Introduction
Green growth is a process that aims to maintain
sustainable development while maintaining and improving the global
environment; thus, it is of great social interest and importance
(1). An essential part of
achieving green growth involves the recycling of agricultural waste
generated during the production of agricultural products (2). The by-products from agricultural
activities are abandoned during the processing steps. These
abandoned agricultural by-products are rich sources of various
functional materials and are now actively evaluated for their
efficacy (3). Previous studies
have focused on high-value agricultural by-products and
bioenergetics (4) and
eco-friendly materials (5), where
functional improvement using bioconversion is a representative
example. Therefore, the present study aimed to conduct a
bioconversion process (fermentation) to increase the use of
bioresources in the production of functional food materials.
Bioconversion is a technique that is used to produce
a desired product from specific precursors with the help of
microorganisms. It includes bioprocessing, biosynthesis and
biocatalysis (6). The difference
between bioconversion and the conventional fermentation process is
that the latter starts from a simple raw material, whereas the
former produces a product from precursors using the selectivity of
a microbial or enzyme substrate (7). In this regard, the bioconversion
process is an energy-saving advanced technology (8). The bioconver-sion process may
particularly contribute to improved usability and effectiveness in
the development of pharmaceuticals and cosmetics (9). In a previous study, it was confirmed
that bioconversion increased the production of active ingredients
including β-glucan and γ-oryzanol compounds in rice bran, and
lignan compounds in sesame seed cake (10). Additionally, it was confirmed that
soybean cake glycosides (daidzin, glycitin and genistin) were
converted to aglycones (daidzein, glycitein and genistein)
(11). Furthermore, bioconversion
increased the antioxidant activity of rice bran, soybean cake and
sesame seed cake, and altered the anti-allergic activities of
sesame seed cake (12).
The skin is the largest organ of the body that
controls metabolite excretion and the body temperature. It is
constantly exposed to the outside environment and protects the body
from external stimuli (13).
Ultraviolet (UV) rays, a type of typical external stimulus, have
beneficial effects on vitamin D synthesis and sterilization
(14,15). Although UV radiation, through
sufficient sun exposure, is necessary for humans, a high frequency
of external activities and long-term exposure to UV rays may result
in skin aging (16).
Based on its wavelength, UV light may be classified
as UVA (320-400 nm), UVB (290-320 nm) or UVC (200-290 nm) (17). The ozone layer in the atmosphere
absorbs UVC rays completely; however, UVA and UVB rays penetrate
through to the Earth's surface (18). Excessive UVB exposure, in
particular, induces changes in the epidermis (19). Photodamaged skin is characterized
by the development of erythema, edema, hyperplasia,
hyperpigmentation, sunburns, drying, reduced elasticity and the
formation of wrinkles (20).
Furthermore, skin aging may occur due to increased reactive oxygen
species (ROS) production in the skin caused by UVB. Although the
skin has developed a defense system against ROS, the continuous
production of ROS disrupts the enzymatic and non-enzymatic
antioxidant defense system in the skin (21). Furthermore, UVB aggravates the
inflammatory response by activating and promoting the inflammatory
cell infiltration that causes skin damage (22). ROS generated by UVB may affect
mitogen-activated protein kinase (MAPK) signaling and activate
nuclear factor-κB and activation protein 1 to release inflammatory
cytokines including tumor necrosis factor-α (TNF-α), interleukin
(IL)-1β and IL-6 (23). In
addition, the increase in matrix metalloproteinase (MMP) activity
in response to ROS production results in the destruction of the
structure and function of the extracellular matrix (ECM) via
collagen degradation (24). UV
exposure also stimulates the production of cyclooxygenase-2
(COX-2), which aggravates skin inflammation by catalyzing the
synthesis of prostaglandin E2 and inducing the expression of
inducible nitric oxide synthase (iNOS), which may result in skin
inflammation (25).
However, the modern lifestyle choices of people from
different age groups place a great emphasis on skin health and
beauty (26). A number of
cosmetics and foods have been developed to delay and prevent the
aging process and to maintain healthy skin (27,28). A study aiming for the alleviation
and improvement of skin wrinkles is currently being performed
(29). Therefore, it is a
worthwhile endeavor to improve the value of agricultural products
while meeting the needs of modern people. The present study was
undertaken to investigate the anti-photoaging effect of fermented
agricultural by-products including fermented rice bran (FRB),
soybean cake (FSB) and sesame seed cake (FSC) on UVB-irradiated
hairless mouse skin.
Materials and methods
Care of animals and UV irradiation
Seven-week-old female HR-1 hairless mice (weight,
27.9-33.8 g) were provided by the Central Lab. Animal Inc. (Seoul,
Korea). The trial was ethically approved by the Institutional
Animal Care and Use Committee of Kangwon National University
(approval no. KW-170417-1; 15 May, 2017). These mice (n=30) were
randomly divided into six groups of five animals per group
(Table I). They were housed in a
climate-controlled room (22°C at 50% humidity) in 12/12 h
light/dark cycles. All the mice had ad libitum access to
water and food. The oral administration of FRB, FSB and FSC, which
were dissolved in 500 µl vehicle (saline solution) and
administered once a day for a total of 8 weeks, was calculated and
performed according to the body weight of the mice. In order to
equalize the conditions with the sample treatment groups, the UVB
vehicle group was orally administered the vehicle alone. The body
weight of each mouse was measured every week. The food efficiency
rate was calculated and expressed as a percentage using the
following formula: Food efficiency ratio (%)=[total weight gain
(g)/total food intake (g) ×100]. UV light equipped with a 100
µW/cm2 UVB lamp that had a maximum emission
wavelength of 312 nm (Jiatian Trading Co., Ltd.) was used. To
determine the 1 minimal erythemal dose (MED), the dorsal skin of
the mice was exposed to different doses of UV light and erythema
formation was detected after 24 h. Skin aging was performed via 1
MED irradiation three times a week for 8 weeks. HR-1 hairless mice
were irradiated with 100 mJ/cm2 of UVB radiation (1
MED=100 mJ/cm2) daily for the first week, followed by
UVB irradiation thrice a week at 200 mJ/cm2 from week 2
to 8. Following 8 weeks once the radiation treatment was over,
vehicle, topically applied 0.01% retinoic acid (cat. no. R2625;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), FRB, FSB and FSC
treatments commenced, and the animals were sacrificed immediately
using CO2 gas to acquire skin tissue samples.
 | Table IDesign and treatment of HR-1 hairless
mice groups. |
Table I
Design and treatment of HR-1 hairless
mice groups.
| Groups | Treatment | Number |
|---|
| HR-1 normal | HR-1 without any
treatment (control) | 5 |
| UVB vehicle | UVB irradiation
vehicle | 5 |
| UVB RA | UVB + 0.01%
retinoic acid (topical ointment) | 5 |
| UVB FRB | UVB + FRB 40 mg/kg
(oral administration) | 5 |
| UVB FSB | UVB + FSB 40 mg/kg
(oral administration) | 5 |
| UVB FSC | UVB + FSC 40 mg/kg
(oral administration) | 5 |
Preparation of FRB, FSB and FSC
The samples used in the present study were provided
by STR Biotech Co., Ltd. (Chuncheon, Korea). Rice, soybean and
sesame seeds were harvested in Geochang, Korea, between August 2014
and October 2014. The samples were identified by Dr Sea Kwan Oh at
the National Institute of Crop Science (Milyang, Korea). These were
fermented with Lentinula edodes (Shiitake mushrooms) using a
fermentation system. Fermentation was performed as previously
described (12). In brief, L.
edodes mycelia were isolated from the mushroom fruit body and
cultured in potato dextrose agar (PDA) medium (Difco; BD
Biosciences, Franklin Lakes, NJ, USA). The genetic identity of the
fungus was confirmed by the Korean Center of Microorganisms (Seoul,
Korea). The mycelia cultured on PDA media were inoculated in 50 ml
of a liquid medium containing 2% glucose, 0.5% yeast extract, 0.5%
soy peptone, 0.2% mono-potassium phosphate
(KH2PO4), 0.05% magnesium sulfate
(MgSO4) and 0.002% ferrous sulfate (FeSO4).
The experiments were conducted in 250 ml Erlenmeyer flasks at 28°C
for 5 days in a rotary shaker, and the resulting broth was used to
seed the main liquid culture. Liquid culture media containing
agricultural by-products (rice bran, soybean cake and sesame seed
cake) were treated with amylase and cellulase at 60°C for 60 min to
enzymatically digest the articulate materials containing
carbohydrates. The culture mass was subsequently adjusted by
bringing the pH to 6.0 using hydrochloric acid (HCl), followed by
sterilization in an autoclave. Further experiments were performed
in a 5 l fermenter (working volume of 3 l) at 28°C and 1,050 × g by
inoculating the media with the cultured mycelia (10%). Subsequent
to 7 days, the culture was treated with an enzyme mixture
containing cellulase, hemicellulase, pectinase, glucanase, mannase
and arabinase (Sigma-Aldrich; Merck KGaA) at 50°C for 60 min to
lyse the cell walls. The enzyme-treated culture mass was extracted
at 90°C following 1 h and freeze-dried to make a powder.
Measurement of wrinkle formation
The degree of UVB-induced skin aging was measured by
investigating wrinkle formation. HR-1 hairless mice were
anesthetized with intraperitoneal injections of 0.1 ml 8% chloral
hydrate (320 mg/kg) for up to 8 weeks at 2-week intervals,
following which the UVB-irradiated dorsal area was photographed at
a magnification of ×400 using a USB digital Optical microscope.
Skin wrinkles were investigated using the DETAX System 2 and
Double-Stick Dis (3M Deutschland GmbH, Walheim, Germany). The
wrinkles were also analyzed using a Dermo Bella wrinkle analyzer
(Chowis Co. Ltd., Seongnam, Korea). Wrinkle formation was assessed
according to the scoring system established by Bissett et al
(30): Grade 0, no coarse
wrinkles; grade 1, few shallow coarse wrinkles; grade 2, some
coarse wrinkles; and grade 3, several deep coarse wrinkles.
Measurement of transepidermal water loss
(TEWL)
TEWL was measured following a previously reported
method (30). Mice were
maintained in a climate-controlled room (22°C and 50% humidity) for
30 min. The dorsal skin of each mouse was examined using a
Corneometer® CM825 probe (Courage + Khazaka electronic
GmbH, Koln, Germany), which was placed in close contact with the
surface of the dorsal skin and lightly pressed to record the skin
moisture content.
Measurement of β-glucosidase
activity
To measure β-glucosidase activity, the separated
epidermis was pulverized using phosphate-buffered saline (1x, pH
7.2) supplemented with 100 µM phenylmethanesulfonyl fluoride
(PMSF) and centrifuged at 10,000 × g for 5 min at 4°C. The
separated supernatant was reacted with citrate-phosphate buffer (pH
5.6, 5 mM sodium taurocholate) containing 4-methylumbellifery-β-D-
glucopyranoside (4-MUG) at 37°C for 60 min. The reaction was
terminated by adding 200 mM carbonate-bicarbonate buffer (pH 10.5)
and the fluorescence intensity of 4-methylumbelliferone (4-MU)
converted from 4-MUG was measured using a spectrofluorometer
(Hitachi, Ltd., Tokyo, Japan) at excitation and emission
wavelengths of 360 and 450 nm, respectively. The 4-MU
concentrations ranging from 0 to 300 nM were used as the standard
for fluorescence measurements.
Analysis of histological staining
Histological analysis was performed to determine
epidermal thickness, collagen fiber structure and mast cells.
Dorsal skin tissues from each experimental group were fixed in 10%
formalin at 21°C for 48 h. These were washed, dehydrated, permeated
and embedded using the Paraffin Embedding Station (Leica
Microsystems GmbH, Wetzlar, Germany). Hematoxylin & eosin
(H&E) staining (each 25°C for 3 and 1 min, respectively) was
performed to measure epidermal thickness. Masson's trichrome (M-T)
and toluidine blue staining (each 25°C for 10 and 30 min,
respectively) were also used to analyze the collagen fiber
structure and mast cell infiltration, respectively.
Measurement of MMP-2 using an
enzyme-linked immunosorbent assay (ELISA)
To measure the MMP-2 protein expression levels in
the dorsal skin tissue extracted from HR-1 hairless mice, an MMP-2
ELISA kit (QC126; R&D Systems, Inc., Minneapolis, MN, USA) was
used according to the manufacturer's protocol. Skin tissue samples
were homogenized for 2 min on ice using Tissue-Tearor (BioSpec
Products, Inc., Bartlesville, OK, USA) and resuspended in 100 mg/ml
Tris-buffered saline (1x, pH 7.6) containing 1 mM PMSF, 1 mM EDTA
and protease inhibitors (Sigma-Aldrich; Merck KGaA). The
homogenates were centrifuged at 10,000 × g for 30 min at 4°C. To
determine the protein concentration, a Bradford protein assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used according
to the manufacturer's protocol. The MMP-2 ELISA kit was used to
measure protein expression and a monoclonal antibody specific for
total MMP-2 was pre-coated onto 96-well microplate. The 200
µl assay diluent was added to the wells and incubated at
room temperature for 1 h. Subsequent to diluting the standard
solution and supernatants 20 times, the microplate was washed,
followed by the addition of 100 µl standard and supernatant
to each well and incubation for 2 h at room temperature. The plate
was further incubated at room temperature for 1 h with a 200
µl Biotin-labeled antibody (detection antibody), followed by
treatment with a 200 µl tetramethylbenzidine substrate
solution for 1 h at room temperature in the dark. Stop solution was
added to each well and the absorbance was measured at 450 nm using
a microplate spectrophotometer. All of the antibodies and solutions
used to measure MMP-2 protein levels were included in the MMP-2
ELISA kit.
Measurement of MMPs and cytokines using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
RT-qPCR was performed by modifying a previously
published protocol (25) to
determine the effect of FRB, FSB and FSC (40 mg/kg) on MMPs
(MMP-2,9,3 and 13) and inflammation-associated cytokines including
TNF-α, COX-2, iNOS, IL-6 and IL-1β. Total cellular RNA was
extracted from skin tissue using the phenol-chloroform method using
RNAzol (Tel-Test, Inc., Friendswood, TX, USA). In brief, 3
µg total RNA was used to synthesize cDNA using the ReverTra
Ace® qPCR RT kit (Toyobo Life Science, Osaka, Japan).
The 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used for RT-qPCR
(according to the manufacturer's protocol) along with the primer
sequences mentioned in Table II.
TaqMan probes containing carboxyfluorescein dye were used to
determine mRNA expression. A mouse GAPDH probe set (cat no.
4352339E, VIC/MGB Probe, Primer Limited; Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used as an internal standard. The
final concentration of the primer was 200 nM. The standard PCR
conditions were 50°C for 2 min and 94°C for 10 min, followed by 40
cycles of 94°C for 1 min and 60°C for 1 min. The number of cycles
at which the emission intensity of the sample rose above the
baseline value represented the relative quantity (RQ), which was
proportional to the target concentration. The RQ value of the
target group was used as the internal control and fold changes in
the relative abundance of transcripts were calculated using the
2-∆∆cq method (31).
 | Table IIPrimer sequences for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table II
Primer sequences for reverse
transcription-quantitative polymerase chain reaction analysis.
| Target gene | Sequences
(5′-3′) |
|---|
| MMP-2 | F:
CAGGGAATGAGTACTGGGTCTATT |
| R
ACTCCAGTTAAAGGCAGCATCTAC |
| MMP-3 | F:
TGGACCTGGAAATGTTTTGG |
| R:
ATCAAAGTGGGCATCTCCAT |
| MMP-9 | F:
AATCTCTTCTAGAGACTGGGAAGGAG |
| R:
AGCTGATTGACTAAAGTAGCTGGA |
| MMP-13 | F:
CCTCTTCTTCTCCGGAAACC |
| R:
GGTAGTCTTGGTCCATGGTATGA |
| TNF-α | F:
TTCTGTCTACTGAACTTCGGGGTGATCGGTCC |
| R:
GTATGAGATAGCAAATCGGCTGACGGTGTGGG |
| COX-2 | F:
AGTGATCGAAGACTACGTGCAA |
| R:
GGGATTTCCCATAAGTCCTTTC |
| iNOS | F:
CGAAACGCTTCACTTCCA |
| R:
TGAGCCTATATTGCTGTGGCT |
| IL-6 | F:
TCCAGTTGCCTTCTTGGGAC |
| R:
GTGTAATTAAGCCTCCGACTTG |
| IL-1β | F:
CAACCAACAAGTGATATTCTCCATG |
| R:
AGATCCACACTCTCAGCTGCA |
| GAPDH | F:
GTGAGGCCGGTGCTGAGTAT |
| R:
CATCCTGCACCACCAACTGCTTAGCC |
Statistical analysis
Statistical analyses were performed using the
statistical package SPSS version 24.0 (IBM Corp., Armonk, NY, USA).
For continuous variables normality was checked. The appropriate
nonparametric test was selected for the variables not normally
distributed. One-way analysis of variance and Kruskal-Wallis tests
were used for comparing multiple groups with Scheffe's and
Bonferroni's post-hoc comparisons, respectively. Results were
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
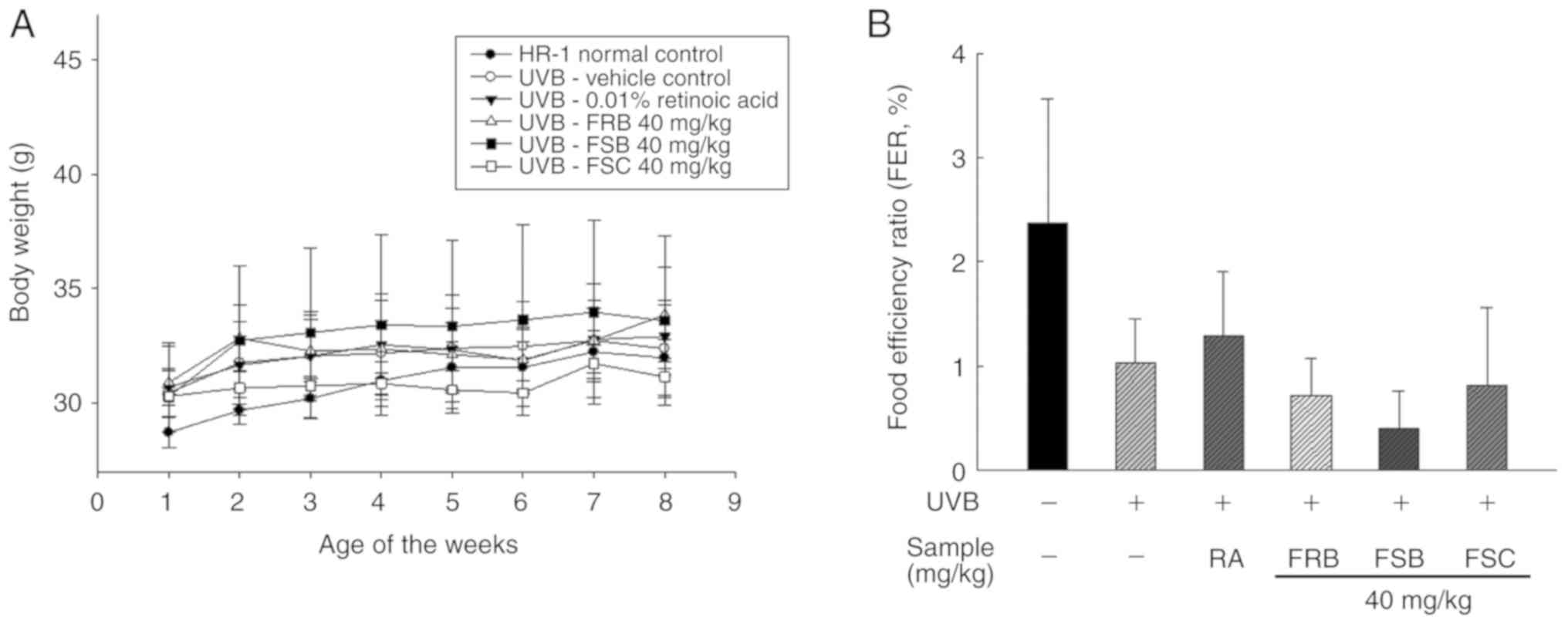
Effects of FRB, FSB and FSC on body
weight and food efficiency rate
Body weights were measured weekly from week 1 to 8
(Fig. 1A). No significant
difference in the mean body weight of mice was observed between the
six groups following 3 weeks. The initial mean body weight in the
normal control group was 28.7±0.66 g, which was lower compared with
that of the other groups. However, the UVB vehicle group exhibited
no significant difference in body weight when compared with the
other groups subsequent to 3 weeks. Evaluation of the food
efficiency ratio [total weight gain (g)/total food intake (g) ×100]
revealed no significant differences between the normal control
group and UVB-irradiated groups during the 8 experimental weeks
(Fig. 1B). All UVB-irradiated
groups did not significantly differ compared with that of the
normal control group; however, the mean values decreased to
1.03±0.42% following UVB treatment from 2.37±1.19 (control group).
Additionally, the intake of FRB, FSB and FSC (40 mg/kg) groups were
decreased to 0.72±0.36, 0.40±0.36 and 0.82±0.74%, respectively.
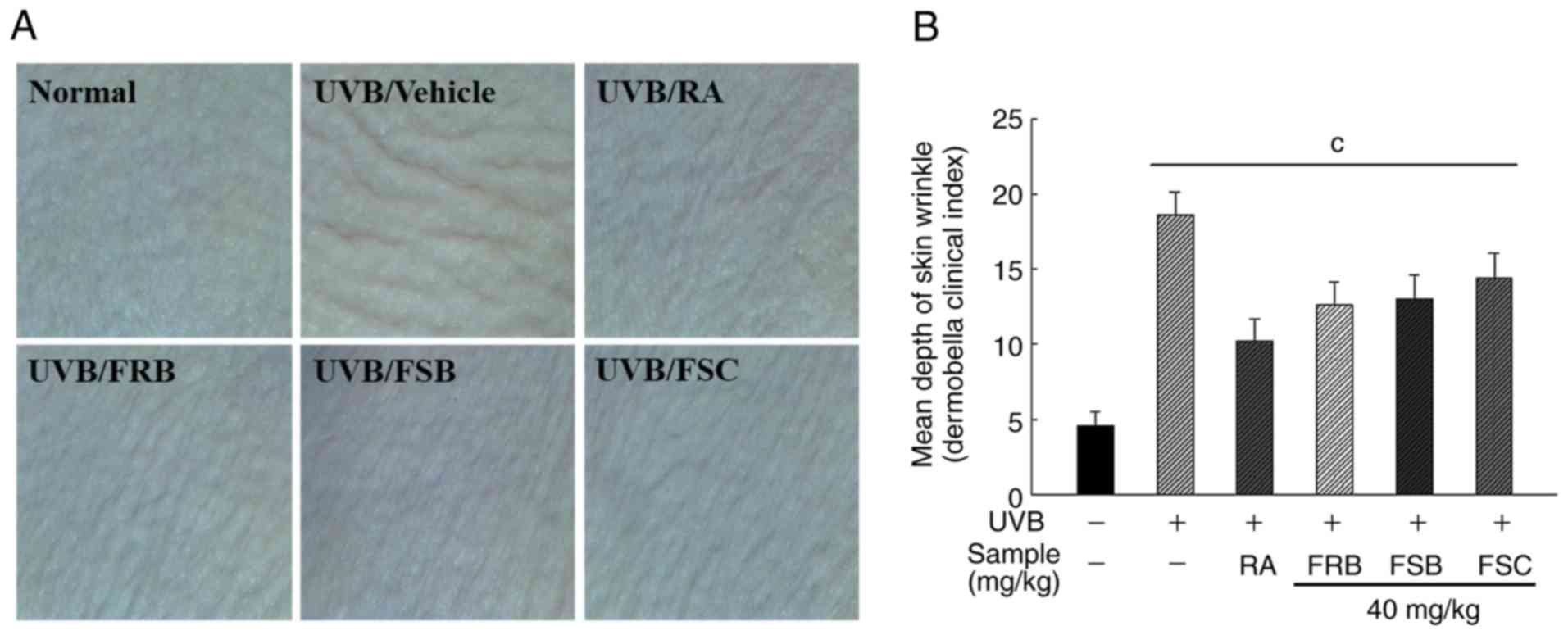
Inhibition of UVB-induced wrinkle
formation by FRB, FSB and FSC
To measure the effect of FRB, FSB and FSC (40 mg/kg
each) on wrinkle formation, HR-1 hairless mice were irradiated with
UVB in the dorsal region for 8 weeks to induce photoaging. Skin
wrinkle depth was measured using a Dermo Bella 3D analyzer
(Fig. 2). Wrinkle formation and
depth due to photoaging was observed to significantly increase
(P<0.001) in the UVB vehicle group (18.60±1.52) when compared
with the normal group (4.60±0.89). However, the group treated with
retinoic acid exhibited a significant decrease (P<0.001) in the
Dermo Bella clinical index value (10.20±1.48) when compared with
the UVB vehicle group. The oral administration of FRB, FSB and FSC
(40 mg/kg) also reduced the Dermo Bella clinical index to
12.6±1.52, 13.00±1.58 and 14.40±1.67, respectively.
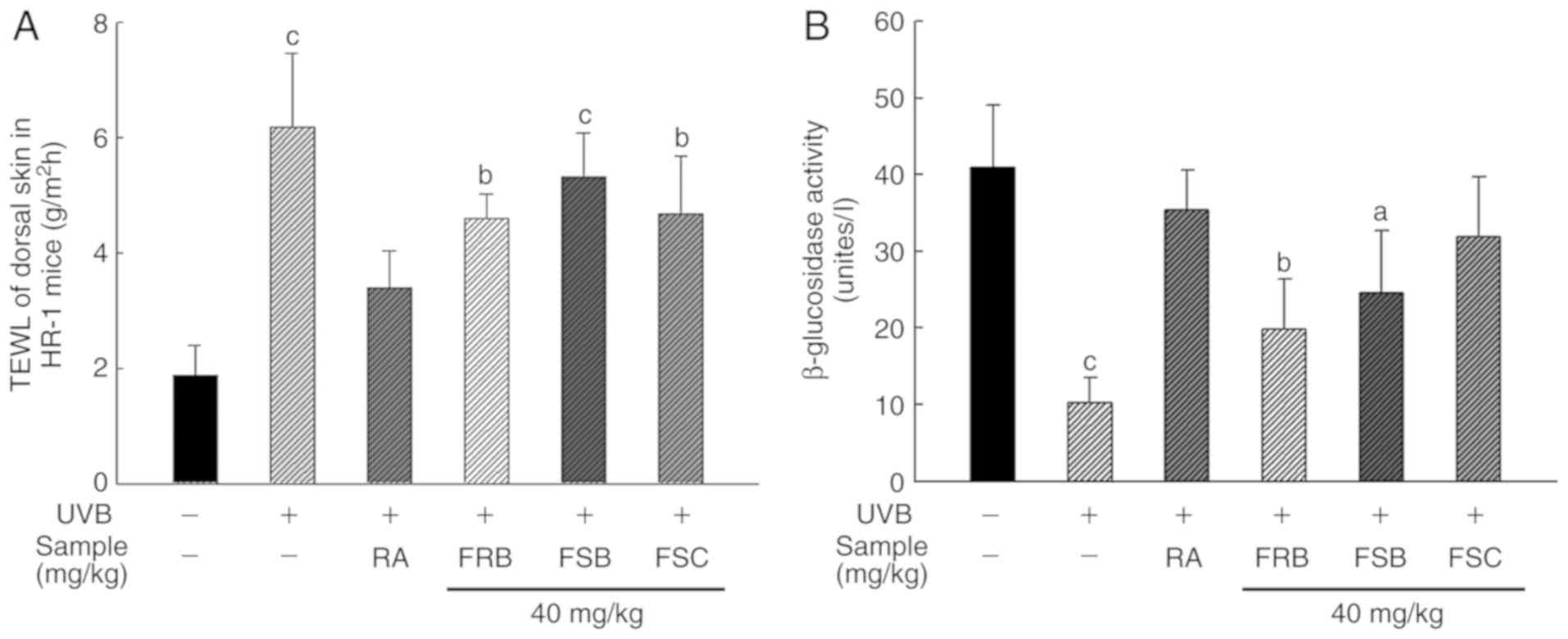
Effects of FRB, FSB and FSC on TEWL and
β-Glucosidase activity
The stratum corneum of the skin is the outermost
layer that prevents the excessive loss of moisture from the body.
It is comprised of 10-20% moisture, which prevents the skin surface
from drying and reduces skin barrier anomalies (32,33). Ceramide is a lipid component that
constitutes the stratum corneum and is known to have a skin
moisturizing effect, while β-glucosidase is an enzyme that
regenerates ceramide from glycosylceramide and acyl
glycosylceramide (34,35). Therefore, the moisture content of
the stratum corneum is an index used to monitor skin damage. To
evaluate the protective effect of FRB, FSB and FSC (40 mg/kg)
against water loss, the UVB-irradiated dorsal skin of mice was
analyzed. As presented in Fig.
3A, UVB irradiation caused a 3.3-fold increase in the TEWL in
the skin compared with the normal group. However, retinoic acid
treatment decreased the TEWL value by 1.3-, 1.2- and 1.3-fold in
mice orally administered with FRB, FSB and FSC, respectively
compared with the UVB vehicle group. β-glucosidase was used as an
index to evaluate skin aging in the present study. As presented in
Fig. 3B, β-glucosidase activity
reduced 4.0-fold following UVB irradiation when compared with the
normal group, which was recovered following retinoic acid
treatment. β-glucosidase activity was also restored following the
oral administration of FRB, FSB and FSC (40 mg/kg). FSC treatment
particularly restored activity by ~1.3-fold. Therefore, FRB, FSB
and FSC (40 mg/kg) may prevent photoaging, as observed from the
TEWL and β-glucosidase assay results.
Effects of FRB, FSB and FSC on
histological change
To evaluate the positive effect of FRB, FSB and FSC
(40 mg/kg) on skin wrinkles, the epidermal thickness of the dorsal
skin of UVB-irradiated HR-1 mice was measured via H&E staining
(Fig. 4A and B). The epidermal
thickness was observed to increase in the UVB vehicle group when
compared with the normal control group. However, the mice orally
administrated with FRB, FSB and FSC exhibited a significant
decrease (P<0.01) in epidermal thickness compared with the UVB
vehicle group. As presented in Fig.
4C, additional changes were observed in the collagen fiber
structures in dorsal skin tissue via M-T staining. The intensity of
M-T staining decreased in the UVB vehicle group when compared with
the normal control group. This result indicates an acceleration in
the wrinkle formation process owing to collagen degradation.
However, the collagen fiber numbers increased in mice orally
administered FRB, FSB and FSC when compared with those in the UVB
vehicle group. Mast cell distribution and the degree of
degranulation in the dermis and subcutaneous layer are presented in
Fig. 4D. Mast cell infiltration
around the dermis substantially increased in the UVB vehicle group
when compared with the normal control group, whereas this effect
was reduced in the tissues of mice treated with FRB, FSB and FSC
(40 mg/kg). These results demonstrate that FRB, FSB and FSC inhibit
UVB-induced changes in epidermal thickness, collagen degradation
and mast cell infiltration.
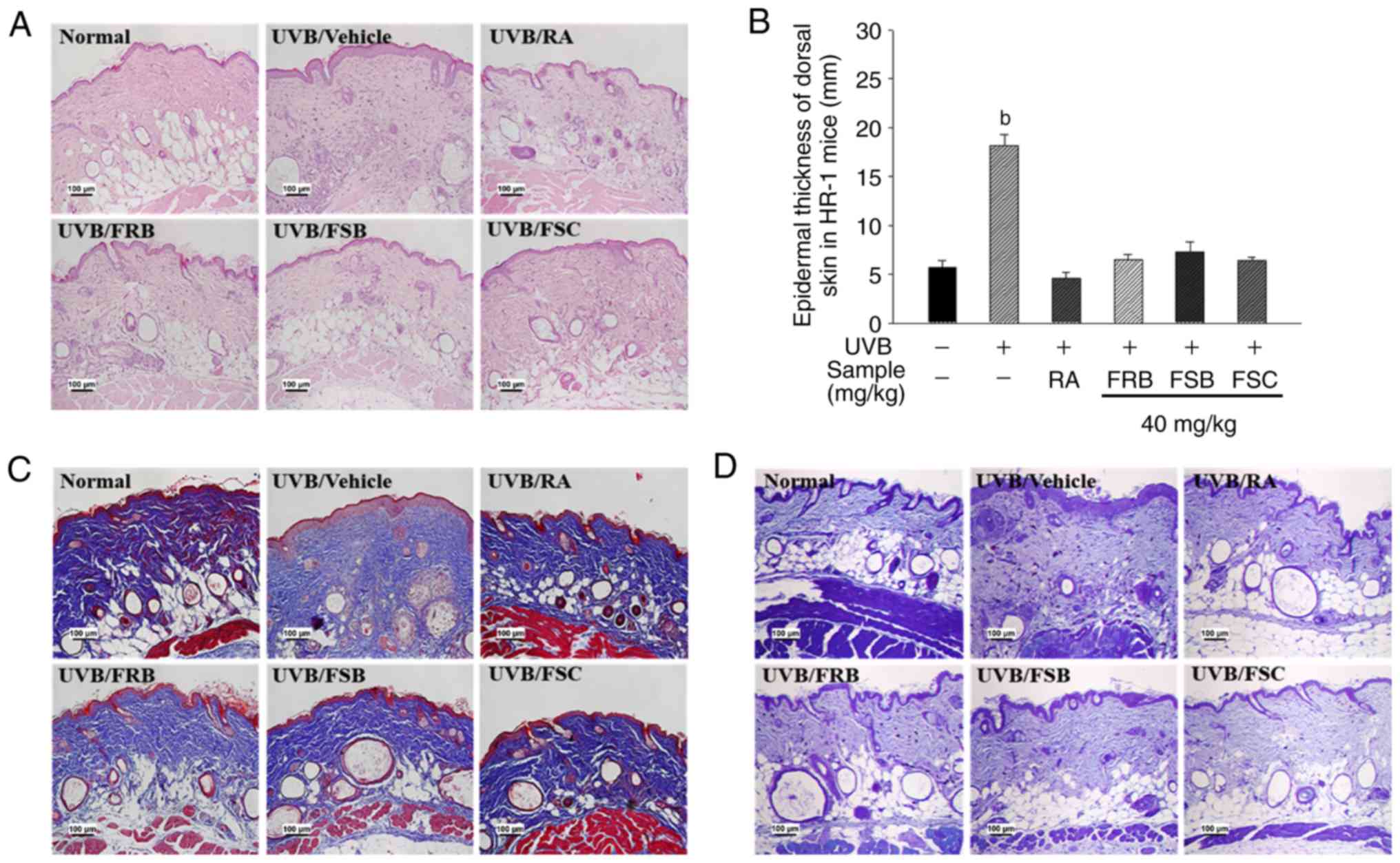 | Figure 4Inhibition of UVB-induced
histological alteration by FRB, FSB and FSC in hairless mice.
Histological alterations of epidermal thickness, collagen
degradation and mast cell infiltration were analyzed using (A)
haemotoxylin and eosin staining, (B) epidermal thickness of dorsal
skin, (C) Masson's trichrome staining and (D) toluidine blue
staining, respectively. All values are presented as the mean ±
standard deviation. A Kruskal-Wallis nonparametric test and
Bonferroni's post-hoc tests were used to control for multiple
comparisons. bP<0.01 vs. normal control mice. FRB,
fermented rice bran; FSB, fermented soybean cake; FSC, fermented
sesame seed cake; UVB, ultraviolet B; RA, retinoic acid. |
Effects of FRB, FSB and FSC oral intake
on MMP expression
MMPs are zinc-dependent endopeptidases that are
formed via the UVB irradiation-induced activation of MAPK signaling
pathway transcription factors. Functionally, MMPs are classified as
stromelysins (including MMP-3, -10 and -11), gelatinases (MMP-2 and
-9) and collagenases (MMP-1, -8 and -13) on the basis of their
substrate specificity (36).
Stromelysins may cleave type IV collagen, in addition to
proteoglycans, laminin and fibronectin (37). Collagenases specifically degrade
collagen type I, II and III into characteristic 3/4 and 1/4
fragments (38). Subsequent to
the initial cleavage, the collagen triple helix becomes denatured,
and the dissociated polypeptide chains (gelatin molecules) may then
be degraded by gelatinases (39).
Gelatinase enzymes are able to break down type IV, V and VII
collagen and exert activity against denatured collagen molecules
(gelatin) (40). To determine the
effect of FRB, FSB and FSC (40 mg/kg) on MMP expression, HR-1
hairless mice were irradiated with UVB to induce wrinkles, and the
expression of wrinkle-associated genes, including collagenases
(MMP-13), gelatinases (MMP-2 and -9), and stromelysins (MMP-3),
were analyzed. As presented in Fig.
5A, MMP-2 and MMP-9 mRNA expression levels increased in the UVB
vehicle group when compared with the normal control group; however,
FSB and FSC (in MMP-9 only; 40 mg/kg) treatment significantly
decreased (P<0.05) the expression levels of these mRNAs when
compared with the UVB vehicle group. No significant difference in
MMP-3 and MMP-13 mRNA expression levels were observed between the
different groups; however, the mean values decreased in mice orally
treated with FRB, FSB and FSC when compared with those in the UVB
vehicle group. Similar to the MMP-2 mRNA gene expression results,
MMP-2 protein expression levels were significantly inhibited
(P<0.05) in mice treated with FSB and FSC compared with the UVB
vehicle group (Fig. 5B). These
results indicate that FRB, FSB and FSC (40 mg/kg) blocked collagen
and gelatin degradation by inhibiting MMP activity. These results
were sufficient to explain the inhibition of collagen and gelatin
degradation, but the measurement of MMP-1 expression may provide
more definite results. The skin is composed of collagen type II in
addition to type I and III (41).
MMP-13 cleaves collagen type II in preference to collagens type I
and III (42). To complement
these results, the determination of MMP-1 expression and
immunohistochemistry for MMPs-induced wrinkle formation will be
performed in future studies.
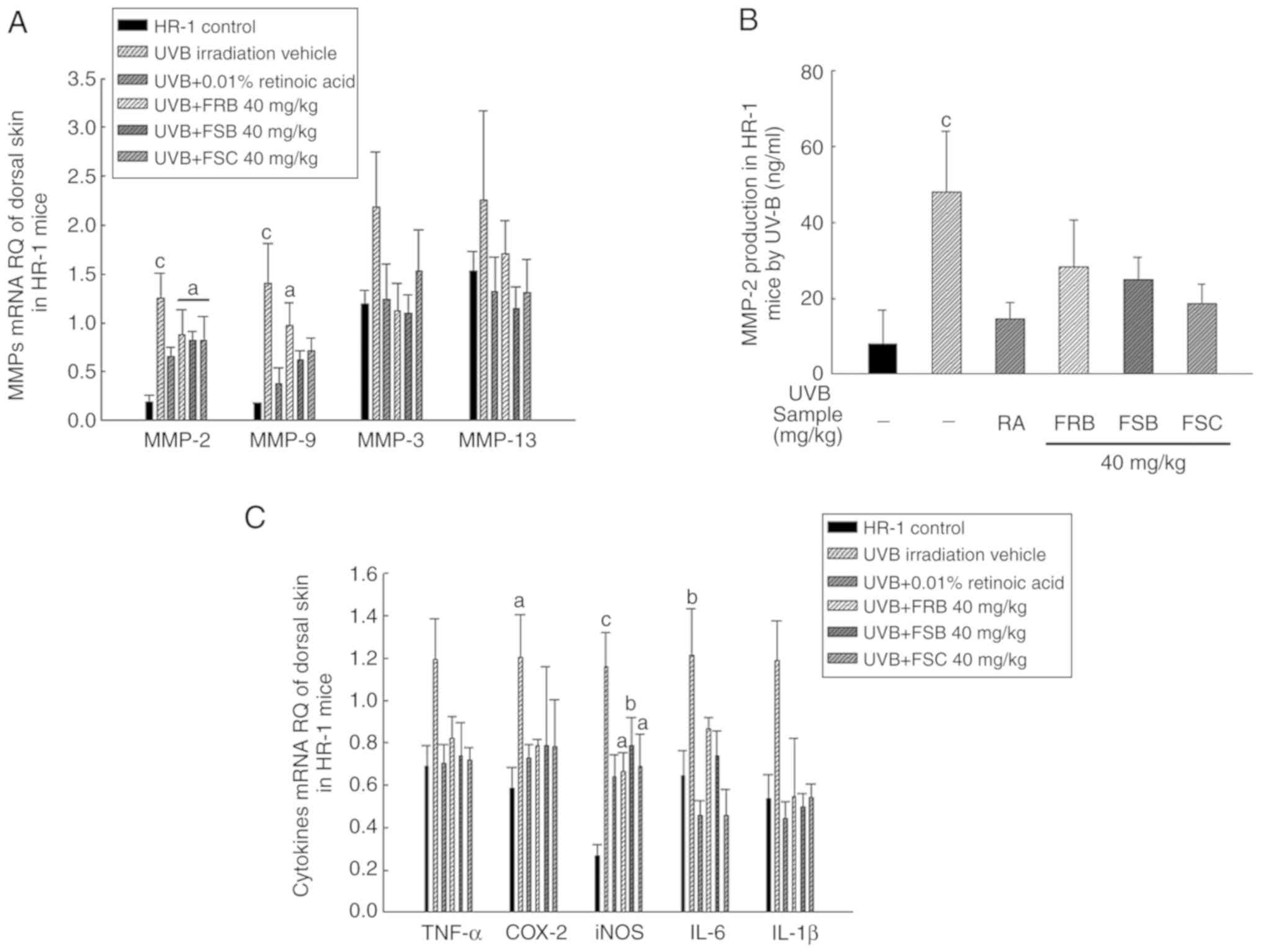 | Figure 5Effects of FRB, FSB and FSC on MMP
and cytokine expression in UVB-induced hairless mice. (A) MMP-2,
MMP-9, MMP-3 and MMP-13 expression levels determined using RT-qPCR.
Total cellular RNA was extracted using RNAzol from skin tissues.
Next, 3 µg total RNA was used for cDNA synthesis via RT-qPCR
with the appropriate primer sequences. (B) Enzyme-linked
immunosorbent assay kit-mediated determination of the MMP-2 protein
expression. (C) Measurement of inflammation-associated cytokines
using RT-qPCR. All values are presented as the mean ± standard
deviation. A Kruskal-Wallis nonparametric test and Bonferroni's
post-hoc tests were used for MMP-3, MMP-13, TNF-α and IL-1β groups
and one-way analysis of variance and Scheffe's post-hoc test were
used to control for multiple comparisons. aP<0.05,
bP<0.01 and cP<0.001 vs. normal control
mice. RQ, MMP, matrix metalloproteinase; TNF-α, tumor necrosis
factor-α; COX-2, cyclooxygenase-2; iNOS, inducible nitric oxide
synthase; IL, interleukin; FRB, fermented rice bran; FSB, fermented
soybean cake; FSC, fermented sesame seed cake; UVB, ultraviolet B;
RA, retinoic acid; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
Effect of FRB, FSB and FSC oral intake on
inflammatory cytokine expression
UVB-induced skin inflammation is a major cause of
skin aging (43). Exposure of the
skin to UVB radiation is known to enhance the levels of
proinflammatory cytokines including TNF-α, IL-1β and IL-6 (44). Interestingly, a number of natural
compounds have been demonstrated to exert anti-photoaging effects
by inhibiting inflammatory cytokines (45). In the present study, a similar
increase was observed in inflammatory cytokine levels following UVB
irradiation, which were reduced following FRB, FSB and FSC
treatments (40 mg/kg). To determine the effect of FRB, FSB and FSC
(40 mg/kg) on inflammatory cytokine levels, HR-1 hairless mice were
irradiated with UVB to induce skin inflammation, following which
the inflammation-associated cytokines including TNF-α, COX-2, iNOS,
IL-6 and IL-1β, were measured. The mRNA expression level of these
inflammatory cytokines were observed to increase following UVB
irradiation when compared with the normal group. However, the
inhibition of UVB-induced cytokine expression was also confirmed
subsequent to FRB (TNF-α, COX-2, iNOS, IL-1β, IL-6 all P<0.05),
FSB (TNF-α and IL-6, P<0.05; IL-1β, P<0.001) and FSC (TNF-α
and iNOS, P<0.05; IL-6 and IL-1β, P<0.001) treatments when
compared with the UVB vehicle group (Fig. 5C). These observations suggest that
FRB, FSB and FSC effectively inhibit the inflammatory response.
Discussion
Agricultural waste biomass is currently one of the
most important challenges facing environmental protection, which
has gained attention in the past several decades (46). The recycling of abandoned
agricultural waste is meaningful not only for environmental
protection but also for its potential value as functional food
ingredients. The current study therefore assessed the possibility
of FRB, FSB and FSC as ingredients for anti-photoaging. Skin is
naturally composed of antioxidant defence systems against
UV-induced ROS generation. However, these antioxidant defences are
insufficient when exposed to solar radiation (47). ROS generated by UVB may affect
MAPK signaling and inflammatory cytokines (23). Activated MAPK signaling increases
the activity of various MMPs, leading to the structural and
functional disruption of the ECM (24).
Wrinkle formation is caused by the decreased
elasticity of the skin and is characteristic of aging (48). UVB is a typical extrinsic aging
factor that reduces skin elasticity via wrinkle formation and is
known to cause acute and chronic reactions in human skin (49). Exposure to UV light causes skin
symptoms of skin inflammation, including erythema, edema, epidermis
thickening, keratin thickening and increased skin pigmentation
(50). Long-term exposure to UVB
may also cause severe skin lesions, resulting in cell death and
malignancy (51). The dermis is
composed of collagen, elastin and hyaluronic acid (52). Photodamaged skin exhibits
decreased elasticity due to decreased fibroblast function (53). Furthermore, photo-damage causes
elastin fibers to reduce in number and diameter, and exhibit
morphological changes (54). UV
radiation also causes the degranulation of mast cells and the
infiltration of chronic inflammatory cells (55,56). MMP expression also results in
wrinkle formation via the decomposition of collagen and elastin,
which are the main components of ECM (57).
The present study demonstrated that fermented
agricultural by-products (FRB, FSB and FSC) may attenuate
UVB-induced inflammatory cytokine production, collagen breakdown
and mast cell infiltration in hairless mice. These products were
also demonstrated to substantially reduce UVB-induced TEWL and
wrinkle formation in mouse skin and upregulate β-glucosidase
expression. Furthermore, the mRNA expression of a certain MMPs were
revealed to be notably regulated by the fermented agricultural
by-products. In conclusion, the results of the present study
indicated that discarded agricultural by-products may be recycled
via bioconversion and used as functional materials in the
prevention of UVB-induced skin damage. However, the present study
did not distinguish the efficacy of Lentinula edodes
(shiitake) used for fermentation. Thus, future studies should
determine the effect of fermentation with Lentinula edodes
(shiitake) and assess the changes in bioconversion mechanisms that
are involved with antioxidants and anti-photoaging.
Abbreviations:
|
ECM
|
extracellular matrix
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
H&E
|
hematoxylin and eosin
|
|
MMP
|
matrix metalloproteinase
|
|
UV
|
ultraviolet
|
|
TNF-α
|
tumor necrosis factor-α
|
|
COX-2
|
cyclooxygenase-2
|
|
iNOS
|
inducible nitric oxide synthase
|
|
TEWL
|
transepidermal water loss
|
|
M-T
|
Masson's trichrome
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the Korea
Institute of Planning and Evaluation for Technology in Food,
Agriculture, Forestry and Fisheries, the High Value-added Food
Technology Development Program (grant no. 314076-3) and a research
grant from Kangwon National University in 2017. Additionally, the
present study was supported by the National Research Foundation of
Korea grant funded by the Korean Government (grant no. NRF-2018
H1A2A1062634; Fostering Core Leaders of the Future Basic Science
Program/Global Ph.D. Fellowship Program).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
OHL, YCK and SJL designed the current study. SIC,
TDJ, BYC, SHC, WSS and XH performed the experiments. SIC and TDJ
wrote the manuscript. All authors performed data analysis, and
drafted/critically revised the manuscript. All authors approval the
version final version of the manuscript and agree to be accountable
for all aspects of the study.
Ethics approval and consent to
participate
The use of animals in the current study was approved
by the Institutional Animal Care and Use Committee of Kangwon
National University (approval no. KW-170417-1; 15 May 2017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests, financial or otherwise, associated with this
publication.
References
|
1
|
Schmalensee R: From 'Green Growth' to
sound policies: An overview. Energy Econ. 34(Suppl 1): S2–S6. 2012.
View Article : Google Scholar
|
|
2
|
Jänicke M: 'Green growth': From a growing
eco-industry to economic sustainability. Energ Policy. 48:13–21.
2012. View Article : Google Scholar
|
|
3
|
Peschel W, Dieckmann W, Sonnenschein M and
Plescher A: High antioxidant potential of pressing residues from
evening primrose in comparison to other oilseed cakes and plant
antioxidants. Ind Crop Prod. 25:44–54. 2007. View Article : Google Scholar
|
|
4
|
Asadullah M: Barriers of commercial power
generation using biomass gasification gas: A review. Renew Sust
Energ Rev. 24:201–215. 2013.
|
|
5
|
Iwata T: Biodegradable and bio-based
polymers: Future prospects of eco-friendly plastics. Angew Chem Int
Ed Engl. 54:3210–3215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perkins C, Siddiqui S, Puri M and Demain
AL: Biotechnological applications of microbial bioconversions. Crit
Rev Biotechnol. 36:1050–1065. 2016. View Article : Google Scholar
|
|
7
|
Cho YH, Cho JS and Lee GW: Antioxidant
activity of wood vinegar by bioconversion. J Korea Acad Industr
Coop Soc. 12:4434–4442. 2011. View Article : Google Scholar
|
|
8
|
Kiran EU, Trzcinski AP, NG WJ and Liu Y:
Bioconversion of food waste to energy: A review. Fuel. 134:389–399.
2014. View Article : Google Scholar
|
|
9
|
Sanchez S and Demain AL: Enzymes and
bioconversions of industrial, pharmaceutical, and biotechnological
significance. Org Process Res Dev. 15:224–230. 2011. View Article : Google Scholar
|
|
10
|
Jung TD, Shin GH, Kim JM, Choi SI, Lee JH,
Lee SJ, Park SJ, Woo KS, Oh SK and Lee OH: Comparative analysis of
γ-oryzanol, β-glucan, total phenolic content and antioxidant
activity in fermented rice bran of different varieties. Nutrients.
9:E5172017. View Article : Google Scholar
|
|
11
|
Jung TD, Shin GH, Kim JM, Oh JW, Choi SI,
Lee JH, Lee SJ, Heo IY, Park SJ, Kim HT, et al: Assessment of
validation method for bioactive contents of fermented soybean
extracts by bioconversion and their antioxidant activities. J
Korean Soc Food Sci Nutr. 45:680–689. 2016. View Article : Google Scholar
|
|
12
|
Jung TD, Choi SI, Choi SH, Cho BY, Sim WS,
Han-Xionggao, Lee SJ, Park SJ, Kim DB, Kim YC, et al: Changes in
the anti-allergic activities of sesame by bioconversion. Nutrients.
10:E2102018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kruk J and Duchnik E: Oxidative stress and
skin diseases: Possible role of physical activity. Asian Pac J
Cancer Prev. 15:561–568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Webb AR and Engelsen O: Calculated
ultraviolet exposure levels for a healthy vitamin D status.
Photochem Photobiol. 82:1697–1703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taylor GJ, Bannister GC and Leeming JP:
Wound disinfection with ultraviolet radiation. J Hosp Infect.
30:85–93. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imokawa G: Mechanism of UVB-induced
wrinkling of the skin: Paracrine cytokine linkage between
keratinocytes and fibroblasts leading to the stimulation of
elastase. J Investig Dermatol Symp Proc. 14:36–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matts PJ: Solar ultraviolet radiation:
Definitions and terminology. Dermatol Clin. 24:1–8. 2006.
View Article : Google Scholar
|
|
18
|
Algaba I and Riva A: In vitro measurement
of the ultraviolet protection factor of apparel textiles. Color
Technol. 118:52–58. 2002. View Article : Google Scholar
|
|
19
|
Kim DB, Shin GH, Kim JM, Kim YH, Lee JH,
Lee JS, Song HJ, Choe SY, Park IJ, Cho JH and Lee OH: Antioxidant
and anti-ageing activities of citrus-based juice mixture. Food
Chem. 194:920–927. 2016. View Article : Google Scholar
|
|
20
|
Afaq F and Mukhtar H: Botanical
antioxidants in the prevention of photocarcinogenesis and
photoaging. Exp Dermatol. 15:678–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jurkiewicz BA, Bissett DL and Buettner GR:
Effect of topically applied tocopherol on ultraviolet
radiation-mediated free radical damage in skin. J Invest Dermatol.
104:484–488. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weichenthal M, Godorr M, Altenhoff J,
Neuber K and Breitbart EW: Effects of whole-body UVB irradiation on
cytokine production by peripheral blood mononuclear cells from
stage I melanoma patients. Arch Dermatol Res. 292:348–353. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muthusamy V and Piva TJ: The UV response
of the skin: A review of the MAPK, NFkappaB and TNFalpha signal
transduction pathways. Arch Dermatol Res. 302:5–17. 2010.
View Article : Google Scholar
|
|
24
|
Miyachi Y: Photoaging from an oxidative
standpoint. J Dermatol Sci. 9:79–86. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi SH, Choi SI, Jung TD, Cho BY, Lee JH,
Kim SH, Yoon SA, Ham YM, Yoon WJ, Cho JH and Lee OH:
Anti-photoaging effect of jeju putgyul (unripe citrus) extracts on
human dermal fibroblasts and ultraviolet B-induced hairless mouse
skin. Int J Mol Sci. 18:E20522017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar S: Exploratory analysis of global
cosmetic industry: Major players, technology and market trends.
Technovation. 25:1263–1272. 2005. View Article : Google Scholar
|
|
27
|
Landriscina A, Rosen J and Friedman A:
Nanotechnology, inflammation and the skin barrier: Innovative
approaches for skin health and cosmesis. Cosmetics. 2:177–186.
2015. View Article : Google Scholar
|
|
28
|
Jeong SC, Park JH and Kim JH: The
development trend of skin beauty food with skin protection effects
from natural source. Asian J Beauty Cosmetol. 11:203–212. 2013.
|
|
29
|
Yaar M and Gilchrest BA: Aging versus
photo aging: Postulated mechanisms and effectors. J Investing
Dermatol Symp Proc. 3:47–51. 1998.
|
|
30
|
Bissett DL, Hannonand DP and Orr TV: An
animal model of solar-aged skin: Histological, physical, and
visible changes in UV-irradiated hairless mouse skin. Photochem
Photobiol. 46:367–378. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Kwon SB, Lee GT, Choi SJ, Lee NK, Park HW,
Lee KS, Lee KK, Ahn KJ and An IS: The effect of glycerin,
hyaluronic acid and silicone oil on the hydration, moisturization
and transepidermal water loss in human skin. Asian J Beauty
Cosmetol. 11:761–768. 2013.
|
|
33
|
Nasir A: Diseases associated with
cutaneous barrier dysfunction: Basic science aspects and clinical
perspectives. Toxicology of the Skin. Monteiro-Riviere NA: Informa
Healthcare; New York: pp. 203–279. 2010
|
|
34
|
Kitatani K, Sheldon K, Rajagopalan V,
Anelli V, Jenkins RW, Sun Y, Grabowski GA, Obeid LM and Hannun YA:
Involvement of acid beta-glucosidase 1 in the salvage pathway of
ceramide formation. J Biol Chem. 284:12972–12978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sirikudta W, Kulthanan K, Varothai S and
Nuchkull P: Moisturizers for patients with atopic dermatitis: An
overview. J Allergy Ther. 4:1–6. 2013. View Article : Google Scholar
|
|
36
|
Gkouveris I, Nikitakis N, Aseervatham J,
Rao N and Ogbureke KUE: Matrix metalloproteinases in head and neck
cancer: Current perspectives. Metalloproteinases Med. 4:47–61.
2017. View Article : Google Scholar
|
|
37
|
Chin JR, Murphy G and Werb Z: Stromelysin,
a connective tissue-degrading metalloendopeptidase secreted by
stimulated rabbit synovial fibroblasts in parallel with
collage-nase. Biosynthesis, isolation, characterization, and
substrates. J Biol Chem. 260:12367–12376. 1985.PubMed/NCBI
|
|
38
|
Sunami E, Tsuno N, Osada T, Saito S,
Kitayama J, Tomozawa S, Tsuruo T, Shibata Y, Muto T and Nagawa H:
MMP-1 is a prognostic marker for hematogenous metastasis of
colorectal cancer. Oncologist. 5:108–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fini ME and Girard MT: The pattern of
metalloproteinase expression by corneal fibroblasts is altered by
passage in cell culture. J Cell Sci. 97:373–383. 1990.PubMed/NCBI
|
|
40
|
Murphy G, Hembry RM, McGarrity AM,
Reynolds JJ and Henderson B: Gelatinase (type IV collagenase)
immunolocalization in cells and tissues: Use of an antiserum to
rabbit bone gelatinase that identifies high and low Mr forms. J
Cell Sci. 92:487–495. PubMed/NCBI
|
|
41
|
Meigel WN, Gay S and Weber L: Dermal
architecture and collagen type distribution. Arch Dermatol Res.
259:1–10. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Robichaud TK, Steffensen B and Fields GB:
Exosite interactions impact matrix metalloproteinase collagen
specificities. J Biol Chem. 286:37535–37542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pillai S, Oresajo C and Hayward J:
Ultraviolet radiation and skin aging: Roles of reactive oxygen
species, inflammation and protease activation, and strategies for
prevention of inflammation-induced matrix degradation-a review. Int
J Cosmet Sci. 27:17–34. 2005. View Article : Google Scholar
|
|
44
|
Nichols JA and Katiyar SK: Skin
photoprotection by natural polyphenols: Anti-inflammatory,
antioxidant and DNA repair mechanisms. Arch Dermatol Res.
302:71–83. 2010. View Article : Google Scholar
|
|
45
|
Chen CC, Chiang AN, Liu HN and Chang YT:
EGb-761 prevents ultraviolet B-induced photoaging via inactivation
of mitogen-activated protein kinases and proinflammatory cytokine
expression. J Dermatol Sci. 75:55–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fo KY and Hameed BH: Utilization of rice
husk ash as novel adsorbent: A judicious recycling of the colloidal
agricultural waste. Adv Colloid Interfac Sci. 152:39–47. 2009.
View Article : Google Scholar
|
|
47
|
Puglia C, Offerta A, Saija A, Trombetta D
and Venera C: Protective effect of red orange extract
supplementation against UV-induced skin damages: Photoaging and
solar lentigines. J Cosmet Dermatol. 13:151–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Davies KJ: Protein damage and degradation
by oxygen radical. I. general aspects. J Biol Chem. 262:9895–9901.
1987.PubMed/NCBI
|
|
49
|
Bissett DL, Chatterjee R and Hannon DP:
Photoprotective effect of superoxide-scavenging antioxidants
against ultraviolet radiation-induced chronic skin damage in the
hairless mouse. Photodermatol Photoimmunol Photomed. 7:56–62.
1990.PubMed/NCBI
|
|
50
|
Korać RR and Khambholja KM: Potential of
herbs in skin protection from ultraviolet radiation. Pharmacog Rev.
5:164–173. 2011. View Article : Google Scholar
|
|
51
|
Choi WH, Ann HS, Choi TY, Jin SY and Ahn
RM: Effects of natural extracts on UVB-induced pigmentation and
inflammation in C57BL/6 mouse skin. Korean J Environ Health Sci.
32:492–498. 2006.
|
|
52
|
Nanashima N, Horie K, Maeda H, Tomisawa T,
Kitajima M and Nakamura T: Blackcurrant anthocyanins increase the
levels of collagen, elastin and hyaluronic acid in human skin
fibroblasts and ovariectomized rats. Nutrients. 10:E4952018.
View Article : Google Scholar
|
|
53
|
Varani J, Schuger L, Dame MK, Leonard C,
Fligiel SE, Kang S, Fisher GJ and Voorhees JJ: Reduced fibroblast
interaction with intact collagen as a mechanism for depressed
collagen synthesis in photodamaged skin. J Investig Dermatol.
122:1471–1479. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Frances C and Robert L: Elastin and
elastic fibers in normal and pathologic skin. Int J Dermatol.
23:166–179. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kligman LH and Kligman AM: The nature of
photoaging: Its prevention and repair. Photodermatol. 3:215–227.
1986.PubMed/NCBI
|
|
56
|
Foote CS: Photosensitized oxidation and
singlet oxygen; consequences in biological systems. Free Radicals
in Biology. Pryor WA: Academic Press; New York: pp. 85–133. 1976,
View Article : Google Scholar
|
|
57
|
Hwang BM, Noh EM, Kim JS, Kim JM, You YO,
Hwang JK, Kwon KB and Lee YR: Curcumininhibits UVB-induced matrix
metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK
pathways in human dermal fibroblasts. Exp Dermatol. 22:371–374.
2013. View Article : Google Scholar : PubMed/NCBI
|