Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant
tumor of the head and neck, with a high incidence in South Asia and
Southern China (1). At present,
the primary treatment methods of NPC are radiotherapy and
chemotherapy, which have clinically-demonstrated antitumor effects;
however, the 5-year overall survival rate of patients with NPC
remains low (1,2). The mechanism of NPC has not yet been
elucidated, although an accelerated cell cycle and enhanced cell
invasion are considered to be closely associated with the
occurrence and development of NPC (3). A variety of genes are involved in
the regulation of cell proliferation and invasion in NPC (3). However, appropriate target genes for
NPC treatment have not been identified.
Fibroblast growth factor receptors (FGFRs) belong to
a family of transmembrane tyrosine kinase receptors with
autophosphorylation activity (4).
A total of 4 family members, FGFR1, FGFR2, FGFR3 and FGFR4, have
been identified (4).
Overexpression, mutations and abnormal transcriptional regulation
of FGFRs may cause abnormalities in the FGFR downstream signaling
pathway, leading to tumor formation (5). The FGFR downstream signaling pathway
has been identified to serve a critical role in the development of
prostate and skin cancer, transitional cell carcinoma and
hematological malignancies (5).
FGFR2 is a product of the expression of the oncogene BEK, which is
located on chromosome 10 (10q26), and induces dimerization of
FGFR2, autophosphorylation of intracellular kinases and
conformational changes of the receptor by binding to fibroblast
growth factors (FGFs). In addition, it has been demonstrated to
cause the activation of a series of downstream cascade signaling
pathways, including the mitogen-activated protein
kinase/extracellular signal-regulated kinase and
phosphatidylinositol 3-kinase/RAC-alpha serine/threonine-protein
kinase pathways (6,7). Overexpression and missense mutations
of the FGFR2 gene have been identified in a variety of human
tumors, including gastric, breast and ovarian cancer (8-10).
However, to the best of our knowledge, there have been few studies
investigating FGFR2 in NPC.
Cisplatin has been widely used in the treatment of a
variety of tumors following approval by the United States of
America Food and Drug Administration in 1978 (11). Patients usually experience a good
therapeutic effect in the early stages of cisplatin chemotherapy;
however, it is common for drug resistance to develop gradually
during treatment and severely limit the efficacy of subsequent
cisplatin therapy (12). In
addition, certain patients have been observed to exhibit intrinsic
resistance to cisplatin (12).
Cisplatin resistance has also been identified in NPC cells, and is
becoming a serious public health concern (13,14).
In light of these data, we hypothesized that FGFR2
served a critical role in the effect of cisplatin on the viability
and apoptosis of NPC cells. Therefore, the present study aimed to
explore whether the expression levels of FGFR2 in NPC tissues and
cell lines were altered, and whether the efficiency of cisplatin
was improved following the downregulation of FGFR2, in order to
reveal the potential of FGFR2 in improving the efficacy of
cisplatin treatment.
Materials and methods
Collection of cancer and adjacent tissues
from NPC patients
The study protocol was approved by the Ethics Board
of Zhejiang Provincial People's Hospital, People's Hospital of
Hangzhou Medical College (Hangzhou, China). Samples from 55
patients were collected, including 25 females and 30 males. The age
of these patients ranged from 18-80 years old, with an average of
53.8 years. All patients were diagnosed with NPC by pathological
examination of biopsy specimens from September 2017 to September
2018 in Zhejiang Provincial People's Hospital, People's Hospital of
Hangzhou Medical College. Patients without distant metastasis were
classified according to the 7th edition of Union for International
Cancer Control Staging System for NPC (15). Biopsy samples of cancer and
adjacent tissues were placed in liquid nitrogen immediately, and
then stored at −80°C until use. All patients provided written
informed consent prior to the initiation of the study.
Cell culture, experimental grouping and
transfection
NP69, SUNE1, C666-1, 6-10B and HNE-3 cell lines were
obtained from The Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China), and were cultured in
RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The NP69 cell line was used as the control group, which was
derived from epithelial cells of the human nasopharynx. Cells were
supplied with 10% fetal bovine serum (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) and cultured at 37°C with 5%
CO2. Cells were sub-cultured when cell density reached
80-90% confluence. The small interfering RNA (siRNA) against FGFR2
(siFGFR2) and negative control (NC)-siRNA were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequences of
the FGFR2 siRNA were 52032-CAATAGGACAGTGCTTATT-3′ and
5′-CTCTCTATGTCATAGTTGA-3′, and the sequence of the NC-siRNA was
5′-AATTCTCCGAACGTGTCACG-3′. SUNE1 and C666-1 cells were transfected
with siFGFR2 (40 nM) or NC-siRNA using Lipofectamine™ 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. After 48 h, the cells
were collected and used for subsequent experiments.
To observe the effect of siFGFR2, SUNE1 and C666-1
cells were divided into three groups: Control (blank); NC
(transfection with the NC-siRNA); and siFGFR2 groups (transfection
with siFGFR2). Subsequently, to explore the effect of cisplatin,
SUNE1 and C666-1 cells were divided into an additional four groups:
Control (blank); CIS-1 (treatment with 1 μg/ml cisplatin);
CIS-2 (treatment with 10 μg/ml cisplatin); and CIS-3
(treatment with 20 μg/ml cisplatin) groups. Finally, in
order to investigate the effect of cisplatin with concomitant FGFR2
downregulation, SUNE1 and C666-1 cells were divided into six
groups: Control (blank); NC (transfection with the NC-siRNA);
siFGFR2 (transfection with siFGFR2); CIS (treatment with 10
μg/ml cisplatin); NC+CIS (treatment with 10 μg/ml
cisplatin and transfection with the NC-siRNA); and siFGFR2+CIS
groups (treatment with 10 μg/ml cisplatin and transfection
with siFGFR2).
Immunohistochemistry (IHC)
FGFR2 expression in tissues of the 55 cases was
examined using an immunohistochemical streptomycin
avidin-peroxidase (SP) kit (KIT-9706; Fuzhou Maxim Biotech, Co.,
Ltd., Fuzhou, China). Tissues were fixed with 4% paraformaldehyde
for 24 h at room temperature (RT), embedded in paraffin and then
cut into 4 μm sections. The paraffin sections were incubated
at 60°C for 2 h, treated with xylene at RT for 20 min, and then
immersed in 100% ethanol for 2 min, 95% ethanol for 2 min, 90%
ethanol for 2 min, 80% ethanol for 2 min, 70% ethanol for 2 min,
and then placed in distilled water. The sections were placed in an
incubator containing citrate antigen repair solution (DAS-0010;
Fuzhou Maxim Biotech, Co., Ltd.), and were then placed in an
autoclave (126°C) to be steamed for 2 min. Avidin (25 μg/ml)
was added to the sections and incubated for 10 min at RT. Following
this, sections were washed 3 times for 5 min with PBS. D-biotin
solution was added at RT for 10 min. Sections were washed with PBS
3 times for 5 min again. An endogenous peroxidase blocker (3%
hydrogen peroxide) was added to the sections and incubated at RT
for 15 min, and then washed 3 times for 3 min. Goat serum (10%;
Abcam, Cambridge, MA, USA) was used to block the sections, and
sections were incubated for 10 min at RT. An anti-FGFR2 antibody
(cat. no. ab10648; Abcam) was diluted to 1:1,000, and incubated
with the sections overnight at 4°C. Following washing with PBS 3
times for 5 min, a biotin-labeled goat anti-rabbit IgG secondary
antibody (cat. no. ab205718; 1:2,000; Abcam) was added to sections
for 10 min at RT. Sections were then washed with PBS 3 times for 3
min, and the SP solution was added and the mixture was incubated at
RT for 10 min. Sections were then rinsed with PBS 3 times for 3 min
and fresh 3,3′-diaminobenzidine solution was added. Sections were
incubated for 5-10 min until a color change was observed, and then
were rinsed in distilled water immediately. The slides were
counterstained with 0.5% hematoxylin for 3 min at RT, and then
washed. The sections were immersed in 70% ethanol for 2 min, 80%
ethanol for 2 min, 90% ethanol for 2 min, 100% ethanol for 2 min,
and then treated with 100% xylene for 2 min, and then observed
under a light microscope (magnification, ×100 and ×200).
The positive proportions of tissue were determined
according to the following classification: 0, no staining; 1,
<33% cell staining; 2, 33-66% cell staining; 3, >66% cell
staining. The staining intensity was then determined according to
the following classification: 0, without staining; 1, light yellow;
2, brownish yellow; 3, dark brown. The final classification scores
were calculated by combining the 2 scores: 0, negative; 2-3, weak
positive (+); 4, positive (++); and 5-6, positive (+++).
Survival curves of FGFR2
Complete clinical and follow-up records from the 55
NPC cases were gathered. According to the IHC results of FGFR2
expression, the cases with final classification scores ≥4 were
grouped as the high FGFR2 expression cases, and the cases with
final classification scores <4 were grouped as the low FGFR2
expression cases. The overall survival curves of the two groups
were then analyzed.
Cell viability assay
Cell vitality of SUNE1 and C666-1 cells was examined
using a Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Procedures were performed according the
manufacturer's protocols. The cells were then incubated at 37°C for
1 h, and the optical density (OD) was measured at 450 nm using a
Multiskan™ microplate reader (Thermo Fisher Scientific, Inc.). The
assays were performed subsequent to culturing for 24 and 48 h
post-transfection.
Assessment of cell cycle rates
Cell cycle rates were measured by Vybrant™ DyeCycle™
Violet Stain reagent (Thermo Fisher Scientific, Inc.). Briefly,
flow cytometry tubes each containing 1 ml cell suspension in
complete media at a concentration of 1×106 cells/ml was
prepared. Then, 1 μl Vybrant DyeCycle™ Violet stain was
added to each tube and mixed well. The stain concentration was
adjusted to 5 μM. Cells were incubated at 37°C for 30 min in
the dark. Cells were maintained at 37°C until analysis. Cells were
analyzed without washing or fixing on a flow cytometer at
excitation and emission wavelengths of 405 and 440 nm,
respectively.
Determination of mRNA levels by reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted by TRIzol® (Thermo Fisher
Scientific, Inc.) from SUNE1 and C666-1 cells and cDNA was
synthesized using an iScript™ cDNA Synthesis kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). In brief, 1 μg RNA
of each sample was transcribed into cDNA following the
manufacturer's protocol. A Fast Start Universal SYBR-Green Master
kit Roche Diagnostics GmbH (Mannheim, Germany) was used to perform
the qPCR. The primers used are listed in Table I. The reaction system was set as
the following: 12.5 μl 2X SYBR-Green master mix; 2 μl
cDNA template; 1 μl forward primer (10 μM); 1
μl reverse primer (10 μM); and 8.5 μl
ddH2O. The PCR thermocycling conditions were set as
follows: Initial denaturation at 95°C for 10 min, followed by 40
cycles of three-step PCR (denaturation at 95°C for 15 sec,
annealing at 60°C for 1 min, elongation at 72°C for 3 min), and
final extension at 75°C for 10 min. A CFX96 Touch™ (cat. no. 6093;
Bio-Rad Laboratories, Inc.) machine was used to conduct the PCR
assay. Relative mRNA levels of samples were calculated using the
2−ΔΔCq method (16).
 | Table IPrimers used in reverse
transcription-quantitative PCR assay. |
Table I
Primers used in reverse
transcription-quantitative PCR assay.
| Gene name | Primer sequence
(5′-3′) |
|---|
| FGFR2 | (F)
TTAGAGCCAGAAGAGCCACC |
| (R)
TACAAGCATAGAGGCCGGAG |
| Bcl-2 | (F)
TTGAGGAAGTGAACATTTCGGTG |
| (R)
AGGTTCTGCGGACTTAGGTC |
| Bax | (F)
GCGAGTGTCTCAAGCGCATC |
| (R)
CCAGTTGAAGTTGCCGTCAGAA |
| Cyclin D1 | (F)
CCCTCGGTGTCCTACTTCAA |
| (R)
CTTAGAGGCCACGAACATGC |
| CDC25A | (F)
CTGTTTGACTCCCCTTCCCT |
| (R)
GGGGAAGATGCCAGGGATAA |
| β-actin | (F)
CACCATTGGCAATGAGCGGTTC |
| (R)
AGGTCTTTGCGGATGTCCACGT |
Extraction and measurement of total
protein content and western blot analysis
SUNE1 and C666-1 cells were collected and washed
with PBS, and then lysed by radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. The supernatant of cells was then
collected following centrifugation at 4°C and 6,000 × g for 15 min.
The density of total proteins was measured by the Pierce™ BCA
Protein Assay kit (Thermo Fisher Scientific, Inc.). Briefly, 2
μl samples and standard reference proteins, which were
diluted to 1, 0.5, 0.25, 0.125 and 0.0625 g/ml, respectively, were
added to a 96-well plate. BCA reagent was added to the plate and
cells were incubated at 37°C for 30 min. The standard curve was
generated and the density of the total protein was calculated
according to the OD measured at 562 nm using a Multiskan™
microplate reader (Thermo Fisher Scientific, Inc.). Then, 20
μg total protein of each sample was denatured at 95°C for 10
min and separated by 10% SDS-PAGE electrophoresis at 100 V for 2 h.
Proteins were transferred onto a PVDF membrane by wet transferring
at 90 V for 2 h, and then blocked with 5% non-fat milk for 1 h at
RT. Anti-FGFR2 (cat. no. ab10648; Abcam; dilution, 1:1,000),
anti-cleaved caspase-3 (Asp175; cat. no. 9664; Cell Signaling
Technologies, Danvers, MA, USA; dilution, 1:1,000), anti-B-cell
lymphoma 2 (Bcl-2)-associated X protein (Bax; cat. no. ab32503;
Abcam; dilution, 1:1,000), anti-Bcl-2 (cat. no. ab32124; Abcam;
dilution, 1:1,000) and anti-β-actin (cat. no. ab8227; Abcam;
dilution, 1:1,000) primary antibodies were added onto the membrane
separately. The membrane was then placed on a shaker at 4°C
overnight. A horseradish peroxidase-conjugated IgG (H&L)
secondary antibody (cat. no. ab6721; Abcam; dilution, 1:2,000) was
added following washing of the membrane 3 times with PBS/0.05%
Tween-20 (PBST; Beijing Solarbio Science & Technology, Co.,
Ltd., Beijing, China) for 5 min. The membrane was then incubated
for 1 h at RT and washed 3 times in PBST. Protein expression levels
were detected by Pierce™ ECL and a western blot analysis substrate
(Thermo Fisher Scientific, Inc.). The densitometric analysis was
performed by ImageJ software (v.1.46; National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
GraphPad Prism v.7.0 software (GraphPad Software,
Inc., La Jolla, CA, USA was used for statistical analysis. Data was
presented as the mean ± standard deviation. Differences were
performed using one-way analysis of variance test followed by
Tukey's post-hoc test. Survival analysis was performed using the
Kaplan-Meier method, and the Breslow test was used to compare
survival curves. P<0.05 was considered to indicate a
statistically significant difference.
Results
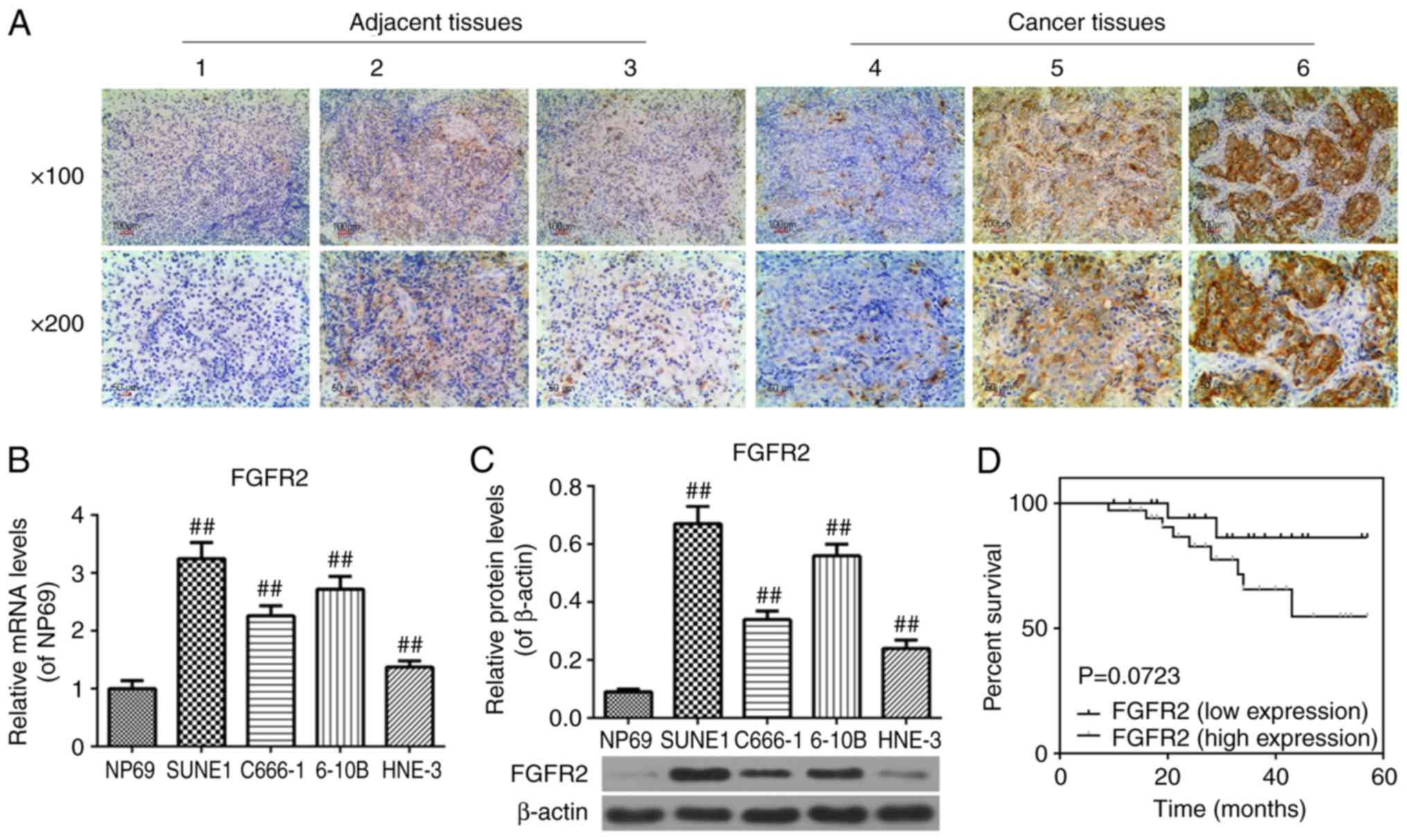
FGFR2 is overexpressed in cancer tissues
from patients with NPC, and in SUNE1, C666-1, 6-10B and HNE-3 cell
lines
In order to identify whether FGFR2 was overexpressed
in NPC, the expression of FGFR2 in adjacent and cancer tissues from
patients with NPC was measured by immunohistochemistry and in NPC
SUNE1, C666-1, 6-10B, HNE-3 cell lines using RT-qPCR and western
blot analysis. Representative images of each level are demonstrated
at magnification ×100 and ×200 in Fig. 1A. Adjacent tissues exhibited
decreased scores (<3) compared with the cancer tissues (>4;
Fig. 1A), and the expression of
FGFR2 was increased in the SUNE1, C666-1, 6-10B and HNE-3 cell
lines compared with the NP69 cell line (P<0.01; Fig. 1B and C). These data indicated that
FGFR2 was overexpressed in NPC.
Overexpression of FGFR2 leads to
unfavorable prognoses in patients with NPC
To determine whether the expression of FGFR2
affected the prognosis of patients with NPC, the survival curves of
patients with NPC with low and high FGFR2 expression levels were
analyzed. According to the Kaplan-Meier analysis, the difference
between these groups was not statistically significant (P=0.0723);
however, these data provide evidence to suggest that changes in the
expression of FGFR2 may affect the prognosis of patients with NPC
to a certain extent (P=0.0723; Fig.
1D). However, additional studies are required to confirm this
hypothesis.
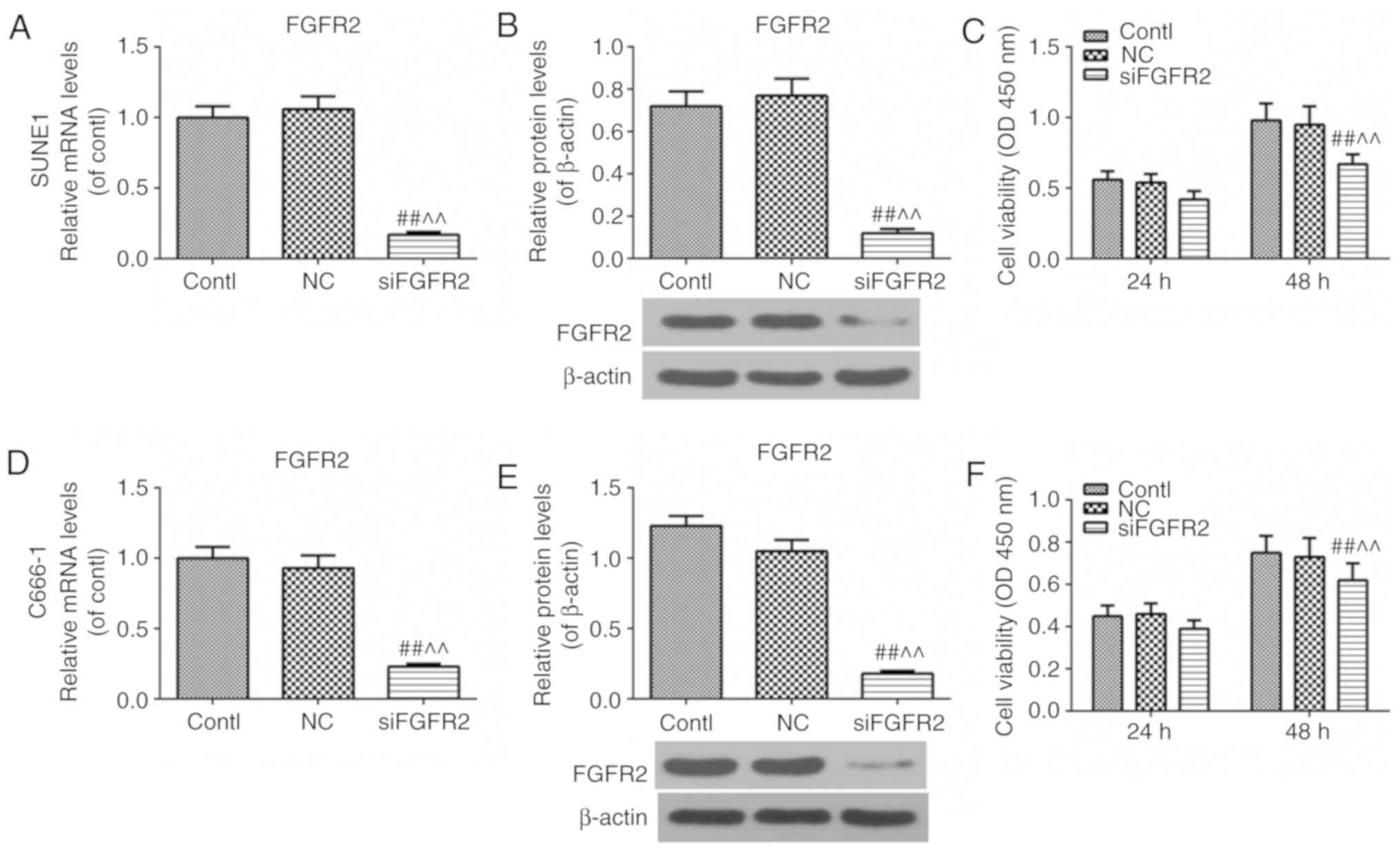
FGFR2 silencing decreases the cell
viability of SUNE1 and C666-1 cell lines
To investigate whether the cell viability was
affected due to the downregulation of FGFR2, cell viability was
measured using a CCK-8 assay, and the expression of FGFR2 was
examined using RT-qPCR and western blot analysis, following
silencing of the expression of FGFR2 using siFGFR2. The expression
of FGFR2 was downregulated successfully and cell viability was
decreased at 48 h after siFGFR2 transfection compared with the
control or NC groups in the SUNE1 cell line (Fig. 2A-C). The results were similar in
the C666-1 cell line (Fig. 2D-F),
suggesting that the downregulation of FGFR2 may decrease the
viability of SUNE1 and C666-1 cell lines.
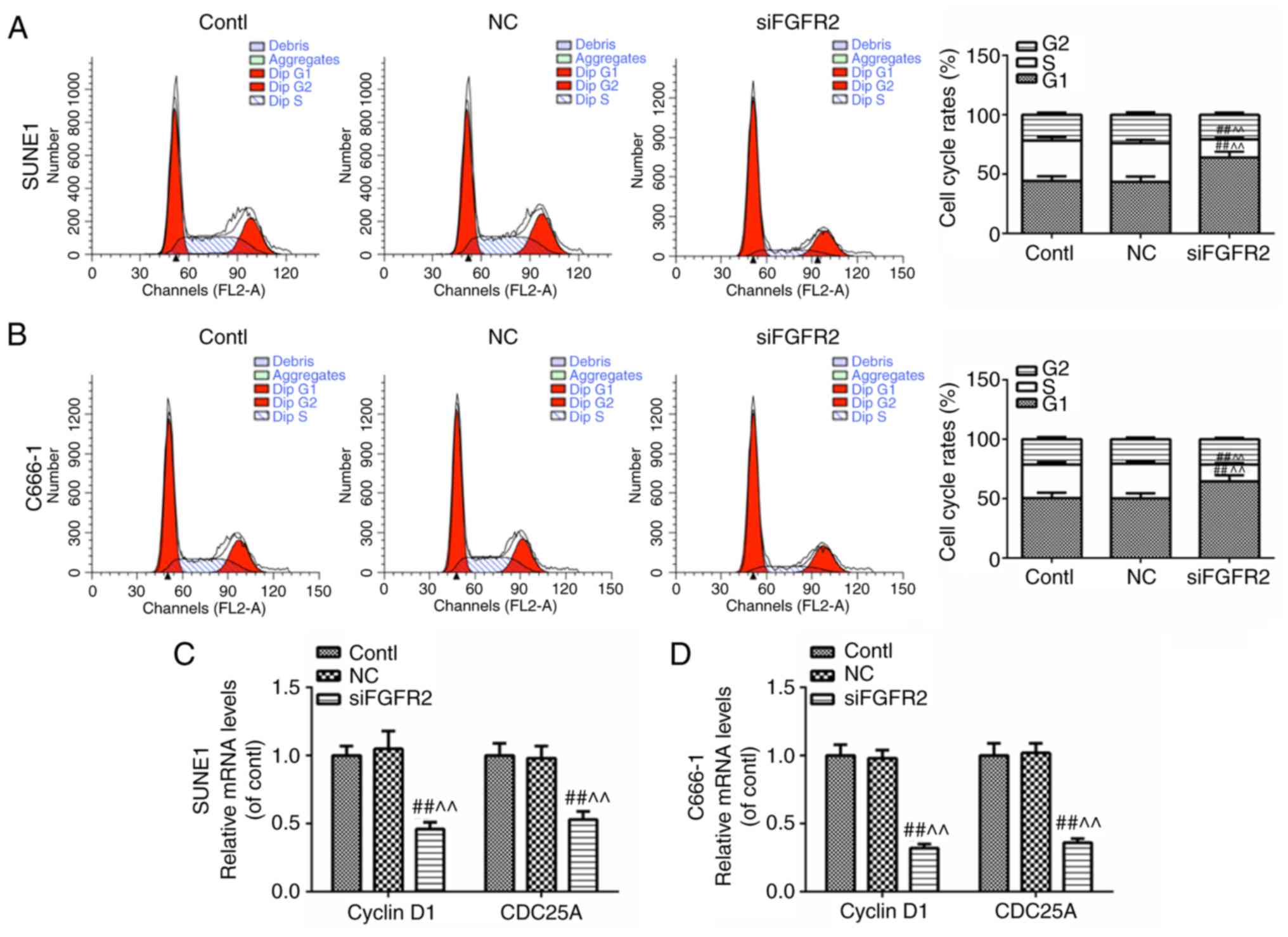
FGFR2 silencing induces cell cycle
arrest
To observe whether the cell cycle was affected by
FGFR2 silencing, changes to the phases of the cell cycle, and the
mRNA expression levels of cyclin D1 and cell division cycle gene
25A (CDC25A) were investigated by flow cytometry and RT-qPCR,
respectively. The G1 phase was prolonged and the S phase was
shortened (Fig. 3A and B), and
the expression levels of cyclin D1 and CDC25A were decreased in the
SUNE1 and C666-1 cell lines (Fig. 3C
and D). These data implied that the silencing of FGFR2 may
induce cell cycle arrest in the G1 phase.
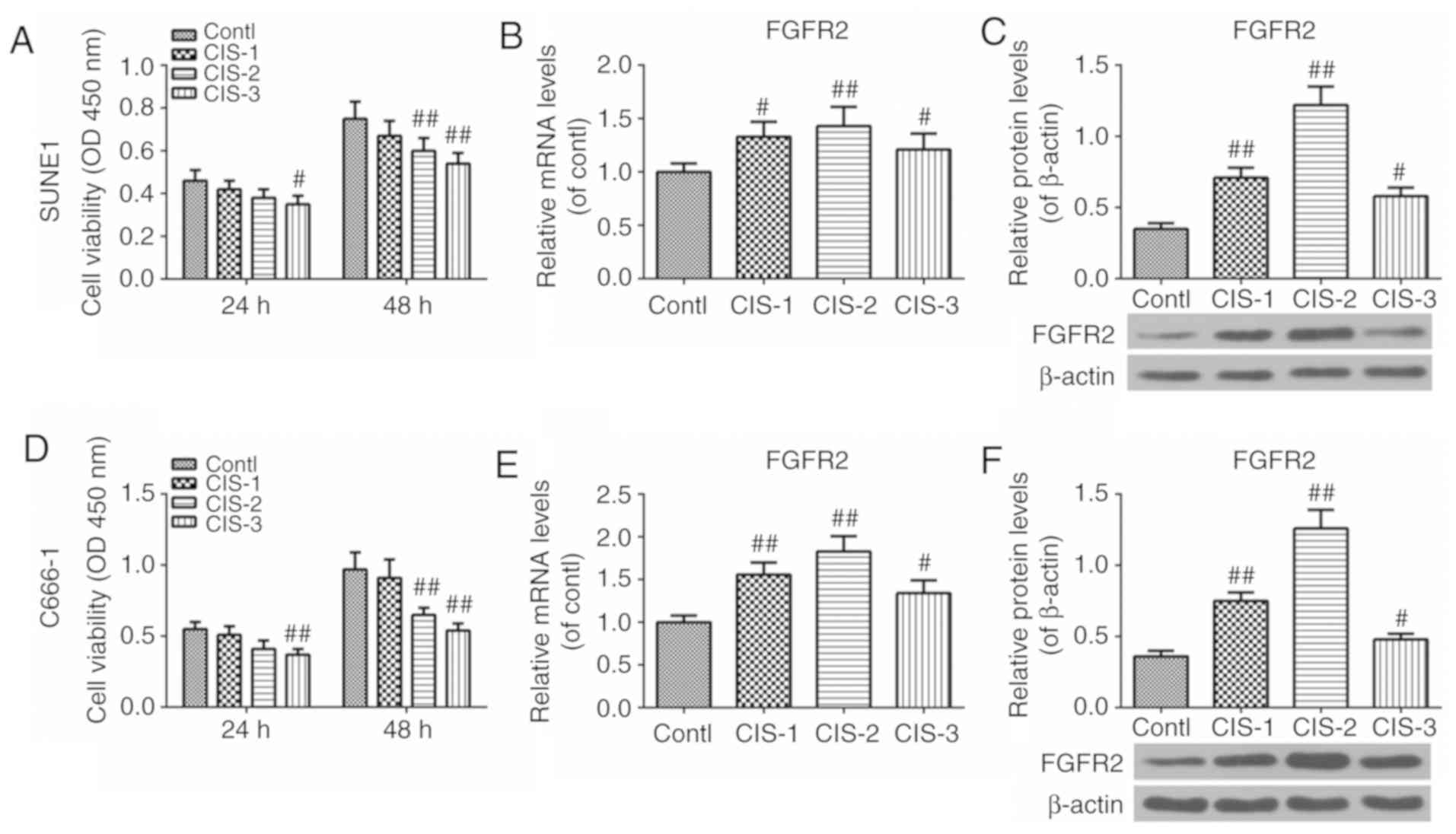
Cisplatin decreases cell viability and
increases FGFR2 expression
Cell viability, FGFR2 mRNA and FGFR2 protein
expression levels following treatment with cisplatin were examined
by CCK-8 assay, RT-qPCR and western blot analysis, respectively. As
demonstrated in Fig. 4, the cell
viability was decreased after 24 h in the CIS-3 group, and
decreased in the CIS-2 and CIS-3 groups after 48 h when compared
with the control groups (Fig.
4A). In addition, the expression levels of FGFR2 in the CIS-1,
2, 3 groups were increased compared with the control group; levels
were highest in CIS-2 group (Fig. 4B
and C). Similar results were observed in the SUNE1 and C666-1
groups (Fig. 4D-F). These results
revealed that cisplatin may decrease the viability of NPC cells and
increase the expression of FGFR2.
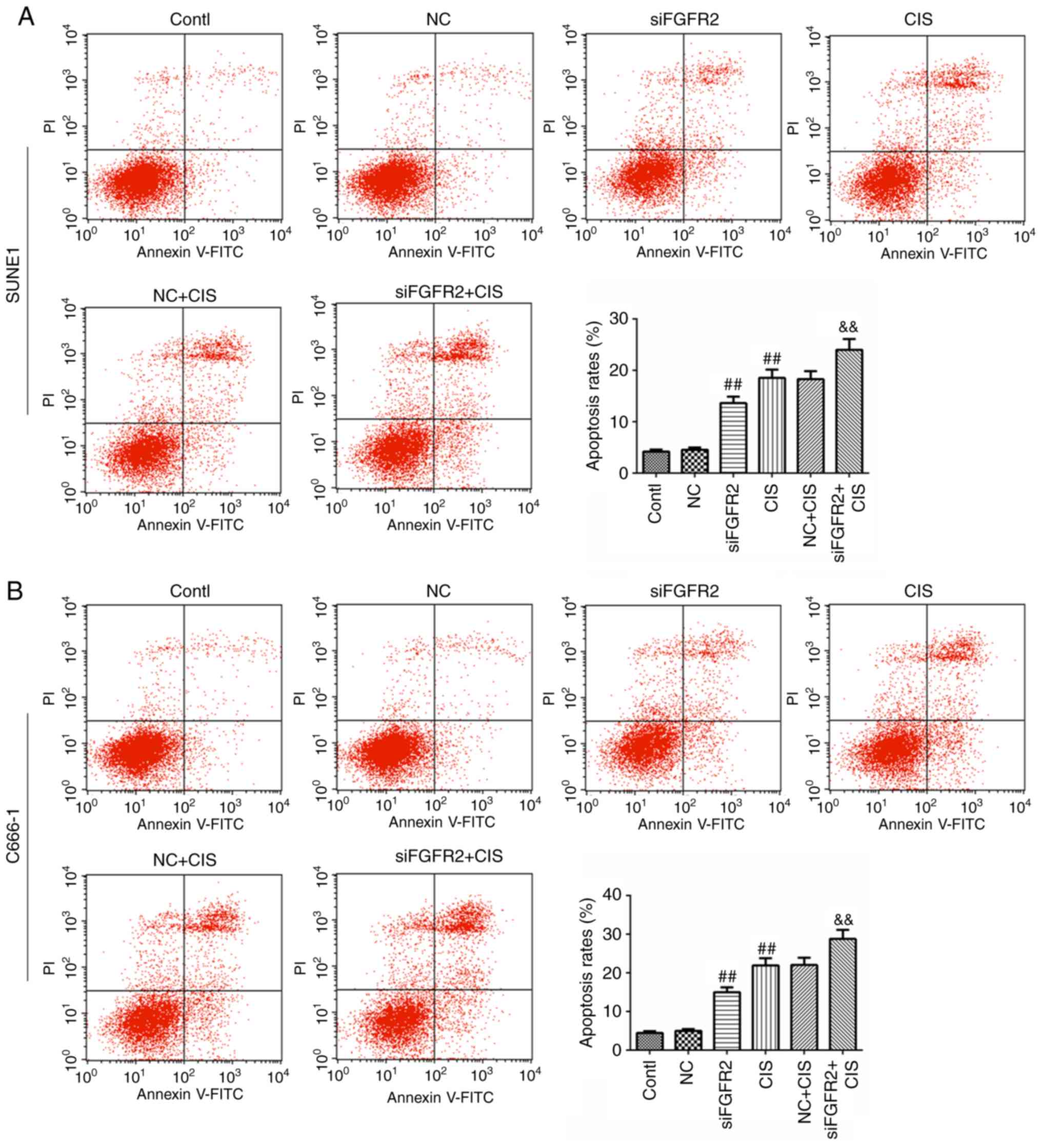
FGFR2 silencing increases the apoptosis
induced by cisplatin
To determine whether the overexpression of FGFR2 was
involved in the apoptosis effect induced by cisplatin, the
apoptosis rate was measured by flow cytometry. As expected, the
apoptosis rates in siFGFR2 and CIS groups were increased compared
with the control or NC groups (Fig.
5A). Notably, the apoptosis rate in the siFGFR2+CIS group was
signifi-cantly increased compared with the CIS group (Fig. 5A). Similar results were observed
in the SUNE1 and C666-1 cell lines (Fig. 5B). We hypothesized that the
downregulation of FGFR2 may increase the effectiveness of
cisplatin-induced apoptosis.
FGFR2 silencing enhances the intrinsic
apoptosis pathway induced by cisplatin
To understand the mechanism underlying the improved
effect of apoptosis induced by the combination of siFGFR2 and
cisplatin, the protein levels of key effector molecules involved in
the activation of the intrinsic apoptosis pathway were determined.
Significantly, the expression of FGFR2 in the siFGFR2 and
siFGFR2+CIS groups was suppressed, but increased in the CIS group
compared with the control or NC groups (Fig. 6A). The expression level of cleaved
caspase-3 and Bax were increased in the siFGFR2, CIS, and
siFGFR2+CIS groups, with the highest levels observed in siFGFR2+CIS
group, compared with the control or NC groups (Fig. 6A). The expression levels of Bcl-2
in the siFGFR2, CIS and siFGFR2+CIS groups were decreased compared
with the control or NC groups; notably, the lowest level was
observed in the siFGFR2+CIS group (Fig. 6A). The results in the SUNE1 cells
were confirmed by similar results in the C666-1 cell line (Fig. 6B). Taken together, these data
indicated that the intrinsic apoptosis pathway may be activated by
FGFR2 silencing and cisplatin; however, the combination of these
two interventions was demonstrated to be more effective compared
with each intervention alone, suggesting that the downregulation of
FGFR2 may enhance the activation of intrinsic apoptosis pathway
induced by cisplatin.
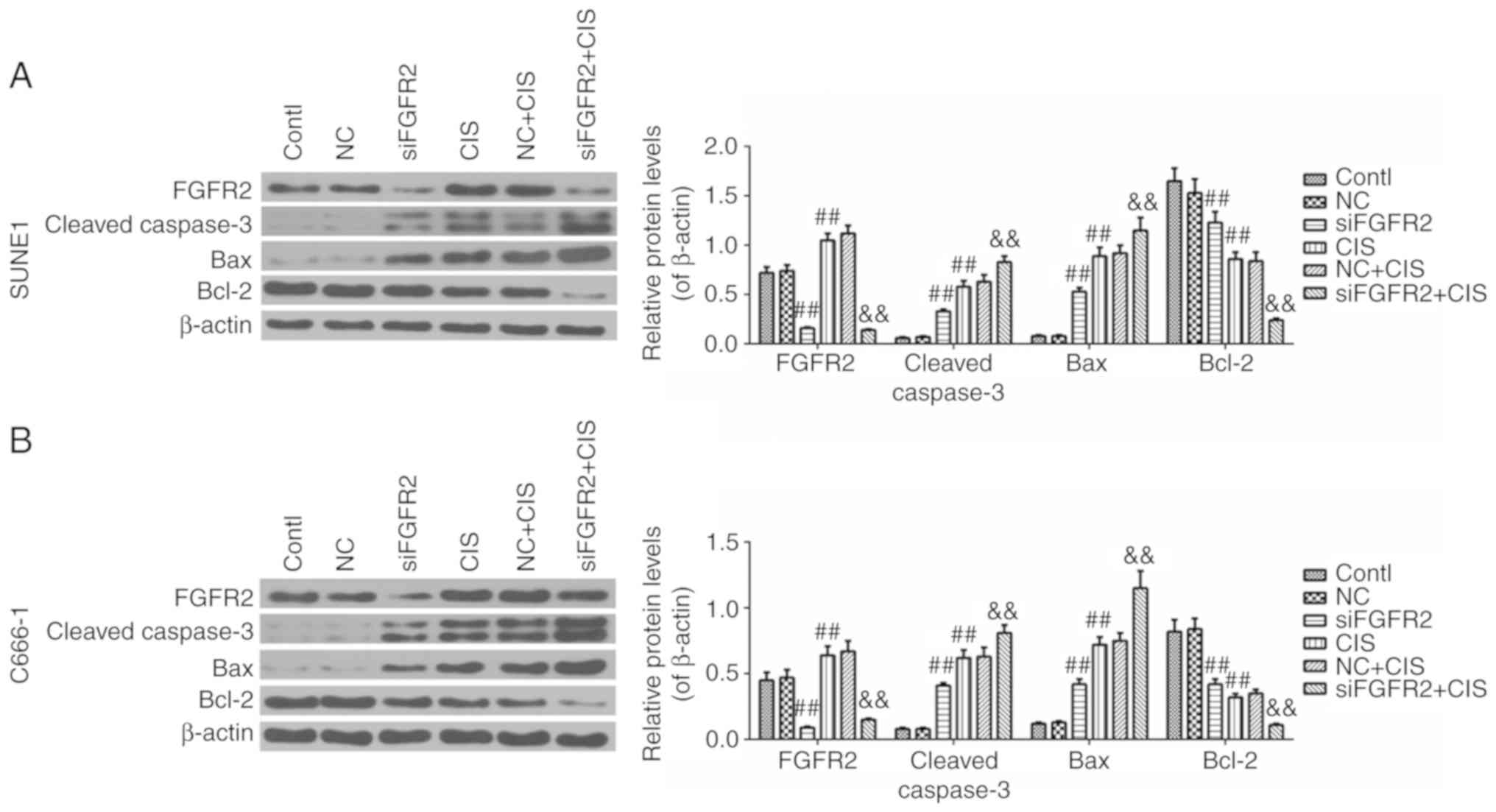 | Figure 6Expression levels of FGFR2, cleaved
caspase-3, Bax and Bcl-2 in control, NC, siFGFR2, CIS, NC+CIS and
siFGFR2+CIS groups of SUNE1 and C666-1 cell lines. (A) The
expression of FGFR2, cleaved Caspase-3, Bax and Bcl-2 in the SUNE1
cell line (B) The expression of FGFR2, cleaved Caspase-3, Bax and
Bcl-2 in the C666-1 cell line. Data are presented as mean ±
standard deviation. ##P<0.01 vs. control or NC group;
&&P<0.01 vs. CIS group. FGFR2, fibroblast
growth factor receptor 2; si, small interfering; Bcl-2, B-cell
lymphoma 2; Bax, Bcl-2-associated X protein; contl, control; NC,
negative control; CIS, cisplatin. |
Discussion
The present study revealed that FGFR2 was
overexpressed in cancer tissues from patients with NPC and multiple
NPC cell lines, and that the downregulation of FGFR2 decreased the
cell viability and induced cell cycle arrest in SUNE1 and C666-1
cell lines. An increased expression of FGFR2 was also observed in
cisplatin-treated cells, and the use of cisplatin with siFGFR2
demonstrated increased effectiveness in increasing the apoptosis
rate and activating the intrinsic apoptosis pathway of SUNE1 and
C666-1 cells compared with the treatment of siFGFR2 or CIS alone,
suggesting a potential mechanism for improving the efficacy of
cisplatin in the treatment of NPC.
The present study also demonstrated that FGFR2 was
highly expressed in the tumor tissues of patients with NPC, and
that this level was increased compared with that in adjacent
tissues. No significant difference in the outcomes of the survival
analyses of patients with low or high expression of FGFR2 was
observed; however, the data suggested that changes in the
expression of FGFR2 may affect the prognosis of patients with NPC.
To the best of our knowledge, the present study was the first to
demonstrate the marked increase in FGFR2 expression in NPC tissues
and cell lines. Therefore, FGFR2 may be a promising target in the
study of NPC.
To additionally understand the role of FGFR2 in NPC,
the expression of FGFR2 was downregulated, and a decrease in cell
viability 48 h after transfection was observed in the SUNE1 and
C666-1 cell lines. Zhang et al (17) demonstrated that FGFR2 was
essential for cell proliferation and differentiation in corneal
epithelial cells during embryonic development. Zhao et al
(18) also revealed that microRNA
(miR)-494 was able to inhibit proliferation and promote apoptosis
of ovarian cancer cells by targeting FGFR2. Given these data, we
hypothesized that the downregulation of FGFR2 may decrease the
viability of SUNE1 and C666-1 cell lines.
One of the most basic biological characteristics of
malignant tumors is the malignant transformation of cells caused by
disruptions in cell cycle regulation and the uncontrolled
proliferation of tumor cells. Understanding the expression of cell
cycle-associated genes may reveal key effector molecules and
biological pathways involved in basic tumor biology (19). During the proliferation of cancer
cells in NPC, the acceleration of cell cycle has been demonstrated
to be closely associated with cell proliferation (3). Cyclin D1 is a member of the cyclin
family that regulates cell cycle progression by binding to protein
kinases; abnormalities in its expression may interrupt the cell
cycle, which is one of the key mechanisms of cell malignant
transformation (20). In
eukaryotic cells, the G1 cell cycle checkpoint initiates the cell
cycle and promotes cell proliferation, and is considered the
primary regulatory point (21).
The G1 phase is arrested by the rapid degradation of cyclin D1
protein, if DNA damage is detected in the G1/S phase (22). CDC25A is the most important member
of the cell division cycle gene 25 (CDC25) (23). It has been demonstrated to
function in all stages of the cell cycle, and to serve important
roles in the cell cycle, mitosis and various physiological
activities (23,24). It is also a regulatory factor in
cell apoptosis, and the primary regulatory molecule responsible for
maintaining DNA damage checkpoint pathways (24). Degradation of CDC25A may cause
cell cycle arrest in the G1 or G2 phases, allowing cells to address
DNA damage or abnormalities (25,26). A high expression level of CDC25A
protein signifies checkpoint dysfunction, causing the cell cycle to
continue in the presence of DNA damage, which is a mechanism that
is considered to be important in the occurrence and development of
malignant tumors (25,26). In addition, the G1 phase is
considered to be the phase most sensitive to cisplatin. The present
study demonstrated that the proportion of cells in G1 and S phases
were increased and decreased, respectively, and that the expression
levels of cyclin D1 and CDC25A were decreased following the
downregulation of siFGFR2 in SUNE1 and C666-1 cell lines. Lee et
al (27), suggested that a
single-point mutation in FGFR2 may affect cell cycle in
osteoblasts. Yin et al (28), demonstrated that miR-19b-1 may
target FGFR2 mRNA and inhibit cell cycle progression from the S
phase to the G2/M phase transition by regulating the expression of
cyclin D1. Gredler et al (29), also revealed that FGFR2 regulated
cell cycle progression in the urethral and surface ectoderm. These
data implied that the downregulation of FGFR2 may induce cell cycle
arrest in NPC cells in the G1 phase, inhibiting cell proliferation
and increasing the duration of the cell cycle phase that is more
sensitive to cisplatin, which may result in an increased efficacy
of cisplatin treatment in NPC.
Notably, the present study identified that increased
concentrations (at least 10 μg/ml) of cisplatin decreased
cell viability, and that this decrease in cell viability was
observed to be cisplatin concentration-dependent. It was also
identified that the FGFR2 mRNA and protein expression levels were
increased by cisplatin; the highest levels were exhibited in the 10
μg/ml cisplatin group. Chen et al (30) demonstrated that cisplatin
inhibited proliferation and promoted apoptosis in TW03 cells. Huang
et al (31) also suggested
that cisplatin increased the cell apoptosis and inhibited the cell
proliferation of HNE1 cells. Considering these aforementioned data,
the results of the present study verified that cisplatin inhibited
the viability of NPC cells, and it was hypothesized that the
increased expression of FGFR2 was associated with the efficacy of
cisplatin treatment in NPC.
Based on the aforementioned data, the present study
explored the effects of siFGFR2, cisplatin and the combination of
these two interventions on apoptosis. Following the stimulation of
intracellular apoptotic factors, Bcl-2 and Bax are activated and
bind to the mitochondrial outer membrane, forming pores between the
mitochondria and the cytoplasm, which leads to the release of
cytochrome c into the cytoplasm and the activation of caspase-3 or
-7, thereby initiating the intrinsic apoptosis pathway (32). The results from the present study
indicated that the rate of apoptosis was increased following
treatment with siFGFR2 and cisplatin; however, the simultaneous
treatment of cisplatin with siFGFR2 markedly increased the
apoptosis rate of SUNE1 and C666-1 cells compared with the use of
siFGFR2 or cisplatin alone. In addition, the expression of FGFR2
and Bcl-2 were suppressed by the combined treatment of siFGFR2 and
cisplatin, which was more effective compared with the use of
siFGFR2 or cisplatin alone in SUNE1 and C666-1 cell lines. In
addition, the expression levels of cleaved Caspase-3 and Bax were
markedly increased following the combined treatment of siFGFR2 and
cisplatin, which was also more effective compared with the use of
siFGFR2 or cisplatin alone in SUNE1 and C666-1 cell lines. These
results were supported by the data from Cole et al (33), which suggested that the inhibition
of FGFR2 increased the rate of apoptosis induced by cisplatin in
ovarian cancer. The results from the present study suggested that
the downregulation of FGFR2 improved the efficacy of cisplatin
treatment on the induction of apoptosis through the activation of
intrinsic apoptosis pathway. However, the mechanism by which FGFR2
enhances the sensitivity of NPC cells to cisplatin requires
additional study with appropriate in vivo experiments.
In summary, the present study demonstrated that
FGFR2 was overexpressed in the cancer tissues of patients with NPC
and NPC cell lines, resulting in unfavorable prognoses. The
downregulation of FGFR2 decreased cell viability via cell cycle
arrest at G1 phase, and increased the efficacy of cisplatin on
apoptosis through the intrinsic apoptosis pathway.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LP and XK made substantial contributions to the
conception and design of the study. LS and LP were responsible for
data acquisition, data analysis and interpretation. XK and LS were
involved in drafting the article and critically revising it for
important intellectual content. All authors read and approved the
final manuscript. LP and XK agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics Board
of Zhejiang Provincial People's Hospital, People's Hospital of
Hangzhou Medical College (Hangzhou, China). All procedures
involving human participants were performed in accordance with the
ethical standards of the institutional and/or national research
committee and with the 1964 Helsinki declaration and its later
amendments or comparable ethical standards. All patients provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Liu X, Tang LL, Du XJ, Li WF, Chen L, Zhou
GQ, Guo R, Liu Q, Sun Y and Ma J: Changes in disease failure risk
of nasopharyngeal carcinoma over time: Analysis of 749 p atients
with long-term follow-up. J Cancer. 8:455–459. 2017. View Article : Google Scholar :
|
|
2
|
Wilmot VV and Hathorn I: Surgical
management of nasal stenosis following chemoradiation for
nasopharyngeal carcinoma. J Laryngol Otol. 131:429–432. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strazzulla A, Barreca GS, Giancotti A,
Pisani V, Costa C, Zicca E, La Boria A, Roveda L, Liberto MC, Tucci
L, et al: Nasopharyngeal carcinoma: Review of the literature with a
focus on therapeutical implications. Infez Med. 23:224–229.
2015.PubMed/NCBI
|
|
4
|
Zhang X, Ibrahimi OA, Olsen SK, Umemori H,
Mohammadi M and Ornitz DM: Receptor specificity of the fibroblast
growth factor family. The complete mammalian FGF family. J Biol
Chem. 281:15694–15700. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grose R and Dickson C: Fibroblast growth
factor signaling in tumorigenesis. Cytokine Growth Factor Rev.
16:179–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katoh M and Katoh M: FGFR2 and WDR11 are
neighboring oncogene and tumor suppressor gene on human chromosome
10q26. Int J Oncol. 22:1155–1159. 2003.PubMed/NCBI
|
|
7
|
Turner N and Grose R: Fibroblast growth
factor signalling: From development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung EJ, Jung EJ, Min SY, Kim MA and Kim
WH: Fibroblast growth factor receptor 2 gene amplification status
and its clinicopathologic significance in gastric carcinoma. Hum
Pathol. 43:1559–1566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsumoto K, Arao T, Hamaguchi T, Shimada
Y, Kato K, Oda I, Taniguchi H, Koizumi F, Yanagihara K, Sasaki H,
et al: FGFR2 gene amplification and clinicopathological features in
gastric cancer. Br J Cancer. 106:727–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Byron SA, Gartside MG, Wellens CL,
Goodfellow PJ, Birrer MJ, Campbell IG and Pollock PM: FGFR2
mutations are rare across histologic subtypes of ovarian cancer.
Gynecol Oncol. 117:125–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riddell IA: Cisplatin and oxaliplatin: Our
current understanding of their actions. Met Ions Life Sci.
18:2018.PubMed/NCBI
|
|
12
|
Köberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: Preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
13
|
Wu P, Tang Y, He J, Qi L, Jiang W and Zhao
S: ARC is highly expressed in nasopharyngeal carcinoma and confers
X-radiation and cisplatin resistance. Oncol Rep. 30:1807–1813.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song Y, Zhou X, Bai W and Ma X: FBW7
increases drug sensitivity to cisplatin in human nasopharyngeal
carcinoma by downregulating the expression of multidrug
resistance-associated protein. Tumour Biol. 36:4197–4202. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Zhang J, Upadhya D, Lu L and Reneker LW:
Fibroblast growth factor receptor 2 (FGFR2) is required for corneal
epithelial cell proliferation and differentiation during embryonic
development. PLoS One. 10:e01170892015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao X, Zhou Y, Chen YU and Yu F: miR-494
inhibits ovarian cancer cell proliferation and promotes apoptosis
by targeting FGFR2. Oncol Lett. 11:4245–4251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar
|
|
20
|
Feldt M, Bjarnadottir O, Kimbung S,
Jirström K, Bendahl PO, Veerla S, Grabau D, Hedenfalk I and
Borgquist S: Statin-induced anti-proliferative effects via cyclin
D1 and p27 in a window-of-opportunity breast cancer trial. J Transl
Med. 13:1332015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neumann J, Boerries M, Köhler R, Giaisi M,
Krammer PH, Busch H and Li-Weber M: The natural anticancer compound
rocaglamide selectively inhibits the G1-S-phase transition in
cancer cells through the ATM/ATR-mediated Chk1/2 cell cycle
checkpoints. Int J Cancer. 134:1991–2002. 2014. View Article : Google Scholar
|
|
22
|
Roque T, Haton C, Etienne O,
Chicheportiche A, Rousseau L, Martin L, Mouthon MA and Boussin FD:
Lack of a p21waf1/cip-dependent G1/S checkpoint in neural stem and
progenitor cells after DNA damage in vivo. Stem Cells. 30:537–547.
2012. View Article : Google Scholar :
|
|
23
|
Boutros R, Lobjois V and Ducommun B: CDC
25 p hosphatases in cancer cells: Key players? Good targets? Nat
Rev Cancer. 7:495–507. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen T and Huang S: The role of Cdc25A in
the regulation of cell proliferation and apoptosis. Anticancer
Agents Med Chem. 12:631–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang T, Wei Y, Honaker Y, Yamaguchi H,
Appella E, Hung MC and Piwnica-Worms H: GSK-3 beta targets Cdc25A
for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation
correlates with Cdc25A overproduction in human cancers. Cancer
Cell. 13:36–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sur S and Agrawal DK: Phosphatases and
kinases regulating CDC25 activity in the cell cycle: Clinical
implications of CDC25 overexpression and potential treatment
strategies. Mol Cell Biochem. 416:33–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee KM, Santos-Ruiz L and Ferretti P: A
single-point mutation in FGFR2 affects cell cycle and Tgfbeta
signalling in osteoblasts. Biochim Biophys Acta. 1802:347–355.
2010. View Article : Google Scholar
|
|
28
|
Yin R, Bao W, Xing Y, Xi T and Gou S:
MiR-19b-1 inhibits angiogenesis by blocking cell cycle progression
of endothelial cells. Biochem Biophys Res Commun. 417:771–776.
2012. View Article : Google Scholar
|
|
29
|
Gredler ML, Seifert AW and Cohn MJ:
Tissue-specific roles of Fgfr2 in development of the external
genitalia. Development. 142:2203–2212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Liu S, Li Q and Peng J:
Combination of cytosine arabinoside and cisplatin enhances
inhibition of cell proliferation and promotes apoptosis of
resistant nasopharyngeal carcinoma cells. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 31:379–382. 3862015.In Chinese.
|
|
31
|
Huang YY, Pu LJ, Song LL, Ma LY, Liu H and
Jiang CC: Knockdown of GRP78 enhances cell death by cisplatin and
radiotherapy in nasopharyngeal cells. Anticancer Drugs. 27:726–733.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 7:2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cole C, Lau S, Backen A, Clamp A, Rushton
G, Dive C, Hodgkinson C, McVey R, Kitchener H and Jayson GC:
Inhibition of FGFR2 and FGFR1 increases cisplatin sensitivity in
ovarian cancer. Cancer Biol Ther. 10:495–504. 2010. View Article : Google Scholar : PubMed/NCBI
|