Introduction
Evidence (1,2)
shows that mitochondria are the main target following
ischemia/reperfusion (I/R), and the mitochondrial ATP-sensitive
potassium channels (mitoKATP) are located in the mitochondrial
inner membrane of important organs, including the heart, brain and
lungs, serving an important role in the lung following I/R injury.
The mitochondria, which generate reactive oxygen species as a key
medium (3), are the triggering
factor and end effector of I/R injury. I/R lung injury is a typical
manifestation of one-lung ventilation (OLV).
OLV has been widely used in thoracic surgery
anesthesia (4). It can prevent
secretions and blood from the lung undergoing surgery from flowing
into the contralateral lung, ensuring the patency of airways and
collapse of the lung undergoing surgery without cross-infection and
proliferation of lesions. With the extensive application of
thoracoscopic surgical techniques, OLV technique, which facilitates
the visual development of thoracic surgery and makes surgery easier
to perform, has become essential. However, the inhibition of
hypoxemic pulmonary vasoconstriction (HPV), hypoxemia, generation
of oxygen free radicals, ischemia/reperfusion (I/R) injury
(5) and inflammatory reactions
(6) may occur during OLV, and are
mainly found in the lung being operated on, leading to lung injury,
which is difficult to manage. Current solutions are continuous
positive airway pressure and low-flow oxygen insufflation (7,8),
which can reduce the accumulation of inflammatory cytokines and
moisture, improve oxygenation and reduce lung injury. There is a
reasonable selection of anesthetic or vasoactive drugs to protect
the lungs, including dexmedetomidine, isoflurane (9,10),
morphine and phenylephrine (11).
Protective ventilation strategies, including low tidal volume and
high frequency ventilation, represent other ways to reduce lung
injury (7,8).
To date, the protective effect of these methods on
the lungs is far from ideal. Therefore, the investigation of novel
types of protective drug has become important. According to the
basic principles of cell and tissue protection, concern lies on the
focal point of oxidative stress, the mitochondria, and it is
reasonable to focus on its KATP agonists.
Nicorandil, a Food and Drug Administration-approved
mitoKATP-specific opener, activates the opening of the mito-KATP to
protect cells. In the context of cardiology, several reports
(12,13) have highlighted the key function in
protecting the heart (14).
However, only one study (15) has
focused on the reduction of lung injury caused by cyclophosphamide.
In the present study, a mature rabbit OLV model was used to
investigate the protective effect of nicorandil on lung injury and
to examine its mechanisms.
Materials and methods
Animals and groups
A total of 36 healthy three month-old male Japanese
big-ear white rabbits weighing 2.6-3.2 kg were provided by the
Laboratory Animal Center of Nantong University (Nantong, China).
The animals were raised under controlled environmental conditions
of a constant temperature (25±2°C) and a 12/12 h light/dark cycle.
Food for the rabbits was provided by Pizhou Dongfang Breeding Co.,
Ltd. (Xuzhou, China), which was available ad libitum with
water. The physiological status of male animals without fixed
physiological cycle is more stable than that of female animals, and
the difference between individuals is smaller. (16) The hormone levels in the body are
stable and enzyme activity is relatively stable. The investigation
was approved by the Ethics Committee for Animal Experimentation of
Nantong University. The rabbits were randomly divided into six
groups (n=6). Group 1 served as the sham group. Group 2 served as
the control group. Groups 3 and 4 served as the high-dose and
low-dose nicorandil groups, respectively. Group 5 served as the
high-dose nicorandil + glibenclamide group (injected for 1 h at
22°C; 99%; cat. no. G0639; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). LY294002 (injected for 1 h at 22°C; 98%; cat. no. L9908;
Sigma-Aldrich; Merck KGaA) was applied with a high dose of
nicorandil for examining its signaling pathway in group 6 rabbits
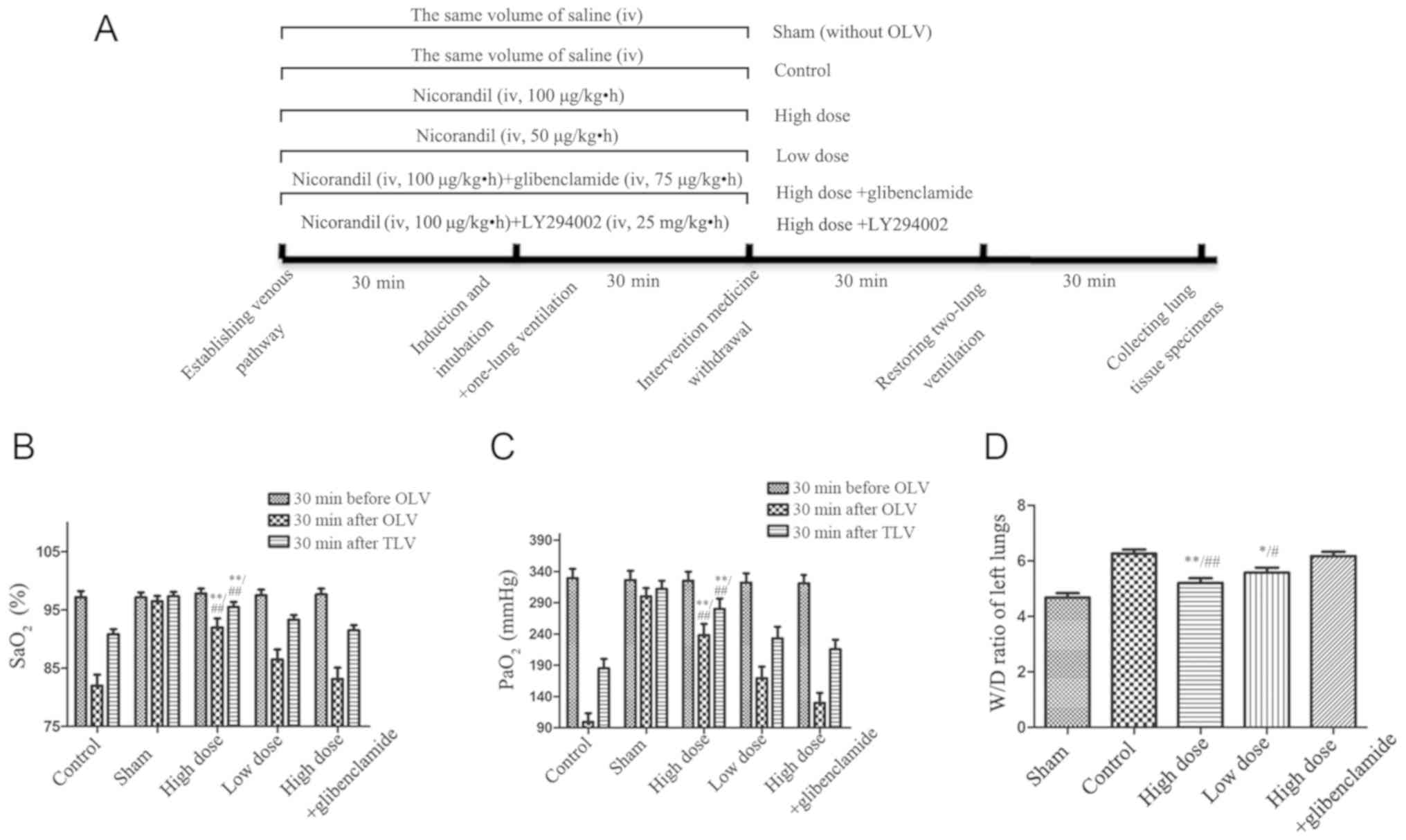
(Fig. 1).
Rabbit OLV model establishment
A rabbit OLV model was established, as previously
described (17,18). Nicorandil is a mature drug used in
cardiovascular medicine, with an advised usual dose of 2 mg/h in
the specifications (19). The
conventional dose for rabbits was then calculated as a high dose
(100 µg/kg·h), half of which was defined as a low dose,
according to the rabbit body and human body surface area conversion
formula. The rabbits were induced with 2% odium pentobarbital (20
mg/kg), (20) and then maintained
under 10% propofol (5 mg/kg·h), 2% sodium pentobarbital (4 mg/kg)
each for 30 min, and vecuronium bromide (0.25 mg/kg·h) (21,22). Following induction, the right
common carotid artery was cannulated to monitor arterial blood
pressure and arterial blood samples were collected. Tracheotomy and
a self-made double-lumen endotracheal tube were used for mechanical
ventilation. The parameters were as follows: FiO2, 1.0;
RR, 40 bpm; VT, 10 ml/kg. Auscultation was used to adjust the
position of the endotracheal tube. The animal was adjusted into a
right lateral position, and the left lumen was closed to establish
the rabbit OLV model; thoracotomy was performed to directly observe
whether the left lung had collapsed. At 1 h post-OLV, both lungs
were ventilated for 30 min. The rabbits were under anesthesia with
propofol and sodium pentobarbital. At the end of each experiment,
the animals were sacrificed by injection of a lethal dose of
pentobarbital sodium (23). In
the absence of mechanical ventilation, 100 mg/kg sodium
pentobarbital was injected through the ear vein. No respiratory
motion, no heartbeat and no detectable SPO2
were observed in the rabbit, which confirmed the rabbit had died.
The operated lung was rapidly excised and washed with ice-cold
saline. One part was dried and weighed, the other was preserved at
−80°C. Changes in SaO2 and PaO2 were observed
in the perioperative period. Following surgery, the wet/dry ratio
was measured. Changes of the micro-structure of the lung tissues
were observed by hematoxylin and eosin (H&E) staining and
transmission electron microscopy. The concentrations of
malondialdehyde (MDA; cat. no. S0131; Beyotime Institute of
Biotechnology, Haimen, China) and tumor necrosis factor-α (TNF-α;
cat. no. PT512; Beyotime Institute of Biotechnology) and the
activity of superoxide dismutase (SOD; cat. no. S0101; Beyotime
Institute of Biotechnology) were measured by ELISA. Terminal
deoxynucleotidyl transferase transfer-mediated dUTP nick
end-labeling (TUNEL) was used to observe the apoptosis of lung
tissues. The expression of phosphatidylinositol-3-kinase (PI3K),
protein kinase B (Akt), phosphorylated (p-)Akt, nuclear factor-κB
(NF-κB) and hypoxia-inducible factor 1α (HIF-1α) were detected by
western blotting. Reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) analysis was used to detect the
transcription of HIF-1α mRNA in lung tissues. The expression of
downstream proteins Bax, Bcl2 and caspase-3 were detected by
western blotting.
Wet/dry (W/D) weight ratio
Following collection, the operated lung was weighed
for its wet weight, and then placed in an oven (80°C, 48 h), and
heated to a constant weight. In the following day, the dry weight
was determined and the W/D weight ratio of the lung was
calculated.
Measurement of the lung injury score
(H&E staining)
For histopathological analysis, the lung tissues
were fixed in 4% formalin at 4°C for 24 h and embedded in paraffin.
Subsequently, sections (5-µm thick) were prepared, stained
with H&E at 22°C for 30 sec for pathological observation, and
then examined using a light microscope (magnification, ×40). The
lung injury score of each slide was assessed by two independent
pathologists in a blinded manner. The score represents the average
of the pathologists scores. Each section was scored according to
the following four criteria (24): i) Alveolar septal congestion; ii)
alveolar hemorrhage; iii) intra-alveolar cell infiltrates; and iv)
intra-alveolar fibrin deposition.
Transmission electron microscopy
Lung tissue sections (1-mm3) were fixed
in 2.5% glutaraldehyde at 4°C for 24 h, followed by 1% osmium
tetroxide and embedded in epoxy resin 618. Subsequently, these
samples were cut into ultrathin sections (0.5 µm) and then
examined using a Hitachi HT-7700 transmission electron microscope
(magnification, ×3,000; HT-7700; Hitachi, Tokyo, Japan).
TUNEL assay
When a cell undergoes apoptosis, it activates a
number of endonucleases that can cut off nucleosomal DNA. When
genomic DNA is disrupted, the exposed 3'-OH can be labeled with
fluorescein-dUTP by terminal deoxynucleotidyl transferase (TdT) so
that it can be detected by fluorescence microscopy. In this way,
TUNEL (cat. no. A112; Vazyme, Piscataway, NJ, USA) was used for the
detection of apop-tosis. The specific procedures for the paraffin
sections were as follows: Dewaxing, in which moisture was increased
with ethyl alcohol; protein dissolved with proteinase K; immersing
DNA in equilibration buffer (DNA incubated with TDT buffer
containing FITC-12-DUTP labeling mix) for 1 h at 37°C; nuclear
staining with Hoechst (20 mg/ml) for 10 min at 22°C; and
observation of slides after mounting in neutral gum with a
fluorescent microscope in at least three different fields.
Western blot analysis
The lung tissues were homogenized with lysis buffer
(cat. no. KPG250; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China), which contained protease inhibitor, phosphatase inhibitor
and phosphorylase inhibitor. The homogenate was incubated at 4°C
for 30 min and then centrifuged at 14,300 × g for 15 min at 4°C.
The supernatant was harvested and stored at -80°C for further
analysis. Total protein concentration in the supernatant was
measured using a BCA protein assay. Subsequently, 300 µg
protein in each group was separated on 5/10% SDS-PAGE gels by
electrophoresis and then transferred onto a polyvinylidene
difluoride membrane (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% fat-free milk for 2 h at 22°C, the membranes were
incubated with primary antibodies against NF-κB (1:500, cat. no.
MAB3026; EMD Millipore) or β-actin (1:1,000; cat. no. 3700, Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Following washing three times with TBST, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. AP124P; EMD Millipore) for 2 h at room
temperature. The protein bands were visualized using an ECL system
(cat. no. K-12045; Advansta, San Jose, CA, USA). The PI3K (1:1,000;
cat. no. AF1966; Beyotime Institute of Biotechnology), Akt
(1:1,000; cat. no. AF0045; Beyotime Institute of Biotechnology),
p-Akt (1:1,000; cat. no. AF1546; Beyotime Institute of
Biotechnology), HIF-1α (1:500; cat. no. NB100-105; Novus
International, Inc., St Charles, MO, USA), Bax (1:1,000; cat. no.
AB026; Beyotime Institute of Biotechnology), Bcl2 (1:1,000; cat.
no. AF0060; Beyotime Institute of Biotechnology) and caspase-3
(1:1,000; cat. no. 9665; Santa Cruz Biotechnology, Inc.) proteins
were assayed using the same method, however, bovine serum albumin
(5 mg/ml; cat. no. P007; Beyotime Institute of Biotechnology) was
used for blocking and as a medium to incubate the primary antibody
for the detection of p-Akt.
RT-qPCR analysis
Total RNA from the lung tissue specimens was
extracted with TRIzol® reagent (Sigma-Aldrich; Merck
KGaA). The cDNA was amplified by PTC-200 according to the RT-PCR
kit instructions. PCR was performed on the basis of cDNA. The
primer sequences were as follows: HIF-1α (NM_001082782), upstream
5'-CAACATCACCACCATACA-3' and downstream 5'-TCAGGAGCAGTAGTTCTTT-3'.
The length of the amplified product was 148 bp. The amplification
reaction conditions were as follows: 94°C pre-denaturation for 3
min, 94°C denaturation for 30 sec, 56°C annealing for 30 sec, 72°C
extension for 45 sec, 32 cycles. The primer sequences for GAPDH
(NM_001082253) were as follows: Upstream 5'-AGAGCACCAGAGGAGGACG-3'
and downstream 5'-CTGGGATGGAAACTGTGAAGAG-3', with a product 105 bp.
The amplification reaction conditions were as follows: 94°C
pre-denaturation for 3 min, 94°C denaturation for 30 sec, 57°C
annealing for 30 sec, 72°C extension for 45 sec, 32 cycles. SYBR
Green PCR Master mix was used to generate fluorescence. The
fluorescence intensity was measured using a real-time fluorescence
qPCR instrument for the results using the 2−ΔΔCq method
(25), which were on the basis of
the sham group.
Statistical analysis
All data are presented as the mean ± standard error
of the mean and each experiment was repeated at least three times.
The results were analyzed by one-way analysis of variance with
Bonferroni's correction for the post-hoc comparisons. Statistical
analysis was performed using GraphPad Prism software (version 5;
GraphPad Software, Inc., La Jolla, CA, USA). For all of the
statistical tests, P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of nicorandil on lung oxygenation
and water accumulation in rabbits with collapse-induced lung
injury
By measuring SaO2 and PaO2,
the respiratory status was evaluated. It was observed that the
pressure in the airway was significantly upregulated in OLV. The
SaO2 and PaO2 in the high-dose group were
significantly higher than those in the control group in the process
of OLV (P= 0.0012 and P= 0.0001, respectively). In accordance with
the previous findings, the W/D weight ratio of the collapsed lung
in the high-dose group was significantly lower than that in the
control group (P=0.0008) (Fig.
1). These data validate the hypothesis that nicorandil can
improve lung oxygenation and decrease edema of the lung.
Effect of nicorandil on collapse-induced
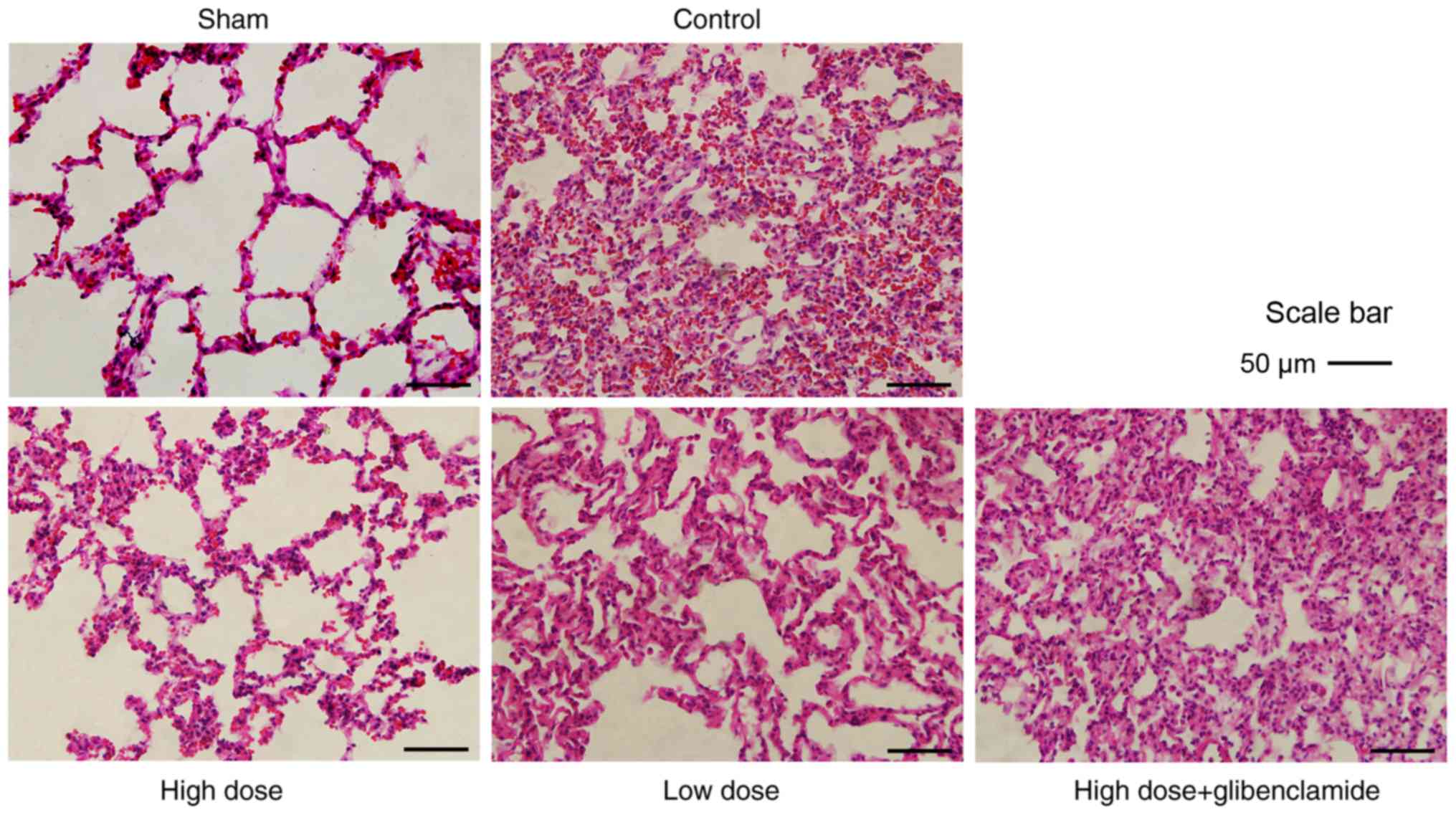
lung injury by improving its microstructure
Based on the H&E staining images, there was
severe damage to the alveolar microstruc-ture, in which some
alveoli had collapsed and disappeared in the control group.
Consequently, marked hemorrhage and hyperemia were observed in the
lung tissue. There was more infiltration of erythrocytes and
inflammatory cells in the alveolar cavity. The alveolar wall was
hyperemic, thickened and with serous exudation, and a transparent
membrane had formed (Fig. 2).
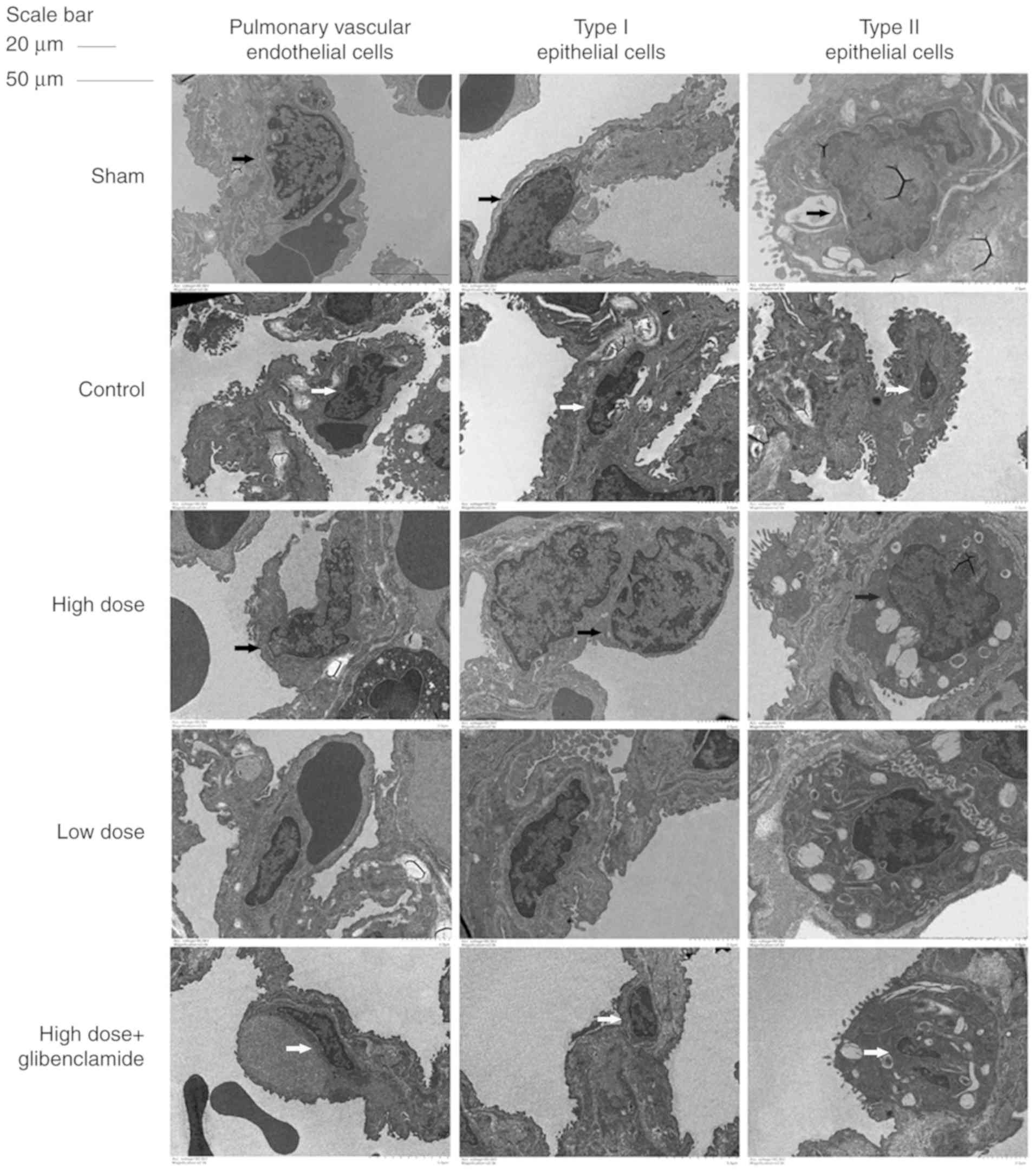
According to results of electron microscopy, the
three types of nuclei exhibited shrinkage, a lobed state and
broadening of the perinuclear space. The type II epithelial cell
lamellar body was significantly evacuated and the microvilli on the
cell membrane became smaller and less numerous (Fig. 3). These results validate the
hypothesis that nicorandil can protect the collapsed lung via
improving its microstructure.
Mechanism underlying the effect of
nicorandil on collapse-induced lung injury by decreasing oxidative
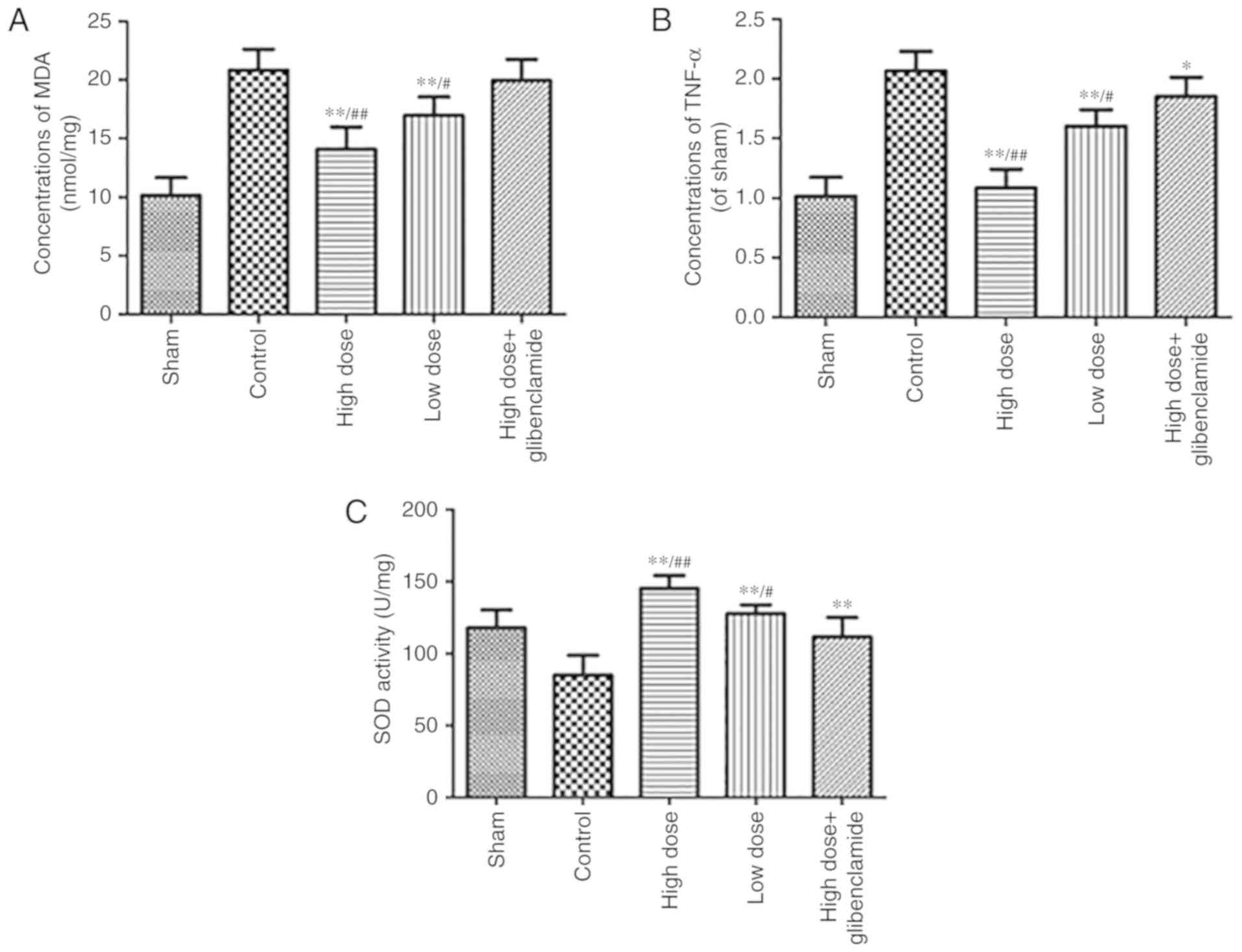
stress
The oxidative stress status, which is a mechanism of
protection, was evaluated by the measurement of MDA, TNF-α and the
activity of SOD. The levels of MDA and TNF-α in the collapsed lung
of the high-dose group were significantly lower than those in the
control group (P=0.0008 and P<0.0001, respectively). The
activity of SOD in the high-dose group was significantly higher
than that in the control group (P<0.0001; Fig. 4). These results confirm the
hypothesis that nicorandil can protect the collapsed lung by
decreasing oxidative stress.
Mechanism underlying the effect of
nicorandil on collapse-induced lung injury by inhibiting
apoptosis
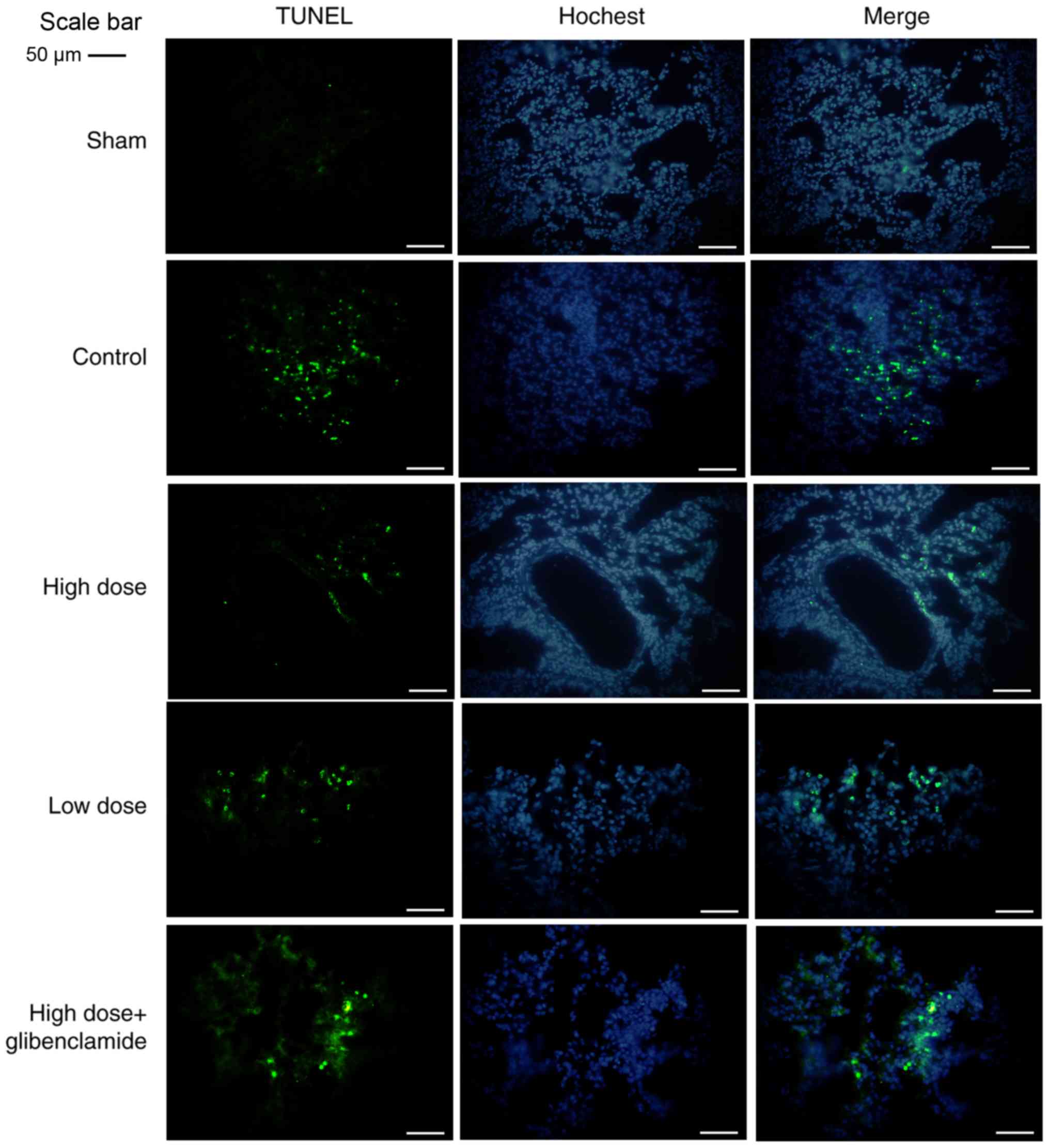
The results of alveolar apoptosis were observed
following a TUNEL assay under a fluorescent microscope. In the
control group and glibenclamide group, there were significant
increases in the number of apoptotic cells, which was consistent
with the positive control group in the TUNEL assay (Fig. 5). Taken together, the results of
the high-dose group showed a significant decrease in apoptosis,
compared with that in the control group, similar to that in the
sham group, and was partially reversed by glibenclamide. A high
dose of nicorandil demonstrated better results compared with a low
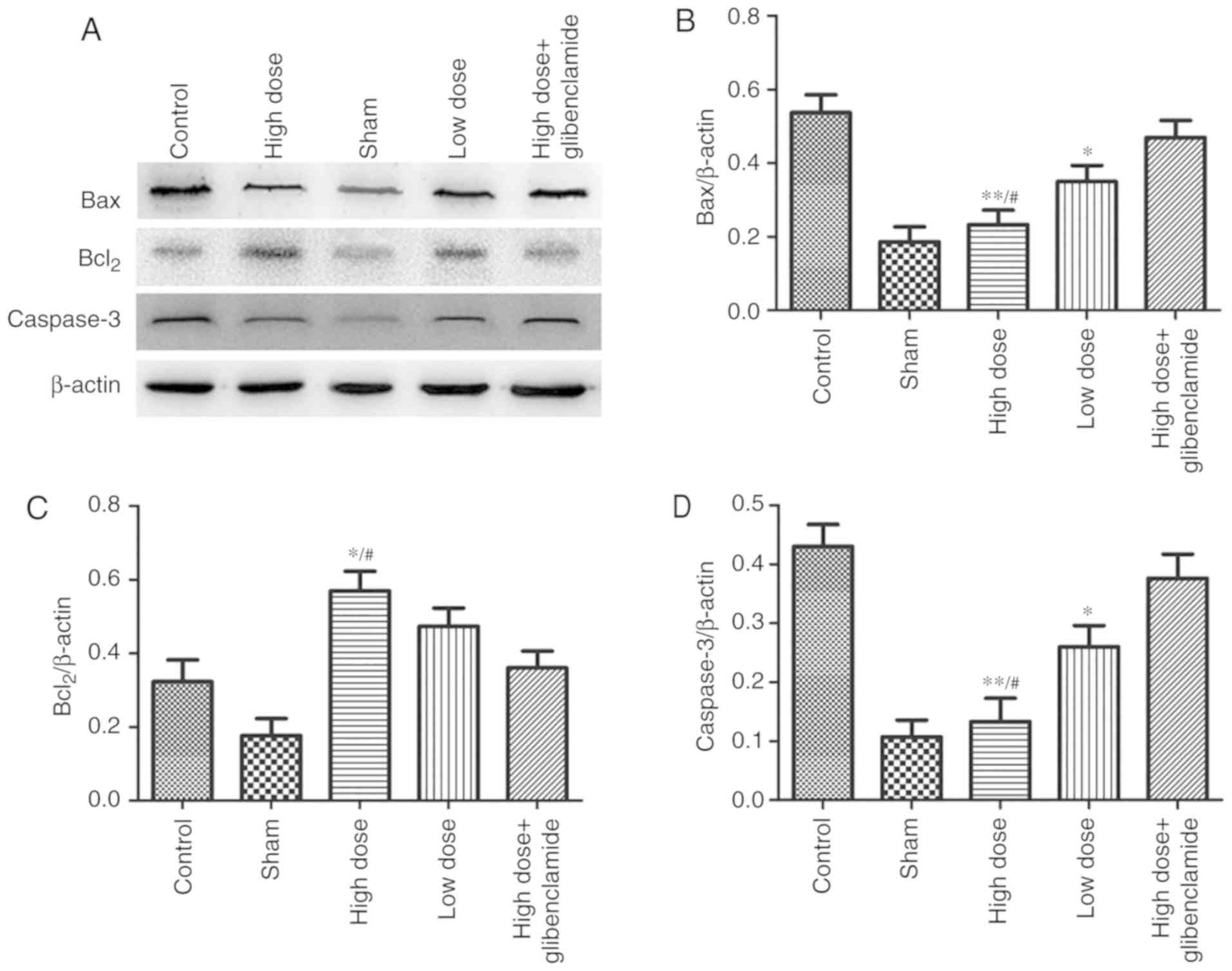
dose of nicorandil. The expression levels of Bax and caspase-3 in
the high-dose group were significantly lower than those in the
control group (P=0.0085 and P=0.0056, respectively). By contrast,
the expression of Bcl2 in the high-dose group was significantly
higher than that in the control group (P=0.036) (Fig. 6A-D). These results were also
reversed by glibenclamide, with a low dose of nicorandil having
less effect.
Signaling pathway involved in the
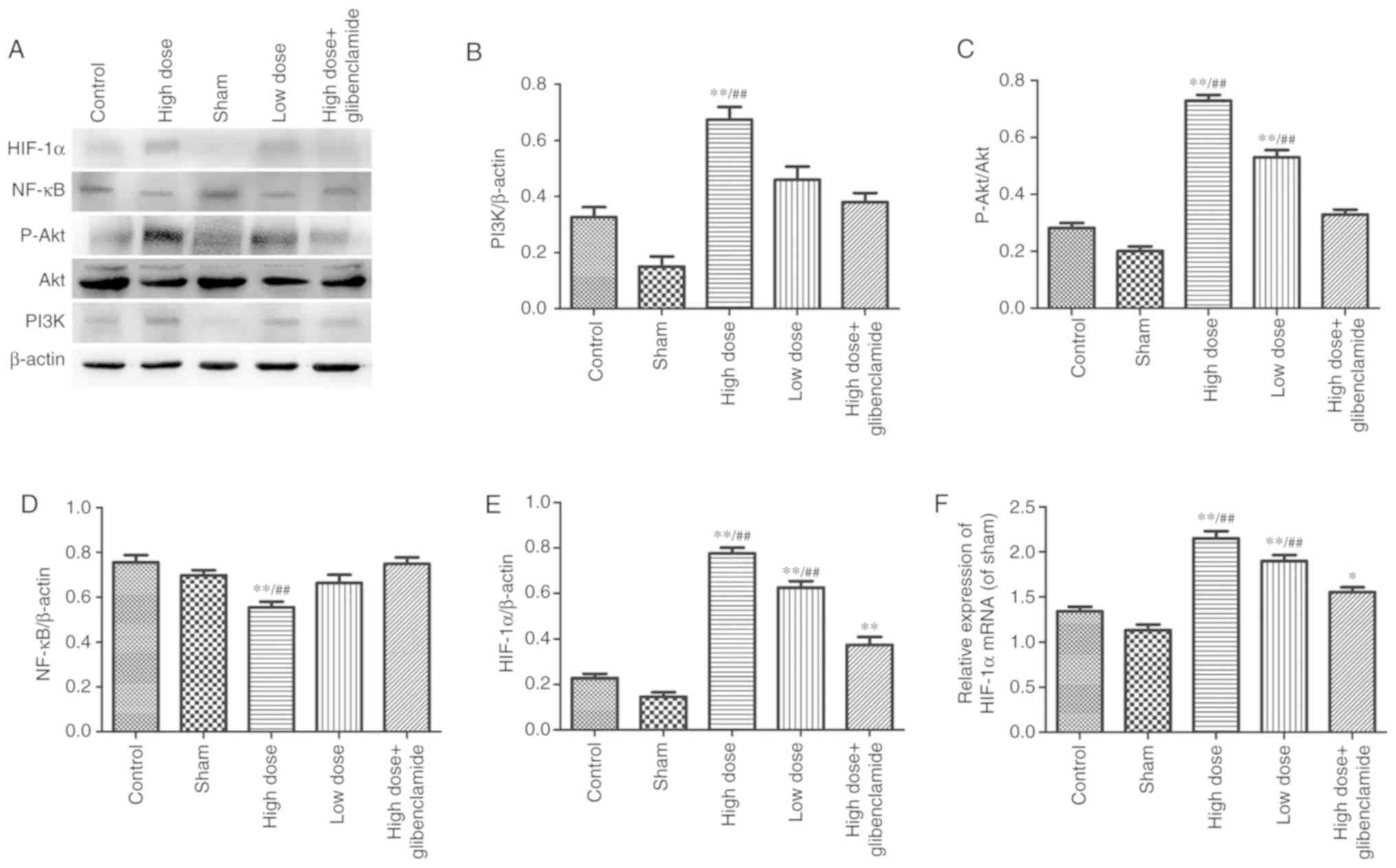
mechanism of nicorandil on collapse-induced lung injury
The PI3K/Akt signaling pathway is a classical signal
transduction pathway. NF-κB and HIF-1α form the bond between Akt
and downstream genes. It was observed that the expression levels of
p-Akt and PI3K in the high-dose group were significantly higher
than those in the control group (P<0.0001 and P=0.0039,
respectively). The results were reversed by LY294002 (P=0.0004 and
P=0.0001, respectively). The protein expression of NF-κB in the
high-dose group was significantly lower than that in the control
group (P=0.0005). The protein and mRNA levels of HIF-1α in the
high-dose group were significantly higher than those in the control
group (P<0.0001 and P<0.0001; respectively; Figs. 7A-F and 8).
Discussion
Nicorandil has been widely used for cardioprotection
in angina pectoris and myocardial infarction (26-28). It has almost no effect on blood
pressure or heart rate when it is applied in clinical practice
(29,30). It also has been used in protection
of the brain (31), liver
(32), lungs (12) and kidneys (33). However, there is currently no
evidence regarding its application in OLV. With the extensive
application of thoracoscopic techniques in surgery, OLV has become
essential. However, the inhibition of HPV, imbalance of
ventilation/perfusion (V/Q), damage from reactive oxygen free
radicals, I/R injury and inflammatory reactions, and even
mechanical and pressure injury, can result in lung injury, which
has a serious impact on patients. Compared with two-lung
ventilation (TLV), an increase in peak airway pressure of ~55%
occurs during OLV (34,35). Szegedi et al (36) firstly demonstrated that the effect
of gravity on V/Q matching, independent of HPV, resulted in more
severe oxidative stress on the operated lung. Misthos et al
(37) reported that reactivation
of the collapsed lung can activate severe oxidative stress, which
was correlated with the time of OLV by reviewing 212 cases between
2001 and 2003 of lung injury caused by re-expansion and
reperfusion. Lung I/R injury, which is difficult to manage, always
occurs during OLV. Although ventilation strategies and drugs have
been found to treat this, the results remain unsatisfactory in
terms of improving the prognosis of patients. The present study may
provide a novel program to protect lung tissues during OLV. The
pathophysiological mechanism in OLV may be multifactorial, and its
further examination requires a suitable animal model to be
established. In the present study, a well-established rabbit OLV
model was used, which has been confirmed to be mature and similar
to clinical procedures. The suitable diameter of the endotracheal
tube is particularly important; the maximum diameter of the left
branch of the double-lumen endotracheal tube was 3.0 mm, which is
consistent with the internal diameter of the left bronchus of the
rabbit. The appropriate pipe length was 20 cm. Its specific shape
was key to ensure the success rate of endotracheal intubation.
However, the double-lumen tube is a 'double-edged sword', which in
turn leads to lung damage. OLV, which is one of the most
challenging techniques for anesthesiologists, is an intraoperative
vital ventilation technique (1).
OLV must ensure the most convenient surgical field while
maintaining adequate gas exchange and minimizing damage to the two
lungs (38). Achieving this
largely depends on how well the non-ventilatory lung is isolated
from the other lung. It is well-known that ventilator-induced lung
injury (VILI) (15) has a
negative effect (39,40) and a significant effect on patient
prognosis (41,42). Ventilation is increasingly
recognized as a harmful intervention as it can cause lung injury,
frequently referred to as VILI (43) and may injure the respiratory
muscles, also termed ventilator-induced diaphragm dysfunction
(VIDD) (44). The occurrence of
hypoxemia in OLV mainly depends on correct placement of the
double-lumen tube, the underlying disease, the setting of the
ventilator and the experience of the anesthesiologist in thoracic
surgery (45,46). Accompanied by the imbalance of
V/Q, HPV is significantly depressed during anesthesia. In addition
to atelectasis, regions with low V/Q also contribute to typical
arterial oxygenation defects during OLV (47), thus, shunting of the
non-ventilatory lung is considered to be the leading cause of
hypoxemia (48). Significant
hypoxemia (22) occurs in 5-20%
of patients undergoing OLV due to increasing V/Q mismatching and
intrapulmonary shunting (49,50).
During OLV, nicorandil was found to significantly
improve lung oxygenation, with notable changes in SaO2
and PaO2 under general anesthesia. The reversal of the
index following the use of glibenclamide (specific antagonist)
demonstrated that the protective effects of nicorandil on the lung
relied on mitoKATP. The result of W/D ratio detection indicated
that the infusion of nicorandil protected the respiratory membrane,
preventing it from thickening. Therefore, the lung had little
edema, which was the protective mechanism of nicorandil. The
results showed that the microstructure of the non-ventilatory lung,
which was improved following the implementation of a high dose of
nicorandil, was seriously damaged in the control group, including
the nuclei, membrane and alveolar wall. H&E staining revealed
that the high-dose group, similar to the sham group, exhibited less
serious damage to the alveolar structure, with minimal hemorrhage,
congestion and infiltration of inflammatory cells. The alveolar
wall exhibited no congestion, thickening or exudation. These
results were partially reversed by glibenclamide. The electron
microscopy images also revealed that the high-dose group, similar
to the sham group, led to no pyknosis or lobulation of nuclei, and
no significant emptying of lamellar bodies or decreasing of
microvilli in type II epithelial cells. These results were also
partially reversed by glibenclamide. Taken together, these results
demonstrated that nicorandil had a protective effect on the
collapsed lung in terms of its microstructure and function.
Therefore, the mechanism underlying its protective role became an
important focus of the present study, which inferred it may affect
oxidative stress in the lungs.
Nieman et al (51) also suggested that mechanical
ventilation can result in lung damage, also caused by OLV, owing to
I/R lung injury from the collapse and re-expansion of the operated
lung during OLV (52). A high
pressure of oxygen in hypoxic-ischemic lung tissue may lead to
reactive oxygen species production, cell damage and local leukocyte
infiltration. The quantity of oxygen free radicals is proportional
to the duration of OLV (37,53). Re-expansion of the collapsed lung
at the end of surgery and high airway pressure during mechanical
ventilation may be other causes of lung injury besides I/R. It has
been reported (54,55) that re-expansion of the operated
lung can lead to increased expression of inflammatory mediators
(56,57). The mechanism of I/R injury, which
is more complex, is currently considered to be associated with
reactive oxygen species, endothelial cell injury, increased
vascular permeability, and activation of neutrophils and the
complement system, which also activates a series of inflammatory
reactions and results in a large accumulation of inflammatory
cytokines. In the present study, it was found that the
concentrations of MDA and TNF-α in the control group were
significantly higher than those in the high-dose group, which
confirmed that the mechanism of nicorandil was to reduce oxidative
stress and inflammatory reaction. SOD, which can eliminate reactive
oxygen species generated by organisms, is important in decreasing
I/R injury. Chen et al (58) found that SOD had protective effect
and led to a significant change in the morphology of myocardial I/R
injury, mainly due to the reduction of oxidative stress. The
present study also found that nicorandil reduced I/R injury to
protect lung function via SOD, which was high in the nicorandil
group.
In addition, TUNEL staining, which revealed another
mechanism, indicated that nicorandil significantly reduced
apoptosis, the start of structural and functional damage. In the
present study, nicorandil had a protective effect, regulated by
apoptosis-related genes. Bcl2 family members, which can be divided
into two categories, are crucial in apoptosis. One category
comprises anti-apoptotic members, including Bcl2 and Bcl-xl. The
other promotes apoptotic rate, including Bax and Bak. Bax protein,
which inhibits Bcl2, can form a heterodimer with Bcl2. Caspase-3 is
a protease identified by Fernandez-Alnemri in 1994, which was named
caspase-3 in 1996. Caspase-3 is now considered the most important
terminal enzyme, which serves an irreplaceable role in the process
of apoptosis. It has been found that the apoptosis (59) in lung injury can be regulated by
the Bax/Bcl2 ratio and the expression of downstream Bax and Bcl2
genes (60), and can be inhibited
by downregulating the main executive protein, caspase-3 (24). The present study found that,
compared with the control group, the gene expression of Bax in the
high-dose group was significantly downregulated, whereas the Bcl2
gene was significantly upregulated. When the Bax/Bcl2 ratio
declined, the protein expression of caspase-3 decreased
significantly. As a result, there was minimal apoptosis in the
high-dose group. This effect was reversed by glibenclamide,
however, the effect in the high-dose of nicorandil group was
significantly higher than that of the low-dose group. These results
demonstrate the important mechanisms comprising the reduced
expression of caspase-3 and downregulation of the Bax/Bcl2 ratio,
which resulted in reduced hypoxemia, oxidative stress and I/R
injury, and improved microstructure in the operated lung in the
high-dose group through decreased apoptosis.
As nicorandil can activate the opening of mitoKATP
to protect cells, it has been confirmed (14,61) that the cytoprotective effect of
nicorandil relies on the PI3K/Akt signaling pathway, which is a
classical pathway involved in cell proliferation, differentiation
and apoptosis. The phosphorylation of Akt (p-Akt) can activate
inhibitor of NF-κB (IκB) kinase, resulting in the degradation of
IκB, an inhibitor of NF-κB, which is released from the cytoplasm
for nuclear translocation and activates its target gene to promote
cell survival. However, the increase of NF-κB following I/R injury
promotes the transcription of downstream genes of additional
cytokines (62,63), including TNF-α (64), interleukin-1β, inducible nitric
oxide synthase and cyclooxygenase 2. In lung injury of mechanical
ventilation, activated NF-κB can lead to the production of a large
number of chemokines and cytokines and the recruitment of
neutrophils, monocytes, macrophages and lymphocytes, which trigger
pulmonary inflammatory responses (65).
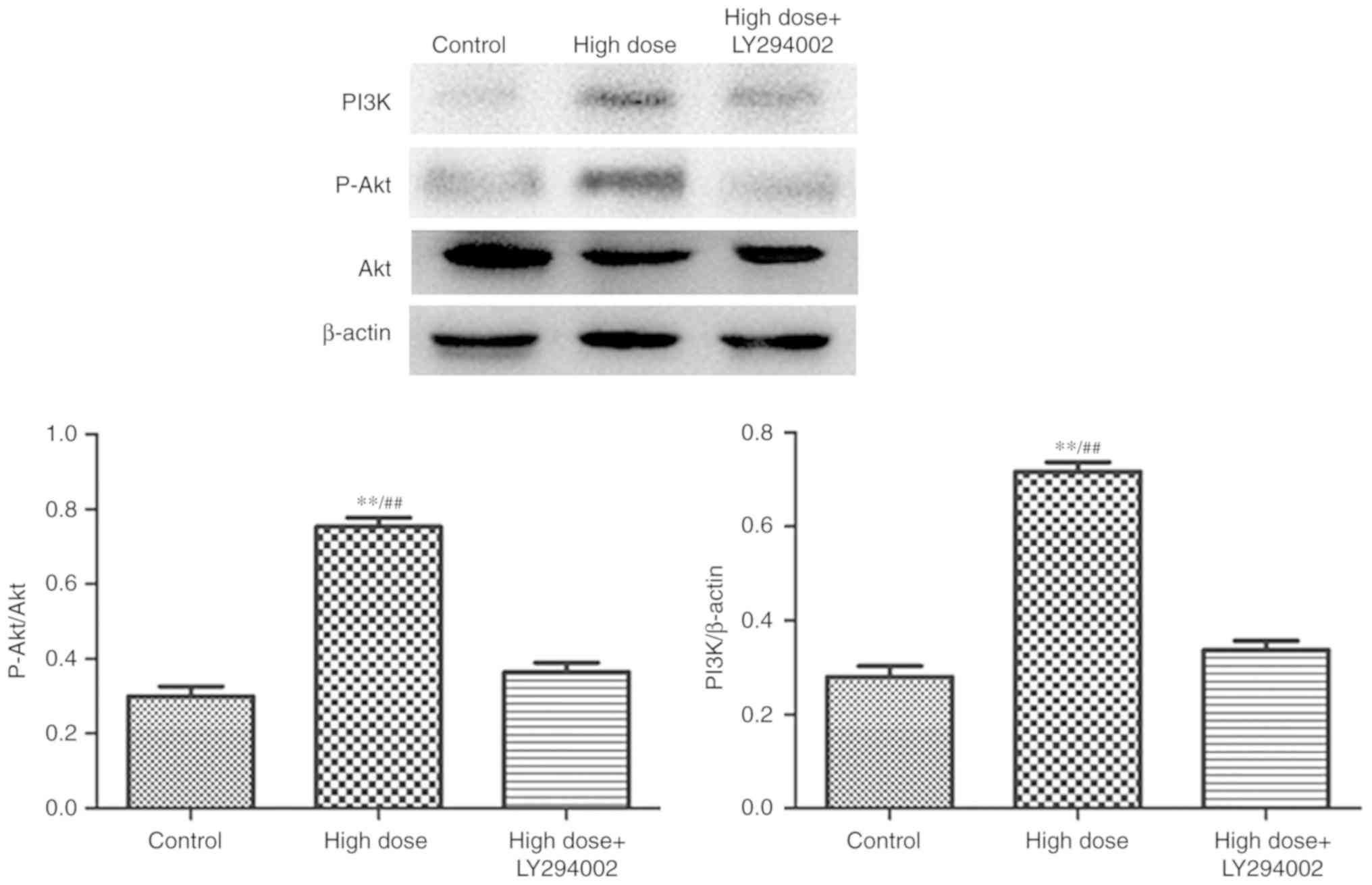
In the present study, the expression levels of PI3K,
p-Akt and the p-Akt/Akt ratio in the high-dose group were
upregulated, and reversed by glibenclamide and LY294002, indicating
that nicorandil acted on mitoKATP through the PI3K/Akt pathway to
inhibit apoptosis in the non-ventilatory lung. In addition, NF-κB,
which is closely associated with the inflammatory reaction, was
downregulated in the high-dose group, whereas HIF-1α, a nuclear
transcription factor, was upregulated, and these effects were
reversed by its antagonist, glibenclamide. It was also demonstrated
that a high dose of nicorandil had better results compared with a
low dose. These results were consistent with the two previous
independent studies (66,67), which demonstrated that the
expression of NF-κB and mitochondrial apoptotic pathway were first
inhibited in pulmonary vascular endothelial cells following the
opening of mitochondrial potassium channels. The expression of
HIF-1α is regulated by oxygen concentration, specifically activated
in hypoxia and initiated firstly in endogenous protective
mechanisms (68). Zhao et
al (69) showed that HIF-1α
is one of the important mechanisms of I/R lung injury following the
application of DMOG, an HIF-1α stabilizing agent. The results of
the present study are partially consistent with those of Wan et
al (70). Hypoxia and I/R in
OLV can lead to the overloading of intracellular calcium, and the
overload of calcium into mitochondria over its translocation limit
causes mitochondrial calcium overload, triggering opening of the
mitochondrial membrane permeability transition pore (mPTP). Opening
of the mPTP (71) uncouples the
mitochondrial respiratory chain, and leads to membrane potential
collapse and the release of cytochrome c and other apoptotic
factors, which ultimately leads to cell death. Nicorandil may serve
a protective role by inhibiting the overload of calcium in
mitochondria, thus triggering activation of the PI3K/Akt pathway,
shutting of the mPTP, downregulating NF-κB, upregulating HIF-1α,
and then downregulating Bax/Bcl2, inhibiting caspase-3 and reducing
apoptosis (72). However, further
investigation of the specific mechanisms and concentration-response
association are required to validate these findings.
In conclusion, nicorandil had a protective effect
via inhibiting apoptosis in the non-ventilated lung, which had been
collapsed and re-expanded during OLV, in the rabbit. This occurred
through acting on mitoKATP through the PI3K/Akt signaling
pathway.
Abbreviations:
|
OLV
|
one-lung ventilation
|
|
TLV
|
two-lung ventilation
|
|
I/R
|
ischemia/reperfusion
|
|
mitoKATP
|
mitochondrial ATP-sensitive potassium
channels
|
|
HPV
|
hypoxemic pulmonary
vasoconstriction
|
|
H&E
|
hematoxylin and eosin
|
|
SaO2
|
arterial oxygen saturation
|
|
SPO2
|
pulse oxygen saturation
|
|
PaO2
|
arterial partial pressure for
oxygen
|
|
MDA
|
malondialdehyde
|
|
TNF-α
|
tumor necrosis factor-α
|
|
SOD
|
superoxide dismutase
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
transfer-mediated dUTP nick end-labeling
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
|
Akt
|
protein kinase B
|
|
NF-κB
|
nuclear factor-κB
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
caspase-3
|
cysteinyl aspartate-specific
proteinase-3
|
|
W/D ratio
|
wet/dry weight ratio
|
|
V/Q
|
ventilation/perfusion
|
|
mPTP
|
mitochondrial membrane permeability
transition pore
|
|
VILI
|
ventilator-induced lung injury
|
|
VIDD
|
ventilator-induced diaphragm
dysfunction
|
Acknowledgments
The authors wish to thank Dr Maorong Jiang of the
Laboratory Animal Center of Nantong University for their assistance
in the preparation of the manuscript.
Funding
The present study was supported by the Natural
Science Foundation of Jiangsu Province, China (grant no.
BK20151268).
Availability of data and materials
The datasets supporting the conclusions of this
study are included within the article.
Authors' contributions
CW and FJ designed the study; CW, HK and JC
performed the experiments; XX and DS analyzed the data; CW and FJ
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experiments were reviewed and approved by the
Ethics Committee for Animal Experimentation of Nantong University
(Nantong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shiva S, Sack MN, Greer JJ, Duranski M,
Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari
N, et al: Nitrite augments tolerance to ischemia/reperfusion injury
via the modulation of mitochondrial electron transfer. J Exp Med.
204:2089–2102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu B, Tewari AK, Zhang L, Green-Church
KB, Zweier JL, Chen YR and He G: Proteomic analysis of protein
tyrosine nitration after ischemia reperfusion injury: Mitochondria
as the major target. Biochim Biophys Acta. 1794:476–485. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pak O, Sydykov A, Kosanovic D, Schermuly
RT, Dietrich A, Schröder K, Brandes RP, Gudermann T, Sommer N and
Weissmann N: Lung ischaemia-reperfusion injury: The role of
reactive oxygen species. Adv Exp Med Biol. 967:195–225. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernasconi F and Piccioni FL: One-lung
ventilation for thoracic surgery: Current perspectives. Tumori.
103:495–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dolch ME, Choukèr A, Hornuss C, Frey L,
Irlbeck M, Praun S, Leidlmair C, Villinger J and Schelling G:
Quantification of propionaldehyde in breath of patients after lung
transplantation. Free Radic Biol Med. 85:157–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ju NY, Gao H, Huang W, Niu FF, Lan WX, Li
F and Gao W: Therapeutic effect of inhaled budesonide
(pulmicort® turbuhaler) on the inflammatory response to
one-lung ventilation. Anaesthesia. 69:14–23. 2014. View Article : Google Scholar
|
|
7
|
Tojo K, Goto T and Kurahashi K: Protective
effects of continuous positive airway pressure on a nonventilated
lung during one-lung ventilation: A prospective laboratory study in
rats. Eur J Anaesthesiol. 33:776–783. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung DM, Ahn HJ, Jung SH, Yang M, Kim JA,
Shin SM and Jeon S: Apneic oxygen insufflation decreases the
incidence of hypoxemia during one-lung ventilation in open and
thoraco-scopic pulmonary lobectomy: A randomized controlled trial.
J Thorac Cardiovasc Surg. 154:360–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia R, Xu J, Yin H, Wu H, Xia Z, Zhou D,
Xia ZY, Zhang L, Li H and Xiao X: Intravenous infusion of
dexmedetomidine combined isoflurane inhalation reduces oxidative
stress and potentiates hypoxia pulmonary vasoconstriction during
one-lung ventilation in patients. Mediators Inflamm.
2015:2380412015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang SQ, Zhang J, Zhang XX, Liu L, Yu Y,
Kang XH, Wu XM and Zhu SM: Can dexmedetomidine improve arterial
oxygenation and intrapulmonary shunt during one-lung ventilation in
adults undergoing thoracic surgery? A meta-analysis of randomized,
placebo-controlled trials. Chin Med J (Engl). 130:1707–1714. 2017.
View Article : Google Scholar
|
|
11
|
Schloss B, Martin D, Beebe A, Klamar J and
Tobias JD: Phenylephrine to treat hypoxemia during one-lung
ventilation in a pediatric patient. Thorac Cardiovasc Surg Rep.
2:16–18. 2013. View Article : Google Scholar
|
|
12
|
Yang J, Zhang J, Cui W, Liu F, Xie R, Yang
X, Gu G, Zheng H, Lu J, Yang X, et al: Cardioprotective effects of
single oral dose of nicorandil before selective percutaneous
coronary intervention. Anatol J Cardiol. 15:125–131. 2015.
View Article : Google Scholar
|
|
13
|
Saha KK, Kumar A, Deval MM, Saha KK, Jacob
RV, Jagdale L and Kaul SK: Nicorandil infusion during off-pump
coronary artery bypass grafting reduces incidence of intra-aortic
balloon pump insertion. Innovations (Phila). 11:123–127. 2016.
View Article : Google Scholar
|
|
14
|
Su Q, Li L, Zhao J, Sun Y and Yang H:
Effects of nicorandil on PI3K/Akt signaling pathway and its
anti-apoptotic mechanisms in coronary microembolization in rats.
Oncotarget. 8:99347–99358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed LA, El-Maraghy SA and Rizk SM: Role
of the KATP channel in the protective effect of nicorandil on
cyclophosphamide-induced lung and testicular toxicity in rats. Sci
Rep. 5:140432015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asarian L and Geary N: Sex differences in
the physiology of eating. Am J Physiol Regul Integr Comp Physiol.
305:R1215–R1267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu ZP, Gu LB, Bian QM, Li PY, Wang LJ,
Chen XX and Zhang JY: A novel method for right one-lung ventilation
modeling in rabbits. Exp Ther Med. 12:1213–1219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
You Z, Feng D, Xu H, Cheng M, Li Z, Kan M
and Yao S: Nuclear factor-kappa B mediates one-lung
ventilation-induced acute lung injury in rabbits. J Invest Surg.
25:78–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ito H, Taniyama Y, Iwakura K, Nishikawa N,
Masuyama T, Kuzuya T, Hori M, Higashino Y, Fujii K and Minamino T:
Intravenous nicorandil can preserve microvascular integrity and
myocardial viability in patients with reperfused anterior wall
myocardial infarction. J Am Coll Cardiol. 33:654–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olson ME, McCabe K and Walker RL:
Guaifenesin alone or in combination with ketamine or sodium
pentobarbital as an anesthetic in rabbits. Can J Vet Res.
51:383–386. 1987.PubMed/NCBI
|
|
21
|
Pérez-Martínez A, Gonzálvez-Piñera J,
Marco-Macián A, Carpintero-Moreno F and Moya-Marchante M: Propofol
in continuous perfusion as anesthetic in experimental surgery in
the rabbit. Rev Esp Anestesiol Reanim. 42:253–256. 1995.In
Spanish.
|
|
22
|
Bellis DJ, Day S and Barnes PK: The
chronotropic effect of acetylcholine in the presence of vecuronium
and atracurium. A study in the isolated perfused rabbit heart
Anaesthesia. 45:118–119. 1990.
|
|
23
|
Shinozawa E and Kawamura M:
Anti-thrombotic effect of a factor Xa inhibitor TAK-442 in a rabbit
model of arteriove-nous shunt thrombosis stimulated with tissue
factor. BMC Res Notes. 11:7762018. View Article : Google Scholar
|
|
24
|
Lin L, Zhang L, Yu L, Han L, Ji W, Shen H
and Hu Z: Time-dependent changes of autophagy and apoptosis in
lipopolysaccharide-induced rat acute lung injury. Iran J Basic Med
Sci. 19:632–637. 2016.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Kostic J, Djordjevic-Dikic A, Dobric M,
Milasinovic D, Nedeljkovic M, Stojkovic S, Stepanovic J, Tesic M,
Trifunovic Z, Zamaklar-Tifunovic D, et al: The effects of
nicorandil on microvascular function in patients with ST segment
elevation myocardial infarction undergoing primary PCI. Cardiovasc
Ultrasound. 13:262015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang YP, Zhang Y, Sun YR, Sun ZG, Zuo ZK,
Feng ZR, Chang FY, Xu YC, Chen BZ and Ye YY: Effect of nicorandil
on ventricular arrhythmia in patients with acute ST-segment
elevation myocardial infarction underwent emergent percutaneous
coronary intervention treatment. Zhonghua Xin Xue Guan Bing Za Zhi.
45:701–705. 2017.In Chinese. PubMed/NCBI
|
|
28
|
Suleimani HF, Eshraghi A, Daloee MH,
Hoseini S and Nakhaee N: Effect of nicorandil on QT dispersion in
patients with stable angina pectoris undergoing elective
angioplasty: A triple-blind, randomized, placebo-controlled study.
Electron Physician. 9:4934–4941. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka K, Kato K, Takano T, Katagiri T,
Asanoi H, Nejima J, Nakashima M, Kamijo T and Sakanashi M: Acute
effects of intravenous nicorandil on hemodynamics in patients
hospitalized with acute decompensated heart failure. J Cardiol.
56:291–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Minami Y, Nagashima M, Kajimoto K, Shiga T
and Hagiwara N: Acute efficacy and safety of intravenous
administration of nicorandil in patients with acute heart failure
syndromes: Usefulness of noninvasive echocardiographic hemodynamic
evaluation. J Cardiovasc Pharmacol. 54:335–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kong J, Ren G, Jia N, Wang Y, Zhang H,
Zhang W, Chen B and Cao Y: Effects of nicorandil in neuroprotective
activation of PI3K/AKT pathways in a cellular model of Alzheimer's
disease. Eur Neurol. 70:233–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamazaki H, Oshima K, Sato H, Kobayashi K,
Suto Y, Hirai K, Odawara H, Matsumoto K and Takeyoshi I: The effect
of nicorandil on ischemia-reperfusion injury in a porcine total
hepatic vascular exclusion model. J Surg Res. 167:49–55. 2011.
View Article : Google Scholar
|
|
33
|
Shimizu S, Saito M, Kinoshita Y, Ohmasa F,
Dimitriadis F, Shomori K, Hayashi A and Satoh K: Nicorandil
ameliorates ischaemia-reperfusion injury in the rat kidney. Br J
Pharmacol. 163:272–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Szegedi LL, Bardoczky GI, Engelman EE and
d'Hollander AA: Airway pressure changes during one-lung
ventilation. Anesth Analg. 84:1034–1037. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kilpatrick B and Slinger P: Lung
protective strategies in anaesthesia. Br J Anaesth. 105(Suppl 1):
i108–i116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Szegedi LL, D'Hollander AA, Vermassen FE,
Deryck F and Wouters PF: Gravity is an important determinant of
oxygenation during one-lung ventilation. Acta Anaesthesiol Scand.
54:744–750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Misthos P, Katsaragakis S, Milingos N,
Kakaris S, Sepsas E, Athanassiadi K, Theodorou D and Skottis I:
Postresectional pulmonary oxidative stress in lung cancer patients.
The role of one-lung ventilation. Eur J Cardiothorac Surg.
27:379–382; discussion 382-383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Licker M, Fauconnet P, Villiger Y and
Tschopp JM: Acute lung injury and outcomes after thoracic surgery.
Curr Opin Anaesthesiol. 22:61–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeon K, Yoon JW, Suh GY, Kim J, Kim K,
Yang M, Kim H, Kwon OJ and Shim YM: Risk factors for
post-pneumonectomy acute lung injury/acute respiratory distress
syndrome in primary lung cancer patients. Anaesth Intensive Care.
37:14–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tusman G, Böhm SH, Warner DO and Sprung J:
Atelectasis and perioperative pulmonary complications in high-risk
patients. Curr Opin Anaesthesiol. 25:1–10. 2012. View Article : Google Scholar
|
|
41
|
Gajic O, Dara SI, Mendez JL, Adesanya AO,
Festic E, Caples SM, Rana R, St Sauver JL, Lymp JF, Afessa B and
Hubmayr RD: Ventilator-associated lung injury in patients without
acute lung injury at the onset of mechanical ventilation. Crit Care
Med. 32:1817–1824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Serpa Neto A, Cardoso SO, Manetta JA,
Pereira VG, Espósito DC, Pasqualucci Mde O, Damasceno MC and
Schultz MJ: Association between use of lung-protective ventilation
with lower tidal volumes and clinical outcomes among patients
without acute respiratory distress syndrome: A meta-analysis. JAMA.
308:1651–1659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Slutsky AS and Ranieri VM:
Ventilator-induced lung injury. N Engl J Med.
370:9802014.PubMed/NCBI
|
|
44
|
Levine S, Nguyen T, Taylor N, Friscia ME,
Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, et
al: Rapid disuse atrophy of diaphragm fibers in mechanically
ventilated humans. N Engl J Med. 358:1327–1335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Karzai W and Schwarzkopf K: Hypoxemia
during one-lung ventilation: Prediction, prevention, and treatment.
Anesthesiology. 110:1402–1411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ishikawa S and Lohser J: One-lung
ventilation and arterial oxygenation. Curr Opin Anaesthesiol.
24:24–31. 2011. View Article : Google Scholar
|
|
47
|
Kozian A, Schilling T, Schütze H, Heres F,
Hachenberg T and Hedenstierna G: Lung computed tomography density
distribution in a porcine model of one-lung ventilation. Br J
Anaesth. 102:551–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Benumof JL: One-lung ventilation and
hypoxic pulmonary vasoconstriction: Implications for anesthetic
management. Anesth Analg. 64:821–833. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rozé H, Lafargue M and Ouattara A: Case
scenario: Management of intraoperative hypoxemia during one-lung
ventilation. Anesthesiology. 114:167–174. 2011. View Article : Google Scholar
|
|
50
|
Lee SM, Kim WH, Ahn HJ, Kim JA, Yang MK,
Lee CH, Lee JH, Kim YR and Choi JW: The effects of prolonged
inspiratory time during one-lung ventilation: A randomised
controlled trial. Anaesthesia. 68:908–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nieman GF, Satalin J, Andrews P, Aiash H,
Habashi NM and Gatto LA: Personalizing mechanical ventilation
according to physiologic parameters to stabilize alveoli and
minimize ventilator induced lung injury (VILI). Intensive Care Med
Exp. 5:82017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
de Perrot M, Liu M, Waddell TK and
Keshavjee S: Ischemia-reperfusion-induced lung injury. Am J Respir
Crit Care Med. 167:490–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cheng YJ, Chan KC, Chien CT, Sun WZ and
Lin CJ: Oxidative stress during 1-lung ventilation. J Thorac
Cardiovasc Surg. 132:513–518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Riva DR, Contador RS, Baez-Garcia CS,
Xisto DG, Cagido VR, Martini SV, Morales MM, Rocco PR, Faffe DS and
Zin WA: Recruitment maneuver: RAMP versus CPAP pressure profile in
a model of acute lung injury. Respir Physiol Neurobiol. 169:62–68.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rzezinski AF, Oliveira GP, Santiago VR,
Santos RS, Ornellas DS, Morales MM, Capelozzi VL, Amato MB, Conde
MB, Pelosi P and Rocco PR: Prolonged recruitment manoeuvre improves
lung function with less ultrastructural damage in experimental mild
acute lung injury. Respir Physiol Neurobiol. 169:271–281. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Silva PL, Moraes L, Santos RS, Samary C,
Ornellas DS, Maron-Gutierrez T, Morales MM, Saddy F, Capelozzi VL,
Pelosi P, et al: Impact of pressure profile and duration of
recruitment maneuvers on morphofunctional and biochemical variables
in experimental lung injury. Crit Care Med. 39:1074–1081. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Arnal JM, Paquet J, Wysocki M, Demory D,
Donati S, Granier I, Corno G and Durand-Gasselin J: Optimal
duration of a sustained inflation recruitment maneuver in ARDS
patients. Intensive Care Med. 37:1588–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen C, Lu W, Wu G, Lv L, Chen W, Huang L,
Wu X, Xu N and Wu Y: Cardioprotective effects of combined therapy
with diltiazem and superoxide dismutase on myocardial
ischemia-reperfusion injury in rats. Life Sci. 183:50–59. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu G, Zhang J, Chen H, Wang C, Qiu Y, Liu
Y, Wan J and Guo H: Effects and mechanisms of alveolar type II
epithelial cell apoptosis in severe pancreatitis-induced acute lung
injury. Exp Ther Med. 7:565–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wu Z, Dai F, Ren W, Liu H, Li B and Chang
J: Angiotensin II induces apoptosis of human pulmonary
microvascular endothelial cells in acute aortic dissection
complicated with lung injury patients through modulating the
expression of monocyte chemoattractant protein-1. Am J Transl Res.
8:28–36. 2016.PubMed/NCBI
|
|
61
|
Yu D, Fan C, Zhang W, Wen Z, Hu L, Yang L,
Feng Y, Yin KJ and Mo X: Neuroprotective effect of nicorandil
through inhibition of apoptosis by the PI3K/Akt1 pathway in a mouse
model of deep hypothermic low flow. J Neurol Sci. 357:119–125.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Broucek JR, Francescatti AB, Swanson GR,
Keshavarzian A, Brand MI and Saclarides TJ: Unusual thrombotic
complications. Am Surg. 78:728–729. 2012.PubMed/NCBI
|
|
63
|
Xia Z, Peng W, Cheng S, Zhong B, Sheng C,
Zhang C, Gong W, Cheng S, Li J and Wang Z: Naoling decoction
restores cognitive function by inhibiting the neuroinflammatory
network in a rat model of Alzheimer's disease. Oncotarget.
8:42648–42663. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ambros JT, Herrero-Fresneda I, Borau OG
and Boira JM: Ischemic preconditioning in solid organ
transplantation: From experimental to clinics. Transpl Int.
20:219–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Suzuki T, Yamashita K, Jomen W, Ueki S,
Aoyagi T, Fukai M, Furukawa H, Umezawa K, Ozaki M and Todo S: The
novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin, prevents
local and remote organ injury following intestinal
ischemia/reperfusion in rats. J Surg Res. 149:69–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang H, Zuo X, Wang Q, Yu Y, Xie L, Wang
H, Wu H and Xie W: Nicorandil inhibits hypoxia-induced apoptosis in
human pulmonary artery endothelial cells through activation of
mito-KATP and regulation of eNOS and the NF-κB pathway. Int J Mol
Med. 32:187–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sakamoto N, Ishibashi T, Sugimoto K,
Sawamura T, Sakamoto T, Inoue N, Saitoh S, Kamioka M, Uekita H,
Ohkawara H, et al: Role of LOX-1 in monocyte adhesion-triggered
redox, Akt/eNOS and Ca2+ signaling pathways in
endothelial cells. J Cell Physiol. 220:706–715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao X, Jin Y, Li H, Wang Z, Zhang W and
Feng C: Hypoxia-inducible factor 1 alpha contributes to pulmonary
vascular dysfunction in lung ischemia-reperfusion injury. Int J
Clin Exp Pathol. 7:3081–3088. 2014.PubMed/NCBI
|
|
70
|
Wan J, Wu W, Chen Y, Kang N and Zhang R:
Insufficient radio-frequency ablation promotes the growth of
non-small cell lung cancer cells through PI3K/Akt/HIF-1α signals.
Acta Biochim Biophys Sin (Shanghai). 48:371–377. 2016. View Article : Google Scholar
|
|
71
|
Gomez L, Li B, Mewton N, Sanchez I, Piot
C, Elbaz M and Ovize M: Inhibition of mitochondrial permeability
transition pore opening: Translation to patients. Cardiovasc Res.
83:226–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Forgiarini LA Jr, Grun G, Kretzmann NA, de
Muñoz GA, de Almeida A, Forgiarini LF and Andrade CF: When is
injury potentially reversible in a lung ischemia-reperfusion model?
J Surg Res. 179:168–174. 2013. View Article : Google Scholar
|