Introduction
Spinal cord injury (SCI) is one of the most
heterogeneous injuries occurring in the central nervous system for
its diverse symptoms and treatment outcomes, because of variability
in the external mechanical forces causing the injury. Based on the
epidemiological data, the World Health Organization has predicted
that SCI is most likely to surpass numerous diseases as the major
cause of death and disability worldwide by 2020 (1). Moreover, a statistical study by the
National Spinal Cord Injury Statistical Center has indicated that
the global incidence of SCI is roughly 23 SCI cases per million
annually (2,3). Chronic complications after primary
SCI are quite common and severe (4–9),
leading to reduced life expectancy and enhanced morbidity. This not
only creates a physically and emotionally debilitating condition
but also generates a prominent financial burden for individuals,
families, and society (10,11). Thus, there is an urgent need in
research for developing new therapeutics.
SCI can cause immediate cellular death, resulting
from direct mechanical impact or compression and followed by
numerous types of secondary and neurodegenerative processes
(12–14). Those secondary processes may cause
progressive and fundamental alterations of the cellular structure
and function. These effects are most likely to occur on the injured
sites, where the cells are extremely susceptible to free radical
overproduction and lipid peroxidation as well as glutamate-calcium
(Ca2+)- and potassium (K+)-related
neurotoxicity, followed by a local or systemic inflammatory
responses (12,13,15,16). Consequently, all these drastic
alterations at the cellular and subcellular level inevitably result
in not only accumulative progression of the microglial inflammatory
response but also Wallerian degeneration and reactive astrogliosis.
Eventually, the fibrotic core surrounded by reactive astrocytes
will form glial scars, resulting in progressive and severe loss of
motor and sensory function due to structural changes at the
neuronal micro-circuitry level (17–19).
Unlike the immediate primary injuries caused by
structural deconstruction, secondary injuries, caused by numerous
inflammatory mediators, particularly cytokines and chemokines, are
progressive, leading to further damage beyond the primary injury.
Those chemokines are secreted by resident cells in the central
nervous system (CNS) and by infiltrating cells, recruited to the
CNS through blood vessels (20,21). As a result of the extensive
association of inflammatory mediators with CNS function,
chemokines, designated as a novel subtype of neurotransmitters and
neuromodulators, have previously received attention under
physiological or pathological conditions and, hence, are also known
as neurochemokines (22,23). In previous years, several studies
independently uncovered that the secondary injury of SCI is closely
correlated with immunological components (24). Moreover, the corresponding
therapeutic approaches targeting inflammatory responses are quite
promising, with enhanced neuroprotection and neuro-regeneration
(25,26).
In particular, C-X3-C motif chemokine ligand
1(CX3CL1) also known as fractalkine has so far been regarded as the
only member of the CX3Cδ subfamily that has both soluble and
membrane-anchored forms. Thus, its dual roles are uniquely
fulfilled as both a chemoattractant and a cell adhesion molecule.
The latter role is mediated by its binding to the C-X3-C motif
chemokine receptor 1 (CX3CR1), a G protein-coupled receptor
(27).
Given its role in mediating communication among
neuronal, microglial and astroglial populations, CX3CL1/CX3CR1
signaling plays an important role in hippocampal synaptic
plasticity and maturation (28),
and this signaling exhibits a remarkable effect on the modulation
of human temporal lobe epilepsy (29), glioblastoma (30,31) and CNS injury (32). Previous evidence (28,32) has implicated the role of the
CX3CL1/CX3CR1 axis in the pathophysiology of neuroinflammatory
processes after CNS injuries such as traumatic brain injury and
SCI. The role of the CX3CL1/CX3CR1 axis has not been widely
recognized for its presence and potential function in the
pathophysiology of SCI-related phenomena, and its importance in
systemic and direct local immune responses is still under
investigation. The quality of the evidence and the safety of its
application has been continually debated. The microglial
inflammatory response, in addition to subsequent Wallerian
degeneration and reactive astrogliosis, can dramatically vary
following SCI (33–35). Until now, there is little data on
how to treat SCI in experimental animals or human patients. A
favorable profile of the corresponding treatment via the
CX3CL1/CX3CR1 axis is thus far from being considered definitive.
Thus, this study focuses on developing a novel therapeutic approach
to selectively target the CX3CL1/CX3CR1 axis following SCI and
explore the detailed molecular mechanisms for the role of
CX3CL1/CX3CR1 in the pathogenesis of secondary injury in SCI.
Materials and methods
Animal models
Adult male Sprague-Dawley rats (n=75), weighing
220–280 g, were purchased from the Animal Facilities of Soochow
University. A total of 65 of them eventually qualified for final
statistical analyses. They were kept in cages with controlled
temperature (22°C) and humidity (50–70%), where food and water were
offered ad libitum, along with 12-h light and dark cycle.
All experimental procedures were approved and supervised by the
Animal Care and Use Committee of the Soochow University, which were
practiced in compliance with the guidelines regarding the Care and
Use of Laboratory Animals from the National Institutes of
Health.
The rat SCI model was generated with a clip
compression method as previously described (36). Following anesthesia via
intraperitoneal injection with 4% chloralhydrate (400 mg/kg), the
rat skulls were fixed by a stereotactic instrument. Following a
posterior median incision at T10, the paravertebral muscles were
pulled aside from both sides to expose the T9–T11 lamina. The T10
lamina was trimmed and, hence, the bilateral edges of the dura were
clearly exposed. After satisfactory hemostasis, a clamp (Lawton;
Huanxi) with a closing pressure of 30 g, was applied to directly
clamp the spinal cord and then released carefully after 20 sec.
After surgery, the muscles and the skin were sutured. According to
each experimental design as follows, the T10 lamina was resected
and the dura mater was exposed. However, no clamp was used in the
sham operated rats. For drug treatment, the CX3CR1 inhibitor
AZD8797 (80 μg/kg) (37)
dissolved in DMSO, was injected intraperitoneally after SCI in rats
from subset II, once per day until the rats were sacrificed,
whereas methylprednisolone was injected intraperitoneally (30
mg/kg) (38) within 30 min after
SCI in rats from the subset II. A bladder massage was given twice a
day after the operation until normal urination was restored. The
pads were replaced every 2 days after the operation to keep the
limbs dry and the limbs with pressure sores were disinfected with
an iodophor.
A total of 4% chloral hydrate (400 mg/kg) was
injected intraperitoneally, blood samples were collected after
anaesthesia, then an assistant held the back of the rat, exposing
the neck, the rats’ head were removed with scissors and the needed
spinal cord tissues were collected.
A total of 25 out of 30 surviving rats (subset I),
which had no statistical difference concerning their weight, intake
and motor ability, were randomly divided into 5 groups, namely
sham, SCI 3 day (D), SCI 7D, SCI 10D, and SCI 14D groups (Fig. 1B). The tissues from the spinal
lesion area were collected and longitudinally split into two equal
aliquots, one of which were used for quantitative(q)PCR and the
other for western blot analyses. Similarly, 40 out of 45 surviving
rats (subset II), which had no statistical difference in weight,
intake and motor ability, were randomly divided into 4 groups,
namely sham, SCI, SCI + inhibitor (AZD8797), and SCI +
methylprednisolone (Me) groups (Fig.
1C). A total of 10 days after SCI, the locomotive recovery was
assessed via Basso Beattie Bresnahan (BBB) scoring in all groups
before sacrificing. The blood (~3 ml) was then collected from the
inferior vena cava after anesthesia in each of those 40 rats.
Finally, the rats were sacrificed to collect their spinal cord
tissues. In addition, other evaluations, such as terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) staining and fluoro-jade B (FJB) staining, were also
carried out accordingly (Fig.
1C). All experiments were strictly adhered to blinded methods
during the analyses. Meanwhile, all data that was associated with
the corresponding samples was recorded by an independent
researcher.
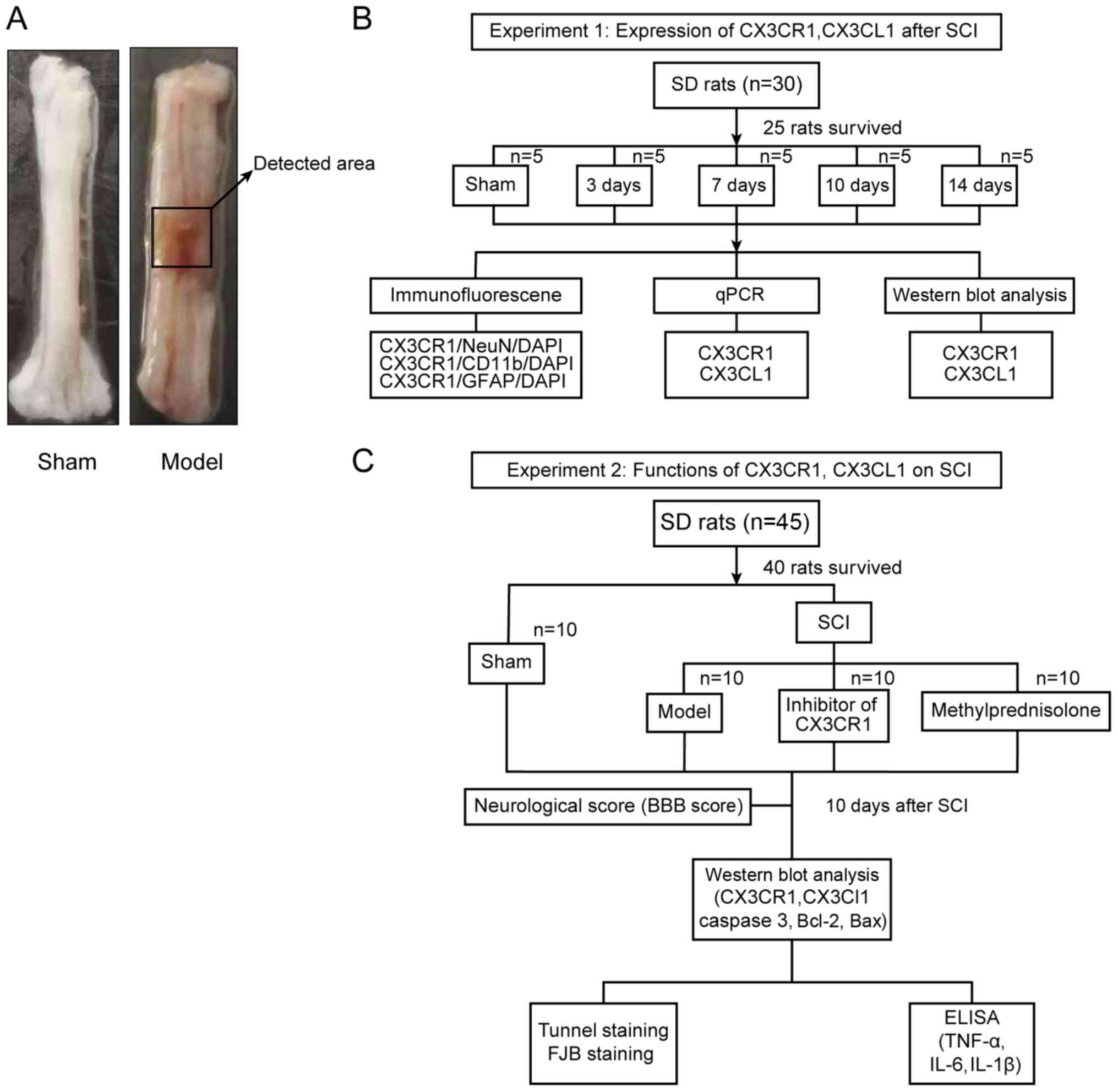 | Figure 1Study design. (A) The representative
areas obtained from the injured rat spinal cords for further
analysis. (B) Experiment subset I was employed for the time course
expression analyses of CX3CL1/CX3CR1 after SCI. (C) Experiment
subset II was employed to establish the functional role of
CX3CL1/CX3CR1 signaling in SCI rats. SCI, spinal cord injury;
CX3CL1, C-X3-C motif chemokine ligand 1CX3CR1, C-X3-C motif
chemokine receptor 1; SD, Sprague-Dawley; q, quantitative; IL,
interleukin; TNF, tumor necrosis factor; BBB, Basso Beattie
Bresnahan; TUNEL, terminal deoxynucleotidyl transferase-mediated
dUTP nick-end labeling; FJB, fluoro jade B; DAPI,
4′,6-diamidino-2-phenylindole. |
qPCR
Using TRIzol reagent (Invitrogen; ThermoFisher
Scientific, Inc.), the total RNA was obtained from the spinal cord
tissues. The extracted RNA was reverse-transcribed as follows:
First, the oligo(dt) primer, mRNA and nuclease-free water were
added into a sterile, nuclease-free tube on ice-cold water to make
a total volume 12 μl. The solution was mixed gently, centrifuged
briefly (860 × g; 4°C; 30 sec) and incubated at 65°C for 5 min. The
solution was then chilled on ice, spun down (860 × g; 4°C; 30 sec)
and the resulting vial placed back on ice. The 5× Reaction Buffer,
RiboLock RNase Inhibitor, 10 mM dNTP Mix, Revert Aid H Minus M-MuLV
and Reverse Transcriptase were then added to the solution, and it
was mixed gently and then centrifuged (860 × g; 4°C; 30 sec), then
incubated for 60 min at 42°C. The reaction was terminated by
heating the solution at 70°C for 5 min. The reverse transcription
reaction product was used directly in PCR applications or stored at
−20°C for <1 week. For longer storage, −70°C is recommended. The
Revert Aid H Minus First Strange cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) used for the reverse transcription. cDNA was
synthesized from 1 μg of the total RNA. qPCR was performed using a
QuantStudio™ Dx Instrument (Life Technologies; ThermoFisher
Scientific, Inc.) with a PowerUp™ SYBR™ Green Master Mix (Thermo
Fisher Scientific, Inc.). The qPCR protocol is as follows:
Denaturation of templates was initiated at 95°C for 2 min, followed
by 40 cycles of the amplification reaction (95°C for 15 sec, 60°C
for 15 sec and 72°C for 1 min). GAPDH mRNA was employed as an
internal control for each sample tested and relative mRNA
expression levels of all genes tested were quantified using the
2−ΔΔCq method (39)
(n=3). In addition, the primers used in the present studywere as
follows: CX3CR1: Forward, 5′-GCTGAGGCCTGTTATTTGGG-3′; and reverse,
5′-GACCGAACGTGAAGACAAGG-3′; CX3CL1: Forward,
5′-TCATTCAGAAGCTGCCAGGA-3′; and reverse,
5′-AGAGTCCCTTCCAGAACACG-3′; GAPDH: Forward,
5′-TGGCCTTCCGTGTTCCTACC-3′; and reverse,
5′-TCTTCCACCACTTCGTCCGC-3′.
Western blotting
Protein samples were obtained from the spinal cord
tissues lysed for homogenization using RIPA buffer (Beyotime
Institute of Biotechnology), supplemented with a
protease/phosphatase inhibitor cocktail, followed by centrifugation
at 13,000 × g at 4°C for 20 min. The protein concentration in the
supernatants was measured using aPierce™ bicinchoninic acid Protein
Assay kit (Thermo Fisher Scientific, Inc.) and diluted accordingly.
Equal amounts of protein (30 μg) in all samples were used for
electrophoresis with 10% SDS-polyacrylamide gels (Beyotime
Institute of Biotechnology) and then transferred onto
polyvinylidene difluoride membranes (EMD Millipore). After blocking
with 5% non-fat milk in 0.1% TBST for 2 h at room temperature, the
membranes were incubated with the primary antibodies overnight at
4°C with the following dilution: Rabbit anti-GAPDH (1:10,000; cat.
no. PLA 0125; Sigma-Aldrich; Merck KGaA), mouse anti-β-actin
(1:10,000; cat. no. A5316; Sigma-Aldrich; Merck KGaA), rabbit
anti-CX3CL1 (1:1,000; cat. no. ab25088; Abcam), rabbit anti-CX3CR1
(1:1,000; cat. no. ab8021; Abcam), rabbit anti-Bcl2 (1:1,000; cat.
no. ab196495; Abcam), rabbit anti-Bax (1:2,000; cat. no. ab232479;
Abcam) and rabbit anti-caspase 3 (1:500; cat. no. ab49822; Abcam).
The membranes were then incubated with the goat anti-mouse
IgG-horseradise peroxidase (HRP) (cat. no. 31430) or anti-rabbit
IgG-HRP (cat. no. 31431) (both 1:10,000; both from Invitrogen;
Thermo Fisher Scientific, Inc.) secondary antibodies at 4°C for 2
h. Immunoblots were then visualized with a chemiluminescent
substrate (EMD Millipore) using a Bio-Rad imaging system (Bio-Rad
Laboratories, Inc.), followed by image analyses using ImageJ
software (version ImageJ 1.44P; National Institute of Health).
Immunofluorescence
The injured spinal cords were dissected out and
post-fixed with 4% paraformaldehyde for 24 h at 4°C. The samples
were then sequentially dehydrated in 15 and 30% sucrose in PBS (pH
7.4) for 24 h. Then the samples were embedded in OCT compound
(Sakura Finetek USA, Inc.) and frozen at −80°C until use. Coronal
sections (15 μm thick) were obtained by cryosectioning with Leica
DMi8 (Leica Microsystems, GmbH) and transferredonto the slides
pre-coated with poly-L-lysine. After rinsing with 1% Triton in PBS,
the sections were incubated in the blocking buffer containing 10%
goat serum at room temperature for >1 h. Then they were
incubated with the primary antibodies overnight at 4°C as follows:
Mouse anti-cluster of differentiation11b (1:200; cat. no. MABF520;
EMD Millipore), mouse anti-glial fibrillary acidic protein (1:300;
cat. no. SAB1405864; EMD Millipore), rabbit anti-CX3CR1 (1:100;
cat. no. ab8021; Abcam) and mouse anti-NeuN (1:200; cat. no.
MAB377; EMD Millipore), followed by incubation with secondary
antibodies, including donkey anti-rabbit IgG antibody conjugated
with Alexa Fluor 488 (1:1,000; cat. no. R37118; Invitrogen; Thermo
Fisher Scientific, Inc.) or donkey anti-mouse IgG antibody
conjugated with Alexa Fluor 555 (1:1,000; cat. no. A-31570;
Invitrogen; Thermo Fisher Scientific, Inc.), for 1 h at room
temperature. The results were observed under a Leica DMi8 confocal
microscope and captured using LAS X software (version 2.0.1.14392;
Leica Microsystems GmbH).
FJB staining
FJB staining was performed according to the
protocols provided by the manufacturer (EMD Millipore). After
incubating with 1% sodium hydroxide in 80% alcohol for 5 min and
70% alcohol for 2 min, the cryosectioned samples were then
transferred to 0.06% potassium permanganate for 10 min. Afterwards,
they were immersed (room temperature) in 0.0004% fluoro-jade dye
solution containing 0.1% acetic acid for 20 min, followed by
rinsing with deionized water. Then, they were dried in an oven at
50°C for 5–8 min. The sections were then immersed in xylene for at
least 1 min and then mounted with a non-aqueous and non-fluorescent
plastic mounting medium, distyreneplasticiser xylene. The samples
were observed under a Leica DMi8 confocal microscope and the images
were captured using LASX software (Leica Microsystems, GmbH).
TUNEL staining
Apoptosis was detected using TUNEL staining
according to the manufacturer’s protocol (Abcam). Frozen injured
spinal cord tissue sections were soaked in 4% polyformaldehyde for
15 min and then shifted into a protease K working solution and
incubated for 5 min. The samples were immersed in 4%
polyformaldehyde for another 5 min and rinsed with wash buffer
twice for 5 min each. All the slices were incubated (37°C) in DNA
labeling solution and stored in a wet box away from light for 1 h.
After washing the slices, antibody solution was added and kept in a
dark and wet box for 30 min. The samples were then rinsed with
deionizing solution for 5 min. After air drying, they were then
sealed with DAPI (room temperature in the dark for 30 min). All the
slides were observed under a laser confocal microscope Leica DMi8
(Leica Microsystems, GmbH) and images were captured using the LASX
software.
ELISA
The concentrations of interleukin-1β (IL-1β), tumor
necrosis factor-α (TNF-α) and IL-6 in the serially collected serum
samples were measured on the 10th postoperative day using the ELISA
kits for IL-1β (cat. no. MK1198); TNF-α (cat. no. EK0526); and IL-6
(cat. no. EK0412) (all from Wuhan Boster Biological Technology,
Ltd.). The serum was obtained by centrifugation (5,700 × g, for 10
min and 4°C) after the blood was kept overnight at room
temperature. After preparation, the sample and standard (100 μl
each) were added to a 96-well plate and incubated at 37°C for 90
min. Then, the antibodies were labeled with biotin in 100 μl
aliquots in each well and reacted for 60 min at 37°C. Then samples
were rinsed with 0.01 M TBS and 100 μl avidin-biotin complex was
added to each well and allowed to react at 37°C for 30 min,
followed by 5 rinses with 0.01 M TBS. The reaction time was <30
min, after adding tetramethyl benzidine (TMB) at 37°C without light
and then TMB termination solution was added. Using a microplate
reader, the absorbance at 450 nm was measured and the protein
concentrations (ng/ml) were calculated using standard curves.
Statistical analysis
All the collected data were analyzed using SPSS 22.0
software (IBM, Corps.). A one-way analysis of variance was employed
for multiple comparisons among different groups and a Student’s
t-test was employed to compare the results between two groups,
given that all data was normally distributed. The data were
presented as the mean ± standard deviation, Tukey honest
significant difference test was used as a post hoc test. P<0.05
was considered to indicate a statistically significant difference,
while a P<0.01 was considered as indicative of a high level of
significance.
Results
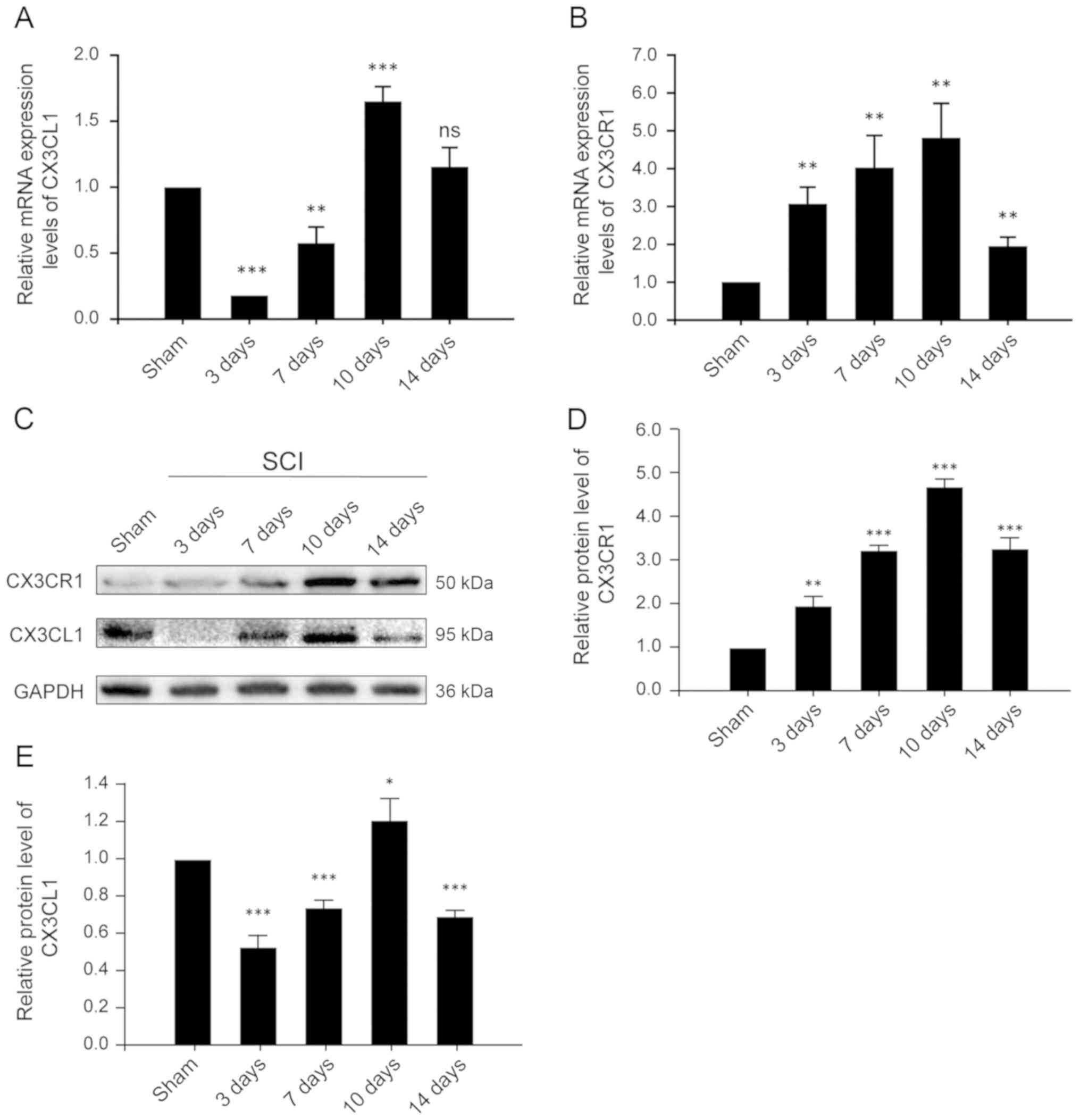
CX3CR1 and CX3CL1 are upregulated at both
the mRNA and protein levels in the injured spinal cords after
SCI
CX3CL1, also known as fractalkine, is a chemokine
that is uniquely anchored to the plasma membrane. The CX3CL1/CX3CR1
axis has also been frequently described for its role in the
pathogenesis and progression of numerous CNS diseases and injuries.
Thus, in order to evaluate the time course of changing
CX3CL1/CX3CR1 signaling, samples were collected at different time
points after SCI and assessed accordingly. CX3CR1, at both the mRNA
and protein levels, increased after 3 days of SCI, reached its peak
on the 10th day, and decreased significantly on the 14th day
(Fig. 2B and D). These results
indicate that CX3CR1 expression increased, mediating a local
inflammatory response and decreased significantly 14 days after
SCI. Similarly, the expression of CX3CL1 at the mRNA level
decreased significantly on the 3rd day, then increased gradually,
reaching its peak on the 10th day and decreasing significantly on
the 14th day after SCI (Fig. 2A).
These results suggest that CX3CL1 exists as a membrane-bound as
well as a secretory protein. After SCI, the expression of CX3CL1
decreased for a short period of time, potentially due to local
injury and being released into the blood as a chemokine. It then
gradually increased, possibly due to enhanced expression, adhesion
and aggregation. Its expression then again decreased following a
constant pattern, indicative of a relatively stable level of
protein on the 14th post-SCI day.
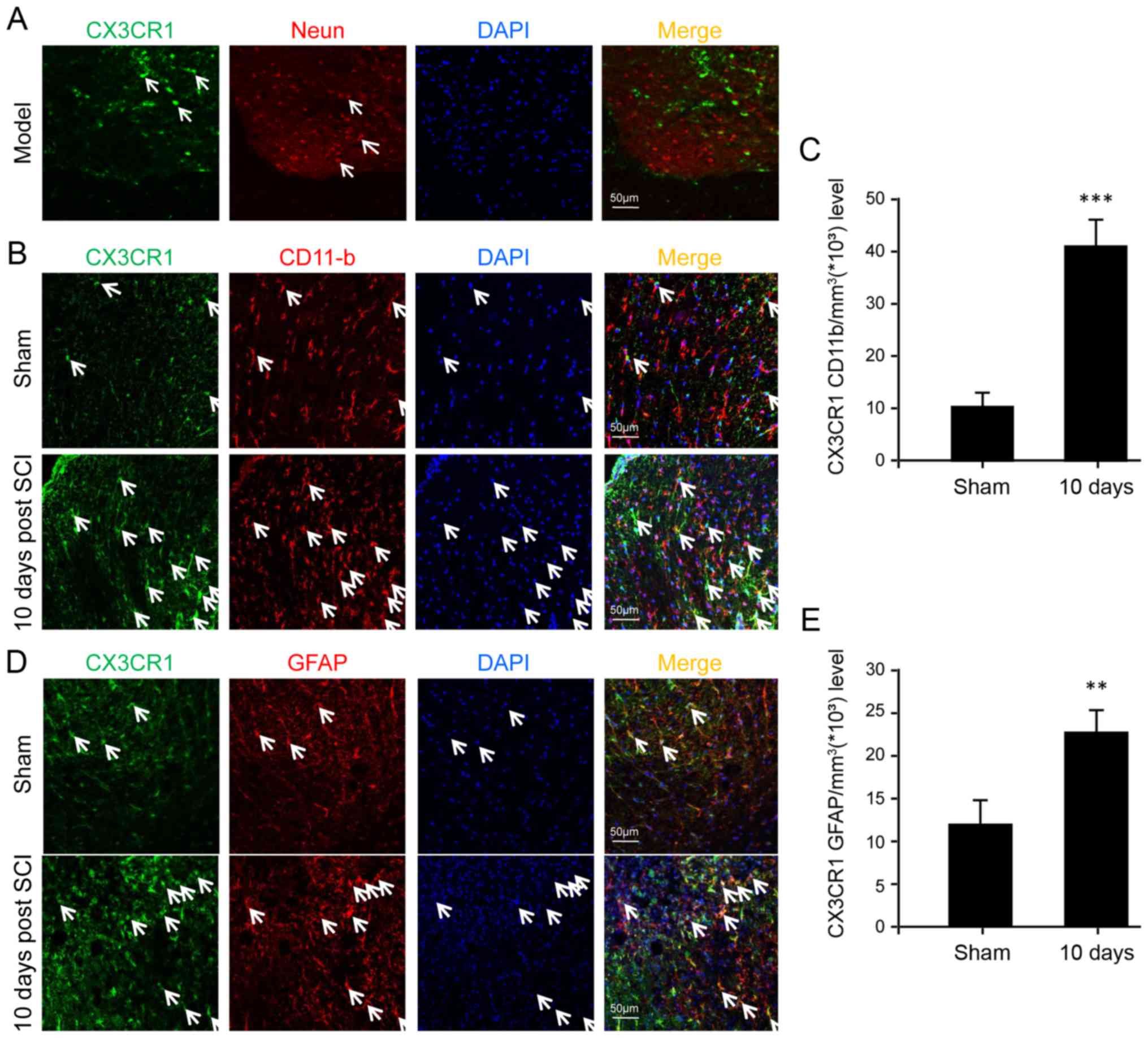
CX3CR1 is upregulated in microglia,
astrocytes and neurons after SCI
In order to determine the specific cell types
implicated in CX3CL1/CX3CR1 signaling, immunofluorescence was
performed to identify certain cell types expressing CX3CR1 among
neurons, microglia and astrocytes. The results show that CX3CR1 was
not detectable in neurons at all (Fig. 3A). In contrast, both microglia and
astrocytes displayed the expression of CX3CR1 at the protein level
(Fig. 3B and D). Furthermore,
they were upregulated significantly after SCI, especially in the
microglia, suggesting a major role of microglia following SCI
(Fig. 3C).
AZD8797 treatment enhances the early
behavioral recovery after SCI
Considering extensive involvement of CX3CL1/CX3CR1
signaling in CNS diseases and injuries, the effect of AZD8797
treatment on SCI was yet to be examined, and, hence, evaluation of
locomotive recovery via BBB scoring was performed on the 10th day
after the operation. As shown in Table I, the BBB score was reduced nearly
4 times in the SCI group compared with the sham control. In
contrast, the treatment with AZD8797 significantly improved the BBB
score when compared to the untreated SCI group. Similarly,
treatment with methylprednisolone also significantly improved the
BBB score. However, there was no statistical difference between the
BBB scores in the AZD8797 treated group and the methylprednisolone
treated group. Thus, the results clearly indicate that AZD8797
treatment enhances early behavioral recovery after SCI and is as
effective as the methylprednisolone treatment.
 | Table IAssessment of locomotive recovery of
rats from sham, injury, inhibitor, or methylprednisolone
administration groups 10 days after operation via BBB scoring. |
Table I
Assessment of locomotive recovery of
rats from sham, injury, inhibitor, or methylprednisolone
administration groups 10 days after operation via BBB scoring.
| BBB score and
difference | Group |
|---|
|
|---|
| Sham | Model | Inhibitor | Me |
|---|
| BBB score | 19.30±0.95 | 5.40±0.84 | 7.70±0.82 | 7.40±0.70 |
| P-value | | <0.001a | <0.001a | <0.001a |
| P-value | | | <0.001b | <0.001b |
| P-value | | | 0.391c | |
AZD8797 treatment suppresses the
activation of apoptosis machinery after SCI
To explore the underlying mechanism for CX3CR1
inhibitor-mediated enhanced behavioral recovery, the expression
levels of apoptosis-related molecules, including caspase 3, Bcl-2
and Bax were evaluated, in addition to the expression levels of
CX3CL1/CX3CR1 signaling after AZD8797 treatment. Cleaved caspase 3
has two molecular weight forms of 17 and 19 kDa. In the present
experiment, cleaved caspase 3 with the weight form of 17 kDa was
tested as the expression of cleaved caspase 3 with the molecular
weight form of 19 kDa was weak (Fig.
4A). The total caspase 3 in each group is equivalent to that of
the reference β-actin, so the ratio between cleaved caspase 3 and
β-actin was calculated to reflect apoptosis. For the CX3CR1 level,
the results showed that there was a significant increase in the SCI
model group compared with the sham control (P<0.001). However,
treatment with AZD8797 (P=0.861) or with methylprednisolone
(P=0.771) did not result in a significant change in CX3CR1 levels
(Fig. 4A and C). Comparatively,
CX3CL1 levels were significantly elevated in the SCI model group
when compared with the sham control (P<0.001). AZD8797 treatment
resulted in a significant increase in CX3CL1 levels when compared
to the untreated model (P=0.012) and to the methylprednisolone
treated group (P=0.007). In contrast, methylprednisolone treatment
did not have any effect on CX3CL1 levels when compared to the
untreated model group (P=0.643; Fig.
4A and B).
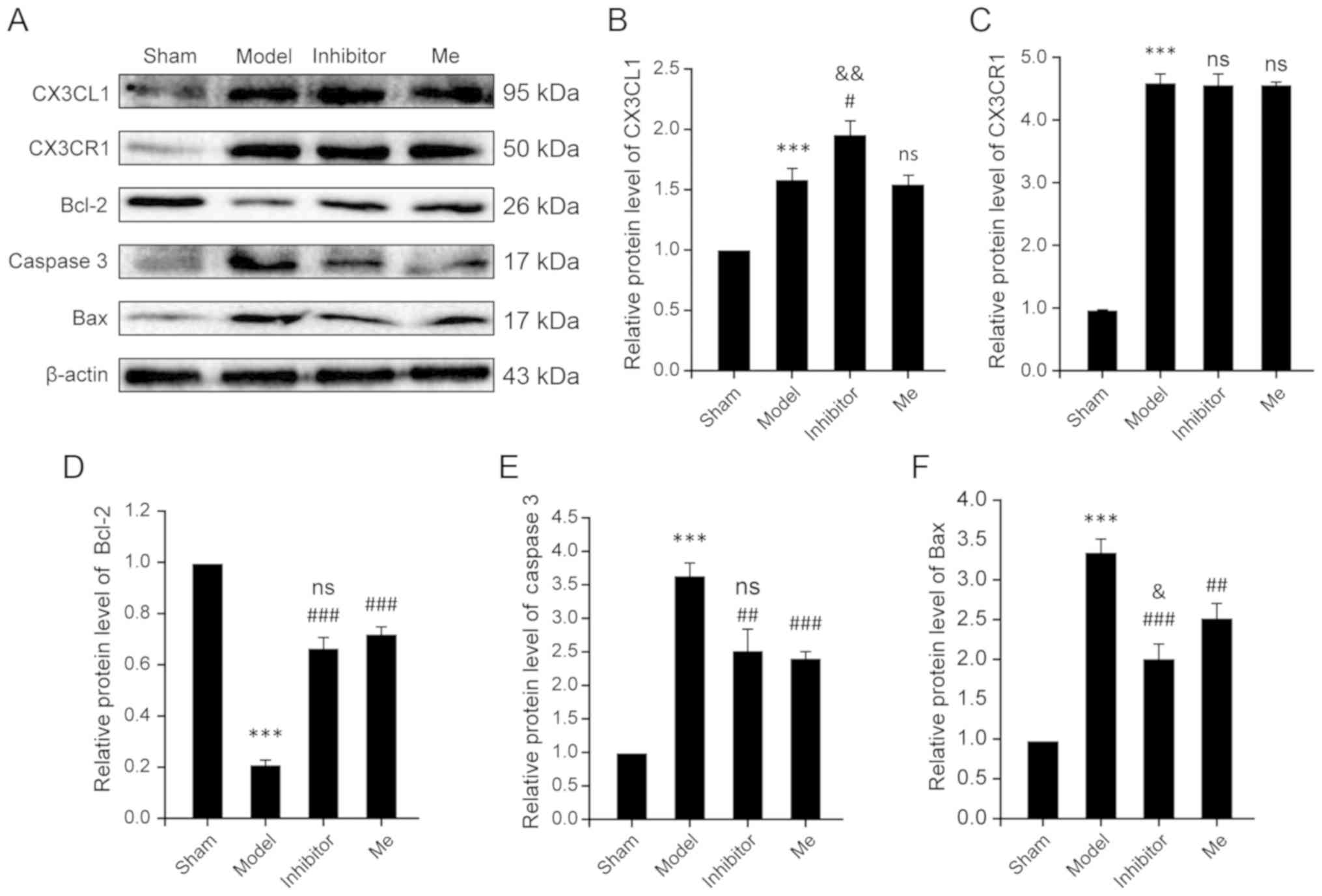 | Figure 4AZD8797 reduces neuronal apoptosis
after rat SCI. (A) The representative examples of the western blot
analysis results for the expression of CX3CL1, CX3CR1, caspase 3,
Bcl-2 and Bax in four groups as indicated 10 days after SCI. (B)
Quantitative analysis of (B) CX3CL1, (C) CX3CR1, (D) Bcl2, (E)
caspase 3 and (F) Bax expression in the western blotting in the
sham, Model, Inhibitor and Me groups 10 days after SCI. Data is
presented as means ± standard deviation. ***P<0.001
vs. sham, nsP>0.05 vs. Injury,
&P<0.05 and &&P<0.01 vs.
Me. #P<0.05, ##P<0.01 and
###P<0.001 vs. Injury. n=10. Me, methylprednisolone;
ns, not significant; GFAP, glial fibrillary acidic protein; SCI,
spinal cord injury; CX3CL1, C-X3-C motif chemokine ligand 1CX3CR1,
C-X3-C motif chemokine receptor 1. |
For the apoptosis machinery-related molecules,
including caspase 3, Bax and Bcl-2, the results consistently show
significant differences between the SCI model and the sham control
(P<0.001; Fig. 4D–F).
Treatment with AZD8797 or methylprednisolone significantly reversed
the changes when compared to the untreated SCI group (P<0.01;
Fig. 4D–F). Treatment with
AZD8797 was even more effective in reducing the elevated levels of
Bax in the SCI model when compared to the methylprednisolone
treatment (P=0.029; Fig. 4F),
but, for Bcl-2 and caspase 3, treatment with AZD8797 was as
effective as that with methylprednisolone (P=0.158; Fig. 4D; P=0.586; Fig. 4E). Accordingly, given the
activation of apoptosis machinery after SCI through the
CX3CL1/CX3CR1 signaling pathway, significantly enhanced cell
necrosis was also noted in the SCI model compared to the sham
control (P<0.001; Fig. 5A and
B). AZD8797 treatment significantly reduced cell necrosis when
compared to the untreated SCI group (P<0.01). The
methylprednisolone treatment also reduced necrosis when compared to
the untreated SCI group (P<0.01) but was not as effective as the
AZD8797 treatment (P<0.05). Note that the Bax level was also
different between these two groups (Fig. 4F).
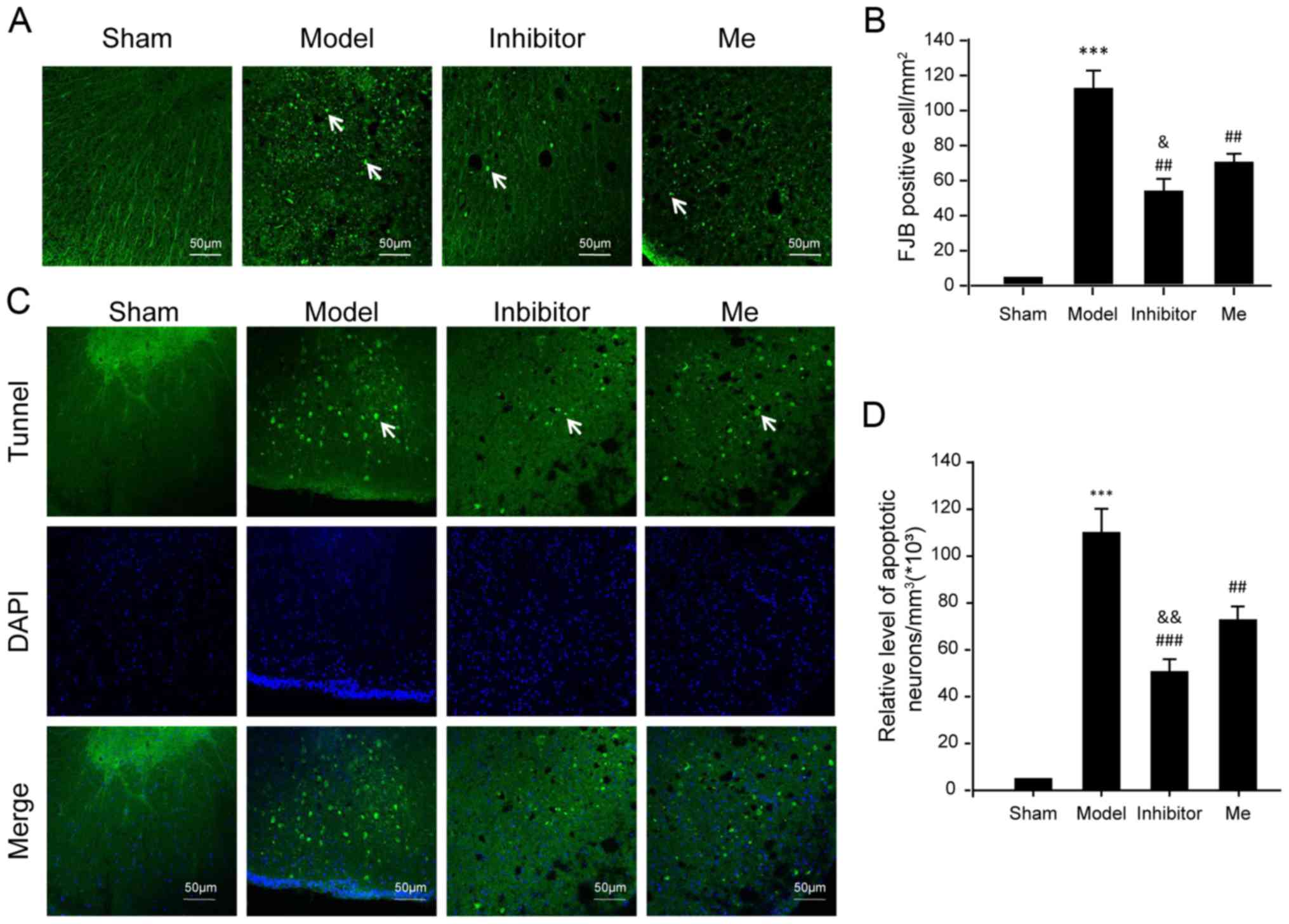 | Figure 5Evaluation of neuronal death. (A)
Neuronal death was examined by FJB staining. FJB-positive (green)
cells per mm2 were quantified accordingly. (B) The data
was represented as mean ± SD. ***P<0.01 vs. sham;
##P<0.01 vs. Injury; and &P<0.05
vs. Me. Scale bar, 50 μm. n=10. (C) Neuronal apoptosis was examined
by a TUNEL assay using immunofluorescence. In each section,
apoptotic neurons were labeled by TUNEL (green), while nuclei were
labeled with DAPI (blue). TUNEL-nuclei positive neurons per
mm3 were (D) quantified accordingly. The data are
presented as the mean ± SD. White arrows indicate positive signals.
***P<0.01 vs. sham; ##P<0.01 and
###P<0.001 vs. injury; and
&&P<0.01 vs. Me. Scale bar, 50 μm. n=10.
TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP nick-end
labeling; FJB, fluoro jade B; DAPI, 4′,6-diamidino-2-phenylindole;
SD, standard deviation; SCI, spinal cord injury; CX3CL1, C-X3-C
motif chemokine ligand 1CX3CR1, C-X3-C motif chemokine receptor 1;
Me, methylprednisolone. |
Intriguingly, the pattern of neuronal death caused
by apoptosis was consistent with the evaluation for neuronal
necrosis (Fig. 5C and D).
Overall, these results have demonstrated that AZD8797 treatment
suppresses the activation of apoptosis machinery following SCI.
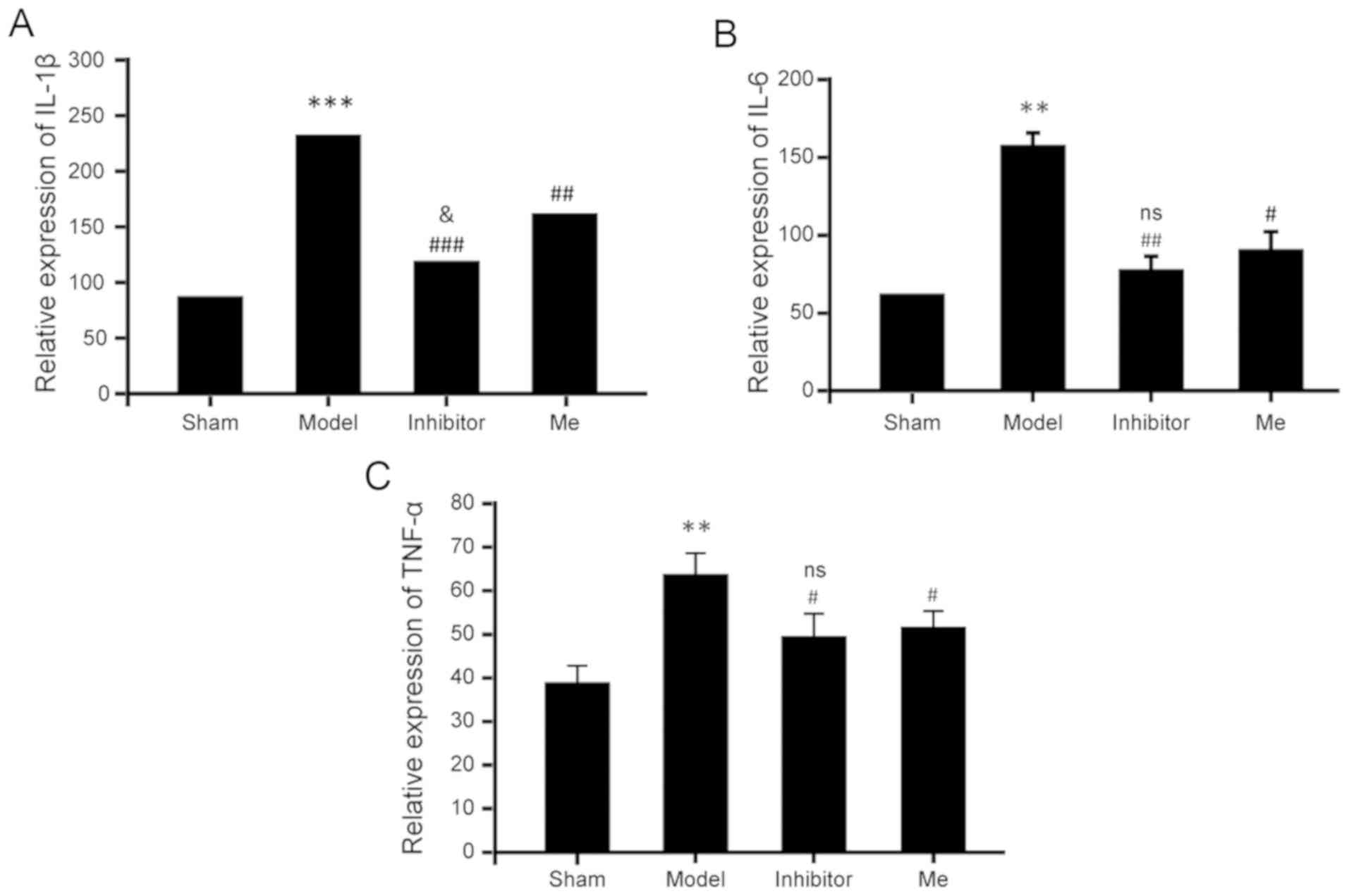
AZD8797 treatment inhibits the
inflammatory response after SCI
Due to the widespread influence of CX3CL1/CX3CR1
signaling during the inflammatory response, the inhibition of this
pathway by AZD8797 could suppress numerous types of chemokines and
cytokines. Thus, the serum levels of multiple cytokines (e.g.,
IL-1β, IL-6 and TNF-α) needed to be determined. As shown in
Fig. 6, on the 10th postoperative
day, the serum levels of these three cytokines were significantly
increased in the SCI group compared to the sham control. However,
the SCI treated with AZD8797 resulted in a significant reduction of
the elevated levels for these three cytokines when compared with
the SCI group without treatment. Methylprednisolone treatment
resulted in a similar reduction in TNF-α and IL-6 when compared to
the AZD8797-treated group, but with a slightly smaller, yet
significant, reduction of IL-1β when compared to the
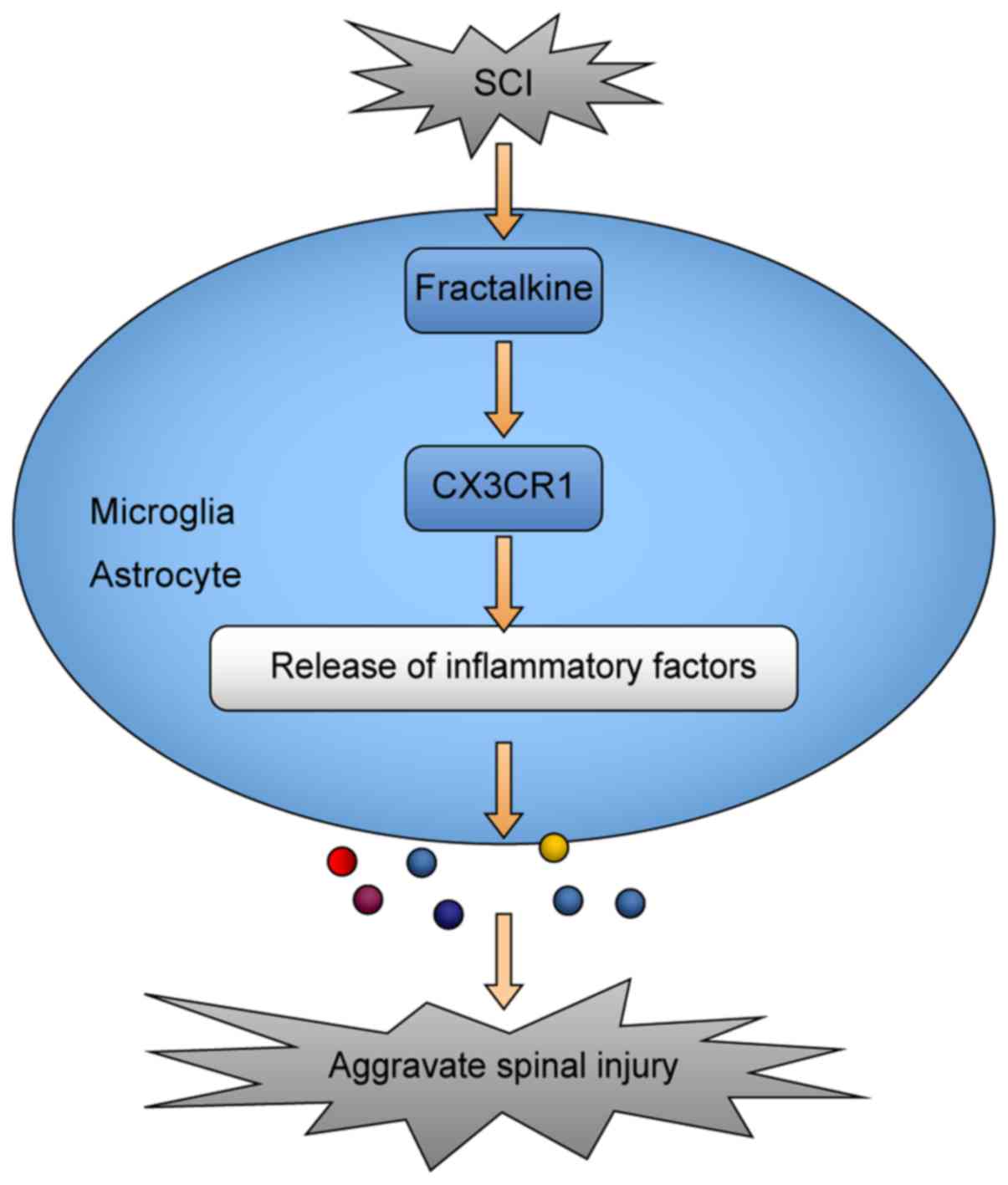
AZD8797-treated group. As regards the potential mechanisms of the
CX3CR1-mediated inflammatory response after SCI, microglia and
astrocytes play an important role (Fig. 7).
Therefore, these results suggest that this
particular CX3CR1 inhibitor can suppress the overall inflammatory
response after SCI and it is as effective as, or even better than,
methylprednisolone.
Discussion
Currently, the precise role of the
fractalkine/CX3CR1 signaling pathway following SCI remains largely
unknown. To this end, using an SCI rat model, the association of
the CX3CL1/CX3CR1 signaling pathway with inflammation was explored,
as well as necrosis after SCI. Combining evaluations for the
expression of both CX3CL1 and CX3CR1 at both the mRNA and protein
levels, it was found that CX3CR1 and CX3CL1 expression levels
consistently increase after 3 days of SCI, reaching a peak after 10
days, and decrease after 14 days. This pattern of short-term
alteration is consistent with its role as a chemokine during the
inflammatory response after SCI. This pattern also indicates a
gradual increase in its role of adhesion and aggregation, whereas a
stable decrease in its expression following 14 days matches the
short-term release of most chemokines. Interestingly, the
suppression of the CX3CL1/CX3CR1 signaling pathway by AZD8797 led
to improved recovery after SCI as shown by BBB scoring, compared
with the sham group within the same period. However, this CX3CR1
inhibitor did not change the expression level of the CX3CR1 protein
but enhanced the expression level of the CX3CL1 protein. AZD8797 is
a non-competitive inhibitor for CX3CR1. The above result suggests
that it has no influence on the CX3CR1 protein itself except for
affecting the activation of its downstream signaling pathway. More
intriguingly, AZD8797 increases the expression level of CX3CL1,
indicating that there is a feedback loop, probably a compensatory
effect after blocking the signaling pathway, which has been pointed
out previously but is yet to be further defined (40).
Unlike CNS diseases in most cases, SCI creates a
short term but usually drastic opening of the blood-brain barrier.
It certainly cannot be ignored that CX3CL1 functions as an adhesion
molecule in a subset of leukocytes, that allows these cells to
cross the blood vessel wall by binding to CX3CR1 expressed in
vascular endothelial cells (41).
Some clinical trials have demonstrated that, in cases of ischemic
stroke, endovascular therapy is effective, especially for those
caused by a proximal intracranial occlusion within the anterior
circulation (42–44). Given that the short-term
alteration pattern of CX3CL1/CX3CR1 signaling matches the time
window of reactive astrogliosis (45,46), microglia, known as resident immune
cells of the CNS, are thus considered to generally play a major
role in inflammatory responses in the CNS, following either CNS
injuries or diseases. Specifically, microglia express CX3CR1 at a
high level and are an activated cell type detectable through
phenotypic transformation following ischemia (47,48). The deficiency in microglial CX3CR1
can cause communication defects among neurons, microglia and
astrocytes during numerous CNS diseases or even injuries (49). In addition, CX3CL1 activation is
correlated with the specific type of neuropathic pain induced by
multiple sclerosis through interaction with CX3CR1 (50). Moreover, the levels of CX3CL1 are
potentially regulated by diverse neurotoxic stimuli and its
signaling is correlated with several types of CNS diseases,
including HIV infection, epilepsy, and cerebral tumors among other
neuropathologies (51).
Methylprednisolone is a glucocorticosteroid commonly
used in the clinic with strong immunosuppressive and
anti-inflammatory effects. A phase III clinical study (52–53) of the National Association of Acute
Spinal Cord Injury Study has confirmed that it is effective to use
high-dose methylprednisolone to treat the early stage of acute SCI.
The known mechanisms for methylprednisolone’s effect in the
treatment of SCI include i) inhibition of lipid peroxidation,
apoptosis, inflammation and the release of inflammatory substances,
and improvement of spinal microcirculation, ii) modification of
vascular permeability and tissue edema, and iii) inhibition of
excitatory amino acid toxicity, promotion of neurotrophic factors
and the release of other cytokines. However, the detailed molecular
mechanism behind methylprednisolone’s effect is not clear. Notably,
previous studies demonstrated that in vitro
lipopolysaccharide- and interferon γ-induced release of multiple
cytokines (i.e., IL-1β, TNFα, and IL-6) in cultured microglia can
be effectively suppressed through the activity of CX3CL1 signaling.
This suggests that CX3CL1/CX3CR1 signaling is vital in modulating
upstream production and cytokine release from microglia (51), thereby contributing to the
feedback loop as well. By inhibiting the cx3cl1/CX3CR1 pathway,
AZD8797 can suppress the role of microglia and astrocytes, thus
preventing the development of the inflammatory response (54,55). The present study shows that
blocking the CX3CR1 signaling with AZD8797 results in, not only a
more effectively reduced concentration of the inflammatory
cytokine, IL-1β (which is associated with a better recovery after
injury), but also lower apoptosis and necrosis levels when compared
to methylprednisolone treatment.
In conclusion, the present study has demonstrated
that treatment with the CX3CR1 specific inhibitor, AZD8797, can
facilitate the recovery of neurological function in the acute phase
of SCI through suppression of CX3CL1/CX3CR1 signaling. The current
findings provide a novel, practical approach for SCI treatment.
Nevertheless, further investigation is required for elucidating the
combined effect of CX3CL1/CX3CR1 signaling, especially considering
its extensive involvement and potential side effects, as well as
analyzing its efficacy and safety in preclinical and clinical
trials.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data used to support the findings of this study
are available from the corresponding author upon request.
Authors’ contributions
GC and ZZ constructed the SCI model, performed
western blot analysis, reverse transcription-quantitative PCR and
immunofluorescence analyses. WS, LW and FY determined the FJB
staining. XY and XQ performed the TUNEL staining. ST and DL
analyzed and interpreted the data. MW finished the assessment of
locomotive recovery of rats (BBB score). GC revised the manuscript
critically for important intellectual content. ST, DL, GC and ZZ
designed the study, supervised the research group and gave final
approval of the version to be published. The final version of the
manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
The present study certifies that all applicable
institutional and governmental regulations concerning the ethical
use of animals were followed during the course of this
research.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that they have no
competing interests.
References
|
1
|
Hyder AA, Wunderlich CA, Puvanachandra P,
Gururaj G and Kobusingye OC: The impact of traumatic brain
injuries: A global perspective. NeuroRehabilitation. 22:341–353.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee BB, Cripps RA, Fitzharris M and Wing
PC: The global map for traumatic spinal cord injury epidemiology:
Update 2011, global incidence rate. Spinal Cord. 52:110–116. 2014.
View Article : Google Scholar
|
|
3
|
Fitzharris M, Cripps RA and Lee BB:
Estimating the global incidence of traumatic spinal cord injury.
Spinal Cord. 52:117–122. 2014. View Article : Google Scholar
|
|
4
|
Oertel M, Kelly DF, McArthur D, Boscardin
WJ, Glenn TC, Lee JH, Gravori T, Obukhov D, McBride DQ and Martin
NA: Progressive hemorrhage after head trauma: Predictors and
consequences of the evolving injury. J Neurosurg. 96:109–116. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen H, Guo Y, Chen SW, Wang G, Cao HL,
Chen J, Gu Y and Tian HL: Progressive epidural hematoma in patients
with head trauma: Incidence, outcome and risk factors. Emerg Med
Int. 2012:134905. 2012. View Article : Google Scholar
|
|
6
|
Cuenca PJ, Tulley EB, Devita D and Stone
A: Delayed traumatic spinal epidural hematoma with spontaneous
resolution of symptoms. J Emerg Med. 27:37–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khuyagbaatar B, Kim K and Kim YH: Effect
of bone fragment impact velocity on biomechanical parameters
related to spinal cord injury: A finite element study. J Biomech.
47:2820–2825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Persson C, McLure S, Summers J and Hall R:
The effect of bone fragment size and cerebrospinal fluid on spinal
cord deformation during trauma: An ex vivo study. J Neurosurg
Spine. 10:315–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilcox RK, Boerger TO, Allen DJ, Barton
DC, Limb D, Dickson RA and Hall RM: A dynamic study of
thoracolumbar burst fractures. J Bone Joint Surg Am. 85:2184–2189.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scholten AC, Haagsma JA, Panneman MJM, Van
Beeck EF and Suzanne P: Traumatic brain injury in the Netherlands:
Incidence, costs and disability-adjusted life years. PLoS One.
9:e1109052014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcia-Altes A, Pérez K, Novoa A, Suelves
JM, Bernabeu M, Vidal J, Arrufat V, Santamarina-Rubio E, Ferrando
J, Cogollos M, et al: Spinal cord injury and traumatic brain
injury: A cost-of-illness study. Neuroepidemiology. 39:103–108.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guha A: Management of traumatic brain
injury: Some current evidence and applications. Postgrad Med J.
80:6502004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borgens RB and Liu-Snyder P: Understanding
secondary injury. Q Rev Biol. 87:89–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oyinbo CA: Secondary injury mechanisms in
traumatic spinal cord injury: A nugget of this multiply cascade.
Acta Neurobiol Exp (Wars). 71:281–299. 2011.
|
|
15
|
Maas AIR, Nino S and Ross B: Moderate and
severe traumatic brain injury in adults. Lancet Neurol. 7:728–741.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silva NA, Sousa N, Rui LR and Salgado AJ:
From basics to clinical: A comprehensive review on spinal cord
injury. Prog Neurobiol. 114:25–57. 2014. View Article : Google Scholar
|
|
17
|
Bazarian JJ, Cernak I, Noblehaeusslein LJ,
Potolicchio S and Temkin N: Long-term neurologic outcomes after
traumatic brain injury. J Head Trauma Rehabil. 24:4392009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang YH, Yang TM, Lin WC, Ho JT, Lee TC,
Chen WF, Rau CS and Wang HC: The prognosis of acute blunt cervical
spinal cord injury. J Trauma. 66:1441–1445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawano H, Kimura-Kuroda J, Komuta Y,
Yoshioka N, Li HP, Kawamura K, Li Y and Raisman G: Role of the
lesion scar in the response to damage and repair of the central
nervous system. Cell Tissue Res. 349:169–180. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ziebell JM and Morganti-Kossmann MC:
Involvement of pro- and anti-inflammatory cytokines and chemokines
in the pathophysiology of traumatic brain injury.
Neurotherapeutics. 7:22–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sandhir R, Puri V, Klein RM and Berman NE:
Differential expression of cytokines and chemokines during
secondary neuron death following brain injury in old and young
mice. Neurosci Lett. 369:28–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Haas AH, van Weering HR, de Jong EK,
Boddeke HW and Biber KP: Neuronal chemokines: Versatile messengers
in central nervous system cell interaction. Mol Neurobiol.
36:137–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rostène W, Dansereau MA, Godefroy D, Van
Steenwinckel J, Reaux-Le Goazigo A, Melik-Parsadaniantz S, Apartis
E, Hunot S, Beaudet N and Sarret P: Neurochemokines: A menage a
trois providing new insights on the functions of chemokines in the
central nervous system. J Neurochem. 118:680–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Estes ML and Kimberley MAA: Alterations in
immune cells and mediators in the brain: It’s not always
neuroinflammation! Brain Pathol. 24:623–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morganti-Kossmann MC, Satgunaseelan L, Bye
N and Kossmann T: Modulation of immune response by head injury.
Injury. 38:1392–1400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bowes AL and Yip PK: Modulating
inflammatory cell responses to spinal cord injury: All in good
time. J Neurotrauma. 31:1753–1766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huhtinen A, Hongisto V, Laiho A,
Löyttyniemi E, Pijnenburg D and Scheinin M: Gene expression
profiles and signaling mechanisms in
α2B-adrenoceptor-evoked proliferation of vascular smooth
muscle cells. Bmc Syst Biol. 11:652017. View Article : Google Scholar
|
|
28
|
Paolicelli RC, Bisht K and Tremblay MÈ:
Fractalkine regulation of microglial physiology and consequences on
the brain and behavior. Front Cell Neurosci. 8:1292014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roseti C, Fucile S, Lauro C, Martinello K,
Bertollini C, Esposito V, Mascia A, Catalano M, Aronica E, Limatola
C and Palma E: Fractalkine/CX3CL1 modulates GABA, currents in human
temporal lobe epilepsy. Epilepsia. 54:1834–1844. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee HW, Lee K, Kim DG, Yang H and Nam DH:
Facilitating tailored therapeutic strategies for glioblastoma
through an orthotopic patient-derived xenograft platform. Histol
Histopathol. 31:269–283. 2016.
|
|
31
|
Marchesi F, Locatelli M, Solinas G, Erreni
M, Allavena P and Mantovani A: Role of CX3CR1/CX3CL1 axis in
primary and secondary involvement of the nervous system by cancer.
J Neuroimmunol. 224:39–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poniatowski ŁA, Wojdasiewicz P, Krawczyk
M, Szukiewicz D, Gasik R, Kubaszewski Ł and Kurkowska-Jastrzębska
I: Analysis of the role of CX3CL1 (Fractalkine) and its receptor
CX3CR1 in traumatic brain and spinal cord injury: Insight into
recent advances in actions of neurochemokine agents. Mol Neurobiol.
54:2167–2188. 2017. View Article : Google Scholar :
|
|
33
|
de Pablos RM, Herrera AJ, Espinosa-Oliva
AM, Sarmiento M, Muñoz MF, Machado A and Venero JL: Chronic stress
enhances microglia activation and exacerbates death of nigral
dopaminergic neurons under conditions of inflammation. J
Neuroinflammation. 11:342014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gwak YS, Kang J, Unabia GC and Hulsebosch
CE: Spatial and temporal activation of spinal glial cells: Role of
gliopathy in central neuropathic pain following spinal cord injury
in rats. Exp Neurol. 234:362–372. 2012. View Article : Google Scholar :
|
|
35
|
Zhang L, Zhang J and You Z: Switching of
the microglial activation phenotype is a possible treatment for
depression disorder. Front Cell Neurosci. 16:3062018. View Article : Google Scholar
|
|
36
|
Joshi M and Fehlings MG: Development and
characterization of a novel, graded model of clip compressive
spinal cord injury in the mouse: Part 1. Clip design, behavioral
outcomes, and histopathology. J Neurotrauma. 19:175–190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu YZ, Wang C, Wang Q, Lin YZ, Ge YS, Li
DM and Mao GS: Role of fractalkine/CX3CR1 signaling pathway in the
recovery of neurological function after early ischemic stroke in a
rat model. Life Sci. 184:87–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fehlings MG, Wilson JR, Harrop JS, Kwon
BK, Tetreault LA, Arnold PM, Singh JM, Hawryluk G and Dettori JR:
Efficacy and safety of methylprednisolone sodium succinate in acute
spinal cord injury: A systematic review. Global Spine J. 7(Suppl
3): S116–S137. 2017. View Article : Google Scholar
|
|
39
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
40
|
Cederblad L, Rosengren B, Ryberg E and
Hermansson NO: AZD8797 is an allosteric non-competitive modulator
of the human CX3CR1 receptor. Biochem J. 473:641–649. 2016.
View Article : Google Scholar :
|
|
41
|
Stavros A and Demetrios S: Chemokines and
atherosclerosis: Focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol
Sin. 34:1251–1256. 2013. View Article : Google Scholar
|
|
42
|
Berkhemer OA, Fransen PS, Beumer D, van
den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn
PJ, Wermer MJ, et al: A randomizedtrial ofintraarterial treatment
for acute ischemicstroke. N Engl J Med. 372:11–20. 2015. View Article : Google Scholar
|
|
43
|
Campbell BC, Mitchell PJ, Kleinig TJ,
Dewey HM, Leonid C, Nawaf Y, Bernard Y, Dowling RJ, Parsons MW,
Oxley TJ, et al: Endovascular therapy for ischemic stroke with
perfusion-imaging selection. N Engl J Med. 372:1009–1018. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Goyal M, Demchuk AM, Menon BK, Eesa M,
Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL,
et al: Randomized assessment of rapid endovascular treatment of
ischemic stroke. N Engl J Med. 372:1019–1030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sofroniew MV: Molecular dissection of
reactive astrogliosis and glial scar formation. Trends Neurosci.
32:638–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hara M, Kobayakawa K, Ohkawa Y, Kumamaru
H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima
Y and Okada S: Interaction of reactive astrocytes with type I
collagen induces astrocytic scar formation through the
integrin-N-cadherin pathway after spinal cord injury. Nat Med.
23:818–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen Y, Won SJ, Xu Y and Swanson RA:
Targeting microglial activation in stroke therapy: Pharmacological
tools and gender effects. Curr Med Chem. 21:2146–2155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stoll G, Jander S and Schroeter M:
Inflammation and glial responses in ischemic brain lesions. Prog
Neurobiol. 56:149–171. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sungho L, Varvel NH, Konerth ME, Guixiang
X, Cardona AE, Ransohoff RM and Lamb BT: CX3CR1 deficiency alters
microglial activation and reduces beta-amyloid deposition in two
Alzheimer’s disease mouse models. Am J Pathol. 177:2549–2562. 2010.
View Article : Google Scholar
|
|
50
|
Zhu W, Acosta C, MacNeil B, Cortes C,
Intrater H, Gong Y and Namaka M: Elevated expression of fractalkine
(CX3CL1) and fractalkine receptor (CX3CR1) in the dorsal root
ganglia and spinal cord in experimental autoimmune
encephalomyelitis: Implications in multiple sclerosis-induced
neuropathic pain. Biomed Res Int. 2013:480702. 2013. View Article : Google Scholar
|
|
51
|
Limatola C and Ransohoff RM: Modulating
neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci.
8:2292014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bracken MB, Shepard MJ, Holford TR,
Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW,
Marshall LF, et al: Administration of methylprednisolone for 24 or
48 h or tirilazad mesylate for 48 h in the treatment of acute
spinal cord injury. Results of the Third National Acute Spinal Cord
Injury Randomized Controlled Trial National Acute Spinal Cord
Injury Study. JAMA. 277:1597–1604. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bracken MB, Shepard MJ, Holford TR,
Leo-Summers L, Aldrich EF, Fazl M, Fehlings MG, Herr DL, Hitchon
PW, Marshall LF, et al: Methylpredniso-lone or tirilazad mesylate
administration after acute spinal cordinjury: 1-year follow up.
Results of the third National Acute Spinal Cord Injury randomized
controlled trial. J Neurosurg. 89:699–706. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Biber K, Owens T and Boddeke E: What is
microglia neurotoxicity (Not)? Glia. 62:841–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kasama T, Wakabayashi K, Sato M, Takahashi
R and Isozaki T: Relevance of the CX3CL1/fractalkine-CX3CR1 pathway
in vasculitis and vasculopathy. Transl Res. 155:20–26. 2010.
View Article : Google Scholar
|