Introduction
Ovarian cancer (OC) is the most lethal malignant
gynecological cancer that constitutes 4% of all cancers diagnosed
in women worldwide (1,2). The majority of patients are
diagnosed at an advanced stage and the 5-year overall survival rate
of patients with OC is <40% (3-6).
Unfortunately, despite the current standard treatment of combining
surgery with chemotherapy being efficient, the majority of patients
ultimately develop recurrence (7-9).
Therefore, clinical biomarkers and more efficient therapeutic
targets are urgently required to treat OC as well as the
elucidation of the molecular pathogenesis of OC.
MicroRNAs (miRNAs/miRs) are endogenous non-coding,
single-stranded small regulatory RNA molecules (21-23 nucleotides)
(10). A body of evidence has
suggested that miRNA is an important regulatory factor of gene
expression and miRNA dysregulation is frequently associated with
biological processes, including cell proliferation (11), differentiation (12), angiogenesis (13,14), apoptosis (15) and the adhesion of tumor cells,
including OC (16-19). miR-183 is located on chromosome
7q32 and has been reported to be overexpressed in the different
types of tumors, including prostate cancer and breast cancer
(20-22). In addition, a previous study has
also reported that miR-183 was implicated in the regulatory
mechanisms of tumor invasion and metastasis (23). However, the potential role of
miR-183 in the pathogenesis of OC remains unclear. Based on these
results, it was hypothesized that the aberrant expression of
miR-183 may be an important mediator of cell growth, invasion and
apoptosis.
Transforming growth factor-β (TGF-β) is a secreted
homodimeric protein and the TGF-β signaling pathway serves pivotal
roles in a variety of biological processes. It’s essential
biological and pathological activities are mediated by mothers
against decapentaplegic homolog (Smad) signaling pathways (24). Smad4 is a central-mediator that
serves complex and contradictory roles, and cooperates with other
transcription factors to regulate the TGF-β signaling pathway
during tumourigenesis (25).
Accumulating data has reported that Smad4 is involved in various
cellular responses and that dysregulation of Smad4 is closely
associated with a variety of human cancers, including prostate,
pancreatic, colorectal and thyroid cancer (26-29).
The aim of the present study was to investigate the
association between miR-183 and the development and progression of
OC. It was observed that miR-183 was markedly upregulated in the OC
clinical samples and cell lines. In addition, functional assays
revealed that downregulation of miR-183 could inhibit cell
proliferation and invasion, and induce apoptosis in OC. In
addition, it was demonstrated that Smad4 is a direct target of
miR-183 via a luciferase activity assay. Taken together, the
results of the present study suggested that miR-183 may serve an
efficient regulatory role and thus, may be a novel strategy and
prognostic marker for the diagnosis and prognosis of OC.
Materials and methods
Tissue samples and cell lines
Tissues were obtained from 30 female patients, who
were histopathologically and clinically diagnosed at Zhongnan
Hospital of Wuhan University (Hubei, China) between January 2016
and December 2017 and their average age was 57±12 years. The
present study was approved by the Ethics Committee of Zhongnan
Hospital of Wuhan University and every patient provided written
informed consent. The tissues were collected prior to chemotherapy
and radiotherapy, and all fresh specimens were stored at −80°C
until the reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) assay.
Human OC cell lines (SKOV-3 and OVCAR3) and normal
human ovarian surface epithelial (HOSE) cells were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were grown in RPMI-1640 containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
then maintained at 37°C in a humidified incubator with 5%
CO2.
Cell transfection
miR-183 mimics, miR-183 inhibitors, negative control
(NC) and the luciferase reporter plasmid were designed and
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. The
sequences were as follows: miR-183 mimics forward, 5′-UGA GGU AGU
AAG UUG UAU UGU U-3′ and reverse, 5′-CAA UAC AAC UUA CUA CCU CAU
U-3′; and miR-183 inhibitors, 5′-TAT GGC ACT GGT AGA ATT CAC T-3′;
NC forward, 5′-CAG UAC UUU UGU GUA GUA CAA-3′ and reverse, 5′-UUG
UAC UAC ACA AAA GUA CUG-3′. The SKOV3 and OVCAR3 cells were
transfected with miR-183 mimics, miR-183 inhibitors or NC using
Lipofectamine® 2000™ (Invitrogen; Thermo Fisher
Scientific, Inc.) at a final concentration of 50 nM according to
the manufacturer’s protocol. Subsequently, cells were cultured with
fresh medium containing 10% FBS for 48 h prior to further
experiments.
RT-qPCR
Total RNA was extracted from 5.0×103
cells and tissues using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and cDNA was synthesized using the
SYBR® PrimeScript™ RT-PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China) at 37°C for 30 min according to the
manufacturer’s instructions. RT-qPCR was performed using the SYBR
Premix ExTaq (Takara Bio, Inc., Otsu, Japan). U6 small RNA was used
as an endogenous control. The thermocycling conditions for qPCR
were as follows: 95°C for 5 min followed by 40 cycles of 95°C for
15 sec and 60°C for 60 sec. Fold changes were quantified using the
2−ΔΔCq method (30).
Primers were as follows: miR183 forward, 5′-CGC GGT ATG GCA CTG GTA
GA-3′ and reverse, 5′-AGT GCA GGG TCC GAG GTA TTC-3′; TGF-β
forward, 5′-GAAG TGG ATC CAC GAG CCC AAG-3′ and reverse, 5′-GCT GCA
CTT GCA GGA GCG CAC-3; Smad4 forward, 5′-GTC GAA AAC AAC GCT ATT
GAC T-3′ and reverse, 5′-CAG AGA ATT GTC GCC GTA CAT-3′; β-actin
forward, 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′ and reverse,
5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3′.
Western blot analysis
Total protein was extracted from cells using 3 ml of
lysis buffer comprised of well-mixed solution containing 7 mol/l
urea, 2 mol/l thiourea, 5 ml/l isocratic pH gradient buffer (pH
3-10), 65 mmol/l dithiothreitol, 40 g/l 3-(3-cholamidopropyl)
dimethylammonio]-1-propanesulfo-nate, 5 mg/l protease inhibitor and
10 ml/l trypsin inhibitor, and homogenized on ice. The protein
concentration was measured using the BCA method and total protein
(20 µg) was separated by 10% SDS-PAGE. Proteins were then
transferred to polyvinylidene difluoride membranes and blocked with
5% skim milk in TBST solution containing 5% bovine serum albumin
(Gibco; Thermo Fisher Scientific, Inc.) and 0.1% Tween-20, at room
temperature for 1 h. The membranes were probed with primary
antibodies at room temperature overnight. The specific primary
antibodies were as follows: Smad4 (cat. no. ab40759; 1:1,000
dilution; Abcam, Cambridge, MA, USA), TGF-β (cat. no. ab64715;
1:1,000 dilution; Abcam), p21 (cat. no. ab109520; 1:1,000 dilution;
Abcam), p27 (cat. no. ab32034; 1:1,000 dilution; Abcam), Cyclin D1
(cat. no. ab16663; 1:1,000 dilution; Abcam) and GAPDH (cat. no.
ab9485; 1:1,000 dilution; Abcam). Then the membranes were incubated
with the horseradish peroxidase (HRP)-conjugated anti-rabbit
secondary antibody (cat. no. ab6721; 1:2,000; Abcam) for 1 h at
room temperature. GAPDH was used as the loading control. Protein
bands were visualized with the Amersham Enhanced Chemiluminescence
system (Amersham; GE Healthcare, Chicago, IL, USA) and the results
were measured using ImageJ software 1.48 (National Institutes of
Health, Bethesda, MD, USA).
Measurement of cell viability by MTT
Cell proliferation was assessed using an MTT assay.
Briefly, cells were seeded into 96-well plates at
5.0×103 cells/well in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and incubated at 37°C
in 5% CO2. Cell proliferation was measured using an MTT
assay kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
dimethyl sulfoxide used to dissolve the purple formazan. The
optical density of cells was determined at 490 nm using a
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Migration and invasion assays
Cell migration was assessed using a 24-well
Transwell chamber with a non-coated membrane in the top chamber and
5×104 cells were plated in RPMI-1640 medium in the top
chamber of inserts. For the Transwell invasion assay,
5×104 cells were plated in the top chamber of inserts
that were coated with Matrigel (BD Biosciences, San Jose, CA, USA).
Subsequently, the lower chambers were filled with RPMI-1640 medium
supplemented with 10% FBS. Following incubation for 24 h, the
non-migrating and non-invading cells on the upper membrane surface
were removed using a cotton swab. The cells in the lower membrane
surface were washed with PBS three times, fixed with 4%
paraformaldehyde at room temperature for 30 min and stained with
0.5% crystal violet for 4 h at room temperature. The cells were
counted under a light microscope in five random fields
(magnification, ×200). Each experiment was performed in
triplicate.
Wound healing assay
A wound healing assay was used to evaluate the
migratory ability of the transfected cells. A total of
5×104 cells were seeded in 6-well plates until the cells
grew to 100% confluence. The scratch wound was created in the
surface of the plates using a pipette tip. The cells were washed
with PBS and replaced with DMEM. Cells were then observed at 0, 24
and 48 h under a microscope.
Colony formation assay
Following transfection for 48 h, SKOV3 and OVCAR3
cells were seeded in 6-well plate with complete growth medium and
incubated for 2 weeks at 37°C in a humidified incubator with 5%
CO2. Following this incubation period, the cells were
washed with PBS, fixed with 4% paraformaldehyde at room temperature
for 30 min and stained with 0.5% crystal violet for 4 h at room
temperature. Cell colonies were counted and photographed using a
light microscope. The experiment was repeated in triplicate.
Flow cytometry analysis
A total of 5×104 cells were seeded in
6-well plates, cultured for 48 h and then cells were collected.
Then the cells were fixed with precooled 70% ethanol for 30 min at
room temperature, and collected following centrifugation at 12,000
x g for 5 min at room temperature. Next, the cells were resuspended
in PBS containing 50 mg/ml propidium iodide and 50 mg/ml RNaseA
(cat. no. 40711ES10; Shanghai Yeasen Biotechnology, Co., Ltd.,
Shanghai, China) for 30 min. Then cells were incubated for 1 h at
37°C in the dark, and analyzed using flow cytometry (FACSCalibur;
BD Biosciences) and analyzed using FlowJo 10.06 software (FlowJo
LLC, Ashland, OR, USA). The experiment was repeated three
times.
Immunofluorescence analyses
A total of 1×105 cells were plated onto
coverslips in 6-well plates and transfected with miR-183 mimics or
miR-183 inhibitors. Following transfection for 48 h, the coverslips
were fixed with 4% formaldehyde for 24 h at room temperature and
stained with the primary antibodies overnight at 4°C after blocking
cells with 3% bovine serum albumin for 20 min at room temperature
(Gibco; Thermo Fisher Scientific, Inc.). The specific primary
antibodies were as follows: Smad4 (cat. no. ab40759; 1:1,000
dilution) and TGF-β (cat. no. ab64715; 1:1,000 dilution; both
Abcam). Then the coverslips were incubated with the HRP-conjugated
anti-rabbit secondary antibody (cat. no. ab205718; 1:2,000; Abcam)
for 1 h at room temperature in the dark. Coverslips were
counterstained with 4′,6-diamidino-2-phenylindole (Molecular
Probes; Thermo Fisher Scientific, Inc.) for 20 min at room
temperature for the visualization of nuclei. The results were
observed using a fluorescence microscope (Leica Microsystems GmbH,
Wetzlar, Germany) and analyzed using ImageJ software version 1.48
(National Institutes of Health).
Luciferase reporter assays
A search for putative targets of mi-183 was
performed with TargetScan Human 7.2 (www.targetscan.org/vert_72/) and miRanda software
(www.microrna.org/). Cells (1×105) were
seeded in 96-well plates and grown in RPMI-1640 containing 10% FBS
at 37°C in a humidified atmosphere containing 5% CO2.
Following 24 h, psiCheck-2 with the 3′-untranslated region (UTR) of
Smad4 was cotransfected with miR-183 mimics wild-type; (WT)], or
mutated miR-183 mutant; (MUT). mimics using Lipofectamine 3000™
(Invitrogen; Thermo Fisher Scientific, Inc.). Following
transfection for 48 h, luciferase activity was detected via a
luciferase assay using a Dual Luciferase Reporter Assay kit
(Promega Corporation, Madison, WI, USA). Normalized luciferase
activity was reported as luciferase activity/Renilla
luciferase activity. Three independent experiments were
performed.
Statistical analysis
All data in the study were assessed using SPSS 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). Comparisons
between groups for statistical significance were performed with
Student’s t-test and multiple group comparisons were conducted via
one-way analysis of variance with Tukey’s post hoc test. Data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-183 is upregulated in OC tissues and
cell lines
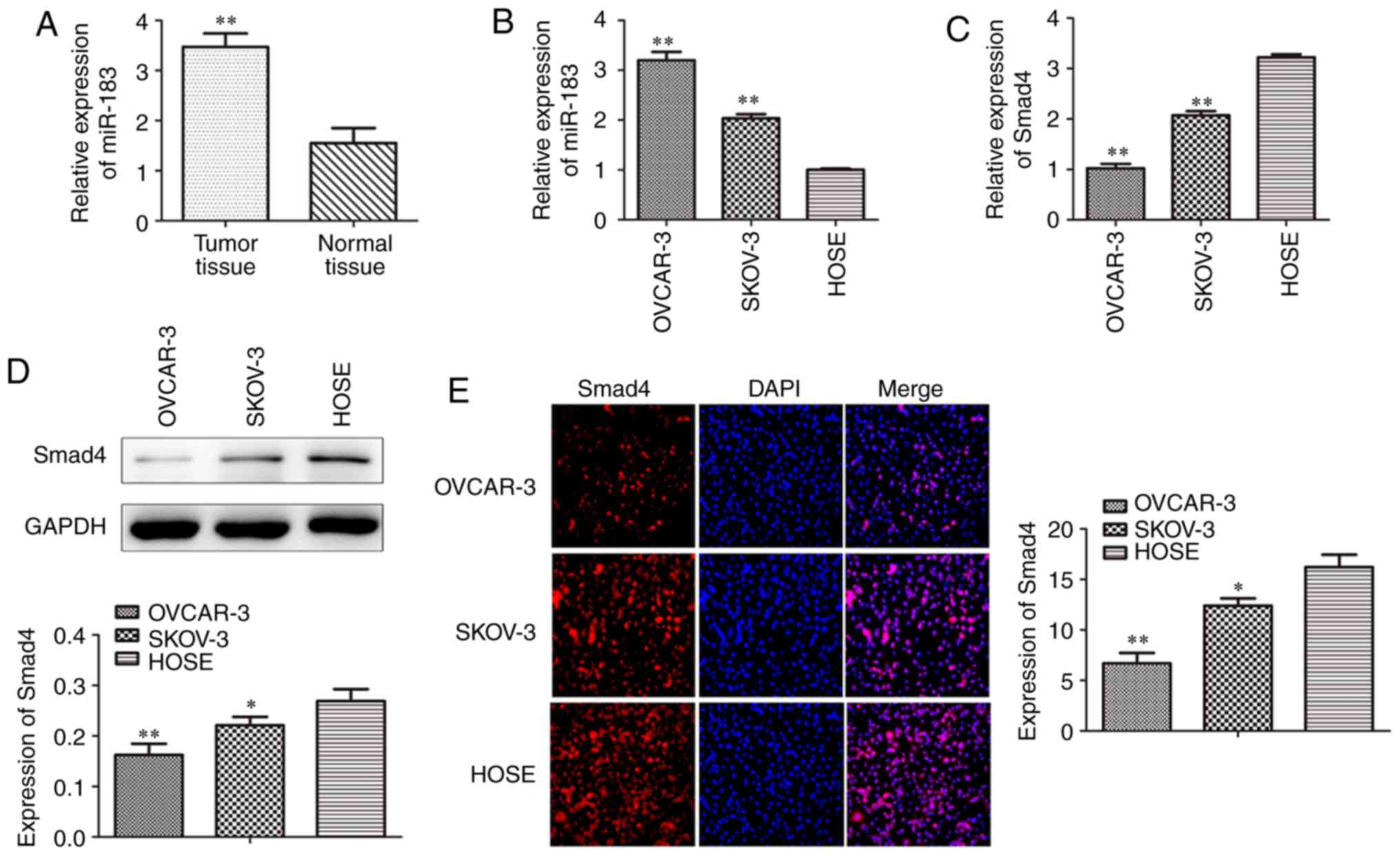
To investigate whether miR-183 is associated with
the progression of OC, the present study determined the expression
levels of miR-183 in OC tissues and cell lines by RT-qPCR. The
results revealed that the expression of miR-183 was increased in
the OC tissues when compared with the normal tissues (Fig. 1A). In addition, SKOV3 and OVCAR3
cells were also investigated and the results indicated that the
miR-183 expression levels were markedly higher in OC cell lines
than in the HOSE cell line (Fig.
1B). The present study also assessed the levels of Smad4 in
cell lines using RT-qPCR, western blotting and an
immunofluorescence assay. The data implied that Smad4 expression in
the OC cell lines was markedly lower when compared with HOSE cells
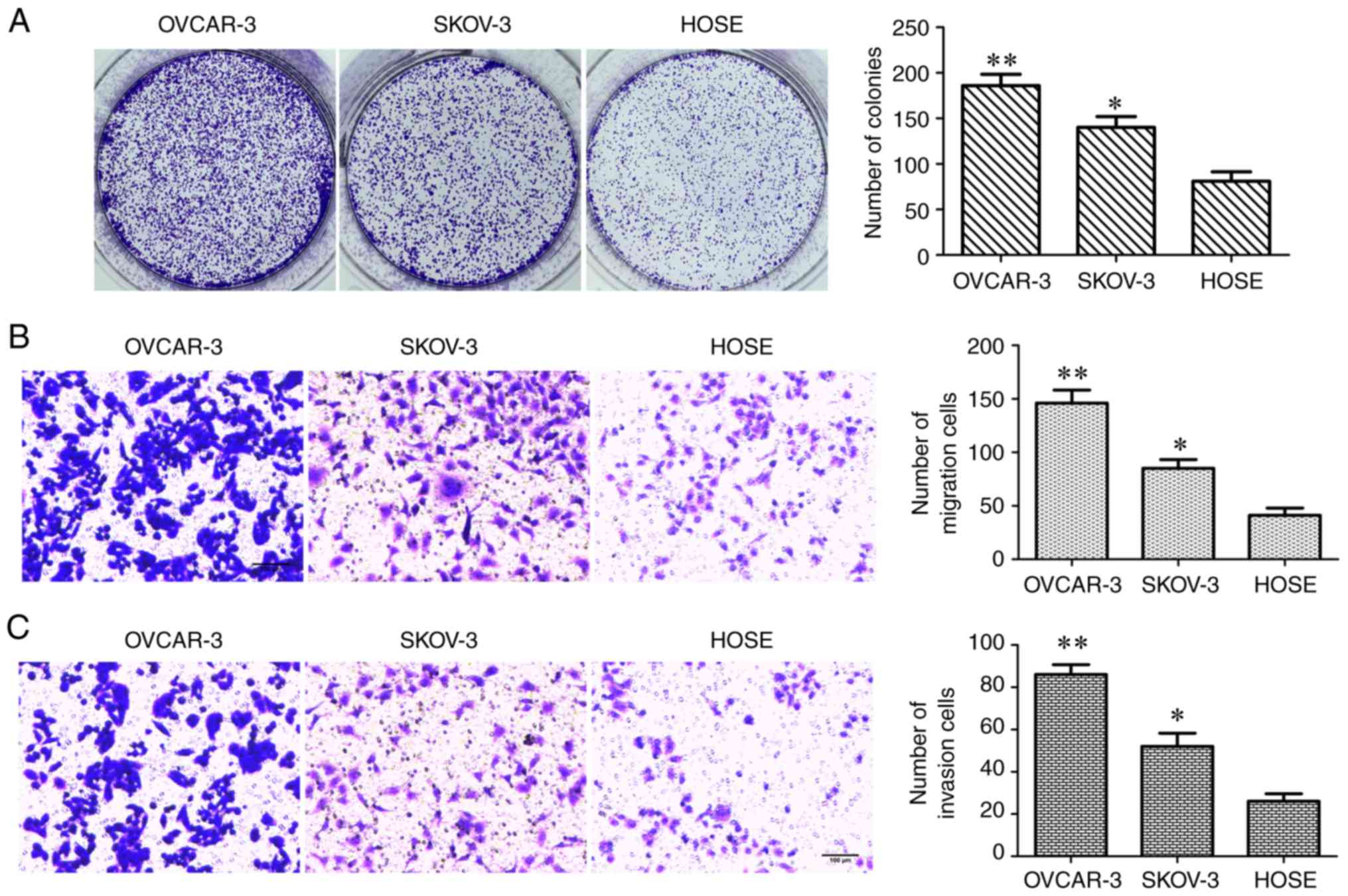
(Fig. 1C-E). The colony formation
and Transwell assays were conducted to assess cell proliferation,
migration and invasion abilities, the number of colonies formed,
and the number of migrating and invading cells in each group
(Fig. 2A-C). These results
indicated that all of these measures were significantly increased
in OC cell lines when compared with HOSE cells.
Effects of miR-183 on OC cell
proliferation
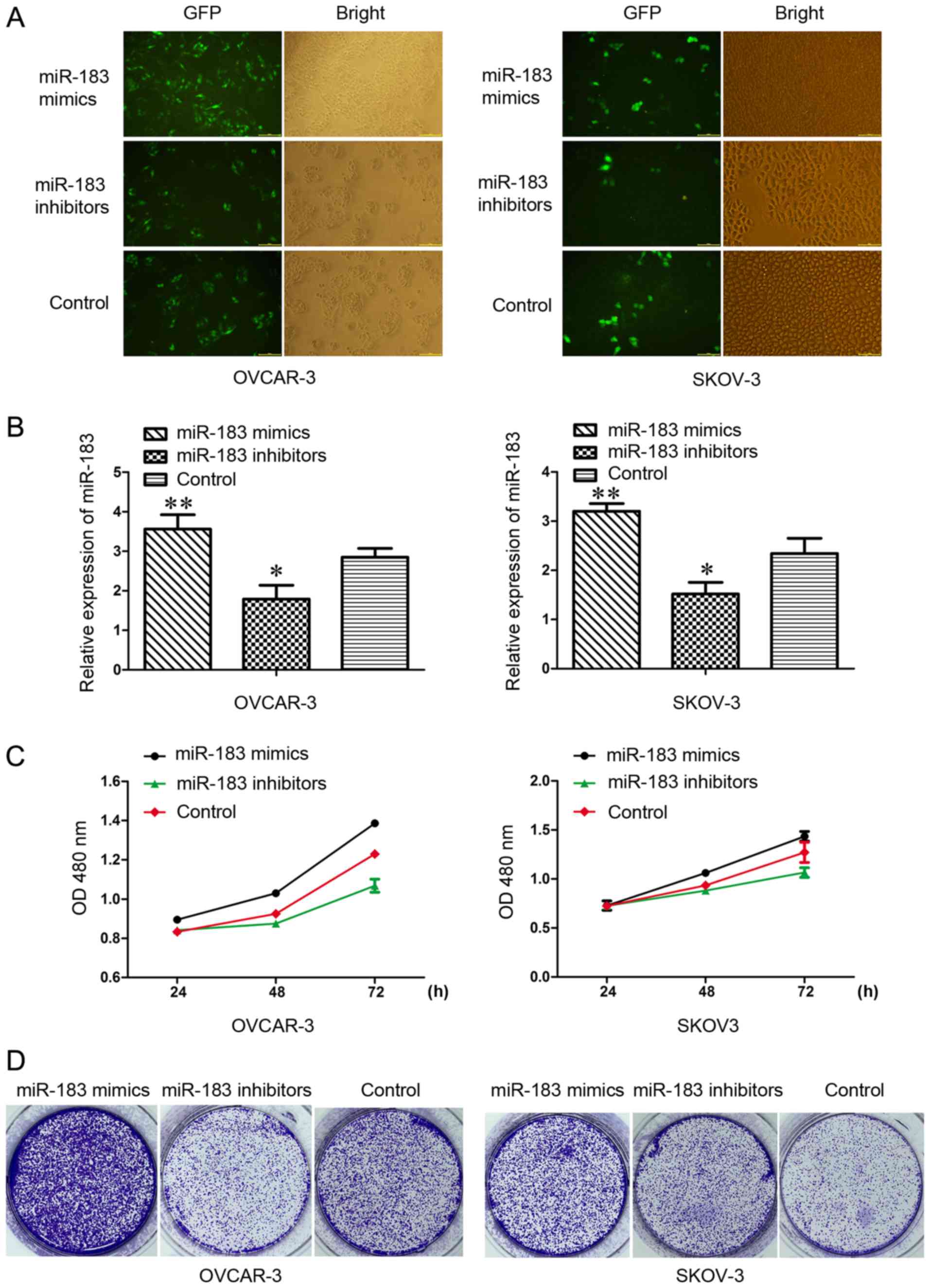
The present induced overexpression of miR-183 and
anti-miR-183 via transfection with lentivirus in SKOV3 and OVCAR3
cells to explore the biological functions of miR-183 in OC. The
success of transfection was validated by fluorescence microscopy
and RT-qPCR (Fig. 3A and B). The
MTT and colony formation assays were conducted to investigate the
effects of miR-183 on cell proliferation. The results suggested
that overexpression of miR-183 markedly increased the growth rate
of SKOV3 and OVCAR3 cells (Fig.
3C). Increased and decreased colony formation was observed in
the miR-183 mimics and miR-183 inhibitors groups, respectively,
when compared with the control group (Fig. 3D). These results indicated that
miR-183 may be involved in the regulation of OC cell growth.
miR-183 mediates the cell cycle and
apoptosis in OC cells
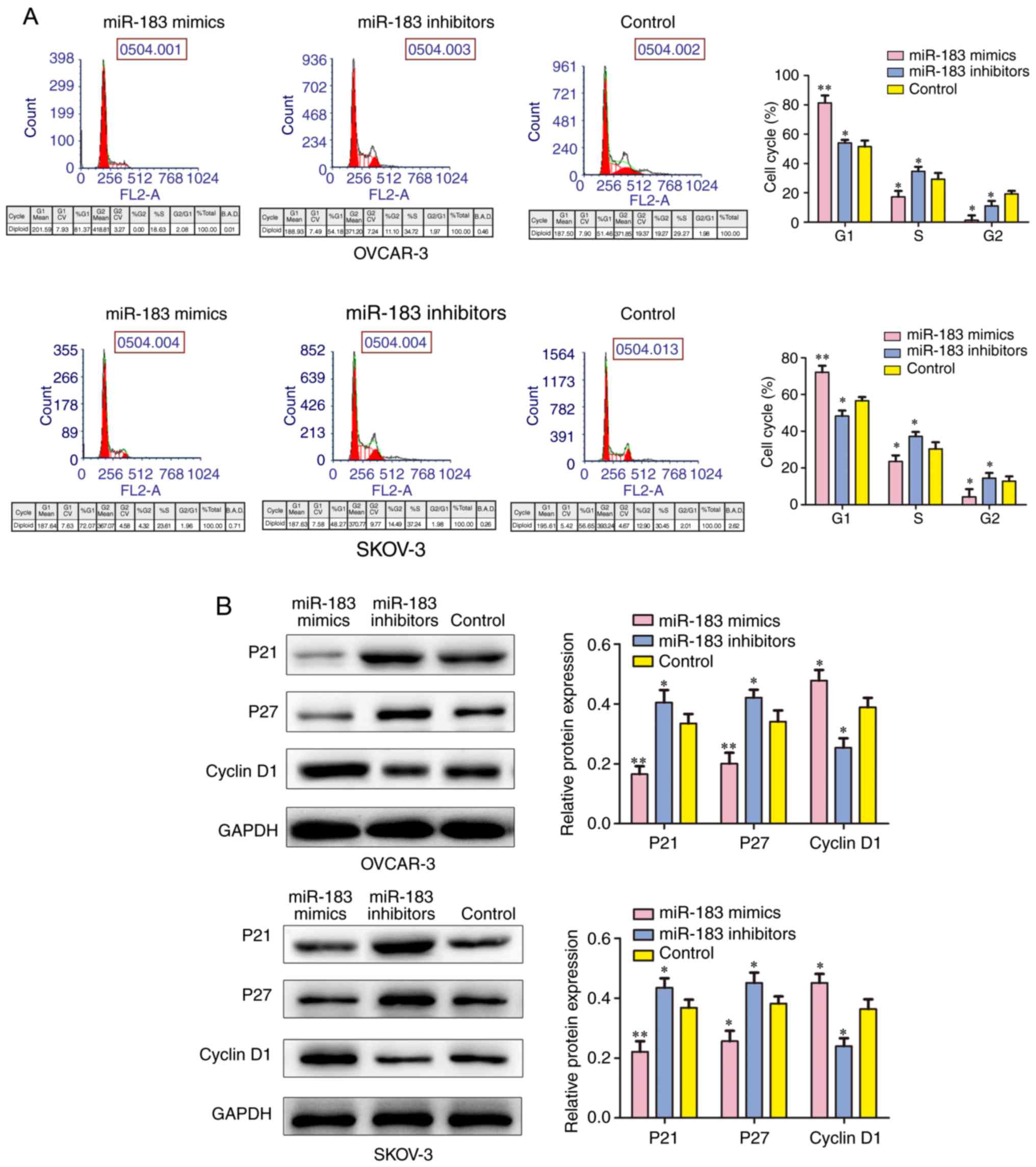
To determine whether miR-183 is able to influence
the cell cycle, the present study performed a flow cytometry assay
and observed that the downregulation of miR-183 could markedly
arrest a greater number of cells in the S phase when compared with
the control group, whereas the cells in the G1 phase were
decreased. Furthermore, the number of cells in the S phase were
reduced when transfected with miR-183 mimics when compared with the
control group (P<0.05; Fig.
4A) and the number of cells in the G1 phase was increased when
transfected with miR-183 mimics when compared with the control
group (P<0.01). The western blotting assay was conducted to
detect several cell cycle-associated factors. The expressions of
p21 and p27 protein were revealed to be reduced by miR-183 mimics
when compared with the control group. By contrast, the expression
of Cyclin D1 was reduced in the miR-183 inhibitors group when
compared with the control group (Fig.
4B). In order to further investigate the influence of miR-183
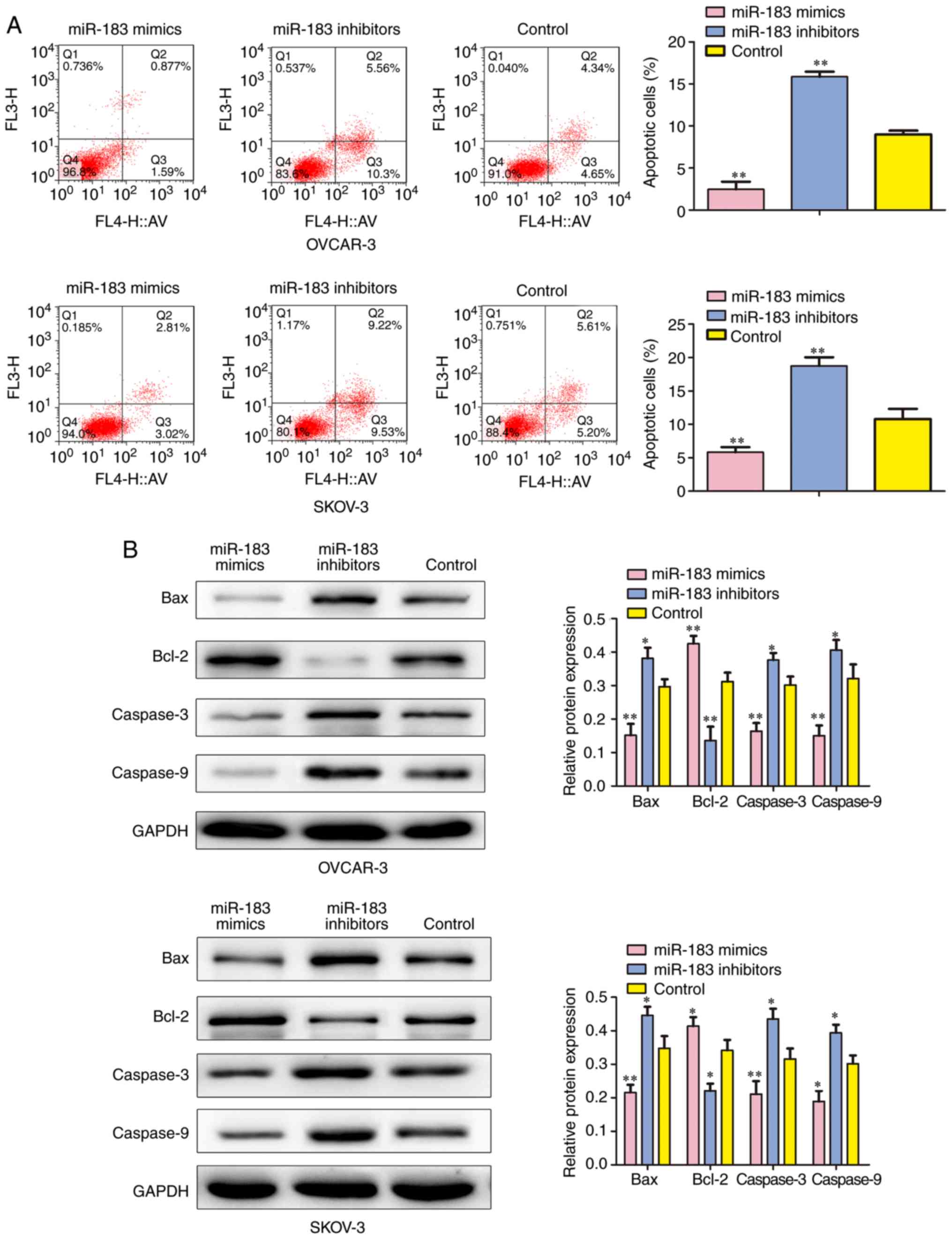
expression on cell apoptosis, the percentage of apoptotic cells was
measured by flow cytometry. The results indicated that the
percentage of apoptosis was significantly increased in the cells
transfected with miR-183 inhibitors. In addition, overexpression of
miR-183 markedly decreased the number of apoptotic cells when
compared with the control group (P<0.01; Fig. 5A). A western blot assay was
employed to detect the protein expression of apoptosis-associated
factors. The results demonstrated that downregulation of miR-183
markedly suppressed the expression of B-cell lymphoma 2 (Bcl-2),
and induced Bcl-2-associated X protein (Bax), Caspase-3 and
Caspase-9 levels (Fig. 5B).
Therefore, these results demonstrated that miR-183 could induce
cell cycle progression and suppress the apoptosis of OC cells.
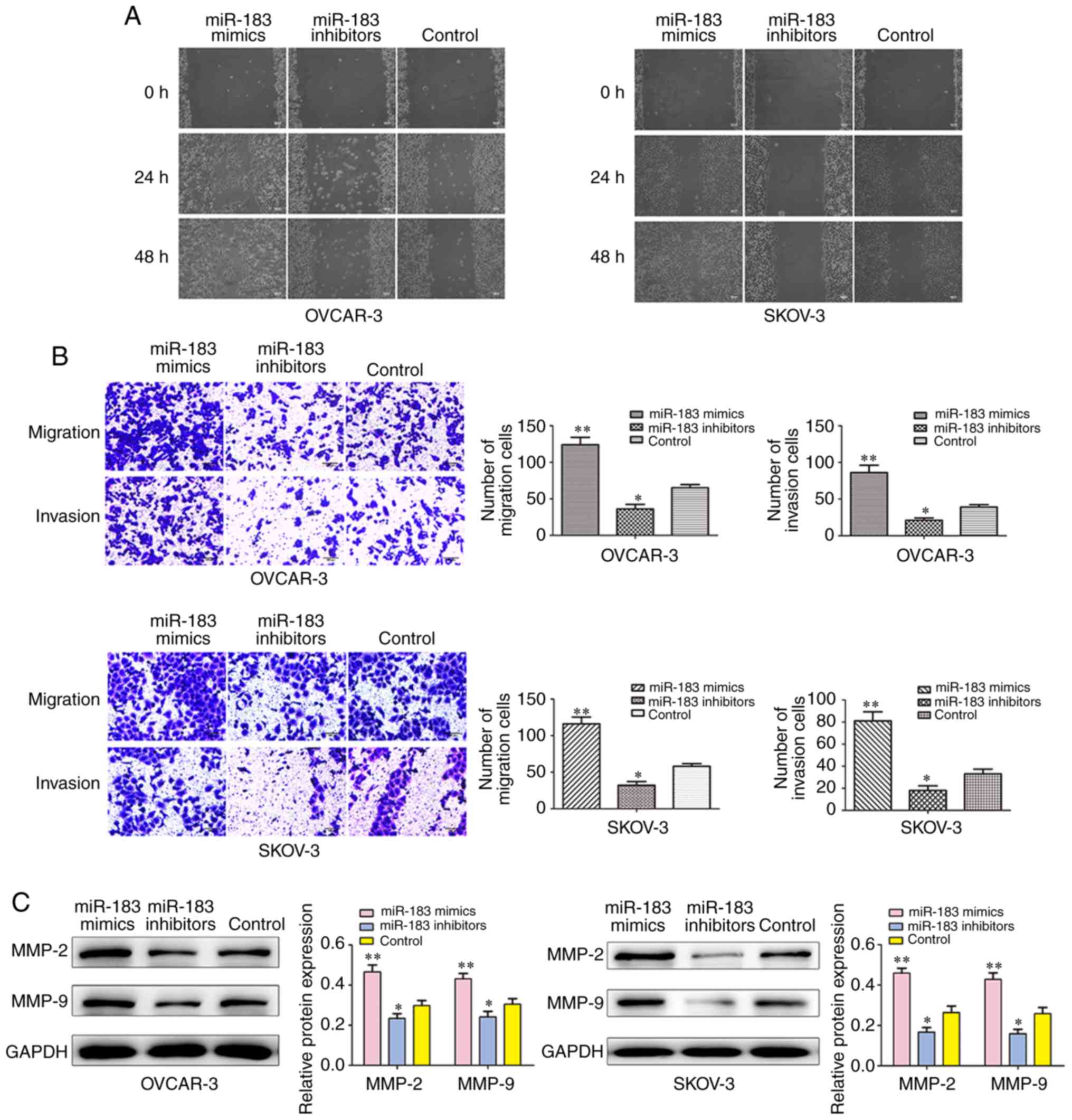
miR-183 promotes cell migration and
invasion
To explore the role of miR-183 in the migration and
invasion of OC cells, wound healing and Transwell invasion assays
were performed to investigate the migration and invasion abilities
of SKOV3 and OVCAR3 cells following transfection with miR-183
mimics and inhibitors. The wound healing assay revealed that the
cells in the control group migrated less so than the miR-183 mimics
group but more so than the miR-183 inhibitor group (Fig. 6A). As shown in Fig. 6B, the cells that were transfected
with the miR-183 mimics had promoted migratory and invasive
abilities when compared with the cells in the control group. The
results of the Transwell assay indicated that overexpression of
miR-183 lead to significant enhancements in cell migration. In
addition, western blot analysis was performed to determine whether
the protein levels of invasion-associated markers were also
affected by miR-183. Overexpression of miR-183 significantly
increased the expressions of matrix metalloproteinase (MMP)-2 and
MMP-9 (Fig. 6C). These results
indicated that the downregulated expression of miR-183 inhibited
the cell migration and invasion of OC cells.
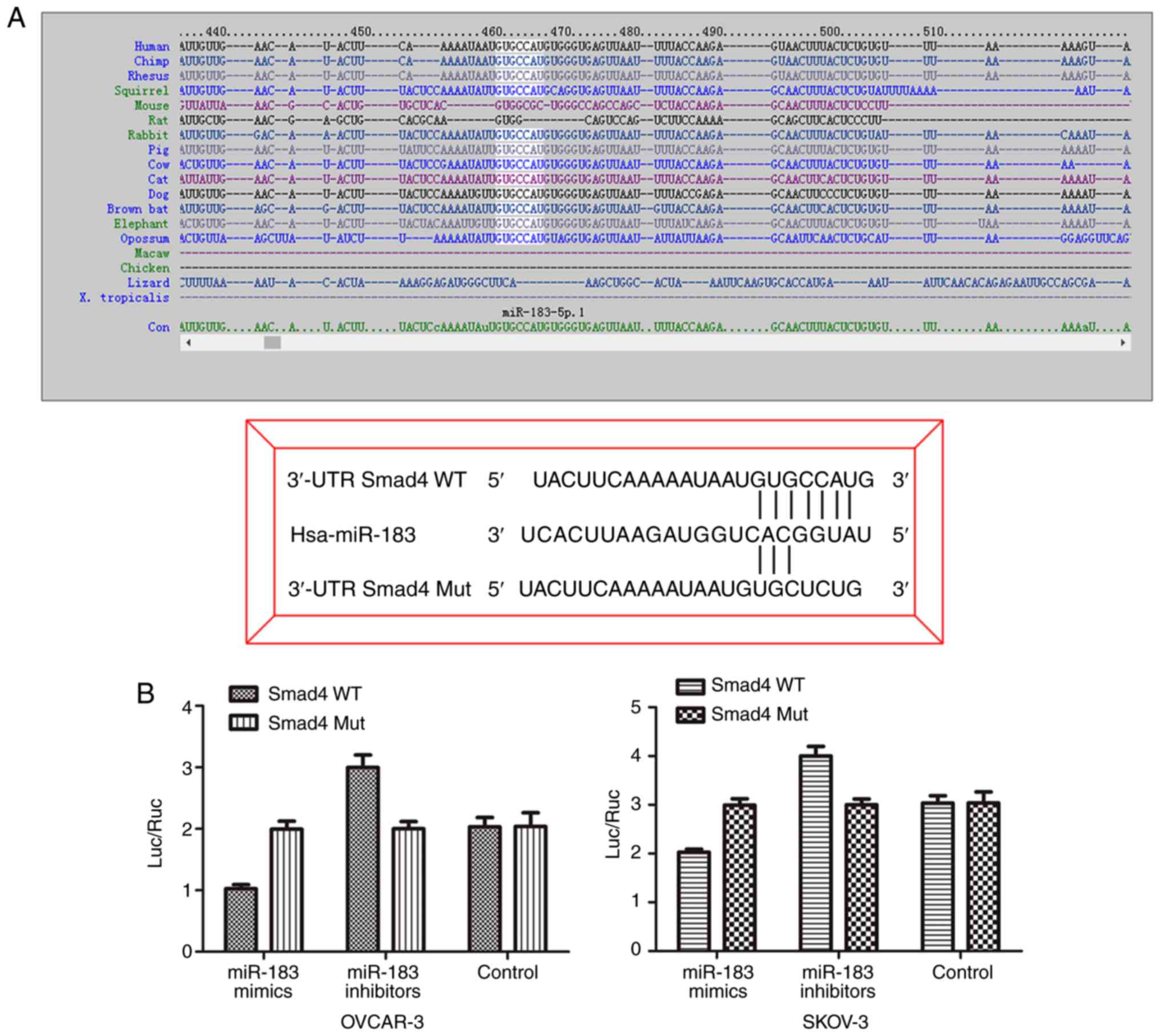
miR-183 directly regulates biological
function via the TGF-β/Smad4 signaling pathway in OC cells
To determine the underlying mechanism of miR-183 in
OC, the present searched for the potential targets of miR-183 by
using prediction programs including TargetScan and miRanda. As
shown in Fig. 7A, Smad4 3′-UTR
was predicted to be a potential target of miR-183. To determine
whether Smad4 is a direct target of miR-183, luciferase reporter
constructs were combined with the 3′-UTR of Smad4 mRNA containing
the miR-183 binding sites and the mutant. The results of the
luciferase reporter assay indicated that the luciferase activity in
the Smad4 3′-UTR WT group that was transfected with miR-183 mimics,
was markedly decreased when compared with the control group
(Fig. 7B). However, this effect
was abolished among the three groups cotransfected with the
Smad4-Mut vector. These results were further supported via
assessment of the protein levels of SMAD4 in the OC cell lines
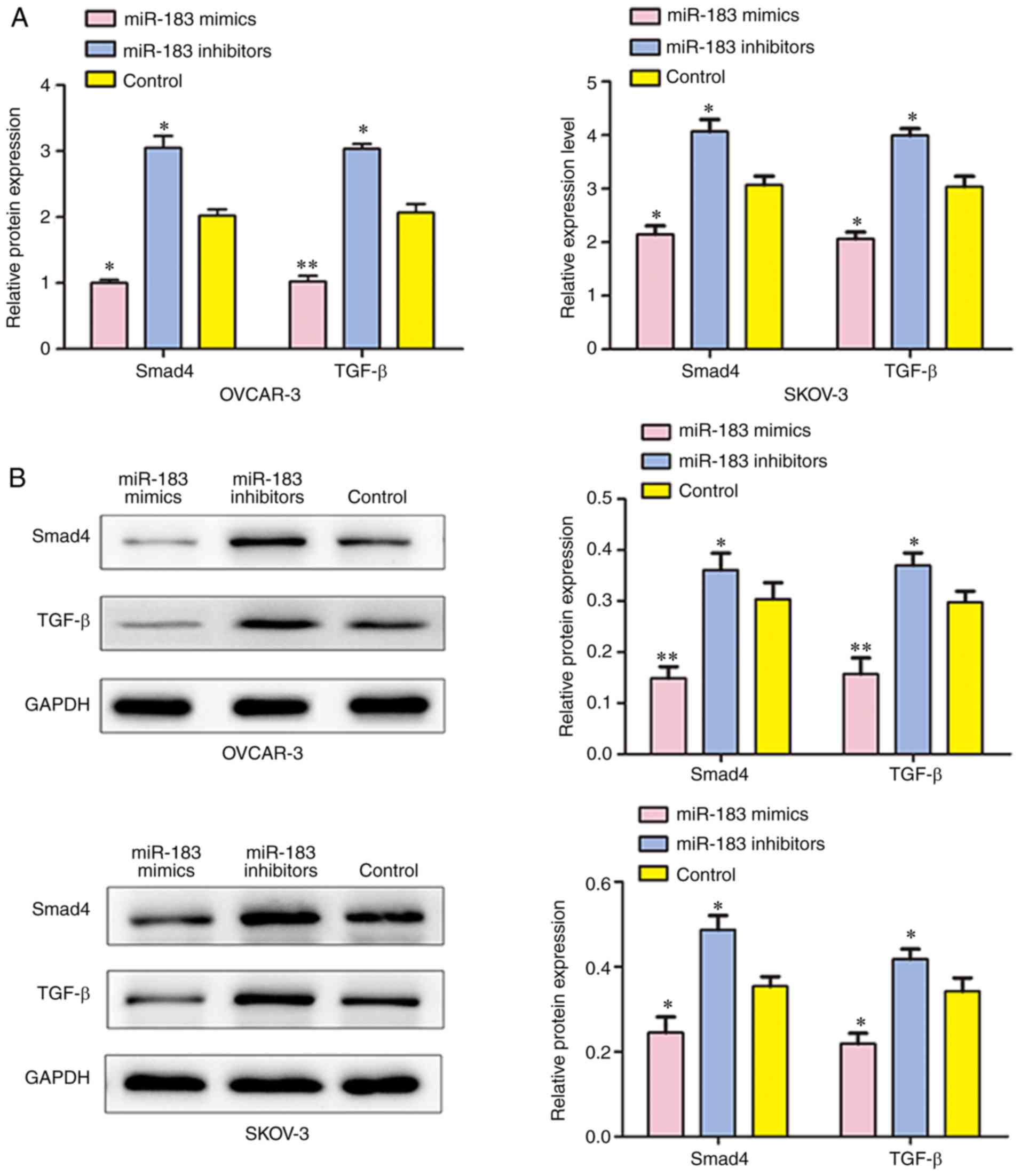
treated with miR-183 mimics and miR-183 inhibitors (Fig. 8B).
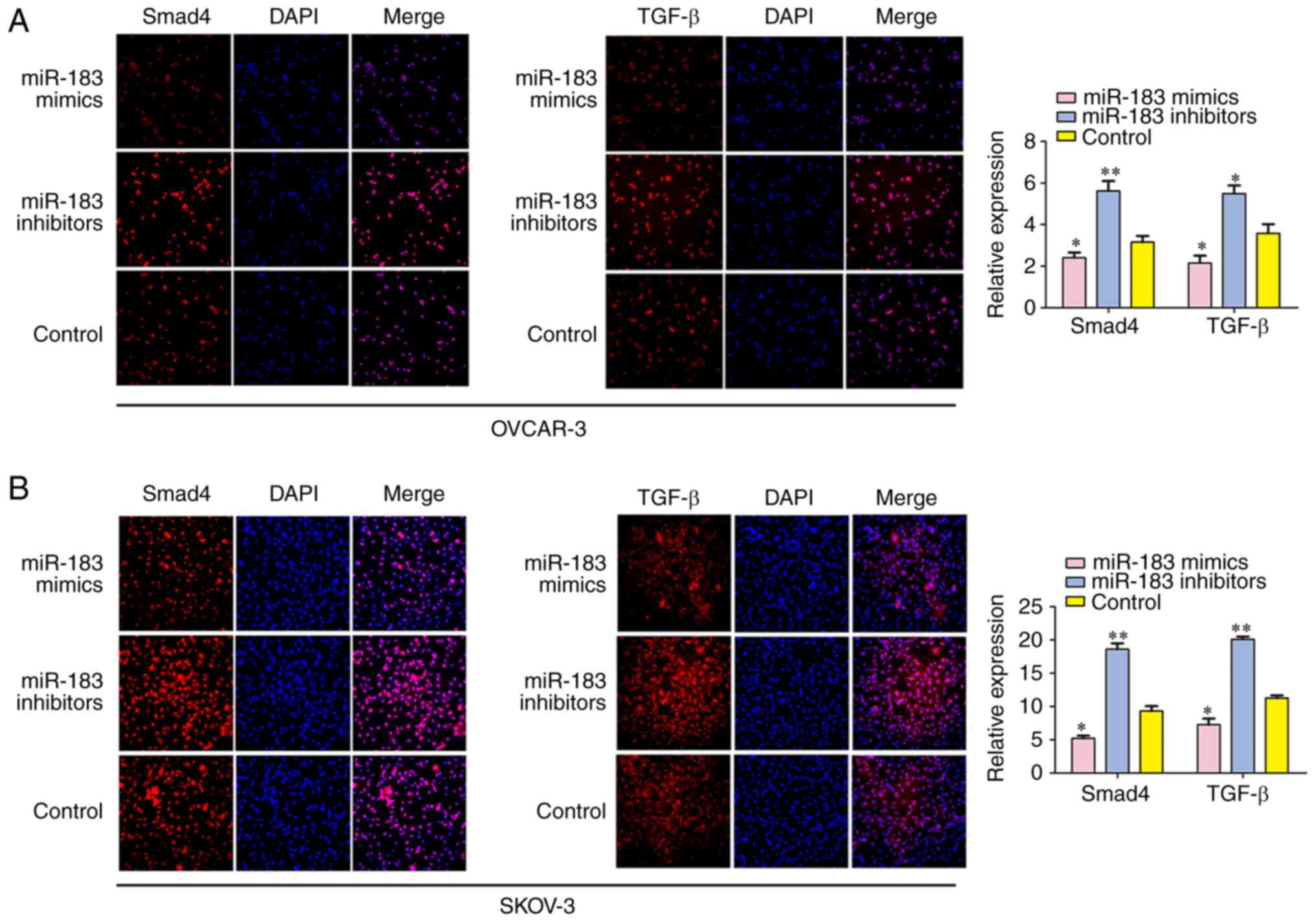
Additionally, the expressions of TGF-β and SMAD4
were detected by RT-qPCR, western blotting and immuno-fluorescence
analyses following transfection. The results demonstrated that the
mRNA and protein expressions of TGF-β and SMAD4 were significantly
suppressed in cells that were transfected with miR-183 mimics when
compared with the control group, and cells transfected with miR-183
inhibitors had significantly upregulated TGF-β and SMAD4
expressions in SKOV3 and OVCAR3 cells (Figs. 8 and 9). All of these results demonstrated
that SMAD4 may be a direct target of miR-183 and suggest that
miR-183 may participate in the regulation of cell biological
function, at least in part through the TGF-β/Smad4 signaling
pathway in OC.
Discussion
An increasing body of evidence has suggested that
miRNAs are important regulators in different cellular processes
(31) and are frequently
dysregulated in various types of cancer, including OC (32). miR-183 was overexpressed and acted
as an oncogene in several types of cancers, including gastric,
bladder and colon cancers (33-35). Ren et al (36) identified that miR-183 was markedly
overexpressed in OSCC. In addition, Li et al (33) reported that miR-183 was markedly
upregulated in gastric cancer. This may suggest that miR-183 is
involved in the pathogenesis of OC and that it may be a biomarker
for the diagnosis and treatment of OC. Therefore, the present study
was conducted to further investigate the functional role of miR-183
in OC. The results revealed that miR-183 was upregulated in OC
tissues and cell lines. Furthermore, SKOV3 and OVCAR3 were used as
an in vitro model to examine the functional impact of
miR-183 inhibition or overexpression on cell behavior.
The results revealed that downregulation of miR-183
suppressed proliferation, migration and invasion, and promoted cell
apoptosis in SKOV3 and OVCAR3 cells, while overexpression of
miR-183 enhanced cell growth, migration and invasion. Cell cycle
analysis demonstrated that downreg-ulation of miR-183 could
markedly arrest more cells in the S phase when compared with the
control group. Furthermore, when compared with the control group,
the number of cells in the S phase was reduced when cells were
transfected with miR-183 mimics. In addition, a luciferase reporter
assay was performed and the results revealed that SMAD4 was a novel
target gene of miR-183. These results were further supported by the
protein expression analyses of SMAD4 in the OC cell lines treated
with miR-183 mimics. SMAD4 was demonstrated to be the key mediator
of the TGF-β signaling pathway (37,38). In addition, the miR-183 level was
inversely correlated with the expression of Smad4 (39). Smad4 was also confirmed to be the
pivotal mediator of the TGF-β signaling pathway and recent evidence
has suggested that SMAD4 has dual roles with tumor-suppressive and
tumor-promoting effects in different types of cancers (40-46). Accordingly, the present results
revealed that miR-183 overexpression significantly downregulated
Smad4 and activation of the TGF-β/Smad4 signaling pathway.
In conclusion, to the best of our knowledge, the
present study for the first time, demonstrated that miR-183 was
increased in OC tissues and cells. Notably, the results revealed
that miR-183 serves an oncogene-like function by regulating the
TGF-β/Smad4 signaling pathway, thereby promoting cell
proliferation, migration and invasion in OC. Taken together, the
present results provide insight into the potential contribution of
miR-183 in the progression of OC, and downregulation of miR-183 and
activation of Smad4 could be a promising approach for the diagnosis
and treatment of OC.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors’ contributions
JZ and DJ conceived and designed the study. CZ and
BZ performed the experiments. JZ wrote the manuscript. All authors
have read and approved the manuscript and agree to be accountable
for all aspects of the research in ensuring that the accuracy and
integrity of any part of the study are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zhongnan Hospital of Wuhan University and every
patient provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vargas AN: Natural history of ovarian
cancer. Ecancermedicalscience. 8:4652014.PubMed/NCBI
|
|
3
|
Tomao F, Papa A, Rossi L, Strudel M, Vici
P, Lo Russo G and Tomao S: Emerging role of cancer stem cells in
the biology and treatment of ovarian cancer: Basic knowledge and
therapeutic possibilities for an innovative approach. J Exp Clin
Cancer Res. 32:482013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhattacharya R, Nicoloso M, Arvizo R, Wang
E, Cortez A, Rossi S, Calin GA and Mukherjee P: MiR-15a and MiR-16
control Bmi-1 expression in ovarian cancer. Cancer Res.
69:9090–9095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Edwards BK, Brown ML, Wingo PA, Howe HL,
Ward E, Ries LA, Schrag D, Jamison PM, Jemal A, Wu XC, et al:
Annual Report to the nation on the status of cancer, 1975–2002,
featuring population-based trends in cancer treatment. J Natl
Cancer Inst. 97:1407–1427. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu HY, Wang Y, Zhang H, et al: The long
non-coding RNA UCA1, as a prognostic biomarker for high grade
serous ovarian carcinoma. Eur J Gynaecological Oncol. 38:883–889.
2017.
|
|
7
|
Chekerov R, Braicu I, Castillo-Tong DC,
Richter R, Cadron I, Mahner S, Woelber L, Marth C, Van Gorp T,
Speiser P, et al: Outcome and clinical management of 275 patients
with advanced ovarian cancer international federation of obstetrics
and Gynecology II to IV inside the european ovarian cancer
translational research consortium-OVCAD. Int J Gynecol Cancer.
23:268–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peiretti M, Bristow RE, Zapardiel I,
Gerardi M, Zanagnolo V, Biffi R, Landoni F, Bocciolone L, Aletti GD
and Maggioni A: Rectosigmoid resection at the time of primary
cytoreduction for advanced ovarian cancer. A multi-center analysis
of surgical and oncological outcomes. Gyneco l. Oncol. 126:220–223.
2012.
|
|
9
|
Chen CY, Chang HP, Ng KK, Wang CC, Lai CH
and Chao A: Long-term disease-free survival in three ovarian cancer
patients with a single relapse. Eur J Gynaecol Oncol.
33:3212012.PubMed/NCBI
|
|
10
|
Yuan B, Liang Y, Wang D and Luo F: MiR-940
inhibits hepatocellular carcinoma growth and correlates with
prognosis of hepatocellular carcinoma patients. Cancer Sci.
106:819–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou CK, Chen RF, Chou FF, Chang HW, Chen
YJ, Lee YF, Yang KD, Cheng JT, Huang CC and Liu RT: miR-146b is
highly expressed in adult papillary thyroid carcinomas with high
risk features including extrathyroidal invasion and the BRAF(V600E)
mutation. Thyroid. 20:489–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuehbacher A, Urbich C, Zeiher AM and
Dimmeler S: Role of Dicer and Drosha for endothelial microRNA
expression and angiogenesis. Circ Res. 101:59–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Würdinger T, Tannous BA, Saydam O, Skog J,
Grau S, Soutschek J, Weissleder R, Breakefield XO and Krichevsky
AM: miR-296 regulates growth factor receptor overexpression in
angiogenic endothelial cells. Cancer Cell. 14:382–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun
S, Hong L, Liu J and Fan D: miR-15b and miR-16 modulate multidrug
resistance by targeting BCL2 in human gastric cancer cells. Int J
Cancer. 123:372–379. 2010. View Article : Google Scholar
|
|
16
|
Mitamura T, Watari H, Wang L, Kanno H,
Hassan MK, Miyazaki M, Katoh Y, Kimura T, Tanino M, Nishihara H, et
al: Downregulation of miRNA-31 induces taxane resistance in ovarian
cancer cells through increase of receptor tyrosine kinase MET.
Oncogenesis. 2:e402013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: Discovery, function and future
perspectives. Cell Death Differentiation. 17:2152010. View Article : Google Scholar
|
|
18
|
Chung YW, Bae HS, Song JY, Lee JK, Lee NW,
Kim T and Lee KW: Detection of microRNA as novel biomarkers of
epithelial ovarian cancer from the serum of ovarian cancer
patients. Int J Gynecol Cancer. 23:673–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shapira I, Oswald M, Lovecchio J, Khalili
H, Menzin A, Whyte J, Dos Santos L, Liang S, Bhuiya T, Keogh M, et
al: Circulating biomarkers for detection of ovarian cancer and
predicting cancer outcomes. Br J Cancer. 110:976–983. 2014.
View Article : Google Scholar :
|
|
20
|
Ouyang M, Li Y, Ye S, Ma J, Lu L, Lv W,
Chang G, Li X, Li Q, Wang S and Wang W: MicroRNA profiling implies
new markers of chemoresistance of triple-negative breast cancer.
PLoS One. 9:e962282014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mihelich BL, Khramtsova EA, Arva N,
Vaishnav A, Johnson DN, Giangreco AA, Martens-Uzunova E, Bagasra O,
Kajdacsy-Balla A and Nonn L: miR-183-96-182 cluster is
overexpressed in prostate tissue and regulates zinc homeostasis in
prostate cells. J Biol Chem. 286:44503–44511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lumayag S, Haldin CE, Corbett NJ, Wahlin
KJ, Cowan C, Turturro S, Larsen PE, Kovacs B, Witmer PD, Valle D,
et al: Inactivation of the microRNA-183/96/182 cluster results in
syndromic retinal degeneration. Proc Natl Acad Sci USA.
110:E507–E516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kundu ST, Byers LA, Peng DH, Roybal JD,
Diao L, Wang J, Tong P, Creighton CJ and Gibbons DL: The miR-200
family and the miR-183~96~182 cluster target Foxf2 to inhibit
invasion and metastasis in lung cancers. Oncogene. 35:173–186.
2016. View Article : Google Scholar
|
|
24
|
Lan HY: Diverse Roles of TGF-β/Smads in
Renal Fibrosis and Inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar :
|
|
25
|
Massagué J: TGFbeta in Cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biankin AV, Morey AL, Lee CS, Kench JG,
Biankin SA, Hook HC, Head DR, Hugh TB, Sutherland RL and Henshall
SM: DPC4/Smad4 expression and outcome in pancreatic ductal
adenocarcinoma. J Clin Oncol. 20:4531–4542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang
J, Perry SR, Labrot ES, Wu X, Lis R, et al: SMAD4-dependent barrier
constrains prostate cancer growth and metastatic progression.
Nature. 470:269–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alazzouzi H, Alhopuro P, Salovaara R,
Sammalkorpi H, Järvinen H, Mecklin JP, Hemminki A, Schwartz S Jr,
Aaltonen LA and Arango D: SMAD4 as a prognostic marker in
colorectal cancer. Clin Cancer Res. 11:2606–2611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geraldo MV, Yamashita AS and Kimura ET:
MicroRNA miR-146b-5p regulates signal transduction of TGF-β by
repressing SMAD4 in thyroid cancer. Oncogene. 31:1910–1922. 2012.
View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using realtime quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:4024082001. View Article : Google Scholar
|
|
31
|
Tsai MM, Wang CS, Tsai CY, Huang HW, Chi
HC, Lin YH, Lu PH and Lin KH: Potential diagnostic, prognostic and
therapeutic targets of MicroRNAs in human gastric cancer. Int J Mol
Sci. 17:pii: E9452016. View Article : Google Scholar
|
|
32
|
Kasinski AL and Slack FJ: MicroRNAs en
route to the clinic: Progress in validating and targeting microRNAs
for cancer therapy. Nat Rev Cancer. 11:849–864. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li C, Deng L, Zhi Q, Qian A, Sang H, Li X
and Xia J: MicroRNA-183 functions as an oncogene by regulating
PDCD4 in gastric cancer. Anticancer Agents Med Chem. 16:447–455.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gu W, Gao T, Shen J, Sun Y, Zheng X, Wang
J, Ma J, Hu XY, Li J and Hu MJ: MicroRNA-183 inhibits apoptosis and
promotes proliferation and invasion of gastric cancer cells by
targeting PDCD4. Int J Clin Exp Med. 7:2519–2529. 2014.PubMed/NCBI
|
|
35
|
Bi DP, Yin CH, Zhang XY, Yang NN and Xu
JY: MiR-183 functions as an oncogene by targeting ABCA1 in colon
cancer. Oncol Rep. 35:2873–2879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren LH, Chen WX, Li S, He XY, Zhang ZM, Li
M, Cao RS, Hao B, Zhang HJ, Qiu HQ and Shi RH: MicroRNA-183
promotes proliferation and invasion in oesophageal squamous cell
carcinoma by targeting programmed cell death 4. Br J Cancer.
111:2003–2013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Torbenson M, Marinopoulos S, Dang DT,
Choti M, Ashfaq R, Maitra A, Boitnott J and Wilentz RE: Smad4
overexpression in hepatocellular carcinoma is strongly associated
with transforming growth factor beta II receptor immunolabeling.
Hum Pathol. 33:871–876. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee DK, Park SH, Yi Y, Choi SG, Lee C,
Parks WT, Cho H, de Caestecker MP, Shaul Y, Roberts AB and Kim SJ:
The hepatitis B virus encoded oncoprotein pX amplifies TGF-beta
family signaling through direct interaction with Smad4: Potential
mechanism of hepatitis B virus-induced liver fibrosis. Genes Dev.
15:455–466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao W, Yang H, Li J, Chen Y, Cao J, Zhong
T, Wang L, Guo J, Li L and Zhang H: MiR-183 promotes preadipocyte
differentiation by suppressing Smad4 in goats. Gene. 666:158–164.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ang CW, Nedjadi T, Sheikh AA, Tweedle EM,
Tonack S, Honap S, Jenkins RE, Park BK, Schwarte-Waldhoff I,
Khattak I, et al: Smad4 loss is associated with fewer
S100A8-positive mono-cytes in colorectal tumors and attenuated
response to S100A8 in colorectal and pancreati. cancer cells
Carcinogenesis. 31:1541–1551. 2010. View Article : Google Scholar
|
|
41
|
Argani P, Shaukat A, Kaushal M, Wilentz
RE, Su GH, Sohn TA, Yeo CJ, Cameron JL, Kern SE and Hruban RH:
Differing rates of loss of DPC4 expression and of p53
overexpression among carcinomas of the proximal and distal bile
ducts. Cancer. 91:1332–1341. 2015. View Article : Google Scholar
|
|
42
|
Torbenson M, Marinopoulos S, Dang DT,
Choti M, Ashfaq R, Maitra A, Boitnott J and Wilentz RE: Smad4
overexpression in hepatocellular carcinoma is strongly associated
with transforming growth factor beta II receptor immunolabeling.
Human Pathol. 33:871–876. 2002. View Article : Google Scholar
|
|
43
|
Ouyang L, Liu P, Yang S, Ye S, Xu W and
Liu X: A three-plasma miRNA signature serves as novel biomarkers
for osteosarcoma. Med Oncol. 30:3402013. View Article : Google Scholar
|
|
44
|
Novello C, Pazzaglia L, Cingolani C, Conti
A, Quattrini I, Manara MC, Tognon M, Picci P and Benassi MS: MiRNA
expression profile in human osteosarcoma: Role of miR-1 and
miR-133b in proliferation and cell cycle control. Int J Oncol.
42:667–675. 2013. View Article : Google Scholar
|
|
45
|
Maire G, Martin JW, Yoshimoto M,
Chilton-MacNeill S, Zielenska M and Squire JA: Analysis of
miRNA-gene expression-genomic profiles reveals complex mechanisms
of microRNA deregulation in osteosarcoma. Cancer Genet.
204:138–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xue J, Lin X, Chiu WT, Chen YH, Yu G, Liu
M, Feng XH, Sawaya R, Medema RH, Hung MC and Huang S: Sustained
activation of SMAD3/SMAD4 by FOXM1 promotes TGF-β-dependent cancer
metastasis. J Clin Invest. 124:564–579. 2014. View Article : Google Scholar : PubMed/NCBI
|