Introduction
In both Western and traditional Chinese medicine,
arsenic has been used in antiseptics, antispasmodics and sedatives
and for the treatment of ulcers and cancer since ancient times.
There are mainly three types of mineral arsenicals; these include
orpiment (As2S3), realgar (largely
As4S4) and arsenolite (largely
As2O3, arsenic trioxide, ATO). In the last
decade, ATO has been proven to be effective in the treatment of
malignant diseases, especially acute promyelocytic leukemia (APL).
APL is a distinct subtype of acute myeloid leukemia (AML), and 98%
of these patients have balanced reciprocal translocations between
chromosomes 15 and 17 that result in the fusion of the
promyelocytic leukemia (PML) and retinoic acid receptor α
(RARα) gene.
Historically, APL was the most malignant form of
acute leukemia due to the tendency for severe bleeding in patients.
The complete remission (CR) rate following chemotherapy (CT) alone
was 75% to 80% in newly diagnosed patients, and the 5-year
disease-free survival (DFS) was as low as 35% (1–3).
However, targeted therapy against the causative PML-RARα molecule
has changed APL from a highly fatal disease to a highly curable
one. All-trans retinoic acid (ATRA) was found to be the most
effective differentiation therapy for APL, and this treatment
raised the CR rate to between 90% and 95% and the 5-year DFS to 74%
when combined with CT (4). ATO was
found to improve the clinical outcome for newly diagnosed patients
as well as for those who were relapsed, refractory to treatment or
resistant to ATRA. When ATO was used as a single agent, the CR rate
was 72.7% and 85.1% in the newly diagnosed and relapsed patients,
respectively (5). When combined
with ATRA in newly diagnosed APL patients, ATO increased the CR
rate to 94.1% and the 5-year event-free survival (EFS) to 89.2%
(6). Nevertheless, ATO is toxic
and has severe side effects with long-term use, such as
prolongation of the corrected QT interval and liver damage
(7). Accordingly, ATO must be
administered intravenously due to the potential for severe liver
damage when given orally (7).
The efficacy of realgar has also been established
for the treatment of APL and chronic myelogenous leukemia (CML)
(8,9). Realgar is significantly less toxic
than ATO and can be administered orally (10). Hence, realgar may provide an
effective, safe and convenient way to treat malignant hematological
diseases. The basis for the therapeutic effects of realgar,
however, still needs to be fully elucidated. In this study, the
effects of realgar on cellular cytotoxicity, proliferation,
apoptosis and differentiation were comprehensively investigated
using the PML-RARα-positive APL-derived ATRA-sensitive
NB4 and ATRA-resistant MR2 cell lines. These
data contribute to an understanding of the underlying mechanism
responsible for the therapeutic effects of realgar in the clinical
treatment of APL.
Materials and methods
Reagents
Highly purified realgar was prepared from mined
natural realgar. The purity of As4S4 in our
realgar preparation was not below 98.0%, which was confirmed by
repeated X-ray powder diffraction analyses (in collaboration with
the Research Center at the Xi'an Institute of Geology and Mineral
Resources). These results were compatible with pure
As4S4 standards and excluded the potential
for trace amounts of ATO and other arsenic compounds that could
influence the results. The high-purity realgar was dissolved in
RPMI-1640 and sterilized by filtration. The content of As was
determined by atomic absorption spectrometry. A 142.2 mg/l (near to
0.5 mM) stock solution was made by dilution in RPMI-1640 medium.
According to the blood arsenic levels from
As4S4-treated patients, the stock solution
was appropriately diluted between 100 and 1000 times in RPMI-1640
for a working solution (8).
Cell culture
The NB4 cell line was derived from a
PML-RARα-positive APL patient, who was sensitive to ATRA.
MR2, a subclone of NB4, is resistant to ATRA.
Both cell lines were cultured in RPMI-1640 containing 10% fetal
bovine serum (FCS; Gibco BRL, Gaithersburg, MD) in a humidified
atmosphere with 5% CO2 at 37°C.
LDH release assay
Following treatment with 355 μg/l realgar for 24 h,
cell culture supernatants were collected for the LDH release assay
(Cytotoxicity Detection kit; Roche, Indianapolis, IN, USA).
Untreated NB4 cells that had been repeatedly
freeze-thawed were set as the positive control for LDH release.
Cells without any treatment or freeze-thaw cycle were used as the
negative control.
MTT assay
Briefly, cells were plated at a density of
2×104 cells/100 μl/well in 96-well microtiter plates,
and there were four replicates for each sample. Realgar (100 μl) at
various concentrations was added to each well and was incubated for
24 h to 72 h. Cell culture medium without realgar was added to the
control wells, and wells without cells were used as blank controls.
Cells were then fed 20 μl/well MTT (5 mg/ml in PBS) and incubated
for 4 h. After removing the supernatants, the MTT-formazan crystals
were dissolved in 150 μl DMSO per well and the absorbance was
measured at 570 nm using a multi-well plate reader (Model Anthos
Labtec 2010.7 reader). The percent viability of each well was
calculated as follows: The growth inhibition rate =
(1-At/Ac) ×100%; (At, average
absorbance of test; Ac, average absorbance of
control).
Cell morphology by light microscopy and
transmission electron microscopy
For light microscopy, the cells were stained with
Wright or hematoxylin-eosin (H&E) after they had been loaded
onto slides by cytospin centrifugation (100 g, 4 min; Shandon,
Runcorn, UK). One million cells were collected and prepared for
transmission electron microscopy in the following order: cells were
washed twice with phosphate-buffed saline (PBS, 0.01 M, pH 7.4),
fixed in 2.5% glutaraldehyde (containing formamint) at 4°C for
greater than 2 h, rinsed, post-fixed in 1% osmium tetroxide
(OsO4) for 2 h, washed, dehydrated using a stepwise
ethanol gradient and embedded in Epon 812 after permeation. The
sections were stained with uranyl acetate followed by lead citrate
and were observed using a transmission electron microscope.
Annexin V staining
Following treatment with 355 μg/l of realgar for 36
h, 1×106 cells were collected. The cells were washed in
PBS and resuspended in 200 μl of staining solution containing 10 μl
of annexin V-fluorescein isothiocyanate (FITC) and 5 μl of 20 μg/ml
propidium iodide (PI). To distinguish live cells (negative for both
fluorochromes) from apoptotic cells (positive for annexin V but
negative for PI) and necrotic cells (positive for PI), 5,000 cells
were analyzed by flow cytometry (Coulter EPICS Elite). All data
were collected, stored and analyzed by Multigraph software
(Coulter, Miami, FL).
DNA content analysis
NB4 and MR2 cells were
collected following treatment with 0, 177 μg/l, 355 μg/l or 711
μg/l of realgar for 24, 36, 48, 60 or 72 h. Cells were washed in
PBS and fixed in 70% cold ethanol for at least 1 h at 4°C. Shortly
before flow cytometry analysis, cells were rinsed with PBS, treated
with 100 mg/l RNase A for at least 15 min at 37°C and stained with
20 mg/l PI. The distribution of cells having different DNA contents
was analyzed by flow cytometry (Coulter EPICS Elite). Gating was
performed to remove debris and doublets before collection. Data
were measured using MultiCycle software (Phoenixm Flow Systems, San
Diego, CA).
NBT reduction assay
One million cells were harvested from control wells
and wells treated with 0, 177 μg/l or 355 μg/l of realgar and 1 μM
ATRA for 24, 48 or 72 h. NB4 cells treated with 1 μM
ATRA were used as a positive control. Cells were washed and
incubated in 1 ml PBS containing 1 mg/ml NBT and 5 μg/ml TPA for 30
min at 37°C. Cells containing intracellular black-blue formazan
deposits were counted by microscopic examination and a minimum of
200 cells were examined in duplicate from four separate
experiments. Following centrifugation, 600 ml DMSO was added to the
cell pellets to solubilize the formazan deposits, and the amount of
formazan was determined by recording the absorbance at 568.5
nm.
CD11b and CD33 expression by flow
cytometry
CD11b and CD33 expression was analyzed for
NB4 and MR2 cells by flow cytometry. A volume
of 100 μl of culture medium containing approximately
5×105 cells was collected from control wells and from
those treated with 177 μg/l or 355 μg/l of realgar and 1 μM ATRA
for 48 h. NB4 cells treated with 1 μM ATRA were used as
a positive control. Next, 15 μl of mouse anti-human CD11b-FITC or
CD33-FITC antibody (Immunotech) was added to the cell medium and
incubated for 30 min at room temperature. Mouse IgG1 isotype
control-FITC (Immunotech) antibody was used as a negative control.
Cells were analyzed by flow cytometry (Coulter EPICS Elite)
following a wash with PBS and fixation in paraform.
Immunofluorescence analysis of PML-RARα
in NB4 cells
NB4 cells from untreated wells or from
wells treated for 12 h with 1 μM realgar, ATO,
As2S2, As2S3 and ATRA
were centrifuged onto slides. The cells were fixed with
methanol/acetone (1:1) at −20°C for 5 min, washed with PBS, blocked
with BSA and incubated with mouse anti-human PML antibody (Santa
Cruz, CA) and rabbit anti-mouse IgG-FITC (Dako, Denmark).
Immunofluorescent images were recorded using a confocal microscope
(Zeiss LSM 510, Germany).
Oligonucleotide microarray analysis of
NB4 cells
NB4 cells treated with 355 μg/l realgar
for 4 h were compared to untreated controls. Then these two groups
were further compared when pretreated with 10 mg/l cycloheximide
for 1 h before any realgar treatment. The Biostar-1024D (HGEC-10d)
microarrays consisted of 1003 novel or known genes (provided by
United Gene Holdings Ltd., Shanghai). Construction of the
microarray and the gene list used for this study followed the
guidelines on the website http://www.unitedgene.com. RNA was isolated from NB4
cells. Purified mRNA was labelled with Cy3 or Cy5, then hybridized
with microarray. The chip was scanned after rinsed. Each ratio of
Cy5 to Cy3 was computed. Overall intensities were normalized for
the correction coefficient of the natural logarithm ratio. To
minimize artifacts arising from low-expression values, only genes
with raw intensity values for both Cy3 and Cy5 >200 or those
with >800 counts were chosen for differential analysis. A 2-fold
difference in the ratio was used to represent differently expressed
genes.
Statistical analysis
All experiments were performed at least in
triplicate, and the results were expressed as the mean ± SD.
Statistical analyses were performed with the t-test, χ2
test and the multiple comparisons test using SPSS12.0 software.
Results
Cytotoxicity assay
An increase in LDH release indicates a breakdown of
the cell membrane that can lead to cell death. In this study, we
compared the LDH activity of NB4 cells treated for 24 h
with 355 μg/l realgar with that of the untreated controls. However,
there was no significant difference in activity between these two
groups (67±3.5 U/l vs. 69±2.7 U/l, P>0.05). The activity from
the positive control was 282±7.2 U/l, while that from the negative
control was 48 U/l. These results suggest that there was no
increase in membrane permeability in NB4 cells following
treatment with 355 μg/l realgar.
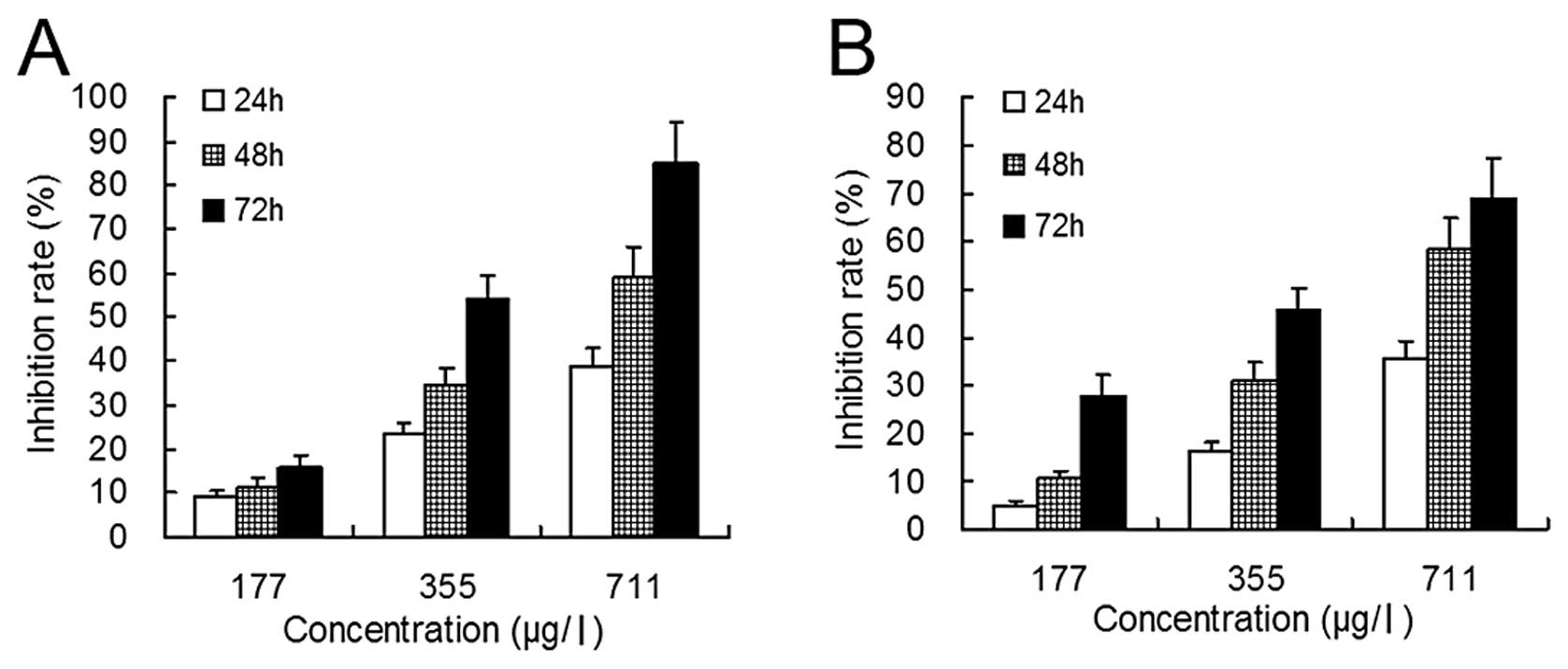
Cell proliferation
Cell proliferation was evaluated by the MTT assay.
When NB4 and MR2 cells were treated with
realgar at concentrations of 177 μg/l, 355 μg/l and 711 μg/l for 24
h, the growth inhibition rates were 9.4±1.0%, 23.4±2.4% and
38.9±4.1% for NB4 cells (P<0.01) and were 4.9±1.0%,
16.2±1.8% and 35.5±3.7% for MR2 cells (P<0.01),
respectively (Fig. 1). However,
following treatment with 44–88 μg/l realgar, the inhibition rates
of these two groups of cells were negligible (data not shown).
Cell apoptosis and differentiation
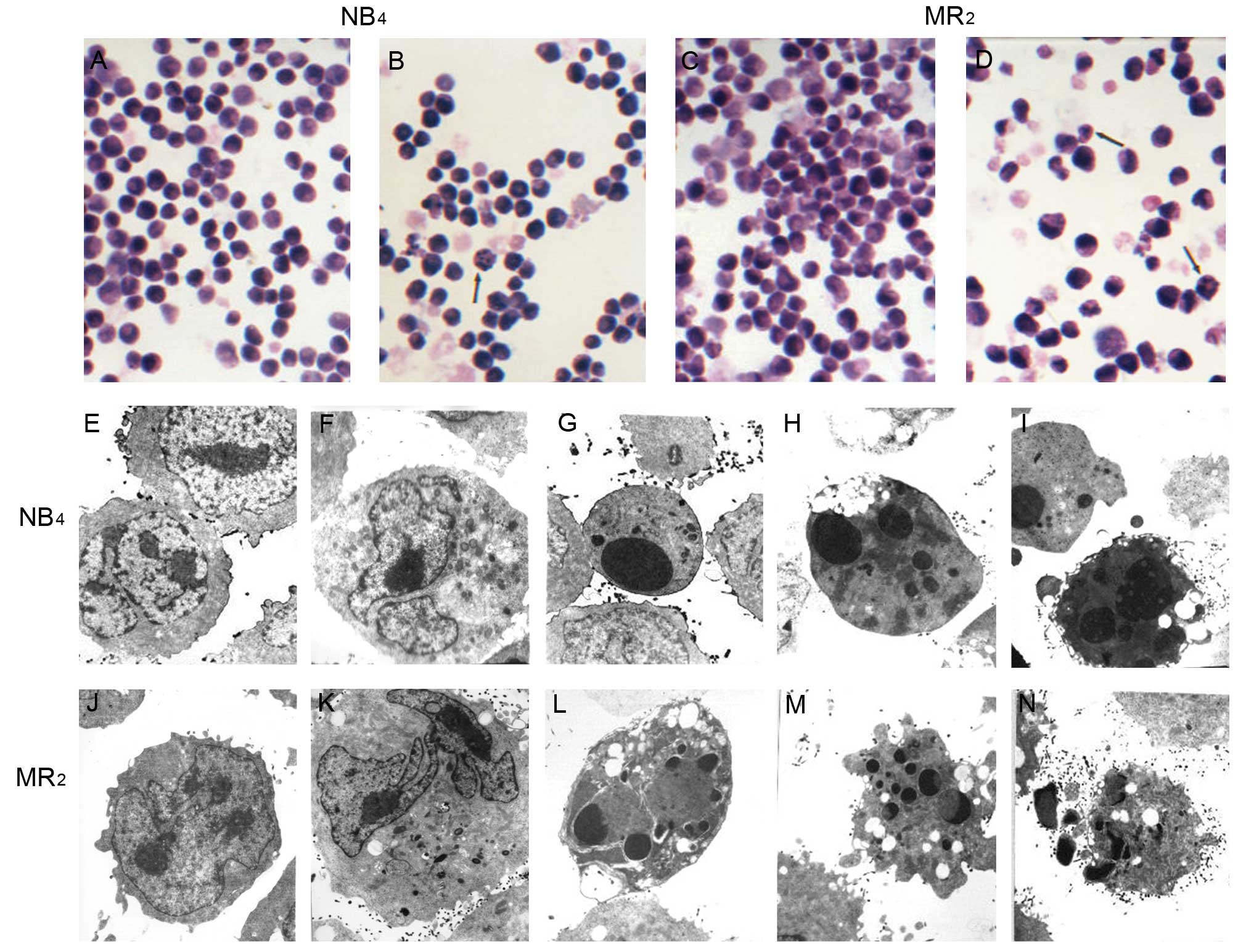
Cell morphology
By light microscopy, NB4 and
MR2 cells showed reduced cell size, condensed nuclei and
dark staining, which are indicative of apoptotic changes to cell
morphology. The condensed nuclei broke into scattered small lumps
surrounding the cell membrane. Following treatment with 177 μg/l of
realgar, both cell populations began to differentiate towards
having larger areas of clear cytoplasm and hollow nuclei. This
effect was more obvious in 60 h for both cell lines (Fig. 2B and D). By transmission electron
microscopy, apoptotic changes in NB4 (Fig. 2G-I) and MR2 (Fig. 2L-N) cells were obvious after
exposure to 355 μg/l realgar for 48 h and 65 h, respectively. These
changes were characterized by the presence of apoptotic bodies,
cell shrinkage, vacuole formation and undulations of the plasma
membrane. Additionally, a certain extent of differentiation was
suggested by the decreased ratio of nuclei to plasma, by the
emergence of some cellular particles, by the partial disappearance
of nucleoli and by nuclear remodeling, which ranged from simple
indentations to polylobular nuclei (Fig. 2F and K).
Annexin V staining
Annexin V is expressed on the membranes of apoptotic
cells in the early stages of apoptosis. After treatment with 355
μg/l of realgar for 36 h, the percents of NB4 and
MR2 cells expressing Annexin V (PI−) were
10.4±0.5% and 16.2±0.6%, while those of the blank controls were
0.5±0.1% and 0.3±0.1%, respectively (both P<0.005).
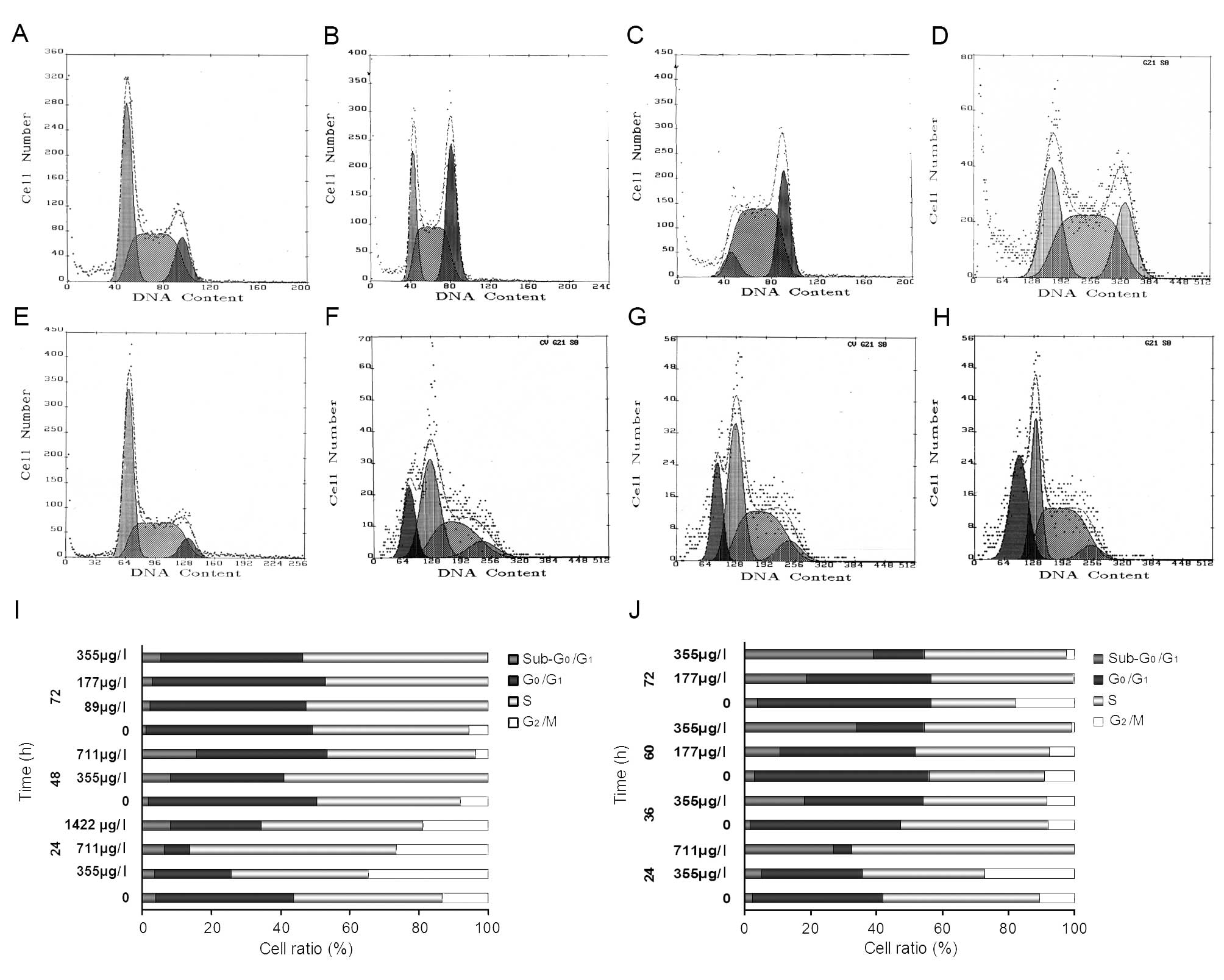
DNA content analysis
After NB4 cells were treated for 24 h, we
found evidence for significant cell cycle arrest at the
G2/M stage with 355 μg/l or 711 μg/l realgar. Compared
with the untreated group, this change in the cell cycle
distribution was significant (P<0.005). But it did not increase
in significance for the treatment of 1422 μg/l realgar (Fig. 3A-D). In MR2 cells
treated with 177 μg/l or 355 μg/l realgar for 36 h or 60 h, the
fraction of cells in the G0/G1 stage was
sharply reduced, while that in S phase was remarkably increased
(P<0.005) (Fig. 3E-H).
Additionally, in both cell lines, the
sub-G0/G1 cells increased in a time- and
dose-dependent manner (Fig. 3I and
J).
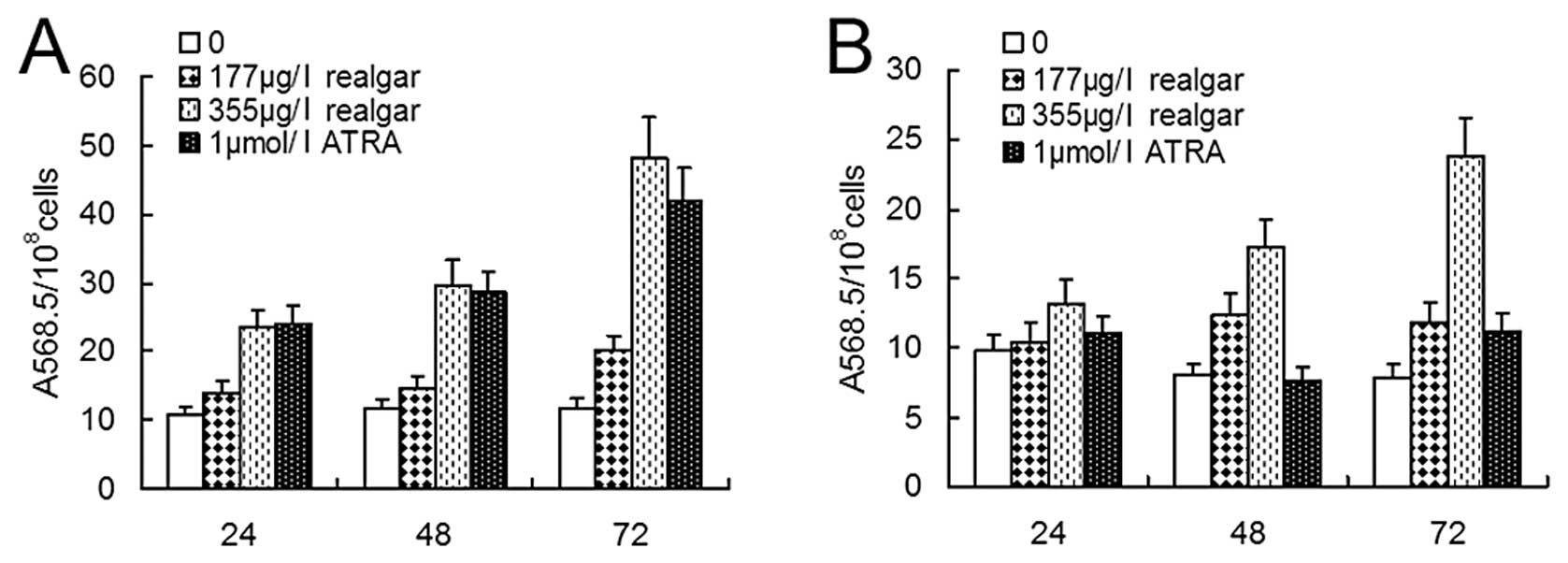
NBT reduction assay
Realgar-induced differentiation was assessed by the
NBT reduction assay. Among NB4 cells, NBT-positive cells
were greatly increased following treatment with 1 μM ATRA, 177 μg/l
or 355 μg/l realgar for 24, 48 and 72 h (all P<0.005) (Fig. 4A). Compared with 1 μM ATRA
treatment, however, the increase was not significant for the
treatment with 177 μg/l realgar at these time points (P<0.005).
For cells treated with 355 μg/l realgar, there was no difference at
24 h or 48 h (P>0.05) in comparison to those given ATRA
treatment, but the percentage of NBT-positive cells was
significantly greater at 72 h (P<0.0005). For MR2
cells, no changes occurred following 1 μM ATRA treatment for 24, 48
or 72 h (P>0.05). In samples treated with 177 μg/l realgar,
NBT-positive cells were not increased at 24 h (P>0.05) but were
increased at 48 h (P<0.01) and 72 h (P<0.05). However,
treatment of cells with 355 μg/l realgar increased the NBT positive
percentages at 24 h (P<0.05), 48 h (P<0.001) and 72 h
(P<0.0005) (Fig. 4B).
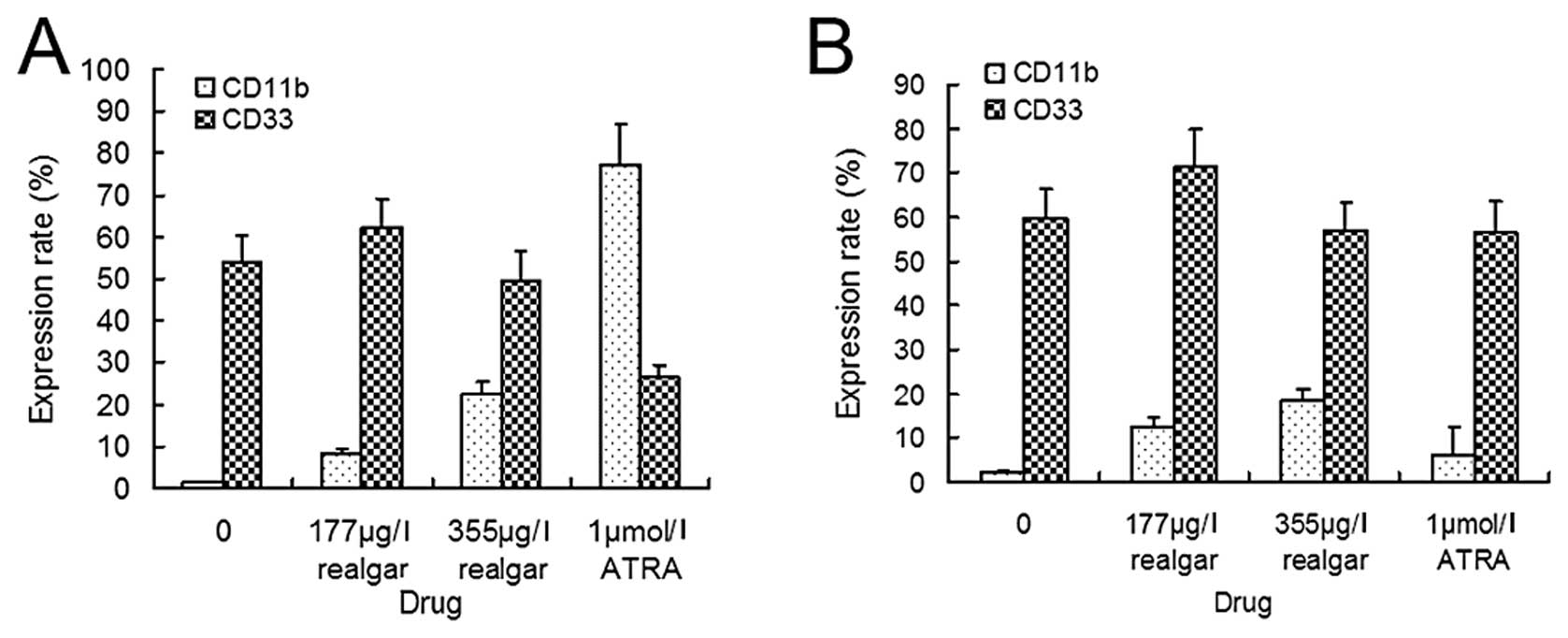
CD11b and CD33 expression
CD11b is expressed later in monocytic or
granulocytic differentiation, whereas CD33 is associated with
primitive myeloid cell populations in APL. FACS analysis of
NB4 cells, following treatment with 1 μM ATRA, found the
expression of CD11b to be significantly increased (P<0.005) but
that of CD33 to be decreased (P<0.01). Treatment with 177 μg/l
or 355 μg/l realgar was also found to increase the expression of
CD11b (P<0.05 and P<0.005, respectively). However, treatment
with either of these concentrations of realgar resulted in weaker
expression than did treatment with ATRA (P<0.005). Unlike ATRA,
realgar treatment did not cause a significant decrease in the
proportion of cells that expressed CD33 (P>0.05) (Fig. 5A). In the ATRA-resistant
MR2 cells, 1 μM ATRA neither increased the expression of
CD11b significantly (P>0.05) nor had an effect on the expression
of CD33 (P>0.05). Following treatment with 177 μg/l and 355 μg/l
realgar, however, the expression of CD11b was significantly
increased (P<0.005). Additionally, realgar treatment did not
decrease the expression of CD33 (P>0.05) (Fig. 5B).
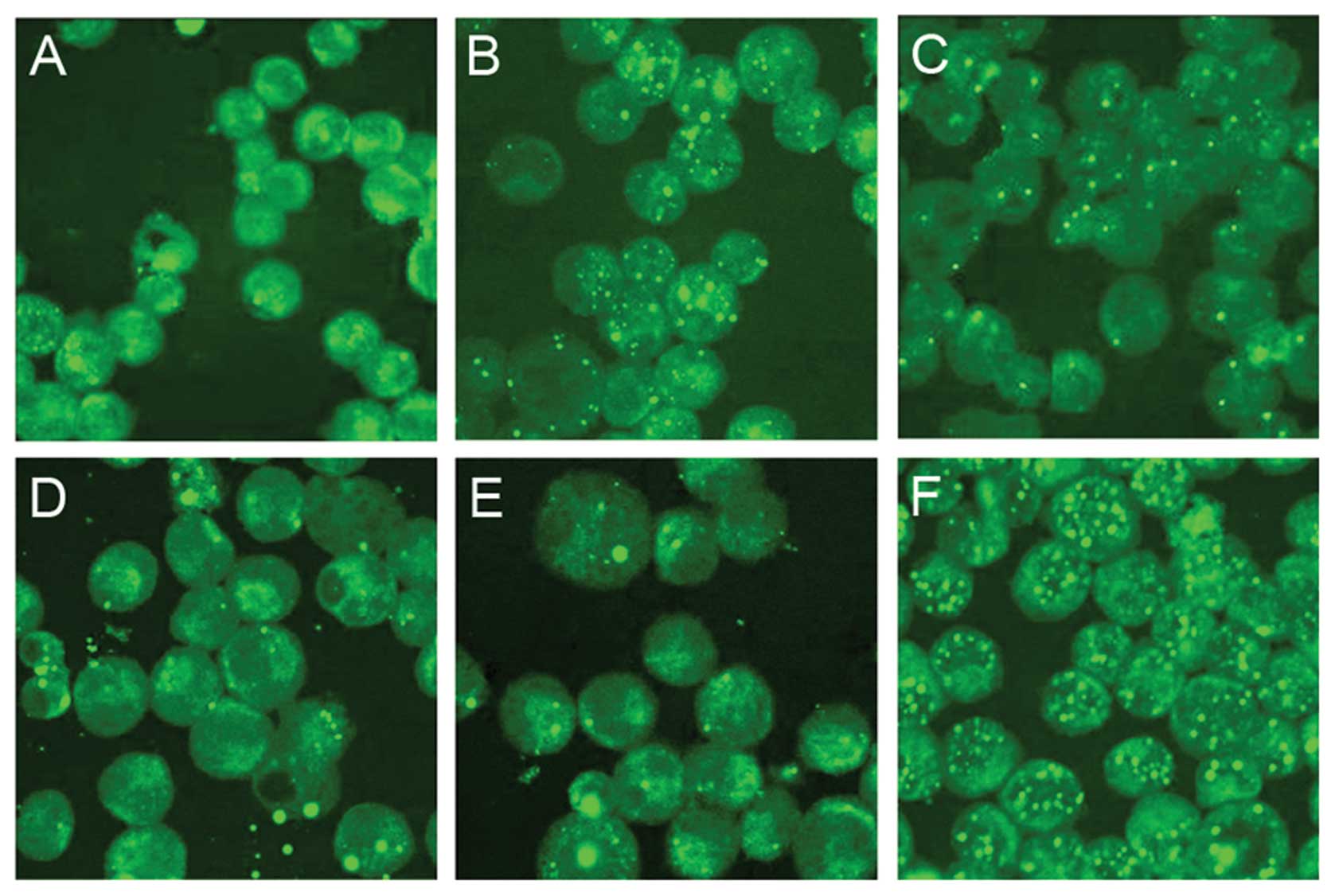
Localization of PML-RARα protein in
realgar-treated NB4 cells
Using confocal microscopy, the localization of
PML-RARα protein was found to be altered in realgar-treated
NB4 cells. In these cells, most of the PML-RARα protein
had entered the nuclei and PML-positive particles had accumulated.
Similar relocalization was also observed in cells treated with ATO,
As2S2, As2S3 and ATRA
(Fig. 6).
Gene expression profile in
NB4 cells
We performed microarray analyses to evaluate the
global gene expression profiles of NB4 cells treated
with 355 μg/l realgar for 4 h. Compared with the untreated
controls, gene expression profiling revealed 13 aberrantly
expressed genes in treated cells. Of these, the following 11 genes
were down-regulated: the signal transduction genes U51903
(IQGAP2), L42572 (IMMT) and Y00483 (GPX1); the
protein translation genes M86752 (STIP1) and U58048
(CHMP1A); the metabolism genes X66435 (HMGCS1) and
D16480 (HADHA); the DNA binding and transcription factor
gene AF036613 (GTF2IP1); the immune-related gene NM_006995
(BTN2A2); and two unclassified genes, AF052108
(LOC157627) and D21261 (TAGLN2) (Table I). In comparison, Z22533
(ACVRL1), a signal transduction gene, and NM_007057
(ZWINT), were up-regulated in treated cells. When these two
groups were compared after pretreatment with 10 mg/l cycloheximide
for 1 h, we found that the genes U51903 (IQGAP2), X66435
(HMGCS1) and AF036613 (GTF2IP1) were down-regulated,
whereas the gene Z22533 (ACVRL1) was up-regulated. These
changes in gene expression were independent of the synthesis of new
protein.
 | Table IDifferentially expressed genes in
NB4 cells following treatment with realgar. |
Table I
Differentially expressed genes in
NB4 cells following treatment with realgar.
| | | | Ratio
(Cy5/Cy3a) |
|---|
| Gene ID | GenBank ID | Definition | Category | Chip1 | Chip2 |
|---|
| 10788 | U51903b | IQGAP2, IQ motif
containing GTPase activating protein 2 [Homo sapiens] | Signal
transduction | 0.236 | 0.276 | 0.261 | 0.268 |
| 2970 | AF036613b | GTF2IP1, general
transcription factor IIi pseudogene 1 [Homo sapiens] | DNA transcription,
TF | 0.276 | 0.265 | 0.461 | 0.453 |
| 10989 | L42572 | IMMT, inner
membrane protein, mitochondrial [Homo sapiens] | Signal
transduction/cytoskeletons | 0.324 | 0.333 | 0.492 | 0.513 |
| 3157 | X66435b | HMGCS1,
3-hydroxy-3-methylglutaryl-CoA synthase 1 (soluble) [Homo
sapiens] | Metabolism | 0.351 | 0.320 | 0.227 | 0.248 |
| 8407 | D21261 | TAGLN2, transgelin
2 [Homo sapiens] | | 0.367 | 0.395 | 0.637 | 0.652 |
| 10963 | M86752 | STIP1,
stress-induced-phosphoprotein 1 [Homo sapiens] | Translation,
synthesis of protein | 0.380 | 0.411 | 0.561 | 0.678 |
| 2876 | Y00483 | GPX1, glutathione
peroxidase 1 [Homo sapiens] | Signal
transduction | 0.409 | 0.427 | 0.779 | 0.886 |
| 157627 | AF052108 | LOC157627,
uncharacterized LOC157627 [Homo sapiens] | | 0.403 | 0.456 | 0.553 | 0.531 |
| 3030 | D16480 | HADHA,
hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA
hydratase (trifunctional protein), α-subunit [Homo
sapiens] | Metabolism | 0.422 | 0.439 | 0.622 | 0.686 |
| 5119 | U58048 | CHMP1A, charged
multivesicular body protein 1A [Homo sapiens] | Transportation of
proteins | 0.466 | 0.500 | 0.785 | 0.800 |
| 10385 | NM_006995 | BTN2A2
butyrophilin, subfamily 2, member A2 [Homo sapiens] | Immune
response | 0.487 | 0.481 | 0.657 | 0.631 |
| 11130 | NM_007057 | ZWINT, ZW10
interacter [Homo sapiens] | | 2.103 | 2.008 | 0.958 | 0.995 |
| 94 | Z22533b | ACVRL1, activin A
receptor type II-like 1 [Homo sapiens] | Signal
transduction | 2.262 | 2.170 | 2.749 | 2.672 |
Discussion
Realgar has been used in Chinese traditional
medicine for more than 1500 years. In recent years, its promising
anti-cancer potential has attracted attention at home and abroad,
especially in the therapy of APL. According to a pilot report on
As4S4 levels in APL by Lu et al
(8), realgar used alone as therapy
was effective in inducing hematological CR (HCR) in both newly
diagnosed and relapsed APL patients. Additionally, cytogenetic and
molecular CR was obtained for 87.5% of the newly diagnosed patients
and for 5 of the 7 patients with hematologic relapses. Furthermore,
realgar treatment is highly effective for CR maintenance, as there
was a 3-year DFS of 76.6% for the newly diagnosed patients and a
6-year DFS of 87.4% for the HCR patients.
To fully elucidate the therapeutic basis of realgar
in APL, PML-RARα+ cell line ATRA-sensitive NB4 and
ATRA-resistant MR2 were investigated in this study.
After being treated with realgar in MTT assay, both cells were
significantly inhibited in a dose- and time-dependent manner. An
LDH-releasing assay demonstrated that realgar had no direct
cytotoxic effect on the cell membrane, which was consistent with
the clinical observation that no obvious myelosuppression was
observed in APL patients treated with realgar (8).
The mechanism of action of realgar was also studied
in NB4 and MR2 cells. ATO treatment was found
to exert dual effects on APL cells in a dose-dependent manner in
vitro. At greater concentrations (0.5–2.0 μM), ATO has been
shown to trigger apoptosis, while at lower concentrations (0.1–0.5
μM), it can induce partial differentiation (11). The dual effects of ATO were
reported in the bone marrow of APL patients and in the PML-RARα/APL
mouse model (12). In this study,
the dual effects of realgar were demonstrated in ATRA-sensitive
NB4 and ATRA-resistant MR2 cells. In contrast
to ATO treatment, the dual effects of realgar treatment were
exerted simultaneously. Morphologically, we found that with greater
realgar concentration or culture time, more apoptotic cells were
observed. Simultaneously, partial differentiation was indicated by
the decreased nuclear to cytoplasm ratio, the partial disappearance
of nucleoli and the nuclear remodeling ranging from indentation to
polylobular nuclei. Apoptotic effects of treatment were demonstated
by the significant increase in the percentage of cells expressing
Annexin V (PI−) and the sub-G0/G1
cells increased in a time- and dose-dependent manner in DNA content
assay. As evidence of cellular differentiation, the NBT reduction
values were enhanced in NB4 and MR2 cells
treated with realgar. Realgar treatment did not reduce the
expression of CD33. However, it did significantly induce the
expression of CD11b in NB4 and MR2 cells,
which suggested the partial differentiation of these two APL cell
lines. Compared to ATRA treatment, realgar treatment exerted a
weaker differentiation effect on NB4 cells, while ATRA
did not have any differentiation effect on MR2 cells.
Similar to ATO treatment, realgar treatment effectively changed the
localization of PML-RARα protein to the nuclei and increased PML
positive particles in NB4 cells (13). This relocalization was also
detected in the bone marrow cells of APL patients treated with
arsenic sulfides, although this occurred before any morphologic
change was detected (8).
Cai et al reported that 0.1–0.5 mM ATO did
not induce differentiation in ATRA-resistant cell lines (14). In our study, we found that realgar
induced differentiation in MR2 cells. This result
indicated that some different mechanisms may underlie between the
differentiation effect of ATO and realgar. Based on the
simultaneous dual effect of realgar, we hypothesized that apoptosis
and differentiation might share some initiating steps or signal
transduction in APL cells.
To further define the molecular mechanisms at work
following realgar treatment, microarrays from NB4 cells
found 11 genes down-regulated and two genes up-regulated. The
activity of 4 of these 13 genes was not blocked by the addition of
cycloheximide, which suggests that these 4 genes were of importance
because their modulation was independent of new protein synthesis.
These 13 genes are known to be involved in the modulation of signal
transduction, translation, transcription and metabolism and the
immune response. Among them, U51903 encodes the Ras
GTPase-activating-related human protein IQGAP2, which inhibits both
the intrinsic and the RhoGAP-stimulated GTP hydrolysis rates of
Cdc42 and Rac1 by binding Cdc42 and Rac1, but not RhoA (15). The down-regulation of U51903 by
realgar led to the decreased expression of IQGAP2, which may
up-regulate Cdc42 and Rac1 to activate the JNK pathway leading to
apoptosis. In ATO-induced apoptosis, JNK signaling is also
activated (16). The gene Z22533
encodes the activin receptor-like kinase ALK1, a member of
serine/threonine protein family, which is in the transforming
growth factor-β (TGF-β) receptor superfamily (17). Realgar treatment up-regulated
Z22533 and the expression of ALK1, which may inhibit cell
proliferation by regulating TGF-β/Smad signaling pathway. Recently,
a proteomic investigation of cellular differentiation caused by
As4S4 was performed in the retinoic acid
(RA)-resistant cell line NB4-R1 using high-solution
two-dimensional electrophoresis and mass spectrometry. These
results suggested that the proteins SET, RPP2 and PHB may be novel
effective therapeutic targets for RA-resistant APL (18).
In patients, realgar treatment was well tolerated
and had only moderate side effects, such as transient liver injury
and elongated QT intervals without symptoms (8). No obvious myelosuppression or serious
cardiac events were observed in APL patients treated with realgar.
However, treatment with ATRA and ATO was found to induce an ATRA
syndrome that was severe and was responsible for deaths early
during remission induction (8).
Recently, realgar nanoparticles have been studied to enhance
bioavailability (19,20). Accordingly, realgar may provide an
effective, safe and convenient way to treat APL patients.
In conclusion, in the present study, realgar was
found to mediate both apoptotic and differentiation effects in the
ATRA-sensitive NB4 and ATRA-resistant MR2
PML-RARα + APL cell lines. Dual effects were exerted simultaneously
and included the modulation of signal transduction, translation,
transcription, metabolism and immune response genes. Given its low
toxicity, realgar is a promising alternative reagent for the
therapy of APL. Our data contribute to the understanding of the
underlying mechanisms of realgar treatment and its clinical
application for APL.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (no. 30300139, 30801062 and 30900548), Key
Project of Shanghai Municipal Commission for Education (07zz43),
Natural Science Foundation of Shanghai Municipal Commission for
Science and Technology (11ZR1423400) and Postdoctoral Science
Foundation of Shanghai Municipal Commission for Science and
Technology. We are grateful to Dr Zhenyi Wang, Dr Saijuan Chen and
Dr Fangyuan Chen for their valuable advice on the study.
References
|
1
|
Cunningham I, Gee TS, Reich LM, Kempin SJ,
Naval AN and Clarkson BD: Acute promyelocytic leukemia: treatment
results during a decade at Memorial Hospital. Blood. 73:1116–1122.
1989.PubMed/NCBI
|
|
2
|
Ribeiro RC and Rego E: Management of APL
in developing countries: epidemiology, challenges and opportunities
for international collaboration. Hematology Am Soc Hematol Educ
Program. 162–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanz MA, Jarque I, Martin G, et al: Acute
promyelocytic leukemia. Therapy results and prognostic factors.
Cancer. 61:7–13. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niu C, Yan H, Yu T, et al: Studies on
treatment of acute promyelocytic leukemia with arsenic trioxide:
remission induction, follow-up, and molecular monitoring in 11
newly diagnosed and 47 relapsed acute promyelocytic leukemia
patients. Blood. 94:3315–3324. 1999.
|
|
6
|
Hu J, Liu YF, Wu CF, et al: Long-term
efficacy and safety of all-trans retinoic acid/arsenic
trioxide-based therapy in newly diagnosed acute promyelocytic
leukemia. Proc Natl Acad Sci USA. 106:3342–3347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Lu Y, Wu Q, Goyer RA and Waalkes
MP: Mineral arsenicals in traditional medicines: orpiment, realgar,
and arsenolite. J Pharmacol Exp Ther. 326:363–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu DP, Qiu JY, Jiang B, et al:
Tetra-arsenic tetra-sulfide for the treatment of acute
promyelocytic leukemia: a pilot report. Blood. 99:3136–3143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mao JH, Sun XY, Liu JX, et al: As4S4
targets RING-type E3 ligase c-CBL to induce degradation of BCR-ABL
in chronic myelogenous leukemia. Proc Natl Acad Sci USA.
107:21683–21688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu J, Liang SX, Lu YF, Miao JW, Wu Q and
Shi JS: Realgar and realgar-containing Liu-Shen-Wan are less
acutely toxic than arsenite and arsenate. J Ethnopharmacol.
134:26–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen GQ, Shi XG, Tang W, et al: Use of
arsenic trioxide (As2O3) in the treatment of acute promyelocytic
leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL
cells. Blood. 89:3345–3353. 1997.PubMed/NCBI
|
|
12
|
Chen Z, Zhao WL, Shen ZX, et al: Arsenic
trioxide and acute promyelocytic leukemia: clinical and biological.
Curr Top Microbiol Immunol. 313:129–144. 2007.PubMed/NCBI
|
|
13
|
Chen GQ, Zhu J, Shi XG, et al: In vitro
studies on cellular and molecular mechanisms of arsenic trioxide
(As2O3) in the treatment of acute promyelocytic leukemia: As2O3
induces NB4 cell apoptosis with downregulation of Bcl-2 expression
and modulation of PML-RAR alpha/PML proteins. Blood. 88:1052–1061.
1996.
|
|
14
|
Cai X, Shen YL, Zhu Q, et al: Arsenic
trioxide-induced apoptosis and differentiation are associated
respectively with mitochondrial transmembrane potential collapse
and retinoic acid signaling pathways in acute promyelocytic
leukemia. Leukemia. 14:262–270. 2000. View Article : Google Scholar
|
|
15
|
Brill S, Li S, Lyman CW, et al: The Ras
GTPase-activating-protein-related human protein IQGAP2 harbors a
potential actin binding domain and interacts with calmodulin and
Rho family GTPases. Mol Cell Biol. 16:4869–4878. 1996.
|
|
16
|
Davison K, Mann KK and Miller WH Jr:
Arsenic trioxide: mechanisms of action. Semin Hematol. 397:3–7.
2002. View Article : Google Scholar
|
|
17
|
Ten Dijke P, Ichijo H, Franzen P, et al:
Activin receptor-like kinases: a novel subclass of cell-surface
receptors with predicted serine/threonine kinase activity.
Oncogene. 8:2879–2887. 1993.PubMed/NCBI
|
|
18
|
Qi J, He P, Chen W, Wang H, Wang X and
Zhang M: Comparative proteome study of apoptosis induced by As4S4
in retinoid acid resistant human acute promyelocytic leukemia
NB4-R1 cells. Leuk Res. 34:1506–1516. 2010. View Article : Google Scholar
|
|
19
|
Wu JZ and Ho PC: Evaluation of the in
vitro activity and in vivo bioavailability of realgar nanoparticles
prepared by cryo-grinding. Eur J Pharm Sci. 29:35–44. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xi RG, Huang J, Li D, Wang XB and Wu LJ:
Roles of PI3-K/Akt pathways in nanoparticle realgar powders-induced
apoptosis in U937 cells. Acta Pharmacol Sin. 29:355–363. 2008.
View Article : Google Scholar : PubMed/NCBI
|