Introduction
Proliferation of vascular smooth muscle cells
(VSMCs) forms the common basis of the pathology of cardiovascular
diseases, including hypertension, coronary artery disease and
angiographic restenosis, and it has an important role in
development of these diseases (1). Studies have demonstrated that
phenotypic transformation of VSMCs is also pivotal for
cardiovascular diseases (2,3).
When blood vessels are damaged or VSMCs cultured in vitro
are stimulated by growth factors, VSMCs are rapidly transformed
through phenotypic transformation, characterized by alteration of
gene expressions. VSMCs are transformed from a contractile
phenotype to a secretory phenotype (or dedifferentiated VSMCs) and
acquire proliferation ability, and this process is termed
phenotypic transformation (4).
Therefore, how to control and reverse the phenotypic transformation
of VSMCs is the key measure to control the abnormal proliferation
of VSMCs. No methods or drugs are particularly effective in
preventing the phenotypic transformation of VSMCs. Generally, it is
hypothesized that endogenous active substances in the body have a
spontaneous regulatory role in the proliferation or phenotypic
transformation of VSMCs; thus, it is important to identify novel
endogenous regulatory proteins mediating the phenotype of VSMCs.
Nucleolin is the most abundant RNA-binding protein in the
nucleolus. Its major functions are binding and transporting
ribosomal RNAs, and regulating the assembly of ribosomes (5,6).
Previous studies have demonstrated that nucleolin is involved in
cell growth, proliferation, apoptosis, inflammation, immune and
other physiological and pathological processes (7-9).
Nucleolin consists of three major functional domains: Amino
terminus, central region and carboxyl terminus. The central region
is composed of four conserved and consistent RNA binding domains
[RBDs; consensus sequence type RBD (CS-RBD)]. Currently, it is
hypothesized that the nuclear localization signals of nucleolin
N-terminal, the central area of the RBD and the C-terminal glycine
rich region determine the nucleolar localization of nucleolin, and
are also required for the bidirectional shift of the protein
between the cytoplasm and nucleus (10). The cellular shuttle function of
nucleolin is involved in the regulation of cell proliferation,
growth, apoptosis and other biological processes. It remains
unknown whether nucleolin regulates VSMC phenotypic transformation
and its underlying mechanism. A large number of studies have
revealed that the RNA binding properties nucleolin of are important
for a variety of biological functions, and the specific nucleic
acid binding element is ‘(T/G) CCCG (A/G)’ (11-16). Nucleolin shuttles between the
nucleus and cytoplasm in different types of cells and under
different stimulation. In the majority of cells, nucleolin is
mainly expressed in the nucleus and can also be present in the cell
membrane or cytoplasm, as glycosylated or phosphorylated forms
(17,18). All of these findings indicate that
nucleolin has an important role in regulating cell proliferation,
growth, phenotypic transformation and apoptosis, and the cellular
shuttle function of nucleolin participates in various biological
processes.
In the present study, it was aimed to investigate
the role of nucleolin in the transformation from a contractile
phenotype to a secretory phenotype, and to investigate the
endogenous active substances mediating VSMC phenotypic
transformation. Angiotensin II (Ang II) was used to induce the
phenotypic transformation of VSMCs. Gene overexpression and RNA
interference techniques were used to assess the effect of cellular
nucleolin on Ang II-mediated VSMC phenotypic transformation and its
influence on the expressions of VSMC phenotypic
transformation-associated genes. Furthermore, the spatial and
temporal expression patterns of nucleolin in VSMCs were also
investigated. Protein-RNA co-immunoprecipitation was used to
investigate the possible target genes regulated by nucleolin in
phenotypic transformation of VSMCs. Finally, the decay of target
gene mRNA and the effect of nucleolin on the expression of target
gene at the protein level were assayed. The findings provide a new
perspective on the regulatory mechanism of VSMC phenotypic
transformation.
Materials and methods
Cell culture and treatment
Rat VSMCs were obtained from Shanghai Tiancheng Life
Technologies (Shanghai, China; American Type Culture
Collection® no. CRL-1476) and maintained in Dulbecco’s
modified Eagle’s medium supplemented with 10% heat-inactivated
fetal bovine serum, 2 mM glutamine and antibiotic-antimycotic mix
in a humidified incubator with 5% CO2 and 95% air at
37°C. VSMCs were stimulated with Ang II at different concentrations
(10−5, 10−6, 10−7 and
10−8 mmol/l) for 48 h or 10−5 mmol/l Ang II
for different durations (19).
Cell viabilities were analyzed using the MTT method. Briefly, VSMCs
were seeded into 96-well microtiter plates at a density of
2×104 cells/well in 200 µl culture medium. The
VSMCs were treated with different concentrations for various
durations. Subsequently, 100 µl MTT solution (5 mg/ml) was
added to each well, and the cells were incubated for 4 h at 37°C,
followed by addition of 150 µl dimethyl sulfoxide into each
well. The absorbance was measured at 570 nm, and the values were
used to calculate the relative ratio of viable cells to total
cells.
Extraction of cytoplasmic and nuclear
proteins
The cell membrane, cytoplasmic and nuclear protein
fractions were collected and stored at −70°C using the NE-PER™
nuclear and cytoplasmic extraction regent kit (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Extraction of total cell proteins
Following the corresponding treatments, VSMCs were
washed with pre-cooled PBS three times. According to the cell
confluence, 50-80 µl 12X SDS lysis buffer (100 mM Tris-HCl,
200 mM dithiothreitol, 40 g/l SDS, 20% glycerol; pH 6.8) was added
to each well. Subsequently, the cell fragments and lysates were
transferred into the 1.5 ml centrifuge tubes, denatured at 100°C
for 10 min and then centrifuged at 12,000 × g for 10 min at 4°C.
The supernatant was transferred to 0.5 ml centrifuge tubes and
stored at −70°C. The protein concentration was determined using a
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer’s
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using the RNeasy kit
(Qiagen, Inc., Valencia, CA, USA) according to the manufacturer’s
instructions, and 2 µg purified RNA was reverse transcribed
into cDNA (37°C for 15 min, followed by 85°C for 5 sec using RT
kit; Fermentas; Thermo Fisher Scientific, Inc.). The expressions of
target genes were examined using the 7500 Fast Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) using a
QuantiTect SYBR-Green PCR kit (Qiagen, Inc.). Briefly, after an
initial denaturation step at 95°C for 10 sec, amplifications were
performed for 40 cycles at a melting temperature of 95°C for 5 sec
and an annealing temperature of 60°C for 30 sec and extension at
72°C for 30 sec. Primer sequences used in the present study are
listed in Table I. PCR products
were detected by 1.5% agarose gel electrophoresis, stained with
ethidium bromide and imaged on a UV transilluminator. The relative
expressions of target genes were calculated by 2−ΔΔCq
method (20), and β-actin was
selected as the reference gene. Each experiment was conducted in
triplicate.
 | Table IPrimer sequences used in the present
study. |
Table I
Primer sequences used in the present
study.
| Genes | Primer
sequences |
|---|
| α-smooth
muscle-actin | Forward:
5′-ACTGGGACGACATGGAAAAG-3′ |
| Reverse:
5′-CATCTCCAGAGTCCAGCACA-3′ |
| Calponin | Forward:
5′-ACTTCATGGATGGCCTCAAG |
| Reverse:
5′-GTGCCAGTTCTGGGTTGACT |
| Smooth muscle
protein 22α | Forward:
5′-TTCTGCCTCAACATGGCCAAC-3′ |
| Reverse:
5′-CACCTTCACTGGCTTGGATC-3 |
| Osteopontin | Forward:
5′-ATGGCTTTCATTGGAGTTGC-3′ |
| Reverse:
5′-CCTCGCCTTTGCCGATCC-3′ |
| β-actin | Forward:
5′-CCTCGCCTTTGCCGATCC-3′ |
| Reverse:
5′-GGATCTTCATGAGGTAGTCAGTC-3′ |
| Nucleolin | Forward:
5′-CAATCAGGCTGGAGTTGCAAG-3′ |
| Reverse:
5′-TGGCCCAGTCCAAGGTAACTT-3′ |
| Epiregulin | Forward:
5′-GTGTCAATAACAAAGTGTAGC-3′ |
| Reverse:
5′-ATTACAGAAAGAAGTGTTCACATCG-3′ |
| Fibroblast growth
factor 2 | Forward:
5′-GCAGAAGAGAGAGGAGTGTGT-3′ |
| Reverse:
5′-CCCAGTTCGTTTCAGTGC-3′ |
| Tropoelastin | Forward:
5′-ATGATCCCAGGTGTTGGGGGC-3′ |
| Reverse:
5′-TCCAAGATCACCA GGTACAAGG-3′ |
Amplification and extraction of
recombinant plasmids
The recombinant plasmids pcDNA3.1-Nuc and PsiRNA-Nuc
were kindly gifted by Professor Kangkai Wang (Department of
Pathophysiology, Xiangya School of Medicine, Central South
University, Changsha, China). The recombinant vector PsiRNA-Nuc
containing the nucleolin small interfering RNA (siRNA) was
constructed. Using nucleolin as the target, the siRNA sequences
were as follows: Nucleolin siRNA, 5′-ACCTGCCTTCGCGAGCTTCACCAT-3′;
the scramble siRNA, 5′-CATGGTGAAGCTCGCGAAGGCAGGT-3′. Nucleolin
expression plasmid (pcDNA3.1-Nuc) and RNA interference fragment of
nucleolin (PsiRNA-Nuc) were transformed into competent cells, and
then monoclonal colonies were inoculated into 5 ml LB medium
containing corresponding antibiotics and maintained at 37°C on a
rotary bed (250 rpm) overnight. Subsequently, the culture
suspension was transferred to 200 ml LB medium containing the
corresponding antibiotics. When the turbidity reached the standard,
the bacteria were collected for the extraction of plasmids.
PsiRNA-Nuc and pcDNA3.1-Nuc were extracted using a QIAGEN Plasmid
Maxi kit (Qiagen, Inc.) according to the manufacturer’s
instructions, and the DNA concentration of purified plasmids was
determined using spectrophotometer. Finally, isolated plasmids were
stored at −70°C.
Transient transfection
Transfection of cells was performed using MegaTran
1.0 following the manufacturer’s instructions (OriGene
Technologies, Inc., Rockville, MD, USA). Briefly, 5×105
cells were cultured in 5 ml appropriate complete growth medium at
37°C in a CO2 incubator until the cells reached 70-80%
confluence (24 h). Subsequently, cells were washed with serum-free
and antibiotic-free medium and transfected with
pcDNA3.1-Nuc/PsiRNA-Nuc (experimental) or pcDNA3.1/PsiRNA (vector
control) by mixing 6 µl MegaTran1.0 containing 2 µg
DNA, and the mixture was placed at room temperature for ~10 min.
Subsequently, the mixture was added to 6-well plate, followed by
gentle agitation and incubation at 37°C for 24 h in a
CO2 incubator.
Western blot analysis
Following various treatments, VSMCs cells were lysed
with radioimmunoprecipitation assay lysis buffer (Shanghai
Biyuntian Biotechnology, Ltd., Shanghai, China). The protein
concentration was measured using a Bicinchoninic Acid Protein Assay
kit (Beyotime Institute of Biotechnology). Equal amounts of
proteins (20 µg/lane) were subjected to SDS-PAGE using 10%
gels and transferred onto polyvinylidene fluoride membranes. The
membrane was blocked with Tris-buffered saline-Tween containing 5%
bovine serum albumin (BSA; Gibco; Thermo Fisher Scientific, Inc.)
by incubating at room temperature for 6 h. Following removal of the
blocking solution, the blots were incubated with respective primary
antibodies against nucleolin (1:1,000; cat. no. N2662; rabbit
polyclonal antibody; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), α-smooth muscle-actin (α-SM-actin; 1:500; cat. no.
BM0002; mouse monoclonal antibody; Wuhan Boster Biological
Technology, Ltd, Wuhan, China), smooth muscle protein 22α (1:1,000;
cat. no. ab14106; SM22a; rabbit polyclonal antibody; Abcam,
Cambridge, UK), calponin (1:1,000; cat. no. ab700; mouse monoclonal
antibody; Abcam), osteopontin (OPN; 1:1,000; cat. no. c-21742;
mouse monoclonal antibody; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), tropoelastin (1:1,000; cat. no. ab21600; rabbit
polyclonal antibody; Abcam), β-actin (1:1,000; cat. no. ab49900;
mouse monoclonal antibody; Abcam), β-tubulin (1:1,000; cat. no.
ab18207; rabbit polyclonal antibody; Abcam), and proliferating cell
nuclear antigen (PCNA; 1:1,000; cat. no. 610664; mouse monoclonal
antibody; BD Biosciences, San Jose, CA, USA) at 25°C for 2 h.
Subsequently, blots were incubated with peroxidase-conjugated goat
anti-rabbit (1:5,000; cat. no. sc-2004) or anti-mouse IgG (1:5,000;
cat. no. sc-516180) secondary antibody (Santa Cruz Biotechnology,
Inc.) for 1 h at 37°C. Immunoreactive bands were visualized using
an Enhanced Chemiluminescence Detection kit (Beyotime Institute of
Biotechnology) according to the manufacturer’s instructions, and
the densitometry analysis was performed using ImageJ (1.48v)
software (National Institutes of Health, Bethesda, MD, USA).
Immunocytochemical analysis
VSMCs were seeded into 6-well plates with sterile
coverslips at 80% confluence, fixed in 4% formaldehyde at room
temperature for 20 min and permeabilized with 0.1% Triton X-100 at
4°C for 10 min. Coverslips were blocked with 2% BSA (Sigma-Aldrich;
Merck KGaA) at room temperature for 1 h and processed for
immunofluorescence staining with rabbit anti-nucleolin polyclonal
antibody (1:100; cat. no. N2662; Sigma-Aldrich; Merck KGaA),
followed by incubation with fluorescein isothiocyanate-conjugated
sheep anti-rabbit IgG (1:500; cat. no. BA1105; Wuhan Boster
Biological Technology, Ltd.). Nuclear morphology was analyzed with
0.5 µg/ml Hoechst 33258 staining at room temperature for 20
min. Between all incubation steps, cells were washed with PBS
containing 0.2% BSA three times (3 min for each). Images were
acquired using a fluorescence microscope (ECLIPSE 80i; Nikon
Corporation, Tokyo, Japan).
Bioinformatics analysis identification of
nucleolin binding elements
The mRNA sequences of 12 vascular smooth muscle cell
phenotype-associated genes, such as epiregulin, were searched in
PubMed (ncbi.nlm.nih.gov/pubmed/) and UCSC Genome Browser gene
databases (genome.ucsc.edu/), and the mRNA sequences of these genes
were screened for nucleolin binding elements, such as ‘(T/G) CCCG
(A/G)’ and analysed whether these binding elements were located in
the 5′ untranslated region (UTR), 3′ UTR or coding region.
Co-immunoprecipitation of nucleolin
protein and tropoelastin mRNA
Following treatment, VSMCs were homogenized in 1 ml
radioimmunoprecipitation assay lysis buffer (Shanghai Biyuntian
Biotechnology, Ltd.) containing a variety of protease inhibitor
mixtures (10 µl/0.1 g tissue weight; Sigma-Aldrich; Merck
KGaA). Soluble proteins were collected following centrifugation at
12,000 × g for 15 min at 4°C. Following quantitative analysis by
using a bicinchoninic acid assay, protein supernatant (500
µl each) was divided into three equal fractions as follows:
Input sample; control IgG for immunoprecipitation; and the antibody
against nucleolin monoclonal antibody. An aliquot (500 µl)
of cell lysate was pre-cleared by incubation with 200 µl
protein A/G beads on ice for 60 min, followed by centrifugation at
12,000 × g for 10 min at 4°C. Then, 15 µg anti-nucleolin
monoclonal antibody (1:1,000; cat. no. sc-8031; Santa Cruz
Biotechnology, Inc.) was added to the pre-cleared cell lysate, and
the mixture was incubated at 4°C for 1 h, followed by the addition
of 200 µl protein A/G beads. The lysate was incubated at 4°C
with shaking, and the immune complexes were separated by
centrifugation at 10,000 × g for 30 sec at 4°C. RNA was extracted
from the immunoprecipitate, and cDNA was prepared using a reverse
transcription kit (Fermentas; Thermo Fisher Scientific, Inc.) and
subjected to PCR (as described). Western blot analysis was
performed using the other half of the precipitate to detect the
content of nucleolin in the cell extracts and the sediments, which
confirmed the effectiveness of the nucleolin antibody.
Measurement of tropoelastin mRNA
stability
VSMCs were treated with either 10−6 mM
Ang II for 48 h or nucleolin siRNA plasmid/pcDNA3.1-Nuc plasmid for
the indicated periods, and the cells were then incubated with
either 0.5% ethanol or 5 µg/ml actinomycin D in 0.5%
ethanol. Aliquots were removed from the cultures at 30 min
intervals over a 3-h time course. Actinomycin D at this
concentration induced no DNA fragmentation during this period. At
the indicated time points, 2×105 cells were harvested,
and total RNA was isolated using the RNeasy kit (Qiagen, Inc.).
RT-qPCR amplification of the pooled cDNA was performed as
described.
Statistical analysis
Data are expressed as the mean ± standard error
based on at least three independent experiments. Statistical
analysis was performed by one-way analysis of variance for multiple
testing, followed by post hoc testing (least significant difference
test). P<0.05 was considered to indicate a as statistically
significant difference.
Results
Effect of Ang II on phenotypic
transformation of VSMCs
RT-qPCR revealed that the mRNA expression levels of
contractive phenotypic markers α-SM-actin, calponin and SM22a, and
synthetic phenotypic marker, OPN, were negatively and positively
associated with the Ang II concentration, respectively, and the
most evident effect was observed when VSMCs were treated with Ang
II at 10−5 mmol/l for 48 h (Fig. 1A). Additionally, the mRNA
expressions of α-SM-actin, calponin and SM22a, and OPN were
negatively and positively associated with the duration of Ang II
induction, respectively, and the most evident effect was observed
when VSMCs were treated with 10−5 mmol/l Ang II for 72 h
(Fig. 1B). Similarly, western
blotting demonstrated that the protein expression of α-SM-actin,
calponin and SM22a, and OPN was negatively and positively
correlated with the Ang II concentration, respectively, and the
most evident effect was observed when VSMCs were treated with Ang
II at 10−5 mmol/l for 48 h (Fig. 1C). In addition, the protein
expression of α-SM-actin, calponin and SM22a, and OPN was
negatively and positively correlated with the duration of Ang II
induction, respectively, and the most evident effect was observed
when VSMCs were treated with 10−5 mmol/l Ang II for 72 h
(Fig. 1D). These results
suggested that Ang II significantly promoted the phenotypic
transformation of VSMCs. Dose-response analysis of VSMCs treated
with various concentrations of Ang II and time-response analysis of
VSMCs treated with Ang II for 0-72 h demonstrated that the
proliferation of VSMCs was increased in a dose- and time-dependent
manner (Fig. 1E). It indicated
that Ang II had no obvious cytotoxic effect on cell viabilities of
VSMCs, and therefore the cytotoxicity of Ang II at experimental
doses could be excluded in this study.
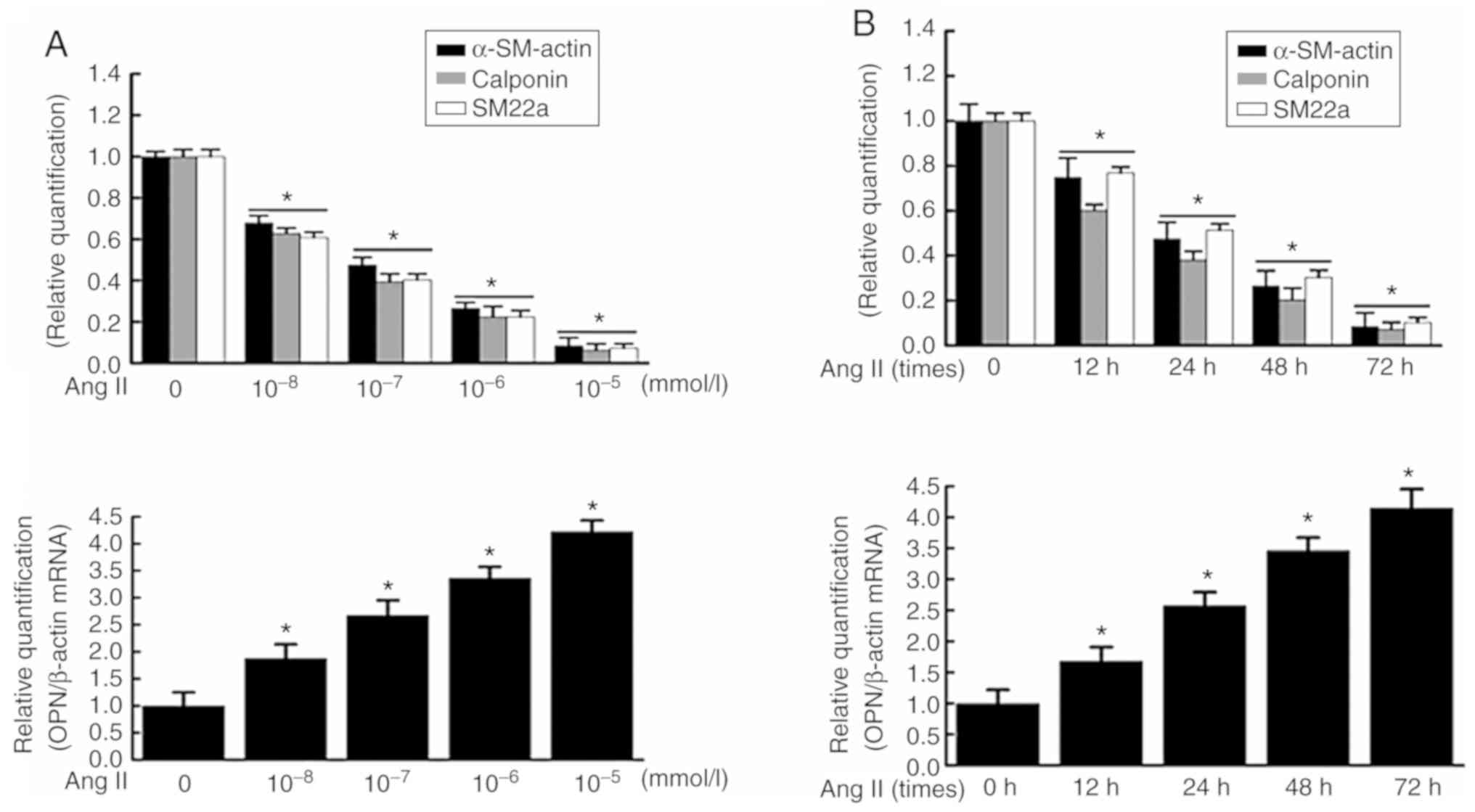 | Figure 1Effect of Ang II on the expressions
of VSMC phenotypic transformation markers α-SM-actin and OPN. VSMCs
were stimulated with (A) different concentrations of Ang II for 48
h and (B) Ang II (10−5 mmol/l) for different durations;
reverse transcription-quantitative polymerase chain reaction was
used to detect the expressions of contractile phenotype of VSMCs
(α-SM-actin, calponin, SM22a) and synthetic phenotype of VSMCs OPN
at the mRNA level. VSMCs were stimulated with (C) different
concentrations of Ang II for 48 h and (D) Ang II (10−5
mmol/l) for different durations; the total protein was extracted,
and the expressions of contractile phenotype of VSMCs (α-SM-actin,
calponin, SM22a) and synthetic phenotype of VSMCs OPN at the
protein level were analyzed by western blotting. (E) Dose-response
study of VSMCs treated with Ang II of various concentrations and
time-response study of VSMCs treated with Ang II for 0-72 h showed
that there was a dose and time-dependent increase in the
proliferation of VSMCs. Data are expressed as the mean ± standard
error. *P<0.05 vs. control. VSMCs, vascular smooth
muscle cells; Ang II, angiotensin II; α-SM-actin, α-smooth
muscle-actin; SM22a, smooth muscle protein 22 α; OPN, osteopontin;
OD, optical density. |
Effect of Ang II on the expression and
subcellular localization of nucleolin in VSMCs
The mRNA expression of nucleolin in VSMCs was
increased by Ang II treatment at different concentrations for 48 h,
and the most evident effect was observed when VSMCs were treated
with 10−6 mmol/l Ang II for 48 h, with an increase of up
to 4.26-fold compared with the normal level. The mRNA expression of
nucleolin in VSMCs was increased by treatment with 10−6
mmol/l Ang II for different durations, and the most evident effect
was observed at 48 h (Fig. 2A).
Similarly, the protein expression of nucleolin was gradually
increased by treatment with Ang II at different concentrations for
48 h, and the most evident effect was observed when VSMCs were
treated with 10−6 mmol/l Ang II for 48 h, exhibiting an
increase of up to 3.86-fold compared with the normal level. The
protein expression of nucleolin was increased by treatment with
10−6 mmol/l Ang II for different durations, the most
evident effect was observed at 48 h, and such an effect lasted to
72 h (Fig. 2B).
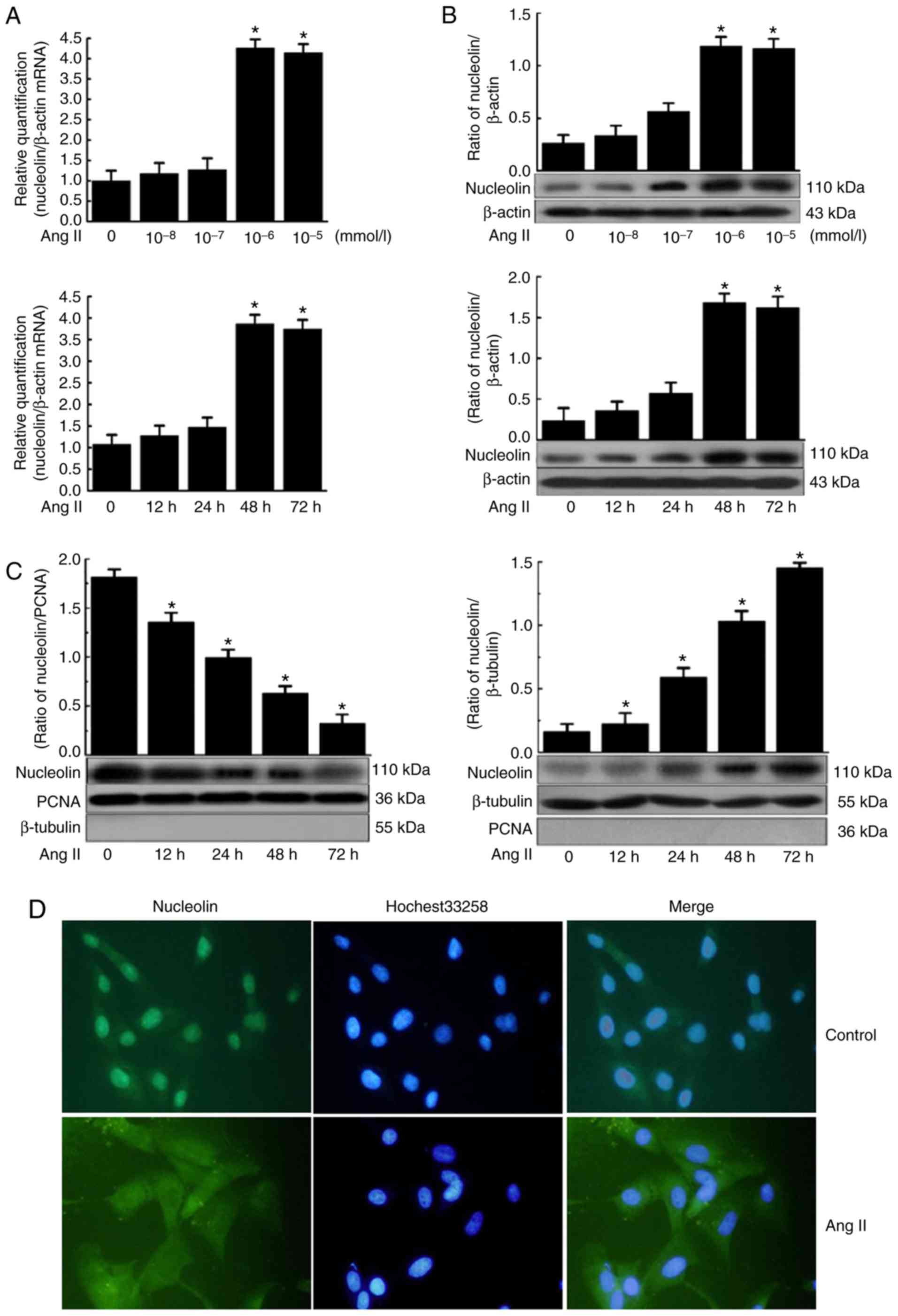 | Figure 2Effect of Ang II on the expression
and subcellular localization of nucleolin in VSMCs. (A) VSMCs were
stimulated with different concentrations of Ang II for different
durations, the total RNA of cells was extracted, and cDNA was
obtained after reverse transcription. Quantitative polymerase chain
reaction was used to detect the expression of nucleolin.
*P<0.05 vs. control group (0, 10−8 and
10−7 mmol/l). (B) VSMCs were stimulated with different
concentrations of Ang II for different durations, the total protein
was extracted, and the expression of nucleolin was analyzed by
western blotting. *P<0.05 vs. control group (0,
10−8 and 10−7 mmol/l). (C) VSMCs were
stimulated with Ang II (10−6 mmol/l) for 12, 24, 48 and
72 h, nuclear protein and cytoplasmic protein were extracted,
western blotting was used to detect the expression of nucleolin,
and β-tubulin and PCNA were used as the internal controls of
cytoplasmic protein and nuclear protein, respectively. Data are
expressed as the mean ± standard error, n=5. (D) VSMCs were
stimulated with Ang II (10−6 mmol/l) for 48 h, and
indirect immunofluorescence was used to observe the subcellular
localization of nucleolin. Nucleolin, analysis of nucleolin with
fluorescein isothiocyanate-labeled antibody (green); Hochest33258,
nuclei were counterstained with Hoechst 33258 (violet); Merge,
overlap of cytoplasmic and nuclear fractions. Magnification, ×400.
VSMCs, vascular smooth muscle cells; Ang II, angiotensin II; PCNA,
proliferating cell nuclear antigen. |
Nucleolin was predominantly localized in the nucleus
of VSMCs under normal conditions, and only a small portion was
present in the cytoplasm. However, it was translocated from the
nuclear to cytoplasm following Ang II-induced phenotypic
transformation, exhibiting increased content of nucleolin in the
cytoplasm and gradually decreased content of nucleolin in the
nucleus (Fig. 2C). In addition,
β-tubulin and PCNA were used as the internal controls for
cytoplasmic protein and nuclear protein, respectively, indicating
no obvious contamination between the components. The aforementioned
results were further confirmed by indirect immunofluorescence
analysis. In the control cells, the majority of the nucleolin
(green fluorescence) was localized in the nucleus, while only a
small portion was present in the cytoplasm. However, the expression
of nucleolin was increased in the cytoplasm, while its expression
was decreased in the nucleus following the treatment of VSMCs with
10−6 mmol/l Ang II for 48 h (Fig. 2D). These findings suggested that
Ang II induces nucleolin translocation from the nucleus to
cytoplasm.
Effect of nucleolin overexpression and
silencing on Ang II-induced phenotypic transformation of VSMCs
Results demonstrated that nucleolin was
overexpressed in pcDNA3.1-Nuc-transfected cells compared with the
control plasmid pcDNA3.1 and untransfected group cells.
Furthermore, the expression of nucleolin at the protein level was
significantly increased in the untransfected and control plasmid
groups following 10−6 mmol/l Ang II stimulation for 48
h, while the nucleolin overexpression further increased the Ang
II-induced expression of nucleolin (Fig. 3A). Additionally, western blot
analysis demonstrated that the expression of OPN was significantly
increased in the untransfected and control plasmid groups by Ang II
stimulation, while nucleolin overexpression further increased the
Ang II-induced expression of OPN. By contrast, the protein
expressions of α-SM-actin, calponin and SM22a were significantly
decreased by Ang II stimulation of untransfected and control
plasmid groups, while nucleolin overexpression further decreased
the Ang II-induced expressions of α-SM-actin, calponin and SM22a
(Fig. 3B).
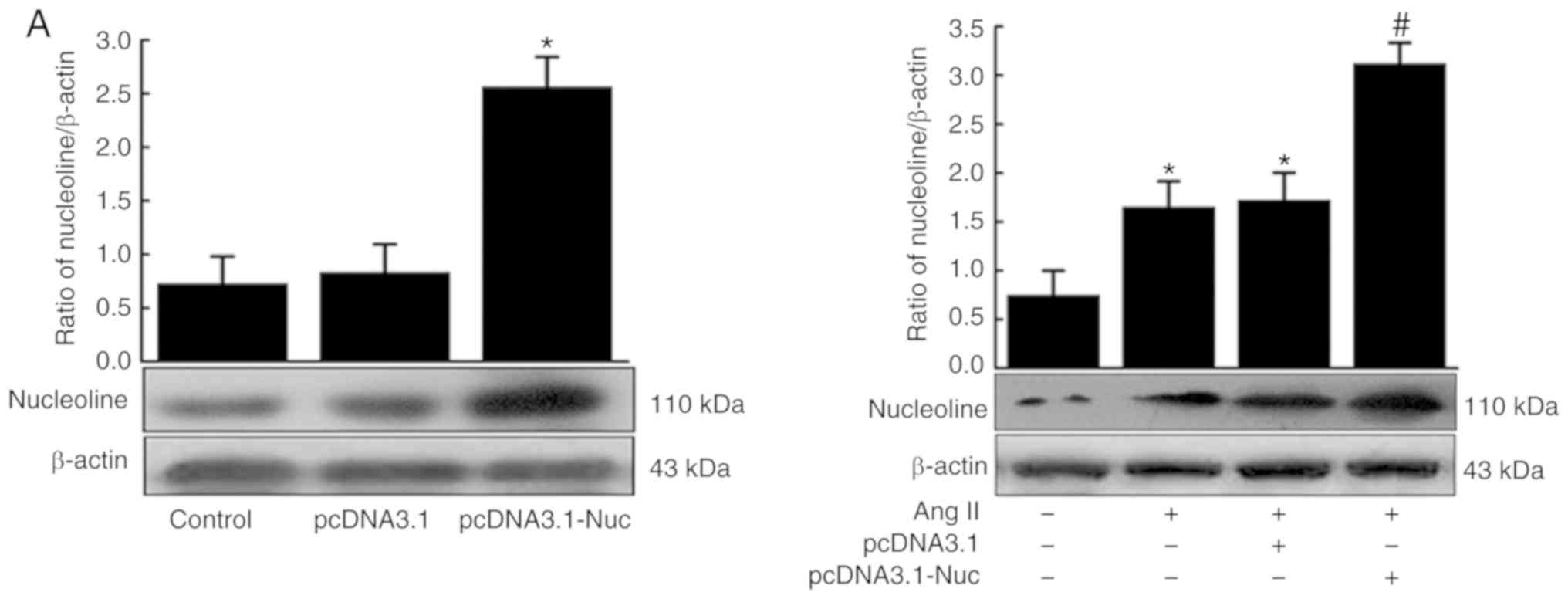 | Figure 3Effect of nucleolin overexpression
and silencing on Ang II-induced phenotypic transformation of VSMCs.
(A) Top panel, effect of nucleolin overexpression on the expression
of nucleolin in VSMCs; VSMCs were transfected with the control
plasmid (pcDNA3.1) and the recombinant plasmid pcDNA3.1-Nuc; total
protein was extracted from the transfected cells and western
blotting was performed (data are expressed as the mean ± standard
error, n=5; *P<0.05 vs. control and pcDNA3.1 group).
Bottom panel, effect of nucleolin overexpression on Ang II-induced
expression of nucleolin in VSMCs; VSMCs were transfected with
pcDNA3.1 and pcDNA3.1-Nuc, and then cells were treated with
10−6 mmol/l Ang II for 48 h. (B) Effect of nucleolin
overexpression on Ang II-induced expressions of VSMC phenotypic
transformation markers α-SM-actin, calponin, SM22a and OPN. VSMCs
were transfected with pcDNA3.1 and pcDNA3.1-Nuc, and then cells
were treated with 10−6 mmol/l Ang II for 48 h. (C) Left
panel, effect of silencing of nucleolin on expression of nucleolin
in VSMCs. VSMCs were transfected with the control plasmid (PsiRNA)
and nucleolin siRNA plasmid (PsiRNA-Nuc); total protein was
extracted from the transfected cells, and then western blotting was
performed. Right panel, effect of silencing of nucleolin on Ang
II-induced expression of nucleolin in VSMCs. VSMCs were transfected
with PsiRNA and PsiRNA-Nuc, and then cells were treated with
10−6 mmol/l Ang II for 48 h. (D) Effect of silencing of
nucleolin on Ang II-induced expressions of VSMC phenotypic
transformation markers α-SM-actin, calponin, SM22a and OPN. VSMCs
were transfected with PsiRNA and PsiRNA-Nuc, and then cells were
treated with 10−6 mmol/l Ang II for 48 h (data are
expressed as the mean ± standard error, n=5; *P<0.05
vs. normal control group; #P<0.05 vs. untransfected
cell group and pcDNA3.1 or PsiRNA group). VSMCs, vascular smooth
muscle cells; control, untransfected cell group; Ang II,
angiotensin II treatment; pcDNA3.1, control plasmid group;
pcDNA3.1-Nuc, nucleolin overexpression plasmid; OPN, osteopontin;
α-SM-actin, α-smooth muscle-actin; SM22a, smooth muscle protein
22α; PsiRNA, control plasmid group; PsiRNA-Nuc, nucleolin RNA
interference plasmid. |
The expression of nucleolin was significantly
inhibited in PsiRNA-Nuc-transfected cells compared with control
plasmid PsiRNA group and control group. Nucleolin at the protein
level was significantly increased in the untransfected and control
plasmid groups following Ang II stimulation, while the expression
of nucleolin was significantly inhibited in PsiRNA-Nuc-transfected
cells were treated with 10−6 mmol/l Ang II for 48 h
compared with the other Nag II treated groups (Fig. 3C). Additionally, the expression of
OPN was significantly increased in the untransfected and control
plasmid groups after Ang II stimulation, while the expression of
OPN was significantly inhibited in PsiRNA-Nuc-transfected cells
treated with 10−6 mmol/l Ang II for 48 h compared with
the other Ang II-treated groups. By contrast, the expressions of
contractive phenotype markers α-SM-actin, calponin and SM22a were
significantly decreased in the untransfected and control plasmid
groups following Ang II stimulation, while such downregulation was
reduced in PsiRNA-Nuc-transfected cells treated with
10−6 mmol/l Ang II for 48 h by comparison (Fig. 3D).
Effects of nucleolin on Ang II-induced
expression of phenotypic transformation-associated genes and the
binding of nucleolin protein with tropoelastin, epiregulin, and
fibroblast growth factor 2 (b-FGF) mRNA in VSMCs
Previous studies have demonstrated that the RBD in
nucleolin can bind to the corresponding binding elements in target
mRNAs, (T/G) CCCG (A/G). Subsequently, the mRNA stability of the
target gene is modulated by nucleolin, mediating
post-transcriptional regulation of certain genes. Therefore, mRNA
sequences of phenotypic transformation-associated genes in VSMCs
were screened by bioinformatics analysis, and multiple phenotypic
transformation-associated genes containing nucleolin binding
elements ‘(T/G) CCCG (A/G)’ were identified. Table II lists the mRNA sequences of 12
genes containing nucleolin binding elements, including epiregulin.
The PsiRNA-Nuc interference vector and control plasmid PsiRNA were
transiently transfected into VSMCs, and then cells were treated
with 10−6 mmol/l Ang II for 48 h. The results
demonstrated that the expression of phenotypic
transformation-associated genes, tropoelastin, epiregulin and
b-FGF, were significantly increased in the untransfected and
control plasmid groups following Ang II stimulation, while their
expressions were significantly inhibited in PsiRNA-Nuc-transfected
cells compared with the other Ang II stimulation groups (Fig. 4A). However, nucleolin
overexpression further increased the Ang II-induced expressions of
tropoelastin, epiregulin and b-FGF (Fig. 4A).
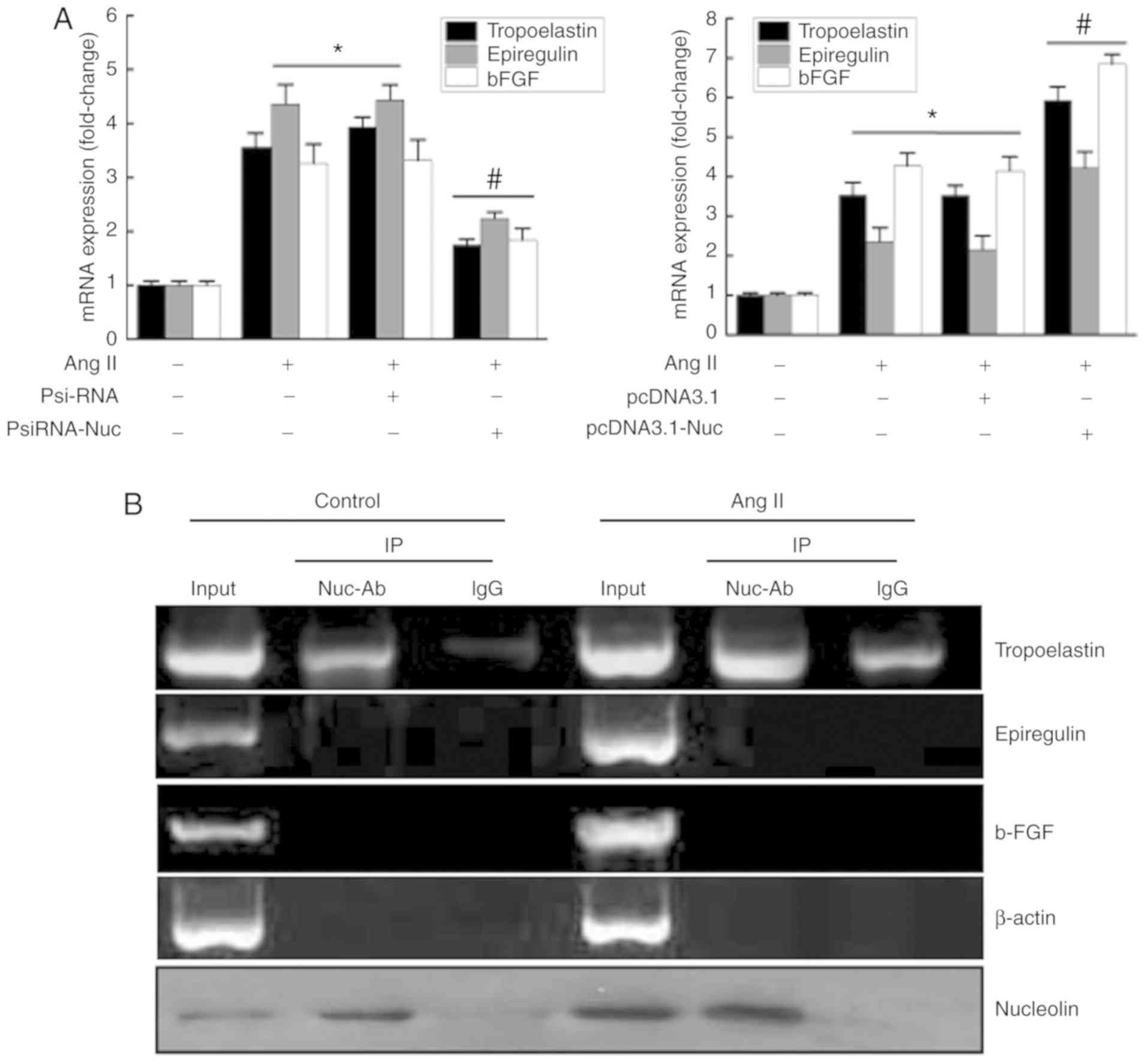 | Figure 4Effects of nucleolin on Ang
II-induced expressions of phenotypic transformation-associated
genes and the binding of nucleolin protein with tropoelastin,
epiregulin and b-FGF mRNA in VSMCs. (A) Left, effects of silencing
of nucleolin on Ang II-induced expressions of phenotypic
transformation-related genes tropoelastin, epiregulin and b-FGF in
VSMCs. VSMCs were transfected with PsiRNA and PsiRNA-Nuc, the total
RNA was extracted from the transfected cells after 48 h, and then
RT-qPCR was performed. Right, effect of nucleolin overexpression on
Ang II-induced expressions of phenotypic transformation-associated
genes tropoelastin, epiregulin and b-FGF in VSMCs. VSMCs were
transfected with pcDNA3.1 and pcDNA3.1-Nuc, the total RNA was
extracted from the transfected cells after 48 h, and then the
RT-qPCR was performed. Data are expressed as the mean ± standard
error, n=5; *P<0.05 vs. normal control group;
#P<0.05 vs. untransfected cell group and pcDNA3.1 or
PsiRNA group). (B) Normal VSMCs and Ang II-treated VSMCs were
collected to prepare the cell extracts, cell extracts were divided
into three equal groups: Input group, negative control IgG group
and nucleolin antibody group. Immunoprecipitation was performed out
using rabbit anti-nucleolin monoclonal antibody, and total mRNA was
extracted from the sediment. Representative of three separate
experiments. VSMCs, vascular smooth muscle cells; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; Ang II,
angiotensin II treatment; pcDNA3.1, control plasmid group;
pcDNA3.1-Nuc, nucleolin overexpression plasmid; PsiRNA, control
plasmid group; PsiRNA-Nuc, nucleolin RNA interference plasmid;
Input, positive control; IgG, immunoglobulin G negative control;
Nuc-Ab, nucleolin antibody; Ctrl, control cells; IP,
immunoprecipitation; b-FGF, fibroblast growth factor 2. |
 | Table IIBioinformatics analysis
identification of 12 phenotypic transformation-associated genes
(mRNA) containing nucleolin binding elements. |
Table II
Bioinformatics analysis
identification of 12 phenotypic transformation-associated genes
(mRNA) containing nucleolin binding elements.
| Gene | Nucleolin-binding
element | Site | Number |
|---|
| Epiregulin | GCCCGG | 3′ UTR | 1 |
| Tropoelastin | (T/G)CCCG(A/G) | Coding region | 5 |
| Thrombospondin | GCCCGG | Coding region | 1 |
| Epidermal growth
factor | TCCCGG/GCCCGG | 5′ UTR and coding
region | 2 |
| Fibroblast growth
factor 2 | TCCCGG/GCCCGG | 5′ UTR | 2 |
| Platelet derived
growth factor-BB | GCCCGG | 5′ UTR | 2 |
| Matrix
metallopeptidase 1 | GCCCGG | Coding region | 1 |
| Insulin-like growth
factor 1 | TCCCGA | Coding region | 1 |
| P38 mitogen
activated protein kinase | GCCCGA/GCCCGG | 5′ UTR, coding
region, 3′ UTR | 3 |
| Nuclear
factor-κB | TCCCGA | Coding region | 1 |
| Angiotensin II | TCCCGG | Coding region | 1 |
| Tumor necrosis
factor-α | GCCCGA/TCCCGG | 5′ UTR and 3′
UTR | 2 |
In order to identify the genes that interact with
nucleolin during phenotypic transformation, protein-RNA
co-immunoprecipitation and RT-qPCR were used. The results
demonstrated that only a small amount of tropoelastin mRNA was
precipitated in normal cell lysate. However, the amount of
tropoelastin mRNA precipitated when using a nucleolin antibody was
increased compared with the control IgG group, suggesting that
nucleolin binds to tropoelastin mRNA. The amount of tropoelastin
mRNA in cell lysates was increased by Ang II stimulation. However,
the amount of tropoelastin mRNA precipitated by nucleolin antibody
was also increased compared with the control IgG group, suggesting
that the binding of nucleolin and tropoelastin mRNA was increased
by Ang II stimulation. However, binding between nucleolin and
epiregulin or b-FGF through was not observed by
immunoprecipitation. In addition, β-actin was used as a control,
and the findings revealed that the binding of nucleolin and
tropoelastin mRNA was specific. Western blot analysis was used to
detect the content of nucleolin in the cell extracts and the
sediments, which confirmed the effectiveness of the nucleolin
antibody pull down of nucleolin (Fig.
4B).
Effect of nucleolin on tropoelastin mRNA
stability and tropoelastin protein expression in VSMCs
Following transfection with pcDNA3.1 and
pcDNA3.1-Nuc for 48 h, cells were treated with actinomycin D (5
µg/ml), and the decay of tropoelastin mRNA was assayed.
Compared with the control group, overexpression of nucleolin slowed
the degradation of tropoelastin mRNA, indicating increased
tropoelastin mRNA stability, However, the control plasmid had no
significant effect on the tropoelastin mRNA stability (Fig. 5A). The effect of Ang II on the
stability of tropoelastin mRNA was also analyzed. Compared with the
control group, Ang II slowed the degradation of tropoelastin mRNA,
indicating increased tropoelastin mRNA stability (Fig. 5B). VSMCs were transfected with
nucleolin siRNA for 24 h before Ang II treatment, and the effect of
Ang II on tropoelastin mRNA stability was relieved. However, the
control plasmid had no significant effect on the tropoelastin mRNA
stability (Fig. 5C). The effect
of nucleolin overexpression and silencing of nucleolin on the
protein expression of tropoelastin was also assessed. The results
demonstrated that the expression of tropoelastin was significantly
increased in the untransfected and control plasmid groups following
Ang II stimulation, while nucleolin overexpression further
increased the Ang II-induced expression of tropoelastin (Fig. 5D). The expression of tropoelastin
was significantly increased in the untransfected and control
plasmid groups following Ang II stimulation, while the expression
of tropoelastin was significantly inhibited in
PsiRNA-Nuc-transfected cells treated with 10−6 mmol/l
Ang II for 48 h (Fig. 5E).
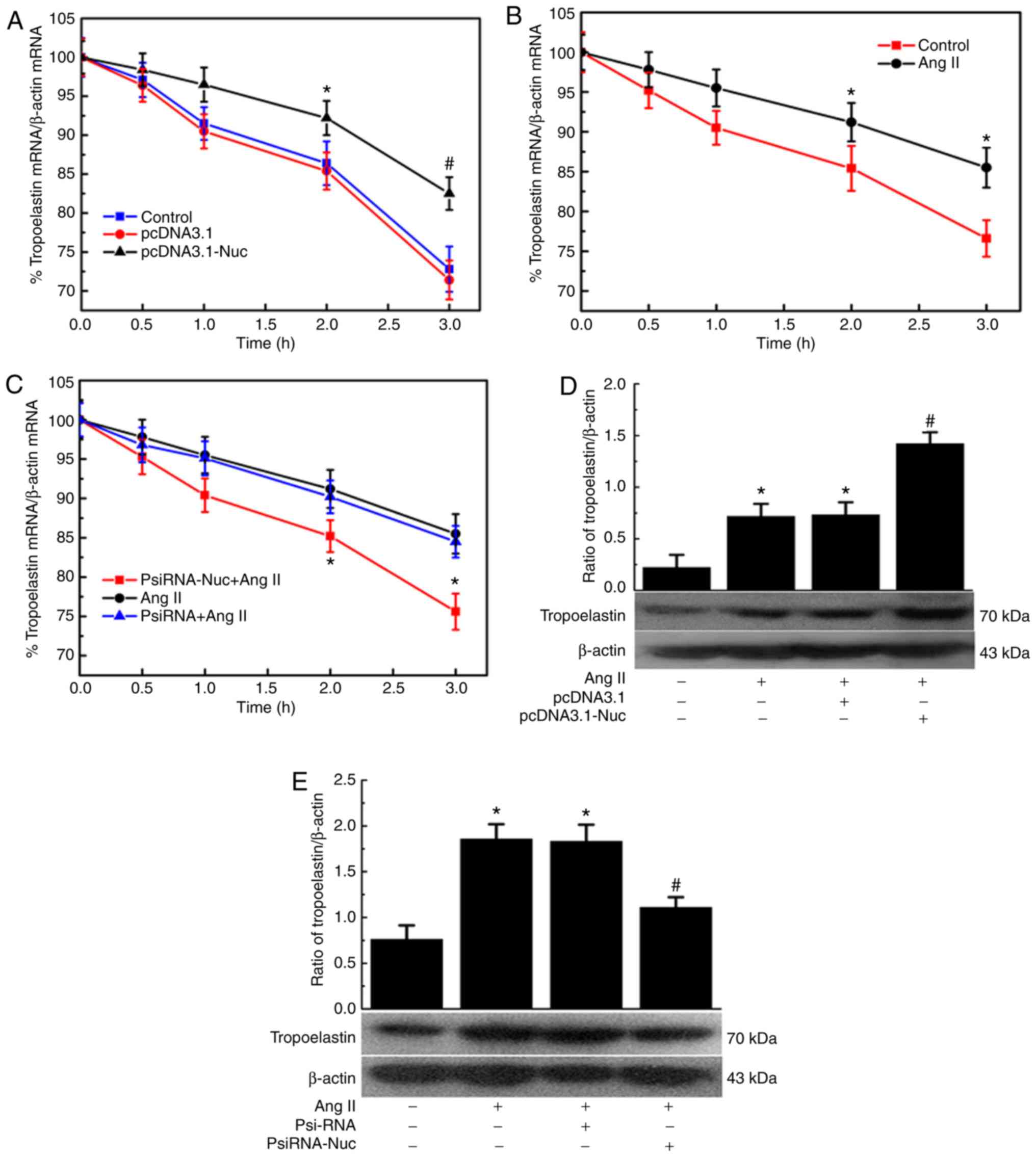 | Figure 5Effect of nucleolin on tropoelastin
mRNA stability and tropoelastin protein expression in VSMCs. (A)
Effect of nucleolin overexpression on tropoelastin mRNA stability
in VSMCs. VSMCs were transfected with pcDNA3.1 and pcDNA3.1-Nuc for
48 h, and then incubated with actinomycin D (5 µg/ml) for
various periods of time (0, 0.5, 1, 2 and 3 h). The mRNA levels of
tropoelastin were determined by RT-qPCR. *P<0.05 vs.
Vect group, #P<0.01 vs. Vect group, n=5. (B) Effect
of Ang II on tropoelastin mRNA stability in VSMCs. VSMCs were
treated with 10−6 mM Ang II for 48 h. The cells were
then incubated with actinomycin D (5 µg/ml) for various
periods of time (0, 0.5, 1, 2 and 3 h). The mRNA levels of
tropoelastin were determined by RT-qPCR. *P<0.05 vs.
Ctrl group, n=5. (C) Effect of low expression of nucleolin on
tropoelastin mRNA stability in VSMCs. VSMCs were transfected with
nucleolin siRNA plasmid for 24 h, cells were treated with
10−6 mmol/l Ang II for 48 h, and then incubated with
actinomycin D (5 µg/ml) for various periods of time (0, 0.5,
1, 2 and 3 h). The mRNA levels of tropoelastin were determined by
RT-qPCR. *P<0.05 vs. PsiRNA group, n=5. Effect of
nucleolin (D) overexpression and (E) low expression on Ang
II-induced expressions of tropoelastin. VSMCs were transfected with
pcDNA3.1, pcDNA3.1-Nuc, PsiRNA and PsiRNA-Nuc, and then cells were
treated with 10−6 mmol/l Ang II for 48 h. Data are
expressed as the mean ± standard error, n=5; *P<0.05
vs. normal control group, #P<0.05 vs. untransfected
cell group and pcDNA3.1 group. VSMCs, vascular smooth muscle cells;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; Ctrl, control; Vect, transfected with pcDNA3.1 plasmid;
Nuc, transfected with pcDNA3.1-Nuc plasmid; Ang II, angiotensin II;
PsiRNA, control plasmid group; psiRNA-Nuc, nucleolin RNA
interference plasmid. |
Discussion
Phenotypic transformation of VSMCs has a pivotal
role in the pathogenesis of cardiovascular diseases, including
hypertension, coronary artery disease and angiographic restenosis.
Great efforts have been made to inhibit the phenotypic
transformation of VSMCs, providing an important experimental and
theoretical basis for the prevention and treatment of
cardiovascular diseases. Measures to potentially prevent and treat
cardiovascular diseases that have been investigated include the
following: Gene transfection, RNA interference, blockade of
transcription factors at the nuclear transcription level, certain
drugs inhibiting the phenotypic transformation of VSMCs, and
replacement, repair or enhancement of vascular endothelial function
in damaged tissues or organs by stem cell transplantation (3,21-24). Although great progress has been
made to uncover the underlying mechanism of VSMC phenotypic
transformation, the specific mechanisms has not been fully
elucidated. Therefore, understanding the molecular mechanisms
regulating VSMC phenotypic transformation has become a key measure
to control the abnormal proliferation of VSMCs, and such efforts
may also provide a novel theoretical approach for angioplasty of
atherosclerotic disease, hypertension and angiographic
restenosis.
Generally, it is believed that endogenous active
substances in the body have a spontaneous regulatory role in the
proliferation or phenotypic transformation of VSMCs. Therefore, to
clarify the mechanism, a key step is to identify novel endogenous
regulatory proteins mediating the phenotype of VSMCs. Although a
variety of cytokines, growth factors and vasoactive substances are
involved in phenotypic transformation of VSMCs (25-27), it remains unclear what type of
factors have a key role in VSMC phenotypic transformation.
Nucleolin (also known as C23) is the most abundant of 271 nucleolar
proteins, accounting for ~10% of the total nucleolar protein
content. It has a fundamental role in the nucleolus of eukaryotic
cells. Nucleolin is involved in the ribosome biosynthesis and
maturation, cell proliferation, growth, embryogenesis, cytokinesis,
chromatin replication and nucleolus function (28,29). A large number of studies have
demonstrated that nucleolin is highly expressed in proliferating
tissues and cells, including stem cells and tumor cells, and it
promotes the regeneration of stem cells and the growth of tumor
cells (12,30,31). The expression level of nucleolin
is positively correlated with the rate of cell division, and
remains at a fairly high level in tumor cells and other rapidly
dividing cells (32). These
results suggest that nucleolin may regulate the phenotypic
transformation of VSMCs under pathological conditions, while it
remains largely unexplored whether and how nucleolin regulates the
phenotypic transformation of VSMCs.
In order to clarify the role of nucleolin in the
phenotypic transformation of VSMCs, Ang II was used to induce the
phenotypic transformation of VSMCs. The results of the current
study demonstrated that the expressions of α-SM-actin, SM22a and
calponin at the mRNA and protein levels were gradually decreased by
Ang II stimulation, while the expression of OPN mRNA and protein
was gradually increased by Ang II stimulation. These results
suggested that Ang II significantly promoted the phenotypic
transformation of VSMCs. An increasing number of studies have
reported that a variety of factors can alter the expression of
nucleolin (6-8,11,14). However, it is unknown whether the
nucleolin expression is changed in cell models of VSMC phenotypic
transformation induced by Ang II. RT-qPCR and western blot analysis
demonstrated that the expression of nucleolin mRNA and protein was
increased by treatment Ang II at different concentrations for 48 h,
and the most evident effect was observed when VSMCs were treated
with 10−6 mmol/l Ang II for 48 h. Furthermore, VSMCs
were treated with 10−6 mmol/l Ang II for different
durations, and the most evident effect was observed at 48 h. Dose-
and time-response experiments demonstrated that the proliferation
of VSMCs was increased by Ang II in a dose-and time-dependent
manner. This indicates that Ang II has no obvious cytotoxic effects
on VSMC within 72 h or at 10−5 mmol/l. Therefore, in
order to avoid the toxic side effects of continuous drug
stimulation on cells, we used time points of 12, 24, 48 and 72 h to
observe the expression of nucleolin after Ang II stimulation. Based
on the expression pattern of nucleolin, it was hypothesized that
upregulation of nucleolin may have a role in Ang II-induced
phenotypic transformation of VSMCs. However, the mechanism
underlying the Ang II-induced upregulation of nucleolin remained
unclear. It has been previously reported that the activation of the
mitogen-activated protein kinase (MAPK) pathway can also upregulate
the expression of nucleolin (33). Furthermore, Ang II-induced
proliferation and phenotypic transformation of VSMCs have been
associated with activation of extracellular signal-regulated kinase
1/2, and activation of c-Jun N-terminal kinase, P38 MAPK and
nuclear factor-кB (NF-кB) (34,35). Therefore, Ang II may upregulate
the expression of nucleolin by activating signaling pathways, such
as MAPK and NF-кB. However, this hypothesis should be further
validated.
It was also observed that in normal untreated and
untransfected cells, the majority of the nucleolin was localized in
the nucleus of VSMCs, and Ang II induced the translocation of
nucleolin from the nucleus to cytoplasm. These results suggested
that nucleolin had a role in the phenotypic transformation of VSMCs
induced by Ang II, and such role may depend on its cytoplasmic
localization. Studies have reported that the translocation of
nucleolin to the cytoplasm depends on the phosphorylation of an
amino-terminal threonine induced by cdc2 kinase, whereas
dephosphorylation promotes its translocation to the nucleus
(36). Therefore, Ang II may have
a role in regulating VSMC phenotypic transformation by activating
certain kinases, leading to the translocation of nucleolin. To
investigate the role of nucleolin in the phenotypic transformation
of VSMCs, rat VSMCs were transfected with the recombinant plasmid
pcDNA3.1-Nuc and control plasmid pcDNA3.1. Results showed that
overexpression of nucleolin promoted the VSMC phenotypic
transformation induced by Ang II. Similarly, we further examined
the effect of low expression of nucleolin on Ang II-induced
phenotypic transformation of VSMCs, and revealed that
downregulation of nucleolin suppressed the promotion of phenotypic
transformation. These findings, for the first time, demonstrated
that the upregulation of nucleolin played a key role in the
phenotypic transformation of VSMCs induced by Ang II. However, the
mechanism of action of nucleolin remains unclear.
It has been reported that the CS-RBD, which binds
the consensus sequence (T/G) CCCG (A/G), is structurally crucial
for the role of RNA-binding proteins in primary transcript splicing
and maturational regulation of ribosomal RNA (10-15). In addition, a large number of
studies have reported that this structural domain also mediates the
post-transcriptional regulation of certain genes by nucleolin
(10,11,13,14,29,37,38). For example, the binding of
nucleolin to the 5′ UTR of interleukin (IL)-2 and growth arrest and
DNA-damage-inducible α mRNA coding region modulates the protein
expressions of target genes by regulating the stability of these
target mRNAs (37,38). The RBD of nucleolin can bind to
the 5′ and 3′ UTRs of mRNAs of Bcl-2, protein kinase B, p53 and
other apoptosis-associated genes, with a crucial role in promoting
cell proliferation and anti-apoptotic effect (10,11,13). Nucleolin regulates the nuclear and
nucleolar localization of telomerase by binding to the telomerase
RNA component human telomerase reverse transcriptase through four
RBDs, thus mediating cell growth and proliferation (14). Previous studies have also
demonstrated that nucleolin enhances the stability of ATP binding
cassette subfamily A member 1 mRNA, subsequently increasing
cholesterol efflux and inhibiting the formation of foam cells
(29). These studies have
suggested that the RNA binding is the key step for nucleolin to
regulate various biological functions, such as cell growth and
proliferation.
The current study demonstrated that nucleolin may
have a positive modulating effect on the phenotypic transformation
of VSMCs, and its underlying mechanism may be through regulating
the expressions of certain phenotypic transformation-associated
genes in VSMCs. However, it remained largely unexplored how
nucleolin regulated the expressions of these genes. Furthermore, it
also remained unclear whether nucleolin, as an RNA binding protein,
regulated its stability and expression through interacting with
mRNAs associated with phenotypic transformation, and thus, a role
in the phenotypic transformation of VSMCs-induced by Ang II. The
mRNA sequences of phenotypic transformation-associated genes in
VSMCs were analyzed in bioinformatics analysis. Multiple phenotypic
transformation-associated genes containing the nucleolin binding
element were identified. Additionally, in order to investigate
whether nucleolin can upregulate expression by binding to mRNAs
containing the nucleolin binding element, such as tropoelastin,
epiregulin and b-FGF, the effects of overexpression and silencing
of nucleolin on the expressions of phenotypic
transformation-associated genes were investigated. The findings
revealed that overexpression of nucleolin promoted the expressions
of tropoelastin, epiregulin and b-FGF, while silencing of nucleolin
significantly downregulated the expressions of these genes. These
findings suggested that the expressions of tropoelastin, epiregulin
and b-FGF were regulated by nucleolin. Protein-RNA co-precipitation
indicated that there was an interaction between tropoelastin mRNA
and nucleolin protein, promoting the stability of tropoelastin mRNA
and enhancing the expression of tropoelastin at the protein level.
mRNA stability is mainly regulated by cis-acting elements and the
trans-acting factors. Cis-acting elements are part of the structure
of the mRNA itself, including sequences in the 3′ UTR, 5′ UTR and
coding region. Trans-acting factors are proteins that bind to
cis-acting elements. Whether RNA binding protein nucleolin can bind
to the 3′ UTR, 5′ UTR or coding region of tropoelastin mRNA to
regulate mRNA stability requires further investigation. Therefore,
it is necessary to demonstrate the interaction between nucleolin
and tropoelastin or other genes and its specific mechanisms from
multiple perspectives. For example, luciferase reporter gene,
RNA-electrophoretic mobility shift assay and other techniques can
be used to further examine the presence of mutual binding and
stability of target mRNAs following binding.
It is established that the phenotypic transformation
of VSMCs is affected and regulated by a variety of growth factors
(epidermal growth factor, b-FGF, vascular endothelial growth
factor, platelet-derived growth factor, nerve growth factor),
cytokines (transforming growth factor, IL-1, IL-6, tumor necrosis
factor-α), vasoactive substances (Ang II, nitric oxide,
prostacyclin) and extracellular matrix proteins (39-41). When VSMCs undergo phenotypic
transformation, the expressions of synthetic markers, including
OPN, epiregulin, tropoelastin and thrombospondin, are upregulated
in VSMCs (42). In the present
study, it was preliminarily confirmed that nucleolin has an
important role in the phenotypic transformation of VSMCs induced by
Ang II, and nucleolin in combination with tropoelastin mRNA exerted
a positive effect on phenotypic transformation. However, the
association between nucleolin and other phenotypic
transformation-associated genes remain unclear. In addition,
nucleolin is a multifunctional protein. Besides binding to RNA,
nucleolin can also bind to DNA (similar to transcription factors)
to regulate the gene transcription by interacting with the gene
promoter region. Further studies are required explore whether
nucleolin can interact with the promoters of tropoelastin or other
associated genes and regulate the phenotypic transformation of
VSMCs at the transcriptional level. The phenotypic transformation
of VSMCs is extremely complex, and the current study provided a new
perspective for the investigation of the regulatory mechanism of
VSMC phenotypic transformation. Nucleolin may mediate the
post-transcriptional regulation of VSMC phenotypic
transformation-associated mRNAs, affect the stability and protein
expression of associated genes, and have a role in promoting
phenotypic transformation. Therefore, nucleolin could be used as a
target for the treatment of hypertension, atherosclerosis and
angiographic restenosis. However, various contradictions require in
depth investigation, such as if the expression of nucleolin is
inhibited in VSMCs, phenotypic transformation may be inhibited, but
the physiological regulation of nucleolin will be lost in normal
VSMCs. Furthermore, it may have abnormal effects on the
physiological function of cells. Therefore, how to control the
balance between the inhibition of VSMC phenotypic transformation
and the possible side effects of targeting nucleolin needs to be
further investigated.
Funding
This study was supported by the Fund from Bureau of
Science and Technology of Changsha, China (grant no. Kq1701007) and
Hunan Natural Science Foundation, China (grant no. 2018JJ6127).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
All authors contributed extensively to the work
presented in this paper. LF performed the experiments, analyzed
statistical data, and wrote and revised the manuscript. ZXY
designed the current study, acquired data, analyzed and interpreted
the data. KKW provided the plasmids required for the experiment.
KKW and PFZ participated in the design, analyzed the data and
revised the manuscript. ZLX and MY acquired and analyzed the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Rzucidlo EM, Martin KA and Powell RJ:
Regulation of vascular smooth muscle cell differentiation. J Vasc
Surg. 45(Suppl A): A25–A32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong N, Wang W, Tian J, Xie Z, Lv B, Dai
J, Jiang R, Huang D, Fang S, Tian J, et al: MicroRNA-182 prevents
vascular smooth muscle cell dedifferentiation via FGF9/PDGFRβ
signaling. Int J Mol Med. 39:791–798. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi N and Chen SY: Smooth muscle cell
differentiation: Model systems, regulatory mechanisms, and vascular
diseases. J Cell Physiol. 231:777–787. 2016. View Article : Google Scholar
|
|
4
|
Owens GK: Molecular control of vascular
smooth muscle cell differentiation and phenotypic plasticity.
Novart Found Symp. 283:174–191. 2007. View Article : Google Scholar
|
|
5
|
Chen Z and Xu X: Roles of nucleolin. Focus
on cancer and anti-cancer therapy. Saudi Med J. 37:1312–1318. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang B, Zhang B, Liang P, Song J, Deng H,
Tu Z, Deng G and Xiao X: Nucleolin/C23 mediates the antiapoptotic
effect of heat shock protein 70 during oxidative stress. FEBS J.
277:642–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang L, Wang KK, Jiang L, Jiang BM, Wei X,
Song L, Deng GH and Xiao XZ: Role of cell-surface nucleolin in
lipopolysaccharide-stimulated expression and secretion of TNF-alpha
and IL-1beta. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 33:999–1004.
2008.In Chinese. PubMed/NCBI
|
|
8
|
Huang F, Wu Y, Tan H, Guo T, Zhang K, Li D
and Tong Z: Phosphorylation of nucleolin is indispensable to its
involvement in the proliferation and migration of non-small cell
lung cancer cells. Oncol Rep. 41:590–598. 2019.
|
|
9
|
Wang Y, Mao M and Xu JC: Cell-surface
nucleolin is involved in lipopolysaccharide internalization and
signalling in alveolar macrophages. Cell Biol Int. 35:677–685.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iliakis G, Krieg T, Guan J, Wang Y and
Leeper D: Evidence for an S-phase checkpoint regulating DNA
replication after heat shock: A review. Int J Hyperthermia.
20:240–249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Y, Shi H, Zhou H, Song X, Yuan S and
Luo Y: The angiogenic function of nucleolin is mediated by vascular
endothelial growth factor and nonmuscle myosin. Blood.
107:3564–3571. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng Y, Zhao G, Zhang S, Nigim F, Zhou G,
Yu Z, Song Y, Chen Y and Li Y: AS1411-induced growth inhibition of
glioma cells by up-regulation of p53 and down-regulation of Bcl-2
and Akt1 via nucleolin. PLoS One. 11:e01670942016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Chen Y, Luo X, Liang Y, Shi H, Gao
L, Zhan S, Zhou D and Luo Y: The heparin binding motif of
endostatin mediates its interaction with receptor nucleolin.
Biochemistry. 48:11655–11663. 2009. View Article : Google Scholar
|
|
14
|
Wang K, Deng G, Chen G, Liu M, Yi Y, Yang
T, McMillan DR and Xiao X: Heat shock protein 70 inhibits hydrogen
peroxide-induced nucleolar fragmentation via suppressing cleavage
and down-regulation of nucleolin. Cell Stress Chaperon. 17:121–130.
2012. View Article : Google Scholar
|
|
15
|
Khurts S, Masutomi K, Delgermaa L, Arai K,
Oishi N, Mizuno H, Hayashi N, Hahn WC and Murakami S: Nucleolin
interacts with telomerase. J Biol Chem. 279:51508–51515. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang B, Liang P, Wang K, Lv C, Sun L,
Tong Z, Liu Y and Xiao X: Nucleolin involved in myocardial
ischaemic preconditioning via post-transcriptional control of
HSPA1A expression. Cardiovasc Res. 102:56–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tulchin N, Chambon M, Juan G, Dikman S,
Strauchen J, Ornstein L, Billack B, Woods NT and Monteiro AN: BRCA1
protein and nucleolin colocalize in breast carcinoma tissue and
cancer cell lines. Am J Pathol. 176:1203–1214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mosafer J and Mokhtarzadeh A: Cell surface
nucleolin as a promising receptor for effective AS1411
aptamer-mediated targeted drug delivery into cancer cells. Curr
Drug Deliv. 15:1323–1329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimes BW and Brandt BL: Characterization
of two putative smooth muscle cell lines from rat thoracic aorta.
Exp Cell Res. 98:349–366. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Tuan NQ, Lee DH, Oh J, Kim CS, Heo KS,
Myung CS and Na M: Inhibition of proliferation of vascular smooth
muscle cells by cucurbitanes from momordica charantia. J Nat Prod.
80:2018–2025. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Y, Yu S, Liu Y, Zhang J, Han L and Xu
Z: MicroRNA-124 control human vascular smooth muscle cell
phenotypic switch via Sp1. Am J Physiol Heart Circ Physiol.
31:H641–H649. 2017. View Article : Google Scholar
|
|
23
|
Davis-Dusenbery BN, Wu C and Hata A:
Micromanaging vascular smooth muscle cell differentiation and
phenotypic modulation. Arterioscler Thromb Vasc Biol. 31:2370–2377.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma X, Jiang C, Li Y, Feng L, Liu J and
Wang J: Inhibition effect of tacrolimus and platelet-derived growth
factor-BB on restenosis after vascular intimal injury. Biomed
Pharmacother. 93:180–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang L, Chen MF, Xiao ZL, Liu Y, Yu GL,
Chen XB and Xie XM: Calcitonin gene-related peptide released from
endothelial progenitor cell inhibits the proliferation of rat
vascular smooth muscle cells induced by angiotensin II. Mol Cell
Biochem. 355:99–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang L, Chen MF, Xiao ZL, Yu GL, Chen XB
and Xie XM: The effect of endothelial progenitor cells on
angiotensin II-induced proliferation of cultured rat vascular
smooth muscle cells. J Cardiovasc Pharmacol. 58:617–625. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang MJ, Zhou Y, Chen L, Wang YQ, Wang X,
Pi Y, Gao CY, Li JC and Zhang LL: An overview of potential
molecular mechanisms involved in VSMC phenotypic modulation.
Histochem Cell Biol. 145:119–130. 2016. View Article : Google Scholar
|
|
28
|
Andersen JS, Lyon CE, Fox AH, Leung AK,
Lam YW, Steen H, Mann M and Lamond AI: Directed proteomic analysis
of the human nucleolus. Curr Biol. 12:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang S: Building an efficient factory:
Where is pre-rRNA synthesized in the nucleolus. J Cell Biol.
157:739–741. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y, Jiang B, Liang P, Tong Z, Liu M, Lv
Q, Liu Y, Liu X, Tang Y and Xiao X: Nucleolin protects macrophages
from oxLDL-induced foam cell formation through up-regulating ABCA1
expression. Biochem Biophys Res Commun. 486:364–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bose S, Tholanikunnel TE, Reuben A,
Tholanikunnel BG and Spicer EK: Regulation of nucleolin expression
by miR-194, miR-206, and HuR. Mol Cell Biochem. 417:141–153. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galande S: Chromatin (dis)organization and
cancer: BUR-binding proteins as biomarkers for cancer. Curr Cancer
Drug Targets. 2:157–190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reyes-Reyes EM and Akiyama SK:
Cell-surface nucleolin is a signal transducing P-selectin binding
protein for human colon carcinoma cells. Exp Cell Res.
314:2212–2223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen T, Deng S and Lin R: The inhibitory
effect of Isoliquiritigenin on the proliferation of human arterial
smooth muscle cell. BM. Pharmacol Toxicol. 18:572017.
|
|
35
|
Yu XH, Zheng XL and Tang CK: Nuclear
Factor-κB activation as a pathological mechanism of lipid
metabolism and atherosclerosis. Adv Clin Chem. 70:1–30. 2015.
View Article : Google Scholar
|
|
36
|
Webster KA, Discher DJ and Bishopric NH:
Cardioprotection in an in vitro model of hypoxic preconditioning. J
Mol Cell Cardiol. 27:453–458. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen CY, Gherzi R, Andersen JS, Gaietta G,
Jürchott K, Royer HD, Mann M and Karin M: Nucleolin and YB-1 are
required for JNK-mediated interleukin-2 mRNA stabilization during
T-cell activation. Genes Dev. 14:1236–1248. 2000.PubMed/NCBI
|
|
38
|
Zhang Y, Bhatia D, Xia H, Castranova V,
Shi X and Chen F: Nucleolin links to arsenic-induced stabilization
of GADD45alpha mRNA. Nucleic Acids Res. 34:485–495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Izawa Y, Yoshizumi M, Ishizawa K, Fujita
Y, Kondo S, Kagami S, Kawazoe K, Tsuchiya K, Tomita S and Tamaki T:
Big mitogen-activated protein kinase 1 (BMK1)/extracellular signal
regulated kinase 5 (ERK5) is involved in platelet-derived growth
factor(PDGF)-induced vascular smooth muscle cell migration.
Hypertens Res. 30:1107–1117. 2007. View Article : Google Scholar
|
|
40
|
Montezano AC, Callera GE, Yogi A, He Y,
Tostes RC, He G, Schiffrin EL and Touyz RM: Aldosterone and
Angiotensin II synergistically stimulate migration in vascular
smooth muscle cells through c-Src-regulated redox-sensitive RhoA
pathways. Arterioscler Thromb Vasc Biol. 28:1511–1518. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang S, Liu Y, Fan F, Yan J, Wang X and
Chen J: Inhibitory effects of emodin on the proliferation of
cultured rat vascular smooth muscle cell-induced by angiotensin II.
Phytother Res. 22:247–251. 2008. View Article : Google Scholar
|
|
42
|
Takahashi M, Hayashi K, Yoshida K, Ohkawa
Y, Komurasaki T, Kitabatake A, Ogawa A, Nishida W, Yano M, Monden M
and Sobue K: Epiregulin as a major autocrine/paracrine factor
released from ERK- and p38MAPK-activated vascular smooth muscle
cells. Circulation. 108:2524–2529. 2003. View Article : Google Scholar : PubMed/NCBI
|



















