Introduction
Clinically, tooth decay can be easily neglected, and
it has usually already affected the pulp when symptoms appear. A
common treatment for this is often to remove the pulp. Sometimes
the pulp inflammation is mild, although the pulp remains necrotic.
It seems that once decay has invaded the pulp, the pulp's
self-repairing ability is not strong enough to combat its effects.
A number of studies (1,2) have confirmed that pulp contains
highly active and differentiated dental pulp mesenchymal stem
cells, which are a type of somatic stem cells, and the
proliferative and repair regeneration abilities of dental pulp stem
cells are stronger than those of bone marrow stem cells. However,
it remains to be elucidate as to why dental pulp cells are not able
to resist foreign pathogens, and eventually necrosis, when the pulp
becomes inflamed. Researchers are investigating effective methods
with which to prevent caries from further invading the pulp.
Clinically, some indirect capping materials are used to maintain
pulp vitality that is considered to encroach on the pulp. Studies
have shown that the process of repairing dentin can be induced
following mitigation (3-5). However, the process of repair and
regeneration is actually an immune process. Firstly, relatively low
levels of inflammatory molecules are released from the soft and
hard tissues of the teeth. In the meanwhile, the addition of
antioxidants, such as N-acetyl cysteine (NAC) to the Anga material
can limit the activity of the nuclear factor (NF)-κB
proinflammatory pathway by upregulating the p38 mitogen-activated
kinase signaling (MAPK) signaling pathway, and thus releasing the
corresponding cytokines (6). This
process plays an important role in minimizing the inflammatory
response of the pulp. Therefore, it provides a more favorable
environment for promoting dental hard tissue repair.
As is well known, following inflammation and tissue
damage, the complement system immediately provides the signals
needed to eliminate invaders and alter host cells. The activation
of the complement system occurs primarily through infection;
however, it can also be initiated through trauma. Following
complement activation, a series of complementary protein fragments
with important biological effects, such as C3 convertase, C5
convertase, complement 3a (C3a), complement 3b (C3b), complement 4b
(C4b), complement 5a (C5a) and complement 5b (C5b) are formed
(7). C3a, C4a and C5a can cause
the degranulation of basophils/mast cells and a higher release of
histamine, serotonin and vascular active mediators (8-15).
Subsequently, these indirectly alter the vascular permeability and
thereby cause the inflammatory response. C5a is a protein fragment
released from the cleavage of complement component C5 by protease
C5-convertase into C5a and C5b fragments. C5b deposits on the
surface of the pathogen, and C5a is then released into the liquid
phase. As an anaphylaxis, C5a has the most potent effect compared
to C3a and C4a. C5a is 20- to 2,500-fold stronger than C3a and C4a
(16). For this reason, we
selected to C5a for investigation in this study. Complement
activation protects the body against foreign pathogens. However,
sometimes it can damage the body via an overactive immune system as
described above.
In addition, the risk of developing local and
distant organ failure following complement activation depends on
the levels of circulating anaphylatoxins and terminal complement
complex levels, and the duration of complement activation (17). Patients with extensive tissue
damage and massive complement activation are prone to uncontrolled
inflammatory reactions and subsequent multiple organ dysfunction
syndrome (MODS) (18). On the
other hand, clinical trials with experimental animals in trauma
with complement depletion, complement deficiency, or the
administration of exogenous complement inhibitors have demonstrated
that the response of important organs to inflammatory stimuli is
significantly reduced and has a protective effect (19-24). These trials confirm the important
role of complement in the inflammatory response caused by trauma
from the opposite side of the inflammation-repair continuum.
Therefore, an uncontrolled inflammatory response can lead to
pathological damage. Studies have shown that C5a is involved in the
inflammatory response mainly through the C5a receptor (C5aR) of
inflammatory cells. Therefore, blocking the interaction between C5a
and C5aR is expected to inhibit its inflammatory response (17,18). High concentrations of C5a act as
chemotactic agents for related immune cells, and can induce immune
cells to move in the direction of the concentration gradient of
C5a, such as neutrophils, eosinophils, and monocytes (19). High concentrations of C5a can also
stimulate the oxidative metabolism of neutrophils and monocytes,
increase the level of cGMP, promote the fusion of lysosomes and
cell membranes, and release lysosomal enzymes. In addition, C5a
also has the effect of enhancing the immune response, specifically
to induce the secretion of interleukin (IL)-1, IL-6, IL-8 and tumor
necrosis factor (TNF)-α by monocytes (22), and promotes T cell proliferation
and B cell production of antibodies. C5a can be activated by
binding to its corresponding receptor C5aR, and it is involved in
the pathological development processes of a number of inflammatory
diseases, such as cisplatin-associated glomerulonephritis (16), rheumatoid arthritis (25), acute lung injury (26,27), pyemia (21) and cardiovascular diseases, such as
atherosclerosis (24) and the
formation of tumors (23).
Research on the liver have shown that C5a is involved in liver
tissue regeneration and is highly regenerative (28). When the livers of C5-deficient
mice are damaged by toxins, their livers cannot be regenerated when
treated with CCL4/chemokines. However, liver regeneration can be
observed after injecting C5 or C5a into the treated animals
(29). This effect of C5a can
also be found in cardiac tissue regeneration (30). C5a is not only induced by the
implanted progenitor cells, but also promotes the differentiation
of progenitor cells. Following fractures in humans, it has been
found that there is a significant increase in C5aR expression in
mesenchymal progenitors during osteogenic differentiation (31). This also indicates that C5a
induces the migration of mesenchymal progenitor cells, and that C5a
is involved in broken bone regeneration. In recent years, it has
been found that C5a is associated with dental pulp inflammatory
diseases (32-37).
Decay, trauma, and the stimulation of filling
material may cause inflammation in the dental pulp. While severe
tooth decay often leads to pulp necrosis, under mild/moderate
carious decays, a dentin bridge can be observed in vivo,
which protects the healthy pulp and separates it from bacterial
invasion (37,38). During the progression of caries,
odontoblasts are the first cells to face bacteria and their toxins.
Following the stimulation of decay, the dentin is demineralized,
and the released signal molecules in the dentin matrix are
dispersed into the dental pulp cells, so that the secretion
activity of the odontoblasts is stimulated, the activity of the
odontoblasts is upregulated, and reactive dentin is synthesized,
thereby preventing bacteria and their toxins from invading the
pulp. With deep caries, which extend close to or into the pulp, the
fibroblasts in pulp are stimulated and increase its synthetic
capacity. This includes the synthesis of complement proteins and
activation of the complement system to recruit progenitor cells
required for the establishment of dentin bridges. The soluble
fragments C3a, C4a, and C5a produced following complement
activation modify the vascular permeability indirectly via the
induction of basophiles/mastocytes degranulation. This subsequently
leads to a massive liberation of histamine and serotonin, which
increases vascular permeability. C3a, C4a, and C5a also act on
endothelial cell permeability by inducing fiber stress formation,
which facilitates leukocyte diapedesis upon contraction. The
created gradient of these fragments guides leukocyte migration
toward the infected/inflamed tissue. In parallel, C5a activates
pulp progenitor cells, and the produced C5a gradient guides their
migration toward the injured tissue to initiate the dentin-pulp
regeneration process (34). This
clearly shows that any local tissue repair directly leads to dentin
pulp regeneration. It has been reported that the loss of complement
activation is linked to early dentin pulp regeneration. Dental pulp
fibroblasts can be used as non-immune cells with cells that
synthesize all complement proteins and secondary complement
activation (33). It has been
demonstrated that C5a is involved in the early stage of dentin pulp
regeneration; that is, the selective recruitment reaction of dental
pulp progenitor cells to the C5a product site responds to a certain
gradient (36). Tooth repair and
regeneration require odontoblasts, which can synthesize mineralized
tissue in dentinal tubules or around pulp wounds (called
reactive/restorative dentin). This protects the pulp from invading
bacteria. Over the past 5 years, the number of studies on C5a have
increased. The study group of I. About (Chmilewsky et al)
published several articles in the Journal of Dental Research on the
involvement of C5a in pulp inflammation and complement activation.
They proposed that the largest proportion of fibroblasts in dental
pulp cells is made up of non-immune cells with cells that
synthesize all complement proteins and provide secondary complement
activation (33). The recruitment
of dental pulp mesenchyme stem cells induce by C5a is an essential
step in dentin pulp regeneration. In addition, the study group of
I. About presented a hypothesis of the initiation of dentin-pulp
complex regeneration arising after the elimination of cariogenic
bacteria, or at least after the prevention of further progression
of rickets (37). Therefore,
complement activation causes inflammation and regeneration to occur
simultaneously in the pulp.
It is well known that bacteria are closely related
to the onset of dental caries (33). Bacteria are divided into
Gram-negative and Gram-positive species. Most pulp inflammation is
caused by the development of dental caries (36). Gram-positive bacteria consists of
a thick, dense peptidoglycan and teichoic acid. Teichoic acid is a
specific component of Gram-positive bacteria. It is interspersed in
peptidoglycan in a long-chain form. One end is bound to the cell
wall as wall phosphate (WTA), and the membrane or lipoteichoic acid
(LTA) is bound to the plasma membrane. Gram-negative bacteria
consist of a lipopolysaccharide (LPS)-lipoprotein-phospholipid
outer membrane that surrounds a thin peptidoglycan layer and does
not contain teichoic acid. Gram-positive cocci can destroy organic
matter, and the teeth can form cavities after long-term action
(2); while the acid-producing
bacteria are Gram-negative bacteria, which can decompose
carbohydrates to produce acid, which leads to demineralization of
dental minerals (39). Along with
the development of caries, the proportion of bacteria in plaque can
change continuously (32,34). Both LTA and LPS act as antigens,
and activate immune cells to secrete many inflammatory factors
(40-42). There are some studies available
which have used LTA or LPS to stimulate dental pulp cells (32,34,35,42). Although LTA has been less often
reported than LPS, the activation of LTA and LPS as antigens is the
same for immune cells. Therefore, we use both of them to detect the
immune response. The inflammatory response of cells requires the
activation of intercellular signaling pathways, including the
transcription factors nuclear factor (NF)-κB and also those of the
mitogen-activated protein kinase (MAPK) family, for example
extracellular signal-regulated kinase (ERK), JNK and p38. Cytokines
that are typically produced by fibroblasts upon pro-inflammatory
stimuli, such as IL-6 and IL-8. Notably, IL-6 and IL-8 are
associated with pulpal inflammation in caries exposure (43). Thus, the expression of IL-6 or
IL-8 can indicate inflammation of the cells.
It would be of interest to determine the effects on
the secretion of C5a when inflammation occurs and invades the pulp.
Thus, in this study, we aimed to detect the expression of C5a and
its receptor C5aR. Therefore, based on the role of C5a in the
regeneration of other organs and pulpitis, the objective of this
study was to examine the presence of C5a, C5aR and IL-6 in inflamed
pulps and to attempt to elucidate its role in dental pulp
inflammation.
Materials and methods
Primary pulp cell cultures
Human pulp cells were prepared from immature third
molars at the 2/3 root formation stage by the explant outgrowth
method (2). The teeth were
obtained from at least 3 different donors for each experiment
(n=12; 4 molars per donor; age, 18-25 years; ratio, 1:1 male to
female). Surgeries were performed at the Oral and Maxillofacial
Surgery Department of The Second Affiliated Hospital of Harbin
Medical University. The present study was approved by the
Institutional Ethics Committee of The Second Affiliated Hospital of
Harbin Medical University. Written informed consent was obtained
from all patients. The basic cell culture medium used consisted of
Dulbecco's modified Eagle's medium/F-12 (HyClone; GE Healthcare
Life Sciences), supplemented with 15% fetal bovine serum
(Biological Industries) and 1% penicillin and 1% streptomycin
(Beyotime).
Induction of dental pulp cell
differentiation into adipocytes, chondroblasts and osteoblasts
The cells were cultured to the third generation in a
37°C incubator with 5% CO2. All groups of cells were
plated at a density of 1×105 per well. To induce dental
pulp stem cells (DPSCs) to differentiate into adipocytes, the
culture medium was supplemented with 0.5 µM
3-isobutyl-1-methylxanthine, 50 µM indomethacin and 0.5
µM dexamethasone for 3 weeks. The adipogenic cultures were
fixed in 4% paraformaldehyde for 30 min at room temperature and
stained with fresh Oil Red O solution (Sigma-Aldrich) for 1 h at
room temperature. The chondroblast induction medium consisted of 1
µM dexamethasone, 37.5 µg/ml vitamin C-phosphate, 1
mM sodium pyruvate, 10 ng/ml transforming growth factor-β (TGF-β),
1 ng/ml β-fibroblast growth factor (β-FGF), 1X
Insulin-Transferrin-Selenium premix (Sigma-Aldrich; Merck KGaA), in
which the DPSCs were maintained for 3 weeks. The chondrogenic
cultures were fixed in 4% paraformaldehyde for 30 min at room
temperature and stained with toluidine blue (Toluidine Blue O
Cartilage Stain solution, Solarbio Life Sciences) for 30 min in a
37°C incubator. The osteoblast induction medium contained 10 nmol/l
dexamethasone, 5 mmol/l β-glycerophosphate and 50 mg/ml vitamin
C-phosphate, in which the DPSCs were cultured for 3 weeks. The
osteoblast cultures were fixed in 95% ethanol for 30 min at 37°C
and stained with Alizarin Red S (Sigma-Aldrich) for 30 min in a
37°C incubator. The cells were observed under a light microscope
(ZEISS, 37081 Goettingen, Germany) (original magnification,
×40).
Cell groups and culture conditions
The third generation pulp cells (all
8×104) were cultured in 3 (6-well) dishes in a 37°C
incubator with 5% CO2. Prior to grouping, the cells were
washed 3 times with PBS and then incubated in serum-free DMEM (2
ml/well) for 24 h. The cells were cultured by 1% penicillin and 1%
streptomycin, serum-free DMEM, and divided into 4 groups as
follows: i) The 1 µg/ml LTA (Sigma-Aldrich) group; ii) the 1
µg/ml LPS (Sigma-Aldrich) group; iii) the 1 µg/ml LTA
and 1 µg/ml LPS group; and iv) the PBS-only group as
control. There were 5 time points for all 4 groups: 1, 2, 3, 5 and
7 days. The re-addition of equal concentrations of LTA stimulant
for each change.
Cytotoxicity (MTT) assay
The present study employed 96-well plates with
1×103 cells/well. Dimethyl sulfoxide (200 µl) was
added to dissolve the formazan crystals. The absorbance was
measured with an ELISA reader (Thermo Fisher Scientific, Inc.) at a
wavelength of 490 nm. The cell viability ratio was calculated using
the following formula: Inhibitory ratio (%) = [optical density (OD)
control-OD treated)/(OD control)] ×100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
All groups of cells were cultured in mineralized
solution (in a 37°C incubator with 5% CO2) and total RNA
was extracted on days 1, 2, 3, 5 and 7 to detect the gene
expression levels of C5a, C5aR and IL-6. Total RNA was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Subsequently, the RNA
was converted into cDNA using a Transcriptor First Strand cDNA
Synthesis kit (Roche Diagnostics GmbH) using the following
temperature protocol: 50°C for 60 min, 85°C for 5 min and 4°C for
10 min. The expression levels of the genes were quantified using
FastStart Universal SYBR-Green Master Rox mix (Roche Diagnostics
GmbH). The PCR thermocycling conditions were as follows: 95°C for 2
min, followed by 40 cycles of 95°C for 15 sec and 60°C for 30 sec.
PCR results were normalized against the reference gene Actin to
correct for non-specific experimental variation. The method of ΔΔCq
was used to determine the relative quantity of mRNA expression in
samples, and fold change was determined as 2−ΔΔCq
(44). The following primers were
used: C5a forward, 5′-GTT TGT CGT GGC TGT AGT CC-3′ and reverse,
5′-GAC CGC TTT CTG CTG GTGT TT; C5aR forward, 5′-CGT TTG TCG TGG
CTG TAG TCC-3′ and reverse, 5′-GAC CGC TTT CTG CTG GTG TTT-3′; IL-6
forward, 5′-GCT CTG GCT TGT TCC TCA CTA-3′ and reverse, 5′-AAT CAT
CAC TGG TCT TTT GGA G-3; and actin forward, 5′-GGG AAA TCG TGC GTG
ACA TT-3′ and reverse, 5′-GGA ACC GCT CAT TGC CAAT-3′.
Western blot analysis
Total protein was extracted from the 4 groups of
cells on days 1, 2, 3, 5 and 7 using cell lysis buffer [20 mM Tris
(pH 7.5), 150 mM NaCl, 1% Triton X-100, 2.5 mM sodium
pyrophosphate, 1 mM EDTA, 1% Na3CO4, 0.5
µg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride]. The
lysates were collected by scraping from the plates and subsequently
centrifuged at 15,000 x g at 4°C for 5 min. The protein
concentration was quantified using a bicinchoninic acid assay.
Total protein samples (20 µg/lane) were separated by 12%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes.
The membranes were blocked in 1% BSA with 0.05% Tween-20 at room
temperature for 2 h. The membranes were then incubated overnight at
4°C with the following antibodies: Rabbit anti-human C5aR (1:200;
cat. no. 21316-1-AP, Proteintech, Inc.) and β-actin (1:5,000; cat.
no. 20536-1-AP, Proteintech, Inc.). The secondary antibody used
were horseradish peroxidase-conjugated AffiniPure goat anti-rabbit
IgG (cat. no. SA00001-2; Proteintech, Inc.) and goat anti-mouse IgG
(1:10,000; cat. no. SA00001-1, Proteintech, Inc.). Bands were
visualized using a Clarity Max™ Western ECL Substrate (Bio-Rad
Laboratories, Inc.) and a Tanon 1000 digital image gel analytical
system (Tanon Science & Technology Co., Ltd.) was used for
photography and quantification.
Transmission electron microscopy
(TEM)
The DPSCs were collected and fixed in 2.5%
glutaraldehyde 0.1 M cacodylate buffer (pH 7.4) for ~2-3 h at room
temperature. The samples were post-fixed in 1% osmium tetroxide and
dissolved in a 0.1 M phosphate buffer (pH 7.4) for 1 h at room
temperature. The samples were dehydrated in ethanol and embedded in
Epon 812. Ultra-thin sections were contrasted in aqueous uranyl
acetate and lead (II) hydroxide, observed and photographed using a
Hitachi 7000 TEM.
Statistical analysis
All values are expressed as the means ± standard
error of the mean. Statistical analysis was performed by using
one-way analysis of variance followed by Bonferroni multiple
comparisons using SPSS 13.0 software (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of the differentiation
potential of dental pulp cells in vitro
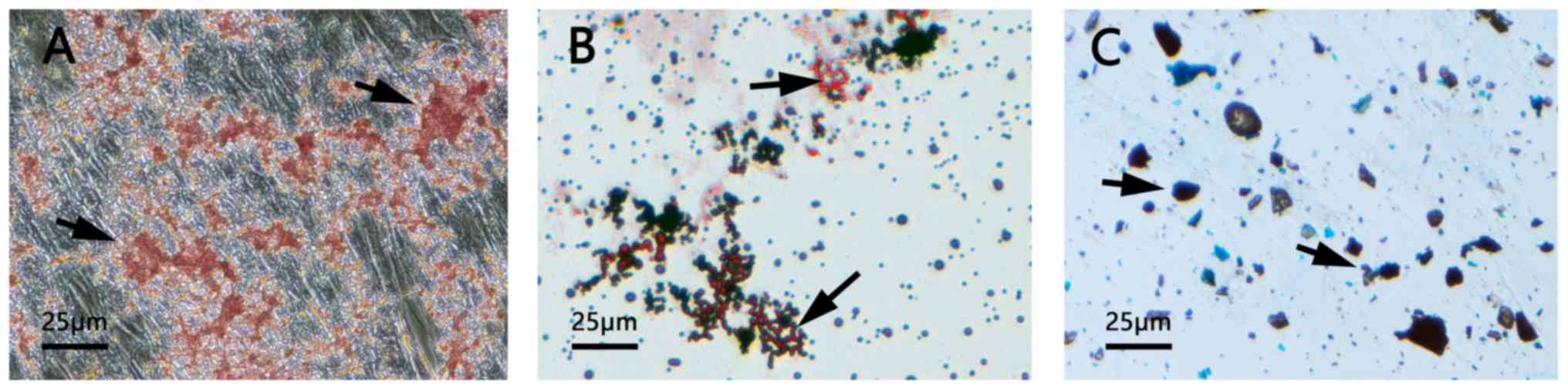
The presence of mineralized nodules demonstrated the
successful osteogenic induction of DPSCs (Fig. 1A). As presented in Fig. 1B, the formation of neutral lipid
vacuoles also indicated the adipogenic potential of the DPSCs.
Furthermore, the DPSCs were observed to differentiate into
chondroblasts following induction (Fig. 1C).
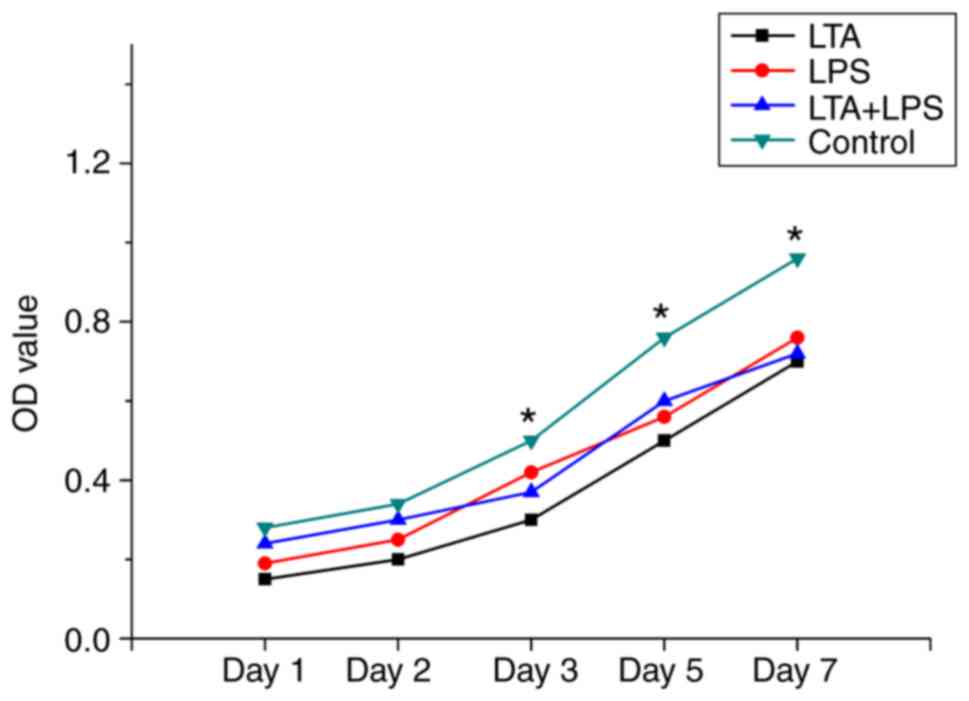
Cytotoxicity of LTA and LPS to the
cells
The data for the control group revealed the highest
level of cell proliferation among the 4 groups. The data for days 1
and 2 revealed no inhibition effect for all 4 groups. Beginning at
day 3, the cells in the LTA, LPS and LTA + LPS groups were less
active than those of the control group (Fig. 2).
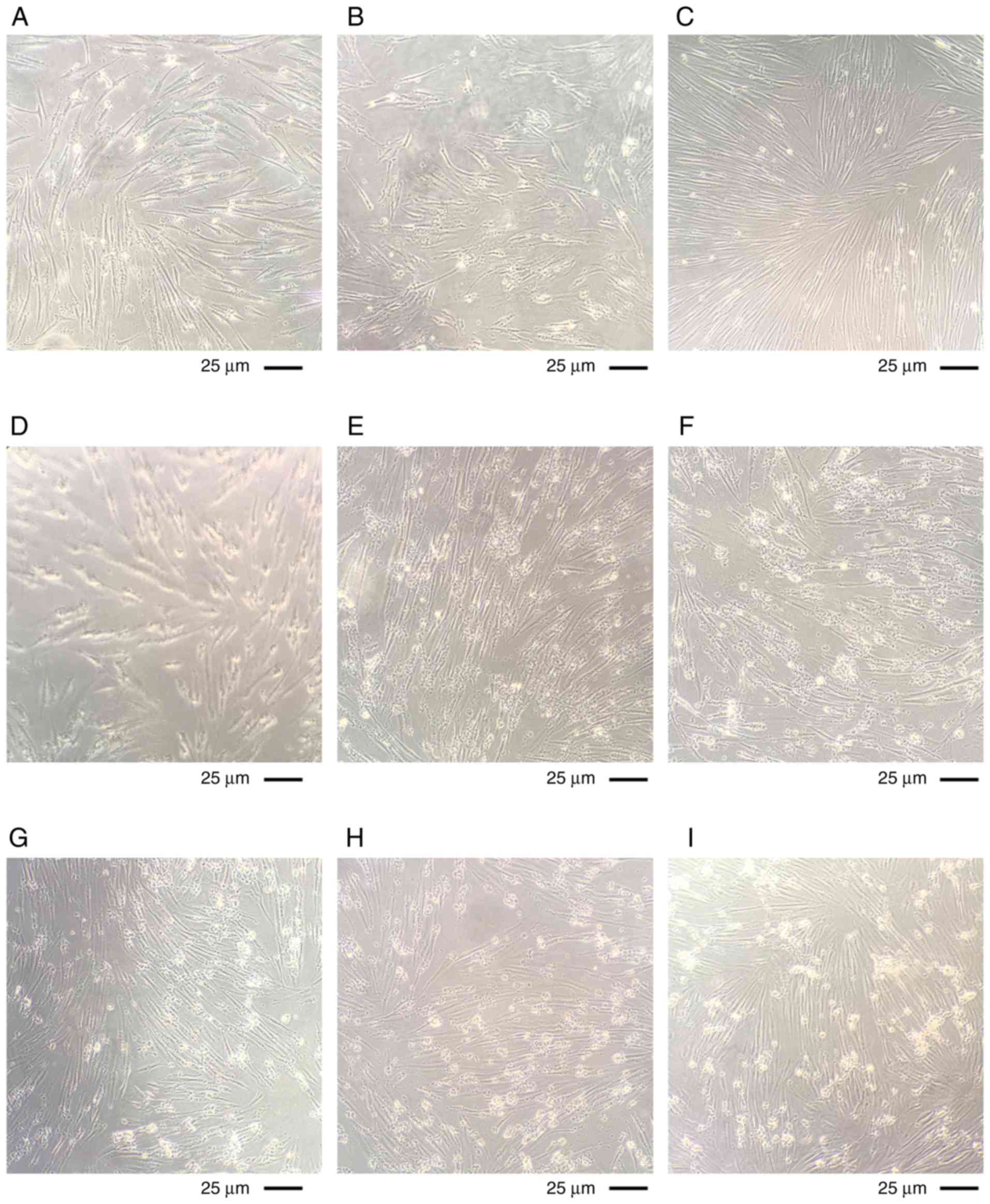
Cell state following stimulation with LTA
and LPS on different days
The cultured cells were observed using a microscope.
The cells in all the stimulated groups were observed to be in a
healthy condition at day 1 (LTA alone in Fig. 3A, LPS alone in Fig. 3B, LTA and LPS co-stimulation in
Fig. 3C). The cells in all the
stimulated groups were still in good condition and a small amount
of cell shrinkage was visible at day 2 (LTA alone in Fig. 3D, LPS alone in Fig. 3E, LTA and LPS co-stimulation in
Fig. 3F). More cell shrinkage was
observed at day 3 (LTA alone in Fig.
3G, LPS alone in Fig. 3H, LTA
and LPS co-stimulation in Fig.
3I). It was observed that the cells had shrunk into pieces and
their condition worsened at day 5 (LTA alone in Fig. 3J, LPS alone in Fig. 3K, LTA and LPS co-stimulation in
Fig. 3L). The cell condition is
not as good as the fifth day, the shrinkage is more serious, and
floating dead cells are visible at day 7 (LTA alone in Fig. 3M, LPS alone in Fig. 3N, LTA and LPS co-stimulation in
Fig. 3O).
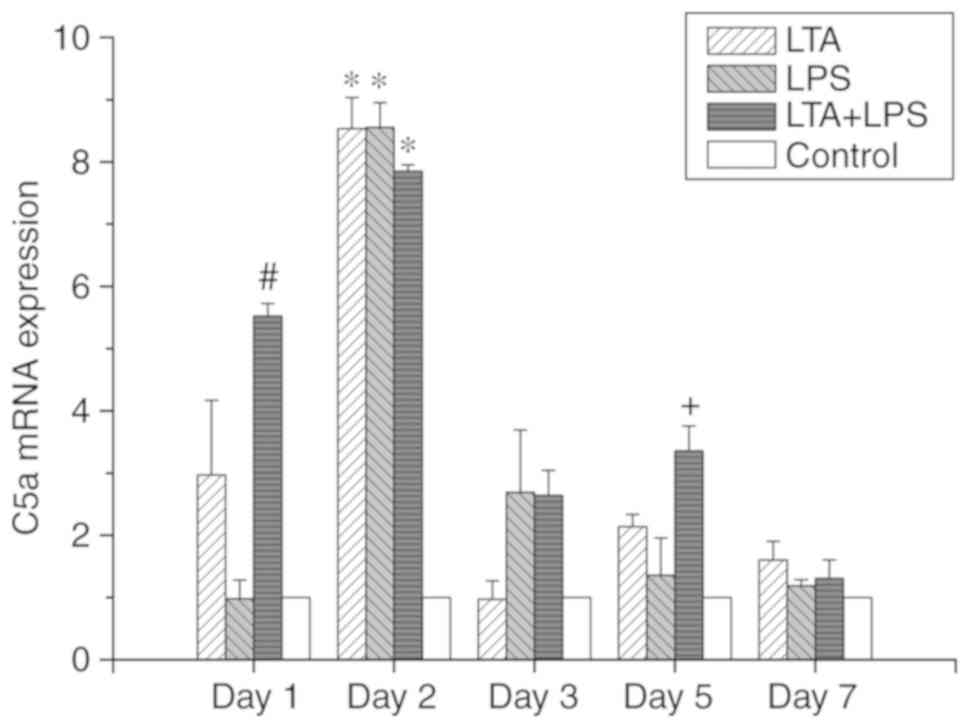
Effects of LTA and LPS and their
combination on C5a and C5aR expression in dental pulp cells
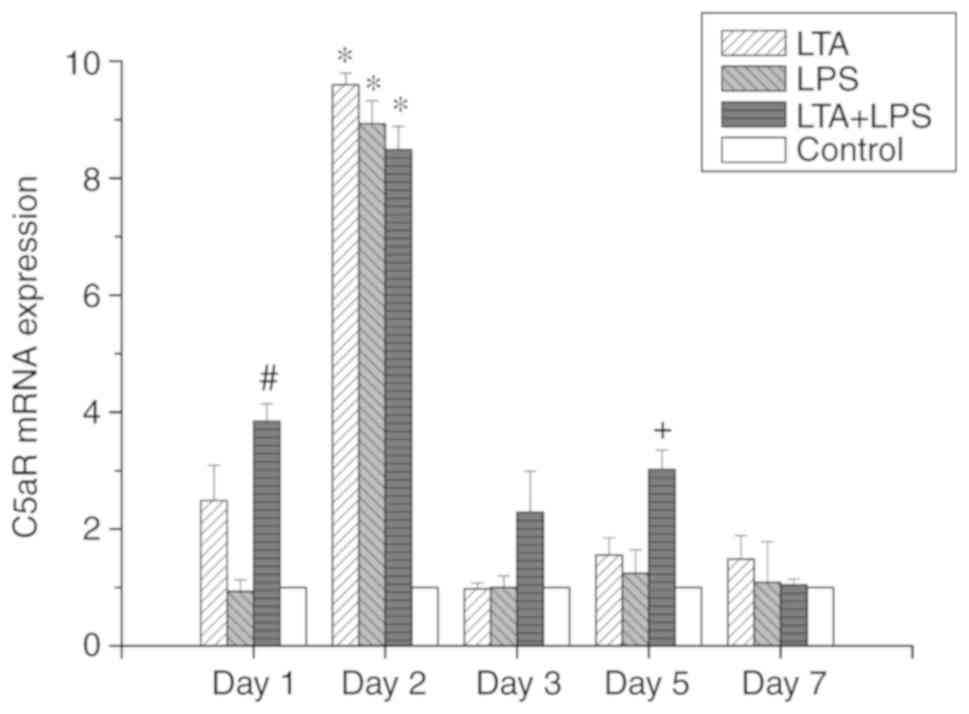
As shown in Figs.
4 and 5, the trends in the
expression levels of C5a and C5aR mRNA were identical. In the
LTA-stimulated group, it was observed that with the progression of
the culture time and the continuation of stimulation, there was a
significant change in the mRNA expression levels of C5a and C5aR.
On the second day of stimulation, the expression levels of C5a and
C5aR mRNA were increased, and the levels were 3-fold greater than
those observed at the other time points examined. The difference
was statistically significant (P<0.05). However, no difference
was observed between the expression of C5a versus C5aR mRNA. In the
LPS-stimulated group, with the progression of the culture time and
the continuation of stimulation, the expression levels of C5a and
C5aR mRNA on the second day were 3-fold higher than those observed
on the other days, and the difference was statistically significant
(P<0.05). Apart from the second day, there was no significant
difference in C5a and C5aR mRNA expression in the remaining days.
In the LTA and LPS co-stimulated group, the expression levels of
C5a and C5aR mRNA increased on the first day, which were 2-fold
higher than those at 3 and 7 days, and the difference was
statistically significant (P<0.05). The expression of C5a and
C5aR was the highest on the second day, which was 2-fold higher
than that at 3 and 7 days. However, there was a rebound in
expression on the fifth day. The difference was statistically
significant (P<0.05). In addition, we examined the expression of
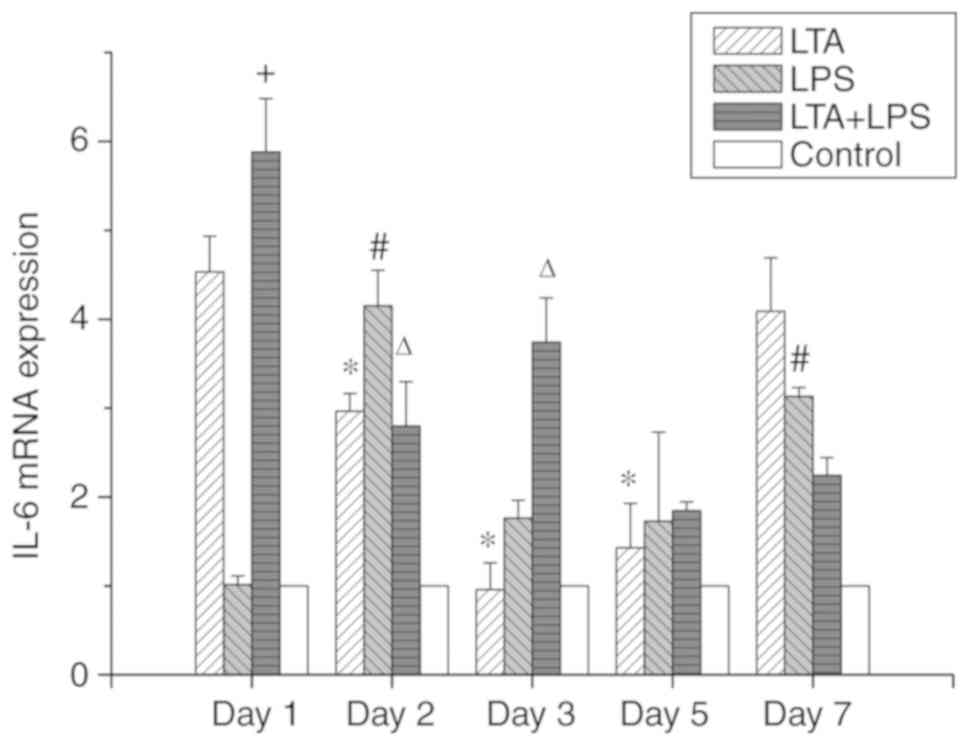
IL-6 mRNA (Fig. 6). In the
LTA-stimulated group, the high expression of IL-6 mRNA was observed
on the first day, and the expression declined after the second day.
However, the expression on day 7 increased again, the same as that
at the first day. In the LPS-stimulated group, on the first day,
IL-6 mRNA expression was low, and the expression increased the
following day; on the third day, its expression decreased, and its
expression on the fifth day was not altered. On the seventh day,
IL-6 expression was slightly increased. Finally, in the LTA and LPS
co-stimulated group, on the first day, IL-6 mRNA expression was
highly expressed, then decreased a little on the second and third
day. The expression of IL-6 mRNA subsequently decreased on the
fifth day. However, no gene expression change was observed on the
seventh day compared to the fifth day.
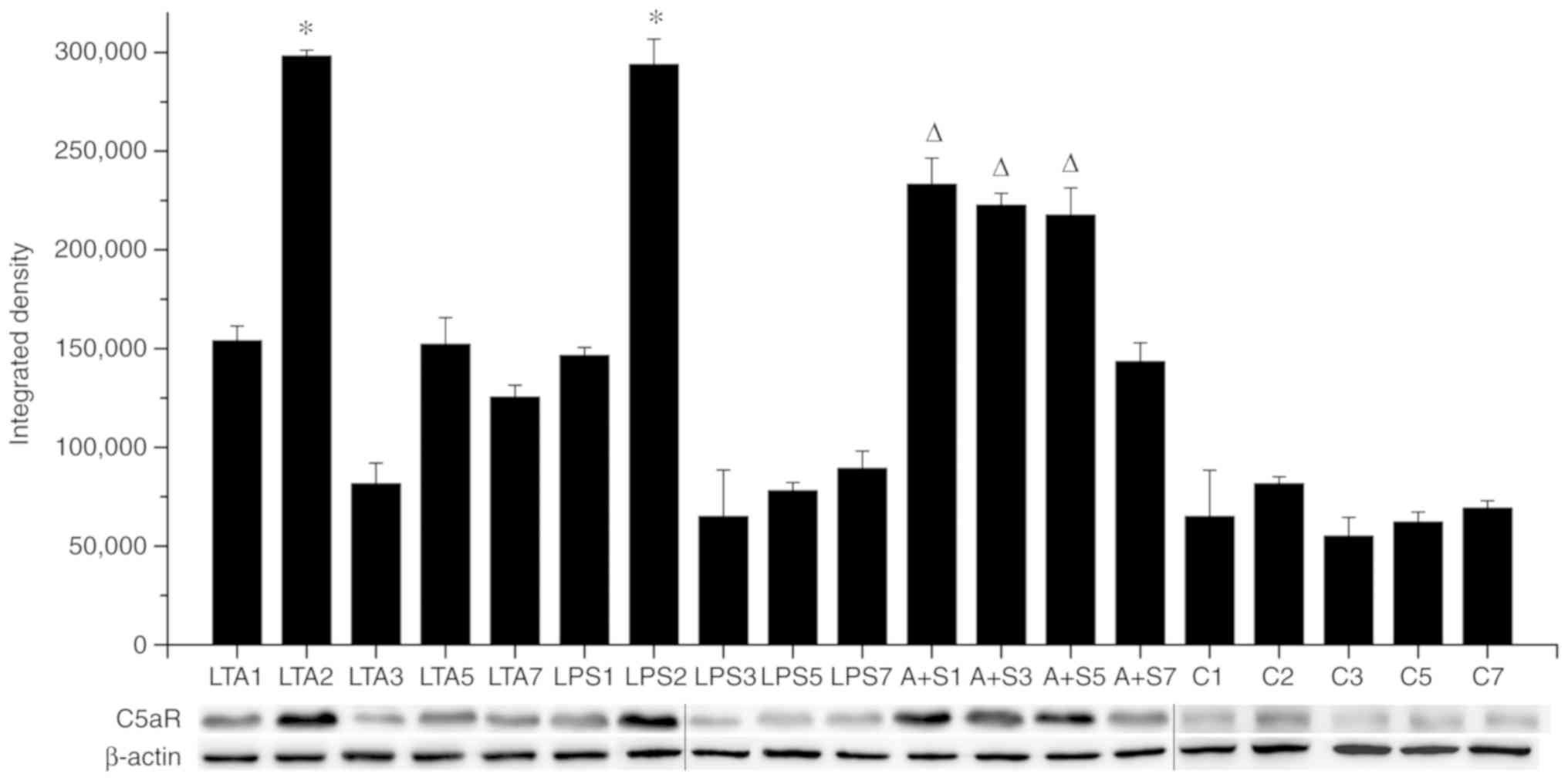
Protein expression of C5aR following
stimulation with LTA and LPS and their combination
This experiment examined the protein expression of
C5aR (Fig. 7). In the
LTA-stimulated group, the expression of C5aR changed significantly
with the progression of the culture time and the continuation of
stimulation (re-addition equal concentrations of LTA stimulant to
each change). On the second day of stimulation, the expression of
C5aR increased, and the gray value of the western blot band was
almost 300,000, which was almost 3-fold higher than that of the
other groups, and the difference was statistically significant
(P<0.05). No differences were observed in C5aR expression on
days 1, 3, 5 and 7. In the LPS-stimulated group, with the
progression of the culture time and the continuation of
stimulation, the gray value of C5aR expression on the second day
was almost 300,000, which was almost 3-fold higher than that on the
other days. The difference was statistically significant
(P<0.05). Apart from the second day, no significant differences
were observed in the expression of C5aR on the remaining days. In
the LTA and LPS co-stimulated group, on the first day, the third
day and the fifth day, the expression of C5aR was high, and the
gray value of the western blot band was almost 250,000, which was
higher than that of the seventh day. The difference was
statistically significant (P<0.05).
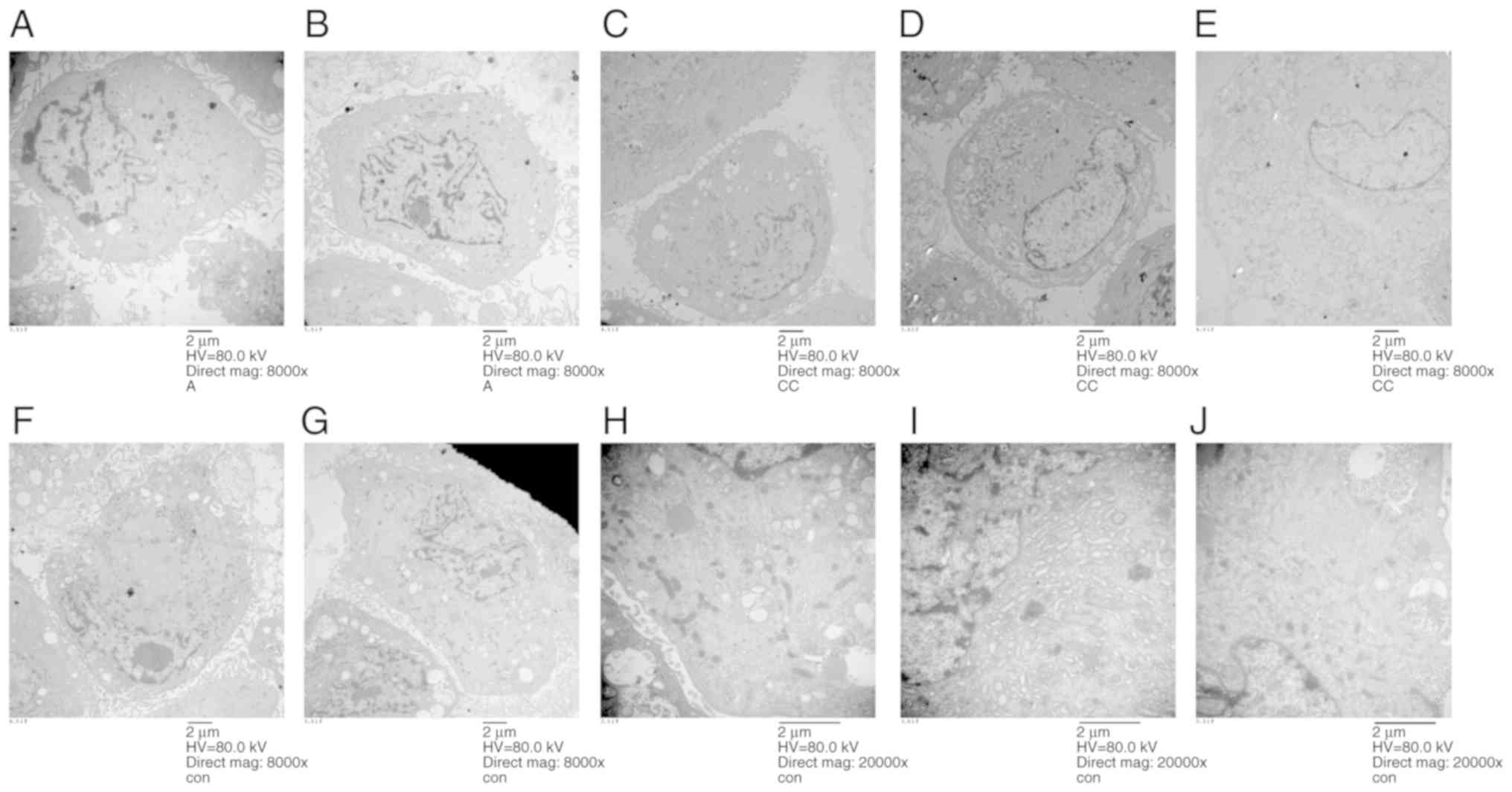
Ultrastructural features of dental pulp
cells
The ultrastructural features of the stimulated
dental pulp cells are shown in Fig.
8. On the first day of stimulation, the number of lysosomes
increased. The microvilli at the cell membrane were normal,
indicating that the cell state was good, and the mitochondria and
endoplasmic reticulum remained unaffected. Autophagy could be
observed inside the cells (Fig.
8A). Following 2 days of stimulation, the cells were in good
condition, but the number of microvilli were reduced (Fig. 8B). On the third day, the cell
state was good, but there were more vacuoles. The numbers of
microvilli were reduced, but the activity of cells was still good
(Fig. 8C). On the fifth day, the
cells were swelled and the cells were about to rupture and die
(Fig. 8D). On the seventh day,
necrotic cells were observable, with the nuclei in the middle, and
the swollen cell edges were unclear (Fig. 8E). The control group displayed
small mitochondria and cells were rich in endoplasmic reticulum,
indicating that the cells were strong enough to synthesize protein.
The ultrastructural features in the control group conformed to the
characteristics of primary cells. Although the mitochondria were
small and underdeveloped, they were numerous in number and
displayed many glycogens. Maybe their energy metabolized through
glycogen. The irregular karyotype may increase metabolic efficiency
in order to increase the contact area with cytoplasm. The control
groups at day 1 are shown in Fig.
8F, at day 2 in Fig. 8G, day
3 in Fig. 8H, day 5 in Fig. 8I, and at day 7 in Fig. 8J.
Discussion
It is well known that bacteria are closely related
to the occurrence of caries. There are, broadly speaking, two
different types of cell walls in bacteria, those of Gram-positive
and those of Gram-negative bacteria. Pulpitis is mainly caused by
bacterial infection, which is itself a secondary development of
caries (tooth decay). LTA is a major constituent of the cell wall
of Gram-positive bacteria. LPS occurs in the outer membrane of
Gram-negative bacteria. In this study, LTA and LPS were used to
stimulate human dental pulp cells, alone or in combination, in
order to observe the immune response of dental pulp cells
stimulated by foreign bacterial cell wall components, instead of
using inflammatory factors directly to stimulate dental pulp cells.
Current research indicates that cells can only interact once they
have accumulated. During the process of aggregation, the cellular
microenvironment and 'soil' are formed, which are required for
morphogenesis and survival. Various factors interact into the
network in this process (35).
Therefore, in this study, fibroblasts and dental pulp stem cells in
dental pulp cells were not isolated separately. We stimulated
dental pulp cells directly to detect complement activation. After
being stimulated by bacterial cell walls, immune-related cells in
dental pulp cells secreted related inflammatory factors and
signaled other immune cells to resist and kill foreign pathogenic
bacteria. LTA and LPS are the best stimulators to study the
activation of C5a by complement activation of dental pulp
cells.
In this study, the results of RT-qPCR revealed that
the expression of C5a mRNA in the LTA-stimulated group and the
LPS-stimulated group were similar. In both these groups, there was
a significant increase in C5a mRNA expression on the second day of
stimulation, and the multiple was the same. This same multiple is
an interesting result, showing that the expression of complement
C5a mRNA was not high at the early stage of stimulation on dental
pulp cells by LTA or LPS. With the progression of the experiment,
C5a mRNA expression was higher, but was subsequently downregulated
and remained at low levels. The reason for the decrease in C5a mRNA
expression was that many immune cells die due to stimulation with
LTA or LPS on the third day of culture. For this reason, other
studies have stimulated cells with LTA or LPS only for 20 min, or
for only 24 h up to 48 h (32,34,35,37,40,42,45). Immune cells would die 3 days
following LTA or LPS stimulation. In this study, the cells were
stimulated for 7 days and were still in good condition. Although
there were some dead cells, they could continue to be cultured.
This indicated that dental pulp cells have a certain resistance. It
is possible that immune cells inside the pulp died following
stimulation, although there were some undifferentiated cells in the
multicellular region of the pulp, which have the ability to
differentiate into immune cells. Furthermore, it also showed the
good vitality of dental pulp cells under stimulation.
When LTA and LPS were used to co-stimulate the human
dental pulp cells, the expression of C5a increased on the first
day, and its expression was 2-fold higher than that at 3 and 7
days. The reason for this may have been due to the more potent
effect of LTA and LPS co-stimulation. Therefore, there must be a
different response process of human dental pulp cells to
co-stimulation. Similarly, mRNA expression was also high on the
second day. Co-stimulation exhibited a distinctly different trend
as regards C5a mRNA expression from stimulation with LTA or LPS
alone. C5a mRNA expression increased only on the second day
following single stimulation, and did not stimulate higher C5a mRNA
expression with prolonged stimulation time. This indicated that
under the condition of the simultaneous stimulation of LTA and LPS,
undifferentiated cells in dental pulp cells may differentiate into
immune cells, and the mRNA of small fragment C5a following
complement activation was detected. Under the condition of single
LTA or LPS stimulation, no such heightened expression occurs. There
are three reasons for this: Firstly, a recently conducted study
demonstrated that dental pulp tissue comprises a unique non-immune
cell type, the human pulp fibroblast, able to produce and
efficiently activate the complement system proteins (35). Secondly, it may be due to the
intense activity of stimulation. This study did not carry out
experiments with longer stimulation times in culture, but C5a
expression with the co-stimulation culture group may have been
greater with thee extended culture time. Thirdly, it is well known
that cell differentiation takes time. The time required to
differentiate into different types of cells varies. For example, it
takes 28 days for dental pulp stem cells to differentiate into
osteoblasts, but only 14 days to differentiate into adipocytes.
Therefore, the undifferentiated cells in the pulp need time to
differentiate into immune cells after stimulation. The decrease in
C5a mRNA expression on the seventh day of co-stimulation may be
because the immune cells die after differentiation, or the secreted
C5a is consumed.
C5aR is a receptor for complement C5a, mainly
expressed in neutrophils, basophils and monocytes. The results of
RT-qPCR revealed that the expression trend of C5aR in each group
was identical to that of C5a, indicating the preciseness and
reproducibility of the experiment. The expression of IL-6 mRNA in
the LPS-stimulated group was low on the first day, then increased
on the second day, which was 20-fold higher than that on the first
day. The expression on the second day was consistent with the
highest level in the LTA-stimulated group. The expression on the
third day was again as low as that on the third day in the
LTA-stimulated group. On the fifth day, IL-6 expression in each
group remained at a low level. IL-6 expression slightly increased
on the seventh day, but it was 2-fold lower than that on the first
day. The expression of IL-6 mRNA in the LTA-stimulated group was
highest on the first day, then decreased with the progression of
time. However, it increased on the seventh day, to levels similar
to those observed on the first day. Although high expression levels
of IL-6 mRNA were observed in both the LTA- and LPS-stimulated
groups on the seventh day, they were both lower than those of the
co-stimulated group on the first day. In addition, the high
expression of IL-6 on the first day was 1.25-fold higher than that
of LTA and LPS alone, which was consistent with the mRNA expression
of C5a and C5aR, indicating the proinflammatory effect of LTA and
LPS. In both the LTA- and LPS-stimulated groups, a rebound in IL-6
expression was observed on the seventh day, probably as
undifferentiated cells in the pulpal cells may have differentiated
into immune cells, thereby secreting inflammatory factors. It can
be seen from the MTT and cell state figures that as the culture
time was prolonged, the cell condition deteriorated and the number
of dead cells increased. This was perhaps due to the stimulating
factor, which are the cell wall components of Gram-positive and
Gram-negative bacteria. The results revealed that the control group
did not express IL-6 mRNA; however, the expression levels of C5a
and C5aR mRNA were not low. As shown in Figs. 4 and 5, the expression levels of C5a and C5aR
mRNA were not significantly higher in the LTA- and LPS-stimulated
groups than in the control group at all time points, apart from day
2. A possible explanation may be that the immunoreaction of dental
pulp cells first inhibits the expression of C5a mRNA, but the
expression levels of C5a and C5aR mRNA were significantly elevated
and higher than those of control group the following day. On the
third day, most of the immune cells may have died due to the
stimulation by LTA and LPS, and thus C5a and C5aR mRNA expression
would both be low past the second day. That is why cells collected
at 24 or 48 h following stimulation with LPS and LTA at a
concentration of 1 µg/ml in other studies were still viable
(32,34,35,37,40,42,45). Our findings are consistent with
those of these studies. The results of cell culture revealed that
the cells remained in a good state until day 7 (Fig. 3). Under such a high concentration
of LTA and LPS stimulation, dental pulp cells could survive to the
seventh day with good activity, which was consistent with other
reports of dental pulp cells. This experiment once again proved the
high viability of dental pulp cells.
As mentioned above, although most immune cells may
die on the third day, some undifferentiated cells still survive in
the pulp, and can differentiate into immune cells following
stimulation. This interpretation is made as IL-6 detection showed
that there was an increase in IL-6 expression on the seventh day.
Scanning electron microscopy also revealed some cell death in the
late culture period, and the expression of C5a and C5aR may be
decreased due to dead cells, while dead cells may cause the release
of inflammatory factors, such as IL-6. Therefore, IL-6 expression
was still high on the seventh day. Our experiment did not detect
C5a expression by western blot, as upon the activation of the
complement, a C5-converting enzyme is formed to cleave C5, which is
the last enzymatic step in the complement cascade. C5 bound to C3b
in C5 convertase, and was then cleaved into C5a and C5b. C5a is
released into the liquid phase, and C5b still binds to the cell
surface. Therefore, western blot analysis could not detect the
expression of C5a.
In this study, the results of C5a and C5aR mRNA
expression indicated that the extracted dental pulp cells had the
ability to encode C5a and C5aR proteins without exogenous
stimulation. This result is consistent with the descriptions in a
previous study by the study group of I. About Chmilewsky et
al (35) describing the
expression of C5a in the LTA-free stimulation group and the control
group by ELISA.
In summary, LTA and LPS stimulated the highest
expression of C5a on the second day, and thus, we recommend the
48-h stimulation condition in the immunological study of dental
pulp cells. From an inflammatory point of view, LPS is milder in
the short time period of investigation. Others have reported that
LTA induces a weaker inflammatory response than LPS. This may be
related to the observation that gram-positive bacteria cause
chronic mastitis more often than do Gram-negative infections
(46). On the first day of
observations, IL-6 mRNA expression was not high in the LPS
stimulation group, although it was high in the LTA stimulation
group. However, the expression in the LPS group increased the
following day, which was 1.5-fold higher than the level in the LTA
group.
Subsequently, from the time progression data, on the
third day and fifth day, all groups expressed low IL-6 mRNA levels,
and these levels increased on the seventh day. This expression in
the LTA stimulation group seemed stronger than that in the LPS
stimulation group. The expression of IL-6 mRNA in the LTA group was
approximately 1.5-fold higher than that in the LPS group.
Therefore, from the perspective of the full stimulation process,
LTA stimulates a higher expression of IL-6 than does LPS, which
indirectly indicates that the proinflammatory effect of LTA is
stronger. From the observations of cell morphology and activity,
the LPS group was superior to the LPS group in cell status and
activity compared to the LPS group (Fig. 3). As for the inflammation of the
LTA and LPS combined stimulation group, it is clear that cell
condition was worse than that of the LTA and LPS single stimulation
groups, as expected (Fig. 3).
Stimulation by LTA differs from that by LPS, with a differential
expression of inflammatory factors, particularly in the timeline
used in this study. While the different expression of inflammatory
factors indicated the different effect of LTA and LPS stimulation.
As we well known, different types of bacteria have different
functions on dental inflammatory diseases. This suggests that the
dominant bacteria in the case of inflammatory pulp are related to
the occurrence and development of rickets. As the results mentioned
above have shown, if LPS are the dominant bacteria, this would lead
to a higher expression of inflammatory factors, such as IL-6, thus
easily causing the inflammation of the pulp. Further research is
required to verify the correlation of pulpitis and
Gram-negative/positive bacteria. However, this study demonstrated
the differences in IL-6 expression following stimulation with LTA
and LPS.
Clinically, Gram-negative bacteria play a role in
the formation of cavities. The pathogenic bacteria involved in pulp
inflammation are more severe. The results of this study are
consistent with these observations. In conclusion, a concentration
of 1 µg/ml of LTA and LPS stimulated human dental pulp cells
to activate the expression of complement C5a, and the expression of
C5a was the highest at 48 h. However, further research is warranted
to confirm the findings of this study. It would be of interest to
determine why C5a is express at low levels along with the
progression of the stimulation process, as well as whether C5a can
promote the dental pulp cells differentiate into odontoblasts. It
would also be of interest to examine how to use the high expression
of C5a in the differentiation process of dental pulp cells. In the
future, we hope to use the immune reaction of dental pulp cells, in
order to develop therapeutic means of combating the bacteria
involved in caries. The development of methods with which to help
pulp cells to protect themselves and to avoid caries would perhaps
prevent the development of irreversible pulpitis.
Funding
The present study was supported by the Heilongjiang
Natural Science Foundation of China under contract No. QC2015104,
the Health and Family Planning Commission of Heilongjiang province.
(grant no. 2017-097) and Heilongjiang Academy of Medical Science
(grant no. 201703).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML designed the study, performed the experiments and
wrote the manuscript. HM performed the experiments and organized
the images. WP and LZ participated in this experiment and made
substantial contributions to the acquisition of data. ZJ analyzed
and interpreted the data. WH and LG organized the images,
interpreted the data, and were involved in drafting the manuscript.
XC performed the surgery to obtain the teeth and was involved in
revising the manuscript. NL and JH helped write the manuscript, and
were responsible for the study funding, and in the conception and
design of the study, as well as providing final approval of the
version to be published. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of The Second Affiliated Hospital of Harbin
Medical University (Harbin, China). Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors are grateful to the Key Laboratory of
Myocardial Ischemia of the Ministry of Education (The Second
Affiliated Hospital of Harbin Medical University, Harbin, China)
for providing facilities to conduct the investigations of the
present study. In addition, the authors are grateful to the office
of Scientific Writing of University of Tennessee Health Science
Center for language editing.
References
|
1
|
Aurrekoetxea M, Garcia-Gallastegui P,
Irastorza I, Luzuriaga J, Uribe-Etxebarria V, Unda F and Ibarretxe
G: Dental pulp stem cells as a multifaceted tool for bioengineering
and the regeneration of craniomaxillofacial tissues. Front Physiol.
6:2892015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu M, Sun Y, Liu Y, Yuan M, Zhang Z and
Hu W: Modulation of the differentiation of dental pulp stem cells
by different concentrations of β-glycerophosphate. Molecules.
17:1219–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Franzon R, Gomes M, Pitoni CM, Bergmann CP
and Araujo FB: Dentin rehardening after indirect pulp treatment in
primary teeth. J Dent Child (Chic). 76:223–228. 2009.
|
|
4
|
Mathur VP, Dhillon JK, Logani A and Kalra
G: Evaluation of indirect pulp capping using three different
materials: A randomized control trial using cone-beam computed
tomography. Indian J Dent Res. 27:623–629. 2016. View Article : Google Scholar
|
|
5
|
Petrou MA, Alhamoui FA, Welk A,
Altarabulsi MB, Alkilzy M and H Splieth C: A randomized clinical
trial on the use of medical Portland cement, MTA and calcium
hydroxide in indirect pulp treatment. Clin Oral Investig.
18:1383–1389. 2014. View Article : Google Scholar
|
|
6
|
Ohnishi T, Bandow K, Kakimoto K, Kusuyama
J and Matsuguchi T: Long-time treatment by low-dose
N-acetyl-L-cysteine enhances proinflammatory cytokine expressions
in LPS-stimulated macrophages. PLoS One. 9:pp. e872292014,
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Beek J, Elward K and Gasque P:
Activation of complement in the central nervous system: Roles in
neurodegeneration and neuroprotection. Ann N Y Acad Sci. 992:56–71.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baumann U, Chouchakova N, Gewecke B, Köhl
J, Carroll MC, Schmidt RE and Gessner JE: Distinct tissue
site-specific requirements of mast cells and complement components
C3/C5a receptor in IgG immune complex-induced injury of skin and
lung. J Immunol. 167:1022–1027. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bürgi B, Brunner T and Dahinden CA: The
degradation product of the C5a anaphylatoxin C5adesarg retains
basophil-activating properties. Eur J Immunol. 24:1583–1589. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Drouin SM, Kildsgaard J, Haviland J,
Zabner J, Jia HP, McCray PB Jr, Tack BF and Wetsel RA: Expression
of the complement anaphylatoxin C3a and C5a receptors on bronchial
epithelial and smooth muscle cells in models of sepsis and asthma.
J Immunol. 166:2025–2032. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
el-Lati SG, Dahinden CA and Church MK:
Complement peptides C3a- and C5a-induced mediator release from
dissociated human skin mast cells. J Invest Dermatol. 102:803–806.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elsner J, Oppermann M, Czech W, Dobos G,
Schöpf E, Norgauer J and Kapp A: C3a activates reactive oxygen
radical species production and intracellular calcium transients in
human eosinophils. Eur J Immunol. 24:518–522. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nilsson G, Johnell M, Hammer CH, Tiffany
HL, Nilsson K, Metcalfe DD, Siegbahn A and Murphy PM: C3a and C5a
are chemotaxins for human mast cells and act through distinct
receptors via a pertussis toxin-sensitive signal transduction
pathway. J Immunol. 157:1693–1698. 1996.PubMed/NCBI
|
|
14
|
Schulman ES, Post TJ, Henson PM and Giclas
PC: Differential effects of the complement peptides, C5a and C5a
des Arg on human basophil and lung mast cell histamine release. J
Clin Invest. 81:918–923. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vogt W: Anaphylatoxins: Possible roles in
disease. Complement. 3:177–188. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan H, Shen Z, Mukhopadhyay P, Wang H,
Pacher P, Qin X and Gao B: Anaphylatoxin C5a contributes to the
pathogenesis of cisplatin-induced nephrotoxicity. Am J Physiol
Renal Physiol. 296:F496–F504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conway EM, Van de Wouwer M, Pollefeyt S,
Jurk K, Van Aken H, De Vriese A, Weitz JI, Weiler H, Hellings PW,
Schaeffer P, et al: The lectin-like domain of thrombomodulin
confers protection from neutrophil-mediated tissue damage by
suppressing adhesion molecule expression via nuclear factor kappaB
and mitogen-activated protein kinase pathways. J Exp Med.
196:565–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van de Wouwer M, Plaisance S, De Vriese A,
Waelkens E, Collen D, Persson J, Daha MR and Conway EM: The
lectin-like domain of thrombomodulin interferes with complement
activation and protects against arthritis. J Thromb Haemost.
4:1813–1824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boshra H, Peters R, Li J and Sunyer JO:
Production of recombinant C5a from rainbow trout (Oncorhynchus
mykiss): Role in leucocyte chemotaxis and respiratory burst. Fish
Shellfish Immunol. 17:293–303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flierl MA, Perl M, Rittirsch D, Bartl C,
Schreiber H, Fleig V, Schlaf G, Liener U, Brueckner UB, Gebhard F
and Huber-Lang MS: The role of C5a in the innate immune response
after experimental blunt chest trauma. Shock. 29:25–31. 2008.
|
|
21
|
Gressner OA, Koch A, Sanson E, Trautwein C
and Tacke F: High C5a levels are associated with increased
mortality in sepsis patients-no enhancing effect by actin-free
Gc-globulin. Clin Biochem. 41:974–980. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo RF and Ward PA: Role of C5a in
inflammatory responses. Annu Rev Immunol. 23:821–852.
3005PubMed/NCBI
|
|
23
|
Marigo I, Dolcetti L, Serafini P,
Zanovello P and Bronte V: Tumor-induced tolerance and immune
suppression by myeloid derived suppressor cells. Immunol Rev.
222:162–179. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Széplaki G, Varga L, Füst G and Prohászka
Z: Role of complement in the pathomechanism of atherosclerotic
vascular diseases. Mol Immunol. 46:2784–2793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hornum L, Hansen AJ, Tornehave D, Fjording
MS, Colmenero P, Wätjen IF, Søe Nielsen NH, Bliddal H and Bartels
EM: C5a and C5aR are elevated in joints of rheumatoid and psoriatic
arthritis patients, and C5aR blockade attenuates leukocyte
migration to synovial fluid. PLoS One. 12:pp. e01890172017,
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Russkamp NF, Ruemmler R, Roewe J, Moore
BB, Ward PA and Bosmann M: Experimental design of complement
component 5a-induced acute lung injury (C5a-ALI): A role of
CC-chemokine receptor type 5 during immune activation by
anaphylatoxin. FASEB J. 29:3762–3772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Xiao H, Guo R, Li Y and Shen B:
The role of C5a in acute lung injury induced by highly pathogenic
viral infections. Emerg Microbes Infect. 4:pp. e282015, View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Michalopoulos GK and DeFrances MC: Liver
regeneration. Science. 276:60–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mastellos D, Papadimitriou JC, Franchini
S, Tsonis PA and Lambris JD: A novel role of complement: Mice
deficient in the fifth component of complement (C5) exhibit
impaired liver regeneration. J Immunol. 166:2479–2486. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lara-Astiaso D, Izarra A, Estrada JC, Albo
C, Moscoso I, Samper E, Moncayo J, Solano A, Bernad A and Díez-Juan
A: Complement anaphylatoxins C3a and C5a induce a failing
regenerative program in cardiac resident cells. Evidence of a role
for cardiac resident stem cells other than cardiomyocyte renewal.
Springerplus. 1:632012. View Article : Google Scholar
|
|
31
|
Ignatius A, Ehrnthaller C, Brenner RE,
Kreja L, Schoengraf P, Lisson P, Blakytny R, Recknagel S, Claes L,
Gebhard F, et al: The anaphylatoxin receptor C5aR is present during
fracture healing in rats and mediates osteoblast migration in
vitro. J Trauma. 71:952–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chmilewsky F, Ayaz W, Appiah J, About I
and Chung SH: Nerve growth factor secretion from pulp fibroblasts
is modulated by complement C5a receptor and implied in neurite
outgrowth. Sci Rep. 6:317992016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chmilewsky F, Jeanneau C, Dejou J and
About I: Sources of dentin-pulp regeneration signals and their
modulation by the local microenvironment. J Endod. 40(Suppl 4): pp.
S19–S25. 2014, View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chmilewsky F, Jeanneau C, Laurent P and
About I: Pulp fibroblasts synthesize functional complement proteins
involved in initiating dentin-pulp regeneration. Am J Pathol.
184:1991–2000. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chmilewsky F, Jeanneau C, Laurent P and
About I: LPS induces pulp progenitor cell recruitment via
complement activation. J Dent Res. 94:166–174. 2015. View Article : Google Scholar
|
|
36
|
Chmilewsky F, Jeanneau C, Laurent P,
Kirschfink M and About I: Pulp progenitor cell recruitment is
selectively guided by a C5a gradient. J Dent Res. 92:532–539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeanneau C, Rufas P, Rombouts C, Giraud T,
Dejou J and About I: Can pulp fibroblasts kill cariogenic bacteria?
Role of complement activation. J Dent Res. 94:1765–1772. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kidd EA and Fejerskov O: What constitutes
dental caries? Histopathology of carious enamel and dentin related
to the action of cariogenic biofilms. J Dent Res. 83:C35–C38. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Section On Oral Health: Maintaining and
improving the oral health of young children. Pediatrics.
134:1224–1229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang W, Lv H, Wang H, Wang D, Sun S, Jia
Q, Wang P, Song B and Ni L: Activation of the NLRP3/caspase-1
inflammasome in human dental pulp tissue and human dental pulp
fibroblasts. Cell Tissue Res. 361:541–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koch L, Frommhold D, Buschmann K, Kuss N,
Poeschl J and Ruef P: LPS- and LTA-induced expression of IL-6 and
TNF-α in neonatal and adult blood: role of MAPKs and NF-κB.
Mediators Inflamm. 2014:2831262014. View Article : Google Scholar
|
|
42
|
Wisithphrom K, Murray PE and Windsor LJ:
Interleukin-1 alpha alters the expression of matrix
metalloproteinases and collagen degradation by pulp fibroblasts. J
Endod. 32:186–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Elsalhy M, Azizieh F and Raghupathy R:
Cytokines as diagnostic markers of pulpal inflammation. Int Endod
J. 46:573–580. 2013. View Article : Google Scholar
|
|
44
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
45
|
Abd-Elmeguid A, Yu DC, Kline LW, Moqbel R
and Vliagoftis H: Dentin matrix protein-1 activates dental pulp
fibroblasts. J Endod. 38:75–80. 2012. View Article : Google Scholar
|
|
46
|
Bulgari O, Dong X, Roca AL, Caroli AM and
Loor JJ: Innate immune responses induced by lipopolysaccharide and
lipoteichoic acid in primary goat mammary epithelial cells. J Anim
Sci Biotechnol. 8:292017. View Article : Google Scholar : PubMed/NCBI
|