Introduction
Acute myocardial infarction is the primary cause of
mortality in patients with coronary heart disease (1). The most commonly accepted and
effective treatment is to restore the blood flow reperfusion as
soon as possible (2). However,
following the resumption of blood flow and reperfusion, ischemic
myocardium aggravates cardiac function and causes additional
structural destruction, leading to the further exacerbation of
myocardial ischemic injury (MIR), known as myocardial
ischemia-reperfusion injury (MIRI) (3). Identifying the key factors involved
in MIRI and developing a rational intervention to reduce apoptosis
following ischemia-reperfusion is a focus of clinical research.
The primary mechanisms of MIRI currently recognized
include the release of excessive calcium, inflammatory factor
release and cell apoptosis (4,5).
In recent years, increasing numbers of scholars have conducted
in-depth studies regarding the mechanism of MIRI (6,7).
Previous studies have confirmed that the induction of apoptosis in
MIR is the main form of acute MIRI (8-12).
At present, three signal transduction pathways that regulate
apoptosis have been identified, including the mitochondrial
pathway, the death receptor pathway and the endoplasmic reticulum
stress (ERS) pathway (13).
Studies have demonstrated that the ERS-mediated apoptosis pathway
serves an important role in the pathogenesis of MIRI (14,15). When cardiomyocytes are affected by
ischemia, the amount of unfolded protein in the lumen of the ER is
significantly increased, which activates the ERS-associated
apoptosis pathway (16,17). Therefore, inhibiting ERS may
significantly improve the progression of MIRI and serve a role in
preventing MIRI.
Aloe is a succulent plant belonging to the family
Liliaceae and has high economic value (18,19). The role of aloe in immunity,
anti-inflammation and anti-oxidation have been demonstrated
previously (18), and has been
used widely clinically (20).
Barbaloin (Bar), the primary active ingredient in aloe has gained
increasing attention (21). A
recent study has demonstrated that Bar controls ventricular
arrhythmia by regulating voltage-gated ion channels (22). Bar pretreatment inhibits
myocardial oxidative stress by activating the AMP-activated protein
kinase (AMPK) signaling pathway, thereby alleviating MIRI (23). However, to the best of our
knowledge, no previous study has investigated whether Bar
pretreatment inhibits myocardial apoptosis induced by MIR to
achieve myocardial protection.
Therefore, based on the current knowledge on
ERS-mediated cardiomyocyte apoptosis and the cardioprotective
effects of Bar, the aim of the present study was to further
evaluate the myocardial protective properties and potential
mechanisms of action of Bar pretreatment in MIRI.
Materials and methods
Main reagents
Barbaloin (Bar, purity >96%) was purchased from
Abcam (Cambridge, UK), and its solvent dimethyl sulfoxide (DMSO)
was obtained from Merck KGaA (Sigma-Aldrich; Darmstadt,
Germany).
Animals and drug treatment
A total of 96 healthy male Sprague-Dawley rats (SD,
8-10 weeks, 250-280 g) were selected and provided by the Tianjin
Animal Center (Tianjin, China). All rats were housed in an
environment of constant temperature (22-25°C) and relative humidity
(50-60%) on a 12-h light/12-h dark cycle, and food and water were
given ad libitum. All experimental protocols in the current
study were performed in strict accordance with the institutional
guidelines and approved by the Laboratory Animal Ethics Committee
of Tianjin Huanhu Hospital (Tianjin, China) consistent with China
Animal Care Committee.
All 96 healthy male rats were randomly divided into
four groups: i) Sham operation + DMSO group (S group), after
repeated intragastric administration of DMSO once a day for 1 week,
the rats were threaded under the left anterior descending coronary
artery (LAD) without ligation; ii) Bar alone group (Bar group),
after repeated intragastric administration of Bar (20 mg/kg) once a
day for 1 week, the rats were threaded under the LAD without
ligation; iii) ischemia and reperfusion (I/R)+DMSO group, after
repeated intragastric administration of DMSO once a day for 1 week,
myocardial ischemia and reperfusion were induced for 30 and 120
min, respectively; iv) I/R+Bar group, after repeated intragastric
administration of Bar (20 mg/kg) once a day for 1 week, myocardial
ischemia and reperfusion were induced for 30 min and 120 min,
respectively. The S and I/R groups were given equal volumes of
DMSO.
Prior to the start of the experiment, SD rats were
adapted to their environment for 1 week. As described previously,
the rat model of MIR was prepared by ligation of the LAD (24). Briefly, rats were anesthetized
with an intraperitoneal injection of 45 mg/kg pentobarbital sodium
and mechanically ventilated using tracheal intubation. By
identifying the origin 2 mm from the LAD, a 5/0 silk thread was
used to surround the LAD. Following stabilization, transient
ligation of LAD-induced ischemia was performed for 30 min, which
was followed by the loosening of the ligature line and reperfusion
for 120 min. Successful ligation of the LAD was confirmed by
observing alterations to the ST segment on the electrocardiogram
and color alterations in the ischemic areas of the heart (anterior
wall and apex of the heart). After 120 min of reperfusion, 5 ml of
abdominal aortic blood was collected for serological examination.
The weight of the experimental rats was 250-280 g. Subsequently,
the heart was removed quickly and the blood remaining in the heart
was cleared with cold physiological saline. The left ventricle was
cut into two parts. One part was fixed in 4% paraformaldehyde for
24 h at room temperature, and the other was stored in a freezer at
-80°C for use in subsequent experimental analysis.
Determination of serum lactate
dehydrogenase (LDH) and creatine kinase (CK)
After 120 min of reperfusion, abdominal aorta blood
was collected and centrifuged at 900 × g for 10 min at 4°C. The
supernatant was subjected to serological analysis by
spectrophotometry. The levels of LDH and CK were detected according
to the manufacturer's protocols (Elabscience Biotechnology Co.,
Ltd., Wuhan, China).
Terminal deoxynucleotidyl transferase
mediated dUTP nick end labeling (TUNEL) assay
At the end of the 120 min reperfusion, cardiomyocyte
apoptosis was detected using a TUNEL assay. According to the
manufacturer's protocol, the paraffin-embedded myocardial slices
were stained using the in situ cell apoptosis detection kit
(POD; Roche Diagnostics, Indianapolis, IN, USA). In the cardiac
ischemic areas, five microscopic fields (×400) from each slice were
randomly selected to determine the percentage of cells positively
stained for apoptosis using a light microscope. The apoptosis rate
was calculated as the number of apoptotic cells/total number of
cells ×100%.
Flow cytometric analysis
Cardiomyocyte apoptosis was analyzed by flow
cytometry, as previously described (25). Double staining of fluorescein
isothiocyanate (FITC)-conjugated Annexin V and propidium iodide
(PI) was performed according to the manufacturer's protocol of the
FITC-Annexin V and PI Apoptosis Detection kit (Beyotime Institute
of Biotechnology, Haimen, China). The rats were anesthetized
following 120 min of ischemia-reperfusion. The rat hearts were
removed, washed with PBS and placed in glassware. The myocardial
tissue was shredded, processed and washed with PBS, followed by
centrifugation at 1,000 × g for 5 min at 4°C. The precipitate was
collected and washed with PBS, and again centrifuged at 1,000 × g
for 5 min at 4°C. The pellet was resuspended in a binding buffer
containing 5 μl of FITC-Annexin V and 10 μl of PI
staining solution. The solution was incubated for 15 min at room
temperature in the dark. The mixture was filtered through a
300-mesh nylon net to remove impurities. Cellular fluorescence was
analyzed using a flow cytometer (Becton, Dickinson and Company,
Franklin, Lakes, NJ, USA). Apoptotic cells were measured as a
percentage of the total number of cells, namely, the apoptotic
index. The experiment was repeated three times.
Immunohistochemistry of protein canopy
homolog 2 (CNPY2) in cardiomyocytes
The expression of CNPY2 in cardiomyocytes was
detected using immunohistochemistry according to the manufacturer's
protocol. Briefly, myocardial specimens were fixed in 4%
formaldehyde for 24 h at room temperature, embedded in paraffin and
sectioned into 4-μm thick slices. The slices were dewaxed
and quenched in 3% H2O2 at room temperature
for 10 min. Next, sections were incubated with anti-CNPY2 rabbit
primary antibodies (1:50; cat. no. 14635-1-AP; ProteinTech Group,
Inc., Chicago, IL, USA) for 2 h at 4°C followed by incubation with
horseradish peroxidase (HRP)-conjugated Affinipure goat anti-rabbit
immunoglobulin (Ig)G antibodies (1:100; cat. no. SA00001-2;
ProteinTech Group, Inc.) at 4°C for 1 h. Thereafter, sections were
incubated in 3,3′-diamino benzidine for 15 min at room temperature
for color rendering. The CNPY2-positive cells were observed at a
×400 magnification under a light microscope. Brown staining
indicated the presence of positive expression. Five areas of each
sample from all experimental groups were randomly captured using
the camera (Leica DM6000; Leica Microsystems GmbH, Wetzlar,
Germany) and analyzed using ImagePro-Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Extraction of myocardial total cell
lysate and ER
After 2 h of reperfusion, left ventricular specimens
from I/R injury were collected for western blot analysis. Protein
isolation and western blotting were performed as previously
described (26). The left
ventricle specimens were lysed with 100 μl of ice-cold RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with 1 μl of 100 mM phenylmethylsulfonyl fluoride (Beyotime
Institute of Biotechnology) for 30 min. Tissue homogenates were
centrifuged at 12,000 × g for 30 min at 4°C and the total cell
lysate was collected. Once the left ventricular samples were fully
lysed, the tissue homogenate was centrifuged (4°C, 800 × g) for 10
min, and the new supernatant was centrifuged (4°C, 10,000 × g) for
20 min; the new supernatant was collected again for centrifugation
(4°C, 100,000 × g) for 1 h, the pellet of which was the ER.
Western blot analysis
The ER was suspended with a lysis solution
containing 1% Triton X-100. The protein concentration of
supernatants was quantified using the Bradford method. Protein
samples (20 μg/lane) were separated on 10% SDS-PAGE gels,
transferred to polyvinylidene difluoride membranes and blocked with
5% skim milk for 1 h at 4°C. The membranes were incubated with
primary antibodies at 4°C overnight: Phosphorylated-PKR endoplasmic
reticulum kinase (p-PERK; 1:1,000; BIOSS, Beijing, China), CNPY2
(1:50; cat. no. 14635-1-AP), PERK (1:1,000; cat. no. 20582-1-AP),
Calnexin (1:400; cat. no. 10427-2-AP) and GAPDH (1:2,000; cat. no.
10494-1-AP; all from ProteinTech Group, Inc.), glucose regulatory
protein 78 (GRP78; 1:3,000; cat. no. ab21685), caspase-12
(1:10,000; cat. no. ab62484), transcriptional activator 4 (ATF4;
1:5,000; cat. no. ab23760), C/EBP-homologous protein (CHOP;
1:2,000; cat. no. ab11419) and caspase-3 (1:1,000; cat. no.
ab13847; all from Abcam). Following washing three times with TBS
with Tween-20, the membranes were incubated with HRP-conjugated
Affinipure goat anti-mouse (cat. no. SA00001-1) or anti-rabbit
(cat. no. SA00001-2; both 1:2,000; ProteinTech Group, Inc.) IgG
antibodies for 1 h at room temperature. The target bands were
visualized using enhanced chemiluminescence (EMD Millipore,
Billerica, MA, USA). The quantification of the blots was measured
using ImageJ software v.1.51u (National Institutes of Health,
Bethesda, MD, USA). All band intensities were normalized to GAPDH
or Calnexin, and expressed as a percentage of the control
sample.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
According to the manufacturer's protocol, the total
RNA was extracted from myocardial samples using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
ReverTra Ace-a kit (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) was used to reverse transcribe the total RNA
(4 g) to cDNA. PCR primers used were as follows: CNPY2 forward,
5′-CAGATTGACCCTTCTACCCACCG-3′ and reverse,
5′-ATGCCGCCATCTTCCTTCCCCTC-3′; GRP78 forward,
5′-TTCACTACTCTTGACCCTGCATCCC-3′ and reverse,
5′-TTTCCTGCTTGAGCCGCTCGTTC-3′; Calnexin forward,
5′-GGCTTTGGGTGGTCTACATTCTG-3′ and reverse,
5′-CATCCTCCTCTGCTTTAGGCTTG-3′. Data were normalized to Calnexin
gene expression and analyzed by the comparative quantification
method (2-ΔΔCq) (27).
Statistical analysis
All data are presented as the mean ± standard
deviation of at least three independent replicates. Multiple groups
were compared by one-way analysis of variance followed by Tukey's
post hoc test using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Data
are presented as the mean ± standard error of the mean. P<0.05
was considered to indicate a statistically significant
difference.
Results
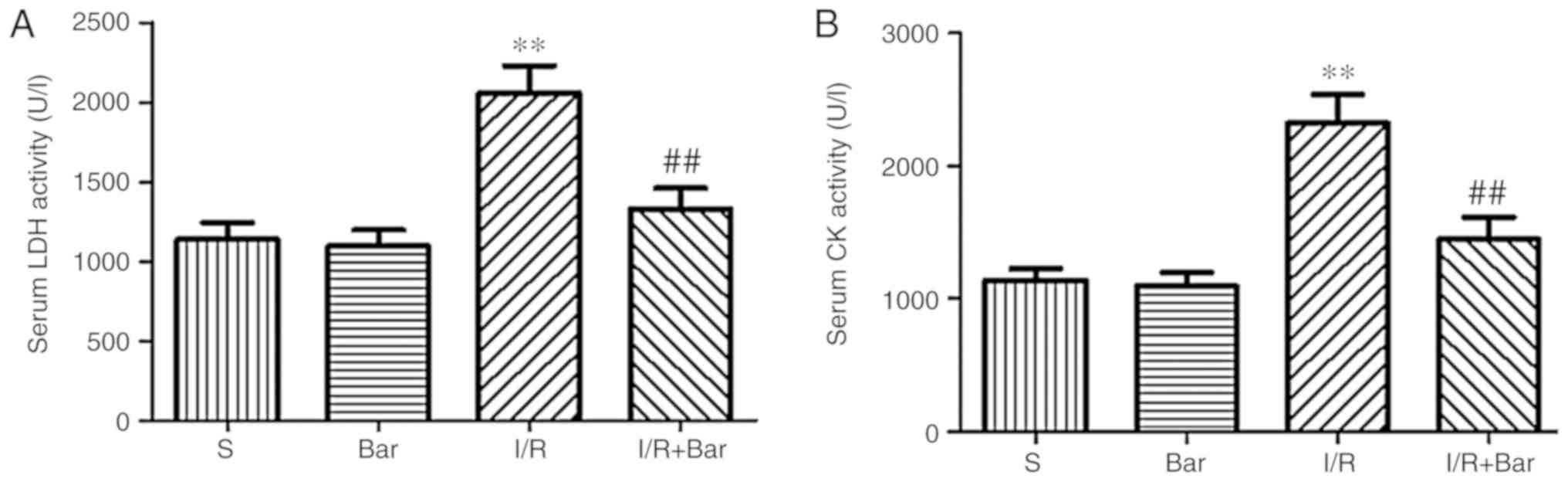
Effects of Bar pretreatment on the serum
levels of LDH and CK induced by MIRI
To investigate the myocardial damage caused by MRI,
alterations in the concentrations of myocardial injury markers LDH
and CK. Compared with the S group, the contents of CK and LDH in
the I/R group increased significantly (Fig. 1). Compared with the I/R, the
contents of CK and LDH in the I/R+Bar group decreased significantly
(Fig. 1). In addition, no
significant difference in the concentrations of CK and LDH between
the S group and the Bar group were observed. The results suggested
that the administration of Bar may inhibit the increase of
myocardial injury markers and may exhibit a protective effect on
cardiac function.
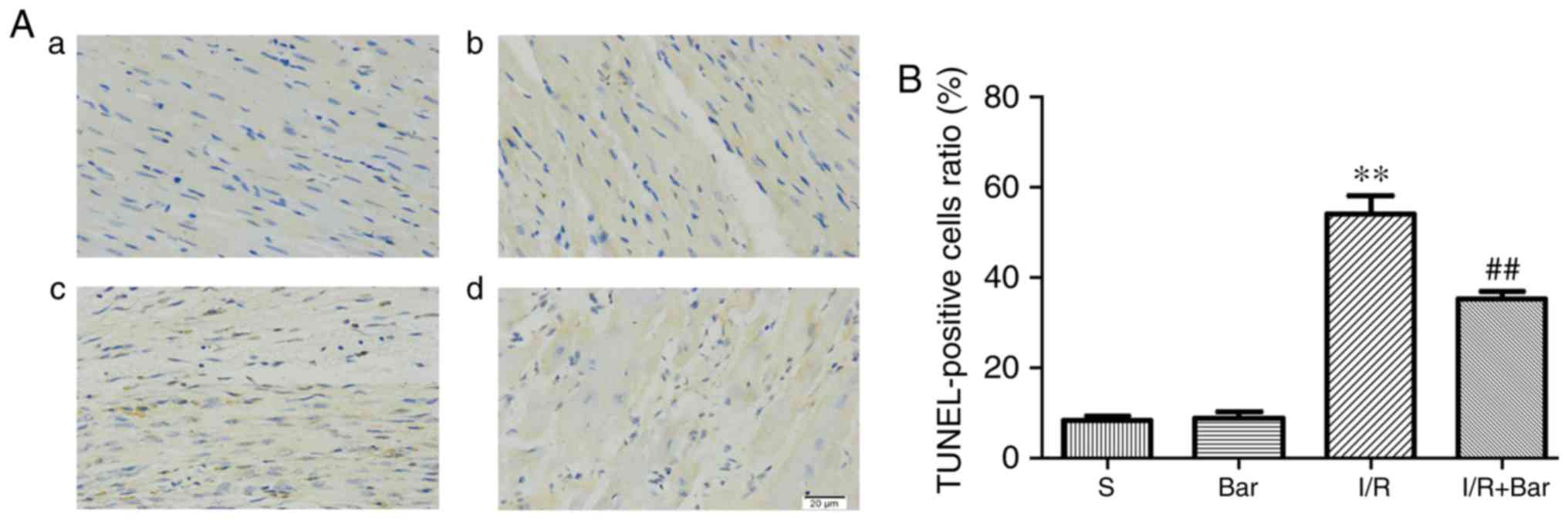
Effects of Bar pretreatment on
cardiomyocyte apoptosis induced by MIRI
To determine whether Bar pretreatment exhibited a
protective effect on the myocardium, a TUNEL assay and flow
cytometry were used to detect cardiomyocyte apoptosis. The results
of the TUNEL assay demonstrated that the apoptosis rate of the I/R
group was significantly higher compared with that of the S group.
The proportion of apoptosis in the I/R+Bar group was significantly
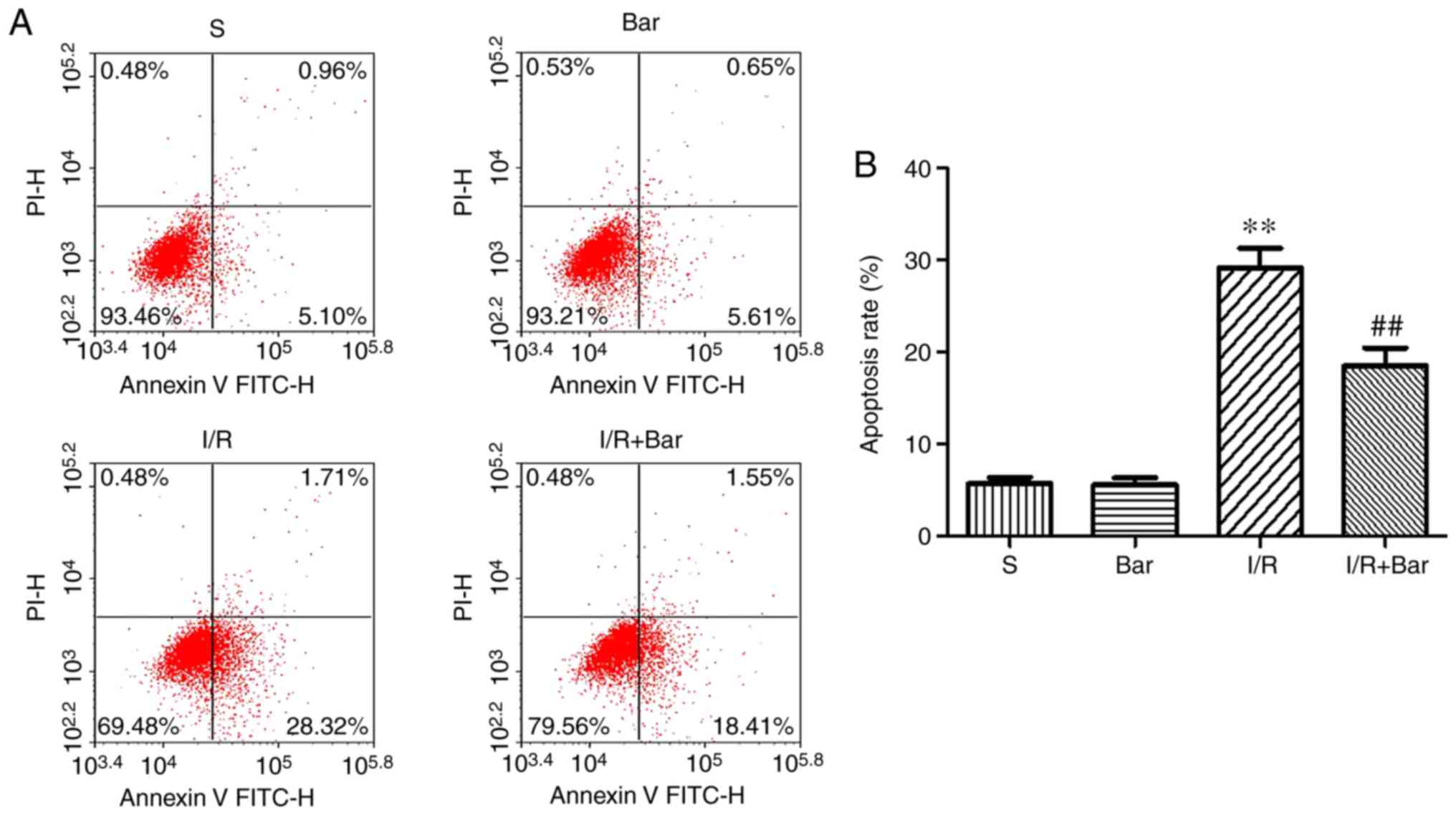
lower compared with that in the I/R group (Fig. 2A and B). Consistent with the
results of the TUNEL assay, the flow cytometry results revealed
that the apoptosis rate in the I/R group was significantly higher
compared with that of the S group, and the percentage of
cardiomyocyte apoptosis in the I/R group was significantly
decreased following treatment with Bar (Fig. 3). There were no significant
differences in apoptotic cells between the S group and the Bar
group. The aforementioned results suggested that Bar pretreatment
significantly inhibited cardiomyocyte apoptosis.
Effects of Bar pretreatment on ERS
induced by MIRI
To further demonstrate whether Bar pretreatment was
able to protect the myocardium by inhibiting ERS, the expression of
cytoplasmic ERS markers GRP78 and caspase-12 were analyzed
(Fig. 4). Compared with the S
group, the expression of GRP78 and cleaved caspase-12 protein in
I/R group increased significantly. However, compared with the I/R
group, the expression of GRP78 and cleaved caspase-12 protein in
the I/R+Bar group was significantly decreased (Fig. 4B-a and -e). Furthermore, to verify
the effect of Bar pretreatment on ERS, the expression levels of
GRP78 mRNA on ERS of cardiomyocytes was detected. The mRNA
expression of GRP78 was significantly higher in the I/R group
compared with that in the S group. Compared with the I/R group, the
mRNA expression of GRP78 in the I/R+Bar group was significantly
decreased (Fig. 5A and B-b). The
results suggested that Bar pretreatment protected the myocardium by
inhibiting ERS.
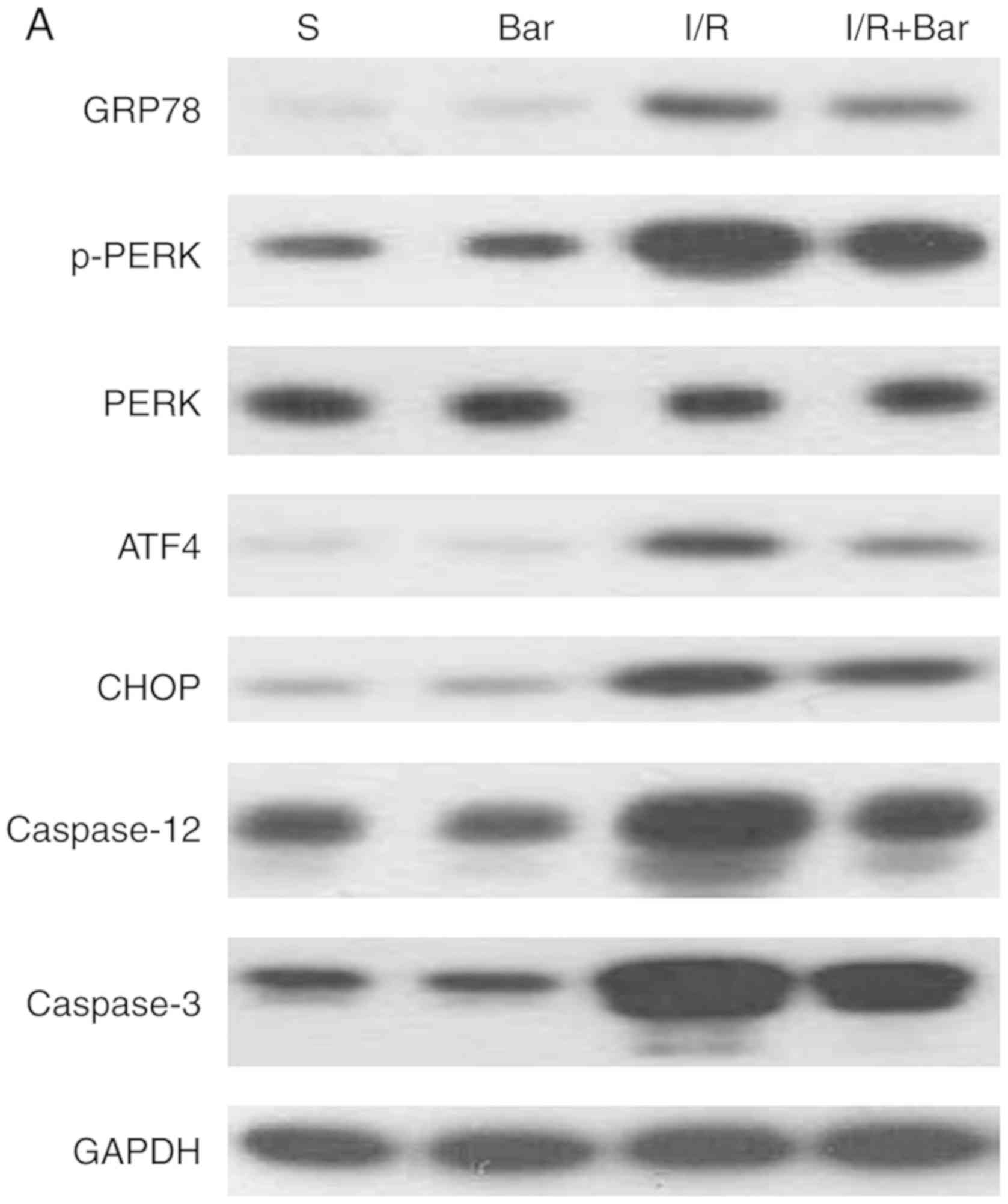 | Figure 4Effects of Bar pretreatment on the
ERS-associated apoptosis pathway following myocardial ischemia
reperfusion injury. (A) Representative western blot analysis and
(B) quantification of the ERS-associated proteins (a) GRP78, (b)
p-PERK, (c) ATF4, (d) CHOP, (e) caspase-12 and (f) caspase-3 from
myocardial cell specimens. Expression of GAPDH was used as the
loading control. The results were normalized to the percentage of
GAPDH expression; n=6. Data are presented as the mean ± standard
error of the mean. **P<0.01 compared with the S
group; ##P<0.01 compared with the I/R group. Bar,
barbaloin; S, Sham operation + DMSO; I/R, ischemia and reperfusion;
ERS, endoplasmic reticulum stress; GRP78, glucose regulatory
protein 78; p-, phosphorylated; PERK, PKR endoplasmic reticulum
kinase; ATF4, transcriptional activator 4; CHOP, C/EBP-homologous
protein. |
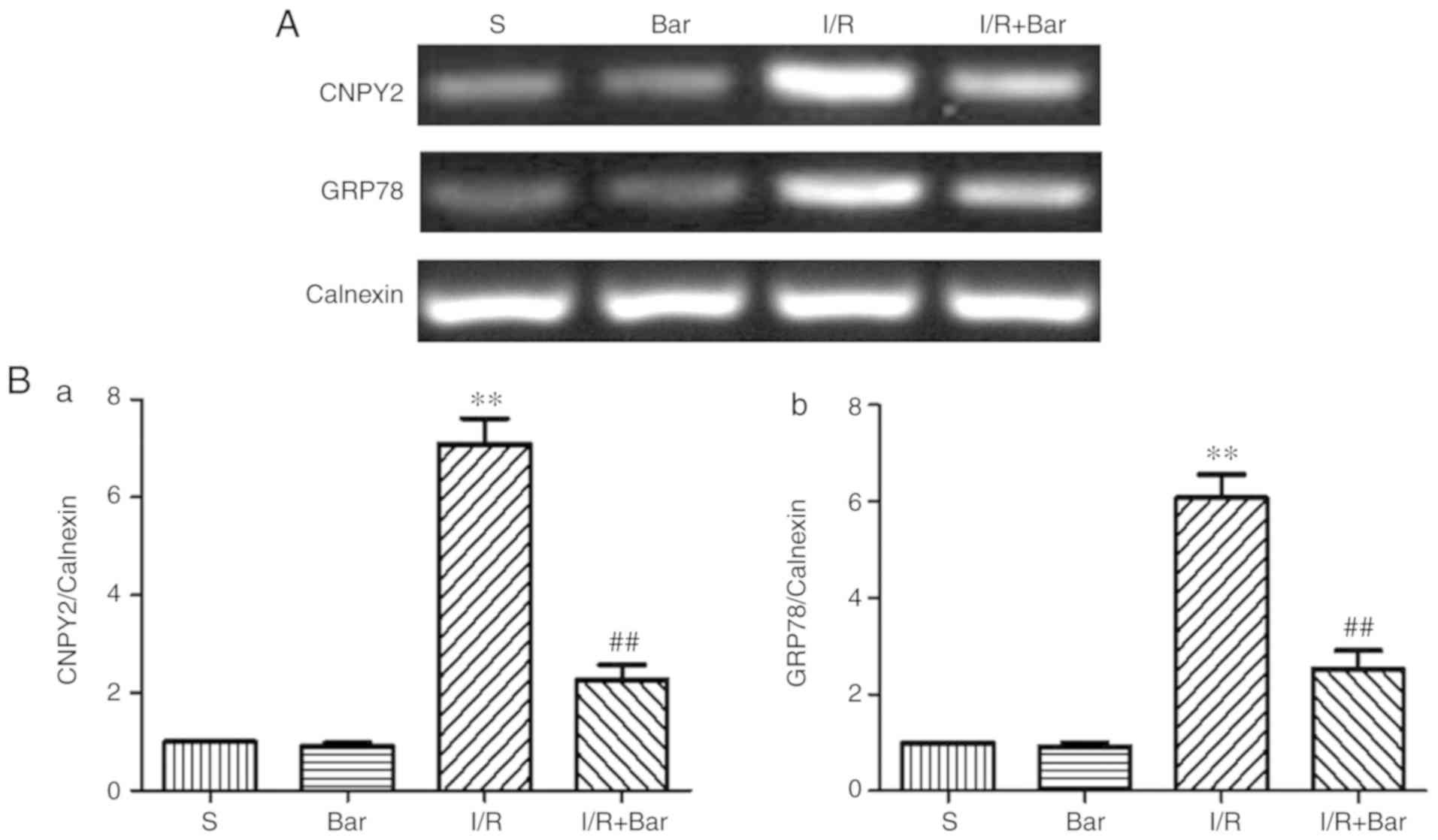
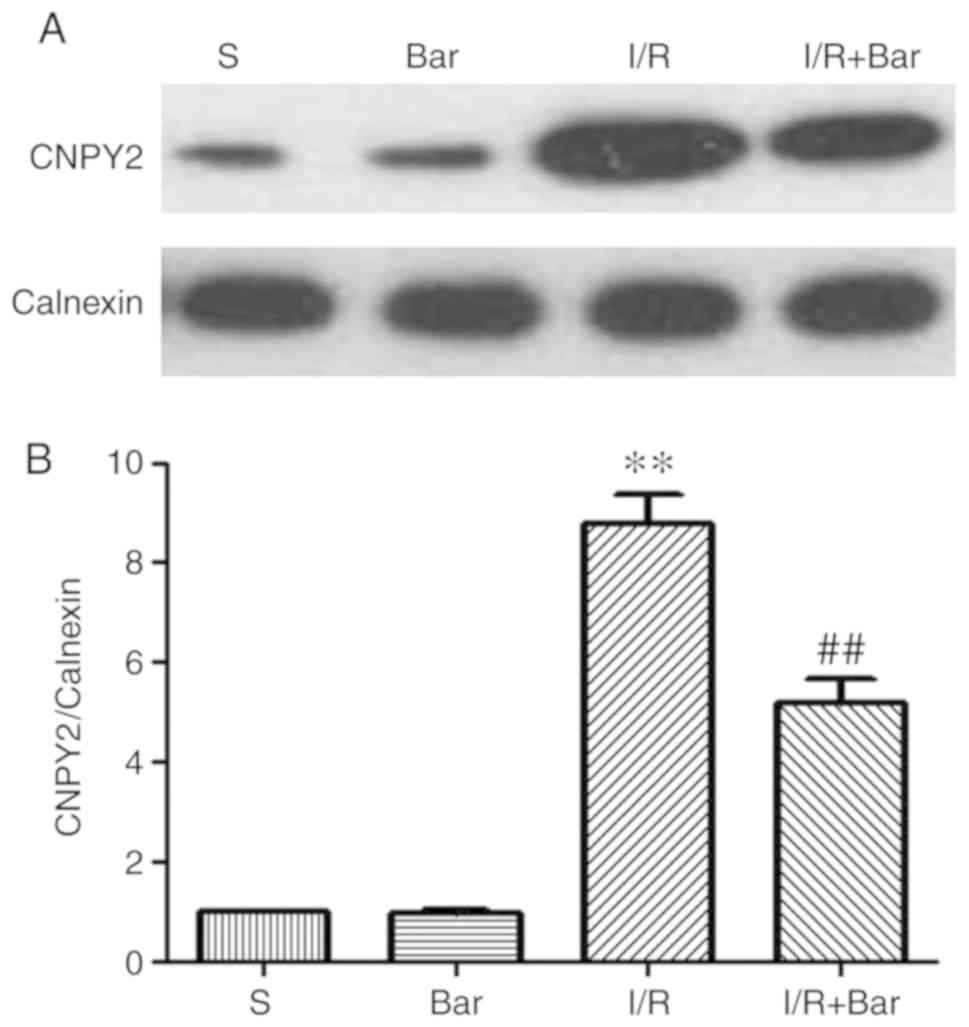
Effects of Bar pretreatment on CNPY2
induced by MIRI
To verify whether Bar affects the expression of
CNPY2, mRNA and protein expression levels of CNPY2 on the ER of
cardiomyocytes were detected (Figs.
5 and 6). Compared with the S
group, the protein expression level of CNPY2 in the I/R group was
significantly higher. Compared with the I/R group, the expression
level of cardiomyocyte CNPY2 protein in the I/R+Bar group was
significantly decreased (Fig. 6A and
B). Compared with the S group, the mRNA level of CNPY2 was
significantly increased in the I/R group. Compared with the I/R
group, mRNA expression of CNPY2 in the I/R+Bar group decreased
significantly, which was consistent with the western blotting
results (Fig. 5A and B-a). The
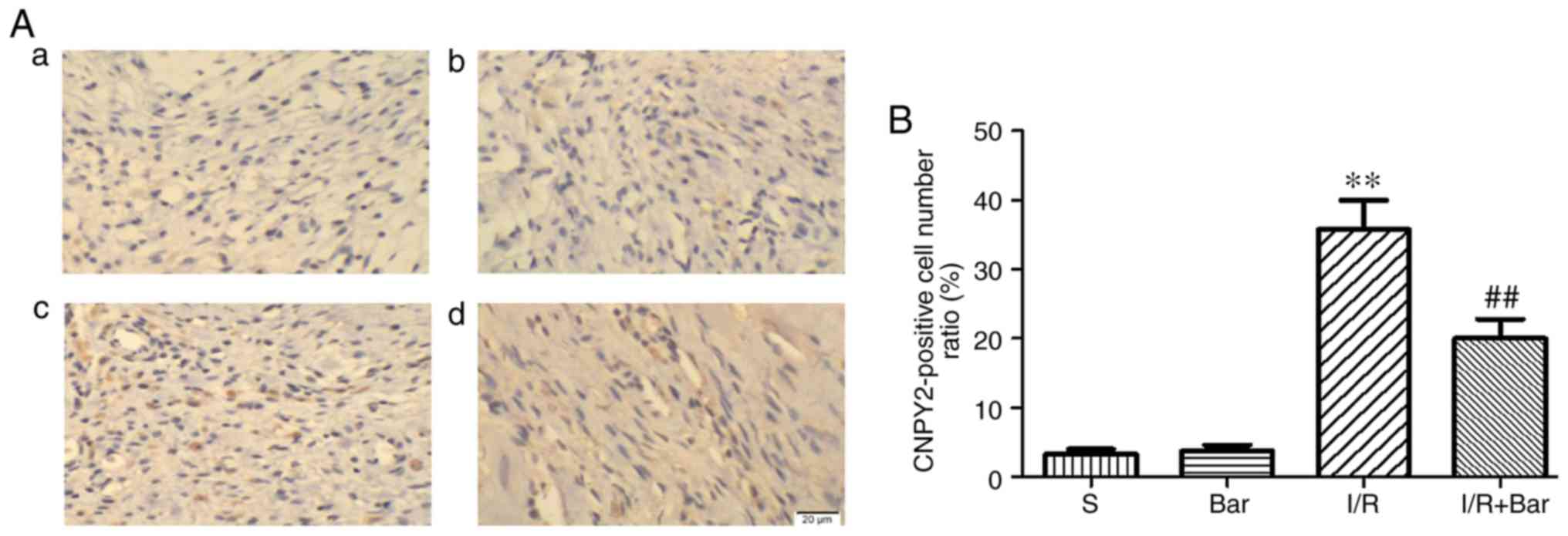
expression of CNPY2 in cardiomyocytes was detected by
immunohistochemistry, as shown in Fig. 7. The expression of CNPY2 in the
I/R group was significantly higher compared with that in the S
group. Compared with the I/R group, the expression of CNPY2
decreased significantly following Bar pretreatment, which was
consistent with western blot analysis and RT-qPCR results (Fig. 7A and B). The aforementioned
results confirmed that CNPY2 is expressed in cardiomyocytes, which
is consistent with previous studies (28,29). Bar pretreatment may protect the
myocardium from ischemia-reperfusion injury by inhibiting the
CNPY2-associated signaling pathway.
Effects of Bar pretreatment on the
ERS-associated apoptosis pathway following MIRI
To further examine the effect of Bar pretreatment on
the ERS-associated apoptosis pathway following MIRI, the expression
of ERS-associated apoptotic pathway proteins was detected. The
western blot analysis results demonstrated no significant
differences in the expression levels of p-PERK, PERK, CHOP, ATF4 or
cleaved caspase-3 between the S and Bar alone groups (Fig. 4). However, the expression of
p-PERK, PERK, ATF4, CHOP and cleaved caspase-3 proteins in the
I/R+Bar group was significantly decreased compared with that of the
I/R group (Fig. 4). These results
indicated that Bar pretreatment protected myocardial cells from
ischemia-reperfusion injury by inhibiting the PERK-CHOP mediated
apoptotic signal pathway.
Discussion
Ischemic heart disease is a serious health problem
worldwide and its incidence is increasing (30,31). Rapidly restoring myocardial blood
flow and relieving tissue ischemia is the only effective treatment
for ischemic heart disease (32,33). However, an important problem in
restoring blood perfusion is MIRI (33). Following an in depth study of the
mechanism of ischemic precondition for myocardial protection, it
may be suggested that other physical stimuli or certain drug
preparations also lead to preconditioning, thereby enhancing the
ability of cells to resist injury (30). Therefore, it is important to
understand the pathogenesis of MIRI and actively identify effective
therapeutic targets to prevent MIRI. In recent years, drug
pretreatment has become an interesting topic in the prevention and
treatment of MIR.
MIRI is a complex pathophysiological process with
the understanding of its pathogenesis and signal transduction
remaining unclear. It is considered that cardiomyocyte apoptosis,
free radical production and intracellular calcium overload are
important pathogenic mechanisms of MIRI (8,9).
Apoptosis is a process of programmed cell death, which is involved
in a variety of physiological and pathological processes (34). Growing evidence from basic and
clinical experiments has demonstrated that cardiomyocyte apoptosis
is associated with the occurrence and development of ischemic
cardiomyopathy (12,34). Apoptosis is an important cause of
myocardial cell injury and cardiac function decline following MIR
(12,35). The results of the present study
demonstrated that MIRI increased the levels of myocardial injury
markers CK and LDH, and increased the proportion of myocardial
cells undergoing apoptosis. This indicates that MIR may cause
myocardial cell apoptosis and exacerbate the damage to cardiac
function.
The classical apoptotic pathways in cells primarily
include the death receptor activation pathway and the mitochondrial
damage apoptotic pathway (36,37). The apoptosis pathway mediated by
ERS is a more recently discovered pathway of apoptosis (9). The ER is the site of protein
folding, calcium homeostasis and lipid biosynthesis. It is an
important organelle for determining the survival of cells (38). The dynamic balance of the
intracellular environment serves a decisive role in the maintenance
of the physiological function of the ER. In a variety of
pathological conditions, the homeostasis of the ER is destroyed,
and its function is disorganized, which results in the accumulation
of a large number of misfolded and unfolded proteins. This process
is called ERS (39). At this
point, the cell starts a highly conservative unfolded protein
response (UPR) to improve the ability of the ER to process and
refold the proteins to maintain the homeostasis of the cells. The
UPR process is primarily mediated by three ER transmembrane
proteins: PERK, inositol dependent enzyme 1 (IRE1) and ATF6. The
binding of these three sentinel proteins to the ER chaperone GRP78
is inactivated (39). However,
when persistent, intense ERS occurs, the overexpression of GRP78 is
induced, which activates the expression of PERK, IRE1 and ATF6 and
triggers the CHOP and caspase-12 apoptosis signaling pathway
(39,40). This experiment showed that the
expression of ERS marker proteins GRP78 and caspase-12 increased
significantly after ischemia-reperfusion, demonstrating that ERS
was involved in the occurrence of MIRI.
Aloe is a kind of plant belonging to
Liliaceae (18,19). It is a traditional Chinese
medicine used to treat wounds and diar-rhea. It has attracted the
attention of many scholars because of its pharmacological
properties (18). It has been
reported that aloe can alleviate neuronal damage and protect the
spinal cord from ischemia-reperfusion injury (41). Aloe has an effective
neuroprotective effect on sciatic nerve ischemia-reperfusion injury
(42). Aloe may serve a
protective role in cerebral ischemia by inhibiting neuronal
apoptosis (43). The gavaging of
aloe has been demonstrated to protect kidney and lung tissues from
oxidative damage caused by ischemia-reperfusion (44). Bar is the main active ingredient
of aloe and has received increasingly more attention (21). A recent study demonstrated that
Bar controlled ventricular arrhythmia by regulating voltage-gated
ion channels (22). Bar
pretreatment inhibits myocardial oxidative stress by activating the
AMPK signaling pathway, thereby alleviating MIRI (23). However, to the best of our
knowledge, no previous studies have demonstrated that Bar
pretreatment inhibits myocardial apoptosis induced by MIR to
achieve myocardial protection. In the present study, following
pretreatment with Bar, the concentration of myocardial injury
markers CK and LDH decreased significantly, and the apoptosis of
myocardial cells was significantly reduced. The experimental
results suggested that the expression of ERS marker proteins GRP78
and caspase-12 were markedly decreased following the application of
Bar. The aforementioned experimental results suggest that Bar
pretreatment exhibited a protective effect on MIRI and ERS
participated in this protection mechanism.
ER is a membranous organelle. It is a specific site
for monitoring protein synthesis, folding, modification and
aggregation (45). These proteins
serve an important role in regulating cell activity in the ER,
golgi apparatus and the cell membrane. Correct protein folding is
key to cell survival (39).
Therefore, UPR is an important protein reaction for the function
and maintenance of normal physiological activities of the ER
(37,39). The ER has a complex monitoring
system that ensures the correct folding, modification and assembly
of proteins through a variety of regulatory mechanisms.
Ischemia-reperfusion injury disrupts the homeostasis of the ER,
causing unfolded or misfolded proteins, thereby activating UPR and
promoting cell survival (37,39). The UPR pathway is continuously
regulated under normal and stress conditions. However, how UPR
sensors are triggered by molecules remains unclear. A previous
study reported that the deletion of CNPY2 inhibited the PERK-CHOP
signaling pathway and protected mice from UPR-induced liver injury
(46). The PERK-CNPY2 axis
enhanced ER stress-induced cell death in liver disease (47). Recent studies have demonstrated
that CNPY2 is present in cardiomyocytes, and have confirmed that
CNPY2 attenuates the shift from compensatory hypertrophic response
to ventricular dilatation and heart failure (28,29). CNPY2 serves a key role in ERS and
is an important trigger factor for the PERK-CHOP signaling pathway
(46,47). Therefore, we hypothesized that
CNPY2 may also initiate the PERK-CHOP signaling pathway and
participate in the ERS induced by MIRI. The results of the current
study revealed that CNPY2 was expressed in rat cardiomyocytes, and
may activate the PERK-CHOP pathway to trigger the ERS apoptotic
pathway-associated proteins CHOP and caspase-3, eventually leading
to cardiomyocyte apoptosis.
In conclusion, the results of the present study
suggest that CNPY2 is present in cardiomyocytes and may participate
in the development of MIRI by initiating the PERK-CHOP signaling
pathway. Bar pretreatment reduced MIRI by inhibiting the CNPY2-PERK
apoptotic pathway. These findings provide novel insights into the
pathogenesis of MIRI and demonstrate the potential mechanisms by
which Bar treats myocardial infarction, which is important for
developing novel strategies for the prevention and treatment of
myocardial infarction. However, the pathogenesis of myocardial
infarction is complex, and there are other signaling pathways
involved in cardiomyocyte apoptosis. To further clarify the
therapeutic mechanism of Bar for myocardial infarction, further
studies are required.
Funding
The present study was supported by the Foundation of
Tianjin Health and Family Planning Commission (grant no.
14KG101).
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
YC and GL conceived and designed the study. YC
conducted the experiments and wrote the manuscript. YC and YW
analyzed the data. YC and GL revised the manuscript. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols in the current study were
performed in strict accordance with the institutional guidelines
and approved by the Laboratory Animal Ethics Committee of Tianjin
Huanhu Hospital (Tianjin, China) consistent with China Animal Care
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Puaschitz NG, Assmus J, Strand E, Karlsson
T, Vinknes KJ, Lysne V, Drevon CA, Tell GS, Dierkes J and Nygård O:
Adherence to the Healthy Nordic Food Index and the incidence of
acute myocardial infarction and mortality among patients with
stable angina pectoris. J Hum Nutr Diet. 32:86–97. 2019. View Article : Google Scholar
|
|
2
|
Prondzinsky R, Lemm H, Geppert A, Buerke
M, Russ M and Werdan K: Infarct-related cardiogenic shock:
Prognosis and treatment. Med Klin Intensivmed Notfmed. 113:267–276.
2018.In German. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ and
Chen Y: Protective role of melatonin in cardiac
ischemia-reperfusion injury: From pathogenesis to targeted therapy.
J Pineal Res. Epub. Feb 8–2018.Epub ahead of print. View Article : Google Scholar
|
|
4
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muntean DM, Sturza A, Dănilă MD, Borza C,
Duicu OM and Mornoș C: The role of mitochondrial reactive oxygen
species in cardiovascular injury and protective strategies. Oxid
Med Cell Longev. 2016:82549422016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye G, Fu Q, Jiang L and Li Z: Vascular
smooth muscle cells activate PI3K/Akt pathway to attenuate
myocardial ischemia/reperfusion-induced apoptosis and autophagy by
secreting bFGF. Biomed Pharmacother. 107:1779–1785. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Zhang Y, Ding K, Zhang H, Zhao Q,
Liu Z and Xu Y: Involvement of JNK1/2-NF-κBp65 in the regulation of
HMGB2 in myocardial ischemia/reperfusion-induced apoptosis in human
AC16 cardiomyocytes. Biomed Pharmacother. 106:1063–1071. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia P, Liu Y and Cheng Z: Signaling
pathways in cardiac myocyte apoptosis. Biomed Res Int.
2016:95832682016. View Article : Google Scholar
|
|
9
|
Haunstetter A and Izumo S: Apoptosis:
Basic mechanisms and implications for cardiovascular disease.
Circul Res. 82:1111–1129. 1998. View Article : Google Scholar
|
|
10
|
Baines CP and Molkentin JD: STRESS
signaling pathways that modulate cardiac myocyte apoptosis. J Mol
Cell Cardiol. 38:47–62. 2005. View Article : Google Scholar
|
|
11
|
Bernecker OY, Huq F, Heist EK, Podesser BK
and Hajjar RJ: Apoptosis in heart failure and the senescent heart.
Cardiovasc Toxicol. 3:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mattson MP and Kroemer G: Mitochondria in
cell death: Novel targets for neuroprotection and cardioprotection.
Trends Mol Med. 9:196–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minamino T and Kitakaze M: ER stress in
cardiovascular disease. J Mol Cell Cardiol. 48:1105–1110. 2010.
View Article : Google Scholar
|
|
15
|
Zhang C, Tang Y, Li Y, Xie L, Zhuang W,
Liu J and Gong J: Unfolded protein response plays a critical role
in heart damage after myocardial ischemia/reperfusion in rats. PLoS
One. 12:e01790422017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Ischemia/reperfusion. Compr Physiol. 7:113–170. 2016.
View Article : Google Scholar
|
|
17
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singab AN, El-Hefnawy HM, Esmat A, Gad HA
and Nazeam JA: A systemic review on Aloe arborescens
pharmacological profile: Biological activities and pilot clinical
trials. Phytother Res. 29:1858–1867. 2015. View Article : Google Scholar
|
|
19
|
Anuszewska EL: Mechanisms of therapeutic
action of aloe. Wiad Lek. 68:168–172. 2015.In Polish.
|
|
20
|
Akaberi M, Sobhani Z, Javadi B, Sahebkar A
and Emami SA: Therapeutic effects of Aloe spp. in traditional and
modern medicine: A review. Biomed Pharmacother. 84:759–772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel DK, Patel K and Tahilyani V:
Barbaloin: A concise report of its pharmacological and analytical
aspects. Asian Pac J Trop Biomed. 2:835–838. 2012. View Article : Google Scholar
|
|
22
|
Cao ZZ, Tian YJ, Hao J, Zhang PH, Liu ZP,
Jiang WZ, Zeng ML, Zhang PP and Ma JH: Barbaloin inhibits
ventricular arrhythmias in rabbits by modulating voltage-gated ion
channels. Acta Pharmacol Sin. 39:357–370. 2018. View Article : Google Scholar :
|
|
23
|
Zhang P, Liu X, Huang G, Bai C, Zhang Z
and Li H: Barbaloin pretreatment attenuates myocardial
ischemia-reperfusion injury via activation of AMPK. Biochem Biophys
Res Commun. 490:1215–1220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chimenti S, Carlo E, Masson S, Bai A and
Latini R: Myocardial infarction: Animal models. Methods Mol Med.
98:217–226. 2004.PubMed/NCBI
|
|
25
|
Li F, Zheng X, Fan X, Zhai K, Tan Y, Kou J
and Yu B: YiQiFuMai powder injection attenuates
ischemia/reperfusion-induced myocardial apoptosis through AMPK
activation. Rejuvenation Res. 19:495–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi X, Vallentin A, Churchill E and
Mochly-Rosen D: DeltaPKC participates in the endoplasmic reticulum
stress-induced response in cultured cardiac myocytes and ischemic
heart. J Mol Cell Cardiol. 43:420–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
28
|
Guo J, Mihic A, Wu J, Zhang Y, Singh K,
Dhingra S, Weisel RD and Li RK: Canopy 2 attenuates the transition
from compensatory hypertrophy to dilated heart failure in
hypertrophic cardiomyopathy. Eur Heart J. 36:2530–2540. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hatta K, Guo J, Ludke A, Dhingra S, Singh
K, Huang ML, Weisel RD and Li RK: Expression of CNPY2 in mouse
tissues: Quantification and localization. PLoS One. 9:e1113702014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ding S, Fan Z, Lin C, Dai Q, Zhou J, Huang
H, Xu Y and Zhong C: Therapeutic effects of ischemic-preconditioned
exosomes in cardiovascular diseases. Adv Exp Med Biol. 998:271–281.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yasuda S and Shimokawa H: Acute myocardial
infarction: The enduring challenge for cardiac protection and
survival. Circ J. 73:2000–2008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kalra S, Bhatt H and Kirtane AJ: Stenting
in primary percutaneous coronary intervention for acute ST-segment
elevation myocardial infarction. Methodist Debakey Cardiovasc J.
14:14–22. 2018.PubMed/NCBI
|
|
33
|
Piper HM, Kasseckert SA, Schlüter KD and
Abdallah Y: Pathophysiology of myocardial reperfusion injury. Dtsch
Med Wochenschr. 133:586–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Crow MT, Mani K, Nam YJ and Kitsis RN: The
mitochondrial death pathway and cardiac myocyte apoptosis. Circ
Res. 95:957–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu T, Ding W, Tariq MA, Wang Y, Wan Q, Li
M and Wang J: Molecular mechanism and therapy application of
necrosis during myocardial injury. J Cell Mol Med. 22:2547–2557.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khosravi-Far R and Esposti MD: Death
receptor signals to mitochondria. Cancer Biol Ther. 3:1051–1057.
2004. View Article : Google Scholar
|
|
37
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gorman AM, Healy SJ, Jäger R and Samali A:
Stress management at the ER: Regulators of ER stress-induced
apoptosis. Pharmacol Ther. 134:306–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Iurlaro R and Muñoz-Pinedo C: Cell death
induced by endoplasmic reticulum stress. FEBS J. 283:2640–2652.
2016. View Article : Google Scholar
|
|
40
|
Ferri KF and Kroemer G: Organelle-
specific initiation of cell death pathways. Nat Cell Biol.
3:E255–E263. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuksel Y, Guven M, Kaymaz B, Sehitoglu MH,
Aras AB, Akman T, Tosun M and Cosar M: Effects of aloe vera on
spinal cord ischemia-reperfusion injury of rats. J Invest Surg.
29:389–398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guven M, Gölge UH, Aslan E, Sehitoglu MH,
Aras AB, Akman T and Cosar M: The effect of aloe vera on
ischemia-reperfusion injury of sciatic nerve in rats. Biomed
Pharmacother. 79:201–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu ZQ, Deng YJ and Lu JX: Effect of aloe
polysaccharide on caspase-3 expression following cerebral ischemia
and reper-fusion injury in rats. Mol Med Rep. 6:371–374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sahin H, Yener AU, Karaboga I, Sehitoglu
MH, Dogu T, Altinisik HB, Altinisik U and Simsek T: Protective
effect of gel form of gastric gavage applicated aloe vera on
ischemia reperfusion injury in renal and lung tissue. Cell Mol Biol
(Noisy-le-grand). 63:34–39. 2017. View Article : Google Scholar
|
|
45
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hong F, Liu B, Wu BX, Morreall J, Roth B,
Davies C, Sun S, Diehl JA and Li Z: CNPY2 is a key initiator of the
PERK-CHOP pathway of the unfolded protein response. Nat Struct Mol
Biol. 24:834–839. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Urra H and Hetz C: Fine-tuning PERK
signaling to control cell fate under stress. Nat Struct Mol Biol.
24:789–790. 2017. View Article : Google Scholar : PubMed/NCBI
|