Introduction
Approximately 5 million fatalities globally are
attributed to stroke each year. Ischemic stroke accounts for more
than 80% of total stroke cases and is one of the main causes of
mortality and disability worldwide (1-4).
Following a period of ischemia, severe reperfusion injury occurs
when blood returns to the brain. Reperfusion therefore serves a
critical role in cerebral ischemia (5). In clinical treatment, thrombolytic
therapy is the only method approved for the treatment of ischemic
stroke (6,7). However, restoration of blood flow
following thrombolytic treatment may cause severe
ischemia-reperfusion injury (8).
Currently, the neuroprotective approach has become a novel
direction in the treatment of stroke and has been studied in animal
experiments, but the efficacy on patients remains limited (9). Consequently, there is an urgent need
to develop an effective neuroprotective agent for the prevention
and treatment of stroke.
In acute ischemic stroke, apoptosis has been
considered one of the main causes of neuronal death (10,11). Apoptosis is evident in animal
models and in patients of ischemic stroke. Therefore, inhibition of
apoptotic pathways and the creation of a neuroprotective
environment provide a potential therapeutic approach for ischemic
brain damage. Mitogen activated protein kinase (MAPK) is one of the
distinct signaling cascades involved in neuroprotection and is
activated during the ischemic process (12). MAPK signaling pathway is activated
then causes extracellular signal regulated kinase (ERK)
phosphorylation induced by methyl ethyl ketone (MEK) (13). It has been demonstrated that
activation of MEK/ERK elicits an anti-apoptosis effect during
cerebral ischemia (14).
cAMP-response element binding protein (CREB) is a typical protein
that possesses anti-apoptotic properties (15). Down-modulation of the MEK/ERK
pathways inhibits the phosphorylation of CREB, which causes B-cell
lymphoma-2 (Bcl-2) family transcription and expression
downregulation and sensitization to cell apoptosis (16).
Curcumin (Cur) was extracted from the rhizome of
Curcuma genus plants (17). Cur
has anti-inflammatory, antioxidant, anti-apoptotic and other
pharmacological effects (18). In
cerebral ischemia-reperfusion injury, Cur is also known to have
neuroprotective properties (19).
However, the underlying molecular mechanisms are still poorly
understood. In this study, the aim was to investigate whether Cur
protected the brain from ischemic injury by the MEK/ERK/CREB
pathway during in vivo and in vitro experiments. The
results of the present study will inform future investigations into
the neuroprotective effects of curcumin on the molecular level.
Materials and methods
Materials and reagents
Cur was purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Antibodies against phosphorylated
anti-(p)-MEK), anti-MEK, anti-p-ERK, anti-ERK, anti-CREB,
anti-p-CREB, B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein
(Bax), β-actin antibodies and horseradish peroxidase
(HRP)-conjugated secondary antibodies were all provided by
Sigma-Aldrich (Merck KGaA). Terminal dexynucleotidyl transferase
(TdT)-mediate d dUTP nick end labeling (TUNEL) assay kits were
obtained from Roche Diagnostics (Basel, Switzerland). Lactate
dehydrogenase release assay (LDH) kits were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). Dulbecco’s
modified Eagle media: Nutrient Mixture F-12 (DMEM/F12) medium,
fetal calf serum and trypsin were purchased from Hyclone (Logan,
UT, USA). Unless otherwise stated, triphenyl tetrazolium chloride
(TTC) powder, PBS buffer, paraformaldehyde, western blot reagents,
tetrazolium blue tetrazolium bromide (MTT) and all other chemicals
used were provided by Sigma Aldrich; Merck KGaA.
Animals
A total of 60 male Sprague-Dawley (SD) rats (280-320
g) for in vivo experiment and a total of 5 SD rats (1-2 days
old) for primary astrocyte cell culture were provided by the
Experimental Animal Center of Chongqing Medical University
(Chongqing, China) Specific Pathogen Free [animal production
license no. SCXK (Chongqing) 2011-0001]. Animals were kept in a
controlled room with adequate food and water, constant temperature
and humidity (22°C and 55%, respectively) as well as a 12/12 h
light/dark cycle.
Establishment of the cerebral
ischemia-reperfusion model and animal grouping
Experimental protocols were all approved by the
Chongqing Medical University’s Institutional Animal Care and Use
Committee. Using Longa’s method, focal cerebral ischemia and
reperfusion of middle cerebral artery occlusion models of rats were
performed. Rats were anesthetized with 4% chloral hydrate through
intraperitoneal injection, then an incision in the skin was made to
expose the right common carotid artery, internal carotid artery
(ICA) and external carotid artery. The right middle cerebral artery
occlusion model was established by introducing an embolus through
the ICA. The embolus was inserted into the internal carotid artery
until the tip reached the origin of the middle cerebral artery
(18-22 mm). After 120 min of ischemia, the embolus was removed.
After the procedure, the rats recovered in pre-warmed cages. In
total, 60 rats were divided into 4 groups randomly as follows: i)
Sham operation control group (SC) (n=15); ii) ischemia-reperfusion
group (IR) (n=15); iii) Cur-treatment group (IC) receiving cur at
100 mg/kg (n=15); iv) IC receiving Cur at 300 mg/kg (n=15).
Pre-treatment with Cur took place 30 min prior to surgery and daily
via intraperitoneal injection. The sham group and
ischemia-reperfusion group received similar pre-treatment with
normal saline.
Evaluation of neurological deficit
scores
A total of 24 h following ischemia/reperfusion, the
neurological deficit score was examined by referencing the standard
5 point system established by Longa et al (20): i) No obvious signs of deficit
(score 0); ii) failure to extend the right forepaw fully (score 1);
iii) circling to the right (score 2); iv) falling to the right
(score 3); and v) loss of walking abilities (score 4).
Measurement of the brain water
content
After evaluation of neurological deficit scores, 3
rats were anesthetized with 4% chloral hydrate via intraperitoneal
injection and rapidly decapitated. Brain tissue was removed
quickly, followed by the pia mater and brain tissue blood. Tissue
wet weight (A) and dry weight (B, tissue was dried in an oven for
24 h) were weighed (accurate to 0.1 mg). Finally, the brain water
content was calculated in accordance with the Blliot formula: Brain
water content = (A - B)/A ×100%.
Measurement of cerebral infarct
volume
Following evaluation of neurological deficit scores,
3 other rats were anesthetized with 4% chloral hydrate via
intraperitoneal injection and rapidly decapitated. The brain was
removed and coronally sectioned into 2 mm slices, which were placed
into 2% TTC solution at 37°C. After 20 min, slices were stained and
images were captured with a camera. Noninfarcted areas of the brain
tissue were stained red; infarcted areas appeared white. CMIAS-008
image analysis software (Institute of Beijing University of
Aeronautics and Astronautics, Beijing, China) was used to calculate
the ratio of the infarct size/the total area.
Transmission electron microscopy (TEM) to
observe morphological changes of hippocampal neurons
After evaluation of neurological deficit scores, 3
rats were perfused transcardially with 0.9% NaCl and 2.5%
glutaraldehyde and rapidly decapitated. Brain tissue sized 1
mm3 were quickly removed and fixed in 2.5%
glutaraldehyde at 37°C. After 1 h, specimens were dehydrated with
acetone and embedded by Epon812 at 60°C for 24 h. The 60 nm
ultrathin sections of the cubes were stained with uranyl acetate
and lead citrate at 25°C for 30 min, and then observed under TEM
(7100; Hitachi, Tokyo, Japan).
Hematoxylin and eosin staining (H&E)
to observe morphological changes of brain tissue
A total of 3 anesthetized rats were perfused
transcardially with 0.9% NaCl and 4% paraformaldehyde through the
left ventricle and rapidly decapitated. After brains were removed
and embedded in paraffin, they were processed into
5-µm-thick slices. Finally, H&E was applied at 25°C for
15 min to the tissue and the pathological changes of cells in
hippocampal CA1 region were observed under an optical microscope
(Olympus Corporation, Tokyo, Japan).
TUNEL to detect neurons apoptosis in
hippocampal CA1
TUNEL was used to detect neuron apoptosis in
hippocampal CA1 in according to the TUNEL assay kit protocol.
Stained slices with 5-µm-thick were imaged under the optical
microscope. The extent of brain damage was then evaluated by
apoptotic index, which was the arithmetic mean of positive cells
counted in 5 microscopic fields in each CA1 region of the
hippocampus section.
Western blot analysis
A total of 24 h following reperfusion, the
hippocampal CA1 of remaining three rats in each group was removed
and put on ice. Total protein extraction and protein determination
was performed with a protein extraction kit (Beyotime Institute of
Biotechnology, Shanghai, China). Subsequently, using western blot
analysis, the expression of MEK, p-MEK, p-ERK, ERK, CREB, p-CREB,
Bcl-2 and Bax was measured. Protein (30 µl) were separated
by 12% SDS-PAGE and transferred onto a nitrocellulose membrane. The
membrane was blocked with 10% goat serum (Beyotime Institute of
Biotechnology) at 25°C for 2 h and the anti-rabbit antibodies
against MEK (cat. no. SAB4502407), p-MEK (cat. no. HPA026430), ERK
(cat. no. M7927), p-ERK (cat. no. M7927), CREB (cat. no.
SAB4500444), p-CREB (cat. no. AV01026), Bcl-2 (cat. no.
SAB4500003), and anti-mouse antibodies against Bax (cat. no. B8554)
and β-actin (cat. no. A5441) (1:1,000) were added to the membrane
overnight at 4°C. After washing, the membranes were incubated with
anti-rabbit HRP-conjugated secondary antibodies (cat. no. AP182P;
1:2,000) for 40 min at 37°C. Subsequently, the membranes were
processed with an ECL kit (Beyotime Institute of Biotechnology) to
detect immune reactivity. Finally, images were analyzed by a Versa
Doc Model 3000 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Primary astrocyte cell culture
Cerebral cortexes from 1-2 days old SD rats were
separated under sterile conditions. The cell suspensions were
seeded (2×106 cells/cm2) into culture plates
and complete medium was added. Cultured cells were grown in an
incubator at 37°C with 5% CO2 and 100% humidity. After
24 h, astrocytes were replaced with new medium and the medium was
replaced every three days. After 11 days astrocytes were used for
future experiments. The purity of astrocytes was identified as
above 95% by fluorescent antibody technical analysis with
anti-glial fibrillary acidic protein (anti-GFAP).
Establishment of the cerebral
ischemia-reperfusion model in vitro and astrocyte grouping
Astrocytes were seeded at a density of
5×104 into 24-well plates and cultured for 24 h in an
incubator (37°C, 5% CO2). Subsequently, cells were
divided into the normoxic group, oxygen-glucose
deprivation/reoxygenation group (OGD/Reoxy) and OGD/Reoxy + Cur
group (OGD/Reoxy + C group) randomly. There were 6 wells in each
group. In the MTT and LDH experiments, the OGD/Reoxy + C group has
been divided into three doses [5, 10, 20 micromolar
(µmol)/liter (l)], and setting 6 duplicate holes in each
group. The solubilization of Cur was achieved with dimethyl
sulfoxide (DMSO), but the final concentration of DMSO did not
exceed 0.1% in the medium. In the drug-administered group, 1,000
µl of complete medium containing the corresponding drug was
added, the normal group and the model group cells had 1,000
µl of complete medium containing no drug but containing the
same amount of solvent (DMSO) added as the administration group.
Cells were cultivated for 24 h in normal medium, then the model
group and drug-administered group were modeled: Cells were
subjected to hypoxia and hypoglycemia for 2 h and then reoxygenated
for 24 h.
Identification of astrocytes
Cell climbing pieces were washed and fixed by 4%
paraformaldehyde at 25°C for 20 min. Then cell climbing pieces were
blocked with 5% goat serum at 37°C for 1 h, and the primary
antibodies against GFAP (cat. no. G3893) (1:50; Sigma-Aldrich;
Merck KGaA) were added overnight at 4°C. After washing, the cell
climbing samples were incubated with appropriate fluorescein
isothiocyanate fluorescence-labeled secondary antibody (1:200)
(Sigma-Aldrich; Merck KGaA) for 40 min at 37°C.
4′,6-diamidino-2-phenylindole (DAPI) was used to stain the nucleus
of astrocytes at 37°C for 4 min and cell climbing samples were
observed under a fluorescent microscope (Nikon Corporation, Tokyo,
Japan).
Evaluation of cell viability by MTT
analysis
Cell viability was measured by MTT assay. A total of
24 h following reoxygenation, 20 µl MTT solution was added
(5 g/l) to the cell culture plate and after 4 h, 150 µl DMSO
was added then oscillated for 10 min. Absorbance (A) value was
measured by a microplate reader at a wavelength of 490 nm. Finally,
according to the following formula, the relative cell viability can
be calculated: Relative cell viability = experimental group/control
group ×100%.
Evaluation of cell cytotoxicity by LDH
analysis
A total of 24 h following oxygen-glucose
deprivation/reoxygenation, cell cytotoxicity was measured by LDH
assay. Each group had 20 µl cell culture fluid removed and
LDH (U/l) was measured by colorimetry according to the protocol of
the LDH assay kit. Finally, according to the following formula, the
LDH leakage rate can be calculated: Relative cell cytotoxicity =
experimental group (U/l)/control group (U/l) ×100%. Through MTT
experiment and LDH experiment the ideal concentration of Cur was
identified and the optimum concentration was used in the following
experiments.
Western blot analysis
Radioimmunoprecipiration assay lysis solution
(Beyotime Institute of Biotechnology) was used to lyse the cells at
0°C for 20 min, followed by centrifugation at 12,000 × g at 4°C for
10 min to collect protein supernatants. Using western blot
analysis, the expression of MEK, p-MEK, ERK, p-ERK, CREB, p-CREB,
Bcl-2 and Bax were measured. Samples (30 µl) were separated
by 12% SDS-PAGE then transferred to a nitrocellulose membrane. The
membrane was blocked with 10% goat serum at 25°C for 2 h and the
anti-rabbit primary antibodies against MEK (cat. no. SAB4502407),
p-MEK (cat. no. HPA026430), ERK (cat. no. M7927), p-ERK (cat. no.
M7927), CREB (cat. no. SAB4500444), p-CREB (cat. no. AV01026),
Bcl-2 (cat. no. SAB4500003), and anti-mouse antibodies against Bax
(cat. no. B8554) and β-actin (cat. no. A5441) (1:1,000) were added
to the membrane overnight at 4°C. After washing, the membranes were
incubated with anti-rabbit HRP-conjugated secondary antibodies
(cat. no. AP182P; 1:2,000) at 37°C for 40 min. Subsequently, the
membranes were processed with an ECL kit to detect immune
reactivity. Finally, images were analyzed with a Versa Doc Model
3000.
Statistical analysis
All data were expressed as the mean ± standard
deviation of the mean and were analyzed by SPSS version 24.0 for
Windows (IBM Corps., Armonk, NY, USA). A one-way analysis of
variance was used to compare the difference of measurement data
among multiple groups. The post hoc test was performed using
Tukey’s post hoc method. The number of experimental repeats for
each sample was 3. Pairwise comparisons in multiple groups was
conducted with the Student-Newman-Keuls method. P<0.05 was
considered to indicate a statistically significant difference.
Results
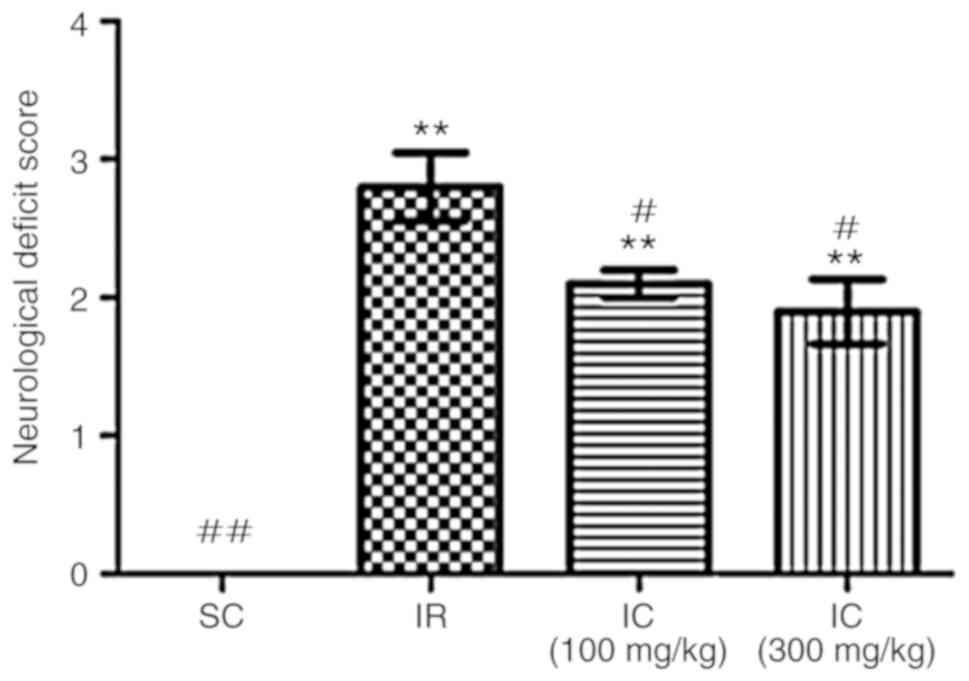
Cur can improve nerve damage symptoms in
rats
A total of 24 h following ischemia/reperfusion, the
neuroprotective effect of Cur was examined by evaluating
neurological deficit scores and brain water content. As presented
in Fig. 1, compared with the IR
group, the IC group can significantly alleviate nerve damage
symptoms (P<0.05), most notably in the IC group of 300
mg/kg.
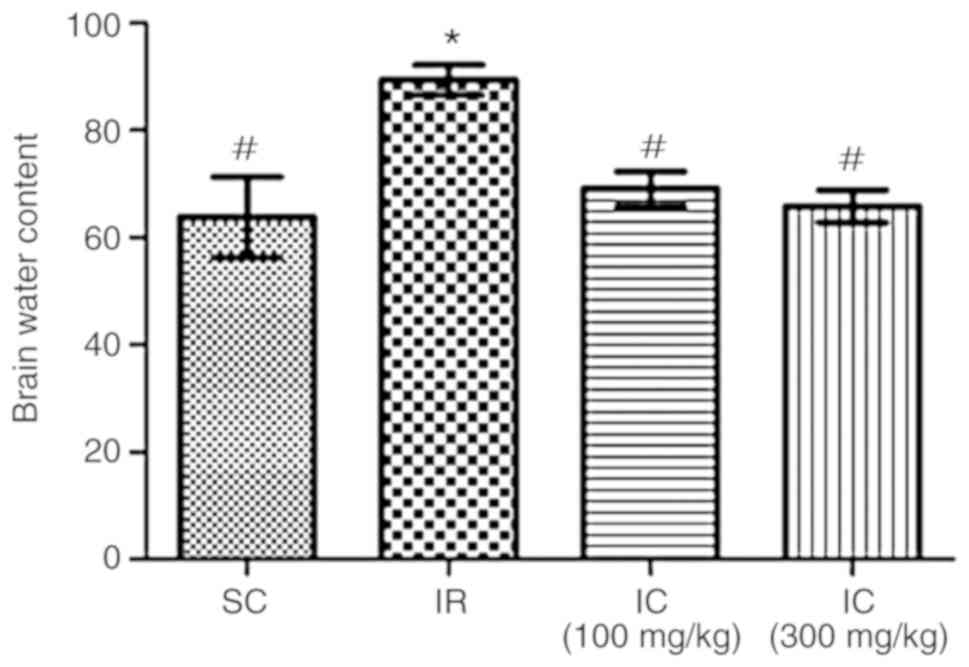
Cur can improve brain water content of IR
rats
A total of 24 h following ischemia/reperfusion, the
effect of Cur was examined by investigating brain water content.
The results demonstrated that Cur can reduce cerebral edema. As
presented in Fig. 2, IC groups
can significantly reduce the brain water content (P<0.05),
particularly the IC group at a dose of 300 mg/kg. Through these two
experiments, it was also demonstrated that neurological deficit
scores and brain water content were decreased in a dose-dependent
manner. In addition, only the IC group of 300 mg/kg were used in
the rest of the experiments.
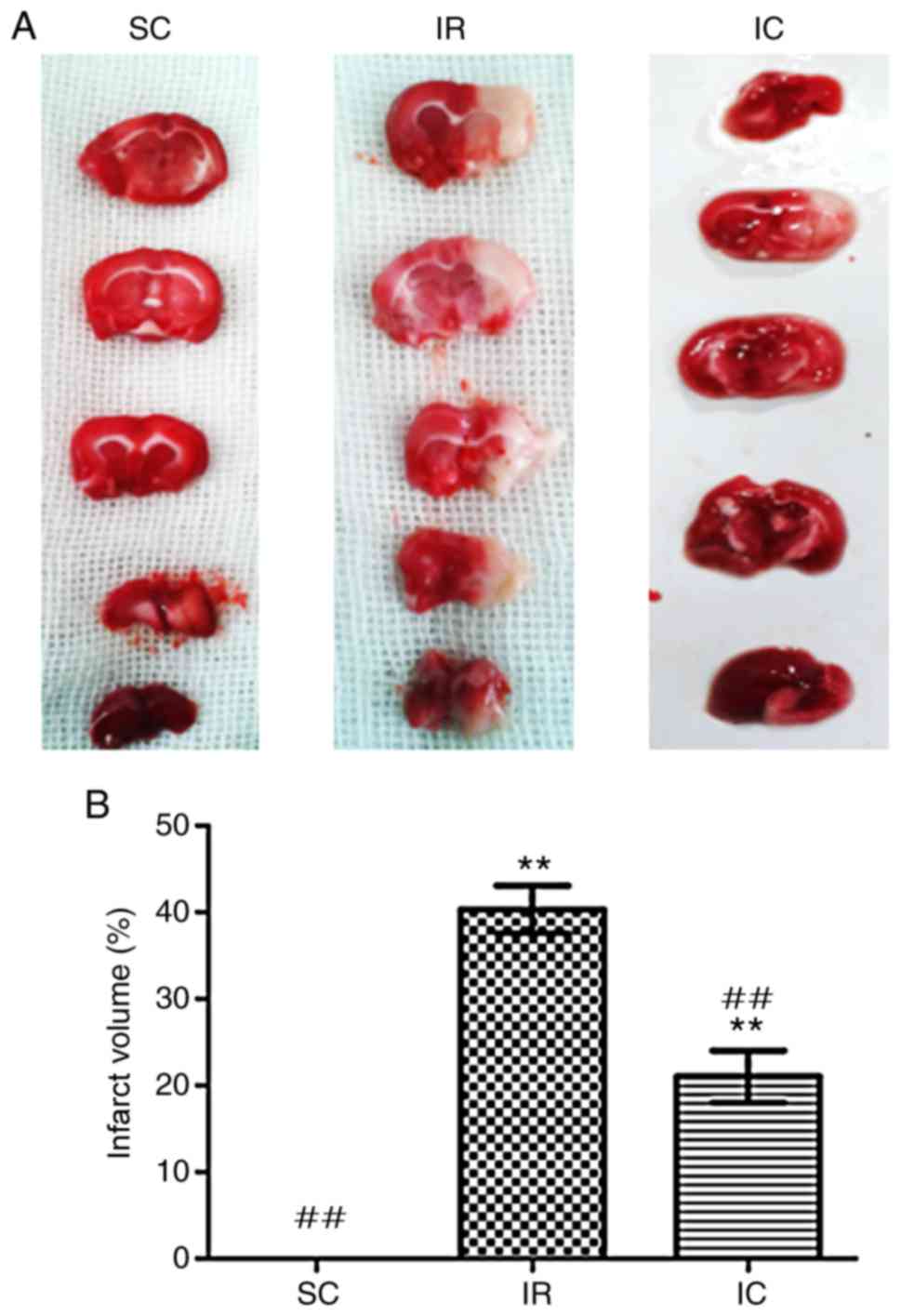
Cur can reduce the volume of a cerebral
infarct in IR rats
A total of 24 h following ischemia/reperfusion, the
infract volume was measured. As presented in Fig. 3, compared with the IR group, the
IC group (IC, 300 mg/kg) displayed significantly decreased
pale-colored regions (P<0.01).
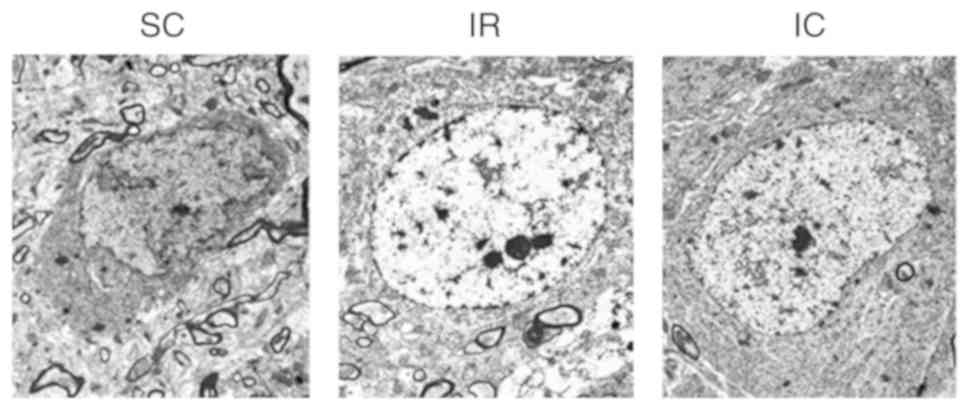
Cur can improve neuronal damage in CA1
area by TEM
A total of 24 h following ischemia/reperfusion,
changes in neurons were observed using TEM. As presented in
Fig. 4, TEM of the SC group
(magnification, ×6,000) displayed normal neuronal structure and no
significant alterations. However TEM of the IR group (×6,000)
revealed serious neuronal damage in the CA1 area, exhibiting
nucleus electron density decreases, dissolution, cavitation,
mitochondrial swelling and endoplasmic reticulum. Compared with the
IR group, the IC group (300 mg/kg; magnification, ×6,000) exhibited
less severe changes.
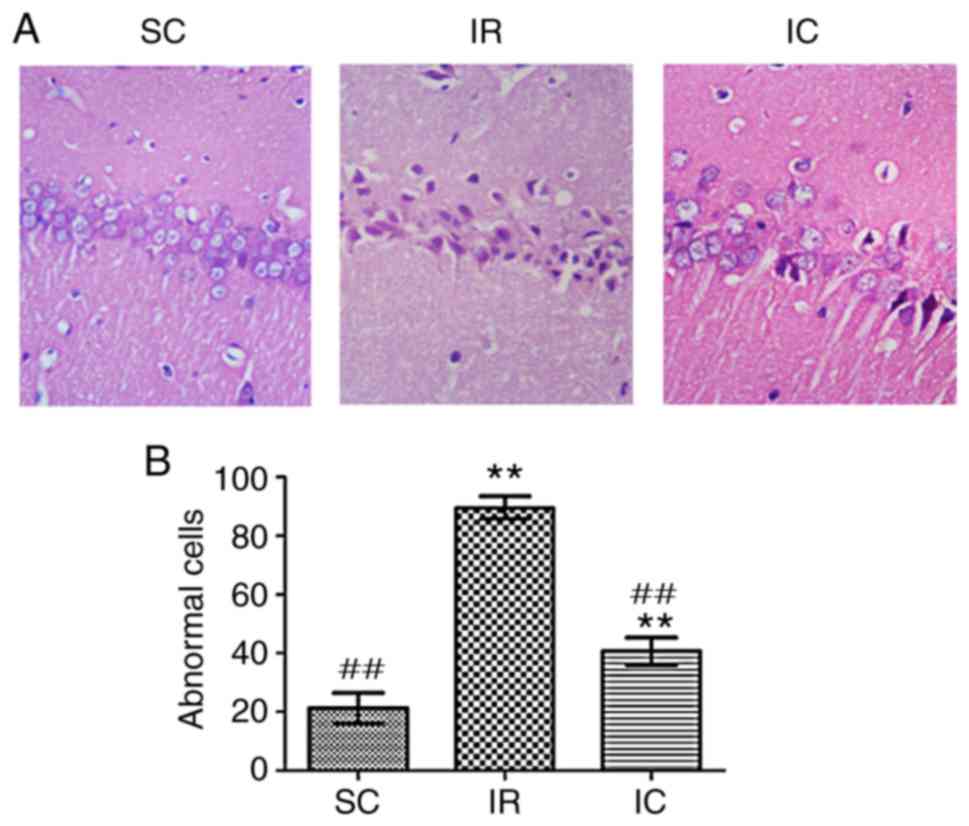
Cur can improve neuronal damage in the
CA1 region as demonstrated by H&E staining
A total of 24 h following ischemia/reperfusion, the
IR group displayed abnormal cell structures and morphology.
Specifically, cell body and nucleus condensation, stained nucleoli,
and gaps around the cells were observed. As presented in Fig. 5, compared with the IR group, the
IC group (300 mg/kg) significantly reduced abnormal cells
(P<0.01).
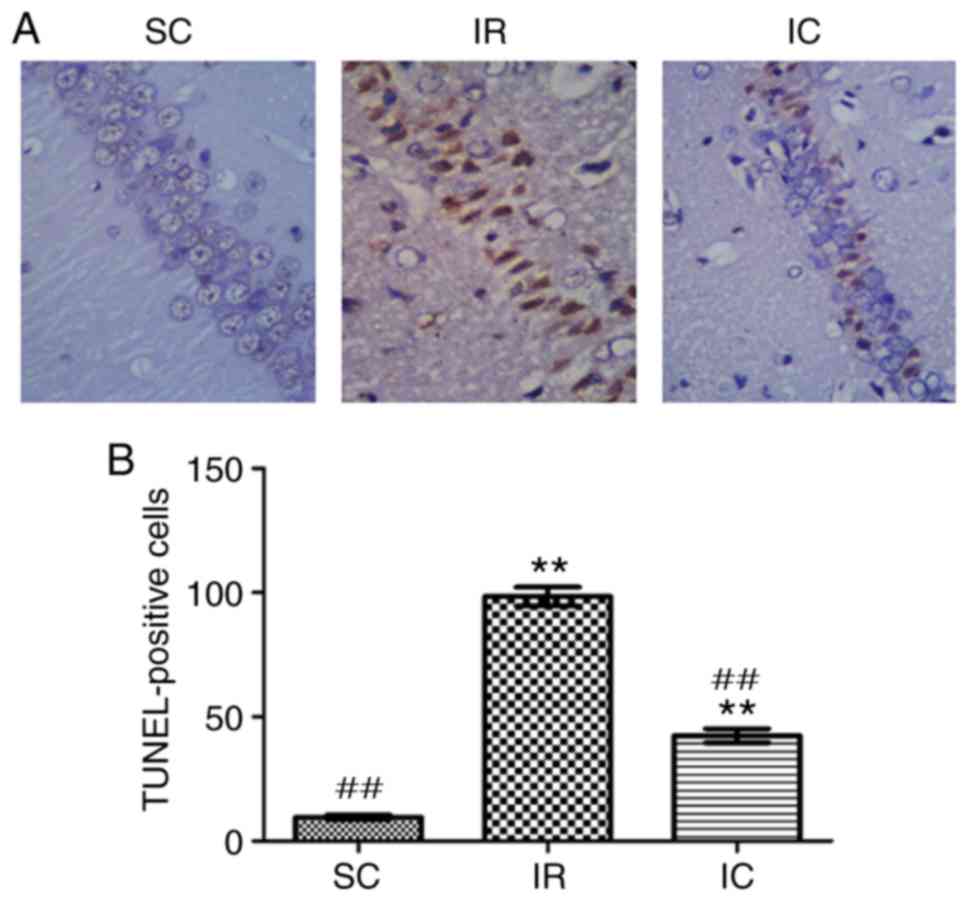
Cur can reduce neuronal apoptosis in the
hippocampal CA1 region as demonstrated by TUNEL staining
A total of 24 h following ischemia/reperfusion, the
TUNEL method was utilized to detect neuronal apoptosis of
hippocampal CA1. In hippocampal CA1, normal cells were stained
blue, but apoptotic cell nuclei were stained brown. As presented in
Fig. 6, in the SC group, almost
no TUNEL-positive cells were present in the hippocampal CA1 region.
Compared with the SC group, the number of apoptotic cells in the IR
group was significantly increased. However the IC group (300 mg/kg)
displayed a significantly lower number of apoptotic cells
(P<0.01).
Cur can increase the expression of p-MEK,
MEK, p-ERK, ERK, p-CREB, CREB, Bcl-2 and reduce the expression of
Bax in vivo
The expression of p-MEK, MEK, p-ERK, ERK, p-CREB,
CREB, Bcl-2 and Bax were detected by western blotting 24 h
following reperfusion in vivo. As presented in Fig. 7, it was demonstrated that the IC
group (300 mg/kg) increased the expression of p-ERK, p-CREB, Bcl-2
and reduced the levels of Bax significantly (P<0.01).
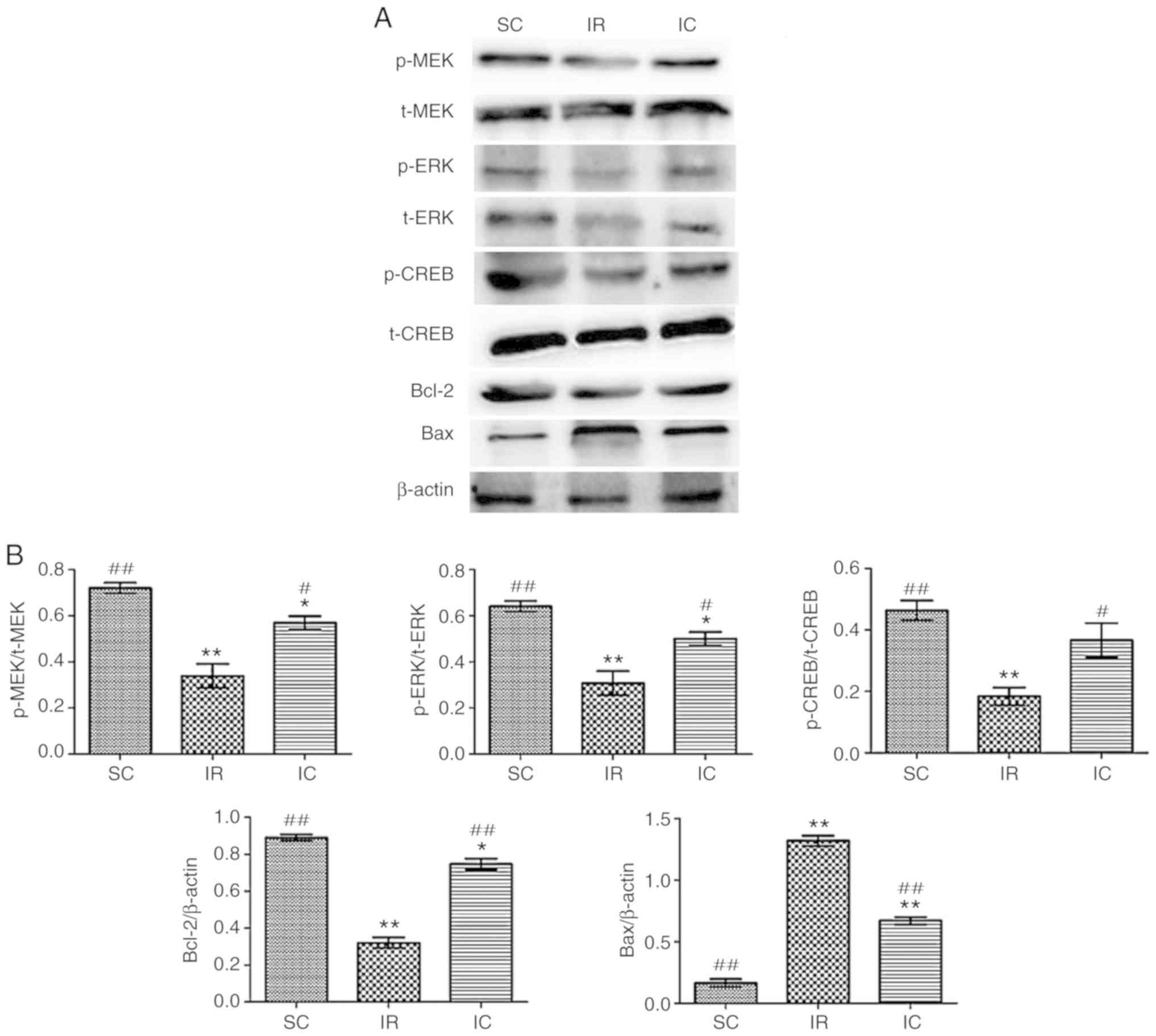 | Figure 7Effect of curcumin on the expression
of p-MEK, MEK, p-ERK, ERK, p-CREB, CREB, Bcl-2 and Bax in
vivo. (A) Western blot analysis of p-MEK, MEK, p-ERK, ERK,
p-CREB, CREB, Bcl-2 and Bax (n=3). For p-MEK, p-ERK and p-CREB,
t-MEK, t-ERK and t-CREB were used as references respectively. For
Bcl-2 and Bax, β-actin was used as an internal reference. (B) The
protein content in the three experiments was analyzed.
*P<0.05 and **P<0.01 vs. the SC;
#P<0.05 and ##P<0.01 vs. IR. SC, sham
group; IR, ischemia-reperfusion group; IC, curcumin treatment
group; p, phosphorylated; Bcl, B-cell lymphoma; CREB, cAMP-response
element binding protein; t, total; MEK, methyl ethyl ketone; ERK,
extracellular signal regulated kinase; Bax, Bcl-2 associated X
protein. |
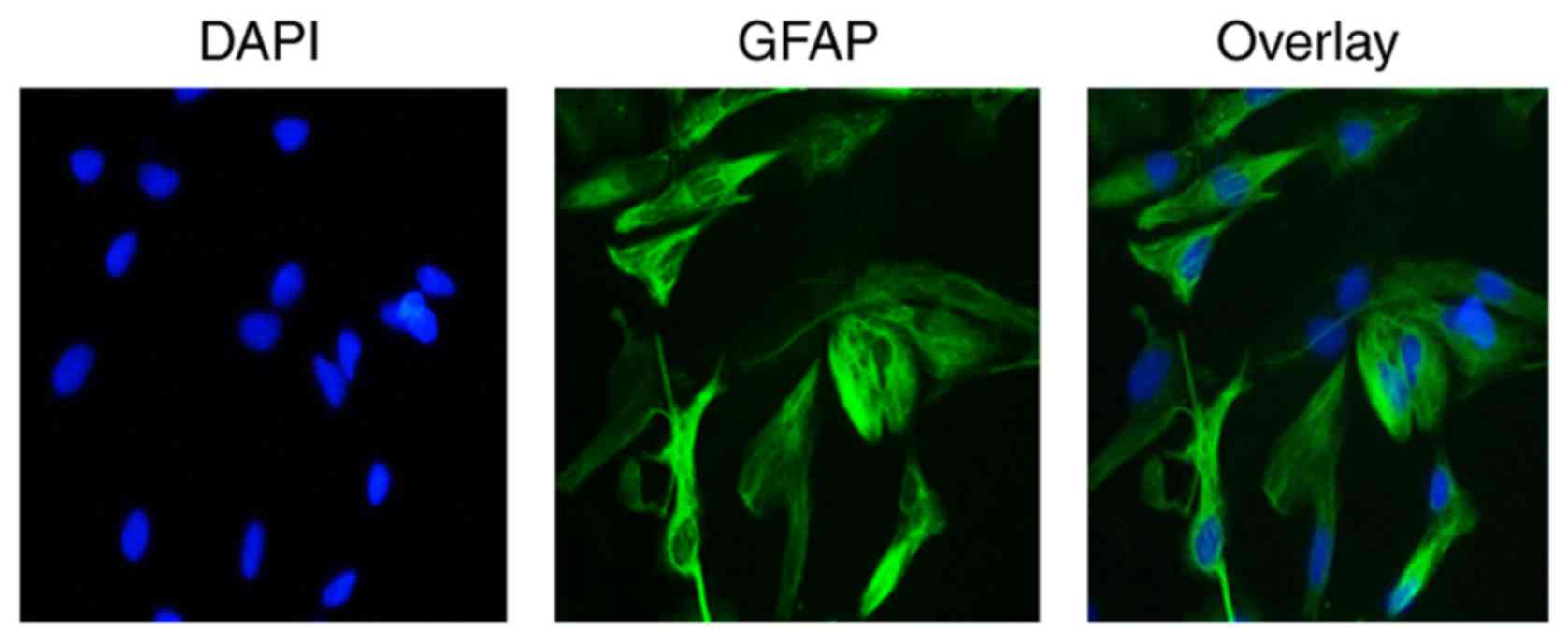
Identification of astrocytes in
vitro
Astrocytes were identified by immunofluorescence
staining with astrocyte-specific marker GFAP antibody. Under a
fluorescent microscope, a notable green fluorescence was visible in
the cell cytoplasm and projections, and nuclei appeared blue by
DAPI staining. Furthermore, cytons were large and irregular with
few cell projections of GFAP-positive cells. These characteristics
were consistent with the morphology of astrocytes and distribution
characteristics of GFAP, demonstrating that astrocytes were present
(Fig. 8).
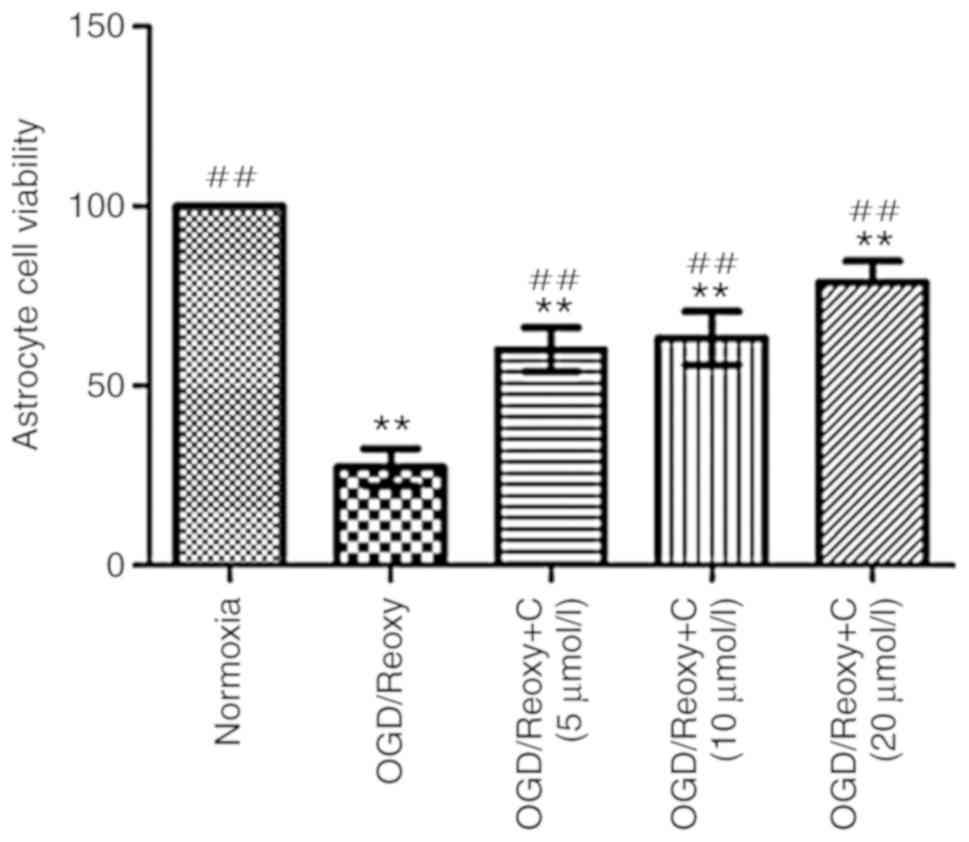
Cur can increase the viability of
astrocytes in vitro
A total of 24 h following oxygen-glucose
deprivation/reoxygenation, the effect of Cur on the viability of
astrocytes was examined. As presented in Fig. 9. compared with the OGD/Reoxy
group, the OGD/Reoxy + C groups significantly increased the
astrocytes’ viability (P<0.01), particularly the OGD/Reoxy + C
group of 20 µmol/l.
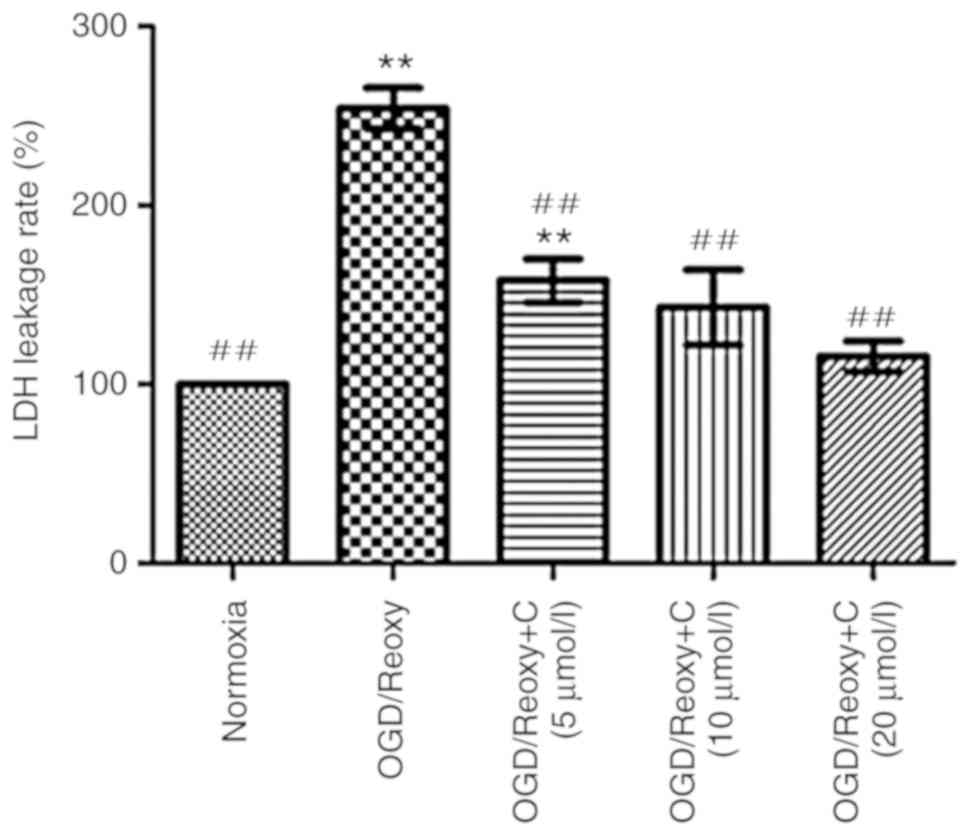
Cur can reduce LDH leakage rates
Following oxygen-glucose deprivation/reoxygenation
for 24 h, the effect of Cur on LDH leakage rates was examined. As
presented in Fig. 10, compared
with the OGD/Reoxy group, the OGD/Reoxy + C groups significantly
reduced the LDH leakage rate (P<0.01), particularly the
OGD/Reoxy + C group of 20 µmol/l. Therefore, only
C-treatment group of 20 µmol/l was used in other
experiments.
Cur can increase the expression of p-MEK,
MEK, p-ERK, ERK, P-CREB, CREB, Bcl-2 and reduce the expression of
Bax in vitro
The expression of p-MEK, MEK, p-ERK, ERK, p-CREB,
CREB, Bcl-2 and Bax were detected by western blotting for 24 h
following reperfusion in vitro. As presented in Fig. 11, the IC groups (20
µmol/l) increased the expression of p-MEK, MEK, p-ERK, ERK,
p-CREB, CREB, Bcl-2 and reduced the Bax levels significantly
compared with the IR group (P<0.01). These results were
consistent with the experimental results in vivo.
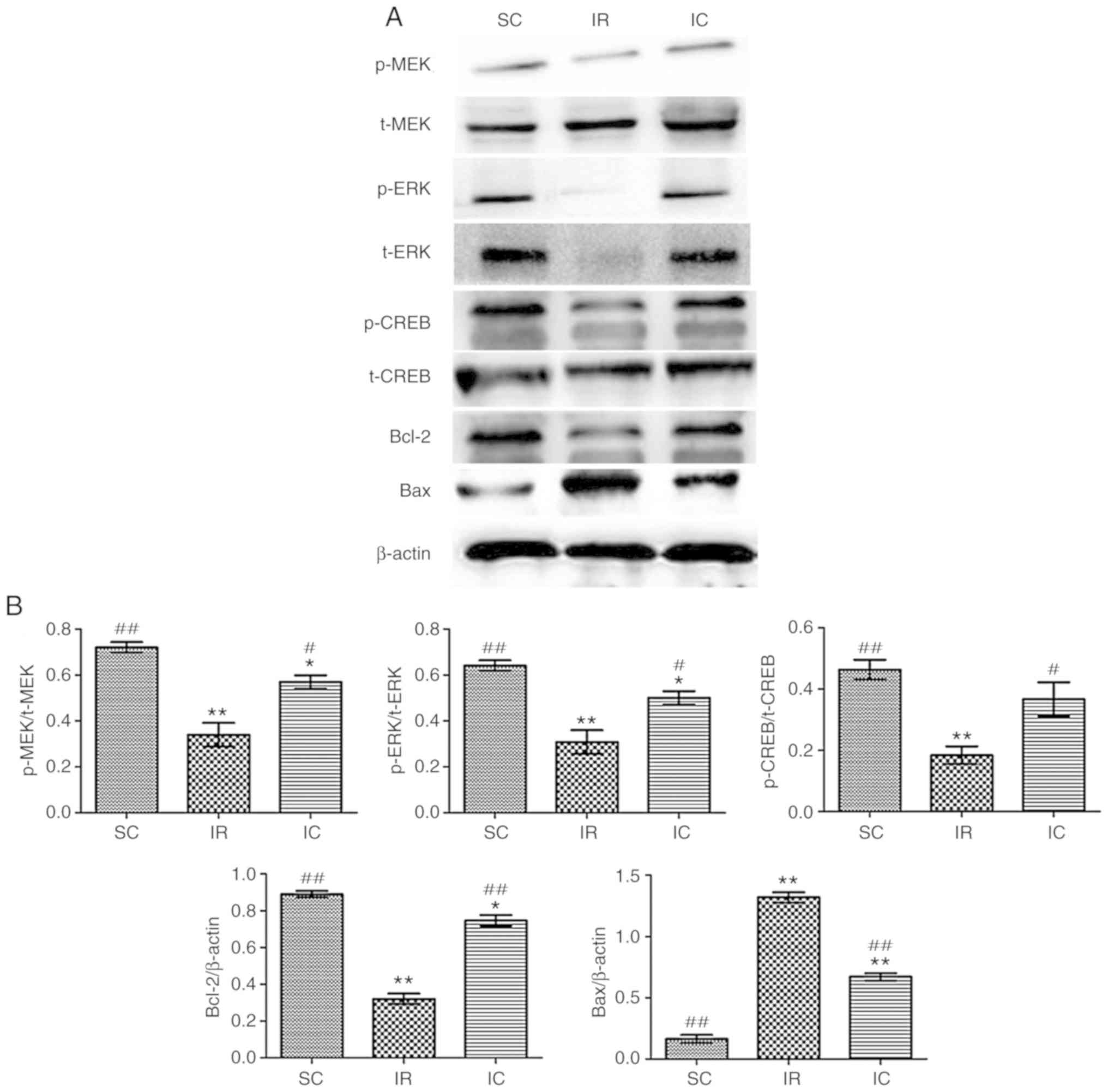 | Figure 11Effect of curcumin on the expression
of p-MEK, MEK, p-ERK, ERK, p-CREB, CREB, Bcl-2 and Bax in
vitro. (A) Western blot analysis of p-MEK, MEK, p-ERK, ERK,
p-CREB, CREB, Bcl-2 and Bax (n=3). For p-MEK, p-ERK and p-CREB,
t-MEK, t-ERK and t-CREB were used reference respectively. For Bcl-2
and Bax, β-actin was used as an internal reference. (B) The protein
contents in the three experiments were analyzed.
*P<0.05 and **P<0.01 vs. SC;
#P<0.05 and ##P<0.01 vs. IR. SC, sham
group; IR, ischemia-reperfusion group; IC, curcumin treatment
group; p, phosphorylated; Bcl, B-cell lymphoma; CREB, cAMP-response
element binding protein; t, total; MEK, methyl ethyl ketone; ERK,
extracellular signal regulated kinase; Bax, Bcl-2 associated X
protein. |
Discussion
Glial cells were first identified by Rudolf Virchow
in 1846 (21). In the years
since, a wide range of research has been conducted on the
morphology and function of glial cells, especially astrocytes.
Astrocytes were proved not to be simply inert cells, in contrast,
they serve a very important role in the development of the nervous
system, nerve tissue repair and regeneration, nerve and immune
pathogenesis (22,23).
There are also studies which indicate that
activation of the MEK/ERK or ERK/CREB pathway may protect
astrocytes from ischemic injury (24,25). Cur is known to have
neuroprotective properties in cerebral ischemia-reperfusion injury.
However, the underlying molecular mechanisms are still poorly
understood. The main focus of this study are the in vivo
experiments that utilize the artery occlusion model in rats,
however the hypothesis was further confirmed by conducting
experimental studies in vitro. To the best of our knowledge
this study is the first to investigate the association between Cur
and the MEK/ERK/CREB signaling pathway in cerebral
ischemia-reperfusion.
There is significant evidence demonstrating that
activation of the MEK-ERK1/2 signaling pathway has a
neuroprotective effect in cerebral ischemia-reperfusion injury
(26). Additionally Fu et
al (23) also demonstrated
that increasing the expression of p-CREB through MEK/ERK pathways
can exert neuroprotective effects against ischemia. Previous
studies have also confirmed that Bcl-2, BAD, CREB, glycogen
synthesis kinase3 and brain-derived neurotrophic factor are the
MEK/ERK pathway downstream targets, and they serve a very important
role in neuronal survival, development and maintaining the
plasticity of neurons (27-29) ERK may also improve
microcirculation and reduce neuronal apoptosis.
CREB and the active form p-CREB, are important
nuclear transcription factors, which serve an indispensable role in
the nervous system (30) and CREB
activation is a key factor in neuroprotection against ischemic
reperfusion damage. During a stroke, a variety of apoptosis
regulatory gene products are activated (31). Among them, Bcl-2 is an important
anti-apoptotic proteins and Bax is an important pro-apoptotic
protein (32,33). Enhancing Bcl-2 and reducing Bax
have been demonstrated to promote cell survival and promote a
neuroprotective effect following focal cerebral ischemia (34,35).
The present study suggests that Cur can improve
neurological symptom scores and brain water content, (with the best
effect at 300 mg/kg) and enhance the activation of MEK, ERK and
CREB. The expression of downstream signaling molecules Bcl-2 is
regulated by CREB. As indicated above, the expression of Bcl-2 was
enhanced and Bax was reduced. The results of the TUNEL assay also
verified the neuroprotective effects in terms of pathomorphology.
In hippocampal CA1 of the rats, the results of TUNEL demonstrated
that in the SC group, almost no TUNEL-positive cells were
identified in the hippocampal CA1 region. However in the IR group,
TUNEL-positive cells were increased. Following treatment with Cur
(300 mg/kg), TUNEL-positive cells were reduced. The results of
neurobehavioral scores, TEM and H&E, were also consistent with
these findings.
In conclusion, Cur can protect rats from focal
cerebral ischemia-reperfusion injury and this effect may be carried
out through the MEK/ERK/CREB pathway.
Acknowledgments
The authors would like to thank Professor Zhi Dong
for guidance and the Chongqing Medical University Laboratory of
Biochemistry and Molecular Pharmacology for providing the platform
for the experiment.
Funding
The present study was supported financially by the
Chongqing Science and Technology Commission (grant nos.
CSTC2016jcyjA0268, CSTC2018jcyjAX0821 and CSTC2018jxj1130009) and
the Chongqing Medical and Pharmaceutical College Scientific
Research Projects (grant no. ygz2018302).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors’ contributions
YH and JL conceived and designed the study. LX, LD
and YS performed the experiments. RS analyzed the data. LX wrote
and revised the paper. YH and JL reviewed and edited the
manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Experimental protocols were all approved by the
Chongqing Medical and Pharmaceutical College’s Institutional Animal
Care and Use Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Durai Pandian J, Padma V, Vijaya P, Sylaja
PN and Murthy JM: Stroke and thrombolysis in developing countries.
Int J Stroke. 2:17–26. 2007. View Article : Google Scholar
|
|
2
|
Moskowitz MA, Lo EH and Iadecola C: The
science of stroke: Mechanisms in search of treatments. Neuron.
67:181–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benjamin EJ, Virani SS, Callaway CW,
Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling
FN, Deo R, et al: Heart disease and stroke statistics-2018 update:
A report from the American heart association statistics committee
and stroke statistics subcommittee. Circulation. 137:e67–e492.
2018. View Article : Google Scholar
|
|
4
|
Fogelholm R: Editorial
comment-Explanations for international trends in stroke mortality.
Stroke. 34:1833–1840. 2003. View Article : Google Scholar
|
|
5
|
Nagahiro S, Uno M, Sato K, Goto S, Morioka
M and Ushio Y: Pathophysiology and treatment of cerebral ischemia.
J Med Invest. 45:57–70. 1998.PubMed/NCBI
|
|
6
|
Bates B, Choi JY, Duncan PW, Glasberg JJ,
Graham GD, Katz RC, Lamberty K, Reker D and Zorowitz R; US
Department of Defense and Department of Veterans Affairs: Veterans
affairs/department of defense clinical practice guideline for the
management of adult stroke rehabilitation care: Executive summary.
Stroke. 36:2049–2056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grotta J and Marler J: Intravenous rt-PA:
A tenth anniversary reflection. Surg Neurol. 68:S12–S16. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan J, Konstas AA, Bateman B, Ortolano GA
and Pile-Spellman J: Reperfusion injury following cerebral
ischemia: Pathophysiology, MR imaging, and potential therapies.
Neuroradiology. 49:93–102. 2007. View Article : Google Scholar :
|
|
9
|
Minnerup J, Sutherland BA, Buchan AM and
Kleinschnitz C: Neuroprotection for stroke: Current status and
future perspectives. Int J Mol Sci. 13:11753–11772. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Love S: Apoptosis and brain ischaemia.
Prog Neuropsy chopharmacol Biol Psychiatry. 27:267–282. 2003.
View Article : Google Scholar
|
|
11
|
Sugawara T, Fujimura M, Noshita N, Kim W,
Saito A, Hayashi T, Narasimhan P, Maier CM and Chan Pak H: Neuronal
death/survival signaling pathways in cerebral ischemia. NeuroRx.
1:17–25. 2004. View Article : Google Scholar
|
|
12
|
Zhao Y, Gui W, Niu F and Chong S: The MAPK
signaling pathways as a novel way in regulation and treatment of
parasitic diseases. Disease. 7:E92019. View Article : Google Scholar
|
|
13
|
Selcher JC, Atkins CM, Trzaskos JM, Paylor
R and Sweatt JD: A necessity for MAP kinase activation in mammalian
spatial learning. Learn Mem. 6:478–490. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng CY, Lin JG, Su SY, Tang NY, Kao ST
and Hsieh CL: Electroacupuncture-like stimulation at baihui and
dazhui acupoints exerts neuroprotective effects through activation
of the brain-derived neurotrophic factor-mediated
MEK1/2/ERK/1/2/p90RSK/bad signaling pathway in mild transient focal
cerebral ischemia in rats. BMC Complement Altern Med. 14:922014.
View Article : Google Scholar
|
|
15
|
Li J, Li X, Bi H and Li B: The
MEK/ERK/CREB signaling pathway is involved in atrazine induced
hippocampal neurotoxicity in sprague dawley rats. Ecotoxicol
Environ Saf. 170:673–681. 2019. View Article : Google Scholar
|
|
16
|
Zuo H, Lin T, Wang W, Peng R, Wang S, Gao
Y, Xu X, Zhao L, Wang S and Su Z: RKIP regulates neural cell
apoptosis induced by exposure to microwave radiation partly through
the MEK/ERK/CREB pathway. Mol Neurobiol. 51:1520–1529. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao X, Xu Y, Zhao Q, Chen CR, Liu AM and
Huang ZL: Curcumin exerts antinociceptive effects in a mouse model
of neuropathic pain: Descending monoamine system and opioid
receptors are differentially involved. Neuropharmacology.
62:843–854. 2012. View Article : Google Scholar
|
|
18
|
Kim KT, Kim MJ, Cho DC, Park SH, Hwang JH,
Sung JK, Cho HJ and Jeon Y: The neuroprotective effect of treatment
with curcumin in acute spinal cord injury: Laboratory
investigation. Neurol Med Chir. 54:387–394. 2014. View Article : Google Scholar
|
|
19
|
Gazal M, Valente MR, Acosta BA, Kaufmann
FN, Braganhol E, Lencina CL, Stefanello FM, Ghisleni G and Kaster
MP: Neuroprotective and antioxidant effects of curcumin in a
ketamine-induced model of mania in rats. Eur J Pharmacol.
724:132–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nilsson M, Thorlin T, Blomstrand F and
Hansson E: The star-shaped cells. Astrocytes are involved in the
pathogenesis and progress of neurological diseases. Lakartidningen.
97:3604–3610. 2000.PubMed/NCBI
|
|
22
|
Ridet JL, Malhotra SK, Privat A and Gage
FH: Reactive astrocytes: Cellular and molecular cues to biological
function. Trends Neurosci. 20:570–577. 1997. View Article : Google Scholar
|
|
23
|
Fu J, Xue R, Gu J, Xiao Y, Zhong H, Pan X
and Ran R: Neuroprotective effect of calcitriol on
ischemic/reperfusion injury through the NR3A/CREB pathways in the
rat hippocampus. Mol Med Rep. 8:1708–1714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Z, Zhang Y, Chen X, Lam PY, Yang H,
Xu Q and Yu AC: Activation of Erk1/2 and Akt in astrocytes under
ischemia. Biochem Biophys Res Commun. 294:726–733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou B, Chen D, Xu H and Zhang X:
Proliferation of rabbit chondrocyte and inhibitioof IL-1β-induced
apoptosis through MEK/ERKsignaling by statins. In Vitro Cell Dev
Biol Anim. 53:124–131. 2017. View Article : Google Scholar
|
|
26
|
Yu Z, Cai M, Li X, Zhang J, Wu T, Yang F,
Zhu W, Xiang Y, Zhang W, Xiang J and Cai D: Neuroprotective effects
of Tongxinluo on focal cerebral ischemia and reperfusion injury
inrats associated with the activation of the MEK1/2/ERK1/2/p90RSK
signaling pathway. Brain Res. 1685:9–18. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Annu Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marinissen MJ and Gutkind JS:
G-protein-coupled receptors and signaling. Networks: Emerging
paradigms. Trends Pharmacol Sci. 22:368–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weeber EJ and Sweatt JD: Molecular
neurobiology of human cognition. Neuron. 33:845–848. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sakamoto K, Karelina K and Obrietan K:
CREB: A multifaceted regulator of neuronal plasticity and
protection. J Neurochem. 116:1–9. 2011. View Article : Google Scholar
|
|
31
|
Lipton P: Ischemic cell death in brain
neurons. Physiol Rev. 79:1431–1568. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brambrink AM, Schneider A, Noga H,
Astheimer A, Gotz B, Korner I, Heimann A, Welschof M and Kempski O:
Tolerance-Inducing dose of 3-nitropropionic acid modulates bcl-2
and bax balance in the rat brain: A potential mechanism of chemical
preconditioning. J Cereb Blood Flow Metab. 20:1425–1436. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ajami M, Eghtesadi S, Razaz JM, Kalantari
N, Habibey R, Nilforoushzadeh MA, Zarrindast M and Pazoki-Toroudi
H: Expression of Bcl-2 and Bax after hippocampal ischemia in DHA
+EPA treated rats. Neurol Sci. 32:811–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu Y, Bu Q, Liu X, Hu W and Wang Y:
Neuroprotective effect of TAT-14-3-3epsilon fusion protein against
cerebral ischemia/reperfusion injury in rats. PLoS One.
9:e933342014. View Article : Google Scholar
|
|
35
|
Boucher MJ, Duchesne C, Laine J, Morisset
J and Rivard N: cAMP protection of pancreatic cancer cells against
apoptosis induced by ERK inhibition. Biochem Biophys Res Commun.
285:207–216. 2001. View Article : Google Scholar : PubMed/NCBI
|