Introduction
The renin-angiotensin system (RAS) and its major
effector protein Angiotensin II (Ang II) are known to play a major
role in the regulation of blood pressure and cardiovascular
homeostasis as well as in cell growth, inflammation and
angiogenesis. These physiological actions are not only linked to
the cardiovascular system but also to carcinogenesis and
metastasis. Studies revealed a positive effect of candesartan, a
potent Angiotensin II type 1 receptor (AT1R) antagonist, on tumor
growth, angiogenesis and metastasis in experimental mouse models
suggesting that blockade of AT1R could be an effective anticancer
strategy (1). Furthermore,
findings in WT or AT1aR KO mice support the hypothesis that the
AT1R might play a role in inflammation-related tumor angiogenesis
(2). While the AT1R might
facilitate the steps towards tumor formation, the AT2R is known to
oppose the effect of the AT1R in most physiological situations. But
to date, little is known about the actions of the AT2R in
carcinogenesis and metastasis. However, our group has recently
described the MTUS1 gene, which has now linked the AT2R signaling
cascade to carcinogenesis (3).
MTUS1 has shown not only to act as a tumor suppressor gene in a
wide range of cancers but also to operate as an interaction partner
of the AT2R and seems to act as an early component of the AT2R
signaling pathway in growth inhibition (3,4).
Investigations on MTUS1, which is located at
chromosome 8p21.3–22 and ubiquitously expressed, showed that MTUS1
mRNA was down-regulated in fast proliferating pancreas carcinoma
cell lines, while recombinant expression of MTUS1 reduced cellular
proliferation significantly (3).
Since then, the expression of MTUS1 has been shown to be
down-regulated in breast cancer, colon tumors, prostate cancer cell
lines, ovarian cancer and head and neck squamous cell carcinoma,
implicating a role in a wide range of cancer development (5–10).
As recently shown by our group, MTUS1 is down-regulated in 50% of
the investigated colon cancers and MTUS1 siRNA transfection in
HUVEC cells results in significant increased cell proliferation
(11).
Shortly after the publication of MTUS1 protein, the
identical protein was described as ATIP and ATBP (12,13).
MTUS1 coding sequences are distributed across 18 exons, while
alternative exon usage leads to a family of at least five proteins
(14). MTUS1 isoform 4 protein is
exclusively detected in brain, while the other three proteins are
expressed in most tissues examined (14). The most investigated protein is the
MTUS1 isoform 5 protein, which derives from a coding region of 10
exons, spanning 436 amino-acids with a calculated molecular mass of
50 kDa (3,12,13).
The coild-coil region of the protein was able to bind to the
C-terminal tail of the AT2R, but not to other receptors tested
(12,13). Recombinant expression of the
protein causes anti-proliferative effects while for this effect the
expression but not ligand activation of the AT2R was required
(12). Furthermore, the protein
seemed to traffic the AT2R from the Golgi compartment to the cell
surface, implicating that the protein is required for the cell
surface expression of AT2R (13).
Nevertheless, MTUS1 proteins seem to have more than one signaling
mechanism, since AT2R and MTUS1 are not co-localizing in all
tissues (14).
A recently published study reveals that
Poly(ADP-ribose) polymerase-1 (PARP-1) can activate the
transcription of the MTUS1 gene and represses the AT2R
transcription (15). PARP-1 is
known to be involved in diseases like hypertension and
inflammation, although PARP-1 deficient mice do not develop cardiac
hypertrophy (16).
Investigations of the neural differentiation after
stimulation with Ang II exhibit a new signaling mechanism,
connecting the stimulation of the AT2R with induced MMS2
expression, which enhances neural differentiation and brain
protection via the interaction between MTUS1 and Src homology 2
domain-containing protein-tyrosine phosphatase 1 (SHP-1) (17).
Newly generated mice overexpressing MTUS1 showed
attenuated superoxide anion production, activation of cell
proliferation signaling cascades and expression of tumor necrosis
factor-α (18). After cuff
placement the neointimal formation of the femoral artery was
significantly smaller in mice overexpressing MTUS1 than in WT mice,
implicating an important role of MTUS1 in vascular remodeling
(18).
Although all these results suggest an
anti-proliferative role of MTUS1 in the renin-angiotensin system
and as well in carcinogenesis, additional functions and regulatory
mechanism of the MTUS1 gene and proteins remain largely unknown. We
therefore generated an MTUS1 KO mouse line for further
investigations on cell proliferation, tumor development and
cardiovascular disease.
Materials and methods
Generation of MTUS1 KO mice
The local Ethics Committee approved the generation
of MTUS1 KO mice. Gene trapping is a high-throughput approach used
to introduce insertional mutations across the genome in mouse
embryonic stem (ES) cells. Such cell lines can be used for
generating reporter-tagged, loss-of-function mutations in mice. The
Baygenomic Consortium generated a public library of mutated murine
ES cell lines using a gene trap vector which simultaneously mutates
and reports the expression of the endogenous gene at the site of
insertion and provides a DNA tag for the rapid identification of
the disrupted gene. A data base search revealed that the stem cell
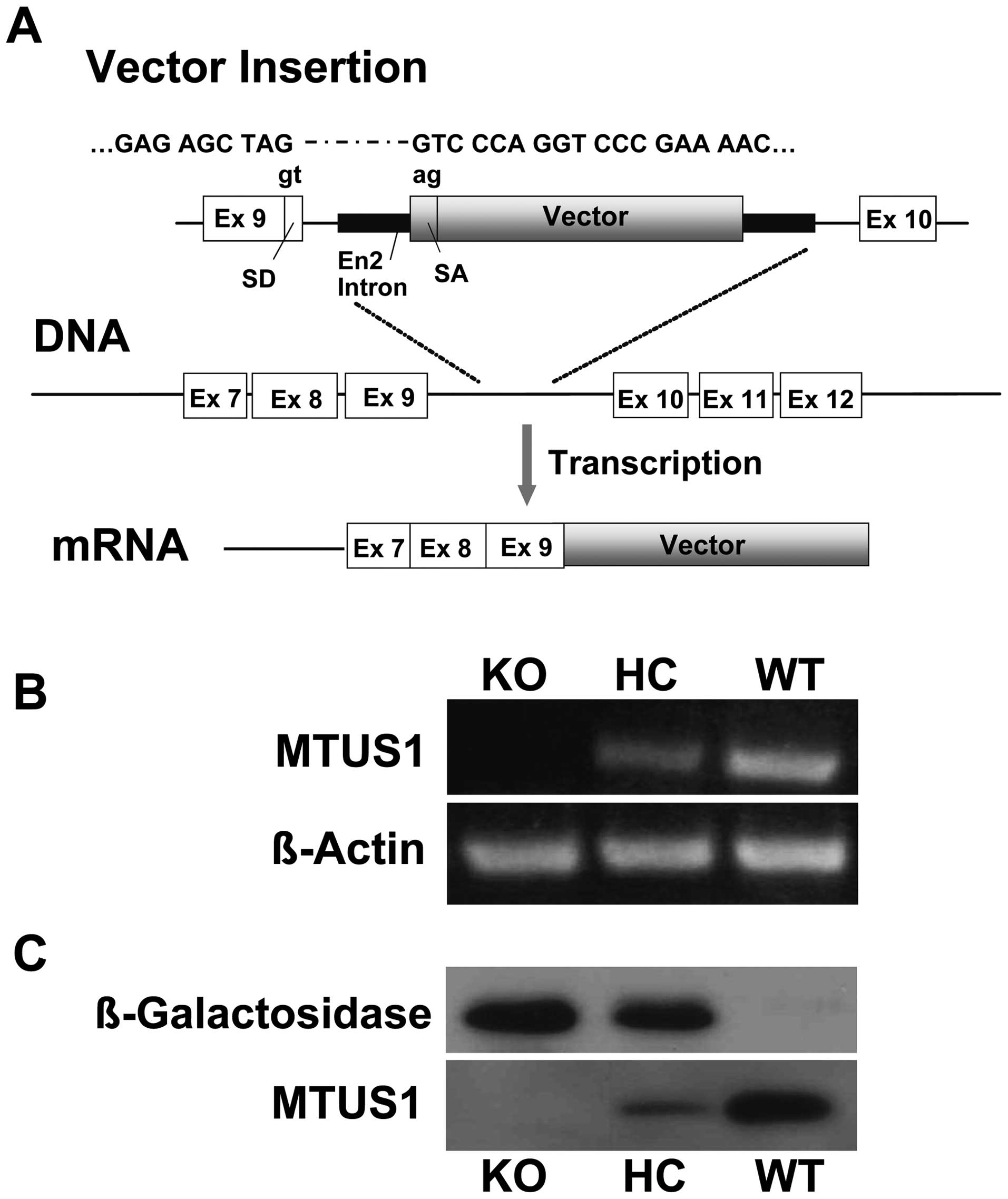
line RRA048 (Baygenomics, San Francisco, USA) contains a trapped
MTUS1 gene by the insertion of a β-galactosidase gene in the intron
region between exon 9 and exon 10. We determined the exact
localization of the vector by sequencing a PCR fragment amplified
with the forward primer 5′-CTGAAGCAACACAAAACCCTCTCTC-3′ and the
reverse primer 5′-CACTCCAACCTCCGCAAACTC-3′ (annealing 66°C, 36
cycles). This stem cell line was used for the generation of MTUS1
KO mice by injection into blastocysts to generate germ line
chimeras. These chimeras were mated with C57Bl/6 WT mice to
generate heterozygote mice, which were then mated to receive
homozygote MTUS1 KO mice. The local Ethics Committee approved the
generation of MTUS1 KO mice (Regierung von Unterfranken, Permit
Number 621-2531.01-70/03).
Treatment and genotyping of MTUS1
deficient mice
All mice were kept in a room where the light was 12
h on and 12 h off and the temperature was kept at 22°C. In each
generation homozygotes, heterozygotes and WT mice were counted to
examine embryonal lethality. For all investigations MTUS1 KO mice
were compared to WT littermates.
MTUS1 KO mice were identified by PCR using
5′-CCCTGCGTTTCCAGAGTCCT-3′ as forward primer and
5′-CACTCCAACCTCCGCAAACTC-3′ as reverse primer for detecting the
gene-trap vector. The WT mice were identified by amplification with
the same forward primer and 5′-GGTTTGATCCCCAACACCAC-3′ as reverse
primer (annealing 60°C, 34 cycles) for detecting the native MTUS1
sequence.
MTUS1 mRNA expression
Total RNA isolation from heart tissue of 12 weeks
old WT and KO mice was performed using the RNeasy Mini kit (Qiagen,
Hilden, Germany) and cDNA synthesis was performed using the First
Strand cDNA Synthesis kit (Fermentas, St. Leon-Roth, Germany).
For the MTUS1 mRNA detection the primers 5′-CTGAAGC
AACACAAAACCCTCTCTC-3′ (forward) and 5′-TGTCTG
ATGCTGCTGGTTTAGTTTC-3′ (reverse) were used. β-actin forward primer
5′-TCTACAATGAGCTGCGTGTG-3′ and reverse primer
5-′TACATGGCTGGGGTGTTGAA-3′ (annealing 60°C, 40 cycles) were used
for the amplification of the β-actin housekeeping gene.
Western blot analysis
Proteins were isolated from heart tissue of 12 weeks
old WT and KO mice. Therefore, tissues were homogenized and
directly lysed in 50 mM hepes pH 7.5, 50 mM NaCl, 20 mM EDTA, 1 mM
MgCl2, 2% Triton X-100, and protease inhibitor (complete
mini, Roche Diagnostics, Mannheim, Germany). Subsequently, protein
concentration was determined with the Bradford reaction. Ten
micrograms of protein were loaded per lane, transferred to a PVDF
membrane (Amersham, Freiburg, Germany) and blocked with 5% non-fat
dried milk. Primary antibodies were polyclonal rabbit anti-MTUS1
(Eurogentec, Seraign, Belgium) diluted 1:2000 and monoclonal rabbit
anti-β-galactosidase (Acris, Hiddenhausen, Germany) diluted 1:2000
in TBS-T containing 5% non-fat dried milk. Secondary antibody was
HRP-conjugated goat anti-rabbit (Dako, Hamburg, Germany), diluted
1:2000. For detection ECL Plus (Amersham) was used.
X-gal staining
In KO mice, the MTUS1 gene is trapped by a
β-galactosidase gene, which is now under the control of the MTUS1
promotor. Staining of β-galactosidase therefore is an easy way to
analyse the expression of MTUS1 in different tissues. Liver,
kidney, lung, heart, brain, spleen, adrenal gland, colon and thymus
of two WT and two KO mice were isolated and tissue slices were
prepared. The X-gal staining was performed as previously reported
(19). The counterstaining was
performed using Nuklear Fast Red (Sigma-Aldrich, Taufkirchen,
Germany) and the slices were dehydrated and embedded with
Histofluid (Marienfeld, Lauda, Germany).
Long-term investigation
Six WT and six KO mice were weighed at the age of
four weeks and ten months. Systolic and diastolic blood pressure
was measured in six WT and six KO mice at the age of 10 months by a
non-invasive tail-cuff system (Föhr, Seeheim, Germany). For each
mouse five values were measured and the mean pressure was
calculated. For long-term investigation, ten WT and 35 KO mice were
kept until the age of 10–12 months. At this age, the organs of all
animals were examined macroscopically and conspicuous organs were
analysed histopathologically after paraffin embedding and H&E
staining. Some sections were also silver stained for reticulin
fibers using Gordon and Sweet's protocol. In addition, blood was
taken and analysed of ten WT and ten KO mice and heart/body weight
was calculated of six WT and six KO mice.
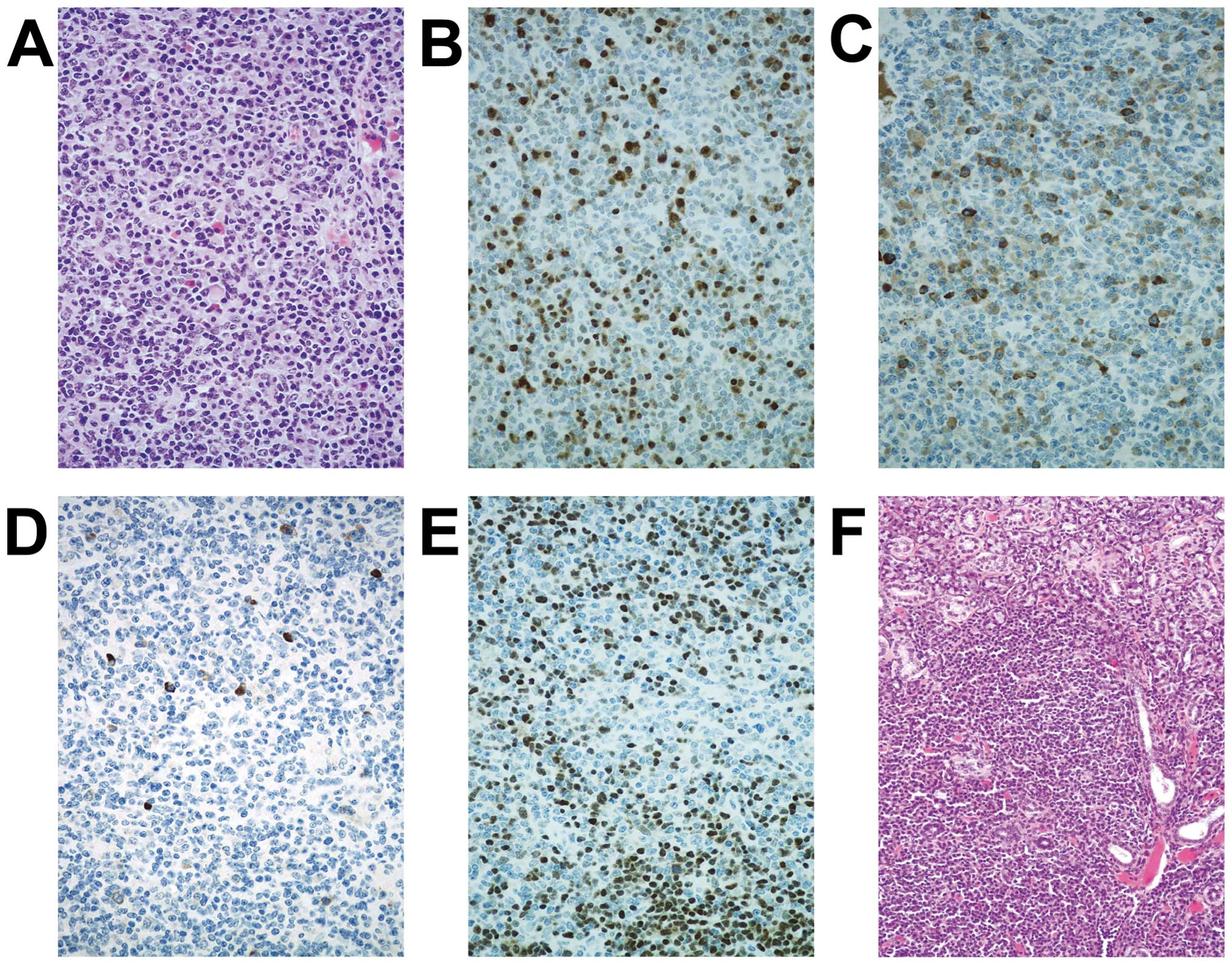
Immunohistochemistry
Immunohistochemistry was performed on formalin-fixed
and paraffin wax-embedded sections. For wet heat-induced antigen
retrieval, dewaxed and re-hydrated sections were heated in
household electric pressure cocker in 1 mmol/l Tris/10 mmol/l EDTA
(pH 9.0). Following the heating, the sections were washed in
Tris-buffered saline (TBS; pH 7.6) containing 10% fetal calf serum
(Gibco Laboratories, Grand Island, NY) for 15 min and incubated
with primary antibodies at room temperature for 75 min. Primary
antibodies were polyclonal goat anti-MUM1/IRF4 (M17) and anti-Pax-5
(C-20) diluted 1:5000 (both from Santa Cruz Biotechnology, Santa
Cruz, CA, USA), polyclonal rabbit anti-λ and anti-κ light chain
(Dako, Glostrup, Denmark) diluted 1:10.000 in TBS containing 10%
FCS and 0.1% (wt/vol) NaN3. Link antibody was
HRP-conjugated goat anti-rabbit (Dako) diluted 1:2000, which was
followed by HRP-conjugated Novolink Polymer (Leica Mycrosystems).
The peroxidase reaction was developed with 3.3′ diaminobenzidine
tetrahydrochloride (Sigma-Aldrich, Steinheim, Germany)/0.03%
(vol/vol) H2O2 and the sections were
counterstained with Gill's hematoxylin.
Isolation and investigation of skin
fibroblasts
The skin of three WT and three same aged homozygote
mice was removed and transferred to sterile PBS (PAA, Linz,
Austria). After cutting into small pieces, the skin was incubated
with 10× Trypsin/EDTA (PAA) for 30 min and suspended in FBM medium
(Cambrex, Walkersville, USA) supplemented with 10% fetal calf serum
(Biochrom, Berlin, Germany), insulin, rhFGF and
Gentamicin/Amphotericin B (Additives FGM2, Cambrex). Three weeks
after isolation the fibroblasts were split for the first time.
X-gal staining was performed as described above. For the
proliferation assay 1×105 cells/well of three WT and
three KO cell lines were plated in a 6-well plate. After 48 h
incubation the cells were collected and cell concentration was
specified by cell counter. Furthermore, the size of the cells was
measured by FACS analysis (Beckton-Dickinson, Heidelberg,
Germany).
For investigation of the proliferation rate of WT
and KO cells in response to various growth factors,
1×103 cells were seeded in a 96-well plate in FBM medium
with all supplements. After 48 h the medium was removed and the
cells were treated with either FBM medium with all supplements, FBM
medium with 1% FCS, FBM medium with 1% FCS and 0.1% rhFGF or FBM
medium with 1% FCS and 0.1% insulin. Cell proliferation was
determined using the Cell Proliferation ELISA (Roche, Mannheim,
Germany) according to the manufacturer's manual after 24 h of
incubation.
Statistical analyses
Data are presented as means ± SEM and analyzed by
Student' t-test. A P<0.05 was considered significant.
Results
Generation and characterization of MTUS1
KO mice
For characterization of the generated KO mice, we
first investigated the correct insertion of the β-galactosidase
vector into the MTUS1 gene and confirmed the functional KO on mRNA
and protein level. Sequencing results confirmed the correct
insertion of the β-galactosidase vector into the intron region
between exon 9 and exon 10 of the MTUS1 (Fig. 1A). Since MTUS1 is known for its
anti-proliferative effect, a possible embryonal lethality had to be
examined. In the F1 generation of the chimeric mice 18
heterozygotes and 14 WT mice were born alive, in the F2 generation
23% WT, 58% heterozygote and 19% homozygote mice were born alive.
Therefore, Mendelian law was confirmed and an obviously higher
embryonal lethality of homozygote or heterozygote mice was not
observed.
RT-PCR analysis of WT and KO mice showed no
detectable MTUS1 expression in the MTUS1 deficient mice, while the
WT mice revealed normal mRNA expression (Fig. 1B). Knockout of MTUS1 protein
expression was confirmed by Western blot analysis in the heart
tissue of MTUS1 KO mice compared to WT mice. MTUS1 KO mice showed
β-galactosidase protein expression instead (Fig. 1C).
Tissue distribution of the MTUS1
expression
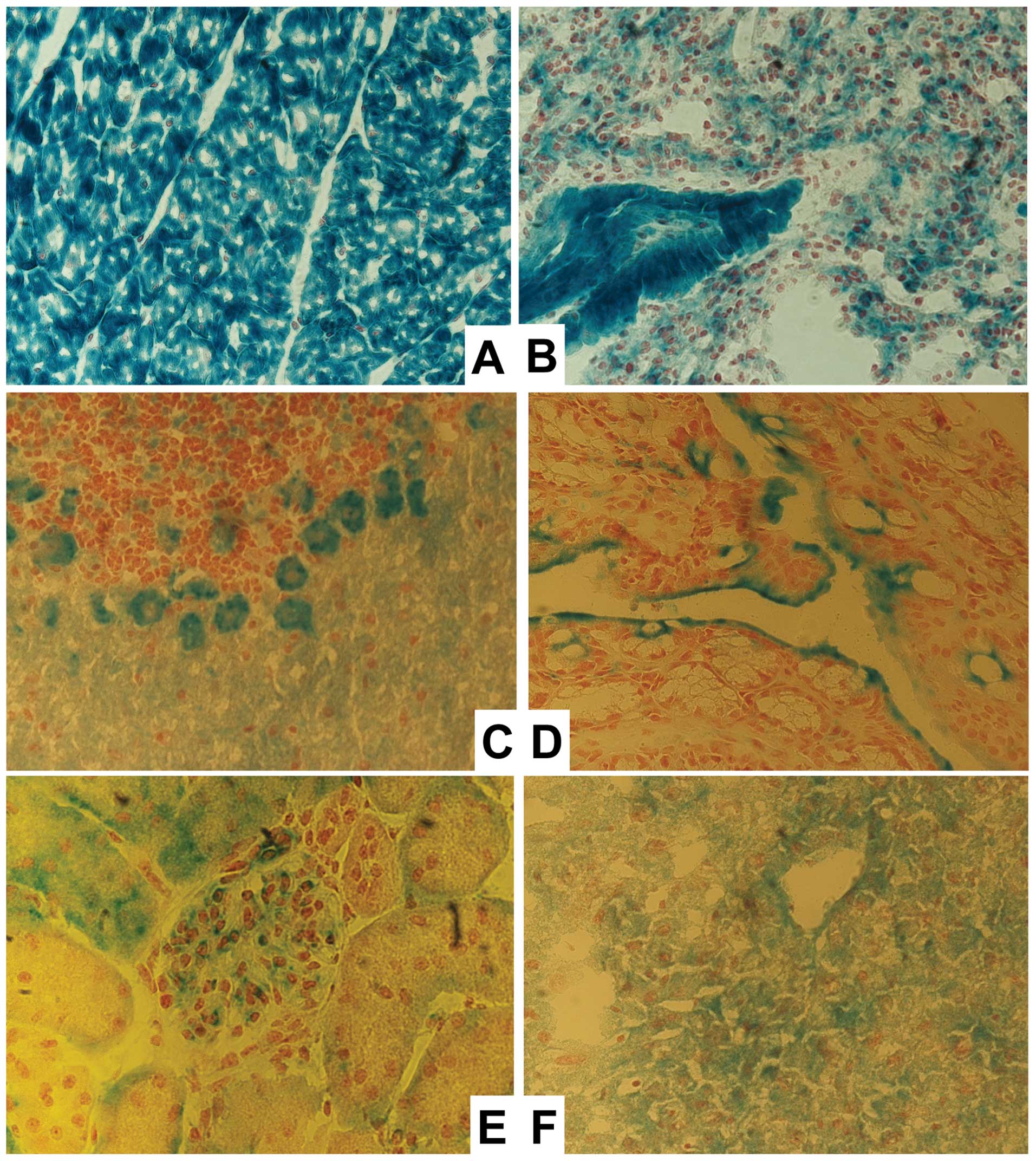
MTUS1 is known to be expressed in various tissues at
moderate or high levels. With the X-gal staining in MTUS1 knockout
mice the tissue distribution could be investigated more precisely,
since in MTUS1 knockout mice the β-galactosidase gene is under the
control of the MTUS1 promoter.
The highest expression was determined in the heart,
where all cardiomyocytes as well as the endothelial cells were
intensively stained. In lung and colon the highest staining was
observed in the epithelial and endothelial cells. In the kidney
especially the glomeruli and the proximal tubulus cells were
clearly stained. The hepatocytes revealed a slight homogeneous
staining, while in brain the Purkinje cells were clearly stained
(Fig. 2). Spleen and adrenal gland
were discretely stained only in the area of the capsule and some
connective tissue (data not shown). The thymus had some net-like
strands of epithelial cells, where staining was also obtained (data
not shown). X-gal staining was performed in WT animals as negative
controls as well, but as expected no staining was detected (data
not shown).
Long-term investigations
For long-term investigations, 10 WT mice and 35 KO
mice were housed for 10–12 months. The behaviour of the KO mice was
not altered during this observation period.
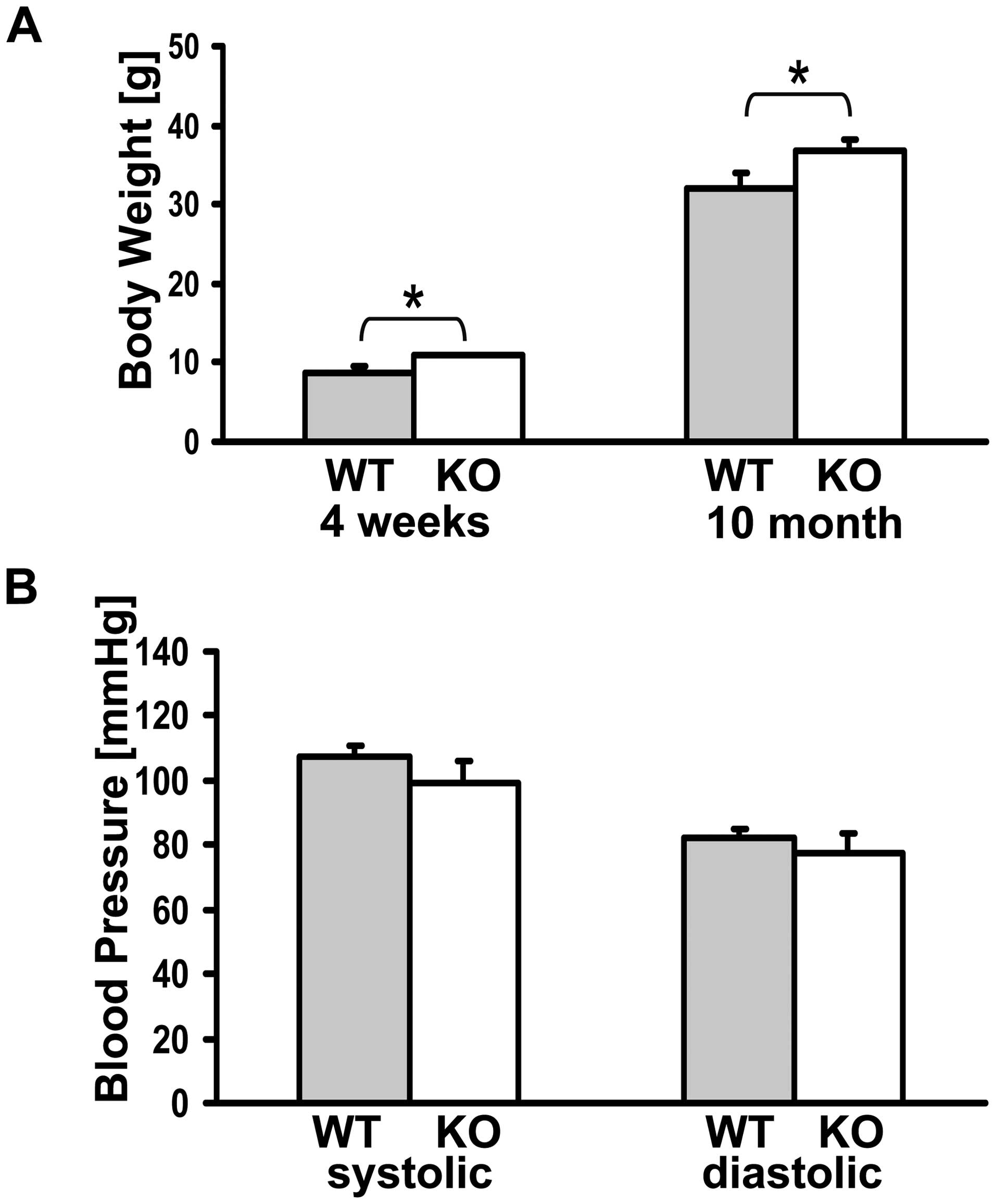
For analysis of the body weight of the mice, six WT
and six KO mice were measured at the age of four weeks and 10
months. Even at the age of four weeks, the KO mice revealed a
significantly (p=0.028) higher body weight, the KO mice weighed
10.8±0.2 g and the WT mice 8.7±0.8 g. At the age of 10 months the
effect was still maintained, the KO weighed 36.7±1.6 g and the WT
mice 32±1.9 g (p=0.036) (Fig.
3A).
Systolic and diastolic blood pressure was compared
in six WT and six MTUS1 KO mice at the age of 10 months. The mean
systolic blood pressure was 100±6 mmHg in KO mice and 107±4 mmHg in
WT mice whereas the mean diastolic blood pressure was 77±6 mmHg in
KO mice and 82±3 mmHg in WT mice. Even if there was a trend towards
lower blood pressure in MTUS1 KO mice, the result was not
significant (p=0.101 for the systolic blood pressure and p=0.087
for the diastolic blood pressure) (Fig. 3B).
For the investigation of the laboratory blood
values, blood samples of 10–12 months old MTUS1 KO mice were
compared to WT littermates. Most serum levels of the investigated
parameters, for example all electrolyte concentrations, did not
significantly differ between the MTUS1 KO and the WT mice. The
hematologic values were noticeably different, with higher leucocyte
and lymphocyte counts in the MTUS1 KO mice compared to WT mice,
while erythrocytes, hemoglobin, hematocrit and thrombocyte levels
were reduced (Table I).
 | Table IBlood values of MTUS1 KO and WT
mice. |
Table I
Blood values of MTUS1 KO and WT
mice.
| WT | KO |
|---|
| Sodium | 150.1 (±0.74)
mmol/l | 148.8 (±0.99)
mmol/l |
| Calcium | 2.85 (±0.03)
mmol/l | 2.71 (±0.05)
mmol/l |
| Magnesium | 1.3 (±0.05)
mmol/l | 1.5 (±0.10)
mmol/l |
| Inorganic
phosphate | 2.65 (±0.13)
mmol/l | 3.03 (±0.27)
mmol/l |
| Chloride | 113.1 (±0.54)
mmol/l | 113.3 (±1.14)
mmol/l |
| Glucose | 364.2 (±25.6)
mg/dl | 286.9 (±33.2)
mg/dl |
| Urea | 36.8 (±4.01)
mg/dl | 68.7 (±18.1)
mg/dl |
| Uric acid | 5.8 (±0.48)
mg/dl | 4.1 (±0.34)
mg/dl |
| Total protein | 6.0 (±0.12)
g/dl | 5.9
(±0.23)g/dl |
| Albumin | 3.6 (±0.08)
g/dl | 3.2
(±0.14)g/dl |
| Cholinesterase | 8033.0 (±424)
U/l | 8174.6 (±458)
U/l |
| Bilirubin | 0.17 (±0.03)
mg/dl | 0.16 (±0.02)
mg/dl |
|
Glutamate-oxalacetate-transferase
(GOT) | 87.7 (±7.52)
U/l | 158.6 (±21.6)
U/l |
|
Glutatmate-pyruvate-transaminase
(GPT) | 48.4 (±3.74)
U/l | 54.3 (±14.5)
U/l |
| Glutamate
dehydrogenase (GLDH) | 9.3 (±1.52)
U/l | 9.1 (±2.13)
U/l |
| Alkaline
phosphatase | 85.5 (±23.8)
U/l | 63.5 (±8.21)
U/l |
| Lactate
dehydrogenase | 427.5 (±77.3)
U/l | 625.5 (±84.4)
U/l |
| Creatine
kinase | 130.3 (±22.5)
U/l | 502.9 (±106)
U/l |
| Lipase | 28.8 (±3.65)
U/l | 20.5 (±1.80)
U/l |
| Cholesterol | 118.8 (±14.5)
mg/dl | 112.3 (±9.64)
mg/dl |
| Triglyceride | 107.6 (±9.75)
mg/dl | 97.5 (±6.40)
mg/dl |
| HDL
cholesterol | 82.0 (±0.00)
mg/dl | 91.4 (±13.2)
mg/dl |
| Iron | 202.6 (±15.2)
μg/dl | 181.8 (±12.7)
μg/dl |
| Leukocyte | 5.7
(±0.64)*1000/μl | 8.7
(±0.72)*1000/μl |
| Erythrocyte | 8.9
(±0.15)*10E6/μl | 7.8
(±0.32)*10E6/μl |
| Hemoglobin | 13.5 (±0.17)
g/dl | 11.3 (±0.67)
g/dl |
| Hematocrit | 47.7 (±0.55)% | 43.0 (±1.70)% |
| Mean corpusculare
volume (MCV) | 53.7 (±0.47)
fl | 55.3 (±0.68)
fl |
| Mean corpusculare
hemoglobin (MCH) | 15.2 (±0.15)
pg | 14.3 (±0.59)
pg |
| Mean corpusculare
hemoglobin concentration (MCHC) | 28.2 (±0.17)
g/dl | 25.9 (±1.03)
g/dl |
| Thrombocyte | 753.3
(±73.4)*1000/μl | 608.4
(±53.3)*1000/μl |
| Lymphocyte | 2.5
(±0.29)*1000/μl | 3.6
(±0.29)*1000/μl |
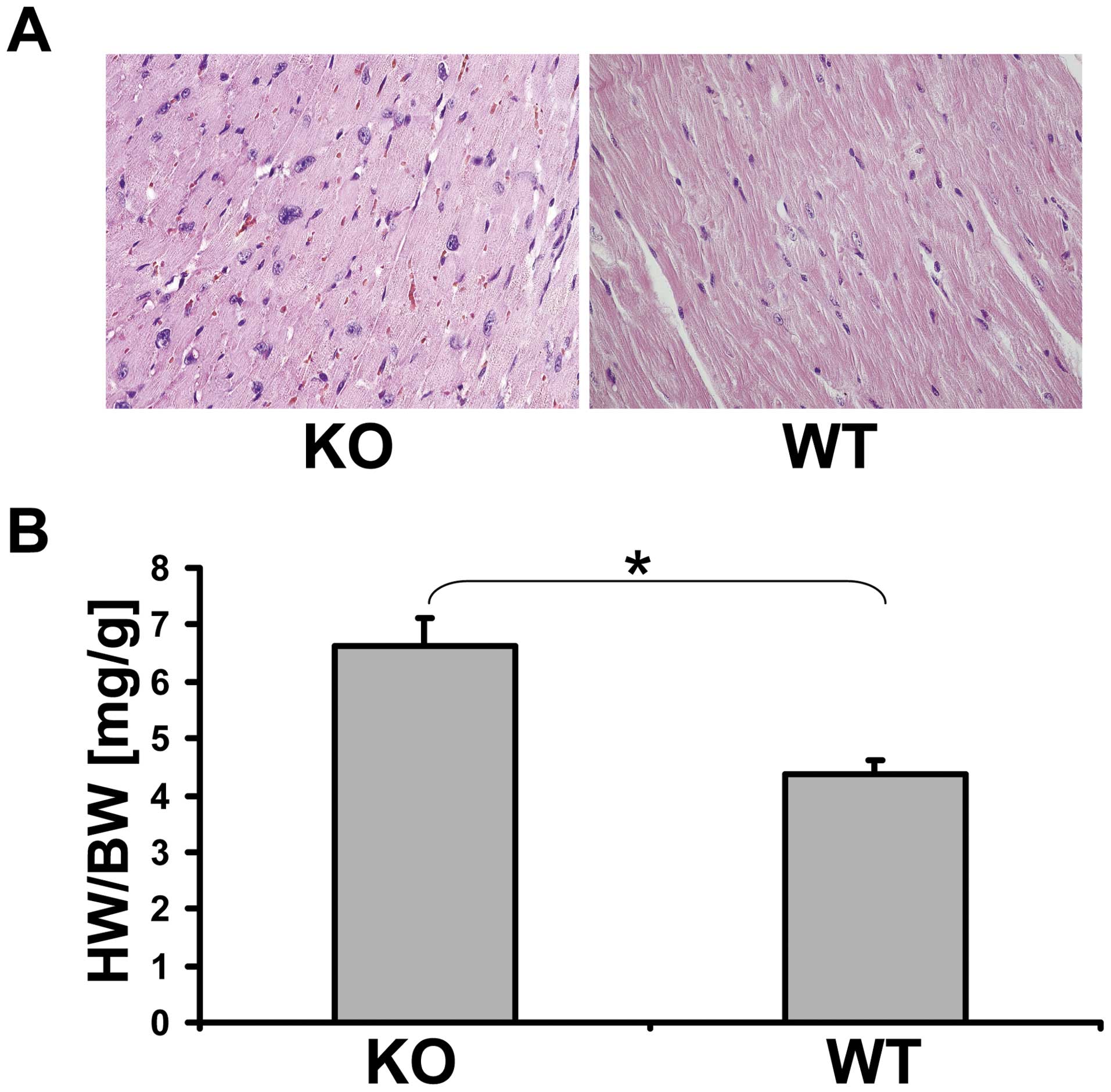
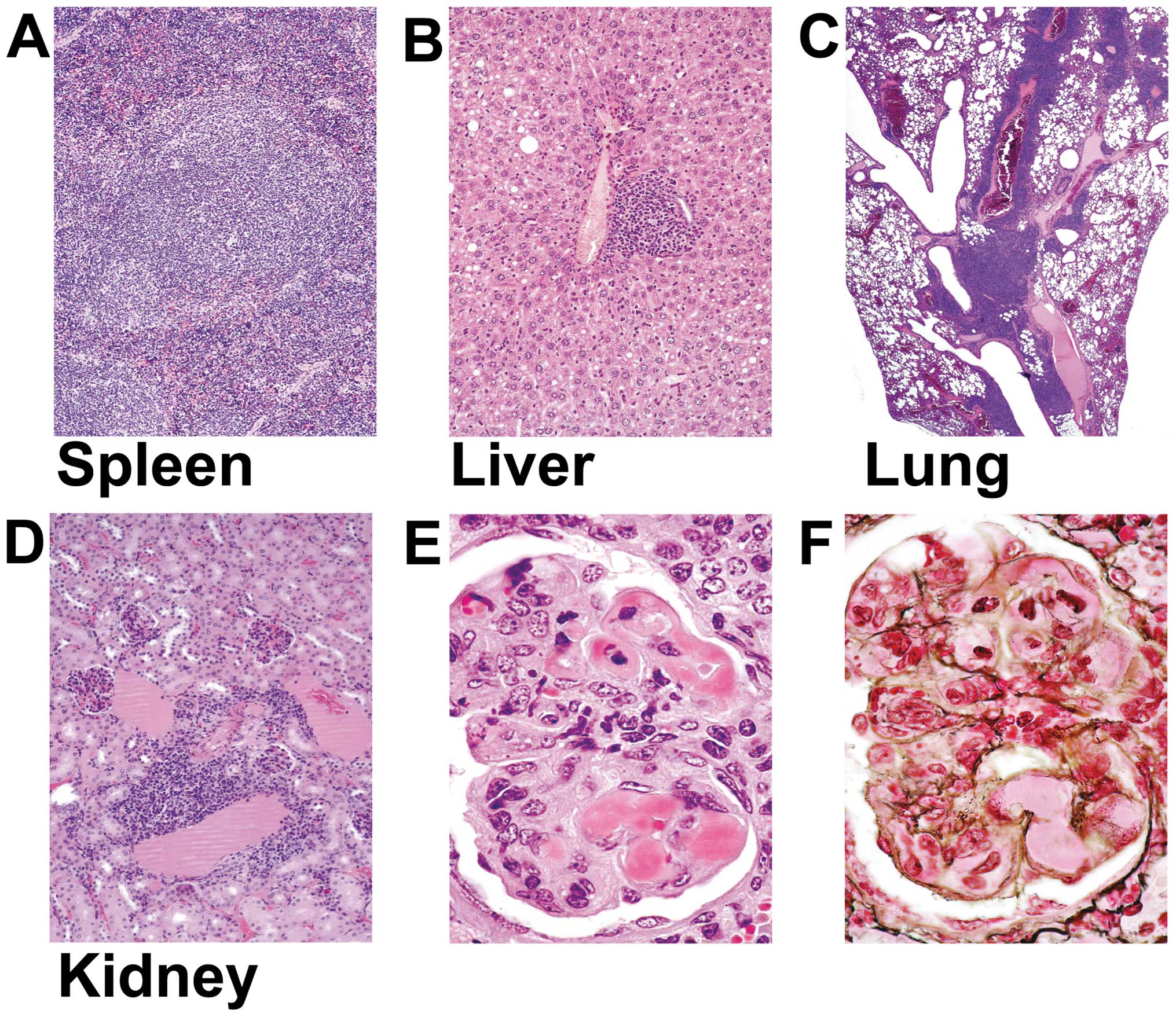
Pathology of the MTUS1 KO mice
None of the 10 WT mice died spontaneously during the
long-term investigation of 12 months. In contrast, 37% of the KO
mice died spontaneously or had to be sacrificed for ethical
reasons. The autopsy of the WT mice at the age of 12 months
revealed no organ anomaly and the histopathology did not
demonstrate any unusual findings. Compared to WT animals, most of
the KO mice revealed organ anomalies. Twenty-eight percent of the
studied MTUS1 KO mice developed heart hypertrophy and 12% developed
glomerulonephritis. The heart/body weight was significantly
(p=0.006) increased in MTUS1 KO mice (Fig. 4). Eighty percent of the nephritic
animals showed diffuse glomerulonephritis with wire-loop lesion in
one and rapidly progressive glomerulonephritis with crescent
formation in another animal (Fig.
5). Forty-three percent of the MTUS1 KO mice revealed
multiorgan lymphoid hyperplasia affecting spleen (20%), kidney
(37%), lung (23%), lymph nodes (17%), and liver (17%) accompanied
with leukocytosis, lymphocytosis, and mild anemia (Fig. 5, Table
I). Eight percent of the animals revealed sialadenitis and 3%
insulitis. One animal (3%) developed a marginal zone B-cell
lymphoma affecting submandibular salivary gland and regional lymph
node (Fig. 6). The symptoms of all
mentioned animals are consistent with a B-cell lymphoproliferative
disease with features of systemic lupus erythematosus.
Investigations on skin fibroblasts
Skin fibroblasts were isolated from three WT and
three MTUS1 KO mice for further in vitro investigation of
cellular proliferation. X-gal staining was positive in fibroblasts
and the MTUS1 protein seemed to be located in the cytoplasm near
the nucleus. Mitotic cells were stained more intensively (data not
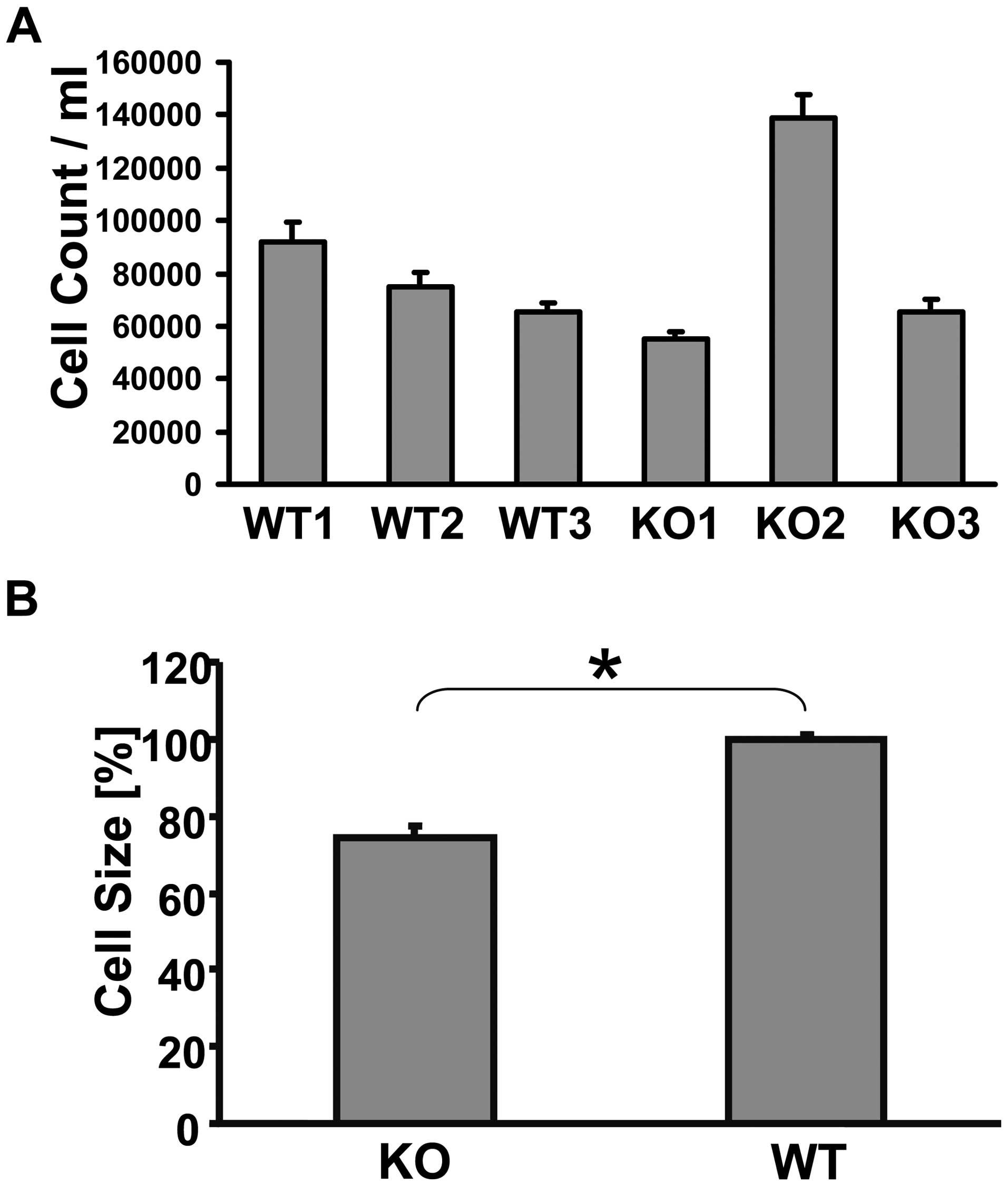
shown). Cell proliferation of the MTUS1 KO fibroblasts were equal
to the WT fibroblasts in two cell lines, but the third MTUS1 KO
cell line was proliferating almost at double speed (Fig. 7A), while the cell volume of all
MTUS1 KO cell lines was significant smaller (p=0.001) than the WT
controls (Fig. 7B). Cell culture
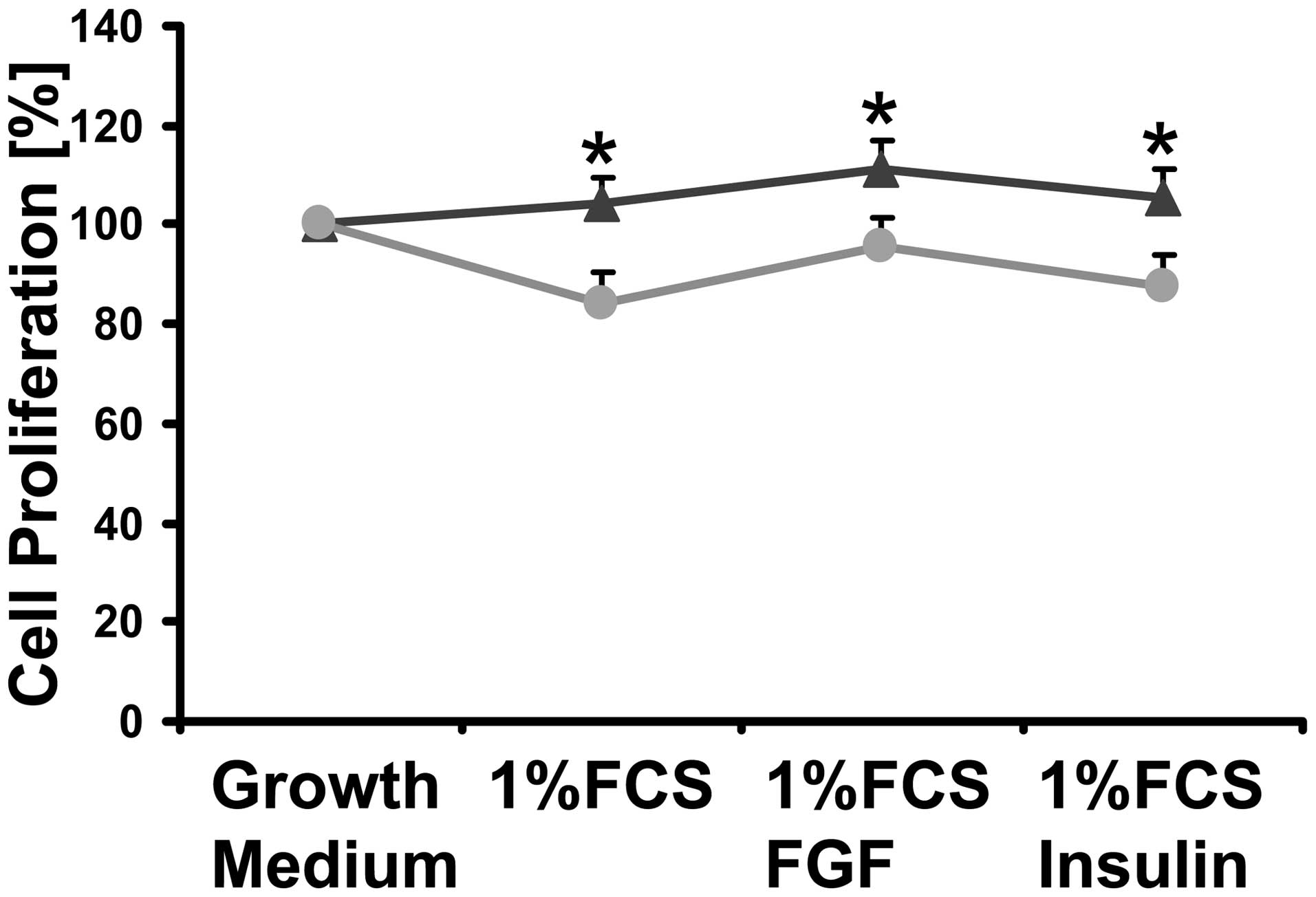
analyses with different growth factors were performed to further
characterize the proliferation characteristics of the MTUS1 KO
fibroblasts. Thereby WT fibroblasts showed much more sensitivity
for FCS depletion and depletion of growth factors, resulting in a
significant lower proliferation (p=0.004, p=0.0023 and p=0.011)
(Fig. 8).
Discussion
The present study reports the generation and
characterization of the first mouse model deficient for MTUS1.
Previously, MTUS1 has been reported to play a role in the cell
proliferation as well as in cancer development (3,11).
Therefore, the main purpose of the study was to prove that MTUS1
can regulate the cell proliferation and to investigate if the lack
of MTUS1 alone can cause cancer development or major pathological
changes.
In the long-term investigations the MTUS1 KO mice
reveal two major diseases. On the one hand the KO mice develop a
significant higher body weight and heart hypertrophy, furthermore,
multiple damage to organs like fatty degeneration of the liver were
observed. The anti-proliferative function of MTUS1, which was
recently linked to its role in the AT2R pathway, may play an
important role in the development of the high body weight and the
hypertrophy of the heart, where X-gal staining shows the highest
and abundant expression of all tested organs (12). As reported previously, the AT2R
antagonizes the accelerating growth effects of the AT1R in
cardiomyocytes (20). In addition,
the development of heart hypertrophy in AT2 KO mice is not or only
in part a consequence of hypertension, because AT2 KO mice
developed hypertension, but no or only a light heart hypertrophy
(21). MTUS1 KO mice show the
tendency for lower blood pressure, while mice overexpressing MTUS1
show a tendency for higher blood pressure without any medical
treatment (18). Both results miss
the significance level, but the data are still surprising. A new
signaling pathway, involving the interaction of the AT2R and PLZF
protein was reported as a possible pathway in the development of
heart hypertrophy (22). After
binding to the AT2R in the heart, PLZF protein translocates to the
nucleus and activates the transcription of p85α, the regulatory
unit of P13 kinase. Growth factors such as EGF activate p85α,
enhancing protein synthesis essential to cardiac hypertrophy
(23). In contrast, the AT2R
linked activation of SHP-1 protein down-regulates growth factors
and p85α (23). A newly published
study in neurons shows that MTUS1 can interact with the SHP-1
protein after AT2R binding, resulting in increased MMS2
transcription and neural differentiation (17). Previous studies show not only an
interaction of MTUS1 with the AT2R but also an MTUS1 mediated
inhibition of insulin, bFGF and EGF signal cascades, which lead to
the activation of ERK2 (12).
MTUS1 could therefore prevent heart hypertrophy in two ways. The
interaction with SHP-1 could lead to a down-regulated transcription
of p85α in the heart and inhibition of EGF could prevent the
activation of p85α. Both issues must be addressed for further
understanding the development of heart hypertrophy in MTUS1 KO
mice.
One out of three MTUS1 KO fibroblast cell lines
shows significantly increased cell proliferation, implicating that
the increased proliferation could differ individually and might be
dependent on other factors as well. Nevertheless, MTUS1 KO
fibroblasts show significant higher proliferation in medium with
depleted FCS concentration and lack of growth factors than control
WT fibroblasts. MTUS1 is known to have anti-proliferative effects
via the inhibition of insulin, epidermal growth factor and
fibroblast growth factor, so the higher proliferation in MTUS1 KO
cells could be due to the obviated inhibition of these growth
factors and therefore a lower sensitivity to reduced growth factors
in the medium (12).
The second major symptomatology in the MTUS1 KO mice
revealed multiorgan lymphoid hyperplasia, splenomegaly, accompanied
with glomerulonephritis, and sialadenitis in some animals. The wire
loop lesion of the kidney and the histopathological investigations
suggest a SLE-like systemic autoimmune disease, which was at least
in one case complicated with a marginal zone B-cell lymphoma
affecting submandibular salivary gland and regional lymph node. The
blood values reveal higher counts of lymphocytes and leucocytes but
mild anemia with enlarged erythrocytes and reduced hemoglobin
levels. These results are not completely in accord with the values
of a SLE disease, but the high level of lymphocytes could be due to
the developement of a lymphoma. To date, nothing is known about the
effects of MTUS1 in hematopoiesis or autoimmune disease like SLE,
but the reported interaction with AT2R could be a hint for similar
effects than the RAS pathway. Interestingly, the ACE KO mice
develop mild anemia and ACE inhibitors and AT1R antagonists are
sporadic reported to cause anemia and bone marrow aplasia (24). In addition, the RAS pathway is
known to play a role in immune responses and inflammation. A new
study in a lupus mouse line with AT1AR deficiency did not show the
expected benefit in lupus nephritis, because the remaining
glomerular AT1BR was stimulated and caused even more severe injury.
This enhanced disease process could be prevented with losartan
treatment (25). Even if
randomised trials in humans are currently missing, RAS inhibition
revealed a general benefit for SLE patients (26). Keeping in mind that the AT2R can
antagonize the AT1R in many cases, there could be a protective
effect of the AT2R and its binding proteins in SLE. MTUS1
down-regulation is now well-described in a wide range of tumor
tissues, but to date there have been no studies on MTUS1
involvement in lymphoproliferative diseases like lymphoma (5–8,27).
At least one animal showed SLE-like disease accompanied with MALT
lymphoma, which can arise at any extranodal site, share histologic
and immunophenotypic characteristics and is usually associated with
chronic inflammation resulting of autoimmune disease or infection
(28). In detail, 10 of 14
separate studies in human SLE patients reported a 3-to 40-fold
increased risk of non-Hodgkin lymphoma and all lymphoma were of the
B-cell subtype (29). In our
study, the autoimmune disease could therefore be the initial step
of lymphoma formation. In general, four chromosomal translocations
are well known to be involved in the development of MALT lymphoma,
which affect the api2, malt1 bcl10, foxp1 and IgH genes and have
partly shown to cause an activation of the nuclear factor kappa B
(NF-κB) pathway, suggesting a common occurance for MALT lymphomas
(30). MTUS1 gene has not yet been
reported to be mutated or translocated in MALT lymphoma, but its
interaction partner AT2R and the RAS were recently described in
various aspects of inflammation, including the NF-κB pathway
(1). AT2R activation oppose the
pro-inflammatory effects of the AT1R by preventing the activation
of NF-κB as well as stabilizing the inhibitory protein κB through
an SHP-1 dependent pathway (31,32).
MTUS1 has been shown to build a complex with SHP-1 upon AT2R
stimulation and to translocate into the nucleus, resulting in
enhanced cell differentiation in neurons (4). In addition, NF-κB showed constitutive
activation not only in SLE but also in some cancers and leukemias,
substantiating the molecular link between chronic inflammation,
autoimmunity and carcinogenesis (33). It is noteworthy that the
microvascular permeability was decreased by activation of AT2R and
increased by blockade of the AT2R (34). MTUS1 has been shown to be localized
in the vascular endothelium and to interact with the AT2R to
cooperate in different functions, so it would be possible that lack
of MTUS1 could effect the microvascular permeability. Taking these
results together, it could be hypothesised that a defiency of MTUS1
could result in an increased activation of NF-κB and increased
microvascular permeability, enabling the infiltration of
inflammatory or metastatic cells and therefore an increased
incidence of autoimmune disease and lymphoma.
In previous studies, wide tissue distribution of
MTUS1 mRNA was reported by quantitative RT-PCR and some new studies
examined the down-regulation of MTUS1 in tumors such as ovarian
cancer, pancreatic carcinoma or breast cancer, but to date nothing
is known about the localisation of MTUS1 within these tissues
(3,5,13,14,27).
The X-gal staining revealed highly restrictive distribution of
MTUS1 proteins. All examined tissues show a similar pattern of
expression and mostly endothelial and epithelial cells were
stained, whereas the stromal cells showed a much lower staining. In
heart and brain the cardiomyocytes and the Purkinje cells were
stained additionally. Most tumors have their origin in endothelial
and epithelial cells, thus the localisation of MTUS1 could be a
hint of its function as tumor suppressor gene. Expression profiles
of MTUS1 proteins and the AT2R are partly overlapping for example
in the endothelial cells of heart and cardiomyocytes or the
epithelial cells in lung, but in colon and kidney the expression
pattern seem to differ (35–38),
suggesting an additional AT2R-independent pathway for MTUS1.
In conclusion, we report here the generation of the
first MTUS1 KO mouse line, which develops spontaneous heart
hypertrophy and SLE-like lymphoproliferative disease. In addition,
skin cells of MTUS1 KO mice reveal higher cell proliferation in
response to depleted FCS and growth factors. Therefore, MTUS1 KO
mice further support the hypothesis of an anti-proliferative
effects of MTUS1 and can serve as a model for further
investigations in autoimmune disease, cardiovascular disease and
carcinogenesis.
Acknowledgements
The excellent skillful technical assistance of Mrs.
Elke Baumeister, Mrs. Carmen Bauer, and Mrs. Margarete Roeder is
gratefully acknowledged. We thank Baygenomics for kindly providing
the stem cell line RRA048.
References
|
1
|
Deshayes F and Nahmias C: Angiotensin
receptors: a new role in cancer? Trends Endocrinol Metab.
16:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Egami K, Murohara T, Shimada T, et al:
Role of host angiotensin II type 1 receptor in tumor angiogenesis
and growth. J Clin Invest. 112:67–75. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seibold S, Rudroff C, Weber M, Galle J,
Wanner C and Marx M: Identification of a new tumor suppressor gene
located at chromosome 8p21.3-22. FASEB J. 02-0934fje2003.
|
|
4
|
Mogi M, Iwai M and Horiuchi M: Emerging
concepts of regulation of angiotensin II receptors: new players and
targets for traditional receptors. Arterioscler Thromb Vasc Biol.
27:2532–2539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frank B, Bermejo JL, Hemminki K, et al:
Copy number variant in the candidate tumor suppressor gene MTUS1
and familial breast cancer risk. Carcinogenesis. 28:1442–1445.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee S, Bang S, Song K and Lee I:
Differential expression in normal-adenoma-carcinoma sequence
suggests complex molecular carcinogenesis in colon. Oncol Rep.
16:747–754. 2006.
|
|
7
|
Tchatchou S and Burwinkel B: Chromosome
copy number variation and breast cancer risk. Cytogenet Genome Res.
123:183–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye HP, Huang N, Muzio BL, et al: Genomic
assessments of the frequent loss of heterozygosity region on
8p21.3-p22 in head and neck squamous cell carcinoma. Cancer Genet
Cytogenet. 176:100–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simon NSL, Laurie C, Linda R, et al:
Expression and function of ATIP/MTUS1 in human prostate cancer cell
lines. Prostate. 70:1563–1574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodrigues-Ferreira S, Di Tommaso A,
Dimitrov A, et al: 8p22 MTUS1 gene product ATIP3 is a novel
anti-mitotic protein underexpressed in invasive breast carcinoma of
poor prognosis. PLoS One. 4:E72392009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuern C, Heimrich J, Kaufmann R, et al:
Down-regulation of MTUS1 in human colon tumors. Oncol Rep.
23:183–189. 2010.PubMed/NCBI
|
|
12
|
Nouet S, Amzallag N, Li JM, et al:
Trans-inactivation of receptor tyrosine kinases by novel
angiotensin II AT2 receptor-interacting protein, ATIP. J Biol Chem.
279:28989–28997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wruck CJ, Funke-Kaiser H, Pufe T, et al:
Regulation of transport of the angiotensin AT2 receptor by a novel
membrane-associated golgi protein. Arterioscler Thromb Vasc Biol.
25:57–64. 2005.PubMed/NCBI
|
|
14
|
Di Benedetto M, Bièche I, Deshayes F, et
al: Structural organization and expression of human MTUS1, a
candidate 8p22 tumor suppressor gene encoding a family of
angiotensin II AT2 receptor-interacting proteins, ATIP. Gene.
380:127–136. 2006.PubMed/NCBI
|
|
15
|
Reinemund J, Seidel K, Steckelings UM, et
al: Poly(ADP-ribose) polymerase-1 (PARP-1) transcriptionally
regulates angiotensin AT2 receptor (AT2R) and AT2R binding protein
(ATBP) genes. Biochem Pharmacol. 77:1795–1805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pillai JB, Gupta M, Rajamohan SB, Lang R,
Raman J and Gupta MP: Poly(ADP-ribose) polymerase-1-deficient mice
are protected from angiotensin II-induced cardiac hypertrophy. Am J
Physiol Heart Circ Physiol. 291:H1545–H1553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li JM, Mogi M, Tsukuda K, et al:
Angiotensin II-induced neural differentiation via angiotensin II
type 2 (AT2) receptor-MMS2 cascade involving interaction between
AT2 receptor-interacting protein and Src homology 2
domain-containing protein-tyrosine phosphatase 1. Mol Endocrinol.
21:499–511. 2007. View Article : Google Scholar
|
|
18
|
Fujita T, Mogi M, Min LJ, et al:
Attenuation of cuff-induced neointimal formation by overexpression
of angiotensin II type 2 receptor-interacting protein 1.
Hypertension. 53:688–693. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bundschu K, Knobeloch KP, Ullrich M, et
al: Gene disruption of Spred-2 causes dwarfism. J Biol Chem.
280:28572–28580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wollert KC and Drexler H: The
renin-angiotensin system and experimental heart failure. Cardiovasc
Res. 43:838–849. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Senbonmatsu T, Ichihara S, Price E Jr,
Gaffney FA and Inagami T: Evidence for angiotensin II type 2
receptor-mediated cardiac myocyte enlargement during in vivo
pressure overload. J Clin Invest. 106:R1–R5. 2000. View Article : Google Scholar
|
|
22
|
Senbonmatsu T, Saito T, Landon EJ, et al:
A novel angiotensin II type 2 receptor signaling pathway: possible
role in cardiac hypertrophy. EMBO J. 22:6471–6482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Landon EJ and Inagami T: Beyond the G
protein: the saga of the type 2 angiotensin II receptor.
Arterioscler Thromb Vasc Biol. 25:15–16. 2005.PubMed/NCBI
|
|
24
|
Park TS and Zambidis ET: A role for the
renin-angiotensin system in hematopoiesis. Haematologica.
94:745–747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wuthrich RP: RAS meets SLE. Nephrol Dial
Transplant. 24:2634–2636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herlitz H, Tarkowski A, Svalander C,
Volkmann R and Westberg G: Beneficial effect of captopril on
systemic lupus erythematosus-like disease in MRL lpr/lpr mice. Int
Arch Allergy Appl Immunol. 85:272–277. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Benedetto M, Pineau P, Nouet S, et al:
Mutation analysis of the 8p22 candidate tumor suppressor gene
ATIP/MTUS1 in hepatocellular carcinoma. Mol Cell Endocrinol.
252:207–215. 2006.PubMed/NCBI
|
|
28
|
Garcia M, Konoplev S, Morosan C, Abruzzo
LV, Bueso-Ramos CE and Medeiros LJ: MALT lymphoma involving the
kidney: a report of 10 cases and review of the literature. Am J
Clin Pathol. 128:464–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smedby KE, Baecklund E and Askling J:
Malignant lymphomas in autoimmunity and inflammation: a review of
risks, risk factors, and lymphoma characteristics. Cancer Epidemiol
Biomarkers Prev. 15:2069–2077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Isaacson PG and Du MQ: MALT lymphoma: from
morphology to molecules. Nat Rev Cancer. 4:644–653. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O,
Ruperez M, Esteban V and Egido J: Inflammation and angiotensin II.
Int J Biochem Cell Biol. 35:881–900. 2003. View Article : Google Scholar
|
|
32
|
Wu L, Iwai M, Li Z, et al: Regulation of
inhibitory protein-kappaB and monocyte chemoattractant protein-1 by
angiotensin II type 2 receptor-activated Src homology protein
tyrosine phosphatase-1 in fetal vascular smooth muscle cells. Mol
Endocrinol. 18:666–678. 2004. View Article : Google Scholar
|
|
33
|
Okamoto T: NF-kappaB and rheumatic
diseases. Endocr Metab Immune Disord Drug Targets. 6:359–372. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Newton CR, Curran B and Victorino GP:
Angiotensin II type 2 receptor effect on microvascular hydraulic
permeability. J Surg Res. 120:83–88. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang ZQ, Moore AF, Ozono R, Siragy HM and
Carey RM: Immunolocalization of subtype 2 angiotensin II (AT2)
receptor protein in rat heart. Hypertension. 32:78–83. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cao Z, Kelly DJ, Cox A, et al: Angiotensin
type 2 receptor is expressed in the adult rat kidney and promotes
cellular proliferation and apoptosis. Kidney Int. 58:2437–2451.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lenkei Z, Palkovits M, Corvol P and
Llorens-Cortes C: Distribution of angiotensin II type-2 receptor
(AT2) mRNA expression in the adult rat brain. J Comp Neurol.
373:322–339. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Gasparo M, Catt KJ, Inagami T, Wright
JW and Unger T: International union of pharmacology. XXIII. The
angiotensin II receptors. Pharmacol Rev. 52:415–472.
2000.PubMed/NCBI
|