Introduction
Insulin resistance is a major pathogenic factor
underlying the development of type 2 diabetes mellitus (T2DM) and
obesity (1). Elevated
concentrations of nonesterified fatty acids, particularly saturated
fatty acids, play a critical role in the induction of insulin
resistance (2). This is because
saturated fatty acids impair insulin signaling via multiple
mechanisms including increases in the activity of stress signaling
kinases [(mitogen-activated protein kinase 8 (JNK), inhibitor of
nuclear factor-κB kinase (IKK), and protein kinase B (PKC)],
enhanced levels of reactive oxygen species, activation of
endoplasmic reticulum stress, perturbations in mitochondrial
function, and the accumulation of lipid intermediates (3-5).
Due to impairments in insulin signaling, C2 myotubes and human
skeletal muscle exposed to saturated fatty acids, such as palmitate
exhibit a failure of the glucose transporter type 4 (GLUT4) to
translocate from the cytosol to the membrane due to impairments in
insulin signaling (6,7).
Glucagon-like peptide-1 (GLP-1) is a hormone
secreted from intestinal L-cells in response to nutrients and
exerts glucose-dependent insulinotropic actions in pancreatic islet
cells (8). Additionally, GLP-1
has extra-pancreatic effects that include the generation of
increased glucose uptake and the regulation of energy homeostasis
in the skeletal muscles of rats and obese humans through the
activation of the phosphoinositide 3 kinase (PI3K)/protein kinase B
(Akt) and mitogen-activated protein kinase pathways (9). Muscle-specific GLP-1-overexpressing
transgenic mice exhibit reduced weight gain and improved lipid
profiles following a high-fat diet challenge (10), and GLP-1 upregulates the
translocation and expression of GLUT4 in the heart and skeletal
muscles of spontaneously hypertensive rats (11).
Sirtuin 1 (SIRT1), a mammalian ortholog of Sir2, is
an NAD-dependent protein deacetylase that is thought to play a
critical role in the adaptation of cells to metabolic alterations
during insulin resistance. The whole-body overexpression of SIRT1
prevents the development of metabolic disorders in mice fed a
high-fat diet (12), and the
pharmacological activation of SIRT1 increases insulin sensitivity
in skeletal muscle in animal models of insulin resistance (13). Forskolin, epinephrine, and oleic
acid stimulate the cyclic adenosine monophosphate (cAMP) and
protein kinase A (PKA) pathway to activate SIRT1 activity, which in
turn increases the rate of fatty acid oxidation in skeletal muscle
cells (14,15). Exenatide, a GLP-1 agonist,
activates the cAMP/PKA pathway and ameliorates hepatic steatosis
via SIRT1 activation in obese C57BL/6J mice fed a high-fat diet
(16). This result is consistent
with investigations of the GLP-1 receptor agonist Exendin-4, which
activates the cAMP/PKA pathway, reduces endoplasmic reticulum
stress, and leads to attenuation of hepatic steatohepatitis in
high-fat diet-induced obese C57BL/6J mice in a SIRT1-dependent
manner (17,18). However, GLP-1 also inhibits
SIRT1-related deacetylase activity during the mass expansion of
pancreatic β-cells (19).
Regardless of the direction, it is evident that there is a link
between the activities of GLP-1 and SIRT1.
The relationship between GLP-1 and SIRT1 and the
effects of GLP-1 on palmitate-induced insulin resistance and GLUT4
regulation in human skeletal muscle remain poorly understood. Thus,
the present study investigated whether GLP-1 has a protective
effect on human skeletal muscle myotubes (HSMMs) exhibiting
palmitate-induced insulin resistance. Moreover, the present study
investigated whether the effects of GLP-1 occur through SIRT1
activation.
Materials and methods
Reagents
The present study utilized recombinant human GLP-1
(ProSpec; Ness Ziona), the SIRT-1 inhibitor EX527 (cat. no. E-7034;
Sigma-Aldrich; Merck KGaA), BSA (cat. no. A9418; Sigma-Aldrich;
Merck KGaA), palmitate (cat. no. P5586; Sigma-Aldrich; Merck KGaA),
H-89 dihydrochloride (cat. no. 127243-85-0; Merck KGaA) and insulin
(cat. no. 19278; Sigma-Aldrich; Merck KGaA), all of which were
dissolved in the appropriate medium or PBS prior to use at the
required working dilution. Recombinant GLP-1 is an active form of
native GLP-1. It consists of 30 amino acids and has a half-life of
<2 min, due to its degradation by the DPPIV enzyme in the
circulation. The palmitate/BSA conjugates were prepared as
described previously (20) and
used to treat the cultured cells. Briefly, a 20-mM solution of
palmitate in 0.01 M NaOH was incubated for 30 min at 70°C, and then
fatty acid soaps were mixed with 5% BSA in PBS at an 8:1 molar
ratio.
Cell cultures
The present study used normal HSMMs (Lonza Group,
Ltd.). The undifferentiated myoblasts were cultured using a
Bulletkit containing basal medium (SkBM; cat. no. CC3161; Lonza
Group, Ltd.) at 37°C in an atmosphere of 5% CO2. The
cells were then induced to differentiate using DMEM (Gibco; Thermo
Fisher Scientific, Inc.; cat. no. 11500416) supplemented with 2%
horse serum (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
10368902), 2 mmol/l glutamine, and 50 U/ml streptomycin and
penicillin; the medium was replaced every other day. The normal
HSMMs were grown for 6 days prior to harvesting for either the
protein or RNA analyses. The differentiated HSMMs were either
treated with palmitate (200 µmol/l) or stimulated with
recombinant GLP-1 (100 or 200 nmol/l) for 24 h. Cell viability and
cytotoxicity under palmitate treatment (100 or 200 µmol/l)
were measured using 2-(2-methoxy-4-nitrophenyl)-3-(4-nitr
ophenyl)-5-(2,4-disulfophenyl)-2 H tetrazolium monosodium salt
(Cell Counting Kit-8; CCK-8) assay (Sigma-Aldrich; Merck KGaA).
Briefly, differentiated HSMMs treated with 100 or 200 µM
palmitate were cultured in a 96-well tissue culture plate. After 24
h, 10 µl CCK-8 solution was added to each well and the cells
were incubated for another 4 h at 37°C. Relative cell viability was
determined by scanning with an ELISA reader with a 450 nm filter
and calculated based on a CCK-8 assay.
Uptake of 2-NBDG by HSMMs
The HSMMs were treated with either palmitate (200
µM) or GLP-1 (200 nmol/l) for 24 h. The cells then were
starved for 16 h and pre-incubated in Krebs-Ringer bicarbonate
buffer (pH 7.4) containing 2% BSA for 30 min at 37°C. Next, the
HSMMs were treated with 500 µmol/l
2-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino-2-deoxyglucose
(2-NBDG; cat. no. N13195; Invitrogen; Thermo Fisher Scientific,
Inc.) in either the presence or absence of insulin (100 nmol/l) for
3 h at 37°C. The cells were then rinsed three times with ice-cold
PBS, lysed with RIPA Lysis and Extraction buffer (cat. no. 89900;
Thermo Fisher Scientific, Inc.), and the lysates were centrifuged
at 12,000 × g for 30 min at 4°C. The supernatants were measured for
fluorescence (excitation: 475 nm; emission: 550 nm) using a
SpectraMax Gemini EM microplate reader (Molecular Devices, LLC).
The protein concentrations were determined using the Bradford assay
(Coomassie Protein Reagent, Pierce; Thermo Fisher Scientific,
Inc.).
Semiquantitative reverse
transcription-PCR (RT-PCR) analysis
The mRNA expression levels were compared using
semi-quantitative RT-PCR performed after RNA extraction using the
TRIzol® method (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized by RT-PCR using the Takara RNA PCR kit
(AMV) ver 3. supplied by Takara Bio, Inc. The first strand cDNA was
synthesized from 1 g total RNA using the AMV reverse transcriptase
and the random 9-mer oligonucleotide. The RT reaction was performed
for 1 cycle under the condition of 30°C for 10 min, 42°C for 30
min, 95°C for 5 min and 5°C for 5 min. Then the PCR reaction was
performed using a Takara Shuzo PCR Amplification kit (cat.
no. R011; Takara Bio, Inc.) with primer sets specific for different
genes. The following PCR primers were used in the present study:
GLUT4, 5′-TGG CTG AGC TGA AGG ATG AG-3′ and 5′-CCA ACA ACA CCG AGA
CCA AG-3′; and GAPDH, 5′-GGT GAA GGT CGG AGT CAA CG-3′ and 5′-CAA
AGT TGT CAT GGA TGA CC-3′. The thermal conditions for the GLUT4,
and GAPDH were denaturation for 30 sec at 95°C, annealing for 30
sec at 56°C, and extension for 30 sec at 72°C. The amplifications
of the GLUT4, and GAPDH were performed using 35, and 25-28 cycles,
respectively. The amplified PCR products were separated by
electrophoresis on a 1.5% agarose gel. Then gel was stained with
ethidium bromide for 30 min at room temperature and washed with
distilled water for 10 min. The levels of amplified cDNA were
quantified by densitometric analysis [Quantity One-4.6.9 (basic)
analysis software; Bio-Rad Laboratories, Inc.] of the stained bands
and compared with those of GAPDH.
GLUT4 promoter activity
The HSMMs (5×106) were trans-fected with
a reporter plasmid (mouse GLUT4 promoter luciferase reporter) using
a pipette-type electroporator (Microporator-Mini; Digital
Biotechnology) according to the manufacturer's protocol. The total
transfected DNA amount was held constant at 50 ng by the addition
of the plasmid vector β-galactosidase (β-gal). For the
co-transfection of hG4-luc and β-gal, 5×106 HSMMs were
mixed with 800 ng mouse GLUT4 promoter luciferase reporter, as
previously described (6) and 50
ng β-gal in 100 µl R buffer by microporation at 1,380 V and
30 m width. Following transfection, the HSMMs were induced to
differentiate by switching to a differentiation medium for 48 h.
Following differentiation, the cells were treated either with or
without palmitate and/or GLP-1 for 24 h, and the cell lysates were
prepared by adding lysis buffer and then scraping, vortexing, and
centrifuging (12,000 × g for 2 min at 4°C) the solution. For all
lysates, β-gal activity was measured using β-Galactosidase Enzyme
Assay System with Reporter Lysis Buffer (cat. no. E2000; Promega
Corporation), and promoter activity was measured using a Luciferase
Assay system (Promega Corporation) and TD-20/20 luminometer (Turner
Design; Promega Corporation). Promoter activity was normal-ized to
β-gal activity and the protein amount, and luciferase activity was
determined by mixing 100 µl luciferase assay reagent with 20
µl lysate and measuring the luminescence.
ELISA for cAMP
cAMP levels in the cell lysates were evaluated using
the Direct cAMP ELISA kit (Enzo Life Sciences, Inc.; cat. no.
BML-AK255) according to the manufacturer's protocol. The maximum
intensity of the bands was converted to 100% and used to calculate
the relative intensity.
Peroxisome proliferator-activated
receptor γ coactivator 1α (PGC1α) acetylation
To assess the acetylation levels of PGC1α, the HSMMs
were transfected using Lipofectamine® 2000 (cat. no.
11668027; Invitrogen; Thermo Fisher Scientific, Inc.) with 3
µg pcDNA3 Flag-PGC1α expression vector. The transfected
cells were treated either with or without recombinant GLP-1 for 24
h, and the acetylation status was determined by immunoprecipitating
1 mg protein lysate in a RIPA lysis buffer [20 mmol/l Tris (pH
8.0), 137 mmol/l NaCl, 1 mmol/l MgCl2, 2 mmol/l
CaCl2, 1% NP-40, 2 mmol/l vanadate, 1 mmol/l
dithiothreitol (DTT) and 2.5 mmol/l phenylmethyl-sulfonyl
fluoride]. The lysates were centrifuged at 12,000 × g for 20 min at
4°C, and aliquots of the supernatant were removed for protein
concentration quantification using the Bradford assay (Coomassie
Protein Reagent, Pierce; Thermo Fisher Scientific, Inc.). All
Flag-tagged proteins were immunoprecipitated with 30 µl of
the 50% slurry of anti-FLAG M2 agarose (15 ul of packed beads; cat.
no. A2220; Sigma-Aldrich; Merck KGaA) and the agarose beads were
washed twice with RIPA buffer. Then, the agarose beads were mixed
with SDS loading buffer and resolved on an 8% gel by SDS-PAGE and
transferred to a polyvinylidene fluoride membrane (EMD Millipore).
After blocking with 3% skimmed milk for 30 min at room temperature,
the membranes were immunoblotted with PGC-1α (1:500; cat. no.
SC-13067; Santa Cruz Biotechnology, Inc.) and acetyl-lysine
antibodies (1:1,000; cat. no. 9441; Cell Signaling Technology,
Inc.) overnight at 4°C and then washed three times with TBS with
Tween-20. The membranes were incubated with peroxidase-conjugated
anti-rabbit IgG secondary antibody (1:3,000; cat. no. SC2357; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. All
immunoreactive bands were visualized using an enhanced
chemiluminescence detection system (Amersham ECL Prime Western
Blotting Detection Reagent; cat. no. RPN2232; GE Healthcare) and
the band intensities were determined using Quantity One-4.6.8.
(basic) analysis software (Bio-Rad Laboratories, Inc.), which is a
one-dimensional image analysis program.
Measurement of SIRT-1 activity
SIRT1 activity was measured using a
fluorescence-based SIRT1 assay kit (Sigma-Aldrich; Merck KGaA)
following the protocol provided. Cell nuclei were isolated in
hypotonic buffer solution (20 mM Tris-HCl, pH 7.4, 10 mM NaCl and 3
mM MgCl2) with 0.5% of NP40, followed by centrifugation
at 1,700 × g for 10 min at 4°C. The reaction was started by mixing
50 µl nuclear extract and 50 µl sirtuin assay buffer
(fluorescence-conjugated human p53 peptides and NAD+).
The reaction was incubated for 30 min at room temperature without
light, after which 10 ml developer was added. The absorbance was
measured at an excitation wavelength of 380 nm and an emission
wavelength of 460 nm.
Measurement of histone deacetylase (HDAC)
activity
HDAC activity in the cell nucleus was measured using
an HDAC colorimetric assay kit (cat. no. N#331-100; BioVision,
Inc.) according to the manufacturer's protocol. Briefly, HSMMS
treated with GLP-1 at various times. Nuclei were isolated in
hypotonic buffer solution (20 mM Tris-HCl, pH 7.4, 10 mM NaCl and 3
mM MgCl2) with 0.5% of NP40, followed centrifugation at 1,700 × g
for 10 min at 4°C. Nuclei protein extracted with HDAC 1X assay
buffer which was supplied by assay kit. Then, 40 µg nuclear
protein from each group was added to a 96-well tissue culture plate
at a final volume of 100 µl per well. Reaction buffer and
enzyme were added according to the manufacturer's recommendations.
After incubation, HDAC activity was measured by scanning with an
ELISA reader with a 450 nm filter.
Quantitative RT-PCR
Total RNA was extracted from the HSMMs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNA was synthesized by RT-PCR using the Takara RNA PCR kit
(AMV) ver 3. (cat. no. RR019) supplied by Takara Bio, Inc. The
first strand cDNA was synthesized from 1 g total RNA using the AMV
reverse transcriptase and the random 9-mer oligo-nucleotide. The RT
reaction was performed for 1 cycle under the condition of 30°C for
10 min, 42°C for 30 min, 95°C for 5 min and 5°C for 5 min. PCR
(Takara Bio, Inc.) was conducted using the SYBR Premix Ex Taq
(Takara Bio, Inc.) and the following primers: GLUT4 forward, 5′-TTC
TCT GCG GTG CTT GG CTC-3′ and reverse 5′-TCC CCA GCC ACG TCT CAT
TG-3′; GAPDH forward, 5′-GAG TCA ACG GAT TTG GAC GT-3′ and reverse
5′-GAC AAG CTT CCC GTT CTC AG-3′; and SIRT1 forward, 5′-TTG GAC TCT
GGC ATG TCC CA-3′ and reverse 5′-CAT TTT CCA TGG CGC TGA GG-3′. The
PCR cycle included a holding stage for 30 sec at 95°C and cycling
stage for 5 sec at 95°C and 30 sec at 60°C. Changes in GLUT4 or
SIRT1 gene expression were normalized to the housekeeping gene
GAPDH and were quantified using the 2−∆∆Cq method
(21).
Small interfering RNAs
The 21-nucleotide small interfering RNA (siRNA)
duplexes for green fluorescent protein (GFP; 5′-GUU CAG CGU GUC CGG
CGA GTT-3′), SIRT1 (5′-GGA UAG AGC CUC ACA UGC AUU-3′), and GLUT4
(5′-GAU UGA ACA GAG CUA CAA U-3′) were designed and created at
Genolution Pharmaceuticals. The cells were transfected with the
siRNA oligonucleotides and cDNA (pCDNA3 plasmid) using a
pipette-type electroporator (Digital Biotechnology) according to
the manufacturer's protocol (pulse voltage, 1,005 V; pulse width,
35 msec; pulse number 2). Briefly, the cells were transfected with
100 pg of each siRNA, cDNA, or 3 µg pcDNA3 in 100 µl
R buer. After transfection, the cells were seeded in six-well
plates, allowed to differentiate for 3 days and treated with or
without GLP-1.
Western blot analysis
The levels of GLUT4 (1:1,000; cat. no. 2213;
overnight at 4°C), GAPDH (14C10; 1:2,000; cat. no. 2118; 2 h at
room temperature), Akt (1:2,000; cat. no. 9272; overnight at 4°C),
and PKA (PKA c-α; 1:2,000; cat. no. 4782; overnight at 4°C) were
determined using antibodies from Cell Signaling Technology, Inc.
SIRT1 (1:10,000; cat. no. SC-15404; overnight at 4°C), and IRS-1
(1:2,000; cat. no. SC-559; overnight at 4°C) antibodies were
obtained from Santa Cruz Biotechnology, Inc. Cytoskeletal actin
antibodies (1:10,000; cat. no. A300-491A; 1 h at room temperature)
were obtained from Bethyl Laboratories, Inc. The levels of IRS-1,
Akt, and PKA were determined using antibodies specific for
phospho-IRS-1 (p-IRS-1; PY612; 1:500; cat. no. MBS624304;
BioSource, Inc.), phospho-Akt (p-Akt; Ser473; 1:1,000; cat. no.
9275; Cell Signaling Technology, Inc.) and phospho-PKA C (p-PKA C;
Thr197; 1:1,000; cat. no. 4781; Cell Signaling Technology, Inc.)
overnight at 4°C. The HSMMs were homogenized at 4°C in lysis buffer
[20 mmol/l Tris (pH 8.0), 137 mmol/l NaCl, 1 mmol/l
MgCl2, 2 mmol/l CaCl2, 1% NP-40, 2 mmol/l
vana-date, 1 mmol/l DTT, 2.5 mmol/l phenylmethylsulfonyl fluoride],
0.12 g/ml nicotinamide, 1 mol/l trichostatin A, and the resulting
lysate was centrifuged for 20 min at 12,000 × g and 4°C. Aliquots
of the supernatant were removed for protein quantification using
the Bradford assay (Coomassie Protein Reagent; Pierce; Thermo
Fisher Scientific, Inc.). The supernatant was incubated in SDS
sample buffer (100 mmol/l DTT and 100 µg sample) for 5 min
at 95°C and resolved by SDS-PAGE on a 10% gel, and the separated
proteins were transferred to a polyvinylidene fluoride membrane
(EMD Millipore). After blocking with 3% skimmed milk for 30 min at
room temperature, the membranes were incubated with primary
antibodies as mentioned above and then a peroxidase-conjugated
anti-rabbit IgG secondary antibody (1:3,000; cat. no. SC2357; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. All
immunoreactive bands were visu-alized using an enhanced
chemiluminescence detection system (Amersham ECL Prime Western
Blotting Detection Reagent; cat. no. RPN2232; GE Healthcare), and
the band intensities were determined using a Quantity One-4.6.8
(basic) analysis software (Bio-Rad Laboratories, Inc.), which is a
one-dimensional image analysis program. The levels of each protein
were normalized to the total protein values.
Statistical analysis
All results are presented as the mean ± standard
deviation consisting of at least three independent experiments, and
all statistical analyses were performed using SPSS (version 19.0;
IBM Corp.). A Student's t-test was used for comparison of two
dependent groups and ANOVA with post-hoc tests (Bonferroni test)
was used for comparison of multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
GLP-1 reverses palmitate-induced insulin
resistance in HSMMs
When treated with palmitate (100 or 200 umol/l) in
HSMMs, no significant viability reduction was noted (Fig. S1). The expression of the GLP-1
receptor in human skeletal muscle tissue was confirmed (Fig. S2) and then analysed further;
there was an increase following exposure to recombinant GLP-1 (200
nmol/l). To demonstrate the effects of GLP-1 in HSMMs exposed to
palmitate, the levels of 2-NBDG uptake, GLUT4 mRNA, and GLUT4
promoter activity were measured in palmitate-treated (200
µmol/l) or palmitate-(200 µmol/l) and GLP-1-treated
(100 or 200 nmol/l) cells. Compared with basal conditions, there
was a significant increase in glucose uptake in HSMMs exposed to
GLP-1 (P<0.05; Fig. 1A) and a
significant decrease in glucose uptake in HSMMs exposed to
palmitate (P<0.05; Fig. 1A).
The co-administration of palmitate and GLP-1 (200 nmol/l)
significantly restored the palmitate-induced reduction in glucose
uptake compared with treatment with palmitate (200 µmol/l)
alone (P<0.05) regardless of whether insulin was present
(Fig. 1A).
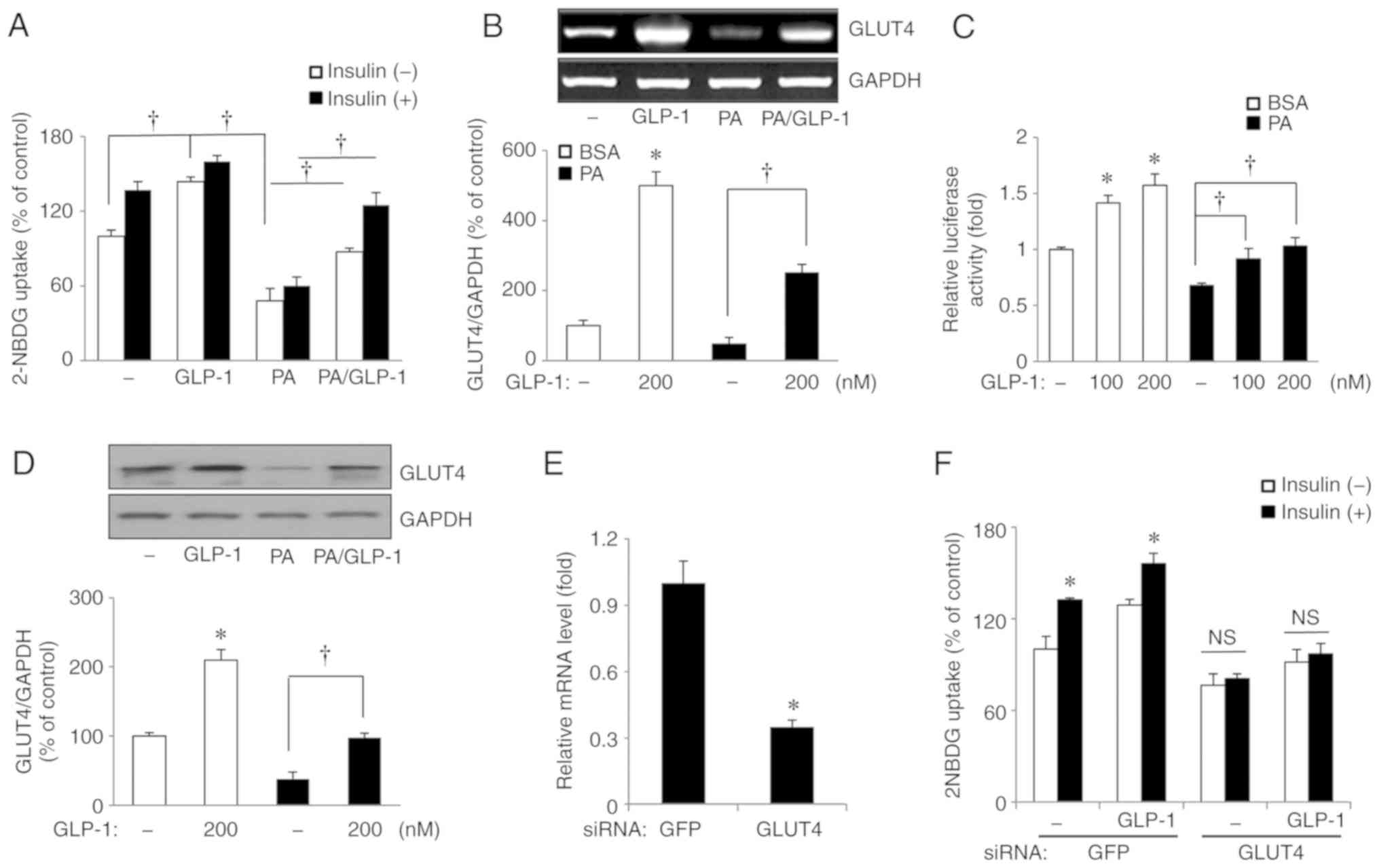 | Figure 1Effects of GLP-1 on glucose uptake
and GLUT4 mRNA expression, promoter activity and protein expression
in human skeletal muscle myotubes with or without exposure to PA.
GLP-1 restores the PA-induced reduction in glucose uptake. GLP-1
reverses GLUT4 mRNA expression, promoter activity levels and GLUT4
protein expression. Knockdown of GLUT4 significantly decreases
glucose uptake by GLP-1. Cells were exposed to 200 µmol/l PA
and 100 or 200 nmol/l GLP-1 for 24 h. (A) Glucose uptake. (B) GLUT4
mRNA expression. (C) GLUT4 promoter activity. (D) GLUT4 protein
expression. (E) Tansfection efficiency for siRNA GLUT4. (F) Glucose
uptake by GLP-1 before and after siRNA transfection.
*P<0.05 vs. basal conditions; †P<0.05.
2-NBDG,
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose;
PA, palmitate; GFP, green fluorescent protein; GLP-1, glucagon like
peptide-1; GLUT4, glucose transporter type 4; siRNA, small
interfering RNA; NS, not significant. |
There was an elevation in GLUT4 mRNA in HSMMs
treated with GLP-1 compared with basal conditions (P<0.05;
Fig. 1B). When palmitate and
GLP-1 were co-administered, GLP-1 significantly restored the
palmitate-induced reductions in GLUT4 mRNA, compared with palmitate
(200 µmol/l) alone (P<0.05; Fig. 1B). GLUT4 promoter activity was
measured using a luciferase assay. Similar to the glucose uptake
and GLUT4 mRNA results, GLP-1 increased GLUT4 promoter activity
(P<0.05 for both 100 and 200 nmol/l GLP-1; Fig. 1C) and palmitate decreased GLUT4
promoter activity (P<0.05; Fig.
1C) compared with basal conditions. When GLP-1 and palmitate
were co-administered, GLP-1 (100 and 200 nmol/l) significantly
restored GLUT4 promoter activity levels, compared with palmitate
(200 µmol/l) alone (P<0.05 for both 100 and 200 nmol/l
GLP-1 with palmitate; Fig. 1C).
There were also consistent results in GLUT4 protein expression in
GLP-1-only, palmitate-only, and GLP-1 and palmitate treatment
conditions (Fig. 1D). To
determine whether the enhanced glucose uptake of GLP-1 by HSMMs was
mediated by GLUT4, glucose uptake in cells treated with siRNA
targeting GLUT4 was measured. The knockdown of GLUT4 significantly
decreased glucose uptake (P<0.05; Fig. 1E and F). These findings suggest
GLP-1 restored palmitate-induced decreases in glucose uptake via
restoration of GLUT4 expression and GLUT4 promoter stimulation in
HSMMs. In other words, GLP-1 influences glucose uptake directly in
human skeletal muscles under conditions of insulin resistance as
well as via insulinotropic actions.
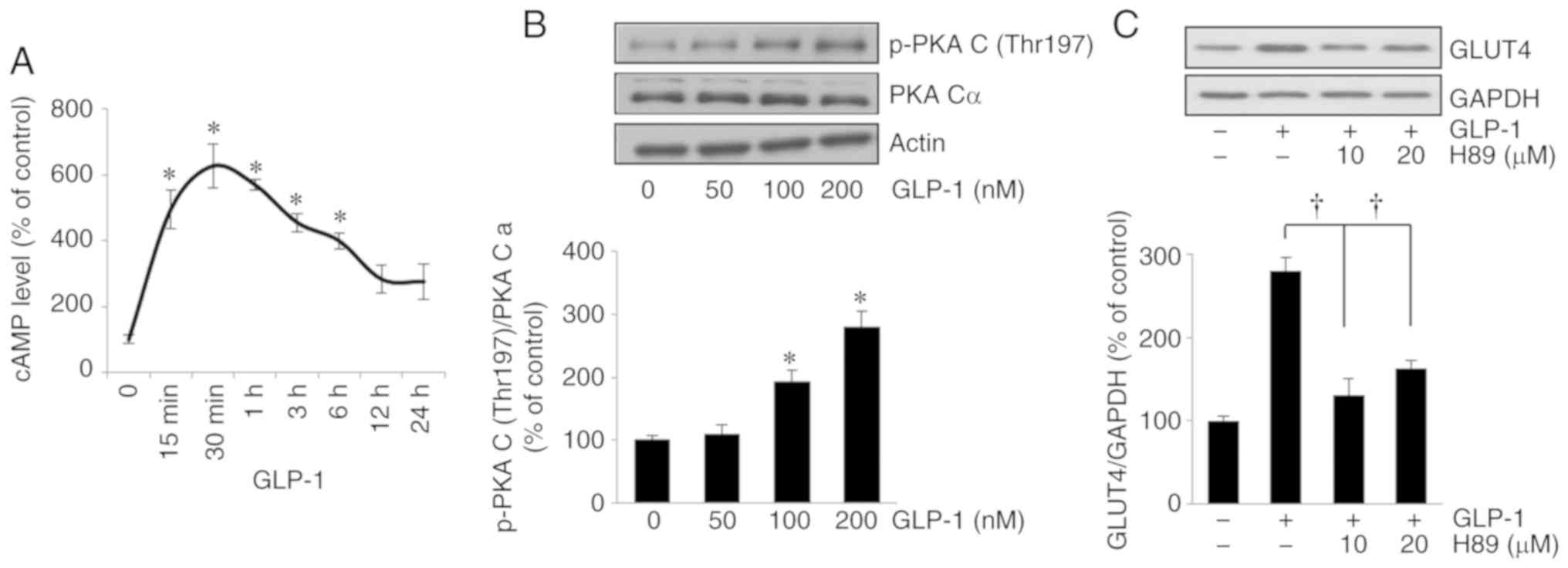
cAMP and PKA signaling cascades acts as
downstream signals of GLP-1 in HSMMs
The classical downstream signals of GLP-1 in
pancreatic β cells, PKA, and cAMP, were investigated in relation to
the activation of GLP-1 in HSMMs. The changes in cAMP were
time-dependent; compared with the baseline, cAMP levels exhibited a
significant elevation 15 min (P<0.05), peaking 30 min
(P<0.05), after treatment with 200 nmol/l GLP-1 (Fig. 2A). Following treatment with GLP-1,
there was a consistent and significant increase in p-PKA C levels
in relation to the GLP-1 concentration, compared with basal
conditions (P<0.05 and P<0.05 for 100 and 200 nmol/l GLP-1,
respectively; Fig. 2B). The
suppression by PKA inhibitor (H89) of the GLP-1-induced increases
in GLUT4 protein (P<0.05 and P<0.05 at 10 and 20
µmol/l H89, respectively; Fig.
2C) suggested that the PKA/cAMP signaling pathway is involved
in the increase in GLUT4 expression induced by GLP-1.
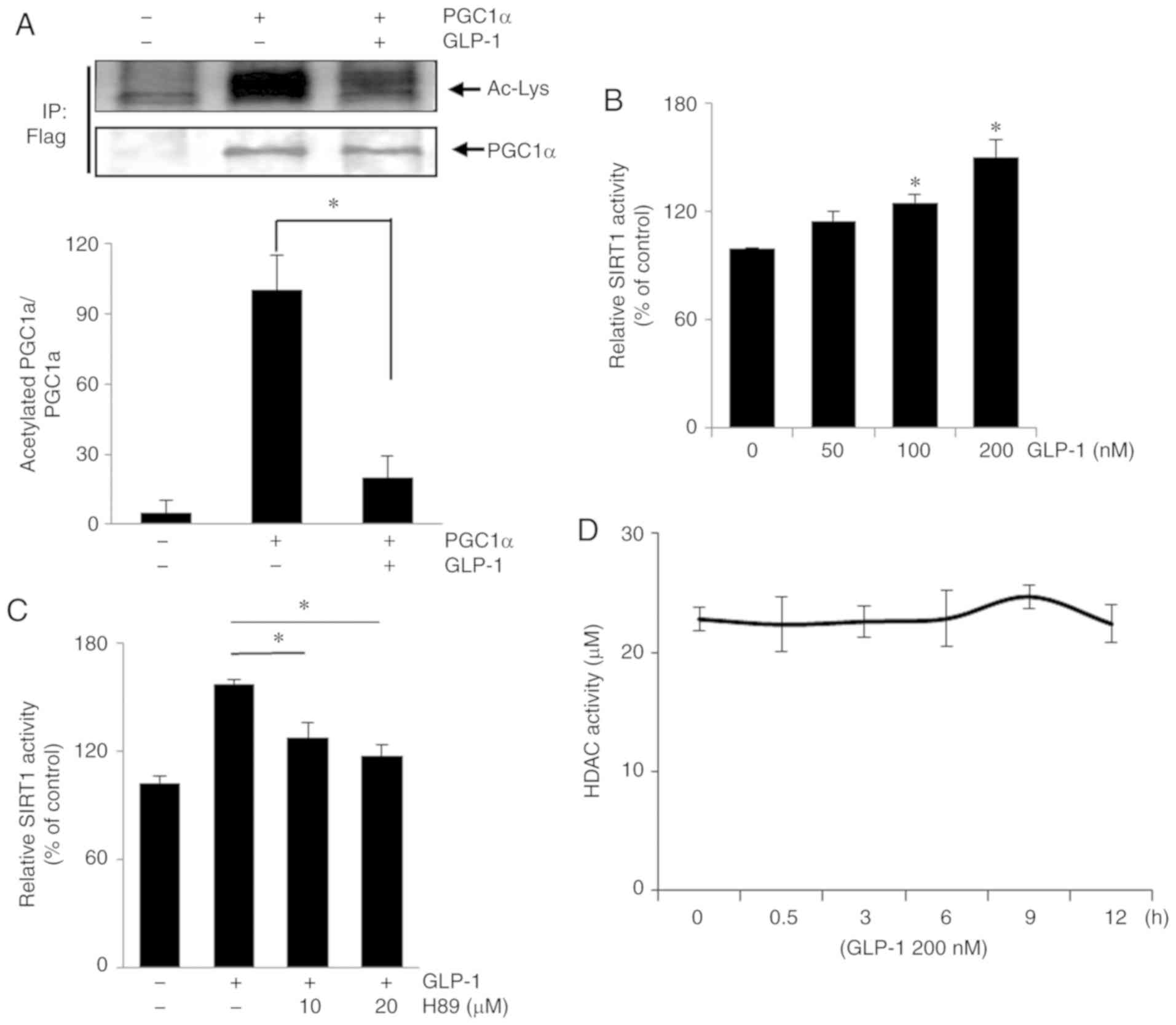
GLP-1 leads to the deacetylation of PGC1α
and increases SIRT1 activity in HSMMs
To determine the linkage between GLP-1 and SIRT1 in
HSMMs, the deacetylation levels of PGC1α were assessed after GLP-1
(200 nmol/l) was added to HSMMs overexpressing Flag-tagged PGC1α.
SIRT1 has been shown to induce PGC1α deacetylation (22). Immunoblot assays using the
indicated antibodies were performed after HSMMs expressing
Flag-tagged PGC-1α were immunoprecipitated by antibodies targeting
the Flag-tag. GLP-1 decreased the levels of acetylated PGC1α
(Fig. 3A), indicating the
presence of GLP-1-activated deacetylases, such as SIRT1. Thus, in
HSMMs treated with GLP-1, SIRT1 activity was proportional to the
GLP-1 concentration (Fig. 3B).
PKA is an enhancer of SIRT1 activity (23) and a downstream signal of GLP-1, as
also demonstrated above. The PKA inhibitor, H89, suppressed SIRT1
activity which was stimulated by GLP-1 (200 nmol/l; Fig. 3C). In a further experiment,
involvement of HDAC-mediated deacetylation, in addition to SIRT1
activity in GLP-1-treated cells was ruled out (Fig. 3D).
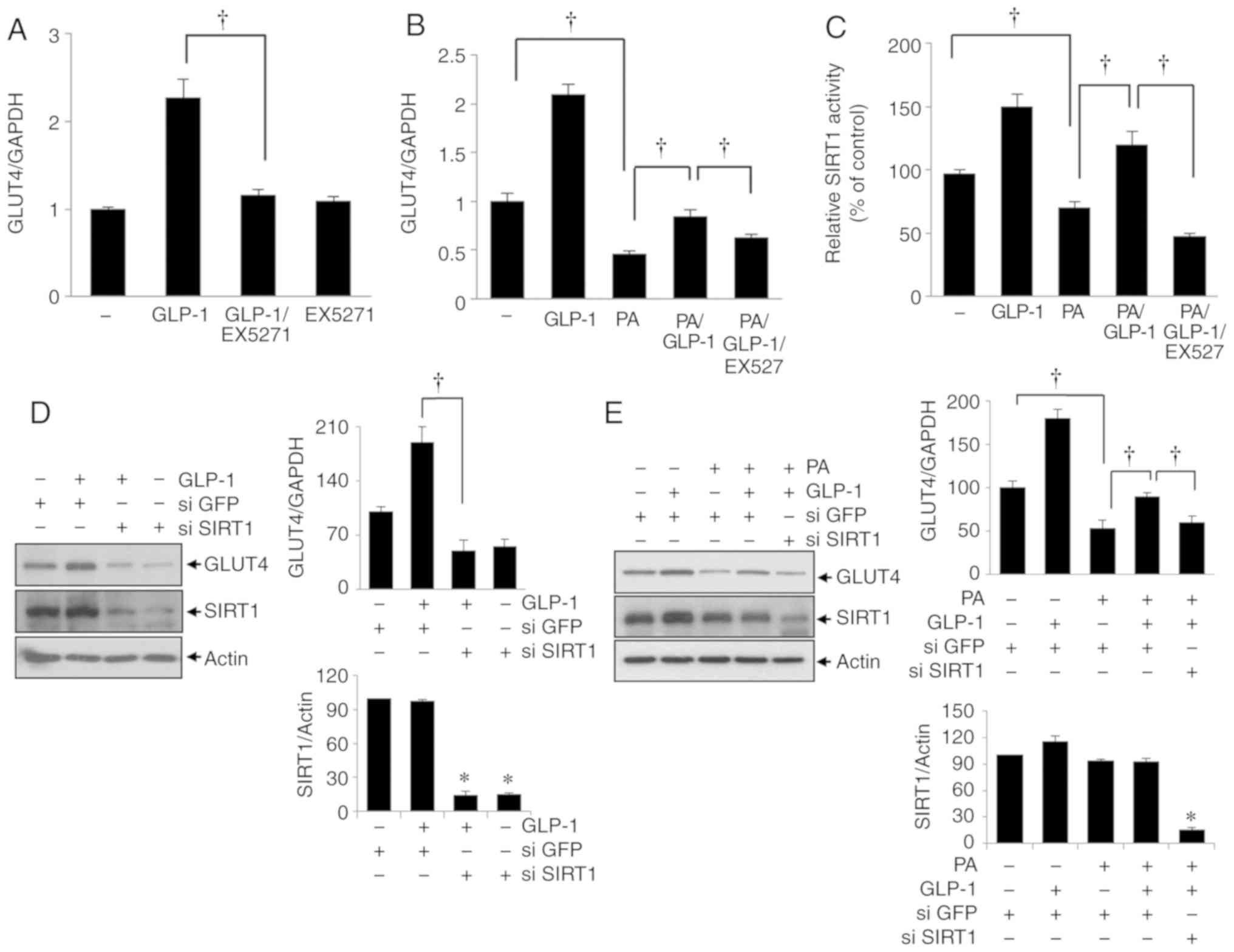
Inhibition of SIRT1 activity suppresses
GLP-1-induced GLUT4 expression in HSMMs
To confirm the role of SIRT1 during GLP-1 activity
in HSMMs, the SIRT1 inhibitor EX527 was applied to the cells, and
GLUT4 mRNA levels were assessed using reverse transcription PCR.
EX527 (10 µmol/l) significantly repressed GLP-1-induced
GLUT4 mRNA expression in HSMMs exposed to GLP-1 alone (200 nmol/l;
P<0.05; Fig. 4A) and with
co-administered palmitate (200 µmol/l) and GLP-1 (200
nmol/l; P<0.05; Fig. 4B).
Simultaneously, SIRT1 activity was measured in cells treated with
GLP-1 and/or palmitate and/or EX527. GLUT4 mRNA expression levels
were correlated with the levels of SIRT1 activity (Fig. 4C). To confirm the SIRT1 dependence
of GLP-1 activity in HSMMs, the cells were treated with SIRT1
siRNA. The transfection efficiency on the expression of SIRT1 was
demonstrated with the si-SIRT1 plasmid (Fig. S3). Consistent with the results
obtained using the SIRT1 inhibitor, SIRT1 siRNA significantly
suppressed the GLP1-induced enhancement of GLUT4 expression in
HSMMs with/without palmitate (P<0.05; Fig. 4D; palmitate, 200 µmol/l,
P<0.05; Fig. 4E). These
findings suggest the restoration of GLP-1-induced GLUT4 expression
was mediated by SIRT1 in HSMMs exposed to palmitate.
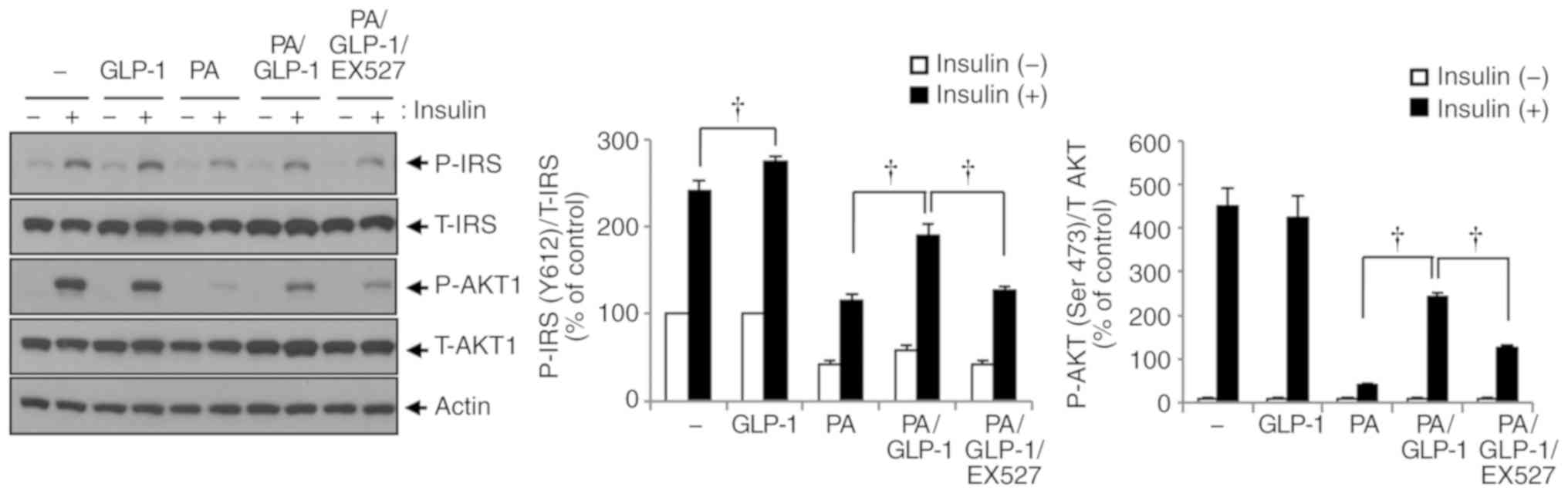
The SIRT1 inhibitor EX527 suppresses the
GLP-1-induced phosphorylation of IRS-1 and Akt in HSMMs exposed to
palmitate
The present study investigated whether GLP-1
influenced the activation of insulin signaling pathways and whether
SIRT1 was involved in the reversal of insulin resistance by GLP-1
in HSMMs exposed to palmitate. The differentiated HSMMs were
treated with palmitate, recombinant GLP-1, and/or EX527 for 24 h
and then stimulated with insulin for 30 min. The phosphorylation
levels of IRS-1 and Akt (proteins related to insulin signaling
pathways) in HSMMs exposed to palmitate (200 µmol/l) and
GLP-1 (200 nmol/l) were simultaneously determined by adding EX527
(10 µmol/l) to the cells. GLP-1 increased, while palmitate
decreased the phosphorylation of IRS-1 in HSMMs exposed to insulin.
Palmitate decreased the phosphorylation of proteins in the insulin
signalling cascade, such as IRS-1 and Akt (Fig. 5). When GLP-1 and palmitate were
co-administered, GLP-1 significantly reversed the palmitate-induced
reduction in insulin signaling in HSMMs exposed to insulin compared
with palmitate (200 µmol/l) and insulin (100 nmol/l;
P<0.05; Fig. 5). Moreover, in
HSMMs exposed to both palmitate and GLP-1 in conjunction with
insulin, EX527 limited the GLP-1-induced enhancement of
phosphorylated IRS-1 and Akt compared with palmitate (200
µmol/l), GLP-1 (200 nmol/l), and insulin (100 nmol/l;
P<0.05; Fig. 5). These
findings suggest that GLP-1 functioned through SIRT1 and improved
the palmitate-induced repression of insulin signaling pathways in
HSMMs. A model of the present study is presented in Fig. 6.
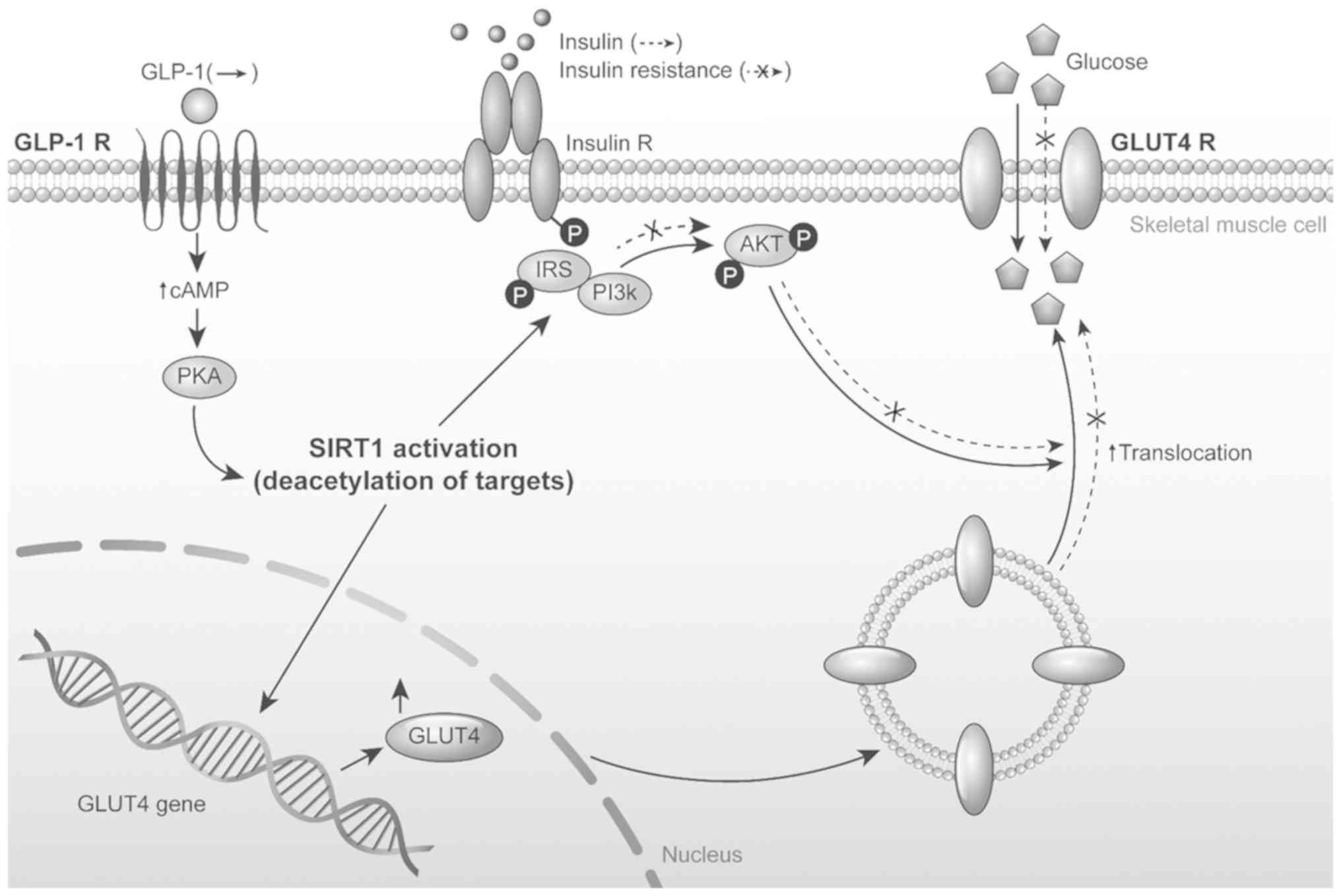 | Figure 6A working model of the GLP-1, SIRT1
and GLUT4 signalling pathway. GLP-1, glucagon like peptide-1; GLP-1
R, glucagon like peptide-1 receptor; insulin R, insulin receptor,
GLUT4 R, GLUT4 receptor; SIRT1, sirtuin 1; PKA, protein kinase A;
PI3K, phosphoinositide 3 kinase; IRS, insulin receptor substrate;
P, phosphate; cAMP, cyclic adenosine monophosphate. |
Discussion
The present study demonstrated that GLP-1 restores
palmitate-induced reductions in glucose uptake and GLUT4
expression, and improves the palmitate-induced repression of
insulin signaling pathways in HSMMs. These actions of GLP-1 were
shown to be mediated by SIRT1. The glucose-lowering effect of GLP-1
involves several mechanisms, the most important of which is
enhancement of glucose-dependent insulin secretion from pancreatic
β cells. GLP-1 also has extra-pancreatic actions, including in
adipose tissue and skeletal muscle, both of which are major
peripheral target organs for glucose control (24,25). Independent of the incretin effect,
GLP-1 plays a role in the stimulation of glucose disposal in
insulin-sensitive tissues (26-28) and increases glucose uptake and
GLUT1/GLUT4 expression in adipose tissue (24). In human skeletal muscle, GLP-1
stimulates glycogen synthesis and glucose uptake (25,29). In the present study, the increased
glucose uptake of GLP-1 in HSMMs was shown to be mediated by GLUT4.
GLP-1 seems to influence glucose uptake by increasing both GLUT4
levels at the plasma membrane and total GLUT4 levels, although this
has yet to be confirmed in other studies (29-31). For example, a previous study
reported that GLP-1 has no direct effect on glucose uptake in human
skeletal muscle (32). However,
short-term treatment (1-2 h) with GLP-1 in individuals with normal
blood glucose may have little effect on GLUT4 translocation and
total GLUT4 expression; this may explain the differences between
studies regarding the effects of GLP-1 on the glucose concentration
(31).
SIRT1 is a promising treatment target for T2DM due
to its roles in the regulation of glucose and lipid metabolism,
insulin sensitization, and stimulation of insulin secretion
(2,33-35). In pancreatic β cells, GLP-1
inhibits SIRT1 activity which stimulates the mass expansion of β
cells, while SIRT1 acts as a negative regulator of β cell
proliferation and prevents the action of GLP-1 (19). In the mouse liver, a GLP-1
analogue activated SIRT1 and improved fat accumulation (16,17). However, the relationship between
GLP-1 and SIRT1 in skeletal muscle cells, is unclear. Thus, in the
present study whether SIRT1 influenced the action of GLP-1 during
glucose uptake was examined. The results of the present study
showed that GLP-1 activated SIRT1 and restored GLUT4 expression in
HSMMs treated with palmitate.
The present study was also able to demonstrate that
GLP-1 ameliorates insulin resistance via the activation of insulin
signaling pathways in insulin-resistant HSMMs. The inhibition of
IRS and Akt phosphorylation by a GLP-1 agonist enhanced GLUT4
translocation in palmitate-treated muscle cells (36). Moreover, in the present study,
SIRT1 was shown to be involved in the GLP-1-induced activation of
insulin signaling pathways, leading to enhancement of IRS and Akt
phosphorylation. In contrast to pancreatic β cells, the cell
signaling cascades associated with GLP-1 in skeletal muscles have
yet to be fully characterized. GLP-1 has been shown to activate the
PI3K/Akt, p70s6k, p42, and p44 mitogen-activated protein kinase
pathways in rat and human skeletal muscles (9,37),
but GLP-1 also influences glucose uptake via PI3K-dependent and
Akt-independent mechanisms (9,30,38). GLP-1 improves insulin sensitivity
via insulin-mediated mechanisms including enhanced glucose disposal
and suppression of endogenous glucose production in patients with
type 2 diabetes, obesity, and normal glucose tolerance (38-40). Additionally, apart from its
incretin effects, GLP-1 potentiates insulin action in
depancreatised dogs (41). The
effects of SIRT1 on insulin sensitivity remain unclear. SIRT1 may
be the mediator that links caloric restriction to enhanced levels
of insulin sensitivity within skeletal muscles (41).
Although GLP-1 receptors are present in skeletal
muscles, they appear to be functionally different from those
located in pancreatic β cells, because muscle-specific GLP-1
receptors do not increase cAMP levels (23,42). The present study reconfirmed that
GLP-1 receptors are expressed in human skeletal muscles, which is
consistent with the first report of GLP-1 receptor expression in
human skeletal muscles (29). In
contrast to previous studies of rat myotubes (43,44), the present study showed that GLP-1
increased glucose uptake and GLUT4 expression through the cAMP/PKA
pathway in HSMMs. cAMP levels rose 15 min after GLP-1 treatment and
peaked at a six-fold increase over basal levels. A PKA inhibitor
also suppressed the GLP-1 effect on GLUT4 expression. Moreover,
downstream involvement of SIRT1 in the signaling pathway of GLP-1
was also determined. The activity of SIRT1 is controlled by various
mechanisms, including expression changes through transcription
factors, post-translational modifications, the formation of
complexes with other proteins, and altered NAD+ levels
(43). SIRT1 activity is
modulated by phosphorylation via the cAMP/PKA pathway (14,15). In another study, SIRT1-mediated
resveratrol was shown to be associated with an improvement of GLUT4
expression in muscles from T2DM mice (44). In conjunction with previous
results, the findings of the present study suggest that SIRT1
mediates the GLP-1 induced enhancement of glucose uptake and GLUT4
expression through the cAMP/PKA pathway. Therefore, the cAMP/PKA
pathway acts upstream of SIRT1, which is also consistent with
previous reports (14,15). The results of the present study
suggest that, in insulin-resistant HSMMs, GLP-1 influences glucose
metabolism through the GLP-1 receptor, cAMP/PKA pathway, SIRT1
activation and increased GLUT4 expression.
The expression of GLP-1 receptor in response to
GLP-1 ligand seems to be different in each target tissue. β-cell
expression of GLP-1 receptor was downregulated by GLP-1 (45). However, in endothelial cells, a
GLP-1 agonist elevated GLP1 receptor level (46). This result from endothelial cells
is consistent with data of the present study from skeletal muscle.
Regulators of GLP-1 receptor expression are not fully understood
although miR-204 and transcription factor 7-like 2 (TCF7L2) in
β-cells and TCF7L2 in endothelial cells were reported to be factors
regulating GLP-1 receptor expression (47,48). Further study is required to reveal
the mechanism regarding GLP-1 receptor expression by GLP-1 in
skeletal muscle.
The effects of SIRT1 on glucose homeostasis differ
among liver, pancreas, and fat tissue (12,34,48-51). There are several possible
underlying mechanisms that may support SIRT-induced improvements in
insulin sensitivity in skeletal muscles. For example, SIRT1
represses the expression of protein tyrosine phosphatase 1B and
increases the efficiency of PI3K signalling in response to caloric
restriction (52,53). In skeletal muscle, SIRT1
deacetylates PGC1α, induces mitochondrial fatty acid oxidation, and
improves insulin sensitivity (14,42,52). The findings of the present study
provided an additional mechanism by which SIRT1 may beneficially
influence glucose metabolism and insulin sensitivity as a mediator
of GLP-1 function in HSMMs.
In conclusion, the present study investigated
whether GLP-1 restores glucose uptake, as mediated by the cAMP/PKA
signaling pathway and GLUT4, in addition to enhancing the activity
of the insulin signaling pathway in palmitate-exposed HSMMs. The
results of the present study provide evidence of the involvement of
SIRT1 in the GLP-1-induced enhancement of glucose disposal and
insulin signaling in insulin-resistant HSMMs.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by the faculty
research fund (JJ) of Ajou University School of Medicine and a
grant (grant. no. NRF-2016-R1D1A1B03930214 to KL) of the National
Research Foundation of Korea.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ, TK, SH, HK, DK, YK and KL contributed to the
study design. JJ, SC, HL and EH performed the experiments for data
acquisition. JJ, SC and EH performed the statistical analyses. TK,
SH, HK, DK, HL and YK interpreted the experimental results. JJ and
SC contributed to the drafting of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeFronzo RA and Del Prato S: Insulin
resistance and diabetes mellitus. J Diabetes Complications.
10:243–245. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boden G: Free fatty acids (FFA), a link
between obesity and insulin resistance. Front Biosci. 3:d169–d175.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Minamino T, Komuro I and Kitakaze M:
Endoplasmic reticulum stress as a therapeutic target in
cardiovascular disease. Circ Res. 107:1071–1082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuzefovych L, Wilson G and Rachek L:
Different effects of oleate vs. palmitate on mitochondrial
function, apoptosis, and insulin signaling in L6 skeletal muscle
cells: Role of oxidative stress. Am J Physiol Endocrinol Metab.
299:E1096–E1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dasu MR and Jialal I: Free fatty acids in
the presence of high glucose amplify monocyte inflammation via
Toll-like receptors. Am J Physiol Endocrinol Metab. 300:E145–E154.
2011. View Article : Google Scholar :
|
|
6
|
Jung JG, Choi SE, Hwang YJ, Lee SA, Kim
EK, Lee MS, Han SJ, Kim HJ, Kim DJ, Kang Y and Lee KW:
Supplementation of pyruvate prevents palmitate-induced impairment
of glucose uptake in C2 myotubes. Mol Cell Endocrinol. 345:79–87.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee MS, Choi SE, Ha ES, An SY, Kim TH, Han
SJ, Kim HJ, Kim DJ, Kang Y and Lee KW: Fibroblast growth factor-21
protects human skeletal muscle myotubes from palmitate-induced
insulin resistance by inhibiting stress kinase and NF-kB.
Metabolism. 61:1142–1151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nauck MA and Meier JJ: Incretin hormones:
Their role in health and disease. Diabetes Obes Metab. 20(Suppl 1):
S5–S21. 2018. View Article : Google Scholar
|
|
9
|
Acitores A, Gonzalez N, Sancho V, Valverde
I and Villanueva-Penacarrillo ML: Cell signalling of glucagon-like
peptide-1 action in rat skeletal muscle. J Endocrinol. 180:389–398.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D'Alessio DA, Prigeon RL and Ensinck JW:
Enteral enhancement of glucose disposition by both
insulin-dependent and insulin-independent processes. A
physiological role of glucagon-like peptide I Diabetes.
44:1433–1437. 1995.
|
|
11
|
Giannocco G, Oliveira KC, Crajoinas RO,
Venturini G, Salles TA, Fonseca-Alaniz MH, Maciel RM and Girardi
AC: Dipeptidyl peptidase IV inhibition upregulates GLUT4
translocation and expression in heart and skeletal muscle of
spontaneously hypertensive rats. Eur J Pharmacol. 698:74–86. 2013.
View Article : Google Scholar
|
|
12
|
Banks AS, Kon N, Knight C, Matsumoto M,
Gutiérrez-Juárez R, Rossetti L, Gu W and Accili D: SirT1 gain of
function increases energy efficiency and prevents diabetes in mice.
Cell Metab. 8:333–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feige JN, Lagouge M, Canto C, Strehle A,
Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ and Auwerx J:
Specific SIRT1 activation mimics low energy levels and protects
against diet-induced metabolic disorders by enhancing fat
oxidation. Cell Metab. 8:347–358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gerhart-Hines Z, Dominy JE Jr, Blattler
SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP and
Puigserver P: The cAMP/PKA pathway rapidly activates SIRT1 to
promote fatty acid oxidation independently of changes in NAD(+).
Mol Cell. 44:851–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim JH, Gerhart-Hines Z, Dominy JE, Lee Y,
Kim S, Tabata M, Xiang YK and Puigserver P: Oleic acid stimulates
complete oxidation of fatty acids through protein kinase
A-dependent activation of SIRT1-PGC1α complex. J Biol Chem.
288:7117–7126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu F, Li Z, Zheng X, Liu H, Liang H, Xu H,
Chen Z, Zeng K and Weng J: SIRT1 mediates the effect of GLP-1
receptor agonist exenatide on ameliorating hepatic steatosis.
Diabetes. 63:3637–3646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee J, Hong SW, Chae SW, Kim DH, Choi JH,
Bae JC, Park SE, Rhee EJ, Park CY, Oh KW, et al: Exendin-4 improves
steato-hepatitis by increasing Sirt1 expression in high-fat
diet-induced obese C57BL/6J mice. PLoS One. 7:e313942012.
View Article : Google Scholar
|
|
18
|
Lee J, Hong SW, Park SE, Rhee EJ, Park CY,
Oh KW, Park SW and Lee WY: Exendin-4 attenuates endoplasmic
reticulum stress through a SIRT1-dependent mechanism. Cell Stress
Chaperones. 19:649–656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bastien-Dionne PO, Valenti L, Kon N, Gu W
and Buteau J: Glucagon-like peptide 1 inhibits the sirtuin
deacetylase SirT1 to stimulate pancreatic β-cell mass expansion.
Diabetes. 60:3217–3222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kharitonenkov A, Wroblewski VJ, Koester A,
Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB and Etgen
GJ: The metabolic state of diabetic monkeys is regulated by
fibroblast growth factor-21. Endocrinology. 148:774–781. 2007.
View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Nemoto S, Fergusson MM and Finkel T: SIRT1
functionally interacts with the metabolic regulator and
transcriptional coacti-vator PGC-1{alpha}. J Biol Chem.
280:16456–16460. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Houtkooper RH, Canto C, Wanders RJ and
Auwerx J: The secret life of NAD+: An old metabolite controlling
new metabolic signaling pathways. Endocr Rev. 31:194–223. 2010.
View Article : Google Scholar :
|
|
24
|
Holz GG: Epac: A new cAMP-binding protein
in support of glucagon-like peptide-1 receptor-mediated signal
transduction in the pancreatic beta-cell. Diabetes. 53:5–13. 2004.
View Article : Google Scholar
|
|
25
|
Luque MA, Gonzalez N, Marquez L, Acitores
A, Redondo A, Morales M, Valverde I and Villanueva-Peñacarrillo ML:
Glucagon-like peptide-1 (GLP-1) and glucose metabolism in human
myocytes. J Endocrinol. 173:465–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Alessio DA, Kahn SE, Leusner CR and
Ensinck JW: Glucagon-like peptide 1 enhances glucose tolerance both
by stimulation of insulin release and by increasing
insulin-independent glucose disposal. J Clin Invest. 93:2263–2266.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Egan JM, Montrose-Rafizadeh C, Wang Y,
Bernier M and Roth J: Glucagon-like peptide-1(7-36) amide (GLP-1)
enhances insulin-stimulated glucose metabolism in 3T3-L1
adipocytes: One of several potential extrapancreatic sites of GLP-1
action. Endocrinology. 135:2070–2075. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miki H, Namba M, Nishimura T, Mineo I,
Matsumura T, Miyagawa J, Nakajima H, Kuwajima M, Hanafusa T and
Matsuzawa Y: Glucagon-like peptide-1(7-36)amide enhances
insulin-stimulated glucose uptake and decreases intracellular cAMP
content in isolated rat adipocytes. Biochim Biophys Acta.
1312:132–136. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Green CJ, Henriksen TI, Pedersen BK and
Solomon TP: Glucagon like peptide-1-induced glucose metabolism in
differentiated human muscle satellite cells is attenuated by
hyperglycemia. PLoS One. 7:e442842012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arnes L, Gonzalez N, Tornero-Esteban P,
Sancho V, Acitores A, Valverde I, Delgado E and
Villanueva-Peñacarrillo ML: Characteristics of GLP-1 and exendins
action upon glucose transport and metabolism in type 2 diabetic rat
skeletal muscle. Int J Mol Med. 22:127–132. 2008.PubMed/NCBI
|
|
31
|
Li Z, Ni CL, Yao Z, Chen LM and Niu WY:
Liraglutide enhances glucose transporter 4 translocation via
regulation of AMP-activated protein kinase signaling pathways in
mouse skeletal muscle cells. Metabolism. 63:1022–1030. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sjøberg KA, Holst JJ, Rattigan S, Richter
EA and Kiens B: GLP-1 increases microvascular recruitment but not
glucose uptake in human and rat skeletal muscle. Am J Physiol
Endocrinol Metab. 306:E355–E362. 2014. View Article : Google Scholar :
|
|
33
|
Rodgers JT, Lerin C, Haas W, Gygi SP,
Spiegelman BM and Puigserver P: Nutrient control of glucose
homeostasis through a complex of PGC-1alpha and SIRT1. Nature.
434:113–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu J and Auwerx J: The role of sirtuins in
the control of metabolic homeostasis. Ann N Y Acad Sci. 1173(Suppl
1): E10–E19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quiñones M, Al-Massadi O, Fernø J and
Nogueiras R: Cross-talk between SIRT1 and endocrine factors:
Effects on energy homeo-stasis. Mol Cell Endocrinol. 397:42–50.
2014. View Article : Google Scholar
|
|
36
|
Li Z, Zhu Y, Li C, Tang Y, Jiang Z, Yang
M, Ni CL, Li D, Chen L and Niu W: Liraglutide ameliorates
palmitate-induced insulin resistance through inhibiting the IRS-1
serine phosphorylation in mouse skeletal muscle cells. J Endocrinol
Invest. 41:1097–1102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gonzalez N, Acitores A, Sancho V, Valverde
I and Villanueva-Penacarrillo ML: Effect of GLP-1 on glucose
transport and its cell signalling in human myocytes. Regul Pept.
126:203–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Egan JM, Meneilly GS, Habener JF and Elahi
D: Glucagon-like peptide-1 augments insulin-mediated glucose uptake
in the obese state. J Clin Endocrinol Metab. 87:3768–3773. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zander M, Madsbad S, Madsen JL and Holst
JJ: Effect of 6-week course of glucagon-like peptide 1 on glycaemic
control, insulin sensitivity, and beta-cell function in type 2
diabetes: A parallel-group study. Lancet. 359:824–830. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Seghieri M, Rebelos E, Gastaldelli A,
Astiarraga BD, Casolaro A, Barsotti E, Pocai A, Nauck M, Muscelli E
and Ferrannini E: Direct effect of GLP-1 infusion on endogenous
glucose production in humans. Diabetologia. 56:156–161. 2013.
View Article : Google Scholar
|
|
41
|
Sandhu H, Wiesenthal SR, MacDonald PE,
McCall RH, Tchipashvili V, Rashid S, Satkunarajah M, Irwin DM, Shi
ZQ, Brubaker PL, et al: Glucagon-like peptide 1 increases insulin
sensitivity in depancreatized dogs. Diabetes. 48:1045–1053. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang H, Egan JM, Wang Y, Moyes CD, Roth J,
Montrose MH and Montrose-Rafizadeh C: GLP-1 action in L6 myotubes
is via a receptor different from the pancreatic GLP-1 receptor. Am
J Physiol. 275:C675–C683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Houtkooper RH, Pirinen E and Auwerx J:
Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol
Cell Biol. 13:225–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yonamine CY, Pinheiro-Machado E, Michalani
ML, Alves-Wagner AB, Esteves JV, Freitas HS and Machado UF:
Resveratrol improves glycemic control in Type 2 diabetic obese mice
by regulating glucose transporter expression in skeletal muscle and
liver. Molecules. 22:pii: E1180. 2017.PubMed/NCBI
|
|
45
|
Fehmann HC, Jiang J, Pitt D, Schweinfurth
J and Goke B: Ligand-induced regulation of glucagon-like peptide-I
receptor function and expression in insulin-secreting beta cells.
Pancreas. 13:273–282. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu L, Liu J, Wong WT, Tian XY, Lau CW,
Wang YX, Xu G, Pu Y, Zhu Z, Xu A, et al: Dipeptidyl peptidase 4
inhibitor sita-gliptin protects endothelial function in
hypertension through a glucagon-like peptide 1-dependent mechanism.
Hypertension. 60:833–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jo S, Chen J, Xu G, Grayson TB, Thielen LA
and Shalev A: miR-204 controls glucagon-like peptide 1 receptor
expression and agonist function. Diabetes. 67:256–264. 2018.
View Article : Google Scholar :
|
|
48
|
Kimura T, Obata A, Shimoda M, Okauchi S,
Hirukawa H, Kohara K, Kinoshita T, Nogami Y, Nakanishi S, Mune T,
et al: Decreased glucagon-like peptide 1 receptor expression in
endothelial and smooth muscle cells in diabetic db/db mice: TCF7L2
is a possible regulator of the vascular glucagon-like peptide 1
receptor. Diab Vasc Dis Res. 14:540–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Frescas D, Valenti L and Accili D: Nuclear
trapping of the fork-head transcription factor FoxO1 via
Sirt-dependent deacetylation promotes expression of glucogenetic
genes. J Biol Chem. 280:20589–20595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bordone L, Motta MC, Picard F, Robinson A,
Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A,
et al: Sirt1 regulates insulin secretion by repressing UCP2 in
pancreatic beta cells. PLoS Biol. 4:e312006. View Article : Google Scholar
|
|
51
|
Lee JH, Song MY, Song EK, Kim EK, Moon WS,
Han MK, Park JW, Kwon KB and Park BH: Overexpression of SIRT1
protects pancreatic beta-cells against cytokine toxicity by
suppressing the nuclear factor-kappaB signaling pathway. Diabetes.
58:344–351. 2009. View Article : Google Scholar :
|
|
52
|
Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X
and Zhai Q: SIRT1 improves insulin sensitivity under
insulin-resistant conditions by repressing PTP1B. Cell Metab.
6:307–319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schenk S, McCurdy CE, Philp A, Chen MZ,
Holliday MJ, Bandyopadhyay GK, Osborn O, Baar K and Olefsky JM:
Sirt1 enhances skeletal muscle insulin sensitivity in mice during
caloric restriction. J Clin Invest. 121:4281–4288. 2011. View Article : Google Scholar : PubMed/NCBI
|