Introduction
Gestational diabetes mellitus (GDM) is defined as
glucose intolerance with onset or first recognition during
pregnancy and is one of the most common complications during
pregnancy (1). Metabolic and
immunological changes can occur during pregnancy and frequently
present clinically as increased insulin resistance and immune
tolerance against the fetus and placenta (2). The risk of adverse pregnancy
outcomes is increased in patients with GDM and affects the mother
and the child; common complications include ischemic heart disease,
hypertension, predisposition to obesity, metabolic syndrome and
type 2 diabetes mellitus (T2DM) following pregnancy (3). Risk factors include pre-pregnancy
weight gain and obesity, a family history of diabetes, an advanced
maternal age, a poor diet and low physical activity (4,5).
In addition, studies have reported that low thyroid hormone levels
during early pregnancy are associated with an increased risk of GDM
and may affect pregnancy outcome and the intellectual level of the
infant (6,7). However, the etiology of and
mechanisms responsible for GDM are unknown for the majority of
patients and thus, the identification of novel signatures or
biomarkers that enhance clinical behavior in the treatment of GDM
are essential.
Long non-coding RNAs (lncRNAs) do not code for
proteins and are pervasive across the genome (8). The dysregulation of lncRNA
expression is associated with various human diseases (9-11).
A previous study suggested metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1) lncRNA expression may be a
novel biomarker to predict GDM (12). Shi et al (13) performed a microarray expression
profile analysis to reveal that lncRNAs were differentially
expressed in the blood of the umbilical cord from patients with GDM
and may play a role in macrosomia development. Recently, an
increasing number of studies have suggested that lncRNAs
participate in competing endogenous RNA (ceRNA) regulatory
processes and in communicating with other RNAs, such as microRNAs
(miRNAs or miRs), coding genes and circular RNAs (circRNAs)
(14,15). lncRNAs compete with miRNA target
genes for binding by sharing common miRNA-binding sites, thus
attenuating miRNA-associated target repression. UICLM lncRNA has
been shown to promote liver metastasis in colorectal cancer by
acting as a ceRNA for miR-215, regulating zinc finger E-box-binding
homeobox 2 (ZEB2) expression (16). MT1JP lncRNA has been shown to
function as a ceRNA in regulating FBXW7 by competitively binding to
miR-92a-3p in gastric cancer (17). In addition, studies have utilized
high-throughput expression profiles and interaction data to
construct a global ceRNA network in diseases (18,19). These data highlight the important
role of lncRNA in interacting with ceRNAs and suggest that the
integration of expression profiles and network analysis contributes
to the identification of problematic lncRNAs and to the elucidation
of the mechanisms of disease. However, a lncRNA-mediated ceRNA
network in GDM has not yet been constructed and analyzed, at least
to the best of our knowledge.
In this study, we used a comprehensive computational
approach to construct a lncRNA-mediated ceRNA network (LCEN) in GDM
by integrating the expression profiles of mRNAs, miRNAs and lncRNAs
from patients with GDM and experimentally verifying the
interactions. We observed that the lncRNAs exhibited specific
topological features in the LCEN, consistent with a regulatory
association with coding mRNAs in GDM. The LCEN in GDM presents
specific and highly competing activity profiles. We further
identified a core GDM-associated subnetwork and 3 modules to
characterize the properties of LCENs. ceRNA expression in these
modules may be able to distinguish between patients that are normal
glucose-tolerant (NGT) and patients with GDM. Functional analysis
revealed that GDM-associated ceRNAs participated in glycolytic and
hormone metabolic processes. Additionally, we identified the
thyroid hormone pathway to be associated with ceRNAs in GDM. On the
whole, these results suggest that GDM-associated LCENs may provide
new insight into the mechanisms of GDM and may aid in the discovery
of novel molecular biomarkers and GDM therapeutic strategies.
Materials and methods
Collection of high-throughput data for
lncRNAs, miRNAs and genes
lncRNA, miRNA and gene expression profiles for GDM
were downloaded from the Gene Expression Omnibus database
(www.ncbi.nlm.nih.gov/geo). The study
collected data for 8 NGT patients and 8 patients with GDM, which
were matched by body mass index and age. Paired data were adjusted
for mid-pregnancy weight gain and by pregnancy week (accession no.
GSE92772; unpublished data). The RNA sequencing of whole blood
cells of these samples were produced by Illumina HiSeq 2500.
Experimental validation of miRNA targets
and GDM-associated genes
Gene-miRNA association data were obtained from a
public database (miRTarBase 7.0) that contained >360,000
miRNA-target interactions (MTIs) (20). Only MTIs supported by strong
experimental evidence were extracted for the current study.
lncRNA-miRNA association data was obtained (RAID 2.0) containing
experimentally and computationally predicted RNA-RNA and
RNA-protein interactions. We further extracted lncRNA-miRNA
association data supported by strong experimental evidence
(21). GDM-associated gene data
were downloaded from DisGeNET, a discovery platform containing one
of the largest publicly available collections of genes and variants
associated with human diseases (22).
Identification of candidate ceRNA
interactions
A hypergeo-metric test was performed to identify
candidate competing mRNA-lncRNA interactions. We evaluated the
significance of shared miRNAs between lncRNAs and mRNAs. The human
genome contains a total number of K miRNAs, with L and M
representing the number of miRNAs associated with the current
lncRNA or mRNA, respectively. The number of common miRNAs shared by
lncRNA and mRNA was defined as ‘i’. P-values were calculated using
the hypergeometric test and by evaluating the enrichment
significance for competing function as follows:
A false discovery rate (FDR) correction was applied.
FDR<0.01 was the threshold to select candidate competing
mRNA-lncRNA pairs.
Identification of GDM-associated
functional ceRNAs by integrating expression and candidate ceRNA
interactions
Pearson correlation coefficients (PCC) were used to
identify GDM-specific mRNA-lncRNA pairs based on the expression
values of competing mRNA-lncRNA pairs. We filtered GDM-associated
functional ceRNAs from expression datasets based on following
rules: PCC (lncRNA, gene) >0.5 and P<0.05; PCC (lncRNA,
miRNA) <−0.5 and P<0.05; and PCC (gene, miRNA) <−0.5 and
P<0.05, with PCC representing interactions based on expression
values. A total of 77 GDM-associated functional ceRNAs, comprising
17 lncRNAs, 14 miRNAs and 40 coding genes, were retained for
further analysis. A GDM-associated ceRNA network was constructed
using Cytoscape 3.0 (http://www.cytoscape.org/).
Functional score of GDM-associated
functional ceRNAs
The correlation of mRNA and miRNA expression is an
effective statistically method for distinguishing between direct
and indirect interactions (23).
The functional score was applied to determine the strength of
competition in GDM-associated functional ceRNAs and was defined as
(|PCC (lncRNA, gene)|+|PCC (lncRNA, miRNA)|+|PCC (gene, miRNA)|)/3.
A higher functional score indicated a greater competition between
lncRNA and gene for miRNA binding.
Dissecting topological features for LCEN
in GDM
We performed topological analyses, including degree
and topological coefficients for all the nodes in LCEN using
Cytoscape 3.0 (http://www.cytoscape.org/).
Identifying core modules from subnetworks
of LCEN in GDM
We extracted a subnetwork form LCEN in GDM following
the verification GDM-associated genes. All the network modules were
identified using ClusterONE with the default parameters based on
the subnetwork of LCEN constructed by GDM-associated genes
(http://apps.cytoscape.org/apps/ClusterONE). ClusterONE
is a tool that clusters a given network based on topology to
identify densely connected regions. A total of 14 modules were
identified and the 3 modules with the highest numbers of nodes were
extracted.
Classification power of the core modules
in GDM
For core modules, the expression data was used to
classify 16 samples (NGT, n=8 and GDM, n=8) applying a consensus
clustering method (24). This
process is performed by ConsensusClusterPlus package in R
(https://www.r-project.org/). We defined
the optimal category number as the smallest increase in the area
under the cumulative distribution function (CDF) curve. Chi-square
tests were used to evaluate whether the disease and control samples
can be classified using this method. A value of P<0.05 was
considered to indicate a statistically significant difference.
Gene, miRNA and lncRNA expression were used to perform this
analysis and the respective expression was integrated.
Functional enrichment analysis
With the online Enrichr tool and by applying default
parameters, a functional enrichment analysis was performed for
genes in LCEN (25). We
identified enriched GO terms (P<0.01) and KEGG pathways
(P<0.05).
Results
Construction and global properties of the
GDM-associated ceRNA network
A total of 480,023 candidate ceRNA interactions
(gene-miRNA-lncRNA) were identified. An integrated pipeline was
used to construct a GDM-associated LCEN based on experimentally
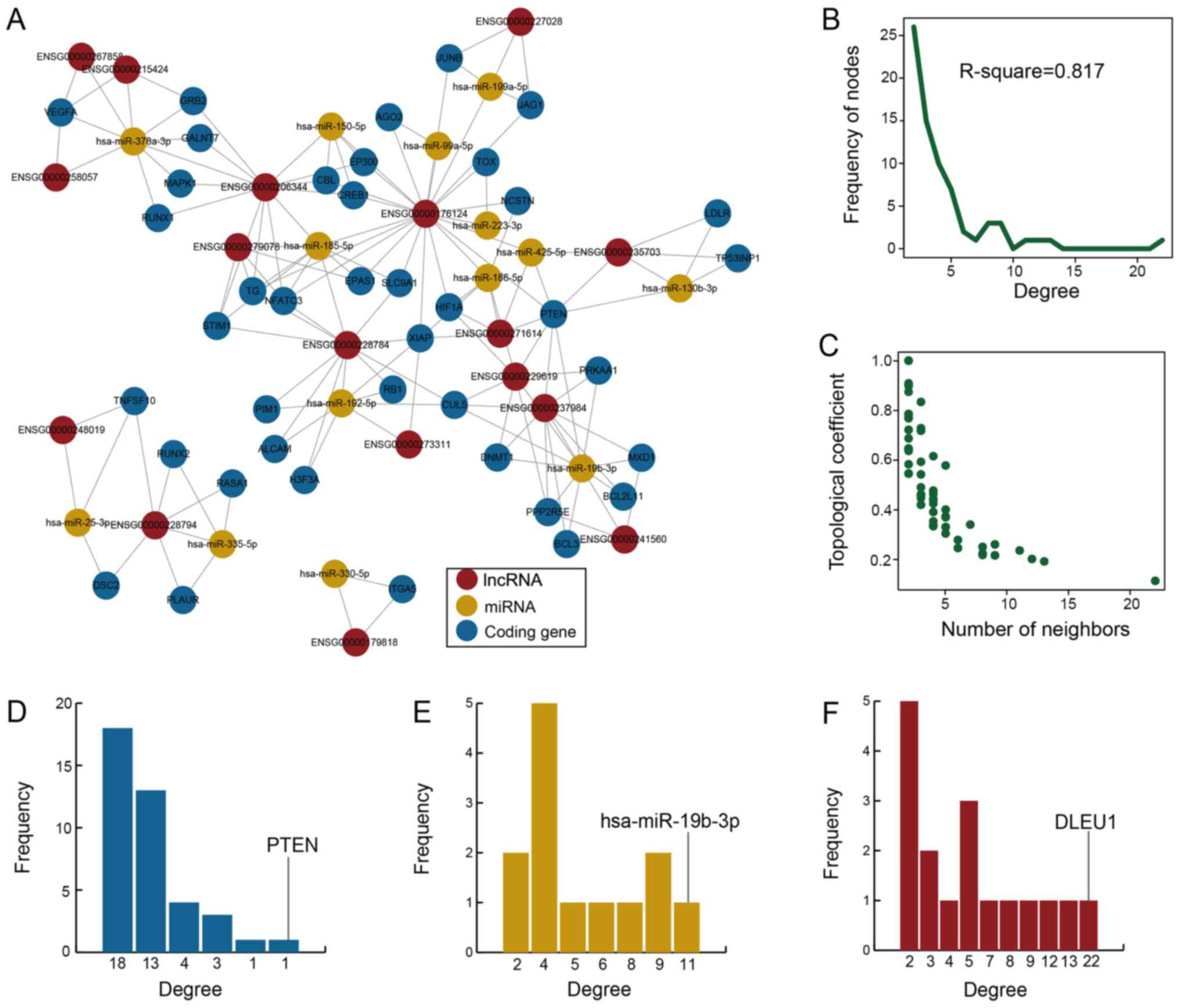
verified RNA interactions and expression data (Fig. 1A). The network consisted of 71
nodes (17 lncRNAs, 14 miRNAs and 40 coding genes) and 150 edges. A
total of 76 gene-lncRNA interactions, 30 miRNA-lncRNA interactions
and 44 gene-miRNA interactions were identified in the
GDM-associated LCEN. The GDM-associated LCEN exhibited a scale-free
distribution (R2=0.817) and was similar to the majority
of biological networks (Fig. 1B).
It was further indicated that this network had a similarity to the
small-world network (26). In
addition, it was discovered that the topological coefficient
decreased when the degree increased, which suggested that the
GDM-associated LCEN described a hierarchical modularity phenomenon
(Fig. 1C) (27). This topological feature has been
presented in ceRNA networks of various types of cancer (19). Furthermore, we determined the
degrees of coding genes, miRNAs and lncRNAs. A node was considered
as a hub, with increased ceRNA interactions representing higher
node degrees. In the current study, lncRNA nodes exhibited higher
mean degrees compared with coding gene and miRNA nodes (Fig. 1D-F). The mean degree of coding for
genes, miRNAs and lncRNAs was 3, 5.54 and 9.40, respectively. These
results suggested that although lncRNA do not code for proteins,
they exhibited more specific topological properties than mRNAs in
the GDM-associated LCEN. The highest degree nodes were determined
for phosphatase and tensin homolog (PTEN), hsa-miR-19b-3p and
deleted in lymphocytic leukemia 1 (DLEU1).
Variable competing activity profiles in
the GDM-associated ceRNA network
To characterize the compactness of each ceRNA
interaction in the GDM-associated LCEN, we evaluated the PCC values
for the gene-lncRNA, miRNA-lncRNA and miRNA-gene interactions.
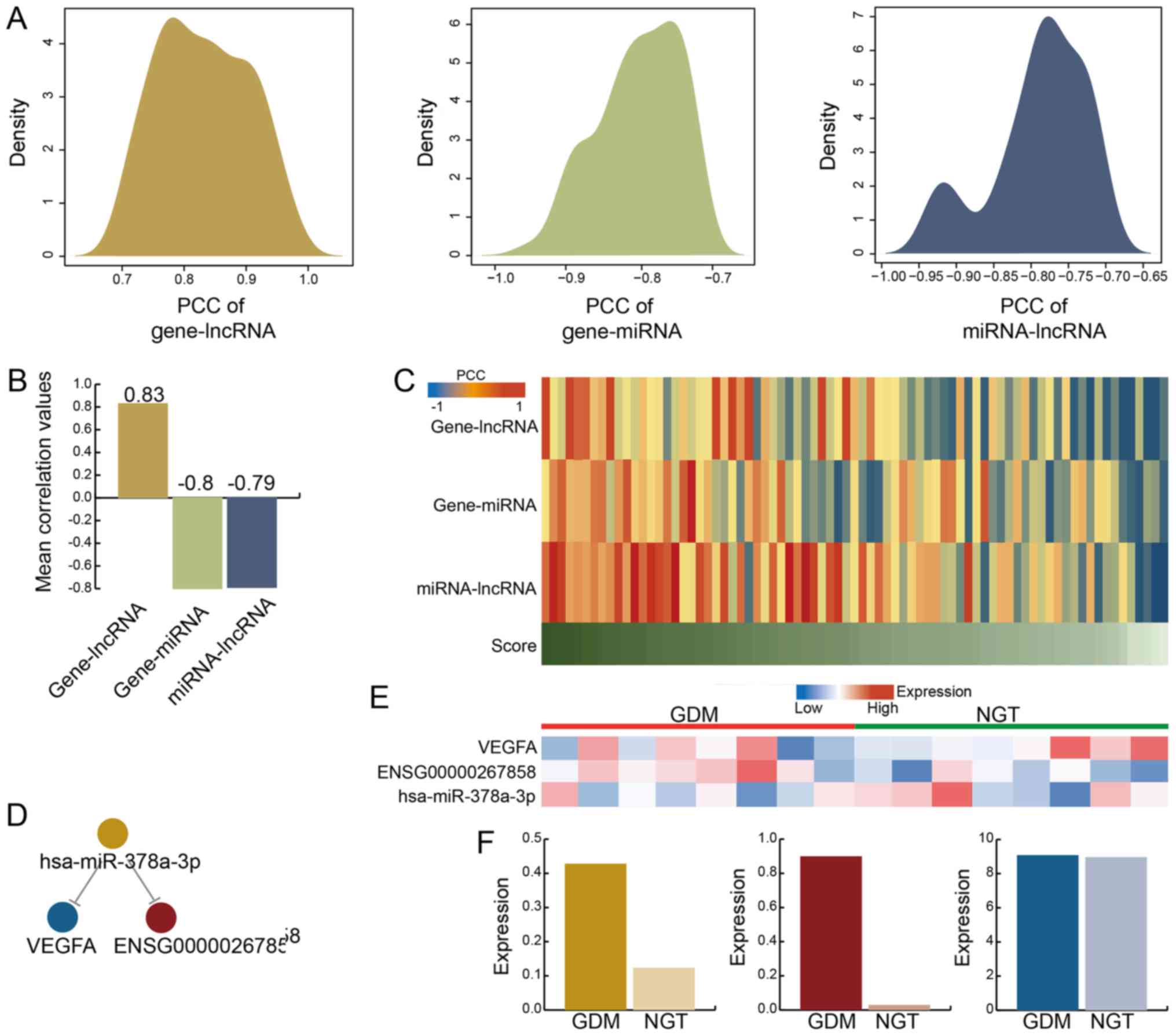
Density curves for these interactions were determined and observed
to be similar (Fig. 2A). The
majority PCC values were concentrated between 0.75-0.80 and the
results indicated that interactions were compact. The mean PCC
value of gene-lncRNA interactions was highest with 0.83 and the
miRNA-gene and miRNA-lncRNA interactions had mean PCC values of
−0.8 and −0.79, respectively (Fig.
2B). In addition, a functional score was proposed to evaluate
the GDM-associated functional ceRNAs. Based on the functional
score, we constructed a competing activity profile for the
GDM-associated LCEN (Fig. 2C). We
discovered that while certain interactions exhibited lower PCC
values, the overall ceRNAs exhibited a high functional score. The
result indicated that the functional score may be used to evaluate
the properties of ceRNA interactions. We further discovered that
the gene expression for VEGFA competed with lncRNA expression of
MZF1-AS1 and hsa-miR-378a-3p expression (Fig. 2D). The expression heatmap
exhibited differences between GDM and NGT (Fig. 2E). The expression levels of VEGFA,
MZF1-AS1 and hsa-miR-378a-3p in GDM were increased compared with
NGT (Fig. 2F). We further
observed that the difference in lncRNA levels between GDM and NGT
was not significant, potentially due to decreased lncRNA expression
in blood. The results suggested that gene and miRNA associations
with ceRNA may potentially be used to explore the role and function
of lncRNA, especially in blood sample.
Characterization of a core GDM-associated
subnetwork
We extracted a subnetwork based on verified
GDM-associated genes to explore how ceRNAs provide insight into GDM
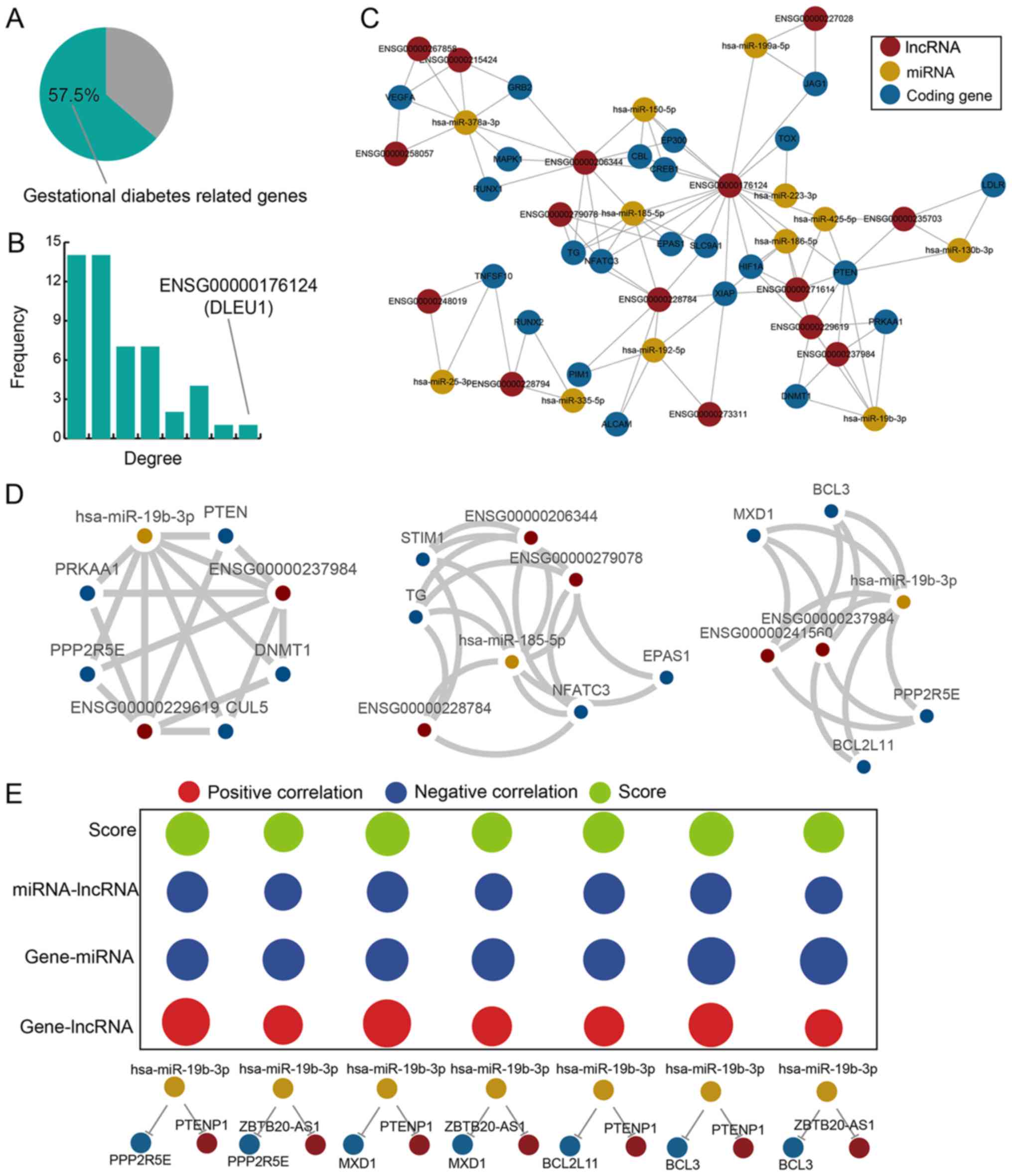
pathogenesis. We observed that 57.5% of the genes in the
GDM-associated LCEN were verified as GDM-associated genes (Fig. 3A). We further counted the degree
of nodes in the subnetwork (Fig.
3B). DLEU1 lncRNA exhibited the highest degree, which indicated
that it may play an essential role in the GDM-associated LCEN. The
subnetwork consisted of 50 nodes (23 coding genes, 12 miRNAs and 15
lncRNAs) and 103 edges (Fig. 3C).
A module analysis of the subnetwork was performed to further
investigate the interactions between various RNA transcripts. Three
modules were extracted, and the number of nodes and features were
analyzed (Fig. 3D). The first
module included 8 nodes (5 coding genes, 1 miRNA and 2 lncRNAs);
the nodes formed 10 ceRNAs. The second module included 8 nodes (4
coding genes, 1 miRNA and 3 lncRNAs); the nodes formed 10 ceRNAs.
The third module included 7 nodes (4 coding genes, 1 miRNA and 2
lncRNAs); the nodes formed 7 ceRNAs. A small quantity of nodes
formed multiple ceRNAs indicating these nodes had strong
associations. We further considered the compactness of each ceRNA
interaction in modules and discovered the ceRNA interactions in
modules were more compact. In the third module, all 7 ceRNAs had
high functional scores (Fig. 3E)
suggesting that the nodes in this module were close.
GDM-associated ceRNA distinguish patients
with NGT and GDM
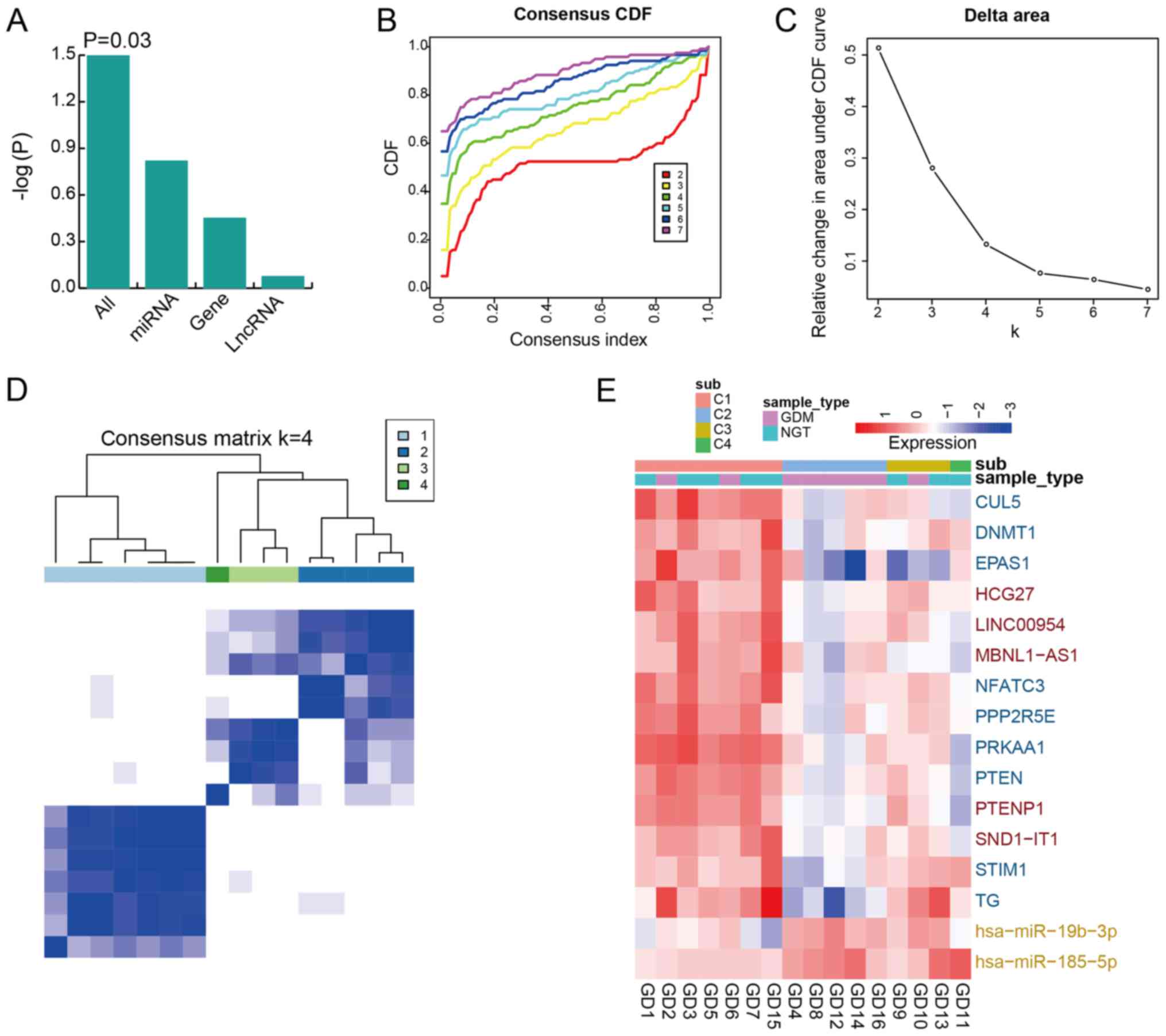
To evaluate whether ceRNAs are potential classifiers
in GDM, we used gene, miRNA and lncRNA expression values from each
module to classify the samples using a consensus clustering method.
We further used a Chi-square test to validate the classification
power of the modules. No significant differences in gene, miRNA or
lncRNA expression between GDM and NGT samples were observed in
these 3 modules; however, significance was determined for the
integration of first and second module (P=0.03; Fig. 4A). The results indicated that
ceRNAs may be an effective classifier for patients with GDM. We
then analyzed the classification power to evaluate the integrated
expression of 9 coding genes [cullin 5 (CUL5), DNA
methyltransferase 1 (DNMT1), endothelial PAS domain protein 1
(EPAS1), nuclear factor of activated T cells 3 (NFATC3), protein
phosphatase 2 regulatory subunit B′ epsilon (PPP2R5E), protein
kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), PTEN,
stromal interaction molecule 1 (STIM1) and thyroglobulin (TG)], 5
lncRNAs (HCG27, LINC00954, MBNL1-AS1, PTENP1 and SND1-IT1) and 2
miRNAs (miR-19b-3p and miR-185-5p). The GDM and NGT samples could
be classified into several groups by the above-mentioned genes,
lncRNAs and miRNAs. According to the CDF and relative change in the
area under the curve (Fig. 4C and
D), we determined that the final number of groups was 4. We
discovered that the 4 sample groups had respective expression
patterns (Fig. 4D). We further
determined that a majority of samples was classified accurately,
particularly in the second group (C2), where all samples were from
GDM (Fig. 4E). Collectively,
these results suggested that the integration of expression in ceRNA
interactions can distinguish between NGT and GDM samples.
GDM-associated ceRNA is associated with
critical biological functions and the thyroid hormone
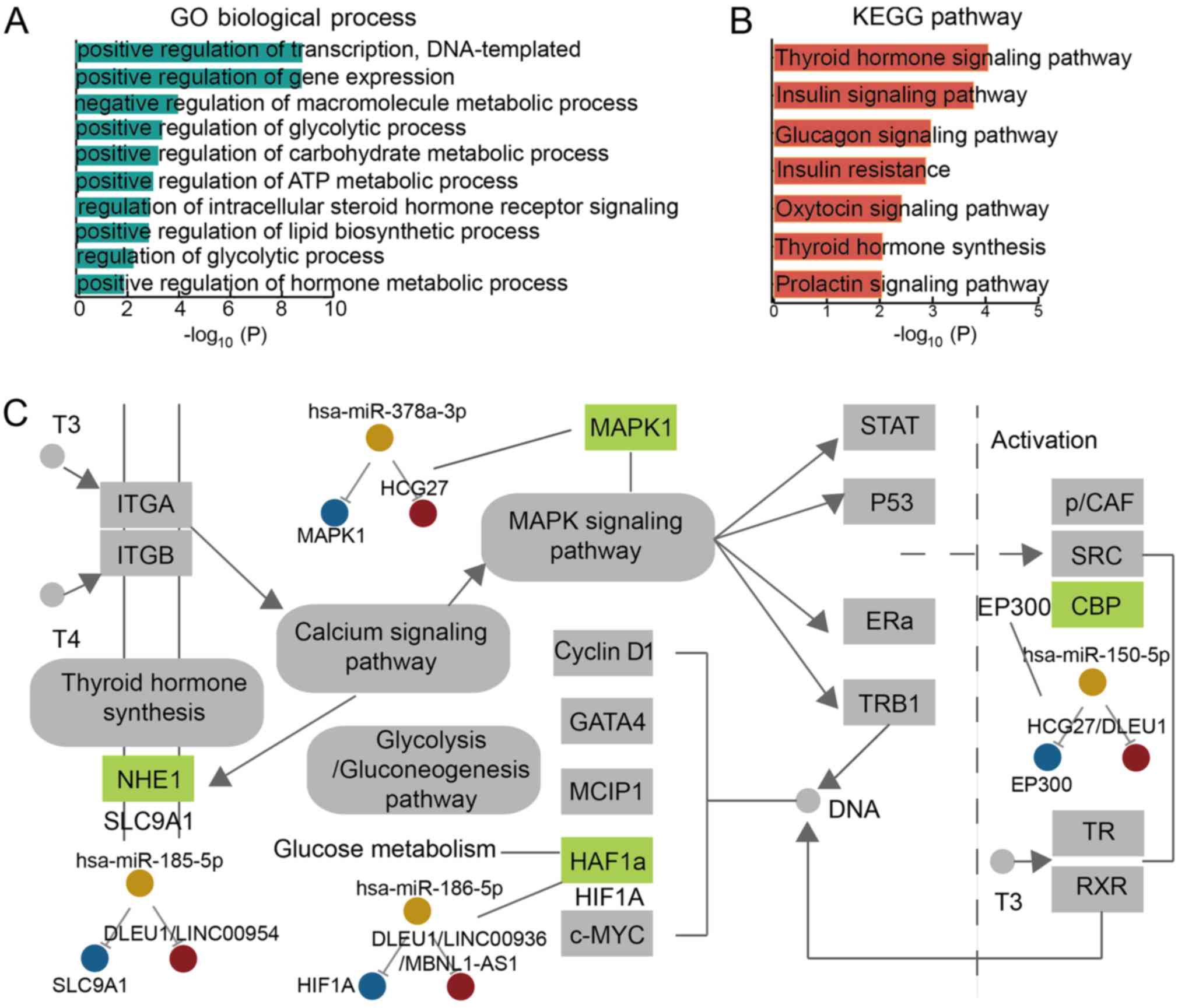
We performed GO enrichment analysis based on the
genes in the GDM-associated LCEN. The enrichment of these genes in
different GO terms was observed (Fig.
5A). Genes were associated with some critical biological
functions, including the positive regulation of gene expression and
the positive regulation of transcription. Certain genes exhibited
metabolism-associated functions, such as the enrichment of the
regulation of macromolecular, carbohydrate and ATP processes. In
addition, we discovered associations to glycolytic processes.
Significantly enriched GO functions were associated with hormones,
including intracellular steroid production and hormone metabolism.
We further performed pathway enrichment analysis and identified
insulin-associated pathways to be relevant in the GDM-associated
LCEN (Fig. 5B). The thyroid
hormone signaling pathway was identified in the analysis and this
hormone may be a potential biochemical marker in predicting GDM and
effects on insulin sensitivity in GDM (28,29). As the thyroid hormone status has
previously been demonstrated to be associated with GDM during
pregnancy (29,30), we further analyzed this signaling
pathway and identified 4 enriched genes (Fig. 5C). These genes participated in 8
ceRNA interactions and the pathway was identified to be associated
with the gluconeogenesis pathway, which is an essential pathway in
diabetes (31). We further
focused on irisin, which is an exercise-induced myokine that drives
brown-fat-type thermogenesis in murine white fat (32). In this analysis, we observed
associations with the MAPK signaling pathway and importance arises
from irisin potentially suppressing obesity and associated T2DM via
the MAPK signaling pathway (33).
A ceRNA module identified in the current study,
MAPK1/miR-378/HCG27, may be associated with irisin based to
literature known MAPK1 and miR-378 associations with irisin
(34,35). Additionally, hyper- and
hypothyroidism are associated with the upregulation of serum irisin
in male rats (36). These results
suggested GDM-associated ceRNAs may participate in the regulation
of the thyroid hormone and irisin and influence GDM
development.
Discussion
Coding genes, miRNAs and lncRNAs can form complex
regulatory clusters, including ceRNAs, which may influence
pathogenic mechanisms in GDM. lncRNAs regulate mRNA expression
through an indirect post-transcriptional mechanism of competing
with miRNA for mRNAs binding sites (14). In various diseases, individual
ceRNA crosstalk interactions were verified and global ceRNA
networks were constructed (37-39). Lin et al (40) constructed a ceRNA network to
reveal the regulatory role of lncRNAs in T2DM. However, Lin et
al (40) solely relied on
disease-associated genes to construct ceRNA network and expression
information was not integrated. The lack of expression information
reduced specificity and accuracy in the disease biomarker analysis.
In this study, a system was created that integrated
experimentally-verified RNA interactions, expression data and
verified GDM-associated genes. A global ceRNA network was
constructed and GDM-associated ceRNAs were identified. A candidate
GDM-associated ceRNA list were provided and some lncRNAs, genes and
miRNAs in this list have not been verified by strong evidence. In
future studies, researchers could extracted these molecules to
construct relations between non-coding RNAs and GDM or identify
more accurate biomakers and treatment targets for GDM.
Collectively, the current study provides a comprehensive resource
for studying the regulation of GDM by non-coding molecules.
Various lncRNAs function as molecular biomarkers of
different types of diseases. lncRNA-HEIH levels in serum and
exosomes are potential biomarkers for HCV-associated hepatocellular
carcinoma (41). Plasma lncRNA
GAS5 is a novel biomarker for coronary artery disease (42). Survival analyses further
demonstrated that ceRNA network modules are potential prognostic
biomarkers in GBM (43). In the
current analysis, we found that although single lncRNAs could not
perfectly distinguish GDM and NGT (Chi-square test, P>0.05),
some combinations among lncRNAs, genes and miRNAs could distinguish
GDM and NGT (Chi-square test, P=0.03). The result also verified the
advantage of considering the ceRNA as specific and effective
biomarkers to distinguish between NGT and GDM. We inferred that
lncRNAs could interact with other molecules (including miRNAs and
genes) to play their roles in some diseases. Blood-based biomarkers
are critical for disease prediction. In this study, it was
suggested that certain candidate ceRNAs in the blood may serve as
circulating biomarkers in GDM and more experiments focused on whole
ceRNA motif in cell lines or animal models should be
constructed.
The frequency of thyroid dysfunction in diabetic
patients is increased compared with the general population and ≤1/3
of patients with type 1 diabetes develop thyroid dysfunction
(44). Thyroid hormones are
positively associated with insulin resistance in the early
development of T2DM (45).
However, little attention has been paid to the diagnosis of thyroid
diseases in diabetics, as they are diagnosed in only approximately
half of the patients (46). In
the current study, we discovered various GDM-associated ceRNAs
associated with the thyroid hormone signaling pathway. For example,
SLC9A1 is a key upstream gene of thyroid hormone signaling pathway
and form two dysregulated ceRNAs with miR-185-5p and lncRNA DLEU1
and LINC00954 in GDM. DLEU1 was associated with BMI-adjusted
adiponectin, which is related to diabetes (47). In this study, lncRNA DLEU1 had the
highest degree in GDM-associated subnetwork (Fig. 3B). We provide novel insight into
how lncRNA DLEU1 influences thyroid hormone synthesis by competing
miR-185-5p with SLC9A1. The MAPK signaling pathway is an essential
part of the thyroid hormone signaling pathway (34). In this study, we found that lncRNA
HCG27 competes for miR-378a-3p with MAPK1 in GDM, and MAPK1 and
miR-378 have both been shown to be associated with irisin in
previous studies (34,35). Irisin is expressed and produced by
human muscle and adipose tissue in association with obesity and
insulin resistance (48). Thus,
we inferred that there were close relations among irisin, thyroid
hormone, obesity and GDM. In summary, novel insight was provided
into the study of the associations between thyroid hormone
dyscrasia and GDM. Further studies are warranted to focus on
investigating an increased number of GDM samples to validate the
accuracy and stability of the method presented in the current
study.
In conclusion, in the present study, we constructed
a GDM-associated LCEN and analyzed its features. A functional score
was used to evaluate the activity of each ceRNA in GDM and certain
ceRNA modules were extracted analyzed for strict interactions. In
addition, these ceRNA modules demonstrated to distinguish between
GDM and NGT samples. We further observed a close association
between thyroid hormone dyscrasia and GDM. The current analysis
provides insight into the identification of novel biomarkers for
GDM.
Funding
The current study was supported by the National
Health and Family Planning Commission Public Welfare Industry
Special (grant no. 201502007).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
LL and QL conceived and designed the present study.
LL and CZ performed the experiments and analyzed the data. LR
validated and improved the computational approach in this study. LL
and LR wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Crusell MKW, Hansen TH, Nielsen T, Allin
KH, Rühlemann MC, Damm P, Vestergaard H, Rørbye C, Jørgensen NR,
Christiansen OB, et al: Gestational diabetes is associated with
change in the gut microbiota composition in third trimester of
pregnancy and postpartum. Microbiome. 6:892018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang C, Bao W, Rong Y, Yang H, Bowers K,
Yeung E and Kiely M: Genetic variants and the risk of gestational
diabetes mellitus: a systematic review. Hum Reprod Update.
19:376–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Daly B, Toulis KA, Thomas N, Gokhale K,
Martin J, Webber J, Keerthy D, Jolly K, Saravanan P and
Nirantharakumar K: Increased risk of ischemic heart disease,
hypertension, and type 2 diabetes in women with previous
gestational diabetes mellitus, a target group in general practice
for preventive interventions: a population-based cohort study. PLoS
Med. 15:e10024882018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bellou V, Belbasis L, Tzoulaki I and
Evangelou E: Risk factors for type 2 diabetes mellitus: an
exposure-wide umbrella review of meta-analyses. PLoS One.
13:e01941272018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang C and Ning Y: Effect of dietary and
lifestyle factors on the risk of gestational diabetes: Review of
epidemiologic evidence. Am J Clin Nutr. 94(Suppl 6): 1975S–1979S.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu C and Zhang Z: Comparative study of
thyroid hormone and antithyroid antibody levels in patients with
gestational diabetes mellitus and pregnant patients with diabetes.
Minerva Endocrinol. 43:126–130. 2018.
|
|
7
|
Yang S, Shi FT, Leung PC, Huang HF and Fan
J: Low thyroid hormone in early pregnancy is associated with an
increased risk of gestational diabetes mellitus. J Clin Endocrinol
Metab. 101:4237–4243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
St Laurent G, Wahlestedt C and Kapranov P:
The Landscape of long noncoding RNA classification. Trends Genet.
31:239–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Gu H, Xu W and Zhou X:
Down-regulation of lncRNA MALAT1 reduces cardiomyocyte apoptosis
and improves left ventricular function in diabetic rats. Int J
Cardiol. 203:214–216. 2016. View Article : Google Scholar
|
|
10
|
Qin J, Bao H and Li H: Correlation of long
non-coding RNA taurine-upregulated gene 1 with disease conditions
and prognosis, as well as its effect on cell activities in acute
myeloid leukemia. Cancer Biomark. Nov 4–2018.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shu J, Li S, Chen YB, Zhu QF and Yu XH:
Long non-coding RNA EPB41L4A-AS2 inhibited non-small cell lung
cancer proliferation, invasion and promoted cell apoptosis.
Neoplasma. 65:664–672. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Wu H, Wang F, Ye M, Zhu H and Bu
S: Long non-coding RNA MALAT1 expression in patients with
gestational diabetes mellitus. Int J Gynaecol Obstet. 140:164–169.
2018. View Article : Google Scholar
|
|
13
|
Shi Z, Zhao C, Long W, Ding H and Shen R:
Microarray expression profile analysis of long non-coding RNAs in
umbilical cord plasma reveals their potential role in gestational
diabetes-induced macrosomia. Cell Physiol Biochem. 36:542–554.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: the Rosetta Stone of a hidden RNA
language. Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ebert MS and Sharp PA: Emerging roles for
natural microRNA sponges. Curr Biol. 20:R858–R861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen DL, Lu YX, Zhang JX, Wei XL, Wang F,
Zeng ZL, Pan ZZ, Yuan YF, Wang FH, Pelicano H, et al: Long
non-coding RNA UICLM promotes colorectal cancer liver metastasis by
acting as a ceRNA for microRNA-215 to regulate ZEB2 expression.
Theranostics. 7:4836–4849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang G, Li S, Lu J, Ge Y, Wang Q, Ma G,
Zhao Q, Wu D, Gong W, Du M, et al: LncRNA MT1JP functions as a
ceRNA in regulating FBXW7 through competitively binding to
miR-92a-3p in gastric cancer. Mol Cancer. 17:872018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu J, Feng L, Han Z, Li Y, Wu A, Shao T,
Ding N, Li L, Deng W, Di X, et al: Extensive ceRNA-ceRNA
interaction networks mediated by miRNAs regulate development in
multiple rhesus tissues. Nucleic Acids Res. 44:9438–9451.
2016.PubMed/NCBI
|
|
19
|
Wang P, Ning S, Zhang Y, Li R, Ye J, Zhao
Z, Zhi H, Wang T, Guo Z and Li X: Identification of
lncRNA-associated competing triplets reveals global patterns and
prognostic markers for cancer. Nucleic Acids Res. 43:3478–3489.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: a resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46(D1): D296–D302.
2018. View Article : Google Scholar :
|
|
21
|
Yi Y, Zhao Y, Li C, Zhang L, Huang H, Li
Y, Liu L, Hou P, Cui T, Tan P, et al: RAID v2.0: An updated
resource of RNA-associated interactions across organisms. Nucleic
Acids Res. 45(D1): D115–D118. 2017. View Article : Google Scholar :
|
|
22
|
Piñero J, Queralt-Rosinach N, Bravo À,
Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F and Furlong LI:
DisGeNET: A discovery platform for the dynamical exploration of
human diseases and their genes. Database (Oxford). 2015:bav0282015.
View Article : Google Scholar
|
|
23
|
Cho S, Jang I, Jun Y, Yoon S, Ko M, Kwon
Y, Choi I, Chang H, Ryu D, Lee B, et al: MiRGator v3.0: A microRNA
portal for deep sequencing, expression profiling and mRNA
targeting. Nucleic Acids Res. 41(D1): D252–D257. 2013. View Article : Google Scholar :
|
|
24
|
Wilkerson MD and Hayes DN:
ConsensusClusterPlus: A class discovery tool with confidence
assessments and item tracking. Bioinformatics. 26:1572–1573. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuleshov MV, Jones MR, Rouillard AD,
Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM,
Lachmann A, et al: Enrichr: a comprehensive gene set enrichment
analysis web server 2016 update. Nucleic Acids Res. 44:W90–W97.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amaral LA, Scala A, Barthelemy M and
Stanley HE: Classes of small-world networks. Proc Natl Acad Sci
USA. 97:11149–11152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simos T, Georgopoulou U, Thyphronitis G,
Koskinas J and Papaloukas C: Analysis of protein interaction
networks for the detection of candidate hepatitis B and C
biomarkers. IEEE J Biomed Health Inform. 19:181–189. 2015.
View Article : Google Scholar
|
|
28
|
Tawfeek MA, Alfadhli EM, Alayoubi AM,
El-Beshbishy HA and Habib FA: Sex hormone binding globulin as a
valuable biochemical marker in predicting gestational diabetes
mellitus. BMC Womens Health. 17:182017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kramer CK, Swaminathan B, Hanley AJ,
Connelly PW, Sermer M, Zinman B and Retnakaran R: Vitamin D and
parathyroid hormone status in pregnancy: Effect on insulin
sensitivity, β-cell function, and gestational diabetes mellitus. J
Clin Endocrinol Metab. 99:4506–4513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serra MC and Ryan AS: Influence of Vitamin
D and parathyroid hormone on bone and metabolic risk in women with
previous gestational diabetes. Horm Metab Res. 48:497–502. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan S and Jena GB: Protective role of
sodium butyrate, a HDAC inhibitor on beta-cell proliferation,
function and glucose homeostasis through modulation of p38/ERK MAPK
and apoptotic pathways: Study in juvenile diabetic rat. Chem Biol
Interact. 213:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee P, Linderman JD, Smith S, Brychta RJ,
Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, et
al: Irisin and FGF21 are cold-induced endocrine activators of brown
fat function in humans. Cell Metab. 19:302–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Li R, Meng Y, Li S, Donelan W,
Zhao Y, Qi L, Zhang M, Wang X, Cui T, et al: Irisin stimulates
browning of white adipocytes through mitogen-activated protein
kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes.
63:514–525. 2014. View Article : Google Scholar
|
|
34
|
Zhang Y, Mu Q, Zhou Z, Song H, Zhang Y, Wu
F, Jiang M, Wang F, Zhang W, Li L, et al: Protective effect of
irisin on atherosclerosis via suppressing oxidized low density
lipoprotein induced vascular inflammation and endothelial
dysfunction. PLoS One. 11:e01580382016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan D, Mao C, Quattrochi B, Friedline RH,
Zhu LJ, Jung DY, Kim JK, Lewis B and Wang YX: MicroRNA-378 controls
classical brown fat expansion to counteract obesity. Nat Commun.
5:47252014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Samy DM, Ismail CA and Nassra RA:
Circulating irisin concentrations in rat models of thyroid
dysfunction - effect of exercise. Metabolism. 64:804–813. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian L, He Y, Zhang H, Wu Z, Li D and
Zheng C: Comprehensive analysis of differentially expressed
profiles of lncRNAs and mRNAs reveals ceRNA networks in the
transformation of diffuse large B-cell lymphoma. Oncol Lett.
16:882–890. 2018.PubMed/NCBI
|
|
38
|
Liu H, Zhang Z, Wu N, Guo H, Zhang H, Fan
D, Nie Y and Liu Y: Integrative Analysis of Dysregulated
lncRNA-Associated ceRNA Network Reveals Functional lncRNAs in
Gastric Cancer. Genes (Basel). 9:92018. View Article : Google Scholar
|
|
39
|
Feng K, Liu Y, Xu LJ, Zhao LF, Jia CW and
Xu MY: Long noncoding RNA PVT1 enhances the viability and invasion
of papillary thyroid carcinoma cells by functioning as ceRNA of
microRNA-30a through mediating expression of insulin like growth
factor 1 receptor. Biomed Pharmacother. 104:686–698. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin Z, Li X, Zhan X, Sun L, Gao J, Cao Y
and Qiu H: Construction of competitive endogenous RNA network
reveals regulatory role of long non-coding RNAs in type 2 diabetes
mellitus. J Cell Mol Med. 21:3204–3213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang C, Yang X, Qi Q, Gao Y, Wei Q and
Han S: lncRNA-HEIH in serum and exosomes as a potential biomarker
in the HCV-related hepatocellular carcinoma. Cancer Biomark.
21:651–659. 2018. View Article : Google Scholar
|
|
42
|
Yin Q, Wu A and Liu M: Plasma long
non-coding RNA (lncRNA) GAS5 is a new biomarker for coronary artery
disease. Med Sci Monit. 23:6042–6048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao Y, Wang P, Ning S, Xiao W, Xiao B and
Li X: Identification of prognostic biomarkers in glioblastoma using
a long non-coding RNA-mediated, competitive endogenous RNA network.
Oncotarget. 7:41737–41747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kadiyala R, Peter R and Okosieme OE:
Thyroid dysfunction in patients with diabetes: Clinical
implications and screening strategies. Int J Clin Pract.
64:1130–1139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lambadiari V, Mitrou P, Maratou E, Raptis
AE, Tountas N, Raptis SA and Dimitriadis G: Thyroid hormones are
positively associated with insulin resistance early in the
development of type 2 diabetes. Endocrine. 39:28–32. 2011.
View Article : Google Scholar
|
|
46
|
Vondra K, Vrbikova J and Dvorakova K:
Thyroid gland diseases in adult patients with diabetes mellitus.
Minerva Endocrinol. 30:217–236. 2005.PubMed/NCBI
|
|
47
|
Li WD, Jiao H, Wang K, Yang F, Grant SF,
Hakonarson H, Ahima R and Arlen Price R: Pathway-based genome-wide
association studies reveal that the rac1 pathway is associated with
plasma adiponectin levels. Sci Rep. 5:134222015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moreno-Navarrete JM, Ortega F, Serrano M,
Guerra E, Pardo G, Tinahones F, Ricart W and Fernández-Real JM:
Irisin is expressed and produced by human muscle and adipose tissue
in association with obesity and insulin resistance. J Clin
Endocrinol Metab. 98:E769–E778. 2013. View Article : Google Scholar : PubMed/NCBI
|