Introduction
Ovarian cancer is one of the most common diseases in
woman. The 5-year survival rates for patients with advanced ovarian
cancer remain at 20-30% (1).
Chemotherapy has been used to treat ovarian cancer for several
decades. Alkylating agents were developed as single chemotherapy
drugs during the 1970s (2).
Cisplatin-based regimen was established as the standard first-line
chemotherapy for patients with ovarian cancer in the late 1980s
(3). Although cisplatin is a
widely used and a highly active chemotherapeutic agent for ovarian
cancer, its use has been limited due to its cumulative toxicities,
in particular its nephrotoxicity (2). In addition, instances of intrinsic
and acquired resistance to cisplatin have been observed in patients
with ovarian cancer (2).
Therefore, novel chemotherapeutic agents are urgently required to
treat ovarian cancer, and to promote the effectiveness and decrease
the side effects of cisplatin treatment.
Adenosine 5′-phosphate (AMP)-activated protein
kinase (AMPK), a nutrient and energy sensor in mammalian cells,
regulates glucose and lipid metabolism (4). AMPK is a heterotrimeric
serine/threonine protein kinase that is composed of a catalytic
α-subunit and 2 regulatory subunits, the β- and γ-subunits. Under
normal physiological conditions, AMPK protects cells against
various metabolic stresses by maintaining homeostatic pools of the
adenosine nucleotides [adenosine 5′-triphosphate (ATP), adenosine
5′-diphosphate (ADP), and AMP] (4). However, the role of AMPK signaling
in cancer has not yet been fully elucidated. Epidemiological
investigations have suggested that treatment with metformin, a drug
that activates AMPK, was associated with decreased incidence of
diseases, including breast, lung, colon, prostate and pancreatic
cancer (5-8). Experimental data have confirmed that
metformin exerts an inhibitory effect on the growth of breast and
pancreatic cancers (9,10). Clinical data have also confirmed
that metformin may improve the overall survival of patients with
diabetes and cancer either alone or in combination with
chemotherapy (11,12). However, conventional AMPK
activators including metformin may cause the toxic side effect of
lactic acidosis with dazzling, muscle pain, tiredness and stomach
pain (13). Therefore, the use of
nontoxic and natural AMPK activators may be preferable to treat
ovarian cancer and its chemoresistance.
The mammalian target of rapamycin (mTOR)
serine/threo-nine kinase, a downstream effector of AMPK, exists in
2 biochemically distinct complexes, mTOR complex 1 and mTOR complex
2. mTOR, similarly to AMPK, serves critical roles not only in cell
growth and cell proliferation but also in metabolism (14). AMPK phosphorylates and activates
tuberous sclerosis 1 protein (TSC1)/tuberous sclerosis 2 protein
(TSC2), thereby inhibiting mTOR. mTOR leads to inhibition of
downstream targets p70S6 kinase (p70S6K) and eukaryotic translation
initiation factor 4E-binding protein 1 (4EBP1), which are involved
in cell growth primarily through the regulation of translation and
protein synthesis (15). AMPK
controls tumor development through the modulation of mTOR activity
(16). Therefore, the AMPK/mTOR
pathway is a promising target for cancer therapy.
Herbal medicine has been widely used to treat
illnesses for centuries. It is the most productive source of lead
compounds for drug development (17). It includes various natural
compounds with biological activities and therapeutic effectiveness,
with minimum side effects (17).
Magnolia officinalis is a species of Magnolia. Its
roots and stem bark have been used for treating thrombotic stroke,
gastrointestinal complaints, anxiety, nervous disturbance, allergic
diseases and cancer (18).
Studies have demonstrated that Magnolia extract is a safe
medicine with low toxicity (18-20). Honokiol is a natural biphenolic
compound derived from the bark of Magnolia trees with
anti-oxidative, anti-inflammatory and anti-tumor properties
(21). Several mechanisms
involved in the anti-tumor activities of honokiol against leukemia,
breast, pancreatic and prostate cancer, oral squamous cell
carcinoma, and skin, gastric, bone and brain cancer have been
suggested (22), including the
induction of cell cycle arrest (23), apoptosis (24), autophagy (25), and anti-proliferative (26) and anti-invasive processes
(27-29).
The present study evaluated the therapeutic
potential of honokiol based on its anticancer properties, including
its effects on apoptosis, migration and invasion in ovarian cancer
cells. Additionally, the potential molecular mechanisms involved in
its anticancer effects were explored.
Materials and methods
Reagents
Honokiol, compound C and
5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Dulbecco's modified eagle's medium (DMEM), McCoy's 5A medium, fetal
bovine serum (FBS) were purchased from Gibco; Thermo Fisher
Scientific, Inc., (Waltham, MA, USA). RPMI-1640 Medium and
Trypsin/EDTA were purchased from HyClone (GE Healthcare Life
Sciences, Logan, UT, USA). The Cell Counting kit-8 was obtained
from Dojindo Molecular Technologies, Inc., (Kumamoto, Japan).
Rabbit polyclonal anti-human caspase-3 (cat. no. 9662), mouse
monoclonal anti-human caspase-7 (cat. no. 9494), rabbit polyclonal
anti-human caspase-9 (cat. no. 9502), rabbit poly-clonal anti-human
poly-(ADP-ribose) polymerase (PARP; cat. no. 9542), rabbit
monoclonal anti-human phospho-AMPK (Thr172; cat. no. 2535), rabbit
polyclonal anti-human AMPK (cat. no. 2532), rabbit polyclonal
anti-human phospho-mTOR (Ser2448; cat. no. 2971), rabbit polyclonal
anti-human mTOR (cat. no. 2972), rabbit polyclonal anti-human
phospho-4EBP1 (Thr70; cat. no. 9455), rabbit polyclonal anti-human
4EBP1 (cat. no. 9452) and rabbit polyclonal anti-human β-actin
(cat. no. 4967) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Horseradish
peroxidase-conjugated anti-mouse (cat. no. 7076) and anti-rabbit
(cat. no. 7074; both 1:3,000) secondary antibodies were purchased
from Cell Signaling Technology, Inc. Super Signal® West
Pico Chemiluminescent substrate was purchased from Pierce; Thermo
Fisher Scientific, Inc.
Cell lines and culture
Human ovary adenocarcinoma SKOV3, Caov-3 and NIH-3T3
cell lines were purchased from the Korean Cell Line Bank, Korean
Cell Line Research Foundation (Seoul, Korea), and grown in McCoy's
5A, DMEM and RPMI-1640 media, respectively, supplemented with 10%
(v/v) FBS. Cells were maintained at 37°C in a humidified 5%
CO2-controlled incubator.
Cell viability assay
Cells were seeded at 5×103 cells/ml in
96-well microplates and were cultured overnight to allow
attachment. Honokiol (1-100 µM), compound C (20 µM),
and AICAR (500 µM) were added to the medium. Following
treatment, cell viability was assessed using the CCK-8 assay. CCK-8
(10 µl) solution was added, and cells were incubated for 3 h
at 37°C. The optical density (OD) was assessed at 450 nm using a
precision microplate reader (Molecular Devices, LLC., San Jose, CA,
USA).
Soft agar colony forming assay
Cells (5×103/ml) were suspended in growth
medium (3 ml) containing 0.3% agar and 10% FBS. They were then
applied to pre-solidified 0.6% agar (3 ml in FBS-free growth media)
in 60 mm culture dishes. After 2-3 weeks of incubation at 37°C,
colonies on soft agar were observed under a phase-contrast
microscope (IX2-ILL100, Olympus Corporation, Tokyo, Japan) at a
magnification of ×40.
Western blot analysis
Cells were harvested using Trypsin-EDTA, and washed
twice in cold PBS. Cells were lysed with lysis buffer (10 mM Tris,
pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% TritonX-100, 0.5% NP-40, 1 mM
PI, 1 mM DTT, 1 mM PMSF) and placed on ice for 1 h. The supernatant
was obtained by centrifugation at 13,000 × g for 10 min at 4°C. A
Pierce BCA Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc.) was used to measure protein concentrations. Equal amounts of
protein (50 µg) were separated by SDS-PAGE and transferred
onto polyvinyli-dene difluoride membranes. Membranes were then
blocked with 5% skim milk in PBS containing 0.05% Tween-20 (PBST)
for 1 h at 25°C to prevent nonspecific antibody binding, then
incubated with caspase-3, caspase-7, caspase-9, PARP, AMPK,
phospho-AMPK, mTOR, phospho-mTOR, 4EBP1, phospho-4EBP1 and β-actin
primary antibodies (1:1,000) overnight at 4°C. Subsequent to
washing with PBST, membranes were incubated with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies
(both 1:3,000) at room temperature for 2 h and visualized with
enhanced chemiluminescence using Super Signal® West Pico
Chemiluminescent substrate. Band intensity was quantified by
densitometry using ImageJ software [version 1.52; National
Institutes of Health (NIH), Bethesda, MD, USA] and was normalized
to loading controls.
Annexin V-PI double staining assay
Cells were cultured at a density of
1×106/ml and treated with honokiol and compound C for 24
h. Cells were centrifuged at 1,000 × g for 5 min at room
temperature, and then the supernatant discarded. The cell pellet
was resuspended in 0.5 ml cold PBS. These cells were processed and
labeled using EzWay™ Annexin V-fluorescein isothiocyanate
(FITC)-propidium iodide (PI) Apoptosis Detection kit (KOMABIOTEC.,
Seoul, Korea). Labeled cells were then analyzed with a BD
FACSCanto™ II flow cytometer using BD FACSDiva™ software (version
6.1.3; BD Biosciences, Franklin Lakes, NJ, USA).
Wound healing assay
Cells were seeded into 6-well plates and incubated
in serum-free medium for 18 h. The cellular mono-layer was wounded
with a 10 µl-pipette tip and washed with serum-free media to
remove cells detached from the plates. These cells were incubated
in the presence and absence of honokiol for 48-72 h in growth
medium containing 10% FBS. The medium was then replaced with PBS
and images of the cells were captured using a phase-contrast
microscope at a magnification of ×40. Results were quantified using
ImageJ software (version 1.52).
Matrigel invasion assay
BD Biocoat™ Transwell Invasion Chambers were used to
perform cell invasion assays. Each insert was equipped with a 6.4
mm diameter PET porous membrane (pore size=8 µm) coated for
6 h at 37°C with Matrigel Matrix (BD Biosciences). Cells
(2.5×104) were suspended in serum-free medium (300
µl) with or without drugs. Cells were placed in the upper
chamber while medium (500 µl) containing 10% FBS was added
to the lower chamber as a chemoattractant. Following incubation for
24 h at 37°C, non-invading cells were removed from the upper
surface of the membrane, while invading cells on the lower surface
of the membrane were stained with 0.1% hematoxylin for 30 min at
room temperature. The membranes were then removed and light
microscopy was used to count invading cells at a magnification of
×40. Results were normalized to control cells, and the relative
invasion is expressed as mean ± standard deviation (SD) of
migrating cells compared with the control cells.
Statistical analysis
Statistical analyses were performed using
IBM® SPSS® Statistics v.24.0 (IBM Corp.,
Armonk, NY, USA). One-way analysis of variance followed by a
Tukey's post-hoc test was used for calculating significance between
different groups. Values are presented as mean ± SD of 3
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
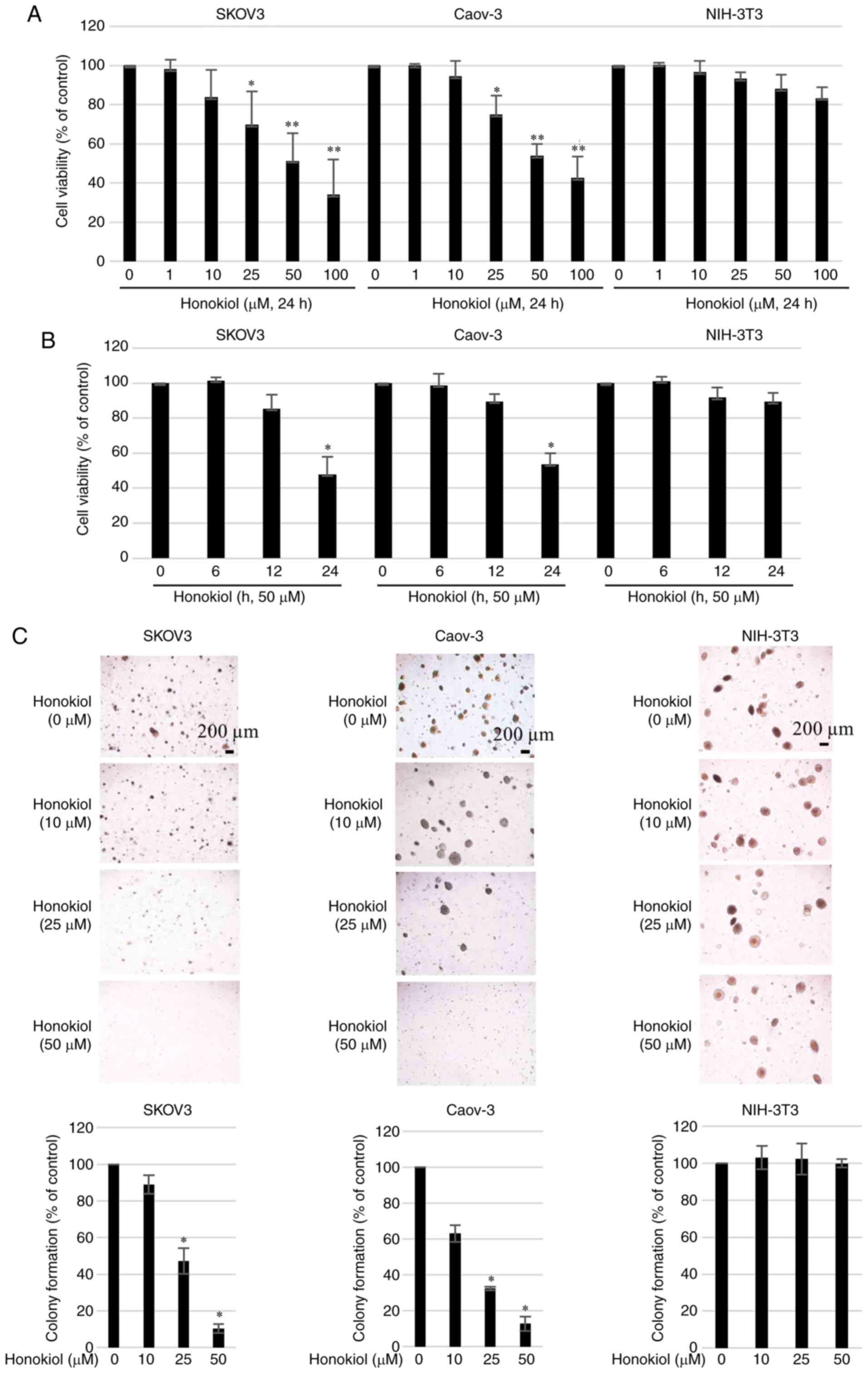
Effect of honokiol on ovarian cancer cell
proliferation and colony formation
To evaluate the therapeutic potential of honokiol
for ovarian cancer treatment, the human ovarian cancer SKOV3 and
Caov-3 cell lines were cultured with increasing concentrations of
honokiol (1-100 µM) for 24 h. Cell viability (%) was then
determined by CCK-8 assay. Honokiol significantly inhibited the
growth of ovarian cancer cells in a dose-(1-100 µM) and
time-(6-24 h) dependent manner (Fig.
1A and B). Honokiol induced a dose-dependent decrease in the
growth of ovarian cancer cells, with a half-maximal inhibitory
concentration (IC50) values of 48.71±11.31 µM for
SKOV3 cells and 46.42±5.37 µM for Caov-3 cells after 24 h of
treatment. Honokiol also exhibited low toxicity in the non-cancer
NIH-3T3 fibroblast cell line. Subsequently, the effect of honokiol
on cell colony formation was investigated. Consistent with its
cancer cell toxicity, honokiol inhibited the colony formation
property of SKOV3 and Caov-3 cells in a dose-dependent manner
(Fig. 1C).
Honokiol activates AMPK in ovarian cancer
cells
To additionally examine the mechanism by which
honokiol induced growth inhibition of ovarian cancer cells, AMPK
signaling in SKOV3 and Caov-3 cells was studied. Honokiol
dose-dependently induced the phosphorylation of the Thr172 subunit
of AMPK in ovarian cancer cells (Fig.
2A). Honokiol-induced AMPK activation was associated with a
decreased level of phosphorylation of mTOR (Fig. 2A). To additionally confirm these
changes, the AMPK inhibitor compound C was used in combination with
honokiol. Compound C is a potent, selective and reversible
ATP-competitive inhibitor of AMPK (inhibitor constant=109 nM in the
presence of 5 µM ATP and absence of AMP). The results
indicated that compound C attenuated honokiol-induced AMPK
activation (Fig. 2B), and rescued
ovarian cancer cells from cell growth inhibition induced by
honokiol (Fig. 2C). Conversely,
treatment with AMPK activator AICAR in combination with honokiol
markedly induced AMPK activation (Fig. 2B) and ovarian cancer cell death
(Fig. 2C). These results
indicated that AMPK regulation modulated honokiol-induced cell
death.
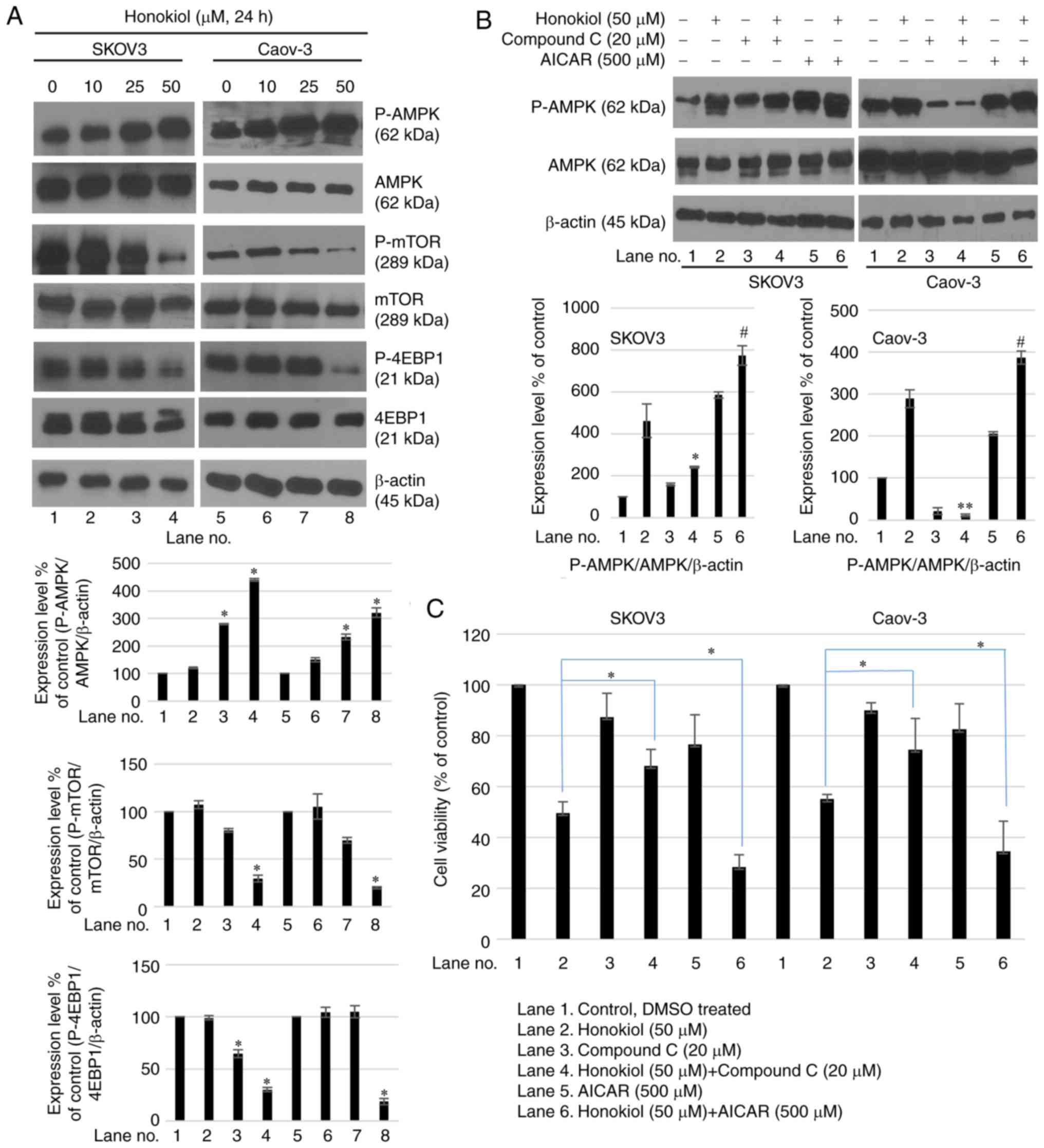 | Figure 2Honokiol activates AMPK in ovarian
cancer cells. (A) Cells were treated with honokiol (10, 25 and 50
µM) for 24 h. Total protein was isolated and equal amounts
of proteins were subjected to SDS-PAGE, and western blot analysis
was performed using specific antibodies for phosphorylated AMPK
(Thr172), AMPK, phosphorylated mTOR (Ser2448) and mTOR. β-actin was
used as a loading control. Values are presented as mean ± standard
deviation of 3 independent experiments. *P<0.05 vs.
the untreated control. (B) Cells were treated with honokiol (50
µM) for 24 h, then with compound C (20 µM) or AICAR
(500 µM) prior to honokiol stimulation. Western blot
analysis was performed to determine protein expression levels of
phosphorylated AMPK (Thr172) and AMPK. *P<0.05 and
**P<0.01 vs. honokiol-only treatment (lane 2).
#P<0.05 vs. honokiol single treatment (lane 2). (C)
Following pre-treatment with compound C (20 µM) or AICAR
(500 µM), cells were treated with honokiol (50 µM)
for 24 h. A CCK-8 assay was used to determine cell viability. Data
are presented as a percentage of the control. *P<0.05
vs. honokiol-only treatment. p, phosphorylated; AMPK, adenosine
5′-phosphate-activated protein kinase; mTOR, mechanistic target of
rapamycin; AICAR, aminoimidazole carboxamide ribonucleotide. |
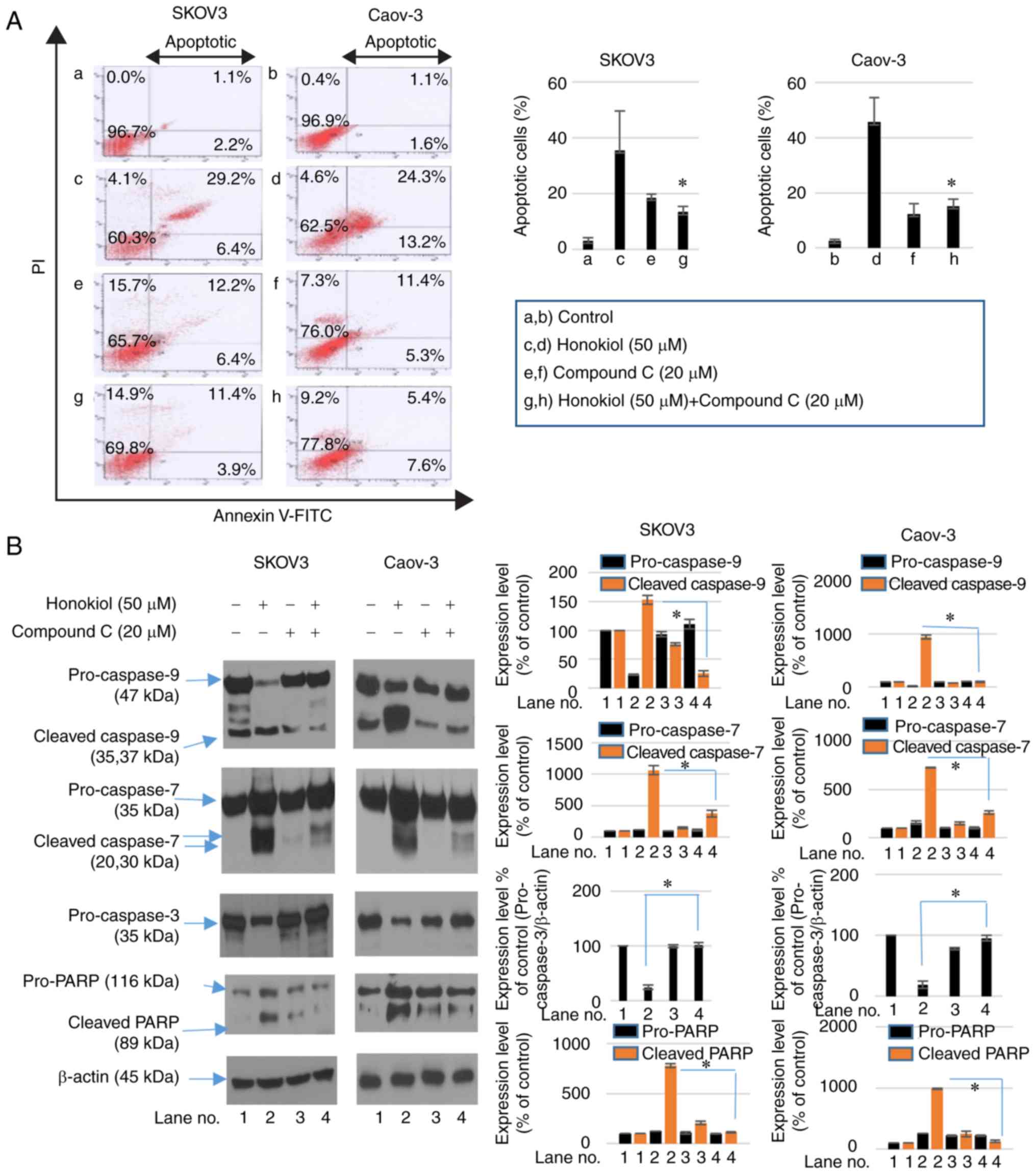
Honokiol induces apoptosis via AMPK
activation in ovarian cancer cells
To examine whether the induction of apoptosis
contributed to the honokiol-mediated decrease in cell viability of
ovarian cancer cells, Annexin V-FITC/PI staining was performed to
analyze the population of apop-totic cells. Treatment with honokiol
for 24 h increased the population of apoptotic cells. However,
compound C treatment with honokiol decreased Annexin V/PI-positive
cell numbers (Fig. 3A). The
mechanism involved in apoptotic cell death induced by honokiol
treatment was then investigated. SKOV3 and Caov-3 cells were
treated with honokiol and compound C for 24 h. Western blot
analysis was used to analyze the activation of caspase-3,
caspase-7, caspase-9, and cleavage of PARP. Following treatment
with honokiol, activation of caspase-3, -7, and -9 and increased
cleavage of PARP were observed. However, treatment with compound C
in combination with honokiol attenuated the activation of
caspase-3, -7 and -9, and increased cleavage of PARP induced by
honokiol alone (Fig. 3B). Cell
cycle analysis by flow cytometry also revealed an accumulation of
sub-G0/G1 cells following honokiol treatment,
while treatment with compound C decreased the proportion of
sub-G0/G1 cells (Fig. 3C). These results demonstrate that
honokiol-induced apoptosis was involved in its AMPK-mediated
anticancer activity.
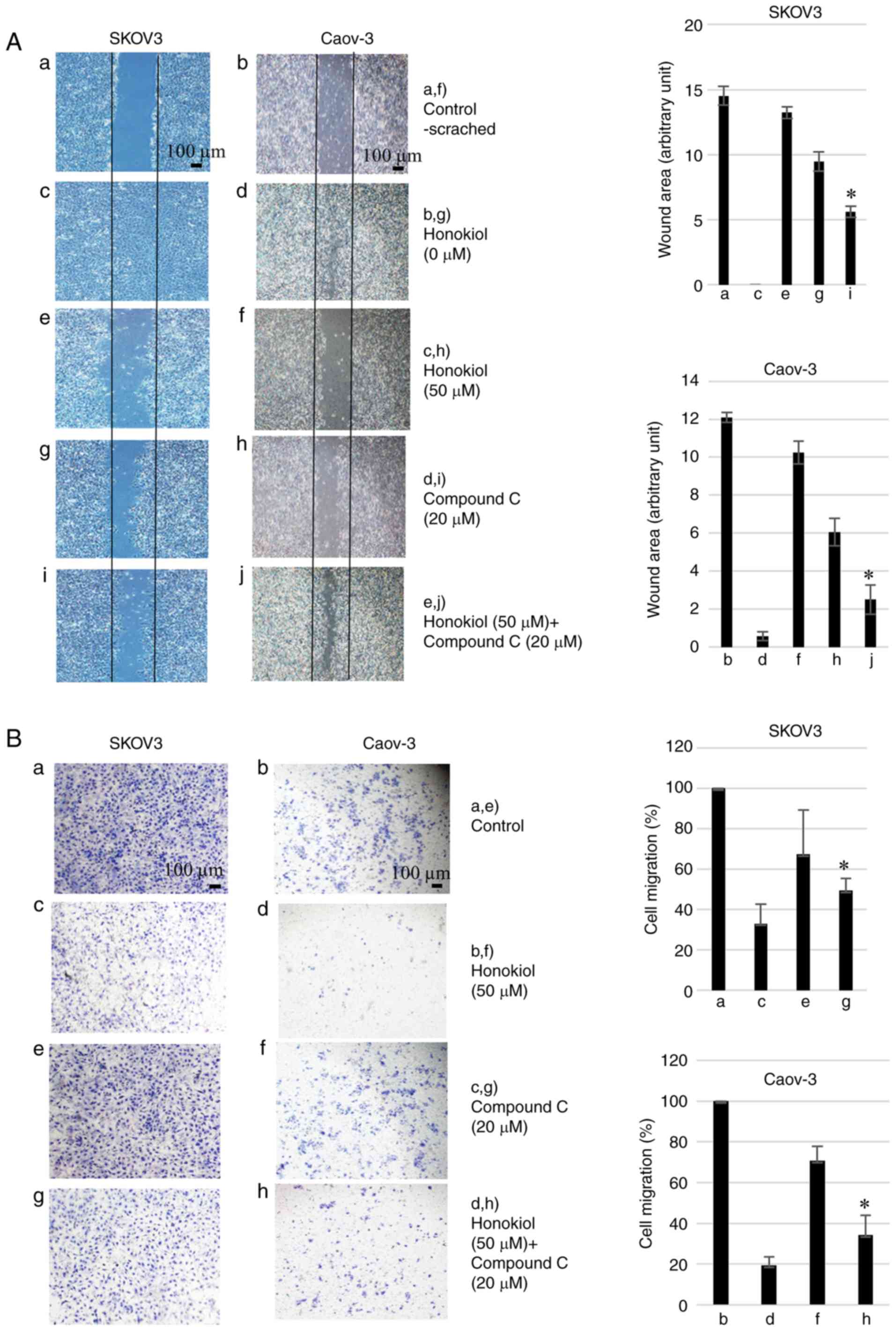
Honokiol inhibits migration and invasion
of ovarian cancer cells
To determine whether honokiol impaired the migration
and invasion of ovarian cancer cells, SKOV3 and Caov-3 were
subjected to honokiol treatment. Wound healing assays indicated
that honokiol treatment significantly inhibited the migration
distance between the leading edge of cells. However, compound C
reversed this activity (Fig. 4A).
The Matrigel invasion assays demonstrated that treatment with 50
µM honokiol resulted in a 66.9 and 80.7% decrease in
migration of SKOV3 and Caov-3 cells, respectively, compared with
the untreated cells. The decrease in Matrigel invasion induced by
honokiol was significantly reversed by compound C treatment
(Fig. 4B). These results
suggested that honokiol-induced AMPK activation inhibited the
migration and invasion of ovarian cancer cells. Based on the
results of the present study, the potential biological activities
of honokiol are illustrated in Fig.
5.
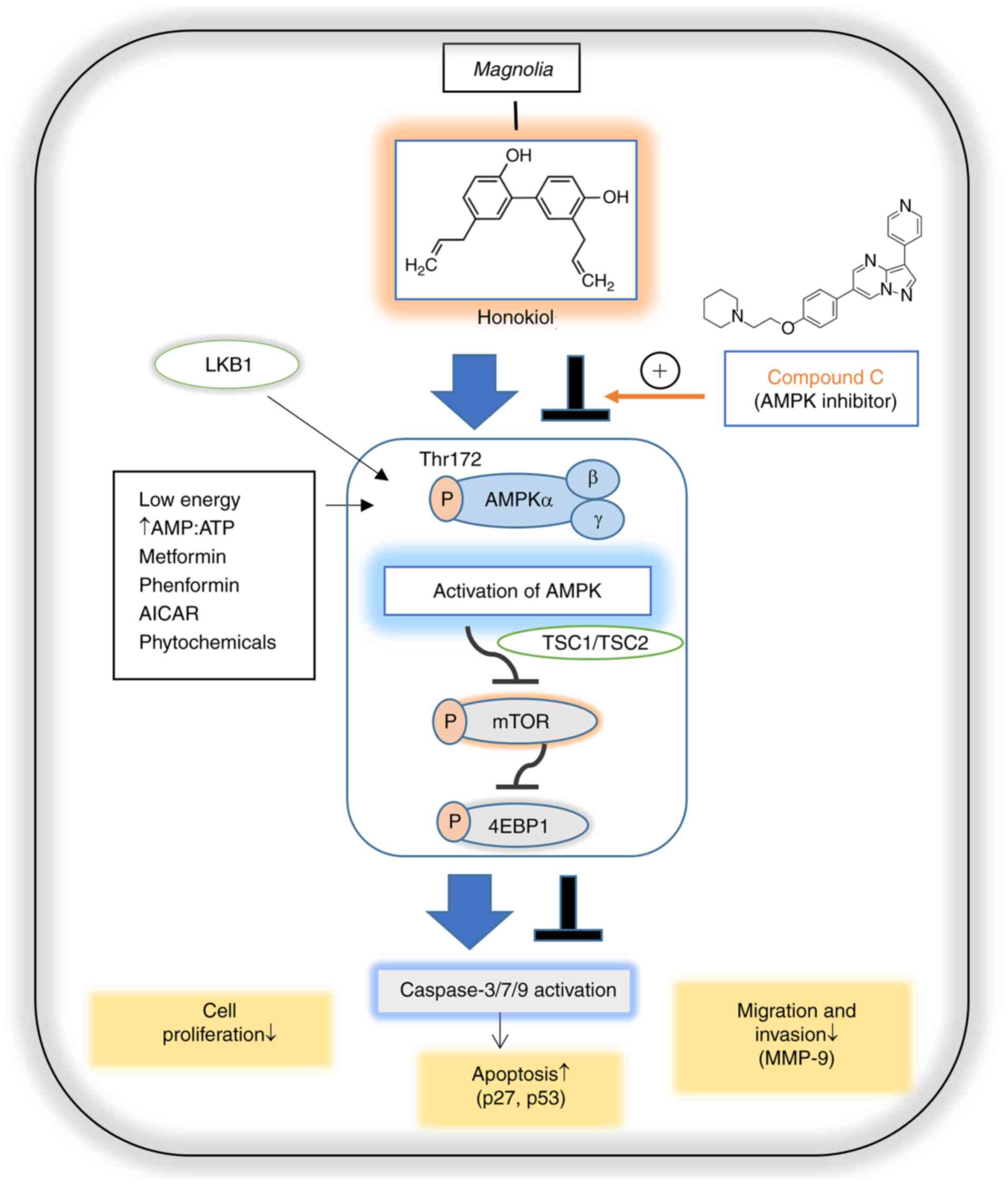 | Figure 5Schematic representation of
AMPK-dependent anticancer activities of honokiol in ovarian cancer
cells. AMPK is activated in response to cellular energy stress by
sensing increasing AMP/ATP ratio, leading to the activation of
LKB1. Metformin, phenformin, AICAR and phytochemicals may also
mimic stressors and lead to AMPK activation in an LKB1-dependent
manner. A specific AMPK pathway involves the TSC1/TSC2 complex,
leading to the downregulation of mTOR, which regulates apoptosis,
cell growth, migration and invasion. The mTOR pathway inhibits
apoptosis via the regulation of the tumor suppressors p27 and p53.
The mTOR/4EBP1 pathway regulates cell migration by regulating
MMP-9. Honokiol activates AMPK, which inhibits mTOR/4EBP1
signaling, leading to anti-proliferative effects and apoptosis in
ovarian cancer cells. Honokiol also inhibits migration and invasion
via AMPK activation. The AMPK inhibitor compound C reverses
honokiol-regulated proliferation, apoptosis, migration and
invasion. Blue arrows indicate activation, T-bars indicate
downregulation. AMP, adenosine 5′-phosphate; ATP, adenosine
5′-triphosphate; AMPK, AMP-activated protein kinase; LKB1, liver
kinase B1; AICAR, 5-aminoimidazole-4-carbox-amide ribonucleotide;
TSC1, tuberous sclerosis 1 protein; TSC2, tuberous sclerosis 2
protein; mTOR, mammalian target of rapamycin; 4EBP1, eukaryotic
translation initiation factor 4E-binding protein 1; MMP-9, matrix
metalloproteinase-9; p27, cyclin-dependent kinase inhibitor 1B;
p53, tumor protein p53. |
Discussion
Honokiol has generated increasing interest in cancer
studies, due to its multi-functional effects, including its
anticancer, anti-angiogenesis and anti-migration properties, which
have been demonstrated in vitro and in vivo using
preclinical models (30).
Previous studies have demonstrated that honokiol may induce growth
inhibition and apoptosis in various types of cancer, including
lung, breast, colon and prostate cancer in vitro and in
vivo (31-34). The present study demonstrated that
honokiol induced cytotoxicity and inhibited proliferation in the
ovarian cancer SKOV3 and Caov-3 cell lines, whereas the normal
NIH-3T3 cell line exhibited low cytotoxicity. These results are
consistent with a previous study that revealed that the
IC50 values of honokiol at 24 h for SKOV3, Coc 1,
Angelen and A2780 cells were 16.7, 19.6, 16.4, and 14.9
µg/ml, respectively (35).
Previous studies have suggested that the upstream
kinases of AMPK, including liver kinase B1 (LKB1), are frequently
mutated and deleted in human cancer (36-38). Genetic deletion of the LKB1
gene results in a loss of AMPK activity that represents a common
event in cancer cell growth (39). Being directly activated by the
tumor suppressor LKB1, AMPK regulates the activation of 2 other
tumor suppressors, TSC1 and TSC2, which are critical regulators of
mTOR (40). AMPK-initiated mTOR
inhibition suppresses downstream effectors p70S6K and 4EBP1,
regulating transcription, translation, protein stability, mRNA
turnover and cell size (40,41). Previous studies have demonstrated
that several AMPK activators, mTOR inhibitors and their
combination, including metformin, AICAR or rapamycin, may suppress
cancer cell growth (42-47). Therefore, AMPK is an essential
target for cancer therapy.
Honokiol targets multiple signaling pathways
including epidermal growth factor receptor, nuclear factor
kappa-light-chain-enhancer of activated B cells B, signal
transducer and activator of transcription 3, and mTOR, which serve
essential roles in cancer initiation and progression (48). Previous data have suggested that
honokiol affects melanoma and breast cancer cell growth by
targeting AMPK signaling (28,49). However, whether AMPK targeting via
honokiol is the cause its anticancer effects in ovarian cancer is
unclear. In the present study, activation of AMPK in
honokiol-treated ovarian cancer cells was observed, which may have
contributed to the cell death pathway.
As honokiol has demonstrated inhibitory effects on
the viability of human ovarian cancer cells, the present study
examined whether it modulated cell cycle progression and induced
apoptotic cell death in the same manner as AMPK activation. The
results indicated that honokiol may lead to the caspase-dependent
apoptotic death of ovarian cancer cells, causing an increase in the
sub-G1 population of apoptotic cells. Induction of
apoptosis was indicated by the elevated expression of apoptotic
markers, including activation of caspase-3, caspase-7 and
caspase-9, and cleavage of PARP. These characteristics of apoptosis
were inhibited by compound C, a pharmacological inhibitor of AMPK.
Previous studies have suggested that AMPK activation may induce
apoptosis and cell cycle arrest in various types of cancer,
including breast, colon and oral cancer (50-52). Therefore, honokiol may induce
apoptosis in ovarian cancer cells through AMPK activation.
Previous studies have revealed that the activation
of AMPK by metformin not only inhibits cell proliferation, but also
decelerates cell migration (53).
Honokiol suppresses metastasis by inhibiting cell migration in
neuroblastoma (25), and breast
(29), and renal cancer (54). However, to the best of our
knowledge, honokiol-induced metastatic activity on ovarian cancer
has never been investigated. To address the functional role of
honokiol in ovarian cancer metastasis, the present study examined
the anti-metastatic effect of honokiol on ovarian cancer cells. It
was identified that AMPK activation by honokiol treatment
suppressed cell migration and the invasive properties of ovarian
cancer cells. These results suggested that honokiol may be a
potential therapeutic target for treating metastatic ovarian
cancer.
In summary, honokiol, a natural compound, exhibited
anticancer activities against the ovarian cancer SKOV3 and Caov-3
cell lines. Honokiol significantly suppressed cell proliferation
and induced apoptosis of ovarian cancer cells by activating AMPK.
Honokiol also inhibited their metastatic and invasive activities,
potentially through AMPK activation. Although honokiol has been
implicated in AMPK signaling in other cancer types, to the best of
our knowledge, this is the first study of the role of AMPK in
ovarian cancer. The results indicated that honokiol has potential
clinical application for preventing and treating ovarian
cancer.
Funding
The present study was supported by a research fund
of Chungnam National University (grant no. 2016168601).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JSL designed the study and prepared the manuscript.
JBP, MSL and EYC performed experiments and analyzed the data. JYS
and YBK were involved in the study conception and design, and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Hoskins WJ, McGuire WP, Brady MF, Homesley
HD, Creasman WT, Berman M, Ball H and Berek JS: The effect of
diameter of largest residual disease on survival after primary
cytoreductive surgery in patients with suboptimal residual
epithelial ovarian carcinoma. Am J Obstet Gynecol. 170:974–979.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu Y, Chen L, He X, Fan L, Yang G, Chen
X, Lin X, DU L, Li Z, Ye H, et al: Enhancement of therapeutic
effectiveness by combining liposomal honokiol with cisplatin in
ovarian carcinoma. Int J Gynecol Cancer. 18:652–659. 2008.
View Article : Google Scholar
|
|
3
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R; Gynecologic Oncology Group: Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
gynecologic oncology group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hardie DG: Sensing of energy and nutrients
by AMP-activated protein kinase. Am J Clin Nutr. 93:891S–896S.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright JL and Stanford JL: Metformin use
and prostate cancer in caucasian men: Results from a
population-based case-control study. Cancer Causes Control.
20:1617–1622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Henderson D, Frieson D, Zuber J and
Solomon SS: Metformin has positive therapeutic effects in colon
cancer and lung cancer. Am J Med Sci. 354:246–251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bodmer M, Meier C, Krähenbühl S, Jick SS
and Meier CR: Long-term metformin use is associated with decreased
risk of breast cancer. Diabetes Care. 33:1304–1308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li D, Yeung SC, Hassan MM, Konopleva M and
Abbruzzese JL: Antidiabetic therapies affect risk of pancreatic
cancer. Gastroenterology. 137:482–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LW, Li ZS, Zou DW, Jin ZD, Gao J and
Xu GM: Metformin induces apoptosis of pancreatic cancer cells.
World J Gastroenterol. 14:7192–7198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He XX, Tu SM, Lee MH and Yeung SC:
Thiazolidinediones and metformin associated with improved survival
of diabetic prostate cancer patients. Ann Oncol. 22:2640–2645.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiralerspong S, Palla SL, Giordano SH,
Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi
GN and Gonzalez-Angulo AM: Metformin and pathologic complete
responses to neoadjuvant chemotherapy in diabetic patients with
breast cancer. J Clin Oncol. 27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirpichnikov D, McFarlane SI and Sowers
JR: Metformin: An update. Ann Intern Med. 137:25–33. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zong H, Yin B, Zhou H, Cai D, Ma B and
Xiang Y: Inhibition of mTOR pathway attenuates migration and
invasion of gallbladder cancer via EMT inhibition. Mol Biol Rep.
41:4507–4512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inoki K, Kim J and Guan KL: AMPK and mTOR
in cellular energy homeostasis and drug targets. Annu Rev Pharmacol
Toxicol. 52:381–400. 2012. View Article : Google Scholar
|
|
16
|
Chapuis N, Tamburini J, Green AS, Willems
L, Bardet V, Park S, Lacombe C, Mayeux P and Bouscary D:
Perspectives on inhibiting mTOR as a future treatment strategy for
hematological malignancies. Leukemia. 24:1686–1699. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Wang S, Zhang Y, Fan HT and Lin HS:
Traditional chinese medicine and cancer: History, present
situation, and development. Thorac Cancer. 6:561–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seo MS, Hong SW, Yeon SH, Kim YM, Um KA,
Kim JH, Kim HJ, Chang KC and Park SW: Magnolia officinalis
attenuates free fatty acid-induced lipogenesis via AMPK
phosphorylation in hepatocytes. J Ethnopharmacol. 157:140–148.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Zhang X, Cui W, Zhang X, Li N, Chen
J, Wong AW and Roberts A: Evaluation of short-term and subchronic
toxicity of magnolia bark extract in rats. Regul Toxicol Pharmacol.
49:160–171. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarrica A, Kirika N, Romeo M, Salmona M
and Diomede L: Safety and toxicology of magnolol and honokiol.
Planta Med. 84:1151–1164. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fried LE and Arbiser JL: Honokiol, a
multifunctional antiangiogenic and antitumor agent. Antioxid Redox
Signal. 11:1139–1148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steinmann P, Walters DK, Arlt MJ, Banke
IJ, Ziegler U, Langsam B, Arbiser J, Muff R, Born W and Fuchs B:
Antimetastatic activity of honokiol in osteosarcoma. Cancer.
118:2117–2127. 2012. View Article : Google Scholar
|
|
23
|
Park EJ, Min HY, Chung HJ, Hong JY, Kang
YJ, Hung TM, Youn UJ, Kim YS, Bae K, Kang SS and Lee SK:
Down-regulation of c-Src/EGFR-mediated signaling activation is
involved in the honokiol-induced cell cycle arrest and apoptosis in
MDA-MB-231 human breast cancer cells. Cancer Lett. 277:133–140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishikawa C, Arbiser JL and Mori N:
Honokiol induces cell cycle arrest and apoptosis via inhibition of
survival signals in adult T-cell leukemia. Biochim Biophys Acta.
1820:879–887. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yeh PS, Wang W, Chang YA, Lin CJ, Wang JJ
and Chen RM: Honokiol induces autophagy of neuroblastoma cells
through activating the PI3K/Akt/mTOR and endoplasmic reticular
stress/ERK1/2 signaling pathways and suppressing cell migration.
Cancer Lett. 370:66–77. 2016. View Article : Google Scholar
|
|
26
|
Dai X, Li RZ, Jiang ZB, Wei CL, Luo LX,
Yao XJ, Li GP and Leung EL: Honokiol inhibits proliferation,
invasion and induces apoptosis through targeting lyn kinase in
human lung adenocarcinoma cells. Front Pharmacol. 9:5582018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen L, Zhang F, Huang R, Yan J and Shen
B: Honokiol inhibits bladder cancer cell invasion through
repressing SRC-3 expression and epithelial-mesenchymal transition.
Oncol Lett. 14:4294–4300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nagalingam A, Arbiser JL, Bonner MY,
Saxena NK and Sharma D: Honokiol activates AMP-activated protein
kinase in breast cancer cells via an LKB1-dependent pathway and
inhibits breast carcinogenesis. Breast Cancer Res. 14:R352012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh T and Katiyar SK: Honokiol, a
phytochemical from magnolia spp., inhibits breast cancer cell
migration by targeting nitric oxide and cyclooxygenase-2. Int J
Oncol. 38:769–776. 2011.PubMed/NCBI
|
|
30
|
Kumar A, Kumar Singh U and Chaudhary A:
Honokiol analogs: A novel class of anticancer agents targeting cell
signaling pathways and other bioactivities. Future Med Chem.
5:809–829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo LX, Li Y, Liu ZQ, Fan XX, Duan FG, Li
RZ, Yao XJ, Leung EL and Liu L: Honokiol induces apoptosis, G1
arrest, and autophagy in KRAS mutant lung cancer cells. Front
Pharmacol. 8:1992017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shigemura K, Arbiser JL, Sun SY, Zayzafoon
M, Johnstone PA, Fujisawa M, Gotoh A, Weksler B, Zhau HE and Chung
LW: Honokiol, a natural plant product, inhibits the bone metastatic
growth of human prostate cancer cells. Cancer. 109:1279–1289. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Zang C, Emde A, Planas-Silva MD,
Rosche M, Kühnl A, Schulz CO, Elstner E, Possinger K and Eucker J:
Anti-tumor effect of honokiol alone and in combination with other
anticancer agents in breast cancer. Eur J Pharmacol. 591:43–51.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng N, Xia T, Han Y, He QJ, Zhao R and
Ma JR: Synergistic antitumor effects of liposomal honokiol combined
with cisplatin in colon cancer models. Oncol Lett. 2:957–962.
2011.
|
|
35
|
Li Z, Liu Y, Zhao X, Pan X, Yin R, Huang
C, Chen L and Wei Y: Honokiol, a natural therapeutic candidate,
induces apoptosis and inhibits angiogenesis of ovarian tumor cells.
Eur J Obstet Gynecol Reprod Biol. 140:95–102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kwan HT, Chan DW, Cai PC, Mak CS, Yung MM,
Leung TH, Wong OG, Cheung AN and Ngan HY: AMPK activators suppress
cervical cancer cell growth through inhibition of DVL3 mediated
Wnt/β-catenin signaling activity. PLoS One. 8:e535972013.
View Article : Google Scholar
|
|
37
|
Gurumurthy S, Hezel AF, Sahin E, Berger
JH, Bosenberg MW and Bardeesy N: LKB1 deficiency sensitizes mice to
carcinogen-induced tumorigenesis. Cancer Res. 68:55–63. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Liu Y, Zhou Y and You H: Molecular
mechanism of LKB1 in the invasion and metastasis of colorectal
cancer. Oncol Rep. 41:1035–1044. 2019.
|
|
39
|
Vazquez-Martin A, Oliveras-Ferraros C,
Lopez-Bonet E and Menendez JA: AMPK: Evidence for an energy-sensing
cytokinetic tumor suppressor. Cell Cycle. 8:3679–3683. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hardie DG: The AMP-activated protein
kinase pathway-new players upstream and downstream. J Cell Sci.
117:5479–5487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duan P, Hu C, Quan C, Yu T, Huang W, Chen
W, Tang S, Shi Y, Martin FL and Yang K: 4-Nonylphenol induces
autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating
AMPK activation in sertoli cells. Toxicol Lett. 267:21–31. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Al-Moujahed A, Nicolaou F, Brodowska K,
Papakostas TD, Marmalidou A, Ksander BR, Miller JW, Gragoudas E and
Vavvas DG: Uveal melanoma cell growth is inhibited by
aminoimidazole carboxamide ribonucleotide (AICAR) partially through
activation of AMP-dependent kinase. Invest Ophthalmol Vis Sci.
55:4175–4185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yung MM, Chan DW, Liu VW, Yao KM and Ngan
HY: Activation of AMPK inhibits cervical cancer cell growth through
AKT/FOXO3a/FOXM1 signaling cascade. BMC Cancer. 13:3272013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Whang YM, Kim MJ, Cho MJ, Yoon H, Choi YW,
Kim TH and Chang IH: Rapamycin enhances growth inhibition on
urothelial carcinoma cells through LKB1 deficiency-mediated
mitochondrial dysregulation. J Cell Physiol. 1–14. 2018.
|
|
46
|
Zhang JW, Zhao F and Sun Q: Metformin
synergizes with rapamycin to inhibit the growth of pancreatic
cancer in vitro and in vivo. Oncol Lett. 15:1811–1816.
2018.PubMed/NCBI
|
|
47
|
Mukhopadhyay S, Chatterjee A, Kogan D,
Patel D and Foster DA:
5-Aminoimidazole-4-carboxamide-1-β-4-ribofura noside (AICAR)
enhances the efficacy of rapamycin in human cancer cells. Cell
Cycle. 14:3331–3339. 2015. View Article : Google Scholar
|
|
48
|
Arora S, Singh S, Piazza GA, Contreras CM,
Panyam J and Singh AP: Honokiol: A novel natural agent for cancer
prevention and therapy. Curr Mol Med. 12:1244–1252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaushik G, Kwatra D, Subramaniam D, Jensen
RA, Anant S and Mammen JM: Honokiol affects melanoma cell growth by
targeting the AMP-activated protein kinase signaling pathway. Am J
Surg. 208:995–1002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Queiroz EA, Puukila S, Eichler R, Sampaio
SC, Forsyth HL, Lees SJ, Barbosa AM, Dekker RF, Fortes ZB and
Khaper N: Metformin induces apoptosis and cell cycle arrest
mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast
cancer cells. PLoS One. 9. pp. e982072014, View Article : Google Scholar
|
|
51
|
Buzzai M, Jones RG, Amaravadi RK, Lum JJ,
DeBerardinis RJ, Zhao F, Viollet B and Thompson CB: Systemic
treatment with the antidiabetic drug metformin selectively impairs
p53-deficient tumor cell growth. Cancer Res. 67:6745–6752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shin JA, Kwon KH and Cho SD:
AMPK-activated protein kinase activation by Impatiens balsamina L.
is related to apoptosis in HSC-2 human oral cancer cells.
Pharmacogn Mag. 11:136–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ferla R, Haspinger E and Surmacz E:
Metformin inhibits leptin-induced growth and migration of
glioblastoma cells. Oncol Lett. 4:1077–1081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cheng S, Castillo V, Eliaz I and Sliva D:
Honokiol suppresses metastasis of renal cell carcinoma by targeting
KISS1/KISS1R signaling. Int J Oncol. 46:2293–2298. 2015. View Article : Google Scholar : PubMed/NCBI
|