Introduction
Sepsis refers to the systemic inflammatory response
syndrome caused by the invasion of pathogenic microorganisms; their
toxins and metabolites invade into the circulating blood,
activating human cells and the humoral immune system to produce
various cytokines and endogenous mediators (1). Myocardial tissue is a common target
organ involved in the course of sepsis, and myocardial tissue
damage is the starting point of multiple organ dysfunction
syndromes (2). Numerous clinical
and animal studies have confirmed the presence of cardiomyocyte
inhibition in sepsis, which is one of the factors contributing to
cardiac insufficiency and hemodynamic instability (3). Cardiac troponin T (CTnT) is a
sensitive indicator of myocardial damage, and 31-85% of patients
have severely elevated serum troponin (4). However, there is no clear
understanding of the mechanism underlying the effects of sepsis on
myocardial injury.
The overactivation of apoptosis is an important
pathological feature of tissue damage during sepsis (5), however, the specific mechanism
underlying the tissue damage and excessive apoptosis of cells
remains to be fully elucidated. The close association between the
oxidative stress response and inflammation has received increasing
attention in recent years, and the effects of inflammation on
mitochondrial function lead to an increase in the production of
reactive oxygen species (ROS), which in turn causes tissue damage
through the activation of oxidative stress (6).
The molecular mechanisms responsible for the
inflammatory reactions caused by inflammatory cytokines, leading to
the inhibition of cardiac function, have been investigated
(7), however, the specific
molecular mechanisms remain unclear. Studies have shown that
inflammasomes are involved in the damage of myocardial tissue
(8). It is known that the
NO-like-binding domain (NOD)-like receptor protein 3 (NLRP3)
inflammasome usually consists of three main components, including
NLRP3, apoptosis-associated speck-like protein (ASC) and aspartate
proteolysis, which is a protein complex composed of
cysteinylaspartate-specific proteases-1 (caspase-1) protein
(9). It was found that
thioredoxin-interacting protein (TXNIP) directly activated
caspase-1 and then activated caspase-1, cleaved caspase-1, cleaved
pro-interleukin (IL)-1β and pro-IL-18. Following cutting, the cells
released physiologically active IL-1β and IL-18, eventually
producing an inflammatory response (10). In addition, ROS have been
identified as one of the mediators that activates the NLRP3
inflammasome (11).
Oxidative stress refers to the imbalance between
oxidation and anti-oxidation, including the excessive production of
ROS (12) in the body.
Malondialdehyde (MDA) is a product of lipid oxidation and is
responsible for the formation of ROS and the extent of oxidative
damage (13). Intracellular ROS
are cleared mainly through antioxidant enzyme substances, including
superoxide dismutase (SOD) and catalase. SOD has three subtypes,
Cu/ZnSOD, FeSOD and MnSOD, among which MnSOD accounts for 70% of
the total SOD in the heart and up to 90% in cardiomyocytes
(14). The expression of MnSOD in
mitochondria regulates the production of O2−
during oxidative phosphorylation, thus, MnSOD serves a key role in
ROS (15).
The present study established a rat and cell sepsis
model and observed changes to myocardial tissue and cells by
detecting changes in CTnT, inflammatory factors, MAD, SOD and
NLRP3/TXNIP in order to provide a promising therapeutic target for
the myocardial protection of clinical sepsis.
Materials and methods
Animals
Twelve adult male Sprague-Dawley (SD) rats (8-weeks
old, weighing 180-250 g) were obtained from the Shanghai SLAC
Laboratory Animal Co., Ltd.; the rats were housed in groups of
three per cage with food and water available ad libitum, and
were maintained in controlled room temperature (22±2°C) and
humidity (60-80%) under a 12 h/12 h light/dark cycle. All animal
protocols were approved by Zhejiang University Animal Committee
(Zhejiang, China).
Reagents
Lipopolysaccharide (LPS) was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). CTnT enzyme-linked
immunosorbent assay (ELISA) kits were purchased from R&D
systems, Inc. (Minneapolis, MN, USA). MDA levels were determined
using the TBA method (Jian Cheng Bioengineering Institute, Nanjing,
China). Small interfering RNA targeting TNXIP (siTNXIP) and
negative control (TNXIP-NC) were purchased from GenePharma
(Shanghai, China).
LPS injection of rats and groups
LPS was dissolved in sterile physiological saline
(0.9% NaCl) at a concentration of 1 mg/ml. The rats were injected
intraperitoneally with 10 mg LPS/kg. The treatment was performed
respectively for 6, 12 and 24 h for pathological analysis. The
control group was injected with the same volume of physiological
saline in the same manner.
MDA measurement via the TBA method
All rats were sacrificed by intraperitoneal
injection of sodium pentobarbital (200 mg/kg) following LPS
treatment for 6, 12 and 24 h. The rats in the control group were
sacrificed using the same method. The absence of sounds of
breathing and heartbeat, namely respiratory arrest and cardiac
arrest, through a stethoscope confirmed death of the rats. Heart
tissues (0.5 g) from the same position were removed from the rats
in the four groups and homogenized with physiological saline.
Subsequently, 0.2 ml homogenate with 0.2 ml 8.1% SOS, 1.5 ml 20%
acetic acid and 1.5 ml 0.8% TBA aqueous solution was placed in a
lidded glass test tube and then diluted to 4 ml with
triple-distilled water, and placed in a water bath for 60 min at
95°C. The samples were removed and cooled to room temperature, and
1 ml distilled water and 5 ml n-butanol pyridine solution were
added to the samples, following which the mixture was shaken for 15
min and separated at 3,000 × g for 15 min at room temperature. The
n-butanol phase at 532 µm was measured for colorimetric
densities. The value of fluorescence was calculated by comparing
with standards prepared from 1,1,3,3-tetraethoxypropane (cat. no.
T9889, Sigma-Aldrich; Merck KGaA).
Hematoxylin and eosin staining
Myocardial tissue was removed and the samples were
fixed with 10% paraformaldehyde solution for >48 h, following
which they were routinely dehydrated and paraffin-embedded to
prepare 5-µm tissue sections. The sections were heated in an
incubator at 68°C for 1-2 h, and were then placed in xylene for
dewaxing three times for 30 min. The sections were then placed in
100, 95, 85 and 75% gradient alcohol for hydration for 5 min.
Following washing the sections for 2 min in tap water, the sections
were stained with hematoxylin for 10 min and with eosin for 30 sec
at room temperature. The sections were then infiltrated with xylene
for 5 sec, and the neutral gum was sealed and observed under a
light microscope (BX51, Olympus Corporation, Tokyo, Japan).
Immunohistochemical analyses
The tissue samples obtained from all rats for
histological and immunohistochemical analyses were fixed in 10%
formalin solution and embedded in paraffin, according to
conventional histological methods. The paraffin-embedded tissue
samples were cut into 5-µm thick sections and the
immunohistochemical expression of TXNIP and NLRP3 in tissue
sections were determined using avidin-biotin-peroxidase complex.
The tissue sections were deparaffinized, rehydrated and treated
with 3% H2O2 to block endogenous peroxidase
activity. Subsequently, the sections were incubated in citrate
buffer (0.1 M, pH 6.0) in a microwave (800 W, 10 min) and washed
with a phosphate buffer solution (PBS; 0.1 M, pH 7.2). The sections
were then incubated in a blocking buffer (5% bovine serum albumin;
cat. no. B2064; Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature., washed with PBS and incubated with anti-TXNIP (cat.
no. ab231966, dilution 1:500, Abcam, Cambridge, MA, USA) and
anti-NLRP3 (cat. no. ab214185, dilution 1:500, Abcam) antibodies
for 1 h at room temperature and washed again with PBS. The sections
were then incubated with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G (1:250; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 30 min at room temperature. Finally, the
sections were washed again and treated with a 3,3-diaminobenzidine
substrate system (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Negative control (NC) samples were used to determine specific
TXNIP and NLRP3 immunoreactivity. The number of positive
microvessels in each section was counted in 10 microscopic fields
(magnifications, ×100 and ×200) under a light microscope (BX51,
Olympus Corporation).
H9C2 cells and culture
The H9C2 cell line was purchased from American Type
Culture Collection (Rockville, MD, USA); the cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere at 5%
CO2 in air. Subsequently, 2×105 cells were
seeded onto culture plates and cultured in medium with LPS (1, 10,
20 and 50 µg/ml) for 24 h at 37°C. siTXNIP was added into
the culture medium for transfection 24 h prior to the LPS (20
µg/ml) treatment. Following incubation, the cells were
collected for the analysis of cellular viability, mRNA and protein
expression, apoptosis and ROS.
Transfection
To investigate the role of TXNIP in sepsis-induced
myocardial dysfunction, the H9C2 cells were divided into five
groups, as follows: Control group, LPS group (cells were treated
with LPS), NC+LPS group (cells were transfected with empty vector
and treated with LPS), siTXNIP+LPS group (cells were transfected
with siTXNIP and treated with LPS) and siTXNIP group (cells were
transfected with siTXNIP). Lipofectamine™ 3000 (LFN) transfection
(Thermo Fisher Scientific, Inc.) was performed according to the
manufacturer's instructions. In brief, the lipid complex was
prepared by combining the reagent of 4 µl LFN Plus with 2
µg of plasmid DNA and then suspended in 1 ml serum-free
medium and incubated at room temperature for 15 min. The solution
was then mixed with 40 µl of LFN in serum-free medium and
incubated at room temperature for 15 min. The lipid compounds were
diluted in serum-free medium to produce a 5-ml volume of the
required concentration, and the cells were incubated at 37°C with
5% CO2 for 24 h.
Cell viability assay
Following transfection of the H9C2 cells with or
without siTXNIP prior to LPS treatment, 10 µl of cell
counting kit (CCK)-8 solution was added to the wells and the cell
were incubated at 37°C for 2 h tin an incubator with 5%
CO2 in the dark. Subsequently, the OD value in each well
from different cell groups at an absorbance of 450 nm was
determined using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Cell viability was detected with the CCK-8
assay kit according to the manufacturer's protocol.
Apoptosis
Following treatment of the H9C2 cells for 24 h,
1×106 cells were collected and 1 ml of trypsin (trypsin)
without ethylenediaminetetraacetic acid was used to digest the
cells, which were shaken gently, and trypsin was removed when the
wall was wet. After 1 min at room temperature, the digestion was
terminated by adding DMEM (Corning) containing 10% FBS. The cells
were centrifuged at 1,000 × g for 3 min at room temperature and the
supernatant was removed. The cells were washed twice with
pre-cooled PBS and resuspended in 1X Annexin V binding buffer.
According to the Annexin V-FITC cell apoptosis detection kit (cat.
no. K201-100, BioVision, Inc., Milpitas, CA, USA), the cells were
stained with 1.25 µl Annexin V-FITC and 10 µl
prop-idium iodide and measured by flow cytometry (version 10.0,
FlowJo, FACSCalibur™, BD Biosciences, Franklin Lakes, NJ, USA).
ROS measurement by flow cytometry
The H9C2 cells were transfected with siTNXIP and
then treated with LPS. The cells were then incubated with
2′,7′-dichloro dihydrogen fluorescein diacetate ester (Beyotime
Institute of Biotechnology, Shanghai, China, 5 µM) for 30
min at 37°C. The excitation wavelength of the flow cytometry
(version 10.0, FlowJo, BD Biosciences) was 480 nm and the emission
wavelength was 525 nm.
Western blot analysis
The tissues and the H9C2 cells were flushed with
cold PBS three times and placed on the ice with protein lysis
buffer (RIPA; Cell Signaling Technology, Inc., Danvers, MA, USA)
for 2 h. The samples were centrifuged at 13,500 × g for 30 min at
4°C and the supernatants were extracted. The concentration of
protein was determined using a BCA protein assay kit (Bio-Rad
Laboratories, Inc.). Subsequently, SDS-PAGE with 10% running gels
was used to separate the proteins (at least 40 µg), which
were then transferred onto a polyvinylidene fluoride membrane
(Bio-Rad Laboratories, Inc.). To block the nonspecific signals, the
membrane was incubated with 5% non-fat milk for at least 2 h at
room temperature. The proteins were then incubated with primary
antibody overnight at 4°C and then washed with 5% bovine serum
albumin (Gibco; Thermo Fisher Scientific, Inc.) in PBS/0.1%
Tween-20 and incubated with secondary antibodies (cat. nos. sc-2004
and sc-2005, 1:2,000; Santa Cruz Biotechnology, Inc. Dallas, TX,
USA) for 1 h at room temperature. The protein bands were developed
with developer (EZ-ECL kit; Biological Industries) and protein
quantity was analyzed using ImageJ software (version 5.0; Bio-Rad
Laboratories). The antibodies used were as follows: Anti-GAPDH
(mouse; cat. no. sc-47724, 1:1,000; Santa Cruz Biotechnology,
Inc.), anti-TXNIP (rabbit, cat. no. ab210826, 1:1,000, Abcam),
anti-NLRP3 (rabbit, cat. no. ab214185, 1:1,000, Abcam),
anti-caspase-1 (rabbit, cat. no. ab1872, 1:1,000, Abcam), and
anti-cleaved caspase-1 (rabbit, cat. no. ab25901, 1:1,000, Abcam),
anti-catalase (rabbit, cat. no. ab16731, 1:1,000, Abcam),
anti-MnSOD (rabbit, cat. no. ab13533, 1:1,000, Abcam) and secondary
antibodies (cat. nos. sc-2004 and sc-2005, 1:2,000; Santa Cruz
Biotechnology, Inc.).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
According to the program provided by the
manufacturer, total RNA of the tissues and H9C2 cells was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Chloroform (Sigma Aldrich; Merck KGaA) was added to the tube and
incubated at room temperature for 5 min and centrifuged at 14,000 ×
g for 20 min at 4°C. The supernatant was transferred to a new tube
and isopropanol was added. The aqueous phase was centrifuged at
14,000 × g for 20 min at 4°C. The precipitate was washed with 70%
ethanol and suspended again in water treated with 0.1% diethyl
carbamate. The purity and concentration of the RNA was assessed
using the NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.), and the absorbance
was read at 260 and 280 nm. According to the program provided by
the manufacturer (Thermo Fisher Scientific, Inc.), a reverse
transcription cDNA kit was used to reverse transcribe 1 µg
total RNA for the synthesis of cDNA (at 42°C for 60 min, at 70°C
for 5 min, and preserved at 4°C). SYBR-Green PCR Master mix (Roche
Diagnostics, Basel, Switzerland) was used to perform the qPCR
experiment using the Opticon real-time PCR detection system (ABI
7500; Thermo Fisher Scientific, Inc.). The thermocycling conditions
were as follows: 40 cycles at 95°C for 15 sec, at 60°C for 1 min).
The relative mRNA quantity was determined using the comparative
cycle threshold (2−ΔΔCq) method (16). The expression of GAPDH was used
for normalization. The primer sequences used for RT-qPCR are listed
in Table I.
 | Table IPrimers for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| IL-1β (mouse) |
TCGCCAGTGAAATGATGGCTTA |
GTCCATGGCCACAACAACTGA |
| IL-18 (mouse) |
GACCTTCCAGATCGCTTCCTC |
GATGCAATTGTCTTCTACTGGTTC |
| IL-1β (human) |
TGCAGAGTTCCCCAACTGGTACATC |
GTGCTGCCTAATGTCCCCTTGAATC |
| IL-18 (human) |
ATCAACCTCAGACCTTCCAG |
GCATTATCTCTACAGTCAG |
| TXNIP (human) |
GCCACACTTACCTTGCCAAT |
TTGGATCCAGGAACGCTAAC |
| MnSOD (human) |
CAGACCTGCCTTACGACTATGG |
CTCGGTGGCGTTGAGATTGTT |
| Catalase
(human) |
CGTGCTGAATGAGGAACAGA |
AGTCAGGGTGGACCTCAGTG |
| GAPDH (mouse) |
GCACCGTCAAGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| GAPDH (human) |
AGGTCGGTGTGAACGGATTTG |
GGGGTCGTTGATGGCAACA |
Statistical analysis
Values are presented as the mean ± standard error of
the mean. GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA) was used to analyze the values. One-way analysis of variance
followed by Turkey's post hoc test was applied to analyze
differences among the experimental groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
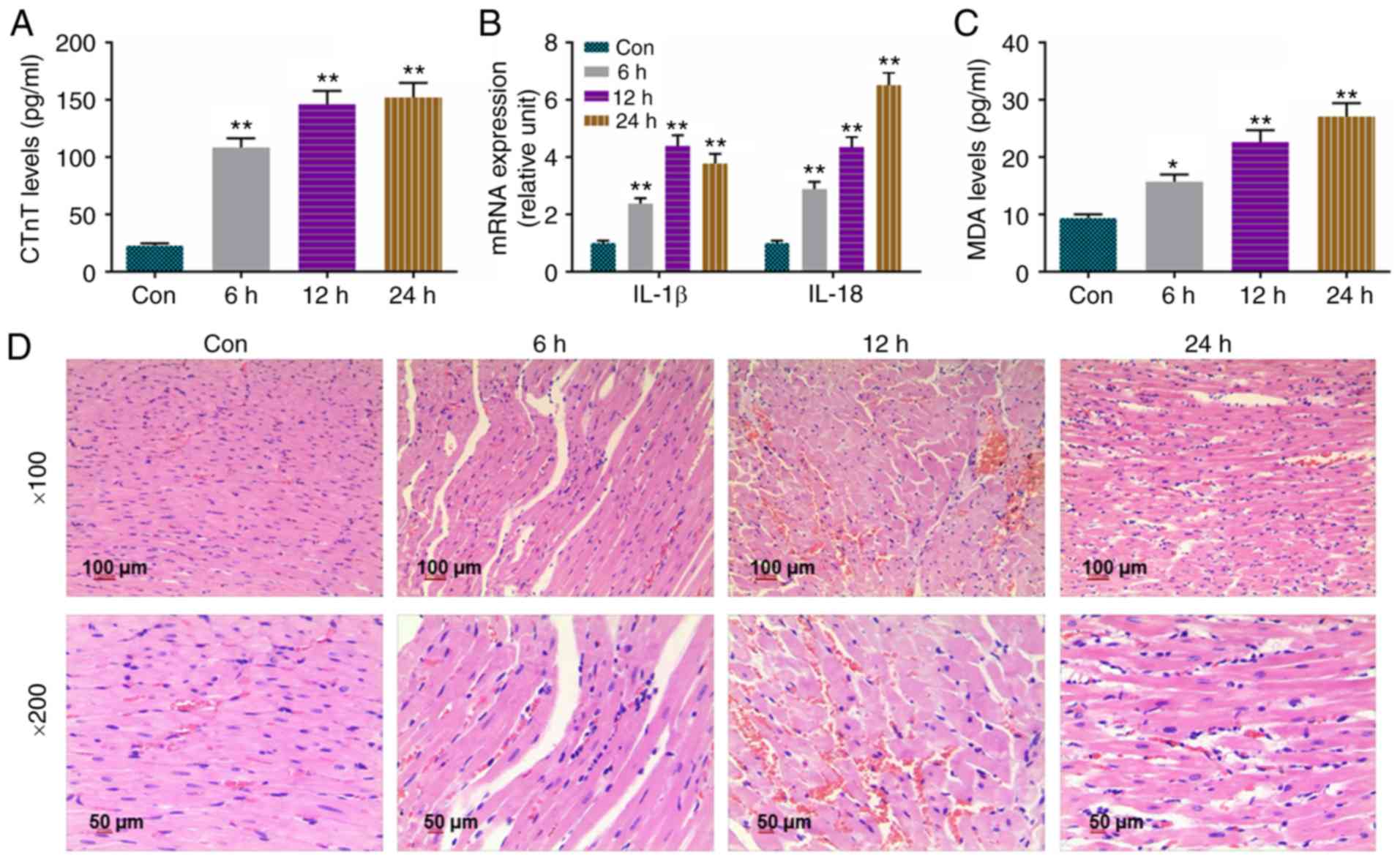
Levels of CTnT, IL-1β, IL-18 and MDA are
increased and pathology is altered in sepsis-induced myocardial
injury rats
The present study determined CTnT levels in the
serum of control rats and in septic rats treated for 6, 12 and 24
h. Quantification of CTnT levels was achieved using the ELISA
method, and the results showed that the levels of CTnT were
significantly higher in the 6, 12 and 24 h groups, compared with
than that in the Con group (Fig.
1A). Detection by RT-qPCR analysis (Fig. 1B) revealed that the expression
levels of IL-1β and IL-18 were increased in the 6, 12 and 24 h
groups, compared with those in the Con group. MDA, a byproduct of
unsaturated fatty acid oxidation, was measured using the TBA
method, and the results revealed that the levels of MDA were
markedly enhanced in the 6, 12 and 24 h groups, compared with that
in the Con group (Fig. 1C).
H&E staining was used to examine the changes in myocardial
tissues, and it was found that myocardial fiber bundles were
loosely arranged, some myocardial fibers were broken and dissolved,
and some cardiomyocytes were degenerated and necrotic. Interstitial
edema was noted and moderate inflammatory cell infiltration was
observed as LPS processing time was prolonged (Fig. 1D).
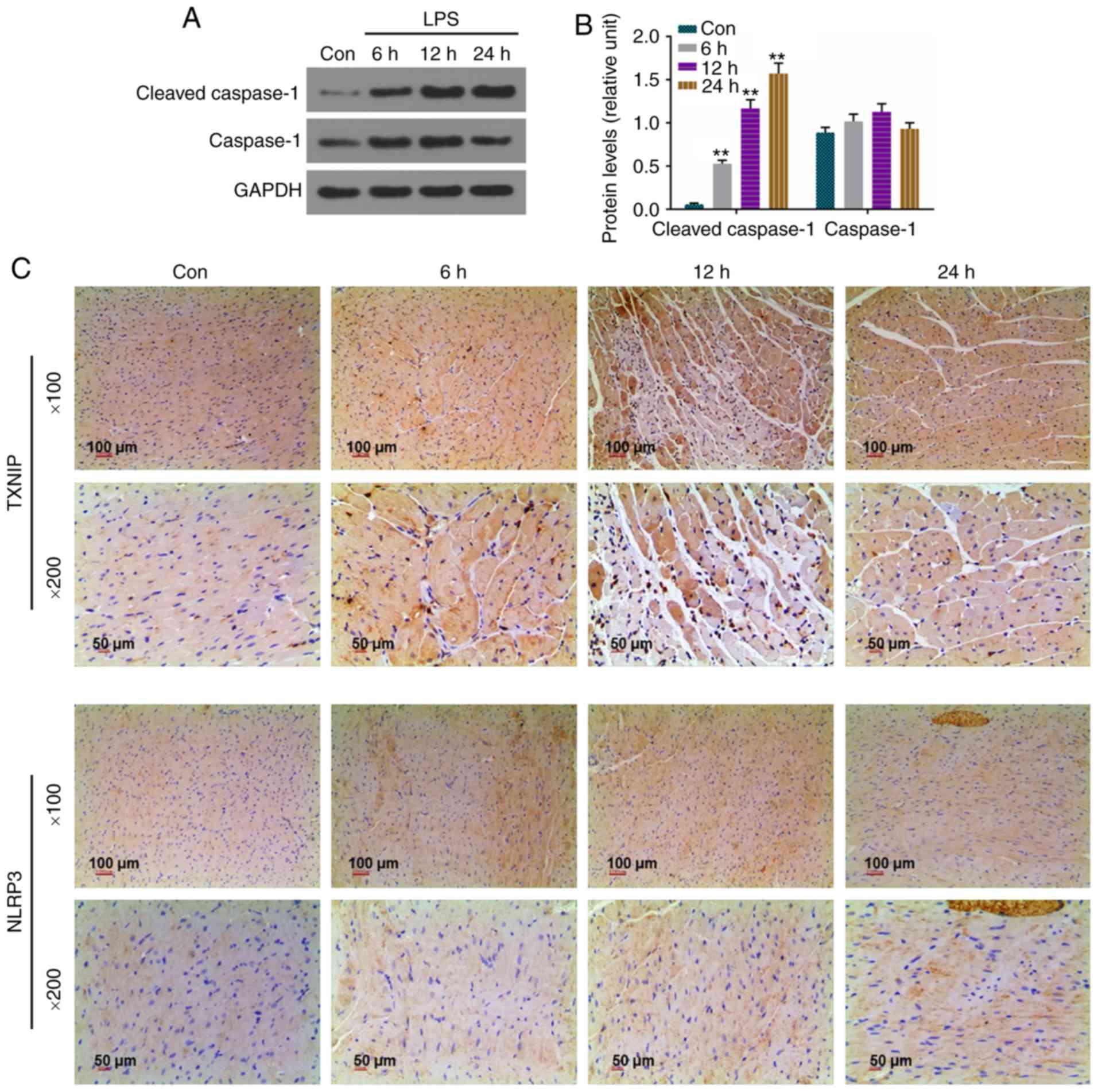
Protein levels of cleaved caspase-1 are
upregulated and strong staining for TXNIP and NLRP3 is present in
sepsis-induced myocardial injury rats
In view of the importance of the TXNIP/NLRP3
signaling pathway in the development of sepsis-induced myocardial
injury, the expression levels of cleaved caspase-1, caspase-1,
TXNIP and NLRP3 were determined by western blot analysis or
immunohistochemistry. It was found that the levels of cleaved
caspase-1 were higher in the 6, 12 and 24 h groups compared with
that in the Con group (Fig. 2A and
B), and the intensity of staining of the rat myocardial
sections treated with LPS was observed in a time-dependent manner
(Fig. 2C).
Levels of IL-1β, IL-18, cleaved
caspase-1, TXNIP and NLRP3 are significantly increased by LPS in
H9C2 cells
In order to elucidate the mechanisms of
sepsis-induced myocardial dysfunction, H9C2 cells were used to
establish a model of sepsis-induced myocardial dysfunction using
LPS (1, 10, 20 and 50 µg/ml) in vitro. The viability
of cells was suppressed by LPS at 20 and 50 µg/ml (Fig. 3A). The mRNA levels of IL-1β, IL-18
(Fig. 3B) and TXNIP (Fig. 3C) were enhanced by LPS, and the
protein levels of cleaved caspase-1, TXNIP and NLRP3 were also
increased by LPS (Fig. 3D and
E).
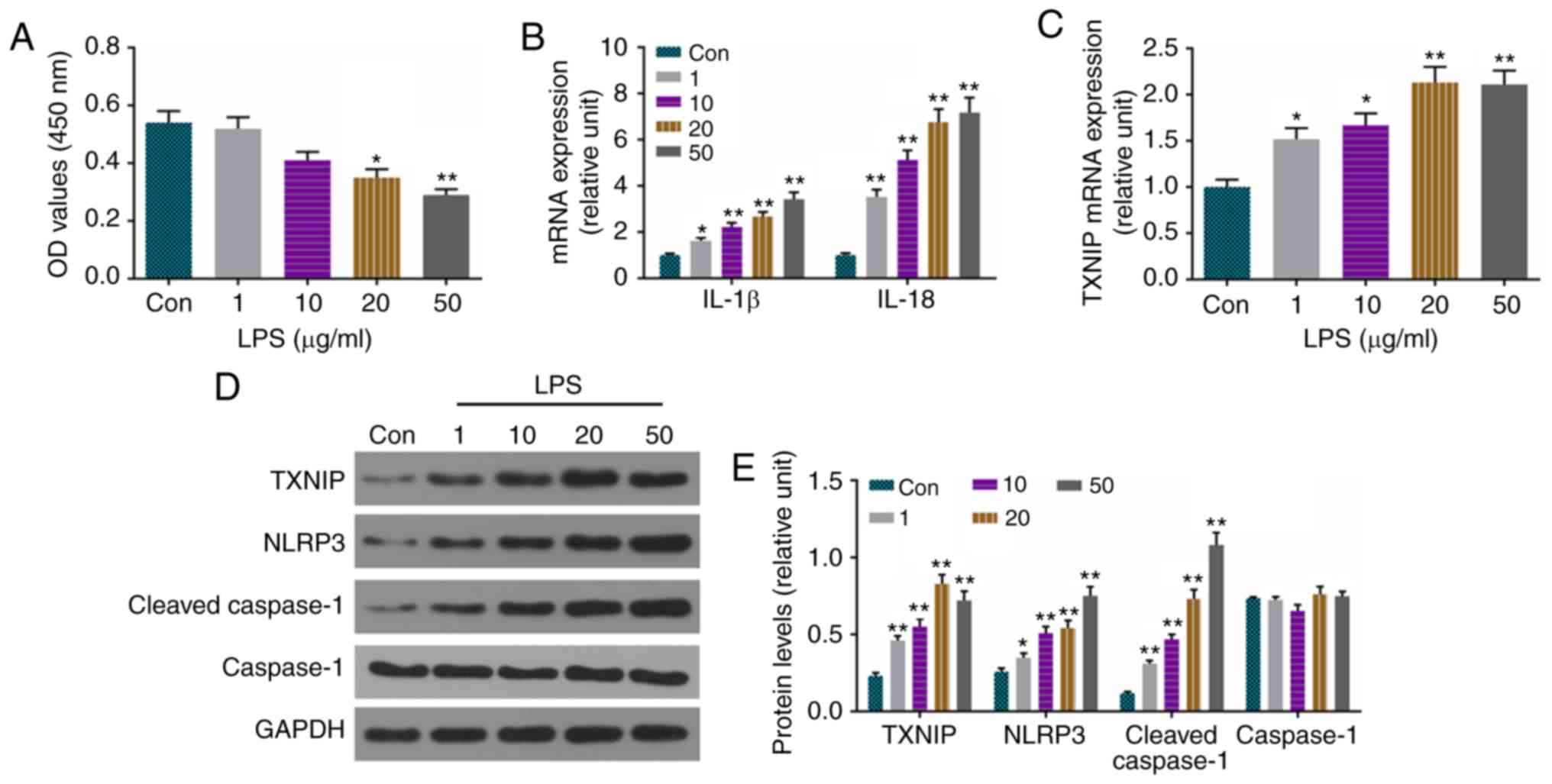 | Figure 3Levels of IL-1β, IL-18, cleaved
caspase-1, TXNIP and NLRP3 are significantly increased by LPS in
H9C2 cells. (A) Viability of cells was detected using a cell
counting kit-8. (B) RT-qPCR analysis was used to detect the mRNA
levels of IL-1β and IL-18. (C) mRNA levels of TXNIP were measured
by RT-qPCR analysis. (D) Protein levels of cleaved caspase-1,
caspase-1, TXNIP and NLRP3 were assessed by western blotting. (E)
Relative levels of proteins in were determined with GAPDH for
normalization. *P<0.05 and **P<0.01 vs.
Con. IL, interleukin; TXNIP, thioredoxin-interacting protein;
NLRP3, NOD-like receptor pyrin domain containing 3; LPS,
lipopolysaccharide; Con, control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
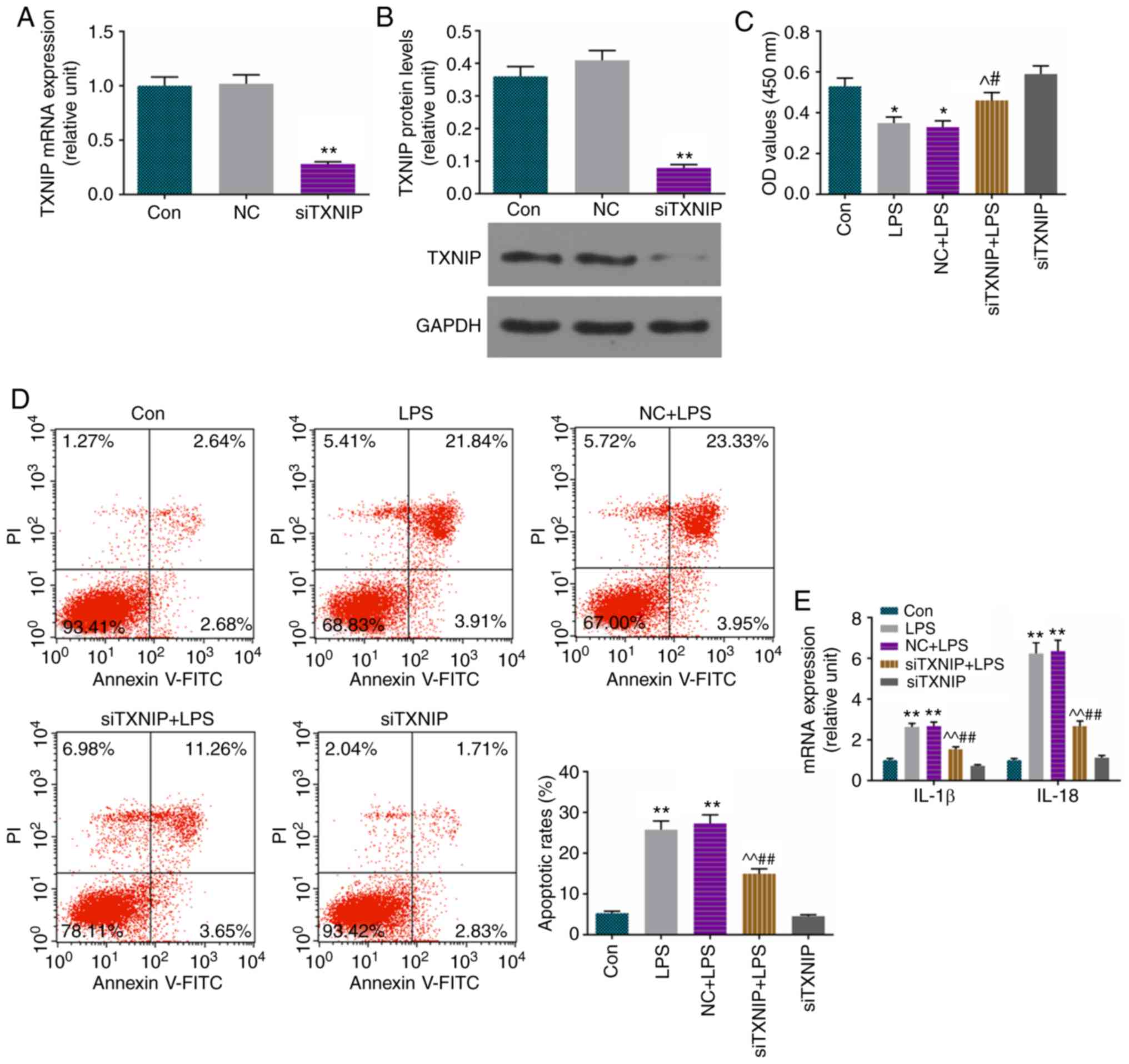
Apoptosis is increased and levels of
IL-1β and IL-18 are improved by LPS, and partially reversed by
siTXNIP
To verify the role of TXNIP in the H9C2 cell model
of sepsis-induced myocardial dysfunction, which was established
using LPS (20 µg/ml), the TXNIP gene was knocked down by
siRNA. Successful transfection using LFN was observed for TXNIP at
the mRNA (Fig. 4A) and protein
(Fig. 4B) levels. The viability
of cells was decreased by LPS and ameliorated by siTXNIP (Fig. 4C). It was also found that the
increase of apoptosis (Fig. 4D)
and augmentation of IL-1β and IL-18 (Fig. 4E) were inhibited by siTXNIP in the
LPS group.
Increases in expression of cleaved
caspase-1, TXNIP and NLRP3 are repressed by siTXNIP
The levels of cleaved caspase-1, TXNIP and NLRP3
were determined by RT-qPCR or/and western blot analysis, and the
results demonstrated that the mRNA level of TXNIP was lower in the
siTXNIP+LPS group than in the LPS and NC+LPS groups (Fig. 5A), and that the protein levels of
cleaved caspase-1, TXNIP and NLRP3 were inhibited by siTXNIP,
compared with levels in the LPS and NC+LPS groups, respectively
(Fig. 5B and C).
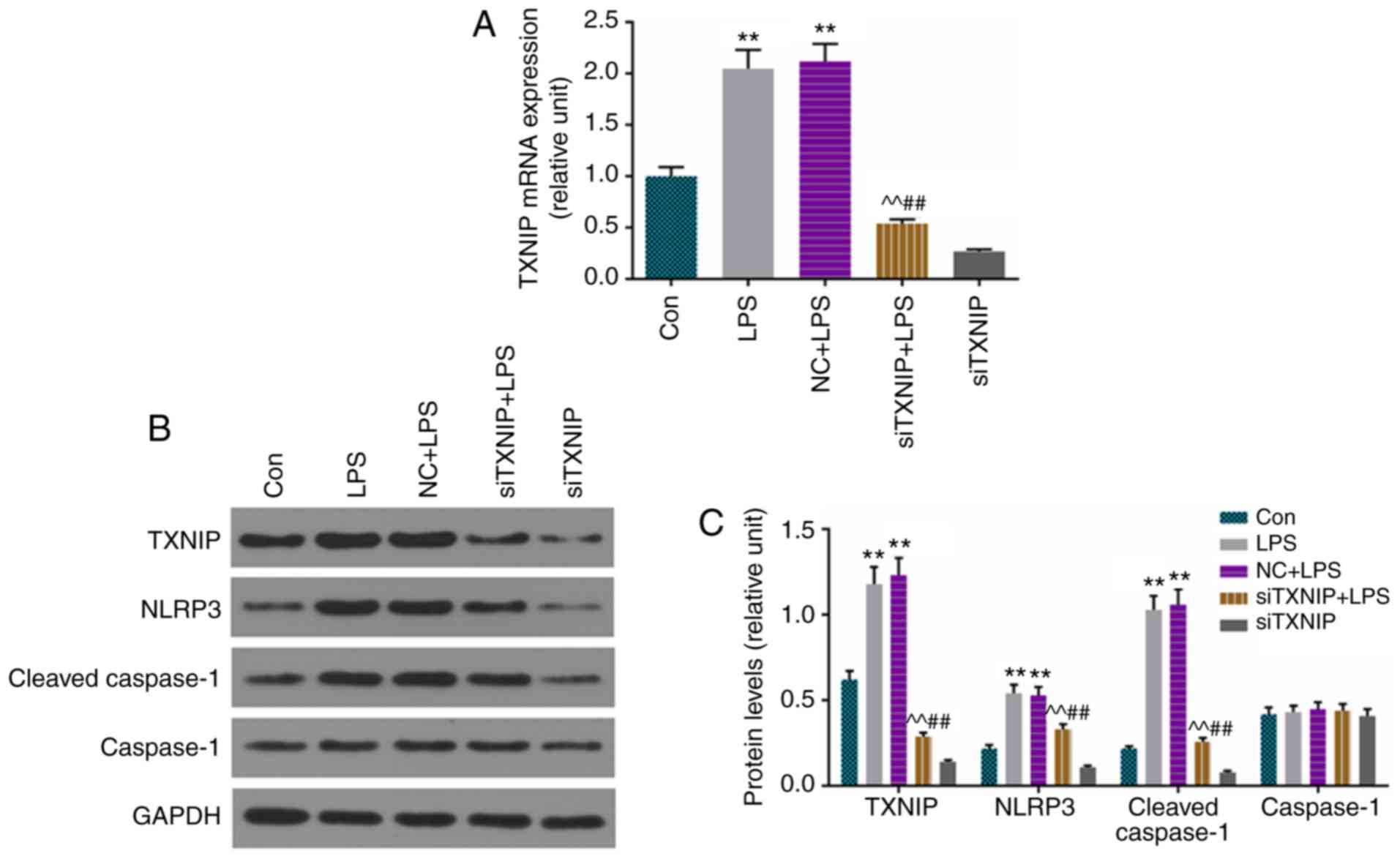 | Figure 5Improvements in cleaved caspase-1,
TXNIP and NLRP3 are inhibited by siTXNIP. (A) Reverse
transcription-quantitative polymerase chain reaction analysis was
used to detect the mRNA levels TXNIP. (B) Protein levels of cleaved
caspase-1, caspase-1, TXNIP and NLRP3 were assessed by western
blotting. (C) Relative levels of proteins described were determined
with GAPDH for normalization. **P<0.01 vs. Con;
^^P<0.01 vs. LPS; ##P<0.01 vs. NC+LPS.
TXNIP, thioredoxin-interacting protein; NLRP3, NOD-like receptor
pyrin domain containing 3; IL, interleukin; siTXNIP, small
interfering RNA targeting TXNIP; NC, negative control; LPS,
lipopolysaccharide; Con, control. |
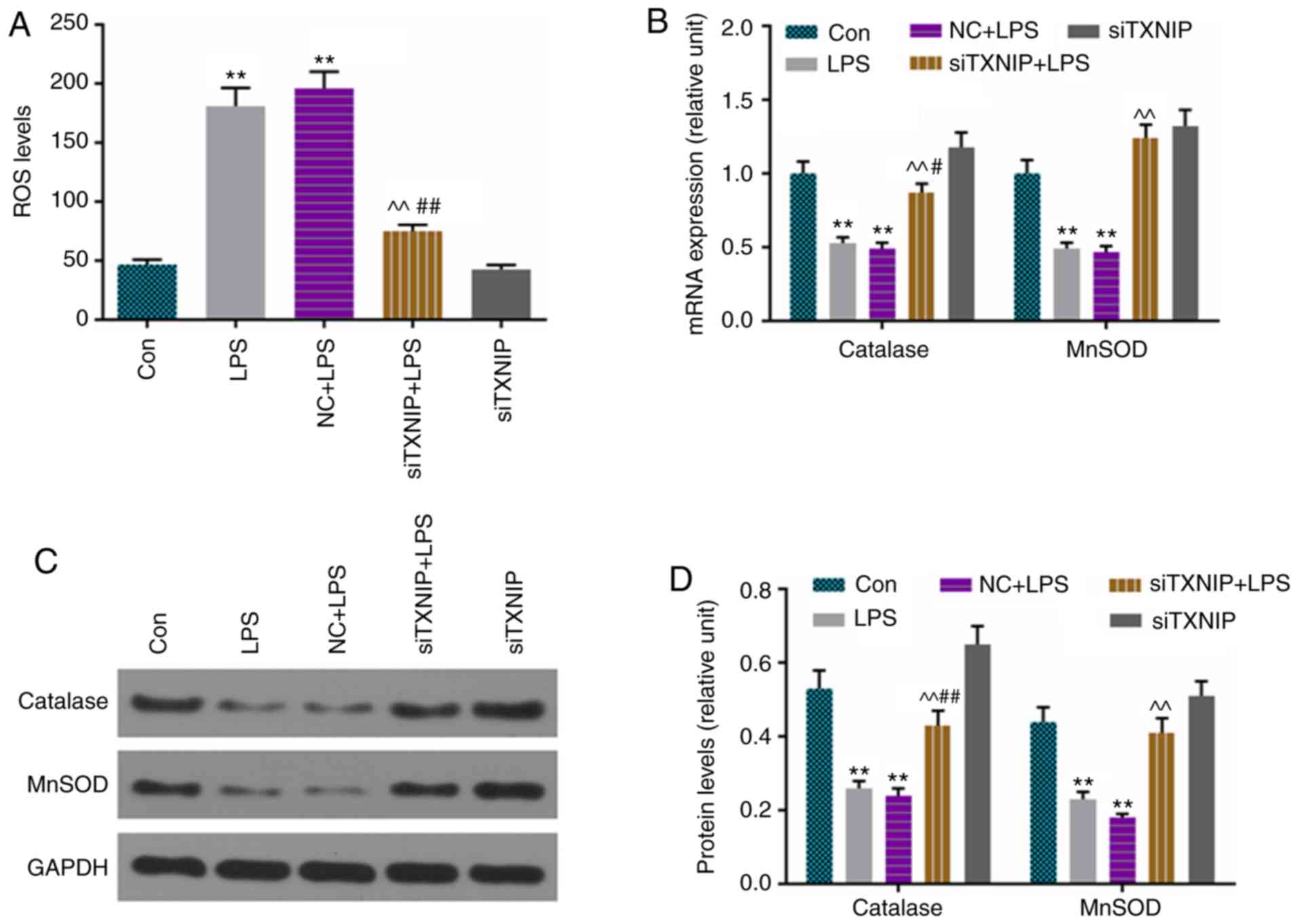
ROS production is elevated by LPS, which
is partially reversed by siTXNIP
ROS levels were assessed by flow cytometry, and
catalase and MnSOD were detected by RT-qPCR and western blot
analyses. The results showed that ROS production was lower in the
siTXNIP+LPS group than in the LPS and NC+LPS groups (Fig. 6A), and the levels of catalase and
MnSOD were enhanced by siTXNIP, compared with levels in the LPS and
NC+LPS groups at the mRNA (Fig.
6B) and protein (Fig. 6C and
D) levels.
Discussion
Sepsis, which is a common complication of severe
trauma, infection, shock and other stress states, has high
incidence and high mortality rates (17). Sepsis-associated mortality is
closely associated with damage, which can easily lead to multiple
organ dysfunction, particularly heart damage (18). Therefore, it is necessary to
examine the pathological mechanism of sepsis-induced myocardial
injury in order to identify an effective therapeutic target.
LPS, which serves an important role in Gram-negative
bacterial infection and disease evolution, is considered to be a
main cause to systemic inflammatory syndrome (19). Sepsis-induced myocardial injury is
one of the manifestations of multiple organ dysfunctions in sepsis,
which has been confirmed in clinical and sepsis animal experiments
(20). LPS is an important
substance causing myocardial damage, which forces the contractile
function in the body to weaken and heart rate to slow down, which
seriously affects heart function (21). Afulukwe et al (22) found that intravenous injection of
LPS (10 mg/kg) helped to prepare an SD rat septic shock model and
create myocardial contractility, myocardial damage; the feasibility
of such a model was confirmed by Cohen et al (23), Iqbal et al (21) and others. Following the
intraperitoneal injection of LPS (l0 mg/kg) in Wistar rats, Chagnon
et al (24) observed a
decrease in left ventricular ejection fraction, which was
consistent with the findings observed in patients with severe
sepsis. Similarly, an animal model of sepsis was prepared by
intraperitoneal injection of LPS (10 mg/kg) into SD rats, and the
results showed that CTnT in the serum increased gradually as time
progressed in the 6 h following LPS injection. CTnT is a sensitive
indicator of myocardial damage (25). In patients with septic shock,
elevated serum CTnT predicts a higher mortality rate and poor
prognosis (26). In the present
study, it was found that myocardial tissue in sepsis rats exhibited
myocardial fiber rupture and lysis, cardiomyocyte eosinophilic
changes, mild edema and inflammatory cell infiltration under a
light microscope. As the treatment duration was prolonged, the
above effects gradually became more marked. Some myocardial tissue
necrosis was observed 24 h following LPS injection, and damage to
myocardial fibers and mitochondrial ultrastructure were observed
under an electron microscope. Therefore, it is feasible to prepare
an animal model of myocardial injury in sepsis by intraperitoneal
injection of 10 mg/kg LPS into SD rats. However, its underlying
mechanisms remain to be fully elucidated.
Previous studies have shown that NLRP3 inflammatory
bodies serve a key role in sepsis-induced myocardial injury
(27-29) and the progression of acute
inflammation (30). As its core
protein, NLRP3 acts as a signal receptor in the cytoplasm, once
activated, NLRP3 recruits ASC and caspase-1 and activates caspase-1
by the cleavage of IL-1β and IL-18 precursors, which mature and are
secreted outside the cell and are involved in the inflammatory
response (31). Yang et al
found that the expression levels of NLRP3 and caspase-1 were
increased in myocardial tissue treated with cecal ligation and
puncture (27). IL-1β increases
in vivo and in vitro in sepsis and septic shock
(32,33). Cardiac contractile function is
preserved and infarct size is reduced in mice deficient in
components of the inflammasome complex (NLRP3) (34). Consistent with previous studies,
the present study performed a systematic assessment of this pathway
in sepsis-induced myocardial dysfunction in vivo and in
vitro. The results showed that the expression levels of NLRP3,
cleaved caspase-1, IL-1β and IL-18 were all significantly
upregulated in sepsis-induced myocardial dysfunction in vivo
and in vitro. These results demonstrated that the NLRP3
inflammasome serves a key role in the progression of sepsis-induced
myocardial dysfunction.
Endotoxins, uric acid and ROS, which are activators
of the NLRP3 inflammasome, have already been identified (35). TXNIP is a key antioxidant in the
human body and is necessary for activation of the NLRP3
inflammasome via direct interaction with NLRP3 (36). Liu et al found that TXNIP
and NLRP3 were significantly increased during myocardial
ischemia/reperfusion injury and that siTXNIP significantly
decreased activation of the NLRP3 inflammasome (37). The role of the TXNIP/NLRP3
signaling pathway in myocardial injury has been reported
previously, however, it has not been reported in sepsis-induced
myocardial dysfunction. In the present study, it was found that the
levels of TXNIP were improved in sepsis-induced myocardial
dysfunction in vivo and in vitro, and that ROS
production was also increased in vitro, which was evident by
decreases in catalase and MnSOD. To further examine the role of
TXNIP in sepsis-induced myocardial dysfunction, siTXNIP was
transfected into H9C2 cells. The data indicated that activation of
NLRP3 was inhibited and ROS production was repressed by siTXNIP,
accompanied by decreased IL-1β and IL-18 levels and increased
catalase and MnSOD levels, respectively. Cell viability was
improved and apoptosis was inhibited in the siTXNIP group, compared
with that in the LPS and NC+LPS groups. These results showed that
TXNIP is essential in the activation of the NLRP3 inflammasome in
sepsis-induced myocardial dysfunction.
In conclusion, the results of the present study
demonstrated that the NLRP3 inflammasome was activated in
sepsis-induced myocardial dysfunction. Additionally, TXNIP was
shown, for the first time, to mediate the activation of NLRP3
inflammasomes in H9C2 cells treated with LPS. siTXNIP inhibited
activation of the NLRP3 inflammasome, and such a result may provide
novel therapies for mitigating sepsis-induced myocardial
dysfunction.
Acknowledgments
Not applicable.
Funding
This study was supported by the Zhejiang Provincial
Medicine and Health Research Foundation (grant no. 2019KY002).
Availability of data and materials
The analyzed datasets generated during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
CY made substantial contributions to conception and
design; WX and XL performed data acquisition, data analysis and
interpretation; JL contributed to drafting and critical revision of
the manuscript for important intellectual content; all authors
approved the final version to be published; AW and CY agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work were appropriately
investigated and resolved. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by Zhejiang
University Animal Committee (Zhejiang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu YB ZH, Chen SZ, Chen GR and Guo S:
Optimization and identification of anti-sepsis potential targets of
palmatine. Chin J Mod Appl Pharm. 35:1602–1605. 2018.
|
|
2
|
Stanzani G, Duchen MR and Singer M: The
role of mitochondria in sepsis-induced cardiomyopathy. Biochim
Biophys Acta Mol Basis Dis. 1865:759–773. 2019. View Article : Google Scholar
|
|
3
|
Rabuel C and Mebazaa A: Septic shock: A
heart story since the 1960s. Intensive Care Med. 32:799–807. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng F, Lai H, Luo Z, Liu Y, Huang X, Chen
J, Liu B, Guo Y, Cai Y and Huang Q: Effect of xuefu zhuyu decoction
pretreatment on myocardium in sepsis rats. Evid Based Complement
Alternat Med. 2018:29393072018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anthonymuthu TS, Kim-Campbell N and Bayir
H: Oxidative lipidomics: Applications in critical care. Curr Opin
Crit Care. 23:251–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Al-Salam S and Hashmi S: Myocardial
ischemia reperfusion injury: Apoptotic, inflammatory and oxidative
stress role of galectin-3. Cell Physiol Biochem. 50:1123–1139.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Su Q, Li L, Sun Y, Yang H, Ye Z and Zhao
J: Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3
inflammasome in coronary microembolization-induced myocardial
injury. Cell Physiol Biochem. 47:1497–1508. 2018. View Article : Google Scholar
|
|
9
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C,
Lu L, Xu X, Guan H, Zheng Z and Qiu Q: Activation of the
TXNIP/NLRP3 inflammasome pathway contributes to inflammation in
diabetic retinopathy: A novel inhibitory effect of minocycline.
Inflamm Res. 66:157–166. 2017. View Article : Google Scholar
|
|
11
|
Zheng R, Tao L, Jian H, Chang Y, Cheng Y,
Feng Y and Zhang H: NLRP3 inflammasome activation and lung fibrosis
caused by airborne fine particulate matter. Ecotoxicol Environ Saf.
163:612–619. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takimoto E and Kass DA: Role of oxidative
stress in cardiac hypertrophy and remodeling. Hypertension.
49:241–248. 2007. View Article : Google Scholar
|
|
13
|
Haileselassie B, Su E, Pozios I, Niño DF,
Liu H, Lu DY, Ventoulis I, Fulton WB, Sodhi CP, Hackam D, et al:
Myocardial oxidative stress correlates with left ventricular
dysfunction on strain echocardiography in a rodent model of sepsis.
Intensive Care Med Exp. 5:212017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Assem M, Teyssier JR, Benderitter M,
Terrand J, Laubriet A, Javouhey A, David M and Rochette L: Pattern
of superoxide dismutase enzymatic activity and RNA changes in rat
heart ventricles after myocardial infarction. Am J Pathol.
151:549–555. 1997.PubMed/NCBI
|
|
15
|
Lone MU, Baghel KS, Kanchan RK,
Shrivastava R, Malik SA, Tewari BN, Tripathi C, Negi MP, Garg VK,
Sharma M, et al: Physical interaction of estrogen receptor with
MnSOD: Implication in mitochondrial O2.− upregulation
and mTORC2 potentiation in estrogen-responsive breast cancer cells.
Oncogene. 36:1829–1839. 2017. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Klingenberg C, Kornelisse RF, Buonocore G,
Maier RF and Stocker M: Culture-negative early-onset neonatal
sepsis-at the crossroad between efficient sepsis care and
antimicrobial stewardship. Front Pediatr. 6:2852018. View Article : Google Scholar
|
|
18
|
Liu H, Sun Y, Zhang Y, Yang G, Guo L, Zhao
Y and Pei Z: Role of thymoquinone in cardiac damage caused by
sepsis from BALB/c mice. Inflammation. 42:516–525. 2019. View Article : Google Scholar
|
|
19
|
Stoll LL, Denning GM and Weintraub NL:
Potential role of endotoxin as a proinflammatory mediator of
atherosclerosis. Arterioscler Thromb Vasc Biol. 24:2227–2236. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakihana Y, Ito T, Nakahara M, Yamaguchi K
and Yasuda T: Sepsis-induced myocardial dysfunction:
Pathophysiology and management. J Intensive Care. 4:222016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iqbal M, Cohen RI, Marzouk K and Liu SF:
Time course of nitric oxide, peroxynitrite, and antioxidants in the
endotoxemic heart. Crit Care Med. 30:1291–1296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Afulukwe IF, Cohen RI, Zeballos GA, Iqbal
M and Scharf SM: Selective NOS inhibition restores myocardial
contractility in endotoxemic rats; however, myocardial NO content
does not correlate with myocardial dysfunction. Am J Respir Crit
Care Med. 162:21–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cohen RI, Wilson D and Liu SF: Nitric
oxide modifies the sarcoplasmic reticular calcium release channel
in endotoxemia by both guanosine-3′,5′ (cyclic) phosphate-dependent
and independent pathways. Crit Care Med. 34:173–181. 2006.
View Article : Google Scholar
|
|
24
|
Chagnon F, Bentourkia M, Lecomte R,
Lessard M and Lesur O: Endotoxin-induced heart dysfunction in rats:
Assessment of myocardial perfusion and permeability and the role of
fluid resuscitation. Crit Care Med. 34:127–133. 2006. View Article : Google Scholar
|
|
25
|
Maxwell MH, Robertson GW and Moseley D:
Potential role of serum troponin T in cardiomyocyte injury in the
broiler ascites syndrome. Br Poult Sci. 35:663–667. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choon-ngarm T and Partpisanu P: Serum
cardiac troponin-T as a prognostic marker in septic shock. J Med
Assoc Thai. 91:1818–1821. 2008.
|
|
27
|
Yang L, Zhang H and Chen P: Sulfur dioxide
attenuates sepsis-induced cardiac dysfunction via inhibition of
NLRP3 inflammasome activation in rats. Nitric Oxide. 81:11–20.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu D, Shi L, Li P, Ni X, Zhang J, Zhu Q,
Qi Y and Wang B: Intermedin1-53 protects cardiac fibroblasts by
inhibiting NLRP3 inflammasome activation during sepsis.
Inflammation. 41:505–514. 2018. View Article : Google Scholar
|
|
29
|
Zhang B, Liu Y, Sui YB, Cai HQ, Liu WX,
Zhu M and Yin XH: Cortistatin inhibits NLRP3 inflammasome
activation of cardiac fibroblasts during sepsis. J Card Fail.
21:426–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lopes de Oliveira GA, Alarcón de la Lastra
C, Rosillo MÁ, Castejon Martinez ML, Sánchez-Hidalgo M, Rolim
Medeiros JV and Villegas I: Preventive effect of bergenin against
the development of TNBS-induced acute colitis in rats is associated
with inflammatory mediators inhibition and NLRP3/ASC inflammasome
signaling pathways. Chem Biol Interact. 297:25–33. 2019. View Article : Google Scholar
|
|
31
|
Shen HH, Yang YX, Meng X, Luo XY, Li XM,
Shuai ZW, Ye DQ and Pan HF: NLRP3: A promising therapeutic target
for autoimmune diseases. Autoimmun Rev. 17:694–702. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clavier T, Besnier E, Lefevre-Scelles A,
Lanfray D, Masmoudi O, Pelletier G, Castel H, Tonon MC and Compère
V: Increased hypothalamic levels of endozepines, endogenous ligands
of benzodiazepine receptors, in a rat model of sepsis. Shock.
45:653–659. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hara Y, Shimomura Y, Nakamura T, Kuriyama
N, Yamashita C, Kato Y, Miyasho T, Sakai T, Yamada S, Moriyama K
and Nishida O: Novel blood purification system for regulating
excessive immune reactions in severe sepsis and septic shock: An ex
vivo pilot study. Ther Apher Dial. 19:308–315. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sandanger Ø, Ranheim T, Vinge LE, Bliksøen
M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G,
Christensen G, et al: The NLRP3 inflammasome is up-regulated in
cardiac fibroblasts and mediates myocardial ischaemia-reperfusion
injury. Cardiovasc Res. 99:164–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin C and Flavell RA: Molecular mechanism
of NLRP3 inflammasome activation. J Clin Immunol. 30:628–631. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishiyama A, Matsui M, Iwata S, Hirota K,
Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y and Yodoi J:
Identification of thioredoxin-binding protein-2/vitamin D(3)
up-regulated protein 1 as a negative regulator of thioredoxin
function and expression. J Biol Chem. 274:21645–21650. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao
C, Xin C, Zhu D, Li Y, Yan W, et al: TXNIP mediates NLRP3
inflammasome activation in cardiac microvascular endothelial cells
as a novel mechanism in myocardial ischemia/reperfusion injury.
Basic Res Cardiol. 109:4152014. View Article : Google Scholar : PubMed/NCBI
|