Introduction
Extrinsic aging is caused by environmental oxidative
factors that can be primarily attributed to exposure to ultraviolet
(UV) radiation (1). A variety of environmental stresses including
UV light can damage sun-exposed areas of the skin including the
face and neck, and accelerate wrinkle formation (2), which is a
cumulative process that depends on the frequency, duration, and
intensity of UV exposure and the degree of natural protection
offered by skin pigmentation (3). In addition to wrinkling, skin
aging manifests as solar elastosis and pigment irregularities
(4).
UVB radiation alters the thickness of the stratum
corneum of the epidermis and consequently, the permeability
barrier, leading to increased transepidermal water loss (TEWL) (5).
Several factors control skin moisturization and elasticity,
including the content of hyaluronan (HA), an extracellular matrix
(ECM) component and transforming growth factor (TGF)-β, in addition
to regulators of the expression of ECM macromolecules (6). Collagen
has been used as a functional ingredient in skin products based on
its efficacy in moisturizing and enhancing elasticity. Furthermore,
involucrin and filaggrin are major proteins that serve an important
role in the formation of the epidermal skin barrier (7). Changes to
the dermal ECM during photoaging involve the abnormal production of
matrix proteins by dermal fibroblasts and increased the activity of
matrix-degrading enzymes such as matrix metalloproteinases (MMPs)
by resident skin cells and infiltrating inflammatory cells (8).
Also involved is decreased procollagen synthesis, resulting in the
loss of skin elasticity, which leads to wrinkle formation (9).
Reactive oxygen species (ROS) produced in the skin
upon UV irradiation cause oxidative damage to the skin (10) by
mediating impaired cellular and ECM functions (11,12). UVB
radiation induces cellular damage, leading to activation of
ROS-sensitive signaling pathways including the mitogen-activated
protein kinase (MAPK) pathway (13), which mediates inflammatory
cytokine production (14). Accordingly, antioxidants can protect the
skin from UVB radiation-induced skin damage (15).
Probiotics have been widely used to regulate
intestinal health and treat medical conditions including allergic
diseases and atopic dermatitis, as well as to prevent dental caries
and respiratory infections (16). The most common microorganisms
used as probiotic bacteria, which include Lactobacillus,
Bifidobacterium and Streptococcus, mainly affect
health and improve gastrointestinal tract homeostasis in the host
by modulating the balance in the gut environment (17). Tyndallized
probiotics, which are processed in their culture media, contain
bacterial walls; moreover, the products released during their cell
death have been demonstrated to positively affect human health and
maintain their immunological effect on the gut barrier (18). It has
been reported that probiotic bacteria may be highly effective in
protecting the skin from photoaging, as oral supplementation prior
to UVB exposure was demonstrated to prevent TEWL, increase
epidermal thickness, and alleviate damage to tight junction
structures and the basement membrane in a mouse model (19,20). The
probiotic gram-positive bacterium Lactobacillus acidophilus
inhabits the intestines and serves an important role in the
maintenance of gut health; however, it is not known whether it can
protect against photoaging induced by UV radiation.
This was addressed in the present study using HaCaT
keratinocytes treated with Lactobacillus acidophilus IDCC
3302 tyndallizate (ACT 3302) prior to UVB exposure.
Lactobacillus acidophilus IDCC 3302 (GenBank accession
number KP325412.1) was originally isolated from the feces of a
Korean breast-fed infant after obtaining oral consent from the
parents. The materials used in the present experiments were not
directly separated from human feces, but were commercially
available materials produced by Il-dong Pharmaceutical. The results
of the present study indicated that ACT 3302 treatment can prevent
photodamage to the skin by inhibiting MMP activation through the
modulation of MAPK signaling.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The CellTiter AQueous One
Solution Cell Proliferation Assay kit and
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-
sulfophenyl)-2H- tetrazolium (MTS) were purchased from Promega
Corporation (Madison, WI, USA). Human HA and MMP-1, MMP-2 and MMP-9
ELISA kits were obtained from R&D Systems, Inc., (Minneapolis,
MN, USA). Antibodies targeting MAPK kinase (MEK), phosphorylated
(p)MEK, extracellular signal-regulated kinase 1 and 2 (ERK1/2),
pERK1/2, p38, pp38, c-Jun N-terminal kinase (JNK), pJNK,
procollagen, and β-actin were purchased from Cell Signaling
Technology, Inc., (Danvers, MA, USA). Secondary antibodies were
purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA,
USA).
Tyndallization process
Bacterial cells were anaerobically cultured in yeast
extract-based medium at 37°C overnight and then centrifuged at
12,500 × g for 1 h at room temperature using a continuous filter
(Alfa Laval, Lund, Sweden). Thereafter, the pellets and the
supernatant were concentrated 5 times using a vacuum concentrator
(Dong Yang Machine Industry, Seoul, Korea) under reduced pressure
at 80°C and then mixed with dry-sterilized cornstarch powder. The
cornstarch was not effective on wrinkle reduction. The mix was then
frozen at −45°C and lyophilized to obtain ACT 3302 powder.
Total bacterial cell numbers
ACT 3302 cell samples were diluted
103-fold in PBS and cell particles in 25 small squares
were counted using a Neubauer counting chamber (Paul Marienfeld
GmbH & Co., KG, Lauda-Königshofen, Germany), with
2.5×10−4-mm3 squares, at a magnification of
×400. The number of cells was calculated as follows: Bacterial cell
number per ml=average number of counted cells per square ×4,000
squares per mm3×103 mm3 per
mlx103 (dilution).
Cell culture and UVB irradiation
HaCaT cells (cell lines service, 300493)-an
immortalized, non-tumorigenic human keratinocyte cell line were
maintained in DMEM supplemented with 10% FBS and 1% antibiotics
(Penicillin-Streptomycin; Gibco; Thermo Fisher Scientific, Inc.) at
37°C in a humidified incubator with 5% CO2. Cells were
seeded (1×104), allowed to adhere for 24 h and treated
with various concentrations (1×105, 1×106,
1×107 and 1×108) of ACT 3302 prior to UVB
irradiation at a dose of 20 mJ/cm2. Normal cells
received no ACT 3302 and were not exposed to UVB radiation. Vehicle
cells received no ACT 3302, but were exposed to UVB radiation.
Cell viability assay
HaCaT cells (1×104) were seeded in
96-well culture plates and exposed to 20 mJ/cm2 UVB in
the presence or absence of ACT 3302 for 24 h. The cell culture
medium was replaced with PBS prior to UVB treatment and cell
viability was assessed immediately following this by incubating
cells with MTS (Promega Corporation) containing serum free medium
for 1 h. MTS containing serum free medium was measured according to
the manufacturer’s protocol; specifically, sample absorbance was
measured at 490 nm using a microplate fluorimeter (Molecular
Devices, LLC, Sunnyvale, CA, USA).
Determination of HA, MMP-1, MMP-2 and
MMP-9 secretion by ELISA
HA (cat. no. DHYAL0), MMP-1 (cat. no. DMP100), MMP-2
(cat. no. MMP200) and MMP-9 (cat. no. DMP900) levels in HaCaT cell
culture supernatant (5×104), following UVB irradiation,
were determined using ELISA kits according to the manufacturer’s
protocol. Briefly, the cells were seeded in 96-well plates and
treated with ACT 3302. After cells were treated with ACT 3302 for
24 h, the medium was removed and then replaced with new medium for
ELISA analysis. After UVB irradiation, the culture supernatant was
collected and centrifuged at 18,928 × g for 5 min at 4°C and HA,
MMP-1, MMP-2, and MMP-9 levels were quantified
colorimetrically.
Antioxidant enzyme activities
Total protein was extracted from HaCaT cells, which
were homogenized in cold lysis buffer, superoxide dismutase (SOD)
and catalase (CAT) activities were measured using colorimetric
assay kits (Cayman Chemical, Ann Arbor, MI, USA) according to the
manufacturer’s protocol. SOD and CAT activities were determined by
measuring the absorbance at 450 and 540 nm, respectively, using a
plate reader (Molecular Devices, LLC).
RNA extraction and Taqman multiplex PCR
assay
Total RNA was extracted from UVB-irradiated HaCaT
cells, using TRIzol reagent according to the manufacturer’s
protocol (Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was
performed using TaqMan assays (Applied Biosystems; Thermo Fisher
Scientific, Inc.) specific for involucrin, filaggrin, TGF-β,
interleukin (IL)-1β, IL-8, and tumor necrosis factor (TNF)-α
(involucrin; Hs00846307_s1, filaggrin; Hs00856927_g1, TGF-β;
Hs00998133_m1, IL-1β; Hs00174097_m1, IL-8; Hs00174103_ m1 and
TNF-α; Hs01113624_g1) with a QuantStudio 6 Flex Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 95°C for 10 min, 40
cycles of denaturation at 95°C for 15 sec, followed by annealing
and extension at 60°C for 1 min. Each sample was assayed in
triplicate and relative mRNA expression levels were calculated
using the ΔΔCq method (21) and normalized to that of β-actin in
each sample.
Western blotting
To extract total protein, HaCaT cells were
homogenized and lysed in RIPA assay buffer containing a protease
inhibitor (ATTO Corporation, Tokyo, Japan). Protein (15 µg)
was separated by SDS-PAGE on a 10% polyacrylamide gel and then
transferred to a polyvinylidene difluoride membrane, which was then
incubated overnight at 4°C with the following primary antibodies
(all, 1:1,000): Procollagen (cat. no. 84336), pERK (cat. no. 9101),
ERK (cat. no. 9102), pMEK (cat. no. 9154), MEK (cat. no. 9126),
pp38 (cat. no. 9215), p38 (cat. no. 9212), pJNK (cat. no. 9251),
JNK (cat. no. 9252), and β-actin (cat. no. 4970). The membrane was
washed three times for 10 min each with PBS containing 0.1% tween
20, which was followed by incubation for 2 h at room temperature
with appropriate horseradish peroxidase conjugated anti-rabbit
secondary antibodies (1:10,000; cat. no. 7074; Cell signaling
Technology, Inc.). Protein bands were visualized using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc.) and analyzed
using ImageQuant LAS 4000 (GE Healthcare).
Statistics
Measurements were performed in triplicate and all
data are presented as the mean ± standard error of the mean. The
significance of differences between groups was evaluated by
performing an analysis of variance with Tukey’s multiple
comparisons test with GraphPad Prism version 7.03 software
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
ACT 3302 increases cell viability and
protects against UVB-induced cell damage in keratinocytes
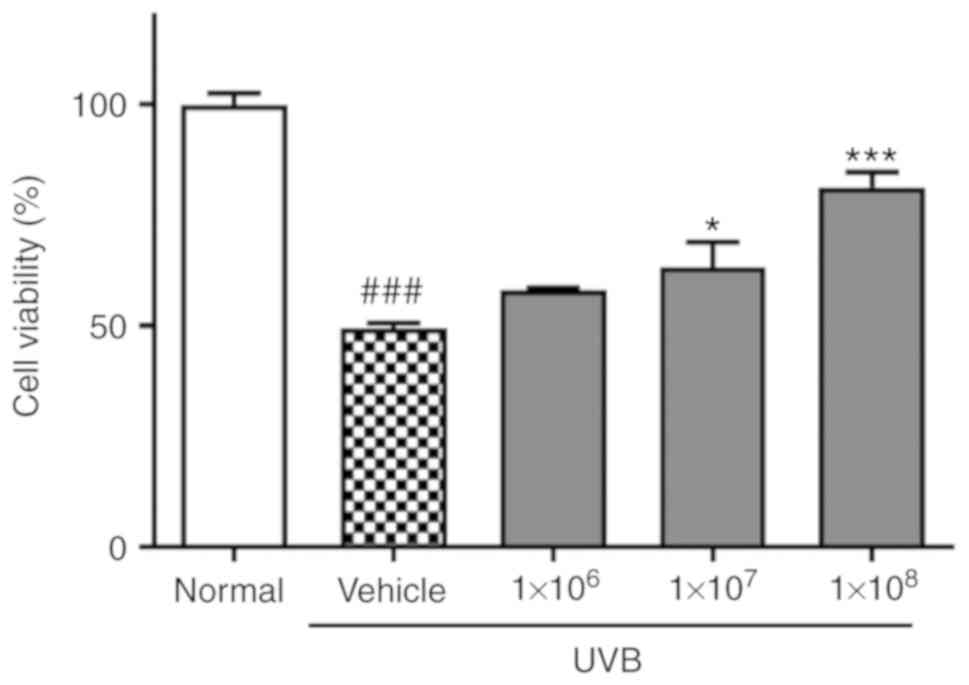
The effect of ACT 3302 on HaCaT cell proliferation
following exposure to UVB was investigated first. Cell viability
was reduced to 49.4% of normal levels by UVB irradiation. In cells
preincubated with 1×108 ACT 3302 cells, cell viability
was 81.4% of normal levels following irradiation in dose-dependent
manner and this difference was significant (P<0.001; Fig. 1).
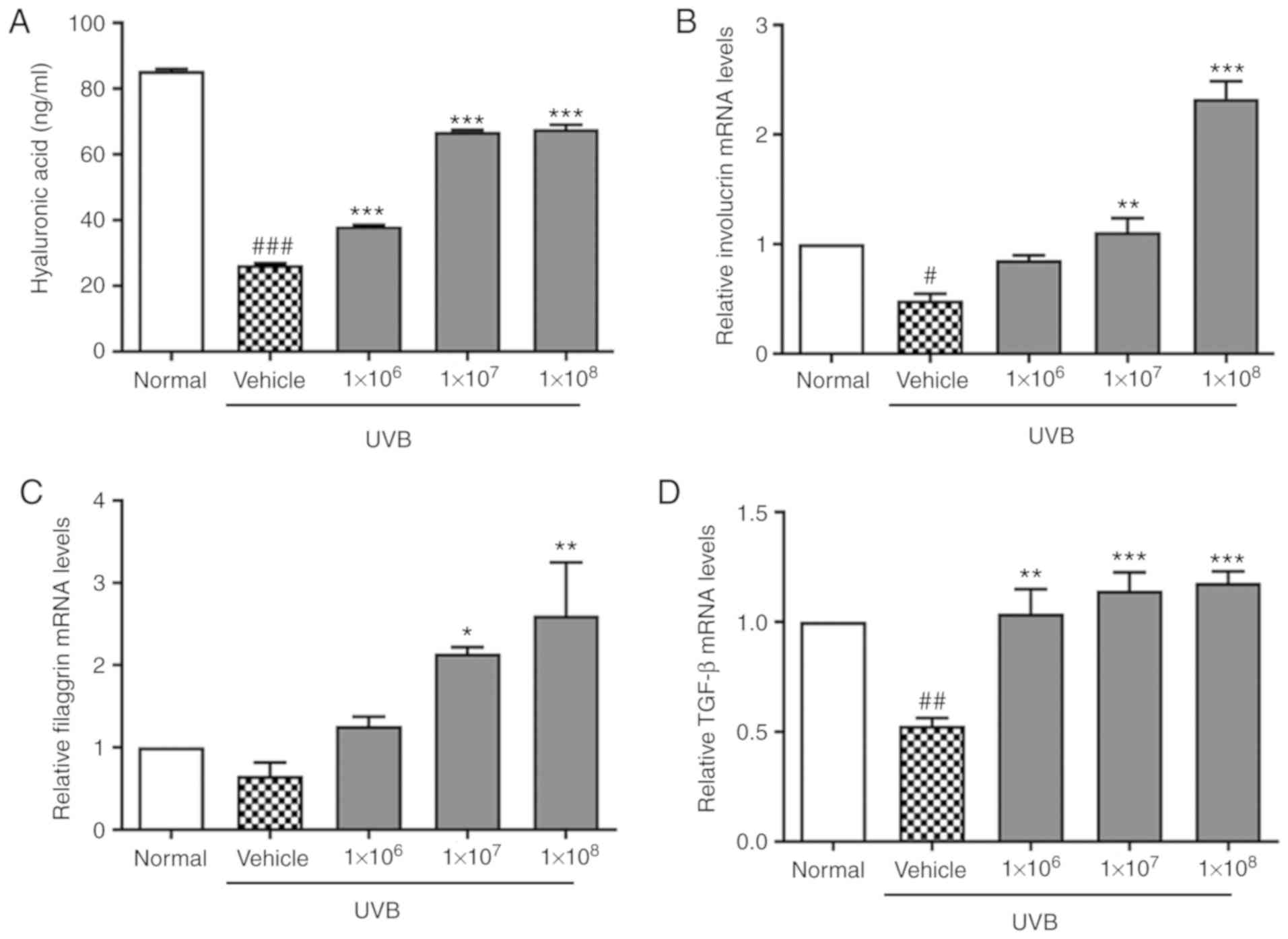
Effect of ACT 3302 on UVB-induced
secretion of skin hydration factors
The effect of ACT 3302 on UVB-induced secretion of
skin hydration factors, namely epidermal ECM components was also
determined. UVB irradiation decreased HA levels, as determined by
ELISA (Fig. 2A), indicating that
ECM breakdown induced by UVB radiation contributes to skin aging.
HA levels were increased significantly (P<0.001) in a dose
dependent manner. In addition, it was demonstrated that the
expression of involucrin, filaggrin, and TGF-β was decreased by
UVB. The mRNA expression of involucrin, filaggrin, and TGF-β in
UVB-induced cells treated with ACT 3302 increased in a
dose-dependent manner, as compared to that in UVB-exposed cells
(Fig. 2B-D).
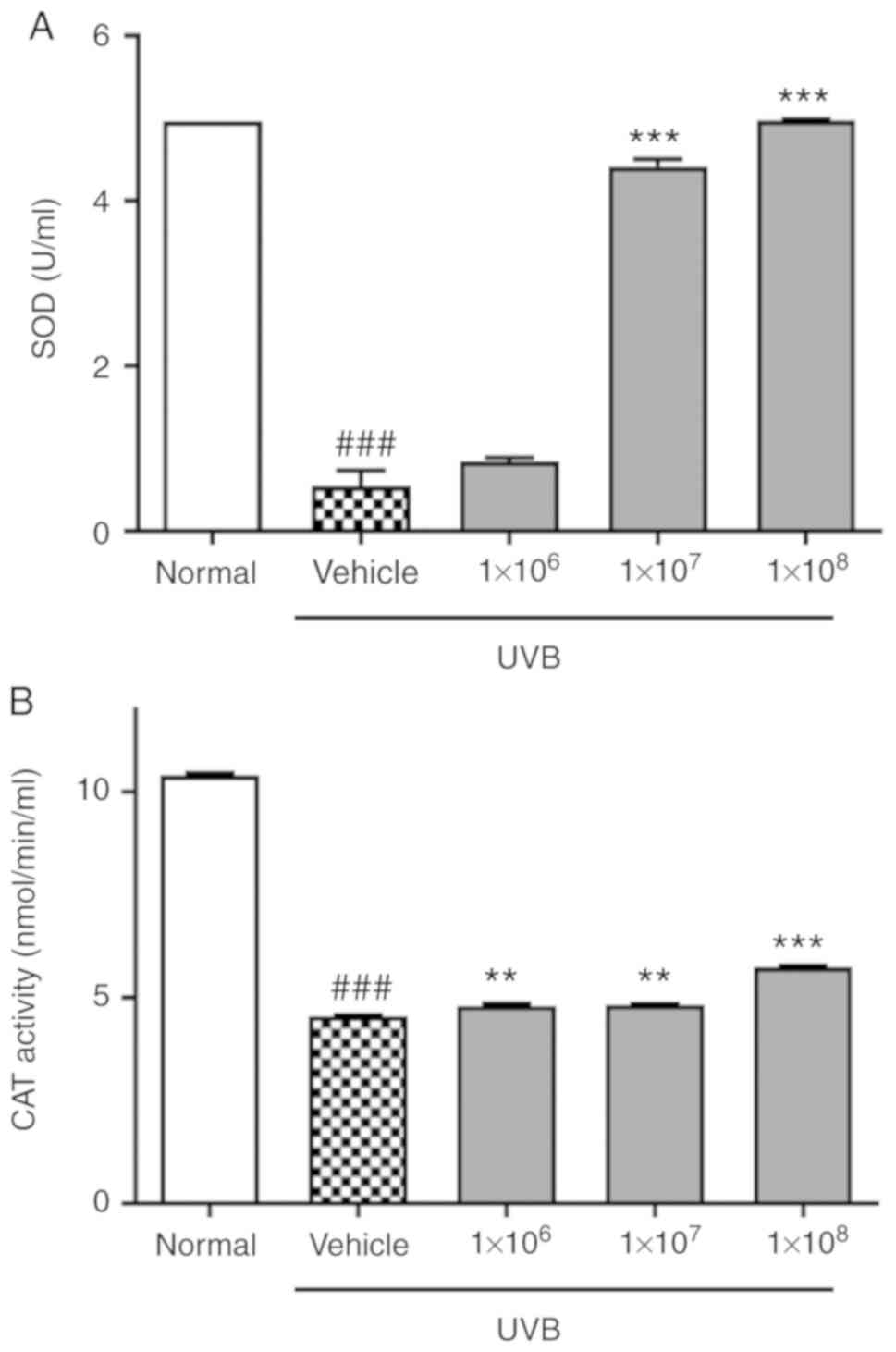
ACT 3302 protects HaCaT cells from
oxidative stress and suppresses inflammation in response to UVB
radiation
To determine whether ACT 3302 can promote radical
scavenging, the activities of antioxidant enzymes in HaCaT cells
exposed to UVB were examined. SOD activity in vehicle group was
reduced compared with the untreated normal group (0.53 vs. 4.96
U/ml; Fig. 3A). Also, CAT was
significantly reduced in UVB-induced cells compared with normal
cells (P<0.01; Fig. 3B).
However, SOD and CAT activity were enhanced by ACT 3302 but SOD was
saturated at 1×108 of ACT 3302. Therefore, ACT 3302
stimulates the activity of antioxidant enzymes that scavenge free
radicals and thereby prevents UVB-induced oxidative stress damage.
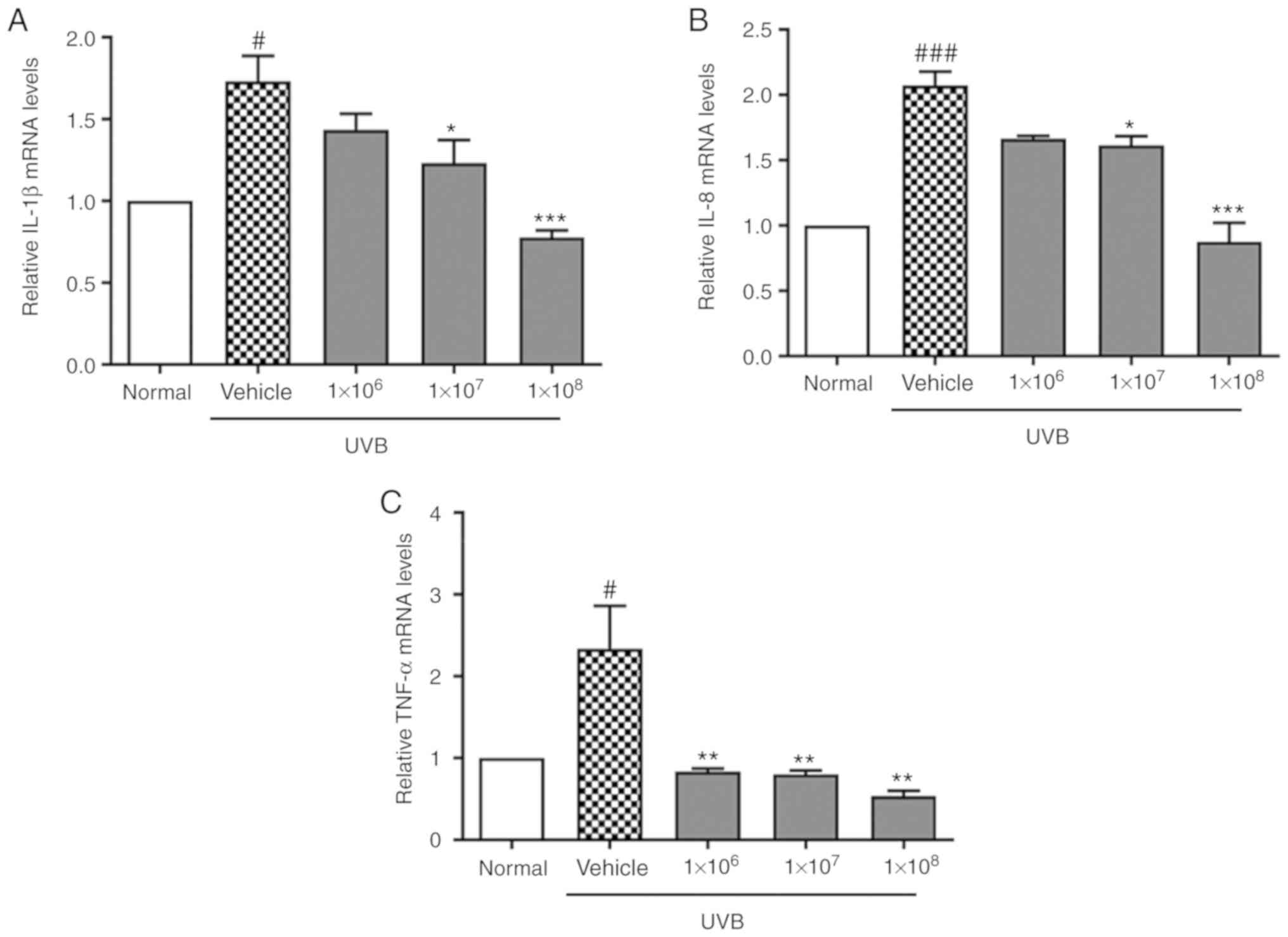
It was also demonstrated that mRNA levels of the pro-inflammatory
cytokines IL-1β, IL-8, and TNF-α were significantly upregulated in
keratinocyte cells upon UVB exposure (P<0.05), which was
reversed by ACT 3302 treatment (Fig.
4). This suggests that ACT 3302 exerts a protective effect by
reducing the inflammatory response to UVB irradiation.
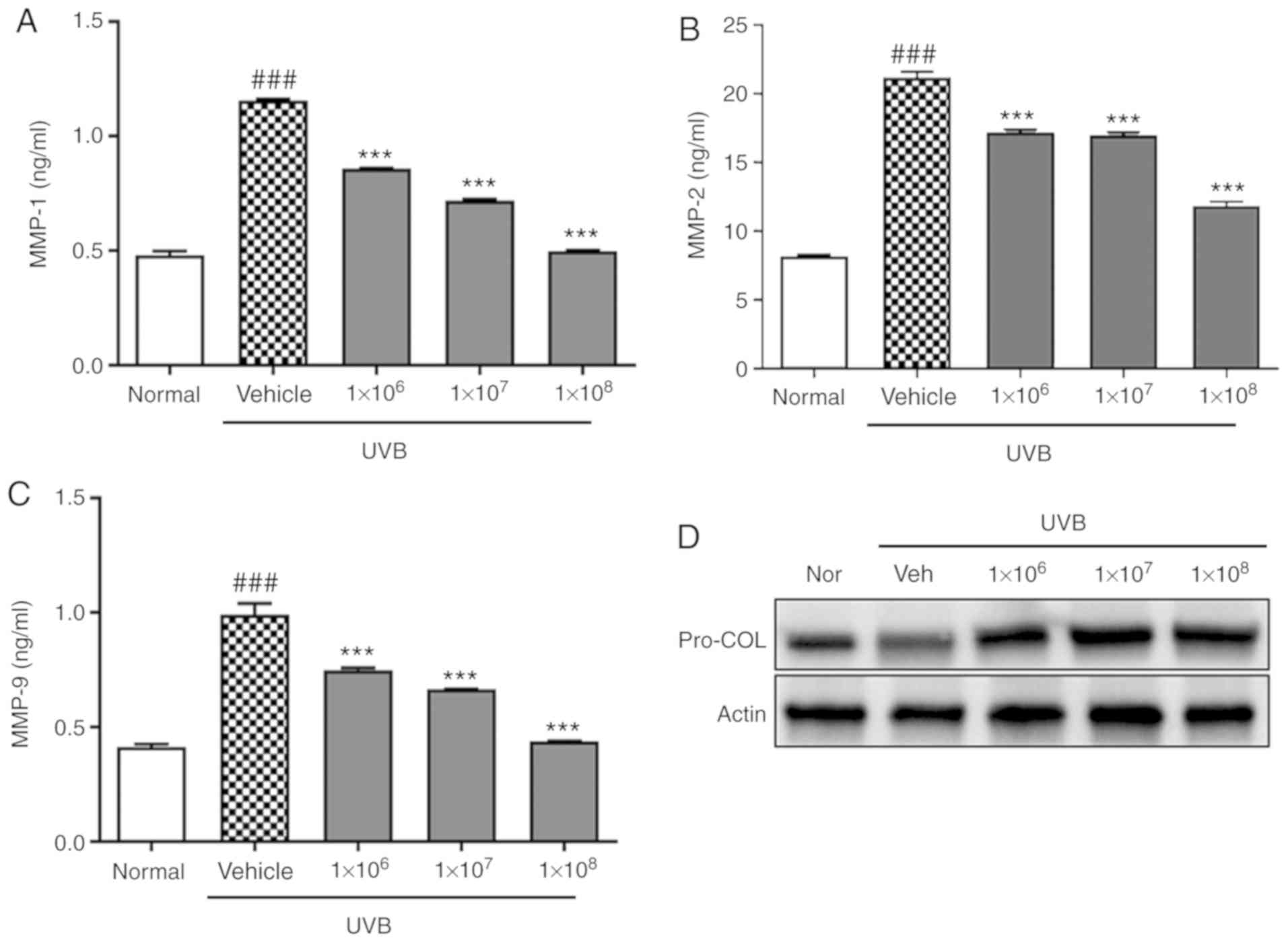
ACT 3302 prevents UVB-induced damage to
keratinocytes by inhibiting MAPK signaling
To clarify the mechanism through which ACT 3302
protects keratinocytes from UVB-induced damage, MMP levels in the
culture supernatant were detected by ELISA following UVB
irradiation. UVB irradiation significantly increased MMP-1, MMP-2,
and MMP-9 levels in HaCaT cells (P<0.001), but this effect was
significantly abolished by pre-treatment with ACT 3302 in a
dose-dependent manner (P<0.001; Fig. 5A-C). Also, ACT 3302 treatment
reversed this trend and increased procollagen phosphorylation
(Fig. 5D).
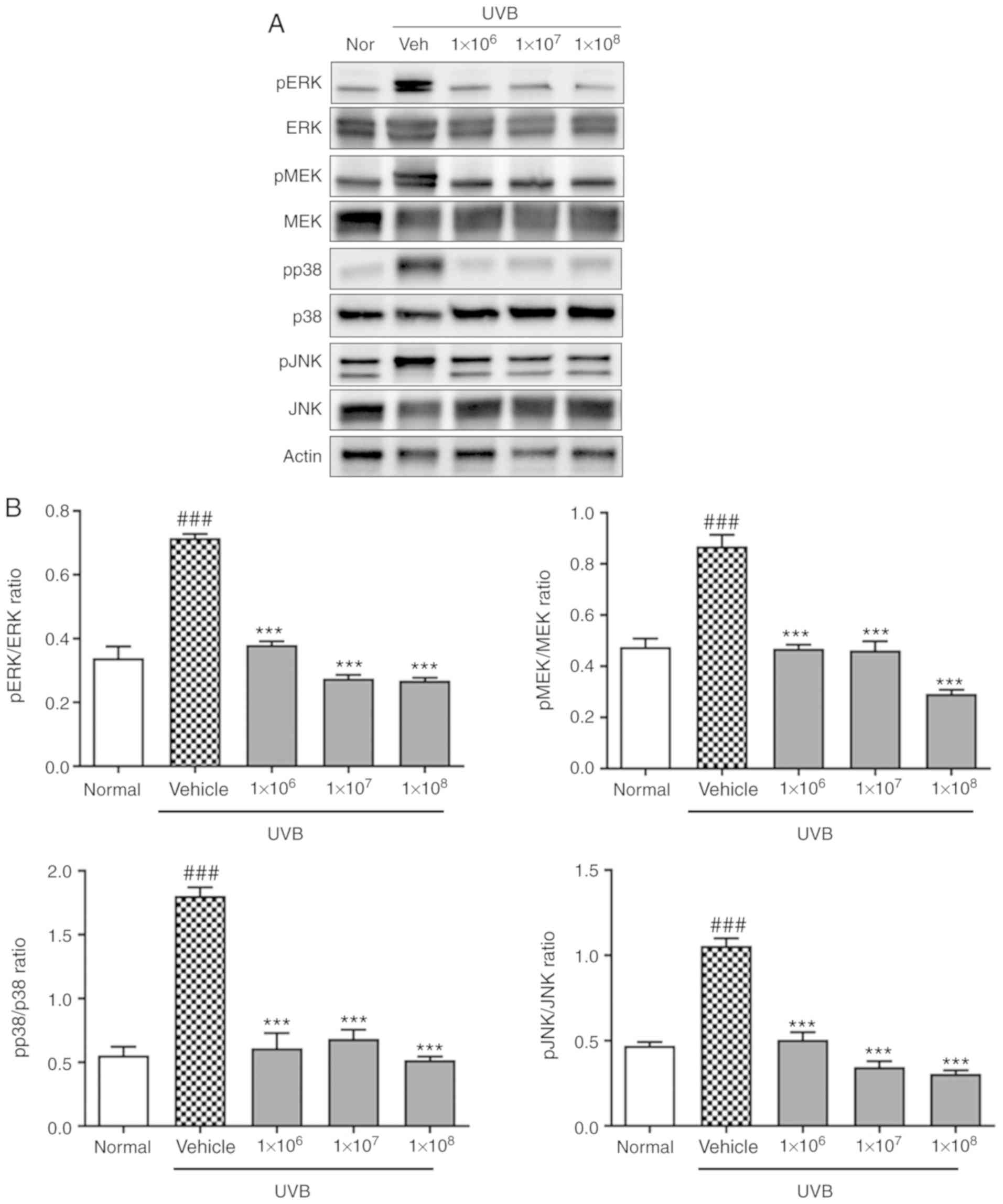
Additionally, the increase in p38, JNK, and MAPK/ERK
phosphorylation induced by UVB irradiation was significantly
decreased by ACT 3302 (P<0.001). These data indicate that ACT
3302 protects HaCaT cells from UVB-induced damage by inhibiting the
activity of ECM-degrading proteins, and this occurs through the
modulation of MAPK signaling (Fig.
6).
Discussion
The present study demonstrated the protective effect
of L. acidophilus IDCC 3302 tyndallizate on HaCaT
keratinocyte damage induced by UVB exposure. Tyndallizate was
analyzed for its nutrients including carbohydrate, crude protein,
crude fat, moisture, and ash (22). For small molecule analysis,
lactic acid was detected as a major chemical component and it is
reported that topical lactic acid has cosmetic benefit (22). In
previous studies, beneficial effects of short-chain fatty acids and
vitamins produced by probiotics have been reported (23,24). In this
study, tyndallizate was hypothesized to be helpful in skin
rejuvenation. The detailed mechanisms regarding anti-wrinkle
activity require further study.
Skin aging is mainly caused by repeated sun exposure
(25). Intrinsically aged skin is characterized by fine wrinkling
and reduced elasticity, whereas extrinsically aged skin exhibits
deep wrinkles, pigment irregularities, and a substantial loss of
elasticity (26,27). Exposure to UV radiation leads to the
deposition of abnormal elastin complexes and the denaturation of
collagen fibers (28), as well as increased epidermal thickness and
alterations in connective tissue organization (29,30). Involucrin
is a differentiation marker normally expressed by irreversibly
differentiated keratinocytes in the stratum corneum; in a previous
study, downregulation of filaggrin was demonstrated to be involved
in skin reconstruction in vitro following UVB exposure
(31,32). In this study, the upregulation of HA, involucrin,
filaggrin, and TGF-β, an epidermal ECM component that serves an
essential role in supporting tissue architecture in the skin and
epidermis and protects skin from dryness caused by UVB exposure was
observed.
Collagen protects skin from photodamage (33). Type I
collagen, which forms the bulk of skin connective tissue in the
dermis, is gradually lost during the aging process (34). Exposure
to UV radiation can cause collagen breakdown, fragmentation, and
disorganization, as well as the inhibition of procollagen
biosynthesis (35). In this study, western blot analysis revealed
that the downregulation of procollagen expression, relative to that
in the normal group, upon UVB irradiation was also reversed by ACT
3302.
Increased activity of MMPs due to chronic sun
exposure promotes dermal ECM fragmentation, causing the aged
appearance of skin (36). Multiple MMPs including MMP-1 and MMP-9 in
the human skin dermis are upregulated during aging, but this is
exacerbated by exposure to UV light from the sun (37). In
particular, MMP-1 serves an important role in the degradation of
dermal collagens in the ECM (38). Indeed, in this study it was
observed that MMP-1, MMP-2, and MMP-9 levels were increased in
HaCaT cells upon UVB irradiation, it was reversed by ACT 3302.
ROS are involved in a variety of physiological
processes including intracellular signaling, cell proliferation,
tumor suppression, immune defense against pathogens, and oxygen
homeostasis (39). Following UVB irradiation, SOD and CAT activities
were suppressed; however, ACT 3302 stimulated antioxidant enzyme
activity and thereby prevented UVB-induced oxidative stress.
Exposing the skin to UV radiation can induce immune
suppression and can lead to inflammation (40). In keratinocytes,
UVB induces the synthesis of various pro-inflammatory cytokines
including TNF-α, IL-1, IL-6, IL-8, and IL-10, leading to the
activation of nuclear factor-κB signaling (41). In the present
study, UVB irradiation increased IL-1β, IL-8, and TNF-α levels in
HaCaT cells, it was abolished by ACT 3302.
UVB irradiation induces the production of ROS and
activates cell surface receptors, leading to the activation of MAPK
signaling, which involves JNK, ERK and p38 (42). MAPK transmits
extracellular signals to the nucleus and is one of the most
important signal transduction pathways activated by UVB radiation
(43). The present study identified that ACT 3302 inhibited the
UVB-induced activation of JNK, p38, MEK and ERK.
In conclusion, it was demonstrated that ACT 3302
reduces skin damage caused by UVB radiation by increasing the
activity of antioxidant enzymes and skin hydration factors, as well
as by suppressing MMP levels along with pro-inflammatory cytokine
production through the inhibition of MAPK signaling pathway. The
results of the present study provide evidence that probiotics are
not only important for maintaining gut health but can have
additional (cosmetic) benefits such as preventing skin aging.
Funding
The present study was supported by the Korea
Institute of Oriental Medicine (grant no. K17300).
Availability of data and materials
All data generated or analyzed during the present
study are available upon reasonable request.
Authors’ contributions
AI, BL, DK and SC conceived and designed the current
study, and acquired the data. AI and BL performed the experiments
and analyzed the data. SC revised and gave final approval of the
current version to be published. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Jenkins G: Molecular mechanisms of skin
ageing. Mech Ageing Dev. 123:801–810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kammeyer A and Luiten RM: Oxidation events
and skin aging. Ageing Res Rev. 21:16–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
D’Orazio J, Jarrett S, Amaro-Ortiz A and
Scott T: UV radiation and the skin. Int J Mol Sci. 14:12222–12248.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rittié L and Fisher GJ: UV-light-induced
signal cascades and skin aging. Ageing Res Rev. 1:705–720. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim SH, Kim SM, Lee YW, Ahn KJ and Choe
YB: Change of biophysical properties of the skin caused by
ultraviolet radiation-induced photodamage in Koreans. Skin Res
Technol. 14:93–102. 2008.PubMed/NCBI
|
|
6
|
Dai G, Freudenberger T, Zipper P, Melchior
A, Grether-Beck S, Rabausch B, de Groot J, Twarock S, Hanenberg H,
Homey B, et al: Chronic ultraviolet B irradiation causes loss of
hyaluronic acid from mouse dermis because of down-regulation of
hyaluronic acid synthases. Am J Pathol. 171:1451–1461. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steinert PM and Marekov LN: The proteins
elafin, filaggrin, keratin intermediate filaments, loricrin, and
small proline-rich proteins 1 and 2 are isodipeptide cross-linked
components of the human epidermal cornified cell envelope. J Biol
Chem. 270:17702–17711. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosset S, Bonnet-Duquennoy M, Barré P,
Charlon A, Kurfurst R, Bonté F, Schnébert S, Le Varlet B and
Nicolas JF: Photoageing shows histological features of chronic skin
inflammation without clinical and molecular abnormalities. Br J
Dermatol. 149:826–835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fligiel SE, Varani J, Datta SC, Kang S,
Fisher GJ and Voorhees JJ: Collagen degradation in
aged/photodamaged skin in vivo and after exposure to matrix
metalloproteinase-1 in vitro. J Invest Dermatol. 120:842–848. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liebel F, Kaur S, Ruvolo E, Kollias N and
Southall MD: Irradiation of skin with visible light induces
reactive oxygen species and matrix-degrading enzymes. J Invest
Dermatol. 132:1901–1907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rinnerthaler M, Bischof J, Streubel MK,
Trost A and Richter K: Oxidative stress in aging human skin.
Biomolecules. 5:545–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pillai S, Oresajo C and Hayward J:
Ultraviolet radiation and skin aging: Roles of reactive oxygen
species, inflammation and protease activation, and strategies for
prevention of inflammation-induced matrix degradation-a review. Int
J Cosmet Sci. 27:17–34. 2005. View Article : Google Scholar
|
|
13
|
Bickers DR and Athar M: Oxidative stress
in the pathogenesis of skin disease. J Invest Dermatol.
126:2565–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davis RJ: The mitogen-activated protein
kinase signal transduction pathway. J Biol Chem. 268:14553–14556.
1993.PubMed/NCBI
|
|
15
|
Sun S, Jiang P, Su W, Xiang Y, Li J, Zeng
L and Yang S: Wild chrysanthemum extract prevents UVB
radiation-induced acute cell death and photoaging. Cytotechnology.
68:229–240. 2016. View Article : Google Scholar :
|
|
16
|
Adams CA: The probiotic paradox: Live and
dead cells are biological response modifiers. Nutr Res Rev.
23:37–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Liu W, Ran C, Hu J and Zhou Z:
Abrupt suspension of probiotics administration may increase host
pathgen susceptibility by inducing gut dysbiosis. Sci Rep.
6:232142016. View Article : Google Scholar
|
|
18
|
Gerritsen J, Smidt H, Rijkers GT and de
Vos WM: Intestinal microbiota in human health and disease: The
impact of probiotics. Genes Nutr. 6:209–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satoh T, Murata M, Iwabuchi N, Odamaki T,
Wakabayashi H, Yamauchi K, Abe F and Xiao JZ: Effect of
Bifidobacterium breve B-3 on skin photoaging induced by chronic UV
irradiation in mice. Benef Microbes. 6:497–504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bouilly-Gauthier D, Jeannes C, Maubert Y,
Duteil L, Queille-Roussel C, Piccardi N, Montastier C, Manissier P,
Piérard G and Ortonne JP: Clinical evidence of benefits of a
dietary supplement containing probiotic and carotenoids on
ultraviolet-induced skin damage. Br J Dermatol. 163:536–543. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Smith WP: Epidermal and dermal effects of
topical lactic acid. J Am Acad Dermatol. 35:388–391. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
LeBlanc JG, Chain F, Martín R,
Bermúdez-Humarán LG, Courau S and Lagella P: Beneficial effects on
host energy metabolism of short-chin fatty acids and vitamins
produced by commensal and probiotic bacteria. Microb Cell Fact.
16:792017. View Article : Google Scholar
|
|
24
|
Preidis GA, Hill C, Guerrant RL,
Ramakrishna BS, Tannock GW and Versalovic J: Probiotics, enteric
and diarrheal disease, and global health. Gastroenterology.
140:8–14. 2011. View Article : Google Scholar
|
|
25
|
Khavkin J and Ellis DA: Aging skin:
Histology, physiology, and pathology. Facial Plast Surg Clin North
Am. 19:229–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Domyati M, Attia S, Saleh F, Brown D,
Birk DE, Gasparro F, Ahmad H and Uitto J: Intrinsic aging vs
photoaging: A comparative histopathological, immunohistochemical,
and ultrastructural study of skin. Exp Dermatol. 11:398–405. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tobin DJ: Introduction to skin aging. J
Tissue Viability. 26:37–46. 2017. View Article : Google Scholar
|
|
28
|
Scharffetter-Kochanek K, Brenneisen P,
Wenk J, Herrmann G, Ma W, Kuhr L, Meewes C and Wlaschek M:
Photoaging of the skin from phenotype to mechanisms. Exp Gerontol.
35:307–316. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chung JH, Seo JY, Lee MK, Eun HC, Lee JH,
Kang S, Fisher GJ and Voorhees JJ: Ultraviolet modulation of human
macrophage metalloelastase in human skin in vivo. J Invest
Dermatol. 119:507–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sherratt MJ: Tissue elasticity and the
ageing elastic fibre. Age (Dordr). 31:305–325. 2009. View Article : Google Scholar
|
|
31
|
Bosset S, Bonnet-Duquennoy M, Barré P,
Chalon A, Lazou K, Kurfurst R, Bonté F, Schnébert S, Disant F, Le
Varlet B, et al: Decreased expression of keratinocyte beta1
integrins in chronically sun-exposed skin in vivo. Br J Dermatol.
148:770–778. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bernerd F and Asselineau D: Successive
alteration and recovery of epidermal differentiation and
morphogenesis after specific UVB-damages in skin reconstructed in
vitro. Dev Biol. 183:123–138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chung JH, Seo JY, Choi HR, Lee MK, Youn
CS, Rhie G, Cho KH, Kim KH, Park KC and Eun HC: Modulation of skin
collagen metabolism in aged and photoaged human skin in vivo. J
Invest Dermatol. 117:1218–1224. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Calleja-Agius J, Brincat M and Borg M:
Skin connective tissue and ageing. Best Pract Res Clin Obstet
Gynaecol. 27:727–740. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yaar M and Gilchrest BA: Photoageing:
Mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Philips N, Auler S, Hugo R and Gonzalez S:
Beneficial regulation of matrix metalloproteinases for skin health.
Enzyme Res. 2011:4272852011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kähäri VM and Saarialho-Kere U: Matrix
metalloproteinases in skin. Exp Dermatol. 6:199–213. 1997.
View Article : Google Scholar
|
|
39
|
Poljšak B and Dahmane R: Free radicals and
extrinsic skin aging. Dermatol Res Pract. 2012:1352062012.
View Article : Google Scholar
|
|
40
|
Clydesdale GJ, Dandie GW and Muller HK:
Ultraviolet light induced injury: Immunological and inflammatory
effects. Immunol Cell Biol. 79:547–568. 2001. View Article : Google Scholar
|
|
41
|
Kirnbauer R, Köck A, Neuner P, Förster E,
Krutmann J, Urbanski A, Schauer E, Ansel JC, Schwarz T and Luger
TA: Regulation of epidermal cell interleukin-6 production by UV
light and corticosteroids. J Invest Dermatol. 96:484–489. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
López-Camarillo C, Ocampo EA, Casamichana
ML, Pérez-Plasencia C, Alvarez-Sánchez E and Marchat LA: Protein
kinases and transcription factors activation in response to
UV-radiation of skin: Implications for carcinogenesis. Int J Mol
Sci. 13:142–172. 2012. View Article : Google Scholar : PubMed/NCBI
|