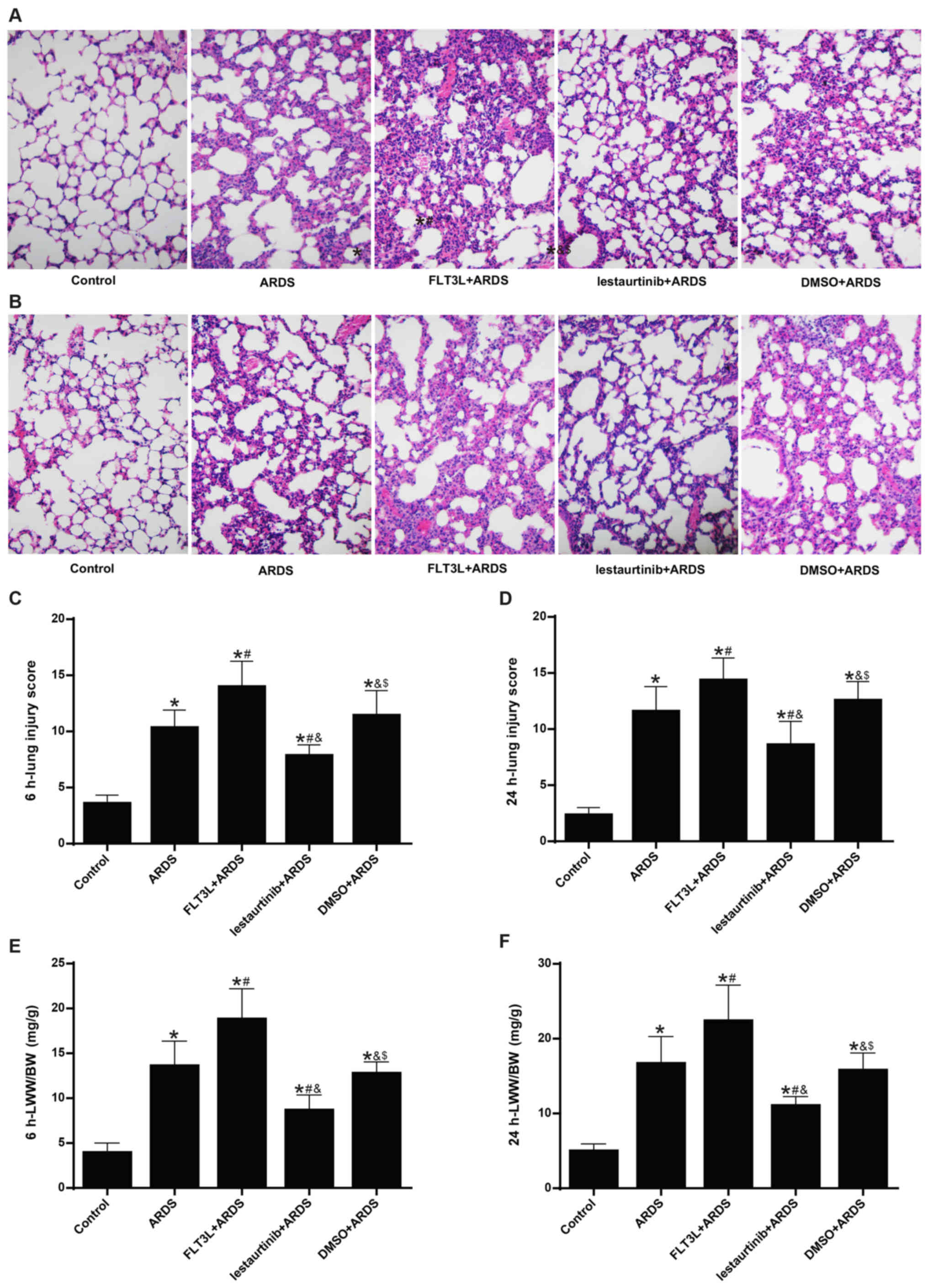
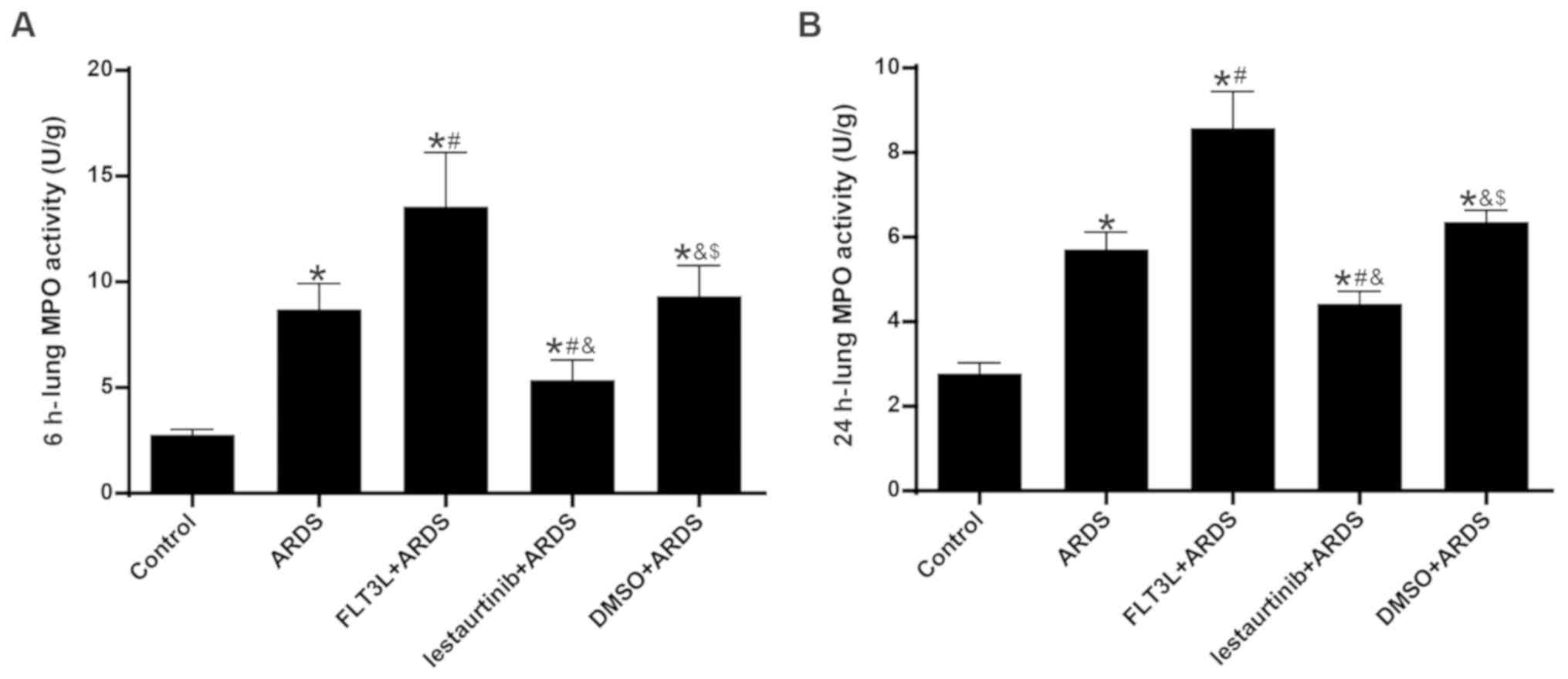
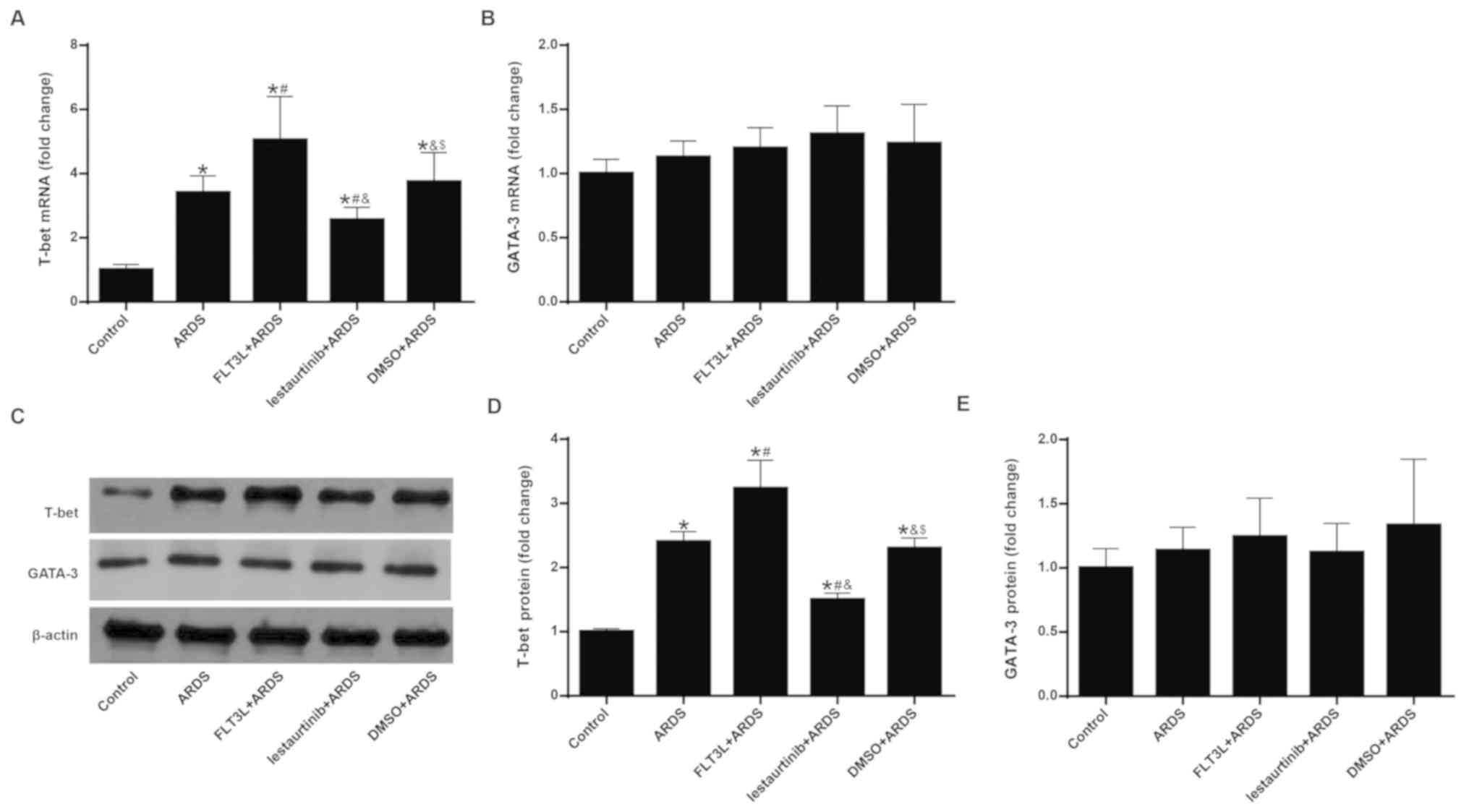
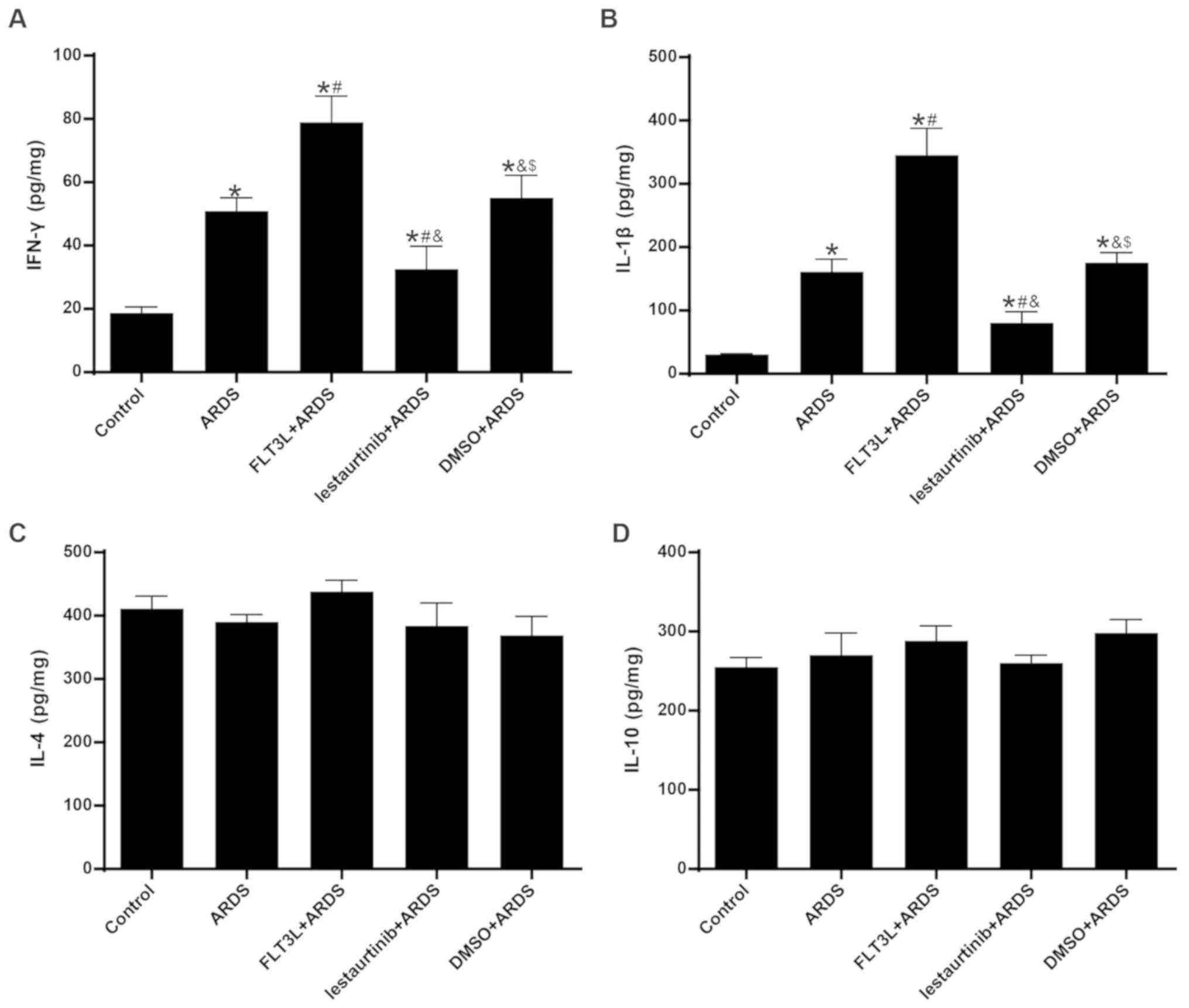
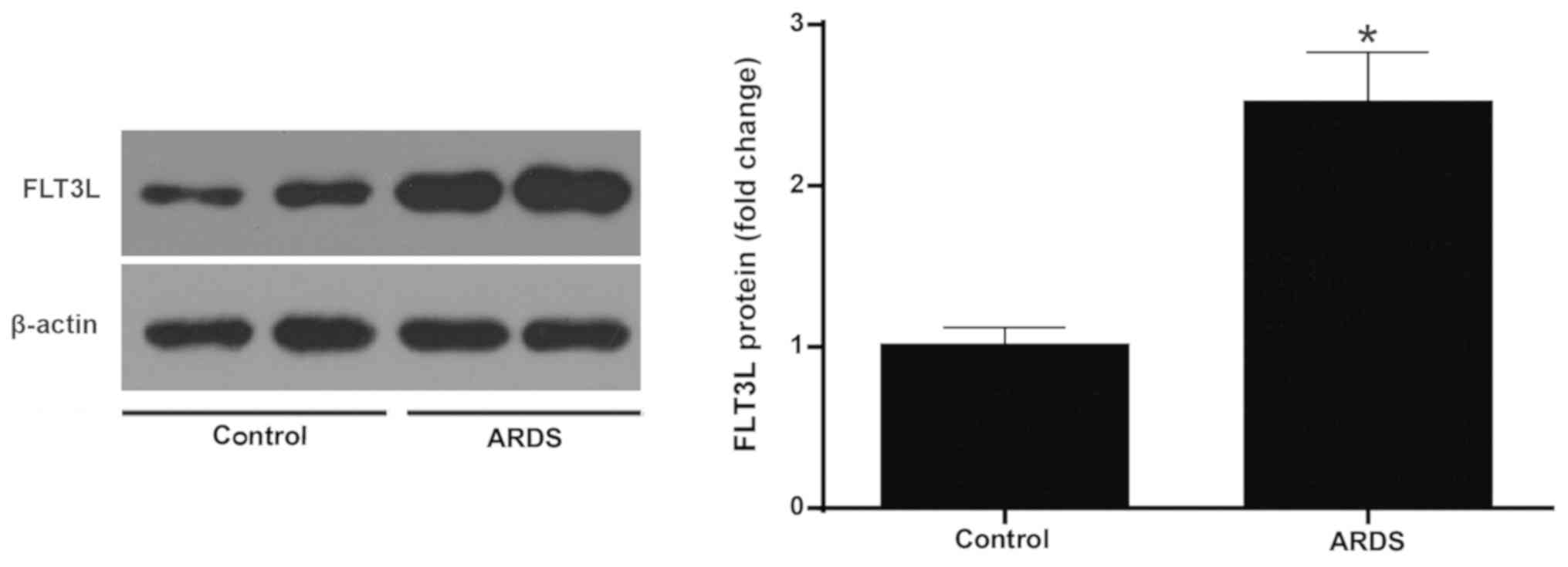
|
1
|
Thompson BT, Chambers RC and Liu KD: Acute
respiratory distress syndrome. N Engl J Med. 377:562–572. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan E, Brodie D and Slutsky AS: Acute
respiratory distress syndrome: Advances in diagnosis and treatment.
JAMA. 319:698–710. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reilly JP, Christie JD and Meyer NJ: Fifty
years of research in ARDS. Genomic contributions and opportunities.
Am J Respir Crit Care Med. 196:1113–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
ARDS Definition Task Force; Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin Definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI
|
|
5
|
Kumar V: Dendritic cells in sepsis:
Potential immunoregulatory cells with therapeutic potential. Mol
Immunol. 101:615–626. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lambrecht BN and Hammad H: The role of
dendritic and epithelial cells as master regulators of allergic
airway inflammation. Lancet. 376:835–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buttignol M, Pires-Neto RC, Rossi E Silva
RC, Albino MB, Dolhnikoff M and Mauad T: Airway and parenchyma
immune cells in influenza A(H1N1)pdm09 viral and non-viral diffuse
alveolar damage. Respir Res. 18:1472017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Wulffen W, Steinmueller M, Herold S,
Marsh LM, Bulau P, Seeger W, Welte T, Lohmeyer J and Maus UA: Lung
dendritic cells elicited by Fms-like tyrosine 3-kinase ligand
amplify the lung inflammatory response to lipopolysaccharide. Am J
Respir Crit Care Med. 176:892–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whartenby KA, Calabresi PA, McCadden E,
Nguyen B, Kardian D, Wang T, Mosse C, Pardoll DM and Small D:
Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune
disease. Proc Natl Acad Sci USA. 102:16741–16746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lau CM, Nish SA, Yogev N, Waisman A,
Reiner SL and Reizis B: Leukemia-associated activating mutation of
Flt3 expands dendritic cells and alters T cell responses. J Exp
Med. 213:415–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SW, Choi SM, Choo YS, Kim IK, Song BW
and Kim HS: Flt3 ligand induces monocyte proliferation and enhances
the function of monocyte-derived dendritic cells in vitro. J Cell
Physiol. 230:1740–1749. 2015. View Article : Google Scholar
|
|
12
|
Sato T, Yang X, Knapper S, White P, Smith
BD, Galkin S, Small D, Burnett A and Levis M: FLT3 ligand impedes
the efficacy of FLT3 inhibitors in vitro and in vivo. Blood.
117:3286–3293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong L, He H, Liu J, Liu L, Yang Y and Qiu
H: The control effects of FLT3 signaling-dependent pulmonary
conventional dendritic cells on the initiation of acute lung
inflammation response to lipopolysaccharide induced acute lung
injury in mice. Zhonghua Ji Zhen Yi Xue Za Zhi. 25:1412–1417.
2016.In Chinese.
|
|
14
|
Mellman I: Dendritic cells: Master
regulators of the immune response. Cancer Immunol Res. 1:145–149.
2013. View Article : Google Scholar
|
|
15
|
Shao Z, Bharadwaj AS, McGee HS, Makinde TO
and Agrawal DK: Fms-like tyrosine kinase 3 ligand increases a lung
DC subset with regulatory properties in allergic airway
inflammation. J Allergy Clin Immunol. 123:917–924.e2. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ludwig IS, Geijtenbeek TB and van Kooyk Y:
Two way communication between neutrophils and dendritic cells. Curr
Opin Pharmacol. 6:408–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dieker J, Tel J, Pieterse E, Thielen A,
Rother N, Bakker M, Fransen J, Dijkman HB, Berden JH, de Vries JM,
et al: Circulating apoptotic microparticles in systemic lupus
erythematosus patients drive the activation of dendritic cell
subsets and prime neutrophils for NETosis. Arthritis Rheumatol.
68:462–472. 2016. View Article : Google Scholar
|
|
18
|
Baudiß K, de Paula Vieira R, Cicko S,
Ayata K, Hossfeld M, Ehrat N, Gómez-Muñoz A, Eltzschig HK and Idzko
M: C1P attenuates lipopolysaccharide-induced acute lung injury by
preventing NF-κB activation in neutrophils. J Immunol.
196:2319–2326. 2016. View Article : Google Scholar
|
|
19
|
McWilliam AS, Nelson D, Thomas JA and Holt
PG: Rapid dendritic cell recruitment is a hallmark of the acute
inflammatory response at mucosal surfaces. J Exp Med.
179:1331–1336. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olguín-Alor R, de la Fuente-Granada M,
Bonifaz LC, Antonio-Herrera L, García-Zepeda EA and Soldevila G: A
key role for inhibins in dendritic cell maturation and function.
PLoS One. 11:e01678132016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schlosser K, Taha M, Deng Y, McIntyre LA,
Mei SHJ and Stewart DJ: High circulating angiopoietin-2 levels
exacerbate pulmonary inflammation but not vascular leak or
mortality in endotoxin-induced lung injury in mice. Thorax.
73:248–261. 2018. View Article : Google Scholar :
|
|
22
|
Aggarwal NR, King LS and D'Alessio FR:
Diverse macrophage populations mediate acute lung inflammation and
resolution. Am J Physiol Lung Cell Mol Physiol. 306:L709–L725.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen H, Hogaboam CM, Gauldie J and Kunkel
SL: Severe sepsis exacerbates cell-mediated immunity in the lung
due to an altered dendritic cell cytokine profile. Am J Pathol.
168:1940–1950. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smit JJ, Lindell DM, Boon L, Kool M,
Lambrecht BN and Lukacs NW: The balance between plasmacytoid DC
versus conventional DC determines pulmonary immunity to virus
infections. PLoS One. 3:e17202008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vermaelen K and Pauwel R: Accurate and
simple discrimination of mouse pulmonary dendritic cell and
macrophage populations by flow cytometry: Methodology and new
insights. Cytometry A. 61:170–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Venet F, Huang X, Chung CS, Chen Y and
Ayala A: Plasmacytoid dendritic cells control lung inflammation and
monocyte recruitment in indirect acute lung injury in mice. Am J
Pathol. 176:764–773. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Masten BJ and Lipscomb MF: Comparison of
lung dendritic cells and B cells in stimulating naive
antigen-specific T cells. J Immunol. 162:1310–1317. 1999.PubMed/NCBI
|
|
28
|
Liu H, Wu J, Yao JY, Wang H and Li ST: The
role of oxidative stress in decreased acetylcholinesterase activity
at the neuromuscular junction of the diaphragm during sepsis. Oxid
Med Cell Longev. 2017:97186152017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li L, Dong L, Wang Y, Zhang X and Yan J:
Lats1/2-mediated alteration of hippo signaling pathway regulates
the fate of bone marrow-derived mesenchymal stem cells. Biomed Res
Int. 2018:43879322018. View Article : Google Scholar
|
|
30
|
Li L, Dong L, Zhang J, Gao F, Hui J and
Yan J: Mesenchymal stem cells with downregulated Hippo signaling
attenuate lung injury in mice with lipopolysaccharide-induced acute
respiratory distress syndrome. Int J Mol Med. 43:1241–1252.
2019.PubMed/NCBI
|
|
31
|
Smith KM, Mrozek JD, Simonton SC, Bing DR,
Meyers PA, Connett JE and Mammel MC: Prolonged partial liquid
ventilation using conventional and high-frequency ventilatory
techniques: Gas exchange and lung pathology in an animal model of
respiratory distress syndrome. Crit Care Med. 25:1888–1897. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hubbell JA, Thomas SN and Swartz MA:
Materials engineering for immunomodulation. Nature. 462:449–460.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lambrecht BN, Salomon B, Klatzmann D and
Pauwels RA: Dendritic cells are required for the development of
chronic eosinophilic airway inflammation in response to inhaled
antigen in sensitized mice. J Immunol. 160:4090–4097.
1998.PubMed/NCBI
|
|
34
|
Riccardi F, Della Porta MG, Rovati B,
Casazza A, Radolovich D, De Amici M, Danova M and Langer M: Flow
cytometric analysis of peripheral blood dendritic cells in patients
with severe sepsis. Cytometry B Clin Cytom. 80:14–21. 2011.
View Article : Google Scholar
|
|
35
|
Schlitzer A, Zhang W, Song M and Ma X:
Recent advances in understanding dendritic cell development,
classification, and phenotype. F1000Res. 7:2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agrawal DK, Hopfenspirger MT, Chavez J and
Talmadge JE: Flt3 ligand: A novel cytokine prevents allergic asthma
in a mouse model. Int Immunopharmacol. 1:2081–2089. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bohannon J, Fang G, Cui W, Sherwood E and
Toliver-Kinsky T: Fms-like tyrosine kinase-3 ligand alters
antigen-specific responses to infections after severe burn injury.
Shock. 32:435–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jankovic D, Liu Z and Gause WC: Th1- and
Th2-cell commitment during infectious disease: Asymmetry in
divergent pathways. Trends Immunol. 22:450–457. 2011. View Article : Google Scholar
|
|
39
|
Abe K, Nguyen KP, Fine SD, Mo JH, Shen C,
Shenouda S, Corr M, Jung S, Lee J, Eckmann L and Raz E:
Conventional dendritic cells regulate the outcome of colonic
inflammation independently of T cells. Proc Natl Acad Sci USA.
104:17022–17027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Beshara R, Sencio V, Soulard D, Barthélémy
A, Fontaine J, Pinteau T, Deruyter L, Ismail MB, Paget C, Sirard
JC, et al: Alteration of Flt3-Ligand-dependent de novo generation
of conventional dendritic cells during influenza infection
contributes to respiratory bacterial superinfection. PLoS Pathog.
14:e10073602018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Madan B, Goh KC, Hart S, William AD,
Jayaraman R, Ethirajulu K, Dymock BW and Wood JM: SB1578, a novel
inhibitor of JAK2, FLT3, and c-Fms for the treatment of rheumatoid
arthritis. J Immunol. 189:4123–4134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kangelaris KN, Prakash A, Liu KD,
Aouizerat B, Woodruff PG, Erle DJ, Rogers A, Seeley EJ, Chu J, Liu
T, et al: Increased expression of neutrophil-related genes in
patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol
Physiol. 308:L1102–L1113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li H, Zhou X, Tan H, Hu Y, Zhang L, Liu S,
Dai M, Li Y, Li Q, Mao Z, et al: Neutrophil extracellular traps
contribute to the pathogenesis of acid-aspiration-induced ALI/ARDS.
Oncotarget. 9:1772–1784. 2017.
|
|
44
|
Udayanga KG, Nakamura Y, Nakahashi-Oda C
and Shibuya A: Immunoreceptor CD300a on mast cells and dendritic
cells regulates neutrophil recruitment in a murine model of sepsis.
Int Immunol. 28:611–615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sallusto F, Palermo B, Lenig D, Miettinen
M, Matikainen S, Julkunen I, Forster R, Burgstahler R, Lipp M and
Lanzavecchia A: Distinct patterns and kinetics of chemokine
production regulate dendritic cell function. Eur J Immunol.
29:1617–1625. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dransfield I and Rossi AG: Granulocyte
apoptosis: Who would work with a 'real' inflammatory cell? Biochem
Soc Trans. 32:447–451. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sharpe AH and Freeman GJ: The B7-CD28
superfamily. Nat Rev Immunol. 2:116–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hathcock KS, Laszlo G, Pucillo C, Linsley
P and Hodes RJ: Comparative analysis of B7-1 and B7-2 costimulatory
ligands: Expression and function. J Exp Med. 180:631–640. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xia W, Pinto CE and Kradin RL: The
antigen-presenting activities of Ia+ dendritic cells
shift dynamically from lung to lymph node after an airway challenge
with soluble antigen. J Exp Med. 181:1275–1283. 1995. View Article : Google Scholar : PubMed/NCBI
|