Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies worldwide, particularly in developing countries
(1). Surgical resection is an
appropriate therapy for early HCC (2). However, the majority of patients are
initially diagnosed with advanced HCC, and, combined with poor
prognosis due to a high intrahepatic or extrahepatic metastatic
potential, survival rates for patients remain low (3). It has been demonstrated that
patients with advanced HCC could benefit from administration of
molecular target drugs that target signaling cascades, such as the
multikinase inhibitor, sorafenib (4). Numerous researchers are interested
in the molecular targeted treatment field (5-7).
Previous studies have demonstrated that several cell signaling
pathways, including the Ras/Raf/mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase (8), Wnt/β-catenin (9) and phosphoinositide 3-kinase
(PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR)
(10) pathways, are involved in
HCC occurrence and metastasis. Furthermore, it is noteworthy that
AKT appears to be activated in >50% cases of HCC occurrence
(11).
The Krüppel-like factor (Klf) family is
characterized by a highly conserved C2H2 zinc finger motif, which
consists of two cysteine and histidine residues (12). The protein family members combine
with the target gene sequence that is complementary to the
GC/GT/CACCC site, serving an important role in regulating specific
gene transcription (13). The
amino-terminal ends of the Klf family members all differ from each
other, and they combine with various activating or inhibiting
factors. This feature leads to the diversity of the Klf family
members (14,15). Klf4, also termed 'gastrointestinal
concentration Klf', is predominantly expressed in gastrointestinal
vascular endothelial cells (16).
Klf4 contains five known AKT phosphorylation sites: Thr-399,
Ser-19, Thr-33, Ser-234 and S326 (17), with the AKT pathway being the
pathway that mediates the expression of the anti-apoptotic protein,
B-cell lymphoma 2 (Bcl-2), and the apoptotic protein,
Bcl-2-associated X protein (Bax) (18).
In various tumor types, Klf4 is considered to be a
cancer suppressor protein that regulates various cell signaling
pathways associated with a number of different processes: i)
Epithelial-mesenchymal transition reversion (19); ii) the oncogenic transforming
growth factor-β/mothers against decapentaplegic homolog signaling
pathway (20); iii) the
PI3K/AKT/p21 pathway (21); and
iv) the Bcl-2/Bax ratio (22).
The present research group has previously reported that the
deregulation of Klf4 is critical for loss of expression of the
vitamin D receptor, which enhances the invasive and metastatic
potential of HCC (23). However,
the molecular mechanisms that are associated with regulating low
expression levels of Klf4 in HCC require further investigation.
microRNAs (miRNAs or miRs) are small, endogenous
non-coding RNAs that are ~22 nucleotides in length, which regulate
gene expression through protein translation and the associated
degradation of mRNA (24). miRNAs
function as oncogenes or tumor suppressors, depending on the target
gene concerned. For example, miR-373 increases proliferation of
cervical cancer cells by directly targeting the highly conserved
deubiquitinating enzyme of the ovarian tumor (otubain) family, YOD1
(25). miR-340, targeting Janus
kinase 1, has been demonstrated to inhibit HCC proliferation and
invasion (26). A study published
previously by our research group revealed that Klf4 expression was
decreased in HCC, and that miR-135a-5p affected the expression of
Klf4 (27).
In the present study, miRNA prediction
bioinformatics analysis was used to demonstrate that miR-9-5p is a
regulator of Klf4. miR-9-5p has a different stem-loop structure
compared with the precursors of miR-9 (28). Overexpression of miR-9 has been
demonstrated in HCC cells (29),
whereas the expression levels of Klf4 are decreased (23). Therefore, the present study aimed
to explore whether miR-9-5p is a functional target of the
3′-untranslated region (3′-UTR) of Klf4 in order to identify novel
targets for HCC treatment.
To meet this aim, the expression levels of miR-9-5p
and Klf4 were initially investigated in clinical samples, and
overall survival (OS) rates of different levels of the miR-9-5p
mRNA expression groups were analyzed in The Cancer Genome Atlas
(TCGA) database. Subsequently, the evidence that miR-9-5p directly
targeted Klf4 in HCC cells was corroborated. Further studies
suggested that miR-9-5p downregulating Klf4, is involved in the HCC
proliferation, apoptosis and migration of HCC cells, potentially
resulting in activation of AKT pathway.
Materials and methods
Cell lines and culture
Human HCC cell lines (SK-Hep-1, HCC-LM3, Huh7 and
Hep-3B), and the human immortalized liver cell line, L02, were
purchased from Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China) and maintained in Invitrogen®
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% Gibco™ fetal bovine
serum (FBS) and 100 U/ml penicillin/streptomycin (Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2.
Clinical specimens
A total of 20 HCC tissue and adjacent normal tissue
samples were obtained from 20 patients at The 5th Department of
Hepatic Surgery, Eastern Hepatobiliary Surgery Hospital, The Second
Military Medical University (Shanghai, China) between March and
July 2017. All patients with pathological diagnosis of primary HCC
and clinical stage of I-III were included. The samples were
snap-frozen in liquid nitrogen and stored at −80°C. The American
Joint Committee on Cancer/International Union Against Cancer
staging system for HCC was used to define the stage of patients
(30,31). The Ethics Committee of the
hospital approved the present study, and patient consent was
obtained prior to tissue collection. All samples following
resection were immediately snap-frozen in liquid nitrogen, and
subsequently stored at -80°C.
Klf4 overexpression plasmid, small
interfering (si)RNA against Klf4 (siKlf4), miRNA mimic and
inhibitor
The Klf4 overexpression Klf4 plasmid was obtained
from the scientific research group of Professor Keping Xie
(University of Texas, MD Anderson Cancer Center, Houston, TX, USA).
siKlf4 (forward primer, 5′-AUCGUUGAACUCCUCGGUCUCUCUC-3′; reverse
primer, 5′-GAGAGAGACCGAGGAGUUCAACGAU-3′) was synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China), as were miR-9-5p
mimic (forward, 5′-UCUUUGGUUAUCUAGCUGUAUGA-3′; reverse,
5′-UUAGAAACCAAUAGAUCGACAUA-3′), miR-9-5p inhibitor
(5′-UCAUACAGCUAGAUAACCAAAGA-3′), miR-9-5p inhibitor negative
control (NC; 5′-CAGUACUUUUGUGUAGUACAA-3′), mimic NC (forward,
5′-UUCUCCGAACGUGUCACGUTT-3′; reverse, 5′-ACGUGACACGUUCGGAGAATT-3′),
miR-128 mimic (forward, 5′-UCACAGUGAACCGGUCUCUUU-3′; reverse,
5′-AGAGACCGGUUCACGGUGAUU-3′), miR-449a mimic (forward,
5′-UGGCAGUGUAUUGUUAGCUGGU-3′; reverse,
5′-CAGCUAACAAUACACUGCAAUU-3′) and miR-214 mimic (forward,
5′-ACAGCAGGCACAGACAGGCAG-3′; reverse,
5′-GCCUGUCUGUGCCUGCUGUUU-3′).
Cell transfection
A total of 2×105 HCC cells per well were
seeded in a 6-well plate prior to the day of transfection. When the
cell density had reached 70-80% confluence, Klf4 over-expression
plasmid, siKlf4, miR-9-5p mimic and miR-9-5p inhibitor were used
for transfection with Lipofectamine® 2000 transfection
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. DMEM without FBS was mixed with
Lipofectamine® 2000, mimic, inhibitor, siRNA or plasmid.
They were incubated at room temperature for 30 min. The mixture was
added into cells. It was then incubated at 37°C and with 5%
CO2 for 48 h. Transfection efficiency was verified using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis. Subsequent experiments were
performed 48 h following transfection.
Western blot analysis
HCC cells were lysed using radioim-munoprecipitation
buffer containing protease inhibitors (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Protein concentrations were determined using
the bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Subsequently, 25
µg protein were resolved on 5 or 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. The membranes
were then treated with 5% non-fat milk blocking solution for 2 h at
room temperature, and incubated overnight at 4°C with the following
primary antibodies (all Cell Signaling Technology, Inc., Danvers,
MA, USA): Anti-Klf4 (cat. no. 4038; 54 kDa; rabbit polyclonal;
1:1,000), anti-AKT Ser-473 (cat. no. 4091; 56 kDa; rabbit
polyclonal; 1:1,000), antibody phosphorylated (p)-AKT Ser-473 (cat.
no. 4069; 56 kDa; rabbit polyclonal; 1:1,000), anti-Bcl-2 (cat. no.
15071; 26 kDa; mouse polyclonal; 1:1,000), anti-Bax (cat. no. 5032;
20 kDa; rabbit polyclonal; 1:1,000), anti-mTOR (cat. no. 2972; 289
kDa; rabbit poly-clonal; 1:1,000), antibody p-mTOR (cat. no. 5536;
289 kDa; rabbit polyclonal; 1:1,000) and GAPDH (cat. no. 5174; 37
kDa; rabbit monoclonal; 1:5,000). Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit (cat. no. 7074) or anti-mouse
(cat. no. 7076; both 1:10,000; Cell Signaling Technology, Inc.) was
used as the secondary antibody and incubated at room temperature
for 1 h. Finally, the protein bands were detected using an enhanced
chemiluminescence (ECL) western HRP substrate reagent (Thermo
Fisher Scientific, Inc.) and images were captured using an Amersham
Imager 600 System (GE Healthcare, Chicago, IL, USA). The band
densitometry analysis was evaluated using ImageJ software, version
1.8.0 (National Institutes of Health, Bethesda, MD, USA).
RT-qPCR
Total RNA was extracted from the cultured cells and
HCC tissue using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol,
and the concentration of RNA was measured using a NanoDrop 1000
spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). Total RNA was used to synthe-size cDNA using a PrimeScript RT
Reagent kit (Takara Bio, Inc., Otsu, Japan). The reverse
transcription reaction conditions were as follows: 37°C for 30 sec,
85°C for 30 sec and 4°C at termination. qPCR was performed using a
SYBR Prime Script™ RT-qPCR kit (Takara Bio, Inc.), according to the
manufacturer's protocol, and an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocy-cling
conditions were as follows: Pre-denaturation at 95°C for 30 sec,
followed by 40 cycles of denaturation at 95°C for 5 sec and
extension at 60°C for 30 sec. miDETEC A Track™ miRNA RT-qPCR
Starter kit (Guangzhou RiboBio Co., Ltd., Guangzhou, China) was
used to detect the expression level of miR-9-5p expression. The
reverse transcription reaction conditions were as follows: 42°C for
1 h, 72°C for 10 min and 0°C at termination. qPCR thermocycling
conditions were as follows: Pre-denaturation at 95°C for 10 min,
followed by 40 cycles of denaturation at 95°C for 10 sec and
extension at 60°C for 20 sec. U6 and GAPDH were used as internal
controls for miRNA and gene expression, respectively. The primers
used in the qPCR analysis were designed as follows: miR-9-5p,
forward 5′-TCTTTGGTTATCTAGCTGTATGA-3′ and reverse
5′-TTCCGCGGCCGCTATGGCCGACGTCGACGGGAATGGGGAAAGGGAA-3′, Klf4, forward
5′-GTCTTGAGGAAGTGCTGAGC-3′ and reverse 5′-ATCGTCTTCCCCTCTTTGGC-3′;
U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse
5′-AACGCTTCACGAATTTGCGT-3′; GAPDH, forward
5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′.
Expression levels were calculated using the 2−ΔΔCq
method (32).
Dual-luciferase reporter assay
The binding site of miR-9-5p on Klf4 was identified
using the bioinformatic tool, miRanda (http://www.microrna.org). The wild-type 3′-UTR, and
mutation-type (MUT) 3′-UTR of Klf4 were cloned into the pGL3-basic
promoter luciferase reporter vector (Promega Corporation, Madison,
WI, USA). Prior to transfection, high-transfection-efficiency
SK-Hep-1 cells were seeded at 2×105 cells per well in
6-well plates. Subsequently, transfection was performed with
Lipofectamine® 2000 transfection reagent (Thermo Fisher
Scientific, Inc.). It was performed according to the experimental
groups (WT-luc group, MUT-luc group, WT-luc + mimic NC group,
MUT-luc + mimic NC group, WT-luc + miR-9-5p mimic group, MUT-luc +
miR-9-5p mimic group). The Renilla luciferase plasmid
(Promega Corporation) was co-transfected as a transfection
efficiency control. Cells in 1×PLB lysis buffer were harvested 48 h
following transfection, and Firefly and Renilla luciferase
activities were quantified and normal-ized using a
dual-Glo® Luciferase Assay system (Promega Corporation)
according to the manufacturer's protocol.
Cell proliferation assay
Transfected cells were seeded at a density of 2,000
cells in 100 µl/well in 96-well plates, and maintained in
culture medium. Cell counting Kit-8 (CCK8; Beyotime Institute of
Biotechnology, Haimen, China) solution (10 µl) was added to
each well, and cells were incubated at 37°C for 2 h. The absorbance
was subsequently read at 450 nm using an ELISA reader. This
experiment was performed daily on days 1-5 following
transfection.
Apoptosis determined by flow cytometric
analysis
At least 10,000 transfected cells were counted in
the treatment group (SK-Hep-1 cells: Control group, mimic group,
Klf4 group, mimic-Klf4 group and HCC-LM3 cell: Control group,
inhibitor group, siKlf4 group, inhibitor-siKlf4 group; SK-Hep-1
cells: Control group, mimic group, AKT inhibitor group, mimic-AKT
inhibitor group and HCC-LM3 cell: Control group, inhibitor group,
AKT inhibitor group, inhibitor-AKT inhibitor group). Transfected
cells were washed twice with ice-cold water, and stained with 5
µl annexin V-fluorescein isothiocyanate for 15 min and 1
µl propidium iodide (PI; 1 mg/ml) for 5 min at room
temperature, the mixture was then incubated at room temperature for
30 min, and subjected to analysis on a flow cytometer (FACS
Calibur; BD Biosciences, San Jose, CA, USA). Results were analyzed
using FlowJo v10 software (Tree Star, Inc., Ashland, OR, USA).
Inhibitor assay
AKT inhibitor (MK-2206,2 HCl; Selleck Chemicals,
Houston, TX, USA) was used in subsequent cell proliferation and
apoptosis assays, and purchased from. It was diluted in DMSO
resulting in a final concentration of 10 µM.
Cell migration assay
Transfected cells were digested into a single cell
suspension using 0.25% trypsin-0.02% EDTA solution in serum-free
medium, and 2×104 cells were seeded into the upper
chamber of 24-well Transwell plates (aperture size, 8 µm;
EMD Millipore, Billerica, MA, USA) in 100 µl medium. FBS
culture medium (10%; 700 µl) was added to the lower chamber,
and the cells were incubated at 37°C for 24 h. The upper cells were
removed with a cotton swab, while the lower cells were fixed in 4%
formaldehyde for 30 min at room temperature. Following washing
twice with PBS (1 ml), the cells that had migrated into the lower
chamber were stained with 0.05% crystal violet for 30 min at room
temperature. Finally, three fields were randomly selected and
images were captured following air-drying under light microscopy
(magnification, ×200).
Wound healing assay
SK-Hep-1 and HCC-LM3 cells were transfected as
indicated in the figures (SK-Hep-1 cells: Control group, mimic
group, Klf4 group, mimic-Klf4 group; and HCC-LM3 cells: Control
group, inhibitor group, siKlf4 group, inhibitor-siKlf4 group).
Following reaching 90% confluence in 24-well plates, a wound was
made in the cell culture using a 10-µl pipette tip, and
images of the closure of the wound were captured at 0, 24 and 48 h
under light microscopy (magnification, ×100).
Statistical analysis and data
profile
GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA) and SPSS 20.0 software (IBM Corp., Armonk, NY, USA)
were used for statistical analysis. Comparisons between two groups
were performed using two-tailed, independent Student's t-test in
cell experimental data processing, whereas a paired Student's
t-test was used in clinical data analysis. Differences among three
or more groups were performed using one-way analysis of variance
followed by the Student-Newman-Keuls post hoc test. TargetScan
(http://www.targetscan.org/vert72/),
miRNAmap (http://mirnamap.mbc.nctu.edu.tw/), and miRanda
(http://www.microrna.org) were used to predict the
miRNA candidates binding to Klf4. TCGA HCC miRNA database was
downloaded (https://portal.gdc.cancer.gov/). A total of 267
records were available in TCGA database. Records with missing
clinical information were excluded, thus 224 individuals were
included in the analysis. For correlation of miR-9-5p and Klf4
expression, the data was analyzed using Spearman's correlation.
Patient survival was evaluated using the Kaplan-Meier method and
log-rank tests. Data are presented as the mean ± standard error of
the mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-9-5p overexpression downregulates
Klf4 expression in HCC
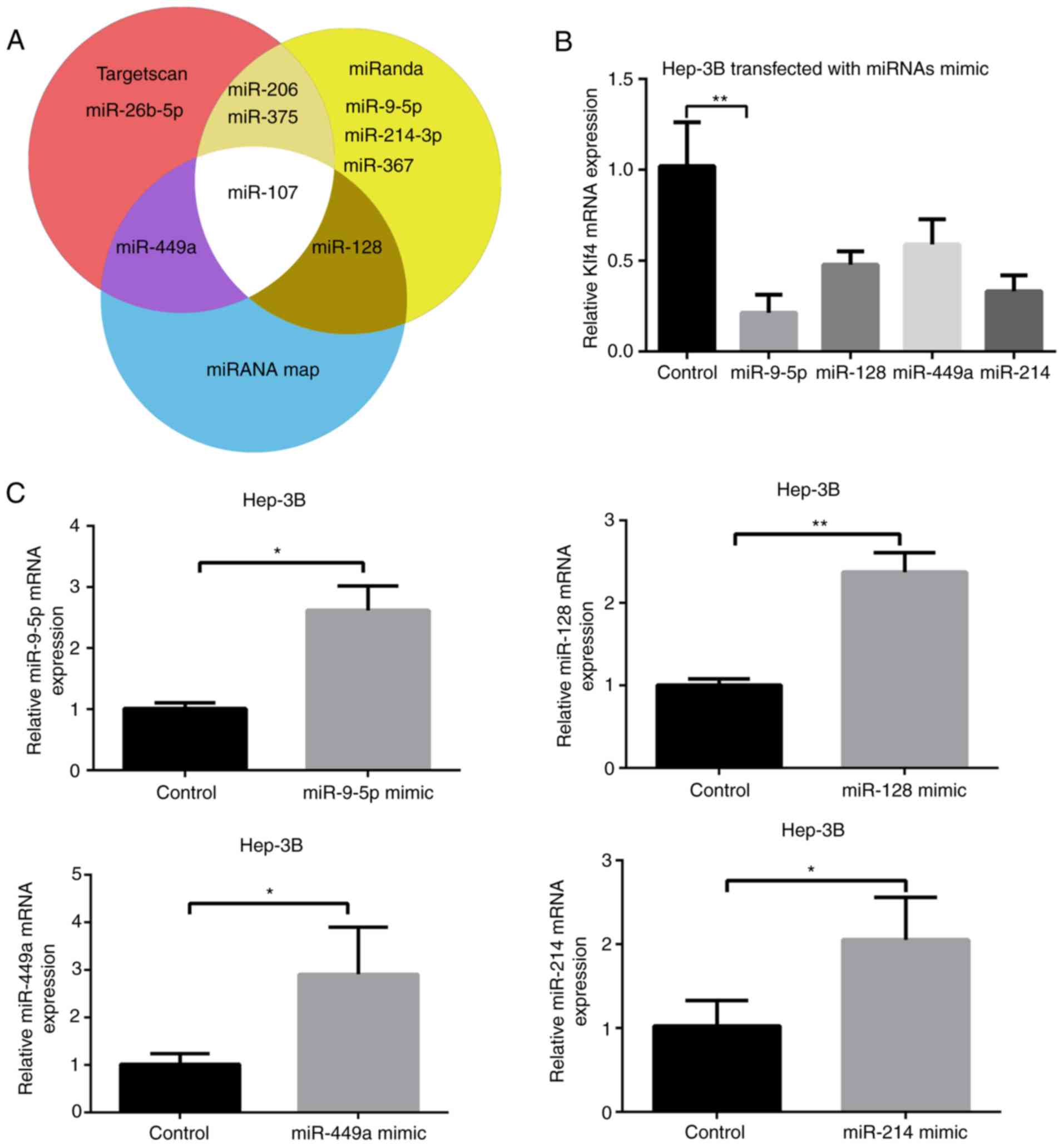
Several miRNA candidates, including miR-26b-5p,
miR-214-3p, miR-375, miR-449a, miR-9-5p, miR-107, miR-206, miR-128
and miR-367, which were predicted to bind to Klf4 using prediction
websites, TargetScan, miRNAmap, and miRanda were selected. Among
these miRNA candidates, miR-26b-5p (33), miR-367 (34), miR-107 (16), miR-375 and miR-206 (35) have been previously reported to
regulate Klf4 (Fig. 1A);
therefore, these miRNAs were not investigated further in the
present study. It was revealed that miR-9-5p mimic markedly reduced
the mRNA expression level of Klf4, suggesting that it may be
critical for the expression of Klf4 (Fig. 1B). Subsequently, the mimics of the
remaining miRNAs' mimics were transfected into Hep-3B cells
(Fig. 1C). The results revealed
that miRNA levels of miR-9-5p, miR-128, miR-449a and miR-214 were
significantly increased following transfection with their
respective mimics.
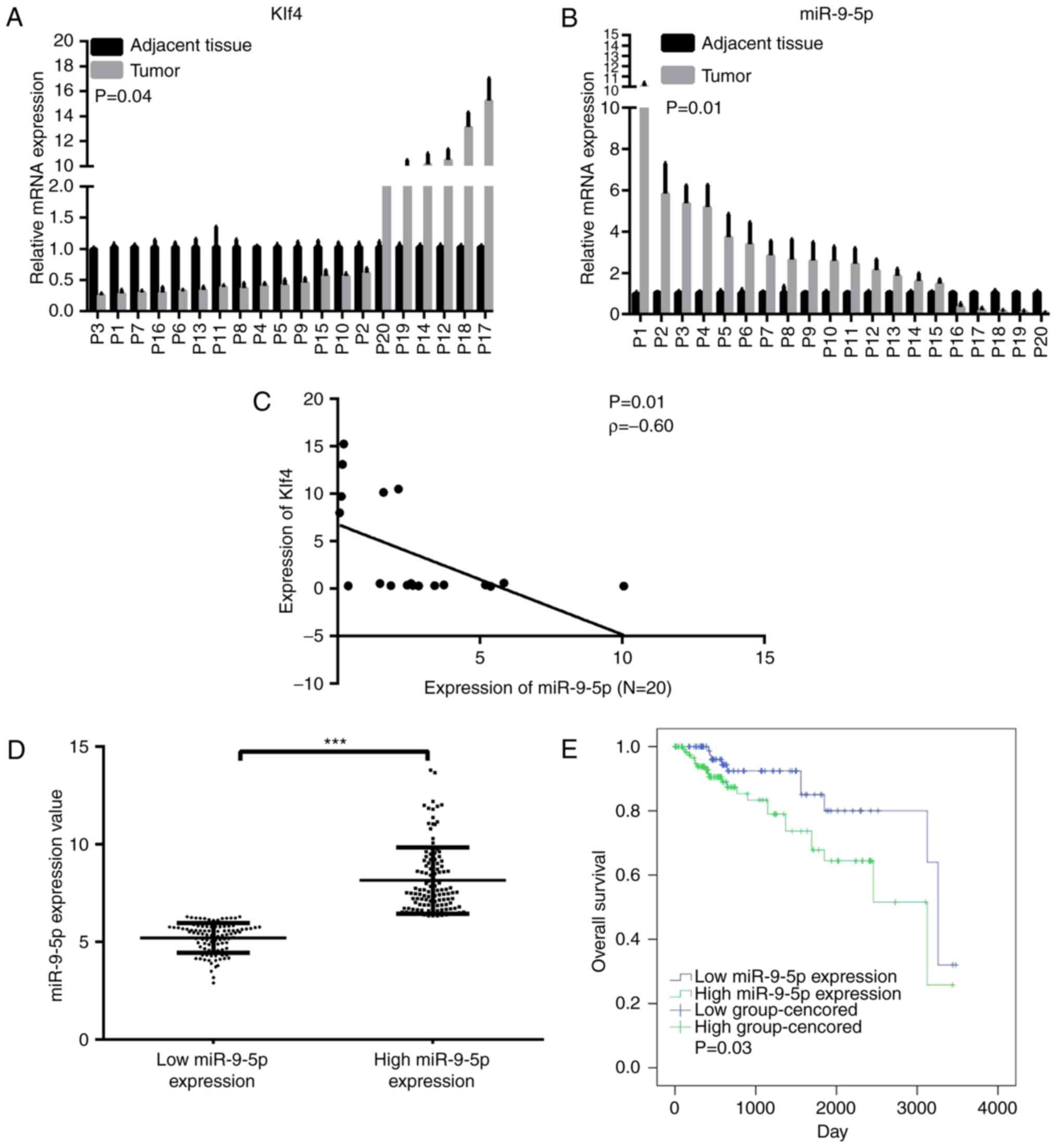
miR-9-5p overexpression in clinical
samples is associated with low expression of Klf4 and poor
prognosis for HCC
Expression levels of Klf4 and miR-9-5p expression in
tumor and paired adjacent normal tissues in 20 patients were
subsequently compared. These results revealed that the miRNA level
of miR-9-5p was significantly increased in 75% of the HCC tissues
(15/20), whereas the mRNA level of Klf4 was down-regulated in 70%
of the tumor samples (14/20; Fig. 2A
and B). Spearman's correlation indicated that miR-9-5p
expression was moderately inversely correlated with Klf4 expression
in these 20 paired specimens, a result that may have been
influenced by the small sample size (Fig. 2C). The correlation between
miR-9-5p and Klf4 expression, and clinicopathological features of
the two groups was subsequently analyzed. These results
demonstrated that there were no significant differences in miR-9-5p
or Klf4 expression associated with age, sex, tumor size, grade or
microvascular invasion (Table I).
Follow-up evaluations did not identify any patients who had
progressive diseases. Therefore, the TCGA HCC database was
consulted, and relevant miRNA-sequencing data were downloaded.
 | Table ICharacteristics of 20 hepatocellular
carcinoma cases. |
Table I
Characteristics of 20 hepatocellular
carcinoma cases.
| Characteristic | Patients | miR-9-5p
| Klf4
|
|---|
| High | Low | P-value | High | Low | P-value |
|---|
| Age, years | | | | 0.423 | | | 0.423 |
| <60 | 7 (35) | 4 (20) | 3 (15) | | 4 (20) | 3 (15) | |
| ≥60 | 13 (65) | 4 (20) | 9 (45) | | 5 (25) | 8 (40) | |
| Sex | | | | 0.081 | | | 0.888 |
| Male | 13 (65) | 4 (20) | 9 (45) | | 6 (30) | 7 (35) | |
| Female | 7 (35) | 5 (25) | 2 (10) | | 3 (15) | 4 (20) | |
| Tumor size, cm | | | | 0.078 | | | 0.064 |
| ≤5 | 9 (45) | 6 (30) | 3 (15) | | 2 (10) | 7 (35) | |
| >5 | 11 (55) | 3 (15) | 8 (40) | | 7 (35) | 4 (20) | |
| Stage | | | | 0.436 | | | 0.436 |
| I, II | 15 (75) | 6 (30) | 9 (45) | | 6 (30) | 9 (45) | |
| III | 5 (25) | 3 (15) | 2 (10) | | 3 (15) | 2 (10) | |
| MVI | | | | 0.582 | | | 0.714 |
| 0 | 12 (60) | 6 (30) | 6 (30) | | 5 (25) | 7 (35) | |
| ≥1 | 8 (40) | 3 (15) | 5 (25) | | 4 (20) | 4 (20) | |
Following separating the data by the median value, a
significant difference was revealed to exist between the high and
low miR-9-5p expression groups (Fig.
2D). The correlation between miR-9-5p expression and
clinicopathological features of the two groups was subsequently
analyzed. These results demonstrated that no significant
differences existed between the expression groups in terms of age,
sex, α-fetoprotein, pathological stage or vascular invasion
(Table II). The 5-year survival
rate was 71.8% in the high miR-9-5p expression group, and 80.2% in
the low miR-9-5p expression group (Table III). Kaplan-Meier analysis
revealed an improved OS rate in the low miR-9-5p expression group
compared with the high miR-9-5p expression group (Fig. 2E).
 | Table IICorrelation of miR-9-5p expression
with clinicopathological features of hepatocellular carcinoma in
The Cancer Genome Atlas database. |
Table II
Correlation of miR-9-5p expression
with clinicopathological features of hepatocellular carcinoma in
The Cancer Genome Atlas database.
| Characteristic | Patients, n | High miR-9-5p
expression
| Low miR-9-5p
expression
| |
|---|
| n | % | n | % | P-value |
|---|
| Age, years | | | | | | 0.328 |
| <60 | 98 | 50 | 51.0 | 48 | 49.0 | |
| ≥60 | 126 | 56 | 44.4 | 70 | 55.6 | |
| Sex | | | | | | 0.290 |
| Male | 155 | 77 | 49.7 | 78 | 50.3 | |
| Female | 69 | 29 | 42.0 | 40 | 58.0 | |
| AFP,
µg/l | | | | | | 0.122 |
| <400 | 88 | 36 | 40.9 | 52 | 59.1 | |
| ≥400 | 136 | 70 | 51.5 | 66 | 48.5 | |
| Pathological
stage | | | | | | 0.263 |
| I/ II | 220 | 103 | 46.8 | 117 | 53.2 | |
| III/IV | 4 | 3 | 75.0 | 1 | 25.0 | |
| Vascular
invasion | | | | | | 0.718 |
| Yes | 66 | 30 | 45.5 | 36 | 54.5 | |
| No | 158 | 76 | 48.1 | 82 | 51.9 | |
 | Table IIISurvival rate of low and high
miR-9-5p expression groups. |
Table III
Survival rate of low and high
miR-9-5p expression groups.
| miR-9-5p
expression | 1-year
survival | 3-year
survival | 5-year
survival |
|---|
| Low | 98.0 | 87.9 | 80.2 |
| High | 95.1 | 86.8 | 71.8 |
miR-9-5p directly targets the
transcription factor Klf4 in HCC cells
Expression levels of Klf4 and miR-9-5p were detected
by RT-qPCR in L02 cells and the four HCC cell lines. The results
revealed that Klf4 was expressed at a lower level in HCC cells
compared with L02 cells (Fig.
3A). By contrast, miR-9-5p was more highly expressed in HCC
cells (Fig. 3B). Subsequently,
the effects of miR-9-5p mimic and inhibitor on Klf4 mRNA and
protein expression were investigated. Transfection effi-ciency was
verified by RT-qPCR analysis (Fig.
3C). The Klf4 expression level was decreased in the HCC cells
upon transfection with the miR-9-5p mimic, whereas the mRNA and
protein expression levels were both increased following
transfection with miR-9-5p inhibitor (Fig. 3D and E). To confirm whether
miR-9-5p directly bound to Klf4, bioinformatics analysis was
utilized (in miRanda) to predict the potential binding site of
miR-9-5p to the 3′-UTR of Klf4. The corresponding wild-type and
mutation type Klf4 3′-UTRs were constructed, and plasmids were
separately cloned into luciferase reporter vector, as presented in
Fig. 3F. The luciferase reporter
assay revealed that the effect of miR-9-5p inhibition was abrogated
upon transfection with the mutation type. These results indicated
that miR-9-5p regulated the expression of Klf4 through a direct
association with the Klf4 3′-UTR.
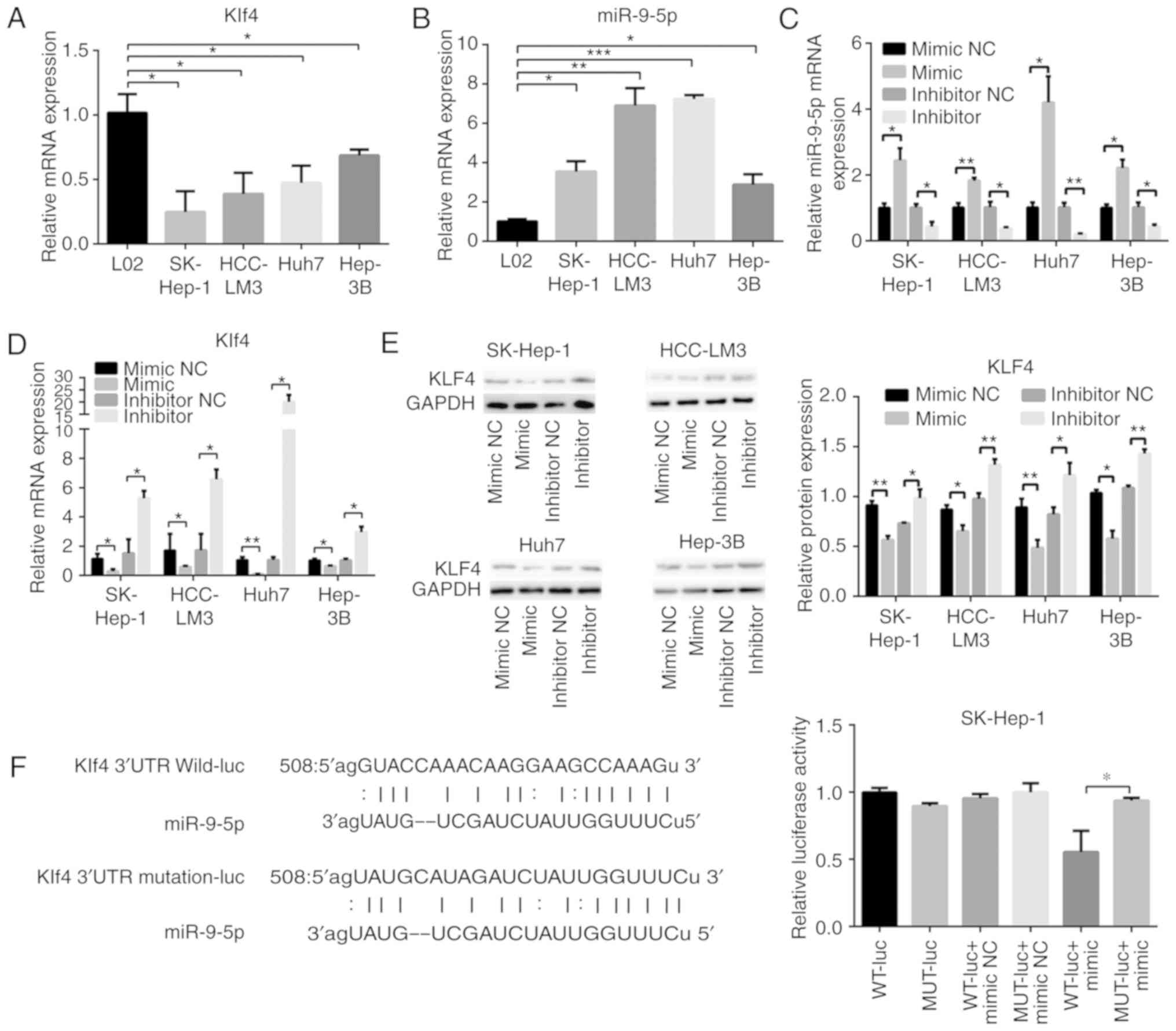 | Figure 3miR-9-5p regulates the transcription
factor Klf4 in HCC cells. RT-qPCR analysis of (A) Klf4 and (B)
miR-9-5p in L02 and HCC cells. (C and D) RT-qPCR and (E) western
blot analysis of mRNA and protein expression levels, respectively,
of Klf4 and miR-9-5p at 48 h following transfection with 100 nM
miR-9-5p mimic or 100 nM inhibitor, and their control groups in HCC
cells. (F) The binding site of miR-9-5p was predicted in the Klf4
3′-UTR. Subsequently, the luciferase reporters of the WT or MUT
miR-9-5p targeting Klf4 3′-UTR sites was constructed. Luciferase
activity was measured 48 h following transfection of miR-9-5p mimic
or negative control, together with WT or MUT Klf4 3′-UTR.
*P<0.05; **P<0.01;
***P<0.001. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; miR,
microRNA; HCC, hepatocellular carcinoma; mimic NC, mimic negative
control; inhibitor NC, inhibitor negative control; Klf4,
Krüppel-like factor 4; 3′-UTR, 3′-untranslated region; WT,
wild-type; MUT, mutation-type. |
miR-9-5p affects cell proliferation and
apoptosis, activating an AKT-associated pathway via downregulation
of Klf4
Transfected cells were seeded to examine their cell
prolifera-tive abilities according to CCK8 assay on days 1-5
following transfection. Transfection efficiency of miR-9-5p mimic
or inhibitor, or siKlf4/Klf4 was verified by RT-qPCR and western
blot analysis (Fig. 4A). The
results demonstrated that miR-9-5p mimic significantly promoted
proliferation compared with the control, and the effect was
reversed by overexpression of Klf4 in the miR-9-5p mimic-treated
HCC cells. The results also revealed that miR-9-5p inhibitor
inhibited the proliferation of HCC cells, and this effect was
circumvented by the addition of siKlf4 to miR-9-5p inhibitor
expressing HCC cells (Fig.
4B).
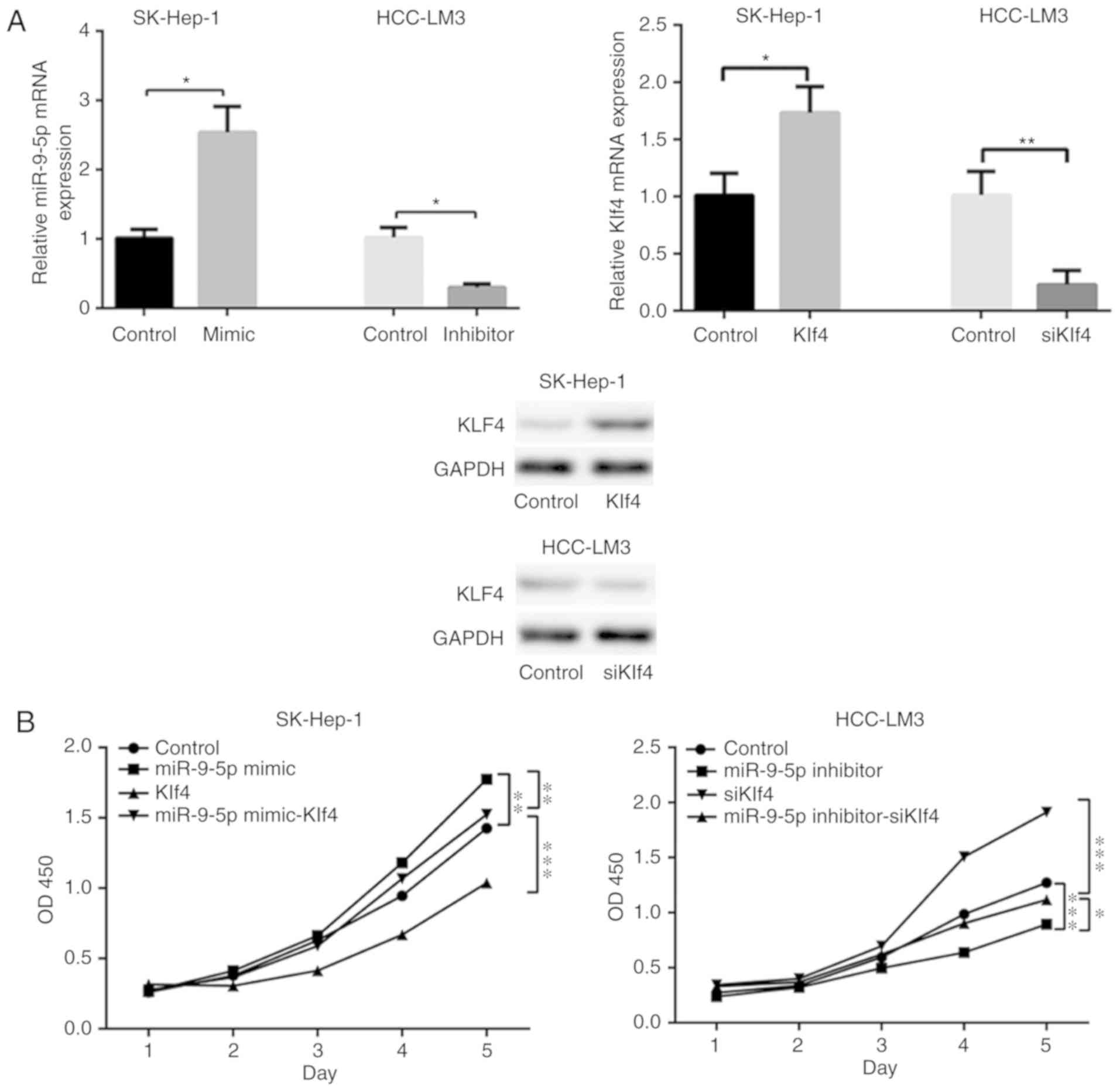 | Figure 4miR-9-5p/Klf4 axis enhances HCC cell
proliferation via an AKT/mTOR-associated pathway. (A) The
transfection efficiency of miR-9-5p mimic or inhibitor, or
siKlf4/Klf4 was measured using reverse transcription-quantitative
polymerase chain reaction and western blot analysis. (B) Cell
proliferation viability was determined by cell counting kit-8
assay. A total number of 2,000 transfected cells dissolved in 100
µl 10% fetal bovine serum were seeded into 96-well plates on
days 1-5, and the absorbance was measured at 450 nm every 24 h to
obtain a cell growth curve. (C) Flow cytometric analysis of
SK-Hep-1 and HCC-LM3 cells treated with miR-9-5p mimic or
inhibitor, or siKlf4/Klf4 for 48 h. Representative data are
featured, presenting the population of living cells (Annexin
V-FITC-/PI-) in the left lower quadrant, early apoptotic cells
(Annexin V-FITC+/PI-) in right lower quadrant, late apoptotic cells
(Annexin V-FITC +/PI+) in the right upper quadrant and necrotic
cells (Annexin V-FITC -/PI+) in the left upper quadrant. (D) Levels
of AKT, p-AKT S473, mTOR, p-mTOR, Bcl-2 and Bax were examined by
western blotting in SK-Hep-1 and HCC-LM3 cells transfected with
miR-9-5p mimic/inhibitor or Klf4/siKlf4. (E) Flow cytometric
analysis of SK-Hep-1 and HCC-LM3 cells treated with miR-9-5p
mimic/inhibitor together with AKTi (10 µl, MK-2206, 2 HCl;
dissolved in DMSO) for 48 h. (F) Cell proliferation analysis of
SK-Hep-1 and HCC-LM3 cells treated with miR-9-5p mimic/inhibitor
with AKT inhibitor (10 µl, MK-2206, 2 HCl; dissolved in
DMSO) for 48 h. *P<0.05; **P<0.01;
***P<0.001. miR, microRNA; p, phos-phorylated; AKT,
protein kinase B; mTOR, mechanistic target of rapamycin; DMSO,
dimethyl sulfoxide; HCC, hepatocellular carcinoma; Klf4,
Krüppel-like factor 4; siKlf4, small interfering RNA against Klf4;
PI, propidium iodide; FITC, fluorescein isothiocyanate; Bcl-2, B
cell lymphoma-2; Bax, Bcl-2-associated X protein; OD, optical
density; AKTi, AKT inhibitor. |
The effects of miR-9-5p and Klf4 on cell apoptosis
were further explored using the annexin V/PI flow cytometric
method. The quantitative apoptosis assay demonstrated that miR-9-5p
mimic inhibited apoptosis via Klf4 in SK-Hep-1 cells, whereas
miR-9-5p inhibitor accelerated cell apoptosis in HCC-LM3 cells
(Fig. 4C). To understand the
mechanisms underpinning how the miR-9-5p/Klf4 axis functions, the
expression levels of total AKT, p-AKT, mTOR and p-mTOR protein were
measured by western blot analysis. The results revealed that the
miR-9-5p/Klf4 axis activated the process of AKT and mTOR
phosphorylation. Subsequently, the expression levels of the
apoptosis-associated proteins Bcl-2 and Bax, were examined. The
results disclosed that the miR-9-5p/Klf4 axis increased the level
of the anti-apoptotic protein Bcl-2 and decreased that of the
apoptotic protein, Bax (Fig. 4D).
On the basis of these results, It was possible to conclude that the
miR-9-5p/Klf4 axis may activate the AKT/mTOR-associated pathway. To
substantiate the hypothesis that the AKT/mTOR-associated pathway
mediates apoptosis of the activated miR-9-5p/Klf4 axis in HCC
cells, whether the cell proliferative capability is reinforced when
AKT is inhibited was investigated. The results obtained revealed
that MK-2206,2 HCl, an AKT inhibitor, notably reduced the
proliferation-promoting apoptosis effect of activated miR-9-5p/Klf4
axis HCC cells (Fig. 4E and
F).
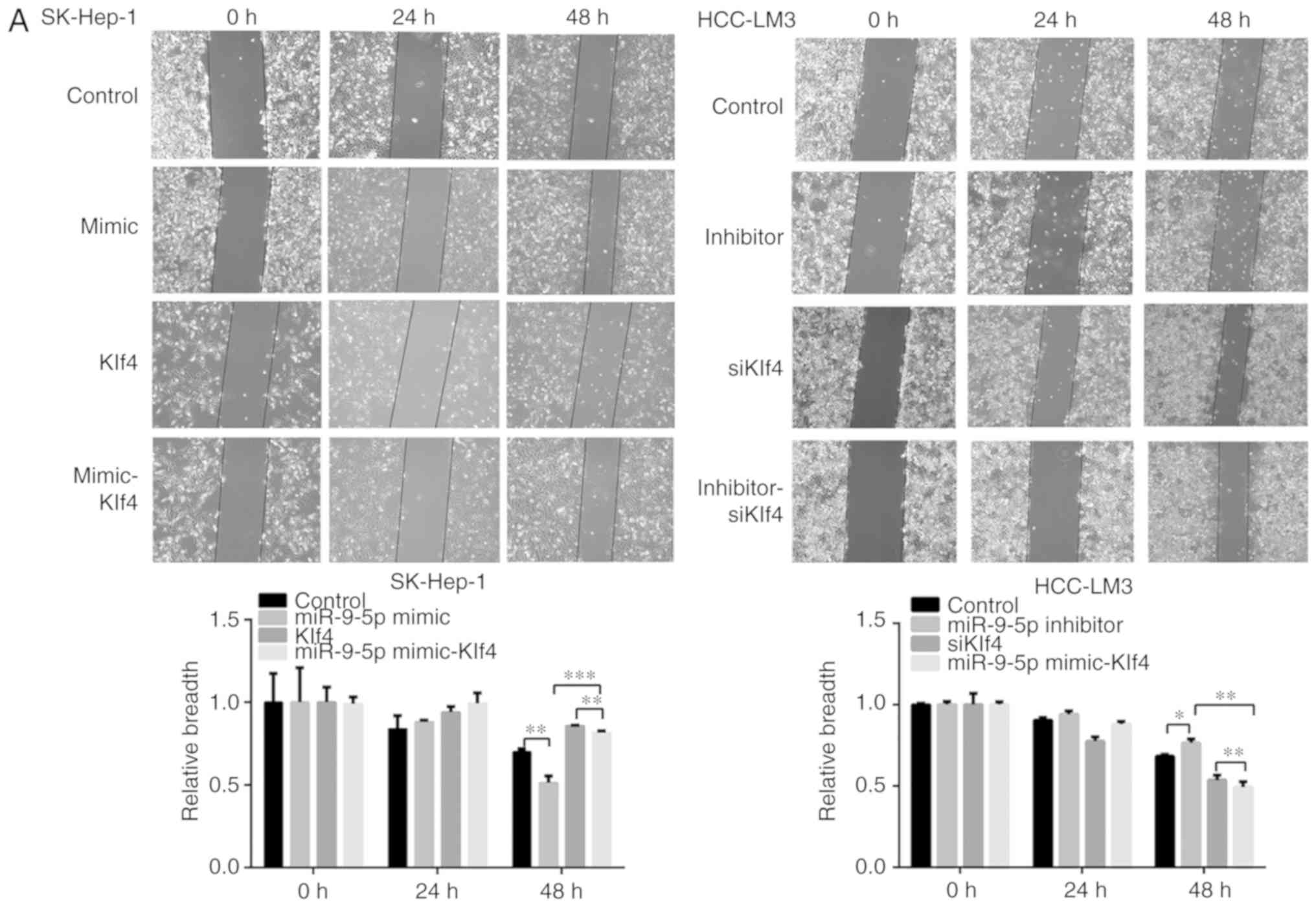
miR-9-5p enhances the HCC migration
ability of HCC cells via downregulating Klf4
To evaluate the effect of miR-9-5p downregulating
Klf4 on cell migration, miR-9-5p mimic and inhibitor, Klf4, and
siKlf4 were respectively transfected into SK-Hep-1 and HCC-LM3
cells. Cell migration rates were increased in the miR-9-5p mimic
group compared with the control, and they were also increased in
the miR-9-5p mimic-Klf4 group compared with the Klf4 group.
Furthermore, the migration rates were decreased in the miR-9-5p
inhibitor group compared with the control; likewise, they were
decreased in the miR-9-5p inhibitor-siKlf4 group compared with the
siKlf4 group. In addition, the Transwell migration assay gave rise
to similar results as those of the wound healing assay (Fig. 5A and B). Taken together, these
results indicated that miR-9-5p promoted the migrational
capabilities of the HCC cells by decreasing Klf4 expression.
Discussion
A characteristic of HCC cells is that they readily
take up therapeutic agents, such as antisense oligonucleotides, a
property that may be exploited in the search for functional
noncoding RNAs (ncRNAs) as potential targets for HCC treatment
(36,37). With numerous studies illustrating
the signaling mechanisms of ncRNAs (38), researchers' attention has been
drawn towards investigating the effects of ectopic miRNA expression
on the proliferation and apop-tosis of HCC cells. A HCC study
(39) have revealed that miRNAs
regulate the expression of various oncogenes and tumor suppressor
genes, contributing to the modulation of diverse biological
processes, including proliferation, apoptosis,
epithelial-to-mesenchymal transition and metastasis. An unusual
feature of miRNAs is their ability to bind to various different
target genes, and to inhibit the process of translation (39-41). For example, an induced increase in
the level of miR-188-5p markedly inhibited fibroblast growth factor
5 (FGF5), whereas the restoration of FGF5 expression reversed the
inhibitory effects on HCC progression (42). miR-130b overexpression reduced the
expression of tumor protein 53-induced nuclear protein 1, which
promoted the growth and self-renewal of CD133+ liver
tumor-initiating cells (43).
miRNA prediction websites have indicated that
miR-9-5p is an upstream gene of Klf4. Previous studies have
reported that miR-9-5p is ectopically regulated in various types of
cancers. miR-9-5p levels are higher in metastatic tumors compared
with non-metastatic tumors and these were demonstrated to be
associated with poor prognosis of rhabdomyosarcomas (44). The homeotic gene miR-9 inhibited
ovarian cancer cell growth via nuclear factor-κβ1 (45). Inhibition of the expression of the
circular RNA, circMTO1, in HCC directly repressed p21, the target
of oncogenic miR-9, and this resulted in the promotion of cell
proliferation and invasion (46).
Klf4 is a transcription factor that is involved in
the pathogenesis and metastasis of digestive tumors (47) and is downregulated in HCC
(23). Klf4 functions as a tumor
suppressor gene, and a decrease in Klf4 expression levels is
strongly associated with poor survival rates in patients with HCC
(23). In our previous study
(20), it was demonstrated that
the expression level of miR-135a-5p was upregulated in clinical HCC
tissues, and this was inversely correlated with the expression of
Klf4. miR-135a-5p promoted proliferation and metastasis in HCC
cells by directly targeting Klf4 in vitro and in
vivo. miRNAs have been confirmed as potential therapeutic
targets for HCC; however, further studies that explore the
mechanisms underpinning the involvement of on miRNAs in HCC are
required. The results of the present study have confirmed that
miR-9-5p expression was inversely associated with Klf4 expression
in HCC clinical samples. Furthermore, a poor prognosis of HCC was
significantly correlated with overexpression of miR-9-5p according
to the TCGA HCC database. However, no comparatively thorough
studies have been performed concerning the role of miR-9-5p as a
regulator of Klf4.
Further experiments performed in the present study
confirmed that Klf4 was directly inhibited by miR-9-5p in the HCC
cells. These findings further supported that miR-9-5p increased
cell proliferation, and inhibited apoptosis, by downregulating
Klf4. AKT-associated signaling pathways fulfill important roles in
HCC (48). miR-7 functions as a
tumor suppressor, and regulates the AKT pathway (49). Klf4 was found to be associated
with phosphorylated sites of AKT, and had a role in AKT-mediated
phosphorylation (17). Via the
suppression of Klf4 and activating AKT/mTOR signaling, miR-9-5p
accelerated the progression of HCC. Apoptosis is perhaps governed
by the Bcl-2 family of proteins, which act as molecular
'sentinels', determining the mitochondrial response to apoptosis
stimuli. Dysregulation of Bcl-2/Bax facilitates tumorigenesis and
tumor progression (50). The
results of the present study suggested that the miR-9-5p/Klf4 axis
had an inhibitory effect on the apoptotic protein, Bax, and served
to activate the anti-apoptotic protein, Bcl-2, by targeting the AKT
pathway.
In conclusion, the findings of the present study
have demonstrated that the high expression level of miR-9-5p in HCC
are associated with poor prognosis of disease, and miR-9-5p
directly targets Klf4, which has been widely demonstrated as a
suppressor gene in HCC. The miR-9-5p/Klf4 axis promoted the
proliferation, and accelerated the migration, of HCC cells, and
inhibited apoptosis by activating the AKT/mTOR pathway. it is to
hoped that studying the miR-9-5p/Klf4 regulation mechanism will
stimulate a greater level of interest in prospective studies, and
these will lead to the discovery of novel and effective therapeutic
targets for HCC treatment. The present study has contributed to
this process by uncovering the role of miR-9-5p as a prognostic
marker and potential therapeutic target.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81272714
and 81572310).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD and FW conducted the experiments and wrote the
manuscript. YX, ZL and WS performed the experiments. XD, NY and QL
analyzed the data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of Eastern Hepatobiliary Surgery Hospital, The
Second Military Medical University. Informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo H, Wu T, Lu Q, Li M, Guo JY, Shen Y,
Wu Z, Nan KJ, Lv Y and Zhang XF: Surgical resection improves
long-term survival of patients with hepatocellular carcinoma across
different Barcelona clinic liver cancer stages. Cancer Manag Res.
10:361–369. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Upregulation of long
noncoding RNA ZEB1-AS1 promotes tumor metastasis and predicts poor
prognosis in hepatocellular carcinoma. Oncogene. 35:1575–1584.
2016. View Article : Google Scholar
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kudo M: Systemic therapy for
hepatocellular carcinoma: Latest advances. Cancers (Basel). 10:pii:
E412. 2018. View Article : Google Scholar :
|
|
6
|
Augello G, Emma MR, Cusimano A, Azzolina
A, Mongiovi S, Puleio R, Cassata G, Gulino A, Belmonte B,
Gramignoli R, et al: Targeting HSP90 with the small molecule
inhibitor AUY922 (luminespib) as a treatment strategy against
hepatocellular carcinoma. Int J Cancer. Nov 29–2018.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harris WP, Wong KM, Saha S, Dika IE and
Abou-Alfa GK: Biomarker-driven and molecular targeted therapies for
hepato-biliary cancers. Semin Oncol. 45:116–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Wei L, Yang H, Yang W, Yang Q,
Zhang Z, Wu K and Wu J and Wu J: Bromodomain containing protein
represses the Ras/Raf/MEK/ERK pathway to attenuate human hepatoma
cell proliferation during HCV infection. Cancer Lett. 371:107–116.
2016. View Article : Google Scholar
|
|
9
|
Chai S, Ng KY, Tong M, Lau EY, Lee TK,
Chan KW, Yuan YF, Cheung TT, Cheung ST, Wang XQ, et al: Octamer
4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in
liver cancer stem cells. Hepatology. 64:2062–2076. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ou DL, Lee BS, Lin LI, Liou JY, Liao SC,
Hsu C and Cheng AL: Vertical blockade of the IGFR- PI3K/Akt/mTOR
pathway for the treatment of hepatocellular carcinoma: The role of
survivin. Mol Cancer. 13:22014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vivanco I and CL S: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 7:489–501. 2002. View
Article : Google Scholar
|
|
12
|
Presnell JS, Schnitzler CE and Browne WE:
KLF/SP Transcription factor family evolution: Expansion,
diversification, and innovation in eukaryotes. Genome Biol Evol.
7:2289–2309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu JH, Navas P, Cao H, Stamatoyannopoulos
G and Song CZ: Systematic RNAi studies on the role of Sp/KLF
factors in globin gene expression and erythroid differentiation. J
Mol Biol. 366:1064–1073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carrano AC, Dillin A and Hunter T: A
Krüppel-like factor downstream of the E3 ligase WWP-1 mediates
dietary-restriction-induced longevity in Caenorhabditis elegans.
Nat Commun. 5:37722014. View Article : Google Scholar
|
|
15
|
Kim CK, He P, Bialkowska AB and Yang VW:
SP and KLF transcription factors in digestive physiology and
diseases. Gastroenterology. 152:1845–1875. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen HY, Lin YM, Chung HC, Lang YD, Lin
CJ, Huang J, Wang WC, Lin FM, Chen Z, Huang HD, et al: miR-103/107
promote metastasis of colorectal cancer by targeting the metastasis
suppressors DAPK and KLF4. Cancer Res. 72:3631–3641. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malak PN, Dannenmann B, Hirth A, Rothfuss
OC and Schulze-Osthoff K: Novel AKT phosphorylation sites
identified in the pluripotency factors OCT4, SOX2 and KLF4. Cell
Cycle. 14:3748–3754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu YY, Liu CM, Tsai HH, Jong YJ, Chen IJ
and Lo YC: KMUP-1 attenuates serum deprivation-induced
neurotoxicity in SH-SY5Y cells: Roles of PKG, PI3K/Akt and
Bcl-2/Bax pathways. Toxicology. 268:46–54. 2010. View Article : Google Scholar
|
|
19
|
Muir KR, Lima MJ, Docherty HM, McGowan NW,
Forbes S, Heremans Y, Forbes SJ, Heimberg H, Casey J and Docherty
K: Krüppel-Like factor 4 overexpression initiates a
mesenchymal-to-epithelial transition and redifferentiation of human
pancreatic cells following expansion in long term adherent culture.
PLoS One. 10:e01403522015. View Article : Google Scholar
|
|
20
|
Sun H, Peng Z, Tang H, Xie D, Jia Z, Zhong
L, Zhao S, Ma Z, Gao Y, Zeng L, et al: Loss of KLF4 and
consequential downregulation of Smad7 exacerbate oncogenic TGF-β
signaling in and promote progression of hepatocellular carcinoma.
Oncogene. 36:2957–2968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang YL, Zhou PJ, Wei L, Li W, Ji Z, Fang
YX and Gao WQ: MicroRNA-7 inhibits the stemness of prostate cancer
stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21
pathway. Oncotarget. 6:24017–24031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Z, Zhao J, Li Q, Yang W, Song Q, Li W
and Liu J: KLF4 promotes hydrogen-peroxide-induced apoptosis of
chronic myeloid leukemia cells involving the bcl-2/bax pathway.
Cell Stress Chaperones. 15:905–912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Q, Gao Y, Jia Z, Mishra L, Guo K, Li Z,
Le X, Wei D, Huang S and Xie K: Dysregulated Krüppel-like factor 4
and vitamin D receptor signaling contribute to progression of
hepatocellular carcinoma. Gastroenterology. 143:799–810.e2. 2012.
View Article : Google Scholar
|
|
24
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang LQ, Zhang Y, Yan H, Liu KJ and Zhang
S: MicroRNA-373 functions as an oncogene and targets YOD1 gene in
cervical cancer. Biochem Biophys Res Commun. 459:515–520. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan J, Ji H, Xiao F, Lin Z, Zhao X, Wang
Z, Zhao J and Lu J: MicroRNA-340 inhibits the proliferation and
invasion of hepa-tocellular carcinoma cells by targeting JAK1.
Biochem Biophys Res Commun. 483:578–584. 2017. View Article : Google Scholar
|
|
27
|
Yao S, Tian C, Ding Y, Ye Q, Gao Y, Yang N
and Li Q: Down-regulation of Krüppel-like factor-4 by
microRNA-135a-5p promotes proliferation and metastasis in
hepatocellular carcinoma by transforming growth factor-β1.
Oncotarget. 7:42566–42578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirihana Arachchilage G, Kharel P, Reid J
and Basu S: Targeting of G-quadruplex harboring Pre-miRNA 92b by
LNA rescues PTEN expression in NSCL cancer cells. ACS Chem Biol.
13:909–914. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Varnholt H, Drebber U, Schulze F,
Wedemeyer I, Schirmacher P, Dienes HP and Odenthal M: MicroRNA gene
expression profile of hepatitis C virus-associated hepatocellular
carcinoma. Hepatology. 47:1223–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
'personalized' approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kamarajah SK, Frankel TL, Sonnenday C, Cho
CS and Nathan H: Critical evaluation of the American joint
commission on cancer (AJCC) 8th edition staging system for patients
with hepatocellular carcinoma (HCC): A surveillance, epidemiology,
end results (SEER) analysis. J Surg Oncol. 117:644–650. 2018.
View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Zawada AM, Rogacev KS, Muller S, Rotter B,
Winter P, Fliser D and Heine GH: Massive analysis of cDNA Ends
(MACE) and miRNA expression profiling identifies proatherogenic
pathways in chronic kidney disease. Epigenetics. 9:161–172. 2014.
View Article : Google Scholar :
|
|
34
|
Wang GC, He QY, Tong DK, Wang CF, Liu K,
Ding C, Ji F and Zhang H: MiR-367 negatively regulates apoptosis
induced by adriamycin in osteosarcoma cells by targeting KLF4. J
Bone Oncol. 5:51–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo Y, An R, Zhao R, Sun Y, Liu M and Tian
L: miR-375 exhibits a more effective tumor-suppressor function in
laryngeal squamous carcinoma cells by regulating KLF4 expression
compared with simple co-transfection of miR-375 and miR-206. Oncol
Rep. 36:952–960. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braconi C and Patel T: Non-coding RNAs as
therapeutic targets in hepatocellular cancer. Curr Cancer Drug
Targets. 12:1073–1080. 2012.PubMed/NCBI
|
|
37
|
Zimmermann TS, Lee AC, Akinc A, Bramlage
B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M,
et al: RNAi-mediated gene silencing in non-human primates. Nature.
441:111–114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Klingenberg M, Matsuda A, Diederichs S and
Patel T: Non-coding RNA in hepatocellular carcinoma: Mechanisms,
biomarkers and therapeutic targets. J Hepatol. 67:603–618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayes CN and Chayama K: MicroRNAs as
biomarkers for liver disease and hepatocellular carcinoma. Int J
Mol Sci. 17:2802016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He S, Zhang DC and Wei C: MicroRNAs as
biomarkers for hepa-tocellular carcinoma diagnosis and prognosis.
Clin Res Hepatol Gastroenterol. 39:426–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang N, Ekanem NR, Sakyi CA and Ray SD:
Hepatocellular carcinoma and microRNA: New perspectives on
therapeutics and diagnostics. Adv Drug Deliv Rev. 81:62–74. 2015.
View Article : Google Scholar
|
|
42
|
Fang F, Chang RM, Yu L, Lei X, Xiao S,
Yang H and Yang LY: MicroRNA-188-5p suppresses tumor cell
proliferation and metastasis by directly targeting FGF5 in
hepatocellular carcinoma. J Hepatol. 63:874–885. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma S, Tang KH, Chan YP, Lee TK, Kwan PS,
Castilho A, Ng I, Man K, Wong N, To KF, et al: miR-130b promotes
CD133(+) liver tumor-initiating cell growth and self-renewal via
tumor protein 53-induced nuclear protein 1. Cell Stem Cell.
7:694–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Missiaglia E, Shepherd CJ, Aladowicz E,
Olmos D, Selfe J, Pierron G, Delattre O, Walters Z and Shipley J:
MicroRNA and gene co-expression networks characterize biological
and clinical behavior of rhabdomyosarcomas. Cancer Lett.
385:251–260. 2017. View Article : Google Scholar :
|
|
45
|
Guo LM, Pu Y, Han Z, Liu T, Li TX, Liu M,
Li X and Tang H: MicroRNA-9 inhibits ovarian cancer cell growth
through regulation of NF-kappaB1. FEBS J. 276:5537–5546. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tang W, Zhu Y, Gao J, Fu J, Liu C, Liu Y,
Song C, Zhu S, Leng Y, Wang G, et al: MicroRNA-29a promotes
colorectal cancer metastasis by regulating matrix metalloproteinase
2 and E-cadherin via KLF4. Br J Cancer. 110:450–458. 2014.
View Article : Google Scholar :
|
|
48
|
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA,
Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, et al:
Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling,
promotes development of human hepatocellular carcinoma.
Gastroenterology. 140:1071–1083. 2011. View Article : Google Scholar :
|
|
49
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phos-phoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|