Introduction
Traumatic brain injury (TBI) has the highest
mortality and disability rate of all types of body trauma. It is
usually caused by accidental injuries and traffic accidents and 10
million new patients are affected each year worldwide (1). According to the pathogenic
mechanism, TBI can be divided into primary ischemic brain injury
and secondary injury. Primary brain tissue injury and intracranial
hemorrhage are irreversible, in which inflammation, brain edema and
neuronal apoptosis usually begin to appear several h after the
injury. However, secondary damage is a pathological process that
gradually develops following TBI and subsequent damage can be
reduced by effective therapy (2,3).
Therefore, exploring the mechanism of secondary injury following
TBI and finding clinically effective interventions following TBI
will help the prognosis of patients.
Apoptosis, inflammatory storm, mitochondrial
dysfunction and destruction of the blood-brain barrier, which
ultimately leads to further brain tissue necrosis, are several
aspects of the secondary injury that occurs following TBI (4). Mitochondrial dysfunction results in
a large number of reactive oxygen species (ROS), free radicals and
malondialdehyde (MDA) released in large enough quantities that the
local expression of antioxidants such as superoxide dismutase (SOD)
is not enough to completely counterbalance their damage (5,6).
ROS and MDA destroy nerve cell membranes, promote lipid
peroxidation and chromatin damage and aggravate oxidative stress.
For example, nicotinamide adenine dinucleotide phosphate (NADP)
oxidase NOX2 causes oxidative damage to neurons
following brain trauma by increasing the production of ROS
(7). In addition, nuclear
factor-erythroid 2 related factor 2 (Nrf2) mediates tissue
resistance to oxidative stress. Combined antioxidant therapy to
inhibit NOX2 and activate Nrf2 decreases secondary brain
damage and improves functional recovery following TBI (8). The use of pramipexole (D2-like
receptor agonist) to activate the transcription process of Nrf2 and
its downstream detoxification enzyme heme oxygenase-1 (HO-1) has a
significant neuroprotective effect following TBI (9). Therefore, it is important to
determine if compounds with antioxidant properties, such as
wogonin, a flavonoid compound extracted from the plant
Scutellaria baicalensis Georgi and used in traditional
Chinese medicine, are effective treatments for reducing secondary
TBI injury.
Wogonin has a variety of biological activities
including anti-inflammatory, antioxidant and anti-tumor properties
(10-12). For example, wogonin alleviates
tubulointerstitial fibrosis and kidney tubular epithelial injury
through the PI3K/Akt/NF-κB signaling pathway that mediates
autophagy and inflammation in diabetic nephropathy (13). In a cell model of lung injury,
wogonin prevents apoptosis of injured lung cells by inhibiting the
expression of the Bcl-2 antagonist of cell death (BAD) gene and
subsequent activation of cleaved caspase-3 through the PI3K/AKT
pathway (14). In the prevention
of TBI, wogonin attenuates the symptoms of TBI in Wistar rats
caused by fluid shock injury (15). In addition, wogonin treatment
improves long-term functional and histological outcomes, reduces
brain edema and attenuates the Toll-like receptor
(TLR)4/NF-κB-mediated inflammatory response in mouse TBI (16). Despite evidence indicating the
benefits of wogonin treatment to neurological recovery in stroke
models, the precise mechanisms of these protective effects remain
to be elucidated. In particular, it is unclear whether wogonin
exerts cognitive enhancing or preservation effects by attenuating
oxidative stress and apoptosis in TBI and whether it regulates
PI3K/Nrf2/HO-1 signaling in the brain.
The present study revealed that wogonin exhibited
neuroprotective effects in a rat model of TBI. In terms of
mechanism, wogonin activated Nrf2/HO-1 signaling in a
PI3K/Akt-dependent manner and attenuated oxidative stress and
apoptosis in the hippocampus of rats following TBI. The present
study provided a new basis for drug treatment and neuroprotection
following TBI.
Materials and methods
Establishment of TBI model
A total of 120 adult male SD rats (body weight
300-330 g; aged 10-12 weeks; purchased from animal Experiment
Center of Hebei Medical University) were provided with food and
water ad libitum and kept in a specific pathogen-free (SPF)
animal room under controlled conditions (12-h light/dark cycle and
22±3°C temperature; humidity 40-70%). The TBI model is induced by
the controlled cortical impingement (CCI) method, as described
previously (17). In short, rats
anesthetized with 10% chloral hydrate (300 mg/kg,
intraperitoneally) were fixed in a stereotactic frame. Rats were
anesthetized ~3 min following injection and all animals exhibited
no side effects, such as peritonitis, pain, or discomfort. A
midline incision was made to expose the skull and then a 6.0 mm
diameter right-hand hole was made through a surgical electric skull
grinder. The opening was centered on the coronal suture and 2.5 mm
outside the sagittal suture. An electromagnetic impactor (round tip
diameter 4 mm; speed 5 m/sec; residence time 100 msec) was used to
inflict CCI injury on rats with an impact depth of 2.0 mm. Finally,
the bone flap was sealed and sutured. For the sham operation group,
the animals received the same anesthesia and surgical scalp
incision, but no impact injury. All animal experiments were
approved by the Ethics Committee of Hebei Medical University
(permit no. 20201086). Humane endpoints were established following
the guideline of assessment for humane endpoints in animal
experiment [People's Republic of China: RB/T 173-2018; bingdian001.com (lascn.net)] in
order to minimize pain or distress to experimental animals. After
the breathing of the rat became slow and weak and it did not
respond to stimulation, the rats was sacrificed by euthanasia under
anesthesia (sodium pentobarbital 40 mg/kg, intraperitoneally).
Cervical vertebra dislocation was used for euthanasia and death was
confirmed by cessation of the heartbeat.
Groups and drug administration
The rats were randomly assigned into three groups:
sham operation group, TBI model group and wogonin treatment group
following TBI. Each sub-group was composed of five rats, testing
after injury was as follows: i) Neurological severity scores at
days 1, 3, 5, 7 and 14; ii) determination of brain water content at
days 3; iii) Morris Water Maze (MWM) test at days 3-7; and iv)
histological analysis, TUNEL, ROS and antioxidant enzymes activity
and western blot analysis at day 1, 3, 7. Wogonin (MilliporeSigma)
was prepared by dissolving in 25% dimethyl sulfoxide (DMSO).
According to the dose provided in a previous study (16), wogonin solution (40 mg/kg) was
injected intraperitoneally at 10 min, 24 and 48 h after the
surgery. The sham group and TBI group were both intraperitoneally
injected with the same amount of normal saline. Sham-operated rats
underwent procedures identical to those of the TBI animals,
including anesthesia and craniotomy surgery, but without TBI. It
should be noted that no adverse effects or mortality were observed
in rats treated with wogonin during the experiments. Therefore,
from the perspective of animal welfare, a blank control group was
not established.
For the study of the mechanism of wogonin affecting
the hippocampus, rats were randomly divided into 5 groups: Sham
operation group, TBI model group, wogonin treatment group following
TBI, wogonin and LY294002 treatment group following TBI and DMSO
administration control following TBI. PI3K inhibitor LY294002 was
dissolved in 25% DMSO in PBS at the time of administration. Rats
were infused with 50 mM LY294002 in one side of the cerebral
ventricle 30 min before TBI. Specifically, according to the
previous study (18), rats under
anesthesia were provided with stepping electric micro-injectors
(Stoelting) to inject LY294002 or DMSO into the left cerebral
ventricle at a rate of 1 µl/min. A total of 120 rats were
used in the present study, of which 2 rats that received CCI died
of brain damage.
Neurological severity score
The neurological severity score (NSS) is a composite
of motor, sensory, reflex and balance tests before and after
treatment in rats, as described previously (19). All rats were assessed for NSS on
days 1, 3, 5, 7 and 14 following TBI. Scores are evaluated by
researchers who are unaware of the grouping, with 1 point for
inability to perform each test or lack of test reflexes. Normal
rats have a score of 2-3, while the maximum injury score is 18.
Evaluation of cerebral edema
The degree of cerebral edema is assessed by relative
brain water content analysis, as described previously (20). Rats were sacrificed by
decapitation under anesthesia (2% sodium pentobarbital, 40 mg/kg,
i.p. injected) on the 3rd day following TBI or sham operation. The
rat brain was harvested and immediately weighed as wet weight (WW).
The brain tissue was then dried at 100°C for 24 h to obtain a dry
weight (DW). The relative brain water content is calculated as
follows: (WW-DW)/WW ×100%.
Morris water maze (MWM)
According to previous research (21), the spatial learning and memory
ability of rats was evaluated in the MWM test 3-7 days following
TBI. Prior to the test, the rats swam freely in the circular pool
for 5 min. In the central area of the target quadrant, a 2 cm
opaque hidden platform (12 cm in diameter and 28 cm in height) was
set up. Prior to the operation, all the rats were guided to the
target platform. In each test, 60 sec were set aside for the rat to
find the platform. The camera hanging above the maze was connected
to a video tracking system (HVS Imaging) and the escape delay time
was recorded. The average escape latency of four repeated trials
was calculated. On the last day of the experiments, the hidden
platform was removed and the percentage of time the rat stayed in
the target quadrant tested. The average swimming speed of rats in
each group was also recorded.
Hematoxylin and eosin staining
(H&E)
The brain tissues were fixed in 4% paraformaldehyde
solution for 24 h at room temperature, washed with running water
for 4 h, then dehydrated with graded alcohol and embedded in
paraffin following standard histological procedures. The paraffin
tissue was sectioned at 4 µm. Sample sections were stained
with H&E according to the usual protocol (hematoxylin for 2 min
and eosin for 30 secs at room temperature). Images of the staining
results of the hippocampal brain slices were captured under a light
microscope (BX51; Olympus Corporation; magnification ×50 and ×200)
and the pyramidal cell density (number/mm2) in the CA1
area was calculated. Cell counts from hippocampus CA1 on each of
the four sections were averaged to provide a single value (number
of neurons/mm2) for each animal. Image Pro Plus 6.0
software (Media Cybernetics) was used for analysis.
Cell apoptosis assay
According to the manufacturer's instruction, the
TdT-mediated dUTP nick-end labeling (TUNEL) apoptosis detection kit
(cat. no. C1088, Beyotime Institute of Biotechnology) was used to
detect neuronal apoptosis in the hippocampus. Briefly, the
hippocampal tissue sections of each group of rats were
deparaffinized and rehydrated. Then the sections were incubated
with 10 µg/ml proteinase K solution at 37°C for 15 min. The
sections were stained with green fluorescein-labeled dUTP solution
for 10 min at room temperature. Finally, the section was sealed in
a mounting medium containing DAPI. The number of TUNEL-DAPI
positive cells was counted using a fluorescence microscope (Olympus
Fluoview FV1000; Olympus Corporation; magnification ×200) according
to a previous study (22). Image
Pro Plus 6.0 software (Media Cybernetics) was used for
analysis.
Immunohistochemistry IHC
The brain tissue sections of TBI rats and wogonin
treatment rats were used for IHC detection. The paraffin sections
were incubated with 3% H2O2 for 20 min at
room temperature and then blocked with goat serum (cat. no. C0265;
Beyotime Institute of Biotechnology) for 1 h at room temperature.
Then the sections were incubated with NOX2 primary
antibody (1:200 dilution; Abcam; cat. no. ab80508) at 4°C
overnight. Following washing with PBS, the sections were incubated
with goat anti-rabbit IgG-HRP secondary antibody (1:500 dilution;
cat. no. sc-2030; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. After counterstaining with hematoxylin, images of the
sections were captured using the AxioVision4Ac microscope system
(Carl Zeiss AG). IHC images were analyzed with optical density (OD)
value by ImageJ software v1.41 (National Institutes of Health). The
deeper the DAB staining, the greater the OD value and protein
expression.
NOX activity determination
NOX activity analysis was carried out according to
previous research (23). The
hippocampal tissue samples were homogenized and lysed and then
centrifuged at 1,000 × g for 10 min at 4°C to collect the
supernatant. The supernatant was centrifuged in an ultracentrifuge
at 13,000 × g at 4°C for 20 min to separate the membrane components
and 50 µg membrane fraction was used to determine NOX enzyme
activity. Relative light units (RLU) were recorded every 1 min
continuously for 5 min by a standard luminometer, as the indicator
of NOX activity (RLU/µg/min).
The activities of SOD, catalase (CAT),
glutathione (GSH) and the levels of MDA and ROS measurement
The hippocampal tissue following TBI or
administration was mechanically homogenized and then 0.5-10% of the
tissue homogenate was used to detect oxidative stress-related
indicators. The measurement of SOD activity was carried out in
accordance with the manufacturer's instructions (cat. no. A001;
Nanjing Jiancheng Bioengineering Institute). The SOD level was
measured by the absorbance of the sample at 550 nm, expressed as a
unit/g protein. CAT activity was measured by ammonium molybdate
spectrophotometry according to the manufacturer's instructions
(cat. no. A007; Nanjing Jiancheng Bioengineering Institute). CAT
decomposed the hydrogen peroxide in the tissue and then the
remaining hydrogen peroxide reacted with ammonium molybdate to form
a yellow complex. The activity of CAT was evaluated by measuring
the content of the yellow complex. Tissue MDA was determined by
using the thiobarbituric acid (TBA) reaction method as described
previously (7). MDA reacted with
TBA to form a pink chromogen and its absorbance at 532 nm could
represent the MDA content (nmol MDA/g tissue). For the measurement
of GSH, the principle is that 5,5′-dithiobis-2-nitrobenzoic acid
oxidizes GSH in tissues to produce 5-thio-2-nitrobenzoic acid. The
GSH measurement was performed according to the instructions of the
manufacturer of the kit (cat. no. 703002; Cayman Chemical Company).
The level of ROS was carried out in accordance with the
manufacturer's instructions (cat. no. E004 Nanjing Jiancheng
Bioengineering Institute). The hippocampal tissue homogenate was
centrifuged at 500 × g for 10 min at 4°C and then 1 ml of 0.01 M
PBS solution was used to resuspend the cell pellet. 10 µl 1
mM 2,7-dichlorodihydrofluorescein diacetate was added to the cells.
After incubating for 30 min at 37°C, the fluorescence intensity was
quantified using a spectrofluorometer (excitation, 500 nm;
emission, 525 nm).
Immunofluorescence
Briefly, after intracardially perfused with 4%
paraformaldehyde solution for 30 min at 4°C, brains tissues were
removed, post-fixed in the same fixative for 1 day at room
temperature and subsequently soaked in 30% sucrose for 2-3 days at
4°C. Tissues were embedded in an optimal cutting temperature
compound (OCT, Sakura Finetek Europe B.V.) and frozen sections cut
at 15 µm. The frozen sections were permeabilized with 0.4%
Triton X-100 for 10 min and then blocked with 10% donkey serum
(cat. no. ab138579; Abcam) for 30 min at room temperature. Sections
with NeuN antibody (dilution 1:100; cat. no. MAB377;
MilliporeSigma), phosphorylated (p-)Akt antibody (dilution 1:100;
cat. no. PA5-104867; Invitrogen; Thermo Fisher Scientific, Inc.) or
Nrf2 antibody (dilution 1:100; cat. no. sc-722; Santa Cruz
Biotechnology, Inc.) were incubated overnight at 4°C. The sections
were incubated with fluorescently-labeled secondary antibody
Alexa-Fluor594 or Alexa-Fluor 488 (diluted 1:200; cat. no.
sc-362281 and sc-362257; Santa Cruz Biotechnology, Inc.) for 2 h in
the dark. Finally, the slices were observed and images captured
under a fluorescence microscope (Olympus Fluoview FV1000; Olympus
Corporation; magnification, ×200). The counting area was located in
the same position in all groups, and the number of p-Akt-positive
or Nrf2-positive NeuN in the hippocampus CA1 was counted for each
section. For each group, quantification was performed in sections
from five different rats.
Western blotting
The hippocampus tissue of the rats in the TBI group
or the administration group was lysed in RIPA lysis buffer (Thermo
Fisher Scientific, Inc.) and the protein concentration was
quantified by BCA reagent (Beijing Solarbio Science &
Technology Co., Ltd.). Protein (30 µg) was used for 12%
SDS-PAGE electrophoresis and then transferred to PVDF membrane
(Bio-Rad Laboratories, Inc.). The membrane was blocked with 5%
skimmed milk for 2 h at room temperature and then incubated with
the primary antibodies overnight at 4°C. The primary antibodies
used included: NOX2 antibody (dilution 1:500; cat. no.
ab80508; Abcam), Nrf2 antibody (dilution 1:500; cat. no. sc-722;
Santa Cruz Biotechnology, Inc.), p-Akt antibody (dilution 1:500;
PA5 -104867; Invitrogen), Akt antibody (dilution 1:500; cat. no.
MA5-14898; Invitrogen), caspase-3 antibody (dilution 1:500; cat.
no. sc-56053; Santa Cruz Biotechnology, Inc.), Bax antibody
(dilution 1:500; cat. no. sc-65532; Santa Cruz Biotechnology,
Inc.), Bcl-2 antibody (dilution 1:500; cat. no. sc-7382; Santa Cruz
Biotechnology, Inc.), HO-1 antibody (dilution 1:500; cat. no.
sc-136960; Santa Cruz Biotechnology, Inc.) and β-actin antibody
(dilution 1:1,000; cat. no. AF7018; Affinity Biosciences). The
membrane was then incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody [goat anti-rabbit IgG-HRP (cat.
no. sc-2030); goat anti-mouse IgG-HRP (cat. no. sc-2005). 1:2,000
dilution; Santa Cruz Biotechnology, Inc.] for 2 h. The western
blotting signal on the membrane was obtained by an enhanced
chemiluminescence (ECL) detection system. ImageJ software v 4.1
(National Institutes of Health) was used to quantify the optical
density signal.
Statistical analysis
SPSS 16.0 software (SPSS, Inc.) was used for data
analysis, and all data were expressed as mean ± standard deviation
(SD). Data comparison among multiple groups was conducted with
one-way analysis of variance (ANOVA), statistical analysis between
two groups were performed with the Newman-Keuls test and between
multiple groups with one-way ANOVA followed by Tukey test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
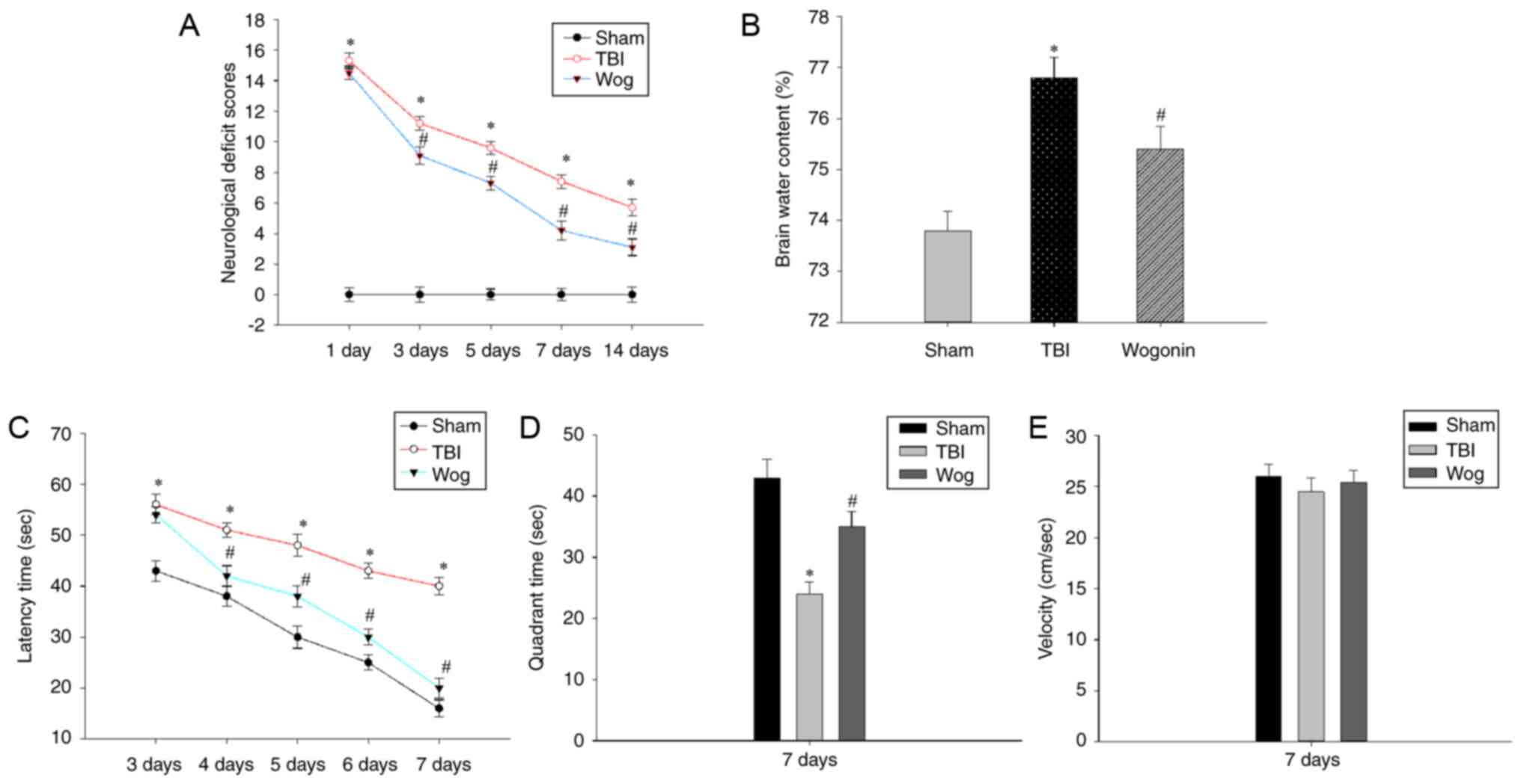
Wogonin attenuates neurological damage
following traumatic brain injury
The present study established a rat TBI model and
verified if wogonin alleviated the neurological damage and
cognitive function caused by TBI. First, the NSS assessment and the
degree of cerebral edema were used to evaluate the neurological
deficit. Compared with sham-operated animals, TBI at 1-14 days
significantly increased the NSS score of rats
(*P<0.05). After treatment with wogonin in TBI rats,
wogonin significantly reduced the NSS score on days 3, 5, 7 and 14
(#P<0.05, Fig.
1A). Brain water content analysis was used to evaluate the
therapeutic effect of wogonin on brain edema in TBI model. TBI
induced a significant increase in brain water content
(*P<0.05), which could be alleviated by wogonin
treatment (#P<0.05, Fig. 1B). Next, the MWM test was used to
evaluate whether wogonin helps to alleviate the spatial memory
impairment induced following TBI. As shown in Fig. 1C, TBI caused a significant
spatial learning deficit compared with the rats in the sham group
and TBI rats spent a longer time searching for the hidden platform
at 3-7 days post-surgery (*P<0.05). However, rats in
the wogonin group displayed a profoundly shorter latency time at
4-7 days as compared to those in the TBI group
(#P<0.05). After removing the hidden platform, TBI
caused the rat to spend an extended time in finding the target
quadrant that previously contained the platform. The administration
of wogonin significantly shortened the time for TBI model mice to
navigate to the target quadrant (Fig. 1D). During this period, there was
no significant difference in the swimming speed of the rats in each
group (Fig. 1E), which proved
that the time they searched for the target was not affected by
their swimming speed. These results prove that wogonin slows down
the neurological damage and reduces brain edema following TBI in
rats and helps to improve the learning and memory ability.
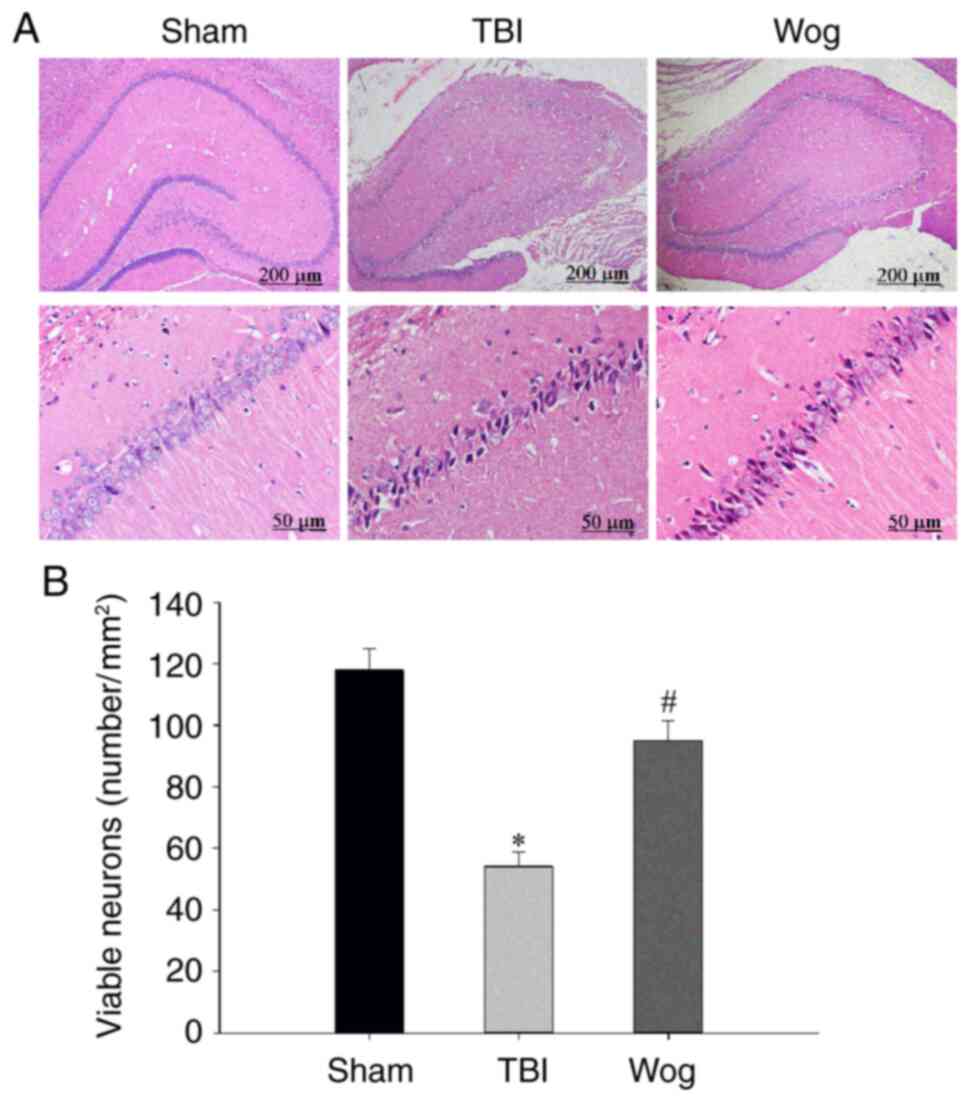
H&E staining was used to measure hippocampal
neurons densities to determine if the behavior in the water maze
correlated with brain recovery of rats treated with wogonin
following TBI. The H&E staining results showed that there was
significant neuronal damage in the hippocampus of rats with TBI,
which was manifested as nuclear pyknosis, decreased number of
neurons and neuron atrophy. However, wogonin treatment reduced
neuronal loss caused by TBI (Fig.
2A). The statistical results of the number of neurons in the
stained visual field showed that TBI significantly induced the
death of pyramidal cells in the hippocampus 24 h after injury,
while wogonin prevented the loss of neurons induced by TBI
(*P<0.05 vs. sham group; #P<0.05 vs.
TBI group; Fig. 2B). These
results proved that wogonin reduces the damage of hippocampal
neurons induced by TBI.
Wogonin promotes anti-oxidation following
TBI
In order to explore the mechanism of wogonin in
alleviating the neurological damage caused by TBI, its role in
anti-oxidation was first tested. The levels of GSH, SOD, CAT, MDA
and ROS in hippocampal tissues 1, 3 and 7 days after the operation
were used as detection indicators. The results showed that TBI
caused a significant decrease in the levels of GSH, SOD and CAT in
the hippocampus. However, administration of wogonin following TBI
increased the amount of these antioxidant factors
(#P<0.05 vs. TBI group; Fig. 3A-C). In addition, compared with
the TBI model group, the administration of wogonin significantly
reduced the levels of oxidative stress products MDA and ROS
(#P<0.05 vs. TBI group; Fig. 3D-E). These results proved that
wogonin had a repairing effect through anti-oxidation in the
hippocampus damaged following TBI.
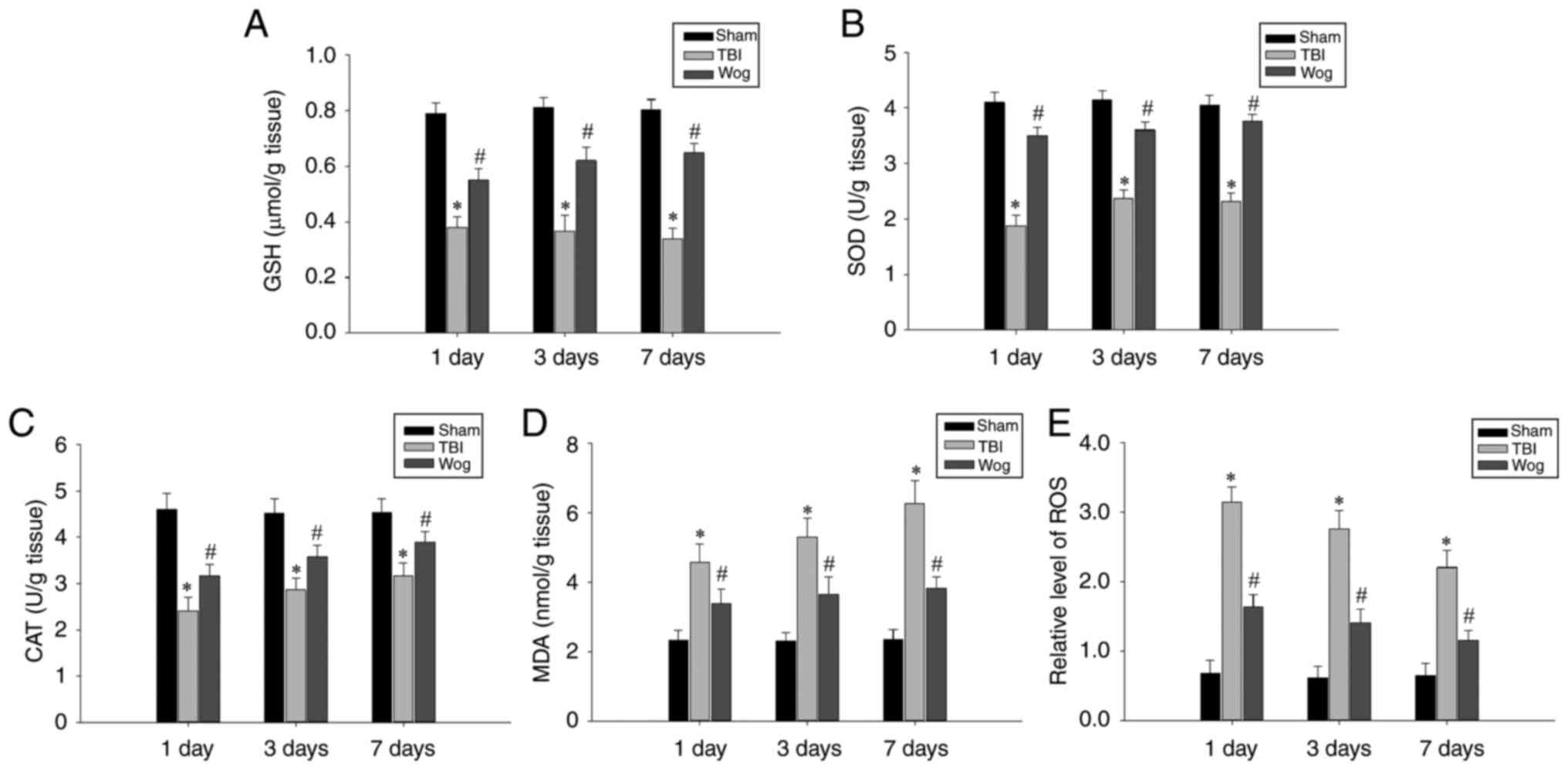 | Figure 3Wogonin regulates the levels of
oxidative stress-related factors. The levels of (A) GSH, (B) SOD,
(C) CAT, (D) MDA and (E) ROS in the hippocampus of rats in the sham
operation group (sham), TBI model group (TBI) and wogonin
administration group (Wog) were detected on the 1, 3 and 7 days
after the operation. Data are expressed as mean ± standard
deviation (n=5/group; *P<0.05 vs. sham group;
#P<0.05 vs. TBI group). GSH, glutathione; SOD,
superoxide dismutase; CAT, catalase; MDA, malondialdehyde; ROS,
reactive oxygen species; TBI, traumatic brain injury. |
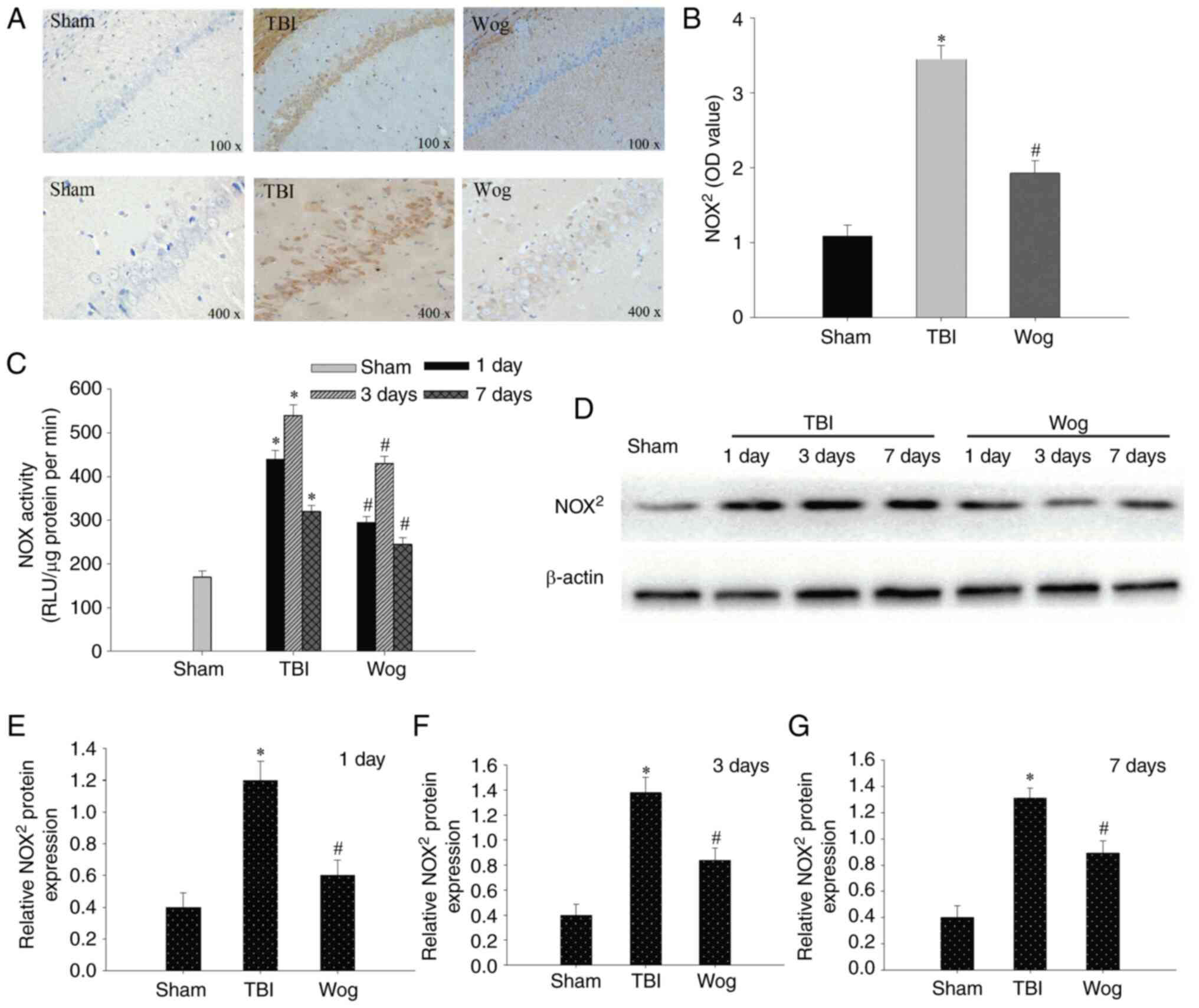
Since NADPH oxidase mediates oxidative
stress-related damage, the expression of NOX2, the main
type of NADPH oxidase, was examined by IHC and western blotting. As
shown in Fig. 4A, occasional
NOX2 positive cells were detected on day 3 in the sham
group. By contrast, NOX2 positive cells were
significantly increased 3 days following TBI as indicated with an
intense color due to enhanced immune reactivity. However, the
immune reactivity of NOX2 in the wogonin group was
significantly weaker than that in the TBI group (Fig. 4B). Compared with the TBI model
group, the administration of wogonin significantly reduced the
enzyme activity of NOX in the hippocampal CA1 region on days 1, 3
and 7 (*P<0.05 vs. sham group; #P<0.05
vs. TBI group; Fig. 4C). western
blotting was used to detect protein expression in hippocampal CA1
area on day 1, 3 and 7 after the operation (Fig. 4D). The results of blotting
optical density analysis showed that, compared with the sham group,
the NOX2 protein expression in the TBI group was
significantly upregulated at 1, 3 and 7 days after the operation.
Compared with the TBI group, the administration of wogonin
significantly reduced the expression of NOX2 protein
(*P<0.05 vs. sham group; #P<0.05 vs.
TBI group; Fig. 4E-G).
Therefore, wogonin exercised its antioxidant function by decreasing
the expression of NOX2 protein.
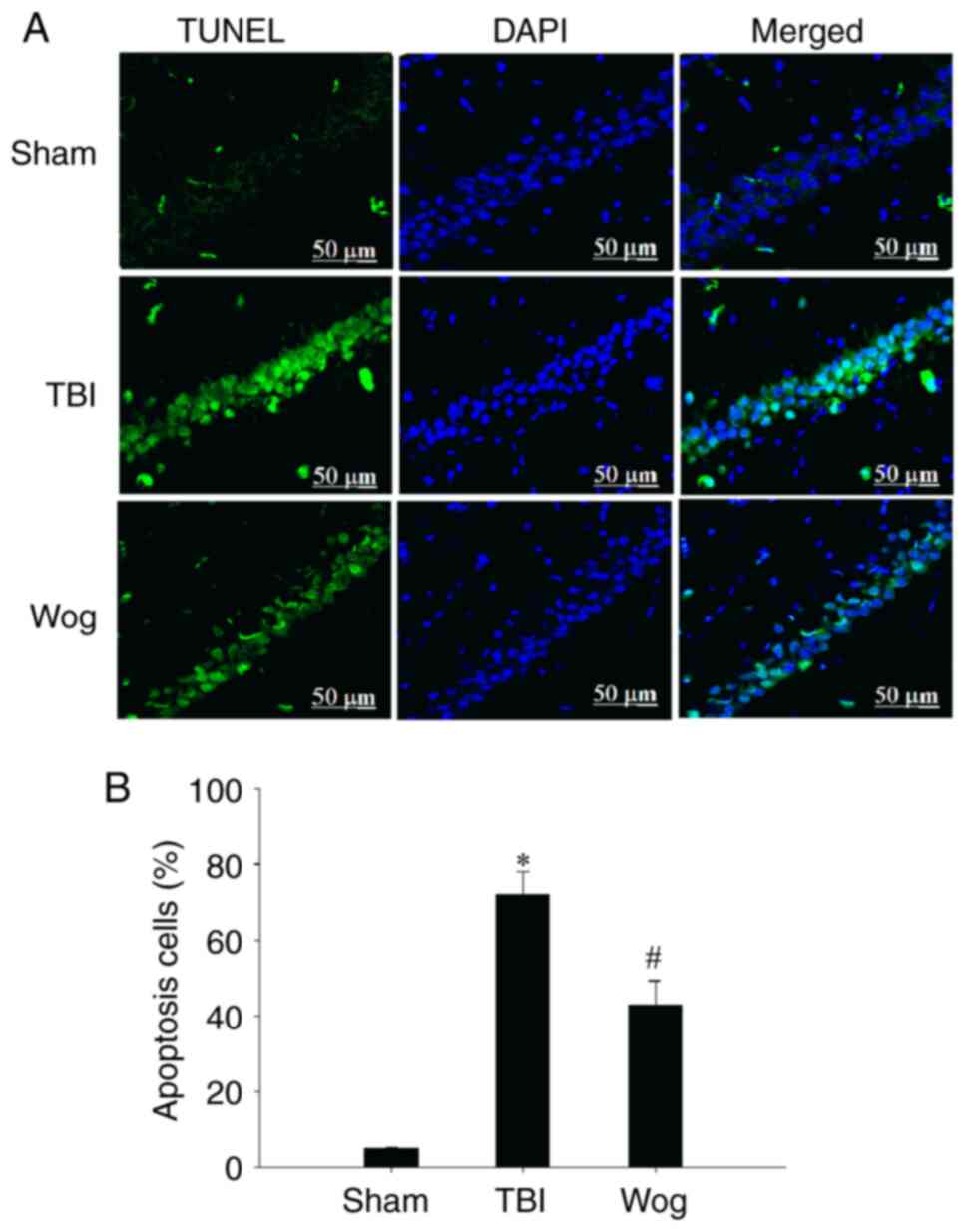
Wogonin inhibits apoptosis of hippocampal
cells induced by TBI
TUNEL assay was used to evaluate the apoptosis in
the CA1 area of the hippocampus following surgery (Fig. 5A). The statistical results showed
that TBI significantly increased the number of TUNEL-positive cells
in the CA1 region of the hippocampus. However, compared with the
TBI group, the apoptotic cells in the wogonin group were
significantly reduced (*P<0.05 vs. sham group;
#P<0.05 vs. TBI group; Fig. 5B). Then the expression of
apoptosis-related proteins were detected by western blotting,
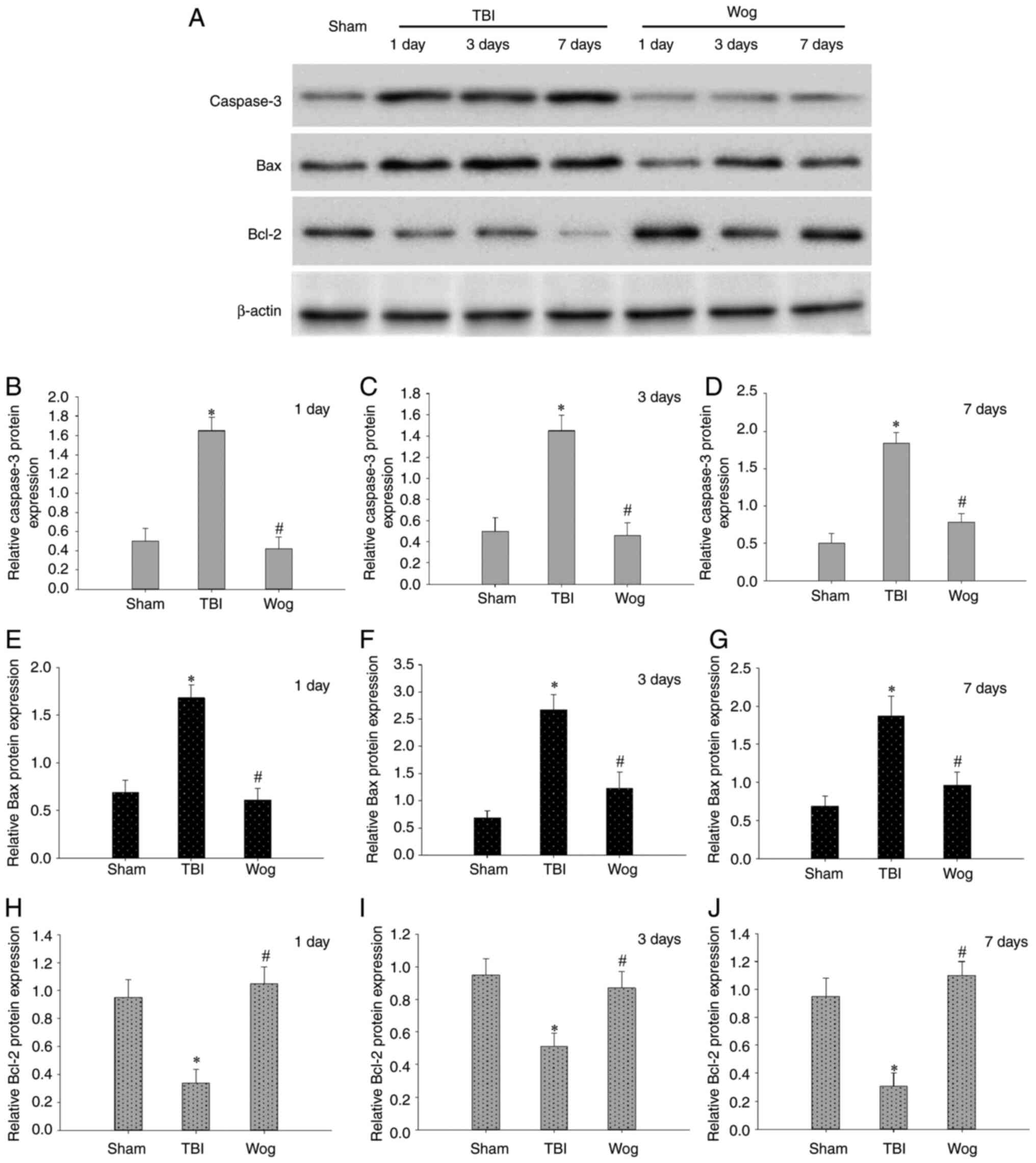
including caspase-3, Bax and Bcl-2 (Fig. 6A). The results of blotting
density analysis showed that TBI increased the expression of
caspase-3 and Bax, but decreased the expression of Bcl-2 on days 1,
3 and 7 after the operation (*P<0.05 vs. sham group;
Fig. 6B-J). However, compared
with the TBI group, wogonin administration significantly reduced
the expression of caspase-3 and Bax and promoted the protein
expression of Bcl-2 (#P<0.05 vs. TBI group; Fig. 6B-J). This suggested that wogonin
effectively inhibited the apoptosis of hippocampal CA1 area
following TBI.
Wogonin promotes PI3K/Akt signaling in
the hippocampus following TBI
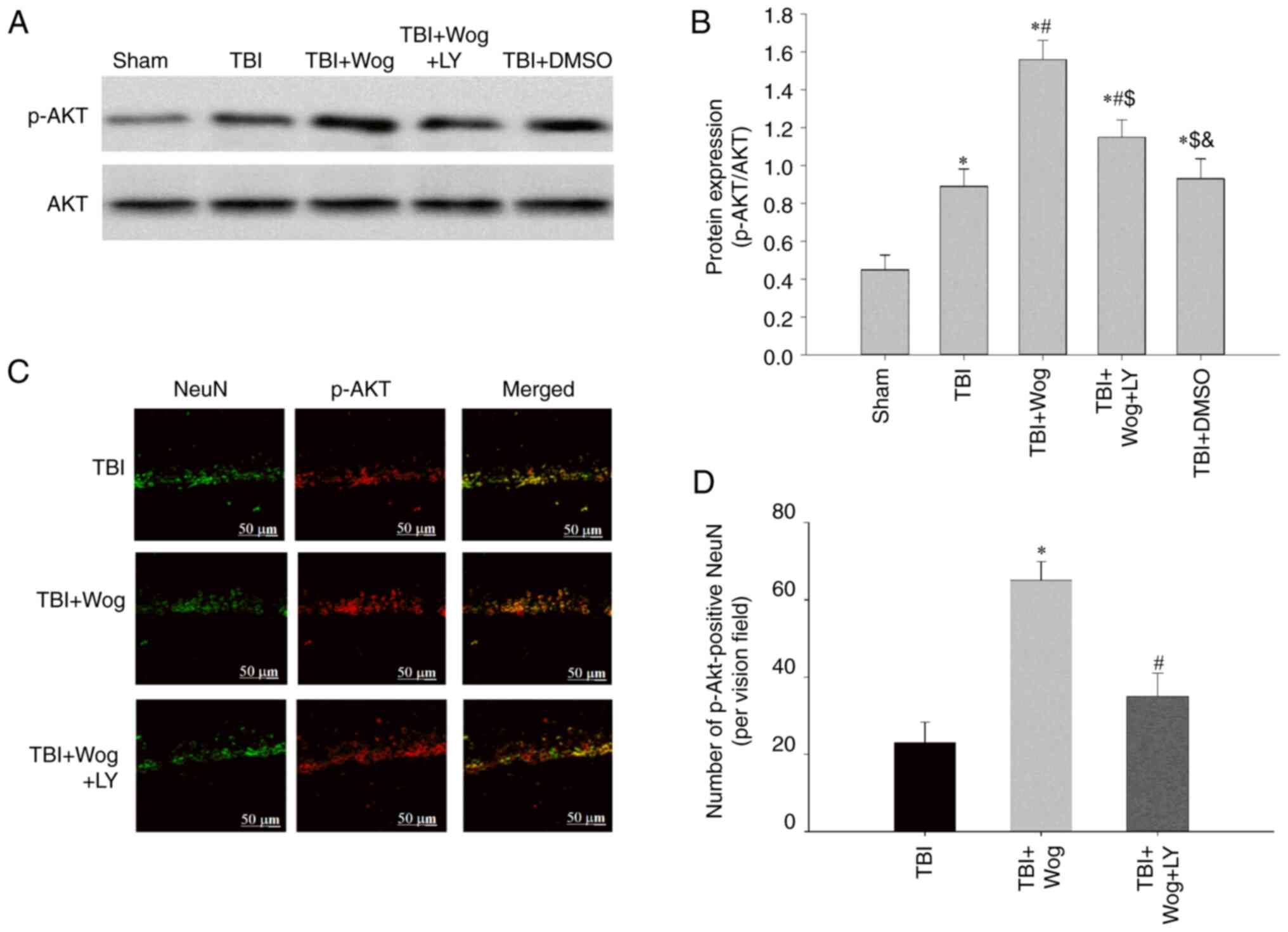
By administering the PI3K inhibitor LY294002, the
PI3K/Akt signal in the hippocampal CA1 area was checked at 24 h
following TBI. The results showed that TBI significantly increased
the expression of p-Akt and the administration of wogonin further
increased the expression of p-Akt following TBI
(*P<0.05 vs. sham group; #P<0.05, vs.
TBI group; Fig. 7A and B).
However, compared with the administration of wogonin, the
additional application of LY294002 reduced the level of p-Akt
($P<0.05 vs. TBI + wogonin group; Fig. 7A and B). This showed that the
regulation of wogonin on the expression of p-Akt is through the
action of PI3K. Immunofluorescence experiments have proved that
p-Akt-positive cells colocalized mainly with NeuN-positive neurons
in the hippocampus of the TBI rats, suggesting that p-Akt was
induced mainly in neurons (24).
The results mirror the western blot results observed in Fig. 7A and B, with a robust increase of
p-Akt immunofluorescence observed in the wogonin group, suggesting
that the level of p-Akt was increased in hippocampal area, which
was reduced by LY294002 pre-treatment (Fig. 7C and D). It was hypothesized that
wogonin serve a key role in the repair of damage following TBI by
inducing PI3K/Akt signaling.
Wogonin promotes the expression of Nrf2
and HO-1 proteins through the PI3K/Akt pathway
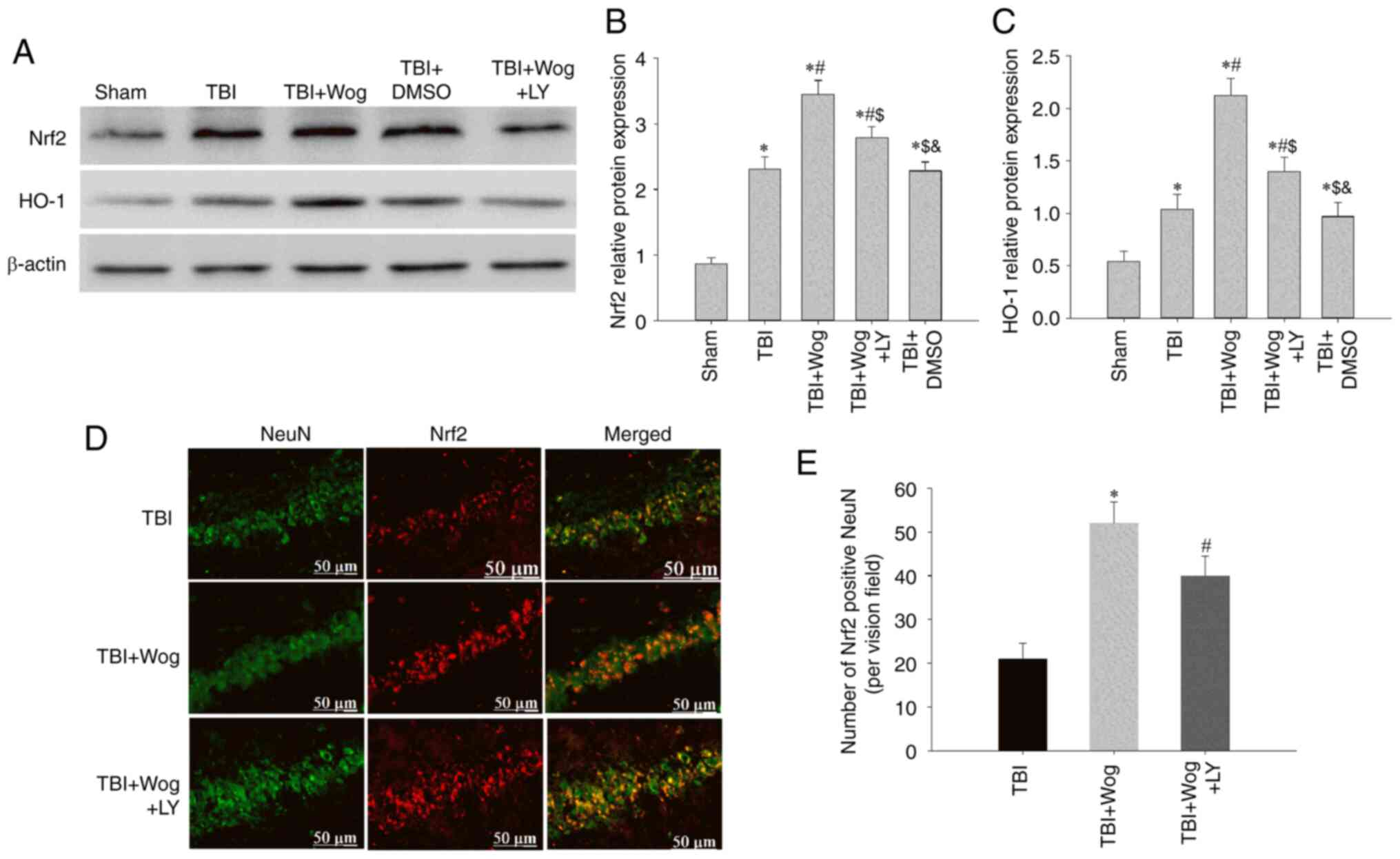
It was considered whether wogonin regulated the
expression of Nrf2 and HO-1, which are considered to be involved in
anti-oxidation, through PI3K/Akt signal following TBI. Western
blotting results showed that the administration of wogonin
increased the expression of Nrf2 and HO-1 following TBI
(*P<0.05 vs. sham group; #P<0.05, vs.
TBI group; Fig. 8A-C). Compared
with the use of wogonin, the additional administration of LY294002
inhibited the expression of Nrf2 and HO-1 induced by wogonin
($P<0.05 vs. TBI + wogonin group; Fig. 8A-C). Furthermore, the western
blot results were supported by double immunofluorescence for Nrf2
and NeuN, showing augmented neuronal Nrf2 immunoreactivity in the
wogonin group, as compared to TBI or LY294002-treated groups at 24
h (Fig. 8D and E). These data
proved that wogonin upregulates the antioxidant proteins Nrf2 and
HO-1 by inducing PI3K/Akt signaling.
Wogonin reduces oxidative damage and cell
apoptosis by regulating PI3K/Nrf2/HO-1 pathway
Since wogonin promotes PI3K/Nrf2/HO-1 signaling
following TBI, whether wogonin could reduce oxidative stress and
apoptosis through this pathway was verified. Western blotting
results showed that the administration of wogonin inhibited the
protein expression of NOX2, caspase-3 and Bax following
TBI (*P<0.05 vs. sham group; #P<0.05,
vs. TBI group; Fig. 9A).
Compared with the use of wogonin, the additional administration of
LY294002 restored the levels of NOX2, caspase-3 and Bax
($P<0.05 vs. TBI + wogonin group; Fig. 9B-D). For Bcl-2, the additional
administration of LY294002 abolished the upregulation effect of
wogonin on Bcl-2 protein expression (Fig. 9E). Therefore, the antioxidant and
anti-apoptotic properties of wogonin after the occurrence of TBI
are achieved by upregulating the PI3K/Akt/Nrf2/HO-1 signal.
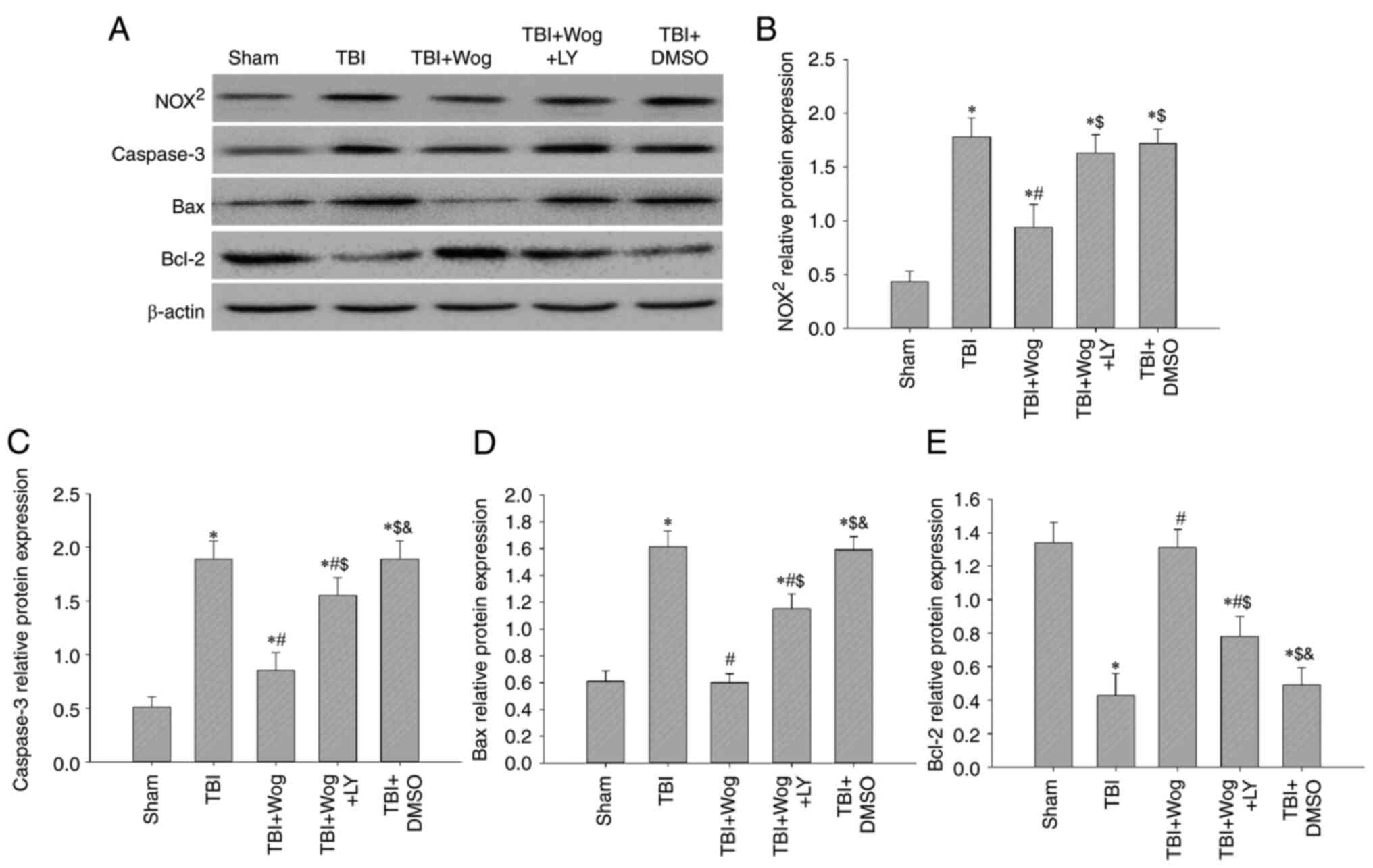 | Figure 9Wogonin reduces oxidative damage and
cell apoptosis by regulating the PI3K/Nrf2/HO-1 pathway. (A)
Western blotting analysis showed NOX2, caspase-3, Bax
and Bcl-2 protein levels in the rat hippocampus at 24 h from the
sham group, TBI group, TBI + Wog group, TBI + Wog + LY group and
TBI + DMSO group. (B-E) The optical density of NOX2,
caspase-3, Bax and Bcl-2 bands relative to β-actin was analyzed.
*P<0.05 vs. sham group; #P<0.05 vs. TBI
group; $P<0.05 vs. TBI + Wog group;
&P< vs. TBI + Wog + LY group. Nrf2, nuclear
factor-erythroid factor 2-related factor 2; HO-1, heme oxygenase-1;
TBI, traumatic brain injury. |
Discussion
As a serious global public health problem, TBI has
causes great pain and an economic burden on patients and society.
The secondary chronic diseases that appear after the occurrence of
TBI are mainly manifested as nerve cell death and nervous system
disorders, accompanied by cerebral ischemia and even
neurodegenerative diseases (25,26). Although some supportive therapies
have been used clinically, including anti-inflammatory, preventing
thrombosis and reducing intracranial pressure, they remain
insufficient to restore normal brain function in patients following
TBI (27,28). Therefore, it is necessary to
explore the pathogenesis of TBI and the means to protect the brain
tissue from damage.
Flavonoids isolated from the traditional Chinese
medicine Scutellaria baicalensis, including baicalin,
baicalein and wogonin, serve anti-oxidant, anti-apoptosis and
tissue repair effects in a variety of diseases (29). The direct cause of tissue damage
following TBI is the increase of local oxygen free radicals and the
occurrence of stress oxidation reaction. Among them, ROS and MDA
are directly involved in brain damage and excessive release of
excitatory neurotransmitters (30). Following TBI, the levels of ROS
and MDA rise sharply, while the activities of biological enzymes
that scavenger oxygen free radicals, such as SOD and CAT, remain
basically unchanged (31,32).
Excessive oxygen free radicals act on tight junction proteins,
destroy the blood-brain barrier and cause brain tissue edema
(33). The present study showed
that TBI promoted the levels of ROS and MDA in rat hippocampus and
induced cerebral edema. The main physiological functions of GSH are
scavenging free radicals, anti-oxidation and anti-aging. The
present study also found that plasma levels of GSH was decreased
significantly in ICH patients from days 1-7, however, this trend
was not statistically significant (34). The results of the present study
also showed that GSH levels declined gradually over time in the TBI
group; notably, there was no statistically significant comparison
between the groups. Wogonin exerted an anti-oxidative stress effect
here. It increased the activity of GSH, SOD and CAT and removed
excess oxygen free radicals in the hippocampus to protect
neurological damage following TBI.
Oxidative stress factors further activates the
caspase family following TBI to initiate programmed death
(apoptosis). After TBI, the expression of pro-apoptosis-related
proteins in the injured brain tissue increases (35). In fact, there is an imbalance
between anti-apoptosis and pro-apoptosis in the occurrence of
apoptosis following TBI. Anti-apoptotic proteins, such as the P53
and Bcl-2 family, have been found to increase their expression
following TBI (36,37). Pro-apoptotic factors, mainly Bax,
Bad and Caspase family proteins, whose expression increased in the
lesion and surrounding brain tissues can inhibit apoptosis
following TBI (38). Data from
the present study confirmed that wogonin act as a neuroprotective
drug to increase Bcl-2 and decrease the expression of caspase 3 and
Bax. NADPH oxidase NOX2 is highly expressed in
hippocampal neurons following TBI and anti-NOX2
strategies help alleviate the damage following TBI (39,40). Therefore, the level of
NOX2 protein in the rat TBI model was used as an
indicator of oxidative stress. The present study revealed that
NOX2 was increased in hippocampus neurons following TBI
by both IHC staining and western blot analysis of NOX2
protein levels of NOX2 protein between 1-7 days
following TBI. Wogonin treatment markedly attenuated NOX activity
and protein levels that would normally be induced by TBI. These
results support the use of specific NADPH oxidase inhibitors in the
treatment of TBI. Mitochondrial pathways including oxidative
stress, apoptosis and NOX2 may be the targets of wogonin
to inhibit neurological damage.
The PI3K/Akt signaling pathway inhibits cell
apoptosis by influencing multiple downstream effector molecules
(41). The neuroprotective
mechanism of PI3K/Akt involves a variety of intracellular signals
and effector proteins, which mainly act on downstream targets
through activated phosphorylated Akt to exert anti-apoptotic
effects. PI3K/Akt signaling pathway can be activated by drugs and
non-drug means, including wogonin, to promote the survival of
neurons and the protection of TBI (24). For example, atorvastatin inhibits
the expression of miR-126 and then upregulates the PI3K/AKT
signaling pathway to promote angiogenesis in the brain tissue of
TBI rats (42). In addition,
scriptaid, a deacetylase HDAC inhibitor, enhances PTEN/PI3K/Akt
signaling and restores microglia function after severe TBI
(43). The adrenergic receptor
agonist dexmedetomidine activates the PI3K/Akt/mTOR signaling
pathway and exerts neuroprotective effects in TBI rats (44). The present study revealed that
the activation of PI3K/Akt signaling pathways was beneficial for
wogonin to exert anti-oxidative effects and to consequently protect
against brain injury. The administration of wogonin significantly
promoted the phosphorylation of Akt and contributed to the
anti-oxidation of hippocampus following TBI. In fact, the effect of
wogonin on the PI3K/Akt pathway has been previously implied in some
other diseases. The mechanism of wogonin in the treatment of lung
cancer involves the PI3K/Akt signaling pathway (45). Wogonin reduces renal tubular
damage by regulating autophagy and inflammation mediated by
PI3K/Akt/NF-κB signaling pathway (13). For nervous system and brain
diseases, wogonin has been found to inhibit the phosphorylation of
Akt and NF-κB and delay the growth of gliomas (46). The present study is the first, to
the best of the authors' knowledge, to reveal the role of wogonin
in promoting neurological function following TBI by regulating the
PI3K/Akt pathway. Following the administration of PI3K inhibitor
LY294002, the antioxidant and anti-apoptotic effects of wogonin on
the hippocampus of rats following TBI were greatly reduced. This
inhibitory effect by LY294002 indicated that the function of
wogonin depended on the PI3K/Akt pathway. LY294002 has also been
used in other studies to reveal the role of wogonin. For example,
LY294002 in breast cancer MCF-7 cells increased wogonin-induced
apoptosis through PI3K/Akt/survivin signaling pathway blockade
(47). Studies have also proved
that LY294002 destroys the blood-brain barrier and causes
neurological dysfunction and cognitive impairment following TBI
(48,49). Therefore, in the follow-up
routine or drug treatment of TBI patients, it is necessary to avoid
mixing the inhibitory components of PI3K/Akt.
Nrf2 plays an important role in the endogenous
antioxidant mechanism following TBI. In the physiological state,
Nrf2 in the cytoplasm binds to the actin binding protein Kelch-like
ECH-associated protein 1 (Keap1) and the activity of Nrf2 is
inhibited. Under TBI pathological stimulation, Nrf2 is
phosphorylated and uncoupled from Keap1 (50). The active Nrf2 is transferred
into the nucleus and the ARE/MAF response element is combined to
initiate the expression of ARE-regulated antioxidant enzyme genes,
such as HO-1 (51). HO-1 is
regulated by Nrf2 and exerts anti-oxidant, anti-inflammatory and
anti-apoptotic effects in different in vitro and in
vivo models (52). A study
showed that in Nrf2 gene-deficient mice, brain damage following TBI
increases, manifesting as neuroinflammation and apoptosis (53). There have been some drugs or
methods used to alleviate TBI-related neurological disorders by
targeting Nrf2 and HO-1. For example, the D2-like receptor agonist
pramipexole activates the Nrf2/HO-1 signaling pathway to serve a
neuroprotective effect following TBI (9). The behavioral changes and reduction
of oxidative stress in Wistar rats treated with tannin following
TBI were attributed to the activation of PGC-1α/Nrf-2/HO-1
signaling pathway (54). Some
natural compound monomers, including wogonin, also regulate
Nrf2/HO-1 signaling to relieve TBI symptoms. Astaxanthin, a
carotenoid pigment, upregulates the expression of Nrf2 and HO-1
following TBI and provides neurological protection (55). Breviscapine treatment from
Erigeron breviscapus upregulates the expression of Nrf2 and
HO-1, reducing TBI-induced neuronal cell apoptosis and improving
neurobehavioral function (56).
The present study revealed that wogonin participated in
neuroprotection in the hippocampus by increasing Nrf2/HO-1
signaling. Wogonin's regulation of Nrf2/HO-1 expression in the
hippocampus following TBI was dependent on PI3K/Akt, because the
administration of LY294002 rescued the biological phenomenon.
Although some previous studies have proved that the
PI3K/Akt-mediated Nrf2/HO-1 signaling pathway directly protects a
number of types of cells from oxidative stress and apoptosis
(57-59), the mechanism following TBI is not
fully understood. The present study revealed the molecular
mechanism of Nrf2/HO-1 activated by PI3K/Akt on neurological
dysfunction following TBI and proposed the therapeutic effect of
wogonin in it.
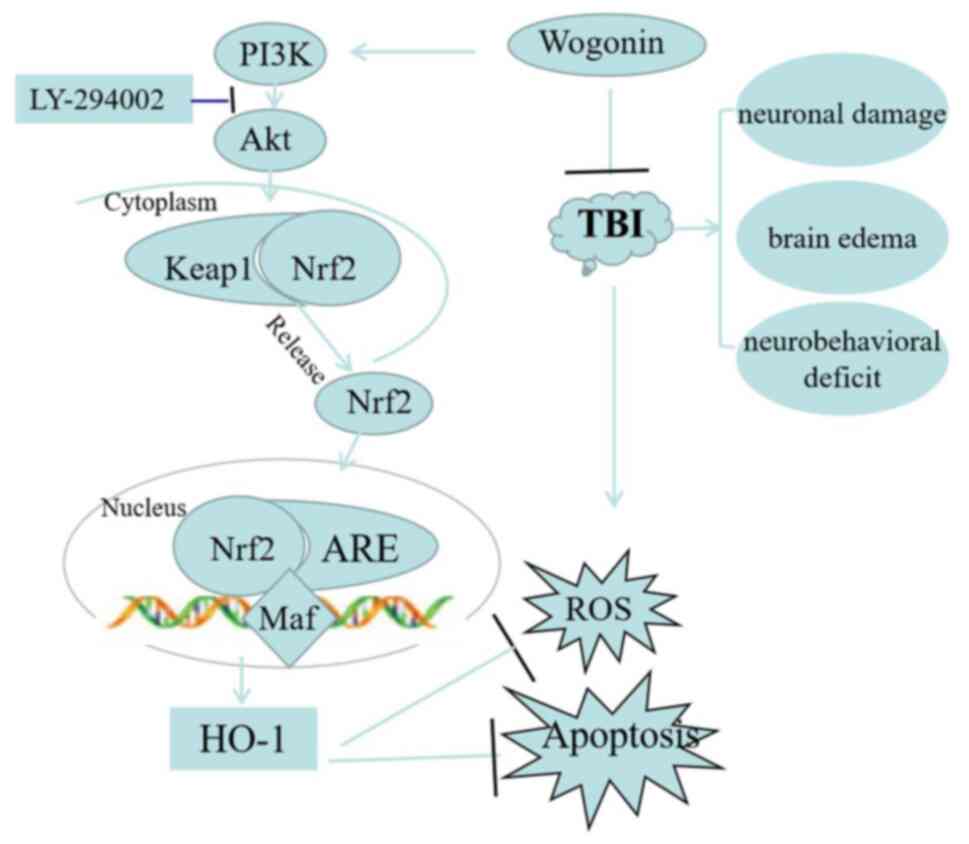
In summary, the present study proposed a
neuroprotective effect and mechanism for wogonin in TBI (Fig. 10). Wogonin improved neurological
deficits and learning and memory abilities and relieved cerebral
edema following TBI. In mechanism, administration of wogonin
reduced the oxidative stress and apoptosis of nerve tissues, which
was attributed to the activation of PI3K/Akt/Nrf2/HO-1 pathway. The
present study revealed the role and mechanism of wogonin in
protecting secondary damage following TBI.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YF and YJ performed the experiments. ZY and MJ
assisted in performing the brain water measurement, MWM and NSS
tests. QW and MY assisted in performing the H&E and
immunofluorescent staining and western blotting. GS was responsible
for designing the study, summarizing the results and writing the
manuscript. LW participated in data analysis and involved in
drafting the manuscript or revising it critically for important
intellectual content. YF and GS confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Hebei Medical University (permit no. 20201086). Humane
endpoints were established following the guideline of assessment
for humane endpoints in animal experiment (People's Republic of
China: RB/T 173-2018) in order to minimize pain or distress to
experimental animals.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
int
Acknowledgments
Not applicable.
Funding
The present study was financially supported by the National
Natural Science Foundation of China (grant no. 81401032), the
Natural Science Foundation of Hebei Province (grant no.
H2020206437), the Medical Science Research Project of Hebei
Province (grant no. 20190063) and the Hebei Medical University
Department-School Consultation Fund-Science and Technology
Innovation-Frontier Cross Discipline Research (grant no.
2020TXJC02).
References
|
1
|
Chowdhury T, Kowalski S, Arabi Y and Dash
HH: Specific intensive care management of patients with traumatic
brain injury: Present and future. Saudi J Anaesth. 8:268–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vidhya V, Gudigar A, Raghavendra U, Hegde
A, Menon GR, Molinari F, Ciaccio EJ and Acharya UR: Automated
detection and screening of traumatic brain injury (TBI) using
computed tomography images: A comprehensive review and future
perspectives. Int J Environ Res Public Health. 18:64992021.
View Article : Google Scholar
|
|
3
|
Schweitzer AD, Niogi SN, Whitlow CJ and
Tsiouris AJ: Traumatic brain injury: Imaging patterns and
complications. Radiographics. 39:1571–1595. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu LM, Dong X, Xue XD, Zhang J, Li Z, Wu
HJ, Yang ZL, Yang Y and Wang HS: Naringenin improves mitochondrial
function and reduces cardiac damage following ischemia-reperfusion
injury: The role of the AMPK-SIRT3 signaling pathway. Food Funct.
10:2752–2765. 2019. View Article : Google Scholar
|
|
5
|
Qian F, Han Y, Han Z, Zhang D, Zhang L,
Zhao G, Li S, Jin G, Yu R and Liu H: In situ implantable,
post-trauma microenvironment-responsive, ROS depletion hydrogels
for the treatment of traumatic brain injury. Biomaterials.
270:1206752021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slavoaca D, Muresanu D, Birle C, Rosu OV,
Chirila I, Dobra I, Jemna N, Strilciuc S and Vos P: Biomarkers in
traumatic brain injury: New concepts. Neurolog Sci. 41:2033–2044.
2020. View Article : Google Scholar
|
|
7
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chandran R, Kim T, Mehta SL, Udho E,
Chanana V, Cengiz P, Kim H, Kim C and Vemuganti R: A combination
antioxidant therapy to inhibit NOX2 and activate Nrf2
decreases secondary brain damage and improves functional recovery
after traumatic brain injury. J Cereb Blood Flow Metab.
38:1818–1827. 2018. View Article : Google Scholar
|
|
9
|
Salman M, Tabassum H and Parvez S:
Nrf2/HO-1 mediates the neuroprotective effects of pramipexole by
attenuating oxidative damage and mitochondrial perturbation after
traumatic brain injury in rats. Dis Model Mech. 13:dmm0450212020.
View Article : Google Scholar :
|
|
10
|
Hassanin M, Mai T, Tadros M, Elmazar M and
Singab AN: Wogonin a promising component of scutellaria
baicalensis: A review on its chemistry, pharmacokinetics and
biological activities. Archives of Pharmaceutical Sciences Ain
Shams University. 3:170–179. 2019. View Article : Google Scholar
|
|
11
|
Huynh DL, Ngau TH, Nguyen NH, Tran BG and
Nguyen CT: Potential therapeutic and pharmacological effects of
Wogonin: An updated review. Mol Biol Rep. 47:9779–9789. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chun W, Lee HJ, Kong PJ, Lee GH, Cheongsu
LY, Park H and Kim SS: Synthetic wogonin derivatives suppress
lipopolysaccharide-induced nitric oxide production and hydrogen
peroxide-induced cytotoxicity. Arch Pharm Res. 28:216–219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei L, Zhao J, Liu XQ, Chen J, Qi XM, Xia
LL and Wu YG: Wogonin alleviates kidney tubular epithelial injury
in diabetic nephropathy by inhibiting PI3K/Akt/NF-κB signaling
pathways. Drug Des Devel Ther. 15:3131–3150. 2021. View Article : Google Scholar :
|
|
14
|
Zhu G, Zhang J, Yang Y, Zhang H, Jin W, Su
F, Liang J, Wang K, Zhang J and Chen C: The key target and
molecular mechanism of the volatile component of scutellaria
baicalensis georgi in acute lung injury based on network
pharmacology. Front Pharmacol. 12:6507802021. View Article : Google Scholar
|
|
15
|
Umemoto Y, Patel A, Huynh T and
Chitravanshi VC: Wogonin attenuates the deleterious effects of
traumatic brain injury in anesthetized Wistar rats. Eur J
Pharmacol. 848:121–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CC, Hung TH, Wang YH, Lin CW, Wang
PY, Lee CY and Chen SF: Wogonin improves histological and
functional outcomes, and reduces activation of TLR4/NF-κB signaling
after experimental traumatic brain injury. PLoS One. 7:e302942012.
View Article : Google Scholar
|
|
17
|
Feng Y, Gao J, Cui Y, Li M, Li R, Cui C
and Cui J: Neuroprotective effects of resatorvid against traumatic
brain injury in rat: Involvement of neuronal autophagy and TLR4
signaling pathway. Cell Mol Neurobiol. 37:155–168. 2017. View Article : Google Scholar
|
|
18
|
Ates O, Cayli S, Altinoz E, Gurses I,
Yucel N, Sener M, Kocak A and Yologlu S: Neuroprotection by
resveratrol against traumatic brain injury in rats. Mol Cell
Biochem. 294:137–144. 2007. View Article : Google Scholar
|
|
19
|
Chen SF, Hsu CW, Huang WH and Wang JY:
Post-injury baicalein improves histological and functional outcomes
and reduces inflammatory cytokines after experimental traumatic
brain injury. Br J Pharmacol. 155:1279–1296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng Y, Cui Y, Gao JL, Li MH, Li R, Jiang
XH, Tian YX, Wang KJ, Cui CM and Cui JZ: Resveratrol attenuates
neuronal autophagy and inflammatory injury by inhibiting the
TLR4/NF-κB signaling pathway in experimental traumatic brain
injury. Int J Mol Med. 37:921–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Changmeng C, Sixin S, Jianzhong C, Yan F,
Junling G and Pei J: Vitamin D receptor activation influences NADPH
oxidase (NOX2) activity and protects against
neurological deficits and apoptosis in a rat model of traumatic
brain injury. Oxid Med Cell Longev. 2017:92457022017.
|
|
22
|
Wang P, Wu Q, Wu W, Li H, Guo Y, Yu P, Gao
G, Shi Z, Zhao B and Chang YZ: Mitochondrial ferritin deletion
exacerbates β-amyloid-induced neurotoxicity in mice. Oxid Med Cell
Longev. 2017:10203572017. View Article : Google Scholar
|
|
23
|
Song SX, Gao JL, Wang KJ, Li R, Tian YX,
Wei JQ and Cui JZ: Attenuation of brain edema and spatial learning
deficits by the inhibition of NADPH oxidase activity using apocynin
following diffuse traumatic brain injury in rats. Mol Med Rep.
7:327–331. 2013. View Article : Google Scholar
|
|
24
|
Du G, Zhao Z, Chen Y, Li Z, Tian Y, Liu Z,
Liu B and Song J: Quercetin attenuates neuronal autophagy and
apoptosis in rat traumatic brain injury model via activation of
PI3K/Akt signaling pathway. Neurol Res. 38:1012–1019. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Netteland DF, Mejlnder-Evjensvold M, Skaga
NO, Sandset EC, Aarhus M and Helseth E: Cerebral venous thrombosis
in traumatic brain injury: A cause of secondary insults and added
mortality. J Neurosurg. 134:1–9. 2020.
|
|
26
|
Robicsek SA, Bhattacharya A, Rabai F,
Shukla K and Doré S: Blood-related toxicity after traumatic brain
injury: Potential targets for neuroprotection. Mol Neurobiol.
57:159–178. 2020. View Article : Google Scholar
|
|
27
|
Khellaf A, Khan DZ and Helmy A: Recent
advances in traumatic brain injury. J Neurol. 266:2878–2889. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gennai S, Monsel A, Hao Q, Liu J, Gudapati
V, Barbier EL and Lee JW: Cell-based therapy for traumatic brain
injury. Br J Anaesth. 115:203–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huynh DL, Sharma N, Singh AK, Sodhi SS,
Zhang JJ, Mongre RK, Ghosh M, Kim N, Park YH and Jeong DK:
Anti-tumor activity of wogonin, an extract from Scutellaria
baicalensis, through regulating different signaling pathways. Chin
J Nat Med. 15:15–40. 2017.
|
|
30
|
Du D, Tang W, Zhou C, Sun X, Wei Z, Zhong
J and Huang Z: Fecal microbiota transplantation is a promising
method to restore gut microbiota dysbiosis and relieve neurological
deficits after traumatic brain injury. Oxid Med Cell Longev.
2021:58168372021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji X, Tian Y, Xie K, Liu W, Qu Y and Fei
Z: Protective effects of hydrogen-rich saline in a rat model of
traumatic brain injury via reducing oxidative stress. J Surg Res.
178:e9–e16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu C, He D and Zhao Q: Licoricidin
improves neurological dysfunction after traumatic brain injury in
mice via regulating FoxO3/wnt/β-catenin pathway. J Nat Med.
74:767–776. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Chen W, Han K, Qi E, Chen R, Yu M,
Hou L and Lv L: Effect of oxidative stress in rostral ventrolateral
medulla on sympathetic hyperactivity after traumatic brain injury.
Eur J Neurosci. 50:1972–1980. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Masomi-Bornwasser J, Kurz E, Frenz C,
Schmitt J, Wesp DMA, König J, Lotz J, Ringel F, Kerz T, Krenzlin H
and Keric N: The influence of oxidative stress on neurological
outcomes in spontaneous intracerebral hemorrhage. Biomolecules.
11:16152021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carteri RB, Kopczynski A, Rodolphi MS,
Strogulski NR, Sartor M, Feldmann M, Bastiani MAD, Wannmacher CMD,
de Franceschi ID, Hansel G, et al: Testosterone administration
after traumatic brain injury reduces mitochondrial dysfunction and
neurodegeneration. J Neurotrauma. 36:2246–2259. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hong MY, Gao JL, Cui JZ, Wang KJ, Tian YX,
Li R, Wang HT and Wang H: Effect of c-Jun NH-terminal
kinase-mediated p53 expression on neuron autophagy following
traumatic brain injury in rats. Chinese Med J (Engl).
125:2019–2024. 2012.
|
|
37
|
Deng H, Yue JK, Zusman BE, Nwachuku EL,
Abou-Al-Shaar H, Upadhyayula PS, Okonkwo DO and Puccio AM: B-cell
lymphoma 2 (Bcl-2) and regulation of apoptosis after traumatic
brain injury: A clinical perspective. Medicina (Kaunas).
56:3002020. View Article : Google Scholar
|
|
38
|
Schober ME, Requena DF, Block B, Davis LJ,
Rodesch C, Casper TC, Juul SE, Kesner RP and Lane RH:
Erythropoietin improved cognitive function and decreased
hippocampal caspase activity in rat pups after traumatic brain
injury. J Neurotrauma. 31:358–369. 2014. View Article : Google Scholar :
|
|
39
|
Jackman KA, Miller AA, de Silva TM, Crack
PJ, Drummond GR and Sobey CG: Reduction of cerebral infarct volume
by apocynin requires pretreatment and is absent in Nox2-defificient
mice. Br J Pharmacol. 156:680–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dohi K, Ohtaki H, Nakamachi T, Yofu S,
Satoh K, Miyamoto K, Song D, Tsunawaki S, Shioda S and Aruga T:
Gp91phox (NOX2) in classically activated microglia exacerbates
traumatic brain injury. J Neuroinflflammation. 7:412010. View Article : Google Scholar
|
|
41
|
Wang M, Zhang J and Gong N: Role of the
PI3K/Akt signaling pathway in liver ischemia reperfusion injury: A
narrative review. Ann Palliat Med. 27:apm-21-32862021.
|
|
42
|
Li Q, Cheng K, Wang AY, Xu QG, Fu ZF, He
SY and Xu PX: microRNA-126 inhibits tube formation of HUVECs by
interacting with EGFL7 and down-regulating PI3K/AKT signaling
pathway. Biomed Pharmacother. 116:1090072019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu
Y, Pu H, Li WW, Tang B, Wang Y, et al: HDAC inhibition prevents
white matter injury by modulating microglia/macrophage polarization
through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA.
112:2853–2858. 2015. View Article : Google Scholar
|
|
44
|
Shen M, Wang S, Wen X, Han XR, Wang YJ,
Zhou XM, Zhang MH, Wu DM, Lu J and Zheng YL: Dexmedetomidine exerts
neuroprotective effect via the activation of the PI3K/Akt/mTOR
signaling pathway in rats with traumatic brain injury. Biomed
Pharmacother. 95:885–893. 2017. View Article : Google Scholar
|
|
45
|
Wang L, Zhang J, Shan G, Liang J, Jin W,
Li Y, Su F, Ba Y, Tian X, Sun X, et al: Establishment of a lung
cancer discriminative model based on an optimized support vector
machine algorithm and study of key targets of Wogonin in lung
cancer. Front Pharmacol. 12:7289372021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Parajuli P, Joshee N, Chinni SR, Rimando
AM, Mittal S, Sethi S and Yadav AK: Delayed growth of glioma by
Scutellaria flavonoids involve inhibition of Akt, GSK-3 and NF-κB
signaling. J Neurooncol. 101:15–24. 2011. View Article : Google Scholar
|
|
47
|
Huang KF, Zhang GD, Huang YQ and Diao Y:
Wogonin induces apoptosis and down-regulates survivin in human
breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int
Immunopharmacol. 12:334–341. 2012. View Article : Google Scholar
|
|
48
|
Wang ZG, Cheng Y, Yu XC, Ye LB, Xia QH,
Johnson NR, Wei X, Chen DQ, Cao G, Fu XB, et al: bFGF protects
against blood-brain barrier damage through junction protein
regulation via PI3K-Akt-Rac1 pathway following traumatic brain
injury. Mol Neurobiol. 53:7298–7311. 2016. View Article : Google Scholar
|
|
49
|
Wu F, Chen Z, Tang C, Zhang J, Cheng L,
Zuo H, Zhang H, Chen D, Xiang L, Xiao J, et al: Acid fibroblast
growth factor preserves blood-brain barrier integrity by activating
the PI3K-Akt-Rac1 pathway and inhibiting RhoA following traumatic
brain injury. Am J Transl Res. 9:910–925. 2017.PubMed/NCBI
|
|
50
|
Liao K, Su X, Lei K, Liu Z, Lu L, Wu Q,
Pan H, Huang Q, Zhao Y, Wang M, et al: Sinomenine protects bone
from destruction to ameliorate arthritis via activating
p62Thr269/Ser272-Keap1-Nrf2 feedback loop. Biomed Pharmacother.
135:1111952021. View Article : Google Scholar
|
|
51
|
Motohashi H, Katsuoka F, Engel JD and
Yamamoto M: Small maf proteins are obligate transcriptional
cofactors for normal keratinocyte differentiation in the Keap1-Nrf2
regulatory pathway. Proc Natl Acad Sci USA. 101:6379–6384. 2004.
View Article : Google Scholar
|
|
52
|
Ndisang JF: Synergistic interaction
between heme oxygenase (HO) and nuclear-factor E2-related factor-2
(Nrf2) against oxidative stress in cardiovascular related diseases.
Curr Pharm Des. 23:1465–1470. 2017. View Article : Google Scholar
|
|
53
|
Bhowmick S, D'Mello V, Caruso D and
Abdul-Muneer PM: Traumatic brain injury-induced downregulation of
Nrf2 activates inflammatory response and apoptotic cell death. J
Mol Med (Berl). 97:1627–1641. 2019. View Article : Google Scholar
|
|
54
|
Salman M, Tabassum H and Parvez S: Tannic
acid provides neuroprotective effects against traumatic brain
injury through the PGC-1α/Nrf2/HO-1 pathway. Mol Neurobiol.
57:2870–2885. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gao F, Wu X, Mao X, Niu F, Zhang B, Dong J
and Liu B: Astaxanthin provides neuroprotection in an experimental
model of traumatic brain injury via the Nrf2/HO-1 pathway. Am J
Transl Res. 13:1483–1493. 2021.PubMed/NCBI
|
|
56
|
Li F, Wang X, Zhang Z, Gao P and Zhang X:
Breviscapine provides a neuroprotective effect after traumatic
brain injury by modulating the Nrf2 signaling pathway. J Cell
Biochem. 120:14899–14907. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhuang Y, Wu H, Wang X, He J, He S and Yin
Y: Resveratrol attenuates oxidative stress-induced intestinal
barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway.
Oxid Med Cell Longev. 2019:75918402019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li ST, Dai Q, Zhang SX, Liu YJ, Yu QQ, Tan
F, Lu SH, Wang Q, Chen JW, Huang HQ, et al: Ulinastatin attenuates
LPS-induced inflammation in mouse macrophage RAW264.7 cells by
inhibiting the JNK/NF-κB signaling pathway and activating the
PI3K/Akt/Nrf2 pathway. Acta Pharmacol Sin. 39:1294–1304. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wen Z, Hou W, Wu W, Zhao Y, Dong X, Bai X,
Peng L and Song L: 6′-O-Galloylpaeoniflorin attenuates cerebral
ischemia reperfusion-induced neuroinflammation and oxidative stress
via PI3K/Akt/Nrf2 activation. Oxid Med Cell Long.
2018:86782672018.
|