Introduction
Human skin, the largest organ of the human body,
plays an important role in biological function and exerts critical
effects as a front-line defense system against various harmful
substances in the environment, including reactive oxygen species
(ROS) and UV-radiation, that can cause premature skin aging
(1). When skin is aged, it
becomes looser, more fragile and wrinkled (2,3).
Cell senescence is caused by multiple mechanisms, such as cell
growth arrest, increased activity of
senescence-associated-β-galactosidase (SA-β-gal), cell cycle arrest
and upregulation of cell cycle inhibitors, including p21 and p16
(4-7).
Skin consists of epidermal, dermal and subcutaneous
tissues, among which fibroblasts, a major cellular component of the
dermal tissue, play an important role in restoring tissue after
dermis injury (8). ROS include
the superoxide anion, hydroxyl radicals and hydrogen peroxide
(H2O2). According to the free radical damage
theory of senescence, premature senescence of human dermal
fibroblasts (hDFs) can be induced by a sublethal dose of
H2O2 (9).
Excessive free radicals can attack cellular components, causing
peroxidation of lipids, proteins and nucleic acids (10). Despite the existence of an
intracellular antioxidant enzyme system, excessive ROS can exceed
the clearance limit of antioxidant enzymes, leading to an
intracellular imbalance in oxidation and antioxidant activities,
and ultimately resulting in cell apoptosis or senescence (11-13). Superoxide dismutase (SOD) and
catalase (CAT) are key intracellular antioxidant enzymes that
contribute to the enzymolysis of superoxide anions and
H2O2 into water and oxygen, thereby delaying
cell senescence (14).
Mitochondrial DNA has a bare ring structure that lacks a protein
protection and damage repair systems, which makes it more
susceptible to damage (15). As a
result, it may directly affect the electron transfer process,
causing mitochondrial dysfunction resulting in increased leakage of
ROS, a byproduct of the respiratory chain and eventually, sustained
damage occurs (16).
Human amniotic epithelial cells (hAECs) and human
amniotic mesenchymal stem cells (hAMSCs) can be isolated from the
human amniotic membrane (hAM) and these cells have low
immunogenicity and secrete numerous growth factors (17). Various reports have shown that
hAM, hAECs and hAMSCs are used to treat wounds, burn lesions and
chronic ulcers (18,19). However, the purpose of the current
study was to compare the therapeutic potency of conditioned medium
(CdM) derived from hAM cells (hAMCs) on delaying senescence in
H2O2-induced premature senescence of hDFs and
to provide an experimental basis for the application of hAM cells
in the beauty industry.
Materials and methods
Isolation and culture of hDFs
Primary hDFs were derived from the healthy dermis of
three adult donors (age, 20-30 years; all male) undergoing surgical
debridement. All samples were negative for human immunodeficiency
virus-1, hepatitis B and hepatitis C virus infection. All samples
were obtained from August to October 2017 at the Shengjing Hospital
of China Medical University and written informed consent was
obtained from all patients. The experiments of the present study
were approved by the Ethics Committee of the Shengjing Hospital of
China Medical University (Shengyang, China). Fresh healthy skin
tissue was washed twice with PBS containing 100 U/ml penicillin and
100 U/ml streptomycin in a culture dish, and subcutaneous fat and
connective tissue was removed with scissors. The skin slice was
then cut into 1×1 cm pieces and placed in 15 ml centrifuge tubes,
followed by the addition of Dispase II enzyme solution (2 mg/ml;
Roche Diagnostics) before incubation at 4°C overnight. On the
second day, the epidermis was removed with tweezers. The dermis was
cleaned twice with sterile PBS, cut into 5×5 mm pieces, moved into
T25 culture flasks and were incubated at 37°C for 2 h to facilitate
tissue adhesion. Following incubation, 4 ml high glucose DMEM
(HyClone; GE Healthcare Life Sciences) containing 10% fetal bovine
serum (FBS; referred to as hDF; HyClone; GE Healthcare Life
Sciences) with penicillin (100 U/ml) and streptomycin (100 U/ml)
was added and plates were incubated at 37°C with 5% CO2.
At 80-90% confluence at the bottom of the flask, cells were
subcultured and the cell passage (P) was recorded as P0. hDFs at
P3-P5 were used for subsequent assays.
hAECs
Human amnions were obtained from healthy patients
undergoing caesarean sections at full term (39-40 weeks). The
procedures were approved by the Ethics Committee of the First
Affiliated Hospital of China Medical University. Written informed
consent was obtained from all patients prior to participation.
Human amnion samples were obtained from 6 females (age, 25-30
years) between December 2016 and April 2018. Amnion samples washed
three times with PBS to remove blood. Then, amnions were cut into
5×5 cm pieces, trypsin (0.25%; Gibco; Thermo Fisher Scientific,
Inc.) was added and samples were digested for 20 min at 37°C. The
digestive liquid was collected and added to DMEM/F12 (HyClone; GE
Healthcare Life Sciences) containing 10% FBS to terminate
digestion. The steps were repeated three times and subsequently,
the obtained single cell suspension was centrifuged at 140 × g for
10 min at room temperature and the supernatant was removed. The
precipitate was suspended in complete hAEC medium, containing
DMEM/F12 containing 10% FBS, 1% nonessential amino acids, 1%
L-glutamine and 10 ng/ml EGF (all Thermo Fisher Scientific, Inc.).
Cells were inoculated at 2.5×109 cells/l in 25
cm2 culture flasks before incubation in saturated
humidity at 37°C with 5% CO2. After 24 h, medium was
replaced and P0 was set when the cells had completely covered
bottom of the flask. hAECs at P2-P3 were used for subsequent
assays.
hAMSCs
Tissues after the isolation of hAECs were incubated
in 1 mg/ml collagenase IV (Sigma-Aldrich; Merck KGaA) at 37°C for
30 min. FBS (10%) was added to terminate digestion and the
supernatant was filtered using a cell strainer (200 μm)
before centrifugation at 140 × g for 10 min at room temperature.
Cells were then cultured in complete hAMSC medium, containing
DMEM/F12 containing 100 U/ml penicillin, 100 U/ml streptomycin and
10% FBS, and medium was replaced every 48 h. P0 was set when cells
completely covered the bottom of the flask. hAMSCs at P3-P6 were
used in subsequent experiments.
Flow cytometric analysis
P2 hAECs and P3 hAMSCs were collected, washed with
PBS, digested with trypsin (0.25%) at 37°C with 5% CO2
for 2 min and resuspended in 100 μl cold PBS at
106 cells/tube. Each samples was mixed with 5 μl
phycoerythrin-conjugated antibody against CD31 (cat. no. 303105),
CD34 (cat no. 343505), CD45 (cat. no. 368509), CD90 (cat. no.
32810), CD105 (cat. no. 323205), CD117 (cat. no. 313204), human
leukocyte antigen (HLA)-DR (cat. no. 307605) or stage-specific
embryonic antigen-4 (SSEA-4; cat. no. 330405; all BioLegend, Inc.)
PE-conjugated nonspecific mouse IgG was used as isotype control
(cat. no. 40011; BioLegend, Inc.). Samples were then incubated at
4°C for 30 min in the dark. After two washes with cold PBS, the
stem cell surface markers were detected using FACSDiva 6.2 (BD
Biosciences); 10,000 cells were used for each test.
Cell immunofluorescence staining
P3 hDFs were seeded in a 12-well plate with a glass
slide at 4×104 cells/well. At 80% confluence, culture
medium was discarded and cells were fixed with 4% paraformaldehyde
at room temperature for 30 min and washed with PBS twice.
Afterwards, Triton X-100 (0.1% in PBS) was allowed to permeate the
cells for 10 min at room temperature. Then, cells were blocked with
bovine serum albumin (5%; Beijing Solarbio Science & Technology
Co., Ltd.) for 30 min at room temperature. Vimentin primary
antibody (rabbit anti-human; cat. no. abp52697; 1:200; Abbkine
Scientific, Co., Ltd.) was added and samples were incubated at 4°C
overnight. Cells were washed three times with PBS on the second
day, followed by addition of the secondary fluorescent antibody
(Alexa 488; donkey anti-rabbit; cat. no. A-11034; 1:200; Thermo
Fisher Scientific, Inc.) and incubation at room temperature for 1 h
in the dark. Cell nuclei were stained with DAPI diluted in PBS
(1:1,000) for 5 min at room temperature, followed by dilution with
PBS (1:1,000). Images were recorded with an inverted fluorescence
microscope (magnification, ×100).
Preparation of CdM
To obtain CdM, hAECs or hAMSCs were cultured in
complete hAEC or hAMSC medium until 60-70% confluence. After two
washes with PBS, cells were cultured in 10% FBS in high glucose
DMEM for 48 h. Cells were then centrifuged at 1,000 × g for 5 min
at room temperature; supernatants were labeled CdM-hAEC or
CdM-hAMSC and mixed with fresh growth hDF medium (1:1) to be
labeled as CdM1/2-hAEC or CdM1/2-hAMSC. CdM was stored in small
sized tubes at −80°C.
H2O2 and CdM
treatments
For establishment of the premature senescence model,
hDFs were treated with 50, 100, 200 and 400 μM
H2O2 (in 10% FBS in high glucose DMEM) at
37°C with 5% CO2 for 1 or 2 h. Cells were then washed
with serum-free high glucose DMEM twice to remove residual
H2O2 (20,21) and cultured in high glucose DMEM
with 10% FBS for 4 days; growth medium changed every 48 h. In the
CdM treatment group, H2O2-treated cells (200
μM, 1 h) were washed with serum free medium and incubated
with CdM for 4 days. To ensure sufficient nutrients were available,
CdM was changed every 24 h.
Cell proliferation assay
hDFs were seeded in 96-well plates at
5×103 cells/well and incubated in growth medium for 24 h
for adherence. Then, H2O2-treated cells (50,
100, 200 and 400 μM, for 1 or 2 h) and cells of the control
group were continued to be cultured in growth medium for 4 or 7
days to test the sustained effect of H2O2 on
cell proliferation, while cells in the CdM group were incubated
with 200 μl CdM for 4 days. A cell proliferation assay was
performed using CellTiter 96® AQueous One Solution Cell
Proliferation assay (Promega Corporation). According to the
manufacturer's instructions, 100 μl serum-free high glucose
DMEM from each plate was mixed with 20 μl MTS and incubated
for 1 h at 37°C with 5% CO2. The OD was measured at 490
nm. Cell viability was assessed according to the following formula:
Viability (%)=OD of experimental group/OD of control group
×100.
Cell viability using fluorescein
diacetate (FDA) staining
FDA (Sigma-Aldrich; Merck KGaA) was used to visually
present living cells. According to the manufacturer's instructions,
5×104 cells/well were seeded in 12-well plates and
incubated in growth medium for 24 h for adherence. Then, cells of
the control and H2O2 group were continued to
grow in growth medium, while cells in the CdM group were incubated
with 1 ml CdM for further 4 days. Then, hDFs were washed twice with
PBS before incubation at 37°C with 5% CO2 for 15 min in
FDA solution (10 μg/ml; in acetone). After that, cells were
washed twice with PBS and cells were observed and five randomly
chosen fields were taken using an inverted fluorescence microscope
(magnification, ×100).
Scratch wound closure assay
A scratch test was used to analyze the migration
abilities of hDFs treated with CdM. P3 hDFs were seeded in a 6-well
plate at 5×104 cells/well. At 80-90% confluence,
scratches with 0.4-0.5 mm width were inflicted using sterile
suction tips. Then, each well was washed twice with PBS to remove
loose cell fragments. After that cells incubated with 10% FBS in
high glucose DMEM were labeled as the control. Cells were cultured
in the respective CdM were labeled as CdM-hAEC and CdM-hAMSC
groups. Cell migration was observed using an inverted microscope
(magnification, ×20) after 0, 12 and 24 h. Results were expressed
as: Migration (%) = [Wound area (initial)-Wound area (final)]/Wound
area (initial) ×100.
SA-β-gal staining
H2O2-treated hDFs were seeded
in 12-well plates at 5×104 cells/well and incubated in
growth medium for 24 h for adherence. Then, cells of the control
and H2O2 group were continued to grow in
growth medium, while cells in the CdM group were incubated with 1
ml CdM for further 4 days. Cells were washed with PBS, cells were
stained using the SA-β-gal staining kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Briefly, cells were immobilized at room temperature with β-gal
fixative for 15 min, followed by three washes with PBS for 3 min
and 500 μl staining working solution were added before cells
were incubated overnight at 37°C in a CO2-free
incubator. Pictures from five consecutive fields of view were
randomly selected; pictures were taken with inverted microscope
(magnification, ×20). The percentage of SA-β-gal-positive cells was
calculated as: SA-β-gal positive cells/total cells ×100.
Cell cycle
Cell cycle distribution was detected using flow
cytometry with PI staining. Briefly,
H2O2-treated hDFs were seeded in 6-well
plates at 1.2×105 cells/well. Cells of the control and
H2O2 group were continued to be cultured in
hDF, while cells in the CdM group were incubated with 2 ml CdM for
further 4 days. Then, cells were suspended, washed with PBS and
fixed in 70% ethanol at 4°C for 24 h. Cells were then suspended in
300 μl dyeing solution (500 μl buffer containing 10
μl RNase A and 25 μl PI) and incubated at 37°C for 30
min in the dark. A total of 1×104 cells/sample were
subjected to cell cycle analysis using FACS with the FACSDiva 6.2
(BD Biosciences).
Detection of SOD, CAT and malondialdehyde
(MDA)
To evaluate the effect of CdM on antioxidant enzyme
activity after H2O2 treatment, the levels of
intracellular total SOD (cat. no. A001-3-2), MDA (cat. no.
A003-4-1) and CAT (cat. no. A007-1-1) in the supernatant of
cultured cells were detected using corresponding kits (all Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
instructions. In brief, cells (1.2×105) were seeded in
6-well plates, control and H2O2 group were
continued to be cultured in hDF, while cells in the CdM group were
incubated with 2 ml CdM for further 4 days. Then, cells were washed
twice with PBS and 200 μl RIPA buffer (Beyotime Institute of
Biotechnology) was added to each well of a 6-well plate to lyse the
cells on ice for 10 min. Then, cells were collected and centrifuged
at 3,000 × g for 10 min at 4°C. The OD was detected at 450 nm using
20 μl supernatant for SOD, at 530 nm using 100 μl for
MDA and at 405 nm using 100 μl for CAT. A total of 25
μl supernatant was used for protein content determination
(BCA Protein Assay kit; Tiangen Biotech Co., Ltd.). Enzyme activity
was calculated based on the manufacturer's instructions: SOD
activity (U/mg protein)=inhibition ratio/50% × reaction
system/dilution multiple/protein concentration (mg/ml). MDA content
(nmol/mg protein)=OD value-blank OD value/standard OD value-blank
OD value × standard concentration (10 nmol/ml)/protein
concentration (mg/ml). CAT activity (U/ml) = (control OD
value-measured OD value) × 235.65 × 1/(60 × sampling quantity) ×
dilution multiple before testing.
Detection of ROS levels
Intracellular ROS levels in hDFs were measured with
using the Reactive Oxygen Species Assay kit (Beyotime Institute of
Biotechnology). According to the manufacturer's instructions, cells
were suspended in 10 μmol/l 2′,7′-dichlorodihydrofluorescein
diacetate (DCFH-DA; in serum-free medium) and incubated for 20 min
at 37°C with 5% CO2; cells were mixed every 5 min during
incubation. After three washes with serum-free medium, fluorescence
images were taken with an inverted fluorescence microscope
(magnification, ×100).
Reverse transcription-quantitative
(RT-q)PCR analysis
Total RNA was isolated from hDFs after exposure to
H2O2 for 1 h and co-culture with CdM for 4
days using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA with an A260/A280 of
1.8-2.0 was considered pure and used for subsequent assays. cDNA
was synthesized from 1 μg total RNA using GoScript™ RT kit
(cat. no. A2791; Promega Corporation); the following conditions
were applied: 70°C for 5 min followed by 25°C for 5 min, 42°C for
60 min and 70°C for 15 min. qPCR was performed using the SYBR
Premix Ex Taq™ II kit (Takara Bio, Inc.) and the thermocycling
conditions were as follows: 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec and 60°C for 34 sec. GAPDH was used as internal
control. Results were calculated using the 2−ΔΔCq method
(19). Each sample was analyzed
in triplicate. Primer sequences were as follows: p21, forward,
5′-CTG GAG ACT CTC AGGG TCGA A-3′ and reverse, 5′-CCA GGA CTG CAG
GCT TCC T-3′; GAPDH, forward, 5′-ACC CAC TCC TCC ACC TTT GAC-3′ and
reverse, 5′-GTC CAC CAC CCT GTT GCT G-3′.
Western blotting
The control, H2O2 and CdM
groups were washed three times with cold PBS and lysed on ice with
RIPA buffer containing a protease inhibitor (Roche Diagnostics).
The BCA Protein Assay kit was used for protein quantification.
Samples were separated on SDS-PAGE gels and transferred to
polyvinylidene fluoride membranes. Membranes were blocked at room
temperature for 2 h with 5% non-fat milk and then incubated with
primary antibodies: p21 and H2AX (cat. nos. 10355-1-AP and
10856-1-AP, respectively; 1:1,000; ProteinTech Group, Inc.), p16
(cat. no. WL01418; 1:1,000; Wanleibio, Co., Ltd.), γ-H2AX (22) and β-actin (cat. nos. ab2893 and
ab8226, respectively; 1:1,000; Abcam) at 4°C overnight. Membranes
were then incubated with an HRP-conjugated goat anti-rabbit IgG
(cat. no. SA00001-2; 1:2,500) or goat anti-mouse IgG (cat. no.
SA00001-1; 1:2,500; all ProteinTech Group, Inc.) for 1 h at room
temperature. Immunoreactive bands were visualized using the
SuperSignal West Pico Chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc.) using LAS-3000 mini (Fuji). β-actin was
used as an internal control. The bands were analyzed by using Image
J software version 1.48 (National Institutes of Health).
ELISA
8-hydroxydeoxyguanosine (8-OHdG) was detected as a
marker of DNA damage using the 8-OHdG ELISA kit (cat. no. BYE10099;
Shanghai Bangyi Biotechnology). The control,
H2O2 group and CdM groups were washed twice
with PBS followed by the addition of 100 μl RIPA buffer
(Beyotime Institute of Biotechnology) and centrifugation at 3,000 ×
g for 20 min at 4°C. A total of 40 μl sample diluent
provided with the kit and 10 μl sample were added to the
enzyme-labeled coating plate before sealing and incubation at 37°C
for 30 min. According to the manufacturer's instructions, the OD at
450 nm was determined.
Statistical analysis
Data are presented as the mean ± standard deviation.
All assays were repeated three times. Comparison between two groups
was performed using an independent sample t-test or for multiple
groups one-way ANOVA followed by Tukey's test. All statistical
analyses were performed with Graph Pad Prism 5.0 (GraphPad
Software, Inc.) and SPSS 19.0 (IBM Corp.). P<0.05 was considered
to indicate statistical significant difference.
Results
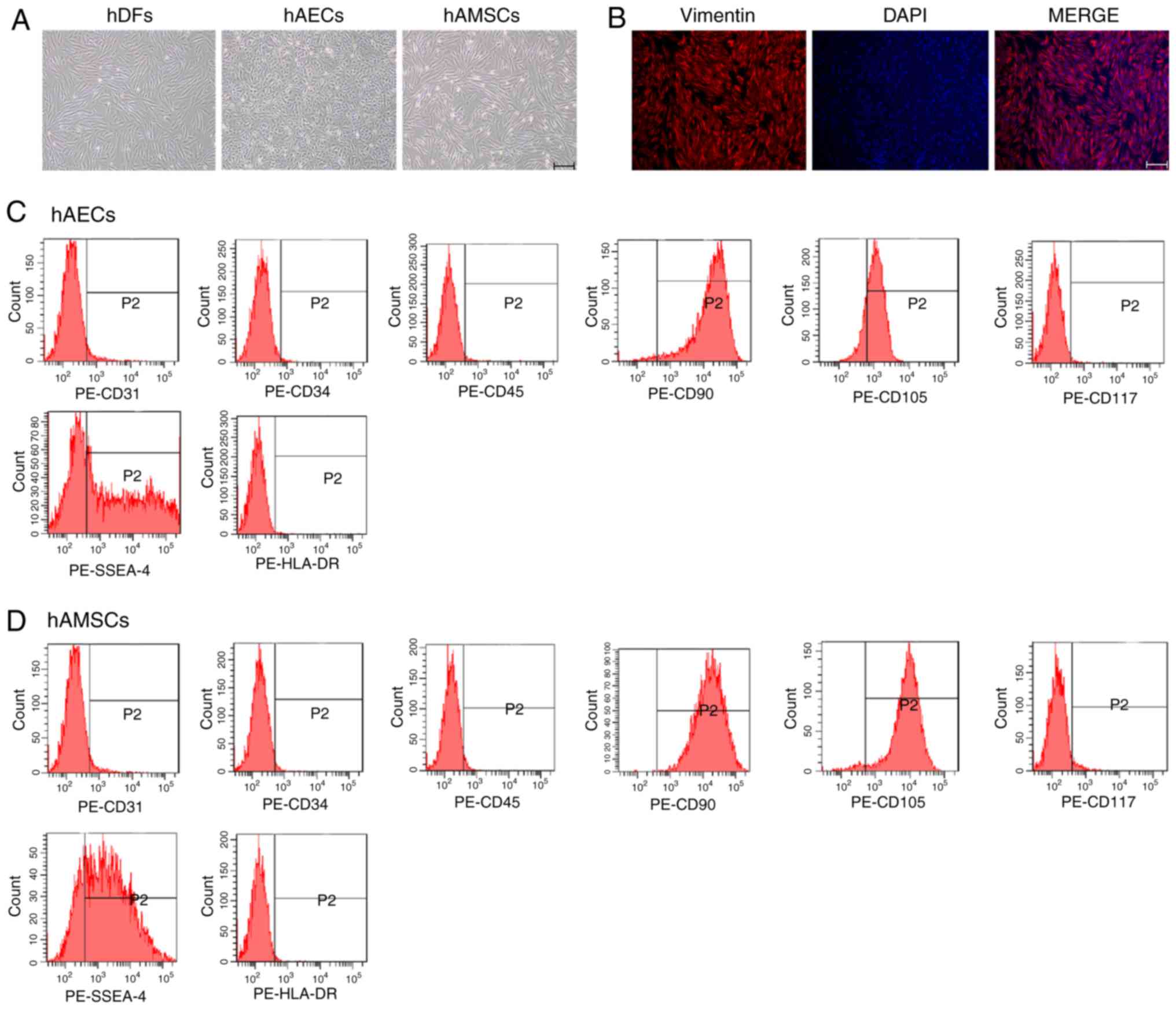
Characterization of hDFs, hAECs and
hAMSCs
hDFs were spindle shaped and showed typical
fibroblast morphology (Fig. 1A).
Immunofluorescence analysis demonstrated that the cells were
positive for the hDF surface marker vimentin (Fig. 1B) (23). hAECs and hAMSCs were isolated from
hAMs and hAECs exhibited cobblestone-like morphology (Fig. 1A), the typical morphology of hAECs
(19). The morphology of hAMSCs
was similar to that of fibroblasts, as fibrous cell morphology was
observed in the plastic plates (Fig.
1A). Flow cytometry was used to detect the expression of
surface markers on hAECs and hAMSCs. hAECs and hAMSCs expressed
CD90 and CD105, but did not express CD31, CD34, CD45 or the
hematopoietic progenitor cell marker CD117. In addition, SSEA-4 was
expressed by hAMSCs but not by hAECs, and immune-related marker
HLA-DR was not expressed by either cells (Fig. 1C and D). These results were
consistent with observations from previous reports (24,25).
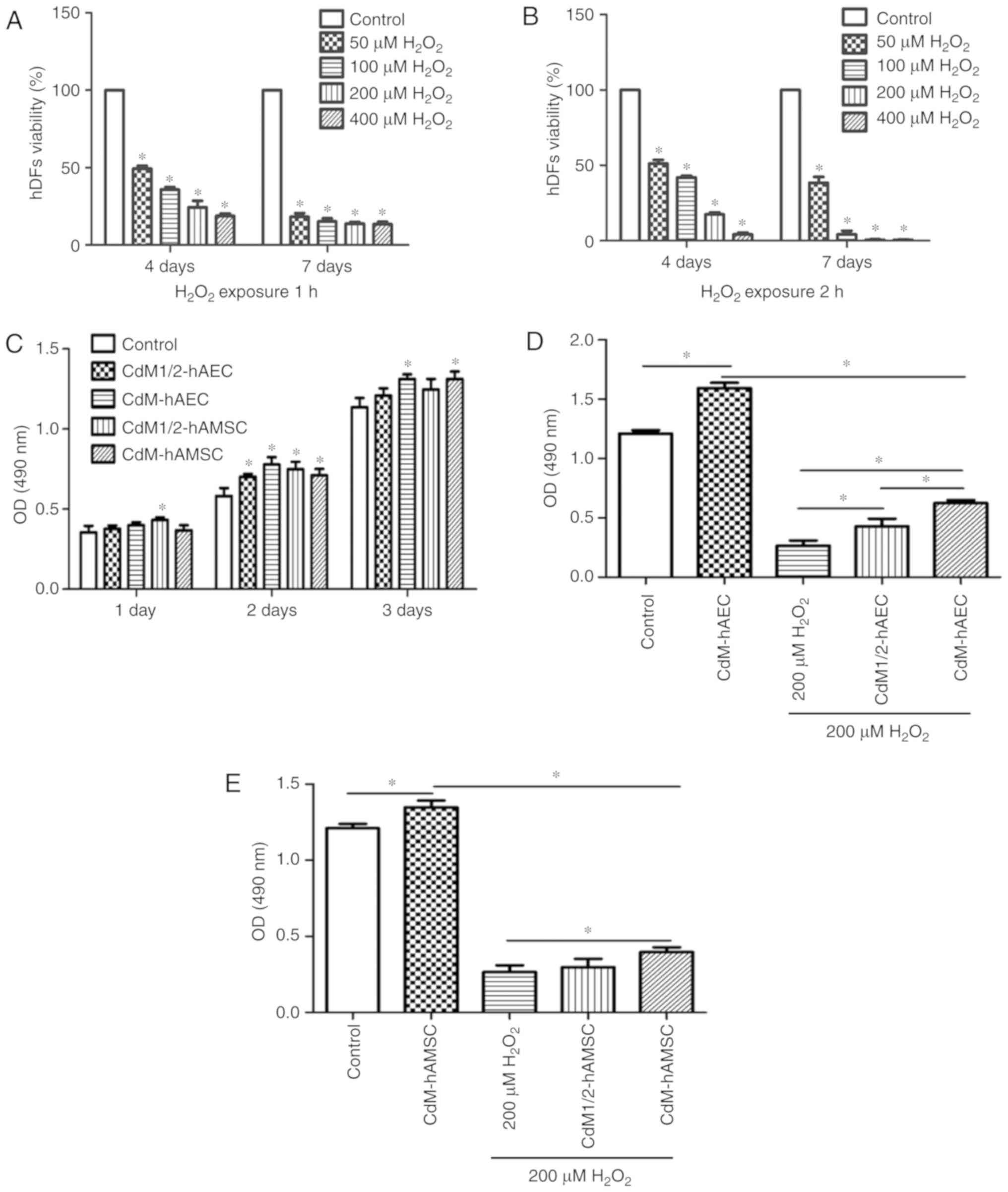
CdM alleviated the inhibitory effect of
H2O2 on hDFs growth
To evaluate effects of H2O2 on
hDFs, 50, 100, 200 and 400 μM H2O2 was
used to treat hDFs for 1 or 2 h, followed by 4 and 7 day culture.
Based on MTS assay results, after 1 and 2 h
H2O2 treatment at varying concentrations, the
cell viability was significantly reduced compared with the control
(P<0.05; Fig. 2A and B). After
culturing for 4 days following 1 h treatment, cell viability was
49.30±1.57, 35.87±1.20, 24.30±3.46 and 18.71±1.31% in the 50, 100,
200 and 400 μM groups, respectively. Following 7 days of
culture after 1 h H2O2 treatment, cell
viability was 18.21±1.91% in the 50 μM group, 15.23±1.56% in
the 100 μM group, 13.78±0.89% in the 200 μM group and
13.50±1.15% in the 400 μM group. After
H2O2 treatment for 2 h followed by 7 days of
culturing, for 50, 100, 200 and 400 μM
H2O2 groups the cell viability was
51.14±2.01, 41.94±0.88, 17.49±1.12, and 4.21±0.95%, respectively.
Following seven-day culture, cell viability was 38.41±3.24,
4.22±1.94, 0.44±0.34 and 0.38±0.31% for the 50, 100, 200 and 400
μM groups, respectively. The results revealed that
H2O2 treatment for 1 h and 2 h caused damage
even after prolonged periods of culturing.
To study the effect of CdM derived from hAM cells on
the proliferation of hDFs, cells were cultured with CdM1/2 or CdM
from hAMSCs or hAECs for 1 day. Results indicated that the
CdM1/2-hAMSC group exhibited significantly greater cell
proliferation compared with the control group at the 1 and 2 day
measurements (P<0.05; Fig.
2C). In the 2 day experiments, the determined OD values for
CdM1/2-hAEC, CdM-hAEC, CdM1/2-hAMSC and CdM-hAMSC were 0.70±0.02,
0.78±0.04, 0.75±0.04 and 0.71±0.03, respectively, all significantly
increased compared with the control group (0.58±0.04; P<0.05;
Fig. 2C). In the 3 day
measurement, OD values of CdM-hAEC and CdM-hAMSC were significantly
higher compared with the control (P<0.05; Fig. 2C), while the 50% CdM showed no
signifi-cant improvement compared with the control. To explore
whether CdM alleviated the damage caused by
H2O2, two concentrations of CdM were tested
as medium for cells following 200 μM
H2O2 exposure for 1 h. The results showed
that the effect in the 200 μM
H2O2+CdM-hAEC group was significantly
improved compared with the 200 μM
H2O2+CdM1/2-hAEC group and cell proliferation
was significantly promoted compared with the 200 μM
H2O2 control group (P<0.05; Fig. 2D). In comparison with the 200
μM H2O2 group, cell proliferation was
significantly promoted in the 200 μM
H2O2+CdM-hAMSC group (P<0.05; Fig. 2E), while 1/2CdM-hAMSC did not
exhibit a significant improvement.
Effect of CdM on living cell density is
assessed using FDA probes
FDA staining was used to trace density changes in
living cells to verify results of proliferation experiments and
account for influences of cells with poor viability. CdM was found
to promote proliferation of hDFs after H2O2
treatment (Fig. 3A). Fluorescence
images revealed that the cell density was markedly higher in the
200 μM H2O2+CdM-hAEC and the 200
μM H2O2+CdM-hAMSC group compared with
the 200 μM H2O2 group. The strongest
effect was observed for the 200 μM
H2O2+CdM-hAEC group. These results were
consistent with cell proliferation data.
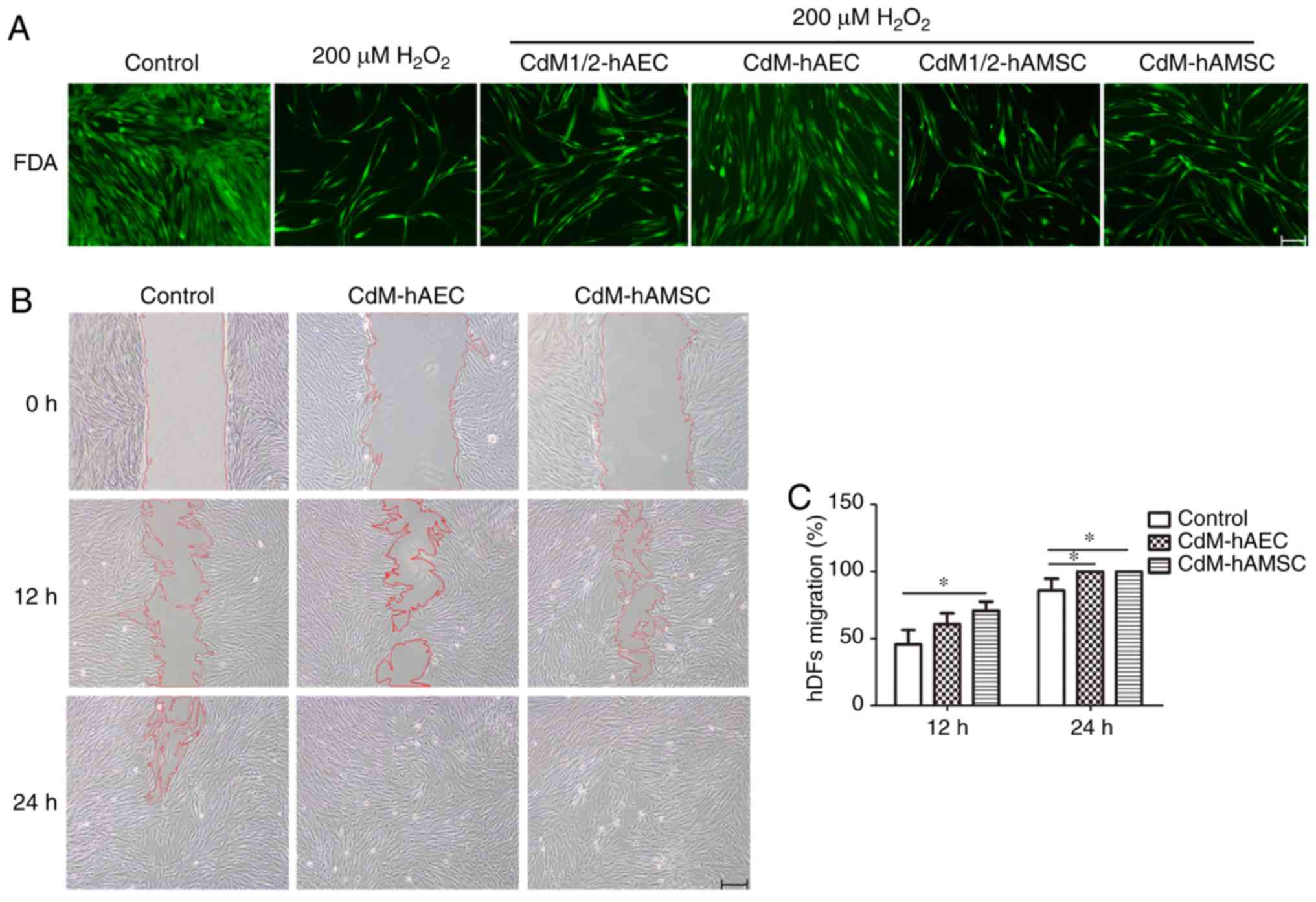 | Figure 3Effect of CdM on hDF proliferation
and migration. (A) hDFs were treated with 200 μM
H2O2 for 1 h and cultured in CdM1/2-hAEC,
CdM-hAEC, CdM1/2-hAMSC or CdM-hAMSC for 4 days. Cell density was
observed in FDA stained fluorescence images. Scale bar, 50
μm. (B) Migration of hDFs following scratch at 0, 12 and 24
h of culture in CdM-hAEC or CdM-hAMSC. Scale bar, 200 μm.
(C) Rate of wound healing. *P<0.05.
H2O2, hydrogen peroxide; CdM, conditioned
medium; hDF, human dermal fibroblast; hAEC, human amniotic
epithelial cell; hAMSC, human amniotic mesenchymal stem cell;
CdM1/2, 50% CdM; FDA, fluorescein diacetate. |
CdM enhances the migration ability of
hDFs
To examine whether CdM-hAEC and CdM-hAMSC exhibited
biological effects relevant to hDF migration, a scratch assay was
conducted. hDFs were imaged after 12 and 24 h in CdM-hAEC and
CdM-hAMSC and results are shown in Fig. 3B. The migration into the wound was
accelerated compared with that of the control group. After 12 h of
culture, the migration percentage in the CdM-hAMSC group was
significantly higher compared with the control. After 24 h of
culture, the migration percentage in the CdM-hAEC and the CdM-hAMSC
groups were significantly higher compared with the control
(P<0.05; Fig. 3C) and the
observed effect was superior compared with the CdM-hAEC group.
CdM from hAECs affects cell distribution
in the S phase after H2O2 exposure
Cell cycle distribution was determined by flow
cytometry. The results showed that the percentage of cells in the S
phase significantly decreased in the 200 μM
H2O2 group compared with the control
(P<0.05; Fig. 4A and B).
CdM-hAEC significantly increased the percentage of cells in the S
phase compared with the 200 μM H2O2
group (P<0.05); effects of CdM-hAMSC were not significant. No
significant changes in the G0/G1 or the G2/M phases were
observed.
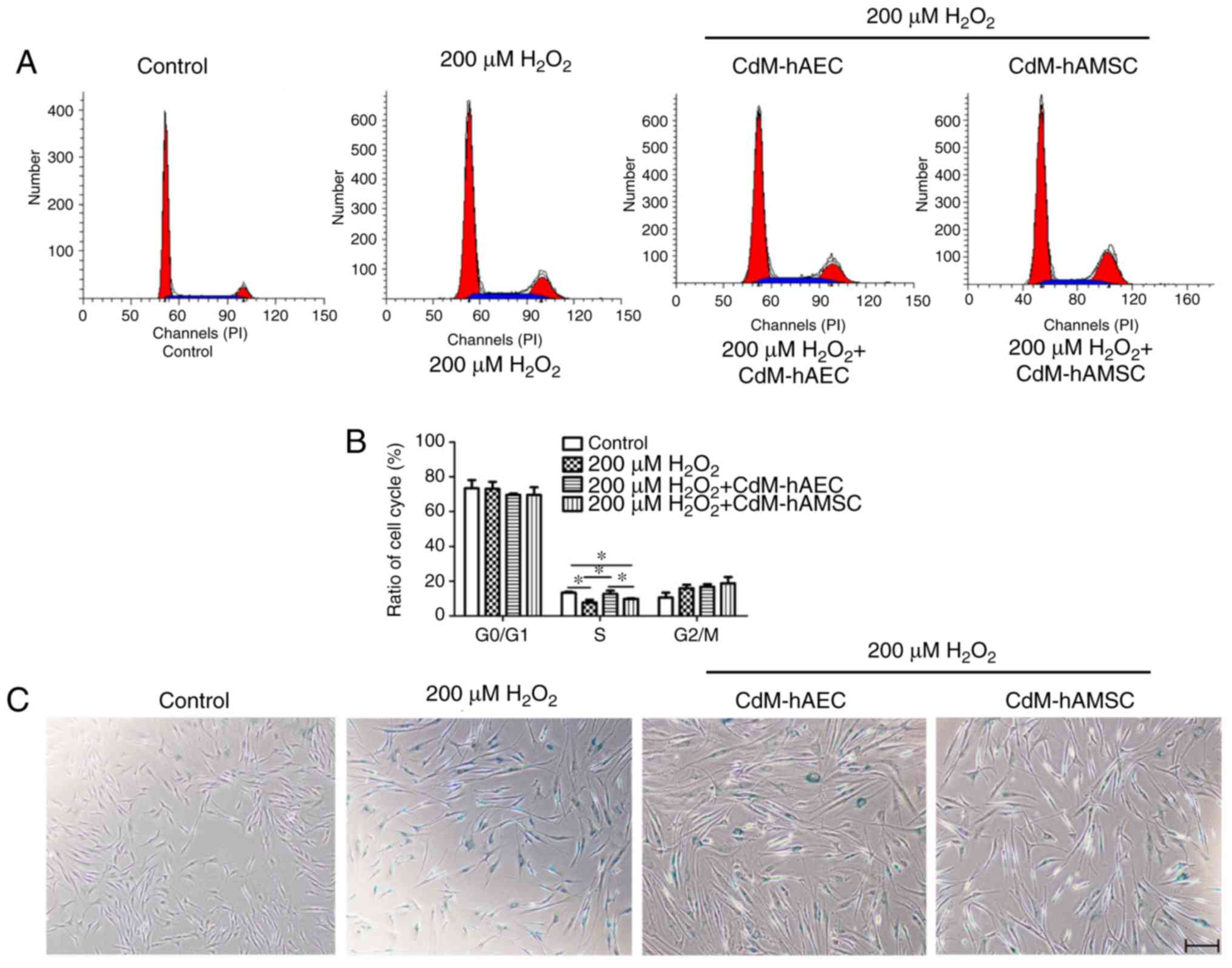 | Figure 4Treatment with CdM alleviate
H2O2-induced premature senescence. hDFs were
treated with 200 μM H2O2 for 1 h and
cultured in CdM-hAEC or CdM-hAMSC for 4 days. (A) Flow cytometric
analysis of the cell cycle and (B) determined cell cycle
distribution of hDFs. (C) Representative images for SA-β-gal
staining (blue, cells positive for senescence). Scale bar, 200
μm. (D) Quantification of SA-β-gal-positive cells. (E) ROS
levels determined using DCFH fluorescence. Scale bar, 50 μm.
(F) Quantified ROS fluorescence intensity. Data are presented as
the mean ± standard deviation (n=3). *P<0.05.
H2O2, hydrogen peroxide; CdM, conditioned
medium; hDF, human dermal fibroblast; hAEC, human amniotic
epithelial cell; hAMSC, human amniotic mesenchymal stem cell;
SA-β-gal, senescence-associated-β- galactosidase; ROS, reactive
oxygen species. |
CdM from hAECs and hAMSCs reduce
H2O2 induced HDFs senescence
A senescence-specific SA-β-gal staining assay was
conducted to observe hDFs treated with H2O2.
The results showed that the number of positive cells was
significantly increased in the 200 μM
H2O2 group compared with the control group
(67.0±4.87 vs. 1.0±0.16%; P<0.05; Fig. 4C and D). After treatment with 200
μM H2O2 for 1 h and culturing in
CdM-hAEC or CdM-hAMSC for 4 days, the percentage of positive cells
were 41.75±2.49 and 44.43±3.20%, respectively (Fig. 4C and D), both significantly
decreased compared with the 200 μM
H2O2 group (P<0.05).
CdM from hAECs and hAMSCs alleviates
oxidative stress induced by H2O2
The generation of ROS was determined using the
ROS-specific fluorescent dye DCFH-DA. The results showed that
treatment of hDFs with H2O2 for 1 h, ROS
levels were significantly higher compared with the control group
(P<0.05; Fig. 4E and F). In
addition, using CdM-hAEC and CdM-hAMSC as culture medium after
H2O2 exposure significantly reduced the level
of ROS compared with the 200 μM H2O2
group (P<0.05; Fig. 4E and F).
Furthermore, ELISA results showed that for hDFs treated with
H2O2 for 1 h, activities of SOD and CAT were
significantly decreased and the concentration of MDA was
significantly increased compared with the control group (P<0.05;
Table I).
 | Table ILevels of total-SOD, CAT and MDA in
hDFs. |
Table I
Levels of total-SOD, CAT and MDA in
hDFs.
| Group | Total-SOD (U/mg
protein) | CAT (U/ml) | MDA (nmol/mg
protein) |
|---|
| Control | 78.4±2.5 | 1.29±0.07 | 18.2±2.2 |
| 200 μM
H2O2 | 32.7±2.4a | 0.15±0.05a | 101.0±1.1a |
| 200 μM
H2O2+CdM-hAEC | 82.1±8.4b |
1.62±0.11a,b | 8.5±1.8b |
| 200 μM
H2O2+CdM-hAMSC |
43.1±2.9a,c |
0.60±0.08a-c |
44.2±8.0a-c |
Next, 8-OHdG and γ-H2AX levels were assessed,
reflecting the extent of DNA damage caused by oxidation (26,27). H2O2
treatment significantly increased 8-OHdG levels compared with the
control group (P<0.05; Fig.
5A). Culturing in CdM-hAEC and CdM-hAMSC after
H2O2 treatment significantly decreased the
8-OHdG levels in hDFs detected by ELISA compared with the 200
μM H2O2 group (P<0.05; Fig. 5A). Levels of γ-H2AX and H2AX were
measured by western blot to analyze the therapeutic efficacy of CdM
after H2O2 treatment. Results showed that
γ-H2AX levels were significantly upregulated after
H2O2 treatment compared with the control. For
hDFs cultured in CdM-hAEC and CdM-hAMSC after peroxide challenge,
γ-H2AX levels were significantly decreased and H2AX levels were
markedly upregulated compared with the 200 μM
H2O2 group (P<0.05; Fig. 5C and F). The ratio of γ-H2AX/H2AX
is indicative of the phosphorylation level and 41.0±4.2, 476.0±8.5,
80.0±1.8 and 98.0±5.7% were determined for the control, the 200
μM H2O2, the CdM-hAEC and the
CdM-hAMSC groups (Fig. 5G).
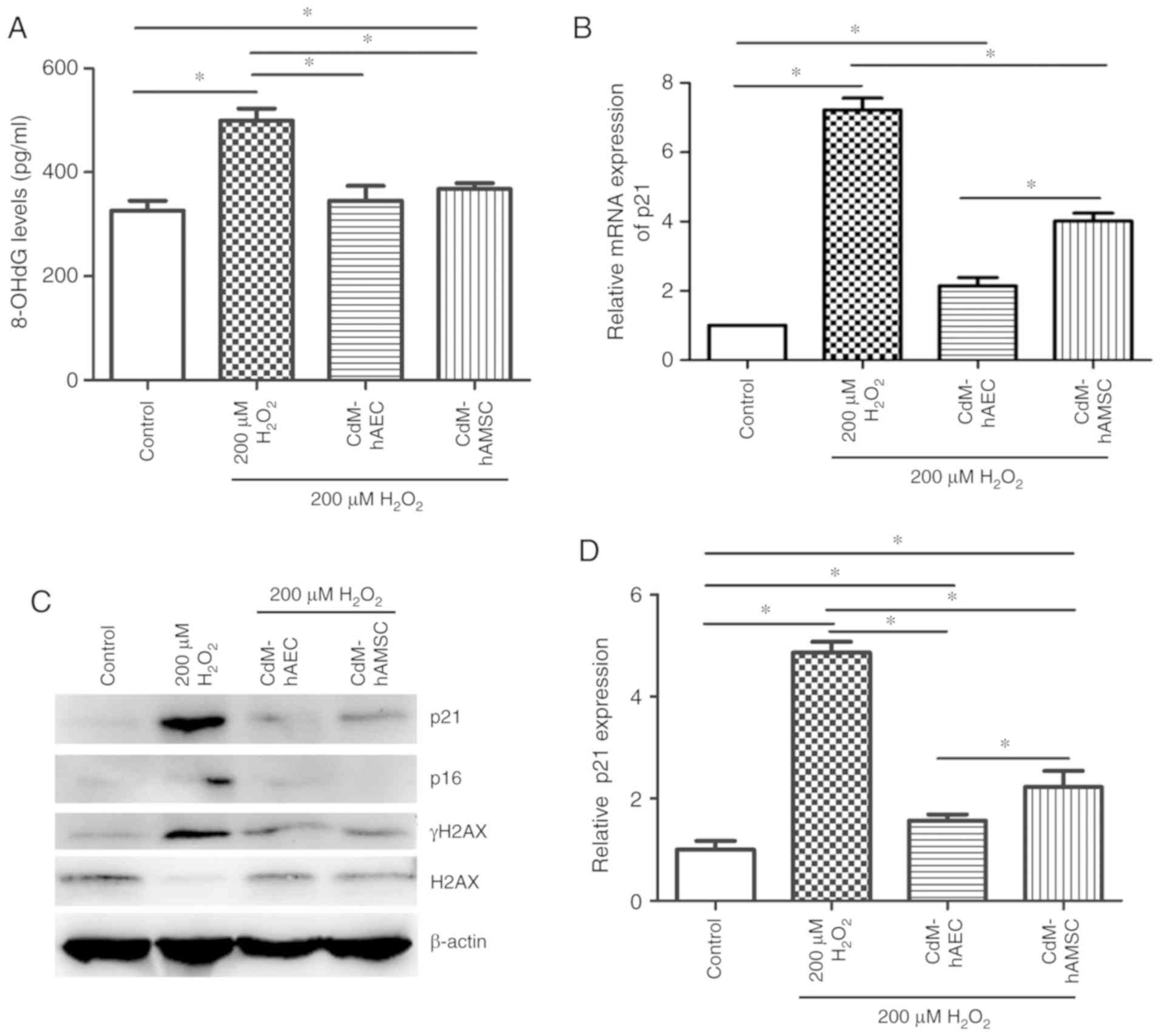 | Figure 5CdM affects DNA damage, and mRNA and
protein expression in H2O2-treated hDFs. (A)
8-OHdG levels in hDFs measured using ELISA. (B) p21 mRNA expression
determined by reverse transcription-quantitative PCR. (C) Western
blot images and quantification of (D) p21, (E) p16, (F) γ-H2AX
levels, as well as (G) the ratio of γ-H2AX to H2AX. β-actin was
used as the internal control. Data are presented as the mean ±
standard deviation (n=3). *P<0.05.
H2O2, hydrogen peroxide; CdM, conditioned
medium; hDF, human dermal fibroblast; hAEC, human amniotic
epithelial cell; hAMSC, human amniotic mesenchymal stem cells;
8-OHdG, 8-hydroxydeoxyguanosine. |
CdM from hAECs and hAMSCs reduces p21 and
p16 expres- sion in H2O2-induced premature
senescence in hDFs
p21 and p16 are transcription factors and
overexpression of p21 and p16 has previously shown to cause
premature cell senescence (28).
Expression of p21 and p16 was assessed by RT-qPCR and western
blots. The results of RT-qPCR and western blot analysis showed that
p21 and p16 expression was significantly upregulated in 200
μM H2O2-treated group compared with
control untreated group (P<0.05; Fig. 5B-E). mRNA levels of p21 and
protein levels of p21 and p16 were significantly reduced after CdM
treatment when compared with the 200 μM
H2O2 group (P<0.05; Fig. 5B-E).
Discussion
Stem cells derived from hAM have several advantages
for beauty therapy, including that hAM is usually not utilized
after delivery, small pieces of membrane can contain high levels of
stem cells with easy expandability and hAM has low immunogenicity
(29,30). These points provide favorable
conditions for the application of hAM cells in cosmetic
products.
In this study, effects of CdM-hAEC and CdM-hAMSC on
H2O2-induced premature senescence in hDFs
were examined. Previous reports have shown that hAMSCs protect
against UVA irradiation-induced hDF senescence (31). The results of the presents study
demonstrated that treatment with CdM reversed cellular senescence
induced by H2O2. Cell proliferation and
migration experiments were performed to evaluate effects of CdM on
the biological function of hDFs. CdM promoted hDF proliferation in
a dose-dependent manner and significantly promoted cell migration
(24,32).
Oxidative and antioxidant systems co-exist in cells
to maintain ROS balance, which may damage the cells, weaken cell
function and result in premature cell senescence (33). SOD and CAT hydrolyze ROS and
attenuate cell damage caused by free radicals, and MDA is an
indicator of lipid peroxidation, which amplifies the ability of ROS
to cause cell damage (34,35).
Numerous studies have indicated that H2O2
increases the level of ROS in hDFs and attenuates the activity of
antioxidant enzymes (36,37). In this study, it was discovered
that using CdM after H2O2 treatment increased
the activity of total-SOD and CAT, and decreased the levels of MDA
and intercellular ROS in hDFs. Additionally, the oxidative
stress-induced premature senescence phenotype was ameliorated by
retaining the oxidation-reduction equilibrium.
Previous research has shown that activation of the
DNA damage response (DDR) pathway is triggered by a continued high
concentration of ROS (38). In
case of failure to repair DSBs in time, this pathway activates the
downstream signaling pathways, resulting in cell apoptosis or
senescence (39). In addition,
sustained DSB damage signals activate the ATM-Chk2-p53-p21-pRb and
the p38-p16INK4α signaling pathways (40), which maintain the cell senescence
phenotype and block cell cycle progression (41). Results of the present study showed
that a significant reduction in intracellular ROS after CdM
treatment compared with the 200 μM
H2O2 group. p21 and p16 inhibit cell cycle
regulation by affecting the activity of various cyclin-dependent
kinases, which eventually block the cell cycle (42). In addition, mRNA levels of p21
were also significantly reduced. 8-OHdG and γ-H2AX are sensitive
damage markers of DSBs and both markers are commonly used to detect
DNA base and nucleotide damage (43,44). H2AX is an important member of the
H2A histone family. The H2A protein family includes H2A1-H2A2, H2AZ
and H2AX. H2AX phosphorylation is a key step in the DDR, playing a
role in signaling and initiating the repair of DSBs (45). H2AX phosphorylation creates an
epigenetic signal that is recognized by specific domains on
downstream DDR proteins. Accumulation of these proteins depends on
H2AX phosphorylation but the accumulation rate can differ (46). The current study showed that H2AX
phosphorylation increased in the presence of
H2O2 and CdM reversed the observed
phosphorylation potentially through enhanced dephosphorylation. One
mechanism relies on the dephosphorylation of γ-H2AX by phosphatase
2A and phosphatase 4C (47,48). The other mechanism of γ-H2AX
removal is through redistribution in the chromatin involving
histone exchange and replacing γ-H2AX with H2AZ during chromatin
remodeling, mediated by histone acetyltransferases (49,50). Previous studies suggested that
transplanted AMSCs increase the activity of antioxidant enzymes and
reduce DNA damage in a premature ageing model (51). According to results of this study,
CdM alleviated DNA damage caused by hydrogen peroxide. Therefore,
CdM may delay aging caused by oxidative stress in cells.
In conclusion, utilizing CdM was a novel cell-free
treatment strategy for potential anti-aging applications, supported
by evidence indicating that healing effects of stem cells are
associated with their paracrine properties (52). The extraction of secretory
proteins from the CdM from adipose stem cells has previously been
injected into human skin for facial anti-aging (53). CdM from hAECs and hAMSCs used here
contained various growth factors, cytokines and proteins that
improved cell function. Other reports have shown that hAM cells
promote senescent hDF function (31). However, little is known about the
components in the CdM that make it resistant to damage from
H2O2 and delay cell aging. Future work may
concern high-throughput sequencing of hAM derived cells coupled to
simultaneous mass spectrometry analyses of the CdM to identify
differentially expressed proteins. The present results demonstrated
that CdM-hAEC and CdM-hAMSC delayed
H2O2-induced premature senescence of hDFs and
the CdM potentially can be applied in the field of anti-aging.
Acknowledgements
Not applicable.
Funding
This study was supported by the Shenyang Key
R&D and Technology Transfer Program (grant no. 250039).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
XP and FZ conceived and designed the experiments.
HL contributed to hAMSC, hDF and hAEC collection. HL, TZ, RW, XL
and PS contributed reagents, materials and the analysis of the
data. CP performed experiments and prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital and Shengjing Hospital
of China Medical University (Shenyang, China). Written informed
consent was obtained from all patients.
Patient consent for publications
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Varani J: Fibroblast aging: Intrinsic and
extrinsic factors. Drug Discov Today Ther Strateg. 7:65–70. 2010.
View Article : Google Scholar
|
|
2
|
Makrantonaki E and Zouboulis CC: Molecular
mechanisms of skin aging: State of the art. Ann N Y Acad Sci.
1119:40–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seo MY, Chung SY, Choi WK, Seo YK, Jung
SH, Park JM, Seo MJ, Park JK, Kim JW and Park CS: Anti-aging effect
of rice wine in cultured human fibroblasts and keratinocytes. J
Biosci Bioeng. 107:266–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marcotte R and Wang E: Replicative
senescence revisited. J Gerontol A Biol Sci Med Sci. 57:B257–B269.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serrano M and Blasco MA: Putting the
stress on senescence. Curr Opin Cell Biol. 13:748–753. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen QM: Replicative senescence and
oxidant-induced premature senescence. Beyond the control of cell
cycle checkpoints. Ann N Y Acad Sci. 908:111–125. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng H, Qiu L, Ma J, Zhang H, Cheng M, Li
W, Zhao X and Liu K: Replicative senescence of human bone marrow
and umbilical cord derived mesenchymal stem cells and their
differentiation to adipocytes and osteoblasts. Mol Biol Rep .
38:pp. 5161–5168. 2011, View Article : Google Scholar
|
|
8
|
Jiang B, Li Y, Liang P, Liu Y, Huang X,
Tong Z, Zhang P, Huang X, Liu Y and Liu Z: Nucleolin enhances the
proliferation and migration of heat-denatured human dermal
fibroblasts. Wound Repair Regen. 23:807–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng B, Fang Y and Wei SM: Effect and
mechanism of epigallocatechin-3-gallate (EGCG). Against the
hydrogen peroxide-induced oxidative damage in human dermal
fibroblasts. J Cosmet Sci. 64:35–44. 2013.PubMed/NCBI
|
|
10
|
Hernández-García D, Wood CD,
Castro-Obregón S and Covarrubias L: Reactive oxygen species: A
radical role in development? Free Radic Biol Med. 49:130–143. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Halliwell B and Gutteridge JMC: Free
radicals in biology and medicine. J Free Radic Biol Med. 1:331–332.
2007. View Article : Google Scholar
|
|
12
|
Gutteridge JMC and Halliwell B:
Mini-review: Oxidative stress, redox stress or redox success?
Biochem Biophys Res Commun. 502:183–186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ames BN, Shigenaga MK and Hagen TM:
Oxidants, antioxidants, and the degenerative diseases of aging.
Proc Natl Acad Sci USA. 90:7915–7922. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Djamali A: Oxidative stress as a common
pathway to chronic tubulointerstitial injury in kidney allografts.
Am J Physiol Renal Physiol. 293:F445–F455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Deursen JM: The role of senescent
cells in ageing. Nature. 509:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harman D: The biologic clock: The
mitochondria. J Am Geriatr Soc. 20:145–147. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Díaz-Prado S, Muiños-López E,
Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ
and Blanco FJ: Multilineage differentiation potential of cells
isolated from the human amniotic membrane. J Cell Biochem.
111:846–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castellanos G, Bernabé-García Á, Moraleda
JM and Nicolás FJ: Amniotic membrane application for the healing of
chronic wounds and ulcers. Placenta. 59:146–153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao B, Liu JQ, Zheng Z, Zhang J, Wang SY,
Han SC, Zhou Q, Guan H, Li C, Su LL and Hu DH: Human amniotic
epithelial stem cells promote wound healing by facilitating
migration and proliferation of keratinocytes via ERK, JNK and AKT
signaling pathways. Cell Tissue Res. 365:85–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hahn HJ, Kim KB, An IS, Ahn KJ and Han HJ:
Protective effects of rosmarinic acid against hydrogen
peroxide-induced cellular senescence and the inflammatory response
in normal human dermal fibroblasts. Mol Med Rep. 16:9763–9769.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdul Hisam EE, Rofiee MS, Khalid AM,
Jalaluddin AF, Mohamad Yusof MI, Idris MH, Ramli S, James RJ, Jack
Yoeng W, Lay Kek T and Salleh MZ: Combined extract of Moringa
oleifera and Centella asiatica modulates oxidative stress and
senescence in hydrogen peroxide-induced human dermal fibroblasts.
Turk J Biol. 42:33–44. 2018. View Article : Google Scholar
|
|
22
|
Borodkina A, Shatrova A, Abushik P,
Nikolsky N and Burova E: Interaction between ROS dependent DNA
damage, mitochondria and p38MAPK underlies senescence of human
adult stem cells. Aging (Albany NY). 6:481–495. 2014. View Article : Google Scholar
|
|
23
|
Priya D, Selokar NL, Raja AK, Saini M,
Sahare AA, Nala N, Palta P, Chauhan MS, Manik RS and Singla SK:
Production of wild buffalo (Bubalus arnee) embryos by interspecies
somatic cell nuclear transfer using domestic buffalo (Bubalus
bubalis) oocytes. Reprod Domest Anim. 49:343–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Wang Z, Wang R, Zhao F, Shi P,
Jiang Y and Pang X: Direct comparison of the potency of human
mesenchymal stem cells derived from amnion tissue, bone marrow and
adipose tissue at inducing dermal fibroblast responses to cutaneous
wounds. Int J Mol Med. 31:407–415. 2013. View Article : Google Scholar
|
|
25
|
Wang G, Zhao F, Yang D, Wang J, Qiu L and
Pang X: Human amniotic epithelial cells regulate osteoblast
differentiation through the secretion of TGFβ1 and microRNA-34a-5p.
Int J Mol Med. 41:791–799. 2018.
|
|
26
|
Flach J, Bakker ST, Mohrin M, Conroy PC,
Pietras EM, Reynaud D, Alvarez S, Diolaiti ME, Ugarte F, Forsberg
EC, et al: Replication stress is a potent driver of functional
decline in ageing haematopoietic stem cells. Nature. 512:198–202.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sperka T, Wang J and Rudolph KL: DNA
damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol
Cell Biol. 13:579–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan
G, Cui W, Luo ZP, Pei M, Yang H and He F: Melatonin reverses
H2O2-induced premature senescence in
mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal
Res. 59:190–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Díaz-Prado S, Muiños-López E,
Hermida-Gómez T, Rendal-Vázquez ME, Fuentes-Boquete I, de Toro FJ
and Blanco FJ: Multilineage differentiation potential of cells
isolated from the human amniotic membrane. J Cell Biochem.
111:846–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim EY, Lee KB and Kim MK: The potential
of mesenchymal stem cells derived from amniotic membrane and
amniotic fluid for neuronal regenerative therapy. BMB Rep.
47:135–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang C, Yuchi H, Sun L, Zhou X and Lin J:
Human amnion-derived mesenchymal stem cells protect against UVA
irradiation-induced human dermal fibroblast senescence, in vitro.
Mol Med Rep. 16:2016–2022. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim WS, Park BS, Sung JH, Yang JM, Park
SB, Kwak SJ and Park JS: Wound healing effect of adipose-derived
stem cells: A critical role of secretory factors on human dermal
fibroblasts. J Dermatol Sci. 48:15–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao H, Xiong L, Song X, Jin P, Chen L,
Chen X, Yao H, Wang Y and Wang L: Angelica sinensis polysaccharides
ameliorate stress-induced premature senescence of hematopoietic
cell via protecting bone marrow stromal cells from oxidative
injuries caused by 5-fluorouracil. Int J Mol Sci. 18:pp. pii
E22652017, View Article : Google Scholar
|
|
34
|
Faraci FM and Didion SP: Vascular
protection: Superoxide dismutase isoforms in the vessel wall.
Arterioscler Thromb Vasc Biol. 24:1367–1373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin J, Lv X, Chen L, Zhang W, Li J, Wang
Q, Wang R, Lu X and Miao D: Bmi-1 plays a critical role in
protection from renal tubulointerstitial injury by maintaining
redox balance. Aging Cell. 13:797–809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park MJ and Bae YS: Fermented acanthopanax
koreanum root extract reduces UVB- and H2O2-induced senescence in
human skin fibroblast cells. J Microbiol Biotechnol. 26:1224–1233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi SI, Lee JH, Kim JM, Jung TD, Cho BY,
Choi SH, Lee DW, Kim J, Kim JY and Lee OH: Ulmus macrocarpa hance
extracts attenuated H2O2 and UVB-Induced skin
photo-aging by activating antioxidant enzymes and inhibiting MAPK
pathways. Int J Mol Sci. 18:pp. pii E12002017
|
|
38
|
Lombard DB, Chua KF, Mostoslavsky R,
Franco S, Gostissa M and Alt FW: DNA repair, genome stability, and
aging. Cell. 120:497–512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boyette LB and Tuan RS: Adult stem cells
and diseases of aging. J Clin Med. 3:88–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bockeria L, Bogin V, Bockeria O, Le T,
Alekyan B, Woods EJ, Brown AA, Ichim TE and Patel AN: Endometrial
regenerative cells for treatment of heart failure: A new stem cell
enters the clinic. J Transl Med. 11:562013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Savickienė J, Baronaitė S, Zentelytė A,
Treigytė G and Navakauskienė R: Senescence-associated molecular and
epigenetic alterations in mesenchymal stem cell cultures from
amniotic fluid of normal and fetus-affected pregnancy. Stem Cells
Int. 2016.2019498:2016.
|
|
43
|
Kasai H and Nishimura S: Hydroxylation of
deoxyguanosine at the C-8 position by ascorbic acid and other
reducing agents. Nucleic Acids Res. 12:2137–2145. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mah LJ, El-Osta A and Karagiannis TC:
gammaH2AX: A sensitive molecular marker of DNA damage and repair.
Leukemia. 4:679–686. 2010. View Article : Google Scholar
|
|
46
|
Bhogal N, Jalali F and Bristow RG:
Microscopic imaging of DNA repair foci in irradiated normal
tissues. Int J Radiat Biol. 9:732–746. 2009. View Article : Google Scholar
|
|
47
|
Downs JA, Allard S, Jobin-Robitaille O,
Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP and Côté J:
Binding of chromatin-modifying activities to phosphorylated histone
H2A at DNA damage sites. Mol Cell. 6:979–990. 2004. View Article : Google Scholar
|
|
48
|
Ikura T, Tashiro S, Kakino A, Shima H,
Jacob N, Amunugama R, Yoder K, Izumi S, Kuraoka I, Tanaka K, et al:
DNA damage-dependent acetylation and ubiquitination of H2AX
enhances chromatin dynamics. Mol Cell Biol. 20:7028–7040. 2007.
View Article : Google Scholar
|
|
49
|
Altaf M, Auger A, Covic M and Côté J:
Connection between histone H2A variants and chromatin remodeling
complexes. Biochem Cell Biol. 1:35–50. 2009. View Article : Google Scholar
|
|
50
|
Kusch T, Florens L, Macdonald WH, Swanson
SK, Glaser RL, Yates JR, Abmayr SM, Washburn MP and Workman JL:
Acetylation by Tip60 is required for selective histone variant
exchange at DNA lesions. Science. 306:2084–2087. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xie C, Jin J, Lv X, Tao J, Wang R and Miao
D: Anti-aging effect of transplanted amniotic membrane mesenchymal
stem cells in a premature aging model of Bmi-1 deficiency. Sci Rep.
5:139752015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li F and Zhao SZ: Mesenchymal stem cells:
Potential role in corneal wound repair and transplantation. World J
Stem Cells. 6:296–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu Y, Guo S, Wei C, Li H, Chen L, Yin C
and Zhang C: The comparison of adipose stem cell and placental stem
cell in secretion characteristics and in facial antiaging. Stem
Cells Int. 2016.7315830:2016.
|