Introduction
Primary human hepatocytes (PHHs) represent the gold
standard for drug development procedures, including the evaluation
of human hepatic drug metabolism, drug clearance, drug interaction,
transporter activity and hepatotoxicity (1-3).
However, the use of PHHs for these purposes must be carefully
considered due to the limited supply of those cells, and PHHs can
be cultured for only short time periods owing to difficulties in
maintaining their functionality and viability (4). Beigel et al performed genomic
and proteomic analyses of cultured primary rat hepatocytes and
revealed that, after 24 h, a number of important phase I and II
enzymes involved in xenobiotic metabolism (e.g., cytochrome P450s,
glutathione S-transferases and sulfotransferases), as well as
antioxidant enzymes (e.g., catalase, superoxide dismutase and
glutathione peroxidase), were downregulated (5). They concluded that primary
hepatocytes represent a suitable model for time points up to 24 h,
whereas for medium- to long-term toxicology studies, suitable cell
culture systems must be urgently developed to ensure faster,
cost-effective, and more sensitive toxicity predictions (5). Although induced pluripotent stem
(iPS) cells or embryonic stem cell-derived hepatocyte-like cells
(HLCs) are promising alternatives owing to their unlimited
proliferation and differentiation capacity, HLCs have an immature
phenotype and their functions may not be similar to those of PHHs
(6,7). In the phenotypic and functional
analyses reported by Baxter et al, stem cell-derived HLCs
more closely resembled fetal hepatocytes rather than adult cells;
in other words, 81% of phase I enzymes in HLCs were markedly
upregulated, and the expression of half of these enzymes in HLCs
did not differ statistically significantly from their expression in
fetal hepatocytes. In addition, HLCs secrete ALB and metabolize
testosterone [cytochrome P450 (CYP) 3A] and dextrorphan (CYP2D6) in
a manner similar to fetal hepatocytes (7). Therefore, PHHs are essential for
in vitro studies of liver molecular mechanisms as well as
drug development. Thus, improvements in PHH culturing are
crucial.
Collagen is an extracellular matrix (ECM) protein
commonly used to culture PHHs. Coating culture dishes with collagen
type I enhances hepatocyte attachment and survival, and also
promotes the maintenance of liver-specific phenotypes (2). Conventional 2D culture of
hepatocytes is easily achievable and represents the most common
culture strategy (3). The
conventional collagen coat comprises extremely small amounts (in
the order of µg/cm2) of collagen (1,8).
PHHs cultured on conventional collagen-coated dishes rapidly
dedifferentiate and lose their hepatic function within a few days
(5,9,10).
Several reports have indicated that hepatic function can be
improved by co-culture with non-parenchymal cells (11-13) or by using collagen gel sandwich
configuration models, which can maintain hepatic function, polarity
and viability (8,14,15). However, the uppermost collagen
matrix layer in the sandwich culture system prevents access to test
compounds on PHHs, and co-culture adds additional complications
compared with conventional culture. Therefore, without complicating
culture methods, we sought to improve the conventional
collagen-coated monolayer cultures, which are currently the gold
standard for drug development procedures.
In atelocollagen, telopeptides are enzymatically
depleted, resulting in low immunogenicity. In addition,
atelocollagen has the same typical triple helix structure as native
collagen, and it can form collagen fibrils under physiological
conditions; therefore, it is compatible with both in vivo
and in vitro applications, such as regenerative medicine
(16,17) and drug delivery systems (18,19). We developed a new scaffold
comprising higher amounts (in the order of mg/cm2) of
atelocollagen compared with conventional atelocollagen coating
(µg/cm2) and induced cross-linking via exposure
to ultraviolet (UV) radiation to improve stability.
Several thick collagen scaffolds, such as
conventional collagen gel or collagen membrane vitrigel (20,21), which are composed of high-density
collagen fibrils, have been described previously. Takezawa et
al characterized collagen vitrigel using scanning electron
microscopy (SEM) and found that both conventional collagen gel and
collagen vitrigel display a reticular network architecture composed
of numerous collagen fibrils, as well as characteristic
cross-striations every ~70 nm for individual collagen fibrils
(20). Our scaffold differs from
these scaffolds in terms of the presence of a micro-dimpled surface
(MDS). In the present study, we first compared a conventional
atelocollagen coat with one featuring large amounts of
atelocollagen scaffolds; this coat was also referred to as MDS
atelocollagen, had a characteristic structure and maintained PHH
functionality. Moreover, MDS atelocollagen suppressed the abnormal
expression of cell adhesion molecules observed on conventional
atelocollagen coats, suggesting that cells grown on MDS
atelocollagen may behave similar to in vivo hepatocytes. We
herein describe a novel culture model developed using MDS
atelocollagen to maintain PHHs in culture using the conventional
atelocollagen coat culture without a complicated culture
method.
Materials and methods
Preparation of atelocollagen coats
A 5-mg/ml solution of type I atelocollagen (IPC-50;
Koken) derived from bovine dermis was used for atelocollagen
coating. The atelocollagen solution was added to 96-well cell
culture plates to final densities of 0.04, 0.2, 1 and 2
mg/cm2 following air-drying for 5 days on a clean bench.
The atelocollagen solution was diluted using 1 mM HCl to obtain two
dilutions as follows: 0.25 mg/ml for 0.04 mg/cm2 and
1.25 mg/ml for 0.2 mg/cm2. One millimolar HCl was
selected as the dilution buffer, as it was also used to prepare
IPC-50 atelocollagen. The diluted solutions (50 µl) were
added to the 96-well plates. For the 1- and 2-mg/cm2
atelocollagen solutions, the 5-mg/ml atelocollagen solution was
directly added to 96-well plates without dilution (64 µl for
1 mg/cm2 and 128 µl for 2 mg/cm2).
During air-drying, the plate lids were opened, and UV radiation was
stopped to prepare a non-uniform coat and regulate UV-induced
cross-linking. Furthermore, atelocollagen was cross-linked using
254-nm UV radiation at 0.5 J/cm2 delivered on two
separate occasions using a UV cross-linker (CL-1000; UVP). The
atelocollagen-coated plates were stored at room temperature.
SEM
Atelocollagen samples were fixed with 2%
glutaraldehyde, sequentially dehydrated in ethanol, and embedded in
tert-butyl alcohol. The samples were then freeze-dried and
sputter-coated with osmium using an osmium coater (Neoc-STB;
Meiwafosis Co., Ltd.). Images were obtained using a scanning
electron microscope (SU6600; Hitachi High-Technologies). The
micro-roughness of the samples was assessed via ISO 25178-compliant
motif analysis (bottom detection) using MountainsMap®
software (Digital Surf) based on the SEM images. The method,
presently called segmentation, is based on the application of a
watershed algorithm associated with an algorithm for simplifying
graphs that describe the relationships between individual points
(Wolf pruning) (22). Details on
feature parameters were described by Blateyron (23).
Transmission electron microscopy
(TEM)
Atelocollagen samples were fixed with 2%
glutaraldehyde, sequentially dehydrated in ethanol and embedded in
Epon. The samples were cut into 70-nm sections using an
ultramicrotome (Leica EM UC7; Leica Microsystems) and stained with
uranium acetate. Images were captured using a transmission electron
microscope (JEM-1400Plus; JEOL Ltd.).
PHH cultures
One day before the cells were cultured, 96-well
atelocollagen-coated plates were washed and pre-incubated with
medium to ensure sufficient reconstruction of atelocollagen and a
smooth cell culture. Briefly, the wells were washed twice with
Dulbecco's phosphate-buffered saline (DS Pharma Biomedical),
incubated with 50 µl/well hepatocyte culture medium (HCM;
Lonza Bioscience) supplemented with 2% fetal bovine serum (FBS),
and then incubated at 37°C overnight. The next day, cryopreserved
human hepatocytes (HUCPI; Lonza Bioscience) were cultured according
to the manufacturer's instructions with minor modifications. All
the series of PHHs from this supplier are produced from a single
donor. PHHs, which demonstrated 87% viability using trypan blue
staining, were seeded directly into pre-incubated
atelocollagen-coated 96-well plates at a density of
1×105 cells/100 µl/well (total, 150
µl/well) and incubated under a humidified atmosphere of 5%
CO2 at 37°C. For the first 24 h, HCM supplemented with
2% FBS was used as the culture medium; thereafter, this medium was
replaced daily with HCM without FBS.
LIVE/DEAD assay
The medium was replaced with HCM containing 2
µM calcein AM, 4 µM ethidium homodimer (LIVE/DEAD™
Viability/Cytotoxicity kit for mammalian cells; Thermo Fisher
Scientific, Inc.), and 0.5 µg/ml Hoechst 33342 (Dojindo
Molecular Technologies, Inc.). The cells were then incubated at
37°C under a humidified atmosphere of 5% CO2 for 5 min.
Thereafter, the medium was replaced with fresh HCM. Fluorescence
was imaged using an IX83 microscope (Olympus Corporation).
Human ALB ELISA
The ALB level in the culture medium was measured
using ELISA kits (Bethyl Laboratories, Inc.), following the
manufacturer's instructions. Briefly, the culture medium was
changed 1 day prior to sampling. The conditioned medium in which
PHHs were cultured for 24 h was then collected and centrifuged at
625 × g for 1 min at 4°C to eliminate cell debris. The supernatant
was stored at -80°C until ELISA was performed. Total RNA was
extracted using an RNeasy micro kit (Qiagen) on day 3 of culture
according to manufacturer's instructions, and measured using a
NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific,
Inc.).
CYP activity
CYP3A4 activity was measured using a P450-Glo™
CYP3A4 Assay with Luciferin-IPA (Promega Corporation), following
the manufacturer's instructions. Luminescence was measured in
duplicates using an ARVO MX (PerkinElmer, Inc.) plate reader.
CYP3A4 activity values were corrected using total RNA amount
values. Total RNA was extracted using an RNeasy micro kit (Qiagen)
according to the manufacturer's instructions, from wells different
from those used for the CYP assay on day 3, and was measured using
NanoDrop 2000c (Thermo Fisher Scientific, Inc.). For the CYP3A4
induction assay, the culture medium was replaced with HCM
containing 25 µM rifampicin (Sigma-Aldrich; Merck KGaA) in
0.1% dimethyl sulfoxide (DMSO) or vehicle control (0.1% DMSO alone)
on day 1. Rifampicin is the drug most commonly used to induce CYP3A
enzyme activity. In addition, this induction route may be used by
different medications, such as phenobarbital (24). On the following day, the medium
was replaced with fresh medium containing the inducer or vehicle.
After 48 h of treatment, CYP3A4 activity was measured as described
above.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using an RNeasy micro kit
(Qiagen), following the manufacturer's instructions. Total RNA was
reverse-transcribed using a PrimeScript™ RT reagent kit (Perfect
Real Time; Takara Bio, Inc.), following the manufacturer's
instructions. RT-qPCR was performed using a LightCycler 1.5
(LightCycler3 software; Roche) and SYBR® Premix Ex Taq™
(Tli RNaseH Plus; Takara Bio, Inc.). The primer sequences used are
listed in Table SI. The PCR conditions were as follows:
Denaturation at 95°C for 60 sec; 40 cycles at 95°C for 5 sec and
60°C for 20 sec; melting at 65°C for 15 sec; and cooling at 40°C
for 30 sec. The final concentrations of the reagents used were as
follows: SYBR® Premix Ex Taq (1X); forward primer (0.2
µM); reverse primer (0.2 µM); and cDNA (<100 ng).
The threshold cycle was measured and normalized to the levels of
glyceraldehyde 3-phosphate dehydrogenase. Analysis was performed
using the ΔΔCq method (25).
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). Statistical significance was assessed using one-way analysis
of variance with Tukey's post hoc test or unpaired t-test.
P<0.05 was considered to indicate statistically significant
differences. Data were analyzed using the Prism 7 software
(GraphPad Software Inc.). This experiment was repeated three
times.
Results
Characterization of MDS
atelocollagen
To characterize the structure of the newly developed
atelocollagen scaffold, we compared it with a conventional
atelocollagen coat (0.04 mg/cm2). SEM and TEM images are
shown in Fig. 1. The surface of
the conventional 0.04-mg/cm2 atelocollagen coat
displayed a fine, flat isotropic structure (Fig. 1A). The 0.2-mg/cm2
atelocollagen coat was similar to the conventional atelocollagen
coat (Fig. 1B). By contrast, the
surface of the 1- and 2-mg/cm2 atelocollagen coats
displayed rough anisotropic structures (Fig. 1C and D). The collagen fibrils were
observed in the 1- and 2-mg/cm2 atelocollagen coats
(Fig. 1C and D, arrows), but not
in the 0.04- and 0.2-mg/cm2 atelocollagen coats. The
characteristic dimpled structure with dimple diameters of ~2-3
µm was observed only on the surfaces of the 1- and
2-mg/cm2 atelocollagen coats (Fig. 1C and D, arrowheads).
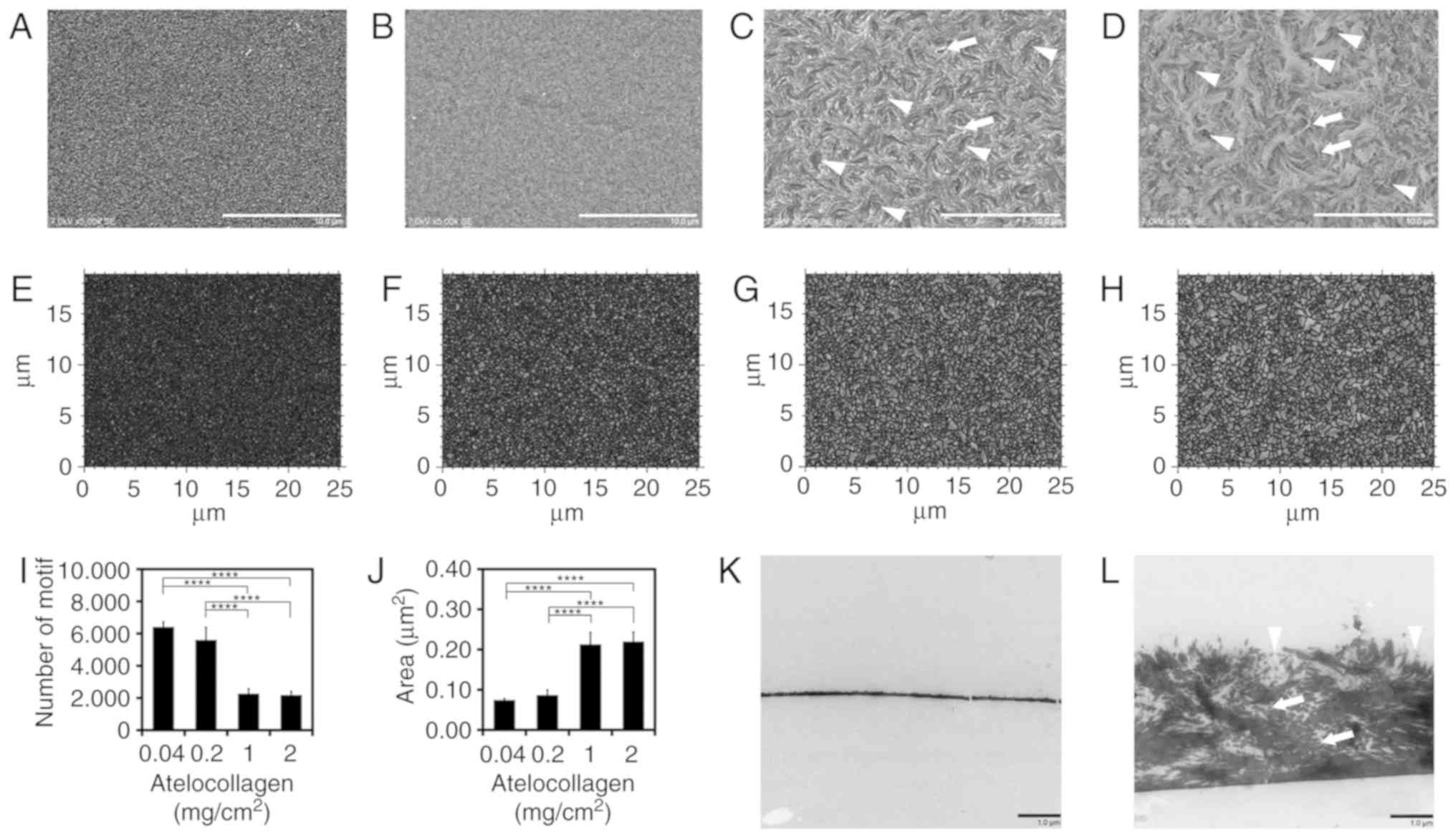 | Figure 1Characteristics of micro-dimpled
surface (MDS) atelocollagen. (A) Atelocollagen (0.04
mg/cm2, conventional atelocollagen coat), (B) 0.2
mg/cm2 atelocollagen, (C) MDS atelocollagen (1
mg/cm2), and (D) MDS atelocollagen (2 mg/cm2)
observed using scanning electron microscopy. Arrows, representative
collagen fibril. Arrowheads, representative MDS. Scale bars: 10
µm. (E) Motif analysis of 0.04 mg/cm2
conventional atelocollagen, (F) 0.2 mg/cm2, (G) 1
mg/cm2, and (H) 2 mg/cm2 atelocollagen using
the Digital Surf MountainsMap® analytical software. (I)
Number of motifs (n=4). (J) Average area of motif (n=4).
Transmission electron microscopy observation of (K) 0.04
mg/cm2 conventional atelocollagen and (L) MDS
atelocollagen (2 mg/cm2). Arrows, representative
collagen fibril. Arrowheads, representative MDS. Scale bars: 1
µm. All images were taken near the center of the well. (K)
Two cracks observed in collagen are artifacts. Statistical analysis
was performed using one-way analysis of variance and Tukey's post
hoc test. The confidence level was set at ****P<0.0001. |
To analyze the micro-roughness of the surface, ISO
25178-compliant motif analysis was performed using
MountainsMap® analytical software (Fig. 1E-J); using an ISO 25178-compliant
segmentation algorithm, this analysis detects an isolated area of
the surface as a motif. The motif numbers of the 0.04-, 0.2-, 1-
and 2-mg/cm2 atelocollagen coats were 6,410±312,
5,607±772, 2,274±295 and 2,187±222, respectively (Fig. 1I). The 1- and 2-mg/cm2
atelocollagen coats comprised fewer motifs compared with the
conventional and 0.2-mg/cm2 atelocollagen coats, thereby
indicating that the surface of MDS atelocollagen coat is rougher
compared with that of the conventional atelocollagen coat. The
average motif areas of the 0.04-, 0.2-, 1- and 2-mg/cm2
atelocollagen coats were 0.07±0.00, 0.09±0.01, 0.21±0.03 and
0.22±0.02 µm2, respectively (Fig. 1J). This indicates that the 1- and
2-mg/cm2 atelocollagen coats comprised a motif
approximately thrice the size of that comprised by the conventional
atelocollagen coat. As shown in Fig.
1I and J, statistically significant differences (P<0.0001)
were observed between the low-density groups of 0.04 and 0.2
mg/cm2 and the high-density groups of 1 and 2
mg/cm2 for all combinations. As was the case for the
comparison between the groups of 0.04 and 0.2 mg/cm2,
there was no significant difference between the groups of 1 and 2
mg/cm2. Therefore, there may be structural differences
between the low- and high-density groups.
Collagen molecules have a rod-like structure, with a
length and width of 300 and 1.5 nm, respectively (26). TEM was used to determine the
arrangement of collagen molecules. In the conventional
atelocollagen coat, no fibril structure or spaces were observed
(Fig. 1K). By contrast, the MDS
atelocollagen coat comprised randomly arranged collagen fibrils
(Fig. 1L, arrows) surrounded by
spaces (Fig. 1L). Consistent with
the SEM image (Fig. 1D), dimpled
structures were observed on the surface of the MDS atelocollagen
coat (Fig. 1L, arrowheads).
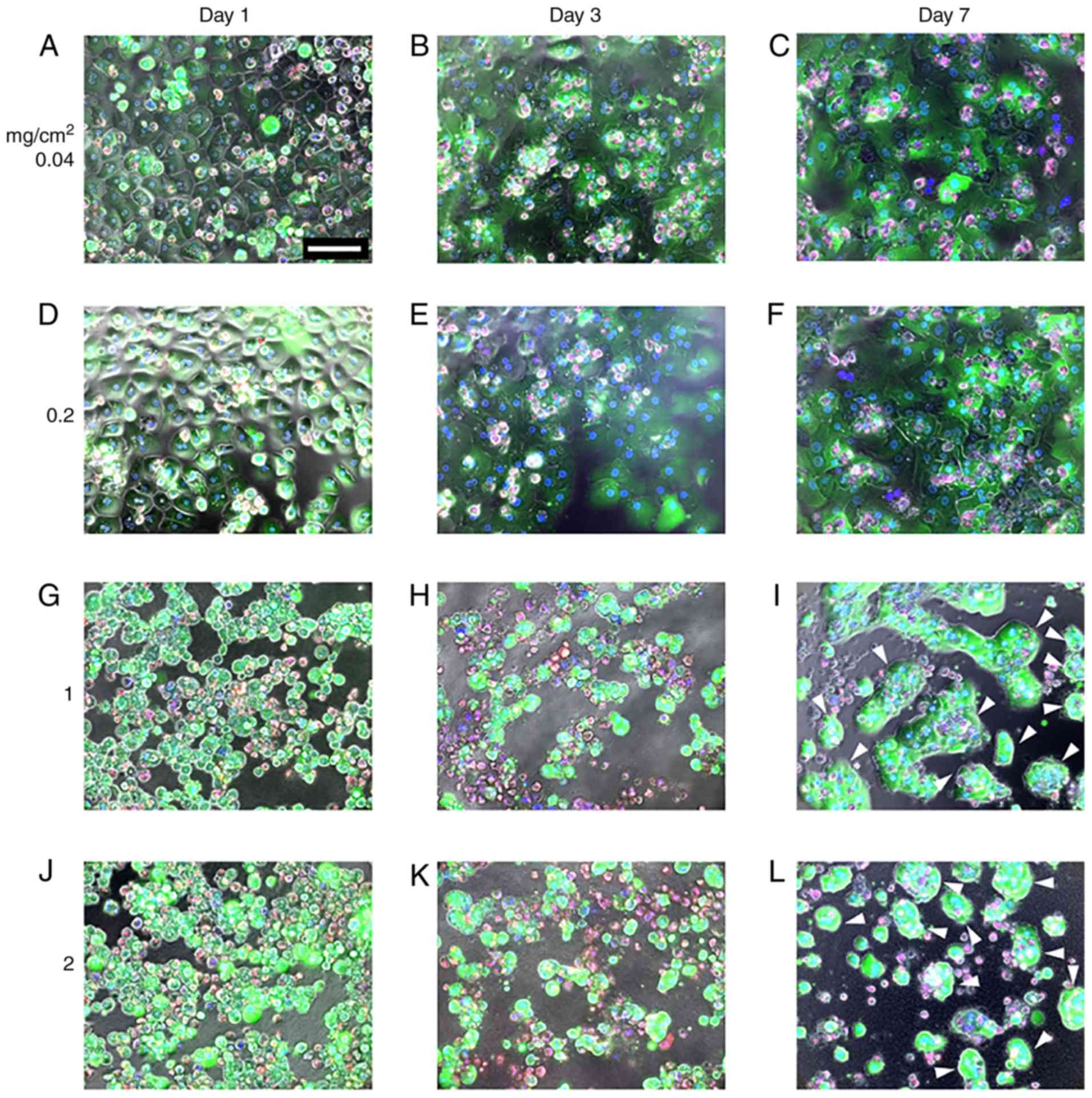
MDS atelocollagen alters the
morphological characteristics of PHHs
To evaluate the impact of the atelocollagen content
on PHH morphology and viability, PHHs were cultured on different
amounts of atelocollagen and their morphology and viability were
observed using LIVE/DEAD staining. LIVE/DEAD and phase contrast
merged images are presented in Fig.
2. PHHs grown on the 0.04-mg/cm2 atelocollagen coat
exhibited the typical polygonal shape on day 1 (Fig. 2A) that gradually extended on days
3 and 7 (Fig. 2B and C),
indicating dedifferentiation. PHHs grown on the
0.2-mg/cm2 atelocollagen coat were slightly swollen on
day 1 (Fig. 2D), and on days 3
and 7 the morphology was similar to that of cells grown on the
0.04-mg/cm2 atelocollagen coat (Fig. 2E and F). By contrast, PHHs
cultured on 1- or 2-mg/cm2 MDS atelocollagen coats were
round with an extremely compact cytoplasm (Fig. 2G and J). Cells cultured on MDS
atelocollagen were markedly smaller compared with those grown on
0.04- or 0.2-mg/cm2 atelocollagen coats (Fig. 2G and J). The round shape of the
cells grown on MDS atelocollagen was maintained until day 3
(Fig. 2H and K). Motile and
islet-like morphologies were observed on day 7 (Fig. 2I and L), suggesting higher
cell-cell interactions compared with those observed on day 3,
confirming lack of dedifferentiation (Fig. 2I and L).
As revealed by the viability of almost all cells,
cell survival on day 1 was not affected by the atelocollagen
scaffolds (Fig. 2A, D, G and J).
Cell viability declined over time on all scaffolds, with cell death
rates increasing with increasing atelocollagen content. By
contrast, for lower commercial-grade PHHs (HUCPM; Lonza
Bioscience), only few viable cells were observed on the
conventional 0.04-mg/cm2 atelocollagen coat on day 7,
whereas more cells remained on MDS atelocollagen (data not shown).
On MDS atelocollagen, a large number of dead cells was temporally
observed on day 3 (Fig. 2H and K,
red color); however, the dead cells were removed by medium
replacement, and most cells were viable on day 7 (Fig. 2I and L). During the culture
period, green fluorescence intensity (calcein AM) was low on the
0.04- and 0.2-mg/cm2 atelocollagen coats and high on the
1- and 2-mg/cm2 MDS atelocollagen coats, indicating that
the latter exhibited enhanced esterase activity.
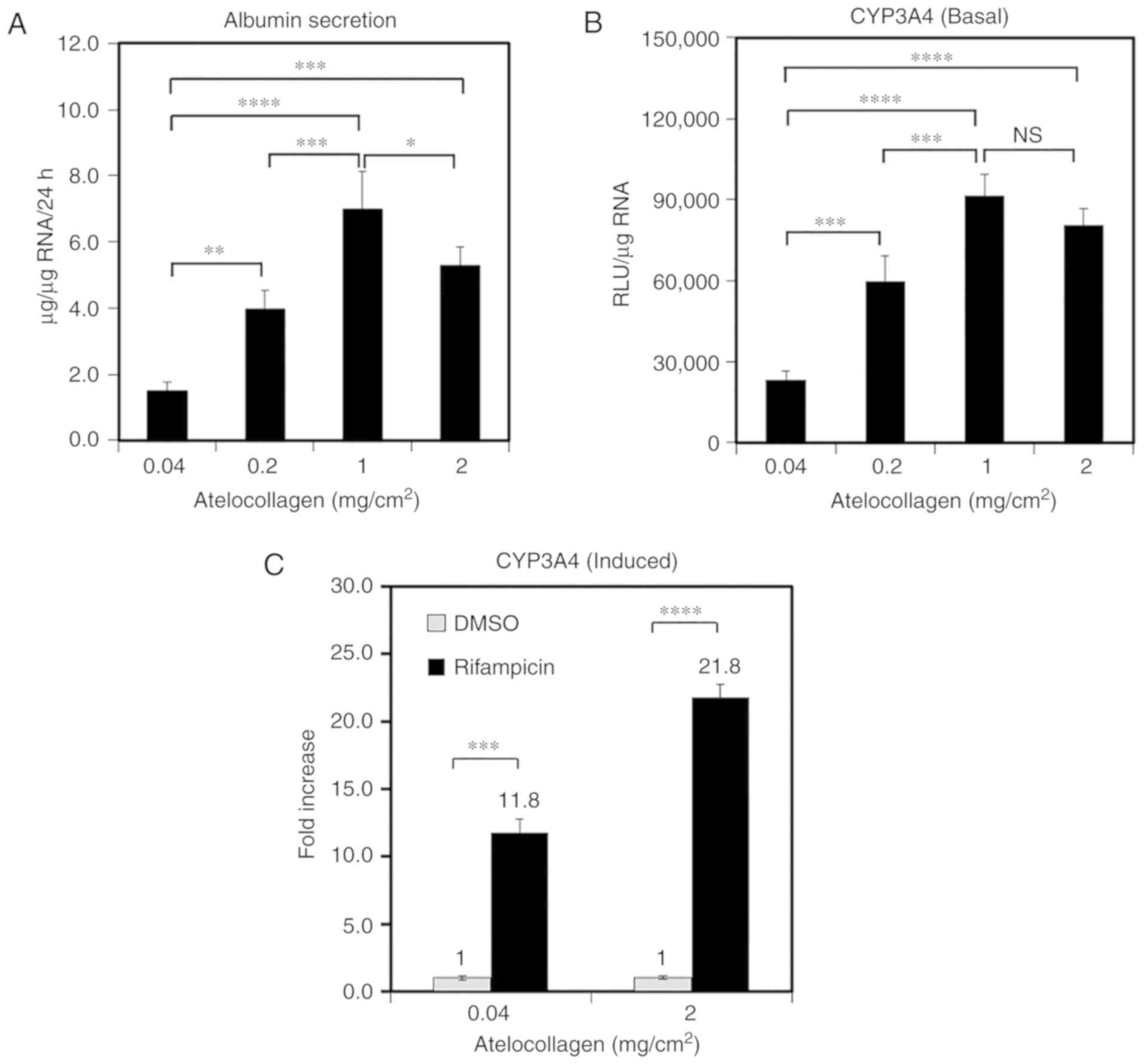
MDS atelocollagen enhances ALB secretion
and CYP activity
ALB secretion and CYP activity are generally used as
markers of hepatocyte function. Therefore, to investigate the
impact of atelocollagen content on PHH function, ALB secretion and
CYP3A4 activity were compared among cells grown on various
matrices. ALB secretion into the medium increased with increasing
atelocollagen content (Fig. 3A).
The cells grown on the 1-mg/cm2 atelocollagen coat
demonstrated 4.7-fold higher 24-h ALB secretion compared with those
grown on the 0.04-mg/cm2 atelocollagen coat (7.0±1.1 vs.
1.5±0.3 µg/µg RNA, respectively; Fig. 3A). Furthermore, the CYP3A4
activity was observed to increase with increasing atelocollagen
content (Fig. 3B). The CYP3A4
activity in PHHs grown on the 1-mg/cm2 atelocollagen
coat was 3.4-fold higher compared with that in cells grown on the
conventional 0.04-mg/cm2 atelocollagen coat (Fig. 3B). CYP3A4 induction was analyzed
using rifampicin; CYP3A4 activity increased by 11.8±2.9-fold in the
presence of rifampicin relative to the effects of DMSO (mean ± SD)
in the cells grown on the conventional 0.04-mg/cm2
atelocollagen coat (Fig. 3C). By
contrast, the CYP3A4 activity increased by 21.8±1.9-fold in the
cells grown on the 2-mg/cm2 atelocollagen coat. These
results indicate that MDS atelocollagen promotes protein production
and drug metabolism.
To further investigate the effect of atelocollagen
levels on PHH function, we analyzed the expression of 15
hepatocyte-related genes using RT-qPCR. The expression of ALB,
α1-antitrypsin (AAT) and tryptophan 2,3-dioxy-genase (TO)
significantly increased in the cells grown on the
2-mg/cm2 MDS atelocollagen coat compared with those
grown on the 0.04-mg/cm2 atelocollagen coat (2.1±0.2-,
1.5±0.1- and 3.6±0.3-fold, respectively, Fig. 4A). CYP expression (1A2, 2C9, 2C19
and 2D6) also significantly increased in the cells grown on the MDS
atelocollagen coat (3.0±0.2-, 2.0±0.2-, 1.7±0.1- and 3.0±0.1-fold,
respectively; Fig. 4B). By
contrast, the expressions of CYP3A4 (Fig. 4B), the phase II conjugation
enzymes glutathione S-transferase A4 (GSTA4) and UDP-glucuronosyl
transferase 1A1 (UGT1A1) (Fig.
4C), and the phase III transporter multidrug
resistance-associated protein 2 (MRP2) (Fig. 4D) were not affected by MDS
atelocollagen. Expression of the nuclear receptor aryl hydrocarbon
receptor (AhR) significantly decreased in the cells grown on the
MDS atelocollagen coat; by contrast, the expression of the nuclear
receptors constitutive androstane receptor (CAR) and pregnane X
receptor (PXR) significantly increased depending on the
atelocollagen levels (Fig. 4E).
Although not statistically significant, an increasing trend was
observed for hepatocyte nuclear factor 4α (HNF4α) expression based
on the atelocollagen levels (Fig.
4E). These results indicate that MDS atelocollagen specifically
enhances hepatocyte-related protein production and CYP
activity.
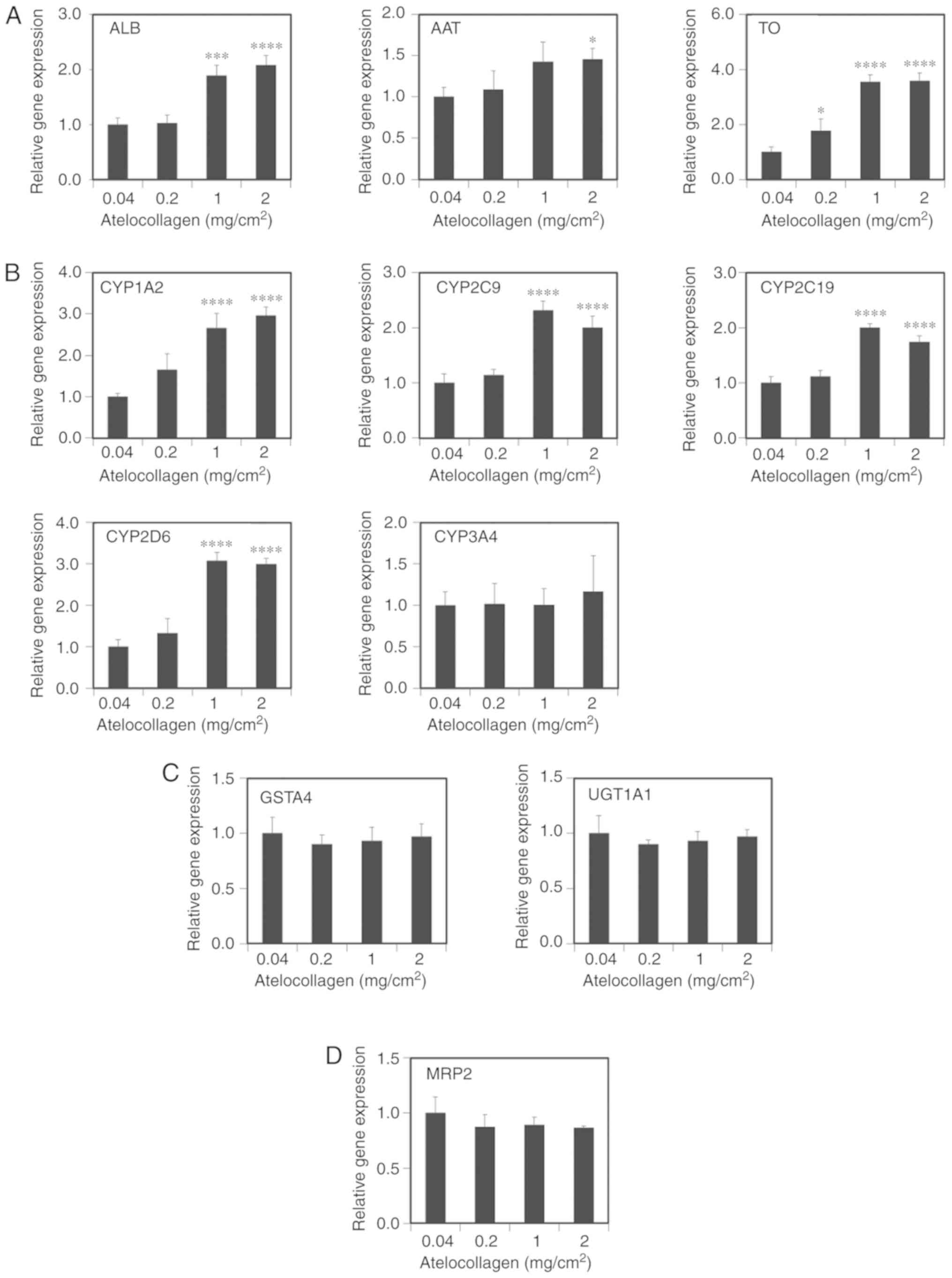 | Figure 4Hepatic gene expression based on
atelocollagen content. RT-qPCR analysis on day 3 of culture. (A)
Hepatocyte-related proteins. (B) Cytochrome P450s. (C) Phase II
enzymes. (D) Phase III transporter. Statistical analysis was
performed using one-way analysis of variance followed by Tukey's
post hoc test. The post hoc test was used for comparison with 0.04
mg/cm2. *P<0.05, ***P<0.001,
****P<0.0001. Hepatic gene expression based on
atelocollagen content. RT-qPCR analysis on day 3 of culture. (E)
Nuclear receptors. The data are presented as relative gene
expression normalized to that in cells grown on conventional 0.04
mg/cm2 atelocollagen (n=4; 1 mg/cm2 of HNF4α
is considered as n=3 due to the lack of cDNA). Statistical analysis
was performed using one-way analysis of variance followed by
Tukey's post hoc test. The post hoc test was used for comparison
with 0.04 mg/cm2. *P<0.05,
***P<0.001, ****P<0.0001. RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; ALB,
albumin; AAT, α1-antitrypsin; TO, tryptophan 2,3-dioxygenase; CYP,
cytochrome P450; GSTA4, glutathione S-transferase A4; UGT1A1,
UDP-glucuronosyl transferase 1A1; MRP2, multidrug
resistance-associated protein 2; AhR, aryl hydrocarbon receptor;
CAR, constitutive androstane receptor; HNF4α, hepato-cyte nuclear
factor 4α; PXR, pregnane X receptor. |
MDS atelocollagen is associated with the
maintenance of hepatocyte-related gene expression
As PHHs immediately lose function during culturing,
the maintenance of hepatic function was investigated in cells grown
on various scaffolds (Fig. 5).
ALB gene expression was decreased by 6.4±0.4% of the initial level
on day 7 in the cells cultured on the conventional atelocollagen
coat. By contrast, its expression level was maintained at 17.4±1.5%
of the control level in the cells cultured on the MDS atelocollagen
coat (Fig. 5A). AAT and TO gene
expressions were elevated on day 7 in the cells grown on the MDS
atelocollagen coat [2.2-fold (454.4±97.5%) and 1.7-fold
(20.7±2.6%), respectively, Fig.
5A]. The expression of CYP1A2, CYP2C9, CYP2C19 and CYP2D6 in
the cells grown on the MDS atelocollagen coat was elevated compared
with that in the cells grown using conventional culture [3.3-fold
(797.9±73.0%), 1.4-fold (13.9±0.9%), 1.4-fold (15.6±0.8%), and
6.3-fold (9.6±0.5%), respectively; Fig. 5B]. These results indicate that MDS
atelocollagen can maintain hepatic function more effectively
compared with conventional culture.
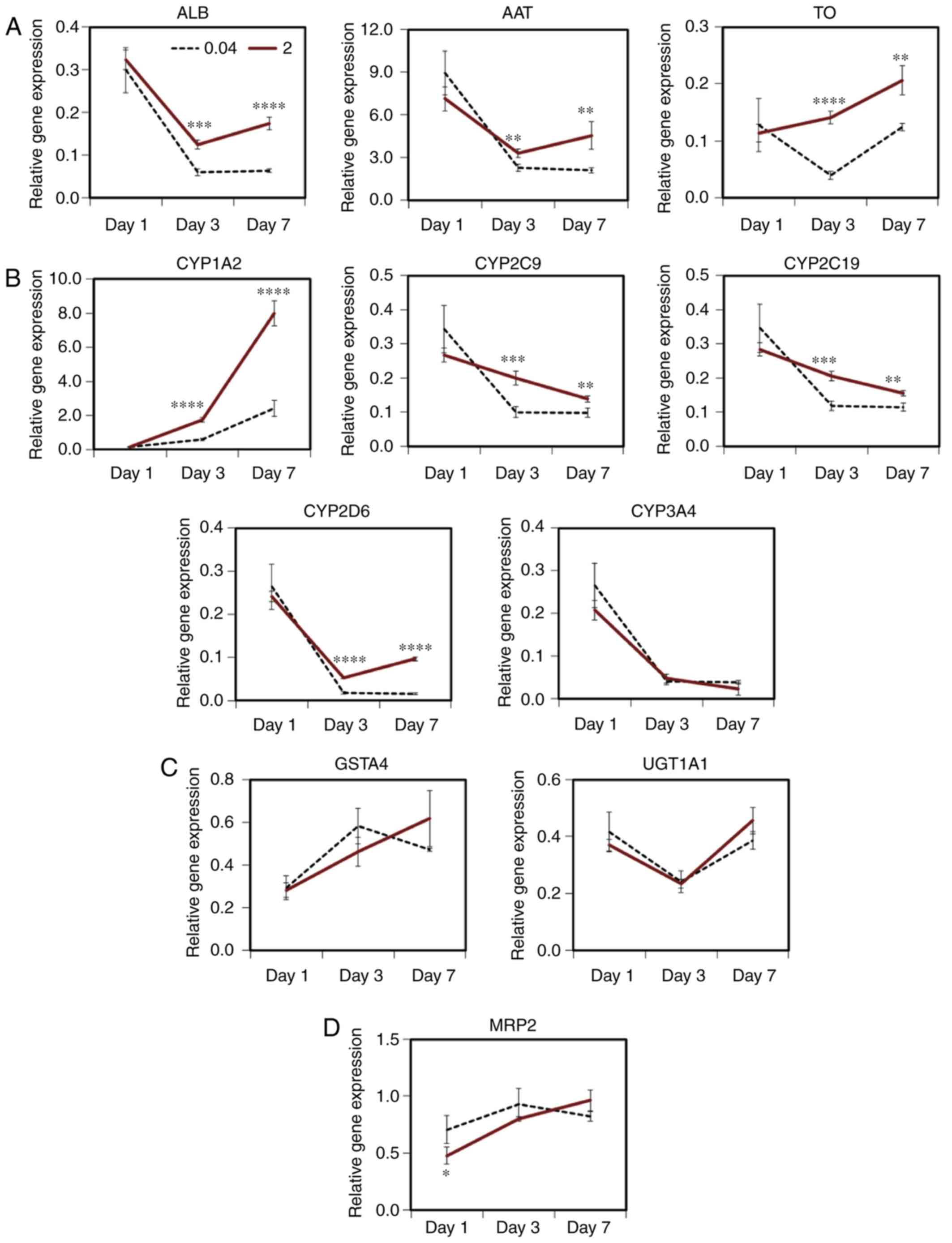 | Figure 5Maintenance of hepatic function in
cells grown on micro-dimpled surface (MDS) atelocollagen. RT-qPCR
analysis of gene expression in primary human hepatocytes (PHHs)
cultured on conventional 0.04 mg/cm2 atelocollagen
(----) or 2 mg/cm2 MDS atelocollagen (----). (A)
Hepatocyte-related genes. (B) Cytochrome P450s. (C) Phase II
enzymes. (D) Phase III transporter. n=4. Statistical analysis was
performed between the two groups using t-test.
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. Maintenance
of hepatic function in cells grown on micro-dimpled surface (MDS)
atelocollagen. RT-qPCR analysis of gene expression in primary human
hepatocytes (PHHs) cultured on conventional 0.04 mg/cm2
atelocollagen (----) or 2 mg/cm2 MDS atelocollagen (—).
(E) Nuclear receptors. The data are presented as the relative gene
expression normalized to that in freshly thawed cryopreserved PHHs.
n=4. Statistical analysis was performed between the two groups
using t-test. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. RT-qPCR,
reverse transcription-quantitative polymerase chain reaction; ALB,
albumin; AAT, α1-antitrypsin; TO, tryptophan 2,3-dioxygenase; CYP,
cytochrome P450; GSTA4, glutathione S-transferase A4; UGT1A1,
UDP-glucuronosyl transferase 1A1; MRP2, multidrug
resistance-associated protein 2; AhR, aryl hydrocarbon receptor;
CAR, constitutive androstane receptor; HNF4α, hepatocyte nuclear
factor 4α; PXR, pregnane X receptor. |
Furthermore, the expression levels of CYP3A4
(Fig. 5B), phase II enzymes
(GSTA4 and UGT1A1) and MRP2 were not increased by MDS atelocollagen
(Fig. 5C and D). In addition, AhR
expression was lower in the cells grown on the MDS atelocollagen
coat compared with those cultured on conventional atelocollagen;
moreover, the AhR expression level of these cells was similar to
that in freshly thawed cryopreserved PHHs (Fig. 5E). In addition, the expression
levels of CAR, PXR and HNF4α genes in the cells grown on the MDS
atelocollagen coat were significantly higher on day 7 compared with
those in cells grown on conventional atelocollagen [6.6-fold
(6.1±0.8%), 1.5-fold (42.1±5.7%), and 1.4-fold (57.0±2.9%),
respectively; Fig. 5E].
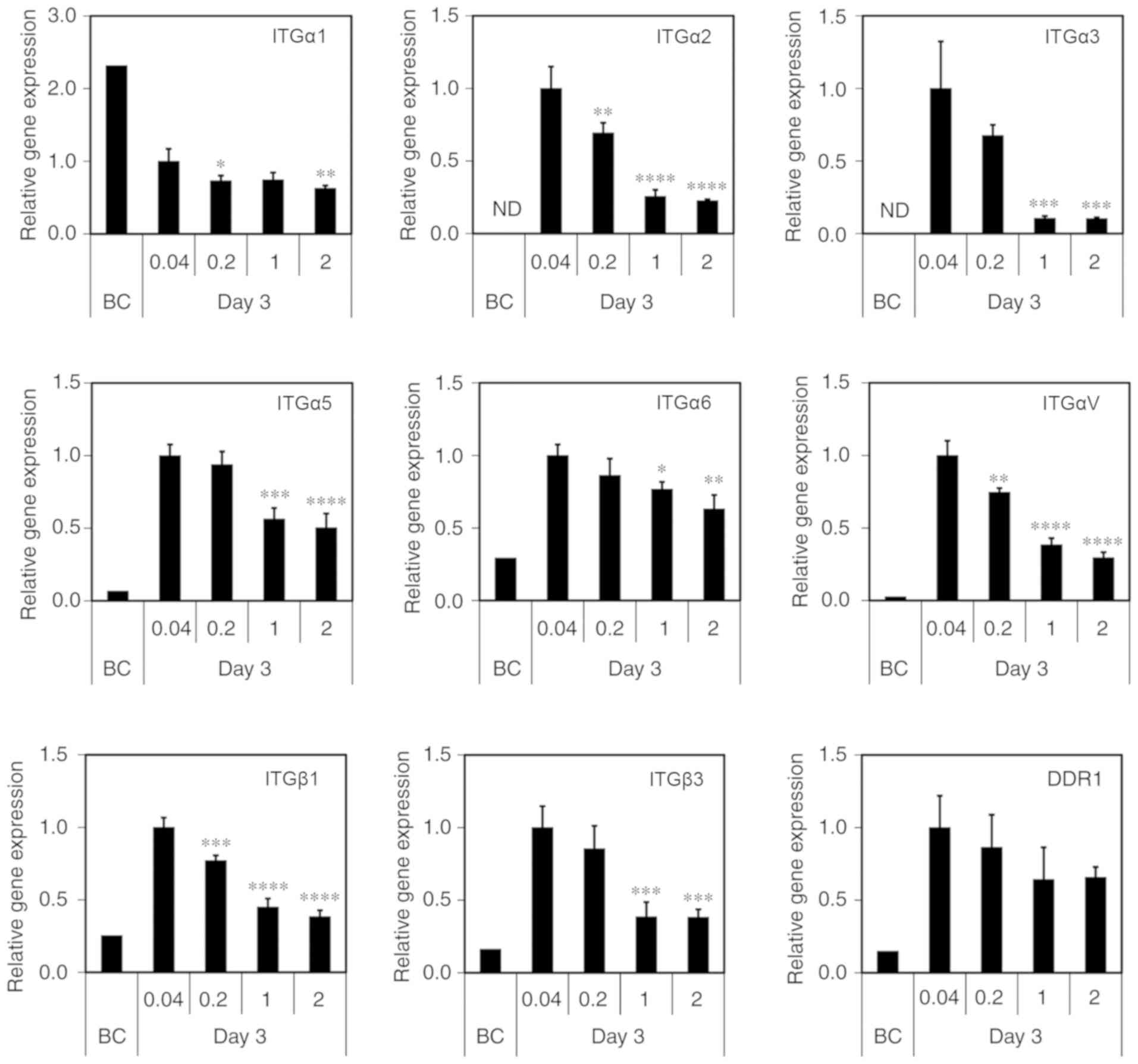
MDS atelocollagen maintains integrin
(ITG) gene expression
ITGs are transmembrane cell adhesion proteins that
link the cellular cytoskeleton and ECM. Hepatocytes reportedly
interact with collagen via ITGα1 and β1 (27). The discoidin domain receptor (DDR)
is a known collagen receptor. Therefore, to investigate the
cell-ECM interaction, we analyzed ITG and DDR expression using
RT-qPCR. Consistently with previous findings, the expression level
was low or undetectable for all genes, excluding ITGα1, prior to
culturing on the scaffolds [before culture (BC); Fig. 6]. Compared with BC, the
conventional 0.04-mg/cm2 atelocollagen coat induced an
abnormal increase in the expression of the ITGα2, α3, α5, α6, αV,
β1 and β3, as well as DDR1, after 3 days of culture (Fig. 6). By contrast, low gene expression
was maintained in the cells grown on the MDS atelocollagen coat
(Fig. 6). Although DDR1
expression did not differ significantly between the scaffolds, its
levels tended to be lower in the cells grown on the MDS
atelocollagen coat compared with those grown on the conventional
atelocollagen coat.
Discussion
The present study compared the morphology and
function of PHHs during culturing on different concentrations of
atelocollagen. Culturing cells in a three-dimensional environment
is beneficial for the maintenance of hepatocyte dedifferentiation
(28). In some cases, hepatic
morphology and functionality are preserved for up to 4 weeks in
culture. However, to use these methods, we have to resolve some of
the major obstacles encountered in drug toxicity screening, such as
the need for expensive equipment, large cell numbers and low
throughput. In the present study, some improvements were achieved
compared with monolayer cultures of primary hepatocytes on a
collagen-coated dish, delaying by a few days the rapid loss of
morphology and liver-specific functions, such as activities of
drug-metabolizing enzymes and transporters. To the best of our
knowledge, this is the first report presenting the observations of
cross-sections of thin atelocollagen coats using TEM. Conventional
0.04-mg/cm2 atelocollagen comprised a fine, flat
isotropic surface (Fig. 1A), and
no collagen fibrils were observed in its cross-section images
(Fig. 1K). By contrast, a 50-fold
higher amount of atelocollagen (2 mg/cm2) exhibited a
characteristic MDS structure (Fig.
1D). This MDS structure was also observed in
1-mg/cm2 atelocollagen, but not in 0.2-mg/cm2
atelocollagen. Therefore, 0.2- and 1-mg/cm2
atelocollagen coats are significantly different, as revealed by
one-way analysis of variance (ANOVA); this was also confirmed via
the findings of motif analysis (Fig.
1E-J) and cell culture experiments. The MDS atelocollagen
surface structure largely affects cell behavior. Using ANOVA, we
reanalyzed the data sets shown in Fig. 4; the results revealed significant
differences between 0.2 and 1 mg/cm2 in terms of the
expression of numerous genes, such as ALB, TO, CYP1A2, CYP2C9,
CYP2C19, CYP2D6, AhR and CAR. Equivalent data for the genes ITGα2,
ITGα3, ITGα5, ITGαV, ITGβ1 and ITGβ3 are shown in Fig. 6. In those cases, there were no
statistically significant differences between 1 and 2
mg/cm2. These findings are strongly correlated with the
data from the structural SEM analysis presented in Fig. 1. This suggests that the expression
of hepatocyte-related genes is strongly affected by the structure
of the cell culture surface. Due to its characteristic collagen
structure, MDS atelocollagen is noticeably different from collagen
gel and vitrigel (20,21), which are composed of homogeneous
collagen fibrils. Therefore, MDS atelocollagen is a novel collagen
scaffold featuring fibrous collagen and a characteristic MDS.
The structural differences of the MDS atelocollagen
coat affected the morphology and function of PHHs. Our observations
corroborated a previous study that reported that mouse primary
hepatocytes cultured on soft matrix were round and small, and that
their function improved compared with cells grown on a stiff matrix
(29). In fact, PHHs grown on
stiff 2-mg/cm2 atelocollagen treated with UV radiation
10-fold higher (10 J/cm2) compared with that used in
this study exhibited an elongated shape and decreased function,
similar to cells cultured on conventional 0.04-mg/cm2
atelocollagen (Fig. S1). These
findings suggest that the rigidity of MDS atelocollagen affects
hepatic cell function and morphology. The small, round shape of
PHHs is attributed to the density of MDS atelocollagen. MDS
atelocollagen may contain abundant cell-binding ligands, such as
the ITG-recognition sequence
glycine-phenylalanine-hydroxy-proline-glycine-glutamate-arginine
(GFOGER) or the RGD sequence. Therefore, the cells can easily
attach to atelocollagen without elongation. Taken together, our
findings indicate that the structure and rigidity of atelocollagen
may affect the morphology and function of PHHs.
The functional characteristics of PHHs are more
enhanced on MDS atelocollagen compared with conventional
atelocollagen. The function of CYP3A4 was greatly increased on 1-
and 2-mg/cm2 atelocollagen compared with that on lower
atelocollagen concentrations; however, gene expression remained
unchanged (Figs. 3B and 4B). This indicates that 1- and
2-mg/cm2 atelocollagen may enhance CYP3A4 activity at
the protein level. CYP3A4 is the CYP most highly implicated in drug
metabolism; therefore, the MDS atelocollagen culture model can
improve the sensitivity of drug metabolism assessments and the risk
of drug-induced liver injury evaluations; this is supported by the
finding that CYP3A4 induction by rifampicin increased on 1- and
2-mg/cm2 atelocollagen (Fig. 3C), suggesting that CYP3A4
induction is closely associated with the atelocollagen content.
CYP1A2, CYP2C9, CYP2D6, CYP2C19 and CYP3A4 collectively metabolize
90% of the currently marketed drugs; furthermore, their gene
expressions are better preserved in cells grown on MDS
atelocollagen compared with conventional atelocollagen. Therefore,
MDS atelocollagen may contribute to several research fields,
including pharmacology, toxicology, tissue engineering and clinical
hepatocyte transplantation.
Intracellular focal adhesion kinase (FAK) and its
downstream signaling are activated by cell attachment to collagen
via ITG. FAK is activated via Src in mouse primary hepatocytes
cultured on stiff and dry collagen, thereby leading to the
activation of ERK and Akt signaling. Activation of the Ras/Raf/ERK
pathway causes dedifferentiation and epithelial-to-mesenchymal
transition, whereas activation of the phosphoinositide-3-kinase/Akt
pathway causes resistance to apoptosis (30). FAK activation was suppressed in
cells grown on MDS atelocollagen compared with those grown on
conventional atelocollagen. The association between ITG and FAK
activation has been reported previously; for example, blocking
ITGβ1 was shown to inhibit FAK phosphorylation in human lens
epithelial cells (31), and FAK
phosphorylation was found to be significantly decreased in ITGβ1
conditional knockout mice (32).
Thus, MDS atelocollagen may reduce FAK activation via low ITG
expression, thereby suppressing dedifferentiation and resistance to
apoptosis. This hypothesis is supported by a previous study
reporting that FAK suppression was observed in mouse primary
hepatocytes and various cell lines cultured on soft collagen gel
(29,33). Time-course analysis using the stem
cell marker Lgr5 as well as several ducts/progenitors, including
Cd44, Prom1, Krt19 and Sox9, may shed more light on these findings
(34). In addition,
immunohistochemical analysis of CD44 and Sox9 may enable the
characterization of the cell state (35).
The present study had certain limitations. We were
unable to compare long-term cultures over the past week. Due to the
culture on the conventional collagen coat to be compared, the
enzymatic activity is abruptly lowered, rendering the use of the
assay within 3 days difficult. Although useful methods for
long-term culture, such as sandwich culture method, have previously
been reported (28), the culture
conditions are significantly different. It is essential to devise
methods to ensure that similar conditions are maintained in the
comparative control groups. The staining with annexin V may be
effective in the evaluation of cell death in the culture. Annexin V
has been recognized as a useful sensitive probe for detecting
phosphatidylserine exposure on the cell membrane. The detection of
annexin V bound to the cell surface indicates the initial stage of
apoptosis. Therefore, it is also useful for distinguishing the two
types of cell death, apoptosis and necrosis. We intend to use MDS
atelocollagen in different cell culture methods, including sandwich
culture and 3D cell culture. To achieve this, we need to change the
culture protocol and the detection assay. In various long-term
culture methods, wherein the number of cells suitable for each
condition, the composition of the culture medium, and the exchange
timing of the culture medium, as well as the exchange method
employed are different, it is crucial to identify the best aligned
conditions to facilitate the evaluation.
In conclusion, our results indicate that the
structure of atelocollagen scaffolds may be altered by the
atelocollagen content, thereby leading to changes in the function
and morphology of PHHs. Furthermore, MDS atelocollagen is more
useful compared with conventional atelocollagen for enhancing
hepatic function. Optimization of the ECM and the presence of
non-parenchymal cells or other factors may be required for
obtaining completely functional PHHs in vitro. We believe
that co-culture and/or sandwich culture may further improve the
maintenance of hepatic cell function; however, this may lead to the
formation of a complex model. MDS atelocollagen provides a simple
culture model with similar utility as conventional
atelocollagen-coated cultures; furthermore, MDS atelocollagen
provides a clear superiority for maintaining PHHs in culture.
Therefore, MDS atelocollagen may be used as an alternative scaffold
for PHH culture for drug development procedures, particularly for
the evaluation of human hepatic drug metabolism, drug clearance,
drug-drug interactions, transporter activity and
hepatotoxicity.
Supplementary Materials
Funding
The present study did not receive any specific grant
from funding agencies in the public, commercial, or not-for-profit
sectors.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS conceived the project, performed research,
analyzed the data, and was a major contributor to the preparation
of the manuscript. KS and IF supervised the study. IF contributed
to the preparation of the manuscripts. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations
Abbreviations:
|
AAT
|
α1-antitrypsin
|
|
ALB
|
albumin
|
|
AhR
|
aryl hydrocarbon receptor
|
|
CAR
|
constitutive androstane receptor
|
|
CYP
|
cytochrome P450
|
|
DDR
|
discoidin domain receptor
|
|
GSTA4
|
glutathione S-transferase A4
|
|
HLC
|
hepatocyte-like cell
|
|
HNF4α
|
hepatocyte nuclear factor 4α
|
|
ITG
|
integrin
|
|
MDS
|
micro-dimpled surface
|
|
MRP2
|
multidrug resistance-associated
protein 2
|
|
PHH
|
primary human hepatocyte
|
|
PXR
|
pregnane X receptor
|
|
SEM
|
scanning electron microscopy
|
|
TO
|
tryptophan 2,3-dioxygenase
|
|
TEM
|
transmission electron microscope
|
|
UGT1A1
|
UDP-glucuronosyl transferase 1A1
|
Acknowledgments
The authors would like to thank Toshihiro Nagai
(Keio University) for his assistance with SEM and TEM and Professor
Shoen Kume (Tokyo Institute of Technology) for the helpful
discussion.
References
|
1
|
Godoy P, Hewitt NJ, Albrecht U, Andersen
ME, Ansari A, Bhattacharya S, Bode JG, Bolleyn J, Borner C, Böttger
J, et al: Recent advances in 2D and 3D in vitro systems using
primary hepatocytes, alternative hepatocyte sources and
non-parenchymal liver cells and their use in investigating
mechanisms of hepatotoxicity, cell signaling and ADME. Arch
Toxicol. 87:1315–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeilinger K, Freyer N, Damm G, Seehofer D
and Knöspel F: Cell sources for in vitro human liver cell culture
models. Exp Biol Med (Maywood). 241:1684–1698. 2016. View Article : Google Scholar
|
|
3
|
Fraczek J, Bolleyn J, Vanhaecke T, Rogiers
V and Vinken M: Primary hepatocyte cultures for
pharmaco-toxicological studies: At the busy crossroad of various
anti-dedifferentiation strategies. Arch Toxicol. 87:577–610. 2013.
View Article : Google Scholar
|
|
4
|
Lee SY, Kim HJ and Choi D: Cell sources,
liver support systems and liver tissue engineering: Alternatives to
liver transplantation. Int J Stem Cells. 8:36–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beigel J, Fella K, Kramer PJ, Kroeger M
and Hewitt P: Genomics and proteomics analysis of cultured primary
rat hepatocytes. Toxicol In Vitro. 22:171–181. 2008. View Article : Google Scholar
|
|
6
|
Godoy P, Schmidt-Heck W, Natarajan K,
Lucendo-Villarin B, Szkolnicka D, Asplund A, Björquist P, Widera A,
Stöber R, Campos G, et al: Gene networks and transcription factor
motifs defining the differentiation of stem cells into
hepatocyte-like cells. J Hepatol. 63:934–942. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baxter M, Withey S, Harrison S, Segeritz
CP, Zhang F, Atkinson-Dell R, Rowe C, Gerrard DT, Sison-Young R,
Jenkins R, et al: Phenotypic and functional analyses show stem
cell-derived hepatocyte-like cells better mimic fetal rather than
adult hepatocytes. J Hepatol. 62:581–589. 2015. View Article : Google Scholar :
|
|
8
|
Tuschl G, Hrach J, Walter Y, Hewitt PG and
Mueller SO: Serum-free collagen sandwich cultures of adult rat
hepatocytes maintain liver-like properties long term: A valuable
model for in vitro toxicity and drug-drug interaction studies. Chem
Biol Interact. 181:124–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rowe C, Goldring CEP, Kitteringham NR,
Jenkins RE, Lane BS, Sanderson C, Elliott V, Platt V, Metcalfe P
and Park BK: Network analysis of primary hepatocyte
dedifferentiation using a shotgun proteomics approach research
articles. J Proteome Res. 9:2658–2668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baker TK, Carfagna MA, Gao H, Dow ER, Li
Q, Searfoss GH and Ryan TP: Temporal gene expression analysis of
monolayer cultured rat hepatocytes. Chem Res Toxicol. 14:1218–1231.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhatia SN, Balis UJ, Yarmush ML and Toner
M: Effect of cell-cell interactions in preservation of cellular
phenotype: Cocultivation of hepatocytes and nonparenchymal cells.
FASEB J. 13:1883–1900. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skett P: Problems in using isolated and
cultured hepatocytes for xenobiotic metabolism/metabolism-based
toxicity testing-Solutions. Toxicol In Vitro. 8:491–504. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Skett P and Bayliss M: Time for a
consistent approach to preparing and culturing hepatocytes.
Xenobiotica. 26:1–7. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi HJ and Choi D: Successful mouse
hepatocyte culture with sandwich collagen gel formation. J Korean
Surg Soc. 84:202–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dunn JCY, Tompkins RG and Yarmush ML:
Hepatocytes in collagen sandwich: Evidence for transcriptional and
translational regulation. J Cell Biol. 116:1043–1053. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida J, Oshikata-Miyazaki A, Yokoo S,
Yamagami S, Takezawa T and Amano S: Development and evaluation of
porcine atelocollagen vitrigel membrane with a spherical curve and
transplantable artificial corneal endothelial grafts. Investig
Ophthalmol Vis Sci. 55:4975–4981. 2014. View Article : Google Scholar
|
|
17
|
Kim BS, Yang SS and Lee J: Precoating of
biphasic calcium phosphate bone substitute with atelocollagen
enhances bone regeneration through stimulation of osteoclast
activation and angiogenesis. J Biomed Mater Res A. 105:1446–1456.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sano A, Maeda M, Nagahara S, Ochiya T,
Honma K, Itoh H, Miyata T and Fujioka K: Atelocollagen for protein
and gene delivery. Adv Drug Deliv Rev. 55:1651–1677. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fujimoto I and Takei Y:
Atelocollagen-mediated siRNA delivery: Future promise for
therapeutic application. Ther Deliv. 5:369–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takezawa T, Takeuchi T, Nitani A, Takayama
Y, Kinooka M, Taya M and Enosawa S: Collagen vitrigel membrane
useful for paracrine assays in vitro and drug delivery systems in
vivo. J Biotechnol. 131:76–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calderón-Coló X, Xia Z, Breidenich JL,
Mulreany DG, Guo Q, Uy OM, Tiffany JE, Freund DE, McCally RL,
Schein OD, et al: Structure and properties of collagen vitrigel
membranes for ocular repair and regeneration applications.
Biomaterials. 33:8286–8295. 2012. View Article : Google Scholar
|
|
22
|
Wolf A: The Jordan watershed: Past
attempts at cooperation and lessons for the future. Water Int.
18:5–17. 1993. View Article : Google Scholar
|
|
23
|
Blateyron F: The areal feature parameters.
Characterisation of Areal Surface Texture. Leach R: Springer Berlin
Heidelberg; Berlin, Heidelberg: pp. 45–65. 2013, View Article : Google Scholar
|
|
24
|
Czekaj P: Phenobarbital-induced expression
of cytochrome P450 genes. Acta Biochim Pol. 47:1093–1105. 2000.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Yamauchi M and Sricholpech M: Lysine
post-translational modifications of collagen. Essays Biochem.
52:113–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zelts C and Gulberg D: The
integrin-collagen connection-a glue for tissue repair? J Cell Sci.
129:653–664. 2016. View Article : Google Scholar
|
|
28
|
Burkhardt B, Martinez-Sanchez JJ, Bachmann
A, Ladurner R and Nüssler AK: Long-term culture of primary
hepatocytes: New matrices and microfluidic devices. Hepatol Int.
8:14–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Desai SS, Tung JC, Zhou VX, Grenert JP,
Malato Y, Rezvani M, Español-Suñer R, Willenbring H, Weaver VM and
Chang TT: Physiological ranges of matrix rigidity modulate primary
mouse hepatocyte function in part through hepatocyte nuclear factor
4 alpha. Hepatology. 64:261–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Godoy P, Hengstler JG, Ilkavets I, Meyer
C, Bachmann A, Muller A, Tuschl G, Mueller SO and Dooley S:
Extracellular matrix modulates sensitivity of hepatocytes to
fibroblastoid dedifferentiation and transforming growth factor
β-induced apoptosis. Hepatology. 49:2031–2043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Yan XL, Zheng XL, Mei L, Wang S,
Han J and Yan H: Electric field exposure promotes
epithelial-mesenchymal transition in human lens epithelial cells
via integrin β1-FAK signaling. Mol Med Rep. 16:4008–4014. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Terrell AM, Riggio BA, Anand D,
Lachke SA and Duncan MK: β1-integrin deletion from the lens
activates cellular stress responses leading to apoptosis and
fibrosis. Investig Ophthalmol Vis Sci. 58:3896–3922. 2017.
View Article : Google Scholar
|
|
33
|
Wang YK, Wang YH, Wang CZ, Sung JM, Chiu
WT, Lin SH, Chang YH and Tang MJ: Rigidity of collagen fibrils
controls collagen gel-induced down-regulation of focal adhesion
complex proteins mediated by alpha2beta1 integrin. J Biol Chem.
278:21886–21892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuijk EW, Rasmussen S, Blokzijl F, Huch M,
Gehart H, Toonen P, Begthel H, Clevers H, Geurts AM and Cuppen E:
Generation and characterization of rat liver stem cell lines and
their engraftment in a rat model of liver failure. Sci Rep.
6:221542016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S, Lee Y, Kim J, Hyun J, Lee K, Kim Y
and Jung Y: Potential role of Hedgehog pathway in liver response to
radiation. PLoS One. 8:pp. e741412013, View Article : Google Scholar : PubMed/NCBI
|