Introduction
Diabetes mellitus is a metabolic disorder
characterized by consistent hyperglycemia and is a global public
health concern (1). The vascular
complications of type 2 diabetes can lead to microvascular and
macrovascular damage and are major causes of disability and death
in type 2 diabetes patients (2,3).
Endothelial dysfunction renders diabetics vulnerable to limb
infections and end-organ damage, such as nephropathy, neuropathy
and retinopathy (4). In addition,
diabetic macrovascular disease resembles atherosclerotic lesions
both morphologically and functionally (5), and oxidized low-density lipoprotein
(ox-LDL) is a key component involved in the genesis of
atherosclerotic lesions and is cytotoxic to various cell types,
such as endothelial cells (ECs), and is therefore suggested to
contribute to endothelial dysfunction (6). Therefore, preventing ox-LDL-induced
endothelial injury has received considerable attention as a
potential therapeutic target for the treatment of diabetic vascular
complications (7).
Astragaloside IV (ASV), known as a purified small
molecular saponin, is one of the main active components of Radix
Astragali that possesses comprehensive biological properties,
including anti-inflammatory, immunoregulatory, antioxidant and
antiaging properties (8-10). Our previous findings demonstrated
that ASV significantly inhibited epithelial-mesenchymal transition
induced by transforming growth factor-β1 during the progression of
lung fibrosis (11). In addition,
previous findings suggested that ASV induced vasodilation by
regulating nitric oxide production in the endothelium (12) and it improvesd vascular
endothelial dysfunction induced by hyperglycemia via the toll-like
receptor 4/nuclear factor (NF)-κB signaling pathway (13). Although the protective effects of
ASV on endothelial dysfunction have been reported, the detailed
molecular mechanisms remain unclear. microRNAs (miRNAs) have been
proposed to serve crucial roles in diverse pathophysiological
processes, including endothelial injury (14). A previous study demonstrated that
miR-26a expression was downregulated in atherosclerotic mice and
ox-LDL-stimulated human aortic ECs, and miR-26a overexpression
prevented ox-LDL-induced EC apoptosis (15). In addition, Yin et al
(16) reported that miR-338-3p
downregulation increased cell viability and inhibited cell
apoptosis in ox-LDL-induced human umbilical vein endothelial cells
(HUVECs). The above findings indicated the involvement of miRNAs in
regulating ox-LDL-induced EC damage. However, further studies are
required to elucidate the involvement of miRNAs in mediating the
protective effects of ASV on ox-LDL-induced ECs.
Based on the above results, the present study
conducted RNA sequencing (RNA-Seq) analysis to screen for
dysregulated miRNAs in HUVECs under ox-LDL stimulation. Next, the
effect of miR-140-3p, one of the most strongly downregulated miRNAs
induced by ox-LDL, in the protective role of ASV in ox-LDL-induced
HUVEC apoptosis was explored. The mechanisms underlying the effects
of ASV in HUVECs were also investigated.
Materials and methods
Cell culture and reagents
HUVECs (Clonetics; Lonza Group, Ltd.) were incubated
in Dulbecco's modified Eagle medium (DMEM; HyClone; GE Healthcare
Life Sciences) with 5 mM glucose, 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin, and 100 mg/ml
streptomycin (Beyotime Institute of Biotechnology). Cells were
incubated at 37°C with 5% CO2 in an incubator (Thermo
Fisher Scientific, Inc.). ASV (Sigma-Aldrich; Merck KGaA) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA).
HUVECs were treated with 100 µg/ml ox-LDL (Beijing Solarbio
Science & Technology Co., Ltd.) for varying incubation periods
(6, 12 and 24 h), as previously described (17,18).
Cell transfection
An overexpression vector (pcDNA3.1/+) containing the
human Krüppel-like factor 4 (KLF4) gene and an empty
pcDNA3.1/+vector were purchased from Guangzhou RiboBio Co., Ltd.
miR-140-3p mimics (5′-UAC CAC AGG GUA GAA CCA CGG-3′), miR-negative
control (miR-NC; 5′-UGC AAG CAC GAA UUA AUU GGC G-3′), miR-140-3p
inhibitors (5′-CCG UGG UUC UAC CCU GUG GUA-3′), as well as
inhibitor control (5′-UGA CCG AUC GUA CUU AUA GUC UG-3′), were
purchased form Guangzhou RiboBio Co., Ltd. Cells were cultured in
six-well plates at the density of 2×105 cells/well. A
total of 200 nM pcDNA3.1-KLF4 plasmid or empty vector pcDNA3.1 was
transiently transfected into HUVECs using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). miR-140-3p mimics,
miR-NC, miR-140-3p inhibitors and control inhibitors (50 nM) were
transiently transfected into HUVECs using Lipofectamine 2000,
according to manufacturer's instructions. Changes in mRNA and
protein expression levels were assessed at 24 h
post-transfection.
RNA-seq
Normal HUVECs and ox-LDL-stimulated HUVECs were
analyzed using RNA-seq. Four separate samples were prepared for
each group. Total RNA was isolated from cells using RNAios Plus
reagent (Takara Bio, Inc.) and purified using the RNeasy Plant Mini
kit (Qiagen GmbH). Sequencing was performed at Guangzhou RiboBio
Co., Ltd. Total RNA was sequenced on the Illumina HiSeq 2500
system. RNA-seq reads were aligned to the human transcriptome
(UCSC, hg19) using bowtie (http://bowtie-bio.sourceforge.net/index.shtml) and
RSEM (https://deweylab.github.io/RSEM/), as previously
described (19). P<0.05 was
considered statistically significant.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
miR-140-3p levels and KLF4 mRNA expression levels
were analyzed by RT-qPCR. Total RNA was isolated from HUVECs using
RNAios Plus reagent (Takara Bio, Inc.), according to the
manufacturer's protocol. To evaluate miR-140-3p expression, cDNA
was synthesized from total RNA using a miScript Reverse
Transcription kit (Qiagen GmbH). Subsequently, qPCR was conducted
using a TaqMan® MicroRNA Reverse Transcription kit
(Qiagen GmbH). To measure KLF4 mRNA expression, total RNA was
reverse-transcribed to cDNA using a PrimeScript RT reagent kit
(Takara Bio, Inc.). Next, qPCR was performed using SYBR Premix Ex
Taq™ (Takara Bio, Inc.), following the manufacturer's instructions.
Relative miR-140-3p levels and KLF4 mRNA expression levels were
normalized to those of U6 RNA and β-actin, respectively. The
following primers were designed: miR-140-3p, forward 5′-ACA CTC CAG
CTG GGA GGC GGG GCG CCG CGG GA-3′ and reverse 5′-CTC AAC TGG TGT
CGT GGA-3′; U6, forward 5′-CTC GCT TCG GCA GCA CA-3′ and reverse
5′-AAC GCT TCA CGA ATT TGC GT-3′; KLF4, forward 5′-GAA CTC ACA CAG
GCG AGA AA-3′ and reverse 5′-GAA CTC ACA CAG GCG AGA AA-3′; and
β-actin, forward 5′-ATT TCT GAA TGG CCC AGG T-3′ and reverse 5′-CTG
CCT CAA CAC CTC AAC C-3′. The thermocycling conditions were 95°C
for 5 min, followed by 35 cycles of 95°C for 5 sec, 60°C for 30 sec
and 70°C for 10 sec. Relative miRNA levels and mRNA expression
levels were determined using the 2−ΔΔCq method (20).
Cell proliferation assay
Cell proliferation was evaluated using a Cell
Counting Kit-8 (CCK-8) reagent (Dojindo Molecular Technologies,
Inc.). Following transfection, HUVECs (5×104/ml) were
seeded into 96-well plates and allowed to grow for 0, 1, 2 and 3
days. The experiment was repeated thrice. Five parallel wells were
set in each group after incubation. CCK-8 reagent (10 µl)
was added to each well and incubated at 37°C with 5% CO2
for another 2 h. Finally, the optical density of each well was
measured at 450 nm using a microplate reader (model 680; Bio-Rad
Laboratories, Inc.).
Apoptosis analysis
Cell apoptosis was evaluated using a fluorescein
isothiocyanate (FITC)-conjugated Annexin V Apoptosis Detection kit
I (BD Biosciences). Following cell transfection, HUVECs were seeded
into six-well plates and exposed to ox-LDL for 24 h. Apoptotic
cells were analyzed using FACScan (BD Biosciences) with CellQuest
software version 0.9.3.1 (BD Biosciences).
Luciferase reporter assay
The 3′ untranslated region (UTR) fragments of KLF4
containing the predicted binding sites of miR-140-3p were
synthesized and cloned into the psiCHECK-2 dual luciferase reporter
plasmid (Promega Corporation); this reporter plasmid was designated
KLF4 wild-type. Mutation in the putative miR-140-3p target
sequences in the 3′UTR of KLF4 was generated using a site-directed
gene mutagenesis kit (Takara Bio, Inc.); this reporter plasmid was
designated KLF4 mutant-type. For the luciferase reporter assay,
HUVECs were seeded into six-well plates and co-transfected with 200
ng KLF4 wild-type or KLF4 mutant-type and 100 nM miR-NC or
miR-140-3p mimics using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) reagent, following with the manufacturer's
protocol. After 24 h, the cell lysates were assayed for luciferase
activity using the Luciferase Assay System (Promega Corporation).
Luciferase activity was measured on a luminescence counter (Centro
XS3 LB 960; Berthold Technologies). The relative luciferase
activity was expressed as the ratio of firefly luciferase to
Renilla luciferase activity.
Western blotting
Following treatment, HUVECs were collected and lysed
using 1% RIPA lysis buffer (Thermo Fisher Scientific, Inc.)
supplemented with protease inhibitors (Roche Diagnostics). The
protein concentration was quantified using a bicinchoninic acid kit
(Beijing Solarbio Science & Technology Co., Ltd.), and equal
amounts of proteins (20 µg) were size-fractionated by 12%
sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene fluoride (PVDF) membrane (EMD
Millipore). Following blocking with 5% nonfat skim milk for 1 h at
room temperature, the membranes were incubated overnight at 4°C
with primary antibodies against KLF4 (1:1,000; cat. no. ab215036;
Abcam), phosphorylated (p-) PI3K (1:1,000; Tyr458/Tyr199; cat. no.
4228; Cell Signaling Technology, Inc.), PI3K (1:1,000; cat. no.
4249; Cell Signaling Technology, Inc.), p-Akt (1:1,000; Ser/Thr;
cat. no. 9611; Cell Signaling Technology, Inc.), Akt (1:1,000; cat.
no. 4691; Cell Signaling Technology, Inc.), and β-actin (1:1,000;
cat. no. ab8226; Abcam). Then, the membranes were incubated with
the corresponding secondary horseradish peroxidase-conjugated
secondary antibody (1:5,000; cat. no. ab6721; Abcam) at room
temperature for 1 h. Signals were visualized with the enhanced
chemiluminescence detection reagents (EMD Millipore), and the band
intensities were quantified using Quantity One software version
4.62 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All results were presented as the mean ± standard
deviation of three independent experiments. Differences among
groups were analyzed by one-way ANOVA, followed by Tukey's test
using SPSS version 19.0 software (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-140-3p promotes proliferation and
inhibits apoptosis in HUVECs
To determine the expression profiles of miRNAs in
ox-LDL-induced HUVECs, RNA-Seq analysis was performed to screen for
differentially expressed miRNAs with or without 100 µg/ml
ox-LDL stimulation for 24 h. The miRNA expression profiles of the
ox-LDL-exposed group compared with the control group were
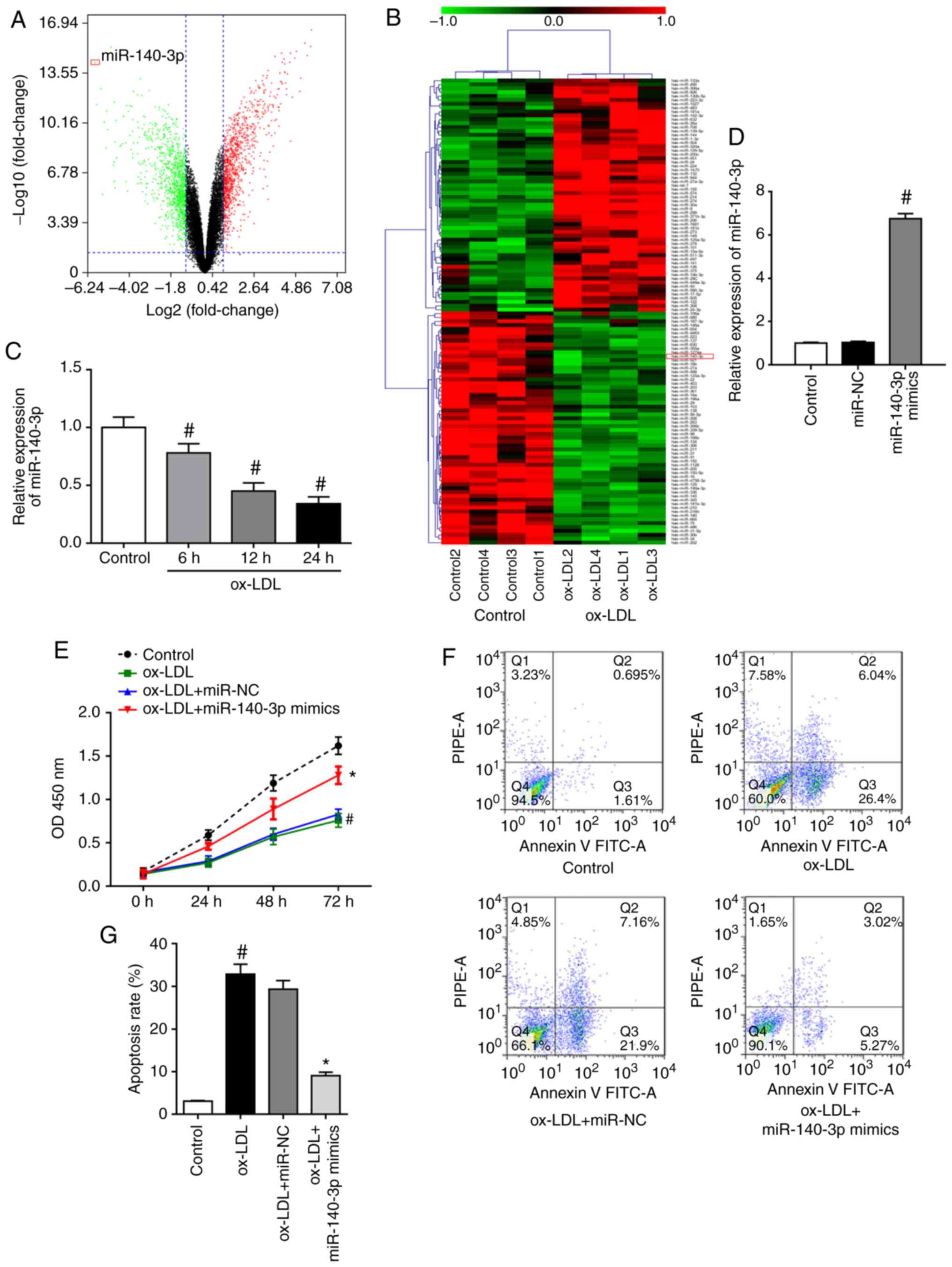
visualized using a volcano plot (Fig.
1A) and heat map (Fig. 1B).
The analysis identified 120 dysregulated miRNAs, including 60
downregulated miRNAs and 60 upregulated miRNAs, using a cutoff of
fold change >2. To investigate the in-depth function of the
dysregulated miRNAs, the most strongly downregulated miRNA,
miR-140-3p, was selected as a target for validation of the RNA-Seq
results. Subsequently, RT-qPCR results confirmed that ox-LDL
stimulation downregulated miR-140-3p expression in HUVECs in a
time-dependent manner (Fig. 1C).
Therefore, the role of miR-140-3p in ox-LDL-induced HUVECs was
further investigated. Transfection of HUVECs with miR-140-3p mimics
results in a significant increase in miR-140-3p levels, as detected
by RT-qPCR (Fig. 1D). Results of
the CCK-8 assay revealed that ox-LDL stimulation suppressed the
proliferation of HUVECs, whereas miR-140-3p overexpression reversed
the inhibitory effects of ox-LDL on cell proliferation (Fig. 1E). Results of flow cytometry assay
in Fig. 1F and G demonstrated
that ox-LDL-induced HUVECs had significantly higher apoptosis rates
compared to those of the control group. However, miR-140-3p
overexpression decreased the apoptotic cell rate compared with
those of the ox-LDL + miR-NC group (Fig. 1F and G). The current findings
suggested that miR-140-3p promoted cell proliferation and inhibited
the apoptosis of ox-LDL-induced HUVECs.
ASV relieves ox-LDL-induced HUVECs
apoptosis by upregulating miR-140-3p expression and suppressing the
PI3K/Akt pathway
Considering that miR-140-3p regulates cell
proliferation and apoptosis in ox-LDL-induced HUVECs and that ASV
is likely to directly influence miRNA expression levels, the
hypothesis that ASV protected HUVECs from ox-LDL by miR-140-3p
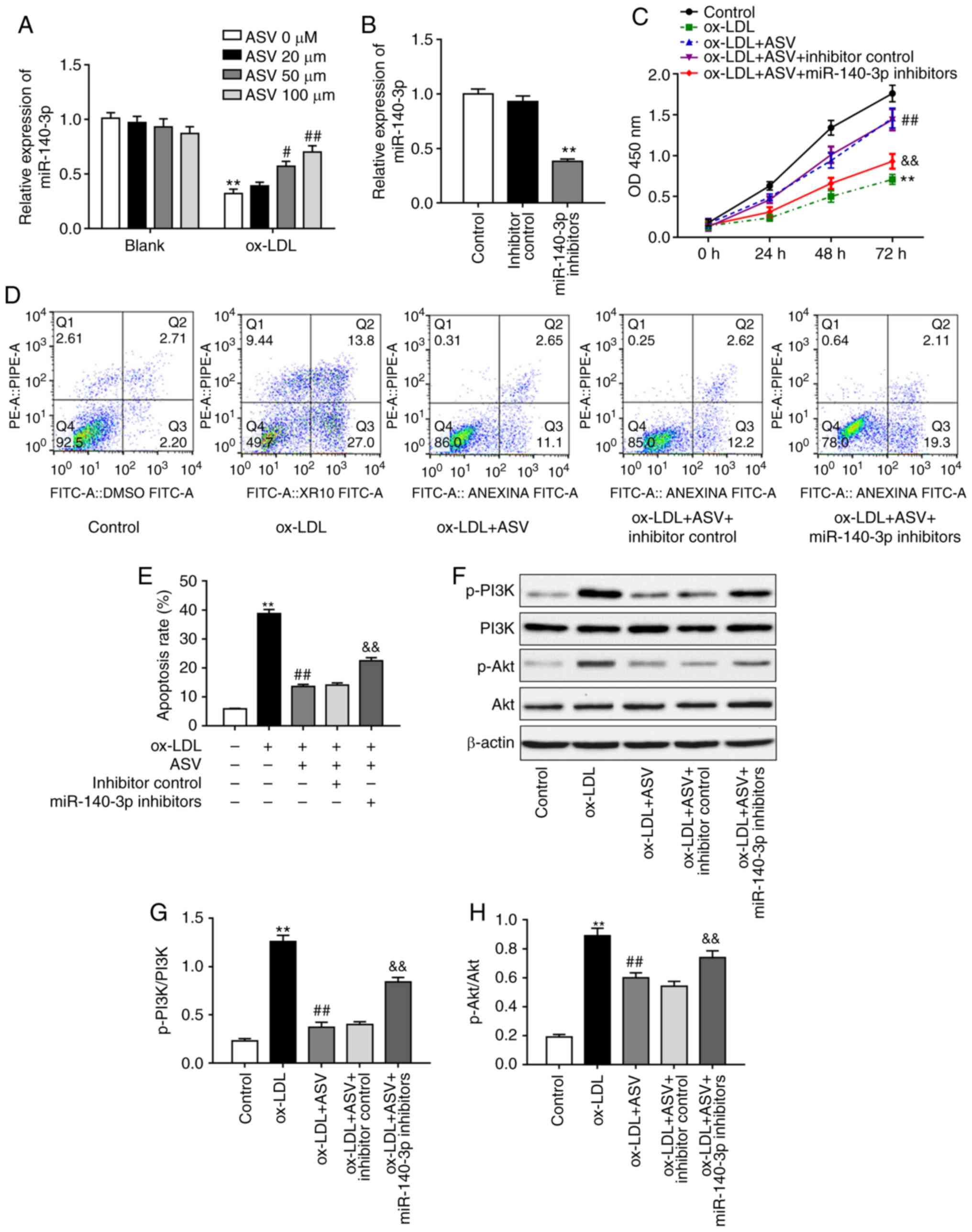
regulation was investigated next. Results demonstrated that ASV did
not affect miR-140-3p levels under normal conditions, but it
significantly upregulated miR-140-3p levels in a
concentration-dependent manner under ox-LDL stimulation (Fig. 2A). To determine whether miR-140-3p
is required for ASV-induced changes in HUVECs, miR-140-3p levels
were downregulated by transfecting HUVECs with miR-140-3p
inhibitors, and the transfection efficiency was validated via
RT-qPCR (Fig. 2B). Based on the
observed effects of ASV on miR-140-3p expression (Fig. 2A), the dose of 100 µM ASV
was selected for the subsequent experiments. Results demonstrated
that miR-140-3p inhibition reversed the protective effects of ASV
on ox-LDL-induced proliferation of HUVECs (Fig. 2C). Results of apoptosis assay
revealed that ASV significantly inhibited ox-LDL-triggered
apoptosis (Fig. 2D and E).
However, inhibition of miR-140-3p expression partly reversed the
effects of ASV on HUVECs (Fig. 2D and
E). In addition, ASV treatment suppressed the ox-LDL-mediated
activation of the PI3K/Akt pathway in HUVECs, as evidenced by the
increased levels of phosphorylated PI3K and phosphorylated Akt
(Fig. 2F-H). Inhibition of
miR-140-3p rescued the ASV-induced downregulation of p-PI3K and
p-Akt levels (Fig. 2F-H). These
results indicated that ASV protected ox-LDL-induced HUVEC injury by
regulating miR-140-3p expression and the PI3K/Akt pathway.
miR-140-3p exerts its function by
regulating KLF4 in HUVECs
To elucidate the mechanisms underlying the role of
miR-140-3p in the effects of ASV on ox-LDL stimulated HUVECs, the
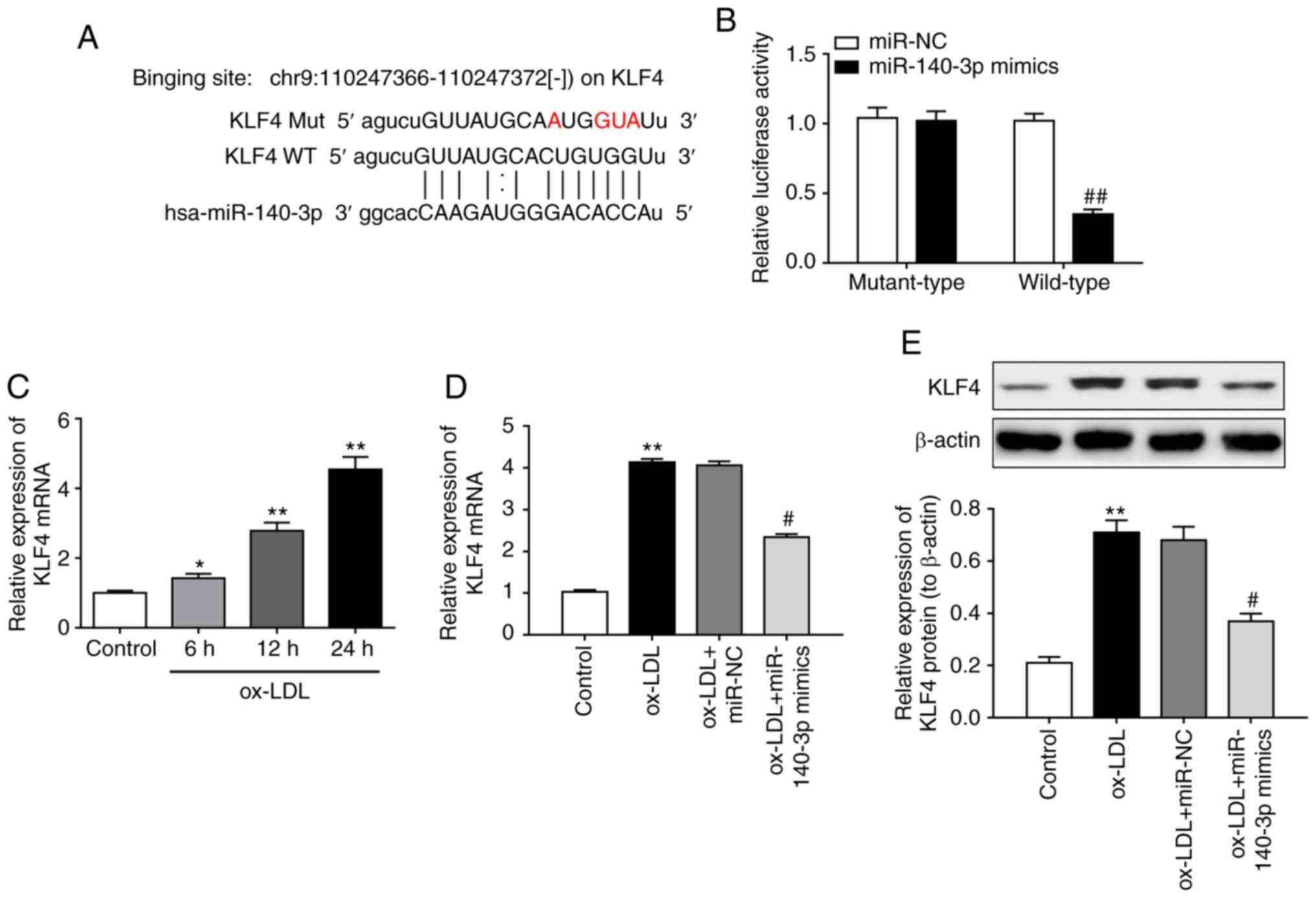
bioinformatics tool StarBase v3.0 (http://star-base.sysu.edu.cn/) was used to predict the
downstream target of miR-140-3p. The complementary binding sites
within miR-140-3p and the 3′UTR of KLF4 are illustrated in Fig. 3A. Subsequently, a luciferase
reporter assay confirmed the direct binding of miR-140-3p at the
KLF4 3′UTR (Fig. 3B). RT-qPCR
analysis revealed that KLF4 mRNA expression levels were upregulated
in ox-LDL-induced HUVECs in a time-dependent manner (Fig. 3C). miR-140-3p overexpression
caused significant downregulation of KLF4 expression in ox-LDL
induced HUVECs both at the mRNA (Fig.
3D) and protein levels (Fig.
3E). Thus, the results indicated that KLF4 is a direct target
of miR-140-3p in HUVECs. Furthermore, KLF4 overexpression using
plasmid transfection significantly upregulated KLF4 expression,
both at the mRNA (Fig. 3F) and
protein levels (Fig. 3G).
Subsequent experiments revealed that KLF4 overexpression reversed
the changes induced by miR-140-3p mimics on ox-LDL-induced cell
proliferation (Fig. 3H) and
apoptosis (Fig. 3I and J) in
HUVECs. Taken together, the present results suggested that
miR-140-3p regulated cell proliferation and apoptosis in
ox-LDL-induced HUVECs by regulating KLF4.
ASV influences ox-LDL-induced HUVEC
damage via the KLF4-dependent PI3K/Akt pathway
The present study further investigated the
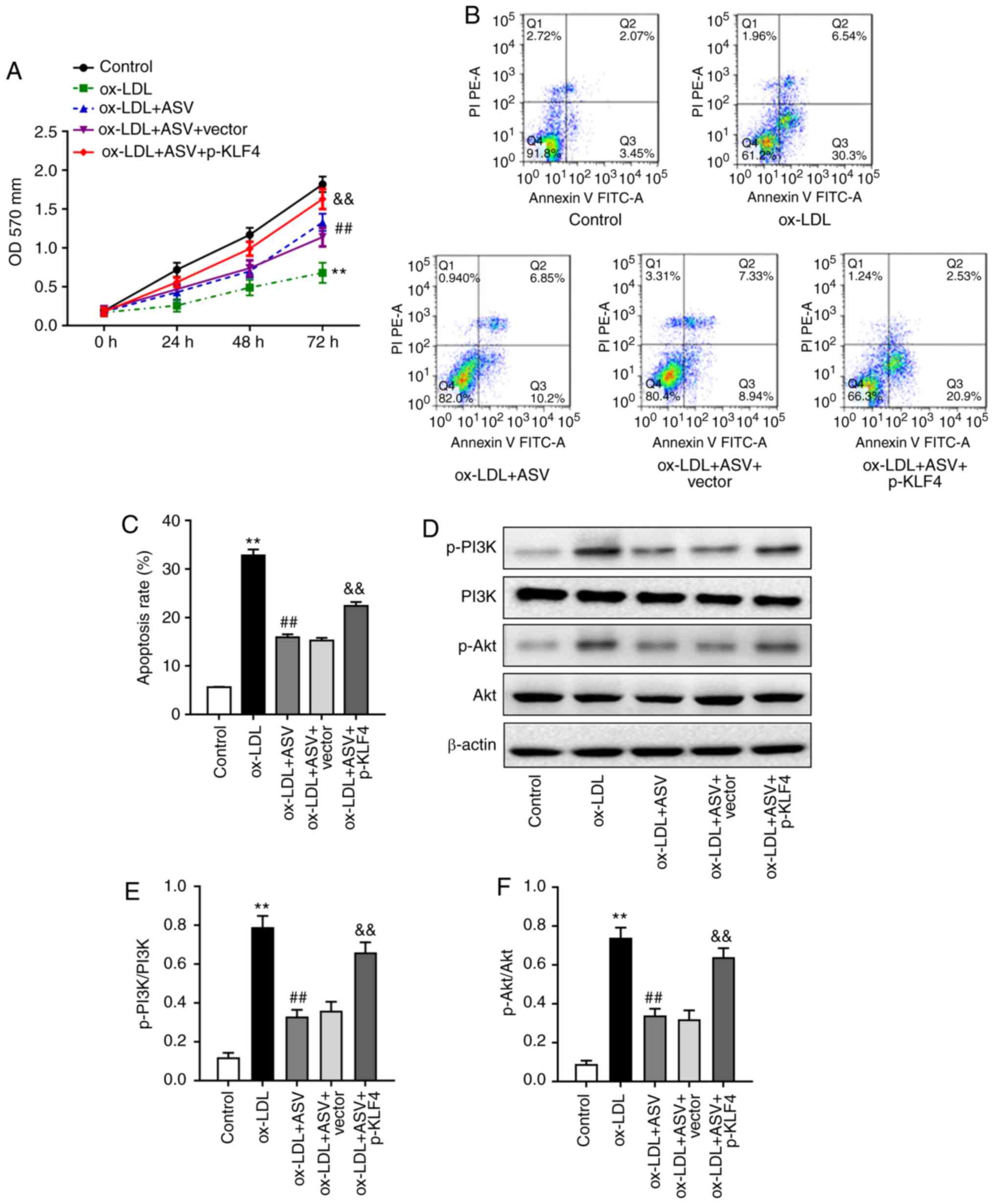
involvement of KLF4 in the protective role of ASV on HUVECs under
ox-LDL stimulation. Functional analyses revealed that KLF4
overexpression partially rescued the effects of ASV on
ox-LDL-induced cell proliferation (Fig. 4A) and apoptosis (Fig. 4B and C). In addition, KLF4
overexpression increased p-PI3K, and p-Akt levels, although the
total protein levels of PI3K and AKT in ASV-treated HUVECs under
ox-LDL condition were not affected (Fig. 4D-F). These results confirmed that
ASV regulated ox-LDL induced cell proliferation and apoptosis in
HUVECs via the KLF4-dependent PI3K/Akt pathway.
Discussion
The present study focused on the molecular mechanism
underlying the protective effects of ASV on HUVECs induced by
ox-LDL. The current integrated analyses revealed that ASV
alleviated ox-LDL-induced HUVEC apoptosis by upregulating
miR-140-3p expression and subsequently inhibiting the KLF4/PI3K/Akt
signaling pathway.
Emerging evidence suggests that ASV has a protective
role against HUVEC injury. A previous study demonstrated that ASV
promoted cell proliferation, reduced apoptosis, and downregulated
the expression levels of tumor necrosis factor-α and interleukin-1β
in HUVECs via inhibition of the JNK pathway (21). In addition, the findings of Ma
et al (22) suggested that
ASV inhibited inflammation induced by phorbol-12- myristate
13-acetate in HUVECs by reducing the phosphorylation levels of JNK
and the p38 pathway. Furthermore, ASV could suppress hydrogen
peroxide-induced oxidative stress by inhibiting the reactive oxygen
species/NF-κB pathway and endothelial nitric oxide synthase
uncoupling (23). The present
results consistently demonstrated that ASV promoted cell
proliferation and inhibited cell apoptosis of ox-LDL-induced
HUVECs. However, further studies will be required to fully
investigate the exact mechanism underlying the beneficial effects
of ASV on HUVECs.
Recently, miRNAs have been reported to have an
important role in HUVEC dysfunction (24-26). Multiple studies indicated that
non-coding RNAs are also involved in the function of ASV on cell
viability (27) and autophagy
(28). However, there is
currently no evidence on the involvement of miRNAs in
anti-apoptosis action of ASV in ox-LDL induced HUVECs. To this end,
the present study performed RNA-seq analysis to screen the
potential miRNAs involved in ox-LDL induced EC injury. The present
results demonstrated that ox-LDL significantly downregulated
miR-140-3p expression in a time-dependent manner. A recent study
demonstrated that ellagic acid could upregulate miR-140-3p
expression and inhibit MAP kinase kinase 6 expression to inhibit
apoptosis in cardiomyocytes (29). However, little is known regarding
the role of miR-140-3p in the apoptosis of HUVECs. The present
study first revealed that miR-140-3p overexpression effectively
reversed ox-LDL-triggered cell apoptosis in HUVECs. In addition,
ASV treatment was demonstrated to upregulate miR-140-3p expression
in ox-LDL-induced HUVECs, and inhibition of miR-140-3p expression
could reverse the protective effects of ASV on ox-LDL-induced
damage in HUVECs. Although Rasheed et al (30) have reported that removal of
epigallocatechin-3-O-gallate could upregulate miR-140-3p expression
in chondrocytes, the present study is the first to provide evidence
that ASV upregulates miR-140-3p expression to alleviate
ox-LDL-mediated cell injury. Previous studies reported that ASV
could inhibit the PI3K/Akt pathway to alleviate cell dysfunction
(9,12,17,31-32). Additionally, a recent study
identified that overexpression of miR-9-5p suppressed the PI3K/Akt
pathway by inhibiting CXC chemokine receptor-4, thereby reducing
high glucose-induced apoptosis in HUVECs (33). The present study identified that
ASV suppressed ox-LDL stimulated activation of the PI3K/Akt pathway
in HUVECs and inhibition of miR-140-3p could reactivate the
PI3K/Akt pathway, which could promote apoptosis. However, the
current study also found that Akt was a survival signaling, which
helps to protect cells from various stimuli inducing cell death
(34,35). Therefore, further studies are
required to verify that ASV inhibits apoptosis via Akt suppression.
Another limitation of the current study is that only one of the
most dysregulated miRNAs during ox-LDL-mediated ECs injury was
confirmed and investigated; further experiments are needed to fully
identify other specific miRNAs involved in EC damage.
Biological analysis and luciferase reporter assay
identified KLF4 as a target of miR-140-3p in ox-LDL-stimulated
HUVECs. KLF4 is a member of the Krüppel-like family of
transcription factors, which serve important roles in regulating
endothelial biology (36). KLF4
has been demonstrated as a downstream effector of ERK5, which
contributes to the protection of endothelial cells from oxidative
stress-induced cell apoptosis (37). Given the importance of KLF4 in
endothelial protection, analyzing the expression profiles in
injured HUVECs and the downstream signaling pathways is of
significant interest. Recent studies demonstrated that KLF4
overexpression reduced cell viability and increased the proportion
of apoptotic cells (38,39). By contrast, another study by Yang
et al (40) suggested that
KLF4 protected cells from ischemic stroke-induced apoptosis via
transcriptional activation of metastasis associated lung
adenocarcinoma transcript 1. In the present study, results
indicated that ox-LDL treatment caused a significant upregulation
of KLF4 expression, whereas miR-140-3p overexpression reduced KLF4
expression levels in ox-LDL-induced HUVECs. In addition,
restoration of KLF4 levels could reverse the anti-apoptosis effect
of miR-140-3p overexpression and ASV treatment in HUVECs. The
conflicting role of KLF4 in cell proliferation and apoptosis could
be attributed to the different types of cells and stimulatory
conditions, and further studies will be required to determine the
role of KLF4 in the pathology of diseases. In addition, KLF4 has
been identified as a regulator of the PI3K/Akt signaling pathway in
cancer cells (41,42). Similarly, the present results
revealed that KLF4 activated the PI3K/Akt signaling pathway in
ox-LDL-induced HUVECs. Taken together, these results revealed that
miR-140-3p regulated ox-LDL-induced EC injury by targeting KLF4 and
the downstream PI3K/Akt pathway.
In summary, the current findings provided evidence
that ASV alleviated ox-LDL-induced apoptosis in HUVECs via the
upregulation of miR-140-3p expression and subsequent inactivation
of the KLF4/PI3K/Akt signaling pathway, thereby shedding light on
the molecular mechanism by which ASV alleviates ox-LDL-induced
HUVEC apoptosis. Thus, ASV may be a promising therapeutic target to
suppress apoptosis of ECs, and fine tuning of the miR-140-3p/KLF4
axis through biological or pharmacological approaches may aid in
relieving ox-LDL-induced EC damage.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81704071), the Key Research
and Development Plan of Shandong province (grant no.
2018GSF119027), the Taishan Scholars Youth Expert Program of
Shandong Province in China (grant no. tsqn201812146), the Young
Elite Scientists Sponsorship Program by the China Association for
Science and Technology (grant no. CACM-2018-QNRC2-B01), the Natural
Science Foundation of Shandong Province (grant nos. ZR2017BH027,
ZR2016HB19 and ZR2012HM093), the Project of Scientific and
Technological Development Program of Shandong Province (grant no.
2010GSF10242), the Project of Scientific and Technological
Development Program of Traditional Chinese Medicine of Shandong
Province (grant nos. 2017-180, 2011-038 and 2009Z004-1), the
Project of Scientific and Technological Development Program of
Jinan (grant nos. 201805081 and 201805009).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WQ, QQ and XC designed the study and performed the
statistical analysis. WQ, XC and RH performed western blot analysis
and data correction. WY, XZ and HZ isolated and identified EPCs. XC
and RZ performed proliferation, migration and tube formation
assays. WQ, XZ and QQ wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All of the animal procedures, including housing,
care and experimental protocols, were approved by the Animal Care
and Use Committee of Shandong University of Traditional Chinese
Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
Akt
|
Akt serine/threonine kinase
|
|
ASV
|
astragaloside IV
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
KLF4
|
Krüppel-like factor 4
|
|
ox-LDL
|
oxidized low-density lipoprotein
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
Acknowledgments
Not applicable.
References
|
1
|
Shore AC, Colhoun HM, Natali A, Palombo C,
Khan F, Östling G, Aizawa K, Kennbäck C, Casanova F, Persson M, et
al: Use of vascular assessments and novel biomarkers to predict
cardiovascular events in type 2 Diabetes: The SUMMIT VIP study.
Diabetes Care. 41:2212–2219. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rao Kondapally Seshasai S, Kaptoge S,
Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal
KJ, Gillum RF, Holme I, et al: Diabetes mellitus, fasting glucose,
and risk of cause-specific death. N Engl J Med. 364:829–841. 2011.
View Article : Google Scholar
|
|
3
|
Tousoulis D, Papageorgiou N, Androulakis
E, Siasos G, Latsios G, Tentolouris K and Stefanadis C: Diabetes
mellitus-associated vascular impairment: Novel circulating
biomarkers and therapeutic approaches. J Am Coll Cardiol.
62:667–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shamsaldeen YA, Ugur R, Benham CD and
Lione LA: Diabetic dyslipidaemia is associated with alterations in
eNOS, caveolin-1, and endothelial dysfunction in streptozotocin
treated rats. Diabetes Metab Res Rev. 34:pp. e29952018, View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gilbert RE: Endothelial loss and repair in
the vascular complications of diabetes: Pathogenetic mechanisms and
therapeutic implications. Circ J. 77:849–856. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu C, Yin D, Nie H and Sun D:
Notoginsenoside R1 protects HUVEC against oxidized low-density
lipoprotein (Ox-LDL)-induced atherogenic response via
down-regulating miR-132. Cell Physiol Biochem. 51:1739–1750. 2018.
View Article : Google Scholar
|
|
7
|
Pollack RM, Donath MY, LeRoith D and
Leibowitz G: Anti-inflammatory agents in the treatment of Diabetes
and its vascular complications. Diabetes Care. 39(Suppl 2): pp.
S244–S252. 2016, View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song MT, Ruan J, Zhang RY, Deng J, Ma ZQ
and Ma SP: Astragaloside IV ameliorates neuroinflammation-induced
depressive-like behaviors in mice via the PPARγ/NF-κB/NLRP3
inflammasome axis. Acta Pharmacol Sin. 39:1559–1570. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu ZH, Liu HB and Wang J: Astragaloside
IV protects against the pathological cardiac hypertrophy in mice.
Biomed Pharmacother. 97:1468–1478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Li H, Fang F, Deng X and Ma S:
Astragaloside IV attenuates cognitive impairments induced by
transient cerebral ischemia and reperfusion in mice via
anti-inflammatory mechanisms. Neurosci Lett. 639:114–119. 2017.
View Article : Google Scholar
|
|
11
|
Qian W, Cai X, Qian Q, Zhang W and Wang D:
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal
transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med.
22:4354–4365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin XP, Cui HJ, Yang AL, Luo JK and Tang
T: Astragaloside IV improves vasodilatation function by regulating
the PI3K/Akt/eNOS signaling pathway in rat aorta endothelial cells.
J Vasc Res. 55:169–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leng B, Tang F, Lu M, Zhang Z, Wang H and
Zhang Y: Astragaloside IV improves vascular endothelial dysfunction
by inhibiting the TLR4/NF-κB signaling pathway. Life Sci.
209:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stępień EŁ, Durak-Kozica M, Kamińska A,
Targosz-Korecka M, Libera M, Tylko G, Opalińska A, Kapusta M,
Solnica B, Georgescu A, et al: Circulating ectosomes: Determination
of angiogenic microRNAs in type 2 diabetes. Theranostics.
8:3874–3890. 2018. View Article : Google Scholar :
|
|
15
|
Liang W, Fan T, Liu L and Zhang L:
Knockdown of growth-arrest specific transcript 5 restores oxidized
low-density lipoprotein-induced impaired autophagy flux via
upregulating miR-26a in human endothelial cells. Eur J Pharmacol.
843:154–161. 2019. View Article : Google Scholar
|
|
16
|
Yin J, Hou X and Yang S: microRNA-338-3p
promotes ox-LDL-induced endothelial cell injury through targeting
BAMBI and activating TGF-β/Smad pathway. J Cell Physiol.
234:11577–11586. 2019. View Article : Google Scholar
|
|
17
|
Wang Y, Che J, Zhao H, Tang J and Shi G:
Paeoniflorin attenuates oxidized low-density lipoprotein-induced
apoptosis and adhesion molecule expression by autophagy enhancement
in human umbilical vein endothelial cells. J Cell Biochem.
120:9291–9299. 2019. View Article : Google Scholar
|
|
18
|
Yu S, Zhang L, Liu C, Yang J, Zhang J and
Huang L: PACS2 is required for ox-LDL-induced endothelial cell
apoptosis by regulating mitochondria-associated ER membrane
formation and mitochondrial Ca2+ elevation. Exp Cell
Res. 379:191–202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B and Dewey CN: RSEM: Accurate
transcript quantification from RNA-Seq data with or without a
reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
You L, Fang Z, Shen G, Wang Q, He Y, Ye S,
Wang L, Hu M, Lin Y, Liu M and Jiang A: Astragaloside IV prevents
high glucose-induced cell apoptosis and inflammatory reactions
through inhibition of the JNK pathway in human umbilical vein
endothelial cells. Mol Med Rep. 19:1603–1612. 2019.PubMed/NCBI
|
|
22
|
Ma Y, Zhao Y, Zhang R, Liang X, Yin Z,
Geng Y, Shu G, Song X, Zou Y, Li L, et al: Astragaloside IV
inhibits PMA-induced EPCR shedding through MAPKs and PKC pathway.
Immunopharmacol Immunotoxicol. 39:148–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Tang F, Lu M, Yang J, Han R, Mei M,
Hu J and Wang H: Pretreatment with Astragaloside IV protects human
umbilical vein endothelial cells from hydrogen peroxide induced
oxidative stress and cell dysfunction via inhibiting eNOS
uncoupling and NADPH oxidase-ROS-NF-κB pathway. Can J Physiol
Pharmacol. 94:1132–1140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin H, Pan S, Meng L, Zhou C, Jiang C, Ji
Z, Chi J and Guo H: MicroRNA-384-mediated Herpud1 upregulation
promotes angiotensin II-induced endothelial cell apoptosis. Biochem
Biophys Res Commun. 488:453–460. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu CY, Zhou ZF, Wang B, Ke ZP, Ge ZC and
Zhang XJ: MicroRNA-328 ameliorates oxidized low-density
lipoprotein-induced endothelial cells injury through targeting
HMGB1 in atherosclerosis. J Cell Biochem. 2018.
|
|
26
|
Zhong X, Li P, Li J, He R, Cheng G and Li
Y: Downregulation of microRNA-34a inhibits oxidized low-density
lipoprotein-induced apoptosis and oxidative stress in human
umbilical vein endothelial cells. Int J Mol Med. 42:1134–1144.
2018.PubMed/NCBI
|
|
27
|
Li Y, Ye Y and Chen H: Astragaloside IV
inhibits cell migration and viability of hepatocellular carcinoma
cells via suppressing long noncoding RNA ATB. Biomed Pharmacother.
99:134–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song Z, Wei D, Chen Y, Chen L, Bian Y,
Shen Y, Chen J and Pan Y: Association of astragaloside IV-inhibited
autophagy and mineralization in vascular smooth muscle cells with
lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol Appl
Pharmacol. 364:45–54. 2019. View Article : Google Scholar
|
|
29
|
Wei DZ, Lin C, Huang YQ, Wu LP and Huang
MY: Ellagic acid promotes ventricular remodeling after acute
myocardial infarction by up-regulating miR-140-3p. Biomed
Pharmacother. 95:983–989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rasheed Z, Rasheed N and Al-Shaya O:
Epigallocatechin-3-O-gallate modulates global microRNA expression
in interleukin- 1β-stimulated human osteoarthritis chondrocytes:
Potential role of EGCG on negative co-regulation of microRNA-140-3p
and ADAMTS5. Eur J Nutr. 57:917–928. 2018. View Article : Google Scholar
|
|
31
|
Wei R, Liu H, Chen R, Sheng Y and Liu T:
Astragaloside IV combating liver cirrhosis through the
PI3K/Akt/mTOR signaling pathway. Exp Ther Med. 17:393–397.
2019.PubMed/NCBI
|
|
32
|
Tang F and Yang TL: MicroRNA-126
alleviates endothelial cells injury in atherosclerosis by restoring
autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem
Biophys Res Commun. 495:1482–1489. 2018. View Article : Google Scholar
|
|
33
|
Yi J and Gao ZF: MicroRNA-9-5p promotes
angiogenesis but inhibits apoptosis and inflammation of high
glucose-induced injury in human umbilical vascular endothelial
cells by targeting CXCR4. Int J Biol Macromol. 130:1–9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kawasaki Y, Fujiki M, Uchida S, Morishige
M, Momii Y and Ishii K: A single oral dose of Geranylgeranylacetone
upregulates vascular endothelial growth factor and protects against
Kainic acid-induced neuronal cell death: Involvement of the
Phosphatidylinositol-3 kinase/Akt pathway. Pathobiology.
84:184–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giordano A, Romano S, D'Angelillo A,
Corcione N, Messina S, Avellino R, Biondi-Zoccai G, Ferraro P and
Romano MF: Tirofiban counteracts endothelial cell apoptosis through
the VEGF/VEGFR2/pAkt axis. Vasc Pharmacol. 80:67–74. 2016.
View Article : Google Scholar
|
|
36
|
Zhou Z, Rawnsley DR, Goddard LM, Pan W,
Cao XJ, Jakus Z, Zheng H, Yang J, Arthur JS, Whitehead KJ, et al:
The cerebral cavernous malformation pathway controls cardiac
development via regulation of endocardial MEKK3 signaling and KLF
expression. Dev Cell. 32:168–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohnesorge N, Viemann D, Schmidt N, Czymai
T, Spiering D, Schmolke M, Ludwig S, Roth J, Goebeler M and Schmidt
M: Erk5 activation elicits a vasoprotective endothelial phenotype
via induction of Kruppel-like factor 4 (KLF4). J Biol Chem.
285:26199–26210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi H and Roh J: Role of Klf4 in the
regulation of apoptosis and cell cycle in rat granulosa cells
during the periovulatory period. Int J Mol Sci. 20:2018. View Article : Google Scholar
|
|
39
|
Wang J, Wang B, Chen LQ, Yang J, Gong ZQ,
Zhao XL, Zhang CQ and Du KL: miR-10b promotes invasion by targeting
KLF4 in osteosarcoma cells. Biomed Pharmacother. 84:947–953. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang H, Xi X, Zhao B, Su Z and Wang Z:
KLF-4 protects brain microvascular endothelial cells from ischemic
stroke induced apoptosis by transcriptionally activating MALAT1.
Biochem Biophys Res Commun. 495:2376–2382. 2018. View Article : Google Scholar
|
|
41
|
Lv S, Ji L, Chen B, Liu S, Lei C, Liu X,
Qi X, Wang Y, Lai-Han Leung E, Wang H, et al: Histone
methyltransferase KMT2D sustains prostate carcinogenesis and
metastasis via epigenetically activating LIFR and KLF4. Oncogene.
37:1354–1368. 2018. View Article : Google Scholar :
|
|
42
|
Liu CH, Huang Q, Jin ZY, Zhu CL, Liu Z and
Wang C: miR-21 and KLF4 jointly augment epithelial-mesenchymal
transition via the Akt/ERK1/2 pathway. Int J Oncol. 50:1109–1115.
2017. View Article : Google Scholar : PubMed/NCBI
|