Introduction
Cardiovascular disease is the leading cause of
mortality globally (1).
Myocardial infarction is a common symptom of ischemic heart
disease, which is associated with the irreversible necrosis of
cardiomyocytes followed by ventricular dysfunction and progressive
heart failure (2-4) due to an acute interruption of blood
supply caused by the limited proliferative capacity of terminally
differentiated cardiomyocytes or coronary artery thrombosis
(2,5). Anywhere from 450,000 to 750,000
patients with heart failure are awaiting heart transplantation,
with only 1,000 recipients per year receiving donor hearts
(6). As such, there is an urgent
requirement for alternative therapeutic options.
One promising strategy to replace lost
cardiomyocytes and regenerate the myocardium is stem cell
(SC)-based therapy. Various SC lines, including embryonic (E)SCs,
induced pluripotent (iP)SCs, multipotent germline (mG)SCs and
mesenchymal SCs, have been investigated for their therapeutic
potential in a number of preclinical studies (7-13).
Pluripotent cell lines are particularly useful cell sources for
cardiac regeneration and cardiomyocyte differentiation; however,
their clinical applicability is limited owing to teratoma formation
following transplantation (14),
despite various attempts to increase the efficiency of cardiac
differentiation (15-17).
The hair follicle (HF) is characterized by cyclical
periods of growth (anagen), regression (catagen) and rest (telogen)
throughout the lifespan of mammals (18). HF SCs/progenitor cells permanently
reside in the upper portion of the HF, known as the bulge area
(19,20) which contains slow-cycling or
label-retaining cells that define the SC population (20-22) that differentiate into various HF
cell types in addition to epidermal cells and generate follicle
structures during anagen (22).
SCs in the bulge area may additionally differentiate into the hair
matrix of the outer-root sheath, inner-root sheath and basal cells
of the sebaceous gland (19,23). One previous study has reported
that precursor cells derived from mammalian skin dermis may produce
neurons, glia, smooth muscle cells and adipocytes (24). Pluripotent epidermal neural crest
SCs are also present in the dermal papillae of HFs (25,26). The neural SC/progenitor cell
marker nestin is selectively expressed in HF bulge cells, which may
be induced to differentiate into blood vessels, neurons, glial
cells, smooth muscle cells, keratinocytes and melanocytes in
vitro (27-31). Furthermore, all three sections
(upper, middle, and lower) of the mouse vibrissa HF harbor
HF-associated PSCs that may generate spontaneously beating cardiac
muscle cells (32). However, a
major drawback of the differentiation protocol is that other cell
types including neurons, glial cells, keratinocytes and smooth
muscle cells are simultaneously produced (32). It is a serious point to overcome
for further practical application. Thus, a cardiac-specific
differentiation protocol should be established.
The present study aimed to determine the conditions
necessary for cardiac-specific in vitro differentiation of
cells isolated from mouse dorsal skin HFs in order to identify
defined culture conditions that may differentiate HF cells into
cardiomyocyte-like cells expressing cardiac-specific markers and
exhibiting spontaneous beating for over 3 months. Thus, HF cells
may be a potential source for generating cells of cardiac lineage
under defined culture conditions.
Materials and methods
Animals
Animal procedures were ethically approved by the
Animal Care and Use Committee of Chung-Ang University (Gyeonggi-do,
Korea; IACUC no. 2018-00073) and were performed in accordance with
the Guidelines for the Care and Use of Laboratory Animals published
by the National Institutes of Health (33). Mice had ad libitum access to a
standard chow diet and water and were housed under controlled
conditions at 21±2°C and 55±10% humidity and subjected to 12:12-h
light/dark cycle conditions. Male heterozygous OG2
[B6;CBA-Tg(Pou5f1-EGFP) 2Mnn/J] mice were purchased from Jackson
Laboratory (Bar Harbor, ME, USA; cat. no. 004654). A total of 8
mice (2-5 months old, 20-35 g) were used in the present study.
Isolation and collection of HF cells
Surgical procedures were performed in a sterile
environment. The OG2 mice were sacrificed and their dorsal skin was
removed and sliced into 1-2-cm pieces that were enzymatically
digested as previously described (34) with some minor modifications. The
piece of dorsal skin was treated overnight at 4°C with 2.5 mg/ml
dispase in Dulbecco's modified Eagle's medium/nutrient mixture F-12
(DMEM/F12; 1:1 mixture; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The following day, whole HFs were gently plucked from the
skin using fine forceps and were enzymatically digested at 37°C for
5 min in a 4:1 solution of 0.25% trypsin-EDTA (Thermo Fisher
Scientific, Inc.) and 7 mg/ml DNase I (Roche Diagnostics, Basel,
Switzerland) in Dulbecco's phosphate-buffered saline (DPBS; Thermo
Fisher Scientific, Inc.). The digestion was terminated by adding
10% (final volume) fetal bovine serum (FBS; GE Healthcare Life
Sciences, Little Chalfont, UK). The cell suspension was filtered
through a nylon mesh having a 40-μm pore size (BD
Biosciences, San Jose, CA, USA) and cell viability was determined
by performing the Trypan Blue dye exclusion test. In brief, a cell
suspension was simply mixed with the Trypan blue dye for a few
second at room temperature and then visually examined immediately
following staining to determine whether cells take up or exclude
the dye under the light micro scope (model no. TS100; Nikon
Corporation, Tokyo, Japan) at a magnification of x100.
HF cell culture and differentiation
HF cells were initially cultured at a density of
1.0×106 cells/well in 6-well culture plates (BD
Biosciences) containing DMEM/F12 (3:1) supplemented with 10% FBS
(GE Healthcare Life Sciences), 20 μl/ml B27 supplement
(Thermo Fisher Scientific, Inc.), 20 ng/ml basic fibroblast growth
factor (bFGF; BD Biosciences), 10 ng/ml glial cell line-derived
neurotrophic factor (GDNF; R&D Systems, Inc., Minneapolis, MN,
USA), 75 ng/ml GDNF family receptor α1 (R&D Systems, Inc.), and
1% penicillin/streptomycin (P/S; Thermo Fisher Scientific, Inc.)
and were incubated at 37°C in a humidified atmosphere of 5%
CO2 (34).
To induce their differentiation into
cardiomyocyte-like cells, HF cells were cultured in α-Minimal
Essential Medium (α-MEM; Thermo Fisher Scientific, Inc.)/FBS medium
(supplemented with 10% FBS and 1% P/S), N2/B27 medium [DMEM-F12
supplemented with 1% B27, 0.5% N2 (Thermo Fisher Scientific, Inc.),
100 μM β-mercaptoethanol (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), 2 mM L-glutamine, and 1% P/S], KnockOut
(KO)-DMEM (Thermo Fisher Scientific, Inc.)/B27 medium (supplemented
with 2% B27 and 1% P/S), or mouse serum-free medium (MSFM) as
previously described (35).
Additional growth factors including 15 μM γ-secretase
inhibitor (GSI; EMD Millipore, Billerica, MA, USA) and 25 ng/ml
bone morpho-genetic protein (BMP)-4, 2 ng/ml activin A, 150 ng/ml
noggin and 5 ng/ml vascular endothelial growth factor (VEGF; all
from R&D Systems, Inc.) were added to the media. HF cells were
seeded on feeder cells, including mitotically inactivated OP9 cells
(bone marrow stromal cells; this cell line was generously provided
by Dr Toru Nakano), SIM mouse embryo-derived thioguanine and
ouabain resistant (STO) cells (cat. no. CRL-1503), C166 cells (cat.
no. CRL-2581; both from American Type Culture Collection, Manassas,
VA, USA) or mouse embryonic fibroblasts (MEFs; this cell line was
isolated from C57BL/6 mice at embryonic day 14.5). Two adult female
C57BL/6 mice [weight, 28-30 g; age, 13-week-old; Korea Animal Tech
(Koatech), Pyeongtaek, Korea] were housed in cages at 21±2°C and
50±20% humidity with a 12-h light/dark cycle; the adult mice
produced 10 mice (sex ratio, 1:1) weighing 232-289 mg. These feeder
cells were maintained prior to mitotic inactivation. Briefly, OP9
cells, STO cells, C166 cells and MEFs were cultured at 37°C in OP9
medium [α-MEM supplemented 20% FBS and 1% P/S], STO medium [DMEM
supplemented 7% FBS, 115 μM β-mercaptoethanol, 2.4 mM
L-glutamine and 1% P/S], C166 medium [DMEM supplemented 10% FBS, 1
mM sodium pyruvate (Sigma-Aldrich; Merck KGaA) and 1% P/S] and MEF
medium [DMEM supplemented 10% FBS, 100 μM non-essential
amino acids (cat. no. 11140050; Sigma-Aldrich; Merck KGaA) and 1%
P/S], respectively. Medium was replaced every 2-3 days and cells
were passaged every 3-5 days.
To inactivate cell lines, cells were incubated in a
medium appropriate to each cell line, as described above,
containing 10 μg/ml mitomycin C for 2-3 h at 37°C. Following
the incubation, they were extensively washed 3 times with
Dulbecco's (D) PBS and collected by trypsinization. Mitomycin
C-inactivated cells were plated onto tissue culture dishes at the
following cell densities: STO, MEF, and OP9 (1×105/2
cm2) and C166 (0.5×105/2 cm2).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was prepared from the HF cells cultured
with OP9 cells, STO cells, C166 cells and MEFs using the PureLink
RNA Mini kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Only RNAs with a 260/280 ratio of 1.8 or
higher were reverse transcribed at 50°C for 50 min and at 85°C for
5 min using Superscript III Reverse Transcriptase (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RT-qPCR
was performed using the TaqMan Gene Expression Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in a 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Thermocycling conditions were as follows: Initial denaturation
stage: 95°C for 20 sec; PCR stage: 40 cycles of 95°C for 1 sec and
60°C for 20 sec; melt curve stage: 95°C for 15 sec, 60°C for 1 min
and 95°C for 15 sec. The level of gene expression was normalized to
that of GAPDH (Assay ID, Mm99999915_g1). Relative transcript
abundance was determined using the ΔΔCq method (36). The following TaqMan probes were
used: Troponin T2, cardiac type 2 (Tnnt2; Assay ID, Mm01290256_m1),
mesoderm posterior bHLH transcription factor 1 (Mesp1; Assay ID,
Mm00801883_g1), islet 1 (Isl1; Assay ID, Mm00517585_m1), myocyte
enhancer factor 2c (Mef2c; Assay ID, Mm01340842_m1), NK2 homeobox
2.5 (Nkx2.5; Assay ID, Mm01309813_s1), and T-box 5 (Tbx5; Assay ID,
Mm00803518_m1; all synthesized by Applied Biosystems; Thermo Fisher
Scientific, Inc.).
Immunocytochemical analysis
To assess the expression of markers in vitro,
HF cells were fixed in 4% paraformalde-hyde for 30 min at room
temperature and then permeabilized with 0.1% Triton X-100
(Sigma-Aldrich; Merck KGaA) in DPBS for 10 min at room temperature.
They were subsequently blocked with DPBS containing 5% (w/v) bovine
serum albumin (BSA; Sigma-Aldrich; Merck KGaA) for 30 min at room
temperature and incubated overnight at 4°C with primary antibodies
against cardiac troponin T (cTnT; cat. no. MS-295-P0; Thermo Fisher
Scientific, Inc.), pan-cadherin (cat. no. sc-1499) and nestin (cat.
no. sc-21248; both from Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), cytokeratin 15 (CK15; cat. no. ab52816; Abcam, Cambridge,
UK), and promyelocytic leukemia zinc finger (PLZF; cat. no. OP128;
EMD Millipore) diluted 1:200 in 5% BSA solution. On the following
day, the cells were washed twice with DPBS and incubated for 1 h at
room temperature in the dark with Alexa Fluor 568-conjugated goat
anti-mouse (cat. no. A11004) or donkey anti-goat (cat. no. A11057;
both from Thermo Fisher Scientific, Inc.) immunoglobulin G (IgG)
secondary antibodies, or with rhodamine (TRITC)-conjugated goat
anti-rabbit IgG (cat. no. 11-025-003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) diluted 1:200 in 5% BSA
solution. Following two washes with DPBS, the cells were mounted
with Vectashield containing 4′,6-diamidino-2-phenylindole for
nuclear counterstaining (cat. no. H-1200; Vector Laboratories,
Inc., Burlingame, CA, USA). The samples were observed using
fluorescence microscopy (model no. TE2000) that employed NIS
Elements imaging software (NIS-Elements BR 5.11.00; both from Nikon
Corporation).
Statistical analysis
All results were presented as mean ± standard error
of the mean. Statistical analyses were performed using SPSS
software (v.23; IBM Corp., Armonk, NY, USA). Analysis of variance
and Tukey's honest significant difference tests were used to assess
the differences between the groups. P<0.05 was considered to
indicate a statistically significant difference. Unless otherwise
stated, all experiments were performed three times.
Results
Effect of culture medium on the cardiac
differentiation of HF cells
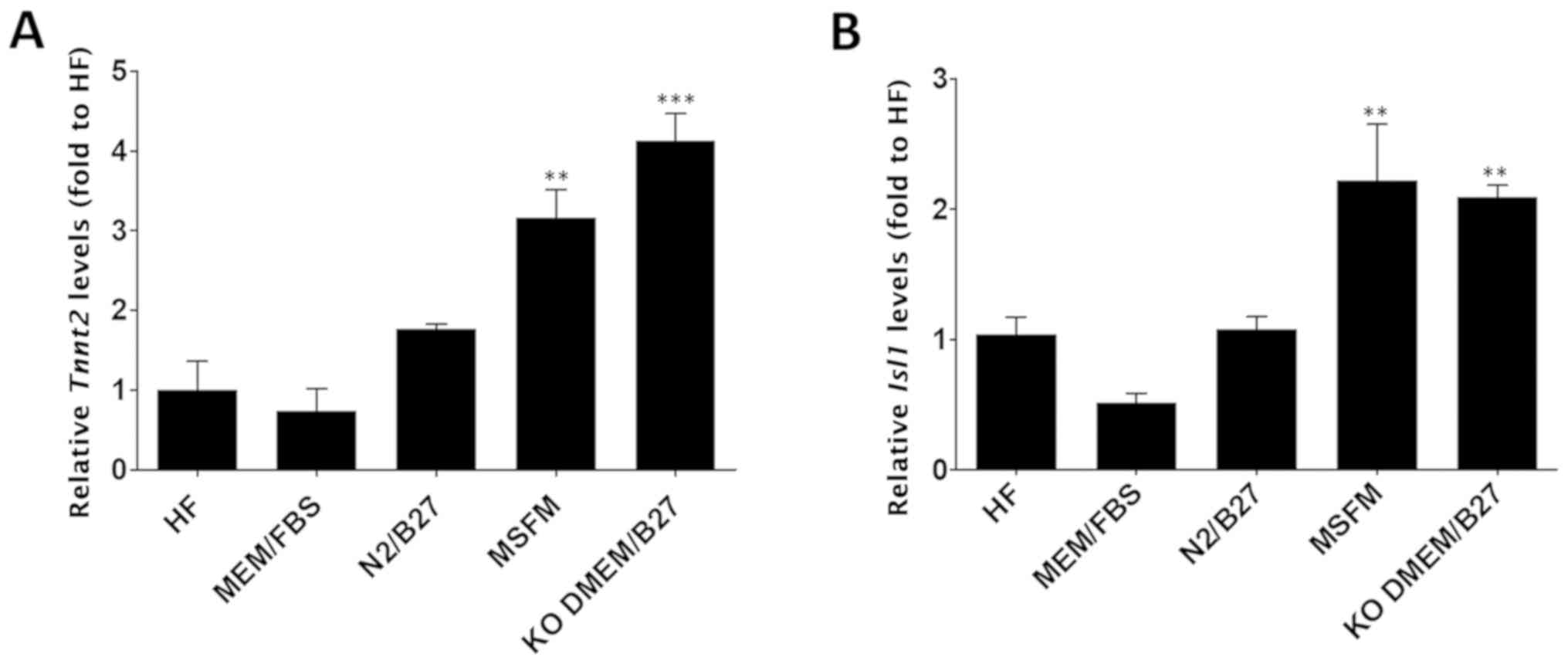
To evaluate the effects of culture medium on cardiac
differentiation, HF cells were cultured for 6 days in a range of
different media, including HF medium, α-MEM/FBS, N2/B27, MSFM and
KO-DMEM/B27, in the absence of growth factors for 6 days.
Subsequent to the induction of cardiac differentiation, the mRNA
levels of Tnnt2, a cardiomyocyte-specific gene, or
Isl1, a cardiac transcription factor, were evaluated using
RT-qPCR. The results of cells cultured in each of the
aforementioned mediums were compared with those of the cells
cultured in the HF medium. Only two groups (cells grown in MSFM and
cells grown in KO-DMEM/B27) exhibited a significant difference
(P<0.01) in Tnnt2 expression levels compared with cells
cultured in HF medium [mean fold difference ± standard error of the
mean (SEM): HF medium, 1.00±0.36; α-MEM/FBS, 0.73±0.29; N2/B27,
1.77±0.07; MSFM, 3.17±0.35; and KO-DMEM/B27, 4.13±0.33; Fig. 1A], although the expression levels
did not differ significantly between the two groups. Isl1
also demonstrated an expression pattern similar to Tnnt2, in
that its expression was significantly higher (P<0.01) in cells
grown either in MSFM or KO-DMEM/B27 compared with in HF cells (mean
fold difference ± SEM: HF medium, 1.05±0.13; α-MEM/FBS, 0.52±0.07;
N2/B27, 1.08±0.1; MSFM, 2.22±0.43; and KO-DMEM/B27, 2.09±0.09;
Fig. 1B). Therefore, MSFM and
KO-DMEM/B27 were selected for use for subsequent experiments.
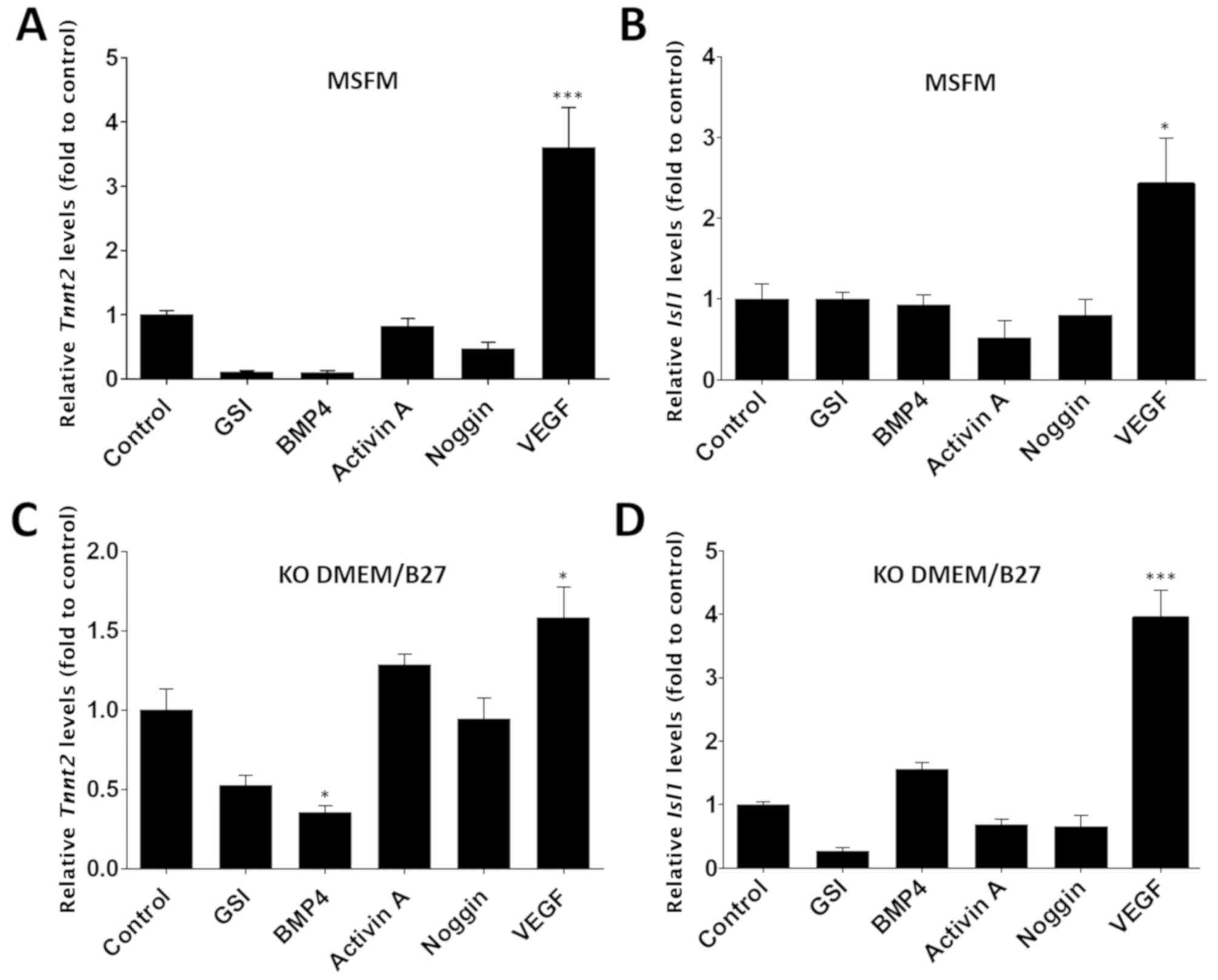
VEGF enhances the cardiac differentiation
potential of HF cells
The effects of soluble growth factors, namely GSI,
BMP-4, activin A, noggin and VEGF, on cultured HF cells were
examined as these factors are known to enhance cardiac
differentiation potential (13,15,17,37-42). HF cells were cultured in MSFM or
KO-DMEM/B27 for 6 days in the presence of each growth factor, and
Tnnt2 or Isl1 mRNA levels were evaluated using
RT-qPCR. Cells cultured in MSFM containing VEGF exhibited a
significant upregulation of Tnnt2 and Isl1 mRNA
levels compared with the untreated cells in the control group
(P<0.05; Tnnt2: 3.6±0.63; Isl1: 2.42±0.58 fold;
Fig. 2A and B). In addition, the
VEGF group demonstrated a higher fold change compared with GSI
(0.11±0.02 fold), BMP-4 (0.11±0.03 fold), activin A (0.82±0.12
fold) and Noggin (0.48±0.1 fold) groups. Similar trends were
observed in HF cells cultured in KO-DMEM/B27 in the presence of
each growth factor; a significant difference was only observed in
the cell population cultured with KO-DMEM/B27 containing VEGF
compared with the control group (P<0.05; Tnnt2:
1.58±0.19; Isl1: 3.94±0.44 fold; Fig. 2C and D). These results demonstrate
that VEGF is highly effective in inducing the cardiac
differentiation of HF cells.
Effect of feeder layers on cardiac
differentiation
To examine the influence of feeder cells on the
induction of cardiac differentiation, HF cells were cultured on
mitotically inactivated feeder cell lines (including OP9, STO, MEF
and C166 feeder cells) in MSFM or KO-DMEM/B27 supplemented with
VEGF. The morphology of the cells during the culture period was
monitored (Fig. 3A and B).
Interestingly, beating cardiomyocyte-like cells under each
condition were observed. To evaluate the efficiency of cardiac
induction in vitro, Tnnt2 or Isl1 expression
levels were compared between different cell populations. The two
genes had significantly higher expression levels in cells cultured
on OP9 feeder cells with MSFM (Tnnt2: 1.00±0.11;
Isl1: 1.00±0.27 fold) compared with those cultured with STO
(Tnnt2: 0.6±0.12; Isl1: 0.6±0.03 fold), MEFs
(Tnnt2: 0.37±0.04; Isl1: 0.05±0.01 fold) or C166
(Tnnt2: 0.6±0.05; Isl1: 0.02±0.01 fold) feeder cells
(P<0.05; Fig. 3C and E).
Additionally, HF cells cultured in KO-DMEM/B27 exhibited
significantly higher mRNA levels of Tnnt2 when OP9 cells
were used as the feeder compared with any other group (OP9,
1.00±0.25; STO, 0.22±0.02; MEF, 0.03±0.0 and C166, 0.01±0.0 fold;
P<0.001; Fig. 3D); similarly,
Isl1 expression was also significantly higher in cells
cultured in KO-DMEM/B27 with OP9 feeder cells compared with any
other group (OP9, 1.00±0.02; STO, 0.26±0.04; MEF, 0.02±0.01; and
C166, 0.06±0.02 fold; P<0.001; Fig. 3F). These data suggest that OP9
feeder cells highly stimulated the cardiac differentiation of HF
cells in the two types of media.
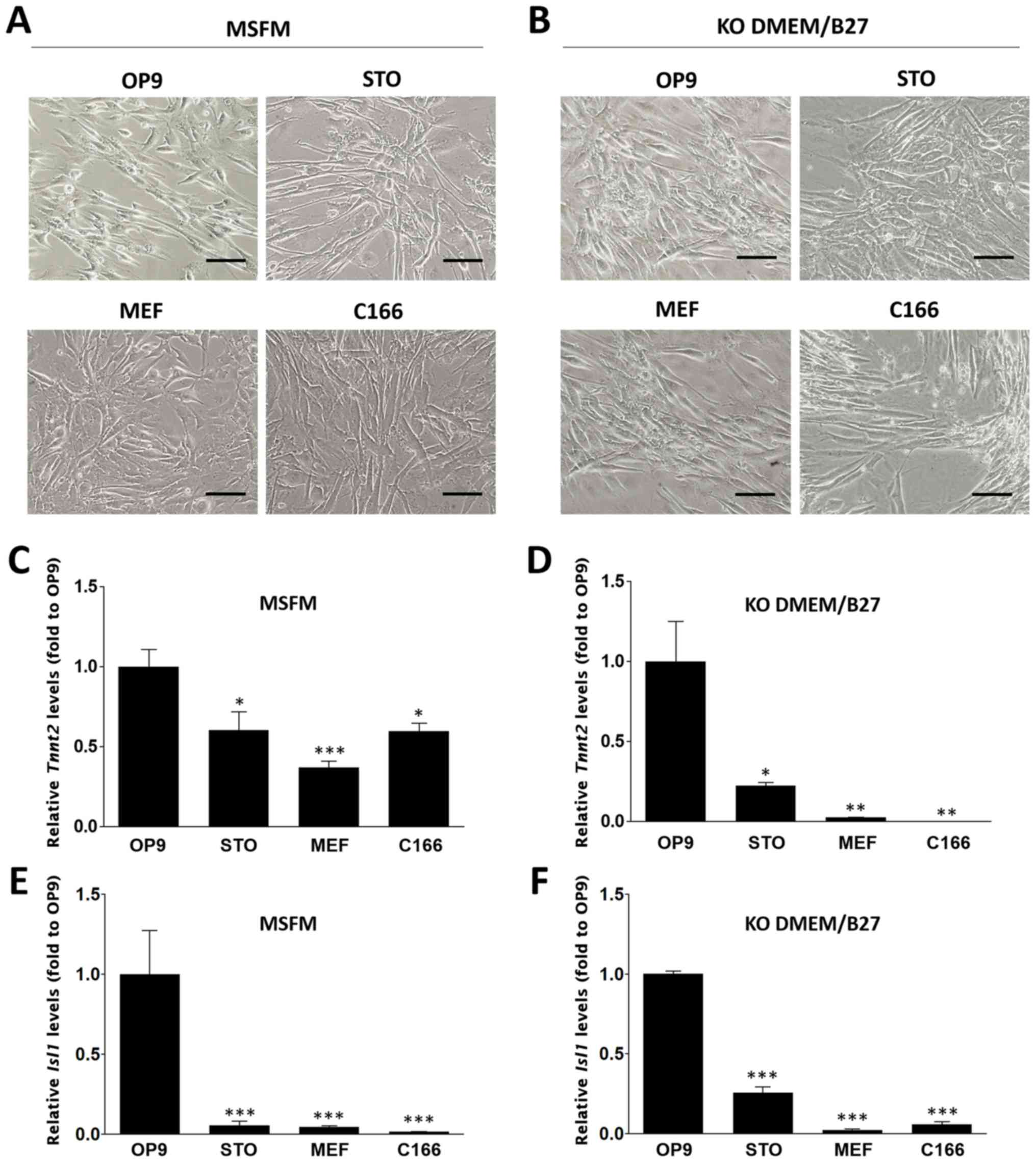 | Figure 3Cardiac differentiation of HF cells
on feeder layers. Bright field micrographs of HF cells cultured on
OP9, STO, MEF and C166 feeder cells in (A) MSFM or (B) KO-DMEM/B27
medium containing VEGF. Bar, 100 μm. Relative mRNA
expression levels of Tnnt2 in (C) MSFM and (D) KO-DMEM/ B27
groups. Relative expression of Isl1 in (E) MSFM and (F)
KO-DMEM/B27 groups. Expression levels were normalized compared with
those in the OP9 feeder cell group. Data is represented as the mean
± the standard error of the mean (n=3). *P<0.05,
**P<0.01 and ***P<0.001 vs. the OP9
group. HF, hair follicles; MSFM, mouse serum-free medium; KO-DMEM,
KnockOut-Dulbecco's modified Eagle's medium; Tnnt2, troponin T2,
cardiac type 2; Isl1, islet 1; VEGF, vascular endothelial growth
factor. |
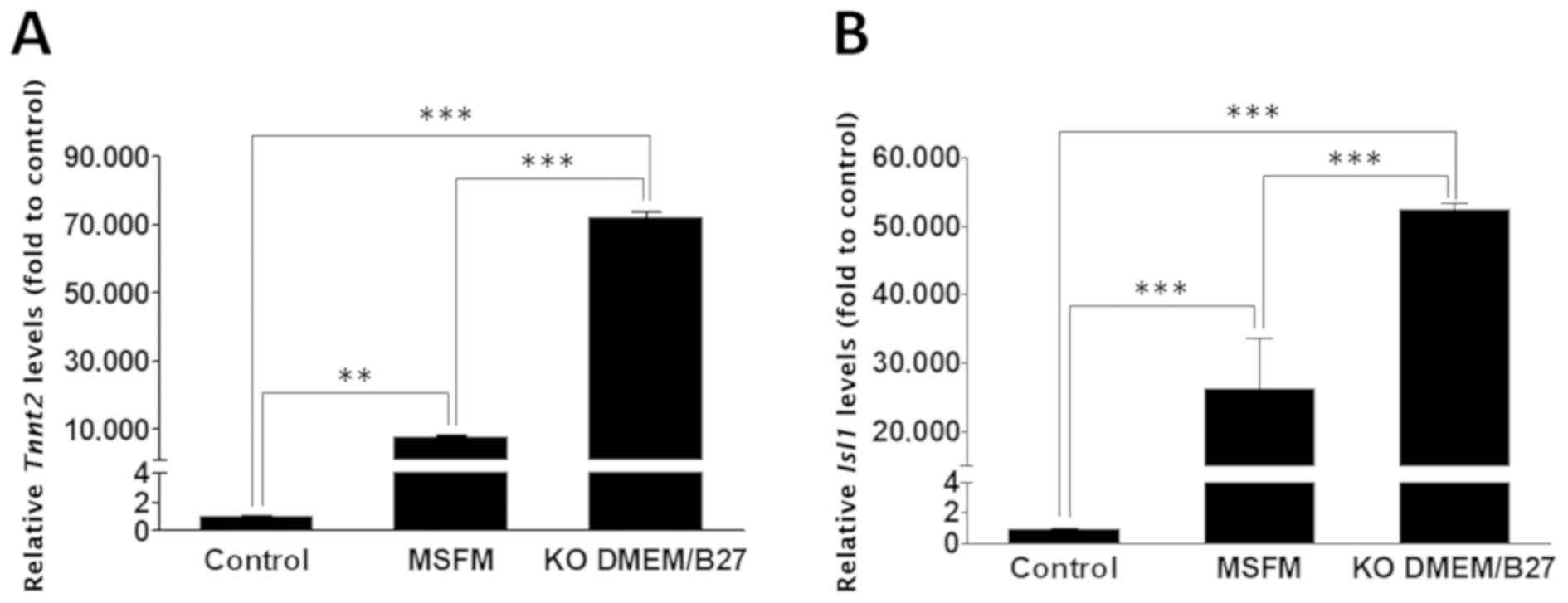
To determine which of the two media (MSFM or
KO-DMEM/B27) is more effective in inducing cardiac differentiation,
HF cells were cultured on OP9 feeder cells with MSFM or KO-DMEM/B27
in the presence of VEGF, and Tnnt2 or Isl1 expression
levels between the two cultures were compared. OP9 feeder cells
alone cultured in MSFM or KO-DMEM/B27 served as the control.
RT-qPCR analysis revealed that Tnnt2 and Isl1 mRNA
levels were significantly upregulated in HF cells cultured on OP9
in MSFM or KO-DMEM/B27 medium compared with the cells grown in the
control medium (P<0.01; Fig.
4); additionally, significantly higher expression levels of
Tnnt2 and Isl1 genes (Tnnt2: 9.43;
Isl1: 1.99 fold) were observed in KO-DMEM/B27 compared with
in the MSFM group (P<0.001). KO-DMEM/B27 was, therefore, used to
analyze the phenotypic characteristics of cardiomyocyte-like
cells.
Phenotype of cardiomyocyte-like cells
derived from HF cells
Following 2 weeks of culturing on OP9 feeder cells
in KO-DMEM/B27 containing VEGF, HF cells were analyzed using
immunocytochemistry. To exclude the expression of non-specific
markers, the present study initially confirmed that the various
markers used were not expressed by OP9 cells (Fig. 5). Numerous cells strongly
expressed the cardiomyocyte marker cTnT and the cardiac
intercellular adherens junction marker pan-cadherin (Fig. 5). On the other hand, these cells
were negative for the neural stem/progenitor cell marker nestin,
the HF bulge marker CK15 and the undifferentiated spermatogonia
marker PLZF (Fig. 5). These
results indicate that the culturing of HF cells on OP9 feeder cells
in KO-DMEM/B27 medium supplemented with VEGF promotes the
expression of genes specific to the cardiac lineage but not to
other lineages including neural, glial, HF and germline cells.
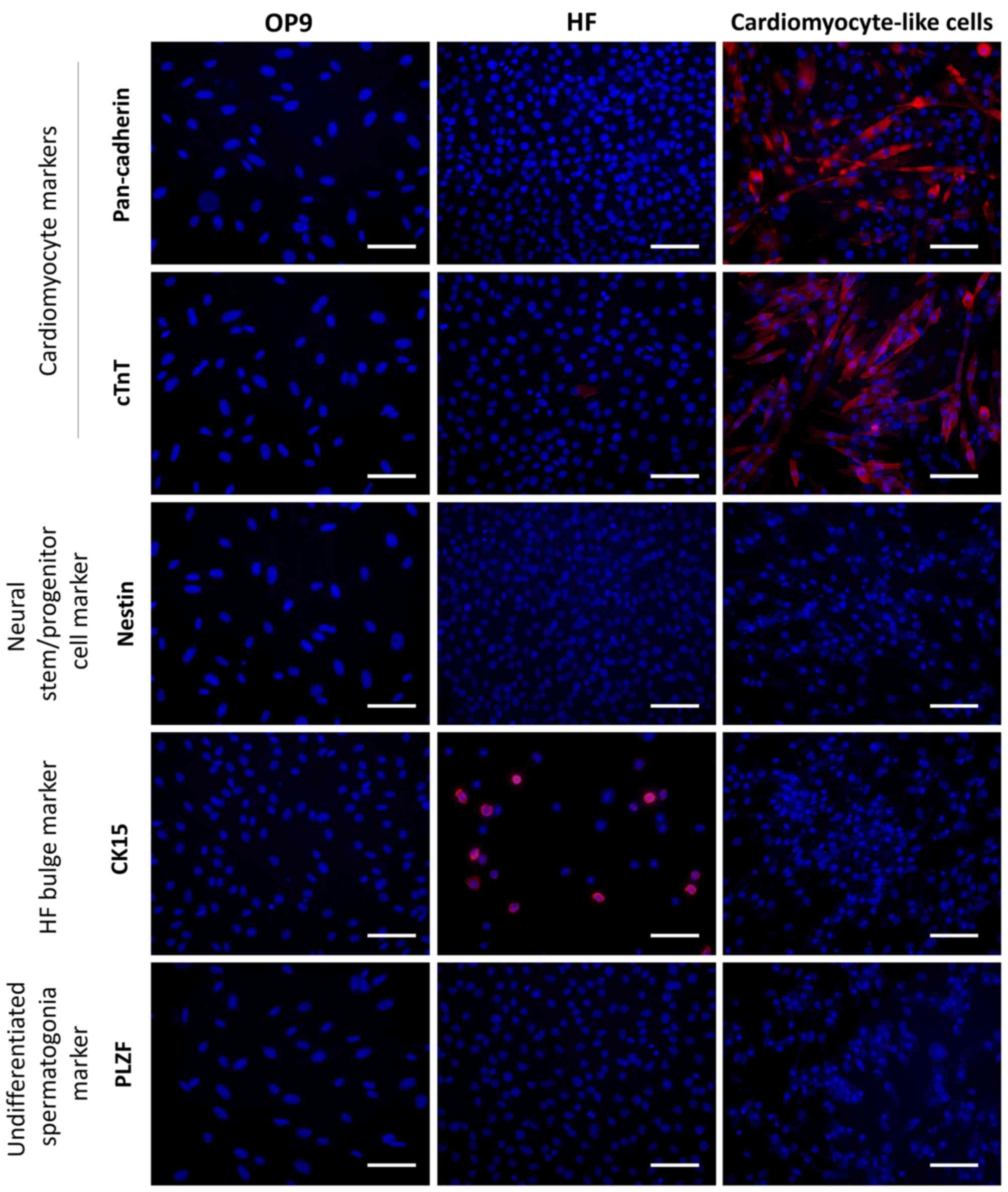 | Figure 5Immunocytochemistry analysis of
lineage-specific marker expression in OP9 feeder cells, HF cells
and cardiomyocyte-like cells. Cells were stained with cardiomyocyte
marker, cTnT, cardiac intercellular adherens junction marker,
pan-cadherin, neural stem/progenitor cell marker, nestin, HF bulge
marker, CK15, or undifferentiated spermatogonia marker, PLZF (red).
Cells were then counterstained with 4′,6-diamidino-2-phenylindole
(blue). Bar, 100 μm. HF, hair follicles; MSFM, mouse
serum-free medium; KO-DMEM, KnockOut-Dulbecco's modified Eagle's
medium; cTnT, cardiac troponin T; CK15, cytokeratin 15; PLZF,
promyelocytic leukemia zinc finger. |
The present study additionally compared HF cells
with cardiomyocyte-like cells in terms of the mRNA expression
levels of cardiac lineage-specific genes including Tnnt2, Mesp1,
Isl1, Mef2c, Nkx2.5 and Tbx5 (9,43-46) and it was revealed that all these
genes were significantly more highly expressed in the latter group
of cells compared with the HF group (P<0.05; Fig. 6), demonstrating that these
differentiated cells exhibited a phenotype that was distinguishable
from that of the parent HF cells.
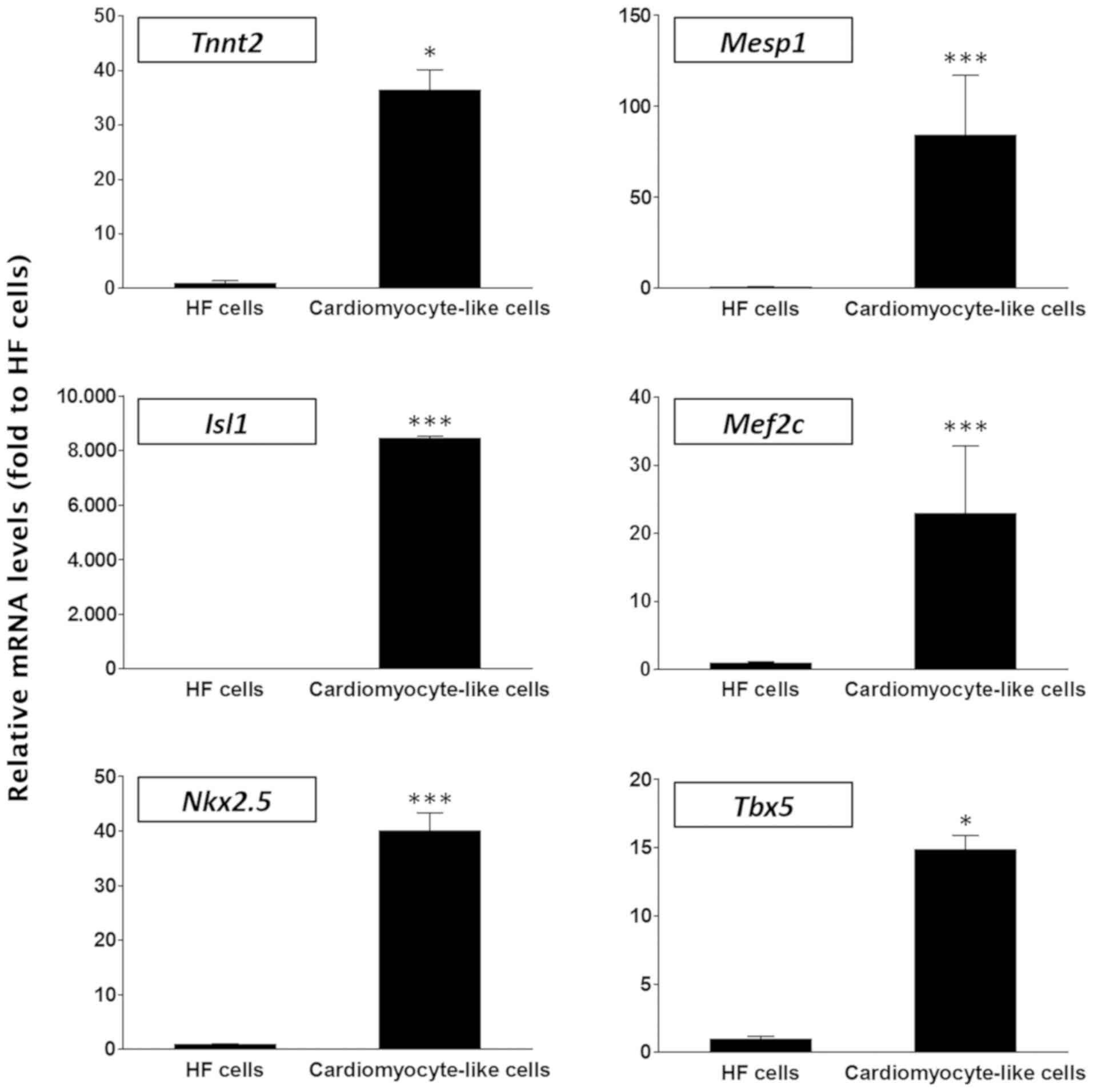 | Figure 6Relative expression levels of cardiac
lineage-specific genes in HF and cardiomyocyte-like cells.
Expression levels of the cardiac lineage-specific genes
Tnnt2, Mesp1, Isl1, Mef2c,
Nkx2.5 and Tbx5 were examined and normalized to the
levels in the HF cell group. Data is represented as the mean ±
standard error of the mean (n=3). *P<0.05 and
***P<0.001 vs. the HF group. HF, hair follicles;
Tnnt2, troponin T2, cardiac type 2; Isl1, islet 1; Mesp1, mesoderm
posterior bHLH transcription factor 1; Mef2c, myocyte enhancer
factor 2c; Nkx2.5, NK2 homeobox 2.5; Tbx5, T-box 5. |
It was observed that cardiomyocyte-like cells had a
spindle- and filament-shaped morphology similar to that of cardiac
muscles and were capable of beating spontaneously for >3 months
without any stimulation (Video
S1). Thus, beating cardiomyocyte-like cells may be derived from
HF cells by culturing on OP9 feeder cells in KO-DMEM/B27 medium
supplemented with VEGF. These cardiomyocyte-like cells may
potentially function normally as spontaneous beating is thought to
be an in vitro indicator of functional cardiomyocytes
(47,48).
Discussion
In the present study, it was postulated that the use
of media, exogenous factors or feeders essential for cardiac
differentiation may be an important first step in priming HF cells
to be receptive to the conversion into the cells of cardiac
lineages. In this regard, it is noteworthy that the differentiation
and/or specification of pluripotent cells into specific cell types
have been frequently demonstrated to require the addition of growth
factors that are specific to the cell type (49,50). For example, ESCs and iPSCs are
differentiated into cardiomyocytes by adding growth factors, which
are implicated in normal cardiac development, into the media
(41,43). In a similar manner, media, growth
factors or feeders that have been implicated in cardiac development
were added to the HF cultures to determine whether they were able
to facilitate reprogramming. Finally, the culture conditions
required for the cardiac-specific differentiation of HF cells
isolated from the mouse dorsal skin were established. The HF cells
were initially cultured in different media (α-MEM/FBS, N2/B27, MSFM
and KO-DMEM/B27) with different growth factors (GSI, BMP-4, activin
A, noggin and VEGF) and feeder cells (OP9, STO, C166 and MEF cells)
to determine the optimal culture conditions for inducing cardiac
differentiation; the cardiac differentiation ability of cultured
cells was evaluated based on their expression of cardiac
lineage-specific genes and beating capacity. It was revealed that
HF cells cultured in KO-DMEM/B27 containing VEGF exhibited the
upregulation of cardiac lineage-specific genes, which was further
enhanced by culturing on OP9 feeder cells.
Numerous studies have been conducted to establish
suitable conditions for inducing the differentiation of PSCs into
specific cell types (13,39,51,52). mGSCs are similar to embryonic stem
and germ cells in terms of phenotype and their capacity of
differentiating into cardiomyocytes and endothelial cells; these
cells were induced to differentiate into mesodermal cells by
culturing in α-MEM containing 10% fetal calf serum and
2-mercaptoethanol on an OP9 stromal cell layer (51). The cardiac differentiation
efficiency of mouse and human ESCs was enhanced by culturing in
DMEM containing 20% FBS or KO-DMEM containing 20% FBS in the
presence of apelin, BMP-4, activin A and bFGF (13). Although efficient differentiation
into mature cardiomyocytes was achieved, the inclusion of FBS
remains controversial as its precise components are unknown. For
this reason, various chemically defined media have been used to
induce the cardiac differentiation of PSCs, including one that
promotes the self-renewal of human ESCs grown on a Matrigel-coated
surface over multiple passages (52). This previous study additionally
selectively induced the differentiation of a human ESC monolayer
into cells of cardiac muscle lineage by transient treatment with
activin A and BMP-4 in N2/B27, a chemically defined medium
comprising DMEM/F12 with N2 and B27 supplements and BSA, amongst
other ingredients. As a result of differentiation, hESCs maintained
in N2/B27 proceeded from the mesendoderm lineage toward the
mesoderm-cardiac muscle lineage. The differentiation of human ESCs
into the cardiac mesoderm and simultaneous suppression of the
neuroectodermal lineage was promoted by culturing in a reduced
volume medium with GSI followed by KO-DMEM. The KO-DMEM/B27 culture
conditions induced the differentiation and maturation of the
cardiac lineage cells (39). MSFM
is another chemically well-defined medium that supports the
proliferation and self-renewal of mouse spermatogonial SCs
(35,53). In the present study, α-MEM
containing FBS and a number of chemically defined media (N2/B27,
KO-DMEM/B27 and MSFM) without FBS were assessed for their ability
to promote cardiac lineage commitment in HF cells. The mRNA
expression levels of the cardiac-specific genes Tnnt2 and
Isl1 were higher in a chemically defined medium when
compared with a serum-containing medium, suggesting that the
presence of serum inhibits cardiac differentiation owing to reasons
that have yet to be determined.
Various growth factors are known to promote
cardiogenesis in PSCs (13,38,39,41,42). Although GSI alone does not affect
the self-renewal and differentiation capacity of human ESCs by
blocking Notch signaling, GSI in a reduced-volume culture medium
may accelerate mesodermal differentiation (37,39,40). A number of other studies have
demonstrated that activin/nodal/transforming growth factor-β, Wnt
and BMP signaling pathways are critical for establishing the
development of the cardiovascular system (15,17,41,54-61). The BMP antagonist noggin is
transiently but notably expressed in the heart-forming region
during gastrulation and functions at the level of mesendoderm
induction to establish conditions conducive to cardiogenesis
(42). Although GSI, BMP-4,
activin A and noggin are known to influence cardiac differentiation
at a number of different stages, the present study did not examine
the positive effects of these growth factors on cardiac-specific
differentiation of HF cells. However, HF cells cultured in the
presence of VEGF demonstrated increased cardiac-specific gene
expression levels. In a previous study, VEGF receptor 2 was
demonstrated to be expressed in a cell population that had the
potential to generate cell types of cardiac lineage (38). Thus, it is likely that VEGF is
necessary for cardiac differentiation.
In the present study, spontaneously beating
cardiomyocyte-like cells were generated using HF cells cultured on
a feeder layer in KO-DMEM/B27 supplemented with VEGF. Specifically,
OP9 feeder cells increased the expression levels of
cardiac-specific genes to a greater extent compared with other
feeder cells. OP9 cells have been used to induce the
differentiation of PSCs into cardiac and endothelial cells,
indicating that OP9 cells have a potential function in the
differentiation of PSCs into contracting cardiac colonies (51,62-64). The results of the present study
are consistent with these earlier reports. However, it may be
difficult to determine the underlying mechanism and number of cells
involved, but with an increase in the expression of specific genes
owing to the implementation of the aforementioned method, the
results of the present study may lay a necessary foundation for
further study. Interestingly, HF cells were induced to
differentiate into beating cardiomyocyte-like cells when cultured
on each of the feeder cell lines, although the precise functions of
the feeder cells in cardiac differentiation remains unclear. Thus,
the precise mechanism and necessity of each growth factor requires
further elucidation.
In conclusion, the results of the present study
demonstrate that cardiomyocyte-like cells may be derived from HF
cells under suitable culture conditions. These may provide a useful
in vitro system for the standardization of the method for
the derivation of cardiomyocytes, and offer a potential cell source
for the development of cell-based therapeutics.
The authors would like to thank Dr Toru Nakano
(Department of Pathology, Medical School, Osaka University, Osaka,
Japan) for the OP9 cell line.
Supplementary Materials
Funding
The present study was supported by the Bio and
Medical Technology Development Program of the National Research
Foundation of Korea (NRF) funded by the Korean government, MSIT
(grant no. 2018M3A9H1023139), the Basic Science Research Program
through the NRF funded by the Ministry of Education (grant no.
2018R1A6A1A03025159) and the Korea Research Institute of Bioscience
and Biotechnology Research Initiative Programs (grant no.
KGM4251824).
Availability of data and materials
All data generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YHK and BYR conceived and designed the project. YHK,
BJK and SMK performed the experiments. YHK, BJK, SUK and BYR
analyzed the data. YHK and BYR wrote the manuscript. BYR supervised
the design of study and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Animal procedures were approved by the Animal Care
and Use Committee of Chung-Ang University (Gyeonggi-do, Republic of
Korea; IACUC no. 2018-00073) and were performed in accordance with
the Guideline for the Care and Use of Laboratory Animals published
by the National Institutes of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mortality GBD and Causes of Death C:
Global, regional, and national life expectancy, all-cause
mortality, and cause-specific mortality for 249 causes of death,
1980-2015: a systematic analysis for the Global Burden of Disease
Study 2015. Lancet. 388:1459–1544. 2016. View Article : Google Scholar
|
|
2
|
Weir RA and McMurray JJ: Epidemiology of
heart failure and left ventricular dysfunction after acute
myocardial infarction. Curr Heart Fail Rep. 3:175–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roger VL, Go AS, Lloyd-Jones DM, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics–2012
update: A report from the American Heart Association. Circulation.
125:e2–220. 2012.
|
|
4
|
Thygesen K, Alpert JS and White HD: Joint
ESC/ACCF/ AHA/WHF Task Force for the redefinition of myocardial
infarction: Universal definition of myocardial infarction. J Am
Coll Cardiol. 50:2173–2195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hunter JJ and Chien KR: Signaling pathways
for cardiac hypertrophy and failure. N Engl J Med. 341:1276–1283.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beaglehole R, Bonita R, Horton R, Adams O
and McKee M: Public health in the new era: Improving health through
collective action. Lancet. 363:2084–2086. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dubois NC, Craft AM, Sharma P, Elliott DA,
Stanley EG, Elefanty AG, Gramolini A and Keller G: SIRPA is a
specific cell-surface marker for isolating cardiomyocytes derived
from human pluripotent stem cells. Nat Biotechnol. 29:1011–1018.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fijnvandraat AC, van Ginneken AC, de Boer
PA, Ruijter JM, Christoffels VM, Moorman AF and Lekanne Deprez RH:
Cardiomyocytes derived from embryonic stem cells resemble
cardiomyocytes of the embryonic heart tube. Cardiovasc Res.
58:399–409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hidaka K, Lee JK, Kim HS, Ihm CH, Iio A,
Ogawa M, Nishikawa S, Kodama I and Morisaki T: Chamber-specific
differentiation of Nkx2.5-positive cardiac precursor cells from
murine embryonic stem cells. FASEB J. 17:740–742. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hattori F, Chen H, Yamashita H, Tohyama S,
Satoh YS, Yuasa S, Li W, Yamakawa H, Tanaka T, Onitsuka T, et al:
Nongenetic method for purifying stem cell-derived cardiomyocytes.
Nat Methods. 7:61–66. 2010. View Article : Google Scholar
|
|
11
|
Baba S, Heike T, Yoshimoto M, Umeda K, Doi
H, Iwasa T, Lin X, Matsuoka S, Komeda M and Nakahata T: Flk1(+)
cardiac stem/progenitor cells derived from embryonic stem cells
improve cardiac function in a dilated cardiomyopathy mouse model.
Cardiovasc Res. 76:119–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guan K, Wagner S, Unsöld B, Maier LS,
Kaiser D, Hemmerlein B, Nayernia K, Engel W and Hasenfuss G:
Generation of functional cardiomyocytes from adult mouse
spermatogonial stem cells. Circ Res. 100:1615–1625. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang IN, Wang X, Ge X, Anderson J, Ho M,
Ashley E, Liu J, Butte MJ, Yazawa M, Dolmetsch RE, et al: Apelin
enhances directed cardiac differentiation of mouse and human
embryonic stem cells. PLoS One. 7:e383282012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu WZ, Hauch KD, Xu C and Laflamme MA:
Human embryonic stem cells and cardiac repair. Transplant Rev
(Orlando). 23:53–68. 2009. View Article : Google Scholar
|
|
15
|
Klaus A, Saga Y, Taketo MM, Tzahor E and
Birchmeier W: Distinct roles of Wnt/beta-catenin and Bmp signaling
during early cardiogenesis. Proc Natl Acad Sci USA.
104:18531–18536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taha MF and Valojerdi MR: Effect of bone
morphogenetic protein-4 on cardiac differentiation from mouse
embryonic stem cells in serum-free and low-serum media. Int J
Cardiol. 127:78–87. 2008. View Article : Google Scholar
|
|
17
|
Ladd AN, Yatskievych TA and Antin PB:
Regulation of avian cardiac myogenesis by activin/TGFbeta and bone
morphogenetic proteins. Dev Biol. 204:407–419. 1998. View Article : Google Scholar
|
|
18
|
Hoffman RM: The hair follicle as a gene
therapy target. Nat Biotechnol. 18:20–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oshima H, Rochat A, Kedzia C, Kobayashi K
and Barrandon Y: Morphogenesis and renewal of hair follicles from
adult multi-potent stem cells. Cell. 104:233–245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cotsarelis G, Sun TT and Lavker RM:
Label-retaining cells reside in the bulge area of pilosebaceous
unit: Implications for follicular stem cells, hair cycle, and skin
carcinogenesis. Cell. 61:1329–1337. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morris RJ and Potten CS: Highly persistent
label-retaining cells in the hair follicles of mice and their fate
following induction of anagen. J Invest Dermatol. 112:470–475.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taylor G, Lehrer MS, Jensen PJ, Sun TT and
Lavker RM: Involvement of follicular stem cells in forming not only
the follicle but also the epidermis. Cell. 102:451–461. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tumbar T, Guasch G, Greco V, Blanpain C,
Lowry WE, Rendl M and Fuchs E: Defining the epithelial stem cell
niche in skin. Science. 303:359–363. 2004. View Article : Google Scholar
|
|
24
|
Toma JG, Akhavan M, Fernandes KJ,
Barnabé-Heider F, Sadikot A, Kaplan DR and Miller FD: Isolation of
multipotent adult stem cells from the dermis of mammalian skin. Nat
Cell Biol. 3:778–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fernandes KJ, McKenzie IA, Mill P, Smith
KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi
NR, Toma JG, et al: A dermal niche for multipotent adult
skin-derived precursor cells. Nat Cell Biol. 6:1082–1093. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sieber-Blum M, Grim M, Hu YF and Szeder V:
Pluripotent neural crest stem cells in the adult hair follicle. Dev
Dyn. 231:258–269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li L, Mignone J, Yang M, Matic M, Penman
S, Enikolopov G and Hoffman RM: Nestin expression in hair follicle
sheath progenitor cells. Proc Natl Acad Sci USA. 100:9958–9961.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yano K, Brown LF and Detmar M: Control of
hair growth and follicle size by VEGF-mediated angiogenesis. J Clin
Invest. 107:409–417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mecklenburg L, Tobin DJ, Müller-Röver S,
Handjiski B, Wendt G, Peters EM, Pohl S, Moll I and Paus R: Active
hair growth (anagen) is associated with angiogenesis. J Invest
Dermatol. 114:909–916. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amoh Y, Li L, Yang M, Moossa AR, Katsuoka
K, Penman S and Hoffman RM: Nascent blood vessels in the skin arise
from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA.
101:13291–13295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amoh Y, Li L, Katsuoka K, Penman S and
Hoffman RM: Multipotent nestin-positive, keratin-negative
hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci
USA. 102:5530–5534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yashiro M, Mii S, Aki R, Hamada Y, Arakawa
N, Kawahara K, Hoffman RM and Amoh Y: From hair to heart:
Nestin-expressing hair-follicle-associated pluripotent (HAP) stem
cells differentiate to beating cardiac muscle cells. Cell Cycle.
14:2362–2366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
34
|
Nath M, Offers M, Hummel M and Seissler J:
Isolation and in vitro expansion of Lgr6-positive multipotent hair
follicle stem cells. Cell Tissue Res. 344:435–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kubota H, Avarbock MR and Brinster RL:
Growth factors essential for self-renewal and expansion of mouse
spermatogonial stem cells. Proc Natl Acad Sci USA. 101:16489–16494.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
37
|
Fox V, Gokhale PJ, Walsh JR, Matin M,
Jones M and Andrews PW: Cell-cell signaling through NOTCH regulates
human embryonic stem cell proliferation. Stem Cells. 26:715–723.
2008. View Article : Google Scholar
|
|
38
|
Kattman SJ, Huber TL and Keller GM:
Multipotent flk-1+ cardiovascular progenitor cells give rise to the
cardiomyocyte, endothelial, and vascular smooth muscle lineages.
Dev Cell. 11:723–732. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jang J, Ku SY, Kim JE, Choi K, Kim YY, Kim
HS, Oh SK, Lee EJ, Cho HJ, Song YH, et al: Notch inhibition
promotes human embryonic stem cell-derived cardiac mesoderm
differentiation. Stem Cells. 26:2782–2790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noggle SA, Weiler D and Condie BG: Notch
signaling is inactive but inducible in human embryonic stem cells.
Stem Cells. 24:1646–1653. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kattman SJ, Witty AD, Gagliardi M, Dubois
NC, Niapour M, Hotta A, Ellis J and Keller G: Stage-specific
optimization of activin/nodal and BMP signaling promotes cardiac
differentiation of mouse and human pluripotent stem cell lines.
Cell Stem Cell. 8:228–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yuasa S, Itabashi Y, Koshimizu U, Tanaka
T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa
S, et al: Transient inhibition of BMP signaling by Noggin induces
cardio-myocyte differentiation of mouse embryonic stem cells. Nat
Biotechnol. 23:607–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim BJ, Kim YH, Lee YA, Jung SE, Hong YH,
Lee EJ, Kim BG, Hwang S, Do JT, Pang MG, et al: Platelet-derived
growth factor receptor-alpha positive cardiac progenitor cells
derived from multipotent germline stem cells are capable of
cardiomyogenesis in vitro and in vivo. Oncotarget. 8:29643–29656.
2017.PubMed/NCBI
|
|
44
|
Org T, Duan D, Ferrari R, Montel-Hagen A,
Van Handel B, Kerényi MA, Sasidharan R, Rubbi L, Fujiwara Y,
Pellegrini M, et al: Scl binds to primed enhancers in mesoderm to
regulate hematopoietic and cardiac fate divergence. EMBO J.
34:759–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bai F, Ho Lim C, Jia J, Santostefano K,
Simmons C, Kasahara H, Wu W, Terada N and Jin S: Directed
differentiation of embryonic stem cells into cardiomyocytes by
bacterial injection of defined transcription factors. Sci Rep.
5:150142015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li G, Plonowska K, Kuppusamy R, Sturzu A
and Wu SM: Identification of cardiovascular lineage descendants at
single-cell resolution. Development. 142:846–857. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Eng G, Lee BW, Protas L, Gagliardi M,
Brown K, Kass RS, Keller G, Robinson RB and Vunjak-Novakovic G:
Autonomous beating rate adaptation in human stem cell-derived
cardio-myocytes. Nat Commun. 7:103122016. View Article : Google Scholar
|
|
48
|
Radaszkiewicz KA, Sýkorová D, Karas P,
Kudová J, Kohút L, Binó L, Večeřa J, Víteček J, Kubala L and
Pacherník J: Simple non-invasive analysis of embryonic stem
cell-derived cardio-myocytes beating in vitro. Rev Sci Instrum.
87:0243012016. View Article : Google Scholar
|
|
49
|
Hayashi K, Ohta H, Kurimoto K, Aramaki S
and Saitou M: Reconstitution of the mouse germ cell specification
pathway in culture by pluripotent stem cells. Cell. 146:519–532.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hayashi K, Ogushi S, Kurimoto K, Shimamoto
S, Ohta H and Saitou M: Offspring from oocytes derived from in
vitro primordial germ cell-like cells in mice. Science.
338:971–975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baba S, Heike T, Umeda K, Iwasa T, Kaichi
S, Hiraumi Y, Doi H, Yoshimoto M, Kanatsu-Shinohara M, Shinohara T,
et al: Generation of cardiac and endothelial cells from neonatal
mouse testis-derived multipotent germline stem cells. Stem Cells.
25:1375–1383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yao S, Chen S, Clark J, Hao E, Beattie GM,
Hayek A and Ding S: Long-term self-renewal and directed
differentiation of human embryonic stem cells in chemically defined
conditions. Proc Natl Acad Sci USA. 103:6907–6912. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ryu BY, Kubota H, Avarbock MR and Brinster
RL: Conservation of spermatogonial stem cell self-renewal signaling
between mouse and rat. Proc Natl Acad Sci USA. 102:14302–14307.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Conlon FL, Lyons KM, Takaesu N, Barth KS,
Kispert A, Herrmann B and Robertson EJ: A primary requirement for
nodal in the formation and maintenance of the primitive streak in
the mouse. Development. 120:1919–1928. 1994.PubMed/NCBI
|
|
55
|
Gadue P, Huber TL, Paddison PJ and Keller
GM: Wnt and TGF-beta signaling are required for the induction of an
in vitro model of primitive streak formation using embryonic stem
cells. Proc Natl Acad Sci USA. 103:16806–16811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Y, Asakura M, Inoue H, Nakamura T,
Sano M, Niu Z, Chen M, Schwartz RJ and Schneider MD: Sox17 is
essential for the specification of cardiac mesoderm in embryonic
stem cells. Proc Natl Acad Sci USA. 104:3859–3864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Marvin MJ, Di Rocco G, Gardiner A, Bush SM
and Lassar AB: Inhibition of Wnt activity induces heart formation
from posterior mesoderm. Genes Dev. 15:316–327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schneider VA and Mercola M: Wnt antagonism
initiates cardio-genesis in Xenopus laevis. Genes Dev. 15:304–315.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schultheiss TM, Burch JB and Lassar AB: A
role for bone morphogenetic proteins in the induction of cardiac
myogenesis. Genes Dev. 11:451–462. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tzahor E and Lassar AB: Wnt signals from
the neural tube block ectopic cardiogenesis. Genes Dev. 15:255–260.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yatskievych TA, Ladd AN and Antin PB:
Induction of cardiac myogenesis in avian pregastrula epiblast: The
role of the hypoblast and activin. Development. 124:2561–2570.
1997.PubMed/NCBI
|
|
62
|
Iida M, Heike T, Yoshimoto M, Baba S, Doi
H and Nakahata T: Identification of cardiac stem cells with FLK1,
CD31, and VE-cadherin expression during embryonic stem cell
differentiation. FASEB J. 19:371–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Murakami Y, Hirata H, Miyamoto Y,
Nagahashi A, Sawa Y, Jakt M, Asahara T and Kawamata S: Isolation of
cardiac cells from E8.5 yolk sac by ALCAM (CD166) expression. Mech
Dev. 124:830–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Misfeldt AM, Boyle SC, Tompkins KL, Bautch
VL, Labosky PA and Baldwin HS: Endocardial cells are a distinct
endothelial lineage derived from Flk1+ multipotent
cardiovascular progenitors. Dev Biol. 333:78–89. 2009. View Article : Google Scholar : PubMed/NCBI
|