Introduction
Overnutrition plays a pivotal role in obesity and
comorbidities including nonalcoholic fatty liver disease (NAFLD),
type 2 diabetes mellitus and cardiovascular disease (CVD) (1). NAFLD is characterized by hepatic
accumulation of fat, particularly triglycerides (TGs), and may
range from simple steatosis to nonalcoholic steatohepatitis (NASH),
cirrhosis and hepatocellular carcinoma (2). In liver cells, excess TGs are stored
in lipid droplets (LDs), and LD-associated proteins, such as the
adipose differentiation-related protein (ADRP), regulate lipid
packing and traffic (3). TG
synthesis is a beneficial response against excess of potentially
toxic fatty acids (FAs), leading to inflammation and reactive
oxygen species (ROS) formation, particularly in mitochondria
(4), which trigger lipid
peroxidation of membranes acting in NAFLD progression (5).
Fructose-enriched food may contribute to the
development of NAFLD (6).
Fructose can enter de novo FA synthesis in liver cells
through the action of fatty acid synthase (FAS). However, the
extent to which fructose contributes to the metabolic disorders
remains unclear, as only a limited number of data reporting its
direct effects on hepatocyte during NAFLD progression are available
(7).
In liver cells, NAFLD is associated with alterations
in lipogenic and lipolytic pathways, which are controlled by a
number of transcription factors, such as peroxisome
proliferator-activated receptor (PPAR) (8), and by microRNAs (miRNAs/miRs),
including miR-122, which is the most abundant hepatic miRNA
(9). Dysregulation of miRNA
expression has been reported in rodent models of NAFLD, and in
certain cases aligned with the changes observed in obese patients
with steatosis (10).
A deeper understanding of the mechanisms underlying
NAFLD progression would help identifying novel cost-effective
therapeutic strategies. It has been reported that plant polyphenols
are promising molecules for the management of NAFLD (11). Silybin, the most relevant
flavonolignan extract from the seeds of milk thistle (Silybum
marianum) (12), exhibited
certain beneficial effects in a preliminary study on NAFLD patients
(13).
In the present study, an in vitro model of
NAFLD progression was established to identify the pathways
sustaining the interference between excess fructose and fatty acids
on dysregulating lipid and radical metabolism in hepatocytes, and
to verify the ability of silybin to reverse these alterations. The
results may have an important translational value for possible
therapy of hepatic steatosis associated with NAFLD.
Materials and methods
Cell treatments
Rat hepatoma FaO cells (European Collection of
Authenticated Cell Cultures, Salisbury, UK; cat. no. 89042701) were
supplied as mycoplasma-free and cultured in Coon's modified Ham's
F12 with 10% fetal bovine serum (South American origin,
EU-approved; Euroclone, Milan, Italy). When 80% confluence was
reached, the cells were incubated in starvation medium containing
0.25% bovine serum albumin (BSA). Subsequently, cells were treated
with an oleate/palmitate mixture (2:1 molar ratio; final
concentration, 0.75 mM) for 3 h (referred to as the FA treatment
group), with 5.5 mM fructose for 72 h (Fru group), or with
sequential combination of fructose for 72 h and FAs for 3 h (Fru/FA
group). Cells in the Fru/FA group were then treated for 24 h with
50 µM silybin (stock solution, 10 mM in dimethyl sulfoxide).
Silybin treatment was also performed on untreated FaO cells, which
served as the control group.
Cell viability and apoptosis
The sulforhodamine B (SRB) assay, relying on the
property of SRB to bind stoichiometrically to proteins, is used to
determine cell density. Briefly, 1.5×104 cells/well were
seeded in 96-well culture plates and treated. Next, the cells were
fixed and incubated with 0.5% SRB in 1% acetic acid for 1 h at
37°C. The dye bound to proteins was extracted with 10 mM Tris-HCl
(pH 10), and quantified in a Varian Cary-50 Bio spectrophotometer
(Agilent Technologies, Inc., Milan, Italy) (14). Caspase 3-like activity is a marker
of apoptosis as it initiates DNA fragmentation (15). Caspase activity was measured in
cell extracts containing 25 µg proteins determined by the
bicinchoninic acid method (16).
Following resuspension in 20 mM HEPES/NaOH (pH 7.5), 250 mM
sucrose, 10 mM KCl, 2 mM MgCl2, 1 mM EDTA, 2 mM
dithiothreitol (DTT) and 100 µM phenylmethylsulfonyl
fluoride, the cell extracts were incubated for 1 h at 37°C in 25 mM
HEPES (pH 7.5), 10% sucrose, 10 mM DTT, 0.1% CHAPS and 100
µM caspase substrate Ac-DEVD-pNA. The released pNA was
measured spectrophotometrically, and the results are expressed as
nmol of pNA released per µg of protein (17).
Lipid quantification and imaging
TGs were extracted from the different cell groups
and spectrophotometrically quantified as previously described
(18). Data are expressed as the
percent TG content relative to the control group. For LD
visualization, cells growing on coverslips were treated as
aforementioned, rinsed with PBS, fixed with 4% paraformaldehyde,
stained by Oil Red O (19) and
then examined with a Leica DMRB light microscope equipped with a
Leica CCD camera DFC420C (Leica, Wetzlar, Germany).
FAS activity
FAS activity in the different cell groups was
measured according to Goodridge (20). Briefly, cell lysate was obtained
by mixing cells with 0.1 M KPi (pH 7.0), 3 mM EDTA and 1 mM DTT via
a syringe needle. Then 20 µg of lysate were mixed to 0.1 M
KPi (pH 7.0), 0.025 mM acetyl coenzyme A (CoA), 0.2 mM NADPH, 3 mM
EDTA, 1 mM DTT, 25 mg/ml BSA and 0.1 mM malonyl-CoA. NADPH
disappearance was followed by spectrophotometric examination. FAS
activity (nmol NADPH/min/mg protein) was expressed as the
percentage relative to the control group.
ROS production and lipid
peroxidation
ROS production was quantified through the oxidation
of 2',7'-dichlorofluorescin diacetate (DCF-DA; Fluka, Germany) to
2',7'-dichlorofluores-cein (DCF), which was measured using a LS50B
fluorimeter (PerkinElmer, Inc., Waltham, MA, USA). Briefly,
suspended cells were loaded with 10 µM DCF-DA at 37°C in the
dark, centrifuged (800 × g for 10 min at 4°C) and resuspended in
PBS (21). The fluorescent
intensity was normalized to the protein content. Lipid peroxidation
was then evaluated through the thiobarbituric acid reactive
substance assay, as previously described (22). Cells were incubated for 45 min at
95°C with 2 vol thiobarbituric acid (TBA) solution, containing
0.375% TBA, 15% trichloroacetic acid and 0.25 N HCl. Subsequently,
1 vol N-butanol was added, and the absorbance of the organic phase
was measured. Values [pmol of malondialdehyde (MDA) per ml/mg
protein] were expressed as the percentage relative to the
controls.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc., Milan, Italy) and quantified
spectrophotometrically. Then, cDNA was synthesized by using
RevertAid H Minus transcriptase according to manufacturer's
instructions (Thermo Fisher Scientific, Inc.); qPCR was performed
in quadruplicate using 1X IQ™ SYBR® Green SuperMix and a
Chromo4™ system (Bio-Rad Laboratories, Inc., Milan, Italy)
(23). Primer pairs for the
assessed genes were designed ad hoc starting from the coding
sequences of Rattus norvegicus (http://www.ncbi.nlm.nih.gov/Genbank/GenbankSearch.html)
and listed in Table I. The
amplification conditions were as follows: 3 min at 95°C, followed
by 40 cycles consisting of 5 sec at 95°C, 30 sec of annealing
(temperatures listed in Table I),
and 40 sec of extension at 72°C. At the end, a melting curve
ranging between 55 and 95°C was measured. The relative quantity of
target mRNA was calculated by using the comparative Cq method and
was normalized for the expression of GAPDH gene (24).
 | Table IPrimer sequences table. |
Table I
Primer sequences table.
| Gene | Forward primer | Reverse primer | Annealing
temperature (°C) | Accession ID |
|---|
| ADRP CC |
GAGCGTGGTGACGAGGG |
GAGGTCACGGTCCTCACTCCC | 64 | AAH85861 |
| GAPDH |
GACCCCTTCATTGACCTCAAC |
CGCTCCTGGGAAGATGGTGATGGG | 60 | DQ403053 |
| IKBIP |
CAGAACAGTGAGCAGGCAAG |
ACGGCATTCTCTATGGTTGG | 60 | NM_001009430.2 |
| PPARα |
CCCCACTTGAAGCAGATGACC |
CCCTAAGTACTGGTAGTCCGC | 60 | NM_013196 |
| PPARγ |
CGGAGTCCTCCCAGCTGTTCGCC |
GGCTCATATCTGTCTCCGTCTTC | 60 | Y12882 |
| PPARδ |
AATGCCTACCTGAAAAACTTCAAC |
TGCCTGCCACAGCGTCTCAAT | 60 | AJ306400.1 |
| CPT1 |
CCGCTCATGGTCAACAGCA |
CAGCAGTATGGCGTGGATGG | 60 | NM_031559 |
| UCP2 |
CGTCGGACCTAGCCGTCTGCA |
CGGAGTCGGGAGGGTGCTTTG | 56 | BC062230 |
In order to measure miR-122 expression, the
High-Capacity cDNA RT kit and the miRNA-specific primers provided
with the TaqMan MicroRNA Assay kit (Thermo Fisher Scientific, Inc.)
were used. Amplification was performed using the StepOnePlus
Real-Time PCR system (Thermo Fisher Scientific, Inc.). Probe and
primers for miR-122-5p (4427975-002245) and miRNA U6
(4427975-001973) were provided by Thermo Fisher Scientific, Inc.
The relative expression of miR-122 and mRNAs was calculated by the
comparative Cq method using miRNA U6 and GAPDH as housekeeping
genes (24).
Oxygen consumption
Oxygen consumption was measured using the Seahorse
XFe96 Extracellular Flux analyzer (Agilent Technologies, Inc.,
Santa Clara, CA, USA) as previously described (25). Briefly, approximately
2×104 cells/well were seeded into 96-well plates. A
final concentration of 3 µM oligomycin, 1 µM FCCP,
and a mixture of 1 µM rotenone and 1 µM antimycin
were added sequentially to cells. The sensor cartridge and the
calibration plate were used for calibration. Three baseline rate
measurements of oxygen consumption rate (OCR) were made using a
3-min mixing and 3-min measure cycle. The compounds were injected
pneumatically by the Seahorse XFe96 analyzer into each well and
mixed, following which the OCR measurements were conducted using
the 3-min mixing and 3-min measure cycle (26).
Statistical analysis
Data are expressed as the mean ± standard deviation
of at least three independent biological experiments performed as
technical triplicates. Statistical analysis was performed using
analysis of variance with Tukey's post-test (GraphPad Software,
Inc., San Diego, CA, USA). Differences with P≤0.05 values were
considered as statistically significant.
Results
Excess FAs and fructose alter lipid
metabolism and cell func- tion
The extent of steatosis was assessed by Oil Red O
staining and TG quantification. The steatosis features were
assessed in terms of the accumulation of cytosolic LDs, whose
number and size markedly increased in all steatotic cells compared
with the control cells (Fig. 1A).
As shown in Fig. 1B,
quantification of intracellular TGs revealed that fructose and
fatty acids alone increased the TG content by 57% (P≤0.01) and 87%
(P≤0.001), respectively, compared with the control group, while
their combination (Fru/FA) led to a larger increase of 277% vs. the
control group (P≤0.001). As markers for LD accumulation and hepatic
cell dysfunction, the mRNA expression levels of ADRP and inhibitor
of nuclear factor-κB kinase subunit β-interacting protein (IKBIP),
respectively, were then assessed (Fig. 1C). ADRP expression was upregulated
by FAs alone (1.85-fold induction vs. control; P≤0.001), with even
greater upregulation induced by the Fru/FA combination (2.08-fold
induction vs. control; P≤0.001). However, IKBIP expression was
significantly upregulated only by the Fru/FA combination (1.61-fold
induction vs. control; P≤0.001). Furthermore, it was observed that
Fru alone, but not FAs, stimulated the FAS activity by 125% as
compared with the control group (P≤0.05), whereas the Fru/FA
combination markedly reduced this Fru-induced activity by 59% (vs.
Fru group; P≤0.05; Fig. 1D). On
the other hand, the expression of miR-122 showed a significant
increase only in Fru/FA-treated cells (1.52-fold induction vs.
control; P≤0.05; Fig. 1E).
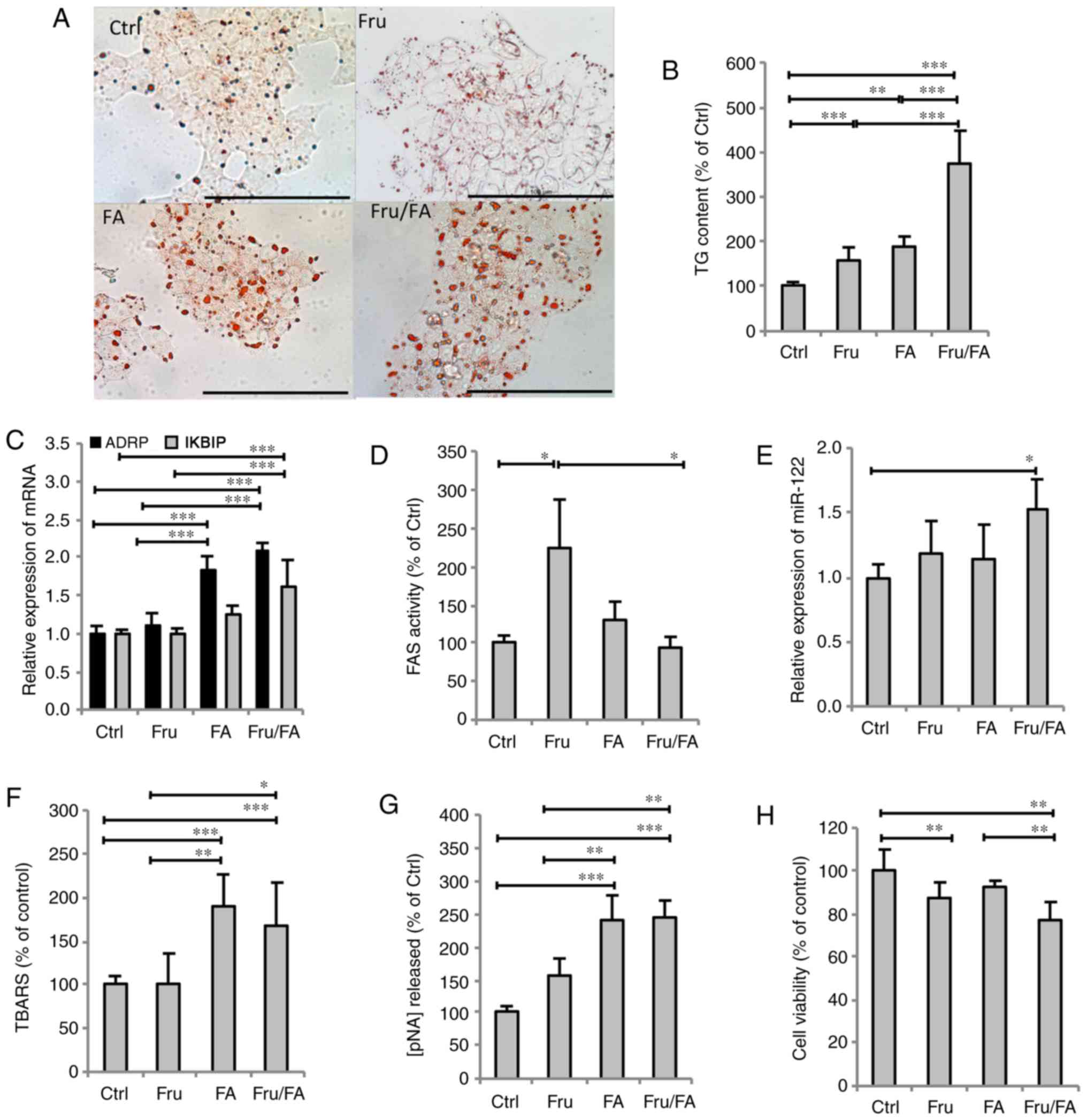 | Figure 1Steatogenic effects of Fru, FAs and
their combination (Fru/Fa) in FaO cells. (A) Lipid droplet
accumulation was visualized by Oil Red O staining (magnification,
×40; bar, 100 µm). (B) TG content was quantified
spectrophotometrically and normalized to total protein level. (C)
Expression levels of ADRP and IKBIP mRNA were evaluated by RT-qPCR
and expressed as the fold induction relative to the control. (D)
FAS activity (nmol NADPH/min/mg protein) was quantified
spectrophotometrically. (E) miR-122 expression was evaluated by
RT-qPCR using U6 as the internal control and is expressed as the
fold induction relative to the control. (F) Malondialdehyde level
(pmol MDA/ml/mg protein) was quantified by TBARS assay. (G)
Activity of caspase 3 (nmol of pNA released/µg protein) was
measured spectrophotometrically. (H) Metabolic activity was
measured by sulforhodamine B assay (percentage relative to the
controls). All values are expressed as the mean ± standard
deviation from at least three independent experiments.
*P≤0.05, **P≤0.01 and ***P≤0.001.
Fru, fructose; FA, fatty acid; Ctrl, control; TG, triglyceride;
ADRP, adipose differentiation-related protein; IKBIP, inhibitor of
nuclear factor-κB kinase subunit β-interacting protein; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; FAS,
fatty acid synthase; TBARS, thiobarbituric acid reactive
substance. |
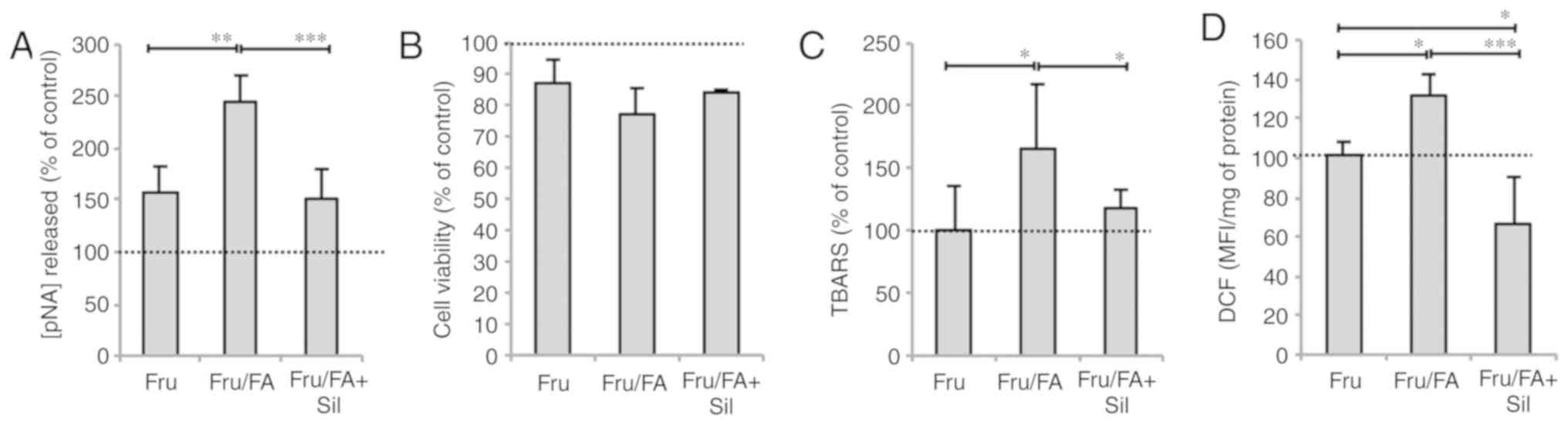
Lipid peroxidation was also assessed as a marker of
oxidative stress. The MDA level increased by 89 and 67% in response
to FAs alone and Fru/FA combination, respectively, as compared with
the control group (P≤0.001; Fig.
1F). This oxidative imbalance was paralleled by changes in
caspase 3-like activity, which increased in cells exposed to FAs
alone or Fru/FA combination (+142 and +145% vs. control,
respectively; P≤0.001; Fig. 1G).
By contrast, cell viability did not change in FA cells, but it was
significantly reduced by 13% in cells exposed to Fru alone as
compared with the control cells (P≤0.01), and further reduced by
23% in the Fru/FA combination group vs. the control (P≤0.01;
Fig. 1H).
Silybin counteracts the steatogenic
effects of fructose and FAs
Exposure of Fru/FA cells to 50 µM silybin for
24 h markedly reduced the steatosis grade by 35% of TG content
(P≤0.01), and the IKBIP upregulation by 32% (P≤0.001) compared with
Fru/FA cells (Fig. 2A and B).
However, silybin did not change the ADRP mRNA level (Fig. 2C). Moreover, silybin treatment led
to changes in the lipogenic transcription factor PPARγ, whose
expression was upregulated in Fru/FA cells (1.55-fold induction vs.
control; P≤0.01) and reduced by 67% upon silybin treatment with
respect to Fru/FA (P≤0.001); by contrast, PPARα and PPARδ levels
were not significantly altered by silybin (Fig. 2D). Exposure to silybin further
increased miR-122 expression (1.84-fold induction vs. control;
P≤0.001) in Fru/FA cells, in which this miRNA was already
overexpressed (Fig. 2E).
Treatment of control cells with silybin had no effects on the
expression of these genes (data not shown).
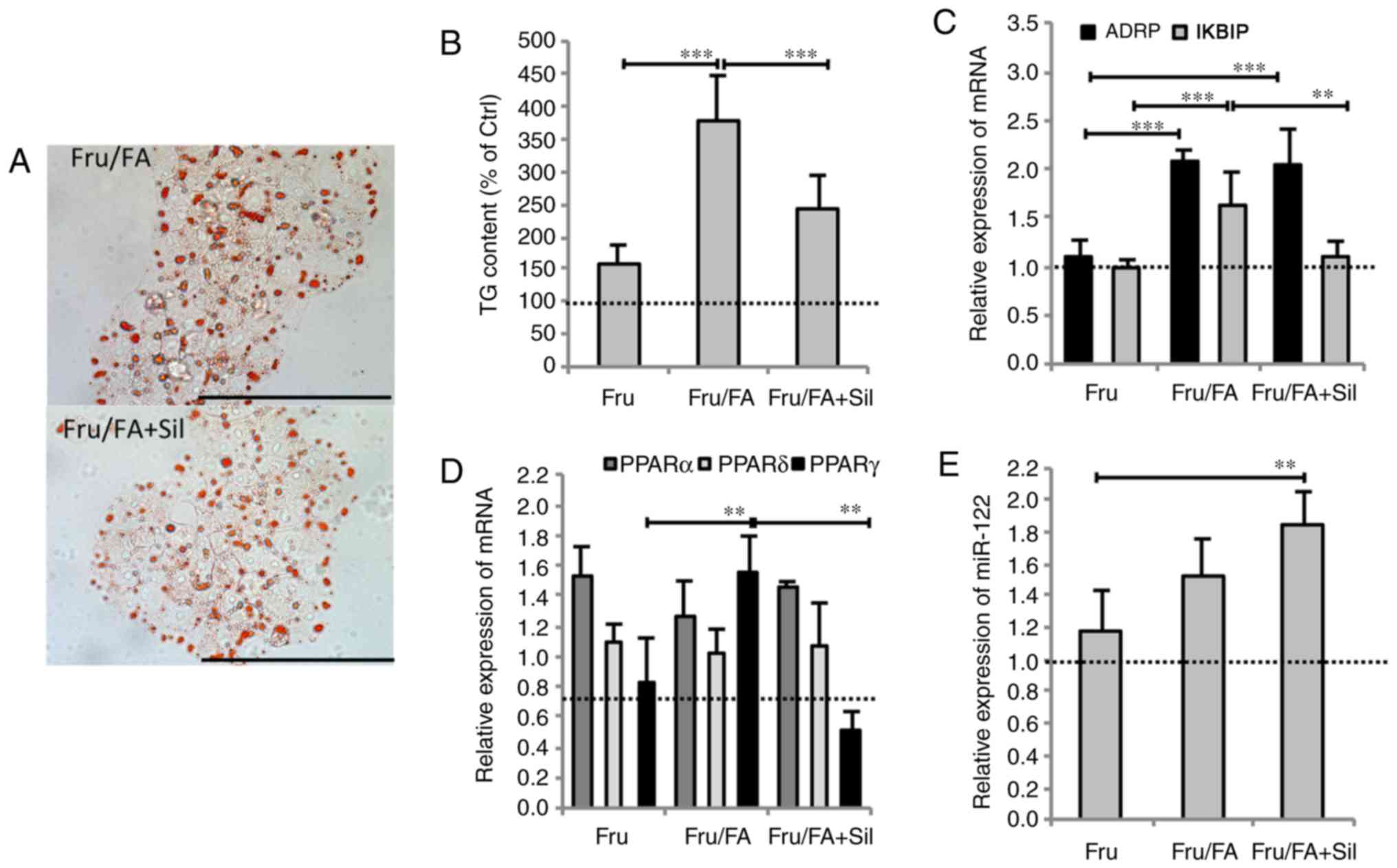 | Figure 2Silybin counteracts lipid metabolism
dysregulation. Cells incubated with Fru/FA were then treated for 24
h with 50 µM silybin. (A) Microphotographs of Oil Red
O-stained cells at a magnification of ×40 (bar, 100 µm), and
(B) histogram of TG content. (C) mRNA expression levels of ADRP and
IKBIP, (D) mRNA expression levels of PPARα, γ and δ, and (E)
miR-122 expression. All values are expressed as the mean ± standard
deviation from at least three independent experiments.
**P≤0.01 and ***P≤0.001. Fru, fructose; FA,
fatty acid; TG, triglyceride; ADRP, adipose differentiation-related
protein; IKBIP, inhibitor of nuclear factor-κB kinase subunit
β-interacting protein. |
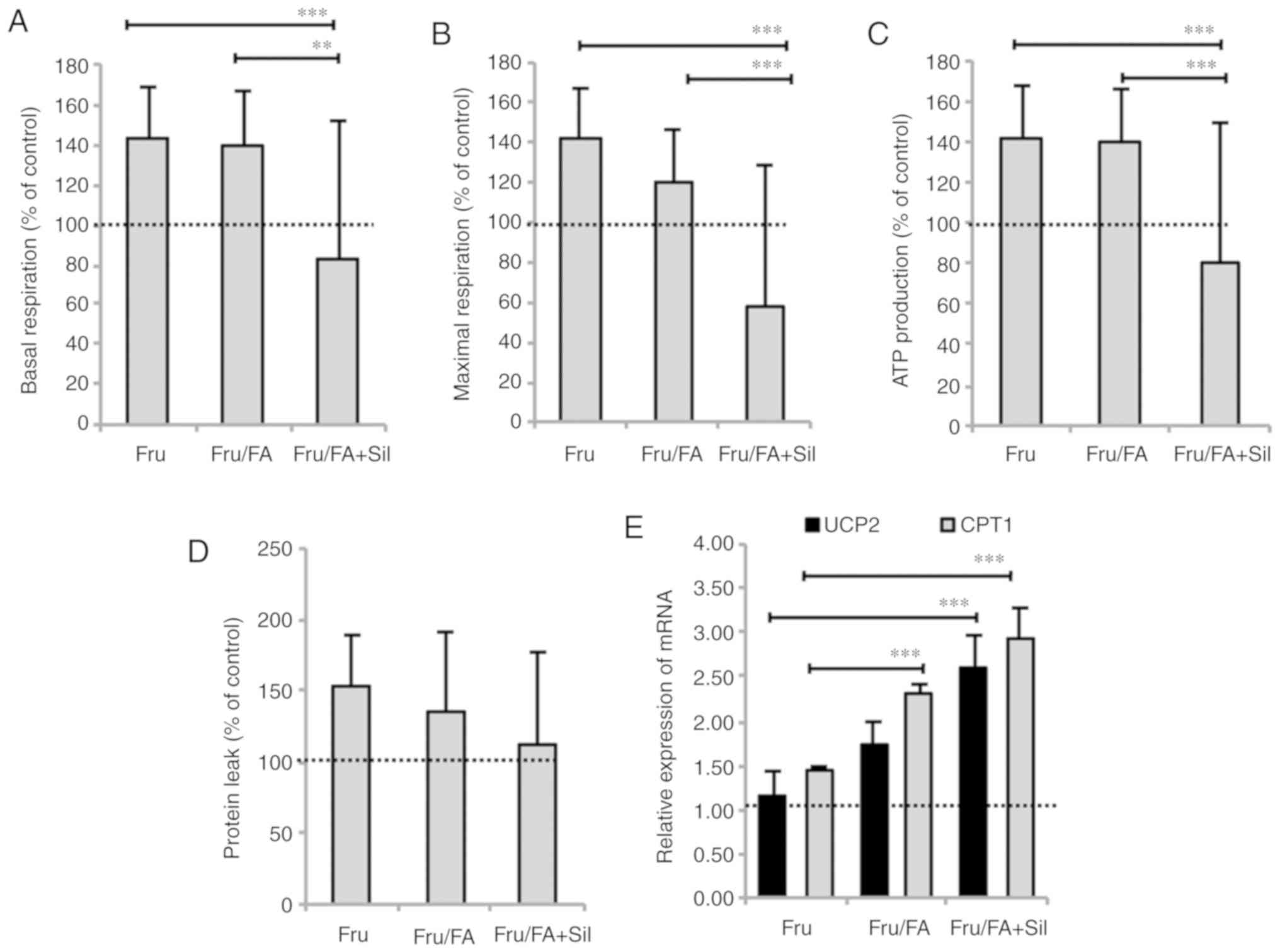
Silybin counteracts apoptosis and
mitochondrial dysfunction
Silybin treatment did not rescue the reduction in
cell viability caused by Fru/FA, but it had anti-apoptotic effects
as indicated by the decrease in the caspase 3-like activity by 38%
compared with the Fru/FA cells (P≤0.001; Fig. 3A and B). Silybin was also able to
counteract the lipid peroxidation (reduction by 30% as compared
with Fru/FA; P≤0.05) and the ROS levels (reduction by 50% as
compared with Fru/FA; P≤0.001; Fig.
3C and D) associated with excess fat in Fru/FA cells.
Steatotic hepatocytes typically stimulate
respiration and ATP production in an attempt to counteract the
excess TGs (21). Silybin reduced
basal respiration by 56% (P≤0.01), maximal respiration by 62%
(P≤0.001) and ATP production by 60% (P≤0.001) in Fru/FA cells,
without significant effects on proton leak (Fig. 4A-D). Moreover, the expression of
the mitochondrial proteins carnitine palmitoyltransferase 1 (CPT1)
and uncoupling protein 2 (UCP2), which is the main regulatory step
of mitochondrial FA oxidation, was increased in Fru/FA cells (2.29-
and 1.74-fold induction, respectively, vs. control; P≤0.001 and
P≤0.01). Further upregulation to the CPT1 and UCP2 levels by 68 and
50%, respectively, was observed upon exposure to silybin (P≤0.001
for both; Fig. 4E). Treatment of
control cells with silybin had no effects on apoptosis, lipid
peroxidation and mitochondrial respiration (data not shown).
Discussion
The present study provided insights into the
molecular mechanisms through which excess fructose impairs the
lipogenic pathways in hepatocytes. In the past, fructose was
considered as a beneficial dietary component since it does not
stimulate insulin secretion; however, the harmful effects of
fructose have recently gained mainstream attention. Studies have
reported that high fructose intake stimulates de novo
lipogenesis (27), and mice fed a
diet of fats and high-fructose corn syrup developed equally severe
NAFLD (28). The findings of the
present study revealed that exposure of FaO cells to a
fructose/fatty acid combination led to larger TG synthesis and
accumulation as compared with the single agents. In addition, it
was observed that the more severe steatosis was associated with
worsening of cell dysfunction parameters, including cell viability,
oxidative stress and mitochondrial respiration.
In the cell model of the current study, the
sequential exposure of hepatocytes to high fructose and fatty acids
mimics the NAFLD progression in vitro. Excess fructose alone
stimulates FAS activity resulting in TG overproduction, and Fru/FA
combination led to more severe cell dysfunction compared with the
single treatments, as confirmed by the following observations: i)
Enhanced steatosis; ii) maximal upregulation of ADRP and IKBIP
expression levels; and iii) enhanced lipid peroxidation, ROS
production and caspase 3-like activity, which are indexes of
oxidative stress and apoptosis. The extensive damaging effect of
Fru/FA combination was also evident when looking at the expression
of miR-122, which is reportedly involved in the onset/progression
of NASH (10).
Mitochondria are the main site for FA degradation,
and steatotic hepatocytes typically enhance mitochondrial
β-oxidation to limit excess fat accumulation (8). Accordingly, the mitochondrial
proteins CPT1 and UCP2 were found to be overexpressed in cells
treated with Fru/FA combination in the present study. Furthermore,
basal and maximal mitochondrial respiration, as well as ATP
production, were stimulated by Fru/FA combination in an attempt to
compensate for the increased FA oxidation. Of note, the increase in
oxidative stress due to the overactive β-oxidation may trigger
proinflammatory pathways sustaining NAFLD progression. The results
of the present in vitro study are in agreement with previous
findings described in patients and animals (29,30). While a 'high-fat' diet results in
obesity, insulin resistance, and hepatic steatosis with minimal
inflammation and no fibrosis, the 'Western' diet that is rich in
fructose leads to steatosis associated with hepatic fibrosis,
inflammation, oxidative stress and apoptosis (31).
The nutraceutical silybin is known as a general
hepatoprotective, anti-steatotic agent (21,25), which has provided promising
results in animal and cellular models of NAFLD (25,30), and in a number of clinical studies
(32-34). Providing further insight into the
effect of this agent, the present study demonstrated that silybin
counteracted the metabolic dysfunctions caused by Fru/FA
combination acting directly on hepatocytes. First, silybin reduced
the large TG accumulation resulting from Fru/FA combination by
down-regulating the expression of PPARγ, the main transcription
factor for lipogenic genes. The hepatoprotective action of silybin
was able to counteract in vitro the Fru/FA-dependent
increase in terms of the following: i) IKBIP expression; ii)
intracellular ROS production and lipid peroxidation; and iii)
apoptosis rate. However, silybin was unable to alleviate the
reduction in cell viability associated with exposure to Fru/FA
combination. The action of silybin appears to be mainly dependent
on its effects on mitochondria, with different mechanisms depending
on the NAFLD grade (25). In our
model mimicking a rather severe NAFLD, silybin exerts beneficial
activity by inhibiting mitochondrial respiration, which is
stimulated in steatosis progression as a consequence of an
increased oxidative metabolism due to stimulation of anabolic
pathways (35).
In conclusion, the cell model used in the present
study, consisting of lipid-loaded hepatocytes mimicking the
progression of NAFLD in vitro, offered new insights into the
harmful steatogenic effects of fructose on liver cells and supports
the hepatoprotective activity of silybin. Further studies can
translate these results into long-term beneficial effects in the
hope that the onset, progression and deterioration of NAFLD/NASH
will be prevented or delayed in patients by using nutraceutical
approaches.
Acknowledgments
The authors would like to thank Dr Moahamed Kalil,
Dr Rita Fabbri and Dr Silvia Carestiato (DISTAV, Genova, Italy) for
their experimental assistance.
Funding
The present study was supported by grants from the
Compagnia di San Paolo (Torino, Italy) and the University of
Genova, and funds from the Foundation for Science and Technology,
Portugal (grant nos. PTDC/DTP-FTO/2433/2014,
POCI-01-0145-FEDER-007440 and POCI-01-0145-FEDER-016659).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors significantly contributed to this study.
LV conceived and designed the study, analyzed and elaborated the
data, and wrote the manuscript. EG carried out the qPCR analysis
for miR-122 and spectrophotometric assay for FAS activity
determination, and participated in writing the manuscript. FB
performed cultures and treatments of FaO cells, fluorimetric and
spectrophotometric assays, and qPCR measurements. GV carried out
apoptosis determination and O2 consumption evaluation.
PJO supplied the Seahorse XFe96 Extracellular Flux Analyzer and
supervised the experiments for mitochondria analyses. VAS
participated in O2 consumption analyses. AV participated
in cell cultures and treatments, and critically revised the
manuscript. PP participated in conceiving and designing the study,
and critically revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted
in the absence of any commercial or financial relationships that
could be construed as a potential conflict of interest.
References
|
1
|
Vecchié A, Dallegri F, Carbone F,
Bonaventura A, Liberale L, Portincasa P, Frühbeck G and Montecucco
F: Obesity phenotypes and their paradoxical association with
cardiovascular diseases. Eur J Intern Med. 48:6–17. 2018.
View Article : Google Scholar
|
|
2
|
Mendez-Sanchez N, Cruz-Ramon VC,
Ramirez-Perez OL, Hwang JP, Barranco-Fragoso B and Cordova-Gallardo
J: New aspects of lipotoxicity in nonalcoholic steatohepatitis. Int
J Mol Sci. 19:2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Listenberger LL, Ostermeyer-Fay AG,
Goldberg EB, Brown WJ and Brown DA: Adipocyte
differentiation-related protein reduces the lipid droplet
association of adipose triglyceride lipase and slows
triacylglycerol turnover. J Lipid Res. 48:2751–2761. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feldstein AE, Werneburg NW, Canbay A,
Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ and Gores GJ: Free
fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha
expression via a lysosomal pathway. Hepatology. 40:185–194. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neuschwander-Tetri BA: Hepatic
lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis:
The central role of nontriglyceride fatty acid metabolites.
Hepatology. 52:774–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ter Horst KW and Serlie MJ: Fructose
consumption, lipogenesis, and non-alcoholic fatty liver disease.
Nutrients. 9:pii: E981. 2017.PubMed/NCBI
|
|
7
|
Gnocchi D, Massimi M, Alisi A, Incerpi S
and Bruscalupi G: Effect of fructose and 3,5-diiodothyronine
(3,5-T(2)) on lipid accumulation and insulin signalling in
non-alcoholic fatty liver disease (NAFLD)-like rat primary
hepatocytes. Horm Metab Res. 46:333–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grasselli E, Canesi L, Portincasa P, Voci
A, Vergani L and Demori I: Models of non-alcoholic fatty liver
disease and potential translational value: The effects of
3,5-L-diiodothyronine. Ann Hepatol. 16:707–719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moore KJ, Rayner KJ, Suárez Y and
Fernández-Hernando C: The role of microRNAs in cholesterol efflux
and hepatic lipid metabolism. Annu Rev Nutr. 31:49–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dongiovanni P, Meroni M, Longo M, Fargion
S and Fracanzani AL: miRNA signature in NAFLD: A turning point for
a non-invasive diagnosis. Int J Mol Sci. 19:2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baselga-Escudero L, Souza-Mello V,
Pascual-Serrano A, Rachid T, Voci A, Demori I and Grasselli E:
Beneficial effects of the Mediterranean spices and aromas on
non-alcoholic fatty liver disease. Trends Food Sci Technol.
61:141–159. 2017. View Article : Google Scholar
|
|
12
|
Loguercio C, Tiso A, Cotticelli G, Blanco
Cdel V, Arpino G, Laringe M, Napoli L, Piccinocchi G, Bonfrate L,
Grattagliano I, et al: Management of chronic liver disease by
general practitioners in southern Italy: Unmet educational needs.
Dig Liver Dis. 43:736–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loguercio C and Festi D: Silybin and the
liver: From basic research to clinical practice. World J
Gastroenterol. 17:2288–2301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar
|
|
15
|
Wolf BB, Schuler M, Echeverri F and Green
DR: Caspase-3 is the primary activator of apoptotic DNA
fragmentation via DNA fragmentation factor-45/inhibitor of
caspase-activated DNase inactivation. J Biol Chem. 274:30651–30656.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiechelman KJ, Braun RD and Fitzpatrick
JD: Investigation of the bicinchoninic acid protein assay:
Identification of the groups responsible for color formation. Anal
Biochem. 175:231–237. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moreira AC, Branco AF, Sampaio SF,
Cunha-Oliveira T, Martins TR, Holy J, Oliveira PJ and Sardão VA:
Mitochondrial apoptosis-inducing factor is involved in
doxorubicin-induced toxicity on H9c2 cardiomyoblasts. Biochim
Biophys Acta. 1842:2468–2478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grasselli E, Voci A, Canesi L, Goglia F,
Ravera S, Panfoli I, Gallo G and Vergani L: Non-receptor-mediated
actions are responsible for the lipid-lowering effects of
iodothyronines in FaO rat hepatoma cells. J Endocrinol. 210:59–69.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grasselli E, Voci A, Pesce C, Canesi L,
Fugassa E, Gallo G and Vergani L: PAT protein mRNA expression in
primary rat hepatocytes: Effects of exposure to fatty acids. Int J
Mol Med. 25:505–512. 2010.PubMed/NCBI
|
|
20
|
Goodridge AG: Regulation of the activity
of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and
citrate. J Biol Chem. 247:6946–6952. 1972.PubMed/NCBI
|
|
21
|
Vecchione G, Grasselli E, Voci A, Baldini
F, Grattagliano I, Wang DQ, Portincasa P and Vergani L: Silybin
counteracts lipid excess and oxidative stress in cultured steatotic
hepatic cells. World J Gastroenterol. 22:6016–6026. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iguchi H, Kojo S and Ikeda M: Lipid
peroxidation and disintegration of the cell membrane structure in
cultures of rat lung fibroblasts treated with asbestos. J Appl
Toxicol. 13:269–275. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Vecchione G, Grasselli E, Cioffi F,
Baldini F, Oliveira PJ, Sardão VA, Cortese K, Lanni A, Voci A,
Portincasa P and Vergani L: The nutraceutic silybin counteracts
excess lipid accumulation and ongoing oxidative stress in an in
vitro model of non-alcoholic fatty liver disease progression. Front
Nutr. 4:422017. View Article : Google Scholar :
|
|
26
|
Deus CM, Zehowski C, Nordgren K, Wallace
KB, Skildum A and Oliveira PJ: Stimulating basal mitochondrial
respiration decreases doxorubicin apoptotic signaling in H9c2
cardiomyo-blasts. Toxicology. 334:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hudgins LC, Parker TS, Levine DM and
Hellerstein MK: A dual sugar challenge test for lipogenic
sensitivity to dietary fructose. J Clin Endocrinol Metab.
96:861–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tetri LH, Basaranoglu M, Brunt EM, Yerian
LM and Neuschwander-Tetri BA: Severe NAFLD with hepatic
necroinflammatory changes in mice fed trans fats and a
high-fructose corn syrup equivalent. Am J Physiol Gastrointest
Liver Physiol. 295:G987–G995. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reina M and Martinez A: Silybin and
2,3-dehydrosilybin flavonolignans as free radical scavengers. J
Phys Chem B. 119:11597–11606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rosso N, Marin V, Giordani A, Persiani S,
Sala F, Cavicchioli L, Rovati LC and Tiribelli C: The pros and the
cons for the use of silybin-rich oral formulations in treatment of
liver damage (NAFLD in particular). Curr Med Chem. 22:2954–2971.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ameer F, Scandiuzzi L, Hasnain S,
Kalbacher H and Zaidi N: De novo lipogenesis in health and disease.
Metabolism. 63:895–902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abenavoli L, Greco M, Nazionale I, Peta V,
Milic N, Accattato F, Foti D, Gulletta E and Luzza F: Effects of
Mediterranean diet supplemented with silybin-vitamin E-phospholipid
complex in overweight patients with non-alcoholic fatty liver
disease. Expert Rev Gastroenterol Hepatol. 9:519–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Federico A, Conti V, Russomanno G, Dallio
M, Masarone M, Stiuso P, Tuccillo C, Caraglia M, Manzo V, Persico
M, et al: A long-term treatment with silybin in patients with
non-alcoholic steatohepatitis stimulates catalase activity in human
endothelial cells. In Vivo. 31:609–618. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wah Kheong C, Nik Mustapha NR and Mahadeva
S: A randomized trial of silymarin for the treatment of
nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol.
15:1940–1949.e8. 2017. View Article : Google Scholar
|
|
35
|
Satapati S, Kucejova B, Duarte JA,
Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu
X, et al: Mitochondrial metabolism mediates oxidative stress and
inflammation in fatty liver. J Clin Invest. 125:4447–4462. 2015.
View Article : Google Scholar : PubMed/NCBI
|