Introduction
The gastrointestinal tract is constantly exposed to
various symbiotic bacteria and potential harmful microorganisms.
Therefore, it is necessary to control the balance of the local
immune system (1). Pathogenic
immune response occurs upon disturbance of the enteric bacterial
homeostasis, which is generally called inflammatory bowel disease
(IBD). This syndrome includes two types of intestinal diseases,
ulcerative colitis (UC) and Crohn's disease (CD) (2). These two diseases are major health
problems in western society, with an incidence of ~200/100,000. In
UC, the inflammation is restricted in the colonic mucous layer,
while CD can affect the entire intestinal tract (3). IBD mainly attacks at the early
adulthood, accompanied with abdominal pain, weight loss, diarrhea
and proctorrhagia. The risk factors of IBD development include
genetic and environmental factors (4). On the one hand, a study with twins
and immediate relatives with high IBD morbidity has demonstrated
the contribution of genetics to disease susceptibility (4). Multiple IBD-related genes have been
identified in recent years. However, environmental conditions,
including high fat diet, sugar diet, smoking, stress and frequent
use of antibiotics, are also significantly associated with higher
risk of IBD. Such findings demonstrate the influence of
environmental factors on IBD (5).
Interleukin (IL)-18 levels are elevated in patients
with active inflammatory disease. However, a mouse study has also
demonstrated a protective role for IL-18, since I1-18−/−
and Il-18 receptor 1 (Il-18R1)−/− mice were more
susceptible to colitis and colitis-related colon tumors (6). Such discrepancy may reflect the
different roles of IL-18 depending on timing and background. During
the dextran sulfate sodium (DSS)-induced inflammatory process,
early production of IL-18 is vital for epithelial restoration and
inflammation recession. However, in the presence of chronic and
dysfunctional inflammation, excessive IL-18 may aggravate disease
progression (7).
IL-22 is a member of the IL-10 cytokine family, and
it is strongly expressed in CD and UC. IL-22 can bind with the
dimer membrane receptor IL-22R that is only expressed in epithelial
cells, rendering IL-22 an important mediator between the immune
system and the epithelial system (8). A mouse model has suggested that
IL-22 serves a key role in restoring enteric dynamic balance during
acute inflammatory colitis (9).
The protection of IL-22 is firstly achieved through inducing the
expression of anti-microbial peptides (AMPs) in epithelial cells
(9). In addition, it can
intensify the epithelial barrier function through mucus production
by goblet cells and restoration of epithelial tight junctions
(10). Secondly, the
IL-22-induced survival and proliferation of epithelial cells can
promote epithelial wound healing (11). However, the deterioration or
uncontrollable effect of IL-22 can result in other pathological
conditions, such as psoriasis and colorectal cancer. IL-22 is
suggested to be soluble, and IL-22 binding protein (IL-22BP) is a
specific and effective inhibitor. Consequently, another hypothesis
for the increased incidence of experimental colon tumors in
Il22bp−/− mice lies in the suppression of IL-22-induced
epithelial cell proliferation (12). Additionally, expression of IL-22
and IL-22BP is regulated in the intestinal tissues during injury
(12).
The present study investigated the dual roles and
the mechanisms of IL-18 in a mouse model of DSS-induced
colitis.
Materials and methods
Experimental model
Animal experiments were approved by the
Institutional Animal Care and Welfare Committee of China
Pharmaceutical University and performed in accordance with
institutional protocols. Six-week-old male C57BL/6 mice (20-21 g)
were obtained from the Laboratory Animal Center of Yangzhou
University (Yangzhou, China), housed under standard conditions at
22±2°C, 50±10% humidity, and 12 h light/dark cycle and fed with
standard diets and tap water ad libitum.
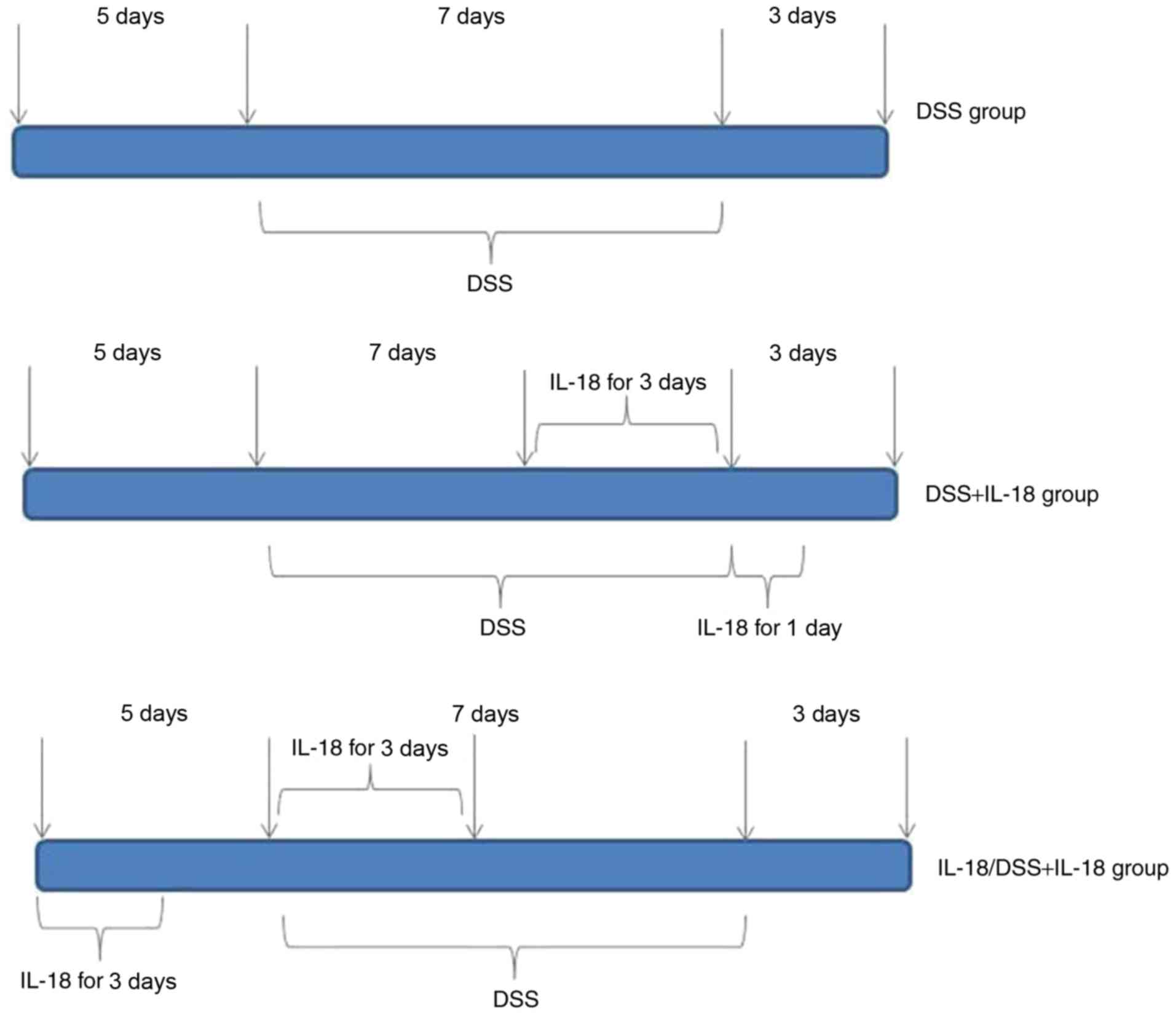
The mice (n=27) were randomly assigned into 4
groups, in order to generate the acute colitis model: Control
(n=6), DSS model (n=7), treatment with IL-18 (n=7), pre-treatment
with IL-18 (n=7). Mice in the control group were given distilled
water for 7 days. In the DSS model group, mice were administered
normal water for 5 days, induced by oral intake of 2% DSS (w/v,
dissolved in drinking water) for 7 days and then given normal water
for an additional 3 days. In the IL-18 treatment group, mice were
on normal water for 5 days, induced by oral intake of 2% DSS (w/v,
dissolved in drinking water) for 7 days and intraperitoneally
injected with 1 µg of IL-18 for the last 3 days of the 7 day
DSS induction period; mice were also intraperitoneally injected
with 1 µg of IL-18 for 1 day at the 3 day normal water
recovery period. Finally, in the IL-18 pre-treatment group, mice
were intraperitoneally injected with 1 µg of IL-18 for 3
days during the initial normal water 5 day period, then
intraperitoneally injected again with 1 µg of IL-18 for 3
days at the beginning of the DSS induction for 7 days, followed by
recovery on normal water for 3 days (Fig. 1).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract total RNA
from cells, according to manufacturer's instructions. cDNA was
synthesized using SuperScript VILO cDNA Synthesis kit (cat. no.
11754250, Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was
performed with SYBR-Green Master Mix (ABI; Thermo Fisher
Scientific, Inc.) on an ABI PRISM 7000 Sequence Detection (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under the following
conditions: 50°C for 5 min, 95°C for 10 min, followed by 40 cycles
at 95°C for 30 sec and 60°C for 30 sec. Relative gene expression
was analyzed using the 2−ΔΔCq method (13). The primers were as follows:
Resistin-like molecule (RELM) β, forward
5′-GCTCTTCCCTTTCCTTCTCCAA-3′ and reverse
5′-AACACAGTGTAGGCTTCATGCTGTA-3′; trefoil factor family (TFF) 3,
forward 5′-CCAAGGACAGGGTGGACTG-3′ and reverse
5′-AAGGTGCATTCTGCTTCCTG-3′; GAPDH, forward
5′-GGGGAAGGTGAAGGTCGGAG-3′ and reverse
5′-CCTGGAGATGGTGATGGGA-3′.
Histopathological, periodic acid-Schiff
(PAS) and Alcian Blue examination
Colon tissue samples were acquired and fixed with 4%
paraformaldehyde for 24 h. Colon tissues were then dehydrated,
embedded in paraffin, and sliced to 5 µm thickness sections.
Sections were stained with hematoxylin and eosin (H&E), PAS and
Alcian Blue for 5 min. Colon tissue samples were observed using an
inverted fluorescence microscope (Zeiss Axio Observer A1; Carl
Zeiss AG, Oberkochen, Germany).
Meta-analysis
Literature search
A comprehensive literature search was conducted in
the platforms of PubMed, Embase, Cochrane Library, and Web of
Science. The last search was performed on June 2018. Words adopted
were as follows: 'IL-18', 'IL-18 gene polymorphism', 'intestinal
disease', 'ulcerative colitis', 'colon cancer', 'Crohn's disease'
and 'inflammatory bowel disease'. The reference lists of the
full-text articles were manually examined to identify any
additional publications relevant to our analysis.
Study selection
The following inclusion criteria were used to select
eligible studies: i) The diagnosis of Crohn's disease or colon
cancer was pathologically confirmed; ii) the prognostic value of
bile acid levels in people with colon cancer, ulcerative colitis or
Crohn's disease were reported; iii) the levels of bile acid were
tested by gaschromatograph, thin-layer chromatography or gas-liquid
chromatography; iv) hazard ratios (HRs) and their 95% confidence
intervals (CIs) for bile acid levels analysis were reported in text
or could be computed from given data. The exclusion criteria were:
i) Abstract, review, case report or comment letter; ii) animal
studies; iii) duplicate publications.
ELISA kits
Colon tissue samples were acquired and lyzed using
RIPA assay (Beyotime Institute of Biotechnology, Haimen, China).
Protein was quantified using a BCA kit (Beyotime Institute of
Biotechnology). A total of 10 µg protein was used per sample
to measure the levels of IL-22 (cat. no EH027-96; ExCell Bio,
Shanghai, China) and IL-22BP (cat. no. EK2506; Nanjing Senberga
Biotechnology Co., Ltd., Nanjing, China) using ELISA kits.
IL-18 recombinant protein
PCR was used to generate an IL-18-expressing
plasmid. The coding sequence (CDS) of IL-18 was 479 bp and the size
of the pET28a vector plasmid was 5,369 bp. A His tag (CAT CAT CAC
CAT CAC CAT) was added into the CDS of IL-18, using the primers
5′-GCTCTAGACATCATCACCATCACCATAAAGATGG-3′ and
5′-CCCAAGCTTTTGCTTCTGATC-3′ for the amplification. The
thermocycling conditions were: 94°C for 5 min, followed by 40
cycles of 94°C for 30 sec, 58°C for 30 sec and 65°C for 30 sec. The
IL-18-expressing plasmid was then transformed into BL21 bacterial
cells, and clones were selected with kanamycin. For protein
expression, BL21 bacteria were grown in LB medium containing 1mM
IPTG at 30°C for 12 h. The His tag protein purification kit (cat.
no. P2226; Beyotime Institute of Biotechnology) was used to extract
and purify the IL-18 recombinant protein.
Immunofluorescence
Colon tissue samples were acquired and fixed with 4%
paraformaldehyde for 24 h. Colon tissues were then dehydrated,
embedded in paraffin, sliced to 5 µm thickness sections.
Sections were permeabilized by 0.25% Triton X-100 for 10 min at
room temperature and blocked with 5% bovine serum albumin in PBS
for 1 h at room temperature. Sections were incubated with mucin
(Muc)-2 targeting antibody (cat. no. sc-59859; 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight and washed
with PBST for 15 min at room temperature. Sections were incubated
with secondary peroxidase-conjugated goat anti-rabbit IgG (cat. no.
sc-362272, 1:100; Santa Cruz Biotechnology, Inc.) antibody for 2 h
at room temperature. Sections were then counterstained with DAPI
for 15 min. Colon tissue samples were observed using an inverted
fluorescence microscope (Zeiss Axio Observer A1; Carl Zeiss
AG).
Western blot analysis
Colon tissue samples were acquired and lyzed with
RIPA buffer (Beyotime Institute of Biotechnology). Protein was
quantified using a BCA kit (Beyotime Institute of Biotechnology). A
total of 100 µg protein per sample was boiled for 5 min
prior to separation by 10% SDS-PAGE, and then transferred onto
nitrocellulose membranes (Pall Life Sciences, Port Washington, NY,
USA). Membranes were blocked with 5% non-fat milk at 37°C for 1 h
and incubated with corresponding primary antibodies: IL-18 (cat.
no. BS6823; 1:2,000; Bioworld Technology, Inc. St. Louis Park, MN,
USA), phosphorylated (p-) signal transducer and activator of
transcription (Stat) 3 (cat. no. 9145; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), total Stat3 (cat. no. 12640;
1:1,000; Cell Signaling Technology, Inc.) and GAPDH (cat. no.
AP0063; 1:5,000; Bioworld Technology, Inc.) at 4°C overnight.
Following 3 washes for 10 min in TBST, the membranes were incubated
with anti-mouse or anti-rabbit horseradish peroxidase-conjugated
secondary antibodies (1:5,000; Santa Cruz Biotechnology, Inc.) at
37°C for 1 h. Enhanced chemiluminescent reagent (Beyotime Institute
of Biotechnology) was used to develop the blots and protein was
analyzed using Image Lab 3.0 (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Statistical analysis
Odds ratio (OR) and 95% confidence intervals (CI)
were applied to continuous outcomes: The fixed-effects model was
used to calculate continuous outcomes. Statistical heterogeneity
was assessed using the I2 statistic, where a value of 50% or
greater indicated heterogeneity. Publication bias was evaluated
using funnel plots. Statistical analyses for the meta-analysis were
performed with Revman (version 5.3). Experimental data were
expressed as the mean ± standard deviation, and analyzed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Differences between groups
were compared with one-way analysis of variance and Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Mutation rate of IL-18 (rs187238,
-137G/C) in patients with colon cancer
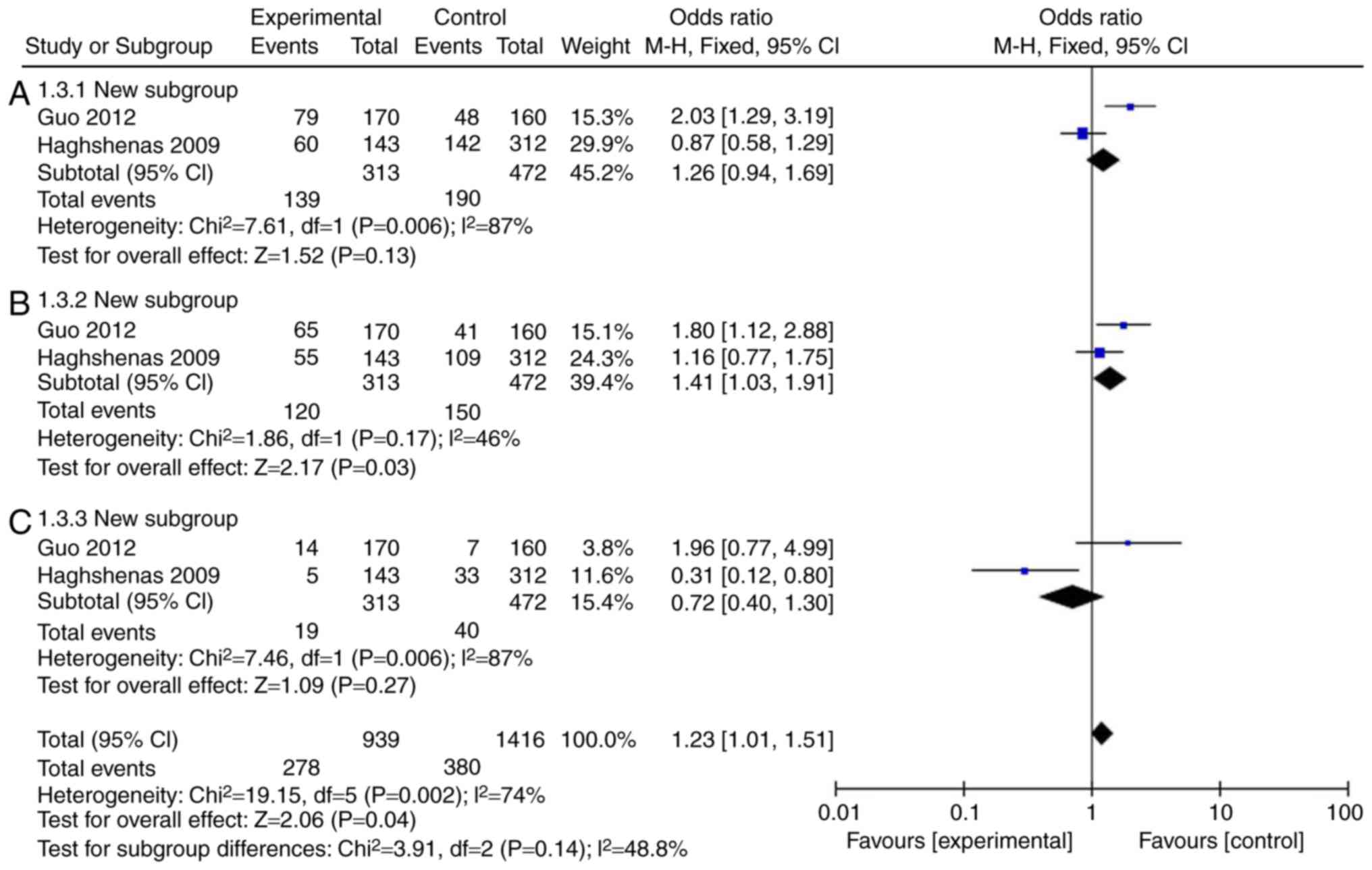
Firstly, a meta-analysis was performed to explore
whether the levels of IL-18 were different in patients with colon
cancer. A total of 11 (14-23) full-text articles were evaluated
for eligibility (Table I), which
were published from 2005 to 2015, with 5,888 patients enrolled. As
illustrated in Fig. 2A and C and
Table II, the incidence rate of
colon cancer in patients was not affected by IL-18 (rs187238,
-137GC+CC or CC), compared with IL-18 -137G/G group. However, IL-18
(rs187238, -137GC) increased the incidence rate of colon cancer in
patients compared with IL-18 -137G/G (Fig. 2B; Table II). Therefore, these results
demonstrated that IL-18 (rs187238, -137G/C) increased the incidence
rate of colon cancer in patients.
 | Table IBasic characteristics of included
studies. |
Table I
Basic characteristics of included
studies.
| Study | Year | Country | Ethnicity | Cases | Group cases | Mutation site |
|---|
| Nikiteas et
al | 2007 | Greece | Europe | 173 | 89/84 | 607 |
| Haghshenas et
al | 2009 | Iran | Europe | 455 | 143/312 | 607, 137 |
| Guo et
al | 2012 | China | Asia | 330 | 170/160 | 607, 137 |
| Ben et
al | 2011 | Tunisia | Africa | 246 | 105/59/100 | 607, 137 |
| Takagawa et
al | 2005 | Japan | Asia | 627 | 210/205/212 | 607, 137 |
| Glas et
al | 2005 | Germany | Europe | 615 | 210/140/254 | 607, 137 |
| Aizawa et
al | 2005 | Japan | Asia | 560 | 158/198/204 | 607, 137 |
| Haas et
al | 2005 | Germany | Europe | 1052 | 470/235/347 | 137 |
| Bank et
al | 2015 | Denmark | Europe | 1830 | 624/411/795 | 607, 137 |
 | Table IIPooled HRs and 95% CIs in
meta-analysis of patients with colon cancer. |
Table II
Pooled HRs and 95% CIs in
meta-analysis of patients with colon cancer.
| Variable | Studies | Heterogeneity
| HR | 95% CI | P-value |
|---|
| I2 (%) | P-value |
|---|
| 137GC+CC | 2 | 87 | 0.006 | 1.26 | 0.94-1.69 | 0.13 |
| 137GC | 2 | 46 | 0.17 | 1.41 | 1.03-1.91 | 0.03 |
| 137CC | 2 | 87 | 0.006 | 0.72 | 0.40-1.30 | 0.27 |
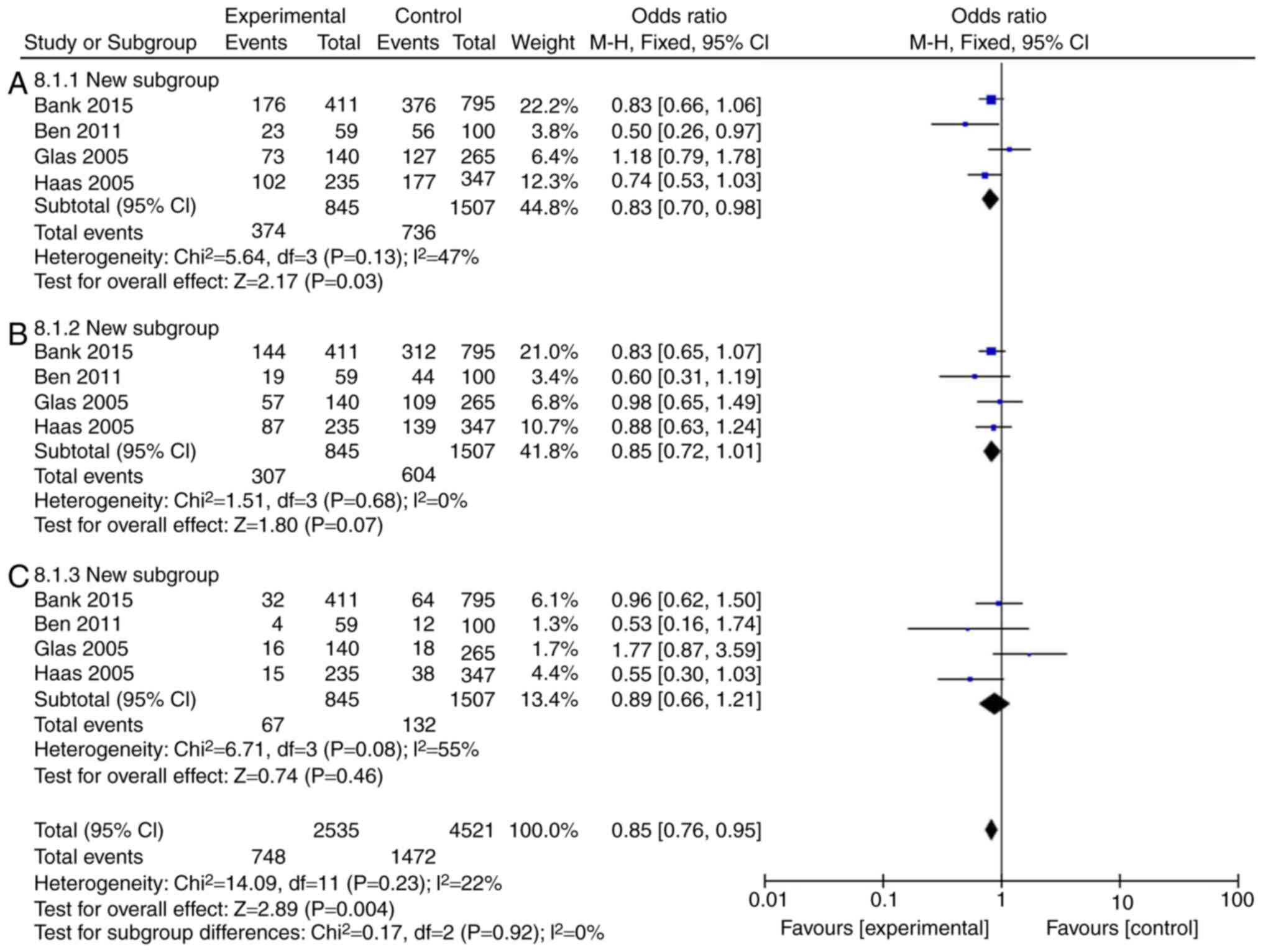
Mutation rate of IL-18 (rs187238,
-137G/C) in patients with Crohn's disease or ulcerative
colitis
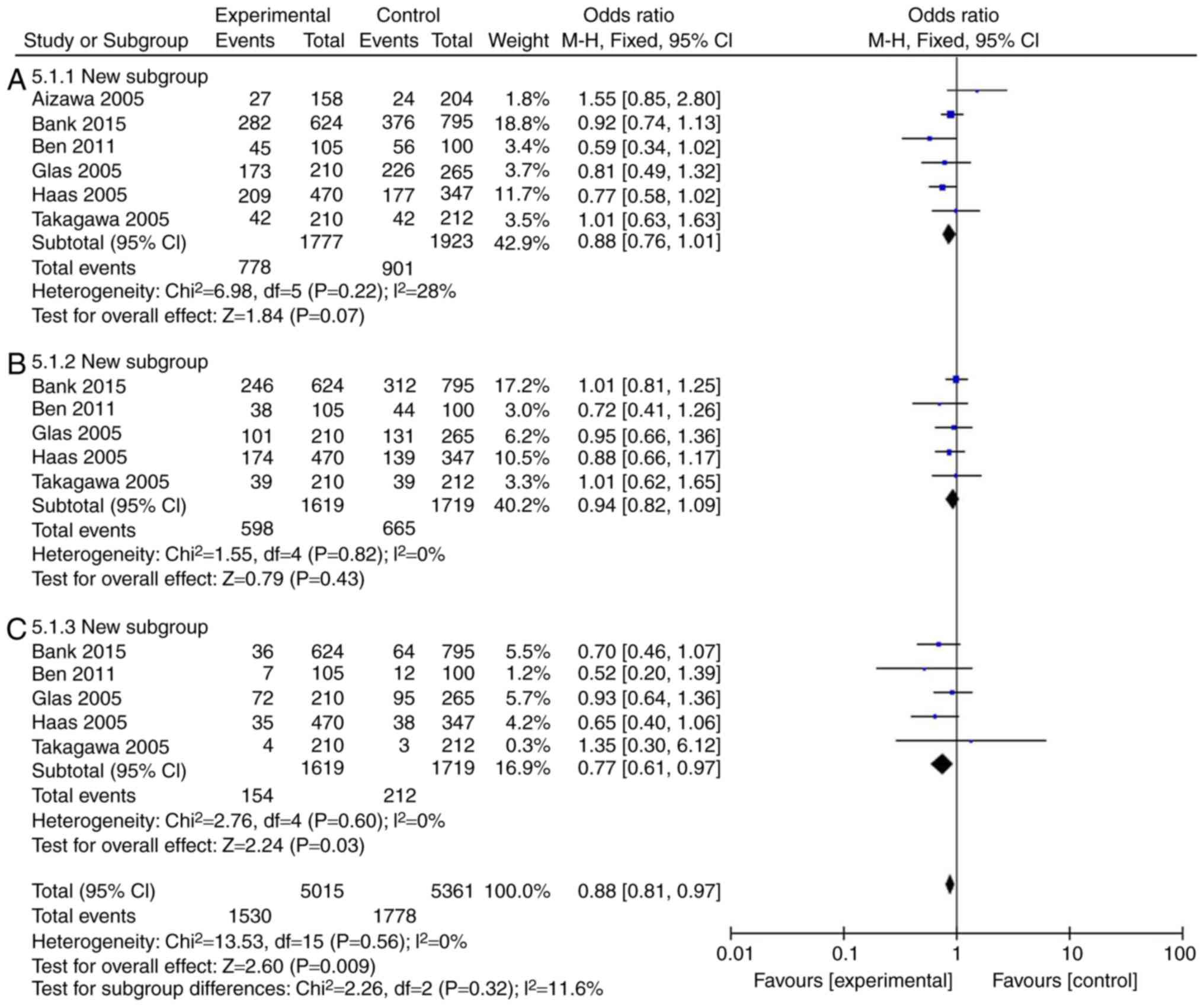
The incidence rate of Crohn's disease in patients
was found to not be influenced by IL-18 (rs187238, -137GC+CC or GC)
compared with the IL-18 -137GG group (Fig. 3A and B; Table III). However, IL-18 (rs187238,
-137CC) reduced the incidence rate of Crohn's disease in patients,
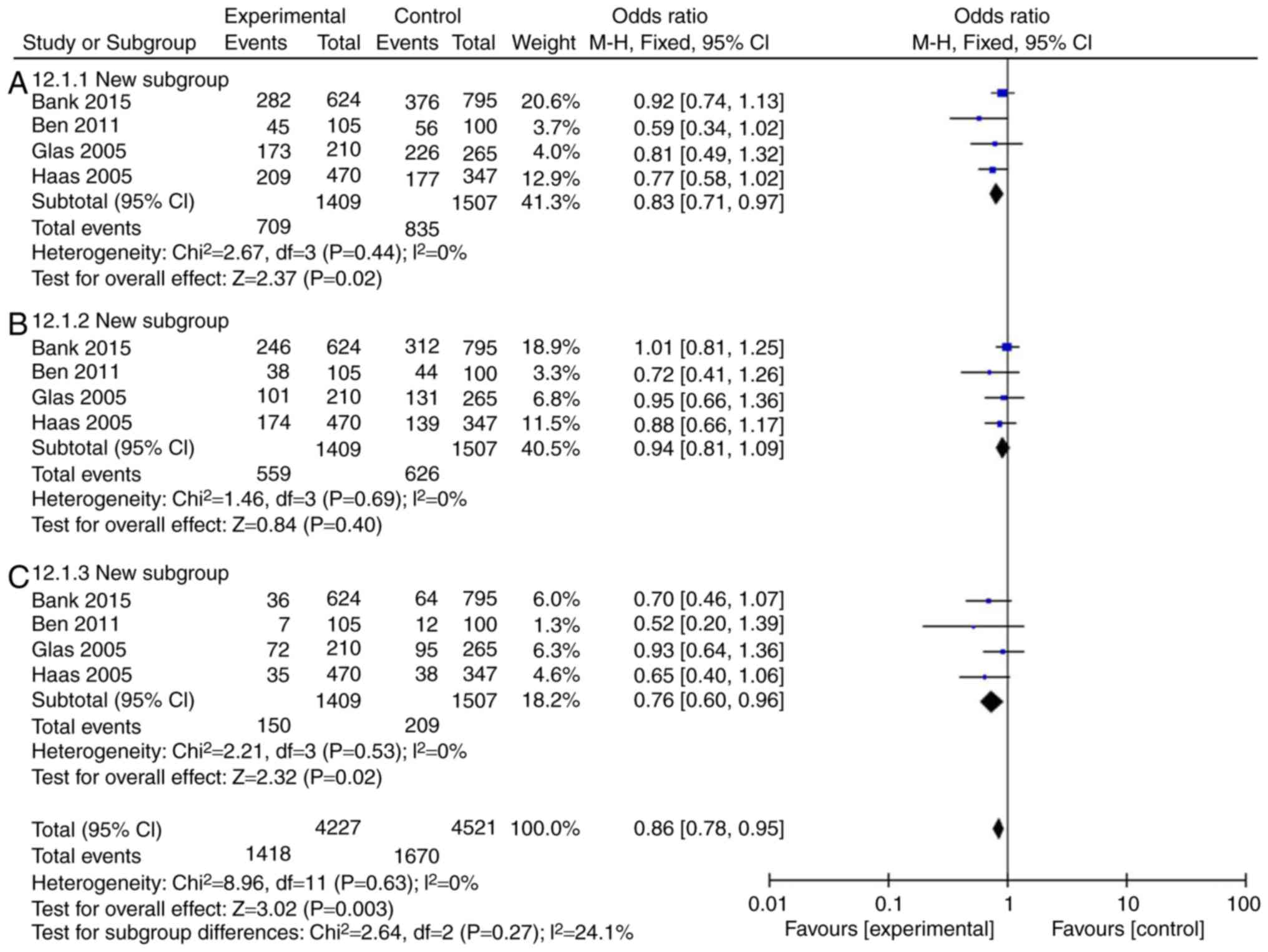
compared with the IL-18 -137GG group (Fig. 3C; Table III). In European continental
ancestry patients, IL-18 (rs187238, -137GC+CC or CC) reduced the
incidence rate of Crohn's disease in patients, compared with the
IL-18 -137GG group (Fig. 4A and
C; Table III). In addition,
the incidence rate of Crohn's disease in patients was not affected
by IL-18 (rs187238, -137GC), compared with the IL-18 -137GG group
(Fig. 4B; Table III). In European continental
ancestry patients, IL-18 (rs187238, -137GC+CC) reduced the
incidence rate of ulcerative colitis in patients, compared with the
IL-18 -137GG group (Fig. 5A;
Table III). However, the
incidence rate of ulcerative colitis in patients was not influenced
by IL-18 (rs187238, -137GC or CC), compared with the IL-18 -137GG
group (Fig. 5B and C; Table III). These results demonstrated
that IL-18 (rs187238, -137G/C) decreased the incidence rate of
ulcerative colitis or Crohn's disease in patients. However, IL-18
(rs187238, -137G/C) may have a dual role in colitis, therefore,
further study was necessary to fully elucidate the mechanism of
IL-18 action in colitis.
 | Table IIIPooled HRs and 95% CIs in
meta-analysis of patients with CD and UC. |
Table III
Pooled HRs and 95% CIs in
meta-analysis of patients with CD and UC.
| Variable | Studies | Heterogeneity
| HR | 95% CI | P-value |
|---|
| I2 (%) | P-value |
|---|
| CD | | | | | | |
| 137GC+CC | 6 | 28 | 0.22 | 0.88 | 0.76-1.01 | 0.07 |
| 137GC | 5 | 0 | 0.82 | 0.94 | 0.82-1.09 | 0.43 |
| 137CC | 5 | 0 | 0.60 | 0.77 | 0.61-0.97 | 0.03 |
| ECA-CD | | | | | | |
| 137GC+CC | 4 | 0 | 0.44 | 0.83 | 0.71-0.97 | 0.02 |
| 137GC | 4 | 0 | 0.69 | 0.94 | 0.81-1.09 | 0.40 |
| 137CC | 4 | 0 | 0.53 | 0.76 | 0.60-0.96 | 0.02 |
| UC | | | | | | |
| 137GC+CC | 4 | 47 | 0.13 | 0.83 | 0.70-0.98 | 0.03 |
| 137GC | 4 | 0 | 0.68 | 0.85 | 0.72-1.01 | 0.07 |
| 137CC | 4 | 22 | 0.08 | 0.89 | 0.66-1.21 | 0.46 |
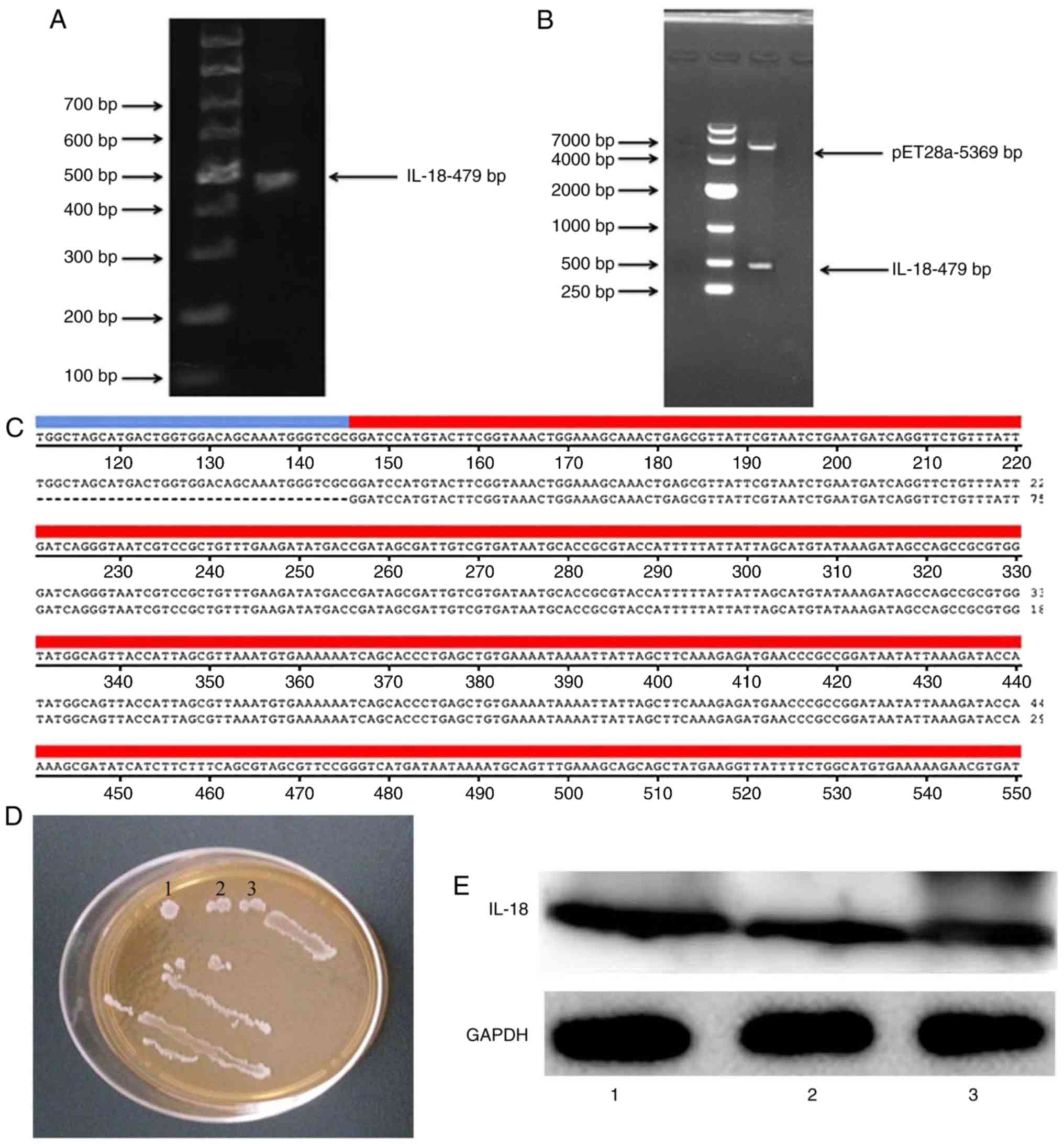
Construction and verification of IL-18
plasmid
An IL-18 expression plasmid was constructed. As
illustrated in Fig. 6A and B, the
coding sequence (CDS) of IL-18 was 479 bp and the size of the
pET28a vector plasmid was 5,369 bp. Gene sequencing was performed
to confirm that the resulting CDS of IL-18 was correct (Fig. 6C). The IL-18-expressing plasmid
was transformed into BL21 bacterial cells, and clones were selected
with kanamycin (Fig. 6D). Western
blot analysis was used to confirm that the generated IL-18 plasmid
successfully expressed the IL-18 protein (Fig. 6E).
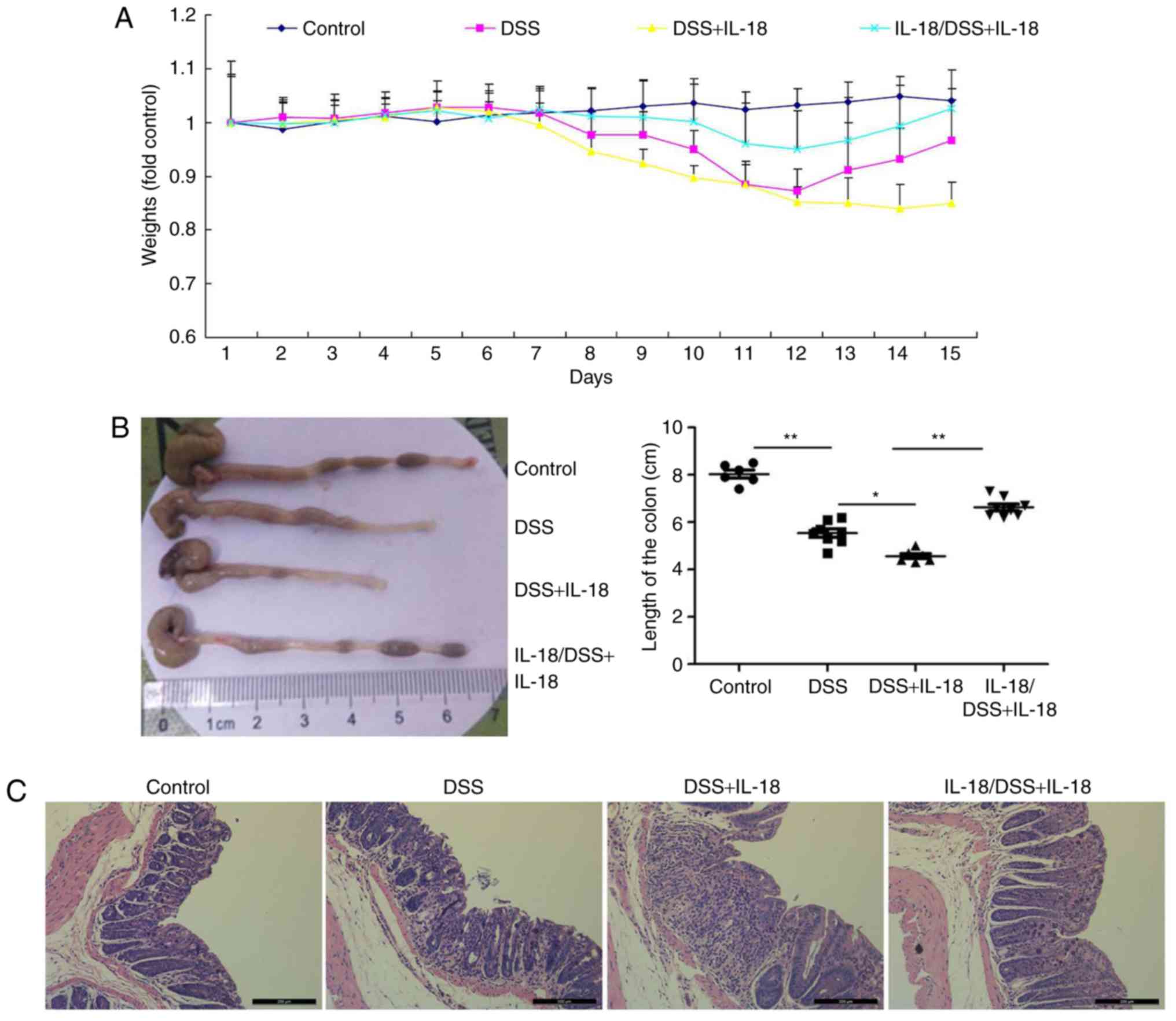
Dual roles of IL-18 in colitis
Next, the effects of IL-18 in colitis were
investigated. Briefly, pre-treatment with IL-18 prior to DSS
administration or treatment with IL-18 concomitant to DSS
administration were employed in DSS-induced colitis in mice, in
order to examine the effects of IL-18 at the early and late stages
of the disease, respectively. As illustrated in Fig. 7, pre-treatment with IL-18
increased body weight, augmented colon length and reduced
inflammatory infiltration in mice with DSS-induced colitis,
compared with the DSS-induced colitis model group. By contrast,
later treatment with IL-18 reduced body weight, reduced colon
length and increased inflammatory infiltration in mice with
DSS-induced colitis, compared with the DSS-induced colitis model
group (Fig. 7). These results
demonstrated that IL-18 had a dual role in colitis. In specific,
IL-18 had an anti-inflammatory effect in the early stage of the
disease, but a pro-inflammatory effect in the later stage.
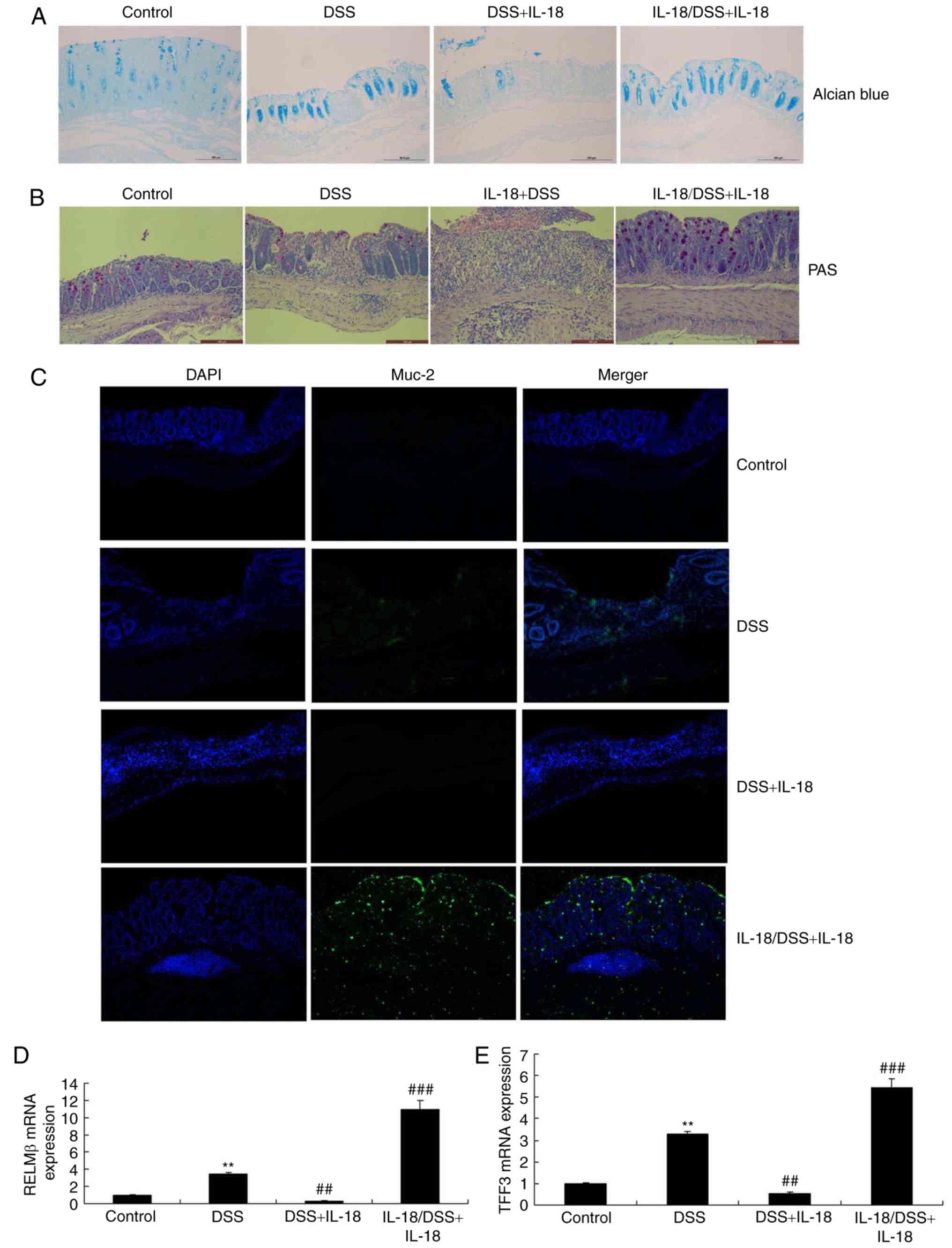
IL-18 regulates the function of goblet
cells in colitis
Next, we explored whether the dual roles of IL-18
regulated the function of goblet cells in colitis. As illustrated
in Fig. 8, Alcian Blue assay, PAS
assay and Muc-2 staining revealed that pre-treatment with IL-18
promoted Muc-2 expression, increased the function and quantity of
goblet cells and enhanced the mRNA levels of resistin-like molecule
(RELM) β and trefoil factor family (TFF) 3 in DSS-induced colitis,
compared with the DSS-induced colitis model group. Treatment with
IL-18 reduced Muc-2 expression, decreased the function and quantity
of goblet cells and decreased the mRNA expression of RELMβ and TFF3
in DSS-induced colitis, compared with the DSS-induced colitis model
group (Fig. 8). Therefore, the
anti-inflammatory effect of IL-18 in the early stage of the disease
promoted the function and quantity of goblet cells, while the
pro-inflammatory effects of IL-18 hindered the function and
quantity of goblet cells in the later stage.
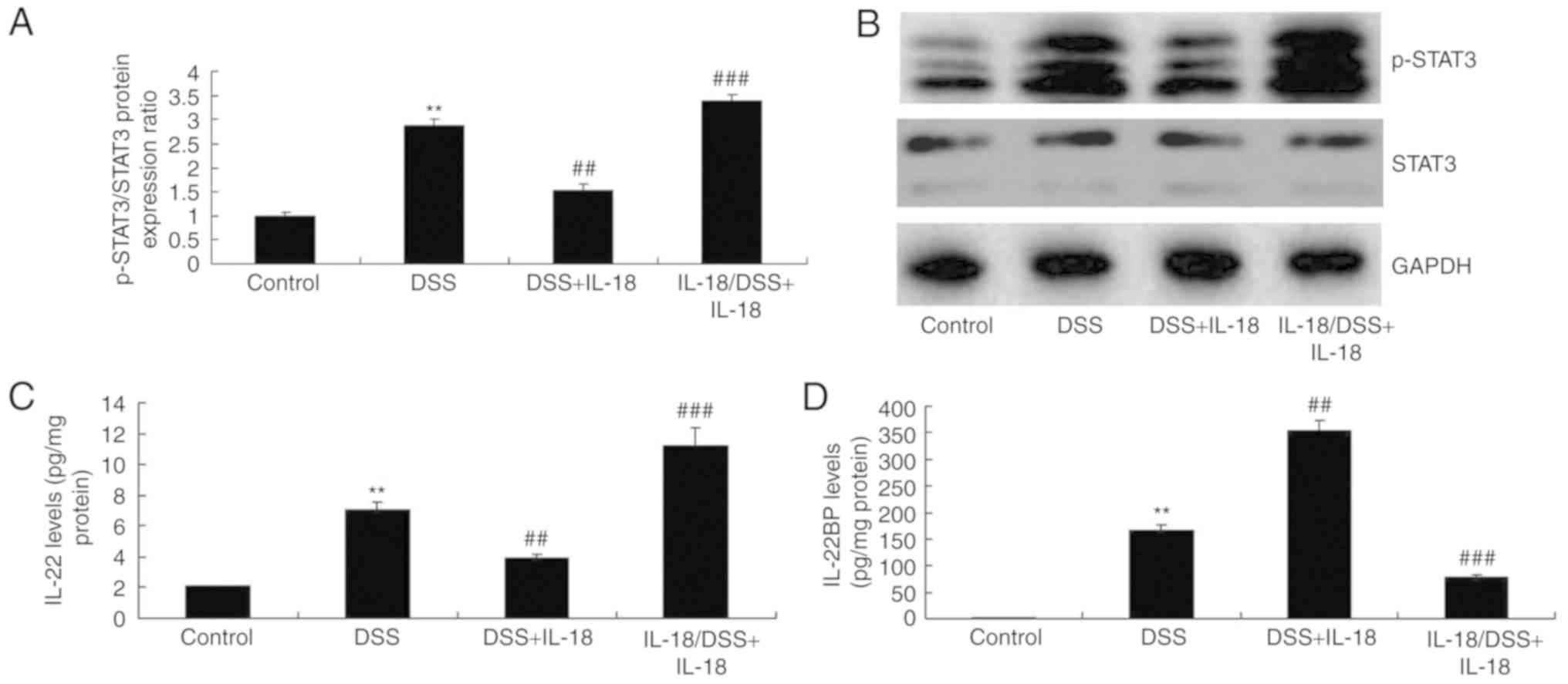
IL-18 regulates IL-22/STAT3 signaling in
colitis
The mechanism by which IL-18 may regulate the
function of goblet cells in colitis was further explored. As
illustrated in Fig. 9,
pre-treatment with IL-18 induced the phosphorylation of Stat3,
increased the levels of IL-22, but reduced the levels of the
IL-22-specific inhibitor IL-22BP in DSS-induced colitis, compared
with the DSS-induced colitis model group. Treatment with IL-18 at
later stages suppressed the phosphorylation of Stat3, decreased the
levels of IL-22 and enhanced the levels of the IL-22BP inhibitor in
DSS-induced colitis, compared with the DSS-induced colitis model
group (Fig. 9). Collectively,
these results suggested that the anti-inflammatory effect of IL-18
in the early stage of the disease induced IL-22/STAT3 signaling,
while the pro-inflammatory effects of IL-18 suppressed the
IL-22/STAT3 signaling pathway in the later stage, by activating
IL-22 BP.
Discussion
IBD is a major health issue, which is associated
with high morbidity and mortality due to its repeated bloody
diarrhea, weight loss and chronic intestinal inflammation (3). At present, the approved IBD therapy
mainly depends on symptom control and inflammation suppression
(24). However, the potential
mechanism underlying the IBD pathogenesis remains unclear (24). The currently popular IBD
pathogenesis model is the imbalance of intestinal microbial
community structure, or the abnormal immune response of symbiotic
bacteria in genetically susceptible host, and/or the genesis of
dysbiosis (24). Therefore, it is
necessary to further elucidate the specific expression of
inflammatory factors in this disease in order to determine the
target treatment. In the present study, IL-18 (rs187238, -137G/C)
was demonstrated to increase the incidence rate of colon cancer in
patients, to decrease the incidence rate of ulcerative colitis or
Crohn's disease in patients, and to have a dual function in a mouse
model of colitis.
The IL-18-mediated protection mechanism remains
unclear. A previous study indicates that IL-18 is mainly expressed
in epithelial cells, and that the epithelium-derived IL-18 is
important to prevent DSS-induced injury (25). IL-18 production is also associated
with upregulation of IL-22, and IL-22 is involved in epithelial
repair (26). The present results
demonstrated that pre-treatment with IL-18 increased body weight,
augmented colon length and reduced inflammatory infiltration in
mice with DSS-induced colitis, while later treatment with IL-18
reduced body weight, decreased colon length and increased
inflammatory infiltration in mice with DSS-induced colitis.
Therefore, IL-18 possessed a dual function in colitis, with
anti-inflammatory effects observed in the early stage of the
disease, and pro-inflammatory effects observed in the later stages.
The present study focused on the dual roles of IL-18 in colitis, so
the exact mechanisms of how IL-18 regulates the function of goblet
cells remain unknown. Further studies will assess in the future
whether IL-18 alone, in the absence of DSS, may be sufficient to
induce inflammation and colitis.
IL-22 is produced by innate lymphoid cells, T-helper
(Th) 17 cells and Th22 cells. The membrane-binding IL-22 receptor 1
(IL-22R1) is not present in immune cells, but is expressed in
tissues, such as in epithelial cells of gastrointestinal tract and
skin (10). IL-22 serves a
critical role in promoting anti-microbial immunity via inducing
AMPs, and in tissue repair via inducing epithelial cell
proliferation and survival (27).
However, IL-22 can also promote skin or intestinal pathological
inflammatory reactions in mouse models (27). Its concentration is increased in
multiple human diseases, including psoriasis, rheumatoid arthritis,
infection and IBD (28). Based on
the multiple effects of IL-22, it is known that such cytokine can
transmit signals through STAT3, which is vital to wound healing and
tumor development. Yet, the role of IL-22 in tumor development
remains controversial, since both suppression and promotion have
been reported (28). The present
study indicated that pre-treatment with IL-18 induced Stat3
activation, increased IL-22 levels and reduced IL-22BP levels in
DSS-induced colitis. Treatment with IL-18 at later stages
suppressed Stat3 activation, decreased IL-22 levels and induced
IL-22BP levels in DSS-induced colitis. These results suggest that
the anti-inflammatory effect of IL-18 in the early stage of
colitis-associated inflammation activated the IL-22/STAT3 signaling
pathway, while the pro-inflammatory effects of IL-18 at the later
stages of the disease suppressed the IL-22/STAT3 signaling pathway,
by activating IL-22 BP. A previous study has demonstrated that AIM2
inflammasome is a central regulator of intestinal homeostasis
through the IL-18/IL-22/STAT3 pathway (29).
UC occurs in the mucin layer and is characterized by
goblet cell consumption. It is manifested with strong expression of
Muc-2 and Muc-4, but low expression of Muc-1 and Muc-3 (30). Therefore, insufficient IL-22
production may promote the consumption of goblet cells and damage
the formation of mucus layer in UC (30). Goblet cells specifically produce
Muc, but can also produce other molecules involved in colitis
regulation and deterioration. Notably, IL-22 downregulates the
expression of goblet cell-derived RELMβ (a potential pathogenic
goblet cell product) (31). The
present findings indicated that pre-treatment with IL-18 promoted
Muc-2 expression, increased the function and quantity of goblet
cells and increased RELMβ and TFF3 mRNA levels in colon tissues
from mice with DSS-induced colitis. Treatment with IL-18 at later
stages reduced Muc-2 expression, decreased the function and
quantity of goblet cells and increased RELMβ and TFF3 mRNA levels
in DSS-induced colitis. The anti-inflammatory effect of IL-18 in
early inflammation promoted the function and quantity of goblet
cell, while the pro-inflammatory effects of IL-18 inhibited the
function and quantity of goblet cells in later stages. Sugimoto
et al (31) reported that
IL-18 inhibited goblet cell maturation by regulating the
transcriptional program instructing goblet cell development.
The present study analyzed IL-18 (rs187238, -137G/C)
in patients with colon cancer, CD or UC, through a meta-analysis of
published cohorts. Then, in vivo experiments demonstrated a
dual function for IL-18 in a mouse model of colitis, through
regulating the function of goblet cells. However, the present study
did not analyze the role of IL-18 on cancer cells or tissues, and
therefore, the effects of IL-18 on colon cancer remain unknown and
will require further research.
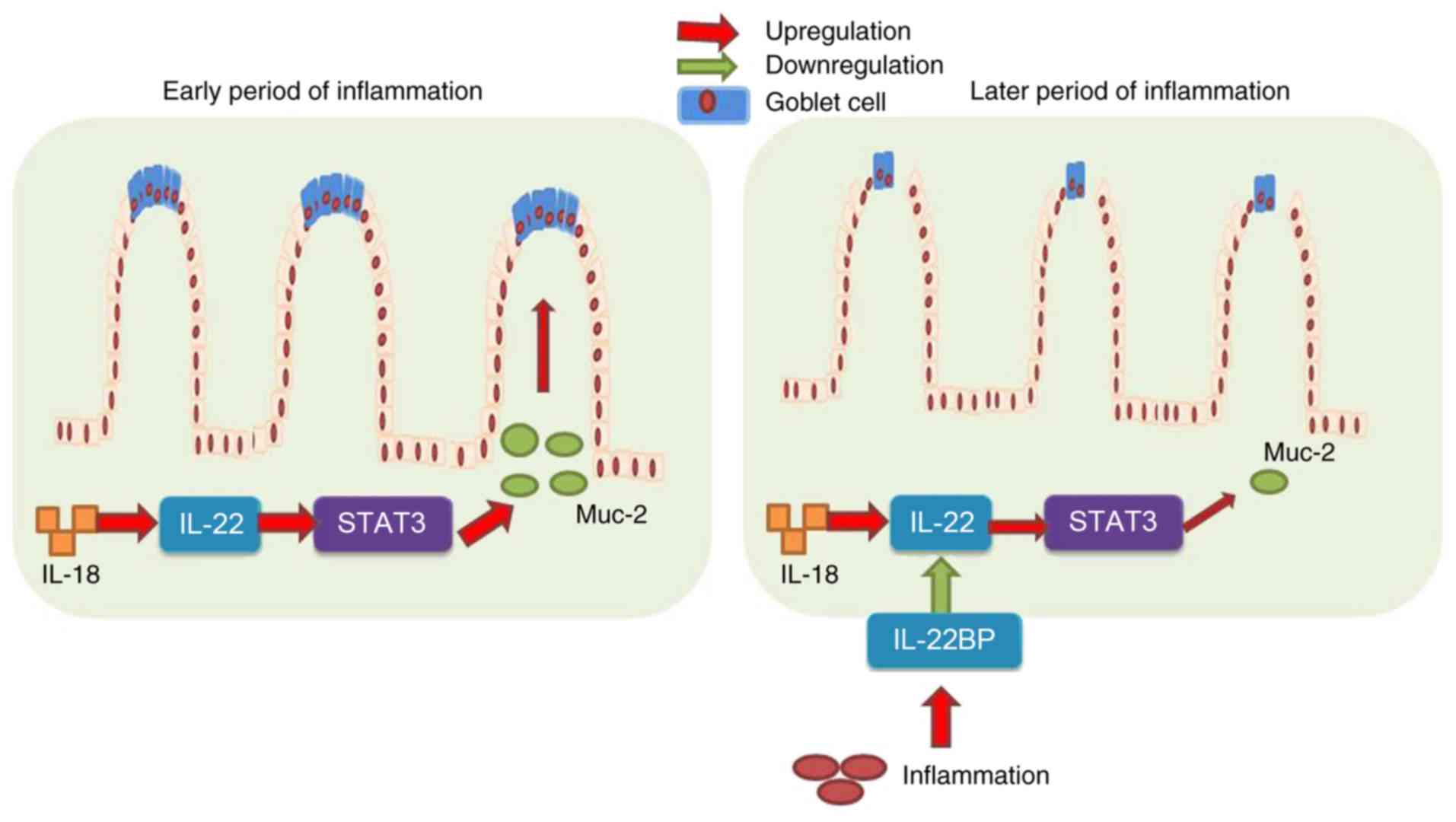
In conclusion, the results of the present study
demonstrated that IL-18 prevented DSS-induced colitis through
recovering goblet cells in the early stages of colitis-associated
inflammation, via IL-22/STAT3 signaling (Fig. 10). However, IL-18 at the later
stages of the disease induced DSS-induced inflammation, increased
IL-22BP expression, and suppressed IL-22/STAT3 signaling to reduce
goblet cells and promote the development and progression of colitis
(Fig. 10). These results
provided evidence that IL-18 may serve as a useful drug candidate
for colitis therapy, which warrants further research in the
application of IL-18 in colitis treatment.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grants nos. 81430091,
81720108032, 91429308, 81603193 and 81421005), the project for
Major New Drugs Innovation and Development (grant no.
2015ZX09501010), the 111 Project (grant no. G20582017001), State
Key Laboratory of Natural Medicines at China Pharmaceutical
University (grant no. SKLNMZZCX201610), and the Fundamental
Research Funds for the Central Universities (grant no.
2016ZPY011).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC and HH conceived and designed the experiments. ZP
performed the experiments. ZP, YC, WZ, HS, HX and TM analyzed the
data. ZP wrote the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Protocols involving animals were approved by the
Institutional Animal Care and Welfare Committee of China
Pharmaceutical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
D'Haens G, Vermeire S, Lambrecht G, Baert
F, Bossuyt P, Pariente B, Buisson A, Bouhnik Y, Filippi J, Vander
Woude J, et al: Increasing infliximab dose based on symptoms,
biomarkers, and serum drug concentrations does not increase
clinical, endoscopic, and corticosteroid-free remission in patients
with active luminal Crohn's disease. Gastroenterology.
154:1343–1351e1. 2018. View Article : Google Scholar
|
|
2
|
Tan B, Li P, Lv H, Yang H, Li Y, Li J,
Wang O and Qian JM: Treatment of vitamin D deficiency in Chinese
inflammatory bowel disease patients: A prospective, randomized,
open-label, pilot study. J Dig Dis. 19:215–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ling XH, Yu X, Kong DJ, Hu CY, Hong Y and
Yang XM: Treatment of inflammatory bowel disease with Chinese drugs
administered by both oral intake and retention enema. Chin J Integr
Med. 16:222–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buckley JP, Kappelman MD, Allen JK, Van
Meter SA and Cook SF: The burden of comedication among patients
with inflammatory bowel disease. Inflamm Bowel Dis. 19:2725–2736.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun CM, Wu J, Zhang H, Shi G and Chen ZT:
Circulating miR-125a but not miR-125b is decreased in active
disease status and negatively correlates with disease severity as
well as inflammatory cyto-kines in patients with Crohn's disease.
World J Gastroenterol. 23:7888–7898. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maerten P, Shen C, Colpaert S, Liu Z,
Bullens DA, van Assche G, Penninckx F, Geboes K, Vanham G,
Rutgeerts P, et al: Involvement of interleukin 18 in Crohn's
disease: Evidence from in vitro analysis of human gut inflammatory
cells and from experimental colitis models. Clin Exp Immunol.
135:310–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reuter BK and Pizarro TT: Commentary: The
role of the IL-18 system and other members of the IL-1R/TLR
superfamily in innate mucosal immunity and the pathogenesis of
inflammatory bowel disease: Friend or foe. Eur J Immunol.
34:2347–2355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang L, Pang Z, Shu W, Wu W, Sun M, Cong Y
and Liu Z: Anti-TNF Therapy induces CD4+ T-cell
production of IL-22 and promotes epithelial repairs in patients
with Crohn's disease. Inflamm Bowel Dis. 24:1733–1744. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang GX, Sun Y, Tsuneyama K, Zhang W,
Leung PS, He XS, Ansari AA, Bowlus C, Ridgway WM and Gershwin ME:
Endogenous interleukin-22 protects against inflammatory bowel
disease but not autoimmune cholangitis in dominant negative form of
transforming growth factor beta receptor type II mice. Clin Exp
Immunol. 185:154–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li LJ, Gong C, Zhao MH and Feng BS: Role
of interleukin-22 in inflammatory bowel disease. World J
Gastroentero. 20:18177–18188. 2014. View Article : Google Scholar
|
|
11
|
Mizoguchi A: Healing of intestinal
inflammation by IL-22. Inflamm Bowel Dis. 18:1777–1784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arj A, Razavizadeh M, Mohammadi H,
Nikoueinejad H and Akbari H: The correlation between the numerical
status of Th22 cells and serum level of IL-22 with severity of
ulcerative colitis. Iran J Allergy Asthma Immuno. 17:78–84.
2018.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
14
|
Nikiteas N, Yannopoulos A,
Chatzitheofylaktou A and Tsigris C: Heterozygosity for
interleukin-18-607 A/C polymorphism is associated with risk for
colorectal cancer. Anticancer Res. 27:3849–3853. 2007.
|
|
15
|
Haghshenas MR, Hosseini SV, Mahmoudi M,
Saberi-Firozi M, Farjadian S and Ghaderi A: IL-18 serum level and
IL-18 promoter gene polymorphism in Iranian patients with
gastrointestinal cancers. J Gastroenterol Hepatol. 24:1119–1122.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo JY, Qin AQ, Li RK, Yang CM, Huang FD,
Huang ZY and Guo HJ: Association of the IL-18 gene polymorphism
with susceptibility to colorectal cancer. Zhonghua Wei Chang Wai Ke
Za Zhi. 15:400–403. 2012.In Chinese. PubMed/NCBI
|
|
17
|
Ben Aleya W, Sfar I, Habibi I, Mouelhi L,
Aouadi H, Makhlouf M, Ayed-Jendoubi S, Najjar T, Ben Abdallah T,
Ayed K, et al: Interleukin-18 gene polymorphisms in tunisian
patients with inflammatory bowel disease. Digestion. 83:269–274.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takagawa T, Tamura K, Takeda N, Tomita T,
Ohda Y, Fukunaga K, Hida N, Ohnishi K, Hori K, Kosaka T, et al:
Association between IL-18 gene promoter polymorphisms and
inflammatory bowel disease in a Japanese population. Inflamm Bowel
Dis. 11:1038–1043. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aizawa Y, Sutoh S, Matsuoka M, Negishi M,
Torii A, Miyakawa Y, Sugisaka H, Nakamura M and Toda G: Association
of interleukin-18 gene single-nucleotide polymorphisms with
susceptibility to inflammatory bowel disease. Tissue Antigens.
65:88–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haas SL, Andreas Koch W, Schreiber S,
Reinhard I, Koyama N, Singer MV and Böcker U: -137 (G/C) IL-18
promoter polymorphism in patients with inflammatory bowel disease.
Scand J Gastroentero. 40:1438–1443. 2005. View Article : Google Scholar
|
|
21
|
Dong YB and Chen GY: Association analysis
of IL-18 gene polymorphisms and ulcerative colitis in Chinese Han
population. Chin J Clin Gastroenterol. 21:21–22. 2009.In
Chinese.
|
|
22
|
Bank S, Andersen PS, Burisch J, Pedersen
N, Roug S, Galsgaard J, Ydegaard Turino S, Brodersen JB, Rashid S,
Kaiser Rasmussen B, et al: Polymorphisms in the toll-like receptor
and the IL-23/IL-17 pathways were associated with susceptibility to
inflammatory bowel disease in a danish cohort. PLoS One.
10:e01453022015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong YB, Bo WL, Feng XB and Chen GY:
Association between single nucleotide polymorphismin of
interleukin-18 gene and ulcerative colitis in Han population of
Shanghai. World Chin J Digestology. 16:2785–2787. 2008.In Chinese.
View Article : Google Scholar
|
|
24
|
Motamed F, Famouri F, Najafi M, Moazzami
K, Farahmand F, Khodadad A, Fallahi GH, Khatami GR and Rezaei N:
Response to induction therapy in a pediatric population of
inflammatory bowel disease. Z Gastroenterol. 48:748–752. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wiercinska-Drapalo A, Flisiak R,
Jaroszewicz J and Prokopowicz D: Plasma interleukin-18 reflects
severity of ulcerative colitis. World J Gastroenterol. 11:605–608.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Striz I: Cytokines of the IL-1 family:
Recognized targets in chronic inflammation underrated in organ
transplantations. Clin Sci. 131:2241–2256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tillack C, Ehmann LM, Friedrich M,
Laubender RP, Papay P, Vogelsang H, Stallhofer J, Beigel F, Bedynek
A, Wetzke M, et al: Anti-TNF antibody-induced psoriasiform skin
lesions in patients with inflammatory bowel disease are
characterised by interferon-gamma-expressing Th1 cells and
IL-17A/IL-22- expressing Th17 cells and respond to anti-IL-12/IL-23
antibody treatment. Gut. 63:567–577. 2014. View Article : Google Scholar
|
|
28
|
Yu LZ, Wang HY, Yang SP, Yuan ZP, Xu FY,
Sun C and Shi RH: Expression of interleukin-22/STAT3 signaling
pathway in ulcerative colitis and related carcinogenesis. World J
Gastroenterol. 19:2638–2649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ratsimandresy RA, Indramohan M,
Dorfleutner A and Stehlik C: The AIM2 inflammasome is a central
regulator of intestinal homeostasis through the IL-18/IL-22/STAT3
pathway. Cell Mol Immunol. 14:127–142. 2017. View Article : Google Scholar :
|
|
30
|
Turner JE, Stockinger B and Helmby H:
IL-22 mediates goblet cell hyperplasia and worm expulsion in
intestinal helminth infection. PLoS Pathog. 9:e10036982013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugimoto K, Ogawa A, Mizoguchi E,
Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ and Mizoguchi
A: IL-22 ameliorates intestinal inflammation in a mouse model of
ulcerative colitis. J Clin Invest. 118:534–544. 2008.PubMed/NCBI
|