Introduction
Cellular senescence is defined as an irreversible
growth arrest of cells after repeated population doublings
(1). Upon senescence, cells have
several characteristic features, such as larger and flattened cell
morphology, protein expression representing cell cycle arrest, and
increased activity of senescence-associated β-galactosidase
(SA-β-Gal) (2). Although cellular
senescence was originally recognized as an antitumor mechanism to
inhibit the proliferation of DNA-damaged cells (3), recent studies have established the
proinflammatory characteristics of senescent cells, termed
senescence-associated secretory phenotype (SASP) (2). Thus, it has been proposed that
senescent cells contribute to the pathogenesis of age-associated
inflammatory diseases (4). For
instance, atherosclerosis is a representative chronic inflammatory
disease of the arteries, which is closely related to aging.
Notably, senescent endothelial cells are detected at the sites of
atherosclerotic lesions, suggesting the involvement of senescent
endothelial cells in atherogenesis (5).
In addition to classical atherogenic conditions,
such as dyslipidemia and hypertension, persistent bacterial
infection can contribute to the pathogenesis of atherosclerosis
(6). Lipopolysaccharide (LPS), an
outer membrane component of Gram-negative bacteria, is a potential
pathogenic factor for atherosclerosis (7), although the mechanism by which LPS
accelerates the formation of atherosclerotic lesions is unclear.
LPS acts on immune cells as well as vascular cells, and induces
proinflammatory or procoagulant responses via the activation of
NF-κB signaling. While the expression of LPS receptors [CD14 and
toll-like receptor 4 (TLR4)] is considerably low in endothelial
cells compared with myeloid cells, TLR4 is upregulated in
endothelial cells localized in atherosclerotic lesions (8), suggesting an augmentation of
LPS-induced inflammatory responses in endothelial cells via
upregulated TLR4.
LL-37 is a human antimicrobial peptide of the
cathelicidin family, consisting of 37 amino acids, and
predominantly produced by neutrophils and epithelial cells
following cleavage from the precursor of human cationic
antibacterial protein of 18 kDa (9). In addition to its broad spectrum of
bactericidal activity (10),
LL-37 binds with host cell surface molecules, such as chemokine
receptors or growth factor receptors, and induces multidirectional
immunomodulatory responses in leukocytes and epithelial cells
(11). LL-37 also acts on
endothelial cells and induces angiogenesis via formyl peptide
receptor 2 (FPR2; also known as FPRL1 and ALX) (12), or increases endothelial cell
stiffness via purinergic receptor P2X 7 (P2X7) (13). Recent studies strongly suggest the
involvement of LL-37 in the pathogenesis of atherosclerosis; LL-37
was found to be highly accumulated on endothelial cells of human
atherosclerotic lesions (14),
and knockout (KO) of cathelin-related antimicrobial peptide (CRAMP;
the mouse homolog of LL-37) reduced atherosclerosis in a
hyperlipidemic ApoE-deficient mice (15). In addition, extrinsic LL-37
promoted monocyte adhesion to endothelial cells in CRAMP KO mice
(16), and CRAMP activated T
cells in atherosclerotic ApoE-deficient mice (17). These observations suggested that
LL-37 may function as a possible pathogenic factor for
atherosclerosis by acting on endothelial cells and immune
cells.
The NF-κB molecule p65 subunit (RelA) has a pivotal
role in the SASP induction (18).
The present study aimed to evaluate the response of senescent
endothelial cells to LPS and LL-37 by assessing putative
atherogenic molecules. Therefore, the present study focused on
NF-κB p65 activation by LPS and LL-37. The results indicated that
both LPS and LL-37 potently induced intercellular adhesion
molecule-1 (ICAM-1) expression and NF-κB p65 activation in
senescent cells compared with non-senescent cells. Furthermore, the
receptors for LPS and LL-37 (TLR4 and P2X7, respectively) were
upregulated in senescent endothelial cells. These observations
suggested that LPS and LL-37 enhanced ICAM-1 expression and p65
activation in senescent endothelial cells, potentially via TLR4 and
P2X7.
Materials and methods
Reagents
LPS from Escherichia coli serotype O111:B4,
was purchased from Sigma-Aldrich (Mer ck KGaA). The 37-mer peptide
LL-37 of the human cathelicidin family (L1L GDF FRK SKE
KIG KEF KRI VQR IKD FLR MLV PRTES37) was synthesized
with the solid phase method on a peptide synthesizer (model PSSM-8;
Shimadzu Corporation) by F-moc chemistry and purified as described
previously (19). The FPR2
antagonist WRW4 peptide (sequence: WRWWWW) was from Alomone Labs;
the P2X7 inhibitor KN-62 was from Sigma-Aldrich (Merck KGaA).
Antibodies
Anti-CD14-phycoerythrin (PE; MY4; cat. no.
CO6603262; Beckman Coulter, Inc.), anti-TLR4-PE (HTA125; cat. no.
12-9917-42; eBioscience; Thermo Fisher Scientific, Inc.),
anti-FPR2-PE (GM1D6; cat. no. sc-57141; Santa Cruz Biotechnology,
Inc.) and isotype control immunoglobulin G (IgG)-PE (eBM2a; cat.
no. 12-4724-42; eBioscience; Thermo Fisher Scientific, Inc.) were
used for flow cytometry. Polyclonal anti-P2X7 (cat. no. APR-008;
Alomone Labs), normal rabbit IgG (cat. no. PM035; Medical and
Biological Laboratories, Ltd.) and PE-conjugated donkey anti-rabbit
IgG (cat. no. 406421; BioLegend, Inc.) were also used for flow
cytometry. Anti-ICAM-1 (H-108; cat. no. sc-7891; Santa Cruz
Biotechnology, Inc.), anti-phosphorylated (p-) NF-κB p65 Ser 536
(93H1; cat. no. 3033; Cell Signaling Technology, Inc.), anti-NF-κB
p65 (D14E12; cat. no. 8242; Cell Signaling Technology, Inc.),
anti-cyclin-dependent kinase inhibitor 1A (CDKN1A; also known as
p21 Waf1/Cip1; 12D1; cat. no. 2947; Cell Signaling Technology,
Inc.), horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
(cat. no. 12-348; Chemicon International; Merck KGaA) and
anti-GAPDH-HRP (5A12; cat. no. 015-25473; Wako Pure Chemical
Industries, Ltd.) were used for western blot analysis.
Cell culture
Human umbilical vein endothelial cells (HUVECs;
tested negative for mycoplasma, bacteria, yeast, fungi, HIV-1,
hepatitis B and hepatitis C) were purchased from Lonza Group, Ltd.
and cultured in endothelial cell growth medium 2 (EGM-2),
containing 2% FBS, endothelial cell growth supplements and
antibiotics (EGM-2 Bullet kit; Lonza Group, Ltd.) at 37°C in a
humidified atmosphere of 5% CO2 and 95% air. Cells were
maintained in 10 cm-diameter tissue culture-treated dishes and
passaged every 3 or 4 days. HUVECs with population doubling level
<4 (PDL; the total number of times the cells in the population
have doubled in vitro since their primary isolation) were
used as non-senescent cells; whereas senescent HUVECs were prepared
by serial passage of the cells (PDL, >32) and exhibited
senescent characteristics, as shown in Fig. 1.
SA-β-Gal assay
SA-β-Gal staining was performed using Senescence
Detection kit (BioVision, Inc.) according to the manufacturer's
protocol. Images were captured using a Leica DM IL LED light
microscope and LAS EZ software (Leica Microsystems GmbH).
Expression analysis by flow
cytometry
Senescent or non-senescent HUVECs were detached with
0.05% trypsin/EDTA, washed with 1% FBS/PBS, suspended in undiluted
Clear Back (a human Fc receptor blocking reagent; Medical and
Biological Laboratories, Ltd.) for 15 min on ice, incubated with
anti-CD14-PE, anti-TLR4-PE, anti-FRP2-PE or isotype IgG-PE (5
µg/ml final) for 30 min on ice, and then analyzed by flow
cytometry (FACSCalibur; Becton, Dickinson and Company) using
CellQuest Pro software (version 6.0; Becton, Dickinson and
Company). For the P2X7 expression analysis, cells were detached
with Cell Dissociation Solution (Sigma-Aldrich; Merck KGaA),
washed, blocked with Clear Back as aforementioned, and incubated
with anti-P2X7 or normal rabbit IgG (5 µg/ml final) for 30
min on ice, followed by anti-rabbit IgG-PE (5 µg/ml final)
for 30 min on ice.
Western blot analysis
Senescent or non-senescent HUVECs in 6-well plates
were incubated with LPS (10 or 100 ng/ml) or LL-37 (2, 5 or 10
µg/ml) for the indicated periods. For the experiments using
receptor antagonists, cells were pre-incubated with WRW4 peptide
(0.1 or 1 µM), KN-62 (0.1 or 1 µM) or DMSO as a
solvent control for 30 min, and then incubated with LL-37 (5
µg/ml) for 24 h. Cells were lysed in RIPA buffer (50 mM
Tris-HCl pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 1% NP-40
and 0.1% SDS; Wako Pure Chemical Industries, Ltd.) with complete
protease inhibitor cocktails and PhosSTOP phosphatase inhibitor
cocktails (Roche Diagnostics). Cell lysates (5 µg
protein/lane, determined by bicinchoninic acid assay) were
subjected to 8% SDS-PAGE and transferred to polyvinylidene fluoride
membranes (Immobilon-P; EMD Millipore) using a semi-dry transfer
system (Trans-Blot; Bio-Rad Laboratories, Inc.). The membranes were
blocked with undiluted BlockAce (DS Pharma Biomedical Co., Ltd.)
for 1 h at room temperature (RT), probed with anti-ICAM-1
(1:1,000), anti-p-NF-κB p65 (Ser536; 1:3,000), anti-total NF-κB p65
(1:3,000), or anti-p21 Waf1/Cip1 (1:1,000) primary antibodies
overnight at 4°C, followed by HRP-conjugated goat anti-rabbit IgG
(1:5,000) for 2 h at RT. Signals were developed with SuperSignal
West Pico/Dura Chemiluminescent Substrate (Pierce; Thermo Fisher
Scientific, Inc.) and detected using FUSION FX luminescent image
analyzer (Vilber Lourmat) and FUSION-Capt Advance software (version
17.03b; Vilber Lourmat). GAPDH was used as an internal control.
NF-κB p65 localization analysis with
immunofluorescence
HUVECs were seeded into Lab Tek II CC2 chamber
slides (Nunc; Thermo Fisher Scientific, Inc.) and cultured
overnight at 37°C and 5% CO2. Cells were then incubated
with LL-37 (10 µg/ml) at 37°C for 4 h, washed, fixed with 2%
paraformaldehyde for 10 min at RT, permeabilized with 0.2% Triton
X-100, blocked with undiluted BlockAce for 1 h at RT, and incubated
with the primary antibody against NF-κB p65 (D14E12; 1:500)
overnight at 4°C. After washing, cells were further incubated with
Alexa Fluor 488-labeled goat anti-rabbit IgG (cat. no. A27034;
1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.) overnight at
4°C, followed by mounting with an aqueous media (Vectashield
Hardset with DAPI; Vector laboratories, Inc.). Images were captured
using a BZ-X710 fluorescence microscope (Keyence Corporation).
Statistical analysis
Data are presented as means ± SD. Statistical
significance was determined by unpaired t-test or two-way ANOVA
followed by Dunnett's multiple comparisons test (GraphPad Prism 8;
GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Preparation of senescent endothelial
cells
To prepare senescent endothelial cells, HUVECs were
serially passaged. A previous report revealed that HUVECs of PDL30
became non-proliferative with larger cell size, and the numbers of
β-Gal-positive cells were significantly higher compared with cells
of PDL9 (20), indicating that
HUVECs at PDL >30 acquire a senescence phenotype. Similarly, in
the present study, the cell proliferation rate appeared decreased
at PDL ~30, and the cells no longer proliferated at PDL40 (data not
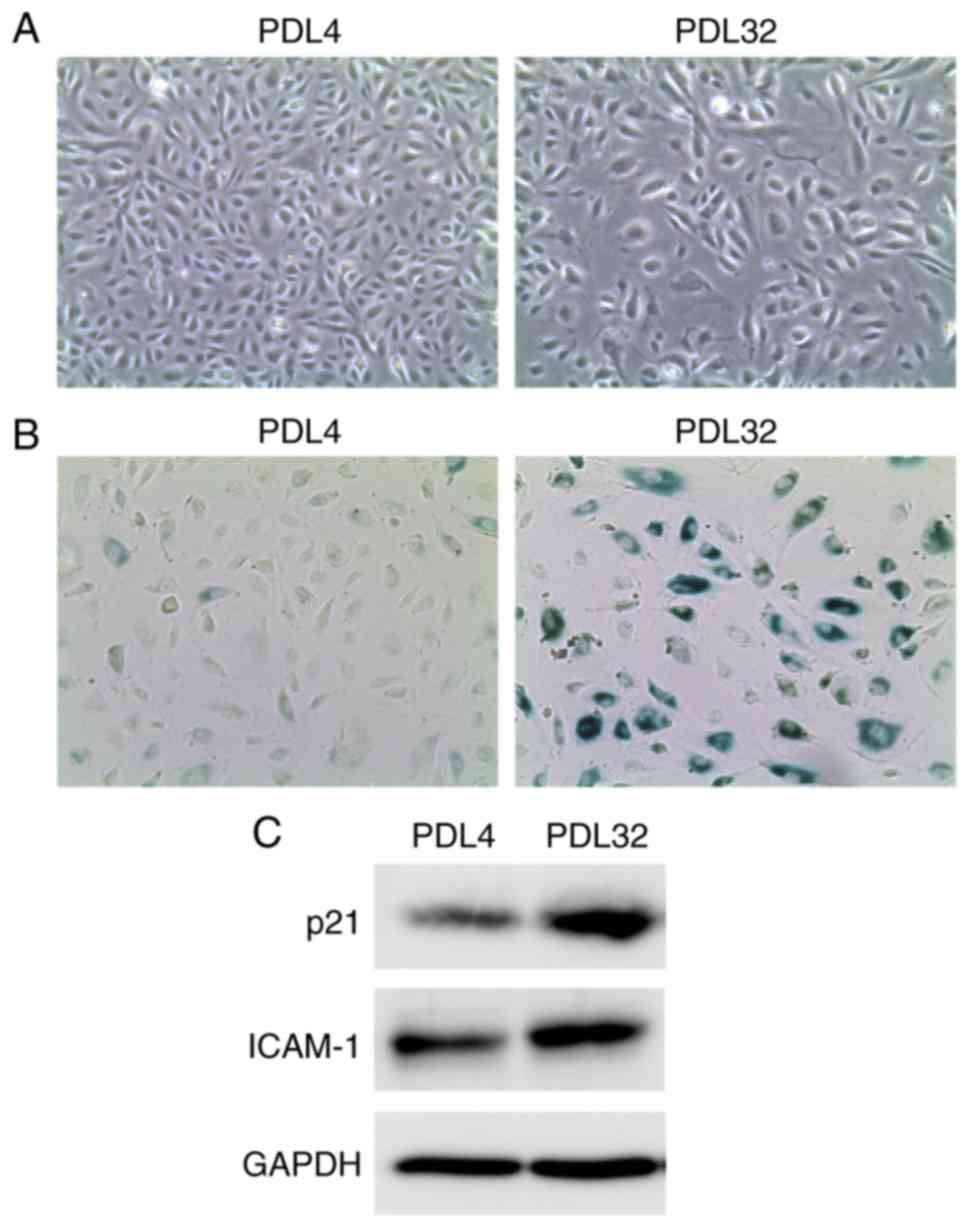
shown). During serial passage, a morphological change of the cells
was also observed from spindle shape (PDL4 cells) to enlarged and
irregular shape (PDL32 cells; Fig.
1A). In addition, a marked increase in SA-β-Gal-positive cell
ratio was found in PDL32 cells compared with PDL4 cells (Fig. 1B). Furthermore, western blot
analysis revealed the upregulation of p21 Waf1/Cip1 (an inhibitory
protein of cyclin-dependent kinase) in the PDL32 cells (Fig. 1C), indicating cell cycle arrest.
Of note, it has been established that cell senescence is
accompanied by the induction of the SASP inflammatory phenotype, as
characterized by the upregulation of proinflammatory cytokines,
adhesion molecules, growth factors or proteases (2). In the PDL32 cells generated in the
present study, ICAM-1 protein expression levels were markedly
increased in the PDL32 cells compared with the PDL4 cells (Fig. 1C), confirming the SASP induction.
These observations indicated that senescence was successfully
induced in the endothelial cells. Thus, in the present study and
for subsequent experiments, cells of PDL >32 were used as
senescent, while cells of PDL <4 were used as non-senescent.
LPS-induced ICAM-1 expression in
senescent endothelial cells
Persistent bacterial infection or increased serum
LPS are associated with the pathogenesis of atherosclerosis
(6,7). Importantly, aging is a major risk
factor for the development of atherosclerosis (4). Therefore, it was hypothesized that
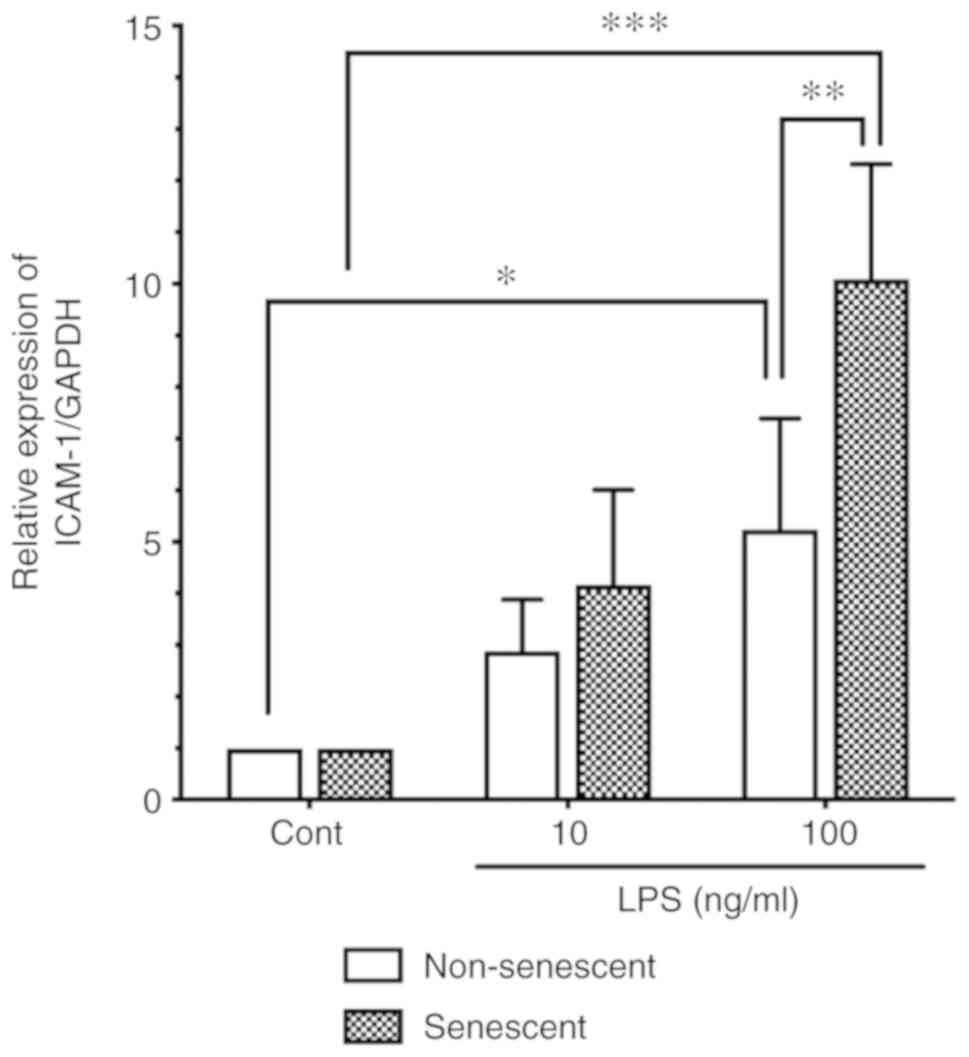
senescent endothelial cells may be more sensitive to LPS. Senescent
HUVECs were incubated with LPS (10 or 100 ng/ml), and the ICAM-1
protein expression levels were compared with that of non-senescent
cells by western blot analysis. As shown in Fig. 2, LPS stimulation (100 ng/ml)
significantly induced ICAM-1 expression in both non-senescent cells
and senescent cells. Of note, ICAM-1 protein expression levels in
LPS-stimulated senescent cells (100 ng/ml) were significantly
higher compared with the levels in LPS-stimulated non-senescent
cells (Fig. 2).
LPS-induced NF-κB p65 activation in
senescent endothelial cells
NF-κB p65 has a pivotal role in the inflammatory
responses, especially in myeloid cells, and is also responsible for
endothelial inflammatory responses, such as LPS-induced ICAM-1
expression (21). To evaluate the
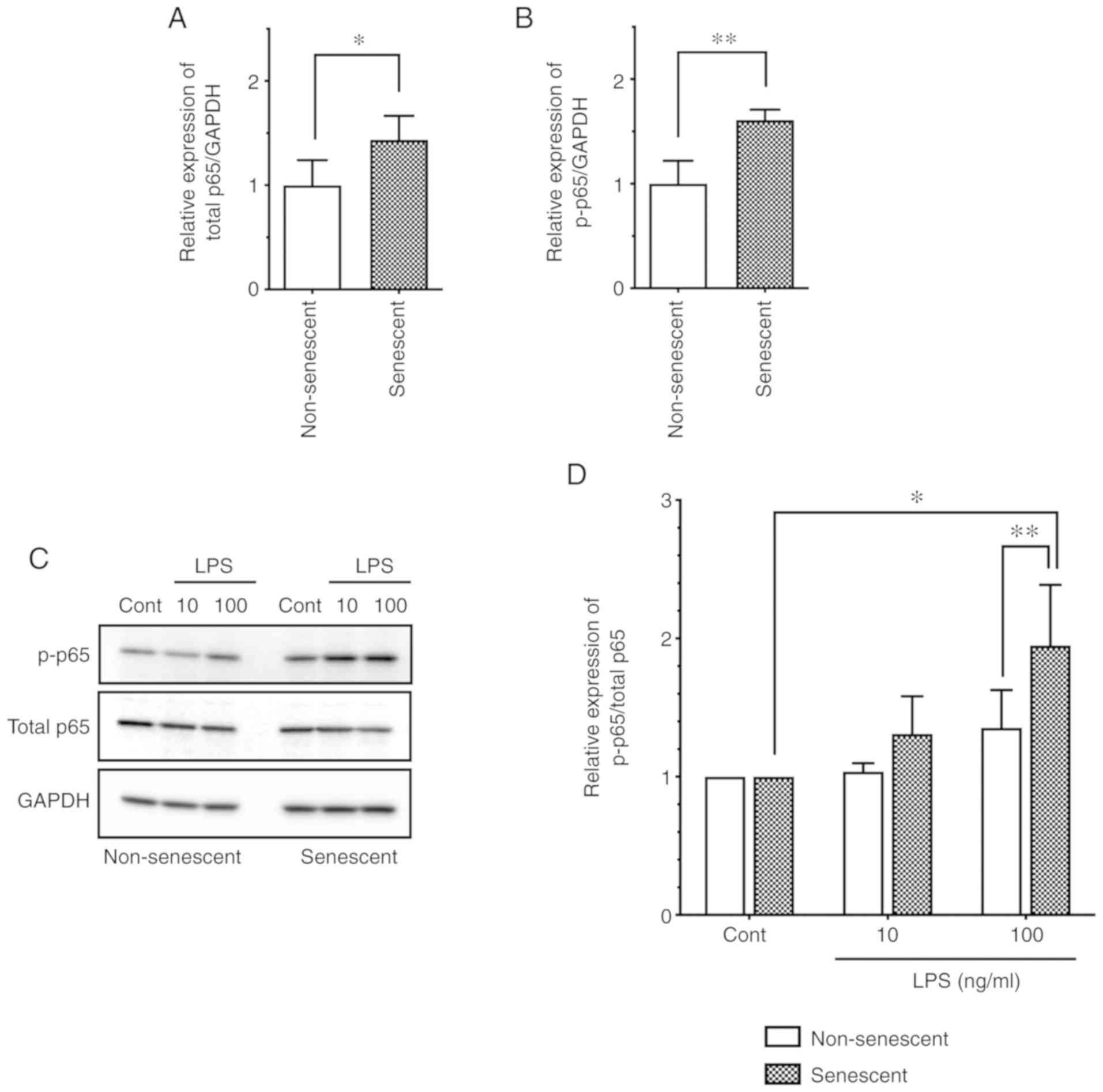
role of NF-κB p65 in senescent endothelial cells, first the basal
expression and phos-phorylation levels were investigated in
senescent HUVECs. Notably, the total protein expression levels of
p65 were increased in senescent cells compared with non-senescent
cells (Fig. 3A). In addition, the
phosphorylated p65 (at Ser536) levels were increased in senescent
cells compared with non-senescent cells (Fig. 3B). Thus, senescent endothelial
cells express intrinsically increased levels of phosphorylated
(activated) NF-κB p65 compared with non-senescent endothelial
cells.
Next, in order to elucidate the mechanism for the
enhanced level of LPS-induced ICAM-1 expression in senescent
endothelial cells (Fig. 2), the
phosphorylation levels of p65 were evaluated following LPS
stimulation. LPS stimulation (100 ng/ml) induced the
phosphorylation of p65 (as evidenced by an increase in the
p-p65/total p65 ratio) in both non-senescent and senescent cells
(Fig. 3C and D); notably, p65
phosphorylation was significantly more pronounced in senescent
cells, compared with non-senescent cells (Fig. 3C and D). These observations
suggested that NF-κB p65 activation is enhanced by LPS stimulation,
which may be associated with the enhanced expression of ICAM-1 in
senescent endothelial cells.
Expression of LPS receptors in senescent
endothelial cells
It has been demonstrated that aging is associated
with changes of TLR expression levels in mice (22) and humans (23). Since the LPS-induced ICAM-1
expression and p65 phosphorylation were enhanced in senescent cells
(Figs. 2 and 3), it was next speculated that an
increase of LPS receptor expression may be present in senescent
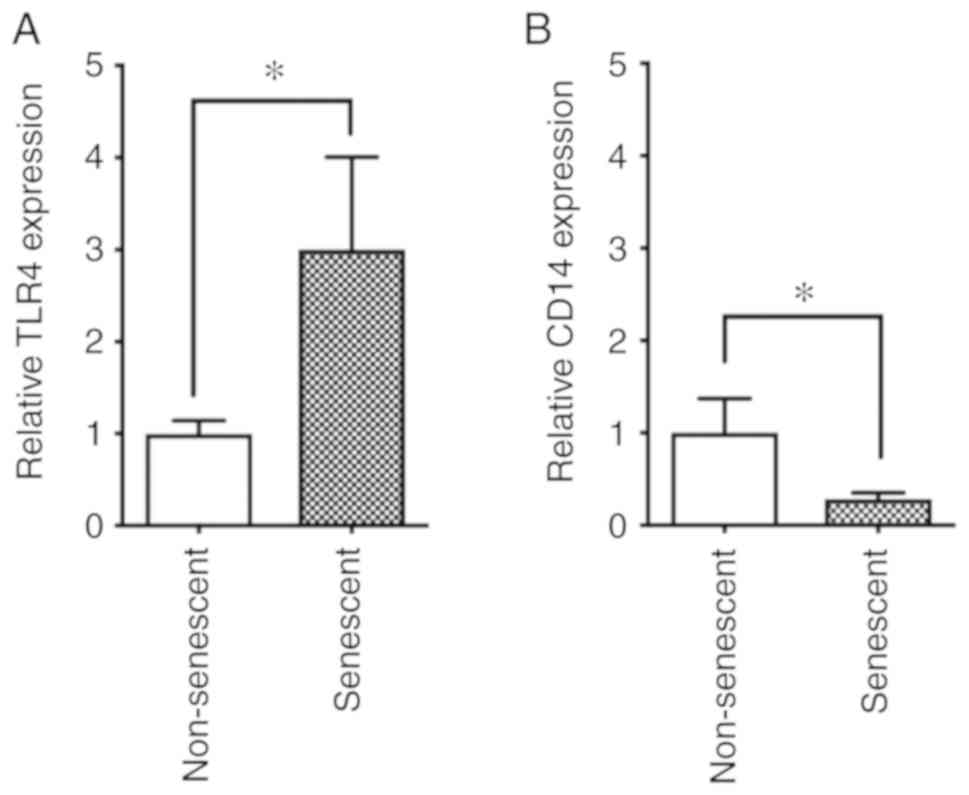
cells. Thus, the cell surface expression levels of the LPS
receptors CD14 and TLR4 were analyzed in non-senescent and
senescent cells by flow cytometry. The results indicated that TLR4
was upregulated in senescent endothelial cells compared with
non-senescent cells (Fig. 4A). By
contrast, CD14 was downregulated in senescent endothelial cells
compared with non-senescent cells (Fig. 4B).
LL-37-induced ICAM-1 expression in
senescent endothelial cells
Human antimicrobial peptide LL-37 has been
speculated to be involved in the pathogenesis of atherosclerosis
(14,15). Moreover, in vitro studies
have demonstrated the direct action of LL-37 on immune or vascular
cells; LL-37 induces integrin activation in monocytes (16), and induces ICAM-1 and monocyte
chemoattractant protein-1 expression in endothelial cells (14). These observations suggest that
LL-37 promotes monocyte/macrophage-endothelial cell interaction in
atherosclerosis. Thus, to assess the effect of LL-37 on senescent
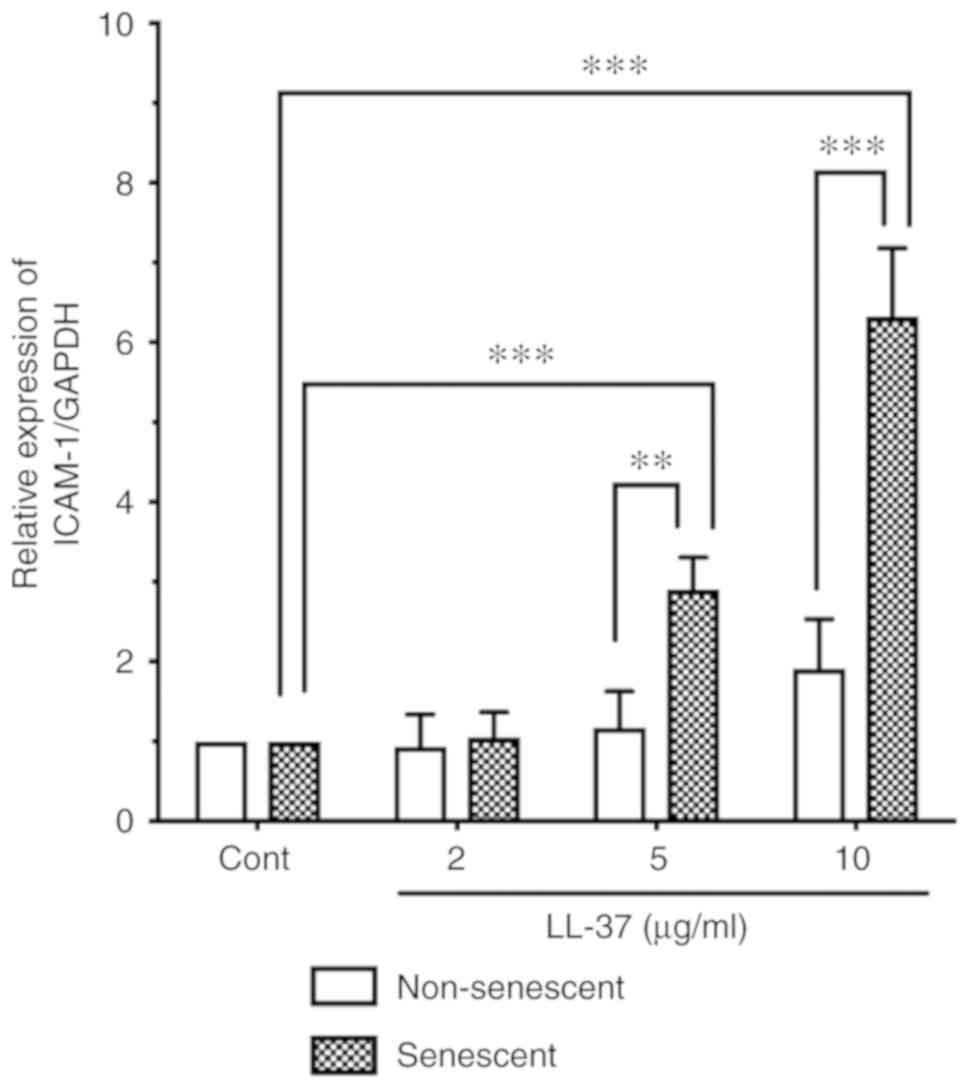
endothelial cells, senescent HUVECs were incubated with LL-37 (2, 5
or 10 µg/ml), and the ICAM-1 protein expression levels were
compared with those of non-senescent cells by western blot
analysis. LL-37 stimulation induced ICAM-1 expression in senescent
HUVECs (Fig. 5). Notably, LL-37
stimulation (5 and 10 µg/ml) more potently induced ICAM-1
expression in senescent cells compared with non-senescent cells
(Fig. 5).
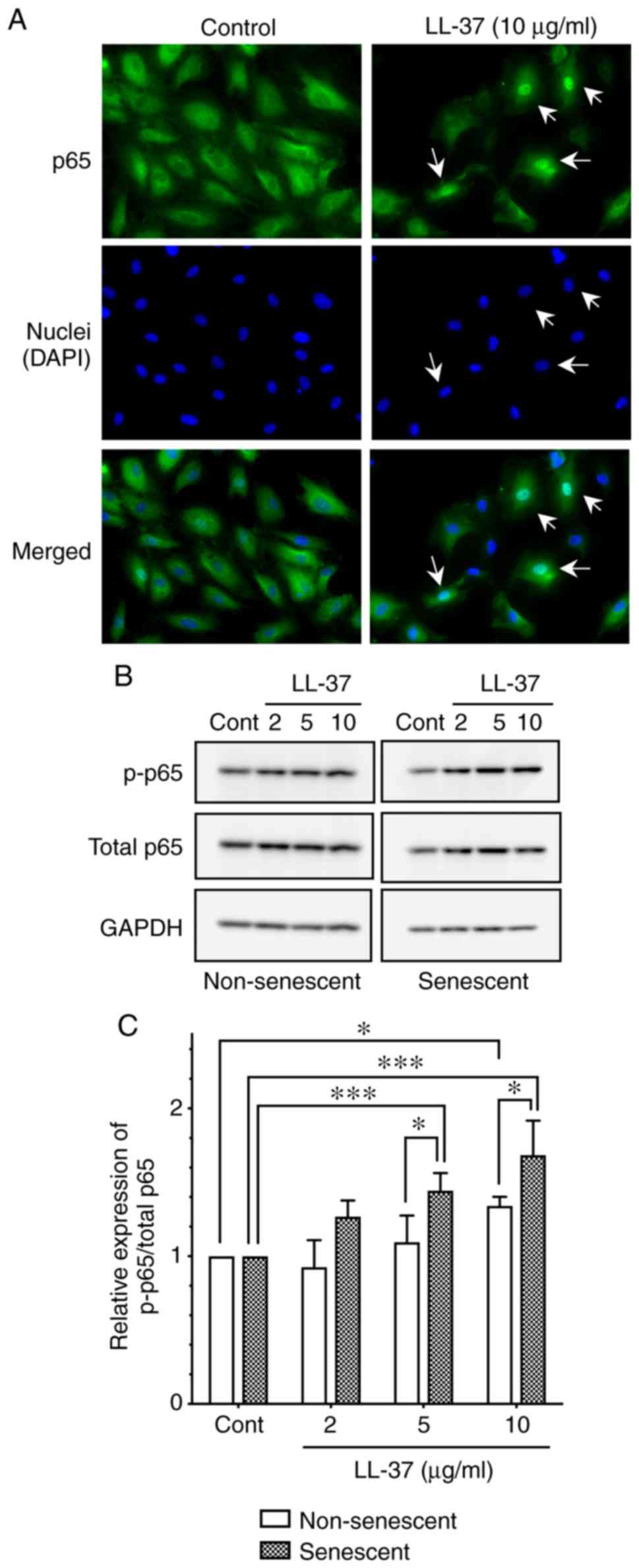
LL-37-induced NF-κB p65 activation in
senescent endothelial cells
The signaling molecules involved in the
LL-37-induced ICAM-1 expression of endothelial cells has not been
elucidated. Koczulla et al (12) reported that NF-κB p65 was involved
in the LL-37-induced endothelial cell proliferation, by
demonstrating nuclear translocation of p65 following LL-37
stimulation (12). Thus, the
present study investigated the localization of p65 in HUVECs
following LL-37 stimulation by immunofluorescence. The results
revealed that LL-37 stimulation (10 µg/ml) induced the
translocation of p65 from cytoplasm to nucleus (Fig. 6A), confirming the activation of
NF-κB signaling by LL-37 in endothelial cells.
Next, to elucidate the mechanism underlying the
enhanced levels of LL-37-induced ICAM-1 expression in senescent
endothelial cells (Fig. 5), the
present study evaluated the activation of p65 following LL-37
stimulation by western blot analysis. LL-37 stimulation (10
µg/ml) induced the phosphorylation of p65 (as evidenced by
the increased ratio of phosphorylated p65/total p65) in both
non-senescent and senescent cells (Fig. 6B and C); notably, p65
phosphorylation was significantly enhanced in senescent cells,
compared with non-senescent cells (Fig. 6B and C). These observations
suggested that NF-κB p65 was more potently activated
(phosphorylated) by LL-37 in senescent HUVECs, which may contribute
to the enhanced expression of ICAM-1 in senescent endothelial
cells.
Expression of LL-37 receptors in
senescent endothelial cells
Several cell surface receptors are involved in the
LL-37-induced host cell activation. FRP2 is known as an LL-37
receptor in endothelial cells; LL-37 induces cell proliferation of
HUVECs via FRP2 (12), or acts on
FRP2 in endothelial cells to induce vascular smooth muscle
relaxation in veins (24).
However, the LL-37 receptor responsible for ICAM-1 induction is
unknown. To identify the LL-37 receptor involved in ICAM-1
induction, the present study first assessed the effect of an FPR2
antagonist on the LL-37-induced ICAM-1 expression. As shown in
Fig. 7A, the FPR2 antagonist WRW4
peptide (1 µM) attenuated the LL-37-induced ICAM-1
expression, suggesting that LL-37 induced ICAM-1 expression via
FPR2. Additionally, P2X7 is also reported as a receptor for LL-37
in immune cells (25) and
endothelial cells (13). In the
present study, the P2X7 antagonist KN-62 (1 µM) attenuated
the LL-37-induced ICAM-1 expression (Fig. 7A), confirming P2X7 as an LL-37
receptor involved in ICAM-1 induction. Of note, the combination of
WRW4 and KN-62 (0.1 µM each) suppressed the LL-37-induced
ICAM-1 expression, although WRW4 or KN-62 at the same concentration
alone did not affect ICAM-1 expression (Fig. 7A), suggesting that FRP2 and P2X7
may cooperatively function as receptors for the LL-37-induced
ICAM-1 expression. These observations indicated that LL-37 induced
ICAM-1 expression in endothelial cells via both FRP2 and P2X7.
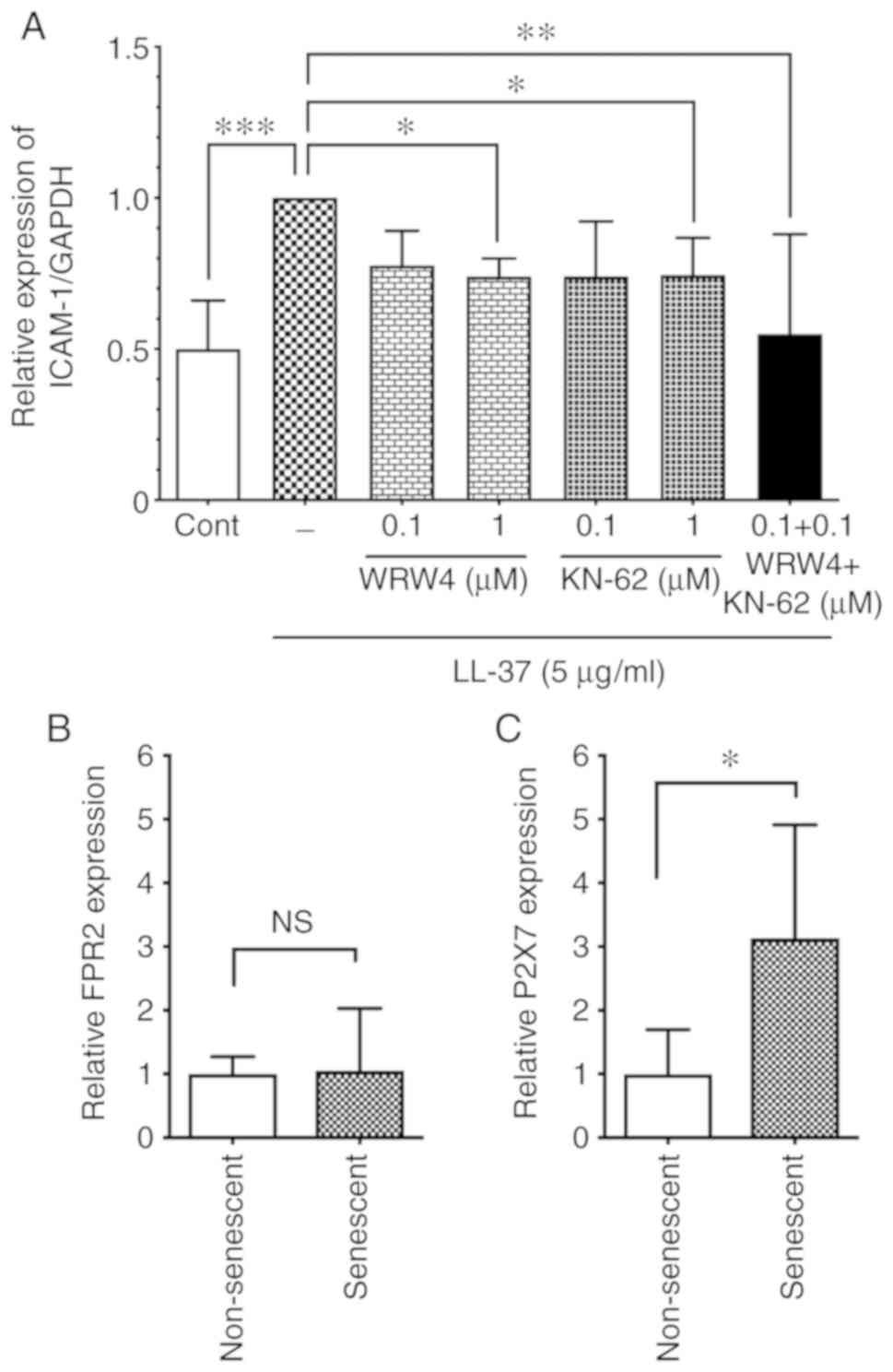 | Figure 7Role of FPR2 and P2X7 receptors in
senescent and non-senescent endothelial cells. (A) Non-senescent
HUVECs were preincubated with WRW4 (0.1 or 1 µM) or KN-62
(0.1 or 1 µM) for 30 min, and then incubated with LL-37 (5
µg/ml) for 24 h. Alternatively, non-senescent HUVECs were
preincubated with a combination of WRW4 (0.1 µM) and KN-62
(0.1 µM) for 30 min, and then incubated with LL-37 (5
µg/ml) for 24 h. ICAM-1 protein expression levels were
analyzed by western blotting. Relative expression of ICAM-1/GAPDH
was calculated as a ratio to LL-37-stimulated cells without
antagonists. Data are presented as the mean ± SD of at least three
independent experiments. (B) Cell surface expression levels of
LL-37 receptors FPR2 and (C) P2X7 were analyzed in senescent and
non-senescent HUVECs by flow cytometry. Relative expression in
senescent cells was calculated as a ratio to non-senescent cells.
Data are presented as the mean ± SD of at least four independent
experiments. *P<0.05, **P<0.01 and
***P<0.001, with comparisons indicated by lines.
FPR2, formyl peptide receptor 2; P2X7, purinergic receptor P2X 7;
HUVECs, human umbilical vein endothelial cells; ICAM-1,
intercellular adhesion molecule-1; Cont, control; NS, not
significant. |
Since LL-37 induced ICAM-1 expression and p65
phos-phorylation more potently in senescent cells compared with
non-senescent cells (Figs. 5 and
6), it was speculated that the
LL-37 receptors may be upregulated in senescent cells. Thus, the
cell surface expression levels of FRP2 and P2X7 were analyzed in
senescent cells by flow cytometry. The results demonstrated that
P2X7 was upregulated in senescent cells compared with non-senescent
cells (Fig. 7C), whereas no
difference was observed in the FRP2 levels between senescent and
non-senescent cells (Fig.
7B).
Discussion
Cellular senescence is associated with the induction
of the proinflammatory phenotype, termed SASP (2). ICAM-1 is recognized as a
representative SASP marker in endothelial cells, since
overexpression of ICAM-1 is commonly observed in several senescent
endothelial cell models, including stress stimulation (26), serial passage (20) and oncogene transfer (5). In the present study, the basal
expression levels of ICAM-1 were compared between senescent and
non-senescent HUVECs, and, as expected, ICAM-1 was demonstrated to
be upregulated in senescent cells (Fig. 1C). Furthermore, p65 activation
(phosphorylation) was compared between senescent and non-senescent
endothelial cells, since NF-κB p65 has a pivotal role in basal SASP
induction (27). The present
results demonstrated that p65 phosphorylation, as well as the total
amount of p65, was upregulated in senescent endothelial cells.
Thus, the present study revealed that senescent endothelial cells
exhibited intrinsic proinflammatory features, as indicated by
higher ICAM-1 expression and p65 activation.
Localization of senescent endothelial cells at the
sites of atherosclerotic lesions suggests the involvement of
senescent endothelial cells in atherogenesis (5). The present study compared the
response to LPS, a potential atherogenic factor, between senescent
and non-senescent HUVECs, and revealed that both ICAM-1 expression
and p65 phosphorylation were increased in senescent endothelial
cells compared with non-senescent endothelial cells. This
observation suggested that the NF-κB p65 pathway was more potently
activated by LPS in senescent endothelial cells, which may result
in the enhanced expression of ICAM-1. Expression of ICAM-1 (an
adhesion molecule) is important for the interaction of
monocyte/macrophage to endothelial cells, the initial step of
vascular inflammation. Thus, the enhanced ICAM-1 expression is
expected to promote monocyte/macrophage adhesion to senescent
endothelial cells and migration of these cells into the
atherosclerotic lesion.
Previously, the effect of LPS on senescent cells was
evaluated using fibroblasts in vitro; LPS from
Campylobacter rectus, which is associated with adult
periodontitis, induces higher production of interleukin (IL)-6 and
plasminogen activator in senescent gingival fibroblasts prepared by
serial passage (28,29). These results are consistent with
the present finding that the LPS response is more enhanced in
senescent cells compared with non-senescent cells.
The important role of TLR4-mediated signaling has
been demonstrated in atherogenesis, based on the finding that the
KO of TLR4 or myeloid differentiation factor 88 (MyD88) reduces the
aortic plaque area in ApoE-deficient atherosclerotic mouse
(30). To elucidate the mechanism
for enhanced p65 activation and ICAM-1 expression in senescent
endothelial cells, the present study compared the expression levels
of LPS receptors TLR4 and CD14 between non-senescent and senescent
cells by flow cytometry. Of note, TLR4 was upregulated in senescent
endothelial cells, whereas CD14 was downregulated in senescent
endothelial cells. Therefore, the LPS-induced enhanced p65
phosphorylation and ICAM-1 expression observed in senescent
endothelial cells may be mediated via the upregulated TLR4.
However, the involvement of CD14 in the LPS response in senescent
cells cannot be excluded, because endothelial cells have also been
reported to use a soluble form of CD14 for the transfer of LPS to
TLR4 (31).
The present study revealed the upregulation of TLR4
in serial passage-induced senescent endothelial cells. Notably,
high glucose and oxidative stress are recognized as inducers of
cellular senescence (32), and
TLR4 is upregulated by high glucose in human retinal vascular
endothelial cells isolated from patients with diabetic retinopathy
(32), and by oxidative stress in
rat and human cerebral endothelial cells (33). Therefore, it can be speculated
that TLR4 is upregulated in senescent endothelial cells under
various conditions including high glucose and oxidative stress.
It is proposed that bacterial infection with
Chlamydia pneumoniae or Porphyromonas gingivaris is
associated with the pathogenesis of atherosclerosis (34). We have previously indicated that
Chlamydiaceae LPS has low affinities for LPS recognition molecules
(such as CD14 and LPS-binding protein) and exhibits weak biological
activities against monocytes, thereby possibly contributing to the
persistent inflammatory response during infection (35). Furthermore, P. gingivaris
LPS, at a higher concentration (1 µg/ml), was demonstrated
to induce proinflammatory responses including ICAM-1 expression in
HUVECs (36). By contrast,
gut-derived bacterial LPS (such as E. coli LPS) possesses a
potent biological activity and can be detected in the
atherosclerotic plaque of carotid arteries (37); in addition, the serum levels of
E. coli LPS is significantly higher in the atherosclerosis
patients (37). Thus, the present
study used E. coli LPS for evaluating the LPS response of
senescent cells, and revealed that E. coli LPS potently
induced ICAM-1 expression and p65 phosphorylation in senescent
endothelial cells.
LL-37 was originally identified as a human
antimicrobial peptide of the cathelicidin family; it is released by
neutrophils and epithelial cells upon infection and participates in
bacterial killing (9,10). However accumulating evidence has
revealed that LL-37 acts on host cells (immune cells, epithelial
cells and endothelial cells) and exhibits immunomodulatory action
(11). Previous studies suggested
the involvement of LL-37 in the pathogenesis of atherosclerosis
(14-17). The current study compared the
response of senescent and non-senescent HUVECs to LL-37, and
revealed that ICAM-1 expression and p65 activation were induced
more by LL-37 in senescent endothelial cells. This observation
suggested that the NF-κB pathway was more potently activated by
LL-37 in senescent endothelial cells to induce the enhanced
expression of ICAM-1.
In the present study, the ICAM-1 induction by LL-37
in senescent endothelial cells was greater compared with
non-senescent cells; LL-37 stimulation (10 µg/ml) increased
ICAM-1 expression by 6.3 times in senescent cells but only 1.9
times in non-senescent cells (Fig.
5). By contrast, the induction of p65 phosphorylation by LL-37
was not apparent between senescent and non-senescent cells (1.7
times in senescent cells and 1.3 times in non-senescent; Fig. 6C). These observations suggested
that signaling molecules other than NF-κB p65 may be involved in
the LL-37-induced ICAM-1 expression. It has been reported that
LL-37 can activate the ERK1/2, p38 MAPK and PI3K pathways, as well
as the NF-κB pathway, in several types of cells (38).
FPR2 and P2X7, the receptors for LL-37, have been
identified to be present in endothelial cells (12,13). To clarify the involvement of FPR2
and P2X7 in the ICAM-1 induction by LL-37, the present study
utilized FPR2 and P2X7 antagonists (WRW4 peptide and KN-62,
respectively), and revealed that the two antagonists inhibited the
LL-37-induced ICAM-1 expression in non-senescent endothelial cells
(Fig. 7A), indicating that LL-37
induced ICAM-1 expression via FPR2 and P2X7 in non-senescent
endothelial cells.
In order to further clarify the contribution of the
two LL-37 receptors to the enhanced ICAM-1 expression in senescent
endothelial cells, the expression levels of FPR2 and P2X7 were
compared between senescent and non-senescent cells, by flow
cytometry (Fig. 7) and western
blot analysis (data not shown). The results demonstrated that both
FPR2 and P2X7 were expressed in non-senescent and senescent cells,
and that P2X7 expression, but not FPR2 expression, was upregulated
in senescent endothelial cells compared with non-senescent
endothelial cells. These observations suggested that upregulated
P2X7 may have a more important role in the enhanced response to
LL-37 in senescent endothelial cells. In this regard, it is
interesting to note that endothelial P2X7 is increased in
atherosclerotic lesions of mouse aorta (39) and is required for inflammatory
signaling in endothelial cells exposed to low shear stress
mimicking atherogenic conditions (39). These observations suggest that
LL-37 localized on endothelial cells of human atherosclerotic
lesion (14) may contribute to
the enhanced inflammatory response during atherogenesis possibly
via the upregulated P2X7.
The effect of LPS or LL-37 on a different type of
senescent endothelial cells (such as aortic endothelial cells) has
not been investigated in the present study. However, the expression
of endothelial TLR4 is augmented in human atherosclerotic aorta
lesions, where senescent endothelial cells have been detected
(8). Additionally, endothelial
P2X7 is increased in atherosclerotic lesions of mouse aorta
(39). Based on these
observations, it can be speculated that enhanced inflammatory
responses could be induced by LPS or LL-37 in different types of
senescent endothelial cells (such as HUVECs and aortic endothelial
cells).
In addition to its broad spectrum of bactericidal
function, LL-37 directly binds to LPS released from Gram-negative
bacteria, and neutralizes its biological activity (40). Due to the LPS-neutralizing
activity, LL-37 suppresses the LPS-induced TNF-α production by
monocytes/macrophages (19),
IL-1β release and pyroptosis of monocytes/macrophages (41) and apoptosis of endothelial cells
(42). Thus, it would be of
interest to examine the effect of LL-37 on the LPS-induced
expression of ICAM-1 in non-senescent and senescent endothelial
cells. The present study evaluated the effect of simultaneous
stimulation with LPS and LL-37, and the results indicated that in
non-senescent endothelial cells, LL-37 stimulation (5 µg/ml)
did not induce the ICAM-1 expression (Fig. 5) and almost completely suppressed
the LPS (100 ng/ml)-induced expression of ICAM-1 due to its
LPS-neutralizing activity (data not shown). By contrast, in
senescent endothelial cells, LL-37 stimulation (5 µg/ml)
itself significantly upregulated ICAM-1 expression (Fig. 5), but suppressed the LPS-induced
expression of ICAM-1; however, the suppression was partial, and
ICAM-1 expression was retained (data not shown). Thus, LL-37 could
be an atherogenic molecule for senescent endothelial cells even in
the presence of LPS.
In conclusion, the present study revealed that
senescent endothelial cells exhibited basal proinflammatory
phenotype, as evidenced by higher ICAM-1 expression and NF-κB p65
phosphorylation. In addition, ICAM-1 expression was potently
enhanced in senescent endothelial cells upon exposure to LPS and
LL-37. Furthermore, NF-κB p65 signaling was more activated by LPS
and LL-37 in senescent endothelial cells compared with
non-senescent cells, possibly via the upregulation of their
respective receptors TLR4 and P2X7. Altogether, the present results
indicated that senescent endothelial cells may contribute to the
pathogenesis of atherosclerosis via the basal proinflammatory
phenotype and the enhanced inflammatory response against
atherogenic factors, including LPS and LL-37.
Acknowledgments
We thank members of the Laboratory of Proteomics and
Biomolecular Science (Dr Yoshiki Miura), the Division of Molecular
and Biochemical Research (Professor Hiroshi Koide), and the
Division of Cell Biology (Drs Akemi Koyanagi and Tamami Sakanishi),
Research Support Center, Juntendo University Graduate School of
Medicine for synthesizing LL-37 peptide or technical assistance. We
are also grateful to Dr Akimasa Someya (Department of Host Defense
and Biochemical Research, Juntendo University Graduate School of
Medicine) for helpful discussion.
Funding
This study was supported by the Strategic Research
Foundation Grant-aided Project for Private Universities, 2014-2018
(grant no. S1411007), and the MEXT KAKENHI (grant no. JP16K08789)
from the Ministry of Education, Culture, Sport, Science, and
Technology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KS designed the study. KS and MO performed the
experiments and analyzed data. KS and IN wrote and edited the
manuscript. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hayflick L: The limited in vitro lifetime
of human diploid cell strains. Exp Cell Res. 37:614–636. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Campisi J and d'Adda di Fagagna F:
Cellular senescence: When bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Childs BG, Durik M, Baker DJ and van
Deursen JM: Cellular senescence in aging and age-related disease:
From mechanisms to therapy. Nat Med. 21:1424–1435. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minamino T, Miyauchi H, Yoshida T, Ishida
Y, Yoshida H and Komuro I: Endothelial cell senescence in human
atherosclerosis: Role of telomere in endothelial dysfunction.
Circulation. 105:1541–1544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aimetti M, Romano F and Nessi F:
Microbiologic analysis of periodontal pockets and carotid
atheromatous plaques in advanced chronic periodontitis patients. J
Periodontol. 78:1718–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pussinen PJ, Tuomisto K, Jousilahti P,
Havulinna AS, Sundvall J and Salomaa V: Endotoxemia, immune
response to periodontal pathogens, and systemic inflammation
associate with incident cardiovascular disease events. Arterioscler
Thromb Vasc Biol. 27:1433–1439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edfeldt K, Swedenborg J, Hansson GK and
Yan ZQ: Expression of toll-like receptors in human atherosclerotic
lesions: A possible pathway for plaque activation. Circulation.
105:1158–1161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagaoka I, Hirata M, Sugimoto K,
Tsutsumi-Ishii Y, Someya A, Saionji K and Igari J: Evaluation of
the expression of human CAP18 gene during neutrophil maturation in
the bone marrow. J Leukoc Biol. 64:845–852. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis SM, Anderson NN, Forsyth WR,
Espiritu C, Conway BD, Greenberg EP, McCray PB Jr, Lehrer RI, Welsh
MJ and Tack BF: Bactericidal activity of mammalian
cathelicidin-derived peptides. Infect Immun. 68:2748–2755. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi KY, Chow LNY and Mookherjee N:
Cationic host defence peptides: Multifaceted role in immune
modulation and inflammation. J Innate Immun. 4:361–370.
2012.PubMed/NCBI
|
|
12
|
Koczulla R, von Degenfeld G, Kupatt C,
Krotz F, Zahler S, Gloe T, Issbrücker K, Unterberger P, Zaiou M,
Lebherz C, et al: An angiogenic role for the human peptide
antibiotic LL-37/hCAP-18. J Clin Invest. 111:1665–1672. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Byfield FJ, Wen Q, Leszczynska K,
Kulakowska A, Namiot Z, Janmey PA and Bucki R: Cathelicidin LL-37
peptide regulates endothelial cell stiffness and endothelial
barrier permeability. Am J Physiol Cell Physiol. 300:C105–C112.
2011. View Article : Google Scholar :
|
|
14
|
Edfeldt K, Agerberth B, Rottenberg ME,
Gudmundsson GH, Wang XB, Mandal K, Xu Q and Yan ZQ: Involvement of
the antimicrobial peptide LL-37 in human atherosclerosis.
Arterioscler Thromb Vasc Biol. 26:1551–1557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doering Y, Drechsler M, Wantha S,
Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C and Soehnlein
O: Lack of neutrophil-derived CRAMP reduces atherosclerosis in
mice. Circ Res. 110:1052–1056. 2012. View Article : Google Scholar
|
|
16
|
Wantha S, Alard JE, Megens RT, van der
Döes AM, Döring Y, Drechsler M, Pham CT, Wang MW, Wang JM, Gallo
RL, et al: Neutrophil-derived cathelicidin promotes adhesion of
classical monocytes. Circ Res. 112:792–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mihailovic PM, Lio WM, Yano J, Zhao X,
Zhou J, Chyu KY, Shah PK, Cercek B and Dimayuga PC: The
cathelicidin protein CRAMP is a potential atherosclerosis
self-antigen in ApoE(−/−) mice. PLoS One. 12:e01874322017.
View Article : Google Scholar
|
|
18
|
Chien Y, Scuoppo C, Wang X, Fang X,
Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, et al:
Control of the senescence-associated secretory phenotype by NF-κB
promotes senescence and enhances chemosensitivity. Genes Dev.
25:2125–2136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagaoka I, Hirota S, Niyonsaba F, Hirata
M, Adachi Y, Tamura H and Heumann D: Cathelicidin family of
antibacterial peptides CAP18 and CAP11 inhibit the expression of
TNF-alpha by blocking the binding of LPS to CD14(+) cells. J
Immunol. 167:3329–3338. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yanaka M, Honma T, Sato K, Shinohara N,
Ito J, Tanaka Y, Tsuduki T and Ikeda I: Increased monocytic
adhesion by senescence in human umbilical vein endothelial cells.
Biosci Biotechnol Biochem. 75:1098–1103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dauphinee SM and Karsan A:
Lipopolysaccharide signaling in endothelial cells. Lab Invest.
86:9–22. 2006. View Article : Google Scholar
|
|
22
|
Renshaw M, Rockwell J, Engleman C, Gewirtz
A, Katz J and Sambhara S: Cutting edge: Impaired Toll-like receptor
expression and function in aging. J Immunol. 169:4697–4701. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qian F, Wang X, Zhang L, Chen S, Piecychna
M, Allore H, Bockenstedt L, Malawista S, Bucala R, Shaw AC, et al:
Age-associated elevation in TLR5 leads to increased inflammatory
responses in the elderly. Aging Cell. 11:104–110. 2012. View Article : Google Scholar :
|
|
24
|
Berkestedt I, Nelson A and Bodelsson M:
Endogenous antimicrobial peptide LL-37 induces human
vasodilatation. Br J Anaesth. 100:803–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagaoka I, Tamura H and Hirata M: An
antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses
neutrophil apoptosis via the activation of formyl-peptide
receptor-like 1 and P2X7. J Immunol. 176:3044–3052. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan SY, Awad EM, Oszwald A, Mayr M, Yin
X, Waltenberger B, Stuppner H, Lipovac M, Uhrin P and Breuss JM:
Premature senescence of endothelial cells upon chronic exposure to
TNFα can be prevented by N-acetyl cysteine and plumericin. Sci Rep.
7:395012017. View Article : Google Scholar
|
|
27
|
Tilstra JS, Clauson CL, Niedernhofer LJ
and Robbins PD: NF-κB in aging and disease. Aging Dis. 2:449–465.
2011.
|
|
28
|
Ogura N, Matsuda U, Tanaka F, Shibata Y,
Takiguchi H and Abiko Y: In vitro senescence enhances IL-6
production in human gingival fibroblasts induced by
lipopolysaccharide from Campylobacter rectus. Mech Ageing Dev.
87:47–59. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mochizuki K, Yamaguchi M and Abiko Y:
Enhancement of LPS-stimulated plasminogen activator production in
aged gingival fibroblasts. J Periodontal Res. 34:251–260. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Michelsen KS, Wong MH, Shah PK, Zhang W,
Yano J, Doherty TM, Akira S, Rajavashisth TB and Arditi M: Lack of
Toll-like receptor 4 or myeloid differentiation factor 88 reduces
atherosclerosis and alters plaque phenotype in mice deficient in
apolipoprotein E. Proc Natl Acad Sci USA. 101:10679–10684. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lloyd-Jones KL, Kelly MM and Kubes P:
Varying importance of soluble and membrane CD14 in endothelial
detection of lipopoly-saccharide. J Immunol. 181:1446–1453. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang L, Wang J, Fang J, Zhou H, Liu X and
Su SB: High glucose induces and activates Toll-like receptor 4 in
endothelial cells of diabetic retinopathy. Diabetol Metab Syndr.
7:892015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagyoszi P, Wilhelm I, Farkas AE, Fazakas
C, Dung NT, Haskó J and Krizbai IA: Expression and regulation of
toll-like receptors in cerebral endothelial cells. Neurochem Int.
57:556–564. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borel N, Pospischil A, Dowling RD, Dumrese
C, Gaydos CA, Bunk S, Hermann C, Ramirez JA and Summersgill JT:
Antigens of persistent Chlamydia pneumoniae within coronary
atheroma from patients undergoing heart transplantation. J Clin
Pathol. 65:171–177. 2012. View Article : Google Scholar
|
|
35
|
Tsutsumi-Ishii Y, Shimada K, Daida H,
Toman R and Nagaoka I: Low potency of Chlamydophila LPS to activate
human mononuclear cells due to its reduced affinities for CD14 and
LPS-binding protein. Int Immunol. 20:199–208. 2008. View Article : Google Scholar
|
|
36
|
An N, Andrukhov O, Tang Y, Falkensammer F,
Bantleon HP, Ouyang X and Rausch-Fan X: Effect of nicotine and
Porphyromonas gingivalis lipopolysaccharide on endothelial cells in
vitro. PLoS One. 9:e969422014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carnevale R, Nocella C, Petrozza V,
Cammisotto V, Pacini L, Sorrentino V, Martinelli O, Irace L,
Sciarretta S, Frati G, et al: Localization of lipopolysaccharide
from Escherichia coli into human atherosclerotic plaque. Sci Rep.
8:35982018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agier J, Efenberger M and
Brzezińska-Blaszczyk E: Cathelicidin impact on inflammatory cells.
Cent Eur J Immunol. 40:225–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Green JP, Souilhol C, Xanthis I,
Martinez-Campesino L, Bowden NP, Evans PC and Wilson HL:
Atheroprone flow activates inflammation via endothelial
ATP-dependent P2X7-p38 signalling. Cardiovasc Res. 114:324–335.
2018. View Article : Google Scholar :
|
|
40
|
Larrick JW, Hirata M, Balint RF, Lee J,
Zhong J and Wright SC: Human CAP18: A novel antimicrobial
lipopolysaccha-ride-binding protein. Infect Immun. 63:1291–1297.
1995.PubMed/NCBI
|
|
41
|
Hu Z, Murakami T, Suzuki K, Tamura H,
Kuwahara-Arai K, Iba T and Nagaoka I: Antimicrobial cathelicidin
peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of
macrophages by dual mechanism. PLoS One. 9:e857652014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suzuki K, Murakami T, Kuwahara-Arai K,
Tamura H, Hiramatsu K and Nagaoka I: Human anti-microbial
cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of
endothelial cells. Int Immunol. 23:185–193. 2011. View Article : Google Scholar : PubMed/NCBI
|