Introduction
The incidence of diabetes is increasing annually
(1). Diabetic nephropathy (DN) is
a common and serious complication of diabetes, and one of the main
causes of mortality in diabetic patients (2). To reduce the risk of DN, it is very
important to study the pathogenesis of DN and implement appropriate
drug control; however, the pathogenesis of DN currently remains
unclear. Previous studies have suggested that inflammatory and
oxidative stress reactions are involved in the development of DN
(2-4). The NOD-like receptor protein 3
(NLRP3) inflammasome activates inflammatory factors, and NLRP3
inflammasome activation induced by reactive oxygen species (ROS)
may be involved in the occurrence and development of kidney
injury.
Elevated levels of ROS are common in diabetic
patients, and these species are necessary for the activation of
NLRP3 inflammasomes. Upon the activation of NLRP3 inflammasomes,
NLRP3 activates caspase-1, and then interleukin (IL)-1 and IL-18
are cleaved and activated, leading to pro-inflammatory responses
(5,6). NLRP3, IL-1 and IL-18 are closely
associated with various diseases, including kidney disease
(7,8). Although the mechanism of NLRP3
inflammasome activation has been one of the research hotspots in
recent years, there are few studies on the activation of NLRP3 in
DN.
Thioredoxin interacting protein (TXNIP) belongs to
the thioredoxin system and is an important participant in
regulating oxidative stress in the body (9). Thioredoxin (TRX) is the main
molecule that resists oxidative stress in cells. TXNIP can bind to
TRX, decreasing TRX activity and increasing oxidative stress
(9). It has been reported that
TXNIP is able to activate NLRP3 inflammasome by binding to NLRP3
(10). However, the association
between TXNIP and NLRP3 inflammasome in DN is not well
understood.
The current study mainly investigated the expression
characteristics of NLRP3 in DN, and examined the effect of TXNIP on
oxidative stress and NLRP3 expression. Furthermore, the role of
TXNIP gene in the occurrence and development of DN was
assessed.
Materials and methods
Animals and establishment of a diabetes
mouse model
A total of 25 5-6 week old male Sprague-Dawley rats
were purchased from Shanghai Laboratory Animal Center (Shanghai,
China). Rats were fed a normal diet with water ad libitum
prior to treatment administration and kept under a 12 h light/dark
cycle in a controlled environment (temperature, 22±2°C; humidity
60-80%). All protocols, including diabetes induction and animal
sacrifice, were approved by the Institutional Animal Care and Use
Committee of the First Hospital of Jilin University (Changchun,
China), and the China Council on Animal Care. The rats received
adaptive feeding.
Rats in DN group (n=15) were administered an
intra-peritoneal injection of streptozotocin (55 mg/kg), while rats
in the control group (n=10) received only citrate buffer.
Biochemical kits (Xibao Biotechnology Co., Ltd., Shanghai, China)
were used to measure the blood glucose levels at 24, 48 and 72 h
after streptozotocin injection. After 72 h of assessment, blood was
collected from the tail vein of rats and fasting blood glucose was
determined. Rats with fasting blood glucose levels of >250 mg/dl
were considered as diabetic. The levels of blood urea nitrogen
(BUN) and serum creatinine (SCr) in rats were detected by
enzyme-linked immunosorbent assay (ELISA), and the corresponding
kits were purchased from Wuhan Huamei Biotech Co., Ltd. (Wuhan,
China).
Hematoxylin and eosin (H&E)
staining
H&E staining was performed to observe the
kidneys in the control and DN groups. After 72 h of injection, the
rats were sacrificed and kidney tissue samples were collected at
the end of week 1, 2 and 3. Kidney tissues from rats were
immediately fixed in formalin for 24 h at room temperature. Next,
tissues were dehydrated with alcohol, embedded in paraffin, cut
into 7-µm uniform sections and placed on glass slides coated
with 3-aminopropyltriethoxysilane. The sections were then
deparaffinized using xylene, hydrated and stained with H&E
reagent (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). For
staining, sections were incubated with hematoxylin at room
temperature for 5 min, washed and then incubated with eosin at room
temperature for 1-3 min. Subsequent to washing with gradient
ethanol, neutral gel was used for sealing. The mean glomerular
volume was calculated by measuring the maximum diameter of the
glomerulus in 10 random fields.
Streptavidin-peroxidase (SP)
staining
SP staining was performed to detect the TXNIP and
NLRP3 protein expression levels. Briefly, tissue specimens were cut
into 4-µm section and deparaffinized in xylene. Next, 0.01
mol/l citrate buffer solution was used for antigen retrieval, and
50 µl peroxidase blocking solution was added to block the
endogenous peroxidase activity. The primary antibodies (anti-NLRP3,
1:500; ab214185; anti-TXNIP, 1:200; ab188865; Abcam, Cambridge, MA,
USA) were added and incubated at 4°C for 12 h. Subsequently, the
horseradish peroxidase conjugated secondary antibody (goat
anti-rabbit IgG; 1:200; ab205718; Abcam) was added and incubated at
room temperature for 10 min. DAB (100 µl) was then added for
5 min, with hematoxylin being used for counterstaining at room
temperature for 2 min, and the staining was observed under a
microscope. The cell staining intensity in three random fields of
view was calculated using a semi-quantitative method (11,12).
Cell culture
Human proximal tubular epithelial HK-2 cells were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The cells were cultured in Dulbecco's modified Eagle's medium
containing 10% fetal bovine serum at 37°C in an incubator with 5%
CO2. HK-2 cells were divided into the control group,
high-glucose group 1 (HG1), HG2 and HG3, and then cultured with
5.5, 15, 30 or 50 mmol/l glucose, respectively. HK-2 cells in each
group were cultured with glucose for 24, 48, 72 or 96 h at 37°C.
These cell groups were subjected to western blot analysis and
ELISA. Based on the results of these analyses, the experimental
conditions of HG3 group (50 mmol/l glucose) were used in subsequent
experiments, and this group was defined as HG. Reagents used in
cell culture were purchased from Thermo Fisher Scientific, Inc.
(Gibco; Waltham, MA, USA).
Small interfering RNA (siRNA)
construction, transfection and grouping
siRNA targeting TXNIP (siTXNIP) was purchased from
GenePharma Co., Ltd. (Shanghai, China). siRNA trans-fection of
cells was performed using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) to obtain the siTXNIP
group. A NC group transfected with NC-siRNA and an untransfected
control group were also established. To assess the effect of TXNIP
gene silencing on HK-2 cells under a high-glucose environment,
cells were divided into five groups as follows: A control group, A
HG group (50 mmol/l glucose), a negative + HG group (NC + HG; cells
transfected with NC-siRNA + 50 mmol/l glucose), an siTXNIP + HG
group (cells transfected with siTXNIP + 50 mmol/l glucose) and an
siTXNIP group (cells transfected with siTXNIP).
Western blot analysis
Protein expression was assessed by western blot
analysis. Briefly, cells were lysed with liquid nitrogen and
blocked with radioimmunoprecipitation assay reagent (Abmole
Bioscience, Inc., Houston, TX, USA), followed by 1% cleavage in
phenylmethane sulfonyl fluoride and phosphatase inhibitors (Abmole
Bioscience, Inc.), and lysis for 30 min at 4°C. The supernatant was
collected by centrifugation at 12,000 × g at 4°C for 15 min, and a
standard curve was constructed using the bicinchoninic acid method
to determine the protein concentration. A 10% SDS-PAGE gel was then
prepared and used for electrophoresis. Next, samples were
transferred to a polyvinylidene difluoride membrane using a
Trans-Blot Transfer Slot (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and blocked with 5% fat-free milk for 2 h at room temperature.
Primary antibodies were added, shaken at room temperature for 2 h
and then incubated at 4°C for 12 h. The primary antibodies used
were as follows: Anti-NLRP3 (ab214185; dilution, 1:800), anti-TXNIP
(ab188865; dilution, 1:600), anti-caspase-1 (ab138483; dilution,
1:800), anti-IL-1 (ab150777; dilution, 1:600), anti-IL-18 (ab52914;
dilution, 1:600), anti-catalase (ab16731; dilution, 1:800) and
anti-manganese superoxide dismutase (MnSOD; ab13533; dilution,
1:800), all purchased from Abcam; and anti-cleaved-caspase-1
(Orbyk310040; dilution, 1:700), supplied by Xiamen Research
Biotechnology Co., Ltd. (Xiamen, China). Subsequently, secondary
antibodies were added and incubated at room temperature for 1.5 h.
The secondary antibodies used were as follows: Goat anti-rat IgG
(ab150160; dilution, 1:10,000), donkey anti-rat IgG (ab175475;
dilution, 1:8,000), rabbit anti-mouse IgG (ab99697; dilution,
1:9,000) and rabbit anti-human IgG (ab6759; dilution, 1:10,000),
all purchased from Abcam; and mouse anti-rabbit IgG (31213;
dilution, 1:7,000), obtained from Thermo Fisher Scientific, Inc.
(Invitrogen). Chemiluminescence detection was subsequently
conducted using an ECL reagent (Huiying Medical Technology Co.,
Ltd., Shanghai, China). Optical density was quantified using ImageJ
software version 1.46 (National Institutes of Health, Bethesda, MD,
USA).
ELISA
The malondialdehyde (MDA), superoxide dismutase
(SOD), IL-1 and IL-18 concentrations of rat serum were assessed by
ELISA assay using kits purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). Briefly, subsequent to
incubation with blocking solution at 4°C for 2 h, the primary
antibody was added and incubated at 4°C overnight. The secondary
antibody was then added and incubated for 1 h at room temperature.
Horseradish peroxidase (100 µl) was added and incubated at
room temperature for 30 min, followed by addition of
3,3′,5,5′-tetramethylbenzidine for 10 min. The absorbance value was
measured at 450 nm by a microplate reader (ELX 800; BioTek
Instruments, Inc., Winooski, VT, USA), and the concentration of
each compound was calculated according to the standard curve.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to detect the mRNA levels. The
cells were triturated and lysed using TRIzol reagent (Thermo Fisher
Scientific, Inc.) at 0°C for 5 min, and the RNAs were extracted by
CHCl3 (Shanghai Aladdin Technology Co., Ltd., Shanghai,
China). Next, the RNA concentration was measured by a UV
spectrophotometer (NanoDrop One Microvolume UV-Vis
spectrophotometer; Thermo Fisher Scientific, Inc.). RT assays were
performed on RNA samples using an RT kit (Takara Bio, Inc.) to
synthesize cDNA. The RT reaction conditions were set to 37°C for 15
min, and reverse transcriptase inactivation was conducted at 85°C
for 15 sec. Subsequently, qPCR experiments were performed with the
SYBR Premix Ex Taq™ Real-Time PCR kit (Takara Bio, Inc.). qPCR was
performed by activating the DNA polymerase at 95°C for 5 min,
followed by 40 cycles of two-step PCR (at 95°C for 10 sec and 60°C
for 30 sec) and a final extension at 75°C for 10 min, held at 4°C.
DNase and RNase-free water were used as the templates of negative
control experiments. All primers were obtained from Genewiz
(Suzhou, China) and are listed in Table I. GAPDH was used as an internal
control. The formula 2−ΔΔCq (13) was implemented to analyze and
quantify the gene expression.
 | Table ISequences of primers used in
polymerase chain reaction. |
Table I
Sequences of primers used in
polymerase chain reaction.
| Gene | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| Catalase | Forward:
GGTGCGGACATTCTACACAAAG | |
| Reverse:
TGTTCTCACACAGGCGTTTCC | 252 |
| MnSOD | Forward:
ACCTGAGCCCTAATGGTGGTGGAGA | |
| Reverse:
ATTGAAACCGAGCCAGCCCCACCCA | 149 |
| GAPDH | Forward:
CCATCTTCCAGGAGCGAGAT | |
| Reverse:
TGCTGATGATCTTGAGGCTG | 222 |
Flow cytometry
Flow cytometry was applied to detect ROS levels
using ROS assay kits purchased from BD Biosciences (BD Pharmingen;
San Jose, CA, USA). Briefly, cells were seeded in 6-well plates
(1×106 cells/well), washed with PBS at 4°C and
resuspended to a concentration of 4×105 cells/ml. Next,
10 µmol/l of the fluorescent probe
2′,7′-dichlorodihydro-fluorescein diacetate was added and incubated
for 20 min. A flow cytometer (BD Biosciences) was applied to
analyze the ROS levels, at an excitation wavelength of 488 nm and
an emission wavelength of 525 nm.
Statistical analysis
All the experimental data are presented as the mean
± standard deviation. Statistical analyses were conducted with SPSS
software, version 20 (IBM, Corp., Armonk, NY, USA). One-way
analysis of variance, followed by Turkey's multiple comparison, was
applied to analyze differences among the experimental groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
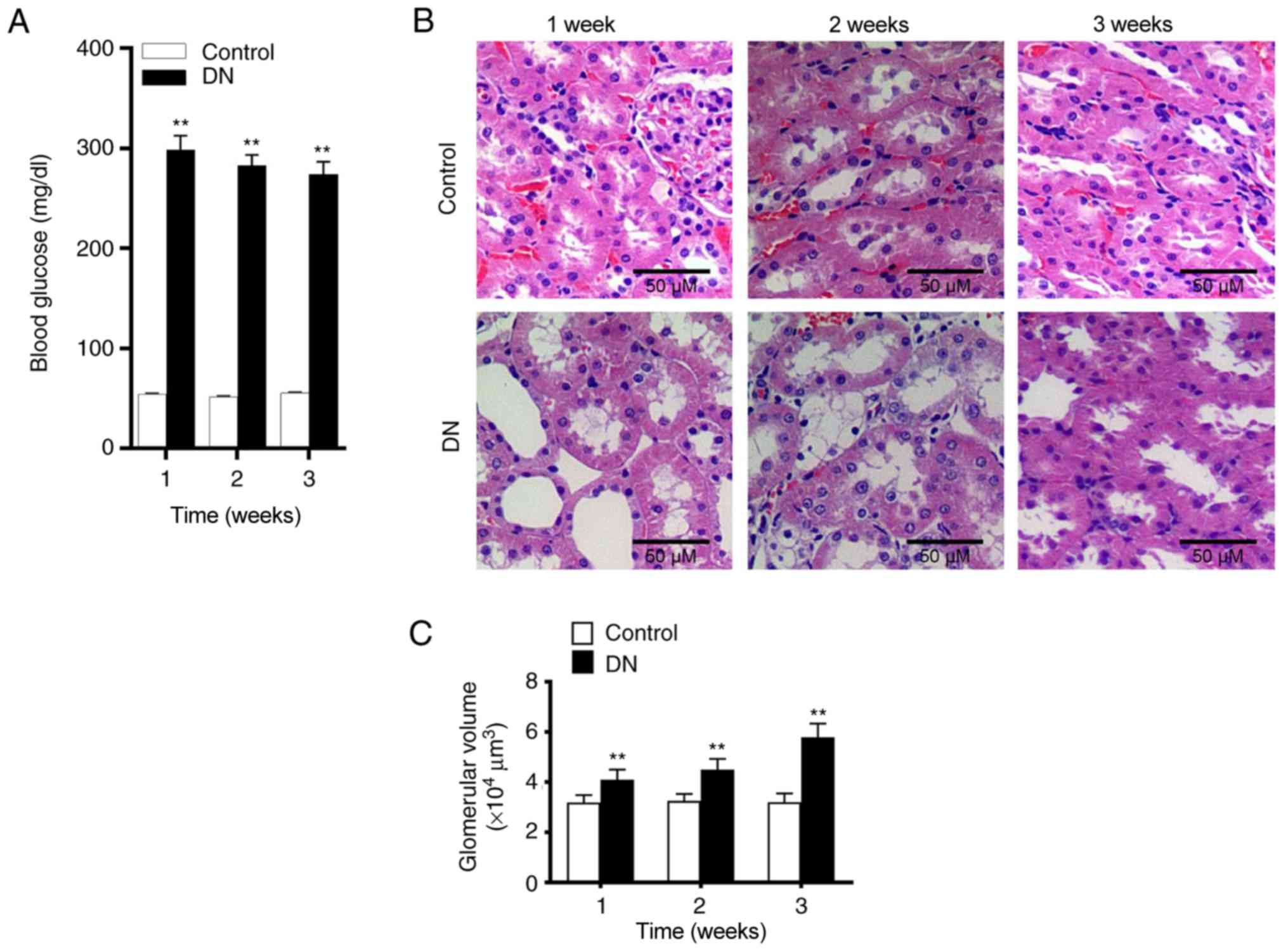
Changes on renal tissue and blood glucose
in DN group
Alterations in rat kidney tissue at 1, 2 and 3 weeks
after streptozotocin injection were subsequently assessed using
H&E staining. In the control group, glomerular and tubular
tissues were observed to be normal. However, in the DN group,
evident necrosis of the kidneys and widening of the mesangial area
were observed. Cystic edema also appeared in the kidney tubular
epithelial cells (Fig. 1B).
Furthermore, significantly increased glomerular volume (Fig. 1C) and vacuolar degeneration were
also observed in DN rats. At 24, 48 and 72 h after streptozotocin
administration, the blood glucose level of rats was significantly
higher compared with that of the control group. Furthermore, the
level of blood glucose in the DN group were >250 mg/dl (Fig. 1A), indicating that the diabetic
rat model was established successfully. As shown in Table II, BUN and SCr levels were
significantly increased in the DN group compared with the control.
These results demonstrated that kidney damage occurred in rats of
the DN group.
 | Table IIChanges in BUN and SCr in rat DN
model. |
Table II
Changes in BUN and SCr in rat DN
model.
| Time | Parameter | Control | DN | P-value |
|---|
| 1 week | BUN (mmol/l) | 7.83±0.73 | 14.96±0.94 | <0.001 |
| SCr
(µmol/l) | 37.44±1.15 | 46.85±1.42 | <0.001 |
| 2 weeks | BUN (mmol/l) | 8.04±0.79 | 18.56±1.67 | <0.001 |
| SCr
(µmol/l) | 38.17±1.72 | 55.79±1.78 | <0.001 |
| 3 weeks | BUN (mmol/l) | 8.21±0.81 | 21.83±1.59 | <0.001 |
| SCr
(µmol/l) | 39.08±2.73 | 62.85±1.94 | <0.001 |
Expression levels of TXNIP, NLRP3 and
inflammatory factors in kidney tissue of DN rats
Western blot analysis was performed to detect the
levels of TXNIP and NLRP3 inflammasome proteins, as well as of a
number of inflammatory factors. The results demonstrated that the
levels of TXNIP, NLRP3, cleaved-caspase-1, IL-1 and IL-18 in the DN
group were significantly higher compared with those in the control
group (P<0.05; Fig. 2A and B).
This indicated that the high-glucose level promoted TXNIP
expression and NLRP3 inflammasome activation. Furthermore, SP
staining results revealed that the expression levels of TXNIP and
NLRP3 in the kidney tissue of DN rats markedly increased (Fig. 2C and D), indicating that the high
glucose promoted TXNIP and NLRP3 protein expression levels.
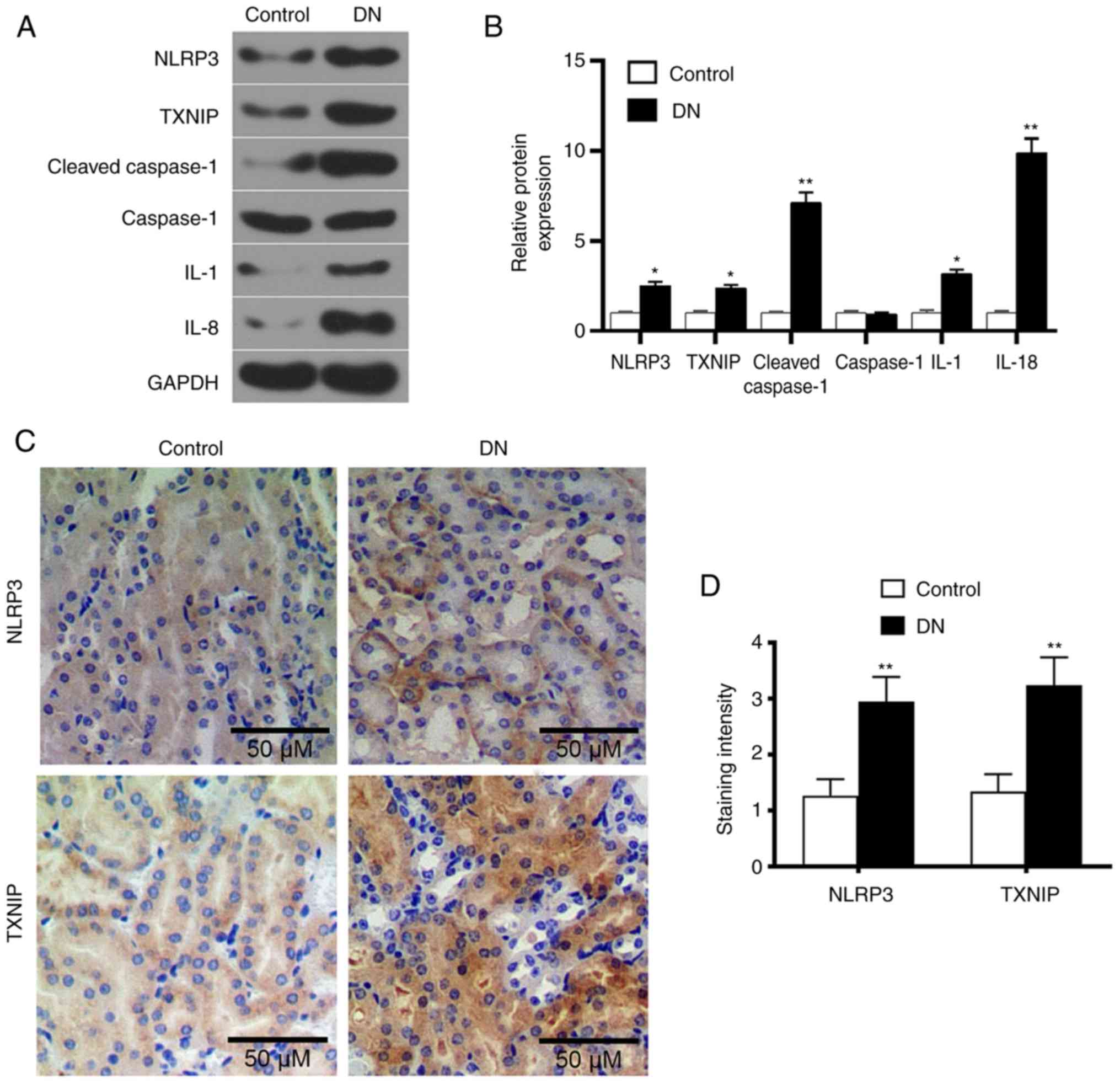 | Figure 2Expression levels of TXNIP and NLRP3
inflammasome-associated proteins in renal tissue of DN rats. (A)
Western blot images and (B) quantified protein levels of TXNIP,
NLRP3, cleaved-caspase-1, caspase-1, IL-1 and IL-18 in the control
and DN groups. (C) Streptavidin-peroxidase staining was used to
detect the TXNIP and NLRP3 proteins expression in kidneys tissues
(magnification, ×100), and (D) the quantified staining intensity is
shown. *P<0.05 and **P<0.01, vs.
control group. DN, diabetic nephropathy; TXNIP, thioredoxin
interacting protein; NLRP3, NOD-like receptor protein 3; IL,
interleukin. |
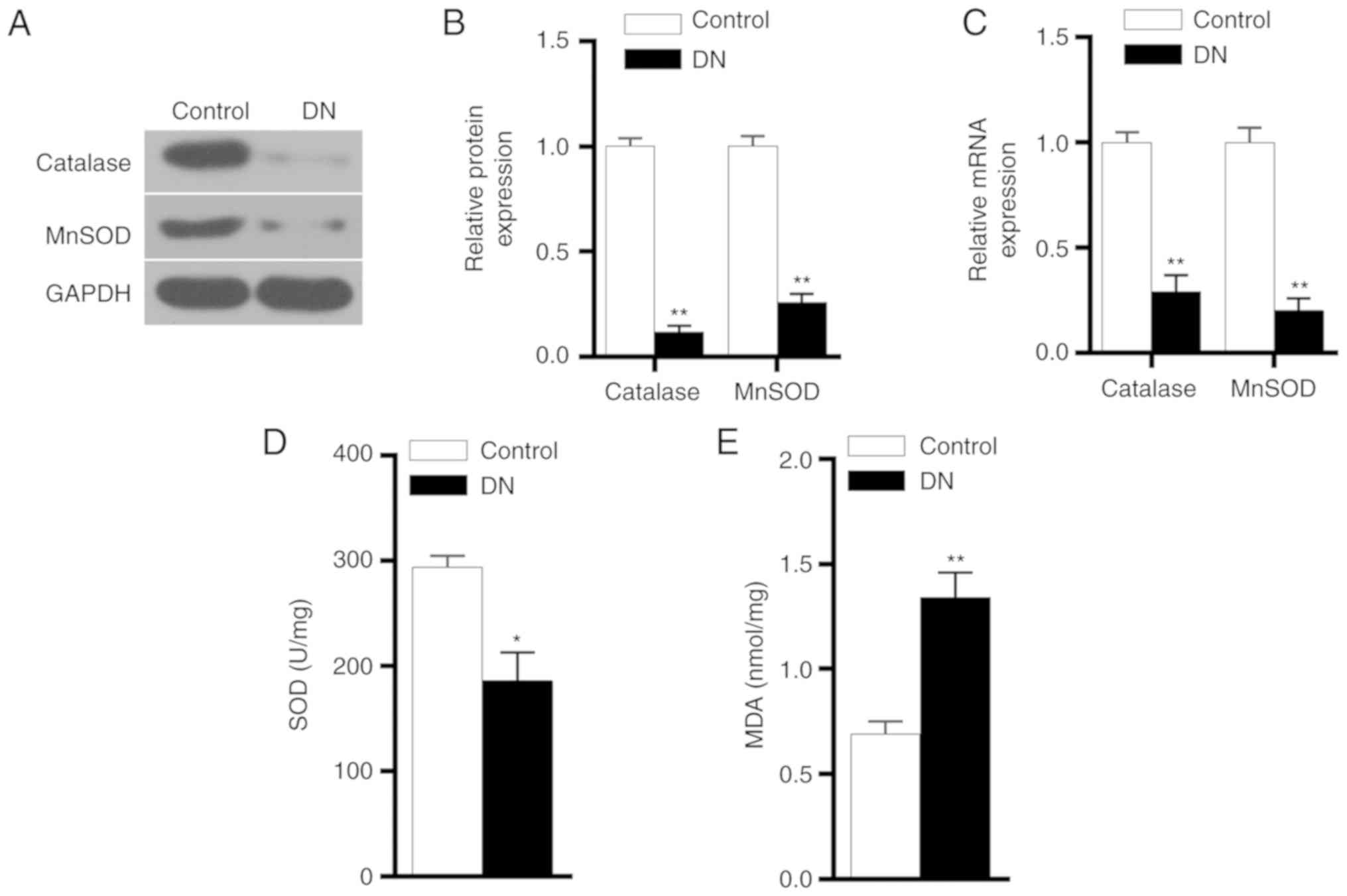
Expression levels of antioxidant genes,
MDA and SOD in DN group
The protein and mRNA expression levels of catalase
and MnSOD were detected by western blot analysis and RT-qPCR,
respectively. The experimental results demonstrated that catalase
and MnSOD protein and mRNA expression levels in the DN group were
significantly lower in comparison with those in the control group
(P<0.01; Fig. 3A-C). ELISA
results also revealed that the SOD expression level was
significantly reduced in the DN group, while MDA level was markedly
increased (Fig. 3D and E). These
results indicated that the antioxidant capacity of kidneys in DN
rats was reduced.
Effect of different glucose
concentrations on HK-2 cells
Western blot analysis was performed to detect the
expression levels of TXNIP, NLRP3, cleaved-caspase-1 and caspase-1
proteins in HK-2 cells in the control, HG1, HG2 and HG3 groups. The
results demonstrated that as the concentration of glucose in the
medium increased, the levels of TXNIP, NLRP3 and cleaved-caspase-1
proteins were gradually increased, while the level of caspase-1
protein decreased (Fig. 4A and
B). The ELISA results also revealed that the levels of IL-1 and
IL-18 secretion increased as the concentration of glucose was
raised in the medium (Fig. 4C and
D). This indicated that glucose promoted the expression of
TXNIP and the activation of NLRP3 inflammasome, as well as enhanced
the inflammatory response, in a dose-dependent manner.
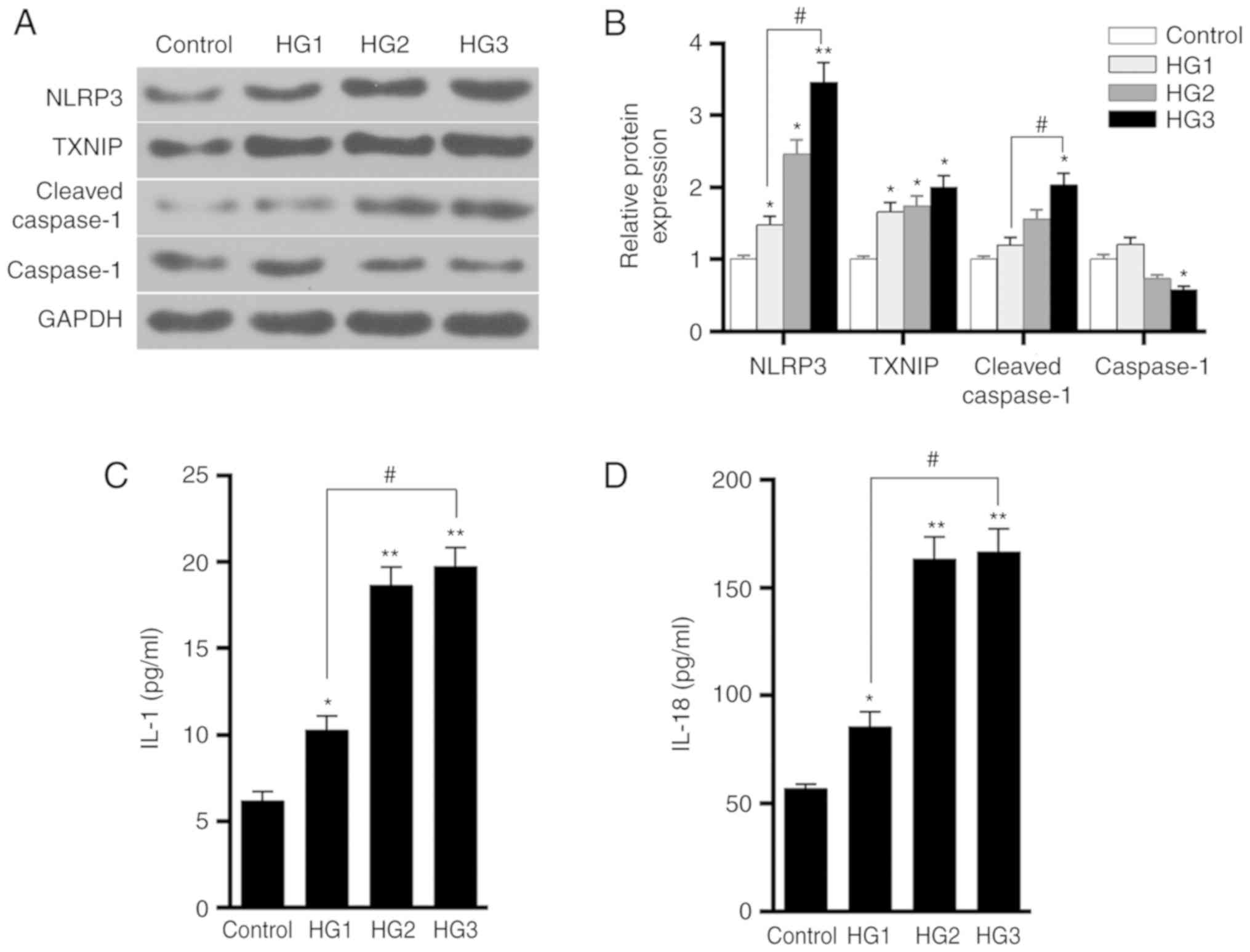 | Figure 4Effects of different concentrations
of glucose on TXNIP expression and the activation of NLRP3
inflammasome in HK-2 cells. (A) Western blots and (B) quantified
expression levels of TXNIP, NLRP3, cleaved-caspase-1 and caspase-1
proteins in the control, HG1, HG2 and HG3 groups, which were
treated with 5.5, 15, 30 and 50 mmol/l glucose, respectively. (C)
IL-1 and (D) IL-18 expression levels in the four groups were tested
using ELISA. *P<0.05 and **P<0.01, vs.
control group; #P<0.05. TXNIP, thioredoxin
interacting protein; NLRP3, NOD-like receptor protein 3; IL,
interleukin. |
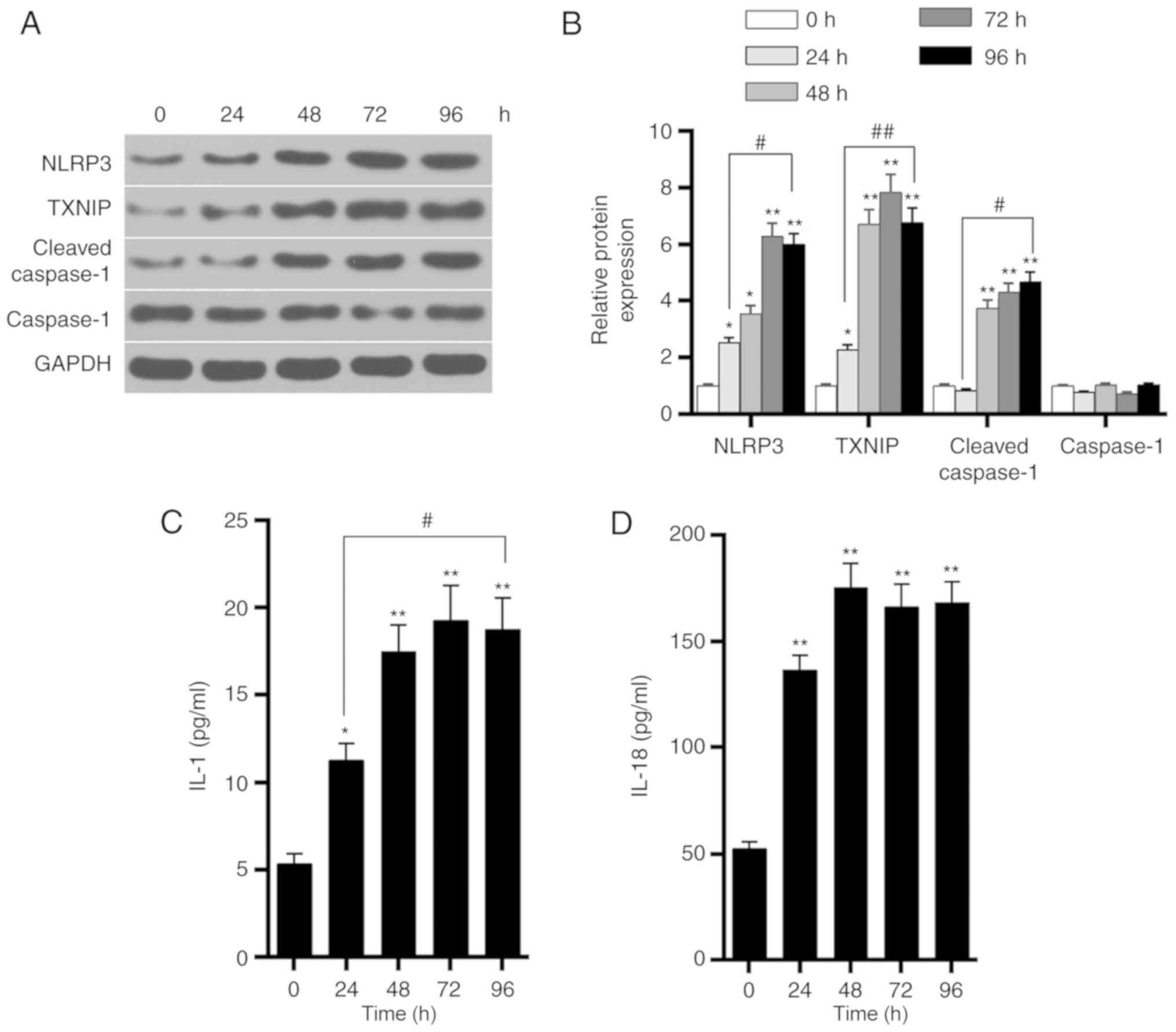
Effects of the high-glucose on HK-2 cells
at different time points
Western blot analysis was further performed to
detect the expression levels of TXNIP, NLRP3, cleaved-caspase-1 and
caspase-1 proteins in HK-2 cells in the HG3 group at 0, 24, 48, 72
and 96 h. The results demonstrated that, as time progressed, the
levels of TXNIP, NLRP3 and cleaved-caspase-1 protein were gradually
increased, reaching a peak value at ~72 h (Fig. 5A and B). By contrast, caspase-1
protein levels were not markedly altered. The ELISA results also
demonstrated that the levels of IL-1 and IL-18 secretion
significantly increased as time progressed (Fig. 5C and D). These findings indicated
that glucose upregulated the expression of TXNIP in a
time-dependent manner, promoted the activation of NLRP3
inflammasome and enhanced the inflammatory response.
Effects of TXNIP gene silencing on HK-2
cells under a high-glucose environment
Western blot analysis was applied to detect the
protein expression levels of TXNIP, NLRP3 inflammasome, and
inflammation-associated factors in the control, HG, NC + HG,
siTXNIP and siTXNIP + HG groups. The results revealed that the
TXNIP, NLRP3 and cleaved-caspase-1 protein levels were upregulated
in the HG and NC + HG groups compared with the control group
(P<0.01). By contrast, the protein levels of TXNIP and
cleaved-caspase-1 were significantly lower in the siTXNIP + HG
group in comparison with those in the NC + HG groups (P<0.05).
No significant differences were detected in caspase-1 protein
expression among the different groups (Fig. 6A and B). Furthermore, the
concentrations of IL-1 and IL-18 in each group were detected using
ELISA. IL-1 and IL-18 expression levels in HG and NC + HG groups
were higher compared with those in control group (P<0.01).
However, IL-1 and IL-18 in the siTXNIP + HG group were
significantly lower than those in the NC + HG groups (P<0.05;
Fig. 6C and D). These
observations suggested that silencing TXNIP gene inhibited the
expression levels of IL-1 and IL-18.
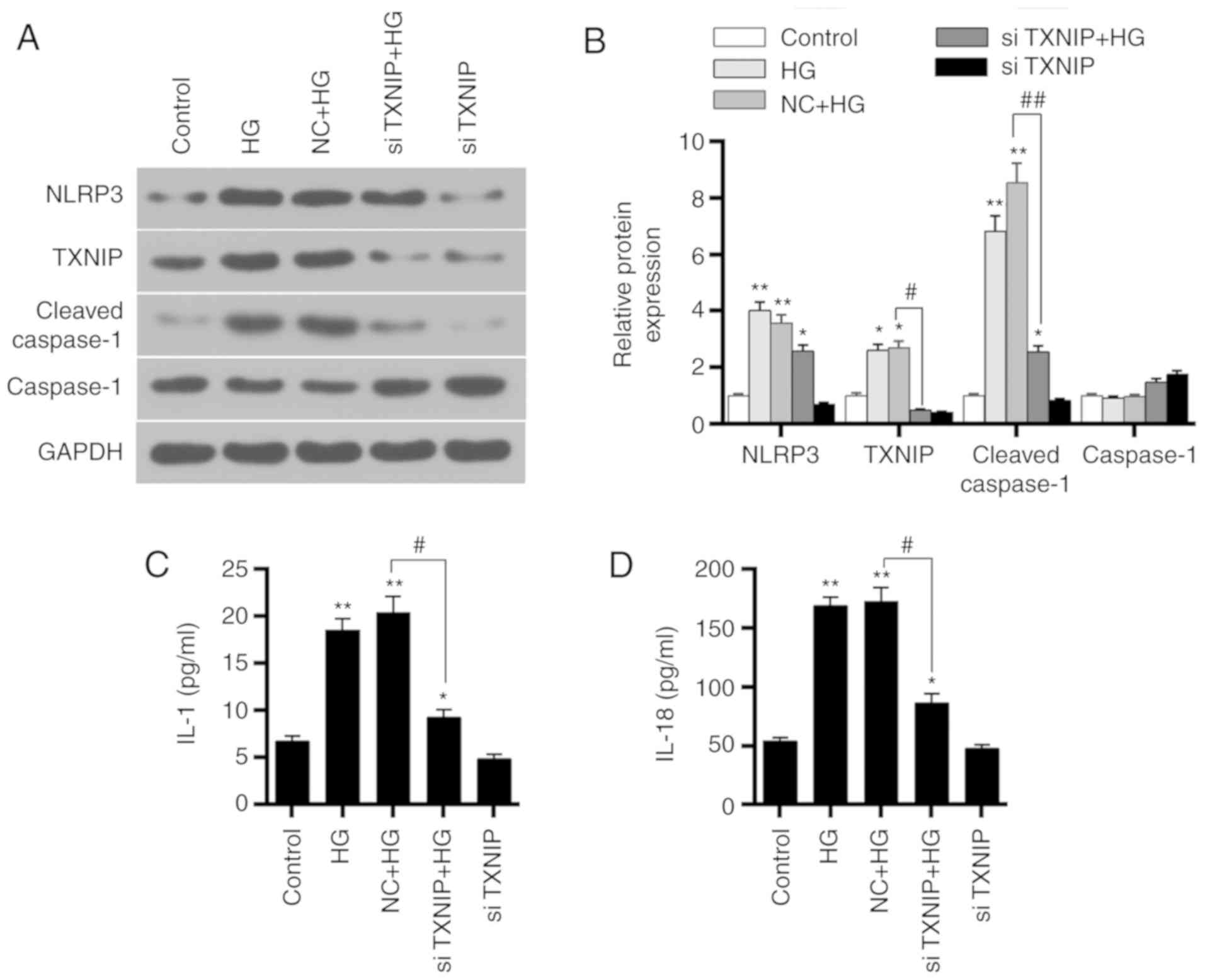 | Figure 6Effects of TXNIP gene silencing on
NLRP3 inflammasome activation-associated proteins and
inflammation-associated factors. (A) Western blots and (B)
quantified expression levels of TXNIP, NLRP3, cleaved-caspase-1 and
caspase-1 proteins in the control, HG, NC + HG, siTXNIP and siTXNIP
+ HG groups. (C) IL-1 and (D) IL-18 expression levels in each group
were detected by ELISA. *P<0.05 and
**P<0.01, vs. control group; #P<0.05
and ##P<0.01. TXNIP, thioredoxin interacting protein;
NLRP3, NOD-like receptor protein 3; IL, interleukin; HG, high
glucose; NC, negative control siRNA; si-, small interfering
RNA. |
Effects of TXNIP gene silencing on
oxidation of HK-2 cells under high-glucose conditions
The expression levels of catalase and MnSOD protein
and mRNA in the control, HG, NC + HG, siTXNIP and siTXNIP + HG
groups were detected by western blot analysis and RT-qPCR,
respectively. The results revealed that the protein and mRNA
expression levels of catalase and MnSOD significantly decreased in
the HG and NC + HG groups compared with the control (P<0.01).
Subsequent to silencing of the TXNIP gene in cells cultured under
high glucose, the protein and mRNA expression levels of catalase
and MnSOD were significantly higher in comparison with those in the
NC + HG group (P<0.05; Fig.
7A-C). This indicated that the high-glucose environment
inhibited the expression levels of antioxidant genes, while
silencing of the TXNIP gene promoted the expression of antioxidant
genes.
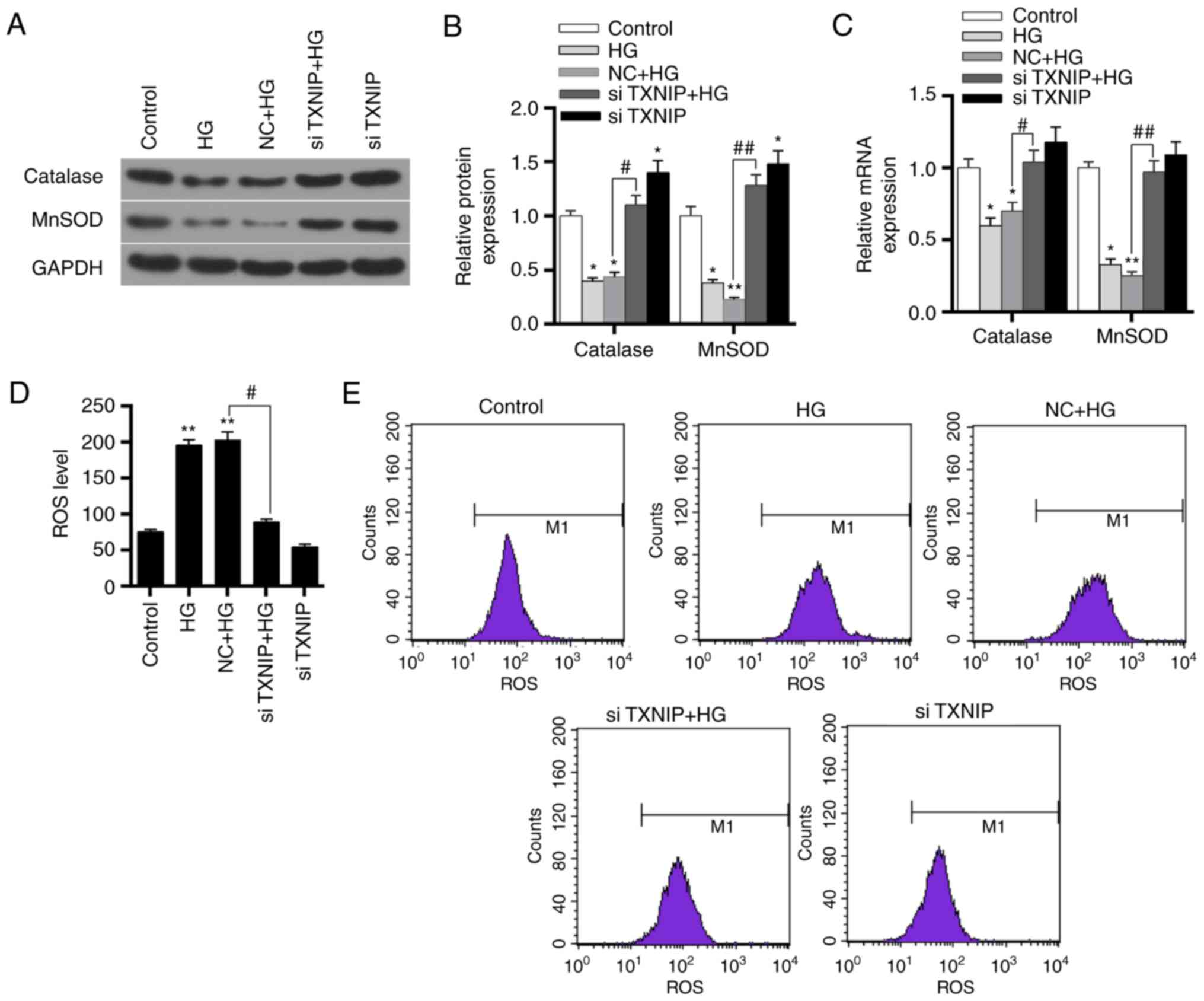 | Figure 7Effects of TXNIP gene silencing on
antioxidant gene expression and ROS levels in HK-2 cells. (A)
Western blots and (B) quantified protein expression levels of
catalase and MnSOD in the control, HG, NC + HG, siTXNIP + HG and
siTXNIP groups, detected using western blot analysis. (C) mRNA
expression levels of catalase and MnSOD in the different groups,
detected using reverse transcription-quantitative polymerase chain
reaction. (D) ROS levels in each group and (E) flow cytometry
applied to detect these levels. *P<0.05 and
**P<0.01, vs. control group; #P<0.05
and ##P<0.01. TXNIP, thioredoxin interacting protein;
ROS, reactive oxygen species; HG, high glucose; NC, negative
control siRNA; si-, small interfering RNA. |
Flow cytometry was subsequently applied to detect
the expression level of ROS in each group. The results revealed
that the ROS level increased in the HG and NC + HG groups, when
compared with the control group (P<0.01). However, the ROS level
in the siTXNIP + HG group was significantly lower in comparison
with that in the NC + HG group (P<0.05; Fig. 7D and E). These results suggested
that the high-glucose environment promoted the production of ROS,
while TXNIP gene silencing was able to markedly reduce the ROS
levels induced by high glucose.
Discussion
Kidney injury is one of the most common
complications of diabetes (14).
Currently, the mechanism of DN is not yet completely clear, and
research has focused on the pathogenesis and treatment of DN in
recent years. Streptozotocin is known to degenerate islet β-cells
and causes diabetes (15), and
streptozotocin-induced diabetic animal models are widely used in
studies conducted on human diabetes (16). The results of the present study
revealed that, following streptozotocin injection, the blood
glucose levels of rats were >250 mg/dl, while H&E staining
also demonstrated kidney damage in the DN group. This suggested
that the streptozotocin-induced diabetic kidney injury model was
successfully constructed and could be used for further
experiments.
Inflammation is an important factor in the
development of diabetes (17),
and extensive inflammatory lesions are the pathological basis of DN
(18,19). Studies have reported that
overexpression of IL-18 increased neutrophil infiltration and
worsened kidney damage (20,21). NLRP3 inflammasome can activate
caspase-1 through ASC, and caspase-1 then further cleaves and
activates IL-1 and IL-18 (22,23). At present, it is known that NLRP3
inflammasome activation participates in the process of DN (4,24).
However, the mechanism underlying the activation of NLRP3
inflammasome in DN remains unclear. ROS generation is considered as
a necessary condition for the activation of NLRP3 inflammasome
(25). TXNIP can regulate renal
tubular and mitochondrial autophagy in DN via the mammalian target
of rapamycin signaling pathway and increase oxidative stress levels
(26).
Therefore, the present study conducted SP staining
and western blot analysis to detect the expression characteristics
of TXNIP and NLRP3 in DN rats. The results demonstrated that TXNIP
and NLRP3 were overexpressed in the kidneys of DN rats, while the
levels of IL-1 and IL-18, which are downstream of NLRP3
inflammasome, were also significantly increased. This is consistent
with previous research results (27), indicating that the occurrence and
development of DN is associated with the overexpression of TXNIP
and activation of NLRP3 inflammasome. To further explore the action
mechanism of TXNIP on the activation of NLRP3 inflammasomes, the
levels of antioxidant genes, SOD and MDA in the kidney of DN rats
were examined. The results revealed that catalase and MnSOD protein
and mRNA expression levels were significantly reduced. In addition,
SOD expression levels were downregulated, whereas MDA levels were
upregulated. Previous studies have demonstrated that NLRP3 is able
to directly act on kidney tubular epithelial cells involved in
kidney injury during renal ischemia-reperfusion (28), and no evident protective effect
was observed on the kidney when mouse NLRP3 gene was knocked out
(29). Another study reported
that elevated blood glucose caused increased levels of oxidative
stress in the body (30). These
observations suggested that the activation of NLRP3 inflammasomes
was associated with the level of oxidative stress.
To investigate the possible activation mechanism of
the NLRP3 inflammasome, the HK-2 cell line was used to mimic the
high-glucose environment in vitro and explore the effect of
the high-glucose concentration on HK-2 cells. The results indicated
that the high-glucose environment was able to upregulate the
expression of TXNIP and the activation of NLRP3 inflammasomes in a
dose-dependent and time-dependent manner, respectively, while it
also promoted the secretion of IL-1 and IL-18 in HK-2 cells. A
previous study has reported that a high-glucose concentration and
lipopolysaccharides significantly induced the mRNA and protein
levels of TXNIP, NLRP3, procaspase-1 and IL-1β in mesangial cells
(31). Another study has also
identified that the high-glucose conditions also caused human
retinal microvascular endothelial cell damage via the TXNIP/NLRP3
pathway (32). This suggested
that the high-glucose environment may act directly on human kidney
tubular epithelial cells and activate NLRP3 inflammasomes, leading
to inflammatory damage, which may be associated with their ability
to promote TXNIP expression levels.
To further study the role of TXNIP gene in DN and
the mechanism of regulating NLRP3 inflammasome, RNA interference
technology was applied to silence TXNIP gene, and the effect of
TXNIP gene silencing on NLRP3 inflammasome in the high-glucose
environment was investigated. The results revealed that TXNIP gene
expression was upregulated in the high-glucose environment and that
NLRP3 inflammasome was overactivated. Interference with siTXNIP
plasmids inhibited the effect of high-glucose on TXNIP, as well as
reduced the activation of NLRP3 inflammasome and the secretion of
downstream IL-1 and IL-18. This suggests that TXNIP silencing
inhibited NLRP3 inflammasome activation-associated proteins.
However, the action mechanism of TXNIP in DN remained unclear.
To further investigate the mechanism through which
TXNIP activates the NLRP3 inflammasome, the effects of TXNIP gene
silencing on the antioxidant genes and ROS were investigated in
HK-2 cells under a the high-glucose condition. The results
demonstrated that the high-glucose environment inhibited the
expression of antioxidant genes catalase and MnSOD in HK-2 cells,
and increased ROS levels. Following the silencing of TXNIP
expression, catalase and MnSOD protein and mRNA levels were
significantly increased and ROS production was inhibited. It has
previously been suggested that TXNIP is the most important
endogenous inhibitor of thioredoxin and constitutes an important
part of the oxygen reduction system in the body (33). A recent study has identified that
ROS was able to activate the NLRP3/IL-1β pathway by upregulating
the level of TXNIP, therefore aggravating the inflammatory response
(34). Zhou et al
(35) observed that ROS induced
TXNIP to directly bind to NLRP3 and activated NLRP3 inflammasome;
however, related research has mainly focused on metabolic diseases
and glomerular diseases. A previous study has reported that TXNIP
upregulated oxidative stress by inhibiting TRX, which leads to an
increase at ROS levels (36).
Another study suggested that, under high-glucose conditions,
downregulation of TXNIP levels inhibited ROS production via the p38
mitogen activated protein kinase and extracellular signal-regulated
kinase pathways (36). The
results of Li et al (37)
demonstrated that overexpression of TXNIP was able to inhibit liver
cancer cell proliferation and induce apoptosis by triggering
mitochondria-mediated ROS production. Combined with the results of
the present study, it is suggested that ROS may interact with TXNIP
in DN. In diabetic rats, high-glucose levels caused TXNIP gene
overexpression and increased ROS levels by inhibiting the
expression of antioxidant genes. Under the action of ROS, TXNIP
further activated the NLRP3 inflammasome and caused kidney damage.
TXINP silencing upregulated the expression of antioxidant genes,
while it reduced not only the level of oxidative stress in the body
and the expression of NLRP3, but also the activation of NLRP3
inflammasomes and the damage of the inflammatory response to the
kidney (38-40).
In conclusion, the findings of the present study
suggest that a high-glucose environment may promote the expression
of TXNIP, increasing the body's oxidative stress level and inducing
DN by activating the NLRP3 inflammasome. TXNIP gene silencing
promoted the expression of antioxidant factors and decreased ROS
levels, reducing the inflammatory levels by inhibiting NLRP3
inflammasome activation. This suggested that the occurrence and
development of DN was closely associated with the activation of
NLRP3 inflammasome caused by TXNIP, and thus TXNIP may provide a
new approach for the treatment of DN.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CG and HW made substantial contributions to the
study conception and design. HW and HD were responsible for data
acquisition, data analysis and interpretation. HD drafted and
critically revised the manuscript for important intellectual
content. SL and HW agreed to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of the work are appropriately investigated and resolved.
All authors approved the final version of the manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Institutional
Animal Care and Use Committee of the First Hospital of Jilin
University, and the China Council on Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
Acknowledgments
Not applicable.
References
|
1
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duran-Salgado MB and Rubio-Guerra AF:
Diabetic nephropathy and inflammation. World J Diabetes. 5:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beisswenger PJ, Drummond KS, Nelson RG,
Howell SK, Szwergold BS and Mauer M: Susceptibility to diabetic
nephropathy is related to dicarbonyl and oxidative stress.
Diabetes. 54:3274–3281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shahzad K, Bock F, Dong W, Wang H, Kopf S,
Kohli S, Al-Dabet MM, Ranjan S, Wolter J, Wacker C, et al:
Nlrp3-inflammasome activation in non-myeloid-derived cells
aggravates diabetic nephropathy. Kidney Int. 87:74–84. 2015.
View Article : Google Scholar :
|
|
5
|
Sun B, Wang X, Ji Z, Li R and Xia T: NLRP3
inflammasome activation induced by engineered nanomaterials. Small.
9:1595–1607. 2013. View Article : Google Scholar
|
|
6
|
Abderrazak A, Syrovets T, Couchie D, El
Hadri K, Friguet B, Simmet T and Rouis M: NLRP3 inflammasome: From
a danger signal sensor to a regulatory node of oxidative stress and
inflammatory diseases. Redox Biol. 4:296–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Granata S, Masola V, Zoratti E, Scupoli
MT, Baruzzi A, Messa M, Sallustio F, Gesualdo L, Lupo A and Zaza G:
NLRP3 inflammasome activation in dialyzed chronic kidney disease
patients. PLoS One. 10:e01222722015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutton HL, Ooi JD, Holdsworth SR and
Kitching AR: The NLRP3 inflammasome in kidney disease and
autoimmunity. Nephrology (Carlton). 21:736–744. 2016. View Article : Google Scholar
|
|
9
|
Ogata FT, Batista WL, Sartori A, Gesteira
TF, Masutani H, Arai RJ, Yodoi J, Stern A and Monteiro HP:
Nitrosative/oxidative stress conditions regulate
thioredoxin-interacting protein (TXNIP) expression and
thioredoxin-1 (TRX-1) nuclear localization. PLoS One. 8:e845882013.
View Article : Google Scholar :
|
|
10
|
Mohamed IN, Hafez SS, Fairaq A, Ergul A,
Imig JD and El-Remessy AB: Thioredoxin-interacting protein is
required for endothelial NLRP3 inflammasome activation and cell
death in a rat model of high-fat diet. Diabetologia. 57:413–423.
2014. View Article : Google Scholar :
|
|
11
|
Sonne SB, Perrett RM, Nielsen JE, Baxter
MA, Kristensen DM, Leffers H, Hanley NA and Rajpert-De-Meyts E:
Analysis of SOX2 expression in developing human testis and germ
cell neoplasia. Int J Dev Biol. 54:755–760. 2010. View Article : Google Scholar
|
|
12
|
Lim J, Goriely A, Turner GD, Ewen KA,
Jacobsen GK, Graem N, Wilkie AO and Rajpert-De Meyts E: OCT2, SSX
and SAGE1 reveal the phenotypic heterogeneity of spermatocytic
seminoma reflecting distinct subpopulations of spermatogonia. J
Pathol. 224:473–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Heneghan HM, Cetin D, Navaneethan SD,
Orzech N, Brethauer SA and Schauer PR: Effects of bariatric surgery
on diabetic nephropathy after 5 years of follow-up. Surg Obes Relat
Dis. 9:7–14. 2013. View Article : Google Scholar
|
|
15
|
Lenzen S: The mechanisms of alloxan- and
streptozotocin-induced diabetes. Diabetologia. 51:216–226. 2008.
View Article : Google Scholar
|
|
16
|
Elias D, Prigozin H, Polak N, Rapoport M,
Lohse AW and Cohen IR: Autoimmune diabetes induced by the beta-cell
toxin STZ. Immunity to the 60-kDa heat shock protein and to
insulin. Diabetes. 43:992–998. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanley AJ, Festa A, D'Agostino RB Jr,
Wagenknecht LE, Savage PJ, Tracy RP, Saad MF and Haffner SM:
Metabolic and inflammation variable clusters and prediction of type
2 diabetes: Factor analysis using directly measured insulin
sensitivity. Diabetes. 53:1773–1781. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wada J and Makino H: Inflammation and the
pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152.
2013. View Article : Google Scholar
|
|
19
|
Elsherbiny NM, Al-Gayyar MM, Abd El and
Galil KH: Nephroprotective role of dipyridamole in diabetic
nephropathy: Effect on inflammation and apoptosis. Life Sci.
143:8–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonul Y, Kazandi S, Kocak A, Ahsen A, Bal
A, Karavelioglu A, Hazman O, Turamanlar O, Kokulu S and Yuksel S:
Interleukin-18 binding protein pretreatment attenuates kidney
injury induced by hepatic ischemia reperfusion. Am J Med Sci.
352:200–207. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhen J, Zhang L, Pan J, Ma S, Yu X, Li X,
Chen S and Du W: AIM2 mediates inflammation-associated renal damage
in hepatitis B virus-associated glomerulonephritis by regulating
caspase-1, IL-1beta, and IL-18. Mediators Inflamm. 2014:1908602014.
View Article : Google Scholar
|
|
22
|
Lin YC, Huang DY, Wang JS, Lin YL, Hsieh
SL, Huang KC and Lin W: Syk is involved in NLRP3
inflammasome-mediated caspase-1 activation through adaptor ASC
phosphorylation and enhanced oligomerization. J Leukoc Biol.
97:825–835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karmakar M, Katsnelson M, Malak HA, Greene
NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR and
Pearlman E: Neutrophil IL-1β processing induced by pneumolysin is
mediated by the NLRP3/ASC inflammasome and caspase-1 activation and
is dependent on K+ efflux. J Immunol. 194:1763–1775. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen K, Zhang J, Zhang W, Zhang J, Yang J,
Li K and He Y: ATP-P2X4 signaling mediates NLRP3 inflammasome
activation: A novel pathway of diabetic nephropathy. Int J Biochem
Cell Biol. 45:932–943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauernfeind F, Bartok E, Rieger A, Franchi
L, Nunez G and Hornung V: Cutting edge: Reactive oxygen species
inhibitors block priming, but not activation, of the NLRP3
inflammasome. J Immunol. 187:613–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang C, Zhang Y, Kelly DJ, Tan CY, Gill
A, Cheng D, Braet F, Park JS, Sue CM, Pollock CA and Chen XM:
Thioredoxin interacting protein (TXNIP) regulates tubular autophagy
and mitophagy in diabetic nephropathy through the mTOR signaling
pathway. Sci Rep. 6:291962016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samra YA, Said HS, Elsherbiny NM, Liou GI,
El-Shishtawy MM and Eissa LA: Cepharanthine and piperine ameliorate
diabetic nephropathy in rats: Role of NF-kappaB and NLRP3
inflammasome. Life Sci. 157:187–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shigeoka AA, Mueller JL, Kambo A, Mathison
JC, King AJ, Hall WF, Correia Jda S, Ulevitch RJ, Hoffman HM and
McKay DB: An inflammasome-independent role for epithelial-expressed
Nlrp3 in renal ischemia-reperfusion injury. J Immunol.
185:6277–6285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HJ, Lee DW, Ravichandran K, O Keys D,
Akcay A, Nguyen Q, He Z, Jani A, Ljubanovic D and Edelstein CL:
NLRP3 inflammasome knockout mice are protected against ischemic but
not cisplatin-induced acute kidney injury. J Pharmacol Exp Ther.
346:465–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang W, Zhao S, Li Y, Peng G and Han P:
Acute blood glucose fluctuation induces myocardial apoptosis
through oxidative stress and nuclear factor-kB activation.
Cardiology. 124:11–17. 2013. View Article : Google Scholar
|
|
31
|
Feng H, Gu J, Gou F, Huang W, Gao C, Chen
G, Long Y, Zhou X, Yang M, Liu S, et al: High glucose and
lipopolysaccharide prime NLRP3 inflammasome via ROS/TXNIP pathway
in mesangial cells. J Diabetes Res. 2016:69731752016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C,
Lu L, Xu X, Guan H, Zheng Z and Qiu Q: Activation of the
TXNIP/NLRP3 inflammasome pathway contributes to inflammation in
diabetic retinopathy: A novel inhibitory effect of minocycline.
Inflamm Res. 66:157–166. 2017. View Article : Google Scholar
|
|
33
|
Devi TS, Hosoya K, Terasaki T and Singh
LP: Critical role of TXNIP in oxidative stress, DNA damage and
retinal pericyte apoptosis under high glucose: Implications for
diabetic retinopathy. Exp Cell Res. 319:1001–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han Y, Xu X, Tang C, Gao P, Chen X, Xiong
X, Yang M, Yang S, Zhu X, Yuan S, et al: Reactive oxygen species
promote tubular injury in diabetic nephropathy: The role of the
mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol.
16:32–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflam-masome activation. Nat Immunol. 11:136–140. 2010.
View Article : Google Scholar
|
|
36
|
Li W, Wu Z, Ma Q, Liu J, Xu Q, Han L, Duan
W, Lv Y, Wang F, Reindl KM and Wu E: Hyperglycemia regulates
TXNIP/TRX/ROS axis via p38 MAPK and ERK pathways in pancreatic
cancer. Curr Cancer Drug Targets. 14:348–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li J, Yue Z, Xiong W, Sun P, You K and
Wang J: TXNIP over-expression suppresses proliferation and induces
apoptosis in SMMC7221 cells through ROS generation and MAPK pathway
activation. Oncol Rep. 37:3369–3376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang A, Ko K and Clark MR: The emerging
role of the inflammasome in kidney diseases. Curr Opin Nephrol
Hypertens. 23:204–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shirasuna K, Takano H, Seno K, Ohtsu A,
Karasawa T, Takahashi M, Ohkuchi A, Suzuki H, Matsubara S, Iwata H
and Kuwayama T: Palmitic acid induces interleukin-1beta secretion
via NLRP3 inflammasomes and inflammatory responses through ROS
production in human placental cells. J Reprod Immunol. 116:104–112.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xia M, Abais JM, Koka S, Meng N, Gehr TW,
Boini KM and Li PL: Characterization and activation of NLRP3
inflammasomes in the renal medulla in mice. Kidney Blood Press Res.
41:208–221. 2016. View Article : Google Scholar : PubMed/NCBI
|