remove DNA
contamination. RNA samples were kept on ice and their
concentrations were measured using a NanoDrop spectrophotometer
(NanoDrop-1000; NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, De, USA). For RNA amplification, the first-strand
cDNA was synthesized from 2 µg total RNA, using Revert Aid™
First Strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd.).
In total, 2 µg total RNA was incubated with 1 µl
oligo (dT) 18 primers and 10 µl diethylpyrocarbonate-treated
water at 70°C for 5 min, followed by cooling on ice. This mixture
was subsequently supplied with 4 µl 5X reaction buffer, 1
µl Riboblock™ Ribonuclease inhibitor and 10 mM dNTP, and
incubated at 37°C for 5 min. For cDNA synthesis, 1 µl Revert
Aid™ M-Mulv Reverse Transcriptase was added to this mixture for a
final incubation at 42°C for 1 h, which was terminated by heating
at 70°C for 10 min. PCR was subsequently conducted using the 2x Mix
SYBR-Green I (Beijing Biosea Biotechnology Co., Ltd., Beijing,
China; 10 µl), primer (0.25 µl; 10 pmol/l), template
DNA (1 µl) and sterile water (8.5 µl). qPCR
amplification was performed at 95°C for 3 min, followed by 39
thermocycling steps consisting of denaturation at 94°C for 30 sec,
annealing at 58°C for 30 sec, and extension at 72°C for 30 sec. The
primer sequences were as follows: GAPDH forward, 5'-CGA GAT CCC TCC
AAA ATC AA-3' and reverse, 5'-TTC ACA CCC ATG ACG AAC AT-3'; and
IGF-1 forward, 5'-GGT GGA TGC TCT TCA GTT C-3' and reverse, 5'-TTT
GTA GGC TTC AGT GGG-3'. The relative quantification cycle (Cq)
method was used to compare target gene expression between samples.
Fold changes in target gene expression were determined relative to
a blank control following normalization to the GAPDH housekeeping
gene using the 2−ΔΔCq method (41).
Western blot analysis
Western blot analysis was performed as previously
described (42,43). The cultured DRG neurons and spared
L5 DRG in rats were harvested and separately homogenized on ice in
10 mM Tris-HCl buffer (pH 7.4), 10 mM EDTA, 30% Triton-1000, 10%
SDS, a protease inhibitor cocktail (Roche Diagnostics,
Indianapolis, IN, USA) and NaCl using a homogenizer. The
homogenates were centrifuged at 12,000 × g for 30 min at 4°C. The
supernatant was collected and the protein concentration was assayed
with bicinchoninic acid reagent (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) according to the manufacturer's protocol. A
total of 50 µg protein was used for 10% SDS-PAGE
(Sigma-Aldrich; Merck KGaA). The protein was subsequently
transferred to a nitrocellulose membrane (Sigma-Aldrich; Merck
KGaA), which was incubated in TBS and Tween-20 containing 5%
non-fat milk powder (both Invitrogen; Thermo Fisher Scientific,
Inc.) at 4°C for 8 h to reduce the nonspecific reaction. The
membrane was subsequently incubated with primary antibodies at 4°C
for 12 h. β-actin (mouse monoclonal anti-β-actin; cat. no.
sc-69879; dilution, 1:800; Santa Cruz Biotechnology, Inc.) was used
as a reference and subtracted for net alterations in expression.
Goat polyclonal anti-IGF-1 antibody (cat. no. ab106836; dilution,
1:1,000; Abcam, Cambridge, UK), mouse monoclonal anti-PI3K antibody
(cat. no. 13666; dilution, 1:1,000), rabbit anti-phosphorylated
(p)-PI3K antibody (cat. no. 4228; dilution, 1:800), and rabbit
polyclonal anti-p-Akt (cat. no. 9275; dilution, 1:1,000) and
anti-Akt antibodies (cat. no. 9272; dilution, 1:1,000; all Cell
Signaling Technology, Inc., Danvers, MA, USA), were used as the
primary antibodies. Horseradish peroxidase-conjugated anti-goat
antibody for IGF-1 (cat. no. PI-9500; dilution, 1:500), horseradish
peroxidase-conjugated anti-mouse antibody for PI3K (cat. no.
PI-2000; dilution, 1:3,000) and horseradish peroxidase-conjugated
anti-rabbit antibody for p-Akt and Akt (cat. no. PI-1000; dilution,
1:2,500; all Vector Laboratories, Inc., Burlingame, CA, USA) were
used as the secondary antibodies. The membranes were subsequently
incubated in the matched secondary antibodies at 20-25°C for 2 h.
Enhanced chemiluminescence luminol reagent (Beyotime Institute of
Biotechnology, Shanghai, China) was used for protein quantity
determination. The densitometric analysis of the target protein
bands were analyzed using Bio-Rad Gel Imagining system (ChemiDoc™
XRS+) with Quantity One software v4.6.6 (all Bio-Rad Laboratories,
Inc., Hercules, CA, USA) for each group in order to quantity the
protein expression levels.
Immunohistochemistry (IHC) detection
The L4 and L5 spinal cord segments of the rats were
collected and post-fixed for 24 h using 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) at 4°C for IHC detection. The spinal
cord tissue sections (20-µm thickness) were processed as
described previously (9,10). The sections were incubated with
rabbit anti-rat polyclonal antibodies, calcitonin gene-related
peptide (CGRP; cat. nos. 57053; dilution, 1:500, Santa Cruz
Biotechnology, Inc.) and growth-associated protein (GAP)43 (cat.
no. ab12274; dilution, 1:1,000; Abcam, Cambridge, MA, USA), at 4°C
overnight. Negative controls were incubated in 2% goat serum (Santa
Cruz Biotechnology, Inc.) instead of the primary antibody. Finally,
the sections were detected by diaminobenzidine staining, according
to the manufacturer's protocol (DAB color development kit; cat. no.
P0203; Beyotime Institute of Biotechnology). In total, 0.5 ml DAB
staining solution A, 0.5 ml DAB staining solution B and 1 ml DAB
staining working solution were prepared and gently mixed. The
sections were incubated with the mixture at 20-25°C for 5 min. The
stain reaction was stopped by adding sterile water to wipe off the
staining solutions. Subsequently, the sections were dried at 45°C
for 2 h, followed by soaking in 75% ethyl alcohol for 10 min, 80%
ethyl alcohol for 10 min, 85% ethyl alcohol for 10 min, 90% ethyl
alcohol for 10 min, 95% ethyl alcohol for 10 min, absolute ethyl
alcohol for 10 min and 100% xylene for 20 min at 20-25°C. The
sections were covered with a clean coverslip. The immunoreactive
staining was observed under a light microscope (Leica Microsystems
GmbH; magnification, ×200 or 400).
Statistical analysis
Each experiment was repeated three times. One-way
analysis of variance or the Student-Newman-Keuls and the least
significant difference or Dunnett's T3 post hoc tests were used to
infer significant differences in the data. SPSS 13.5 covariance
software for Windows (SPSS, Inc., Chicago, IL, USA) was used to
perform these analyses. P<0.05 was considered to indicate a
statistically significant difference. All values are expressed as
the mean ± standard deviation.
Results
Effects of HSV on the expression of
IGF-1
The expression of IGF-1 at the mRNA and protein
levels were detected in all experimental groups. Bilateral dorsal
root ganglionectomies significantly decreased the levels of IGF-1
(sham-operated group vs. Model group; P<0.05), whereas EA
treatment partially rescued the expression of IGF-1 (Model group
vs. EA Model group; P<0.05; Fig.
1A).
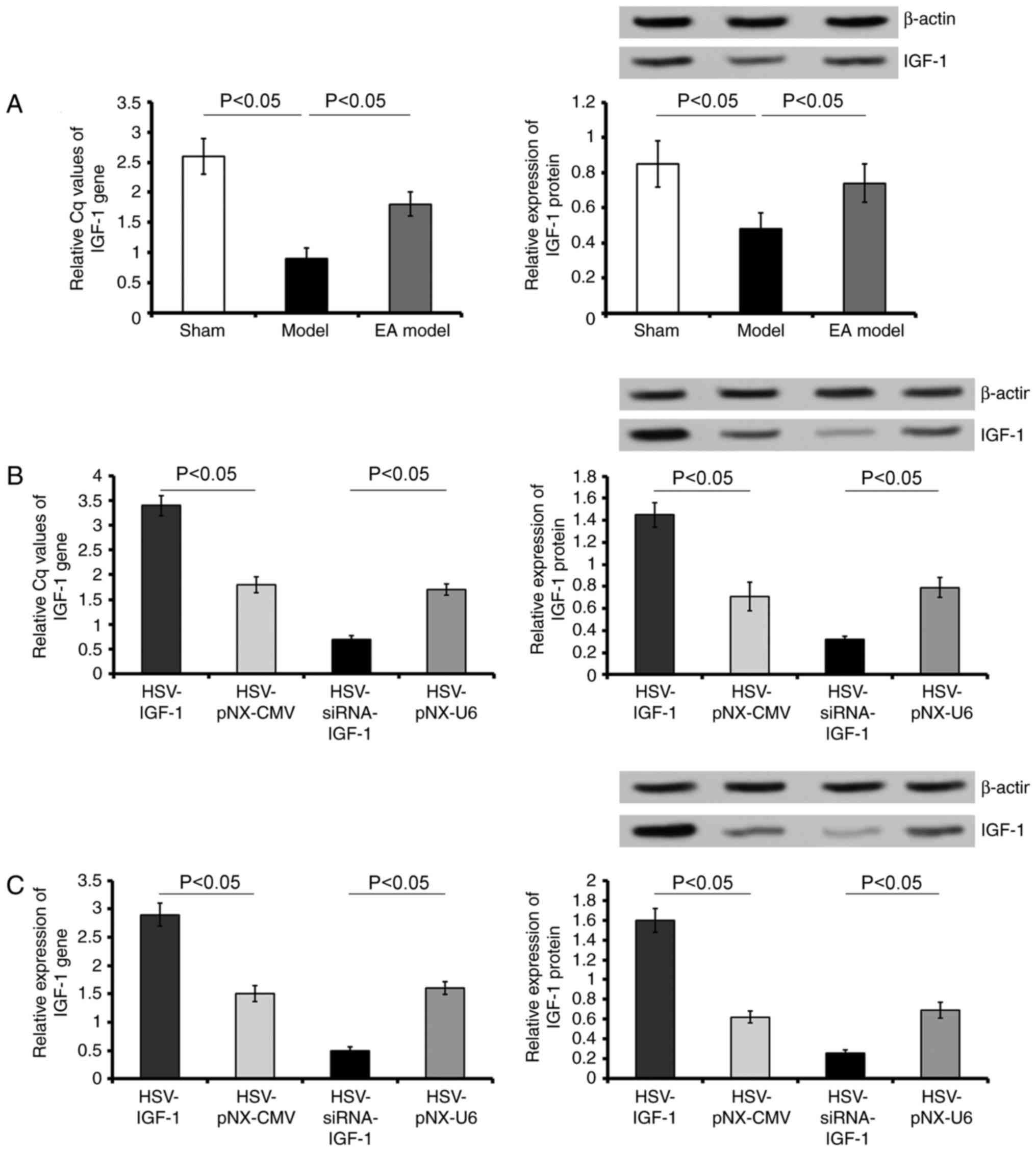 | Figure 1Evaluation of the expression of IGF-1
in vivo and in vitro. (A) Gene and protein levels of
IGF-1 were detected in the L5 spared DRG of rats in the Sham,
Model, and EA Model groups. (B) Gene and protein levels of IGF-1
were detected in EA model rats injected with artificially synthetic
HSV-IGF-1, HSV-siRNA-IGF-1, HSV-pNX-CMV or HSV-pNX-U6 into the
spared L5 DRG. (C) Gene and protein levels of IGF-1 were detected
in cultured DRGs transfected with HSV-IGF-1, HSV-siRNA-IGF-1,
HSV-pNX-CMV or HSV-pNX-U6. Values are plotted as the mean ±
standard deviation (n=7). Sham, sham-operated; Model, bilateral
dorsal root ganglionectomy injury; EA, electro-acupuncture
treatment post-injury; DRG, dorsal root ganglia; IGF-1,
insulin-like growth factor 1; siRNA, small interfering RNA. |
Following HSV-IGF-1 treatment, the expression of
IGF-1 increased 1.6-fold in the spared L5 DRG of the
HSV-IGF-1-injected EA Model group (HSV-IGF-1 vs. HSV-pNX-CMV;
P<0.05; Fig. 1A). By contrast,
HSV-siRNA-IGF-1 injection significantly reduced the expression of
IGF-1 in the spared L5 DRG (HSV-siRNA-I GF-1 vs. HSV-pNX-U6;
P<0.05; Fig. 1B).
In the cultured DRGs, treatment with HSV-IGF-1 also
increased the levels of IGF-1 (HSV-IGF-1 vs. HSV-pNX-CMV;
P<0.05; Fig. 1C), whereas
injection with HSV-siRNA-IGF-1 significantly decreased the
expression of IGF-1 (HSV-siRNA-IGF-1 vs. HSV-pNX-U6; P<0.05;
Fig. 1C). These results
demonstrated that either HSV-IGF-1 or HSV-siRNA-IGF-1 treatment
effectively resulted in the upregulation or downregulation of IGF-1
in the cultured DRGs, respectively.
Locomotor function evaluation
The results demonstrated that the baseline BBB score
of the sham-operated rats was equal to 21. The BBB scores decreased
in the injury model group following bilateral dorsal root
ganglionectomies, and then gradually increased between 7 and 28
dpo. In addition, the BBB scores of the EA group were significantly
elevated between 14 and 28 dpo compared with those of the injured
group (EA model vs. Model; P<0.05). These data suggest that EA
effectively promotes functional locomotor recovery in the hindlimbs
(Fig. 2A).
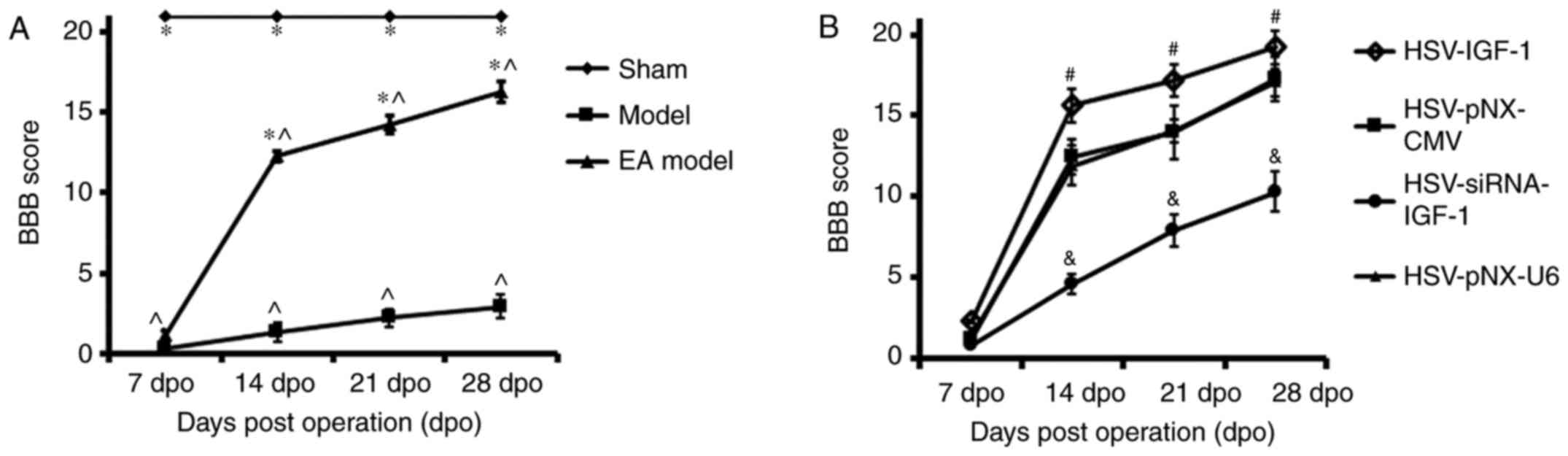 | Figure 2Evaluation of locomotor function in
different groups. (A) BBB score evaluation was performed at 7, 14,
21 and 28 dpo, in the Sham, Model, and EA Model rats.
*P<0.05 vs. Model; ^P<0.05 vs. Sham.
(B) BBB score evaluation in the EA Model injected with artificially
synthetic HSV-IGF-1, HSV-siRNA-IGF-1, HSV-pNX-CMV or HSV-pNX-U6
into the spared L5 DRG. #P<0.05 vs. HSV-pNX-CMV;
&P<0.05 vs. HSV-pNX-U6. Values are plotted as the
mean ± standard deviation (n=7). Sham, sham-operated; Model,
bilateral dorsal root ganglionectomy injury; EA,
electro-acupuncture treatment post-injury; BBB, Basso, Beattie,
Bresnahan; IGF-1, insulin-like growth factor 1; siRNA, small
interfering RNA. |
In the dorsal root ganglionectomy groups of rats
treated with EA (EA Model), the overexpression of IGF-1 by
HSV-IGF-1 injection in the spared L5 DRG significantly increased
the BBB scores between 14 and 28 dpo compared with those in the
blank vector-treated rats (HSV-IGF-1 vs. HSV-pNX-CMV; P<0.05;
Fig. 2B). By contrast,
interference of the endogenous expression of IGF-1 by
HSV-siRNA-IGF-1 injection induced a significant decrease in the BBB
scores of the EA Model rats (HSV-siRNA-IGF-1 vs. HSV-pNX-U6;
P<0.05; Fig. 2B). The results
suggest that the overexpression of IGF-1 enhanced EA-induced
neuroprotection following the dorsal root ganglionectomy procedure,
whereas the inhibition of IGF-1 partially neutralized EA-induced
locomotor functional recovery in the hindlimbs.
MWT and TWL tests
In the sham-operated rats, there were no significant
changes in the MWT or TWL during the entire experimental period.
The MWT was significantly decreased in the bilateral dorsal root
ganglionectomy group (Model vs. sham, P<0.05), and was partially
recovered in the EA Model between 1 and 27 dpo (EA Model vs. Model;
P<0.05; Fig. 3A). The MWT of
the Model group reached its lowest threshold at 9 dpo, and this
decrease was maintained until 13 dpo (Fig. 3A). HSV-IGF-1 injection induced
partial recovery of the MWT in the EA Model group (HSV-IGF-1 vs.
HSV-pNX-CMV, P<0.05). By contrast, MWT showed a further decrease
and reached its lowest threshold at 9 dpo in the
HSV-siRNA-IGF-1-treated rats (HSV-siRNA-IGF-1 vs. HSV-pNX-U6;
P<0.05; Fig. 3B). No
significant differences were observed between the HSV-pNX-U6 and
HSV-pNX-CMV-injected EA Model groups. Similar alterations in TWL in
each experimental group were observed (Fig. 3C and D). These results suggested
that manipulating the expression of endogenous IGF-1 had
significant effects on neuropathic pain following deafferentation
injury.
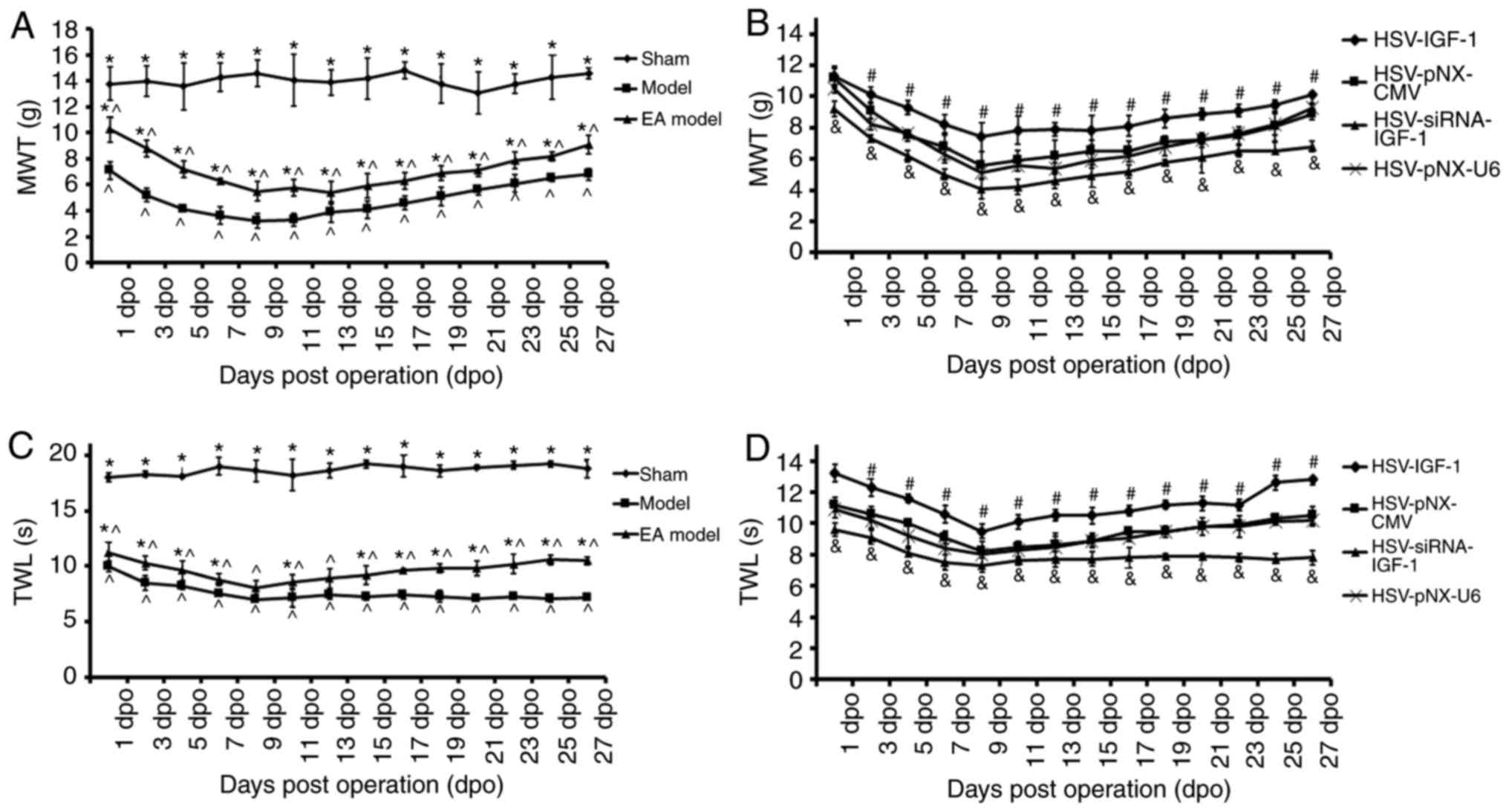 | Figure 3Effects of IGF-1 on neuropathic pain
of rats with dorsal root ganglionectomies treated with EA. (A) MWT
evaluation in rats in the Sham, Model, and EA Model rats.
*P<0.05 vs. Model; ^P<0.05 vs. Sham.
(B) MWT evaluation in the EA model rats injected with artificial
HSV-IGF-1, HSV-siRNA-IGF-1, HSV-pNX-CMV or HSV-pNX-U6 into the
spared L5 DRG. #P<0.05 vs. HSV-pNX-CMV,
&P<0.05 vs. HSV-pNX-U6, P<0.05. (C) TWL test
in rats in the sham, Model, and EA model rats.
*P<0.05 vs. Model; ^P<0.05 vs. Sham.
(D) TWL test in the EA model rats injected with artificial
HSV-IGF-1, HSV-siRNA-IGF-1, HSV-pNX-CMV or HSV-pNX-U6 into the
spared L5 DRG. #P<0.05 vs. HSV-pNX-CMV;
&P<0.05 vs. HSV-pNX-U6. Values are plotted as the
mean ± standard deviation (n=7). Sham, sham-operated; Model,
bilateral dorsal root ganglionectomy injury; EA Model,
electro-acupuncture treatment post-injury; MWT, mechanical
withdrawal threshold; TWL, thermal withdrawal latency; IGF-1,
insulin-like growth factor 1; siRNA, small interfering RNA. |
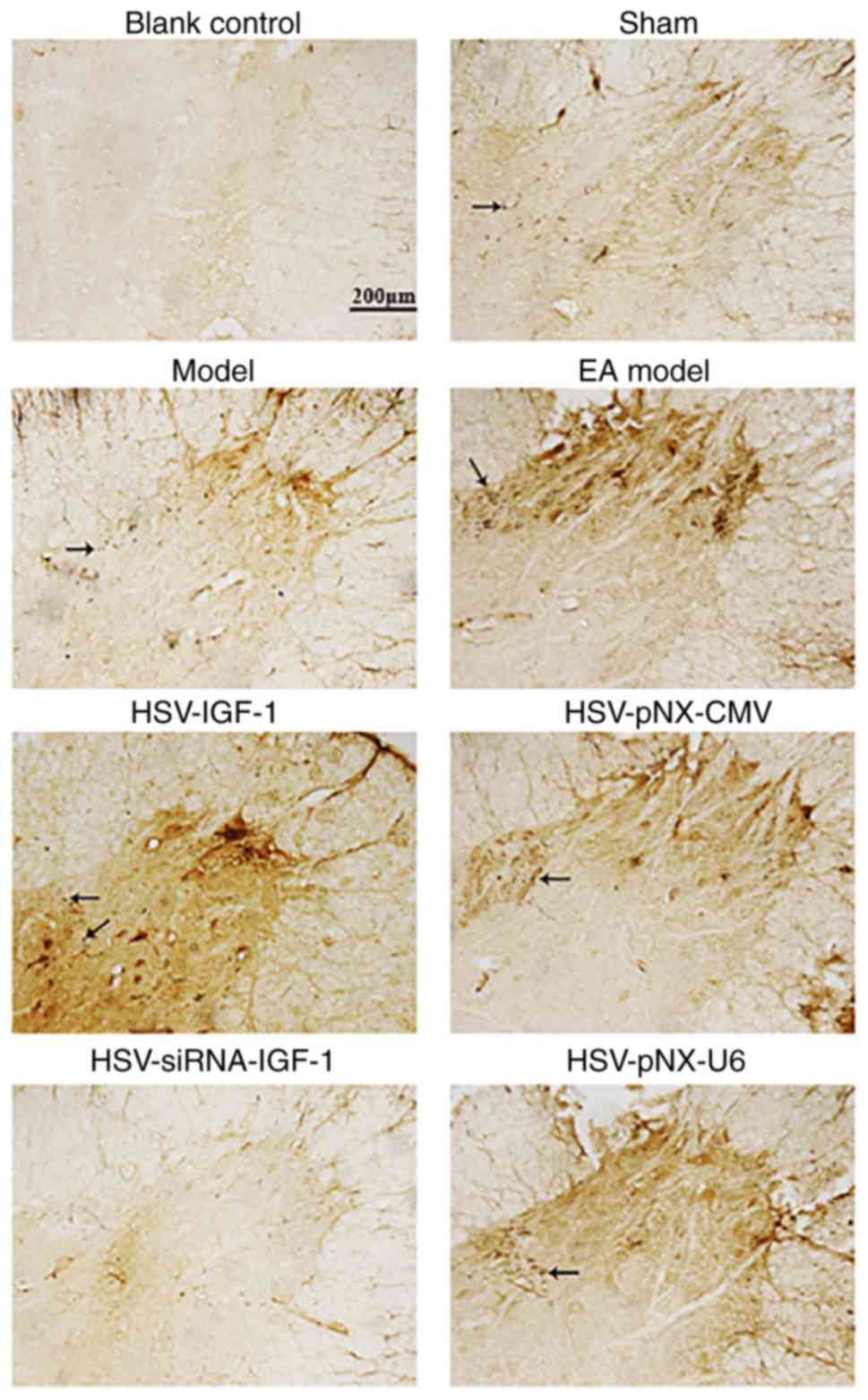
IGF-1 increases CGRP- and
GAP-43-immunopositive reactions (IRs) in the EA Model group via the
regulation of PI3K/Akt
CGRP-positive fibers were observed in the lamina I-V
and motor neurons of spinal ventral horns in the sham-operated and
Model group (Fig. 4),
respectively. CGRP-IRs were observed in the spinal cords of rats in
the EA Model group (Fig. 4).
HSV-IGF-1 treatment enhanced CGRP-immunopositive staining, compared
with that in the HSV-pNX-CMV-treated group (Fig. 4). However, HSV-siRNA-IGF-1
treatment was associated with reduced CGRP-immunopositive staining
(HSV-siRNA-IGF-1 vs. HSV-pNX-U6; Fig.
4).
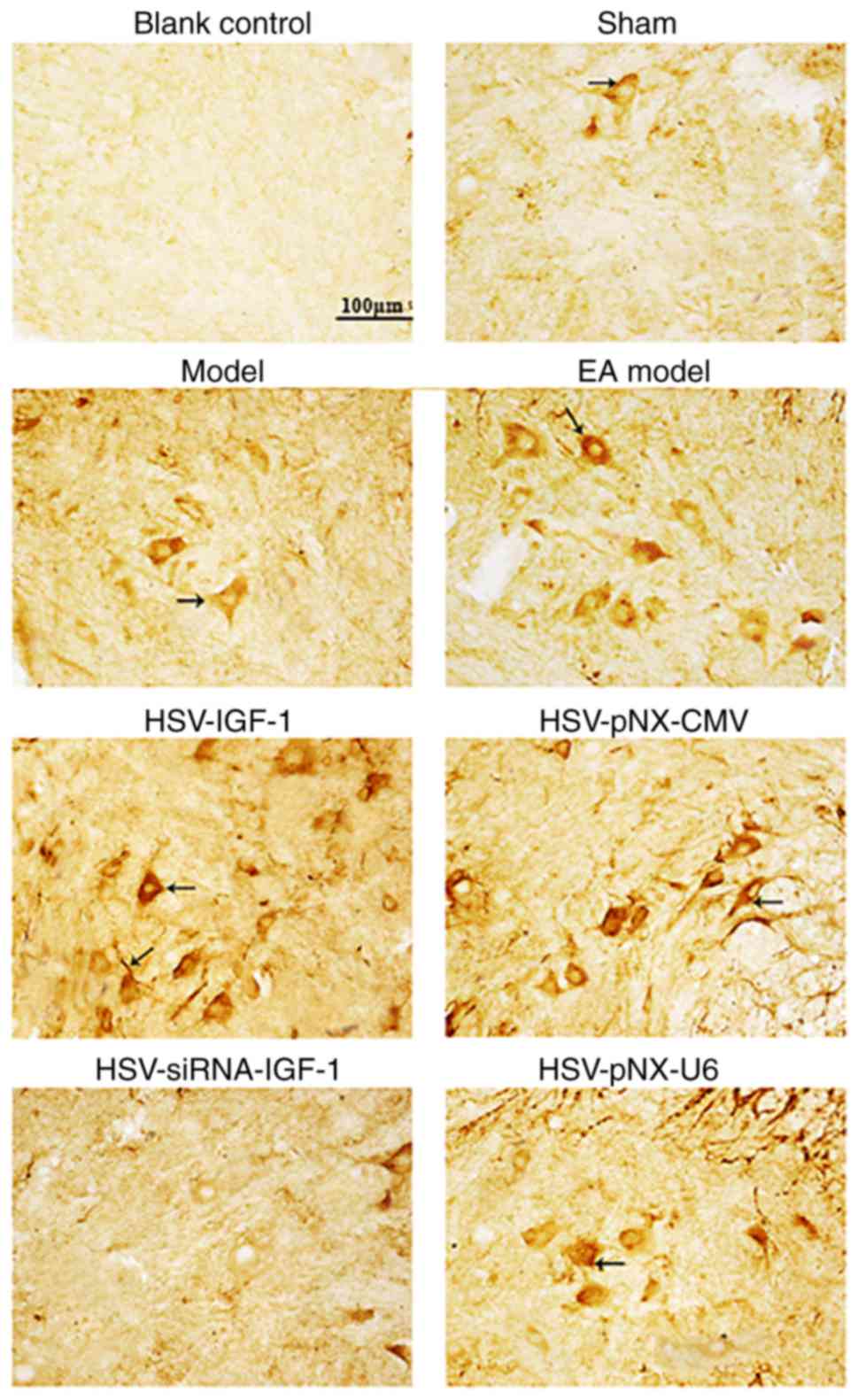
GAP-43-immunopositive staining was additionally
enhanced in the EA Model group relative to that in the
sham-operated group and injured Model group (Fig. 5). GAP-43-IRs were also increased
in the HSV-IGF-1-treated group and reduced in the
HSV-siRNA-IGF-1-treated group, compared with their respective
control groups, HSV-pNX-CMV and HSV-pNX-U6, respectively (Fig. 5).
Similarly, the expression levels of pPI3K and pAkt
were reduced following bilateral dorsal root ganglionectomy
(sham-operated group vs. Model group, P<0.05; Fig. 6). HSV-IGF-1 injection enhanced the
expression of PI3K and pAkt and the ratio of pAkt/Akt in the EA
Model rats (Fig. 6A and B,
P<0.05). However, treatment with HSV-siRNA-IGF-1 reduced the
ratios of p-PI3K/PI3K and pAkt/Akt compared with those in the
HSV-pNX-U6-treated group (P<0.05; Fig. 6A and B).
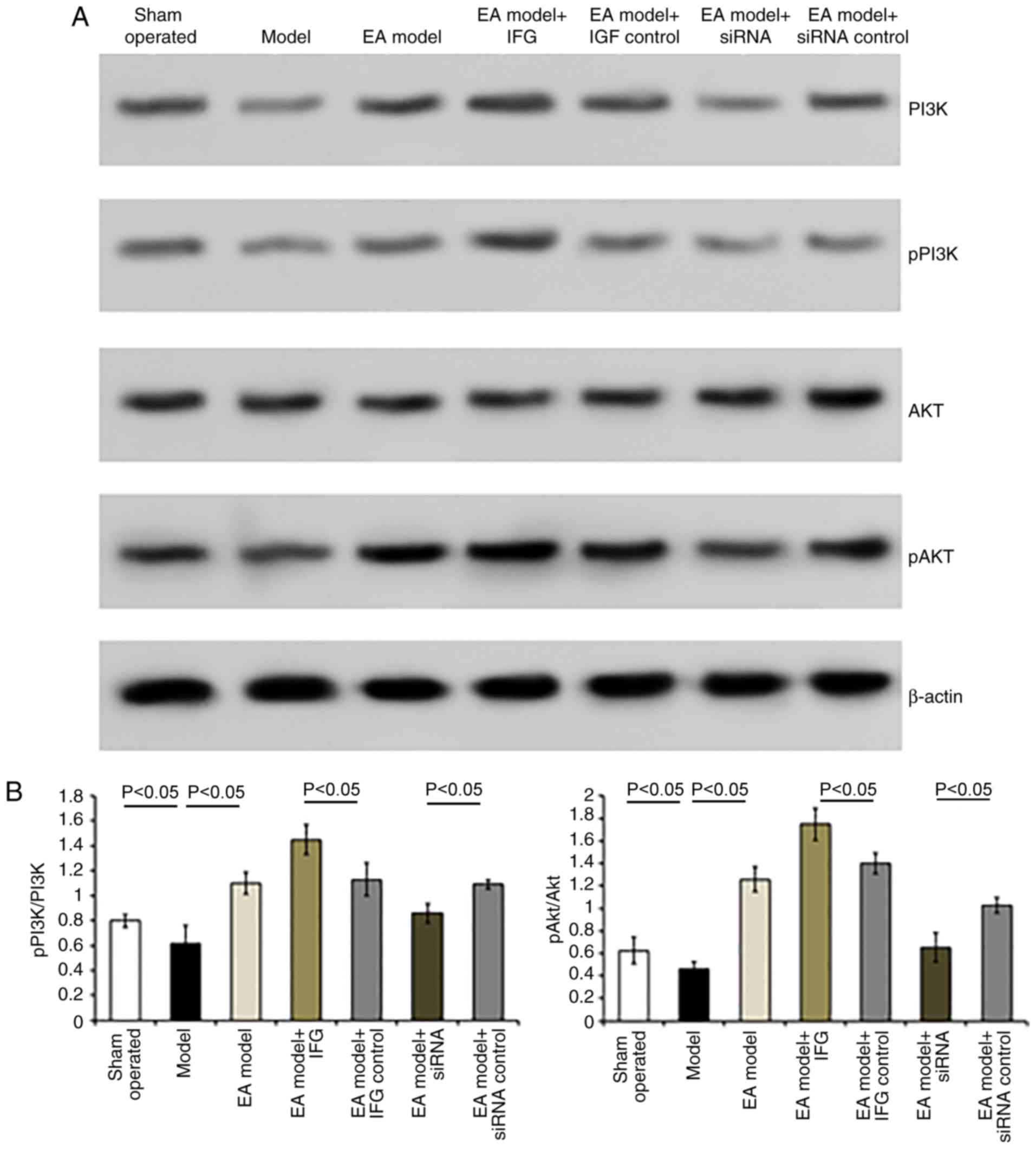 | Figure 6Detection of the expression of PI3K,
pPI3K, Akt and pAkt in the different groups. (A) Representative
blots of PI3K, pPI3K, Akt and pAkt proteins detected by western
blot analysis. (B) Ratios of pPI3K/PI3K and pAkt/Akt. Values are
plotted as the mean ± standard deviation (n=7). Sham,
sham-operated; Model, bilateral dorsal root ganglionectomy injury;
EA Model, electro-acupuncture treatment post-injury; siRNA, small
interfering RNA; PI3K, phosphatidylinositol 3-kinase; pPI3K,
phosphorylated PI3K; pAkt, phosphorylated Akt. |
Overexpression of IGF-1 induces DRG
neuronal growth by activating PI3K/Akt
Treatment with HSV-IGF-1 increased the number and
extension of DRG neurons, compared with the HSV-pNX-CMV-treated
group (P<0.05; Fig. 7A).
HSV-IGF-1 treatment also increased the expression ratios of
pPI3K/PI3K and pAkt/Akt (HSV-IGF-1 vs. HSV-pNX-CMV, P<0.05;
Fig. 7B). However,
HSV-siRNA-IGF-1 treatment reduced the number and extension of DRG
neurons and decreased the pPI3K/PI3K and pAkt/Akt ratios, compared
with those in the matched control group (P<0.05; Fig. 7A and B). In addition, HSV-IGF-1
induced DRG neuron growth, and the activation of PI3K/Akt was
inhibited by pre-treatment with LY294002. Co-treatment with
LY294002 in the HSV-IGF-1-transfected DRG neurons was associated
with decreased DRG neuron numbers and extension, downregulation of
the expression of pPI3K and pAkt, and decreased pPI3K/PI3K and
pAkt/Akt ratios (Fig. 8A and B).
Consistent with these results, Akt siRNA induced a decrease in the
phosphorylation of PI3K/Akt and the pPI3K/PI3K and pAkt/Akt ratios,
and reduced the number and extension of DRG neurons, compared with
those in cells co-transfected with HSV-IGF-1 and control scrambled
siRNA (P<0.05; Fig. 8A and
B).
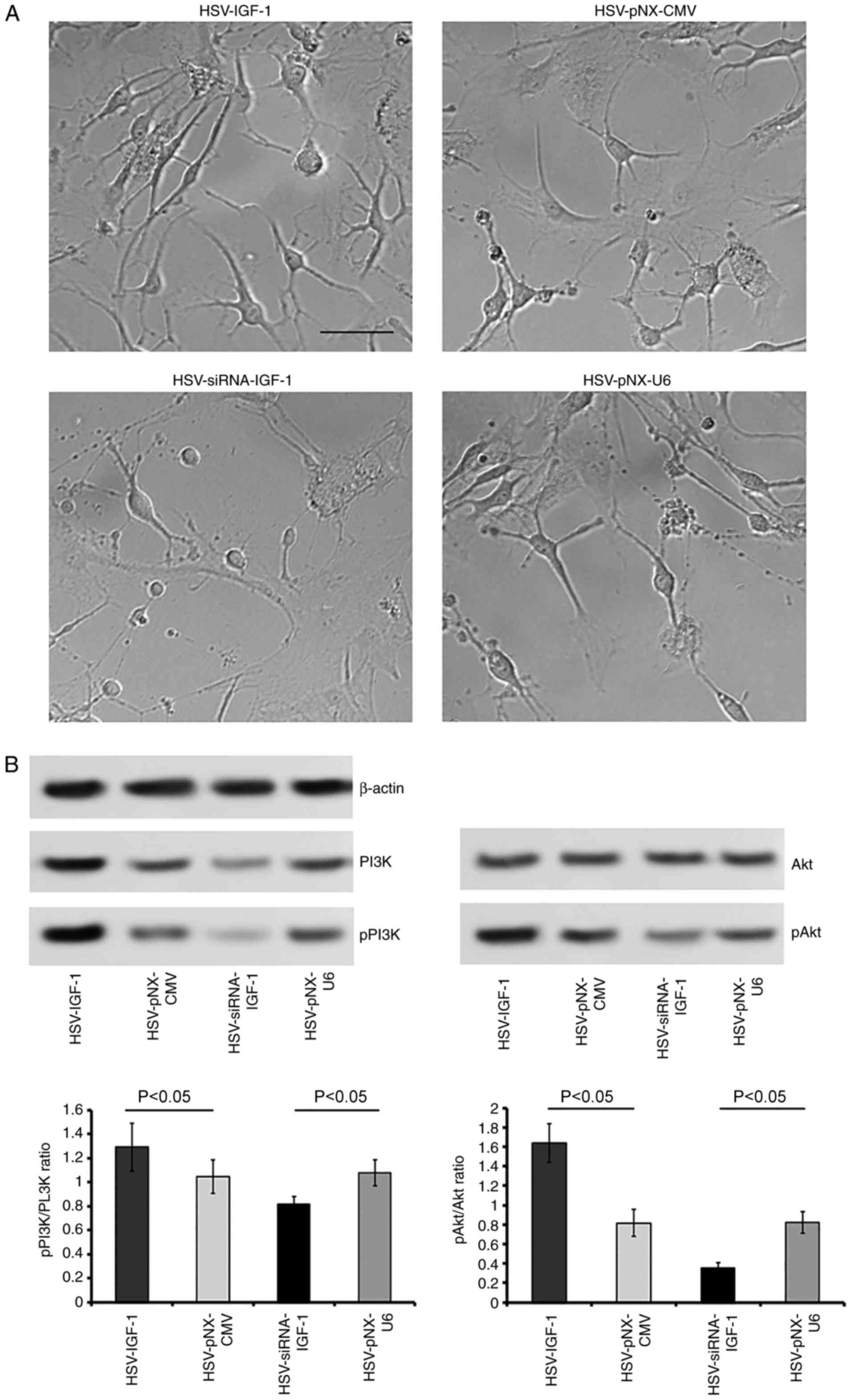 | Figure 7Effects of IGF-1 on cultured DRG
neurons. (A) Morphological changes of cultured DRG neurons
following treatment with artificial HSV-IGF-1, HSV-siRNA-IGF-1,
HSV-pNX-CMV or HSV-pNX-U6 (magnification, ×200). (B) Expression
levels of PI3K and pPI3K, and the ratio of pAkt/Akt were evaluated
by western blot analysis. Values are plotted as the mean ± standard
deviation (n=5). IGF-1, insulin-like growth factor 1; DRG, dorsal
root ganglia; siRNA, small interfering RNA; PI3K,
phosphatidylinositol 3-kinase; pPI3K, phosphorylated PI3K; pAkt,
phosphorylated Akt. |
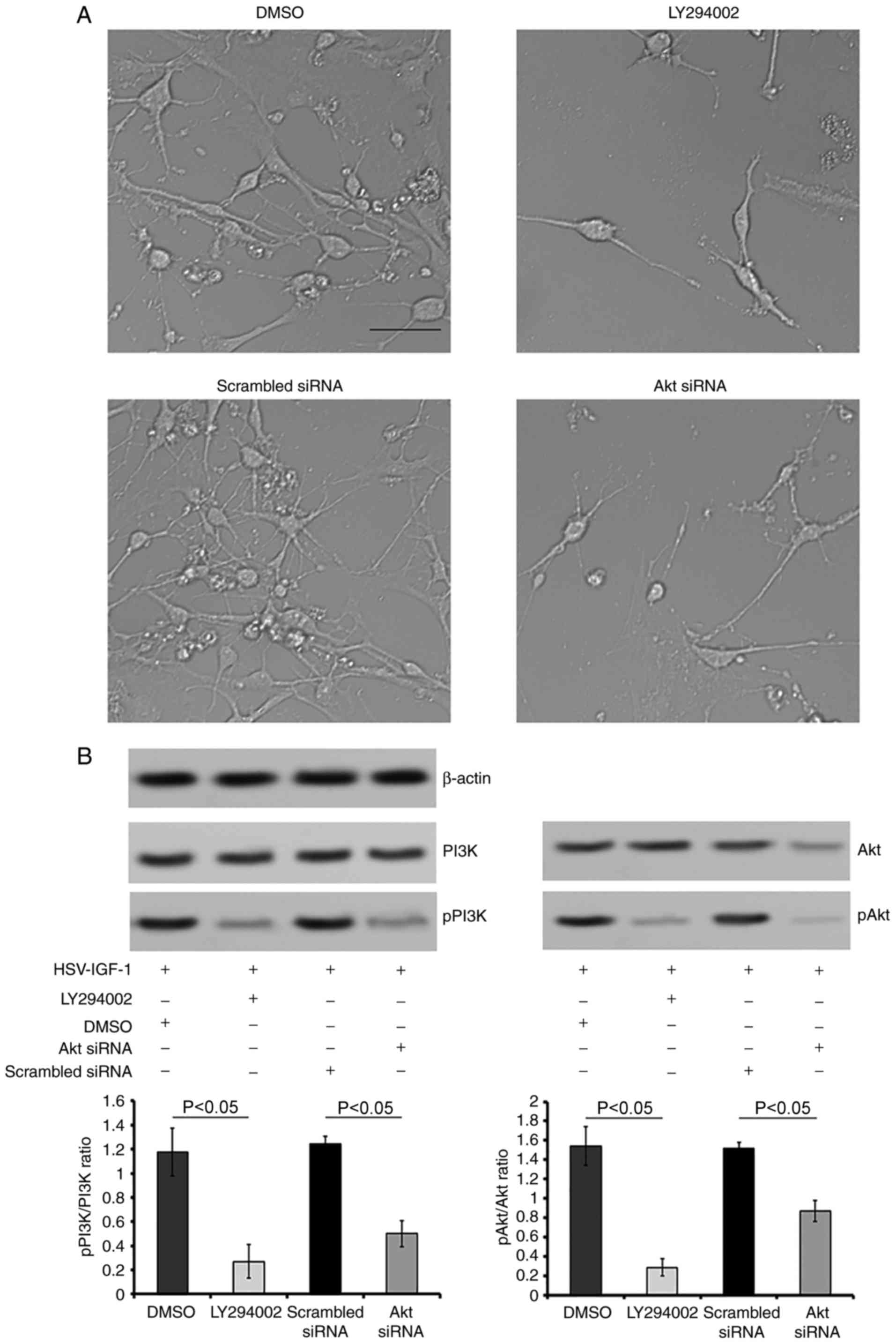 | Figure 8Roles of PI3K/Akt in IGF-1 induced
neuroprotection in vitro. (A) Morphological changes of
cultured DRG neurons following treatment with DMSO (control),
LY294002, scrambled siRNA (control) and Akt siRNA in cultured DRG
neurons transfected with HSV-IGF-1 (magnification, ×200). (B)
Representative blots of PI3K, pPI3K, Akt and pAkt proteins detected
by western blot analysis. The ratios of pPI3K/PI3K and pAkt/Akt are
also shown. Values are plotted as the mean ± standard deviation
(n=5). IGF-1, insulin-like growth factor 1; DRG, dorsal root
ganglia; siRNA, small interfering RNA; PI3K, phosphatidylinositol
3-kinase; pPI3K, phosphorylated PI3K; pAkt, phosphorylated Akt;
DMSO, dimethyl sulfoxide. |
Discussion
Previous studies have demonstrated that improvements
in spinal neuroplasticity induced by EA therapy following SCI are
associated with alterations in the expression of NTFs. Our previous
study demonstrated that EA induced an increase in the expression of
IGF-1 in the spared L6 DRG and associated dorsal horns of cats
subjected to adjacent dorsal root ganglionectomies (26). However, the underlying mechanisms
were unclear.
The results of the present study demonstrated that
EA-induced repair and neuroplasticity following adjacent dorsal
root ganglionectomies in rats were associated with IGF-1 via
PI3K/Akt signaling pathway activation. Bilateral dorsal root
ganglionectomies in rats reduced the expression of IGF-1 in the
spared L5 DRG and impaired motor and sensory function. EA treatment
partially rescued the expression levels of IGF-1, promoted the
rehabilitation of locomotor function (detected using the BBB
scale), remitted neuropathic pain (MWT and TWL test), increased
CGRP- and GAP-43 immunopositivity in the L4-L5 spinal cord, and
upregulated the pPI3K/PI3K and pAkt/Akt ratios in the spared L5 DRG
of the injured rats. The overexpression of IGF-1 by HSV-IGF-1
injection enhanced the effects induced by EA treatment. By
contrast, interference of the expression of IGF-1 using targeted
siRNA sequences neutralized the EA-induced effects in
de-afferentated rats.
CGRP and GAP-43 serve key roles in regenerative
neurite growth following spinal lesions (9). As a well-known marker for sensory
axons transmitting pain sensations, the increased expression of
CGRP is associated with nerve regeneration following lesion
(44). GAP-43, another neuronal
marker, is associated with nerve growth. It is a major component of
the motile ‘growth cones’ that form the tips of elongating axons.
Increases in the number of GAP-43-IRs indicate the regrowth of
cones and synaptic formation in the spinal cord following injury
(9). EA has been demonstrated to
promote the recovery of sensory or motor functions in patients with
SCI (5) and increase
neuroplasticity in de-afferentated spinal cords (6,7).
Additional reports have shown that EA-induced increases in the
expression of IGF-1 in the spared L6 DRG and associated dorsal
horns may be associated with the intraspinal sprouting of DRG
neurons in de-afferentated cats subjected to adjacent dorsal root
ganglionectomies (26).
Therefore, the enhanced CGRP- and GAP-43-IRs observed in the
present study suggests that nerve regeneration, cone regrowth and
synaptic formation were promoted in the de-afferentated spinal cord
following EA treatment, and this may be used to reconstruct local
circuitry for further functional recovery (9,45).
The results of the present study also demonstrated that the
overexpression of IGF-1 promoted EA-induced neuroplasticity and
that IGF-1 siRNA treatment weakened this effect. In addition, EA
exerted a protective effect on de-afferentated spinal cords by
stimulating the expression of IGF-1.
Following these observations, the present study
investigated whether the EA-induced neuroplasticity associated with
IGF-1 was mediated by PI3K/Akt activation. IGF-1 serves a
neuroprotective and proliferative function in the CNS during
development and following injury (15-17). A previous study revealed that
IGF-1 receptor (IGF-1R)-knockout mice have reduced brain size, CNS
hypomyelination, and loss of hippocampal granule cells and striatal
parvalbumin-containing neurons (16). By contrast, transgenic mice
overexpressing IGF-1 showed anti-apoptotic effects in neurons
during early postnatal development of the cerebral cortex, which
was associated with a persistent increase in the total number of
neurons in the adult animal (15). IGF-1 has also been demonstrated to
induce neuroprotection and neuroplasticity in de-afferentated
spinal cords of cats (26).
Initially, IGF-1 exerts its biological functions by binding to the
specific receptor, IGF-1R. During normal insulin signaling,
internalized IGF-1 binds to IGF-1R, which leads to the
phosphorylation of IGF-1R and subsequent initiation of the
downstream substrate, PI3K/Akt. Activated Akt phosphorylates
glycogen synthase kinase-3β (GSK3β) at Ser-9, leading to GSK3β
inhibition and the prevention of pathogenic neuronal death. By
contrast, the inhibition of Akt activates GSK3β and then stimulates
aberrant tau phosphorylation, leading to neuronal death in certain
diseases (46). In addition, the
PI3K/Akt signaling pathway is involved in anti-apoptotic
mechanisms, which influence the balance of anti- and pro-apoptotic
proteins (47,48). The Akt serine/threonine kinase is
known to promote neuronal survival and serves as a key mediator of
several aspects of neurite outgrowth, including elongation,
branching and caliber (49). EA
has been demonstrated to inhibit neuronal apoptosis in the dorsal
root de-afferentated cat spinal cords, by regulating the expression
of apoptosis-related proteins, including B-cell lymphoma 2 (Bcl-2)
and Bcl-2-associated X protein (50).
The results of the present study revealed that EA
treatment increased the expression of PI3K and the pPI3K/PI3K and
pAkt/Akt ratios, and this was enhanced by the upregulation of
IGF-1and weakened following IGF-1 knockdown in the rat model. In
cultured DRGs, the upregulation of IGF-1 induced DRG neuron
outgrowth, increased the expression of pPI3K and pAkt, and
increased the pAkt/Akt ratio. The downregulation of IGF-1 impeded
DRG neuron extension, decreased the expression of PI3K, pPI3K and
pAkt, and decreased the pAkt/Akt ratio. Furthermore, the inhibition
of PI3K or Akt neutralized IGF-1 overexpression-induced
neuroprotection in the cultured DRG neurons. Therefore, these
results suggest that activation of the IGF-1/PI3K/Akt signaling
pathway may be correlated with the EA-induced neuronal survival
following de-afferentated SCI and may occur via activation of the
IGF-1/PI3K/Akt signaling pathway.
As the regulation of neurite outgrowth is crucial
for developing therapies that promote axon regeneration following
nerve injury (49), IGF-1 is
promising for the development of treatments for patients suffering
with SCI.
In conclusion, the present study revealed the
following regarding EA treatment: i) EA partially rescued IGF-1
levels and improved locomotor and sensory function; ii) EA enhanced
CGRP- and GAP-43-IRs in the L4-L5 spinal cord; and iii) EA
upregulated the ratios of pPI3K/PI3K and pAkt/Akt in the spared L5
DRG of rats that underwent bilateral dorsal root ganglionectomies.
The overexpression of IGF-1 induced by the injection of HSV-IGF-1
into the spared L5 DRG reinforced the aforementioned observations
regarding EA-induced neuroprotection. By contrast, downregulation
of the endogenous expression of IGF-1 using specific siRNA IGF-1
target sequences neutralized these EA-induced effects. IGF-1
treatment in cultured DRGs led to neurite outgrowth and increased
expression of pPI3K/PI3K and pAkt/Akt. The results revealed the
crucial role of IGF-1 in EA-induced neuroplasticity via the
activation of PI3K/Akt following adjacent dorsal root
ganglionectomies in rats.
Funding
The present study was supported by Special Fund of
the Applied Basic Research Programs of Yunnan Province associated
with Kunming Medical University in China (grant no. 2015FB001), the
Medical Reserve Talents Cultivation Project of the Health and
Family Planning Commission of Yunnan province (grant no.
H-2017026), the Foundation of Science and Technology innovative
team building of Kunming Medical University (grant no. CXTD201807),
the National Natural Science Foundation of China (grant nos.
81560238 and 81502377) and Yunnan Applied Basic Research Projects
in China (grant nos. 2016FB139 and 2016FB123).
Availability of data and materials
The datasets used and analyzed in the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
TH and YBX contributed to the conception and design
of the study. YBX wrote and critically revised the manuscript. PD,
MNL and BC established the injured model and administered the EA
treatment. JT, MNL, RM, YXT, SSL and TH prepared the herpes simplex
virus construction, injected the HSV vectors into the animals and
performed the cell transfection. MNL, YXT, SSL and YBX determined
the Basso, Beattie, Bresnahan scores rating, and performed the
mechanical withdrawal threshold and thermal withdrawal latency
evaluation. TH, MNL, BC, RM and YBX performed the reverse
transcription-quantitative polymerase chain reactions, western
blotting and immunohistochemical analyses. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Animal use and care were in accordance with the
animal care guidelines, which conformed to the Guide for the Care
and Use of Laboratory Animals published by the US National
Institutes of Health (publication no. 85-23; revised 1996) and the
Care and Use Guidelines of Experimental Animals established by the
Research Ethics Committee of Kunming University of China (permit
no. kmu-eac-2016047).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Morgan JI and Curran T: Immediate-early
gene: Ten years on. Trends Neurosci. 18:66–67. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanner CA, Murray M and Goldberger ME:
Removal of dorsal root afferents prevents retrograde death of
axotomized Clarke's nucleus neurons in the cat. Exp Neurol.
123:81–90. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang B, Goldberger ME and Murray M:
Proliferation of SP- and 5HT-containing terminals in lamina II of
rat spinal cord following dorsal rhizotomy: Quantitative
EM-immunocytochemical studies. Exp Neurol. 123:51–63. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang B, Goldberger ME, Wu LF and Murray
M: Plasticity of complex terminals in lamina II in partially
deafferented spinal cord: The cat spared root preparation. Exp
Neurol. 132:186–193. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong AM, Leong CP, Su TY, Yu SW, Tsai WC
and Chen CP: Clinical trial of acupuncture for patients with spinal
cord injuries. Am J Phys Med Rehabil. 82:21–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu F, Sun WW, Wang Y, Hu LQ, Dai P, Tian
CF and Wang TH: Effects of electro-acupuncture on NT-4 expression
in spinal dorsal cats subjected to adjacent dorsal root
ganglionectomy. Neurosci Lett. 450:158–162. 2009. View Article : Google Scholar
|
|
7
|
Wang XY, Li XL, Hong SQ, Xi-Yang YB and
Wang TH: Electroacupuncture induced spinal plasticity is linked to
multiple gene expressions in dorsal root deafferented rats. J Mol
Neurosci. 37:97–110. 2009. View Article : Google Scholar
|
|
8
|
Zhou HL, Zhang LS, Kang Y, Zhang W and
Wang TH: Effects of electro-acupuncture on CNTF expression in
spared dorsal root ganglion and the associated spinal lamina II and
nucleus dorsalis following adjacent dorsal root ganglionectomies in
cats. Neuropeptides. 42:95–106. 2008. View Article : Google Scholar
|
|
9
|
Wang X, Ju S, Chen S, Gao W, Ding J, Wang
G, Cao H, Tian H and Li X: Effect of electro-acupuncture on
neuroplasticity of spinal cord-transected rats. Med Sci Monit.
23:4241–4251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun WW, Zhao W and Wang TH: Effects of
Electro-acupuncture on PDGF expression in spared dorsal root
ganglion and associated dorsal horn subjected to partial dorsal
root ganglionectomy in cats. Neurochem Res. 33:437–443. 2008.
View Article : Google Scholar
|
|
11
|
Xu DU: Effect of electroacupuncture on
brain derivd neuro-trophic factor and nerve function of spinal cord
injury rats. J Clin Acupuncture Moxibustion. 2:46–48. 2009.In
Chinese.
|
|
12
|
Cowansage KK, LeDoux JE and Monfils MH:
Brain-derived neurotrophic factor: A dynamic gatekeeper of neural
plasticity. Curr Mol Pharmacol. 3:12–29. 2010. View Article : Google Scholar
|
|
13
|
Russo VC, Schütt BS, Andaloro E, Ymer SI,
Hoeflich A, Ranke MB, Bach LA and Werther GA: Insulin-like growth
factor binding protein-2 binding to extracellular matrix plays a
critical role in neuroblastoma cell proliferation, migration, and
invasion. Endocrinology. 146:4445–4455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Galvin J, Eyermann C and Colognato H:
Dystroglycan modulates the ability of insulin-like growth factor-1
to promote oligoden-drocyte differentiation. J Neurosci Res.
88:3295–3307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hodge RD, D'Ercole AJ and O'Kusky JR:
Insulin-like growth factor-I (IGF-I) inhibits neuronal apoptosis in
the developing cerebral cortex in vivo. Int J Dev Neurosci.
25:233–241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beck KD, Powell-Braxton L, Widmer HR,
Valverde J and Hefti F: Igf1 gene disruption results in reduced
brain size, CNS hypomyelination, and loss of hippocampal granule
and striatal parvalbumin-containing neurons. Neuron. 14:717–730.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hung KS, Tsai SH, Lee TC, Lin JW, Chang CK
and Chiu WT: Gene transfer of insulin-like growth factor-I
providing neuroprotection after spinal cord injury in rats. J
Neurosurg Spine. 6:35–46. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung SY, Kim DY, Yune TY, Shin DH, Baek SB
and Kim CJ: Treadmill exercise reduces spinal cord injury-induced
apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med.
7:587–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Craft S, Baker LD, Montine TJ, Minoshima
S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate
SR, et al: Intranasal insulin therapy for Alzheimer disease and
amnestic mild cognitive impairment: A pilot clinical trial. Arch
Neurol. 69:29–38. 2012. View Article : Google Scholar
|
|
20
|
Talbot K, Wang HY, Kazi H, Han LY, Bakshi
KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, et
al: Demonstrated brain insulin resistance in Alzheimer's disease
patients is associated with IGF-1 resistance, IRS-1 dysregulation,
and cognitive decline. J Clin Invest. 122:1316–1338. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hölscher C: Insulin, incretins and other
growth factors as potential novel treatments for Alzheimer's and
Parkinson's diseases. Biochem Soc Trans. 42:593–599. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morris JK, Zhang H, Gupte AA, Bomhoff GL,
Stanford JA and Geiger PC: Measures of striatal insulin resistance
in a 6-hydroxydopamine model of Parkinson's disease. Brain Res.
1240:185–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bosco D, Plastino M, Cristiano D, Colica
C, Ermio C, De Bartolo M, Mungari P, Fonte G, Consoli D, Consoli A
and Fava A: Dementia is associated with insulin resistance in
patients with Parkinson's disease. J Neurol Sci. 315:39–43. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashraghi MR, Pagano G, Polychronis S,
Niccolini F and Politis M: Parkinson's disease, diabetes and
cognitive impairment. Recent Pat Endocr Metab Immune Drug Discov.
10:11–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pang Y, Lin S, Wright C, Shen J, Carter K,
Bhatt A and Fan LW: Intranasal insulin protects against
substantianigra dopaminergic neuronal loss and alleviates motor
deficits induced by 6-OHDA in rats. Neuroscience. 318:157–165.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai P, Wang ZJ, Sun WW, Pang JX, You C and
Wang TH: Effects of electro-acupuncture on IGF-I expression in
spared dorsal root ganglia and associated spinal dorsal horn in
cats subjected to adjacent dorsal root ganglionectomies. Neurochem
Res. 34:1993–1998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fischer G, Kostic S, Nakai H, Park F,
Sapunar D, Yu H and Hogan Q: Direct injection into the dorsal root
ganglion: Technical, behavioral, and histological observations. J
Neurosci Methods. 199:43–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu F, Zou Y, Liu S, Liu J and Wang T:
Electro-acupuncture treatment improves neurological function
associated with downregulation of PDGF and inhibition of
astrogliosis in rats with spinal cord transection. J Mol Neurosci.
51:629–635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vanderah TW, Gardell LR, Burgess SE,
Ibrahim M, Dogrul A, Zhong CM, Zhang ET, Malan TP Jr, Ossipov MH,
Lai J and Porreca F: Dynorphin promotes abnormal pain and spinal
opioid antinociceptive tolerance. J Neurosci. 20:7074–7079. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu FY, Sun YN, Wang FT, Li Q, Su L, Zhao
ZF, Meng XL, Zhao H, Wu X, Sun Q, et al: Activation of satellite
glial cells in lumbar dorsal root ganglia contributes to
neuropathic pain after spinal nerve ligation. Brain Res.
1427:65–77. 2012. View Article : Google Scholar
|
|
33
|
Deuis JR, Dvorakova LS and Vetter I:
Methods used to evaluate pain behaviors in rodents. Front Mol
Neurosci. 10:2842017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding Y, Yao P, Hong T, Han Z, Zhao B, Chen
W and Zhou G: Early hyperbaric oxygen effects on neuropathic pain
and nitric oxide synthase isoforms in CCI rats. Oncotarget.
9:7513–7521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dixon WJ: Efficient analysis of
experimental observations. Annu Rev Pharmacol Toxicol. 20:441–462.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fouillet A, Watson JF, Piekarz AD, Huang
X, Li B, Priest B, Nisenbaum E, Sher E and Ursu D: Characterisation
of Nav1.7 functional expression in rat dorsal root ganglia neurons
by using an electrical field stimulation assay. Mol Pain.
13:17448069–17745179. 2017. View Article : Google Scholar
|
|
38
|
Ren W, Liu Y, Wan S, Fei C, Wang W, Chen
Y, Zhang Z, Wang T, Wang J, Zhou L, et al: BMP9 inhibits
proliferation and metastasis of HER2-positive SK-BR-3 breast cancer
cells through ERK1/2 and PI3K/AKT pathways. PLoS One. 9:e968162014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu WD, Zuo Y, Xu Z and Zhang M: MiR-19a
promotes epithelial-mesenchymal transition through PI3K/AKT pathway
in gastric cancer. World J Gastroenterol. 21:4564–4573. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu T, Li YS, Chen B, Chang YF, Liu GC,
Hong Y, Chen HL and Xiyang YB: Elevated glucose-6-phosphate
dehydrogenase expression in the cervical cancer cases is associated
with the cancerigenic event of high-risk human papillomaviruses.
Exp Biol Med (Maywood). 240:1287–1297. 2015. View Article : Google Scholar
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
42
|
Skup M, Dwornik A, Macias M, Sulejczak D,
Wiater M and Czarkowska-Bauch J: Long-term locomotor training
up-regulates TrkB (FL) receptor-like proteins, brain-derived
neurotrophic factor, and neurotrophin 4 with different topographies
of expression in oligodendroglia and neurons in the spinal cord.
Exp Neurol. 176:289–307. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiyang YB, Liu S, Liu J, Hao CG, Wang ZJ,
Ni W, Wang XY and Wang TH: Roles of platelet-derived growth
factor-B expression in the ventral horn and motor cortex in the
spinal cord-hemisected rhesus monkey. J Neurotrauma. 26:275–287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jang JH, Nam TS, Paik KS and Leem JW:
Involvement of peripherally released substance P and calcitonin
gene-related peptide in mediating mechanical hyperalgesia in a
traumatic neuropathy model of the rat. Neurosci Lett. 360:129–132.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li WJ, Li SM, Ding Y, He B, Keegan J, Dong
H, Ruan JW and Zeng YS: Electro-acupuncture upregulates CGRP
expression after rat spinal cord transection. Neurochem Int.
61:1397–1403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang L, Wang H, Liu L and Xie A: The role
of insulin/IGF-1/PI3K/Akt/GSK3β signaling in parkinson's disease
dementia. Front Neurosci. 12:732018. View Article : Google Scholar
|
|
47
|
Schorey JS and Cooper AM: Macrophage
signaling upon mycobacterial infection: The MAP kinases lead the
way. Cell Microbiol. 5:133–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chan G, Nogalski MT, Bentz GL, Smith MS,
Parmater A and Yurochko AD: Pi3k-dependent upregulation of Mcl-1 by
human cytomegalovirus is mediated by epidermal growth factor
receptor and inhibits apoptosis in short-lived monocytes. J
Immunol. 184:3213–3222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Read DE and Gorman AM: Involvement of Akt
in neurite outgrowth. Cell Mol Life Sci. 66:2975–2984. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhao W, Zhao Q, Liu J, Xu XY, Sun WW, Zhou
X, Liu S and Wang TH: Electro-acupuncture reduces neuronal
apoptosis linked to Bax and Bcl-2 expression in the spinal cords of
cats subjected to partial dorsal root ganglionectomy. Neurochem
Res. 33:2214–2221. 2008. View Article : Google Scholar : PubMed/NCBI
|