Introduction
Intervertebral disc (IVD) degeneration is a typical
and frequently-occurring disease caused by the degeneration of the
IVD, and includes cervical spondylosis, disc herniation and lumbar
instability (1-3). At present, scholars agree that IVD
degeneration occurs under a variety of physiological and
pathological conditions and is affected by a number of factors,
such as heredity, cellular senescence, mechanical load, increased
degradative enzymes, increased levels of inflammatory factors and
apoptosis (4-6). However, the exact mechanisms
responsible for IVD degeneration remain unclear.
The IVD is composed of an outer collagen-rich
annulus fibrosus and a nucleus pulposus (NP) with a gel-like
structure (7). IVD cells,
particularly NP cells, can produce collagen II, aggrecan and other
components to maintain the integrity of the IVD (8). The reduction in the number of NP
cells and the loss of the extracellular matrix are central features
of IVD degeneration (9). Another
previous study also demonstrated that NP cells are indispensable
for maintaining intradiscal balance (10).
It has been widely accepted that the signaling
molecules of Toll-like receptor 4 (TLR4) act through intracellular
and extracellular pathways to activate the nuclear factor κB
(NF-κB) signaling pathway to mediate systemic inflammatory
responses (11). NF-κB is located
in the cytoplasm under physiological conditions, and its p65
subunit binds to IkB monomer to form a NF-κB complex, which is in
an inactive state, and thus it cannot enter the nucleus to play a
regulatory role (12). When the
body is stimulated by an external stimulating factor, IkB is
phosphorylated and dissociated from the NF-κB dimer. NF-κB
activates and shifts into the nucleus, binds to specific sites on
the DNA strand, and initiates gene transcription to affect protein
expression (13), and plays an
important role in regulating the body's inflammatory response.
There is increasing evidence to indicate that the
mechanisms of apoptosis are triggered by a newly defined small
non-coding RNA that triggers translational inhibition or RNA
degradation to control gene expression by binding to the 3′-UTR of
the target gene (14-16). These microRNAs (miRNAs or miRs)
act as key factors in regulating various biological processes
including proliferation, differentiation, apoptosis, organ
development, and inflammatory diseases (17). However, the role of miRNAs in
human IVD degeneration have not been reported in detail. As a
multifunctional miRNA, miR-222 is expressed in different tissues
and is associated with the development of various diseases
(18). It has been found that
miR-222 is upregulate in clinical patients with IVD degeneration
tissue (19), suggesting that
miR-222 may be closely related to the process of IVD degeneration.
Tissue inhibitor of metalloproteinase 3 (TIMP3) is a member of the
metalloproteinase tissue inhibitor family and is an endogenous
inhibitor of aggrecanase, which exerts a strong inhibitory effect
on aggrecanase activity (20).
Previous studies have found that miR-222 can directly target TIMP3
(21-23). Moreover, studies have demonstrated
that increasing the expression of TIMP3 inhibits the degeneration
of the IVD (24,25).
In this study, we detected the level of miR-222, as
well as the expression of TIMP3 in IVD tissues or/and
lipopolysaccharide (LPS)-treated NP cells. With in vitro
experiments, we further determined whether miR-222 targets TIMP3
directly in NP cells, and we examined the effects of miR-222 on
inflammation and on the apoptosis of LPS-treated NP cells, as well
as its association with the TLR4/NF-κB signaling pathway.
Materials and methods
Tissue samples
This study was approved by the Central Hospital
Affiliated to Shenyang Medical College Ethics Committee. From
January, 2013 to January, 2015, 22 intervertebral disc specimens
were collected from the Central Hospital Affiliated to Shenyang
Medical College. The 22 IVD patients underwent intervertebral disc
excision and spinal fusion surgery. In addition, 9 normal tissues
were collected from patients who underwent traumatic lumbar
fracture. Written informed consent was obtained from all patients
that underwent intervertebral disc excision and spinal fusion
surgery, as well as the 9 patients that underwent traumatic lumbar
fracture. All specimens were kept anonymous in accordance with
ethics and the relevant research laws. All tissue samples were
re-evaluated and classified according to the MRI grade and
immediately frozen in liquid nitrogen for RNA extraction. Clinical
information such as age, sex, body mass index and degeneration
level were also collected during follow-up. Clinical follow-up was
available to all patients. At the end of the follow-up (5 years),
22 patients remained alive.
Reagents
miR-222 mimic (5′-AGC UAC AUC UGG CUA CUG GGU-3′),
inhibitor (5′-AGC UAC AUU GUC UGC UGG GUU UC-3′) and mock (5′-UCU
ACU CUU UCU AGG AGG UUG UGA-3′), which was the negative control
used for the transfection of miR-222 mimics and inhibitors, were
obtained from GenePharma. TIMP3-siRNA (cat. no. AM16708) and
TIMP3-siNC (cat. no. AM4611) were obtained from Ambion (Thermo
Fisher Scientific). LPS was purchased from Sigma-Aldrich (cat. no.
L2630).
Cells and cell culture
Human nucleus pulposus (NP, cat. no. 4800) cells
were obtained from ScienCell Research Laboratories and cultured in
Dulbecco's modified Eagle's medium (DMEM, Gibco; Thermo Fisher
Scientific) containing 10% fetal bovine serum (FBS, Gibco; Thermo
Fisher Scientific) and antibiotics (1% penicillin/streptomycin, BBI
Life Sciences) at 37°C in a humidified atmosphere of 5%
CO2.
Cell treatment
miR-222 mimic, inhibitor and TIMP3-siRNA and the
respective controls were used to transiently transfect the NP cells
(5×104 cells/well) in a 6-well plate using Lipofectamine
3000 (Thermo Fisher Scientific). At 24 h following transfection,
the cells were stimulated with 1 µg/ml LPS in serum-free
medium (DMEM) for 24 h at 37°C under 5% CO2. The cells
were then harvested for subsequent experimentation.
Analysis of cell apoptosis
Annexin V-FITC- propidium iodide (PI) apoptosis
detection reagent (BD Biosciences) was used to determine cell
apoptosis according to the manufacturer's instructions. Briefly,
the cells were washed with PBS 3 times, digested with trypsin (1
ml) and follow resuspended in 1X Annexin binding buffer at
1×105 cells/100 µl. At room temperature, the
cells were collected and stained with Annexin V-FITC and PI for 15
min and counted by flow cytometry (version 10.0, FlowJo, FACS
CaliburTM, BD Biosciences). Cells in the lower left
quadrant represent living cells, those in the left upper quadrant
represent mechanically damaged or necrotic cells, those in the
upper right quadrant represent advanced apoptotic cells and those
in the lower right quadrant represent early apoptotic cells.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the IVD tissues and NP
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific).
Small RNA was isolated from the total RNA using a miRcute miRNA
isolation kit (cat. no. DP501, Tiangen Biotech Co.). For mRNA, 1
µg of RNA was used to reverse transcribe the RNA into cDNA
using the reverse transcription cDNA kit (Thermo Fisher
Scientific). SYBR-Green PCR Master Mix (Roche) was used to conduct
the qPCR experiments using the Opticon RT-PCR Detection System (ABI
7500, Life technologies). For miRNA, a miRcute plus miRNA
first-strand cDNA Synthesis kit (cat. no. KR201-02, Tiangen
Biotech) was used to synthesize the cDNA. A miRcute miRNA qPCR
Detection kit (cat. no. FP401, Tiangen Biotech) was used for
quantification. The PCR cycle was as follows: Pre-treatment at 95°C
for 10 min; followed by 40 cycles of 94°C for 15 sec, at 60°C for 1
min finally at 60°C for 1 min and at 4°C for preservation. The
expression levels of the genes were analyzed using the
2-ΔΔCq method (26).
U6 and GAPDH expression was respectively used for miRNA and mRNA
for normalization. The sequences of the primer used are presented
in Table I.
 | Table ISequences of primers used for
RT-qPCR. |
Table I
Sequences of primers used for
RT-qPCR.
| Genes | Forward | Reverse |
|---|
| Collagen II |
CTGGTGATGATGGTGAAG |
CCTGGATAACCTCTGTGA |
| Aggrecan |
GTGGGACTGAAGTTCTTG |
GTTGTCATGGTCTGAAGTT |
| TIMP3 |
GTGCAACTTCGTGGAGAGGT |
AGCAGGACTTGATCTTGCAGT |
| GAPDH H |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| miR-222 |
AGCTACATCTGGCTACTGG |
GTATCCAGTGCAGGGTCC |
| U6 |
CTCGCTTCGGCAGCACA |
TGGTGTCGTGGAGTCG |
Western blot analysis
Total proteins were isolated from the NP cells using
RIPA buffer (Cell Signaling Technology, Inc.). The BCA Protein
Assay kit (Pierce) was applied to measure the protein
concentration, which was adjusted to a concentration of 6
µg/µl using 1X loading buffer and DEPC water.
Electrophoresis was used to separate the samples using a 10%
SDS-PAGE gel followed by transfer onto a polyvinylidene fluoride
membrane (PVDF, Millipore). After blocking in 5% non-fat milk in
PBST [0.1% Tween-20 in phosphate-buffered saline (PBS)] for 1 h,
the membrane was incubated with the primary antibody overnight at
4°C, and subsequently incubated with secondary antibody
[horseradish peroxidase (HRP)-conjugated goat anti-mouse/rabbit
IgG, 1:2,000; sc-516102/sc-2357; Santa Cruz Biotechnology, Inc.] at
room temperature for 2 h. Developer (EZ-ECL kit; Biological
Industries BI) was used for development, and the gray value of the
strips were analyzed and quantified using imageJ software (version
5.0; Bio-Rad). The antibodies utilized included anti-GAPDH (mouse;
1:1,000; LS-B1625; LifeSpan BioSciences, Inc.), anti-TLR4 (rabbit;
1:500; ab13556; Abcam), anti-IκBα (mouse; 1:1,000; #4814),
anti-p-IκBα (mouse; 1:1,000; #9246), anti-p65 (rabbit; 1:1,000;
#8242), anti-p-p65 (rabbit; 1:1,000; #3039) and anti-TIMP3 (rabbit;
1:1,000; #5673) (all from Cell Signaling Technology, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
The serum levels of TNF-α, IL-1β and IL-6 were
measured using ELISA kits (eBioscience) according to the
manufacturer's instructions. All standards and samples were
measured using a microplate reader (SpectraMax M5, Molecular
Devices) at a wavelength of 450 nm, a standard curve was prepared
using computer software, and the corresponding sample concentration
was calculated based on the absorbance value.
Bioinformatics prediction
The potential target genes of miR-222 were predicted
using TargetScan7.2 online software (http://www.targetscan.org/vert_72/), according to the
manufacturer's instructions. 'miR-222' was inserted and 'human' was
selected. The putative target genes of miR-222 were scanned.
Binding ability of TIMP3 to miR-222
293 cells (BeNa Culture Collection) were transfected
with 100 nm TIMP3-3′-UTR plasmid [the TIMP3 mutant (MT) and
wild-type (WT)] (GeneChem), as well as with or without 100 nm
miR-222. Subsequently, the luciferase reporter assay system
(Promega Corp.) was used to measure luciferase activity in Lmax II
luminescence meter (Molecular Devices, LLC) for 48 h following
transfection. Renilla luciferase activity was defined as the
standardization of Firefly luciferase activity.
Statistical analysis
Statistical analysis was carried out using Prism 6
software (GraphPad Software, Inc.). Statistically significant
differences between groups were determined using one-way analysis
of variance (ANOVA), followed by Bonferroni's post hoc test. The
Chi-square test was used for the discontinuous variables shown in
Table II. The results are
presented as the means ± standard deviation (SD) and statistically
significant differences are indicated by P<0.05.
 | Table IIAssociation between miR-222
expression and the clinical characteristics of patients with IVD
degeneration. |
Table II
Association between miR-222
expression and the clinical characteristics of patients with IVD
degeneration.
| Parameters | miR-222 expression
| Total | P-value |
|---|
| Low (%) | High (%) |
|---|
| Age (years) | | | | 0.096 |
| ≤45 | 6 (27.3) | 3 (13.6) | 9 | |
| >45 | 4 (18.2) | 9 (40.9) | 13 | |
| Sex | | | | 0.145 |
| Female | 2 (9.1) | 6 (27.3) | 8 | |
| Male | 8 (36.4) | 6 (27.3) | 14 | |
| Body mass
index | | | | 0.937 |
| ≤24
kg/m2 | 4 (18.2) | 5 (22.7) | 9 | |
| >24
kg/m2 | 6 (27.3) | 7 (31.8) | 13 | |
| Degeneration
level | | | | 0.190 |
| L3/4 | 5 (22.7) | 3 (13.6) | 8 | |
| L4/5 | 2 (9.1) | 7 (31.8) | 9 | |
| L5/S1 | 3 (13.6) | 2 (9.1) | 5 | |
| MRI grade | | | |
0.029a |
| G (I/II) | 8 (36.4) | 4 (18.2) | 12 | |
| G (IV/V) | 2 (9.1) | 8 (36.4) | 10 | |
Results
The expression level of miR-222 is
associated with the clinicopathological characteristics of IVD
degeneration
The association between the expression of miR-222
and the clinicopathologic characteristics of the patients with IVD
were evaluated (Table II). A
high miR-222 expression was associated with a high MRI grade
(P=0.029). However, the miR-222 expression level was not associated
with age, sex, body mass index or the degeneration level
status.
miR-222 inhibitor decreases LPS-induced
NP cell apoptosis
We determined the level of miR-222 in IVD tissues
and an IVD cell model was generated using NP cells treated with
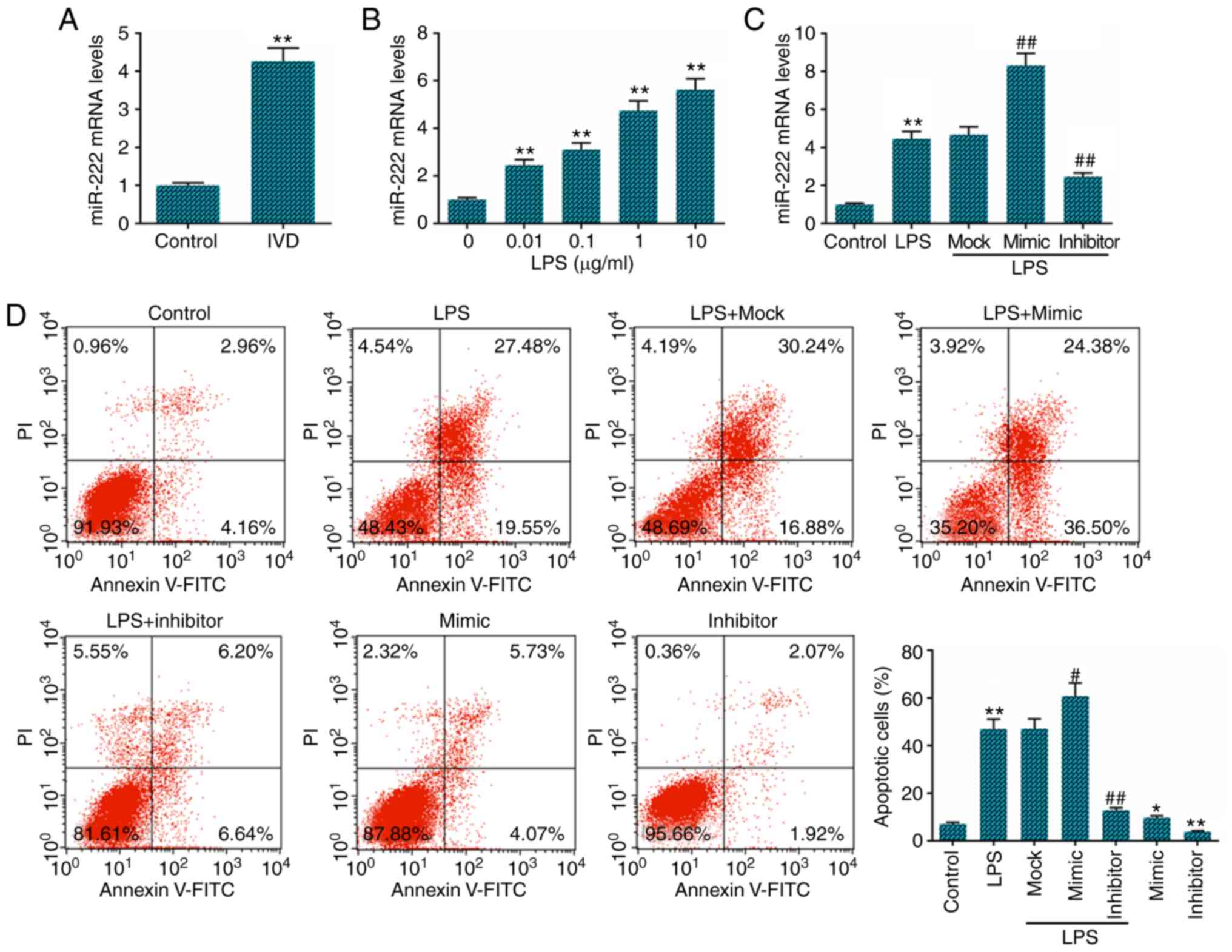
LPS. The results revealed that miR-222 expression was significantly
increased in IVD tissues (Fig.
1A) compared with the normal controls. Its expression was also
increased in a dose-dependent manner in the LPS-treated NP cells
(Fig. 1B). Following the referral
to relevant literature and the experimental results, the
concentration of 1 µg/ml LPS was selected for use in later
experiments (27). To further
examine the effect of miR-222 on LPS-induced NP cell apoptosis,
miR-222 mock, mimics and inhibitor were transfected into the NP
cells, and a high transfection efficiency was observed (Fig. 1C). The results of the flow
cytometric analysis of apoptosis demonstrated that cell apoptosis
was decreased in the miR-222 inhibitor-transfected NP cells
compared with the LPS-treated cells, while miR-222 mimics exerted
the opposite effect (Fig.
1D).
miR-222 inhibitor decreases
pro-inflammatory cytokine levels and enhances collagen II and
aggrecan expression in LPS-stimulated NP cells
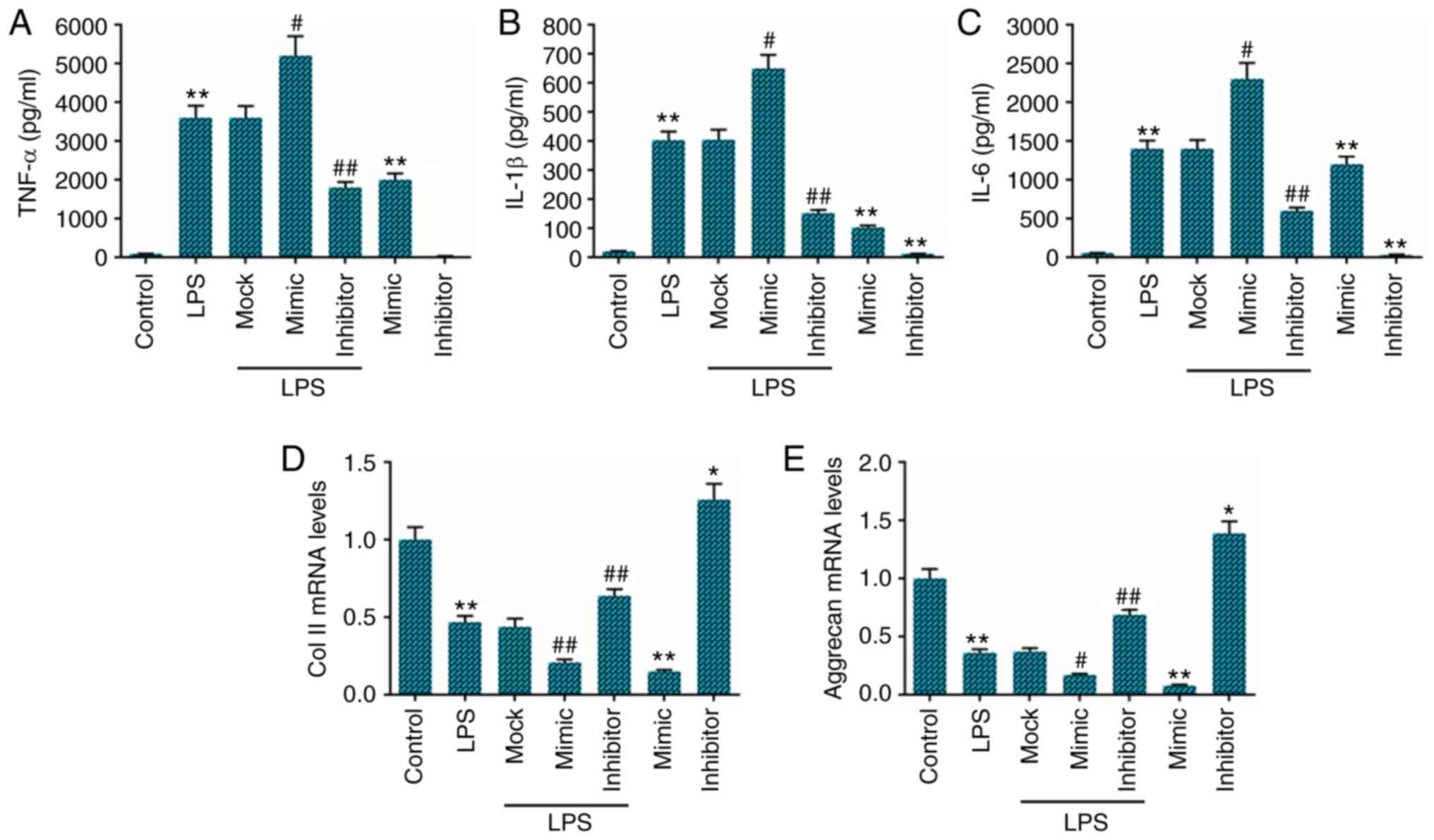
Subsequently, we examined the expression levels of
TNF-α, IL-1β and IL-6 in NP cells stimulated with LPS for 24 h by
ELISA. As expected, we found that compared with the cells
stimulated with LPS alone, transfection with miR-222 inhibitor led
to a marked decrease in the levels of TNF-α, IL-1β and IL-6. By
contrast, transfection with miR-222 mimics markedly increased the
production of TNF-α, IL-1β and IL-6 in the LPS-stimulated cells
(Fig. 2A-C). The effects of
miR-222 on collagen II and aggrecan expression in the NP cells were
also examined by RT-qPCR, and the data indicated that the
expression levels of collagen II and aggrecan were lower in the LPS
group than those in the control group. Compared with the cells
stimulated with LPS only, transfection with miR-222 mimics
significantly inhibited collagen II and aggrecan expression in the
NP cells, while transfection with miR-222 inhibitor markedly
enhanced the collagen II and aggrecan levels (Fig. 2D and E).
The levels of TLR4, p-IκBα and p-p65 are
suppressed by miR-222 inhibitor
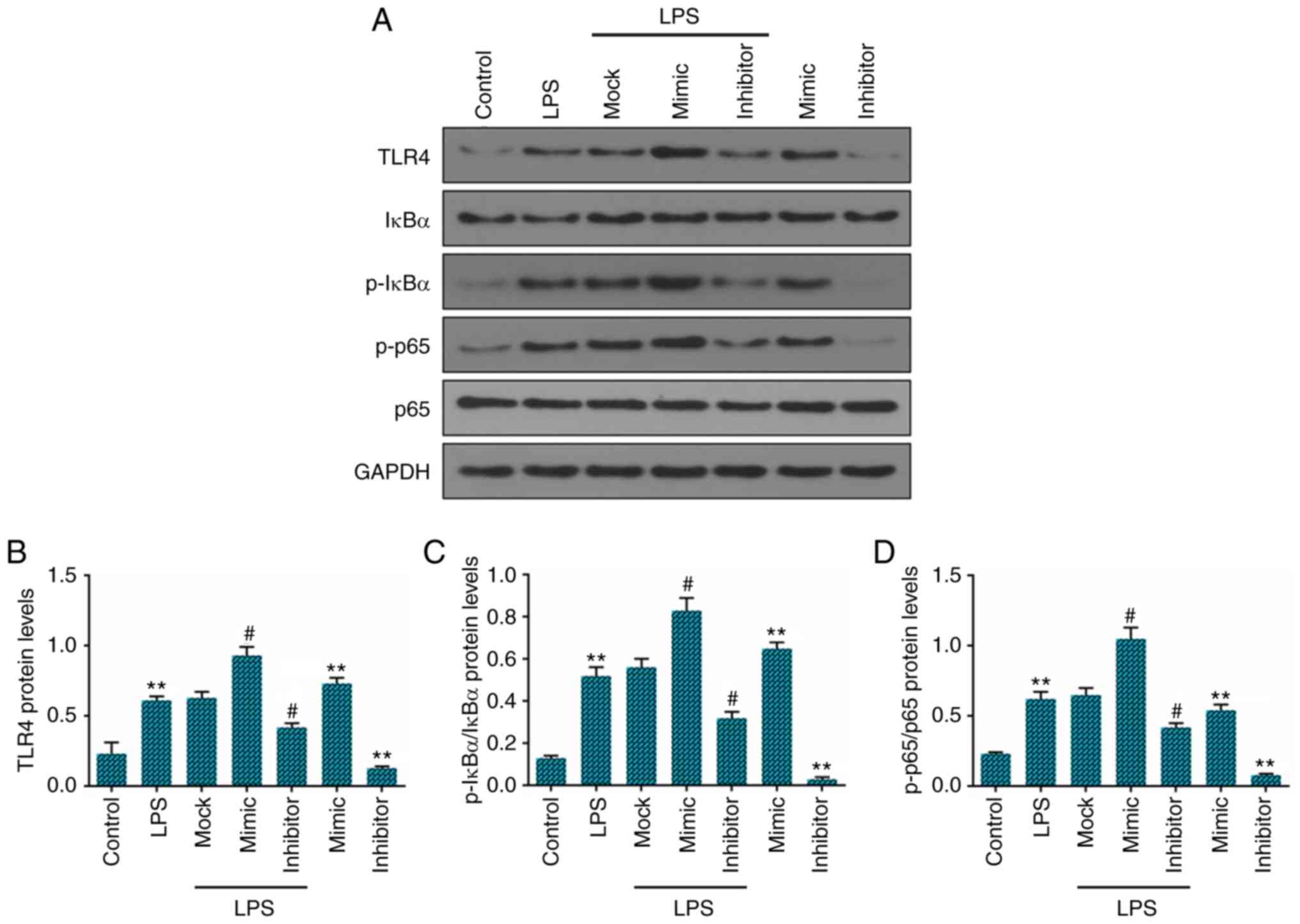
In view of the significant role played by the
TLR4/NF-κB signaling pathway in the progression of IVD, we assessed
the expression of TLR4 and the phosphorylation levels of IκBα and
p65 in human NP cells stimulated with LPS by western blot analysis.
The results demonstrated that the levels of TLR4, p-IκBα and p-p65
were significantly higher in the LPS-treated NP cells than those in
the control NP cells. Transfection with miR-222 mimics
significantly upregulated the TLR4, p-IκBα and p-p65 expression
levels in NP cells, while transfection with miR-222 inhibitor
markedly down-regulated the TLR4, p-IκBα and p-p65 levels, when
compared with the cells stimulated with LPS only (Fig. 3A). The relative protein levels of
TLR4, p-IκBα and p-p65 in NP cells are presented in Fig. 3B-D).
miR-222 increases the expression of TIMP3
by directly binding to TIMP3 3′-UTR
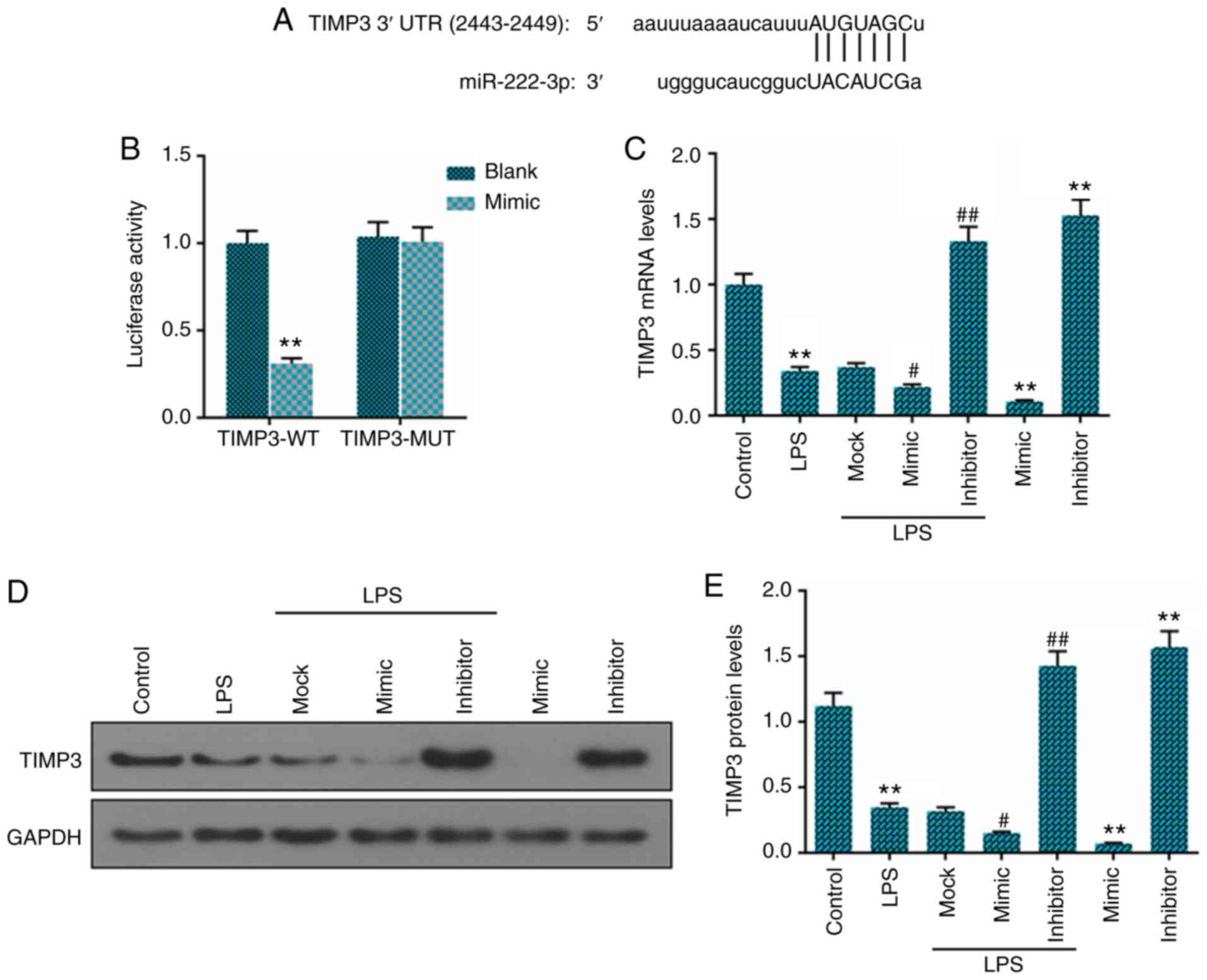
To examine the functions of miR-222 in NP cells, we
first predicted the potential targets of miR-222 using TargetScan
7.2 and found that the 3′-UTR of TIMP3 had a binding site to
miR-222 (Fig. 4A), and dual
luciferase reporter assay was applied to confirm our prediction
(Fig. 4B). The luciferase
activities significantly decreased after miR-222-3p mimic and TIMP3
3′-UTR-wt were co-transfected into the 293 cells. However, the
luciferase activities in the cells co-transfected with TIMP3
3′-UTR-mut and miR-222-3p mimic remained stable. Furthermore, the
expression level of TIMP3 was markedly downregulated in the
LPS-treated NP cells in comparison to the untreated cells, and the
expression level of miR-222 was negatively associated with TIMP3
expression at the mRNA (Fig. 4C)
and protein level (Fig. 4D and
E).
Inhibitory effects of miR-222 inhibitor
on cell apoptosis are reversed by transfection with
TIMP3-siRNA
As the overexpression miR-222 decreased the TIMP3
levels in the LPS-stimulated NP cells, we then wished to determine
whether TIMP3 is involved in the apoptosis of LPS-stimulated NP
cells. The NC-siRNA and TIMP3-siRNA plasmids were transfected into
the NP cells with/without miR-222 inhibitor and cell apoptosis was
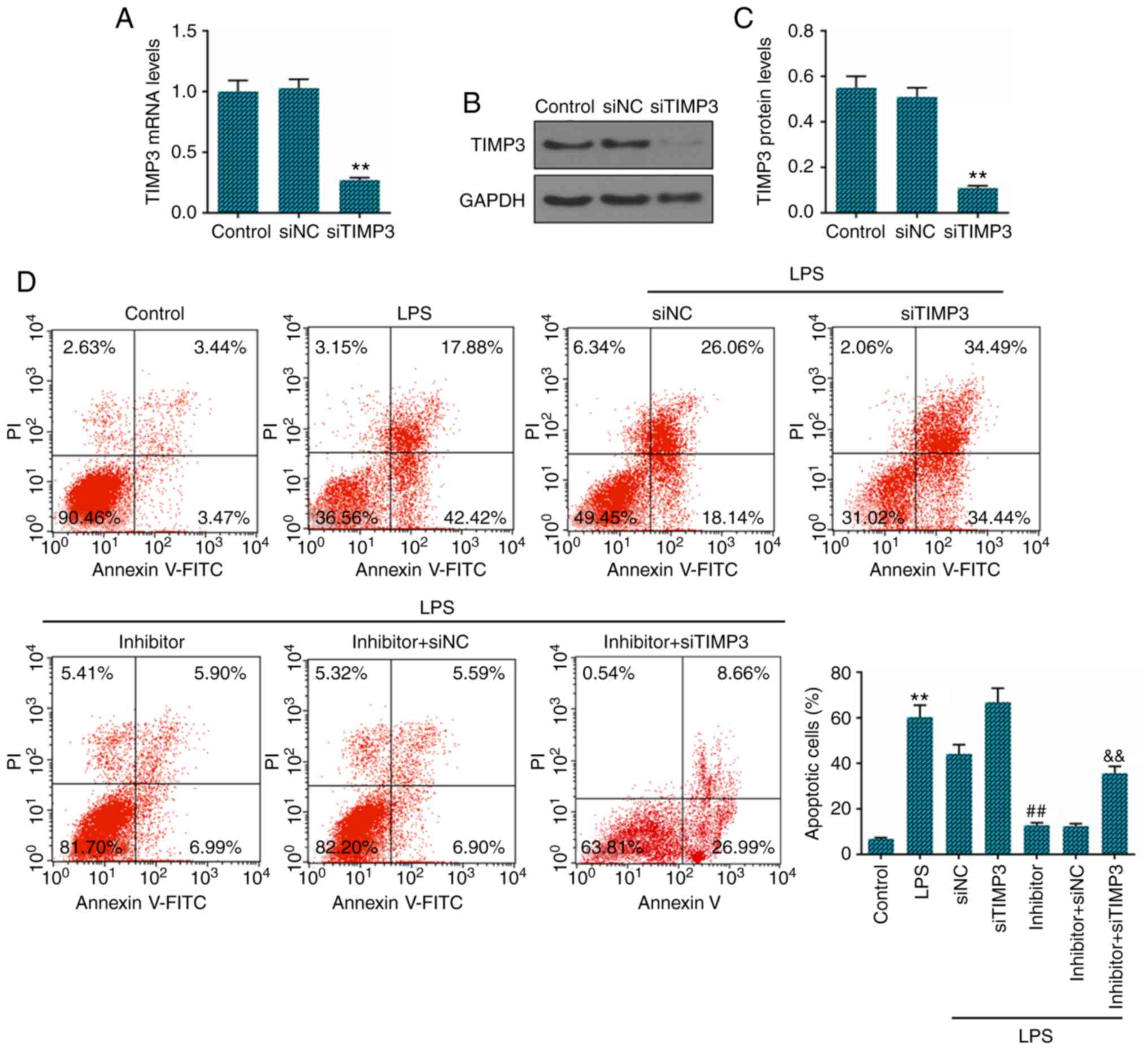
examined. The successful knockdown of TIMP3 was verified by RT-qPCR
(Fig. 5A) and western blot
analysis (Fig. 5B and C). We
further analyzed the apoptosis of LPS-stimulated NP cells, and the
results revealed that the decrease in the expression of TIMP3
effectively attenuated the inhibitory effects on the apoptosis of
LPS-treated NP cells induced by miR-222 inhibition (Fig. 5D).
Discussion
miRNA-222 has been shown to be encoded in the
progression and development of cancer, thereby regulating the
proliferation, migration and apoptosis of cancer cells (28-30). miR-221, the same cluster of
miR-222, also plays a significant role in the progression of cancer
(31,32). Penolazzi et al reported
that miR-221 may play a significant role in the etiology of IVD
degeneration and that its downregulation may play a pivotal role in
the preservation of disc homeostasis and in supporting the
endogenous repair process (33).
However, the regulation of miR-222 in IVD degeneration has not yet
been reported, at least to the best of our knowledge. A recent
study stated that miR-222 was highly expressed in IVD degeneration
and identified this miRNA as the key to IDD development (19). The results of this study also
confirmed that miR-222 was highly expressed in patients with IVD
degeneration. These results suggest that miR-222 plays an important
role in IVD degeneration and that the understanding of the
underlying mechanisms of the regulation of IVD degeneration by
miR-222 may provide a promising strategy for the treatment of IVD
degeneration.
IVD degeneration is accompanied by NP cell
apoptosis, which is primarily induced by TNF-a, and a major
characteristic of IVD degeneration is the reduction of the collagen
II and aggrecan content in NP cells (34,35). LPS, an admitted strong promoter of
inflammation, can reduce the collagen II and aggrecan content, thus
leading to IVD degeneration (36). Thus, in the present study, we used
LPS to generate the IVD degeneration cell model using NP cells for
the further study of miR-222, and found miR-222 was upregulated in
human NP cells stimulated with LPS in a dose-dependent manner.
Moreover, the collagen II and aggrecan levels were markedly
decreased in the LPS-stimulated NP cells, and these effects were
partially reversed by transfection with miR-222 inhibitor, while
transfection with miR-222 mimics exerted opposite effects. Those
outcomes demonstrate that miR-222 plays a role in the progression
of IVD degeneration, at least in LPS-stimulated NP cells.
Related studies have demonstrated that disc
degeneration is associated with a variety of pro-inflammatory
cytokines (37,38). The inhibition of the
IL-1β-mediated inflammatory response can effectively alleviate IVD
degeneration in NP cells (39).
TNF and IL play a key role in the development of intervertebral
disc degeneration (37). The
overexpression of miR-140-5p has been shown to inhibit LPS-induced
human IVD inflammation and degeneration by downregulating TLR4
(27). Previous studies have also
reported that the inhibition of the NF-κB signaling pathway can
protect against IVD degeneration (40,41). It is well known that the
TLR4/NF-κB signaling pathway mediates inflammation cascades to
amplify the inflammatory response (42,43). In this study, the levels of TNF-α,
IL-1β and IL-6 were markedly lower in the LPS + inhibitor group
than that in the LPS-stimulated NP cells, while miR-222 mimics
enhanced the production of TNF-α, IL-1β and IL-6, in comparison to
the LPS group. Moreover, significantly decreased expression levels
of TLR4, p-IκBα and p-p65 were observed following transfection with
miR-222 inhibitor, while transfection with miR-222 mimics increased
the TLR4, p-IκBα and p-p65 expression levels, when compared with
the LPS group. These data suggest that miR-222 may affect the
development of IVD degeneration by aggravating the inflammatory
response.
Apoptosis, as a physiological process, can be used
to remove harmful or severely damaged cells and organelles;
however, when this process becomes excessive, it can lead to
pathological phenomena (44). The
apoptosis of human IVD tissue has been detected and it has been
found that a large part of the human IVD during degeneration had
undergone programmed cell death (45). Zhang et al found that the
injection of the shRNA vector, CHOP shRNA, into the rat IVD
inhibited the apoptosis of IVD cells, thereby attenuating the
degeneration of the IVD (46). In
the present study, NP cells were examined using Annexin V and PI
double staining, which can detect the occurrence of apoptosis
(47). Compared to the NP cells
stimulated with LPS only, apoptosis was inhibited by transfection
with miR-222 inhibitor, while transfection with miR-222 mimics
promoted cell apoptosis. These result prove that miR-222 plays a
key role in IVD degeneration partly by enhancing cell
apoptosis.
miRNAs mostly functions by targeting the 3′-UTR of
their target genes (48). It has
been found that the increased expression of TIMP3 inhibist the
degeneration of the IVD (24,25). Furthermore, TIMP3 has been
reported to be a target of miR-222 (23,49). In the present study, we determined
TIMP3 as the target of miR-222 in IVD degeneration, and a
significantly increased expression of TIMP3 was observed following
transfection with miR-222 inhibitor. However, transfection with
miR-222 mimics decreased TIMP3 expression in the LPS-stimulated NP
cells. Furthermore, to better understand the role of TIMP3 in IVD,
the cells were transfected with TIMP3-siRNA and the results
revealed that TIMP3-siRNA attenuated the inhibitory effects on the
apoptosis of LPS-treated NP cells induced by miR-222 inhibition.
These data demonstrated that miR-222 promoted the progression of
IVD degeneration partly by targeting TIMP3.
Although this study demonstrated that miR-222 was
highly expressed in IVD degeneration and participated in the
process of IVD degeneration, there are several limitations. First,
the function of miR-222 downregulation in protecting IVD against
degeneration by reducing inflammation and inhibiting apoptosis
under LPS stimulation was only supported by in vitro
experiments. Additionally, this research has not been confirmed by
other studies; thus, further experiments using cells and animals,
as well as clinical studies are required to confirm our findings.
Other limitations are, for example, the lacking of anti-apoptotic
factors induced by NF-κB signaling. Therefore, we aim to carry out
a more comprehensive investigation in the future.
Based on the data described above, in this study, we
demonstrated that miR-222 promoted inflammation and apoptosis in
IVD degeneration partly by targeting TIMP3 mRNA, and that the
knockdown miR-222 reversed these effects in LPS-stimulated NP
cells. These results provide further insight into the study of IVD
degeneration and may prove to be of regulatory and diagnostic
importance in the study of IVD degeneration.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
WZ and YaZ made substantial contributions to the
conception and design of the study. JY and XZ were involved in data
acquisition. NW, ZL and DS were involved in data analysis. YuZ and
JF were involved in data interpretation. YaZ and NW were involved
in the drafting of the article. WZ and JF critically revised the
manuscript for important intellectual content. All authors have
read and approved the final manuscript. All the authors agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. This study was approved by the Central Hospital
Affiliated to Shenyang Medical College Ethics Committee. Written
informed consent was obtained from all patients that underwent
intervertebral disc excision and spinal fusion surgery, as well as
the 9 patients that underwent traumatic lumbar fracture.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taher F, Essig D, Lebl DR, Hughes AP, Sama
AA, Cammisa FP and Girardi FP: Lumbar degenerative disc disease:
Current and future concepts of diagnosis and management. Adv
Orthop. 2012:9707522012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chan SC and Gantenbein-Ritter B:
Intervertebral disc regeneration or repair with biomaterials and
stem cell therapy-feasible or fiction? Swiss Med Wkly.
142:w135982012.
|
|
3
|
Park JB, Lee JK, Park SJ, Kim KW and Riew
KD: Mitochondrial involvement in fas-mediated apoptosis of human
lumbar disc cells. J Bone Joint Surg Am. 87:1338–1342.
2005.PubMed/NCBI
|
|
4
|
Solovieva S, Lohiniva J, Leino-Arjas P,
Raininko R, Luoma K, Ala-Kokko L and Riihimäki H: Intervertebral
disc degeneration in relation to the COL9A3 and the IL-1ss gene
polymorphisms. Eur Spine J. 15:613–619. 2006. View Article : Google Scholar
|
|
5
|
Guehring T, Omlor GW, Lorenz H, Bertram H,
Steck E, Richter W, Carstens C and Kroeber M: Stimulation of gene
expression and loss of anular architecture caused by experimental
disc degeneration-an in vivo animal study. Spine (Phila Pa 1976).
30:2510–2515. 2005. View Article : Google Scholar
|
|
6
|
Stokes IA and Iatridis JC: Mechanical
conditions that accelerate intervertebral disc degeneration:
Overload versus immobilization. Spine (Phila Pa 1976).
29:2724–2732. 2004. View Article : Google Scholar
|
|
7
|
Migliorini F, Rath B, Tingart M, Baroncini
A, Quack V and Eschweiler J: Autogenic mesenchymal stem cells for
intervertebral disc regeneration. Int Orthop. 43:1027–1036. 2019.
View Article : Google Scholar
|
|
8
|
Johnson ZI, Shapiro IM and Risbud MV:
Extracellular osmolarity regulates matrix homeostasis in the
intervertebral disc and articular cartilage: Evolving role of
TonEBP. Matrix Biol. 40:10–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aguiar DJ, Johnson SL and Oegema TR:
Notochordal cells interact with nucleus pulposus cells: Regulation
of proteoglycan synthesis. Exp Cell Res. 246:129–137. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsieh AH and Twomey JD: Cellular
mechanobiology of the inter-vertebral disc: New directions and
approaches. J Biomech. 43:137–145. 2010. View Article : Google Scholar
|
|
11
|
McCarty MF: Preclinical studies suggest
complex nutraceutical strategies may have potential for preventing
and managing sepsis. Altern Ther Health Med. 21(Suppl 2): S56–S67.
2015.
|
|
12
|
Gordon JW, Shaw JA and Kirshenbaum LA:
Multiple facets of NF-kB in the heart: To be or not to NF-kB. Circ
Res. 108:1122–1132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jovanovic M and Hengartner MO: miRNAs and
apoptosis: RNAs to die for. Oncogene. 25:6176–6187. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Selbach M, Schwanhausser B, Thierfelder N,
Fang Z, Khanin R and Rajewsky N: Widespread changes in protein
synthesis induced by microRNAs. Nature. 455:58–63. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei T, Ye P, Peng X, Wu LL and Yu GY:
Prognostic value of miR-222 in various cancers: A systematic review
and meta-analysis. Clin Lab. 62:1387–1395. 2016. View Article : Google Scholar
|
|
19
|
Hu P, Feng B, Wang G, Ning B and Jia T:
Microarray based analysis of gene regulation by microRNA in
intervertebral disc degeneration. Mol Med Rep. 12:4925–4930. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balkowiec M, Maksym RB and Wlodarski PK:
The bimodal role of matrix metalloproteinases and their inhibitors
in etiology and pathogenesis of endometriosis (Review). Mol Med
Rep. 18:3123–3136. 2018.PubMed/NCBI
|
|
21
|
Lu Y, Roy S, Nuovo G, Ramaswamy B, Miller
T, Shapiro C, Jacob ST and Majumder S: Anti-microRNA-222
(anti-miR-222) and -181B suppresses growth of tamoxifen-resistant
xenografts in mouse by targeting TIMP3 protein and modulating
mitogenic signal. J Biol Chem. 293:35882018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang C, Zhang J, Hao J, Shi Z, Wang Y,
Han L, Yu S, You Y, Jiang T, Wang J, et al: High level of
miR-221/222 confers increased cell invasion and poor prognosis in
glioma. J Transl Med. 10:1192012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lei Y, Liu Z and Yang W: Negative
correlation of cytoplasm TIMP3 with miR-222 indicates a good
prognosis for NSCLC. Onco Targets Ther. 11:5551–5557. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Li K, Han X, Mao C, Zhang K, Zhao T
and Zhao J: The imbalance between TIMP3 and matrix-degrading
enzymes plays an important role in intervertebral disc
degeneration. Biochem Biophys Res Commun. 469:507–514. 2016.
View Article : Google Scholar
|
|
25
|
Kwon WK, Moon HJ, Kwon TH, Park YK and Kim
JH: The role of Hypoxia in angiogenesis and extracellular matrix
regulation of intervertebral disc cells during inflammatory
reactions. Neurosurgery. 81:867–875. 2017.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Zhang Q, Weng Y, Jiang Y, Zhao S, Zhou D
and Xu N: Overexpression of miR-140-5p inhibits
lipopolysaccharide-induced human intervertebral disc inflammation
and degeneration by downregulating toll-like receptor 4. Oncol Rep.
40:793–802. 2018.PubMed/NCBI
|
|
28
|
Tao Li Z, Wang Y, Jiang X, Li P, Peng J,
Zhang M, Chen X, Liu K, Zhen HP, et al: Tumor-Secreted exosomal
miR-222 promotes tumor progression via regulating P27 expression
and Re-localization in pancreatic cancer. Cell Physiol Biochem.
51:610–629. 2018. View Article : Google Scholar
|
|
29
|
Nie X and Tian H: Correlation between
miR-222 and uterine cancer and its prognostic value. Oncol Lett.
16:1722–1726. 2018.PubMed/NCBI
|
|
30
|
Gu J, Wang Y, Wang X, Zhou D, Shao C, Zhou
M and He Z: Downregulation of lncRNA GAS5 confers tamoxifen
resistance by activating miR-222 in breast cancer. Cancer Lett.
434:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lambertini E, Lolli A, Vezzali F,
Penolazzi L, Gambari R and Piva R: Correlation between Slug
transcription factor and miR-221 in MDA-MB-231 breast cancer cells.
BMC Cancer. 12:4452012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brognara E, Fabbri E, Bazzoli E, Montagner
G, Ghimenton C, Eccher A, Cantù C, Manicardi A, Bianchi N, Finotti
A, et al: Uptake by human glioma cell lines and biological effects
of a peptide-nucleic acids targeting miR-221. J Neurooncol.
118:19–28. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Penolazzi L, Lambertini E, Bergamin LS,
Roncada T, De Bonis P, Cavallo M and Piva R: MicroRNA-221 silencing
attenuates the degenerated phenotype of intervertebral disc cells.
Aging (Albany NY). 10:2001–2015. 2018. View Article : Google Scholar
|
|
34
|
Loreto C, Musumeci G, Castorina A, Loreto
C and Martinez G: Degenerative disc disease of herniated
intervertebral discs is associated with extracellular matrix
remodeling, vimentin-positive cells and cell death. Ann Anat.
193:156–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang WJ, Yu XH, Wang C, Yang W, He WS,
Zhang SJ, Yan YG and Zhang J: MMPs and ADAMTSs in intervertebral
disc degeneration. Clin Chim Acta. 448:238–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Li K, Mao L, Han X, Zhang K, Zhao C
and Zhao J: Cordycepin inhibits LPS-induced inflammatory and matrix
degradation in the intervertebral disc. PeerJ. 4:e19922016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang C, Yu X, Yan Y, Yang W, Zhang S,
Xiang Y, Zhang J and Wang W: Tumor necrosis factor-α: A key
contributor to intervertebral disc degeneration. Acta Biochim
Biophys Sin (Shanghai). 49:1–13. 2017. View Article : Google Scholar
|
|
38
|
Yang W, Yu XH, Wang C, He WS, Zhang SJ,
Yan YG, Zhang J, Xiang YX and Wang WJ: Interleukin-1β in
intervertebral disk degeneration. Clin Chim Acta. 450:262–272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang X, Meng Q, Qiu C, Li P, Qu R, Wang W,
Wang Y, Liu L and Zhao Y: Potential therapeutic role of Co-Q10 in
alleviating intervertebral disc degeneration and suppressing
IL-1β-mediated inflammatory reaction in NP cells. Int
Immunopharmacol. 64:424–431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang G, Huang K, Dong Y, Chen S, Zhang J,
Wang J, Xie Z, Lin X, Fang X and Fan S: Lycorine suppresses
endplate-chondrocyte degeneration and prevents intervertebral disc
degeneration by inhibiting NF-kB signalling pathway. Cell Physiol
Biochem. 45:1252–1269. 2018. View Article : Google Scholar
|
|
41
|
Wang J, Pan H, Li X, Zhang K, Li Z, Wang
H, Zheng Z and Liu H: Hypoxia suppresses serum deprivation-induced
degradation of the nucleus pulposus cell extracellular matrix
through the JNK and NF-kB pathways. J Orthop Res. 35:2059–2066.
2017. View Article : Google Scholar
|
|
42
|
Xing F, Zhang W, Wen J, Bai L, Gu H, Li Z,
Zhang J, Tao YX and Xu JT: TLR4/NF-kB signaling activation in
plantar tissue and dorsal root ganglion involves in the development
of postoperative pain. Mol Pain. 14:17448069188070502018.
View Article : Google Scholar
|
|
43
|
Liao W, He X, Yi Z, Xiang W and Ding Y:
Chelidonine suppresses LPS-Induced production of inflammatory
mediators through the inhibitory of the TLR4/NF-κB signaling
pathway in RAW264.7 macrophages. Biomed Pharmacother.
107:1151–1159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun Z, Jian Y, Fu H and Li B: MiR-532
downregulation of the Wnt/β-catenin signaling via targeting Bcl-9
and induced human intervertebral disc nucleus pulposus cells
apoptosis. J Pharmacol Sci. 138:263–270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gruber HE and Hanley EN Jr: Analysis of
aging and degeneration of the human intervertebral disc. Comparison
of surgical specimens with normal controls. Spine (Phila Pa 1976).
23:751–757. 1998. View Article : Google Scholar
|
|
46
|
Zhang YH, Zhao CQ, Jiang LS and Dai LY:
Lentiviral shRNA silencing of CHOP inhibits apoptosis induced by
cyclic stretch in rat annular cells and attenuates disc
degeneration in the rats. Apoptosis. 16:594–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang W and Liang Z: Comparison between
annexin V-FITC/PI and Hoechst33342/PI double stainings in the
detection of apoptosis by flow cytometry. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 30:1209–1212. 2014.In Chinese. PubMed/NCBI
|
|
48
|
Zhang C, Kang C, Wang P, Cao Y, Lv Z, Yu
S, Wang G, Zhang A, Jia Z, Han L, et al: MicroRNA-221 and -222
regulate radiation sensitivity by targeting the PTEN pathway. Int J
Radiat Oncol Biol Phys. 80:240–248. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu Y, Bei Y, Shen S, Zhang J, Lu Y, Xiao J
and Li X: MicroRNA-222 promotes the proliferation of pulmonary
arterial smooth muscle cells by Targeting P27 and TIMP3. Cell
Physiol Biochem. 43:282–292. 2017. View Article : Google Scholar : PubMed/NCBI
|