Introduction
Experimental animal data are essential to the
development of novel therapeutic compounds, and the observations
are commonly assumed to be predictive of biological responses in
humans. However, ethical issues lead to the need for reducing the
number of animals used in preclinical testing; therefore, there is
a stringent need for the development of alternative assays so as to
allow a selection of compounds for further preclinical development.
Operational primary screens enable researchers to substitute a
significant proportion of animal research (1). This also applies for anticancer
compounds, for which animal testing is still prevalently used. In
the case of anticancer compounds, it is important to have validated
methods for assessing the anti-proliferative effects, before moving
into the next stage, the preclinical one (2).
The functional similarities among living organisms
can be a very useful background for generating biological
information. The extrapolation of results among species can offer
valuable information, particularly in the case of a toxic effect
(3). Some non-animal tests have
proven themselves valuable for pre-screening compounds, and thus
reducing the number of animals used in consequent research. For
example, the Ames test, using strains of the Salmonella
typhimurium to determine whether chemicals cause DNA mutations,
has been successfully validated and is widely employed as
pre-screening to reduce rodent testing for cancer-causing compounds
(4).
Other useful methods are phytobiological tests,
which examine phytotoxicity. Successfully used to evaluate the
environmental effects in contaminated soils (5,6)
and waste-water (7,8), and to assess the cytotoxicity of
pollutants, such as arsenic (9),
pesticides (10) and herbicides
(11), they measure the delay of
seed germination, the inhibition of plant growth or any adverse
effects on plants caused by specific substances (5). In this study, we wished to determine
the relevance of this when testing anticancer compounds. We wished
to determine whether it is possible to establish a direct
association between the impairment of plant growth and the
anti-proliferative effects of cytostatics, thus using
phytobiological tests as pre-screening assays for such
substances.
Several higher plants can be used for the study of
root elongation, ranging from monocotyledons, such as Triticum
aestivum (12,13), Agropyrum repens (14), Allium cepa (15,16), Panicum miliaceum, Avena
sativa (17), to dicotyledons
e.g., Vicia faba (18),
Lycopersicon esculentum (19), Lactuca sativa (20), Cucurbita pepo (21), Raphanus sativus (17), Phaseolus radiatus (13). A previous study demonstrated that
monocots exhibit a greater sensitivity (6).
These plants have been employed as instruments for
the screening of the toxicity of bioactive compounds. Tests using
these plants are very easy to perform, and have a low cost, are
efficient and present good correlations with other tests (6,22)
connected to cytotoxicity and genotoxicity (23). Root growth is regulated by two
processes which are closely linked, cell division and cell
expansion. The inhibition of root elongation can be achieved either
by cytotoxic agents that inhibit the cell cycle in different phases
or by inhibitors of cell expansion (24). However, we wished to determine
whether the Triticum aestivum root elongation test can be
used as an effective method for the biological evaluation of novel
potential anti-proliferative agents and whether a plant-based assay
can predict mammalian efficacy.
It is likely that anti-proliferative drugs can
produce the same effect in the Triticum assay as in higher
living organisms, since a significant number of oncogenic signal
transduction pathways are highly conserved in living organisms
(25). For this purpose, in this
study, we examined the effect produced on the root growth of wheat
seeds by a number of known cytotoxic substances with various
structures and mechanisms of action. To better understand the
limitations of the test, the results were classified and correlated
with the anti-proliferative profiles on human tumor cell lines,
using the data provided by the Developmental Therapeutics Program
(DTP) of the National Cancer Institute (NCI) (26). The NCI60 fingerprint of
anti-proliferative effects can be used to elucidate the mechanisms
of action, or to identify similar known compounds (27). The versatility and usefulness of
chemoinformatic and bioinformatics analyses based on the NCI60
profiles has been demonstrated in various studies (28-31).
The aim of this study was to determine whether the
phytobiological toxicity screening test could provide information
on the anti-proliferative effects in human cells, since this effect
is regulated by similar processes that are highly conserved in the
cell division process of all eukaryotic organisms. Root elongation
is frequently used for the assessment of cytotoxicity, due to its
several already mentioned advantages. The purpose of this study was
to establish its limitations and to validate its use, thus
providing an additional tool for the screening of both natural and
new synthesized compounds with potential anti-proliferative
effects.
Materials and methods
Reagents and chemicals
All reagents and solvents were purchased from
commercial suppliers. The compounds used for screening were
purchased from Sigma-Aldrich and were as follows: Albendazole
(54965-21-8), aminophylline (317-34-0), busulfan (55-98-1),
cantharidin (56-25-7), chlorambucil (305-03-3), cisplatin
(15663-27-1), colchicine (64-86-8), cyclophosphamide (6055-19-2),
epirubicin hydrochloride (56390-09-1), fluorouracil (51-21-8),
hydroxyurea (127-07-1), imatinib mesylate (220127-57-1),
indole-3-acetic acid (87-51-4), irinotecan hydrochloride
(100286-90-6), mercaptopurine (6112-76-1), methotrexate
(133073-73-1), paclitaxel (33069-62-4), podophyllotoxin (518-28-5),
quinine hydrochloride dehydrate (6119-47-7) and verapamil
hydrochloride (152-11-4).
Compound preparation
Triticum aestivum (Boema cultivar), supplied
by SC Adaflor SRL was selected as the test plant. Dry caryopses
were soaked for 24 h in distilled water and 20 caryopses for each
concentration were equally distributed on filter paper disks in
Petri dishes of 90 mm diameter and treated with 5 ml of each test
solution. The bioassay was performed at 25±1°C, 75% relative
humidity in the absence of light, in a plant growth chamber
(MLR-351H; Sanyo). All compounds were dissolved in dimethyl
sulfoxide (DMSO) and diluted with sterilized distilled water until
the concentration of DMSO was 1%. Each compound was tested in
duplicate at concentrations ranging from 0.1 to 500 µM (0.1,
0.5, 1, 5, 10, 50, 100 and 500 µM) depending on the
solubility or the results of preliminary tests. A negative control
sample was prepared with aminophylline due its inactivity on all
NCI60 cell lines, as evidenced by the data downloaded from the NCI
website (https://dtp.cancer.gov/databases_tools/default.htm). A
solvent control with 1% DMSO in distilled water was used.
Indole-3-acetic acid is a well-known plant hormone (32) and was used as positive control.
The length of the embryonic root was measured with the application
ImageJ software version 1.46 r (Wayne Rasband, National Institutes
of Health) and the values of root elongation were expressed in
mm.
Statistical analysis
The D'Agostino Pearson normality test (α=0.5) was
performed on the root elongation data and, due to the abnormal
distribution of the values of radicular elongation, the
non-parametric Kruskall Wallis test with Dunn's post hoc test were
applied in order to evaluate the statistical differences.
The inhibition (I%) was calculated and plotted
against the logarithm of concentrations and the corresponding
curves were calculated using the least squares fit method. The
inhibitory activity was defined as the concentration of compound
(IC50) causing a 50% decrease in root length, relative to the
solvent control, and was calculated using the equation presented in
our previous study (9). The
negative log10 values of the IC50 value expressed as a molar
concentration (pIC50) were calculated for each tested compound.
Whenever the obtained results permitted, the upper and lower limits
of the 95% confidence interval (95% CI) and the correlation
coefficient (r2) were calculated.
NCI uses a panel of 60 human tumor cell lines
representing 9 tissue types (central nervous system, leukemia,
breast, colon, renal, lung, ovary, prostate and melanoma) to screen
novel synthesized compounds and pure natural products. In this
study, we used negative log10 of the 50% growth inhibitory
concentration expressed as molar concentration (pGI50) for each
test compound. All data were collected freely from the DTP website
(https://dtp.cancer.gov/databases_tools/default.htm).
A hierarchical cluster analysis of the root
elongation inhibitory effects was performed using the furthest
neighbor method and Euclidean distance measure. A two-sample t-test
for unpaired data was performed to compare data sets.
Results
Triticum aestivum root elongation
assay
A total of 18 anti-proliferative agents were
selected based on their anti-proliferative mechanistic diversity
and were tested in the Triticum aestivum root elongation
assay. Aminophylline was selected as the negative control, having
no significant effect on all NCI60 cell lines, as well as
indole-3-acetic acid (https://dtp.cancer.gov/databases_tools/default.htm)
selected as the control, since its effect on root elongation is
well known. The values obtained for the inhibitory effects are
presented in Table I. The values
ranged from -41.23, which represent a stimulation of root length,
to an inhibition of 88.03%.
 | Table IThe inhibitory effect on wheat root
elongation after 24 h of exposure to the anti-proliferative
agents. |
Table I
The inhibitory effect on wheat root
elongation after 24 h of exposure to the anti-proliferative
agents.
| Concentration
(µM)
|
|---|
| 500 | 100 | 50 | 10 | 5 | 1 | 0.5 | 0.1 |
|---|
| Compound | | | | I% | | | |
|---|
| Albendazole | 24.68 | 28.17 | 6.76 | 0.99 | 27.22 | 24.33 | 12.86 | NT |
| Aminophylline | 53.04 | 32.63 | −14.74 | −24.60 | −16.68 | −41.23 | −18.33 | −3.06 |
| Busulfan | 10.01 | 6.78 | 3.33 | 29.16 | 15.18 | 6.86 | 3.67 | −0.14 |
| Cantharidin | NT | NT | 80.42 | 66.28 | 17.10 | 32.55 | −10.35 | 4.07 |
| Chlorambucil | 4.09 | 0.24 | 17.92 | 14.20 | −1.67 | 19.72 | 21.53 | NT |
| Cisplatin | 66.21 | 22.60 | 23.45 | −7.67 | 4.11 | 0.74 | 2.29 | −12.94 |
| Colchicine | 88.03 | 85.53 | 81.14 | 17.40 | 22.04 | 16.21 | 15.90 | 2.71 |
|
Cyclophosphamide | 13.70 | 23.74 | 5.46 | 9.33 | −5.90 | −1.48 | −3.98 | −9.77 |
| Epirubicin | 5.72 | 10.84 | −5.15 | −16.05 | −7.65 | −15.24 | −27.41 | −6.11 |
| Fluorouracil | 7.70 | 17.89 | 38.19 | 8.42 | 12.56 | 15.97 | 3.16 | 22.85 |
| Hydroxyurea | 15.05 | 7.35 | 24.88 | 6.70 | 15.62 | 11.23 | 17.66 | 4.90 |
| Imatinib
mesylate | 4.16 | −7.56 | 5.50 | 9.49 | 3.36 | 11.30 | 3.45 | NT |
| Indole-3-acetic
acid | NT | 71.08 | 42.34 | 25.41 | 11.31 | 11.79 | 12.85 | 16.43 |
| Irinotecan | 35.28 | 4.73 | 11.33 | 4.42 | 33.79 | −1.16 | 17.90 | 19.20 |
| Mercaptopurine | 14.22 | 10.12 | 23.01 | −4.83 | −2.72 | −10.09 | −7.00 | −1.67 |
| Methotrexate | NT | NT | 94.39 | 88.82 | 58.75 | 39.36 | −15.71 | −16.63 |
| Paclitaxel | 40.25 | 40.89 | 31.01 | 29.30 | 20.85 | 30.78 | 12.33 | 10.51 |
|
Podophyllotoxin | 44.80 | 24.71 | 35.69 | 28.00 | 20.26 | 19.38 | 34.55 | 1.46 |
| Quinine | 10.23 | 20.32 | −4.92 | −11.57 | −28.97 | −25.35 | −14.12 | −52.48 |
| Verapamil
hydrochloride | 54.98 | 10.45 | −2.94 | −9.94 | −15.91 | −28.29 | −25.50 | −28.99 |
As shown in Table
II, the Kruskal Wallis test revealed statistically significant
differences between the results obtained at various concentrations,
although a linear effect-concentration association was not found
for all determinations. No association was found between
concentration and root elongation for albendazole, chlorambucil,
hydroxyurea, imatinib and irinotecan. Dunn's post-test revealed
statistically significant differences between the results obtained
for each concentration and the solvent control sample, with the
exception of quinine and verapamil, which induced a stimulating
effect, without being statistically significant.
 | Table IIStatistical analysis of the root
elongation. |
Table II
Statistical analysis of the root
elongation.
| Compound | Kruskal-Wallis
test | Dunn's test
[concentration (µM)]
|
|---|
| 500 | 100 | 50 | 10 | 5 | 1 | 0.5 | 0.1 |
|---|
| Albendazole | *** | *** | *** | ns | ns | *** | *** | * | NT |
| Aminophylline | *** | *** | * | ns | ns | ns | ns | ns | ns |
| Busulfan | *** | * | ns | *** | *** | *** | *** | *** | * |
| Cantharidin | *** | NT | NT | *** | *** | ns | ** | ns | ns |
| Chlorambucil | *** | ns | ns | *** | ** | ns | *** | *** | NT |
| Cisplatin | *** | *** | *** | *** | ns | ns | ns | ns | ns |
| Colchicine | *** | *** | *** | *** | ns | * | * | ns | ns |
|
Cyclophosphamide | *** | *** | *** | *** | *** | * | ** | ** | * |
| Epirubicin | * | ns | ns | ns | ns | ns | ns | ** | ns |
| Fluorouracil | *** | ns | *** | *** | ns | * | * | ns | *** |
| Hydroxyurea | *** | * | ns | *** | ** | *** | *** | *** | *** |
| Imatinib | *** | ns | ns | ** | ns | ** | ** | * | NT |
| Indole-3-acetic
acid | *** | NT | *** | *** | ** | ns | ns | ns | * |
| Irinotecan | *** | *** | ns | ns | ns | *** | ns | ns | * |
| Mercaptopurine | *** | * | * | *** | * | ** | ** | ** | ** |
| Methotrexate | *** | NT | NT | *** | *** | *** | * | ns | ns |
| Paclitaxel | *** | *** | *** | *** | *** | * | *** | ns | ns |
|
Podophyllotoxin | *** | *** | *** | *** | ** | * | * | *** | ns |
| Quinine | *** | ns | ns | ns | ns | ns | ns | ns | * |
| Verapamil | *** | *** | ns | ns | ns | ns | ns | ns | ns |
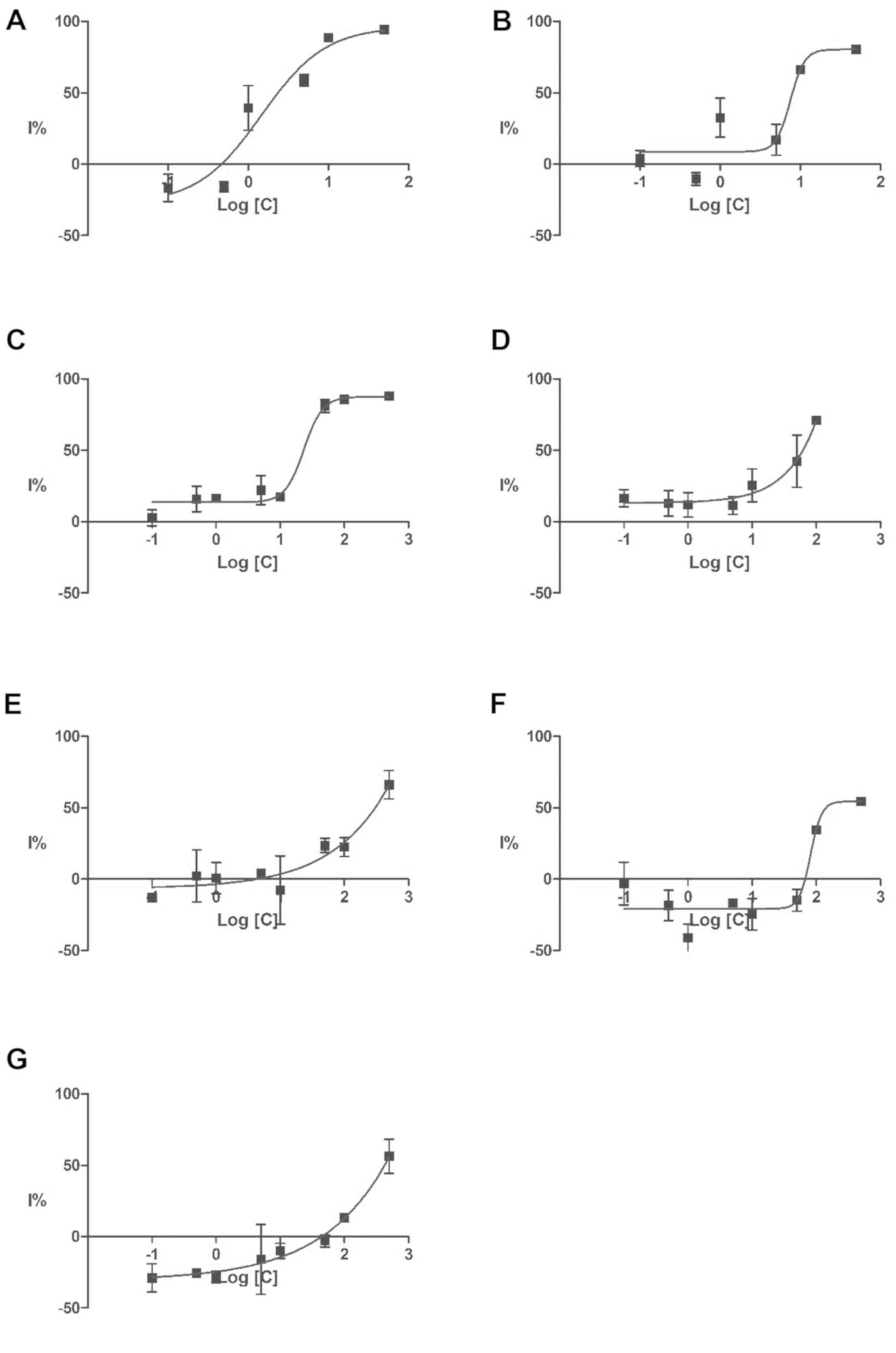
Even after exposure at high doses, only 5 of the
anti-proliferative agents, methotrexate, colchicine, cantharidin,
cisplatin and verapamil produced a growth inhibition over 50%.
Detecting only 5 out of the 18 anti-proliferative compounds tested
suggests a poor predictive power and a high false-negative ratio.
The inhibition threshold of 50% was also achieved for aminophylline
and indole-3-acetic acid. For all these compounds, the half maximal
inhibitory concentration (IC50) values and their corresponding
negative logarithm (pIC50) were computed based on the dose-response
curves presented in Fig. 1. The
IC50 values, their corresponding confidence interval, and
correlation coefficient (r2) were calculated and are
presented in Table III in
ascending order.
 | Table IIIThe inhibitory effect on root
elongation following 24 h of exposure. |
Table III
The inhibitory effect on root
elongation following 24 h of exposure.
| Compound | IC50
(µM) | pIC50 (M) | 95% CI of IC50
(µM) | Goodness of fit
(r2) |
|---|
| Methotrexate | 2.978 | −5.526 | 0.042 to 51.35 | 0.9177 |
| Cantharidin | 9.141 | −5.039 | 1.210 to 46.87 | 0.8254 |
| Colchicine | 20.70 | −4.684 | 8.651 to 63.31 | 0.9668 |
| Indole-3-acetic
acid | 49.204 | −4.308 | NC | 0.8711 |
| Cisplatin | 263.633 | −3.579 | NC | 0.8206 |
| Aminophylline | 409.261 | −3.388 | 41.92 to 160.8 | 0.8560 |
| Verapamil | 441.570 | −3.355 | NC | 0.9156 |
The high rate of false-positives is due to the small
tested set and the deliberate selection of indole-3-acetic acid as
a known root growth inhibitor without an anti-proliferative effect.
The most frequent problem observed was the low water solubility of
some compounds that hindered testing on higher concentrations that
would have produced an inhibition >50%. This is probably the
main reason for the low sensitivity of the test.
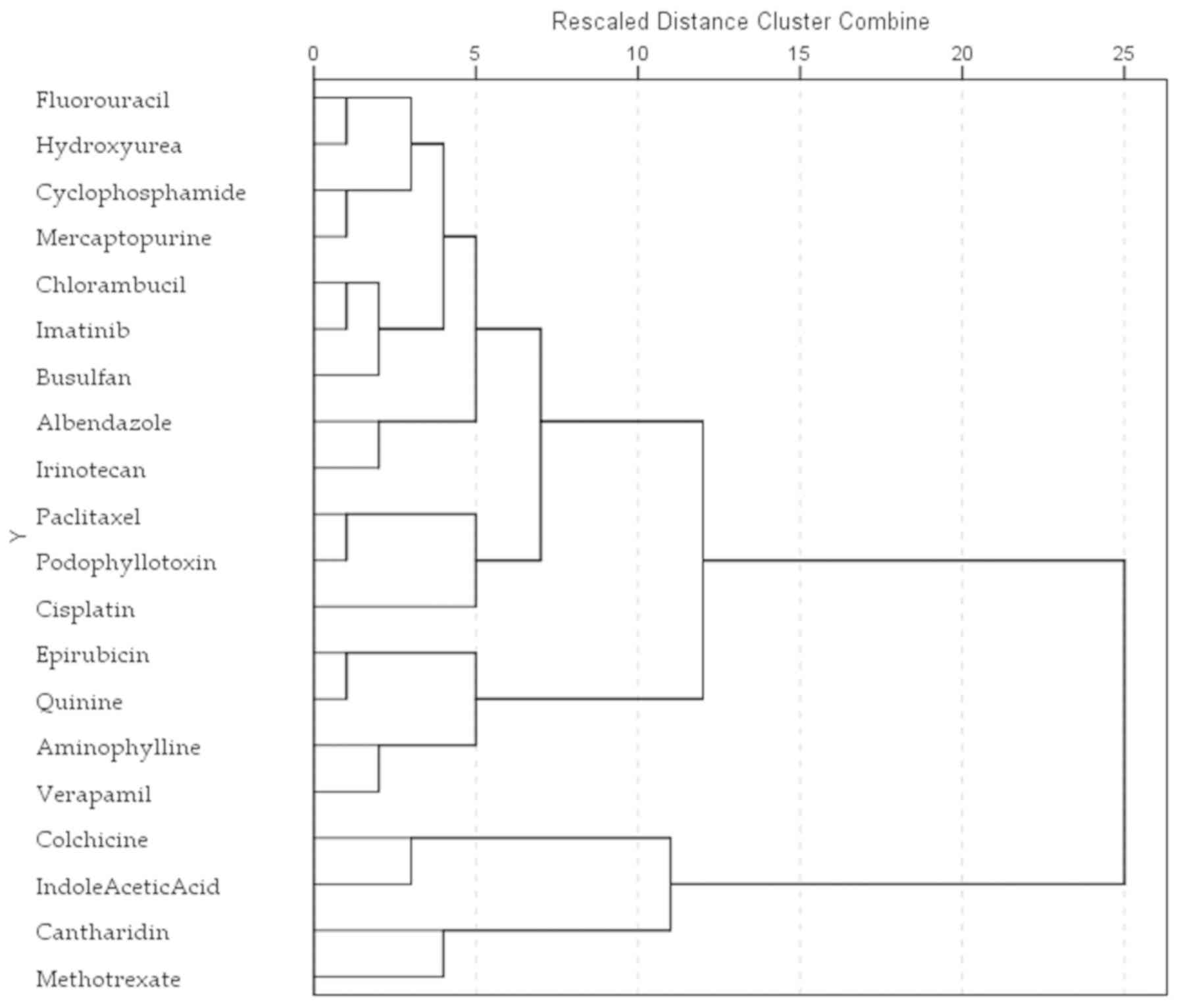
Cluster analysis of the root elongation
inhibitory effects
In order to better determine the wheat root
inhibition profile of all the 20 tested substances, a hierarchical
cluster analysis was performed using the furthest neighbor method
and Euclidean distance measure. Three major clusters were obtained
(Fig. 2). The first is
represented by colchicine, methotrexate, cantharidin and
indole-3-acetic acid as the group of potent root elongation
inhibitors; the second comprises epirubicin, quinine, verapamil,
and aminophylline, compounds with a stimulatory effect at low
concentrations and an inhibitory effect at larger doses. The third
group contains compounds with low inhibitory effects: cisplatin,
podophyllotoxin, paclitaxel, irinotecan, alben-dazole, busulfan,
imatinib, chlorambucil, mercaptopurine, cyclophosphamide,
hydroxyurea and fluorouracil.
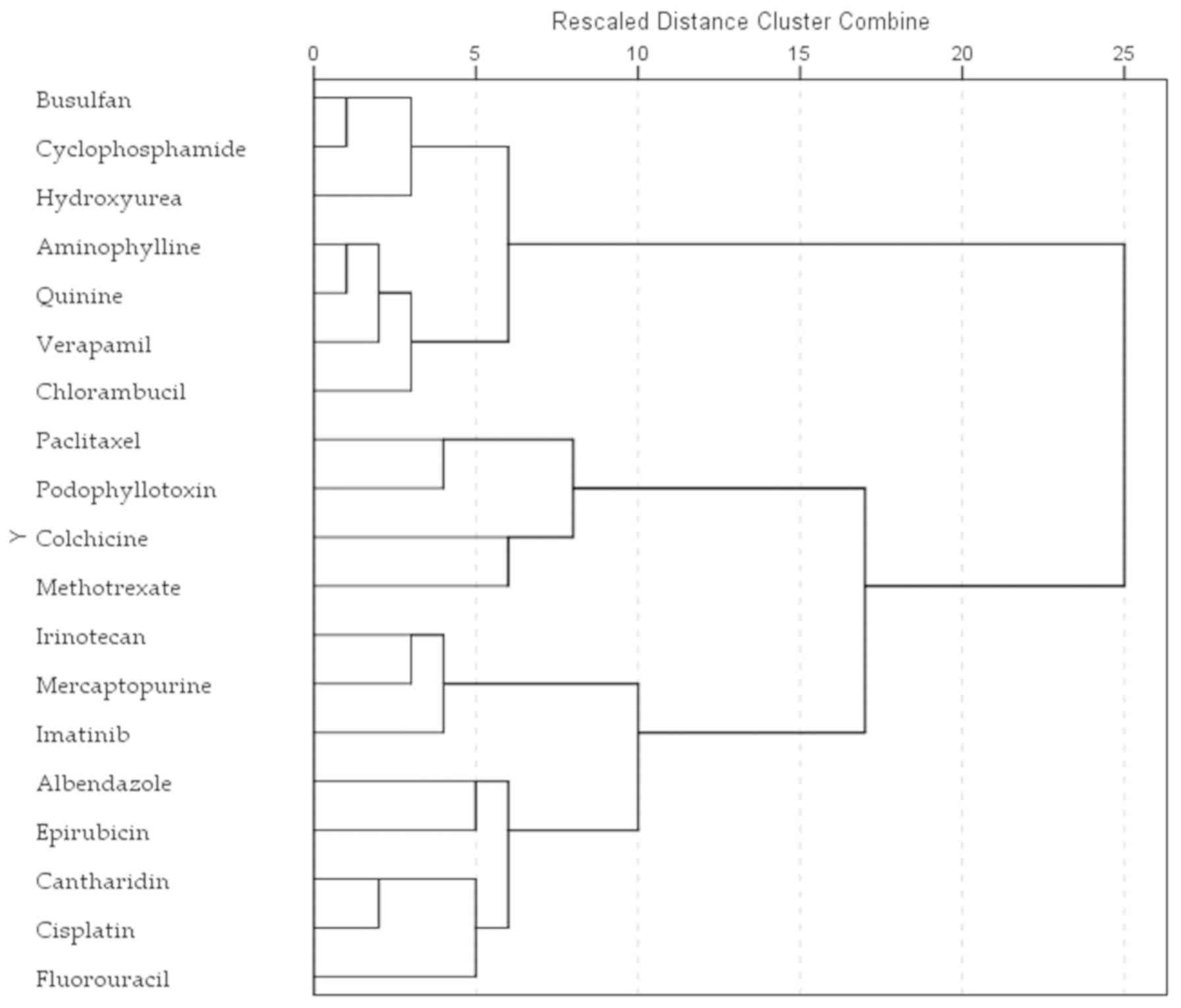
Cluster analysis of the NCI60
anti-proliferative profile
All anticancer agents tested in the Triticum
assay were statistically analyzed based on their anti-proliferative
pattern registered in the NCI60 human tumor cell line anticancer
drug screen. Indole-3-acetic acid was not included in the
classification as it was not tested in the NCI60 5-dose screening.
The NCI60 5-dose assay results in a characteristic fingerprint of
cellular response that can be used to assign a mechanism of action,
or to classify the compounds. NCI defines the half maximal growth
inhibition (GI50) as the molar concentration that causes 50%
inhibition for each cell line, and pGI50 as the negative logarithm
of GI50. The pGI50 values were selected as the end-point for this
study.
The anti-proliferative effect of a compound on a
single cell line offers little information, but an array of effects
across the 60 cell lines gives important strength to the method.
All tested compounds were hierarchically clustered using the
furthest neighbor approach and the Euclidean distance measure on
the 60×19 matrix of pGI50 values (Fig. 3).
The patterns of growth inhibition in the NCI60
5-dose assay have been used in a number of studies to identify the
mechanism for new compounds, or to better determine their effects
on specific oncologic targets (29,31,33). The results of our classification
produced different clusters than the results of Triticum
root assay, but some interesting similarities can be pointed
out.
In both human and plant cells, methotrexate and
colchicine demonstrated comparable profiles. Paclitaxel and
podophyllotoxin produced similar profiles in human cells and in the
root elongation test, in concordance with the known mechanism of
disrupting microtubule/tubulin dynamics. Paclitaxel binds to
polymerized microtubules and promotes tubulin stabilization, while
podophyllotoxin induces microtubule depolymerization (34). Colchicine presented the same
mechanism as podophyllotoxin; however, of note, the
anti-proliferative profile of the two compounds correlated only in
the human cells, while in the root cells its correlated better with
methotrexate. A good correlation of the colchicine and methotrexate
cell susceptibility can also be observed in the NCI60 data.
Correlation of Triticum test results and
NCI60 anti-proliferative profile
Methotrexate had the optimal inhibitory effect on
the Triticum assay, with a pIC50 value of -5.526 (Table III), compared to the values
registered on human cancer cells, ranging from -6.106 to -9.000,
with an average value of -7.895 (data not shown). Cantharidin also
produced a potent inhibitory effect on root elongation, with a
pIC50 value of -5.039, but closer to those in human cells where the
average was -5.780. In the case of colchicine the pIC50 was -4.684,
significantly higher than the average value calculated for the
human cells as -7.211 (data not shown). For all these compounds, as
well as cisplatin and verapamil, the inhibitory effect on the
Triticum test was registered at a higher concentration than
those noted on human cells. In the case of aminophylline, the
results could not be compared as the NCI60 assay protocol begins
with the highest concentration of 10-4 M and the pGI50
values can be anywhere above the -4 threshold.
The calculated pIC50 values were used to compare the
Triticum assay with the array of pGI50 values for all human
tumor cells from the NCI60 panel, in order to identify whether the
root elongation test correlates with a particular type of tumor
cell. The statistical analysis was performed for the methotrexate,
cantharidin, colchicine, cisplatin, aminophylline and verapamil
data arrays on Triticum against each cell line of the NCI60
panel. Indole-3-acetic acid was not used as the pGI50 values are
not registered on the NCI database. The correlation was significant
at the 0.05 level (2-tailed). Of all the 60 cell lines, a
significant correlation was registered for only 12 cell lines.
Plotting the 6 pGI50 values against the IC50, a conversion equation
was computed that can be used for the prediction of the
anti-proliferative effect on those cells based on the
Triticum assay (Table
IV).
 | Table IVCorrelation of the Triticum
assay with the anti-proliferative effects of selected drugs. |
Table IV
Correlation of the Triticum
assay with the anti-proliferative effects of selected drugs.
| Cell ID | Cell line | Tissue type | Pearson's
correlation | Sig.
(2-tailed) | Conversion
equation | r2 |
|---|
| 1004 | A549 | Non-small cell
lung | 0.878 | 0.021 | 1.358*x+0.103 | 0.771 |
| 4003 | HCT-116 | Colon | 0.839 | 0.037 | 1.533*x+0.565 | 0.703 |
| 4015 | HCT-15 | Colon | 0.833 | 0.039 | 1.224*x−0.299 | 0.694 |
| 7003 | CCRF-CEM | Leukemia | 0.813 | 0.049 | 1.397*x+0.143 | 0.661 |
| 7005 | K-562 | Leukemia | 0.832 | 0.040 | 1.605*x+0.886 | 0.693 |
| 9008 | SN12C | Renal | 0.829 | 0.041 | 1.273*x−0.221 | 0.688 |
| 9018 | 786-0 | Renal | 0.859 | 0.028 | 1.404*x+0.272 | 0.738 |
| 9023 | ACHN | Renal | 0.893 | 0.017 | 1.258*x−0.187 | 0.798 |
| 11001 | PC-3 | Prostate | 0.922 | 0.009 | 1.719*x+1.282 | 0.850 |
| 11003 | DU-145 | Prostate | 0.853 | 0.031 | 1.128*x−0.509 | 0.728 |
| 12009 | U251 | Central nervous
system | 0.826 | 0.043 | 1.333*x−0.075 | 0.683 |
| 12014 | SF-268 | Central nervous
system | 0.882 | 0.020 | 1.233*x−0.259 | 0.777 |
The results indicated a similar effect of the tested
6 drugs on the wheat root elongation and on 12 human cancer cell
lines, representing 6 of the 9 tissue types of the NCI60 panel.
There seemed to be no association between the cell origin and the
existence of a correlation with the Triticum test. We
defined these 12 cell lines as the Triticum cell panel.
Comparing these cell lines with those uncorrelated in order to
illustrate the interdependence factors, a simple observation was
made that the cells doubling times were smaller in the
Triticum cell panel, with an average of 25.2 h, compared to
37.45 h in the rest of the cells (data not shown). A two-sample
t-test for unpaired data was performed and demonstrated a
significant difference (P<0.01) between the two set doubling
times. In addition, cells such as HOP-92, NCI-H226, or A498, which
have a doubling time over 60 h, share a Pearson's coefficient under
0.5 with the IC50 array of values (data not shown).
Table IV
describes 12 linear equations that can be used to transform the
IC50 value calculated in any future Triticum assay in order
to predict the pGI50 value of the Triticum cells panel. For
all these cells, the computed equations indicate higher pGI50
values, compared with the IC50 value, arguing to a higher
resistance of the wheat root cells to the effect of the anticancer
drugs. These equations could be reversed to evaluate the
environmental impact of antineoplastic drugs on higher plants.
Discussion
The Triticum root elongation test is a simple
and inexpensive method for screening novel chemical compounds;
however, its use is often empirical. The aim of this study was to
validate this method for assessing the anti-proliferative effects
of synthetic or natural compounds and to use it as an alternative
technique.
The results indicated that when used on its own, the
Triticum test is not a very good choice for the selection of
novel anti-proliferative compounds, due to the high false-negative
ratio. Of the tested 18 anti-proliferative agents, only 5 were
identified as inhibitors of root elongation.
Methotrexate registered the highest impact on the
Triticum root. It is one of the earliest anticancer drugs,
an antifolate that mainly inhibits dihydrofolate reductase, leading
to impaired purine synthesis. As a result, malignant cells are
unable to synthesize DNA and RNA, leading to cell apoptosis
(35). This confirms the findings
of previous studies emphasizing the crucial role of folates in root
development (36,37). Cantharidin is a natural substance,
secreted by blister beetles, particularly Lytta vesicatoria,
which inhibits protein phosphatase 2A. Protein phosphatases are
involved in multiple cellular processes, although the exact pathway
through which they exert growth inhibitory effects and cell death
remains unclear (38). This study
indicated a similar pattern of response for cantharidin and
methotrexate in plants, but different in human cells.
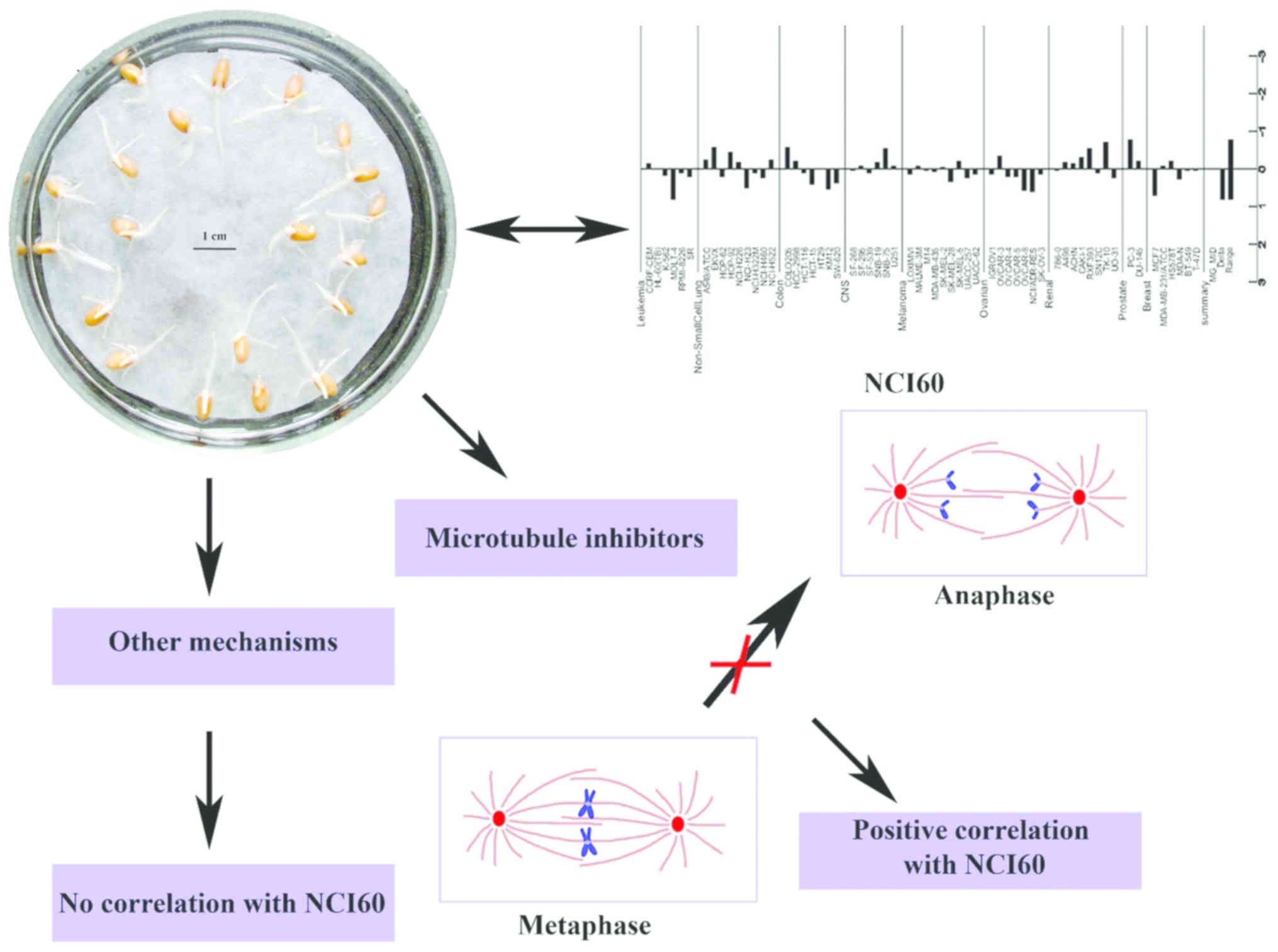
Colchicine, podophyllotoxin and paclitaxel belong to
the larger group of spindle assembly inhibitors or spindle poisons,
compounds that interrupt the mitosis phase of cell division
(25). Previous studies have
indicated that cantharidin also disrupts the organization of
spindles, resulting in a prolonged mitotic arrest (38,39). In this study, all these compounds
inhibited root elongation, indicating that the Triticum root
assay can effectively detect drugs acting as mitotic inhibitors
(Fig. 4).
Cisplatin induces cytotoxicity by interfering with
DNA repair mechanisms, causing DNA damage and inducing the
apoptosis of cancer cells (40).
Cisplatin has a very similar mechanism as busulfan, chlorambucil
and cyclophosphamide (41,42);
however, these compounds had little effect on root elongation. The
most probable explanation is their low potency on human cancer
cells.
The Triticum test can be helpful for future
research, particularly if is coupled with some simple tests to
evaluate the toxicological profile of a new compound or plant
extract, such as the Daphnia assay (43-45). A high inhibitory effect on the
root elongation combined with a low toxic effect can be a good
indicator of an anti-proliferative effect on the cell lines
described as the Triticum panel. Using the conversion
functions presented herein, a good estimation of the GI50 value can
be obtained. The repurposing of plants herbicides as potential
anticancer leads can be an important future direction to use the
Triticum test. Phosphinothricin is such an example emerging
as a novel anticancer agent against MCF-7 breast cancer and A549
lung cancer cell lines (46).
The results of this study suggested that the
Triticum root elongation test can detect several types of
anti-proliferative mechanisms, as described above, particularly
those targeting tubulin. Triticum assay may be an effective
good tool which could be used to identify novel spindle inhibitors.
Some studies (mentioned above) have reported the possibility of
repurposing known herbicides as cytotoxic agents.
In conclusion, the findings of the present study
demonstrate that the Triticum root elongation assay is a
simple and inexpensive method when used empirically for the
screening of novel chemical compounds. The results indicate that on
its own, the Triticum test is not a very good choice for the
selection of novel anti-proliferative compounds, due to the high
false-negative ratio; however, it can be useful if coupled with
simple tests that can evaluate the toxicological profile. This
study indicates that the Triticum assay may prove to be a
good tool which could be used to identify novel spindle inhibitors
and for the repurposing of plant herbicides as potential anticancer
agents. It may prove to be an important assay for future research
direction.
Funding
This study was financially supported by the 'Carol
Davila' University of Medicine and Pharmacy through Contract no.
23PFE/17.10.2018 funded by the Ministry of Research and Innovation
within PNCDI III, Program 1-Development of the National RD system,
Subprogram 1.2-Institutional Performance-RDI excellence funding
projects.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
OTO and GMN were involved in the conceptualization
of the study. CEDP was responsible for funding acquisition. OTO,
AZ, GMN, GN, CEDP, VA, AT, DAS, DM and OCS were all involved in the
study methodology, in writing, review and editing of the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing interests.
The founding sponsors had no role in the design of the study, or in
the collection, analyses, or interpretation of the data, or in the
writing of the manuscript, and in the decision to publish the
results.
Acknowledgments
Not applicable.
References
|
1
|
Festing S and Wilkinson R: The ethics of
animal research. Talking point on the use of animals in scientific
research. EMBO Rep. 8:526–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mak IWY, Evaniew N and Ghert M: Lost in
translation: Animal models and clinical trials in cancer treatment.
Am J Transl Res. 6:114–118. 2014.PubMed/NCBI
|
|
3
|
North M and Vulpe CD: Functional
toxicogenomics: Mechanism-centered toxicology. Int J Mol Sci.
11:4796–4813. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gatehouse D: Bacterial mutagenicity
assays: Test methods. Genetic Toxicology: Principles and Methods.
Parry JM and Parry EM: Springer; New York, New York, NY: pp. 21–34.
2012, View Article : Google Scholar
|
|
5
|
Bagur-González MG, Estepa-Molina C,
Martín-Peinado F and Morales-Ruano S: Toxicity assessment using
Lactuca sativa L. bioassay of the metal(loid)s As, Cu, Mn, Pb and
Zn in soluble-in-water saturated soil extracts from an abandoned
mining site. J Soils Sediments. 11:281–289. 2011. View Article : Google Scholar
|
|
6
|
Czerniawska-Kusza I, Ciesielczuk T, Kusza
G and Cichoń A: Comparison of the Phytotoxkit microbiotest and
chemical variables for toxicity evaluation of sediments. Environ
Toxicol. 21:367–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sánchez-Meza J, Pacheco-Salazar V,
Pavón-Silva T, Guiérrez-García V, Avila-González CJ and
Guerrero-García P: Toxicity assessment of a complex industrial
wastewater using aquatic and terrestrial bioassays Daphnia pulex
and Lactuca sativa. J Env Sci Heal A Tox Hazard Subst Env Eng.
42:1425–1431. 2007. View Article : Google Scholar
|
|
8
|
Pandard P, Devillers J, Charissou AM,
Poulsen V, Jourdain MJ, Férard JF, Grand C and Bispo A: Selecting a
battery of bioassays for ecotoxicological characterization of
wastes. Sci Total Environ. 363:114–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guțu CM, Olaru OT, Purdel CN, Ilie M and
Diacu E: Phytotoxicity of inorganic arsenic assessed by Triticum
test. Rev Chim. 66:333–335. 2015.
|
|
10
|
Ahmed FAW: Cytotoxic and genotoxic potency
screening of WIDE-SPEC pesticide on Allium cepa L. root meristem
cells. J Nat Sci Res. 4:100–108. 2014.
|
|
11
|
Dragoeva A, Koleva V, Hasanova N and
Slanev S: Cytotoxic and genotoxic effects of diphenyl-ether
herbicide GOAL (Oxyfluorfen) using the Allium cepa test. Res J
Mutagen. 2:1–9. 2012. View Article : Google Scholar
|
|
12
|
Șeremet OC, Olaru OT, Ilie M, Negres S and
Balalau D: Phytotoxicity assessment of certain phytochemical
products containing pyrrolizidine alkaloids. Acta Med Marisiensis.
59:250–253. 2013. View Article : Google Scholar
|
|
13
|
Lee WM, An YJ, Yoon H and Kweon HS:
Toxicity and bioavailability of copper nanoparticles to the
terrestrial plants mung bean (Phaseolus radiatus) and wheat
(Triticum aestivum): Plant agar test for water-insoluble
nanoparticles. Environ Toxicol Chem. 27:1915–1921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ashrafi ZY, Sadeghi S and Mashhadi HR:
Inhibitive effects of barley (Hordeum vulgare) on germination and
growth of seedling quack grass (Agropyrum repens). Icel Agric Sci.
22:37–43. 2009.
|
|
15
|
Yuet Ping K, Darah I, Yusuf UK, Yeng C and
Sasidharan S: Genotoxicity of Euphorbia hirta: An Allium cepa
assay. Molecules. 17:7782–7791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pawlowski Â, Kaltchuk-Santos E, Zini CA,
Caramão EB and Soares GLG: Essential oils of Schinus
terebinthifolius and S. molle (Anacardiaceae): Mitodepressive and
aneugenic inducers in onion and lettuce root meristems. S Afr J
Bot. 80:96–103. 2012. View Article : Google Scholar
|
|
17
|
Diaz Napal GN and Palacios SM:
Phytotoxicity of secondary metabolites isolated from Flourensia
oolepis S.F.Blake. Chem Biodivers. 10:1295–1304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furmanowa M, Guzewska J and Bełdowska B:
Mutagenic effects of aqueous extracts of Symphytum officinale L.
and of its alkaloidal fractions. J Appl Toxicol. 3:127–130. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zasada IA, Klassen W, Meyer SL, Codallo M
and Abdul-Baki AA: Velvetbean (Mucuna pruriens) extracts: Impact on
Meloidogyne incognita survival and on Lycopersicon esculentum and
Lactuca sativa germination and growth. Pest Manag Sci.
62:1122–1127. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andrade-Vieira LF, Botelho CM, Laviola BG,
Palmieri MJ and Praça-Fontes MM: Effects of Jatropha curcas oil in
Lactuca sativa root tip bioassays. An Acad Bras Cienc. 86:373–382.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stampoulis D, Sinha SK and White JC:
Assay-dependent phytotoxicity of nanoparticles to plants. Environ
Sci Technol. 43:9473–9479. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jitǎreanu A, Pǎdureanu S, Tǎtǎrîngǎ G,
Tuchilus GC and Stǎnescu U: Evaluation of phytotoxic and mutagenic
effects of some cinnamic acid derivatives using the Triticum test.
Turk J Biol. 37:748–756. 2013. View Article : Google Scholar
|
|
23
|
Radić S, Stipaničev D, Vujcić V, Rajcić
MM, Širac S and Pevalek-Kozlina B: The evaluation of surface and
wastewater genotoxicity using the Allium cepa test. Sci Total
Environ. 408:1228–1233. 2010. View Article : Google Scholar
|
|
24
|
Beemster GTS and Baskin TI: Analysis of
cell division and elongation underlying the developmental
acceleration of root growth in Arabidopsis thaliana. Plant Physiol.
116:1515–1526. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Komlodi-Pasztor E, Sackett DL and Fojo AT:
Inhibitors targeting mitosis: Tales of how great drugs against a
promising target were brought down by a flawed rationale. Clin
Cancer Res. 18:51–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chabner BA: NCI-60 Cell Line Screening: A
radical departure in its time. Natl Cancer Inst. 108:pii: djv388.
2016. View Article : Google Scholar
|
|
27
|
Devinyak O, Havrylyuk D, Zimenkovsky B and
Lesyk R: Computational search for possible mechanisms of
4-thia-zolidinones anticancer activity: The power of visualization.
Mol Inform. 33:216–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soriga SG, Plesu V, Marton A, Bonet-Ruiz
J, Marton GI and Iancu P: Small computer clusters for molecular
modelling. Rev Chim. 65:960–965. 2014.
|
|
29
|
Khan SA, Virtanen S, Kallioniemi OP,
Wennerberg K, Poso A and Kaski S: Identification of structural
features in chemicals associated with cancer drug response: A
systematic data-driven analysis. Bioinformatics. 30:i497–i504.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nitulescu GM, Soriga SG, Socea LI, Olaru
OT and Plesu V: Structure-activity relationships and
chemoinformatic analysis of the anticancer profile of an
aminopyrazole derivative. Rev Chim. 67:162–165. 2016.
|
|
31
|
Nitulescu GM, Iancu G, Nitulescu G, Iancu
RC, Bogdanici C and Vasile D: Brave new hope for breast cancer:
Aminopyrazole derivates between rational design and clinical
efficacy. Rev Chim. 68:754–757. 2017.
|
|
32
|
Fu SF, Wei JY, Chen HW, Liu YY, Lu HY and
Chou JY: Indole-3-acetic acid: A widespread physiological code in
interactions of fungi with other organisms. Plant Signal Behav.
10:e1048052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nitulescu GM, Soriga SG, Socea LI, Olaru
OT and Plesu V: Structure-activity relationships and
chemoinformatic analysis of the anticancer profile of an
aminopyrazole derivative. Rev Chim. 67:162–165. 2016.
|
|
34
|
Lu Y, Chen J, Xiao M, Li W and Miller DD:
An overview of tubulin inhibitors that interact with the colchicine
binding site. Pharm Res. 29:2943–2971. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Papachristou M, Kastis GA, Stavrou PZ,
Xanthopoulos S, Furenlid LR, Datseris IE and Bouziotis P:
Radiolabeled metho-trexate as a diagnostic agent of inflammatory
target sites: A proof-of-concept study. Mol Med Rep. 17:2442–2448.
2018.
|
|
36
|
Giordano D, Reyneri A and Blandino M:
Folate distribution in barley (Hordeum vulgare L.), common wheat
(Triticum aestivum L.) and durum wheat (Triticum turgidum durum
Desf.) pearled fractions. J Sci Food Agric. 96:1709–1715. 2016.
View Article : Google Scholar
|
|
37
|
Gorelova V, Ambach L, Rébeillé F, Stove C
and Van Der Straeten D: Folates in plants: Research advances and
progressin crop biofortification. Front Chem. 5:212017. View Article : Google Scholar
|
|
38
|
Liu Y-P, Li L, Xu L, Dai E-N and Chen WD:
Cantharidin suppresses cell growth and migration, and activates
autophagy in human non-small cell lung cancer cells. Oncol Lett.
15:6527–6532. 2018.PubMed/NCBI
|
|
39
|
Bonness K, Aragon IV, Rutland B,
Ofori-Acquah S, Dean NM and Honkanen RE: Cantharidin-induced
mitotic arrest is associated with the formation of aberrant mitotic
spindles and lagging chromosomes resulting, in part, from the
suppression of PP2Aalpha. Mol Cancer Ther. 5:2727–2736. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun Y, Jiang W, Lu W, Song M, Liu K, Chen
P, Chang A, Ling J, Chiao PJ, Hu Y, et al: Identification of
cisplatin sensitizers through high-throughput combinatorial
screening. Int J Oncol. 53:1237–1246. 2018.PubMed/NCBI
|
|
41
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3. pp.
1351–1371. 2011, View Article : Google Scholar
|
|
43
|
Pahontu E, Socea LI, Barbuceanu SF, Nou
Autor, Octavian-Tudorel Olaru, Aurelian Gulea and Bogdan Socea:
Synthesis, characterization and toxicity evaluation of Cu(II),
Mn(II), Co(II), Ni(II), Pd(II) complexes with ligand derived from
hydrazinecarbothioamide. Rev Chim. 69:2959–2963. 2018.
|
|
44
|
Nitulescu G, Nicorescu IM, Olaru OT,
Ungurianu A, Mihai DP, Zanfirescu A, Nitulescu GM and Margina D:
Molecular docking and screening studies of new natural sortase A
inhibitors. Int J Mol Sci. 18:182017. View Article : Google Scholar
|
|
45
|
Socea LI, Barbuceanu SF, Socea B, Draghici
C, Apostol TV, Pahontu EM and Olaru OT: New heterocyclic compounds
from 1,2,4-triazoles class with potential cytotoxic activity. Rev
Chim. 68:2503–2508. 2017.
|
|
46
|
Sakr MT, Khedr AM, Rashed MH and Mohamed
EM: In silico-based repositioning of phosphinothricin as a novel
technetium-99m imaging probe with potential anti-cancer activity.
Molecules. 23:pii: E496. 2018. View Article : Google Scholar
|