Introduction
A long-lasting and low-grade inflammatory response
may serve a critical role in the pathogenesis of atherosclerosis, a
multi-pathogenic process that occurs within arterial inner and
middle walls (1,2). In addition to macrophages, vascular
smooth muscle cells (VSMCs) contribute to the progression of
atherosclerosis by upregulating inflammatory factors and
pathological proliferation (3).
Lipopolysaccharides (LPS) are primarily located on the outer
membrane of Gram-negative enteric bacteria and can increase the
expression of various types of inflammation-associated cytokines,
including monocyte chemoattractant protein (MCP)-1, interleukin
(IL)-6 and tumor necrosis factor (TNF)-α in VSMCs (4,5).
These proinflammatory factors have all been proven to promote the
progression of atherosclerosis and increase the number of
vulnerable atherosclerotic plaques in several clinical trials
(6). Therefore, controlling
excessive inflammatory responses, especially inhibiting the
proinflammatory cytokines expressed by VSMCs, may be an effective
way to suppress the progression of atherosclerosis and enhance
atherosclerotic plaque stability.
The Toll-like receptor (TLR) family are pattern
recognition receptors that recognize pathogen-associated molecular
patterns. A key member of this family is TLR4, which may be
associated with the innate and adaptive immunity caused by various
stimulants, such as LPS, low-density lipoprotein and heat shock
protein (7). Initially, TLR4 was
revealed to activate the host defense against invasive infection,
but more recent evidence has indicated that activation of TLR4
contributes to the progression of atherosclerosis by inducing
excessive inflammation (8). TLR4
overexpression has been observed in atherosclerotic plaques in
human and animal models, and was accompanied with an increase in
various pro-inflammatory factors, including MCP-1, IL-1β and IL-6
(9,10). By contrast, suppression of TRL4
activation inhibited the progression of atherosclerotic plaques in
TLR4 knockout models (11).
TGF-β-activated kinase 1 (TAK1) belongs to the mitogen-activated
protein kinase (MAPK) family and was initially observed to activate
the phosphorylation of MAPK induced by morphogenetic protein and
transforming growth factor (TGF) (12). As a downstream molecule in the
TLR4 signaling pathway, TAK1 can induce the phosphorylation of
inhibitor of nuclear factor (NF)-κB (IκB) kinase (IKK) and then
cause NF-κB activation, which may be a key mediator of various
inflammatory responses. A previous study has suggested that
inhibition of TAK1 activation suppresses LPS-induced inflammation
(13).
Tanshinone IIA (Tan IIA) is primarily extracted from
the root of Salvia miltiorrhiza Bunge, known as ‘Danshen’ in
Chinese traditional medicine. For centuries Danshen has been used
to treat cardiovascular diseases, including coronary heart disease
(14). Tan IIA is an important
constituent of Tans which are abietane-type norditerpenoid quinone
natural products. Tan IIA has been shown to have various
pharmacological effects, including anti-proliferation (15), anti-inflammation (16), anti-oxidation (17), and anti-tumor influences (18). Fan et al (19) described a Tan IIA
anti-inflammatory effect on LPS-induced RAW264.7 cells, where the
TLR4-MyD88-NF-κB signaling pathway was shown to impede microRNA
expression and regulate the production of a series of cytokines.
However, it is unknown if Tan IIA can inhibit LPS-induced
inflammation in VSMCs or if Tan IIA’s anti-inflammatory effects are
associated with the TLR4/TAK1/NF-κB signaling pathway. Therefore,
the aim of the present study was to define unknown molecular
mechanisms that underpin the anti-inflammatory effects of Tan IIA,
which may be beneficial for the treatment of coronary heart
disease.
Materials and methods
Reagents
Tan IIA was obtained from the National Institute for
the Control of Pharmaceutical and Biological Products (Beijing,
China). The purity of Tan IIA was 99%. Dulbecco’s modified Eagle’s
medium (DMEM) and fetal bovine serum (FBS) were provided by Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Penicillin,
streptomycin, Tris-Buffered saline-0.5% Tween,
ethylenediaminetetraacetic acid (EDTA), LPS from Escherichia
coli 0111:B4, pyrrolidine dithiocarbamate (PDTC),
3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium bromide (MTT)
and the enhanced chemiluminescence (ECL) kit were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Calbiochem (Merck
KGaA) was the commercial provider of 5Z-7-oxozeaenol (cat. no.
499610). Antibodies against TLR4, inducible nitric oxide (NO)
synthase (iNOS), TAK1, phosphorylated (p)-TAK1, α-SMA, osteopontin
(OPN), NF-κB (p65), p47phox, IκBα, p-IκBα, anti-rabbit horseradish
peroxidase (HRP)-conjugated, anti-mouse HRP-conjugated and histone
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). Transzol, EasyScript Reverse Transcriptase, TransStrat Green
Qpcr SuperMix, 2X Power Taq PCR MasterMix and SYBR-Green, and the
β-actin antibody were purchased from Beijing Transgen Biotech Co.,
Ltd. (Beijing, China). MCP-1, IL-6, and TNF-α enzyme-linked
immunosorbent assay (ELISA) kits were purchased from Sigma-Aldrich
(Merck KGaA).
VSMC culture
The present study was carried out in strict
accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
Publication no. 85-23, revised 1996) (20) and was approved by the Ethics and
Animal Welfare Committee of Zhengzhou University (Henan, China). A
total of 6 four-week-old male Sprague-Dawley rats (weighing 150-200
g) were obtained from the Laboratory Animal Institute of Zhengzhou
University and were housed in a temperature (22°C) and humidity
(55%) controlled animal facility maintained with a 12-h light/dark
cycle and ad libitum access to food and water. Experiments
were conducted during the light phase of the cycle. VSMCs were
isolated from the thoracic aorta of rats as previously described
(21). Cells were cultured in
DMEM containing 15% FBS, 100 µg/ml streptomycin and 100 U/ml
penicillin in a humidified atmosphere of 5% CO2 and 95%
air at 37°C. Morphological examination was used to identify VSMCs.
Cells between passage 3 and passage 11 were used for all
experiments. When the cells reached 80-95% confluence, the medium
was replaced with serum-free medium, and cells were cultured for 12
h prior to subsequent experiments.
Cell viability assay
VSMCs were seeded at a density of 5,000 cells/well
in 96-well plates containing 100 µl DMEM with 15% FBS and
incubated for 12 h. Tan IIA was dissolved in dimethylsulfoxide
(DMSO). Cell viability was determined by an MTT reduction assay.
The cells were either treated with Tan IIA at the indicated
concentrations (0, 6.25, 12.5, 25, 50, 100 and 200 µmol/l)
for 24 h at 37°C, or cells were pretreated with Tan IIA (0, 6.25,
12.5, 25, 50, 100 and 200 µmol/l) and then stimulated with
LPS (1 µg/ml) for 24 h at 37°C. The medium was removed and
cells were then incubated with MTT solution (5 mg/ml) for 4 h at
37°C. The dark blue formazan crystals that formed within and on
intact cells were solubilized with 150 µl of DMSO, and then
the absorbance was measured at 490 nm on a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
ELISA
VSMCs were seeded at a density of 5×106
cells/well in 6-well plates. Cells were pretreated with Tan IIA (5,
10 or 30 µmol/l) for 1 h, then LPS (1 µg/ml) was
added to the VSMC culture medium for 24 h. VSMCs were pretreated
with an antibody against TLR4 (1:250; cat. no. 542-0006), the TAK1
inhibitor 5Z-7-oxozeaenol (0.5 µmol/l), and PDTC (100
µmol/l) for 1 h at 37°C and were then incubated with LPS (1
µg/ml) for an additional 24 h at 37°C. The MCP-1, IL-6 and
TNF-α concentrations in the culture medium were measured using the
aforementioned ELISA kits (MCP-1 kit: cat. no. BYK-1021R; IL-6 kit:
cat. no. EK-0411; TNF-α kit: cat. no. BK-0657) at 37°C, according
to the manufacturer’s instructions.
Measurement of NO production
The accumulation of nitrite, a stable precursor of
NO in culture medium, was measured according to the Griess
reaction, as described previously (22). Briefly, 50 µl of the
culture supernatant was mixed with 50 µl of Griess reagent
(0.1% naphthylethylenediamine, 1% sulfanylamide, and 2.5%
phosphoric acid) for 1 min at 37°C. Absorbance was measured at 540
nm using a calibration curve with sodium nitrite standards. Fresh
culture medium was used as a control.
Reactive oxygen species (ROS) production
measurement
The 2′,7′-dichloro-dihydro-fluorescein diacetate
(DCFH-DA) assay kit was purchased from Biyuntian Biotechnology
Research Institute (Jiangsu, China; cat. no. S0076) and was used to
measure intracellular ROS levels, based on the ROS-dependent
oxidation of DCFH to highly fluorescent dichlorofluorescein. DCFH
(10 mmol/l) was dissolved in methanol and then diluted by a factor
of 500 in Hanks’ balanced salt solution (HBSS) to produce a 20
µmol/l DCFH solution. The cells were incubated with DCFH-DA
for 1 h at 37°C and then treated with HBSS containing Tan IIA (25,
50 or 100 µmol/l) or LPS (1 µg/ml) for a further 90
min at 37°C. Fluorescence was measured immediately at a wavelength
of 485 nm for excitation and 528 nm for emission on an iMark™
Microplate Absorbance Reader (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Once the cells were treated with Tan IIA at the
indicated concentrations (25, 50 and 100 µmol/l) for 24 h at
37°C, total RNA was extracted using a TransZol reagent and DNA was
removed using a DNA-free kit (Ambion; Thermo Fisher Scientific,
Inc.). The quality of mRNA was verified by performing denaturing
agarose gel electrophoresis containing 1.5% formaldehyde. The total
RNA concentration and purity were determined through UV-Vis
spectroscopy using the Bio-Rad SmartSpec 5000 system (Bio-Rad
Laboratories, Inc.). To synthesize cDNA, 1 µg of total RNA
was used in a 20 µl reaction using oligo(dT)18 Primer and
EasyScript Reverse Transcriptase kit (Thermo Fisher Scientific,
Inc.). Reverse transcription reaction conditions were as follows:
37°C for 15 min, then 85°C for 5 sec with the reverse transcription
reagent and enzyme, and then kept at 4°C. Primers for rat MCP-1,
IL-6, TNF-α, iNOS, TLR4, p47phox and β-actin were chosen using the
Beacon designer v4.0 (Premier Biosoft International, Palo Alto, CA,
USA; Table I). β-actin was used
as an endogenous control. The mRNA levels of MCP-1, IL-6, TNF-α,
TLR4, iNOS, and β-actin were performed using 2X Power Taq PCR
MasterMix and SYBR-Green (Beijing Transgen Biotech Co., Ltd.), and
the ABI PRISM 7000 sequence detection PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The qPCR thermocycling
conditions for MCP-1, IL-6, TNF-α, TLR4, iNOS, and β-actin were as
follows: 94°C for 5 min, 94°C for 10 sec, 60°C for 20 sec and 72°C
for 30 sec, followed by 40 cycles of denaturation for 150 sec at
72°C, annealing for 90 sec at 40°C and extension from 60 to 94°C,
followed by 1°C for 1 sec. A single melting curve peak confirmed
the presence of a single product. Results were expressed as fold
differences relative to β-actin using the 2−ΔΔCq method
(23).
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Oligonucleotide
primer sequences (5′-3′) |
|---|
| IL-6 | Forward:
GAGAAAAGAGTTGTGCAATGGC |
| Reverse:
ACTAGGTTTGCCGAGTAGACC |
| TNF-α | Forward:
TCCCAACAAGGAGGAGAAGT |
| Reverse:
TGGTATGAAGTGGCAAATCG |
| MCP-1 | Forward:
TCTCACTTGGTTCTGGTCCAGT |
| Reverse:
AGTCAGCGATGGCCCGATAG |
| iNOS | Forward:
CCACGCTCTTCTGTCTACTGAAC |
| Reverse:
ACGGGCTTGTCACTCGAG |
| TLR4 | Forward:
GGCATCATCTTCATTGTCCTTG |
| Reverse:
AGCATTGTCCTCCCACTCG |
| p47phox | Forward:
GGCAGGACCTGTCGGAGAAGGT |
| Reverse:
AAGGATGATGGGGCCTGTGATG |
| GADPH | Forward:
ATCGGCAATGAGCGGTTCC |
| Reverse:
AGCACTGTGTTGGCATAGAGG |
Western blot analysis
Following cell treatment with Tan IIA at the
indicated concentrations (25, 50 and 100 µmol/l) for 24 h at
37°C, VSMC lysates were prepared with 200 µl of ice-cold
lysis buffer (pH 7.4; 50 mmol/l HEPES, 5 mol/l EDTA, 100 mmol/l
NaCl, 1% Triton X-100, and protease inhibitor cocktail; Roche
Diagnostics GmbH, Mannheim, Germany) in the presence of phosphatase
inhibitors (50 mmol/l sodium fluoride, 1 mmol/l sodium
orthovanadate, 10 mmol/l sodium pyrophosphate and 1 nmol/l
microcystin). The activated NF-κB (p65) protein, located in the
nucleus, was extracted using the Pierce NE-PER Nuclear and
Cytoplasmic Extraction kit (Pierce; Thermo Fisher Scientific,
Inc.). A bicinchoninic acid protein assay kit was used to determine
protein concentrations. A total of 30 µg protein was loaded
per lane, then samples were separated by 10% SDS-PAGE and
transferred onto a polyvinylidene difluoride membrane in a semi-dry
system (Bio-Rad Laboratories, Inc.), which was blocked with 5%
fat-free milk in TBST buffer (20 mmol/l Tris-HCl, 137 mmol/l NaCl
and 0.1% Tween-20) for 120 min at 37°C. Membranes were then
incubated with primary antibodies against TLR4 (1:500; cat. no.
sc-2213), TAK1 (1:400; cat. no. sc-2501), p-TAK1 (1:500; cat. no.
sc-1152), p47phox (1:400; cat. no. sc-0032), SMA (1:100; cat. no.
sc-SC19483), OPN (1:100; cat. no. sc-03652), (1:1,000; cat. no.
sc-0096), β-actin (1:2,000; cat. no. sc-21764), histone (1:1,000;
cat. no. sc-1179), IκBα (1:100; cat. no. sc-89320) and p-IκBα
(1:100; cat. no. sc-20076) in TBST buffer overnight at 37°C,
following which membranes were washed and then incubated with
secondary antibodies (1:5,000; anti-rabbit HRP-conjugated; cat. no.
sc-276002; anti-mouse; HRP-conjugated; cat. no. sc-450039) for 110
min at 37°C. The optical densities of bands were quantified using
the ECL kit (Sigma-Aldrich; Merck KGaA) and Gel-Pro Analyzer
software version 4.0 (Media Cybernetics, Inc., Rockville, MD, USA).
β-actin or histone were used as the endogenous control, and the
results were expressed relative to their corresponding control and
ultimately as fold differences compared with the control.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean of six experiments. Differences between groups were
assessed by one-way analysis of variance followed by least
significant difference post hoc tests. Statistical tests were
performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
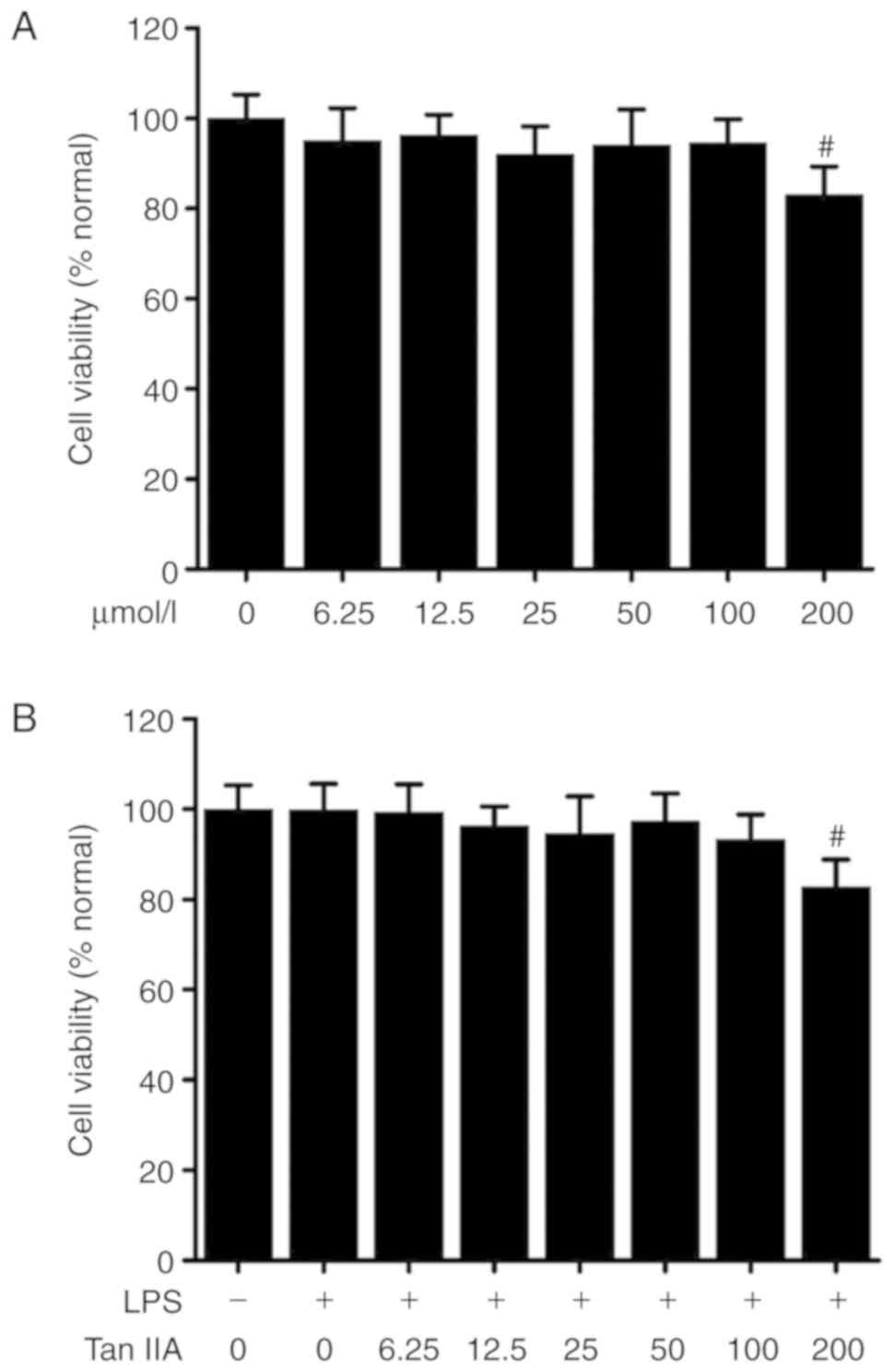
Cytotoxity of Tan IIA in VSMCs
Cells were treated with different concentrations of
Tan IIA (0-200 µmol/l) or LPS (1 µg/ml) for 24 h and
then cytotoxity was measured by an MTT assay. As shown in Fig. 1, 0-100 µmol/l Tan IIA was
not cytotoxic against VSMCs with or without LPS (1
µg/ml).
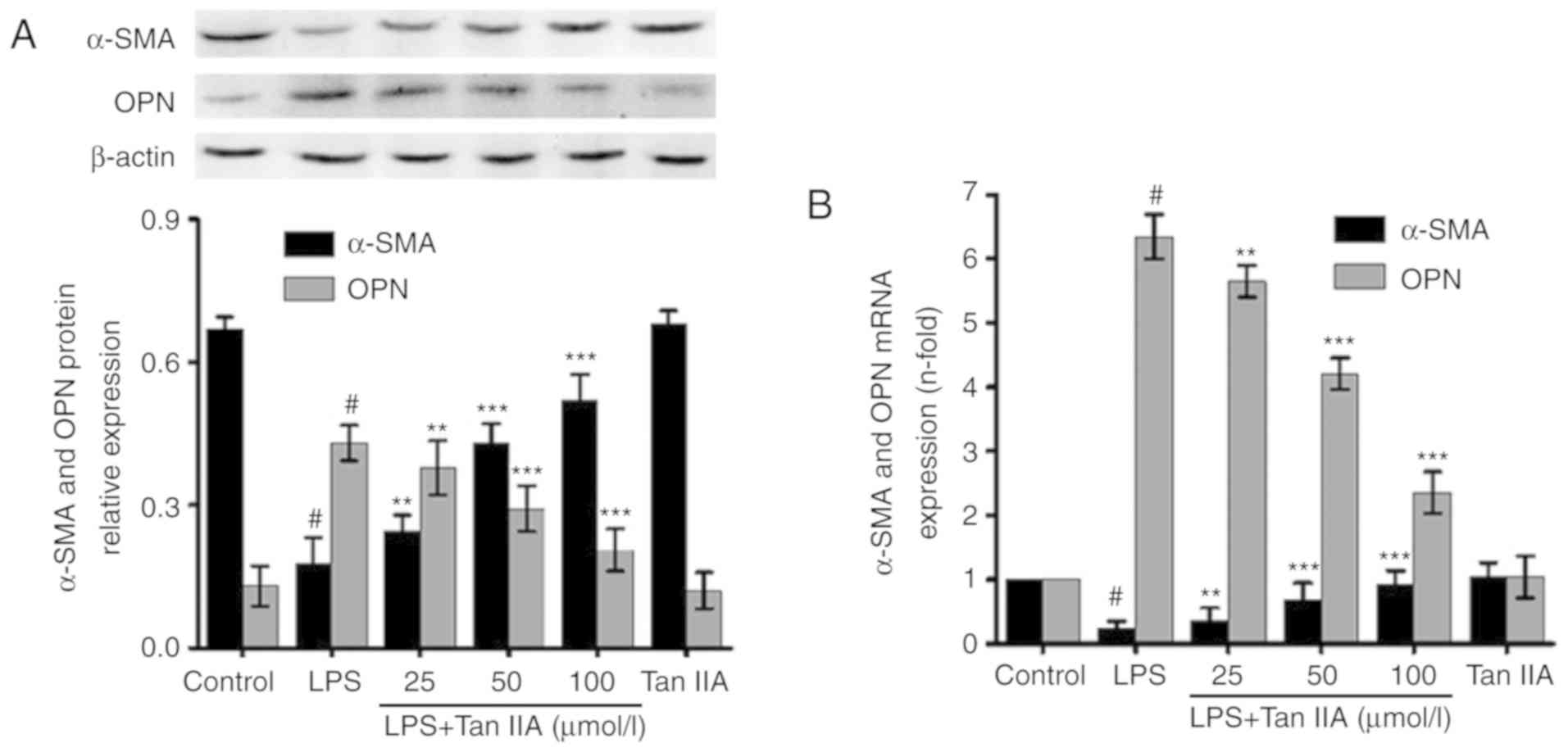
Tan IIA inhibits LPS-induced VSMC
phenotypic switching
VSMC phenotypic switching is involved in the
LPS-induced inflammatory response. When VSMCs transform from the
contractile phenotype to the synthetic phenotype, they produce
lower levels of contractile proteins (24), such as α-SMA, and higher levels of
OPN. As shown in Fig. 2A,
following VSMC treatment with LPS (1 µg/ml) for 24 h, the
protein expression of α-SMA decreased; however, OPN levels
increased. Pretreatment with Tan IIA attenuated the downregulation
of α-SMA and suppressed the upregulation of OPN in LPS-stimulated
VSMCs, in a concentration-dependent manner. Similar results were
obtained from mRNA level measurements via RT-qPCR (Fig. 2B).
Tan IIA inhibits the LPS-induced
expression of MCP-1, IL-6, and TNF-α in VSMCs
A significant increase in the expression of MCP-1,
IL-6, and TNF-α in the conditioned media of VSMCs treated with LPS
(1 µg/ml) for 24 h was observed. Treatment with Tan IIA
inhibited the LPS-induced protein expression of MCP-1, IL-6, and
TNF-α in VSMCs in a concentration-dependent manner (Fig. 3A-C). In addition, Tan IIA also
reduced the mRNA expression of MCP-1, IL-6, and TNF-α in
LPS-stimulated VSMCs in a concentration-dependent manner (Fig. 3D-F). However, treating cells with
Tan IIA (100 µmol/l) alone did not affect either the mRNA or
protein expression of these proinflammatory cytokines.
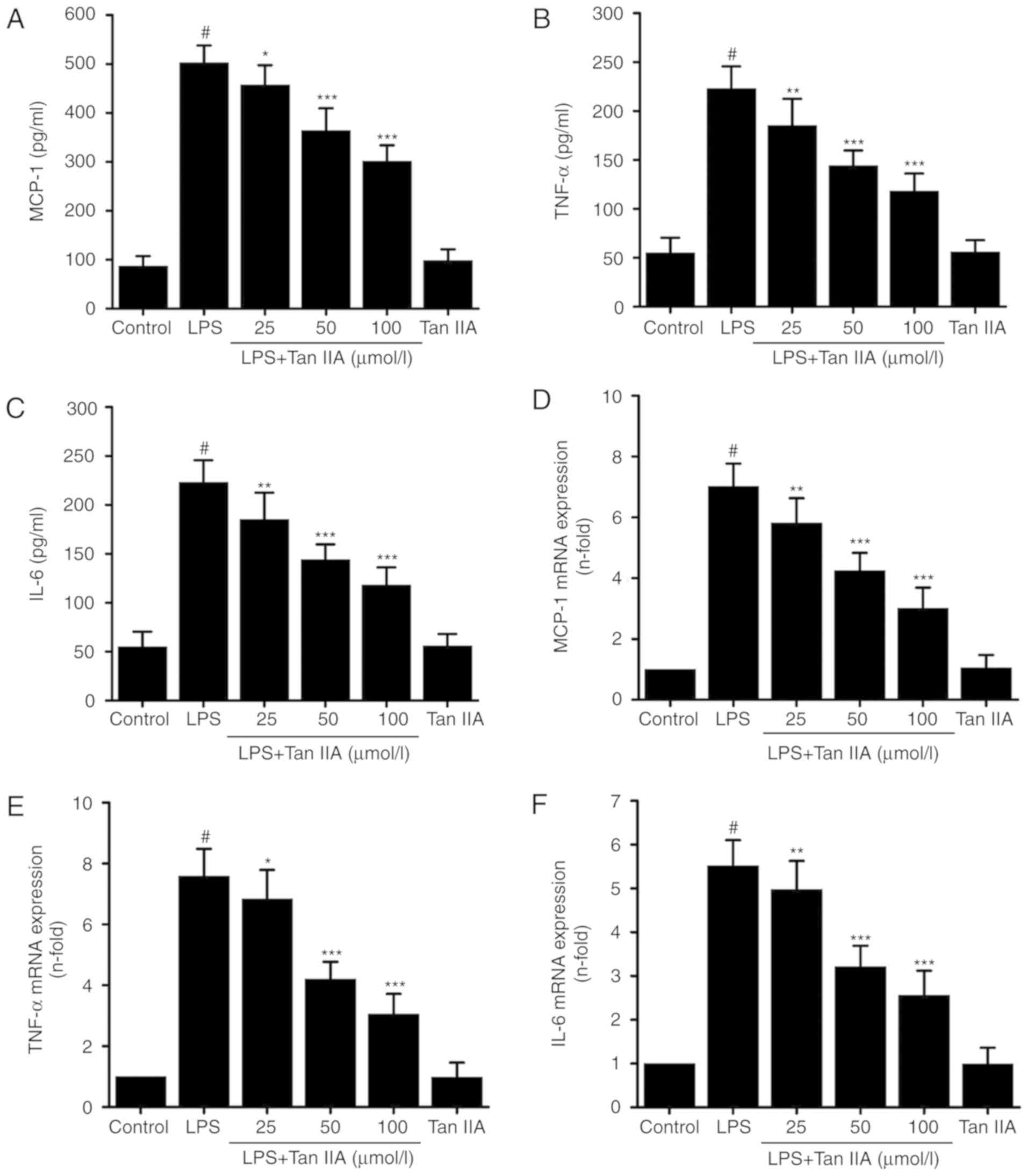 | Figure 3Tan IIA inhibits the LPS-induced
expression of MCP-1, IL-6, and TNF-α in VSMCs. VSMCs were
pretreated with the indicated concentrations of Tan IIA (25, 50 and
100 µmol/l) for 1 h, and then stimulated with LPS (1
µg/ml) for a further 24 h. One group of cells were treated
with Tan IIA (100 µmol/l) alone for 24 h. The protein
expression of (A) MCP-1, (B) TNF-α and (C) IL-6 was measured by an
ELISA assay. Then cells were pretreated with the indicated
concentrations of Tan IIA (25, 50 and 100 µmol/l) for 1 h,
and then stimulated with LPS (1 µg/ml) for a further 6 h.
The mRNA the expression levels of (D) MCP-1, (E) TNF-α and (F) IL-6
was measured by reverse transcription-quantitative polymerase chain
reaction in which GAPDH was used as an internal control. Data are
presented as mean ± standard error of the mean of six independent
experiments. #P<0.001 vs. control;
*P<0.05, **P<0.01 and
***P<0.001 vs. LPS. Tanshinone IIA; VSMCs, vascular
smooth muscle cells; LPS, lipopolysaccharide; IL-6, interleukin-6;
TNF-α, tumor necrosis factor-α; MCP-1, monocyte chemoattractant
protein 1. |
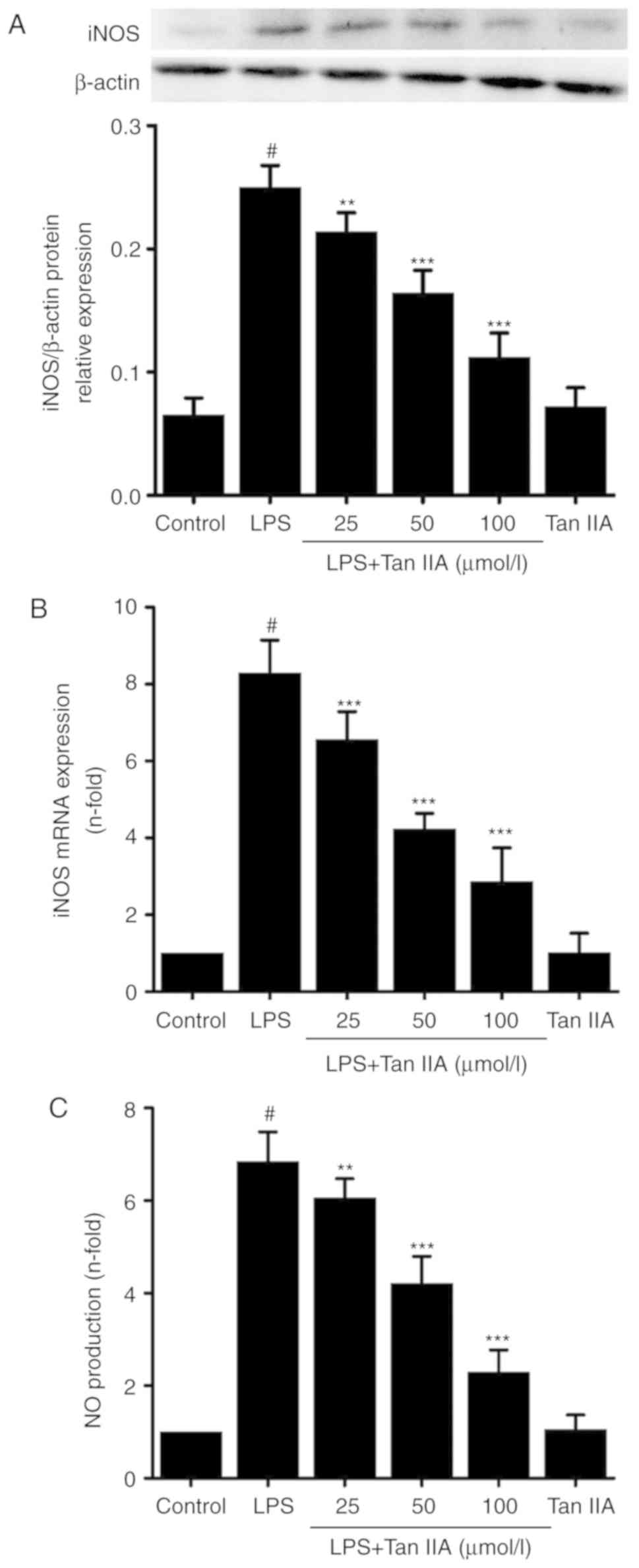
Inhibitory effect of Tan IIA on
iNOS-mediated NO production in LPS-stimulated VSMCs
Results from the Griess assay revealed that Tan IIA
inhibited LPS-induced NO production in VSMCs in a
concentration-dependent manner. However, treating the cells with
Tan IIA (100 µmol/l) alone did not affect NO production
(Fig. 4). To investigate how Tan
IIA decreased LPS-induced NO production, the expression of iNOS was
measured. As shown in Fig. 4A and
B, Tan IIA reduced the LPS-induced expression of iNOS at the
mRNA and protein levels in a concentration-dependent manner.
Treatment with Tan IIA alone did not affect the mRNA or protein
expression of iNOS when compared with control. Similar results were
observed when measuring the NO levels (Fig. 4C). These results suggest that iNOS
activation may serve a role in the inhibitory effects of Tan IIA on
LPS-induced NO production in VSMCs.
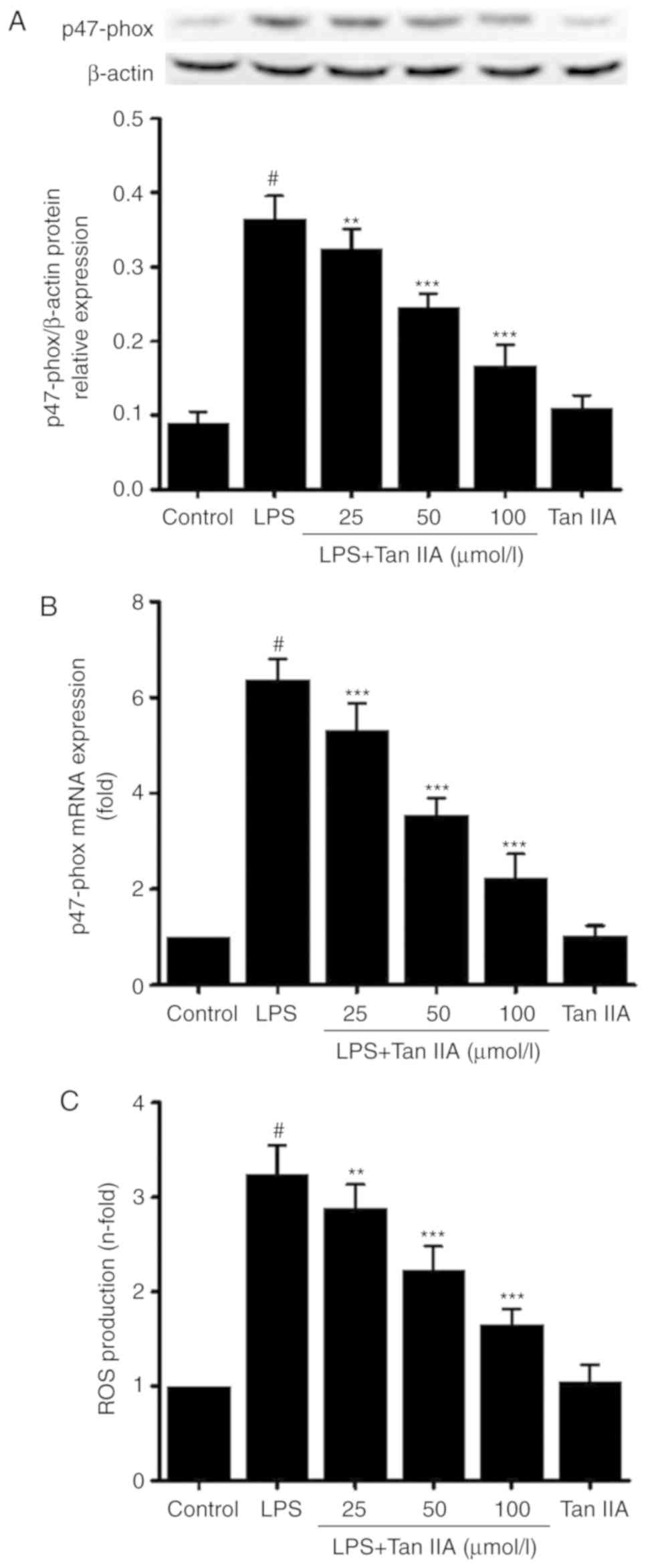
Tan IIA downregulates p47-phox and
decreases ROS levels
p47-phox protein (Fig.
5A) and mRNA (Fig. 5B)
expression, and ROS production (Fig.
5C) in cultured VSMCs were significantly increased by
stimulation with LPS; this effect was dose-dependently inhibited by
co-treatment with Tan IIA.
Associations between the inhibitory
effect of Tan IIA on LPS-induced inflammation and TLR4 expression
in VSMCs
As shown in Fig. 6A
and B, LPS (1 µg/ml) treatment significantly increased
the mRNA and protein expression of TLR4. Pretreatment with Tan IIA
inhibited the LPS-induced expression of TLR4 at the mRNA and
protein level in VSMCs (Fig. 6A and
B). Treating VSMCs with Tan IIA (100 µmol/l) alone did
not affect the expression of TLR4 (Fig. 6A and B). Next, the present study
explored if the anti-inflammatory effect of Tan IIA on the
LPS-induced expression of proinflammatory cytokines may function
partially through TLR4 in VSMCs. Cells were treated with or without
anti-TLR4 neutral-izing antibody (8 µg/ml) for 1 h, and then
Tan IIA (100 µmol/l) was added and incubated for 1 h. This
was followed by LPS (1 µg/ml) stimulation in VSMCs for 24 h.
The TLR4 inhibitor and Tan IIA partially inhibited the LPS-induced
increase in the levels of p-TAK1 (Fig. 6C), MCP-1 (Fig. 6D), TNF-α (Fig. 6E), IL-6 (Fig. 6F), and NO (Fig. 6G) in VSMCs. These results indicate
that the inhibitory effect of Tan IIA on LPS-induced inflammation
may be partially dependent on TLR4 in VSMCs.
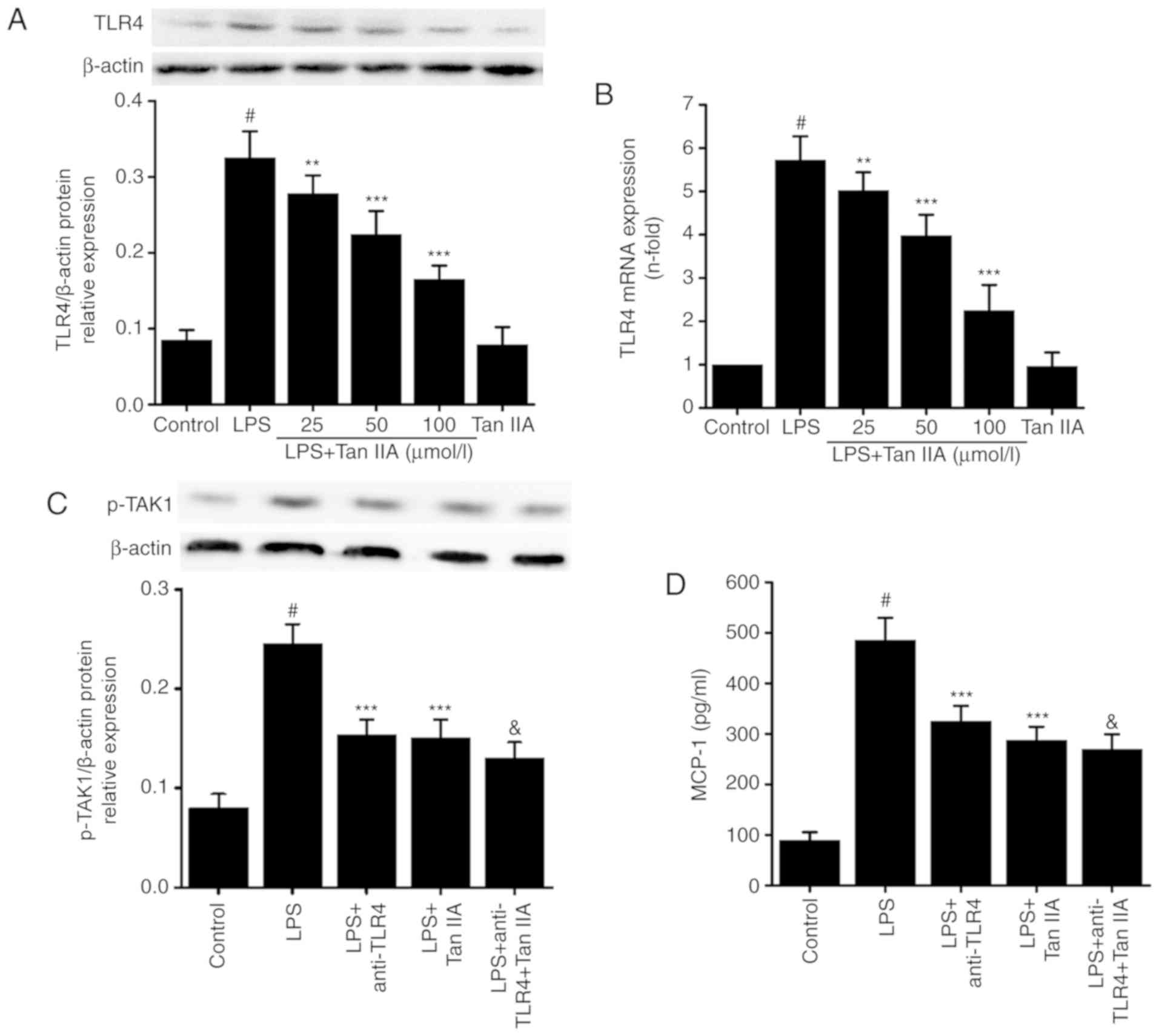 | Figure 6Association between the inhibitory
effect of Tan IIA on LPS-induced inflammation and TLR4 expression
in VSMCs. VSMCs were pretreated with the indicated concentrations
of Tan IIA (25, 50 and 100 µmol/l) for 1 h, and then
stimulated with LPS (1 µg/ml) for a further 24 h (for
western blotting) or 6 h (for RT-qPCR). The (A) protein and (B)
mRNA expression of TLR4 were measured by western blotting and
RT-qPCR, respectively. Cells were then pretreated with anti-TLR4 (5
µg/ml), Tan IIA (100 µmol/l), or anti-TLR4 (5
µg/ml) plus Tan IIA (100 µmol/l) for 1 h, and then
stimulated with LPS (1 µg/ml) for a further 24 h. (C) The
protein expression of p-TAK1 was detected by western blotting. The
expression levels of (D) MCP-1, (E) TNF-α and (F) IL-6 were
measured by an ELISA assay. (G) NO production was detected by the
Griess assay. Data are presented as mean ± standard error of the
mean of six independent experiments. #P<0.001 vs.
control; **P<0.01 and ***P<0.001 vs.
LPS; &P<0.05 vs. LPS+anti-TLR4. Tanshinone IIA;
VSMCs, vascular smooth muscle cells; LPS, lipopolysaccharide;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; NO, nitric oxide; IL-6, interleukin-6; TNF-α, tumor
necrosis factor-α; MCP-1, monocyte chemoattractant protein 1; TLR4,
Toll-like receptor 4; p-, phosphorylated; TAK1, transforming growth
factor-β-activated kinase 1. |
Associations between the inhibitory
effect of Tan IIA on LPS-induced inflammation and TAK1 activation
in VSMCs
To explore which intracellular signaling pathway
downstream of TLR4 is involved in the inhibitory effect of Tan IIA
on LPS-induced inflammation, the phosphorylation of TAK1 was
detected in LPS-stimulated VSMCs. As shown in Fig. 7, treating VSMCs with different
concentrations of Tan IIA (25, 50 and 100 µmol/l) alone did
not affect the phosphorylation of TAK1. However, treatment with LPS
(1 µg/ml) for 30 min significantly increased the levels of
p-TAK1 in VSMCs (Fig. 7B).
Pretreating the cells with Tan IIA attenuated the LPS-induced
phosphorylation of TAK1 in a concentration-dependent manner
(Fig. 7A and B). To determine if
the inhibitory effect of Tan IIA on LPS-induced inflammation is
dependent on the reduced expression of p-TAK1, the VSMCs were
pretreated with or without 5Z-7-oxozeaenol (0.5 µmol/l),
which is a specific inhibitor of TAK1 activation. The results
confirmed that 5Z-7-oxozeaenol significantly decreased p-TAK1
(Fig. 7B). In addition,
5Z-7-oxozeaenol and Tan IIA partially inhibited the LPS-induced
expression of MCP-1, IL-6, TNF-α and NO in VSMCs (Fig. 7C-F). These results suggest that
the reduction in p-TAK1 levels induced by Tan IIA may contribute to
its anti-inflammatory influence on LPS-stimulated VSMCs.
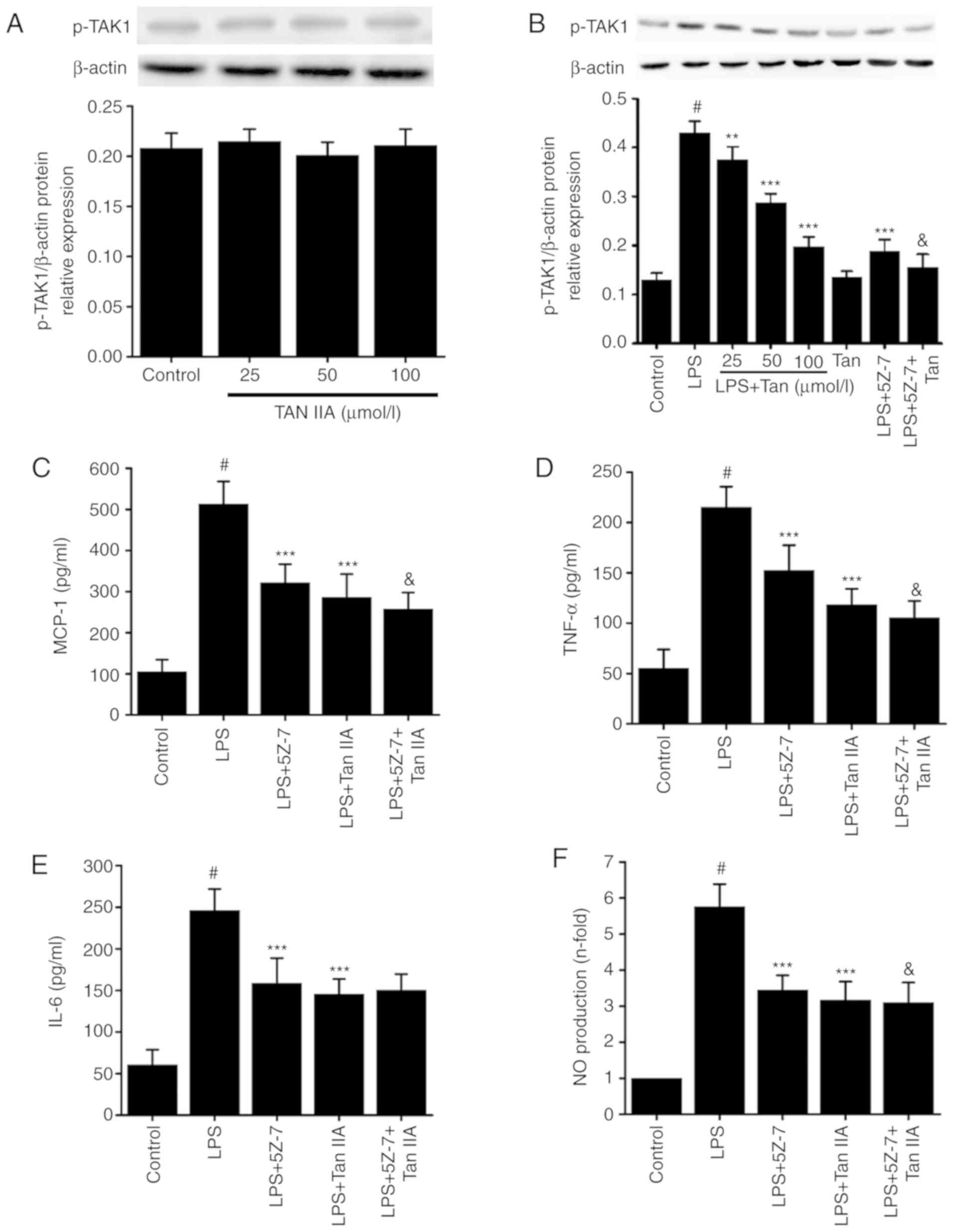 | Figure 7Associations between the inhibitory
effect of Tan IIA on LPS-induced inflammation and TAK1 activation
in VSMCs. (A) One group of VSMCs were only treated with Tan IIA
(25, 50 and 100 µmol/l) for 30 min; (B) another group of
cells were pretreated with the indicated concentrations of Tan IIA
(25, 50 and 100 µmol/l) for 1 h and then stimulated with LPS
(1 µg/ml) for a further 30 min. The levels of p-TAK1 was
measured by western blotting. Cells were pretreated with
5Z-7-oxozeaenol (0.5 µmol/l), Tan IIA (100 µmol/l),
or 5Z-7-oxozeaenol (0.5 µmol/l) plus Tan IIA (100
µmol/l) for 1 h and then stimulated with LPS (1
µg/ml) for a further 24 h. The protein expression of (C)
MCP-1, (D) TNF-α and (E) IL-6 was measured by an ELISA assay. (F)
NO production was detected by the Griess assay. Data are presented
as mean ± standard error of the mean from six independent
experiments. #P<0.001 vs. control;
**P<0.01 and ***P<0.001 vs. LPS;
&P<0.05 vs. LPS+5Z-7-oxozeaenol. Tanshinone IIA;
VSMCs, vascular smooth muscle cells; LPS, lipopolysaccharide; NO,
nitric oxide; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α;
MCP-1, monocyte chemoattractant protein 1; p-, phosphorylated;
TAK1, transforming growth factor-β-activated kinase 1; 5Z-7,
5Z-7-oxozeaenol. |
Tan IIA inhibits LPS-induced NF-κB
activation in VSMCs
The activation of NF-κB serves a key role in
controlling the inflammatory response in various cell types,
including VSMCs, by upregulating the expression of inflammatory
genes. Following stimulation with different proinflammatory
factors, including LPS, NF-κB is activated and translocated from
the cytoplasm to the nucleus (25). As shown in Fig. 8A, treating cells with different
concentrations of Tan IIA alone for 24 h did not affect the nuclear
translocation of NF-κB (p65). Treating VSMCs with LPS (1
µg/ml) for 24 h significantly increased the expression of
NF-κB (p65) in the nucleus. However, pretreatment with Tan IIA
attenuated the LPS-induced nuclear translocation of NF-κB in VSMCs
in a concentration-dependent manner (Fig. 8A). By contrast, cytoplasmic NF-κB
was decreased by LPS, which was reversed by co-treatment with Tan
IIA (Fig. 8A). Furthermore, the
present study measured the levels of total- and p-IκBα, which is a
negative regulator of NF-κB, by western blot analysis. As shown in
Fig. 8B, following VSMC treatment
with LPS (1 µg/ml) for 6 h, the total IκBα levels decreased
while p-IκBα levels significantly increased. Pretreating the cells
with Tan IIA (25, 50 and 100 µmol/l) attenuated the
LPS-induced decrease in total IκBα and increase in p-IκBα in a
concentration-dependent manner (Fig.
8B). Treatment of VSMCs with Tan IIA (100 µmol/l) alone
did not affect the levels of total- and p-IκBα (Fig. 8B).
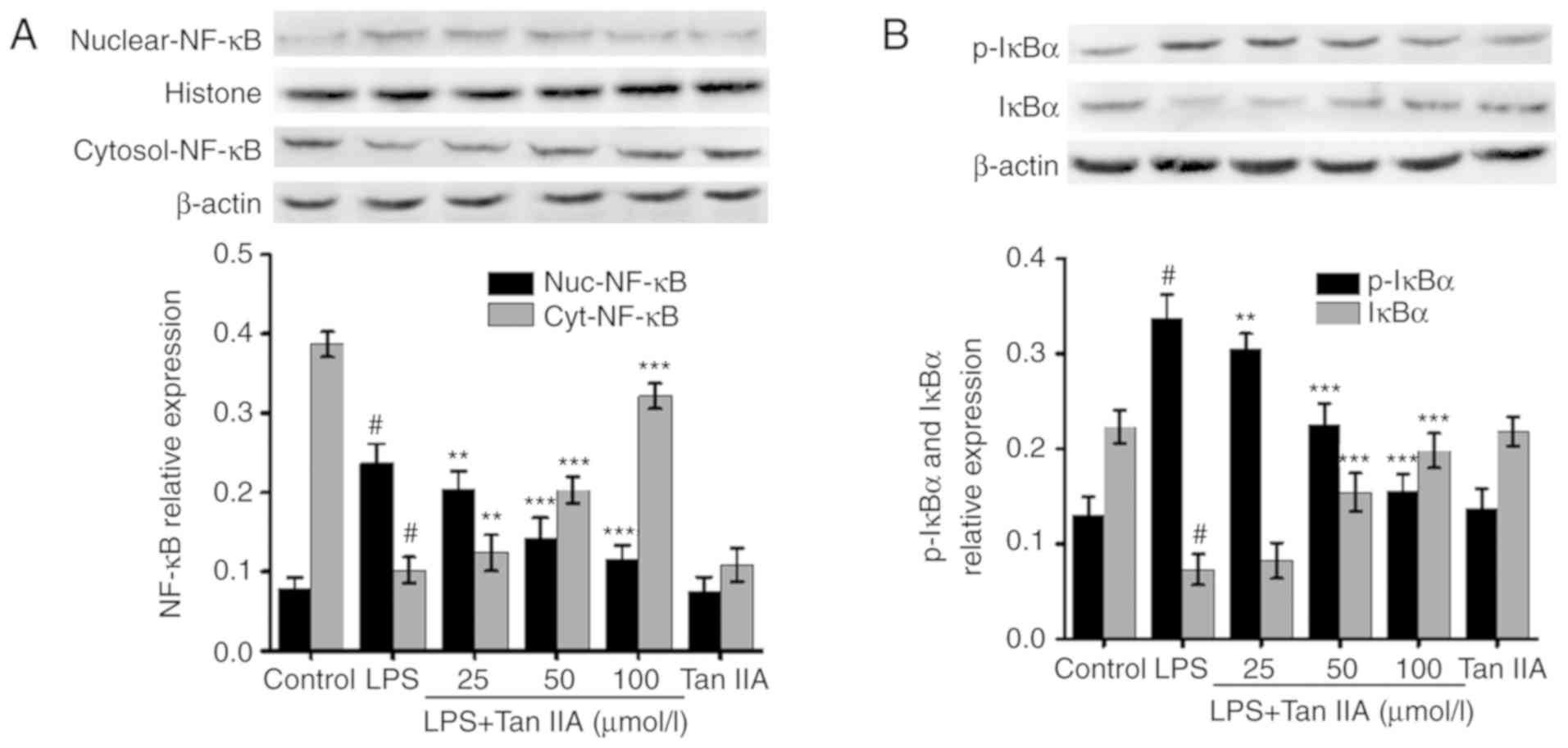 | Figure 8Tan IIA inhibits LPS-induced NF-κB
activation in VSMCs. VSMCs were pretreated with the indicated
concentrations of Tan IIA (25, 50 and 100 µmol/l) for 1 h
and then stimulated with LPS (1 µg/ml) for a further 24 (for
NF-κB) or 6 h (for p-IκBα). The protein expression of (A) NF-κB
(p65) in nuclei and (B) p-IκBα were measured by western blotting,
in which histone and β-actin were used as internal controls,
respectively. Data are presented as the mean ± standard error of
the mean of six independent experiments. #P<0.001 vs.
control; **P<0.01 and ***P<0.001 vs.
LPS. Tanshinone IIA; VSMCs, vascular smooth muscle cells; LPS,
lipopolysaccharide; NF-κB, nuclear factor-κB; p-, phosphorylated;
IκBα, inhibitor of nuclear factor-κB; nuc, nuclear; cyt,
cytoplasmic. |
Discussion
The present study demonstrated that Tan IIA inhibits
LPS-induced MCP-1, IL-6, TNF-α and iNOS-mediated NO production in
VSMCs in vitro. Tan IIA also reduced the LPS-induced
expression of TLR4 and TAK1 and reduced the nuclear translocation
of NF-κB in a concentration-dependent manner. These results provide
a novel association between the anti-inflammatory effect of Tan IIA
and the TLR4/TAK1/NF-κB signaling pathway in LPS-stimulated
VSMCs.
Multiple factors have been shown to contribute to
the progression of atherosclerosis, but chronic low-grade
inflammation may be a critical factor in the acceleration of
atherosclerosis progression and plaque instability. Various
inflammation-associated factors contribute to this process
(1). MCP-1 is a potential
monocyte chemoattractant cytokine that can induce the migration of
monocytes and macrophages to the intima of the arterial walls and
accelerate their development into foam cells (26). LPS is a powerful pro-inflammatory
factor that can induce the expression of various
inflammation-associated cytokines, including MCP-1, IL-6 and TNF-α,
in VSMCs (4). In addition, there
a growing body of evidence that indicates that there is a strong
correlation between LPS and atherosclerosis (27). Tan IIA has been reported to have
diverse pharmacological effects, including anti-inflammation,
anti-apoptosis, and regulation of the immune response (28). Previous studies have indicated
that Tan IIA may inhibit the LPS-induced expression of
inflammation-associated cytokines in various cell types (19,29,30). In the present study, LPS
significantly increased the expression of MCP-1, IL-6, and TNF-α in
VSMCs, which is consistent with a previous study (4). However, Tan IIA treatment could
attenuate the LPS-induced expression of inflammation-associated
cytokines in a dose-dependent manner, which may be a potential
mechanism of the anti-inflammatory activity of Tan IIA. In
addition, Tan IIA also inhibited the oxidized-low-density
lipo-protein-induced inflammatory response in various organs
(31), which suggests that Tan
IIA has multiple anti-inflammatory activities against more than
just LPS.
NO is mainly produced by the endothelium of the
arterial walls, and it serves an important role in regulating
inflammation throughout the process of atherosclerosis (32). While NO is also synthesized by
neuronal NOS and endothelial NOS, NO produced by iNOS is the chief
contributor to the regulation of immunomodulatory activity
(33). Although NO produced by
the endothelium may exhibit some beneficial effects that protect
endothelial function, high concentrations of NO have been revealed
to accelerate the progression of atherosclerosis by increasing
oxidative stress (34). In
addition to atherosclerosis, the overproduction of NO has also been
shown to contribute to the progression of various inflammation- and
immune-associated diseases, such as Alzheimer’s disease (35) and Crohn’s disease (36). Previous studies have demonstrated
that inhibiting NO production through the use of an inhibitor of
iNOS or by knockout of the iNOS gene can reduce the area of
atherosclerotic plaques and attenuate plaque vulnerability in high
fat diet-fed ApoE−/− mice (37). Therefore, in the present study,
the effect of Tan IIA on iNOS-mediated NO production was
investigated. Previous studies have demonstrated that Tan can
inhibit LPS-induced and iNOS-mediated NO production in macrophages
and endothelium (38). In the
present study, Tan IIA significantly inhibited LPS-induced NO
production, which was accompanied by a reduction in iNOS
expression. These results suggest that Tan IIA suppresses
iNOS-mediated NO production in LPS-stimulated VSMCs, which may
provide a novel molecular mechanism for the cardiovascular
protective effect of Tan IIA.
Previous evidence has demonstrated that the
activation of TLR4 serves an important role in the acceleration of
atherosclerosis by inducing an excessive inflammatory response
(9). In human and animal models,
an increase in TLR4 expression has been observed in atherosclerotic
plaques, which was accompanied by an overexpression of various
inflammation-associated factors (39). Our previous study also
demonstrated that LPS increases the expression of TLR4 in VSMCs
(4). However, when given the same
high-cholesterol diet, TLR4-knockout mice exhibited a reduction in
atherosclerotic plaque area and had more stable plaques when
compared with normal ApoE−/− mice (39). In addition, the activation of the
TLR4 signaling pathway serves an important role in NO production in
LPS-stimulated VSMCs (40).
Therefore, it is valid to consider that inhibiting TLR4 activation
may be an effective strategy to impede the progress of
atherosclerosis. A previous study demonstrated that Tans can reduce
the LPS-induced expression of TLR4 in macrophages (19). In the present study, Tan IIA
inhibited the LPS-induced expression of TLR4 at the mRNA and
protein levels in a concentration-dependent manner. This was
accompanied by a reduction in the production of pro-inflammatory
cytokines. Furthermore, the monoclonal antibody of TLR4 exhibited
the same anti-inflammatory effects as Tan IIA, and treatment with a
combination of Tan IIA and the TLR4 monoclonal antibody resulted in
a more powerful inhibitory effect on LPS-induced inflammation.
These results indicate that the inhibition of TLR4 activation may
be involved in the anti-inflammatory effect of Tan IIA in
LPS-stimulated VSMCs.
As an important downstream molecule, TAK1 serves a
critical role in the TLR-mediated inflammatory signaling pathway.
Once LPS binds to the CD14/TLR4 complex, TLR4 is activated. Then,
Myd88 induces the phosphorylation of IL-1 receptor-associated
kinase 4 (IRAK4) and IRAK1, which results in the phosphorylation of
TAK1 (41). Activated TAK1
induces the phosphorylation of MAPKs and IKKs, which are the main
factors that regulate NF-κB activation (42). Tans have been shown to inhibit the
LPS-induced activation of NF-κB by reducing TAK1 expression in
macrophages (19). In addition, a
previous study evaluated the effects of Tan IIA on atherosclerotic
vulnerable plaque stability (43). Tans were reported to also decrease
NF-κB activation in VSMCs, which in turn inhibited VSMC
proliferation and migration (43). As a conventional regulator of
NF-κB, these results indicated that TAK1 may also be involved in
the mechanisms underlying NF-κB inactivation by Tan IIA in VSMCs.
In the present study, Tan IIA induced effects similar to those of
the TAK1 inhibitor. Tan IIA attenuated the LPS-induced expression
of p-TAK1 in a concentration-dependent manner and inhibited the
LPS-induced expression of MCP-1, IL-6, TNF-α, and NO in VSMCs.
These results suggest that the inhibition of TAK1 activation may be
involved in the anti-inflammatory effect of Tan IIA in
LPS-stimulated VSMCs.
NF-κB is a ubiquitously expressed nuclear
transcription factor that is considered to be associated with
inflammation, especially in the expression of cytokines. Activation
of NF-κB occurs when it is isolated from IκBα. Once IκBα is
phosphorylated, it degrades and allows NF-κB to become activated,
and then NF-κB is translocated from the cytosol into the nucleus
where it can control DNA transcription. Following cell stimulation
with LPS, the level of p-IκBα was increased while the level of
total IκBα was decreased, which was consistent with the results of
some previous studies (44,45). Many signaling pathways result in
NF-κB phosphorylation, which causes NF-κB to hydrolyze into several
subunits (46). One of the
subunits is P65, which translocates to the nucleus to bind to DNA.
It then upregulates the expression of cytokines, including MCP-1,
IL-6, TNF-α, and NO (47).
Several studies have indicated that NF-κB may serve an important
role in LPS-induced inflammation in macrophages and VSMCs (48,49). As a downstream molecule of TAK1, a
reduction in NF-κB may contribute to the anti-inflammatory
mechanism of Tan IIA. In the present study, it was observed that
pretreatment with Tan IIA attenuated the LPS-induced nuclear
translocation of NF-κB (p65) in VSMCs in a dose-dependent manner,
which suggests that Tan IIA may inhibit LPS-induced NF-κB
activation in VSMCs.
In conclusion, the present study demonstrated the
anti-inflammatory properties of Tan IIA in VSMCs by inhibiting
MCP-1, IL-6, TNF-α, and NO. These effects may be mediated by
downregulating TLR4 and inhibiting TAK1, which consequently leads
to the inhibition of NF-κB activation and eventually the
suppression of its target genes. The present study provided a novel
experimental basis for using Tan IIA as a therapeutic agent to
treat chronic inflammation-mediated diseases, especially
atherosclerosis.
Acknowledgments
The authors would like to thank Professor Li Ling
(Department of Cardiology, The First Affiliated Hospital of
Zhengzhou University, Zhengzhou, Henan, China) for assisting with
the experimental lab work.
Funding
The present study was supported by the Youth
Foundation of The First Affiliated Hospital of Zhengzhou University
(grant no. YFFAHZZ 2015020105).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
HYL conceived and designed the study. ZM conducted
the majority of the experiments and wrote the paper. CYS, ST and
XHY performed the experiments. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was carried out in strict
accordance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
Publication no. 85-23, revised 1996) and was approved by the Ethics
and Animal Welfare Committee of Zhengzhou University (Henan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hansson GK, Libby P and Tabas I:
Inflammation and plaque vulnerability. J Intern Med. 278:483–493.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Vries MR and Quax PH: Plaque
angiogenesis and its relation to inflammation and atherosclerotic
plaque destabilization. Curr Opin Lipidol. 27:499–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Vascular smooth muscle cell in atherosclerosis. Acta Physiol
(Oxf). 214:33–50. 2015. View Article : Google Scholar
|
|
4
|
Meng Z, Yan C, Deng Q, Gao DF and Niu XL:
Curcumin inhibits LPS-induced inflammation in rat vascular smooth
muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways.
Acta Pharmacol Sin. 34:901–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Son YH, Jeong YT, Lee KA, Choi KH, Kim SM,
Rhim BY and Kim K: Roles of MAPK and NF-kappaB in interleukin-6
induction by lipopolysaccharide in vascular smooth muscle cells. J
Cardiovasc Pharmacol. 51:71–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dickson BC and Gotlieb AI: Towards
understanding acute destabilization of vulnerable atherosclerotic
plaques. Cardiovasc Pathol. 12:237–248. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minguet S, Dopfer EP, Pollmer C,
Freudenberg MA, Galanos C, Reth M, Huber M and Schamel WW: Enhanced
B-cell activation mediated by TLR4 and BCR crosstalk. Eur J
Immunol. 38:2475–2487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roshan MH, Tambo A and Pace NP: The Role
of TLR2, TLR4, and TLR9 in the Pathogenesis of Atherosclerosis. Int
J Inflam. 2016:15328322016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu Z, Zhang X, Li Y, Jin J and Huang Y:
TLR4 antagonist reduces early-stage atherosclerosis in diabetic
apolipoprotein E-deficient mice. J Endocrinol. 216:61–71. 2013.
View Article : Google Scholar
|
|
10
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: A possible pathway for plaque activation. Circulation.
105:1158–1161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michelsen KS, Wong MH, Shah PK, Zhang W,
Yano J, Doherty TM, Akira S, Rajavashisth TB and Arditi M: Lack of
Toll-like receptor 4 or myeloid differentiation factor 88 reduces
atherosclerosis and alters plaque phenotype in mice deficient in
apolipoprotein E. Proc Natl Acad Sci USA. 101:10679–10684. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ajibade AA, Wang HY and Wang RF: Cell
type-specific function of TAK1 in innate immune signaling. Trends
Immunol. 34:307–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao X, Li G, Bai Q, Xu H and Lü C:
Taraxerol inhibits LPS-induced inflammatory responses through
suppression of TAK1 and Akt activation. Int Immunopharmacol.
15:316–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang J, Little PJ and Xu S:
Atheroprotective effects and molecular targets of tanshinones
derived from herbal medicine danshen. Med Res Rev. 38:201–228.
2018. View Article : Google Scholar
|
|
15
|
Fan K, Li S, Liu G, Yuan H, Ma L and Lu P:
Tanshinone IIA inhibits high glucose-induced proliferation,
migration and vascularization of human retinal endothelial cells.
Mol Med Rep. 16:9023–9028. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Jiao Y, Wang H, Shang Q, Lu F, Huang
L, Liu J, Xu H and Chen K: Sodium tanshinone IIA sulfate adjunct
therapy reduces high-sensitivity C-reactive protein level in
coronary artery disease patients: A randomized controlled trial.
Sci Rep. 7:174512017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu H, Zhai C, Qian G, Gu A, Liu J, Ying F,
Xu W, Jin D, Wang H, Hu H, et al: Protective effects of tanshinone
IIA on myocardial ischemia reperfusion injury by reducing oxidative
stress, HMGB1 expression, and inflammatory reaction. Pharm Biol.
53:1752–1758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui H, Zhao J, Zhou L, Wen H, Deng W, Li
C, Ji Q, Liu X, Feng Y, Chai N, et al: Tanshinone IIA inhibits
β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in
normoxic and HIF-1α in hypoxic microenvironments in human
colorectal cancer. Cancer Lett. 403:86–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan G, Jiang X, Wu X, Fordjour PA, Miao L,
Zhang H, Zhu Y and Gao X: Anti-Inflammatory activity of tanshinone
IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and
TLR4-NF-κB PATHWAY. Inflammation. 39:375–384. 2016. View Article : Google Scholar
|
|
20
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. National Academy Press;
Washington, DC: 1996
|
|
21
|
Griendling KK, Taubman MB, Akers M,
Mendlowitz M and Alexander RW: Characterization of
phosphatidylinositol-specific phospholipase C from cultured
vascular smooth muscle cells. J Biol Chem. 266:15498–15504.
1991.PubMed/NCBI
|
|
22
|
Cho JY, Baik KU, Jung JH and Park MH: In
vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene
lactone, from Saussurea lappa. Eur J Pharmacol. 398:399–407. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou W, Wang G, Zhao X, Xiong F, Zhou S,
Peng J, Cheng Y, Xu S and Xu X: A multiplex qPCR gene dosage assay
for rapid genotyping and large-scale population screening for
deletional α-thalassemia. J Mol Diagn. 15:642–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao L, Pan D, Li D, Zhang Y, Chen Q, Xu T,
Li W and Wu W: Relation between anti-atherosclerotic effects of
IRAK4 and modulation of vascular smooth muscle cell phenotype in
diabetic rats. Am J Transl Res. 8:899–910. 2016.PubMed/NCBI
|
|
25
|
Meng Z, Gao P, Chen L, Peng J, Huang J, Wu
M, Chen K and Zhou Z: Artificial zinc-finger transcription factor
of A20 suppresses restenosis in sprague dawley rats after carotid
injury via the PPARα pathway. Mol Ther Nucleic Acids. 15:123–131.
2017. View Article : Google Scholar
|
|
26
|
Chen FL, Yang ZH, Wang XC, Liu Y, Yang YH,
Li LX, Liang WC, Zhou WB and Hu RM: Adipophilin affects the
expression of TNF-alpha, MCP-1, and IL-6 in THP-1 macrophages. Mol
Cell Biochem. 337:193–199. 2010. View Article : Google Scholar
|
|
27
|
Konev IuV and Lazebnik LB: Endotoxin (LPS)
in the pathogenesis of atherosclerosis. Eksp Klin Gastroenterol.
15–26. 2011.In Russian.
|
|
28
|
Li Y, Guo Y, Chen Y, Wang Y, You Y, Yang
Q, Weng X, Li Q, Zhu X, Zhou B, et al: Establishment of an
interleukin-1β-induced inflammation-activated endothelial
cell-smooth muscle cell-mononuclear cell co-culture model and
evaluation of the anti-inflammatory effects of tanshinone IIA on
atherosclerosis. Mol Med Rep. 12:1665–1676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li J, Zheng Y, Li MX, Yang CW and Liu YF:
Tanshinone IIA alleviates lipopolysaccharide-induced acute lung
injury by downregulating TRPM7 and pro-inflammatory factors. J Cell
Mol Med. 22:646–654. 2018. View Article : Google Scholar
|
|
30
|
Cheng J, Chen T, Li P, Wen J, Pang N,
Zhang L and Wang L: Sodium tanshinone IIA sulfonate prevents
lipopolysaccha-ride-induced inflammation via suppressing nuclear
factor-κB signaling pathway in human umbilical vein endothelial
cells. Can J Physiol Pharmacol. 96:26–31. 2018. View Article : Google Scholar
|
|
31
|
Wang B, Ge Z, Cheng Z and Zhao Z:
Tanshinone IIA suppresses the progression of atherosclerosis by
inhibiting the apoptosis of vascular smooth muscle cells and the
proliferation and migration of macrophages induced by ox-LDL. Biol
Open. 6:489–495. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaw CA, Taylor EL, Megson IL and Rossi
AG: Nitric oxide and the resolution of inflammation: Implications
for atherosclerosis. Mem Inst Oswaldo Cruz. 100(Suppl 1): S67–S71.
2005. View Article : Google Scholar
|
|
33
|
Caillaud D, Galinier M, Elbaz M, Carrié D,
Puel J, Fauvel JM and Arnal JF: Atherosclerosis-the role of nitric
oxide. Presse Med. 30:41–44. 2001.In French. PubMed/NCBI
|
|
34
|
Förstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Asiimwe N, Yeo SG, Kim MS, Jung J and
Jeong NY: Nitric oxide: Exploring the contextual link with
Alzheimer’s disease. Oxid Med Cell Longev. 2016:72057472016.
View Article : Google Scholar
|
|
36
|
Heydarpour P, Rahimian R, Fakhfouri G,
Khoshkish S, Fakhraei N, Salehi-Sadaghiani M, Wang H, Abbasi A,
Dehpour AR and Ghia JE: Behavioral despair associated with a mouse
model of Crohn’s disease: Role of nitric oxide pathway. Prog
Neuropsychopharmacol Biol Psychiatry. 64:131–141. 2016. View Article : Google Scholar
|
|
37
|
Detmers PA, Hernandez M, Mudgett J,
Hassing H, Burton C, Mundt S, Chun S, Fletcher D, Card DJ, Lisnock
J, et al: Deficiency in inducible nitric oxide synthase results in
reduced atherosclerosis in apolipoprotein E-deficient mice. J
Immunol. 165:3430–3435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu XH, Wang XL, Xin H, Wu D, Xin XM, Miao
L, Zhang QY, Zhou Y, Liu Q, Zhang Q and Zhu YZ: Induction of heme
oxygenase-1 by sodium 9-hydroxyltanshinone IIA sulfonate derivative
contributes to inhibit LPS-mediated inflammatory response in
macrophages. Cell Physiol Biochem. 36:1316–1330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gargiulo S, Gamba P, Testa G, Rossin D,
Biasi F, Poli G and Leonarduzzi G: Relation between TLR4/NF-κB
signaling pathway activation by 27-hydroxycholesterol and
4-hydroxynon-enal, and atherosclerotic plaque instability. Aging
Cell. 14:569–581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nunes KP, Bomfim GF, Toque HA, Szasz T and
Clinton Webb R: Toll-like receptor 4 (TLR4) impairs nitric oxide
contributing to Angiotensin II-induced cavernosal dysfunction. Life
Sci. 191:219–226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He A, Ji R, Shao J, He C, Jin M and Xu Y:
TLR4-MyD88-TRAF6-TAK1 complex-mediated NF-κB activation contribute
to the anti-inflammatory effect of V8 in LPS-induced human cervical
cancer SiHa cells. Inflammation. 39:172–181. 2016. View Article : Google Scholar
|
|
42
|
Shim JH, Xiao C, Paschal AE, Bailey ST,
Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al:
TAK1, but not TAB1 or TAB2, plays an essential role in multiple
signaling pathways in vivo. Genes Dev. 19:2668–2681. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu WY, Yan H, Wang XB, Gui YZ, Gao F, Tang
XL, Qin YL, Su M, Chen T and Wang YP: Sodium tanshinone IIA silate
inhibits high glucose-induced vascular smooth muscle cell
proliferation and migration through activation of AMP-activated
protein kinase. PLoS One. 9:e949572014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen CL, Chen JT, Liang CM, Tai MC, Lu DW
and Chen YH: Silibinin treatment prevents endotoxin-induced uveitis
in rats in vivo and in vitro. PLoS One. 12:e01749712017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhatia HS, Baron J, Hagl S, Eckert GP and
Fiebich BL: Rice bran derivatives alleviate microglia activation:
Possible involvement of MAPK pathway. J Neuroinflammation.
13:148–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghosh S and Dass JF: Study of pathway
cross-talk interactions with NF-κB leading to its activation via
ubiquitination or phosphorylation: A brief review. Gene.
584:97–109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen XW and Zhou SF: Inflammation,
cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis. Drug
Des Devel Ther. 9:2941–2946. 2015.
|
|
48
|
Kim KJ, Yoon KY, Yoon HS, Oh SR and Lee
BY: Brazilein suppresses inflammation through inactivation of
IRAK4-NF-κB pathway in LPS-induced Raw264.7 macrophage cells. Int J
Mol Sci. 16:27589–27598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang HL, Huang PJ, Liu YR, Kumar KJ, Hsu
LS, Lu TL, Chia YC, Takajo T, Kazunori A and Hseu YC: Toona
sinensis inhibits LPS-induced inflammation and migration in
vascular smooth muscle cells via suppression of reactive oxygen
species and NF-κB signaling pathway. Oxid Med Cell Longev.
2014:9013152014. View Article : Google Scholar
|