Introduction
Hepatocellular carcinoma (HCC) is one of the most
frequently occurring malignant tumors and a common cause of cancer
mortality worldwide (1). The
results of surgical treatments for advanced HCC, such as liver
resection or liver transplantation, have not been satisfactory. To
date, chemotherapy, including transcatheter arterial infusion, is
the first choice for advanced HCC (2,3).
5-Fluorouracil (5-FU) is a commonly used chemotherapeutic agent
that is effective for treating a wide variety of malignant tumors.
However, the effectiveness of the chemotherapy is limited because
of acquired or intrinsic drug resistance (4,5).
The development of resistance to 5-FU appears to be
a major impediment to the successful chemotherapy of human cancers.
5-FU decreases the biosynthesis of pyrimidine nucleotides by
inhibiting thymidylate synthase, which catalyzes the rate-limiting
step in DNA synthesis (6–10). Although the mechanism of resistance
to 5-FU remains unclear, a possible mechanism is that alterations
of plasma membrane proteins reduce the accumulation of 5-FU within
tumor cells (11). Two studies
also reported that epithelial-to-mesenchymal transition (EMT) was
closely related to chemoresistance in the colorectal and pancreatic
cancers (12,13).
EMT is initially observed in embryonic development
in which cells lose epithelial characteristics and gain mesenchymal
properties to increase motility and invasiveness (12,14).
Previous research has suggested that EMT is also important in tumor
progression, metastasis, and chemoresistance (14,15)
and is induced by growth factors, such as hepatocyte growth factor,
transforming growth factor β, and epidermal growth factor,
implicated in these processes (16).
Tissue culture systems have been established to
study the biochemical, physiologic, and genetic bases of
alterations that result in development of multidrug resistance. To
understand drug resistance well, establishing cultured cell lines
resistant to anticancer drugs is necessary. In the current study,
we established two 5-FU-resistant cell lines, HLF-R4 and HLF-R10,
from an HCC cell line and investigated for the first time the
mechanisms of 5-FU resistance including EMT.
Materials and methods
Cell lines
The HLF cell line, derived from undifferentiated
human hepatocellular carcinoma, was obtained from the Japanese
Cancer Research Resources Bank and maintained in Dulbecco's
modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA) supplemented
with 10% fetal bovine serum (FBS) (Gibco) and 100 units/ml
penicillin and streptomycin (Gibco). According to previously
described methods (17–20), two 5-FU-resistant sublines, HLF-Rs,
were established by repeated subcultures in the presence of
stepwise-increases in concentrations of 5-FU (5, 7.5, 10 and 20 μM)
(Wako, Osaka, Japan). Because the chemoresistance of both cell
lines are 4- and 10-fold increased, respectively, the sublines were
named HLF-R4 and HLF-R10, and they are fully resistant to 5-FU and
can grow exponentially in the presence of 10 and 20 μM of 5-FU. The
HLF-Rs showed no loss of resistance even after 2 months of culture
in a drug-free medium.
Cell growth
To investigate cell growth in the HLF, HLF-R4, and
HLF-R10 cells, we performed a cell proliferation assay. The
experiments were carried out for 168 h, and the number of cells
were counted every 24 h. The cells in three samples were
trypsinized and counted using a hemocytometer at the indicated time
points.
Chemosensitivity assay
The cells were seeded at a concentration of
2.0×103 in each well of a 96-well plate in DMEM
containing 10% FBS. After 24 h, culture medium was replaced with
DMEM with 10% FBS and various concentrations of 5-FU (6.25, 12.5,
25, 50, 100, 200, 400, 800 and 1600 μM). After further incubation
for 72 h, a cell viability assay was carried out using the Cell
Counting kit-8 according to the manufacturer's instructions
(Dojindo, Kumamoto, Japan). Six wells were used for each drug
concentration and the experiment was replicated three times. The
50% inhibitory concentration (IC50) was calculated from
the survival curves.
TUNEL assay
The cells were seeded at a concentration of
5.0×105 on Lab-TekII chamber slides (Nalge Nunc
International, Rochester, NY) in DMEM containing 10% FBS. After
incubation for 24 h, the culture medium was replaced with DMEM with
10% FBS and 70 μM of 5-FU. After further incubation for 24 h, the
cells on the chamber slides were washed twice with phosphate
buffered saline, air dried, and fixed in 4% paraformaldehyde at
room temperature for 30 min. The terminal deoxyribonucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay was
performed using an In Situ Apoptosis Detection kit according
to the manufacturer's instructions (Takara, Tokyo, Japan). The
cells were viewed and photographed under a fluorescence
microscope.
Preparation of cDNA
Total RNA was isolated using an RNeasy Mini kit
(Qiagen, Chatsworth, CA) according to the manufacturer's
instructions. cDNA was generated from 5 μg of total RNA using an
Ominiscript RT kit (Qiagen) and random hexamer (Sigma Genosys,
Ishikari, Japan).
mRNA expression analysis
Real-time quantitative reverse transcriptase
polymerase chain reaction (qRT-PCR) was performed to evaluate the
expression levels of five target genes in HLF and its derivatives.
qRT-PCR was carried out with one method using a LightCycler
FastStart DNA Master SYBR Green 1 kit (Roche, Mannheim, Germany).
The specific primers for the target genes are listed in Table I. Amplified products were analyzed
by 3% agarose gel electrophoresis to ascertain size and purity. The
PCR reactions using the LightCycler apparatus were performed in a
final volume of 20 μl of a reaction mixture comprised of 2 μl of
FirstStart DNA Master SYBR Green I mix, 3 mM MgCl2, and
l μM of the primers, according to the manufacturer's instructions.
The reaction mixture was loaded into glass capillary tubes and
subjected to an initial denaturation at 95°C for 10 min, followed
by 45 rounds of amplification at 95°C (10 sec) for denaturation,
62°C (10 sec) for annealing, and 72°C (10 sec) for extension, with
a temperature slope of 20°C/sec. The transcript amounts for the
target genes were estimated from the respective standard curves and
normalized to the glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) transcript amount determined in corresponding
samples.
 | Table ISpecific primers. |
Table I
Specific primers.
| Forward (5′-3′) | Reverse (5′-3′) |
|---|
| RNR-R1 |
AGAGAAGGAGAGGAACACAGCAG |
AGCAAAGCCTTACCACCTCAAG |
| RNR-R2 |
GCCCCTGTTAAGTGGTGAAATC |
GCCAGAATAAGACACTGGGTGAC |
| MRP5 |
TGAGACAGAAGCTCGATTCACC |
AGGGAGGTTTTCTCGGTACCTC |
|
E-cadherin |
CTCTTCCAGGAACCTCTGTGATG |
CCACACTGATGACTCCTGTGTTC |
| Twist-1 |
GCCGGAGACCTAGATGTCATTG |
CTATCAGAATGCAGAGGTGTGAGG |
| GAPDH |
GAGCCAAAAGGGTCATCATCTC |
GGTCATGAGTCCTTCCACGATAC |
Statistical analysis
The statistical significance was evaluated using the
Mann-Whitney U test. P<0.05 was considered statistically
significant.
Results
Cell growth
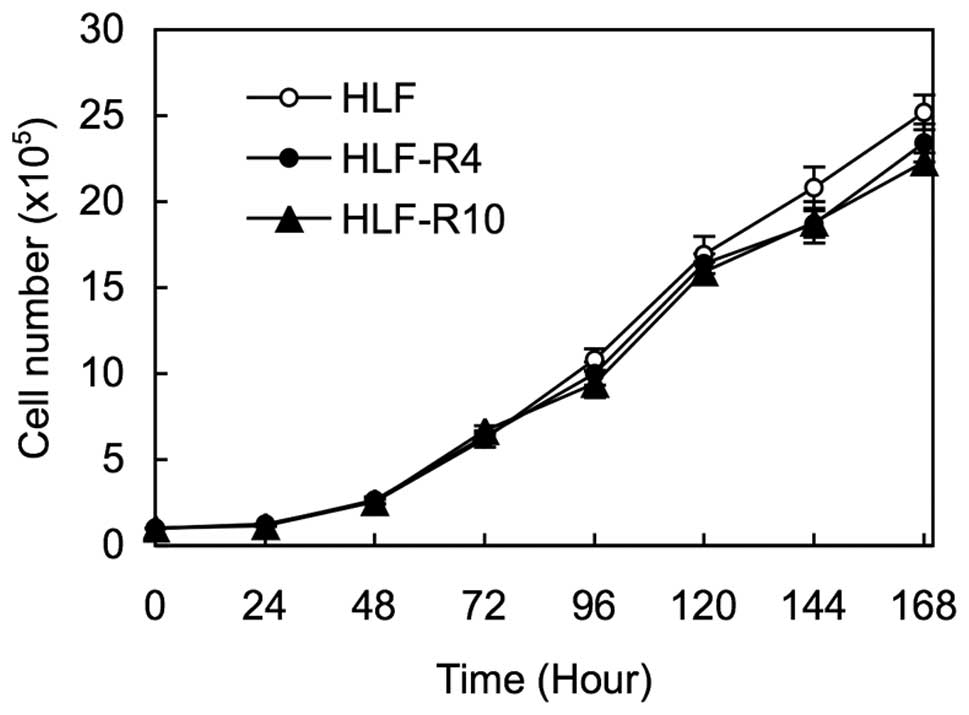
To obtain 5-FU-resistant cells, the HLF cells were
treated with increasing concentrations of 5-FU up to 20 μM, and two
clones, designated HLF-R4 and HLF-R10, were established almost 1.5
years later. There were no significant differences in cellular
proliferation among the HLF, HLF-R4, and HLF-R10 cells (Fig. 1).
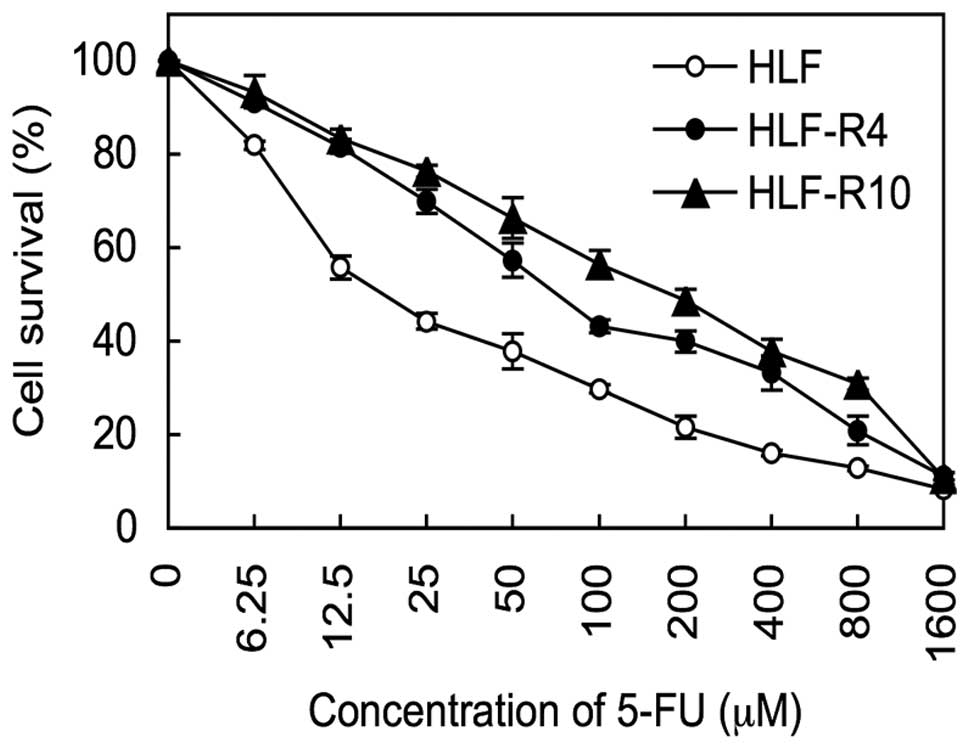
Chemosensitivity assay
The IC50 data indicated that the
5-FU-resistance levels of the HLF-R4 cells (IC50, 69.80
μM) and HLF-R10 cells (IC50, 193.47 μM) were 3.9- and
10.8-fold greater than that of the HLF cells (IC50,
17.92 μM) (Fig. 2).
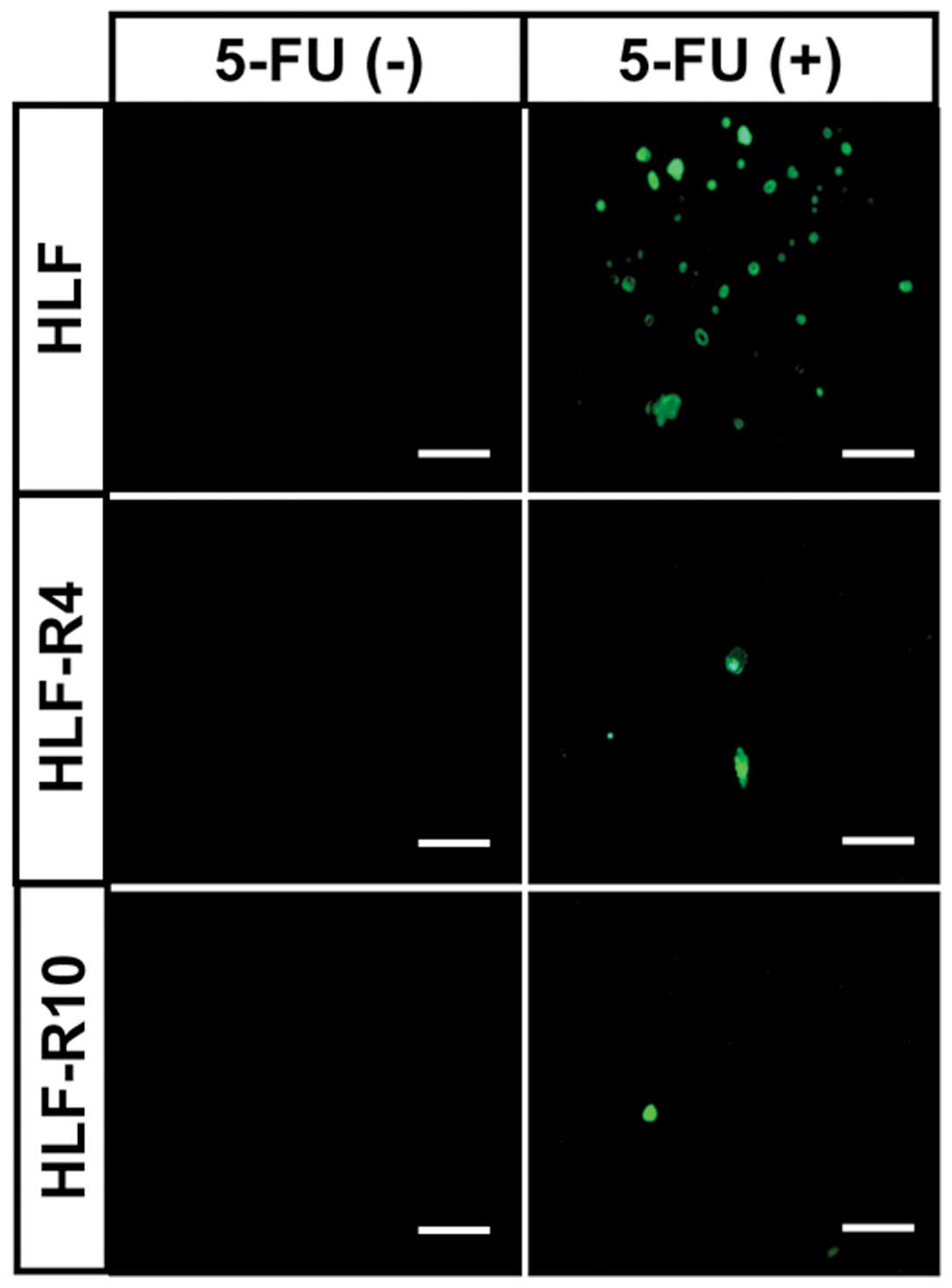
TUNEL assay
TUNEL staining showed a dramatic increase in the
number of cells that were stained green, which indicated that
apoptosis occurred in HLF cells exposed to 5-FU, whereas
5-FU-induced apoptosis was prevented in the HLF-R4 and HLF-R10
cells (Fig. 3).
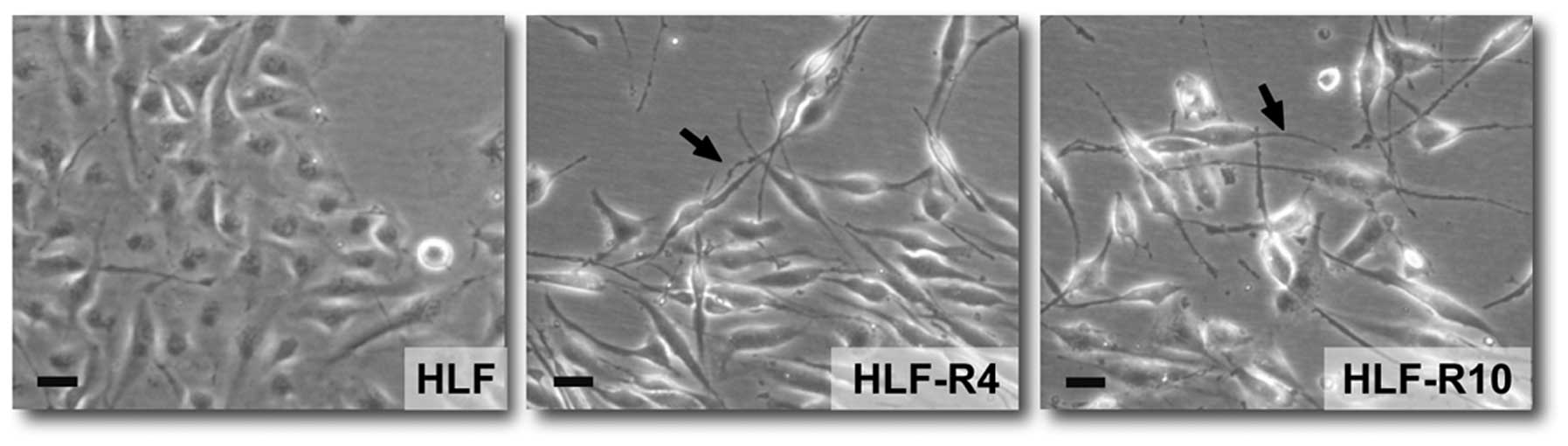
Cell morphology
The 5-FU-resistant cells, HLF-R4 and HLF-R10, were
morphologically distinct from their parental cell line (Fig. 4). The resistant cells had loss of
cell-cell adhesion, spindle-shaped morphology, and increased
formation of pseudopodia. These changes are typical phenotypes of
EMT.
Evaluation of the expression of genes
putatively related to 5-FU resistance and EMT
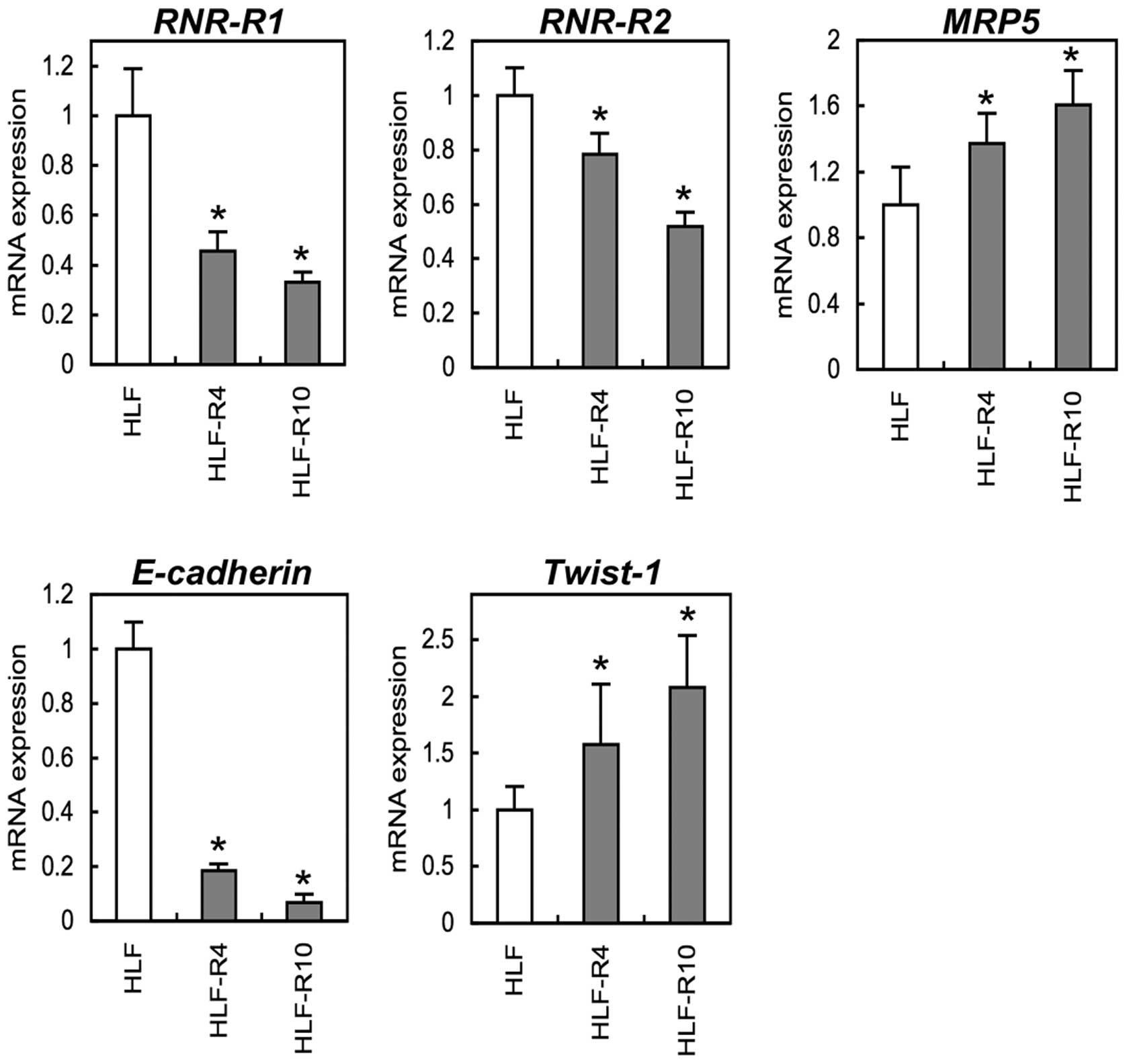
Consistent with our hypothesis that EMT occurs in
5-FU-resistant cells, qRT-PCR showed decreased E-cadherin
and increased Twist-1 by in the HLF-Rs. Among the genes
associated with 5-FU metabolism, we also found that mRNA expression
of ribonucleotide reductases (RNR-R1, RNR-R2)
was down-regulated and multi-drug resistance protein 5
(MRP5) was up-regulated in the HLF-Rs cells in a
5-FU-chemoresistant level-dependent manner (Fig. 5) (p<0.05).
Discussion
5-FU is key anticancer chemotherapy used to treat
solid tumors, such as gastric, colorectal, pancreas, breast, and
lung carcinomas (21–25). 5-FU also has been preferentially
used alone or combined with other chemotherapeutic drugs for HCCs
(2,3). Although several mechanisms of 5-FU
resistance have been investigated, no one mechanism completely
explains the clinical response to 5-FU chemotherapy. Since it is
extremely important to understand the resistance mechanism in order
to develop better treatments, establishing 5-FU-resistant cells was
indispensable to this type of study. Cell lines that are resistant
to 5-FU have been established from several cancers (6,26–28).
However, to date, only the Bel7402/5-FU cell line from HCC has been
reported to be resistant to 5-FU (29). To obtain further detailed
information on 5-FU resistance in HCC, in the current study we
evaluated two new 5-FU-resistant cell lines, HLF-R4 and HLF-R10,
from an undifferentiated HCC cell line that showed different
resistance levels to 5-FU.
Consistent with previous reports that showed that
5-FU metabolism and activity of 5-FU transport were closely related
to 5-FU resistance (30,31), the current data showed
down-regulated RNRs and up-regulated MRP5 genes. RNR,
a key enzyme in 5-FU metabolism, catalyzed 5-fluorouridine
diphosphate to 5-fluorodeoxyuridine diphosphate, the main precursor
of 5-fluorodeoxyuridine monophosphate (FdUMP) (32). Decreased RNR activity leads to a
low FdUMP level followed by inhibited thymidylate synthase cellular
activity, indicating that acquired resistance to 5-FU occurs in the
cells (6–10). MRP5, an energy-dependent
ATP-binding cassette transporter protein (33), mediates the ATP-dependent transport
of various substrates, such as monophosphate metabolites (30), across biologic membranes (34) and is responsible for broad-spectrum
resistance to chemotherapy including 5-FU. 5-FU metabolism may
contribute to a basic understanding of the molecular mechanisms of
chemoresistance. Further study is necessary to identify other
mechanisms.
In addition to typical morphologic phenotypes of EMT
in the HLF-Rs cells, we found decreased E-cadherin expression
caused by Twist-1 expression, which is consistent with the results
of Matsuo et al (35).
These findings reflected an important process by which cancer cells
may potentially become chemoresistant. Even though the relationship
between EMT and chemoresistance remains unclear, induction of EMT
in 5-FU-chemoresistant HCC cells might represent a new potentially
exciting research area into 5-FU-resistance mechanisms. Thus, EMT
is likely to be a potential therapeutic target for development of
anticancer drugs in HCCs. Our data should eventually benefit
patients with advanced HCC or 5-FU-chemoresistant HCCs for whom
there currently are few effective treatment options.
In conclusion, we found that alterations of enzymes
affecting 5-FU transport and metabolism as well as EMT were
observed in the HLF-Rs. Nevertheless, it seems likely that multiple
mechanisms may lead to 5-FU resistance. To the best of our
knowledge, the Bel7402/5-FU cell line showed no EMT changes
(29), 5-FU-resistant cell line
associated with EMT is clearly required in HCC. Therefore, the
HLF-Rs cells might be useful in vitro models for
understanding 5-FU-resistant mechanisms in HCCs. Further, the
HLF-R4 and HLF-R10 cells have different degrees of 5-FU resistance,
so there are advantages to investigating the process of 5-FU
resistance.
Acknowledgements
We thank Dr Mamoru Nukatsuka (Taiho Pharmaceutical
Co., Ltd.) for helpful discussion and Yuhki Suzuki, Chisato
Kitayama, and Rina Ito (Department of Drug Information and
Communication, Graduate School of Pharmaceutical Sciences, Chiba
University) for their considerable contributions to cell culture in
the process of establishment of the HLF-Rs. We also thank Lynda C.
Charters for editing this manuscript.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y
and Peng JY: Enhancement of apoptosis of human hepatocellular
carcinoma SMMC-7721 cells through synergy of berberine and
evodiamine. Phytomedicine. 15:1062–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ming ZJ, Hu Y, Qiu YH, Cao L and Zhang XG:
Synergistic effects of beta-aescin and 5-fluorouracil in human
hepatocellular carcinoma SMMC-7721 cells. Phytomedicine.
17:575–580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Higuchi E, Oridate N, Furuta Y, Suzuki S,
Hatakeyama H, Sawa H, Sunayashiki-Kusuzaki K, Yamazaki K, Inuyama Y
and Fukuda S: Differentially expressed genes associated with
CIS-diamminedichloroplatinum (II) resistance in head and neck
cancer using differential display and CDNA microarray. Head Neck.
25:187–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scotto KW and Bertino JR: Natural and
acquired resistance to chemotherapeutic agents. The Molecular Basis
of Cancer. 2nd edition. W. B. Sanders; Foster City: pp. 407–422.
2001
|
|
6
|
Johnston PG, Drake JC, Trepel J and
Allegra CJ: Immunological quantitation of thymidylate synthase
using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive
and -resistant human cancer cell lines. Cancer Res. 52:4306–4312.
1992.
|
|
7
|
Aschele C, Sobrero A, Faderan MA and
Bertino JR: Novel mechanism(s) of resistance to 5-fluorouracil in
human colon cancer (HCT-8) sublines following exposure to two
different clinically relevant dose schedules. Cancer Res.
52:1855–1864. 1992.
|
|
8
|
Copur S, Aiba K, Drake JC, Allegra CJ and
Chu E: Thymidylate synthase gene amplification in human colon
cancer cell lines resistant to 5-fluorouracil. Biochem Pharmacol.
49:1419–1426. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berger SH, Jenh CH, Johnson LF and Berger
FG: Thymidylate synthase overproduction and gene amplification in
fluorodeoxyuridine-resistant human cells. Mol Pharmacol.
28:461–467. 1985.PubMed/NCBI
|
|
10
|
Berger SH and Berger FG: Thymidylate
synthase as a determinant of 5-fluoro-2′-deoxyuridine response in
human colonic tumor cell lines. Mol Pharmacol. 34:474–479.
1988.PubMed/NCBI
|
|
11
|
Jin S and Scotto KW: Transcriptional
regulation of the MDR1 gene by histone acetyltransferase and
deacetylase is mediated by NF-Y. Mol Cell Biol. 18:4377–4384.
1998.PubMed/NCBI
|
|
12
|
Yang AD, Fan F, Camp ER, van Buren G, Liu
W, Somcio R, Gray MJ, Cheng H, Hoff PM and Ellis LM: Chronic
oxaliplatin resistance induces epithelial-to-mesenchymal transition
in colorectal cancer cell lines. Clin Cancer Res. 12:4147–4153.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiery JP and Chopin D: Epithelial cell
plasticity in development and tumor progression. Cancer Metastasis
Rev. 18:31–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elliott BE, Hung WL, Boag AH and Tuck AB:
The role of hepatocyte growth factor (scatter factor) in
epithelial-mesenchymal transition and breast cancer. Can J Physiol
Pharmacol. 80:91–102. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung YM, Park S, Park JK, Kim Y, Kang Y
and Yoo YD: Establishment and characterization of
5-fluorouracil-resistant gastric cancer cells. Cancer Lett.
159:95–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Negoro K, Yamano Y, Fushimi K, Saito K,
Nakatani K, Shiiba M, Yokoe H, Bukawa H, Uzawa K, Wada T, Tanzawa H
and Fujita S: Establishment and characterization of a
cisplatin-resistant cell line, KB-R, derived from oral carcinoma
cell line, KB. Int J Oncol. 30:1325–1332. 2007.PubMed/NCBI
|
|
19
|
Nakamura M, Nakatani K, Uzawa K, Ono K,
Uesugi H, Ogawara K, Shiiba M, Bukawa H, Yokoe H, Wada T, Fujita S
and Tanzawa H: Establishment and characterization of a
cisplatin-resistant oral squamous cell carcinoma cell line, H-1R.
Oncol Rep. 14:1281–1286. 2005.PubMed/NCBI
|
|
20
|
Nakatani K, Nakamura M, Uzawa K, Wada T,
Seki N, Tanzawa H and Fujita S: Establishment and gene analysis of
a cisplatin-resistant cell line, Sa-3R, derived from oral squamous
cell carcinoma. Oncol Rep. 13:709–714. 2005.PubMed/NCBI
|
|
21
|
Lee JH, Kim MC, Oh SY, Kwon HC, Kim SH,
Kwon KA, Lee S, Jeong JS, Choi SR and Kim HJ: Predictive value of
in vitro adeno-sine triphosphate-based chemotherapy response assay
in advanced gastric cancer patients who received oral
5-Fluorouracil after curative resection. Cancer Res Treat.
43:117–123. 2011. View Article : Google Scholar
|
|
22
|
Gamelin EC, Danquechin-Dorval EM, Dumesnil
YF, Maillart PJ, Goudier MJ, Burtin PC, Delva RG, Lortholary AH,
Gesta PH and Larra FG: Relationship between 5-fluorouracil (5-FU)
dose intensity and therapeutic response in patients with advanced
colorectal cancer receiving infusional therapy containing 5-FU.
Cancer. 77:441–451. 1996. View Article : Google Scholar
|
|
23
|
Murakami Y, Uemura K, Sudo T, Hayashidani
Y, Hashimoto Y, Ohge H and Sueda T: Impact of adjuvant gemcitabine
plus S-1 chemotherapy after surgical resection for adenocarcinoma
of the body or tail of the pancreas. J Gastrointest Surg. 13:85–92.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uday CK, Devarayalu BVSK and Mahmood S:
Overall survival rate in breast cancer patients treated with
5-fluorouracil: a review of randomized clinical trials. Int J
Pharmaceut Biomed Res. 1:132–135. 2010.
|
|
25
|
Huang CL, Yokomise H, Kobayashi S,
Fukushima M, Hitomi S and Wada H: Intratumoral expression of
thymidylate synthase and dihydropyrimidine dehydrogenase in
non-small cell lung cancer patients treated with 5-FU-based
chemotherapy. Int J Oncol. 17:47–54. 2000.
|
|
26
|
Plasencia C, Rooney PH, Taron M,
Martinez-Balibrea E, McLeod HL and Abad A: Chromosomal imbalance
maps of human 5FU-resistant colorectal cancer cell lines:
implications in the analysis of 5FU-acquired resistance mechanisms.
Int J Oncol. 22:945–953. 2003.
|
|
27
|
Tanaka T, Bai T and Toujima S:
Establishment and characterization of monoclonal
5-fluorouracil-resistant cell lines derived from human endometrial
adenocarcinoma. Int J Oncol. 37:731–736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wen J, Zheng B, Hu Y, Zhang X, Yang H, Luo
KJ, Li YF and Fu JH: Establishment and biological analysis of the
EC109/CDDP multidrug-resistant esophageal squamous cell carcinoma
cell line. Oncol Rep. 22:65–71. 2009.PubMed/NCBI
|
|
29
|
Huang M and Liu G: The study of innate
drug resistance of human hepatocellular carcinoma Bel7402 cell
line. Cancer Lett. 135:97–105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pratt S, Shepard RL, Kandasamy RA,
Johnston PA, Perry W III and Dantzig AH: The multidrug resistance
protein 5 (ABCC5) confers resistance to 5-fluorouracil and
transports its monophosphorylated metabolites. Mol Cancer Ther.
4:855–863. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakano Y, Tanno S, Koizumi K, Nishikawa T,
Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T and Kohgo
Y: Gemcitabine chemoresistance and molecular markers associated
with gemcitabine transport and metabolism in human pancreatic
cancer cells. Br J Cancer. 96:457–463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukushima M, Fujioka A, Uchida J, Nakagawa
F and Takechi T: Thymidylate synthase (TS) and ribonucleotide
reductase (RNR) may be involved in acquired resistance to
5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J
Cancer. 37:1681–1687. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hinoshita E, Uchiumi T, Taguchi K,
Kinukawa N, Tsuneyoshi M, Maehara Y, Sugimachi K and Kuwano M:
Increased expression of an ATP-binding cassette superfamily
transporter, multidrug resistance protein 2, in human colorectal
carcinomas. Clin Cancer Res. 6:2401–2407. 2000.
|
|
34
|
Kruh GD and Belinsky MG: The MRP family of
drug efflux pumps. Oncogene. 22:7537–7552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsuo N, Shiraha H, Fujikawa T, Takaoka
N, Ueda N, Tanaka S, Nishina S, Nakanishi Y, Uemura M, Takaki A,
Nakamura S, Kobayashi Y, Nouso K, Yagi T and Yamamoto K: Twist
expression promotes migration and invasion in hepatocellular
carcinoma. BMC Cancer. 9:240–251. 2009. View Article : Google Scholar : PubMed/NCBI
|