Introduction
Osteoarthritis (OA) is a chronic degenerative
osteoarticular disease that is severely damaging to human health
(1). Its degenerative changes
manifest as degeneration and destruction of articular cartilage,
subchondral bone sclerosis or cystic degeneration, hyperostosis in
the joint edge, hyper-trophic synovium, capsular contracture,
ligament laxity, and muscular atrophy and weakness (2). OA is associated with high morbidity,
and is frequently observed in middle-aged and elder patients
(3). Typically, the number of
female cases is greater than that of male cases (3). The morbidity among persons aged
<40, 60-75 or >75 years old is ~5, 50 or 80%, respectively
(3). These findings suggest a
distinctly increasing trend with age. The disability rate of OA is
as high as 53%, and the final outcomes of OA include joint
deformities and dysfunction if it is left untreated (3). Patients with OA partly or completely
lose their ability to live independently. This severely affects the
quality of life of patients and causes tremendous economic and
social burdens. Therefore, investigating the etiology and
pathogenesis of OA is of great value for the early prevention,
early diagnosis and effective intervention of OA.
MicroRNAs (miRs) are a class of highly conserved
non-coding, single-strand, small, molecular RNA that were
identified in recent years (4).
They are 20-25 nucleotides in length (4). Notably, miRs are extensively
distributed in multiple eukaryotes and primarily achieve their
functions through two modes, namely, induction of target mRNA
degradation and targeted inhibition of mRNA translation (5). Furthermore, miRs serve important
regulatory roles in processes such as cell growth and division
(6).
Transforming growth factor (TGF)-β is a polypeptide
factor that can promote cell growth, proliferation and synthesis
(7). In addition, it can regulate
vascular endothelial cell growth, inflammatory cell chemotaxis,
fibroblast proliferation, extracellular matrix synthesis and
degradation (7). Furthermore, it
serves an important role in immune regulation and tissue repair
(7). TGF-β1 is a member of the
TGF-β family that has been extensively studied. Research on the
association between the Smad canonical signal pathway and OA has
been performed. Notably, previous results have emphasized the
importance of the role of the Smad signaling pathway in OA genesis
(7). Thus, Smad signaling pathway
has become a novel hotspot in research on the pathogenesis of OA
(8). Wu et al (9) suggested that melatonin-mediated
miR-590-5p upregulation promotes chondrogenic differentiation of
human mesenchymal stem cells. In the present study, the role of
miR-590 in OA and the underlying mechanism were investigated.
Materials and methods
Induction of OA
A total of 16 male Sprague Dawley rats (200-230 g
and 8-10 weeks old) were purchased from the Animal Experiment
Center of Kunming Medical University (Kunming, China). Rats were
housed under standard conditions (22-23°C with 50-60% humidity) and
had free access to diet and water. All rats were randomly assigned
to two groups: Sham (n=8) and OA (n=8) groups. Rats in the OA group
were injected with 300 μl of chicken CII (100 mg; Chondrex
Inc., Redmond, WA, USA). Rats were sacrificed using decollation at
12 days post-chicken CII injection under 35 mg/kg of pentobarbital
sodium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Animal
experiments were approved by the Institutional Animal Care and
Welfare Committee of Yuxi Municipal Hospital of Traditional Chinese
Medicine (Yuxi, China).
Histopathology
The ankle joints were acquired, washed with PBS and
fixed with 10% formalin for 2-3 days at room temperature. The
tissues samples were decalcified in 10% formic acid and embedded in
paraffin. Following this, tissues samples were cut into 4-μm
thick sections and stained with hematoxylin for 2 min and stained
with eosin for 1 min at room temperature. The tissues were observed
using light microscopy (magnification, ×100). H&E staining was
used to confirm the OA model.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was harvested using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) from
serum samples or cell samples. Subsequently, SuperScript RT system
(Takara Bio Inc., Otsu, Japan) was used to reverse transcribe the
RNA to cDNA. RT-qPCR was performed using the Applied Biosystems
7900 Fast Real-Time PCR system and TaqMan Universal PCR Master Mix
(both Applied Biosystems, Thermo Fisher Scientific, Inc.). Primers
used for RT-qPCR were as follows: miR-590, 5′-GGA ATT CTT CAG TTG
TAA CCC AG-3′ and 5′-CGG GAT CCT TGA GAT GTC ACC AA-3′; and U6,
5′-CTC GCT TCG GCA GCA CA-3′ and 5′-AAC GCT TCA CGA ATT TGC GT-3′.
The reaction condition included pre-denaturation at 95°C for 5 min,
followed by 40 cycles of denaturation at 94°C for 30 sec, annealing
at 60°C for 30 sec and extension at 72°C for 30 sec. Relative
miR-590 expression was calculated using the 2−ΔΔCq
method (10).
Microarray analysis
Total RNA (500 ng) from serum samples or cell
samples was hybridized to the SurePrint G3 Mouse Whole Genome GE
Microarray (G4852A Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA). Data was analyzed using A.10.7.3.1 Agilent Feature
Extraction Software (Agilent Technologies, Inc.).
Culture of bone cells and
transfection
Mouse embryo osteoblast precursor MC3T3-E1 cells
were purchased from the Cell Bank of Chinese Academy of Sciences of
Shanghai, (Shanghai, China) and maintained in Dulbecco's Modified
Eagle Medium with 10% fetal bovine serum (both Invitrogen; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 μg/ml
streptomycin at 37°C in an atmosphere containing 5% CO2.
A total of 100 ng of miR-590 (forward primer, 5′-GGC
GTCGACACAGTTCAGACAGAAGTCACAAAAA-3′ and reverse primer, 5′-GGC TCT
AGA CAC CAT CTA GTA CTT TTG CAA TGA A-3′), 100 ng of anti-miR-590
(5′-GAC GUA AAA UAC UUA UUC GAG-3′ and 5′-GAG CUU AUU CAU AAA AUG
CAG-3′), 100 ng of TGF-β1 (5′-CTC CCC CAT GCC GCC CTC GG-3′ and
5′-AGT GCA GCT GAA GCC CCG CC-3′) and 100 ng of negative mimics
(5′-CCC CCC CCC CCC C-3′ and 5′-CCC CCC CCC CC-3′) were purchased
from Sangon (Shanghai, China). MC3T3-E1 cells were transfected
using Lipofectamine 2,000 (Gibco; Thermo Fisher Scientific, Inc.)
according to the instructions of the manufacturer. Following
anti-miR-590 transfection at 4 h, 100 μg/ml disitertide, a
PI3K inhibitor (MedChemExpress, Shanghai, China), was added to
cells for 44 h.
Cell proliferation assay
MC3T3-E1 cells were transfected for 0, 24, 48 and 72
h and then 20 μl of MTT was added to cells and incubated for
4 h at 37°C. Dimethyl sulfoxide was added to cells once the medium
was removed. Following this, the solution was shaken for 20 min at
37°C. The optical density at 490 nm was measured using a plate
reader (ELX-800; Biotek Instruments, Inc., Winooski, VT, USA).
Lactate dehydrogenase (LDH) activity
levels
LDH activity levels were measured using a LDH kit
(cat. no. C0017; Beyotime Institute of Biotechnology, Haimen,
China) according to the manufacturer's instructions. The optical
density was measured using a plate reader (ELX-800; Biotek
Instruments, Inc.) at 450 nm.
Flow cytometry
MC3T3-E1 cells (1×106 cells/ml) were
washed with PBS, fixed with 4% paraformaldehyde for 10 min at room
temperature and suspended in 1X binding buffer (BD Biosciences, San
Jose, CA, USA). Cell was stained with 5 μl of Annexin
V-fluorescein isothiocyanate and propidium iodide (both BD
Biosciences) in the dark for 15 min. Apoptotic cells were assayed
using a Flow cytometer (C6; BD Biosciences) and quantified using
Flowjo 7.6.1 (FlowJo, LLC, Ashland, OR, USA).
Caspase-3 and caspase-9 activity
levels
MC3T3-E1 cell (1×106 cells/ml) protein
was lysed with radioimmunopre-cipitation assay lysis buffer and the
protein concentration of each sample was quantified via the BCA
protein assay (both Beyotime Institute of Biotechnology). Total
protein (50 μg per lane) was used to measure the activity of
caspase-3 and caspase-9 with caspase-3 and caspase-9 activity kits
(cat. nos. C1115 and C1157; Beyotime Institute of Biotechnology)
according to the manufacturer's instructions.
Dual luciferase report system
The 3′-untranslated region (UTR) of TGF-β1- pMD18-T
vector was designed by GeneCopoeia, Inc. (Guangzhou, China).
MC3T3-E1 cells were co-transfected with the 3′-UTR of TGF-β1, a
dual luciferase reporter vector and miR-590 mimics or negative
mimics using Lipofectamine 2000 (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) according to the manufacturer's instructions.
Following 48 h of transfection, the luciferase activity was
measured using dual luciferase reporter gene assay kit (Beyotime
Institute of Biotechnology). The absolute values of Firefly
luminescence were normalized to those of Renilla luciferase
activity.
Western blot analysis
MC3T3-E1 cells (1×106 cells/ml) were
lysed in radioimmunoprecipitation assaylysis (cat. no. P0013B,
Beyotime Institute of Biotechnology) buffer on ice for 30 min and
centrifuged at 4°C at 10,000 × g for 8 min. Total protein was
centrifuged and quantified with BCA assay reagent (Beyotime
Institute of Biotechnology). A total of 50 μg of Proteins
were electrophoresed on 8-12% SDS gels and transferred to
polyvinylidene difluoride membranes. The membranes were blocked
with 5% bovine serum albumin (Beyotime Institute of Biotechnology)
for 1 h at room temperature and incubated with Collagen I
(sc-25974; 1:1,000), Runt-related transcription factor 2 (Runx2;
sc-8566; 1:1,000), TGF-β1 (sc-13034; 1:1,000; all Santa Cruz
Biotechnology, Inc.), phosphorylated (p)-Smad (ab53100; 1:1,000;
Abcam, Cambridge, MA, USA) and GAPDH (sc-51631; 1:5,000; Santa Cruz
Biotechnology, Inc.) primary antibodies at 4°C overnight.
Horseradish peroxidase-conjugated antibodies against rabbit IgG
(sc-2004; 1:5,000, Santa Cruz Biotechnology, Inc.) was incubated at
4°C for 1 h following a wash step. Following this, bands were
visualized with SuperSignal West Pico chemiluminescencesubstrate
(Thermo Fisher Scientific, Inc.) and analyzed using Image_Lab_3.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence
MC3T3-E1 cells (1×105 cells/ml) were
washed with PBS and fixed with 4% paraformaldehyde for 10 min at
room temperature. MC3T3-E1 cells were treated with 0.1% Triton
X-100 in PBS for 10 min and blocked with 5% bovine serum albumin
(Beyotime Institute of Biotechnology) in PBS for 1 h at 37°C.
MC3T3-E1 cells were then incubated with TGF-β1 (sc-130348; 1:100;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. Following this,
MC3T3-E1 cells were incubated with goat anti-rabbit IgG-CFL 555
(sc-362272; 1:5,000, Santa Cruz Biotechnology, Inc.) for 1 h at
37°C and stained with 4′,6-diamidino-2-phenylindole for 15 min.
Cells were visualized using confocal microscopy (LSM 510 Meta;
Zeiss, Göttingen, Germany).
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical analyses were performed with the Student's
t-test or one-way analysis of variance followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
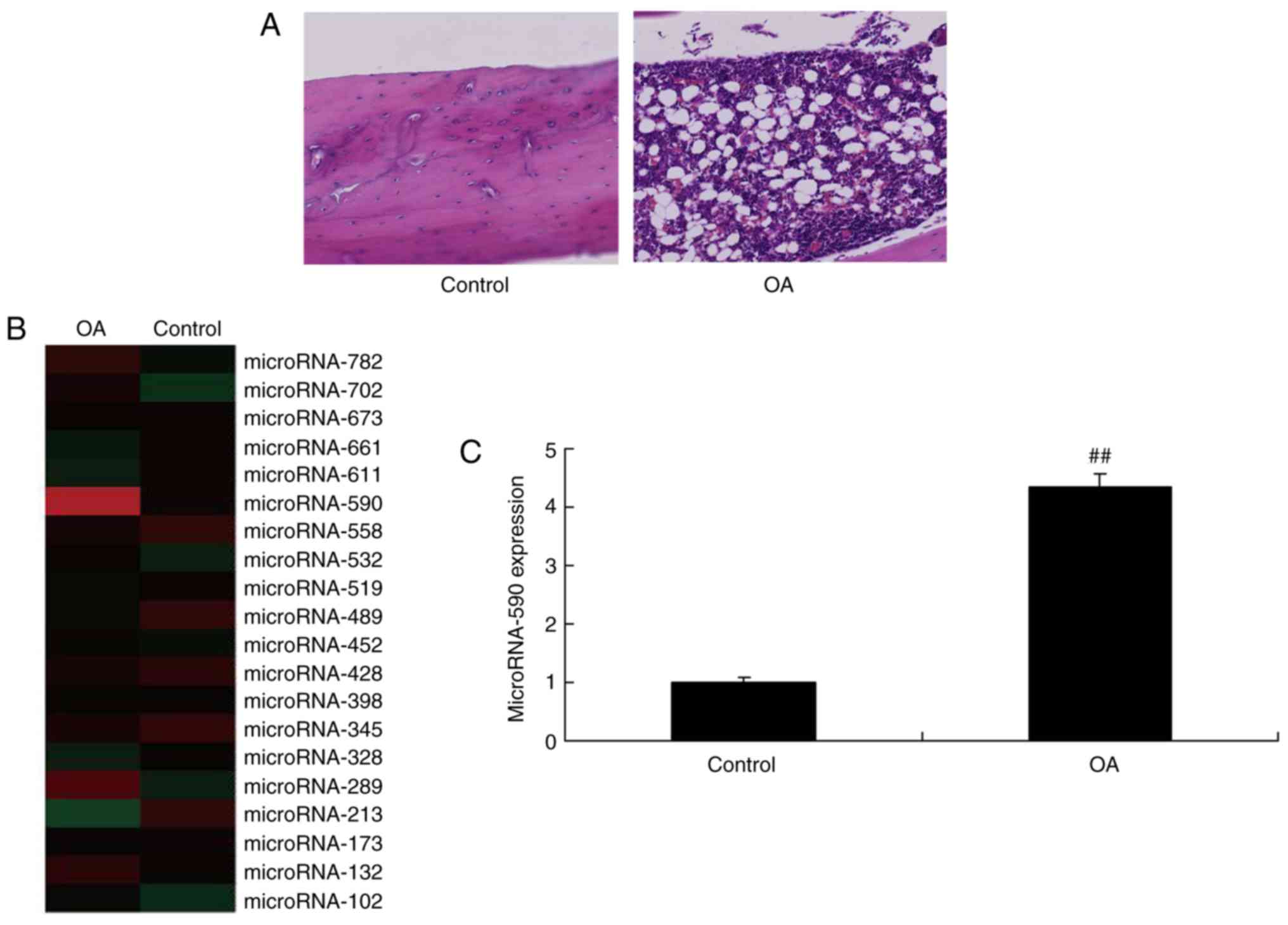
miR-590 is upregulated in the serum of
rats with OA
To evaluate whether miR-590 impacted on the onset of
OA, serum miR-590 expression levels were determined in rats with
OA. As indicated in Fig. 1A,
signs of OA were indicated in rats with OA. Serum miR-590
expression levels were upregulated in rats with OA compared with
the sham group (Fig. 1B and C).
These results indicated that miR-590 may serve an important role in
OA.
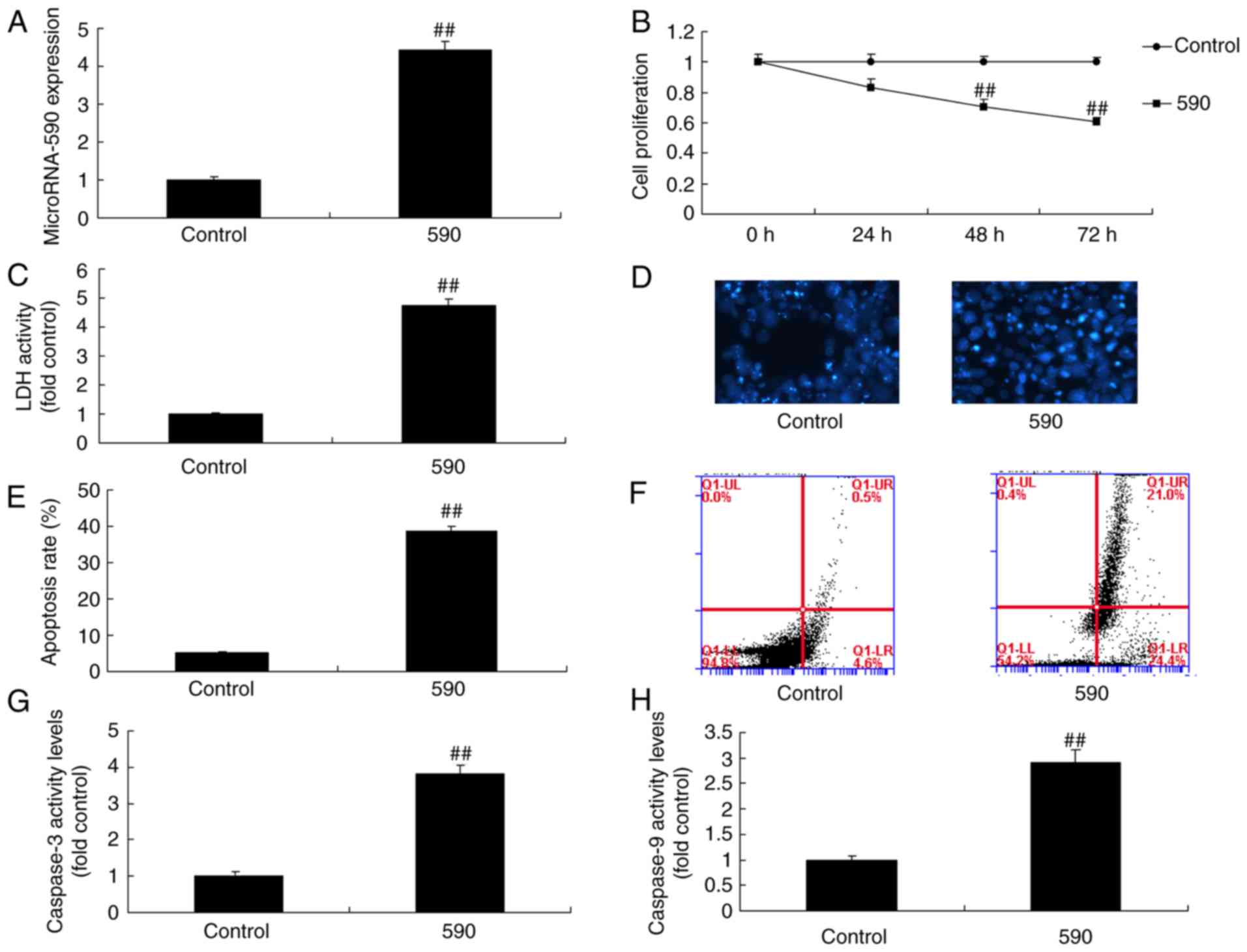
miR-590 regulates cell growth and
apoptosis of MC3T3-E1 cells
To explore the mechanism of miR-590 on apoptosis of
MC3T3-E1 cells, miR-590 mimics were used to increase the expression
of miR-590 in MC3T3-E1 cells. Notably, miR-590 mimics significantly
increased the expression of miR-590, decreased cell proliferation
and increased LDH activity, the apoptotic rate, caspase-3 activity
and caspase-9 activity in MC3T3-E1 cells compared with the control
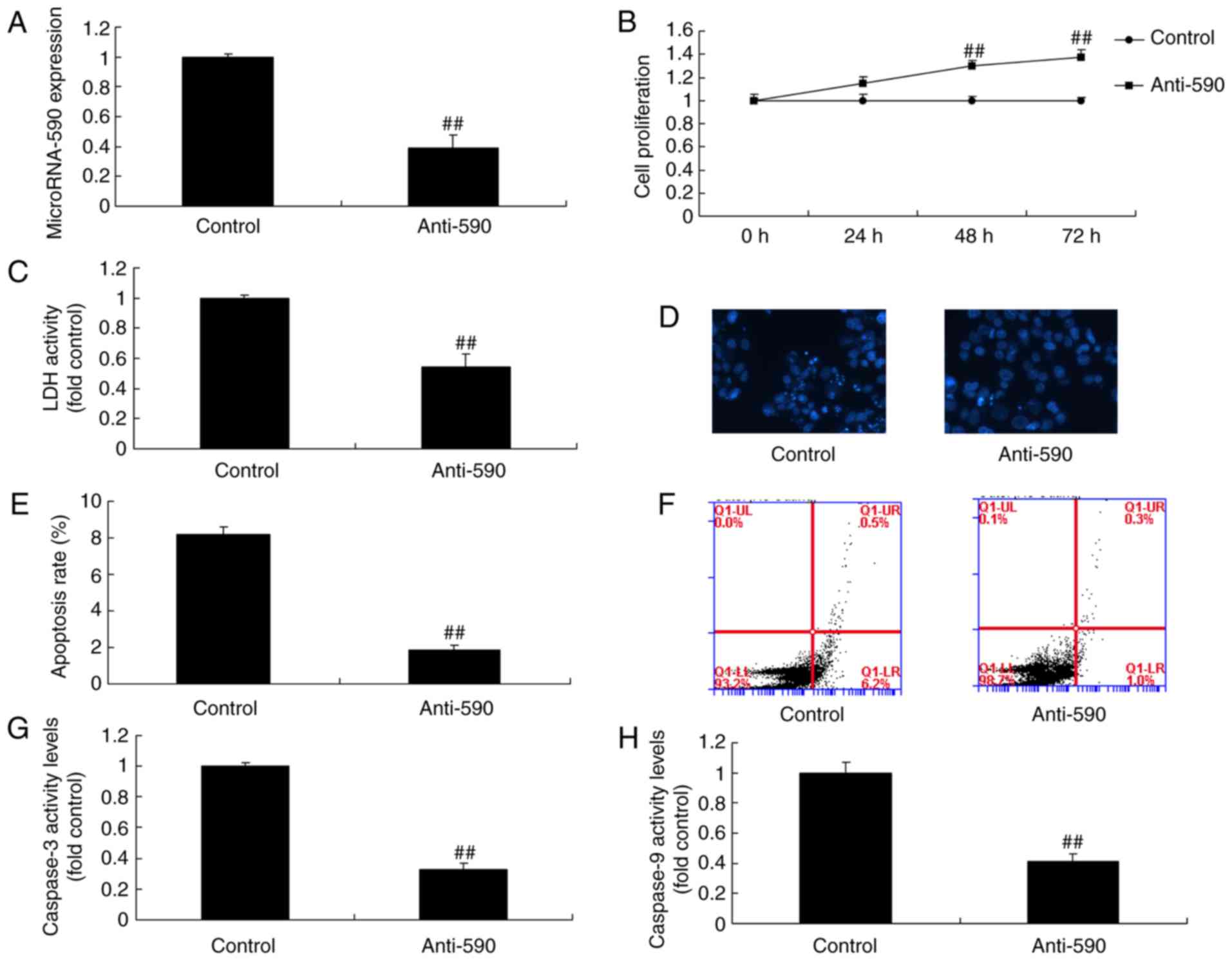
group (Fig. 2). However,
anti-miR-590 mimics significantly inhibited the expression of
miR-590, promoted cell proliferation and decreased LDH activity,
the apoptotic rate, caspase-3 activity and caspase-9 activity in
MC3T3-E1 cells compared with the control group (Fig. 3). Therefore, the findings
indicated that miR-590 could regulate bone cell growth and
apoptosis in MC3T3-E1 cells.
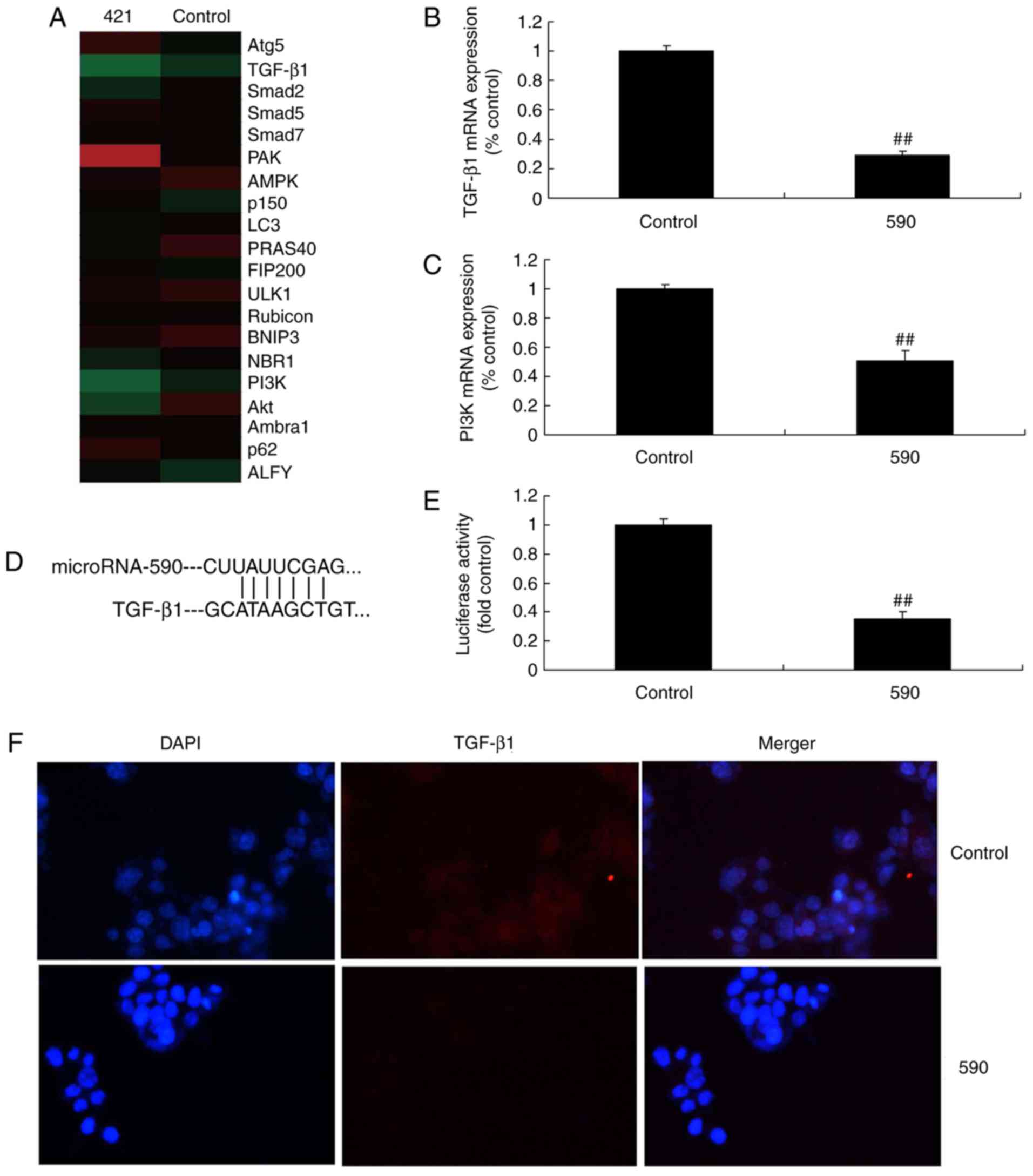
miR-590 regulates the TGF-β1 signaling
pathway of MC3T3-E1 cells
To evaluate the underlying mechanism in
miR-590-mediated bone cell apoptosis in arthritis, gene chip
analysis was used to analyze the expression levels of TGF-β1
signaling pathway components. As indicated in Fig. 4, the mRNA expression levels of
TGF-β1 and PI3K were significantly reduced in MC3T3-E1 cells
treated with miR-590 when compared with the control group.
Furthermore, miR-590 targeted the 3′-UTR of TGF-β1 mRNA. Notably,
miR-590 overexpression led to significantly decreased luciferase
reporter levels compared with the control group (Fig. 4D and E). In addition,
immunofluorescence revealed that overexpression of miR-590
suppressed the protein expression levels of TGF-β1 in MC3T3-E1
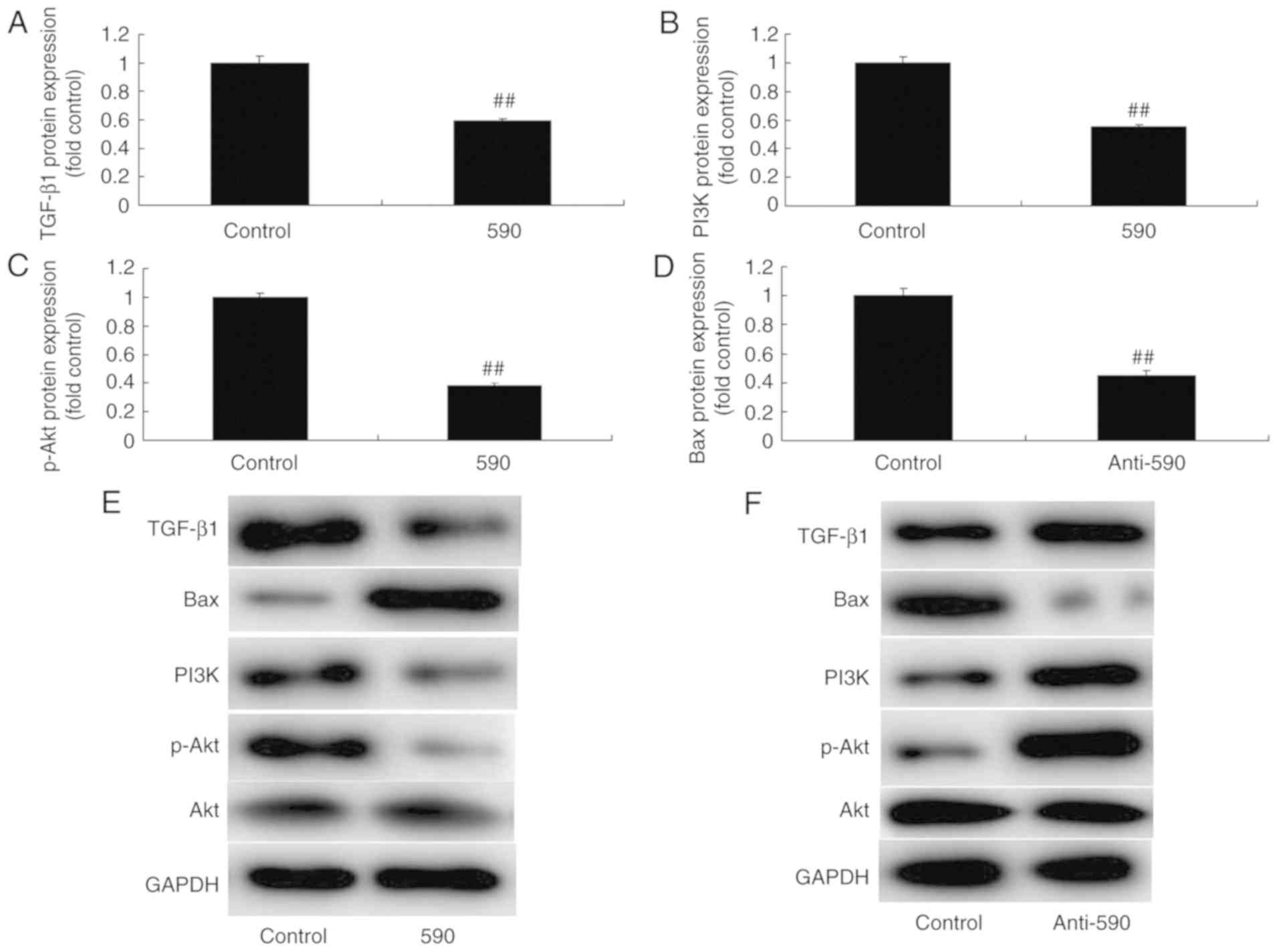
cells compared with the control group (Fig. 4F). Western blot analysis revealed
that upregulation of miR-590 significantly suppressed the protein
expression levels of TGF-β1, PI3K and p-Akt, and induced the
expression levels of B-cell lymphoma-2 associated X protein (Bax)
in MC3T3-E1 cells compared with the control group (Fig. 5A-E). However, downregulation of
miR-590 significantly induced the protein expression of TGF-β1,
PI3K and p-Akt, and suppressed the expression of Bax in MC3T3-E1
cells, compared with control group (Fig. 5F-J). These results indicated that
miR-590 induced bone apoptosis through TGF-β1/PI3K/Akt
signaling.
The promotion of TGF-β1 attenuates the
effects of miR-590 on bone cell apoptosis in MC3T3-E1 cells by the
TGF-β1 signaling pathway
To investigate the role of TGF-β1 on the effects of
miR-590 in bone cell apoptosis in MC3T3-E1 cells, TGF-β1 plasmid
was utilized to increase the protein expression of TGF-β1 in
MC3T3-E1 cells. As indicated in Fig.
6A-E, the promotion of TGF-β1 significantly induced the protein
expression of TGF-β1, PI3K and p-Akt, and suppressed that of Bax in
MC3T3-E1 cells when compared with the miR-590 group. As indicated
in Fig. 6F-L, the promotion of
TGF-β1 significantly attenuated the effects of miR-590 on the
inhibition of cell proliferation and the promotion of LDH activity,
apoptotic rate and caspase-3/9 activity in MC3T3-E1 cells compared
with those in miR-590 group.
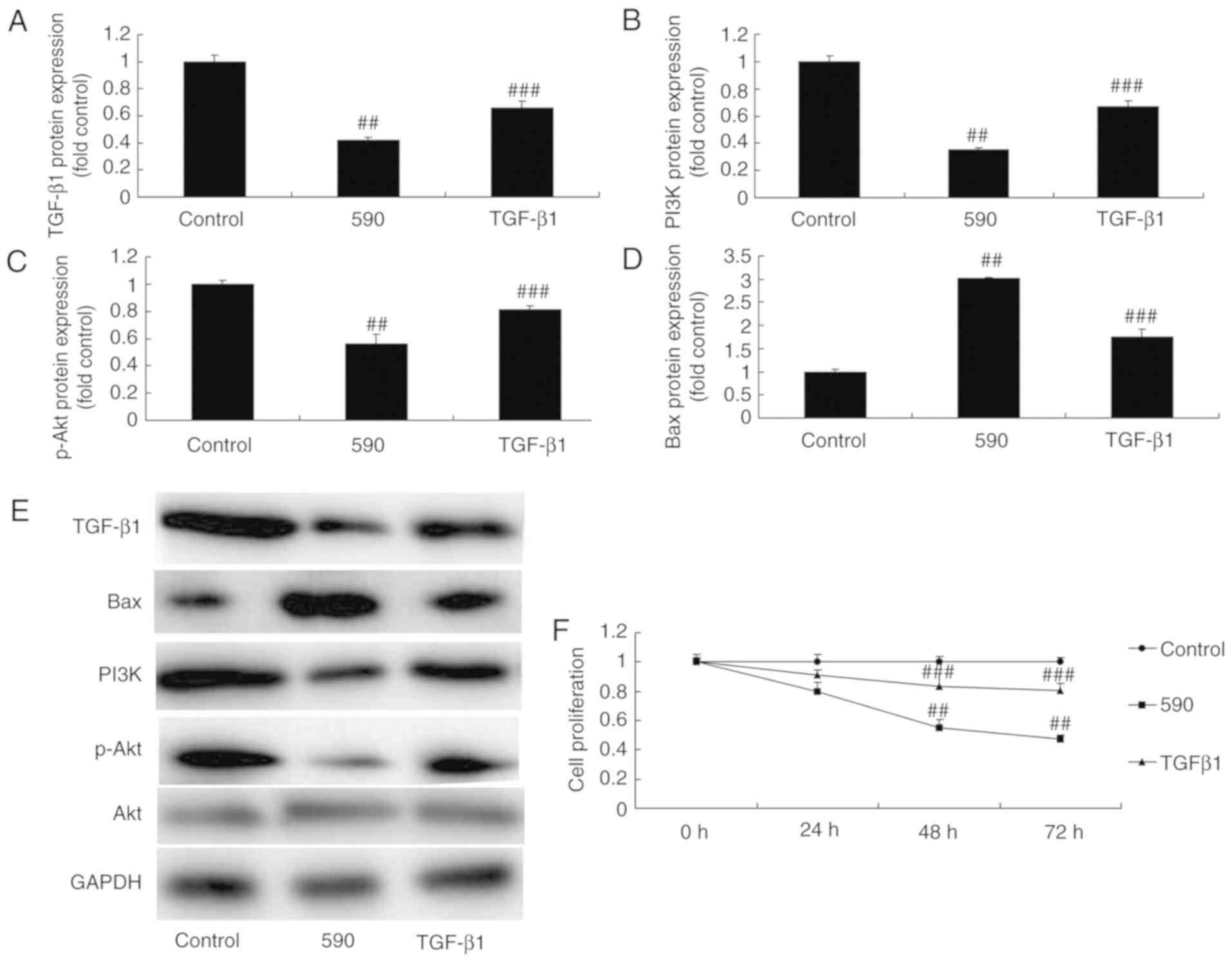 | Figure 6The promotion of TGF-β1 reduces the
effect of microRNA-590 on bone cell apoptosis in MC3T3-E1 cells
through TGF-β1 signaling. (A-E) TGF-β1, PI3K, p-Akt and Bax protein
expression levels were analyzed using western blot analysis. (F)
Cell proliferation, (G) LDH activity, (H) 4′,6-diamidino-
2-phenylindole stained images (magnification, ×100), (I and J)
apoptosis rate, and (K) caspase-3 and (L) caspase-9 activity were
indicated. ##P<0.01 vs. control group,
###P<0.01 vs. microRNA-590 group. 590, microRNA-590
group; TGF-β1, microRNA-590 and TGF-β1 group; PI3K,
phosphoinositide 3-kinase; p, phos-phorylated; TGF, transforming
growth factor; Bax, B-cell lymphoma-2-associated X protein. |
The inhibition of PI3K attenuates the
effects of miR-590 on bone cell apoptosis in MC3T3-E1 cells by the
PI3K signaling pathway
The present study assessed the function of PI3K on
the effects of miR-590 on adipogenic differentiation. Disitertide,
a PI3K inhibitor, significantly suppress the protein expression
levels of PI3K and p-Akt, and induce the protein expression of Bax
in MC3T3-E1 cells compared with the miR-590 group (Fig. 7A-D). Furthermore, PI3K inhibitor
attenuated the effect of miR-590 on cell proliferation and LDH
activity, apoptotic rate and caspase-3/9 activity compared with the
miR-590 group (Fig. 7E-K).
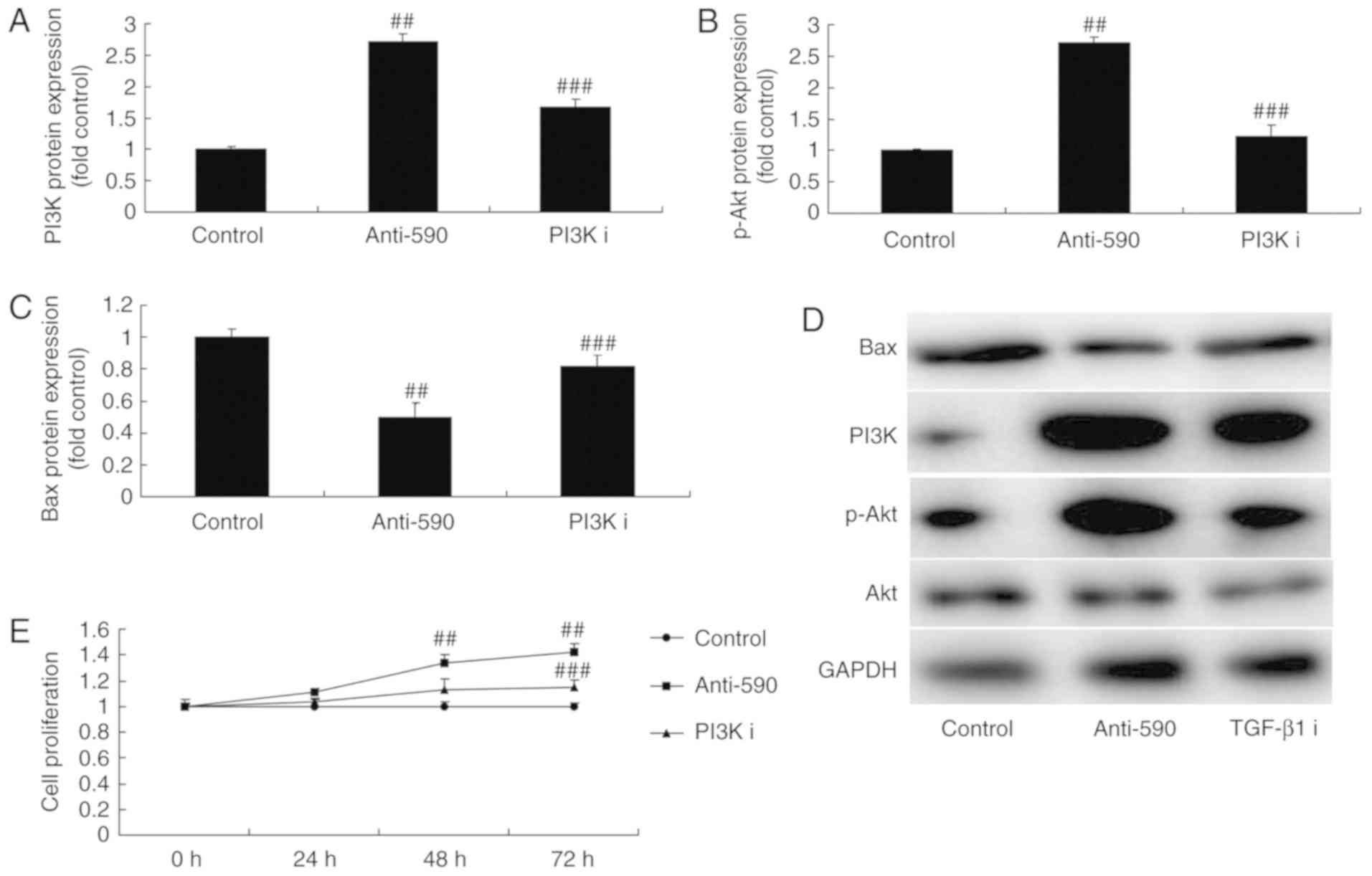 | Figure 7The inhibition of PI3K reduces the
effect of microRNA-590 on bone cell apoptosis in MC3T3-E1 cells
through PI3K signaling. (A-D) PI3K, p-Akt and Bax protein
expression levels were analyzed using western blot analysis. (E)
Cell proliferation, (F) LDH activity, (G)
4′,6-diamidino-2-phenylindole staining images, (H and I) apoptosis
rate, (J) caspase-3 and (K) caspase-9 activity were indicated.
##P<0.01 vs. control group; ###P<0.01
vs. microRNA-590 group. 590, microRNA-590 group; PI3K I,
microRNA-590 and PI3K inhibitor group; TGF, transforming growth
factor p, phosphorylated; TGF, transforming growth factor; Bax,
B-cell lymphoma-2-associated X protein. |
Discussion
OA is the most common degenerative disease in human
axial joints and peripheral dynamic joints (11). It can involve the articular
cartilage, synovial membrane, articular capsule and muscles
surrounding joints (11). OA is a
type of irreversible articular damage induced by different factors.
Major OA-induced pathological changes include cartilage
degeneration and disappearance, reactive hyperplasia of ligament
attachment points at the articular edge and subchondral bone, and
osteophyte formation (12). At
present, no effective treatment is available in clinic, and the
therapeutic effects are inadequate. Therefore, exploring potential
OA treatment options is of great importance (12). Involvement of miRs in the
pathology and physiology of OA has been verified through methods of
gene network and epigenetics (12) Further understanding of the action
of targets and regulatory factors during molecular research on OA
may facilitate the development of novel treatments.
miRs can control joint destruction and stimulate
repair (13,14). Furthermore, miRs regulate protein
expression and can thus can be used to treat and intervene in the
molecular targets of OA (13,14). The present study revealed that
serum miR-590 expression was significantly upregulated in rats with
OA. Additionally, the results indicated that miR-590 mimics
significantly increased the expression of miR-590 in MC3T3-E1
cells, decreased cell proliferation and increased LDH activity, the
apoptosis rate and caspase-3/9 activity in MC3T3-E1 cells. Wu et
al (9) suggested that
melatonin-mediated miR-590-5p upregulation promotes chondrogenic
differentiation in human mesenchymal stem cells. These results
Imply that the upregulation of miRNA-590 in rheumatoid arthritis
promotes apoptosis of bone cells.
TGF-β1 is a type of polypeptide involved in the
formation of bone and cartilage (15). It can also stimulate chondrocytes
to produce proteoglycan and promotes excessive expression of type
II collagen and aggrecan (15).
Previous results have suggested that the expression of TGF-β1,
TGF-β2 and TGF-β3 genes in OA chondrocytes exhibit various degrees
of upregulation (15). This is
consistent with the percentage of positive TGF-β chondrocytes
(15). TGF-β2 can inhibit
chondrocyte hypertrophy and downregulate the expression of
inflammatory cytokines interleukin-1β, tumor necrosis factor-α,
matrix metalloproteinase (MMP)-9 and MMP-13 in OA chondrocytes
cultured in vitro (16,17). Thus, TGF-β2 can prevent the
excessive degradation of type II cartilage collagen. TGF-β1 can
increase the expression of tissue inhibitor of metalloproteinase
(TIMP)-1 in OA chondrocytes, whereas TIMP-1 is also an angiogenic
inhibitor (16,17). Therefore, TGF-β1 can antagonize
vascular invasion of OA (16,17). In the present study, upregulation
of miR-590 significantly suppressed the protein expression of
TGF-β1, PI3K and p-Akt, and induced Bax protein expression in
MC3T3-E1 cells. Notably, Ekhteraei-Tousi et al (18) suggested that miR-590-5p regulates
cardiosphere-derived stem cells differentiation through
downregulation of TGFB signaling. Based on the above information,
it may be concluded that miR-590-5p regulates TGF-β1/PI3K/Akt
signaling in bone cells.
It has been indicated in recent research that the
PI3K/Akt signal transduction pathway is also involved in the
pathogenesis and progression of OA (19). Expression levels of PI3K/Akt are
increased in cartilage in OA. Activation of the PI3K/Akt signaling
pathway can aggravate degradation of cartilage matrix protein
(20). Furthermore, activation of
the PI3K/Akt signaling pathway can also enhance the expression of
MMPs in chondrocytes (20).
Consequently, Smad signaling serves an important role in cartilage
degeneration. Notably, gene knockout can induce increased
expression of PI3K/Akt signaling molecules in nervous system cells
(20). In the present study it
was reported that TGF-β1 significantly reduced the effects of
miR-590 upregulation on bone cell apoptosis. Furthermore, the
inactivation of PI3K significantly inhibited the effects of miR-590
on bone cell apoptosis. Li et al (21) revealed that YKL-40-induced
inhibition of miR-590-3p promotes angiogenesis of endothelial
progenitor cells via PI3K/Akt signaling pathway.
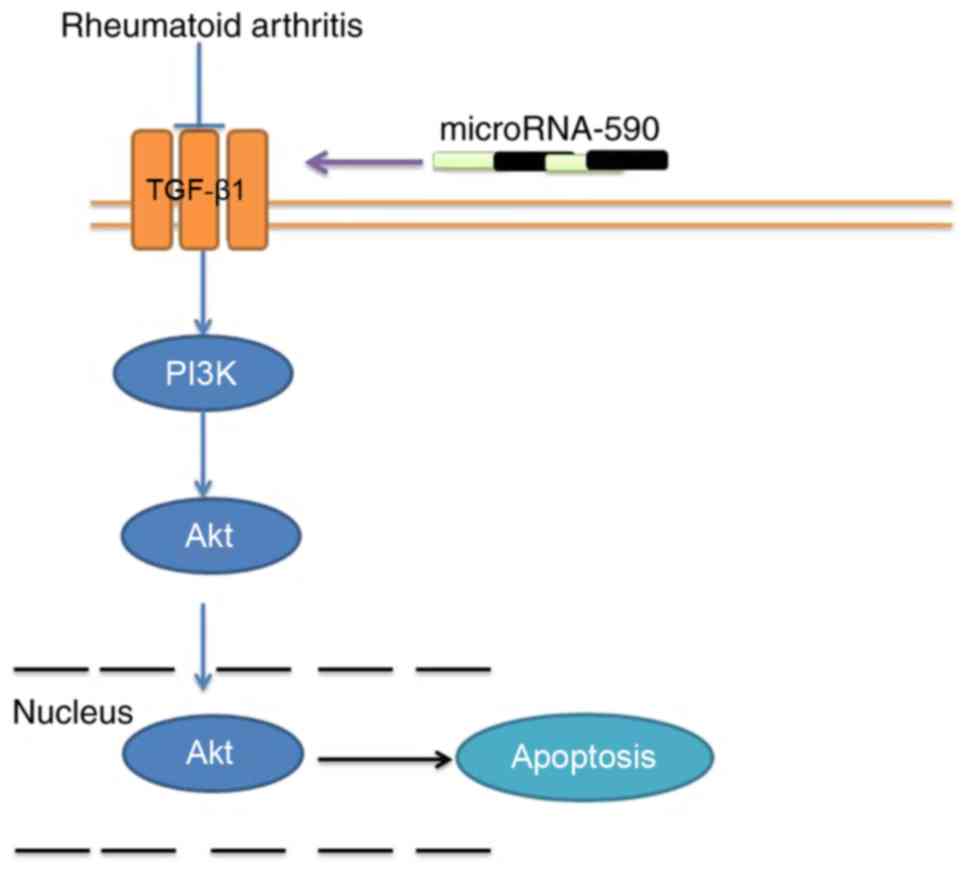
In conclusion, the findings suggest that miR-590 can
mediate OA and regulate bone cell apoptosis through TGF-β1/PI3K/Akt
signaling (Fig. 8), which may be
a critical factor for identifying potential OA treatments in the
clinic. Further clinical studies are warranted to investigate the
effi-cacy of miR-590 in OA.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JY designed the experiment; YZ and JL performed the
experiment; JY and YZ analyzed the data; and JY wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the
Institutional Animal Care and Welfare Committee of Yuxi Municipal
Hospital of Traditional Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Yan H, Su Y, Chen L, Zheng G, Lin X, Chen
B, Zhou B and Zhang Q: Rehabilitation for the management of knee
osteoarthritis using comprehensive traditional Chinese medicine in
community health centers: Study protocol for a randomized
controlled trial. Trials. 14:3672013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skou ST, Roos EM, Simonsen O, Laursen MB,
Rathleff MS, Arendt-Nielsen L and Rasmussen S: The efficacy of
non-surgical treatment on pain and sensitization in patients with
knee osteoarthritis: A pre-defined ancillary analysis from a
randomized controlled trial. Osteoarthritis Cartilage. 24:108–116.
2016. View Article : Google Scholar
|
|
3
|
Cortés Godoy V, Gallego Izquierdo T,
Lázaro Navas I and Pecos Martin D: Effectiveness of massage therapy
as co-adjuvant treatment to exercise in osteoarthritis of the knee:
A randomized control trial. J Back Musculoskelet Rehabil.
27:521–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jingsheng S, Yibing W, Jun X, Siqun W,
Jianguo W, Feiyan C, Gangyong H and Jie C: MicroRNAs are potential
prognostic and therapeutic targets in diabetic osteoarthritis. J
Bone Miner Metab. 33:1–8. 2015. View Article : Google Scholar
|
|
5
|
Sondag GR and Haqqi TM: The role of
MicroRNAs and their targets in osteoarthritis. Curr Rheumatol Rep.
18:562016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rasheed Z, Al-Shobaili HA, Rasheed N,
Mahmood A and Khan MI: MicroRNA-26a-5p regulates the expression of
inducible nitric oxide synthase via activation of NF-κB pathway in
human osteoarthritis chondrocytes. Arch Biochem Biophys. 594:61–67.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akagi R, Akatsu Y, Fisch KM,
Alvarez-Garcia O, Teramura T, Muramatsu Y, Saito M, Sasho T, Su AI
and Lotz MK: Dysregulated circadian rhythm pathway in human
osteoarthritis: NR1D1 and BMAL1 suppression alters TGF-β signaling
in chondrocytes. Osteoarthritis Cartilage. 25:943–951. 2017.
View Article : Google Scholar
|
|
8
|
Finnson KW, Chi Y, Bou-Gharios G, Leask A
and Philip A: TGF-b signaling in cartilage homeostasis and
osteoarthritis. Front Biosci (Schol Ed). 4:251–268. 2012.
View Article : Google Scholar
|
|
9
|
Wu Z, Qiu X, Gao B, Lian C, Peng Y, Liang
A, Xu C, Gao W, Zhang L, Su P, et al: Melatonin-mediated
miR-526b-3p and miR-590-5p upregulation promotes chondrogenic
differentiation of human mesenchymal stem cells. J Pineal Res.
65:e124832018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
11
|
Pascarelli NA, Cheleschi S, Bacaro G,
Guidelli GM, Galeazzi M and Fioravanti A: Effect of mud-bath
therapy on serum biomarkers in patients with knee osteoarthritis:
Results from a randomized controlled trial. Isr Med Assoc J.
18:232–237. 2016.PubMed/NCBI
|
|
12
|
Liao TL, Hsieh SL, Chen YM, Chen HH, Liu
HJ, Lee HC and Chen DY: Rituximab may cause increased hepatitis C
virus viremia in rheumatoid arthritis patients through declining
exosomal microRNA-155. Arthritis Rheumatol. 70:1209–1219. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eshnazarov KE, Seon JK and Song EK:
Comparison of radiological assessments patellar resurfacing with
retention for grade IV osteoarthritis in patellofemoral joint
accomplished total knee arthroplasty. Vestn Rentgenol Radiol.
97:28–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rasheed Z, Rasheed N and Al-Shobaili HA:
Epigallocatechin- 3-O-gallate up-regulates microRNA-199a-3p
expression by down-regulating the expression of cyclooxygenase-2 in
stimulated human osteoarthritis chondrocytes. J Cell Mol Med.
20:2241–2248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corr M: Wnt-beta-catenin signaling in the
pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol.
4:550–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Upregulation of thrombospondin 1
expression in synovial tissues and plasma of rheumatoid arthritis:
Role of transforming growth factor-β1 toward fibroblast-like
synovial cells. J Rheumatol. 44:1312017. View Article : Google Scholar
|
|
17
|
Chemel M, Brion R, Segaliny AI, Lamora A,
Charrier C, Brulin B, Maugars Y, Le Goff B, Heymann D and
Verrecchia F: Bone morphogenetic protein 2 and transforming growth
factor β1 inhibit the expression of the proinflammatory cytokine
IL-34 in rheumatoid arthritis synovial fibroblasts. Am J Pathol.
187:156–162. 2017. View Article : Google Scholar
|
|
18
|
Ekhteraei-Tousi S, Mohammad-Soltani B,
Sadeghizadeh M, Mowla SJ, Parsi S and Soleimani M: Inhibitory
effect of hsa-miR-590-5p on cardiosphere-derived stem cells
differentiation through downregulation of TGFB signaling. J Cell
Biochem. 116:179–191. 2015. View Article : Google Scholar
|
|
19
|
Wu X, Long L, Liu J, Zhang J, Wu T, Chen
X, Zhou B and Lv TZ: Gambogic acid suppresses inflammation in
rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol
Med Rep. 16:7112–7118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Chen JW, Xie X, Tian J, Deng C, Wang
J, Gan HN and Li F: Autophagy inhibitor regulates apoptosis and
proliferation of synovial fibroblasts through the inhibition of
PI3K/AKT pathway in collagen-induced arthritis rat model. Am J
Transl Res. 9:2065–2076. 2017.PubMed/NCBI
|
|
21
|
Li TM, Liu SC, Huang YH, Huang CC, Hsu CJ,
Tsai CH, Wang SW and Tang CH: YKL-40-induced inhibition of
miR-590-3p promotes interleukin-18 expression and angiogenesis of
endothelial progenitor cells. Int J Mol Sci. 18:E9202017.
View Article : Google Scholar : PubMed/NCBI
|