Introduction
In the early stages of pregnancy (<10 weeks),
trophoblasts exist in a hypoxic environment (1-2% O2)
and hypoxia serves an important role in normal trophoblast
differentiation and development (1-4).
In addition, hypoxia has been shown to directly induce trophoblast
apoptosis and represents a significant pathological factor that
contributes to the deficient placentation observed in the early
onset of preeclampsia (PE) (5-8).
PE is one of the most common pathological complications of
pregnancy and is a leading cause of maternal and perinatal
morbidity and mortality (9). As
for the pathogenesis of PE, it is commonly believed that inadequate
migration and the reduced invasive ability of placental
extravillous trophoblasts (EVTs) may contribute to PE development
(10). A recent study with PE
placental explants reported that increased apoptosis and lack of
proliferation stimulation were observed in trophoblasts (11). These studies suggest that
placental hypoxia is critical for trophoblast physiology and
pathology, and apoptosis may be involved in diseases of placental
origin. Gaining greater understanding of these mechanisms and
pathophysiology in the first trimester of pregnancy will be
beneficial for PE treatment and therapy approaches.
p57 [also known as cyclin-dependent kinase inhibitor
1C or kinase inhibitory protein (KIP)-2; p57KIP2] is
ubiquitously expressed in human placental cells (12,13). p57KIP2 is considered to
be a master regulator of the cell cycle during embryogenesis
(14-17). Pregnant mice with
p57KIP2 deficiency presented with symptoms similar to PE
(10). Several studies have shown
the proapoptotic role of p57KIP2 in the pathogenesis of
different cancer cell lines (18-21). An antiapoptotic activity of
p57KIP2 has also been observed during embryogenesis
(19,22-24). A previous study reported that
p57KIP2 was significantly increased in all cell types of
preeclamptic human placentas with the exception of
syncytiotrophoblasts (12). A
decrease in p57KIP2 expression was implicated with
abnormal trophoblast proliferation (25). However, the role of
p57KIP2 in trophoblast function and apoptosis remains
unclear. In the present study, p57KIP2 expression in
trophoblasts (HTR-8/SVneo) under hypoxic conditions was evaluated
and attempts were made to further investigate the effect of
p57KIP2 on migration, invasion and apoptosis in response
to hypoxia. The results showed that overexpression of
p57KIP2 decreased apoptosis and increased cell invasion
and migration in HTR-8/SVneo cells exposed to 2% O2.
This may be mediated by the JNK/stress activated protein kinase
(SAPK) signaling pathway. The present study may provide a novel
molecular mechanism to understand the involvement of
p57KIP2 in the pathogenesis of preeclampsia.
Materials and methods
Cell culture and treatment
The human cell line HTR-8/SVneo was provided by
Professor Wenming Xu (Sichuan University, Chengdu, China) (26), derived from short-lived primary
EVTs, was used in the present study. Cells were cultured in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin.
Cells were plated in 6-wells plates (2×105 cells/well).
After 24 h in non-hypoxic atmosphere (37°C; 5% CO2; 21%
O2), cells were transferred to an incubator with 2%
O2 and 5% CO2 atmosphere for 0, 12, 24, 48 or
72 h at 37°C.
Plasmid constructs and transfection
Small interfering (si)RNA that mediated the
knockdown of human p57KIP2 (si-p57) and the nonspecific
siRNA control (si-ctrl) were obtained from Thermo Fisher
Scientific, Inc. The present study used the following oligos:
si-p57, forward, 5′-GGACCUUCCCAGUACUAGUTT-3′ and reverse,
5′-ACUAGUACUGGGAAGGUCCTT-3′; and si-ctrl, forward,
5′-AAGGGACTTCCTGTAACAATGCA-3′ and reverse,
5′-CTGGAACGGTGAAGGTGACA-3′. To ectopically express
p57KIP2, the synthetic human p57KIP2 sequence
(950 bp) was cloned into the plasmid vector. The overexpression
plasmids of p57KIP2 (pcDNA3.1-p57) and empty vector
plasmid as the control (pcDNA3.1) were purchased from ProteinTech
Group, Inc. Cells (2×105) were cultured in 6-well plates
overnight prior to transfection. At 70% confluence, cells were
transiently transfected with pcDNA3.1 empty vector, pcDNA3.1-p57,
si-p57 or si-ctrl at 50 nmol/l. Cell transfection was performed
using Lipofectamine 3000™ (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instruction. Following transfection
for 24 h, cells were incubated under hypoxic conditions for 24 h
and then harvested to test the knockdown or overexpression
efficiency via RT-qPCR and western blotting, respectively.
Drug treatment
The JNK inhibitor SP600125 was purchased from
Sigma-Aldrich (Merck KGaA) and dissolved in DMSO (final
concentration, 10 µM). Transfected HTR-8/SVneo cells
(2×105) were incubated with SP600125 for 30 min prior to
and throughout hypoxia culturing for 24 h. Cells were then
harvested for subsequent experiments. All cells were compared with
a control group, which received an equal volume of DMSO.
Cell migration and invasion assays
Cell migration and invasion was determined by
Transwell assay as described previously (27). For the migration assay, cells were
resuspended in RPMI-1640 medium containing 1% FBS and placed into
the upper well of a Transwell chamber (pore diameter, 8 µm) at
2.5×105 cells/well. Medium containing 10% FBS was placed
into the lower wells as a chemoattractant. Cells were incubated
under hypoxic conditions for 24 h. Invasion assays were performed
as the migration assays, but the filters were coated with growth
factor-induced Matrigel (BD Biosciences). Following incubation,
cells on the upper surface of the membrane were collected with a
sterile cotton swab. Cells that migrated to the lower surface were
fixed and stained with 0.1% crystal violet for 10 min at room
temperature. The number of migrated and invaded cells was examined
via digital light microscopy (magnification, ×20). A total of ten
visual fields were counted for each sample.
Flow cytometry
HTR-8/SVneo cells were harvested after 24 h hypoxia
using trypsin without EDTA, washed with PBS, resuspended in 1 ml
binding buffer (eBioscience™ Annexin V-FITC Apoptosis kit;
#BMS500FI-100; Invitrogen; Thermo Fisher Scientific, Inc.) and
stained for 15 min with FITC-Annexin V and propidium iodide in the
dark at room temperature. The analysis of the cells was conducted
via flow cytometry (FACScan; BD Biosciences) equipped with
CellQuest V6.0 (BD Biosciences). Cells were sorted into living,
necrotic, early apoptotic and late apoptotic cells. The relative
ratio of early to late apoptotic cells was counted for further
comparison. This assay was repeated >3 times.
TUNEL assays
Apoptosis was validated using the In Situ
Cell Death Detection kit (Roche Diagnostics). Cells
(5×104) on coverslips were fixed with 4% PFA for 30 min
at room temperature and washed twice with PBS (pH 7.0). Cells were
then treated with 3% H2O2 for 10 min at room
temperature, 0.1% Triton X-100 for 2 min at 4°C, incubated with
TUNEL reaction mixture at 37°C for 1 h and then stained with DAPI
(1:100) at room temperature for 10 min. Images were taken using a
fluorescent inverted microscope (magnification, ×40; Nikon
Corporation). The percentage of TUNEL-positive cells to apoptotic
nuclei was determined in three independent samples and quantified
using ImageJ 1.8.0 (National Instituted of Health). The apoptotic
index (AI) was calculated as follows: AI = (number of apoptotic
cells/total number of cells) ×100%.
Western blot analysis
Cells were lysed in cold radioim-munoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology) containing
protease inhibitor cocktails. Bicinchoninic acid assays were used
to quantify protein concentrations. Equal amounts of protein (80
µg) were separated on 10% SDS-PAGE gels and transferred to PVDF
membranes. Membranes were incubated with rabbit monoclonal
antibodies, including anti-p57KIP2 (1:700; #2557; Cell
Signaling Technology, Inc.), anti-p53 (1:1,000; #ab183544; Abcam),
anti-Bax (1:2,000; #50599-2-Ig; ProteinTech Group, Inc.),
anti-Bcl-2 (1:1,000; #12789-1-AP; ProteinTech Group, Inc.),
anti-caspase3 (1:1,000; #9662; Cell Signaling Technology, Inc.),
anti-cleaved caspase3 (1:1,000; #9664P; Cell Signaling Technology,
Inc.), anti-JNK (1:1,000; #9252; Cell Signaling Technology, Inc.),
anti-phosphorylated (p-)Thr183/Tyr18-JNK (p-JNK; 1:1,000; #4671;
Cell Signaling Technology, Inc.) and anti-β-actin (1:10,000;
#AF0003; Zhengneng Biotechnology, Inc.) overnight at 4°C. Membranes
were washed with TBST and incubated with a secondary horseradish
peroxidase-conjugated anti-rabbit or anti-mouse antibody (1:10,000;
#A0208 or #A0208; Zhengneng Biotechnology, Inc.) at room
temperature for 1 h followed by enhanced chemiluminescence assay
for visualization (EMD Millipore). β-actin was used as a loading
control. The optical density of the bands was quantitatively
analyzed using ImageJ 1.8.0 (National Institutes of Health).
RT-qPCR
SYBR-Green-based RT-qPCR (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to examine changes in mRNA levels of
p57KIP2. Total RNA was isolated from the cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). PCR
was performed with the ABI Prism 7500 Sequence Detection system
(Thermo Fisher Scientific, Inc.). The reaction was subjected to
95°C for 60 sec followed by 40 cycles of 95°C for 15 sec and 60°C
for 60 sec. Primers for p57KIP2 were obtained from
Thermo Fisher Scientific, Inc. β-actin was used as the internal
control. All reactions were performed in triplicate. The primer
sequences were used as follows: p57KIP2, forward,
5′-TTCTCAGGCGCTGATCTCTT-3′ and reverse, 5′-AGCTGCACTCGGGGATTT-3′;
and β-actin, forward, 5′-AAGGGACTTCCTGTAACAATGCA-3′ and reverse,
5′-CTGGAACGGTGAAGGTGACA-3′. All results were normalized to β-actin.
Relative expression was calculated using the 2−ΔΔCq
method (27).
Immunofluorescence
Cells (5×104) were grown on a coverslip
and fixed with 4% PFA at room temperature for 30 min. Cells were
probed with rabbit anti-cleaved caspase3 at 4°C overnight. Alexa
Fluor 594 goat anti-rabbit IgG-FITC secondary antibody (1:500;
Invitrogen; Thermo Fisher Scientific, Inc.) was added for 2 h at
room temperature. DAPI (1:100) was used to stain the cell nuclei
for 10 min at room temperature. Cells were mounted with ProLong®
Gold antifade reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Images were obtained by an inverted fluorescence microscope
(magnification, ×60; Nikon Corporation) and analyzed using
Image-Pro Plus 6.0 (Media Cybernetics, Inc.) and Adobe Photoshop
CS5 (Adobe Systems, Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Comparisons between two groups were analyzed by unpaired
Student's t-tests. One-way ANOVA followed by Tukey's test was used
to assess differences among >2 groups. Data were analyzed using
GraphPad Prism 5 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
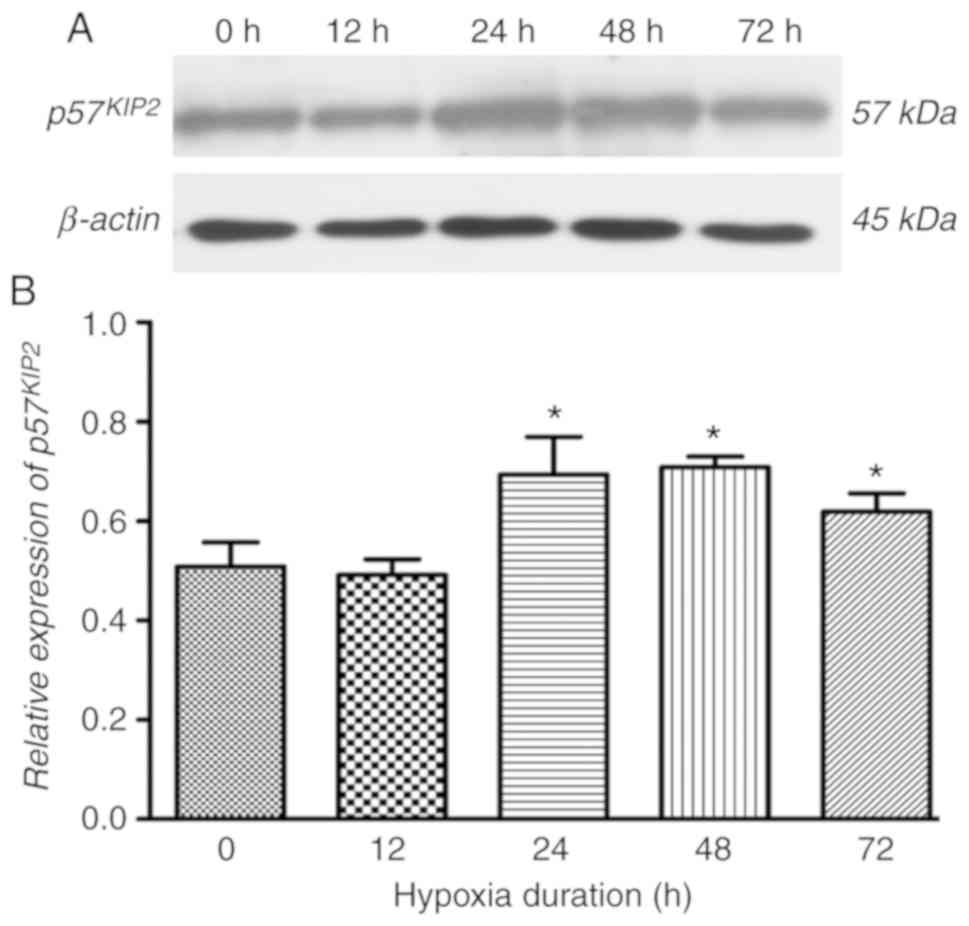
Hypoxia treatment induces
p75KIP2 expression
To investigate the effect of hypoxia on the
expression of p57KIP2 in trophoblasts, HTR-8/SVneo cells
were incubated with 2% O2. It was observed that the
expression of p57KIP2 was significantly increased when
exposed to hypoxia for 24-72 h compared with the cells at 0 h
(Fig. 1). In the following
experiments, hypoxia treatment for 24 h was chosen to elucidate the
mechanism.
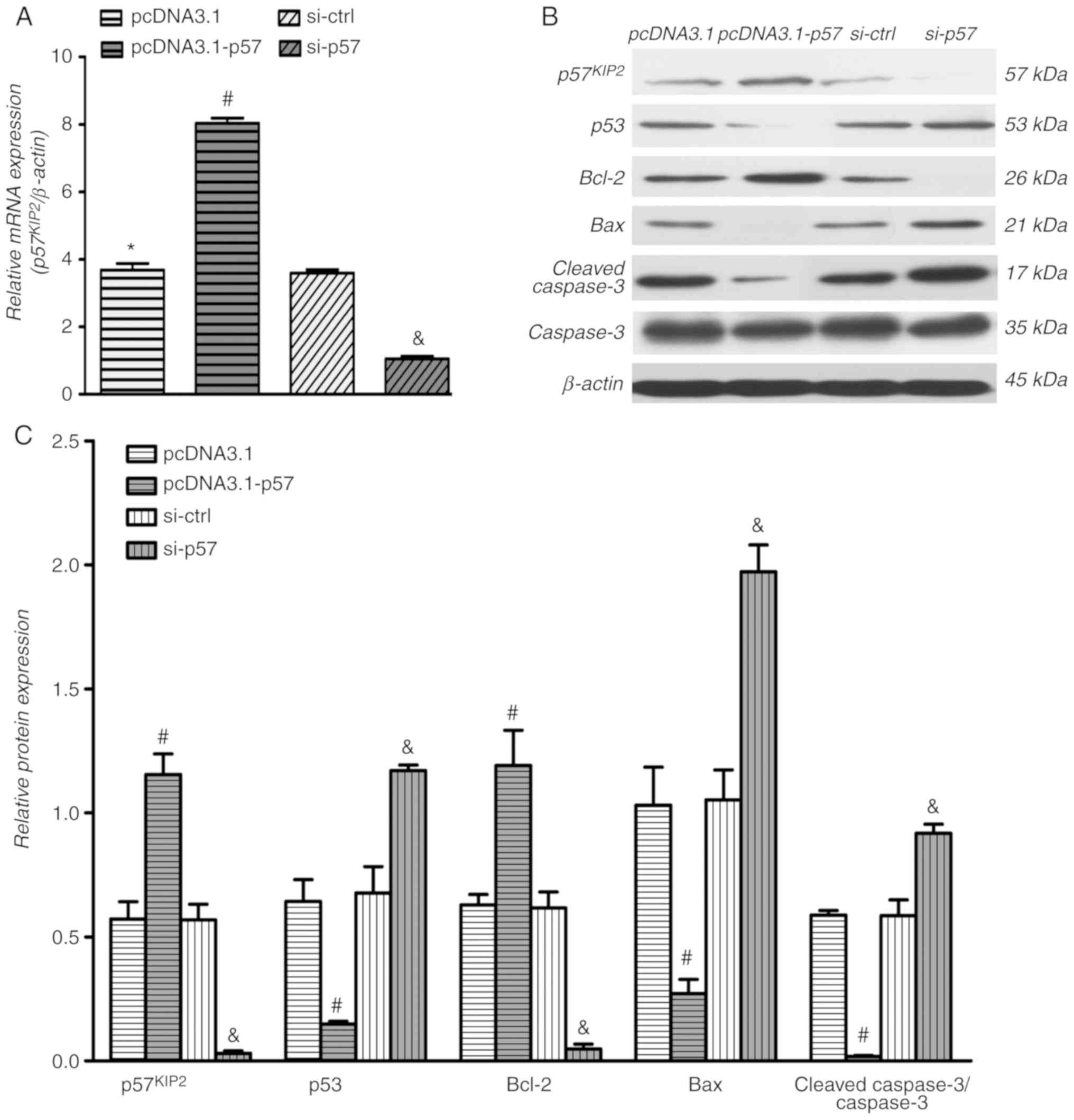
Overexpression and downregulation of
p57KIP2 in human trophoblasts
HTR-8/SVneo cells were transfected with the
pcDNA3.1-p57, si-p57 or respective controls for 24 h. Following
transfection, cells were exposed to hypoxia for further 24 h.
RT-qPCR results demonstrated that p57KIP2 was
significantly overexpressed in the pcDNA3.1-p57 transfected cells
compared with the empty vector control and p57KIP2 was
significantly downregulated in the presence of si-p57 compared with
si-ctrl (Fig. 2A). In addition,
the western blotting results of p57KIP2 were consistent
with the RT-qPCR findings (Fig.
2B).
p57KIP2 expression affects
cell apoptosis under hypoxic conditions
Western blot analysis, flow cytometry and TUNEL
assays were used to obtain comprehensive information regarding the
impact of p57KIP2 on cell apoptosis. As shown in
Fig. 2B, overexpression of
p57KIP2 increased the protein levels of the
antiapoptosis protein Bcl-2 and inhibited expression of the
proapoptosis proteins p53, Bax and cleaved caspase3 compared with
the empty vector control. The analysis further indicated that
expression levels of Bcl-2 decreased and expression of p53, Bax and
cleaved caspase3 increased in cells with p57KIP2
knockdown compared with the si-ctrl transfected cells. In
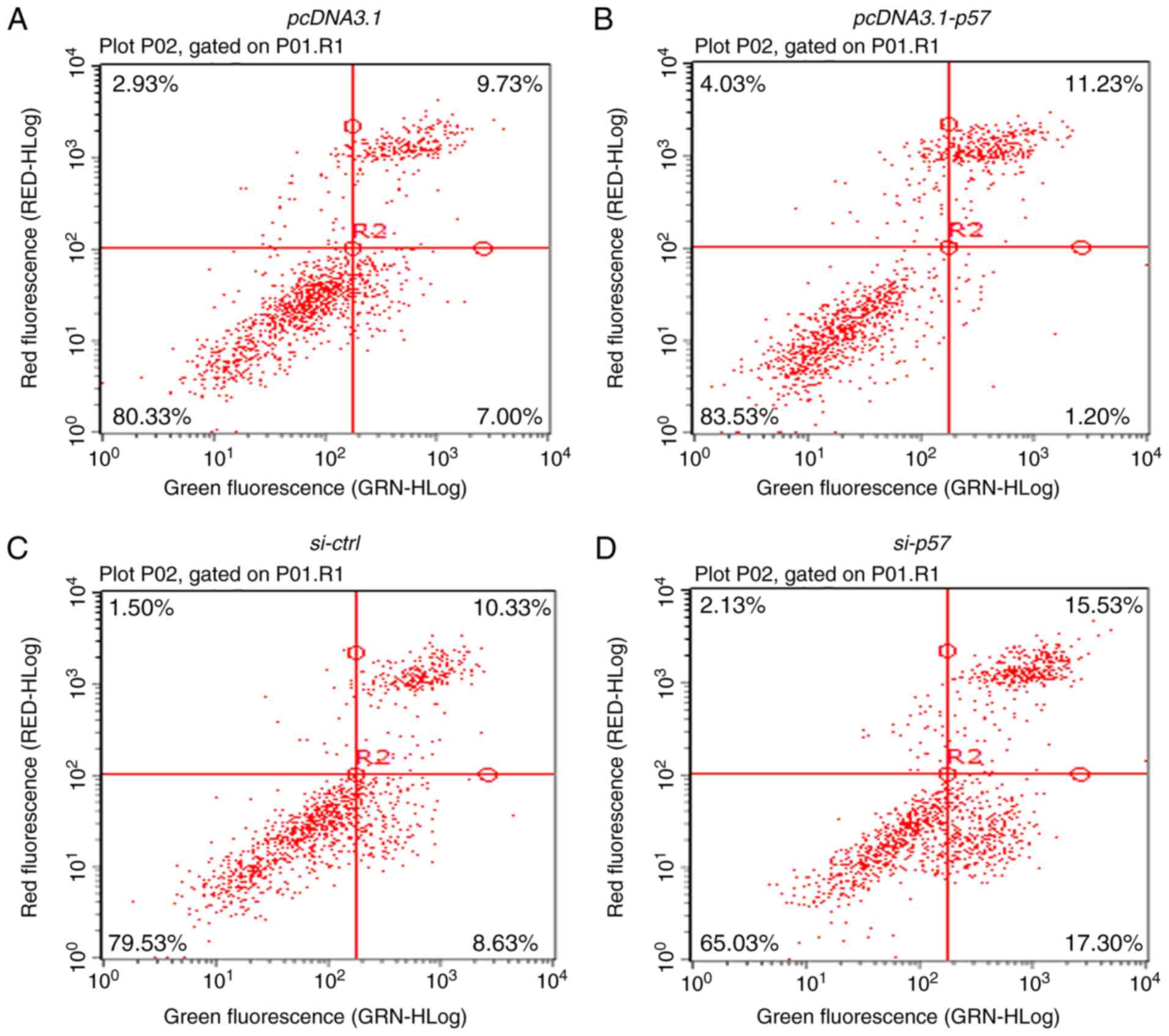
HTR-8/SVneo cells transfected with si-p57 a significant increase in
apoptosis was further observed compared with the si-ctrl cells and
a decrease in apoptosis was observed in the p57KIP2
overexpressing cells compared with the empty vector control
(Fig. 3A-D). Using TUNEL, a
similar rate of cell apoptosis was determined for the different
samples (Fig. 3E and F).
p57KIP2 inhibits the JNK
signaling pathway to affect apoptosis under hypoxia
The present study next explored the potential
mechanisms underlying the regulation of p57KIP2 on
apoptosis under hypoxic conditions. It has been reported that
p57KIP2 interacts with JNK/SAPK through its QT domain,
mediating a variety of cellular activities, including cell
differentiation and survival (28). As shown in Fig. 4, there was a decreasing trend in
p-JNK levels after 24 h of hypoxia in p57KIP2
overexpressing cells compared with the empty vector control.
SP600125, a JNK inhibitor, decreased the ratio of p-JNK/JNK at 24 h
compared with the DMSO-treated control cells. Inhibition of JNK
further decreased the expression of Bax and cleaved caspase3, and
increased the expression of Bcl-2 compared with the DMSO control.
Overexpression of p57KIP2 in combination with JNK
inhibitor treatment markedly decreased the ratio of p-JNK/JNK and
expression of Bax and cleaved caspase3, but increased Bcl-2
expression compared with the empty vector plus DMSO-treated
control. The effect of overexpression of p57KIP2 in
combination with JNK inhibitor treatment on Bax and Bcl-2 appeared
stronger compared with the overexpression of p57KIP2 or
JNK inhibitor treatment alone. The inhibition of JNK had no effect
on p53 expression levels. Cleaved caspase3 proteolytically cleaves
and activates other caspases and is thought to serve an important
role in apoptosis (29). As shown
in Fig. 5, immunofluorescence
results suggested that cleaved caspase3 was mainly located in the
cytoplasm and cell nucleus following 24 h of hypoxia.
Quantification of the data showed that cleaved caspase3 was
significantly increased following si-p57 transfection and 24 h
hypoxia compared with the si-ctrl (Fig. 5I). Overexpression of
p57KIP2 and inhibition of the JNK pathway significantly
decreased the optical density of cleaved caspase3 at 24 h hypoxia
compared with the respective controls. It is suggested that
p57KIP2 may regulate the JNK signaling pathway to
inhibit apoptosis during hypoxic injury.
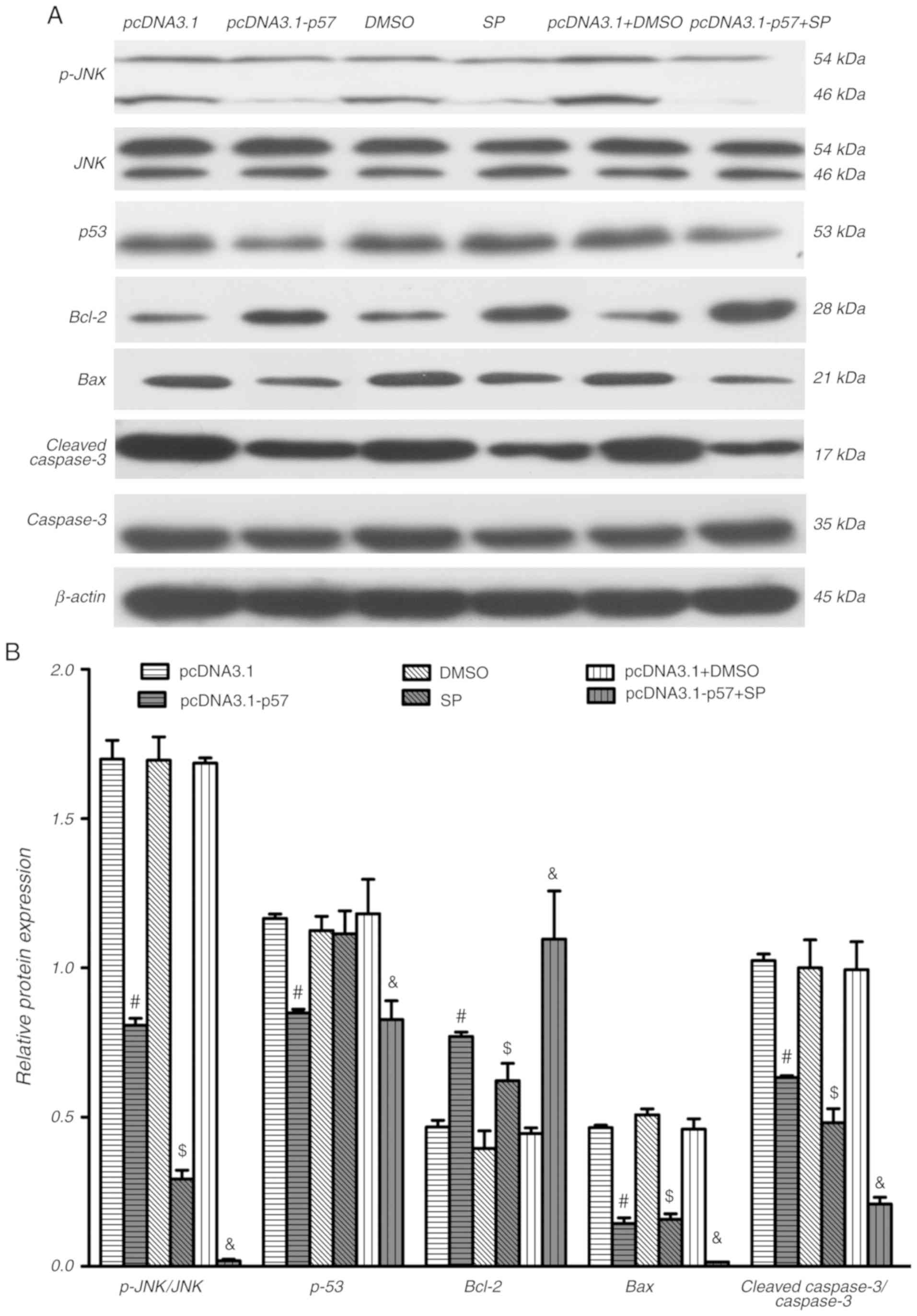 | Figure 4JNK signaling pathway inhibitor and
overexpression of p57KIP2 decrease apoptosis-associated
proteins under hypoxic conditions. HTR-8/SVneo cells were
transfected with pcDNA3.1-p57 or pcDNA3.1, treated with JNK
inhibitor or DMSO, or a combination of both, followed by hypoxia
for 24 h. Western blot (A) images and (B) quantification p-JNK,
JNK, p53, Bcl-2, Bax and cleaved caspase3 levels; β-actin was used
as internal control. #P<0.05 vs. pcDNA3.1; $P<0.05
vs. DMSO; &P<0.05 vs. pcDNA3.1+DMSO. KIP, kinase
inhibitory protein 2; SP, JNK inhibitor SP600125; p-,
phosphorylated. |
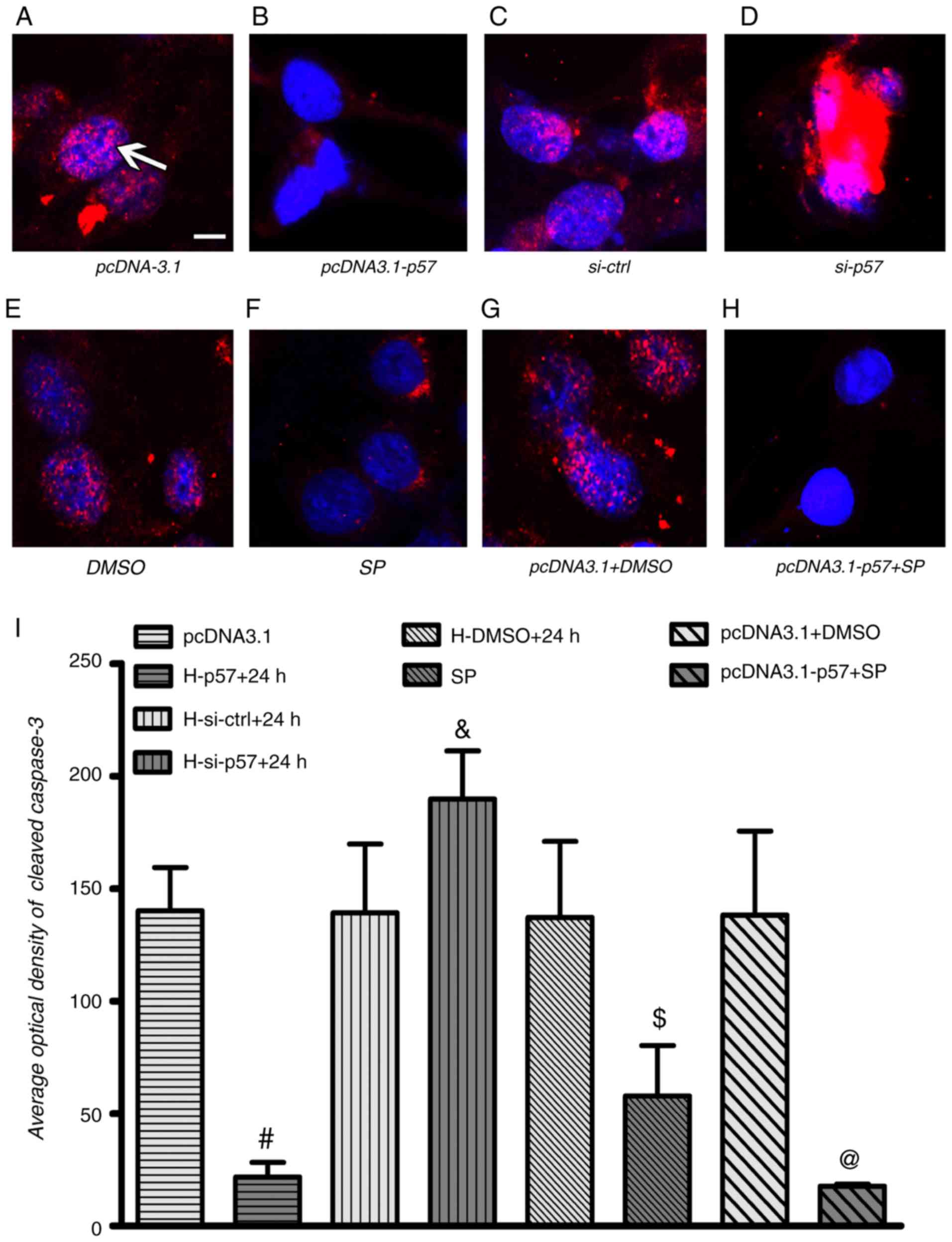 | Figure 5Cleaved caspase3 is affected by
p57KIP2 under hypoxia. HTR-8/SVneo cells were
transfected with pcDNA3.1-p57 or pcDNA3.1, treated with JNK
inhibitor or DMSO, or a combination of both and p57KIP2
knockdown was achieved by transfection with si-p57; all procedures
were followed by hypoxia for 24 h. Immunofluorescence images with
cleaved caspase3 (red; white arrow) and nuclear stain (DAPI; blue)
for (A) pcDNA3.1, (B) pcDNA3.1-p57, (C) si-ctrl and (D) si-p57
transfected cell, for (E) DMSO and (F) SP treated cells, and for
(G) pcDNA3.1 transfected and DMSO and (H) pcDNA3.1-p57 transfected
and SP treated cells; scale bar, 10 µm. (I) Quantified optical
density results for cleaved caspase3 analysis.
#P<0.05 vs. pcDNA3.1; &P<0.05 vs.
si-ctrl; $P<0.05 vs. DMSO; @P<0.05 vs.
pcDNA3.1+DMSO. si-ctrl, siRNA control; si-p58, siRNA targeting
p57KIP2; KIP, kinase inhibitory protein 2; SP, JNK
inhibitor SP600125. |
p57KIP2 expression affects
cell migration and invasion under hypoxic conditions
HTR-8/SVneo cells have been extensively used to
study trophoblast migration and invasion (30). Hypoxia markedly affects
trophoblast biological processes, including cell migration and
invasion (27). To determine the
function of p57KIP2 in trophoblast migration, HTR8/SVneo
cells were transfected with pcDNA3.1-p57, si-p57, JNK inhibitor or
respective controls and exposed to 2% O2 for 24 h. It
was revealed that HTR-8/SVneo cells overexpressing
p57KIP2 exhibited significantly increased migration and
invasion abilities compared with the empty vector control (Figs. 6 and 7). p57KIP2 knockdown
significantly decreased cell migration and invasion following 24 h
of hypoxia compared with the si-ctrl cells. This indicated that
p57KIP2 enhanced migration and invasion abilities of
trophoblasts under hypoxia.
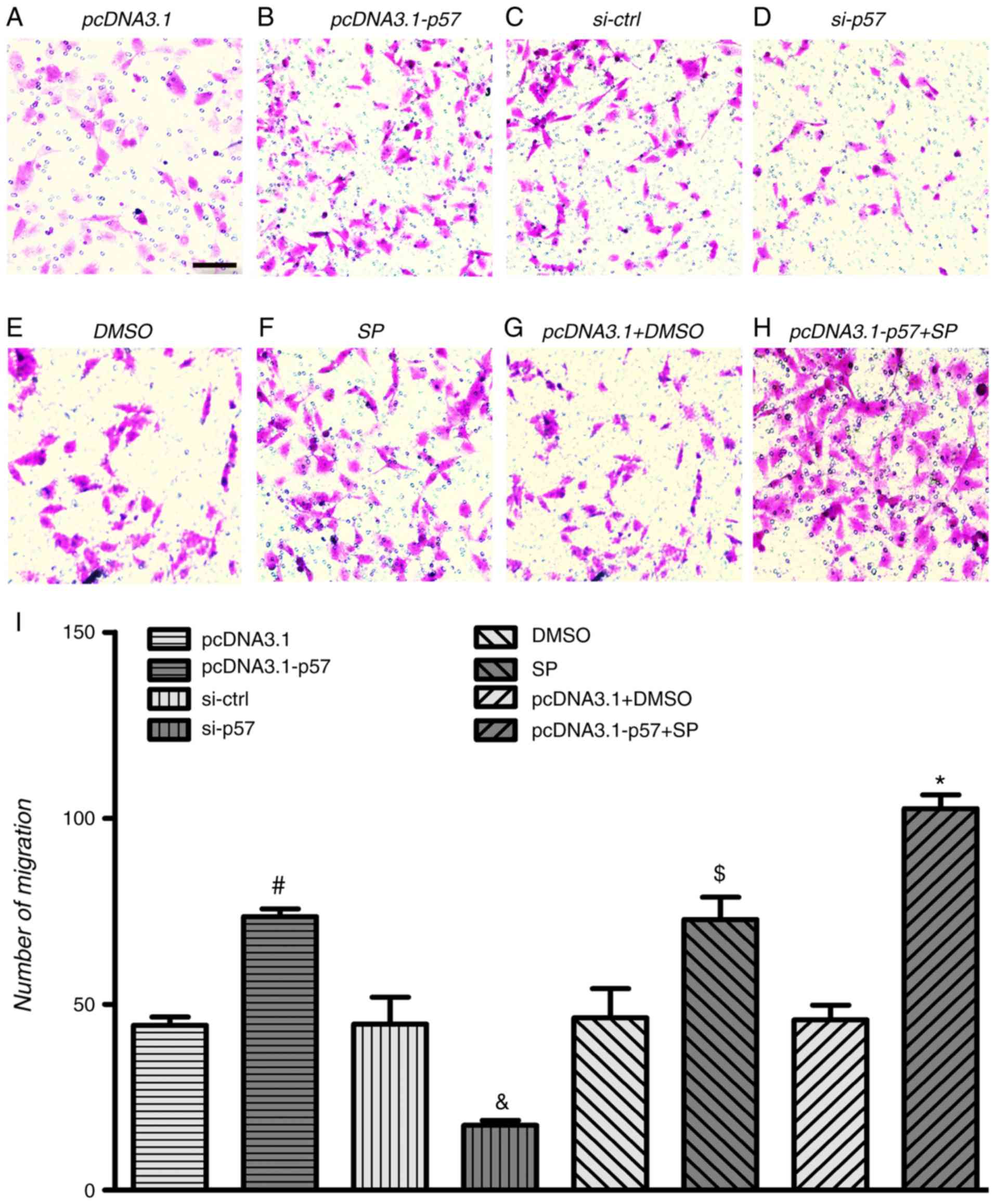 | Figure 6p57KIP2 and JNK inhibitor
affect cell migration under hypoxic conditions. HTR-8/SVneo cells
were transfected with pcDNA3.1-p57 or pcDNA3.1, treated with JNK
inhibitor or DMSO, or a combination of both and p57KIP2
knockdown was achieved by transfection with si-p57; all procedures
were followed by hypoxia for 24 h. Microscopy images if the
Transwell migration assay for (A) pcDNA3.1, (B) pcDNA3.1-p57, (C)
si-ctrl and (D) si-p57 transfected cell, for (E) DMSO and (F) SP
treated cells, and for (G) pcDNA3.1 transfected and DMSO and (H)
pcDNA3.1-p57 transfected and SP treated cells; magnification, ×20;
scale bar, 10 µm. (I) Number of migrated cells.
#P<0.05 vs. pcDNA3.1; &P<0.05 vs.
si-ctrl; $P<0.05 vs. DMSO; *P<0.05 vs.
pcDNA3.1+DMSO. si-ctrl, siRNA control; si-p58, siRNA targeting
p57KIP2; KIP, kinase inhibitory protein 2; SP, JNK
inhibitor SP600125. |
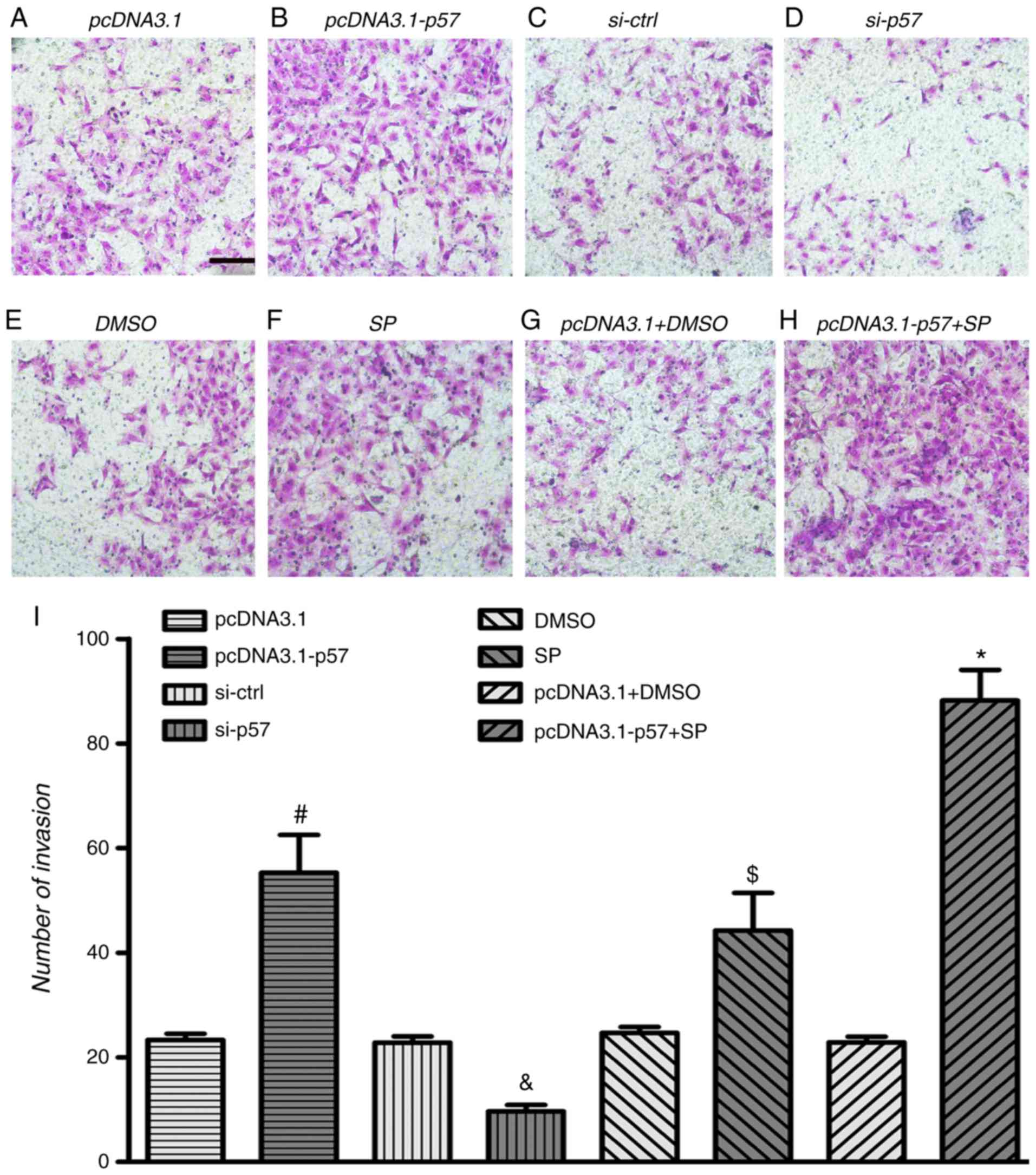 | Figure 7p57KIP2 and JNK inhibitor
affect cell invasion under hypoxic conditions. HTR-8/SVneo cells
were transfected with pcDNA3.1-p57 or pcDNA3.1, treated with JNK
inhibitor or DMSO, or a combination of both and p57KIP2
knockdown was achieved by transfection with si-p57; all procedures
were followed by hypoxia for 24 h. Microscopy images if the
Transwell invasion assay for (A) pcDNA3.1, (B) pcDNA3.1-p57, (C)
si-ctrl and (D) si-p57 transfected cell, for (E) DMSO and (F) SP
treated cells, and for (G) pcDNA3.1 transfected and DMSO and (H)
pcDNA3.1-p57 transfected and SP treated cells; magnification, ×20;
scale bar, 10 µm. (I) Number of invaded cells.
#P<0.05 vs. pcDNA3.1; &P<0.05 vs.
si-ctrl; $P<0.05 vs. DMSO; *P<0.05 vs.
pcDNA3.1+DMSO. si-ctrl, siRNA control; si-p58, siRNA targeting
p57KIP2; KIP, kinase inhibitory protein 2; SP, JNK
inhibitor SP600125. |
As shown in Fig.
6F, the JNK inhibitor significantly increased HTR-8/SVneo cell
migration and invasion at 24 h of hypoxia compared with the DMSO
treated control. Overexpression of p57KIP2 combined with
JNK inhibitor treatment significantly increased HTR-8/SVneo cell
migration and invasion compared with the empty vector transfected
and DMSO treated cells. The results indicated that under hypoxic
conditions p57KIP2 affected HTR-8/SVneo cell migration
and invasion through the JNK signaling pathway.
Discussion
As a member of the CIP/KIP family,
p57KIP2 is considered to be a master regulator of the
cell cycle during embryogenesis, serving a role in cell cycle
control and regulating the induction of apoptosis (31,32). It has been shown that
p57KIP2 has a minor proapoptotic effect on its own,
sensitizing cells to apoptosis (17). A previous study reported that
p57KIP2 expression enhanced apoptosis in HeLa cells and
showed that p57KIP2 was a target of caspase activity
(21). Other studies have
reported an increase in apoptosis and altered differentiation
during mouse development in a p57KIP2 knockout model
(17,33). Previously, it has been shown that
p57KIP2 serves a role in antiapoptosis regulation,
suggesting that whether it promotes or inhibits apoptosis is mainly
cellular context-dependent (24).
In agreement with these previous studies, the present study
revealed that overexpression of p57KIP2 increased
expression of the antiapoptotic protein Bcl-2 following hypoxia and
silencing p57KIP2 induced the expression of the
proapoptosis-associated proteins p53, Bax and cleaved caspase3. It
appeared that in the HTR-8/SVneo hypoxia model, p57KIP2
acted as an antiapoptotic molecule.
The family of mammalian mitogen-activated protein
kinases includes several subgroups, such as extracellular
signal-regulated kinase and JNK/SAPK (34). Accordingly, the JNK/SAPK signaling
pathway is implicated in the control of cell growth,
transformation, survival and death. It has been shown that
p57KIP2 interacts with and inhibits the kinase activity
of JNK/SAPK through its QT domain (29,34). The present study provided evidence
showing that p57KIP2 affected the phosphorylation of JNK
under hypoxic conditions. Furthermore, p57KIP2 modulated
apoptosis by negatively regulating the JNK signaling pathway. In
addition, overexpression of p57KIP2 or treatment with
the JNK inhibitor increased cell migration and invasion under
hypoxic conditions. Treatment of the p57KIP2
overexpressing cells with the JNK inhibitor further affected cell
apoptosis, migration and invasion. These results showed that
p57KIP2 functioned as an inhibitory protein of JNK and
affected HTR-8/SVneo migration and invasion.
In conclusion, the results of the present study
indicated that p57KIP2 served a protective role in
apoptosis and increased cell invasion and migration through the JNK
signaling pathway under hypoxic conditions. These results suggested
that at hypoxia, the p57KIP2-JNK network may serve an
important role in regulating HTR-8/SVneo apoptosis and
function.
Funding
The current work was supported by Science and
Technology Support Projects in Sichuan Province (grant nos.
2013SZ0004, 2014JY0158 and 2019YFS0411).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GQH, WMX and GLH conceived and designed the study.
GQH, GYL and HJL performed the experiments. XHL performed the
analysis of data. GHQ and GLH wrote the manuscript. GLH revised the
article. All authors read and approved the final manuscript.
Ethics approval and consent for
participation
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Mrs. Yan Chen (West
China Second University Hospital, Sichuan University, China) for
her help advice on flow cytometry.
References
|
1
|
Wang K, Chen Y, Ferguson SD and Leach RE:
MTA1 and MTA3 regulate HIF1a expression in hypoxia-treated human
trophoblast cell line HTR8/Svneo. Med J Obstet Gynecol. 1:pii:
1017. 2013.PubMed/NCBI
|
|
2
|
Leslie K, Whitley GS, Herse F, Dechend R,
Ashton SV, Laing K, Thilaganathan B and Cartwright JE: Increased
apoptosis, altered oxygen signaling, and antioxidant defenses in
first-trimester pregnancies with high-resistance uterine artery
blood flow. Am J Pathol. 185:2731–2741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tuuli MG and Longtine MS: Nelson DM.
Review: Oxygen and trophoblast biology-a source of controversy.
Placenta. 32(Suppl 2): pp. S109–S118. 2011, View Article : Google Scholar
|
|
4
|
Highet AR, Khoda SM, Buckberry S, Leemaqz
S, Bianco-Miotto T, Harrington E, Ricciardelli C and Roberts CT:
Hypoxia induced HIF-1/HIF-2 activity alters trophoblast
transcriptional regulation and promotes invasion. Eur J Cell Biol.
94:589–602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma S, Norris WE and Kalkunte S: Beyond
the threshold: An etiological bridge between hypoxia and immunity
in preeclampsia. J Reprod Immunol. 85:112–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou Y, Yu X, Lu J, Jiang Z, Zuo Q, Fan M,
Huang S and Sun L: Decorin-mediated inhibition of human trophoblast
cells proliferation, migration, and invasion and promotion of
apoptosis in vitro. Biomed Res Int. 2015:2016292015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang D, Liu H, Zeng J, Miao X, Huang W,
Chen H, Huang Y, Li Y and Ye D: Glucocorticoid exposure in early
placentation induces preeclampsia in rats via interfering
trophoblast development. Gen Comp Endocrinol. 225:61–70. 2016.
View Article : Google Scholar
|
|
8
|
Heazell AE, Buttle HR, Baker PN and
Crocker IP: Altered expression of regulators of caspase activity
within trophoblast of normal pregnancies and pregnancies
complicated by preeclampsia. Reprod Sci. 15:1034–1043. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saito S and Nakashima A: A review of the
mechanism for poor placentation in early-onset preeclampsia: The
role of autophagy in trophoblast invasion and vascular remodeling.
J Reprod Immunol. 101-102:80–88. 2014. View Article : Google Scholar
|
|
10
|
Kanayama N, Takahashi K, Matsuura T,
Sugimura M, Kobayashi T, Moniwa N, Tomita M and Nakayama K:
Deficiency in p57Kip2 expression induces preeclampsia-like symptoms
in mice. Mol Hum Reprod. 8:1129–1135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kadyrov M, Schmitz C, Black S, Kaufmann P
and Huppertz B: Pre-eclampsia and maternal anaemia display reduced
apoptosis and opposite invasive phenotypes of extravillous
trophoblast. Placenta. 24:540–548. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Unek G, Ozmen A, Mendilcioglu I, Simsek M
and Korgun ET: The expression of cell cycle related proteins PCNA,
Ki67, p27 and p57 in normal and preeclamptic human placentas.
Tissue Cell. 46:198–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Unek G, Ozmen A, Kipmen-Korgun D and
Korgun ET: Immunolocalization of PCNA, Ki67, p27 and p57 in normal
and dexamethasone-induced intrauterine growth restriction placental
development in rat. Acta Histochem. 114:31–40. 2012. View Article : Google Scholar
|
|
14
|
Lee MH, Reynisdóttir I and Massagué J:
Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique
domain structure and tissue distribution. Genes Dev. 9:639–649.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamura T, Hara T, Kotoshiba S, Yada M,
Ishida N, Imaki H, Hatakeyama S, Nakayama K and Nakayama KI:
Degradation of p57Kip2 mediated by SCFSkp2-dependent
ubiquitylation. Proc Natl Acad Sci USA. 100:10231–10236. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Z and Hunter T: Ubiquitylation and
proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2)
CDK inhibitors. Cell Cycle. 9:2342–2352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pateras IS, Apostolopoulou K, Niforou K,
Kotsinas A and Gorgoulis VG: p57KIP2: 'Kip'ing the cell under
control. Mol Cancer Res. 7:1902–1919. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kavanagh E, Vlachos P, Emourgeon V, Rodhe
J and Joseph B: p57(KIP2) control of actin cytoskeleton dynamics is
responsible for its mitochondrial pro-apoptotic effect. Cell Death
Dis. 3:e3112012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan Y, Frisén J, Lee MH, Massagué J and
Barbacid M: Ablation of the CDK inhibitor p57Kip2 results in
increased apoptosis and delayed differentiation during mouse
development. Genes Dev. 11:973–983. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang P, Wong C, Liu D, Finegold M, Harper
JW and Elledge SJ: p21(CIP1) and p57(KIP2) control muscle
differentiation at the myogenin step. Genes Dev. 13:213–224. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Samuelsson MK, Pazirandeh A and Okret S: A
pro-apoptotic effect of the CDK inhibitor p57(Kip2) on
staurosporine-induced apoptosis in HeLa cells. Biochem Biophys Res
Commun. 296:702–709. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu S, Yu FS, Lewis J, Singh B, Borke J,
Osaki T, Athar M and Schuster G: Induction of p57 is required for
cell survival when exposed to green tea polyphenols. Anticancer
Res. 22:4115–4120. 2002.
|
|
23
|
Zhang P, Liégeois NJ, Wong C, Finegold M,
Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA and Elledge
SJ: Altered cell differentiation and proliferation in mice lacking
p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature.
387:151–158. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rossi MN and Antonangeli F: Cellular
response upon stress: p57 contribution to the final outcome.
Mediators Inflamm. 2015:2593252015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi K, Kobayashi T and Kanayama N:
p57(Kip2) regulates the proper development of labyrinthine and
spongiotrophoblasts. Mol Hum Reprod. 6:1019–1025. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu Q, Qiu Y, Wang X, Tang J, Liu Y, Mei L,
Li M, Yang M, Tang L, Gao H, et al: Efficient siRNA transfer to
knockdown a placenta specific lncRNA using RGD-modified
nano-liposome: A new preeclampsia-like mouse model. Int J Pharm.
546:115–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Li P, Wang Y and Yan H:
Hypoxia-induced expression of CXCR4 favors trophoblast cell
migration and invasion via the activation of HIF-1α. Int J Mol Med.
42:1508–1516. 2018.PubMed/NCBI
|
|
28
|
Chang TS, Kim MJ, Ryoo K, Park J, Eom SJ,
Shim J, Nakayama KI, Nakayama K, Tomita M, Takahashi K, et al:
p57KIP2 modulates stress-activated signaling by inhibiting c-Jun
NH2-terminal kinase/stress-activated protein Kinase. J Biol Chem.
278:48092–48098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choudhary GS, Al-Harbi S and Almasan A:
Caspase-3 activation is a critical determinant of genotoxic
stress-induced apoptosis. Methods Mol Biol. 1219:1–9. 2015.
View Article : Google Scholar
|
|
30
|
Yang Y, Zhang J, Gong Y, Liu X, Bai Y, Xu
W and Zhou R: Increased expression of prostasin contributes to
early-onset severe preeclampsia through inhibiting trophoblast
invasion. J Perinatol. 35:16–22. 2015. View Article : Google Scholar
|
|
31
|
Ma J, Li J, Yang S, Huang K, Dong X, Sui C
and Zhang H: P57 and cyclin G1 express differentially in
proliferative phase endometrium and early pregnancy decidua. Int J
Clin Exp Med. 8:5144–5149. 2015.PubMed/NCBI
|
|
32
|
Korgun ET, Celik-Ozenci C, Acar N, Cayli
S, Desoye G and Demir R: Location of cell cycle regulators cyclin
B1, cyclin A, PCNA, Ki67 and cell cycle inhibitors p21, p27 and p57
in human first trimester placenta and deciduas. Histochem Cell
Biol. 125:615–624. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Matsuoka S, Edwards MC, Bai C, Parker S,
Zhang P, Baldini A, Harper JW and Elledge SJ: p57KIP2, a
structurally distinct member of the p21CIP1 Cdk inhibitor family,
is a candidate tumor suppressor gene. Genes Dev. 9:650–662. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Minden A and Karin M: Regulation and
function of the JNK subgroup of MAP kinases. Biochim Biophys Acta.
1333:F85–F104. 1997.PubMed/NCBI
|