Introduction
Retinoblastoma (RB), the most common neoplasm of the
eye, generally develops in childhood and represents 2.5-4% of all
childhood malignancy cases; additionally, two-thirds of patients
are diagnosed before they are 2 years old, and 95% are diagnosed
before they are 5 years old (1).
RB has characteristic clinical features and is classified as
unilateral RB, which accounts for ~75% of cases, or bilateral RB,
which accounts for 25% of cases (2). The incidence of RB is unevenly
distributed in the world, with increased incidence among people in
India, Africa and those of Native American descent in North America
(3). An inverse correlation
between the incidence of RB and socioeconomic index was documented
in a previous study, and it demonstrated that in more
industrialized countries, an increased incidence of RB is
associated with poverty and low levels of maternal education
(2,4). The treatment of RB is
multidisciplinary, including systemic or intra-arterial
chemotherapy, external beam radiation therapy and aggressive focal
treatments (5). A promising study
demonstrated that the inhibition of RB tumor growth can be achieved
by anti-angio-genesis agents (6).
Additionally, in recent years, an increasing number of cases and
accumulating evidence demonstrated that gene therapy may serve an
important role in tumorigenesis and tumor progression (7,8).
Cyclin-dependent kinase regulatory subunit 1B
(CKS1B) is a member of the Cks/Suc1 family of small proteins (9-18
kDa) that bind the catalytic subunit of cyclin-dependent protein
kinases and regulate their function (9). Elevated expression of CKS1B has been
associated with increased p27 (Kip1) turnover and cell
proliferation, and poor prognosis in numerous tumor types,
including oral, gastric, breast and colon carcinoma (10). Overexpression of CKS1B also
activates the mitogen-activated protein kinase kinase
(MEK)/extracellular signal-regulated kinase (ERK) signaling pathway
(11). The MEK/ERK pathway has
been demonstrated to serve an important role in regulating
different biological processes, including proliferation,
differentiation and survival, in numerous different cells (12). A previous study indicated that
therapeutic strategies that target cancer stem cells may benefit
from MEK/ERK inhibition combined with traditional radiotherapy
(13). Another previous
demonstrated that stimulation of the MEK/ERK signaling pathway
partially inhibited cell death and growth inhibition induced by
CKS1B knockdown (14). However,
the role of CKS1B-mediated MEK/ERK signaling in the proliferation,
invasion and angiogenesis of RB cells is poorly understood.
Therefore, in the present study, novel drug targets for RB were
investigated.
Materials and methods
Study subjects
From January 2016 to January 2018, 35 patients
diagnosed with RB who underwent ophthalmectomy in the Pathology
Department of Xiangya Hospital, Central South University (Changsha,
China) were enrolled (male, n=21; female, n=14; mean age, 1.54±1.06
years; range 1-3 years), and their RB tissue and adjacent retina
tissue were resected. In regard to clinical staging, according to
the International Retinoblastoma Staging System (15), 21 patients exhibited stage I, 10
patients presented with stage II and 4 patients exhibited stage
IIIa disease. The histological types included intraocular stage
(n=12), glaucomatous stage (n=15), extra-ocular stage (n=8) and
systemic metastasis (n=0). There were 16 cases with optic nerve
infiltration and 19 without. All cases were confirmed by a
pathology specialist at the Pathology Department of Xiangya
Hospital, Central South University who was blinded to the study.
The study was approved by the Institutional Review Board of Xiangya
Hospital, Central South University. Written informed consent was
obtained from the legal guardian of each participant.
Cell culture and selection
Human normal retinal vascular endothelial cells
(ACBRI-181) and an RB cell line (HXO-RB44) were purchased from the
Cell Center of Xiangya Medical College of Central South University,
the RB cell line Y79 was purchased from American Type Culture
Collection (Manassas, VA, USA), and the RB cell line SO-RB50 was
obtained from the Pathology Laboratory of Zhongshan Eye Hospital
(Zhongshan, China). These four cell lines were incubated in
RPMI-1640 medium (PM150110; Procell, Wuhan, Hubei, China)
containing 10% fetal bovine serum (FBS; Tiandz, Inc., Beijing,
China) with 5% CO2 at 37°C. Culture medium was replaced
every 24 h, and the cells were subcultured every 72 h. The cell
lines with the highest mRNA and protein expression of CKS1B
(SO-RB50 and HXO-RB44 cells) were selected by reverse
transcription-polymerase chain reaction (RT-qPCR) and western blot
analysis, according to the subsequent protocols, for subsequent
experiments.
Construction of the short hairpin RNA
(shRNA) expression vector
The full-length human CKS1B sequence (NC_000001.11)
was obtained from the National Center for Biotechnology Information
(https://www.ncbi.nlm.nih.gov/), and the
CKS1B shRNA (shRNA CKS1B-1, shRNA CKS1B-2 and shRNA CKS1B-3) and
shRNA-negative control (NC) (Table
I) sequences were designed by BLOCK-iT™ RNAi Designer software
(http://rnaidesigner.thermofisher.com/rnaiexpress/)
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The
shRNA-CKS1B sequence and the blank plasmid pRNAT-CMV3.2/Neo (cat.
no. SD1264; Beijing China Ocean Co., Ltd., Beijing, China) were
treated at 37°C for 4 h with BamHI and XhoI, and the
appropriate fragments were ligated. The recombinant shRNA-CKS1B
expression plasmid was transformed into E. coli. After the
bacteria were cultured at 37°C for 1 h, the shRNA-CKS1B expression
plasmid was extracted. Additionally, PCR amplification was
performed, according to the subsequent protocols, to detect
positive colonies using the vector primer CMV-seqF (Taihe
Biotechnology Co., Ltd., Beijing, China) and the target primer R.
Additionally, nucleic acid sequencing was performed to select the
clone with the correct sequence for further culture.
 | Table IshRNA sequence |
Table I
shRNA sequence
| shRNA | Sequence
(5′-3′) |
|---|
| CKS1B-shRNA-1 |
GCGCTGAGAGAGTTGAATATT |
| CKS1B-shRNA-2 |
GCTGAGAGAGTTGAATATTGC |
| CKS1B-shRNA-3 |
GGAGTGTCCTAAGGGCCAATT |
| shRNA-NC |
CTGGACTGGTATTTGGACCAG |
Cell treatment
The selected human RB cell line (SO-RB50) was
cultured in Dulbecco's modified Eagle's medium (DMEM; T&L
Biological Technology, Beijing, China) containing 10% FBS at 37°C
with 5% CO2. After the cells adhered to the well, the
cells were treated at 37°C for 2 min with 0.25% trypsin and placed
in a 24-well plate overnight at 37°C. Cells in the logarithmic
growth phase were selected. A 50 µl aliquot of
double-distilled water was used to dilute 0.8 µg recombinant
plasmid and 2 µl Entranster™-R transfection reagent
(Engreen, Beijing, China), and this dilution was placed at room
temperature for 20 min. The mixture was then added to cells in a
24-well plate in DMEM containing FBS at a final transfection
concentration of 50 nM. Cells in the remaining groups were
sequentially transfected according to the Lipofectamine®
2000 protocols (cat. no. 12566014; Thermo Fisher Scientific, Inc.)
and incubated at 37°C with 5% CO2 for 48 h. The RB cells
were divided into the following groups: Blank (SO-RB50 cells);
negative control (NC; SO-RB50 + empty vector); shRNA-CKS1B-1
(SO-RB50 + shRNA-CKS1B-1 plasmid); shRNA-CKS1B-2 (SO-RB50 +
shRNA-CKS1B-2 plasmid); PMA (SO-RB50 + signaling pathway activator
phorbol 12-myristate 13-acetate); shRNA-CKS1B-1 + PMA (SO-RB50 +
shRNA-CKS1B-1 plasmid + PMA); and shRNA-CKS1B-2 + PMA (SO-RB50 +
shRNA-CKS1B-2 plasmid + PMA). The cells were starved for 24 h after
transfection with 20 mmol/l plasmid for subsequent experiments, and
all experiments were repeated three times. Furthermore, in the
shRNA-CKS1B-1 + PMA and shRNA-CKS1B-2 + PMA groups, the signaling
pathway activator PMA (Beyotime Institute of Biotechnology, Haimen,
China) was added for 12 h treatment at room temperature at a final
concentration of 10 mmol/l. The aforementioned transfection
approach was also applicable to HXO-RB44 cells.
RT-qPCR
Cells in logarithmic growth from each group were
centrifuged at 179 x g at room temperature for 15 min, and then the
supernatant was discarded, and the cells or tissue were retained.
Total RNA was extracted from cells in each group and tissues using
TRIzol® (Beijing Wobisen Technology Co., Ltd., Beijing,
China) according to the manufacturer's protocol. RNA concentration,
purity and integrity were evaluated by spectrophotometry
(Lab-Spectrum Instruments Co., Ltd., Shanghai, China) and agarose
gel electrophoresis (Bewell, Shenzhen, Guangdong, China). The cDNA
was reverse transcribed with a T7 High Yield RNA Transcription kit
(cat. no. R101-01/02; Vazyme Biotech Co., Ltd., Nanjing, Jiangsu,
China) and then amplified with a HiScript II U+ One Step qRT-PCR
Probe kit (cat. no. Q223-01; Vazyme Biotech Co., Ltd.). RT-qPCR
primers (Table II) were designed
by Takara Biotechnology Co., Ltd. (Dalian, China). The reaction
conditions (20 µl total volume) were set at 42°C for 15 min
(reverse transcription reaction) and at 85°C for 2 min (reverse
transcriptase inactivation reaction), according to the protocols of
the TaqMan MicroRNA Assays Reverse Transcription Primer (cat. no.
4366596; Thermo Fisher Scientific, Inc.). Fluorescence qPCR was
performed with reaction solutions according to the protocols of a
mRNA RT-qPCR kit (cat. no. P031-01/02; Vazyme Biotech Co., Ltd.).
PCR was performed in a real-time PCR system (model SLAN-96P;
Shanghai Hongshi Medical Technology Co., Ltd., Shanghai, China).
The results were analyzed by the 2−∆∆Cq method (16), which represents the difference in
target gene expression between the experimental group and the
control group as follows: ΔΔCq=ΔCq (experimental group)-ΔCq
(control group), in which ΔCq=Cq (target gene)-Cq (reference gene).
Additionally, Cq is the number of amplification cycles required for
the quantitative fluorescence intensity of the reaction to reach
the set threshold, during which the amplification occurs in a
logarithmic manner. All experiments were performed in
triplicate.
 | Table IIPrimer sequence for reverse
transcription quantitative polymerase chain reaction. |
Table II
Primer sequence for reverse
transcription quantitative polymerase chain reaction.
| Targeted gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| CKS1B |
CCAGATGAGTGCTCTGTGGA |
TCCATCTGCCAAGTGTGTTC |
| MEK1 |
CAATGGCGGTGTGGTGTTC |
GATTGCGGGTTTGATCTCCAG |
| ERK1 |
TACACCAACCTCTCGTACATCG |
CATGTCTGAAGCGCAGTAAGATT |
| Bcl2 |
CCTTTGTGTAACTGTACGGCC |
CTTTGGCAGTAAATAGCTGATTCGAC |
| VEGF |
GCCTTGCCTTGCTGCTCTA |
GATGTCCACCAGGGTCTCG |
| PCNA |
CCTGCTGGGATATTAGCTCCA |
CAGCGGTAGGTGTCGAAGC |
| Cyclin
D1 |
TGGAGCCCGTGAAAAAGAGC |
TCTCCTTCATCTTAGAGGCCAC |
| bFGF |
AGAAGAGCGACCCTCACATCA |
CGGTTAGCACACACTCCTTTG |
| GAPDH |
ATTCCATGGCACCGTCAAGGCT |
TCAGGTCCACCACTGACACGTT |
Western blot analysis
Extracted cells or tissues in 5 ml cell lysis buffer
(cat. no. hz-3207-1; Shanghai Zhen Biotechnology Co., Ltd.,
Shanghai, China) were homogenized for 10 min on ice at 0°C and then
transferred into a centrifuge tube. Subsequently, the cells were
sonicated 3 times for 15 sec each, centrifuged at 4°C and 258 × g
for 20 min and boiled at 100°C for 10 min. With the supernatant
extracted, the protein concentration was determined with a
bicinchoninic acid protein assay kit, and cell lysates were stored
at -20°C for use. Following this, a 10% separating gel and 5%
spacer gel were prepared, and 10 µg prepared protein from
each group was added into each well. Following electrophoresis at a
constant voltage of 100 V for 90 min and a constant current of 80
mA for 40 min, the separated proteins were transferred to a
nitrocellulose fluoride membrane through the wet method (17). The membrane was washed once with
1X TBS with 0.05% Tween-20 (TBST) buffer for ~1 min and incubated
in 1X TBST buffer (pH 7.6) and 5% skim milk at room temperature for
1 h. Subsequently, the membrane was incubated with the following
primary antibodies overnight at 4°C: Rabbit polyclonal CKS1B (cat.
no. ab72639; 1:500), MEK 1/2 (cat. no. ab96379; 1:1,000), ERK 1/2
(cat. no. ab214362; 1:100), B-cell lymphoma 2 (Bcl2; cat. no.
ab32124; 1:1,000), proliferating cell nuclear antigen (PCNA; cat.
no. ab92552; 1:1,000), cyclin D1 (cat. no. ab134175; 1:1,000),
vascular endothelial growth factor (VEGF; cat. no. ab32152;
1:1,000) and basic fibroblast growth factor (bFGF; cat. no.
ab208687; 1:1,000). All the antibodies aforementioned were
purchased from Abcam (Cambridge, MA, USA). Subsequently, the
membrane was washed with PBS with 0.05% Tween-20 (PBST) three times
for 10 min each. After the secondary anti-goat or anti-rabbit
polyclonal antibody (cat. no. ab205718; 1:2,000; Abcam) was diluted
with 5% skim milk, the membrane was incubated with the secondary
antibody for 1 h at room temperature. The membrane was washed with
PBST three times for 15 min each. The solution A and solution B
from a chemiluminescence fluorescent detection kit (catalog no.
BB-3501; Amersham Pharmacia, Piscataway, NJ, USA) was mixed in the
darkroom, then dripped onto the membranes and exposed in the gel
imager. The images were acquired using a Bio-Rad gel imaging system
(MG6000; Beijing To Morgan Biotech Co., Ltd., Beijing, China) with
GAPDH as the internal control. The ratios of the gray values of the
target bands to the internal control bands were calculated as the
relative quantitative expression of the protein. All experiments
were performed in triplicate.
MTT assay
When the cell density reached ~90%, single-cell
suspensions were prepared with trypsin, and the cell number was
counted. Cells were seeded at a density of 1×104
cells/well in a 96-well plate with 0.2 ml cell suspension in
RPMI-1640 medium containing 10% FBS each well. Each group was set
up with three duplicate wells. Cells were then cultured in the
incubator at 37°C and 5% CO2 for 24, 48, 72 or 96 h.
Subsequently, 5 mg/ml MTT solution (cat. no. M1020; Beijing
Solarbio Technology Co., Ltd., Beijing, China) was added into each
group at the appropriate time. The cells were placed in the 37°C
incubator for 4 h in the dark. After discarding the supernatant,
200 µl dimethyl sulfoxide (cat. no. D5879-100 ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to
dissolve the purple crystals. The 96-well plate was then moved to
the flat-panel oscillator for 10 min of horizontal shaking.
Finally, the optical density of each well was measured at 490 nm
through a microplate reader. The results were recorded and
statistically analyzed.
Transwell migration assay
A total of 100 µl (1×105 cells/ml)
cell dilution diluted by serum-free DMEM was seeded into the upper
chamber of the Transwells, and 500 µl DMEM containing 10%
FBS was added into the lower chambers. Subsequently, the Transwell
chambers were cultured in 5% CO2 at 37°C for 36 h.
Afterwards, the chambers were removed, and cells on the upper layer
of the chamber were removed with a cotton swab. The chambers were
washed two times with PBS, fixed in 4% paraformaldehyde at room
temperature for 30 min, stained with 1% crystal violet at room
temperature for 10 min, inverted on glass slides and dried. The
number of cells that passed through the membrane was determined
under an optical microscope (magnification, ×200), and cells were
counted in 4 randomly selected high-power fields. The experiment
was conducted three times to obtain the mean ± standard
deviation.
Matrigel invasion assay
Precooled serum-free DMEM was used to dilute
Matrigel (BD Biosciences, San Jose, CA, USA) at a ratio of 1:10.
Subsequently, 100 µl diluted Matrigel was added to each
upper chamber, which was allowed to stand at room temperature for 2
h, followed by a rinse with 200 µl serum-free RPMI-1640
medium. The aforementioned cells were detached 24 h after
transfection, resuspended in serum-free DMEM, counted and diluted
to 3×105 cells/ml. Subsequently, 100 µl cell
dilution was added to the upper Transwell chambers (Corning Inc.,
Corning, NY, USA), and 600 µl DMEM containing 10% serum was
added to the lower chambers as the chemotactic factor for a 24-h
incubation. In accordance with the manufacturer's protocols,
crystal violet staining for 10 min at room temperature was
performed. Subsequently, 4 high power fields under an inverted
microscope (magnification, ×200) were selected on a random basis,
and the cells were counted. The experiment was conducted three
times to obtain the mean ± standard deviation.
Flow cytometry
After the cells were transfected for 48 h, according
to the aforementioned protocol, the medium was discarded. The cells
were washed with PBS once, treated at 37°C for 2 min with 0.25%
trypsin and collected. Subsequently, the sample was centrifuged at
179 × g for 5 min at 4°C, and the supernatant was discarded. The
cells were washed with precooled PBS twice and centrifuged at 179 ×
g for 5 min at room temperature, and the supernatant was discarded.
Precooled 70% ethanol was added for fixing the cells at 4°C
overnight. Following being washed two times with PBS and
centrifuged at 179 × g for 5 min at room temperature, the cells
were incubated with 10 µl RNase enzyme at 37°C for 5 min.
The cells were then stained for 30 min at room temperature with 1%
propidium iodide (PI; 40710ES03; Shanghai Qianchen Biotechnology
Co., Ltd., Shanghai, China) in the dark. Cell cycle profiles were
recorded based on red fluorescence at 488 nm detected with a flow
cytometer (FACSCalibur) and analyzed by CellQuest 6.0 (both from BD
Biosciences, San Jose, CA, USA). All experiments were performed in
triplicate.
The cells were seeded in a 6-well plate with
2×105 cells/well. Following transfection for 48 h
according to the aforementioned protocol, the cells were treated at
37°C for 2 min with ethylene diamine tetraacetic acid-free trypsin
and centrifuged at 179 × g at 4°C for 5 min, and the supernatant
was then aspirated. Subsequently, the cells were washed twice with
precooled PBS and centrifuged at 179 × g for 5 min at room
temperature, and the supernatant was aspirated. Apoptosis was
detected by an Annexin-V-fluorescein isothiocyanate (FITC)/PI
apoptosis detection kit (CA1020; Beijing Solarbio Technology Co.,
Ltd). The cells were washed with binding buffer containing 50 mM
N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 700 mM NaCl
and 12.5 mM CaCl2 (pH 7.4), and the mixture of
Annexin-V-FITC and binding buffer (1:40) was prepared.
Subsequently, the cells were resuspended, mixed uniformly, and
incubated at room temperature for 30 min. The mixture of PI and
binding buffer (1:40) was added to the cells, which were thoroughly
mixed and incubated at room temperature for 15 min. Apoptosis was
detected with a flow cytometer. All experiments were performed in
triplicate.
Lumen formation assay
Cells were cultured in a 24-well plate at 37°C and
5% CO2 for 24 h. The Matrigel and the inducer were mixed
according to the manufacturer's protocols (8158; Shanghai Zhongqiao
Xinzhou Biotechnology Co., Ltd., Shanghai, China). Matrigel was
melted on ice, and the pipette tip and the 24-well plate were
precooled at −20°C. Matrigel was added to the 24-well plate with
100 µl/well using the precooled pipette tip. The formation
of bubbles should be avoided during this step. Subsequently,
Matrigel was placed on ice for 5 min to level the surface, and the
plate was moved into an incubator at 37°C for 30 min to allow the
Matrigel to solidify. Simultaneously, the cells were treated at
37°C for 2 min with trypsin. A total of seven groups of cells (NC,
blank, shRNA-CKS1B-1, shRNA-CKS1B-2, PMA, shRNA-CKS1B-1 + PMA,
shRNA-CKS1B-2 + PMA groups) were seeded into a prepared 24-well
plate at 4x104 cells/well and incubated at 37°C for 4-8
h. Cells were allowed to form a lumen on the artificial basement
membrane. The length of the formed lumen was measured in five
randomly selected high-power fields under an inverted microscope
(magnification, x100). The mean ± standard deviation was obtained
to compare the tube formation ability among groups.
Statistical analysis
Statistical analysis was conducted using SPSS 21.0
software (IBM Corp., Armonk, NY, USA). All data demonstrated a
normal distribution and homogeneity of variance. Measurement data
are expressed as the mean ± standard deviation. Differences between
two groups were analyzed using the independent sample t-test, and
statistical analysis among multiple groups was conducted using
one-way analysis of variance, followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
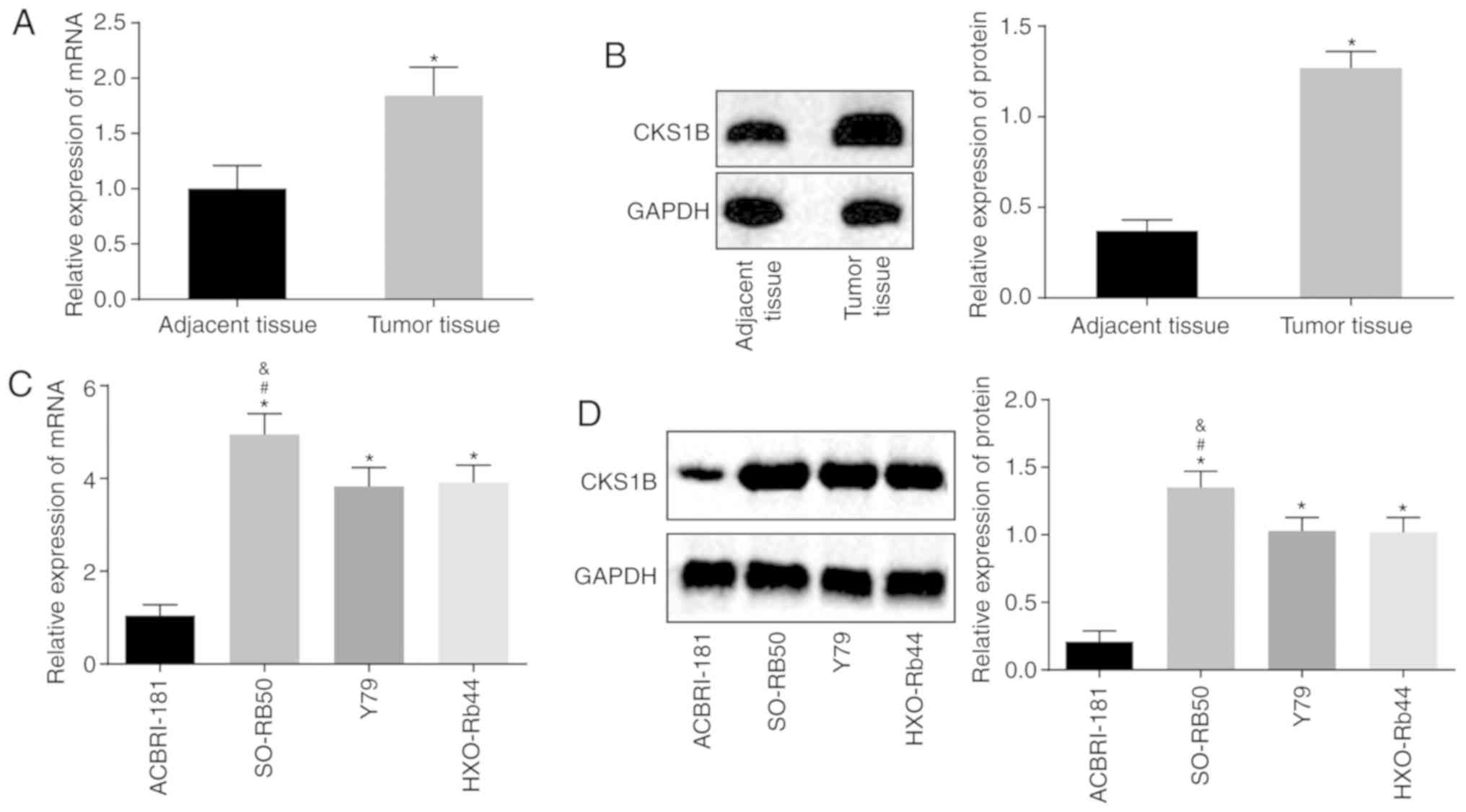
CKS1B is highly expressed in RB
tissue
Firstly, RB and adjacent retina tissues were
collected from participants to quantify the mRNA and protein levels
of CKS1B by means of RT-qPCR and western blot analysis. The results
revealed significantly increased levels of CKS1B in tumor tissue,
compared with adjacent tissue (all P<0.05), indicating a
significant difference in CKS1B expression in clinical RB tissue
(Fig. 1A and B). Subsequently,
RT-qPCR and western blot analyses were conducted to evaluate CKS1B
expression in the ACBRI-181, SO-RB50, Y79 and HXO-RB44 cell lines.
The results demonstrated that compared with the normal human
retinal endothelial cell line ACBRI-181, the SO-RB50, Y79 and
HXO-RB44 cell lines exhibited significantly increased CKS1B
expression (all P<0.05), and the mRNA expression of CKS1B in
SO-RB50 cells was significantly increased, compared with Y79 and
HXO-RB44 cells (P<0.05) (Fig. 1C
and D). Therefore, the human RB cell lines SO-RB50 and HXO-RB44
were selected for the following experiments.
Silencing the CKS1B gene inhibits the
activation of the MEK/ERK signaling pathway
Subsequently, the shRNA transfection efficiency was
detected based on the mRNA expression of CKS1B determined by
RT-qPCR. The results demonstrated that the mRNA expression of CKS1B
was significantly decreased in the shRNA CKS1B-1, shRNA CKS1B-2 and
shRNA CKS1B-3 groups compared with the NC group. Additionally,
CKS1B mRNA expression decreased more significantly in the shRNA
CKS1B-1 and shRNA CKS1B-2 groups (Fig. 2A). Therefore, the shRNA CKS1B-1
and shRNA CKS1B-2 groups were selected for the follow-up
experiments.
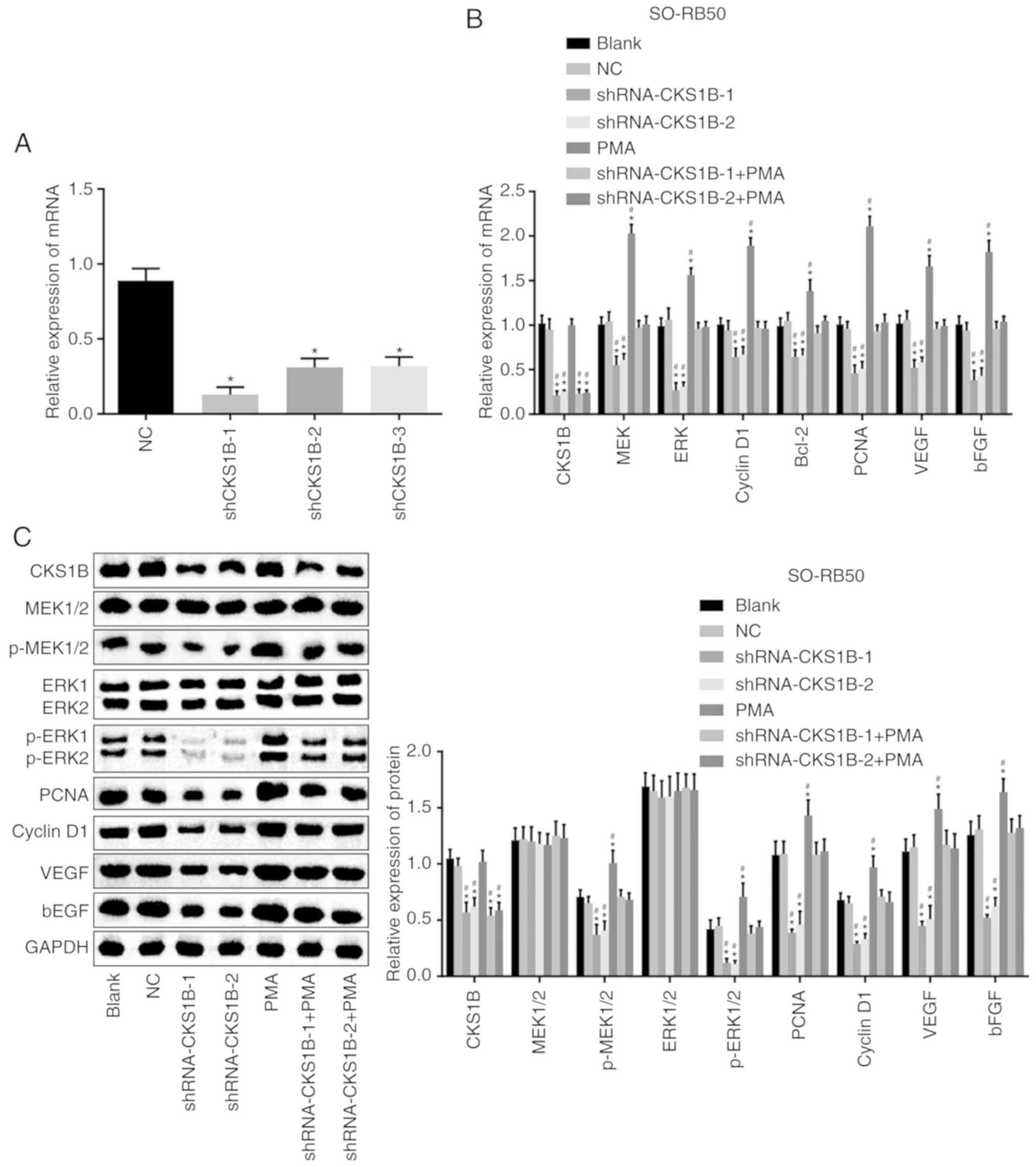 | Figure 2RT-qPCR and western blot analyses
demonstrate that CKS1B silencing inhibits the activation of the
MEK/ERK signaling pathway. (A) mRNA expression of CKS1B following
transfection of shRNA CKS1B. *P<0.05 vs. the NC
group. (B) mRNA expression of CKS1B, MEK, ERK, Bcl2, cyclin D1,
PCNA, VEGF and bFGF in SO-RB50 cells. (C) Gray values and protein
levels of CKS1B, MEK, ERK, Bcl2, cyclin D1, PCNA, VEGF and bFGF
normalized to GAPDH in SO-RB50 cells. *P<0.05 vs. the
blank group; #P<0.05 vs. the NC group. Each
experiment was repeated 3 times. (D) mRNA expression of CKS1B, MEK,
ERK, Bcl2, cyclin D1, PCNA, VEGF and bFGF in HXO-RB44 cells. (E)
Gray values and protein levels of CKS1B, MEK, ERK, Bcl2, cyclin D1,
PCNA, VEGF and bFGF normalized to GAPDH in HXO-RB44 cells.
*P<0.05 vs. the blank group; #P<0.05
vs. the NC group. Each experiment was repeated 3 times. CKS1B,
cyclin-dependent kinase regulatory subunit 1B; MEK,
mitogen-activated protein kinase kinase; ERK, extracellular
signal-regulated kinase; Bcl2, B-cell lymphoma 2; PCNA,
proliferating cell nuclear antigen; VEGF, vascular endothelial
growth factor; bFGF, basic fibroblast growth factor; shRNA, short
hairpin RNA; p-, phospho-; NC, negative control; PMA, phorbol
12-myristate 13-acetate. |
With the use of RT-qPCR and western blot analysis,
the mRNA and protein expression of CKS1B, MEK, ERK, Bcl2, cyclin
D1, PCNA, VEGF and bFGF in SO-RB50 and HXO-RB44 cells was assessed.
The results (Fig. 2B-E)
demonstrated no significant difference in the mRNA and protein
expression of CKS1B, MEK, ERK, Bcl2, cyclin D1, PCNA, VEGF and bFGF
between the blank and NC groups (P>0.05). The mRNA and protein
expression of CKS1B, MEK, ERK, Bcl2, cyclin D1, PCNA, VEGF and bFGF
was significantly decreased in the shRNA-CKS1B-1 and shRNA-CKS1B-2
groups, compared with the blank and NC groups (P<0.05). No
significant difference was determined in the mRNA and protein
expression of CKS1B between the PMA, blank and NC groups
(P>0.05), but a significantly increased expression of MEK, ERK,
Bcl2, cyclin D1, PCNA, VEGF and bFGF mRNA and protein was observed
in the PMA group (P<0.05). The mRNA and protein expression of
CKS1B was significantly decreased in the shRNA-CKS1B-1 + PMA and
shRNA-CKS1B-2 + PMA groups, compared with the blank and NC groups,
but no significant differences were determined in the mRNA and
protein levels of the other factors (P>0.05). These observations
indicated that silencing CKS1B mediated the activation of the
MEK/ERK signaling pathway, as indicated by reduced mRNA and protein
expression levels of MEK, ERK, Bcl2, cyclin D1, PCNA, VEGF and bFGF
in SO-RB50 and HXO-RB44 cells.
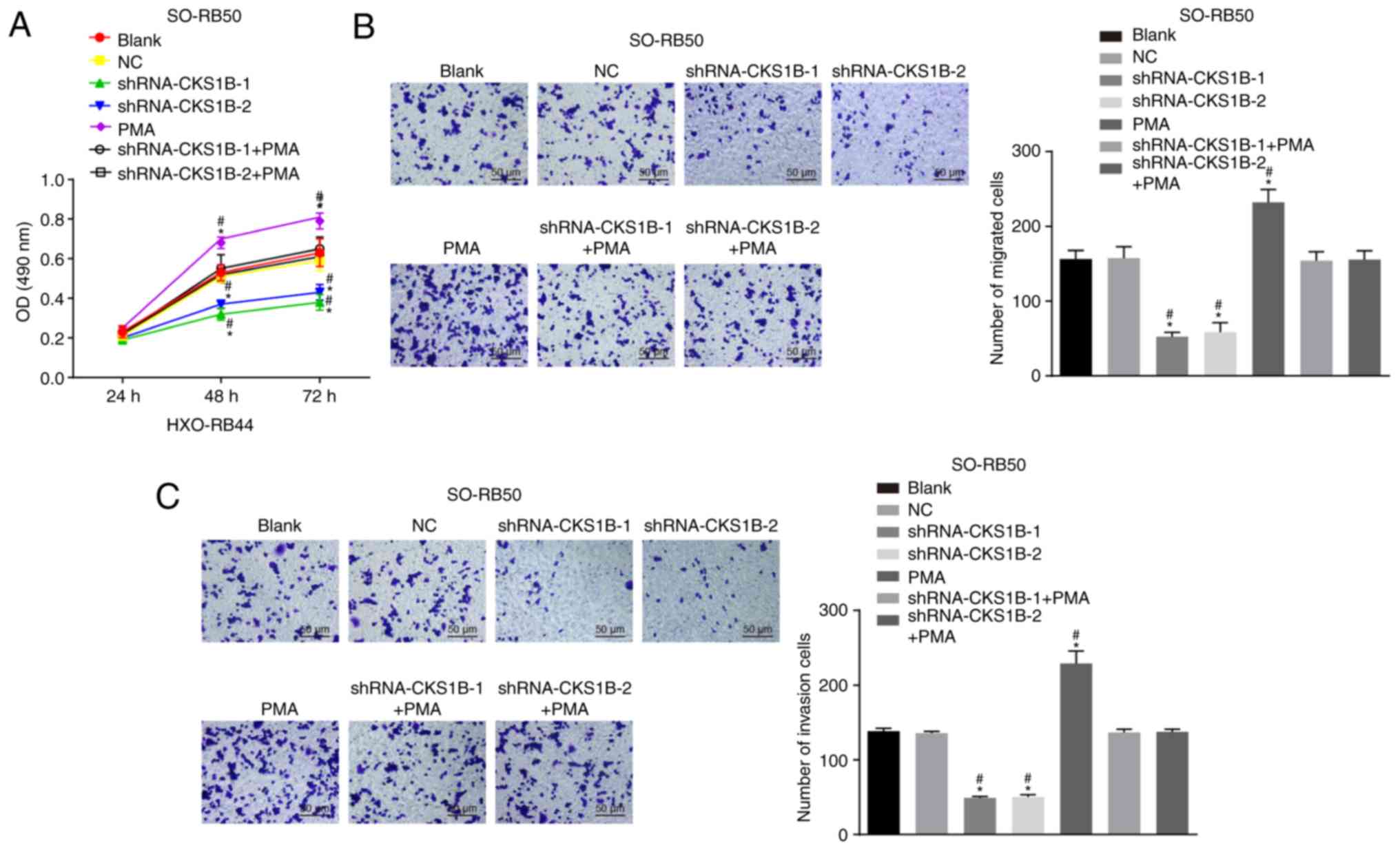
Silencing the CKS1B gene suppresses the
proliferation, migration and invasion of SO-RB50 and HXO-RB44
cells
In the following experiments, the proliferation,
migration, invasion, cell cycle distribution and apoptosis of
SO-RB50 and HXO-RB44 cells were investigated. The results of the
MTT assay (Fig. 3A and D)
demonstrated that cell viability significantly increased over time
under different treatments at 24, 48 and 72 h (all P<0.05). No
significant difference in cell viability was determined in the NC,
shRNA-CKS1B-1 + PMA and shRNA-CKS1B-2 + PMA groups, compared with
the blank group (P>0.05); however, the viability in the
shRNA-CKS1B-1 and shRNA-CKS1B-2 groups decreased gradually over
time (at the 48th and 72nd h) in comparison with the blank and NC
groups, with significant differences apparent after 48 h
(P<0.05), and cell viability decreased as a whole. However, the
opposite results were observed in the PMA group in comparison to
the blank group (P<0.05). These observations indicated that
silencing CKS1B inhibits the proliferation of SO-RB50 and HXO-RB44
cells.
Transwell and Matrigel assays were then utilized to
detect the migration and invasion of SO-RB50 and HXO-RB44 cells.
The results (Fig. 3B, C, E and F)
demonstrated no notable difference in SO-RB50 and HXO-RB44 cell
migration or invasion in the NC, shRNA-CKS1B-1 + PMA and
shRNA-CKS1B-2 + PMA groups, compared with the blank group
(P>0.05). Cell migration and invasion in the shRNA-CKS1B-1 + PMA
and shRNA-CKS1B-2 + PMA groups were significantly suppressed,
compared with those in the blank and NC groups (P<0.05). Cell
migration and invasion were significantly increased in the PMA
group, compared with the blank and NC groups (P<0.05). These
observations indicated that silencing CKS1B weakened the migratory
and invasive potential of SO-RB50 and HXO-RB44 cells.
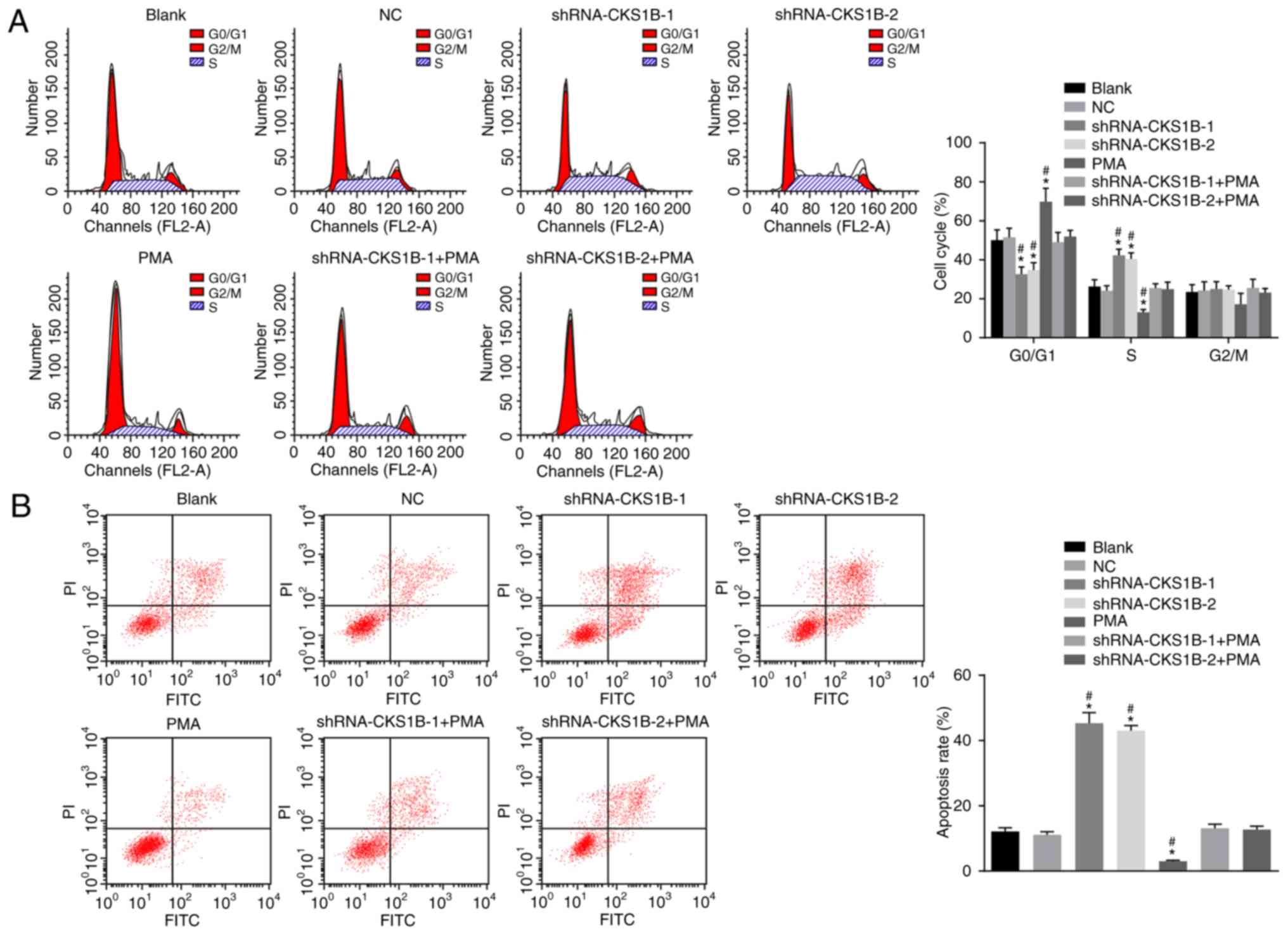
Silencing the CKS1B gene promotes the
apoptosis of SO-RB50 cells
PI staining to detect SO-RB50 cell cycle
distribution demonstrated no significant difference between the
blank NC groups (Fig. 4A).
Compared with the blank and NC groups, the shRNA-CKS1B-1 and
shRNA-CKS1B-2 groups had a significantly shorter G0 or G1 phase
(increased cell proportion) and a significantly prolonged S phase
(decreased cell proportion) (all P<0.05); additionally, the PMA
group had a significantly prolonged G0 or G1 phase (decreased cell
proportion) and a significantly shortened S phase (increased cell
proportion) (all P<0.05). No significant differences were
determined in the shRNA-CKS1B-1 + PMA and shRNA-CKS1B-2 + PMA
groups, compared with the blank and NC groups (P>0.05). The
results demonstrated that silencing CKS1B was responsible for
changes in the SO-RB50 cell cycle distribution by suppressing the
MEK/ERK signaling pathway.
Flow cytometry was used to assess apoptosis
(Fig. 4B). Following
transfection, no significant difference in SO-RB50 apoptosis rate
was determined between the NC and blank groups (P>0.05). The
apoptosis rates of SO-RB50 cells were significantly increased in
the shRNA-CKS1B-1 and shRNA-CKS1B-2 groups, compared with the NC
and blank groups (P<0.05), but the apoptosis rate in the PMA
group was significantly reduced (P<0.05). Compared with the NC
and blank groups, the shRNA-CKS1B-1 + PMA and shRNA-CKS1B-2 + PMA
groups demonstrated no significant difference in the apoptosis rate
(P>0.05). These observations indicated that silencing the CKS1B
gene promoted the apoptosis of SO-RB50 cells.
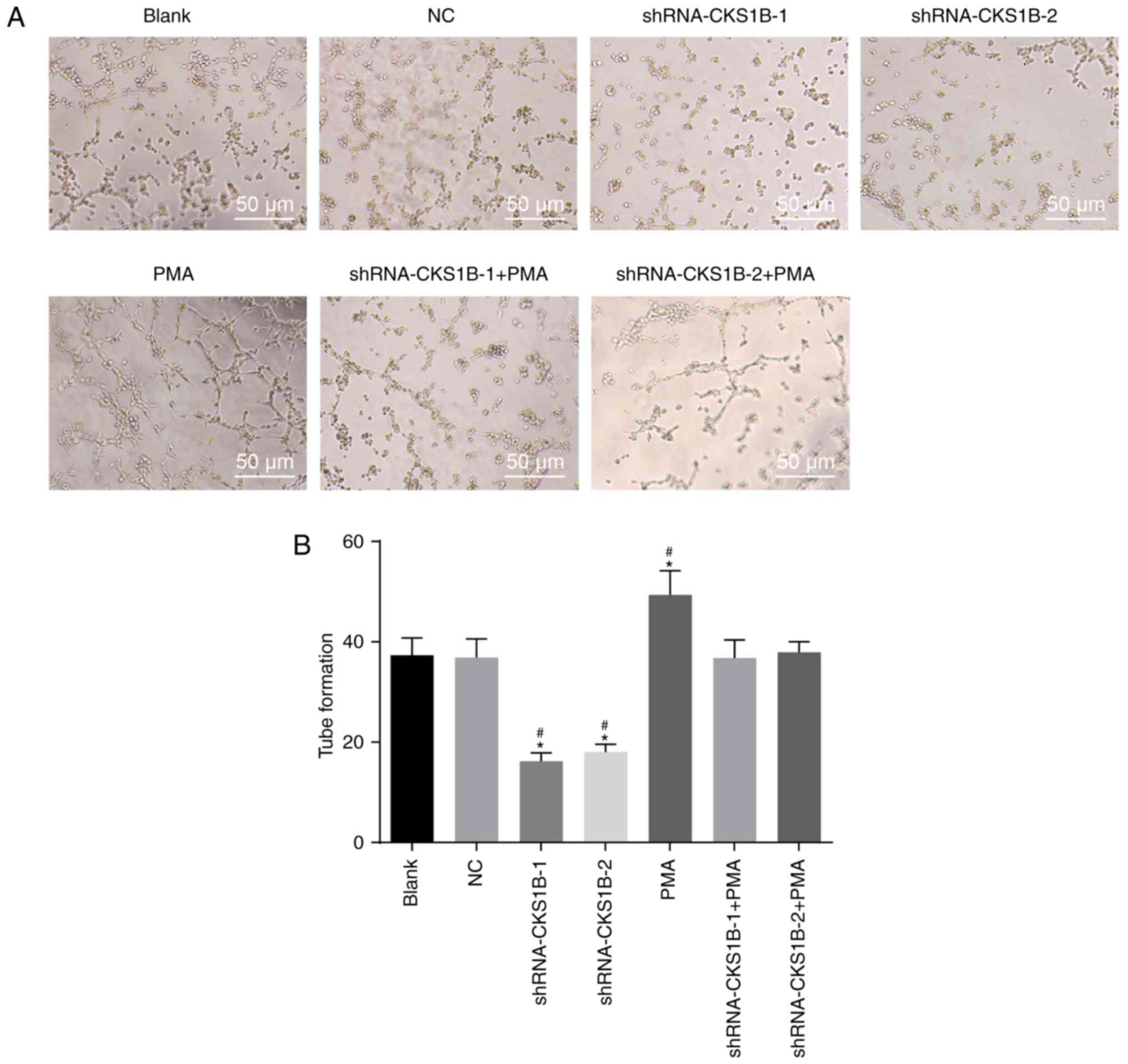
Silencing the CKS1B gene suppresses
angiogenesis in SO-RB50 cells
Lastly, the effects of CKS1B on angiogenesis were
investigated. The lumen formation results (Fig. 5) demonstrated no significant
difference in the number of lumens between the NC and blank groups
(P>0.05). Compared with the blank and NC groups, the
shRNA-CKS1B-1 and shRNA-CKS1B-2 groups indicated a significant
inhibition of lumen formation (P<0.05), while opposite results
were observed in the PMA group (P<0.05). Lumen formation did not
differ significantly in the shRNA-CKS1B-1 + PMA and shRNA-CKS1B-2 +
PMA groups in comparison to the blank and NC groups (P>0.05).
The results demonstrated that silencing the CKS1B gene suppresses
angiogenesis in SO-RB50 cells.
Discussion
RB is a retinal cancer associated with biallelic
loss of the RB1 gene; additionally, a mutation detection
rate of 94.9% has been reported in both blood and tumor samples
(18). Over the last few decades,
notable efforts have been made to search for novel therapeutic
approaches for RB, a potentially curable cancer, yet determining a
safer and more efficient treatment modality to save the eye globe
and preserve functional vision in a child with RB remains a major
challenge (19). In the present
study, the aim was to determine the biological mechanism by which
CKS1B affects RB cells. Consequently, the present study
demonstrated that CKS1B downregulation blocks the MEK/ERK signaling
pathway, thus inhibiting the proliferation, migration, invasion and
angiogenesis of RB cells.
Initially, the present results demonstrated that
CKS1B was overexpressed in RB tissue and cells, and that CKS1B gene
silencing inhibits RB cell growth and invasion, and suppresses
angiogenesis in RB, which indicated that CKS1B has key roles in the
tumorigenesis and malignant progression of RB. A previous study
demonstrated that gene silencing is correlated with RB cell
proliferation and invasion (20),
which shed light on gene silencing for RB treatment. As a member of
the highly conserved CKS1 protein family, CKS1B can interact with
cyclin-dependent kinases and serves an important role in cell cycle
progression (21). It also known
that CKS1B is a tumor promoter that has been largely investigated
in previous studies, which revealed that elevated expression of
CKS1B contributes to increased cell proliferation and a poor
prognosis in oral (21), gastric
(22), and hepatocellular
carcinomas (23), among others,
and that CKS1B ablation strongly induces apoptosis (24).
In the subsequent experiments, it was demonstrated
that downregulation of CKS1B could inhibit the activation of the
MEK/ERK signaling pathway, which exhibited an increased expression
of PCNA, cyclin D1, VEGF and bFGF, thus inhibiting the
proliferation, migration, invasion and angiogenesis of RB cells.
The MEK/ERK signaling pathway couples signals from cell surface
receptors to transcription factors, which regulate gene expression
(25), and regulates the activity
of numerous proteins, including the pro-survival protein myeloid
cell leukemia 1 and caspase-9, involved in apoptosis (26). Previous research demonstrated that
aberrant regulation of the MEK/ERK signaling pathway contributes to
cancer and other human diseases, including human immunodeficiency
virus infection (27), cardiac
hypertrophy (28) and Parkinson's
disease (29), and in particular,
the ERK pathway has been the focus of research and a target of drug
inhibitor development for cancer treatment (30). Consistent with the present study,
observations obtained previously demonstrated that the majority of
RB cells have increased expression levels of VEGF, particularly
VEGF-D; therefore, upregulated VEGF signal transduction serves an
important role in angiogenesis in RB (31). Furthermore, a previous study
demonstrated that since the MEK/ERK signaling pathway is frequently
simultaneously dysregulated in cancer, it is becoming increasingly
more apparent that targeting the MEK/ERK signaling pathway may be
an effective therapeutic intervention for cancer cases with
upstream mutations that result in activation of this pathway
(32). Partially in line with the
present study, another survey demonstrated that norcantharidin
suppresses tumor angiogenesis through blocking the VEGFR2/MEK/ERK
signaling pathways (33). A
previous study also demonstrated that continued activation of the
Raf/MEK/ERK pathway induces growth arrest, accompanied by changes
in cell cycle regulators (decreased RB phosphorylation) (34). Furthermore, partially consistent
with the present study, Shi et al (11) determined that overexpressing CKS1B
could activate the MEK/ERK and Janus kinase/STAT3 signaling
pathways and promote myeloma cell drug resistance. The MEK/ERK
signaling pathway is increasingly used by increasing numbers of
factors and mitogens, including Ras and B-Raf, to transmit signals
from their receptors to regulate gene expression and prevent
apoptosis (35). Notably, another
study indicated that Quercetin contributed to the apoptosis of Y79
RB cells by activating the JNK and p38 MEK/ERK signaling pathways,
providing a novel treatment approach for human RB (36).
In conclusion, it was demonstrated that CKS1B gene
silencing significantly reduces the proliferation, migration and
invasion of RB cells while inhibiting angiogenesis in the retina.
The tumorigenic activity of CKS1B is mediated by inhibition of the
oncogenic MEK/ERK signaling pathway through gene silencing.
Therefore, it was speculated that CKS1B may be a promising novel
target in the development of therapeutic treatments for RB.
However, due to limited funding and time, the specific target of
CKS1B-mediated activation of the MEK/ERK signaling pathway was not
identified in the present study, but it may be the focus of future
research. Additionally, in future studies, animal experiments
should be performed to confirm that CKS1B suppresses metastasis
in vivo.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ, ZLG and ZPZ designed the study. HBJ and CQY
collected the data. JY and XBX designed and developed the database,
and performed data analyses. ZZ, ZLG, ZPZ and HBJ wrote the paper
and conceived and designed the experiments. HBJ, CQY and JY
reviewed and discussed the results and discussions, and prepared
and revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Xiangya Hospital, Central South University
(Changsha, China). Written informed consent was obtained from the
legal guardian of each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Rodriguez-Galindo C, Orbach DB and
VanderVeen D: Retinoblastoma. Pediatr Clin North Am. 62:201–223.
2015. View Article : Google Scholar
|
|
2
|
McEvoy JD and Dyer MA: Genetic and
epigenetic discoveries in human retinoblastoma. Crit Rev Oncog.
20:217–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rangamani S, SathishKumar K, Manoharan N,
Julka PK, Rath GK, Shanta V, Swaminathan R, Rama R, Datta K, Mandal
S, et al: Paediatric retinoblastoma in India: Evidence from the
National Cancer Registry Programme. Asian Pac J Cancer Prev.
16:4193–4198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Truong B, Green AL, Friedrich P, Ribeiro
KB and Rodriguez-Galindo C: Ethnic, racial, and socioeconomic
disparities in retinoblastoma. JAMA Pediatr. 169:1096–1104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wyse E, Handa JT, Friedman AD and Pearl
MS: A review of the literature for intra-arterial chemotherapy used
to treat retinoblas-toma. Pediatr Radiol. 46:1223–1233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Theodoropoulou S, Brodowska K, Kayama M,
Morizane Y, Miller JW, Gragoudas ES and Vavvas DG: Aminoimidazole
carboxamide ribonucleotide (AICAR) inhibits the growth of
retinoblastoma in vivo by decreasing angiogenesis and inducing
apoptosis. PLoS One. 8:e528522013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han G, Lu K, Huang J, Ye J, Dai S, Ye Y
and Zhang L: Effect of Annexin A1 gene on the proliferation and
invasion of esophageal squamous cell carcinoma cells and its
regulatory mechanisms. Int J Mol Med. 39:357–363. 2017. View Article : Google Scholar :
|
|
8
|
Martinez E and Trevino V: Modelling gene
expression profiles related to prostate tumor progression using
binary states. Theor Biol Med Model. 10:372013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stella F, Pedrazzini E, Baialardo E, Fantl
DB, Schutz N and Slavutsky I: Quantitative analysis of CKS1B mRNA
expression and copy number gain in patients with plasma cell
disorders. Blood Cells Mol Dis. 53:110–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu L, Fan S, Zhao J, Zhou P, Chu S, Luo J,
Wen Q, Chen L, Wen S, Wang L and Shi L: Increased expression of
Cks1 protein is associated with lymph node metastasis and poor
prognosis in nasopharyngeal carcinoma. Diagn Pathol. 12:22017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi L, Wang S, Zangari M, Xu H, Cao TM, Xu
C, Wu Y, Xiao F, Liu Y, Yang Y, et al: Over-expression of CKS1B
activates both MEK/ERK and JAK/STAT3 signaling pathways and
promotes myeloma cell drug-resistance. Oncotarget. 1:22–33.
2010.PubMed/NCBI
|
|
12
|
Zarrabi M, Afzal E, Asghari MH, Mohammad
M, Es HA and Ebrahimi M: Inhibition of MEK/ERK signalling pathway
promotes erythroid differentiation and reduces HSCs engraftment in
ex vivo expanded haematopoietic stem cells. J Cell Mol Med.
22:1464–1474. 2018. View Article : Google Scholar
|
|
13
|
Soares HP, Ming M, Mellon M, Young SH, Han
L, Sinnet-Smith J and Rozengurt E: Dual PI3K/mTOR inhibitors induce
rapid overactivation of the MEK/ERK pathway in human pancreatic
cancer cells through suppression of mTORC2. Mol Cancer Ther.
14:1014–1023. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan F, Shi L, Wang S, Xu H, Cao TM, Xu C,
Wu Y, Zangari M, Li G and Tricot GJ: CKS1B Mediates
SKP2/p27Kip1-independent myeloma cell survival and disease
progression through activation of MEK/ERK and JAK/STAT3 signaling
pathways. Blood. 114:1262009.
|
|
15
|
Radhakrishnan V, Kumar R, Malhotra A and
Bakhshi S: Role of pet/ct in staging and evaluation of treatment
response after 3 cycles of chemotherapy in locally advanced
retinoblastoma: A prospective study. J Nucl Med. 53:191–198. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ayuk SM, Abrahamse H and Houreld NN: The
role of photobiomodulation on gene expression of cell adhesion
molecules in diabetic wounded fibroblasts in vitro. J Photochem
Photobiol B. 161:368–374. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Dong P, Hou C, Cao F, Sun S, He F,
Song Y, Li S, Bai Y and Zhu D: Hydroxysafflor yellow A (HSYA)
attenuates hypoxic pulmonary arterial remodelling and reverses
right ventricular hypertrophy in rats. J Ethnopharmacol.
186:224–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tomar S, Sethi R, Sundar G, Quah TC, Quah
BL and Lai PS: Mutation spectrum of RB1 mutations in retinoblastoma
cases from Singapore with implications for genetic management and
counselling. PLoS One. 12:e01787762017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chawla B, Jain A and Azad R: Conservative
treatment modalities in retinoblastoma. Indian J Ophthalmol.
61:479–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Subramanian N, Navaneethakrishnan S,
Biswas J, Kanwar RK, Kanwar JR and Krishnakumar S: RNAi mediated
Tiam1 gene knockdown inhibits invasion of retinoblastoma. PLoS One.
8:e704222013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martín-Ezquerra G, Salgado R, Toll A, Baró
T, Mojal S, Yébenes M, Garcia-Muret MP, Solé F, Quitllet FA,
Espinet B and Pujol RM: CDC28 protein kinase regulatory subunit 1B
(CKS1B) expression and genetic status analysis in oral squamous
cell carcinoma. Histol Histopathol. 26:71–77. 2011.
|
|
22
|
Shrestha S, Yang CD, Hong HC, Chou CH, Tai
CS, Chiew MY, Chen WL, Weng SL, Chen CC, Chang YA, et al:
Integrated MicroRNA-mRNA analysis reveals miR-204 inhibits cell
proliferation in gastric cancer by targeting CKS1B, CXCL1 and
GPRC5A. Int J Mol Sci. 19:E872017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang CW, Lin CY, Huang HY, Liu HW, Chen
YJ, Shih DF, Chen HY, Juan CC, Ker CG, Huang CY, et al: CKS1B
overex-pression implicates clinical aggressiveness of
hepatocellular carcinomas but not p27(Kip1) protein turnover: An
independent prognosticator with potential p27 (Kip1)-independent
oncogenic attributes? Ann Surg Oncol. 17:907–922. 2010. View Article : Google Scholar
|
|
24
|
Zhan F, Colla S, Wu X, Chen B, Stewart JP,
Kuehl WM, Barlogie B and Shaughnessy JD Jr: CKS1B, overexpressed in
aggressive disease, regulates multiple myeloma growth and survival
through SKP2- and p27Kip1-dependent and -independent mechanisms.
Blood. 109:4995–5001. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zassadowski F, Rochette-Egly C, Chomienne
C and Cassinat B: Regulation of the transcriptional activity of
nuclear receptors by the MEK/ERK1/2 pathway. Cell Signal.
24:2369–2377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao N, Budhraja A, Cheng S, Liu EH, Huang
C, Chen J, Yang Z, Chen D, Zhang Z and Shi X: Interruption of the
MEK/ERK signaling cascade promotes dihydroartemisinin-induced
apop-tosis in vitro and in vivo. Apoptosis. 16:511–523. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lieske NV, Tonby K, Kvale D, Dyrhol-Riise
AM and Tasken K: Targeting tuberculosis and HIV infection-specific
regulatory T cells with MEK/ERK signaling pathway inhibitors. PLoS
One. 10:e01419032015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren J, Zhang N, Liao H, Chen S, Xu L, Li
J, Yang Z, Deng W and Tang Q: Caffeic acid phenethyl ester
attenuates pathological cardiac hypertrophy by regulation of
MEK/ERK signaling pathway in vivo and vitro. Life Sci. 181:53–61.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen WF, Wu L, Du ZR, Chen L, Xu AL, Chen
XH, Teng JJ and Wong MS: Neuroprotective properties of icariin in
MPTP-induced mouse model of Parkinson's disease: Involvement of
PI3K/Akt and MEK/ERK signaling pathways. Phytomedicine. 25:93–99.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dobberkau HJ: Problems in the hygienic
evaluation of flowing waters. Z Gesamte Hyg. 21:738–741. 1975.In
German. PubMed/NCBI
|
|
31
|
Wu JH, Xu P, Yi MY, Wang F, Wang XL and
Huang Q: Analysis of angiogenesis associated factors of
retinoblastoma cell line and tumor tissues. Zhonghua Yan Ke Za Zhi.
41:419–422. 2005.In Chinese. PubMed/NCBI
|
|
32
|
Steelman LS, Abrams SL, Shelton JG,
Chappell WH, Bäsecke J, Stivala F, Donia M, Nicoletti F, Libra M,
Martelli AM and McCubrey JA: Dominant roles of the Raf/MEK/ERK
pathway in cell cycle progression, prevention of apoptosis and
sensitivity to chemotherapeutic drugs. Cell Cycle. 9:1629–1638.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L, Ji Q, Liu X, Chen X, Chen Z, Qiu
Y, Sun J, Cai J, Zhu H and Li Q: Norcantharidin inhibits tumor
angiogenesis via blocking VEGFR2/MEK/ERK signaling pathways. Cancer
Sci. 104:604–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong SK, Yoon S, Moelling C, Arthan D and
Park JI: Noncatalytic function of ERK1/2 can promote
Raf/MEK/ERK-mediated growth arrest signaling. J Biol Chem.
284:33006–33018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
36
|
Liu H and Zhou M: Antitumor effect of
Quercetin on Y79 retinoblastoma cells via activation of JNK and p38
MAPK pathways. BMC Complement Altern Med. 17:5312017. View Article : Google Scholar : PubMed/NCBI
|