Introduction
Infantile pneumonia (IP) is pulmonary infection
induced by mycoplasma pneumoniae, which is also called
primary atypical pneumonia that is commonly seen in infants,
school-age children and adolescents (1). In recent years, mycoplasma
pneumoniae pneumonia (MPP) accounts for a gradually
increasing proportion in community acquired pneumonia (CAP), which
may occur all year round and is more frequently seen in winter and
spring. Its incubation period is as long as 2-3 weeks and can
spread through droplet transmission (2). Typically, most cases have good a
prognosis, but this disease is highly recurrent and severe lung
lesion may develop in certain rare cases (2). In addition, it may be accompanied by
severe complications, including encephalitis, myelitis and
nephritis (2). Lipopolysaccharide
(LPS)-induced A549 cells is a common IP model used to research
pulmonary inflammation and injury (3-5).
Inflammation is the defense reaction to the damage
of living tissues in the vascular system, and inflammation can
eliminate hazardous substances that enter the body, but it may also
induce injury to the body (6). IP
has become a common and frequently-occurring disease in children,
which has attracted increasing attention due to its long course,
severe symptoms and possibility of inducing multiple extrapulmonary
complications (7). IP is known to
be caused by mycoplasma infection; nonetheless, the pathogenesis of
IP-induced inflammatory response remains incompletely understood
(6). It is currently suggested
that MPP is a systemic disease involving the immune system and
cytokines serve a leading role in IP-induced immune injury, which
is supported in a number of recent studies (7,8).
Particularly, cytokines and inflammatory mediators, including tumor
necrosis factor (TNF)-α and interleukin (IL)-6, are directly
involved in this process. TNF-α is a dominant cytokine produced by
monocytes and macrophages and is an inflammatory mediator with
important bioactivity (8). It can
induce neutrophil chemo-taxis, local infiltration, phagocytosis and
the killing of pathogens, therefore initiating the inflammatory
response (9). IL-6 is produced by
monocytes and macrophages and lymphocytes spontaneously or under
the stimulation of various factors, and it is an important cytokine
in the inflammatory response and the vital mediator synthesized
during the acute phase (9).
MicroRNA (miRNA) is non-coding RNA that serves a
post-transcriptional regulatory role in gene transcription and
translation (10). It can
regulate post-transcriptional expression of target genes, therefore
serving a vital role in biological development, reproduction, cell
differentiation, apoptosis and pathogenesis. A number of diseases
are recognized to be associated with the inflammatory response;
therefore, it is of crucial importance to study the pathogenesis of
inflammation (11). Recent
studies identified that miRNA can regulate multiple body
activities, including regulation of inflammatory responses and
multiple miRNAs have been demonstrated to be involved in regulating
the inflammatory response and inflammatory diseases (3,11).
RelA is an important member of the NF-κB family,
which can regulate target genes to participate in vital life
activities, including cell proliferation, transformation,
apoptosis, inflammation and immune response (12). In addition, these functions are
regulated by multiple post-translational modifications, like
phosphorylation, acetylation and methylation (12).
Nuclear factor κB (NF-κB), first discovered in B
cells in 1986, is a nuclear protein factor that can specifically
bind with immunoglobulin (Ig)Gκ light chain gene enhancer κB
sequence (GGG ACT TTC) (13). It
is extensively distributed in eukaryocytes and participates in
vital pathophysiological processes, like cell proliferation,
transformation, apoptosis, inflammation and immune responses.
Therefore, it has attracted extensive attention from scholars. At
present, there are 5 members in the NF-κB family, including RelA,
RelB, c-Rel, p105/p50 (NF-κB1) and p100/p52 (NF-κB2) (14). Among them, RelA can regulate the
transcription activity of multiple downstream target genes due to
its special transactivation domain and participate in multiple
vital cellular activities, therefore it has become the research
focus of the NF-κB family (14).
Xiao et al (15) and Peng
et al (16), demonstrated
that the miRNA-302 cluster downregulates the enterovirus 71-induced
innate immune response and inflammation. The present study
investigated the role of miRNA-302e in IP and the potential
protective mechanisms.
Materials and methods
IP model
A total of 1 week old C57BL/6 mice (male, 4-5 g,
n=20) mice was purchased from the Animal laboratory of Shandong
University (Shandong, China). C57BL/6 mice were randomly assigned
to one of two groups: Sham (n=10) and IP group (n=10). C57BL/6 mice
of the sham group were injected with normal saline for 8 h under 50
mg/kg pentobarbital sodium pentobarbital sodium. C57BL/6 mice with
IP were injected with 2 mg/kg of LPS (Sigma-Aldrich Merck KGaA)
under 50 mg/kg pentobarbital sodium (intraperitoneal) (17,18). A total of 8 h later, peripheral
blood (100 μl) was collected from a leg vein under 50 mg/kg
pentobarbital sodium and serum was centrifuged at 1,000 × g for 10
min at 4°C. Mice were sacrificed using decollation under 50 mg/kg
pentobarbital sodium following induction for IP.
Lung tissue samples were collected and fixed with 4%
paraformaldehyde for 24 h at room temperature or preserved at
-80°C. All experiments have been approved by the Ethics Committee
of The People's Hospital of Dongying (Dongying, China).
Hematoxylin and eosin (H&E)
staining
Lung tissue samples fixed with paraformaldehyde were
paraffin-embedded and stored at 4°C. The lung tissue samples were
cut into 10 μm sections using a paraffin slicing machine
(RM2235; Leica Microsystems GmbH, Wetzlar, Germany), stained with
hematoxylin and eosin for 10 min at room temperature and observed
under light microscopy (magnification, ×100; BH3-MJL; Olympus
Corporation, Tokyo, Japan).
ELISA assay kits
Serum samples and transfected cell samples after
induction of infantile pneumonia were collected at 1,000 × g for 10
min at 4°C. Serum samples were used to measure TNF-α, IL-1β, IL-6
and IL-18 levels using ELISA kits. Cell samples was lysed using
radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of
Biotechnology, Haimen, China) and used to measure TNF-α (cat. no.
H052), IL-1β (cat. no. H002), IL-6 (cat. no. H007) and IL-18 (cat.
no. H015) levels using ELISA kits (Nanjing Jiancheng Institute of
Biological Engineering). The optical density value was detected
using a microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA) at 450 nm.
Immunofluorescent staining
Cells were fixed with 4% paraformaldehyde for at
room temperature 15 min, permeabilized using 0.1% Triton X-100 for
15 min and blocked with 5% bovine serum albumin (BSA; Beyotime
Institute of Biotechnology) in PBS for 1 h at 37°C. Cells were
labeled with rabbit-anti-RelA (1:100) antibody (cat. no. ab76228;
Abcam Cambridge, UK) at 4°C overnight. Cells was washed three times
with PBS and incubated with goat anti-rabbit IgG-CFL 555 antibody
(cat. no. sc-362272; 1:100; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 1 h at room temperature. Cells were stained with DAPI
for 15 min and washed with PBS for 15 min. Cell images were
obtained using a Zeiss Axioplan 2 fluorescent microscope (Carl
Zeiss AG, Oberkochen, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and Gene microarray
hybridization
Total RNA was extracted from lung tissue samples or
cell using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and cDNA production was carried out using a
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) at 37°C for 60 min and 82°C for 10 sec. qPCR was
performed with Power SYBR-Green Master Mix (Toyobo Life Science,
Osaka, Japan) on a Prism 7000 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Relative gene
expression was calculated with the 2−ΔΔCq method. The
PCR cycling conditions were as follows: 94°C pre-degeneration for 5
min, 94°C degeneration for 30 sec, 60°C annealing for 30 sec, 72°C
extension for 1 min, for 40 cycles. miRNA-302e forward,
5′-TTGGGTAAGTGCTTCCATGA-3′ and reverse,
5′-GTAATAGCACCTACCTTATAGA-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′, 5′-CTTCGGCAGCACATA
5-CCGCTTCACGAATTTGCGTGTCAT-3′. The relative expression was analyzed
using the 2−ΔΔCq method (19).
Total RNA was labelled using Cyanine-5-CTP and
hybrid-ized to the SurePrint and G3 Mouse Whole Genome GE 8×60 K
Microarray G4852A platform (NEL474001EA; PerkinElmer, Inc.,
Waltham, MA, USA) with an equimolar concentration of
Cyanine-3-CTP-labelled universal mouse (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA). Images were quantified
and feature-extracted using Agilent Feature Extraction Software
(v.A.10.7.3.1; Agilent Technologies, Inc.).
In vitro model
A549 cell was purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
was maintained in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator
at 37°C and 5% CO2. Small interfering (si)-RelA (cat.
no. s11917; Ambion; Thermo Fisher Scientific, Inc.), si-BRD4 (cat.
no. sc-43639) and si-NF-κB (cat. no. sc-44212) were purchased from
Santa Cruz Biotechnology, Inc. miRNA-302e
(5′-UAAGUGCUUCCAUGUUUUGGUGA-3′), anti-miRNA-302e
(5′-AUUCACGAAGGUACAAAACCACU-3′) and negative control mimics
(5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were purchased from Sangon Biotech
(Shanghai) Co., Ltd. (Shanghai, China). A total of 100 ng of
si-RelA (cat. no. sc-61876; Santa Cruz Biotechnology, Inc.), 100 ng
of si-BRD4 (cat. no. sc-43639; Santa Cruz Biotechnology, Inc.), 100
ng of si-NF-κB (cat. no. sc-29410; Santa Cruz Biotechnology, Inc.),
100 ng of miRNA-302e, 100 ng of anti-miRNA-302e and 100 ng of
negative mimics were transfected into cells (1×105
cell/ml) using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.)
for 48 h. Following 48 h, cells were treated with 100 ng/ml of LPS
for 6 h (3,6,17)
and used for western blotting or RT-qPCR.
Luciferase reporter assay
pGL3-RelA-3′ untranslated region (UTR) plasmids were
obtained from Shanghai GeneChem Co., Ltd. (Shanghai, China).
pGL3-RelA-3′UTR plasmid and anti-miRNA-302e were co-transfected
into cells using Lipofectamine-2000 for 48 h. Reporter activity was
quantified at 48 h following transfection using a Dual-Luciferase
Reporter Assay kit (Beyotime Institute of Biotechnology). Readings
were taken with a Lumat LB 9507 instrument (Berthold Technologies,
Bad Wildbad, Germany). pRL-TK (Promega Corporation, Madison, WI,
USA) was transfected as a normalization control. Comparison with
Renilla luciferase activity was performed.
Western blot analysis
Total protein extracts were obtained from
transfected cells at 48 h using cold RIPA buffer (Beyotime
Institute of Biotechnology) and protein concentration was measured
using bicinchoninic acid assay (Beyotime Institute of
Biotechnology). Total protein (50 μg) was loaded on 10%
SDS-PAGE gels, electrophoresed and transferred onto a
polyvinylidene difluoride membrane. Membranes were blocked with 5%
non-fat milk in TBS with 0.1% Tween 20 for 1 h at 37°C and probed
for RelA (1:1,000; cat. no. sc-81622), BRD4 (1:1,000; cat. no.
sc-518021), NF-κB (1:1,000; cat. no. sc-71675) and GAPDH (1:5,000;
cat. no. sc-293335) overnight at 4°C. The membranes were incubated
at room temperature for 1 h with appropriate horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. no. sc-2004; 1:5,000;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) visualized with
enhanced chemiluminescence (Beyotime Institute of Biotechnology)
and analyzed using Image Lab 3.0 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation (n=3) using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Student's t-test or one-way analysis of variance and Tukey's
post-hoc test were used carried out statistical analysis. P<0.05
was considered to indicate a statically significant difference.
Results
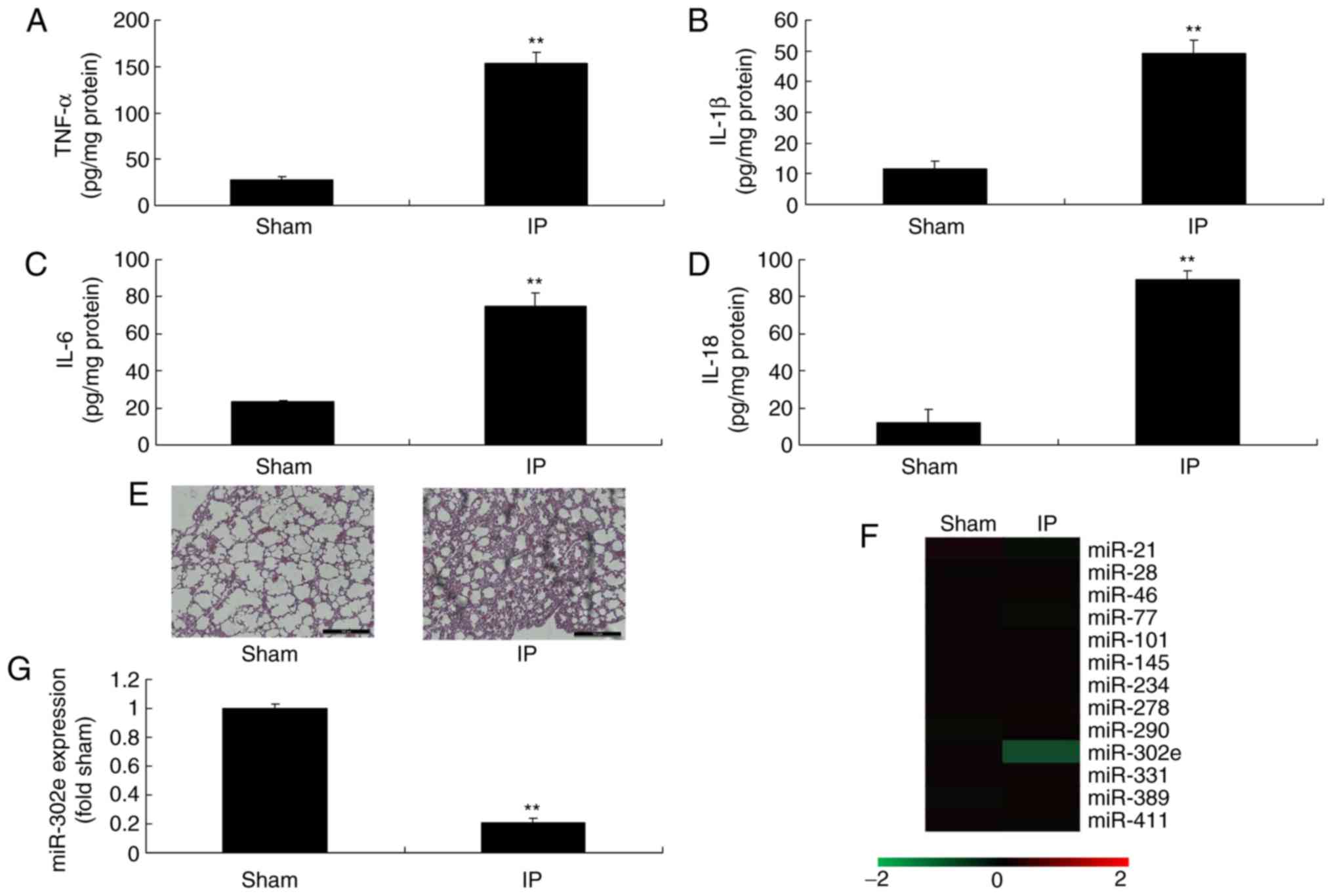
Expression of miRNA-302e in mouse model
of IP
To investigate the roles of miRNAs in a mouse model
of IP, the changes of miRNAs in were analyzed. The levels of TNF-α,
IL-1β, IL-6 and IL-18 were significantly increased in the mouse
model of IP compared with the sham mice (P<0.01; Fig. 1A-D). H&E staining demonstrated
that the pulmonary alveoli were shrunken in the mouse model of IP,
in comparison to sham mice (Fig.
1E). These results of H&E staining, TNF-α, IL-1β, IL-6 and
IL-18 levels indicated that the in vivo model was
successfully established. Moreover, the expression of miRNA-302e
was significantly decreased in a mouse model of IP compared with
the sham group (P<0.01; Fig.
1F-G). Therefore, miRNA-302e may be involved in inflammation in
IP.
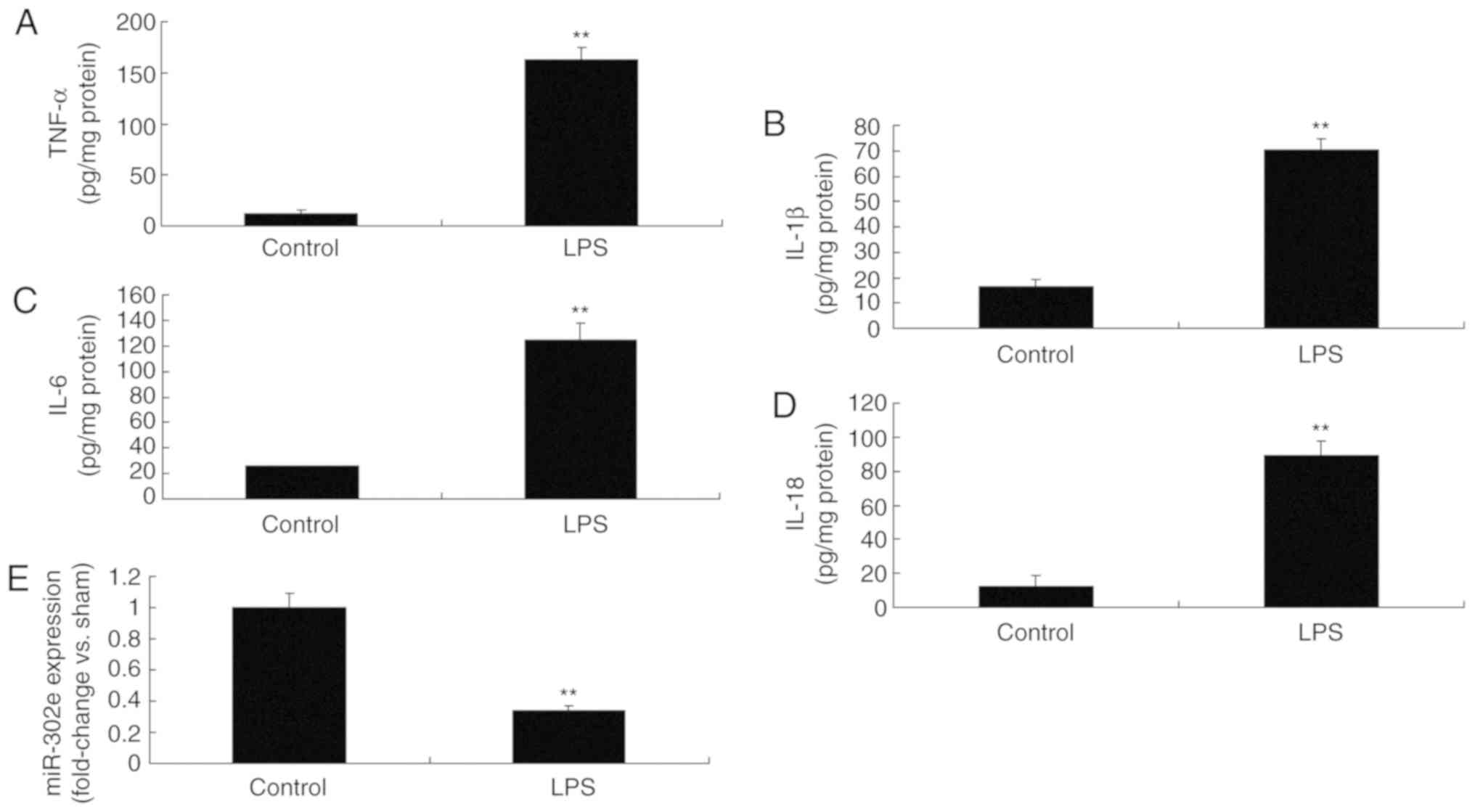
Expression of miRNA-302e in an in vitro
model of IP
Then, it was demonstrated that the levels of TNF-α,
IL-1β, IL-6 and IL-18 were significantly promoted in LPS-induced
A549 cells, compared with the control group (P<0.01; Fig. 2A-D). As expected, the expression
of miRNA-302e was significantly decreased in the LPS-induced group
compared with the control group (P<0.01; Fig. 2E). Therefore, these results
demonstrated that miRNA-302e served a role in the inflammation of
IP in vitro.
Downregulation of miRNA-302a regulates
inflammation in an in vitro model of IP by the RelA/BRD4/NF-κB
signaling pathway
In addition, the expression of miRNA-302e was
significantly downregulated in in vitro model of IP
(P<0.01; Fig. 3A). The levels
of TNF-α, IL-1β, IL-6 and IL-18 were significantly increased in an
in vitro model of IP by down-regulation of miRNA-302a
compared with the control group (P<0.01; Fig. 3B-E). Then, a gene chip was
utilized to analyze the expression of inflammatory signaling
pathways, which revealed that the expression of RelA, BRD4 and
NF-κB was significantly induced in the in vitro model of IP
following downregulation of miRNA-302a, in comparison with the
control group (P<0.01; Fig.
3F-I). As presented in Fig. 4A
and B, the 3-UTR of RelA was complementary to the miRNA-302e
seed sequence and luciferase activity levels also were increased in
the in vitro model of IP following downregulation of
miRNA-302a, compared with the negative group. The protein
expression of RelA, BRD4 and NF-κB was induced in the in
vitro model of IP by downregulation of miRNA-302a, in
comparison to the negative group (Fig. 4C-F). IF demonstrated that the
protein expression of RelA was increased in the in vitro
model of IP by downregulation of miRNA-302a compared with the
negative group (Fig. 4G). These
data indicated that miRNA-302a regulated RelA expression in IP
in vitro.
 | Figure 3Downregulation of miRNA-302a regulates
inflammation in an in vitro model of infantile pneumonia.
(A) quantitative polymerase chain reaction for miRNA-302e
expression, (B) TNF-α, (C) IL-1β, (D) IL-6 and (E) IL-18 levels,
(F) gene chip, qPCR for (G) RelA, (H) BRD4 and (I) NF-κB
expression. **P<0.01 vs. the negative control group.
Negative, negative control group; anti-302a, downregulation of
miRNA-302a group; qPCR, quantitative polymerase chain reaction;
TNF, tumor necrosis factor; IL, interleukin; NF, nuclear factor;
BRD4, bromodomain-containing protein 4; miRNA, microRNA. |
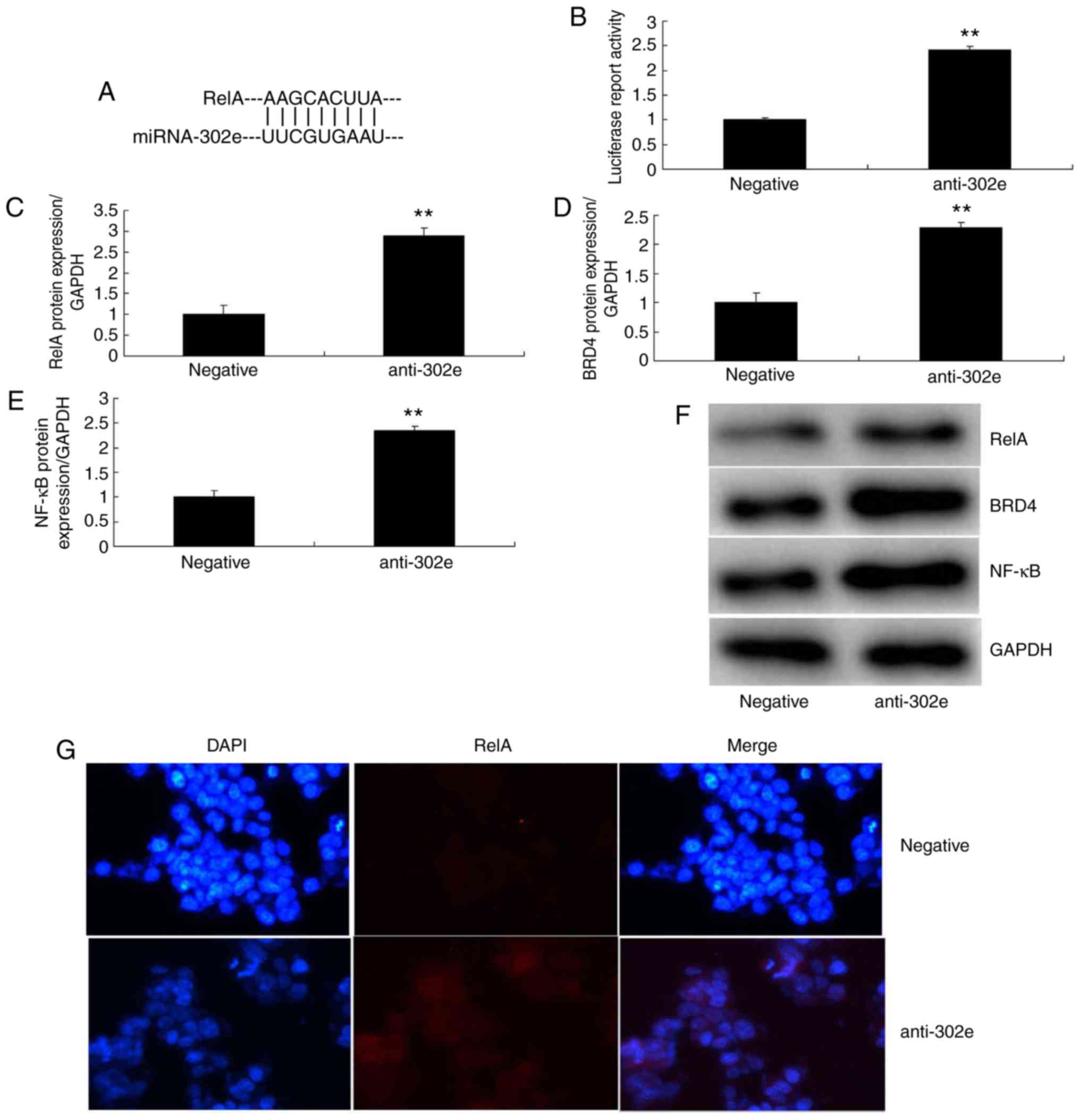 | Figure 4Downregulation of miRNA-302a
regulates RelA/BRD4/NF-κB signaling pathway in an in vitro
model of infantile pneumonia. (A) miRNA-302a matches to the
sequence in the 3-UTR of RelA, (B) luciferase reporter, (C) RelA,
(D) BRD4 and (E) NF-κB protein expression by statistical analysis,
and (F) western blotting analysis, (G) RelA protein expression by
immunofluorescence. **P<0.01 vs. the negative control
group. Negative, negative control group; anti-302a, downregulation
of miRNA-302a group; NF, nuclear factor; BRD4,
bromodomain-containing protein 4; miRNA, microRNA. |
miRNA-302e regulates the RelA/BRD4/NF-κB
signaling pathway in an in vitro model of IP
Next, the function and mechanism of miRNA-302e in IP
was analyzed in vitro. MiRNA-302e mimics were used to
significantly increase the expression of miRNA-302e in the in
vitro model of IP, compared with the negative group (P<0.01;
Fig. 5A). The levels of TNF-α,
IL-1β, IL-6 and IL-18 were significantly reduced in the in
vitro model of IP following over-expression of miRNA-302e, in
comparison with the negative group (P<0.01; Fig. 5B-E). Over-expression of miRNA-302e
significantly suppressed the protein expression of RelA, BRD4 and
NF-κB in IP in vitro, compared with the negative group
(P<0.01; Fig. 5F-I). These
results demonstrated that miRNA-302e could decrease inflammation by
regulating the RelA/BRD4/NF-κB signaling pathway in IP in
vitro.
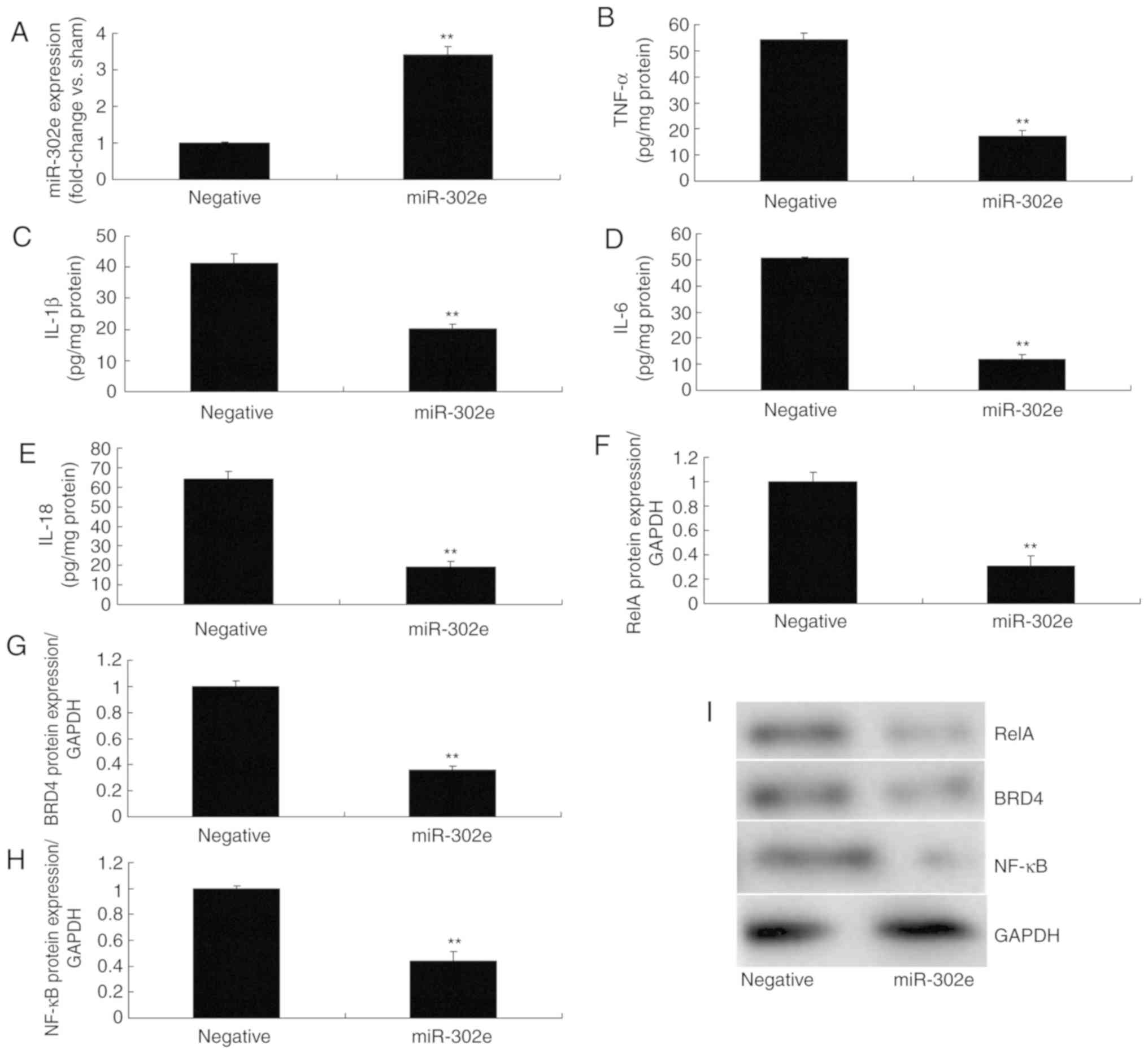 | Figure 5miRNA-302e regulates the RelA/ BRD4/
NF-κB signaling pathway in an in vitro model of infantile
pneumonia. (A) quantitative polymerase chain reaction for
miRNA-302e expression, (B) TNF-α, (C) IL-1β, (D) IL-6 and (E)
IL-18, (F) RelA, (G) BRD4 and (H) NF-κB protein expression by
statistical analysis, and (I) western blotting analysis.
**P<0.01 vs. the negative control group. Negative,
negative control group; miR-302a, upregulation of miRNA-302a group;
TNF, tumor necrosis factor; IL, interleukin; NF, nuclear factor;
BRD4, bromodomain-containing protein 4; miRNA, microRNA. |
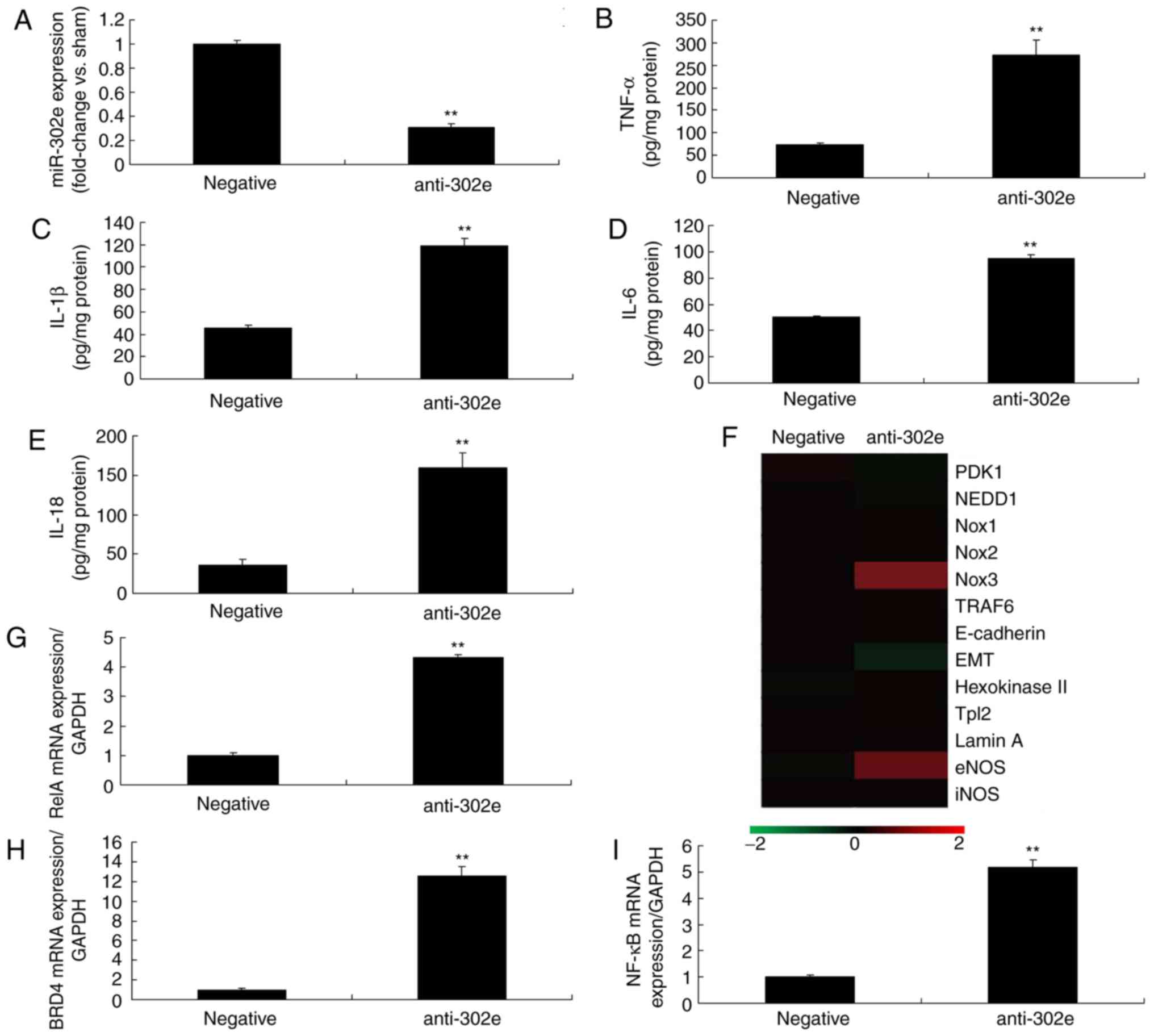
si-RelA attenuates the effects of
miRNA-302e on inflammation in the in vitro model of IP
To further confirm the role of RelA on the effects
of miRNA-302e on inflammation in IP in vitro, si-RelA and
302e inhibitor were transfected in the in vitro model of IP.
The activation of RelA, BRD4 and NF-κB protein expression were
suppressed by si-RelA and the downregulation of miRNA-302e in the
in vitro model of IP, compared with the downregulation of
miRNA-302e group (Fig. 6A-D). The
promotion of TNF-α, IL-1β, IL-6 and IL-18 levels were also reduced
in the in vitro model of IP by downregulation of miRNA-302e
in the si-RelA group, in comparison with the miRNA-302e group
(Fig. 6E-H).
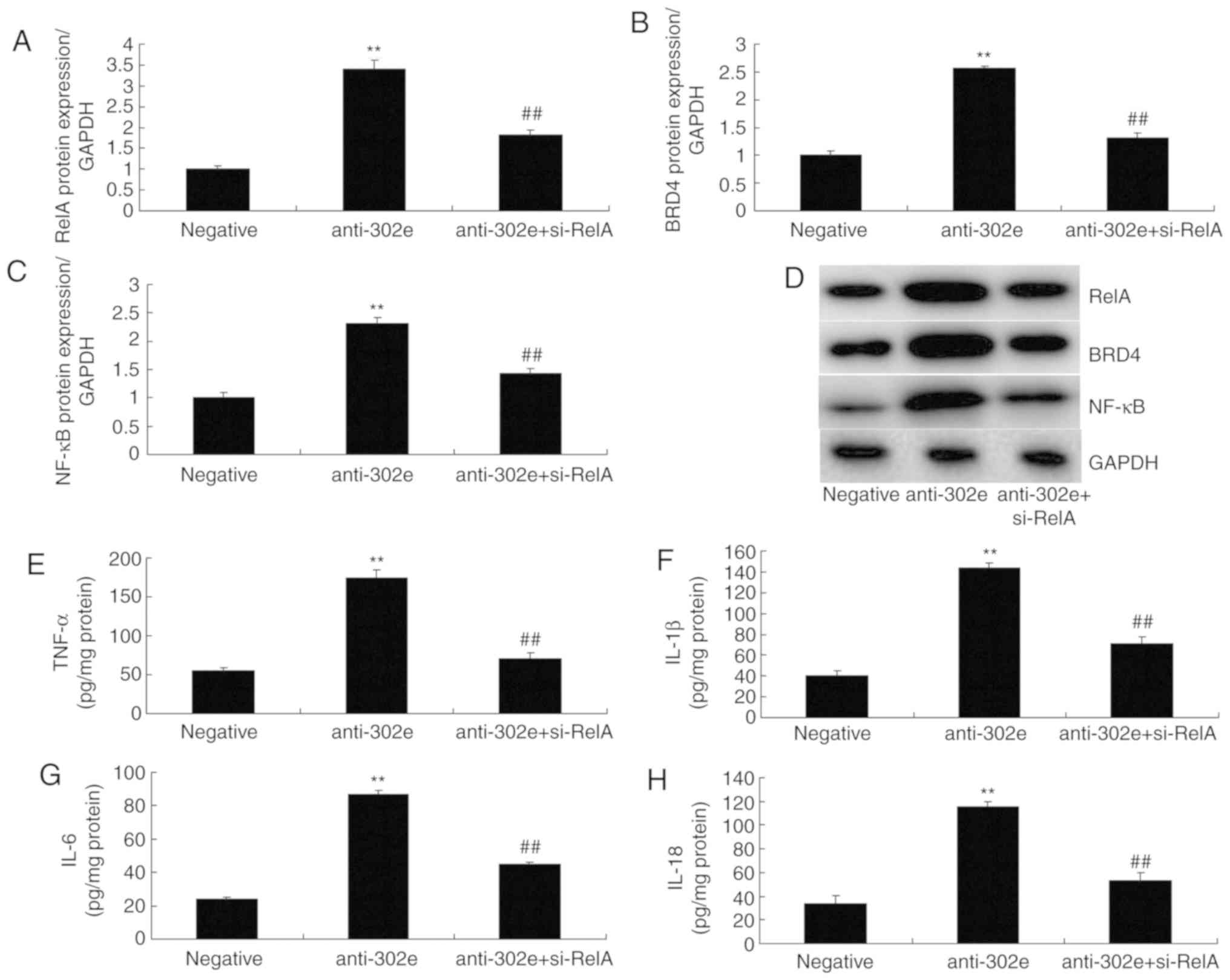 | Figure 6si-RelA reduces the effects of
miRNA-302e on inflammation in an in vitro model of infantile
pneumonia. (A) RelA, (B) BRD4 and (C) NF-κB protein expression by
statistical analysis and (D) western blotting analysis, (E) TNF-α,
(F) IL-1β, (G) IL-6 and (H) IL-18. **P<0.01 vs. the
negative control group; ##P<0.01 vs. downregulation
of miRNA-302e group. Negative, negative control group; anti-302e,
downregulation of miRNA-302e group; anti-302e+si-RelA, si-RelA and
downregulation of miRNA-302a group; Si, small interfering; IL,
interleukin; TNF, tumor necrosis factor; NF, nuclear factor; BRD4,
bromodomain-containing protein 4; miRNA, microRNA. |
si-BRD4 attenuates the effects of
miRNA-302e on inflammation in an in vitro model of IP
To evaluate the function of BRD4 in the effects of
miRNA-302e on inflammation in an in vitro model of IP,
si-BRD4 was administered to reduce the protein expression of BRD4
following downregulation of miRNA-302e in an in vitro model
of IP, compared with the downregulation of the miRNA-302e group.
The induction of BRD4 and NF-κB protein expression were suppressed
in the si-BRD4 and downregulation of miRNA-302e group, compared
with downregulation of miRNA-302e alone group (Fig. 7A-C). The promotion of TNF-α,
IL-1β, IL-6 and IL-18 levels were also decreased in the in
vitro model of IP by downregulation of miRNA-302e and si-BRD4,
in comparison with downregulation of miRNA-302e group (Fig. 7D-G).
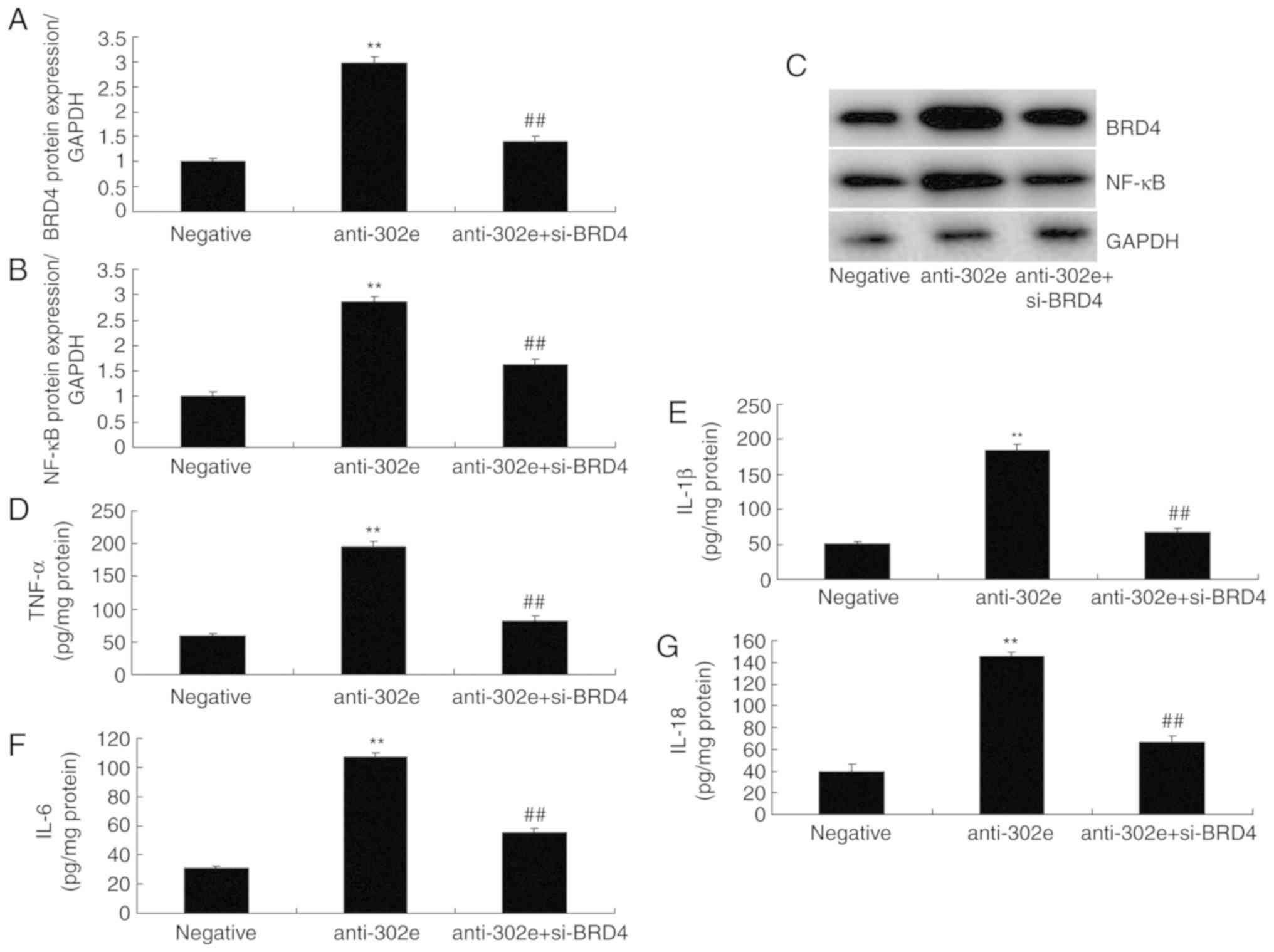 | Figure 7Si-BRD4 reduces the effects of
miRNA-302e on inflammation in an in vitro model of infantile
pneumonia. (A) BRD4 and (B) NF-κB protein expression by statistical
analysis and (C) western blotting analysis, (D) TNF-α, (E) IL-1β,
(F) IL-6 and (G) IL-18. **P<0.01 vs. the negative
control group; ##P<0.01 vs. the downregulation of
miRNA-302e group. Negative, negative control group; anti-302e,
downregulation of miRNA-302e group; anti-302e+si-BRD4, Si-BRD4 and
downregulation of miRNA-302a group; TNF, tumor necrosis factor; IL,
interleukin; NF, nuclear factor; BRD4, bromodomain-containing
protein 4; miRNA, microRNA; si, small interfering. |
si-NF-κB attenuates the effects of
miRNA-302e on inflammation in an in vitro model of IP
Finally, si-NF-κB was used to reduce the protein
expression of NF-κB in an in vitro model of IP following
downregulation of miRNA-302e, in comparison with downregulation of
miRNA-302e group. The activation of NF-κB protein expression was
reduced in the si-NF-κB and downregulation of miRNA-302e group,
compared with the downregulation of miRNA-302e alone group
(Fig. 8A-B). The levels of TNF-α,
IL-1β, IL-6 and IL-18 were reduced in the si-NF-κB and
downregulation of miRNA-302e group, compared with the
downregulation of miRNA-302e alone group (Fig. 8C-F). Collectively, these results
demonstrated that miRNA-302e may serve a critical role in
inflammation IP in vitro.
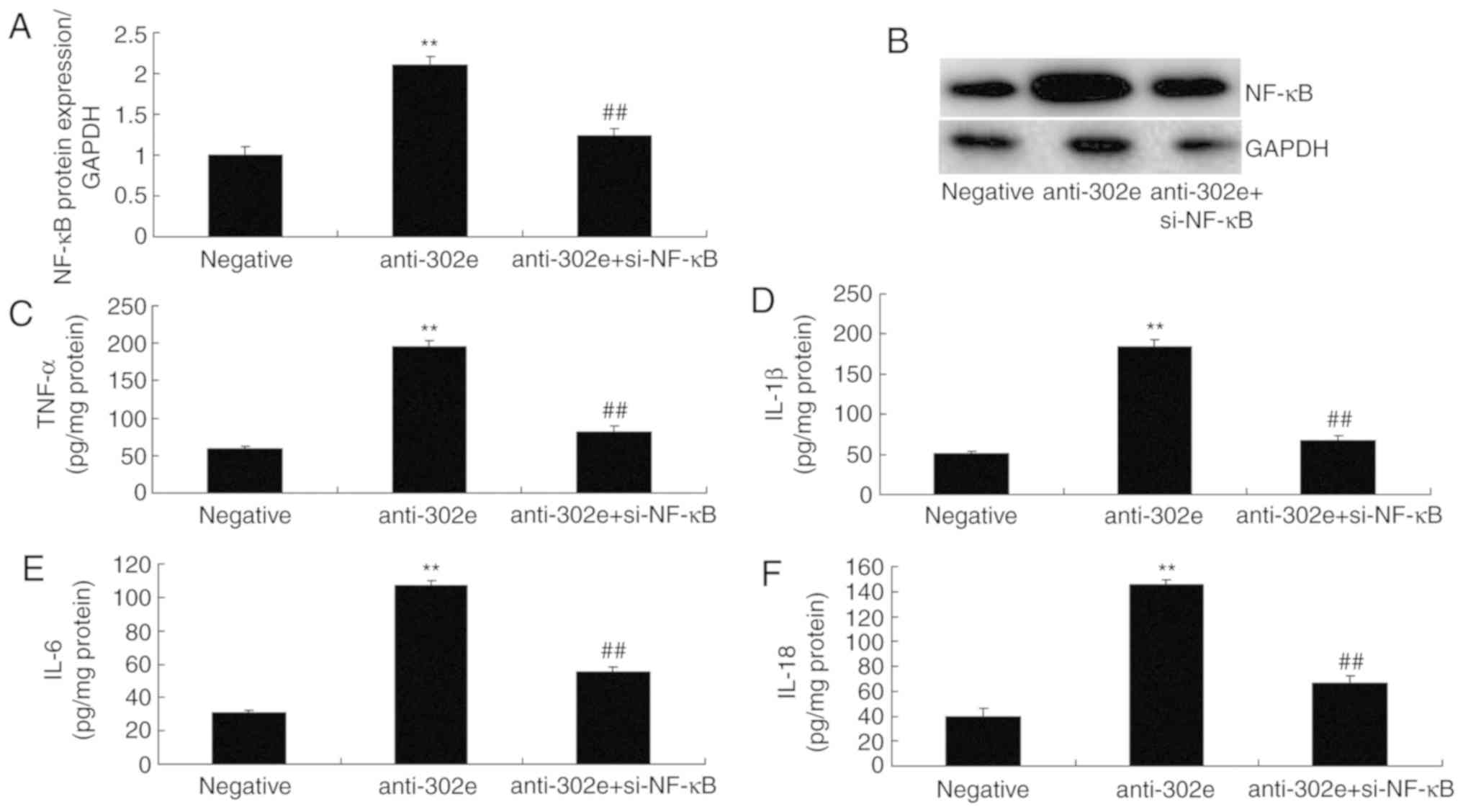 | Figure 8Si-NF-κB reduces the effects of
miRNA-302e on inflammation in an in vitro model of infantile
pneumonia. (A) NF-κB protein expression by statistical analysis and
(B) western blotting, (C) TNF-α, (D) IL-1β, (E) IL-6 and (F) IL-18
protein expression. **P<0.01 vs. downregulation of
miRNA-302e group; ##P<0.01 vs. downregulation of
miRNA-302e group. Negative, negative control group; anti-302e,
downregulation of miRNA-302e group; anti-302e+si-NF-κB, si-NF-κB
and downregulation of miRNA-302a group; TNF, tumor necrosis factor;
IL, interleukin; NF, nuclear factor; BRD4, bromodomain-containing
protein 4; miRNA, microRNA. |
Discussion
IP is one of the major pathogens of CAP in children
and MPP is suggested to account for 10-40% of CAP in children
(1). IP infection will induce
respiratory tract symptoms, but also induce intrapulmonary
complications and extrapulmonary systemic impairment, therefore
severely reducing the quality of life for the children. An
increasing number of studies indicate that immune factors are
involved in IP genesis, particularly, certain cytokine levels are
abnormal (19,20). miRNAs are a class of non-coding
small RNA ~18-24 nucleotides in length, which participate in
regulating multiple vital processes, including cell proliferation,
differentiation and apoptosis (20). A recent study discovered that
miRNA serve a vital regulatory role in inflammation; for instance,
miR-155, miR-146a, miR-221 and miR-192 are involved in the genesis
and development of numerous inflammatory diseases (21). The present study demonstrated that
miRNA-302e expression in mice of IP model was reduced, compared
with the sham group. Xiao et al (15) and Peng et al (16) demonstrated that the microRNA-302
cluster downregulates enterovirus 71-induced innate immune
responses and inflammation. The present study only used one lung
adenocarcinoma cell A549 cell and this is insufficient. The authors
of the present study plan to use non-cancerous cell line in future
studies.
At the acute phase of pneumonia, the action of
pathogenic microorganisms and gas exchange area are reduced, which
will thereby induce hypoxia and infectious poisoning symptoms to
various degrees (22). The
non-specific and specific immune functions in the pediatric
respiratory tract are poor, the respiratory tract can be easily
infected and pulmonary tissue is subjected to inflammation
(22). Moreover, cells suffer
from degeneration or necrosis, along with inflammatory cell
infiltration, which will produce a large amount of reactive oxygen
species (ROS) (23). Furthermore,
the antioxidant capacity of the lung is insufficient and cannot
eliminate these ROS in a timely fashion, so a large amount of lipid
peroxides will damage the tissue and cell membrane, and increase
its permeability, leading to lysosome rupture and tissue necrosis.
Therefore, it will affect the lung function. Therefore, how to
rapidly and accurately diagnose IP at acute phase is of vital
significance to the treatment and rehabilitation of children. In
this study, it was demonstrated that downregulation of miRNA-302e
promoted TNF-α, IL-1β, IL-6 and IL-18 levels in vitro model
of IP. Xiao et al (15)
demonstrated that miRNA-302e attenuates allergic inflammation in
vitro model by targeting RelA/NF-κB activation.
Multiple intracellular regulatory factors can
activate transcription, therefore resulting in reduced apoptosis
and enhanced proliferation (24).
Therefore, it is regarded as an important link during the genesis
and development of gastric cancer. NF-κB is one of these factors,
which can regulate expression of multiple genes and is closely
associated with tumor genesis and development (25). RelA(p65) is one of the NF-κB(Rel)
family members. In the absence of signal stimulation, the inactive
RelA can bind with inhibitor (I)κB and exist in cytoplasm in the
form of a complex. In contrast, IκB will be phosphorylated by the
IκB kinase complex in the presence of a foreign stimulation signal
(13). Therefore, the RelA/IkB
complex will be activated and isolated, and the free RelA will
enter the nucleus to regulate associated gene expression. The
present study demonstrated that downregulation of miRNA-302e
induced RelA, BRD4 and NF-κB protein expression in the in
vitro model of IP. Si-RelA, si-BRD4 or si-NF-κB reduced the
effects of miRNA-302e on inflammation in the in vitro model
of IP. Xiao et al (15)
demonstrated that miRNA-302e attenuates allergic inflammation in an
in vitro model by targeting RelA/NF-κB activation.
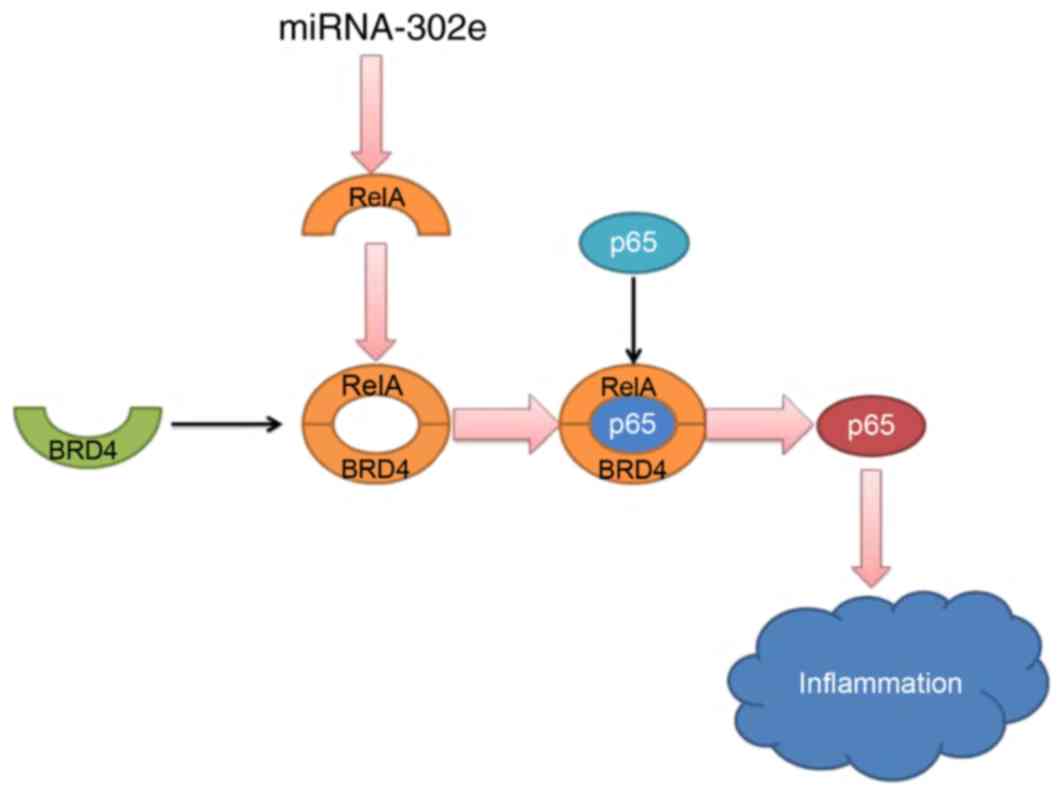
In conclusion, the present study demonstrates that
miRNA-302e expression in mice IP models was reduced. miRNA-302e
attenuates inflammation in IP though the RelA/BRD4/NF-κB signaling
pathway (Fig. 9). These results
reveal a novel anti-inflammatory role of miRNA-302e in activated
mast cells, suggesting that miR-302e may be a promising therapeutic
target for the treatment of IP.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL designed the experiments. QS, YZ, JL and WC
performed the experiments. SL analyzed the data. SL wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experiments have been approved by the Ethics
Committee of The People's Hospital of Dongying (Dongying,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Launay E, Levieux K, Levy C, Dubos F,
Martinot A, Vrignaud B, Lepage F, Cohen R, Grimprel E, Hanf M, et
al: Compliance with the current recommendations for prescribing
antibiotics for paediatric community-acquired pneumonia is
improving: Data from a prospective study in a French network. BMC
Pediatr. 16:1262016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bai D, Han A and Cong S: The effect of
down-regulation of CCL5 on lipopolysaccharide-induced WI-38
fibroblast injury: A potential role for infantile pneumonia. Iran J
Basic Med Sci. 21:449–454. 2018.PubMed/NCBI
|
|
3
|
Cho BO, Yin HH, Park SH, Byun EB, Ha HY
and Jang SI: Anti-inflammatory activity of myricetin from Diospyros
lotus through suppression of NF-κB and STAT1 activation and
Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated
RAW264.7 macrophages. Biosci Biotechnol Biochem. 80:1520–1530.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rocha GR, Florez Salamanca EJ, de Barros
AL, Lobo CIV and Klein MI: Effect of tt-farnesol and myricetin on
in vitro biofilm formed by Streptococcus mutans and Candida
albicans. BMC Complement Altern Med. 18:612018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Revised American Society for Reproductive
Medicine classification of endometriosis: 1996. Fertil Steril.
67:817–821. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim GD: Myricetin inhibits angiogenesis by
inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in
endothelial cells. J Cancer Prev. 22:219–227. 2017. View Article : Google Scholar
|
|
7
|
Zukauskas A, Mrsny RJ, Cortés Barrantes P,
Turner JR, Leong JM and McCormick BA: Transporters MRP1 and MRP2
regulate opposing inflammatory signals to control transepithelial
neutrophil migration during streptococcus pneumoniae lung
infection. mSphere. 3:2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berg AS, Inchley CS, Fjaerli HO, Leegaard
TM, Lindbaek M and Nakstad B: Clinical features and inflammatory
markers in pediatric pneumonia: A prospective study. Eur J Pediatr.
176:629–638. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai JP, Wang QW, Su Y, Gu LM, Zhao Y, Chen
XX, Chen C, Li WZ, Wang GF and Li KS: Emodin inhibition of
influenza A virus replication and influenza viral pneumonia via the
Nrf2, TLR4, p38/JNK and NF-kappaB pathways. Molecules. 22:2017.
View Article : Google Scholar
|
|
10
|
Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu
Z, Wang H, Xu F and Shi L: MiR-127 modulates macrophage
polarization and promotes lung inflammation and injury by
activating the JNK pathway. J Immunol. 194:1239–1251. 2015.
View Article : Google Scholar
|
|
11
|
Fei S, Cao L and Pan L: microRNA3941
targets IGF2 to control LPS-induced acute pneumonia in A549 cells.
Mol Med Rep. 17:4019–4026. 2018.PubMed/NCBI
|
|
12
|
Wang W, Chen M, Jin X, Li X, Yang Z, Lin H
and Xu S: H S induces Th1/Th2 imbalance with triggered NF-κB
pathway 2 to exacerbate LPS-induce chicken pneumonia response.
Chemosphere. 208:241–246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng C, Han J, Ye X and Zhang X: IL-33
treatment attenuates the systemic inflammation reaction in
acinetobacter baumannii pneumonia by suppressing TLR4/NF-κB
signaling. Inflammation. 41:870–877. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li D, Beisswenger C, Herr C, Hellberg J,
Han G, Zakharkina T, Voss M, Wiewrodt R, Bohle RM, Menger MD, et
al: Myeloid cell RelA/p 65 p romotes lung cancer proliferation
through Wnt/β-catenin signaling in murine and human tumor cells.
Oncogene. 33:1239–1248. 2014. View Article : Google Scholar
|
|
15
|
Xiao L, Jiang L, Hu Q and Li Y: MiR-302e
attenuates allergic inflammation in vitro model by targeting RelA.
Biosci Rep. 38:2018. View Article : Google Scholar
|
|
16
|
Peng N, Yang X, Zhu C, Zhou L, Yu H, Li M,
Lin Y, Wang X, Li Q, She Y, et al: MicroRNA-302 cluster
downregulates enterovirus 71-induced innate immune response by
targeting KPNA2. J Immunol. 201:145–156. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto Y, Hosoda K, Imahori T, Tanaka J,
Matsuo K, Nakai T, Irino Y, Shinohara M, Sato N, Sasayama T, et al:
Pentose phosphate pathway activation via HSP 27 p hosphorylation by
ATM kinase: A putative endogenous antioxidant defense mechanism
during cerebral ischemia-reperfusion. Brain Res. 1687:82–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia N, Chen G, Liu M, Ye X, Pan Y, Ge J,
Mao Y, Wang H, Wang J and Xie S: Anti-inflammatory effects of
luteolin on experimental autoimmune thyroiditis in mice. Exp Ther
Med. 12:4049–4054. 2016. View Article : Google Scholar
|
|
19
|
Lee da H and Lee CS: Flavonoid myricetin
inhibits TNF-α-stimulated production of inflammatory mediators by
suppressing the Akt, mTOR and NF-κB pathways in human
keratinocytes. Eur J Pharmacol. 784:164–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shamir R and Garty BZ: Pneumocystis
carinii pneumonia associated with adrenocorticotropic hormone
treatment for infantile spasms. Eur J Pediatr. 151:8671992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu M, Han T, Shi S and Chen E: Long
noncoding RNA HAGLROS regulates cell apoptosis and autophagy in
lipopolysaccharides-induced WI-38 cells via modulating
miR-100/NF-κB axis. Biochem Biophys Res Commun. 500:589–596. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu B, Shen Y, Qiao J and Cui Q: Geniposide
attenuates Staphylococcus aureus-induced pneumonia in mice by
inhibiting NF-κB activation. Microb Pathog. 112:117–121. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roade Tato L, Burgos Cibrian J, Curran
Fábregas A, Navarro Mercadé J, Willekens R, Martín Gómez MT, Ribera
Pascuet E and Falcó Ferrer V: Immune reconstitution inflammatory
syndrome in HIV-infected patients with Pneumocystis jirovecii
pneumonia. Enferm Infecc Microbiol Clin. 36:621–626. 2017.In
English, Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song C, He L, Zhang J, Ma H, Yuan X, Hu G,
Tao L, Zhang J and Meng J: Fluorofenidone attenuates pulmonary
inflammation and fibrosis via inhibiting the activation of NALP3
inflammasome and IL-1β/IL-1R1/MyD88/NF-κB pathway. J Cell Mol Med.
20:2064–2077. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu
C, Li C and Deng G: Plantamajoside ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Int Immunopharmacol. 35:315–322. 2016.
View Article : Google Scholar : PubMed/NCBI
|