Introduction
Lysophosphatidic acid (LPA) is a natural bioactive
phospholipid with growth factor-like activities (1). LPA controls cell proliferation,
motility and differentiation on many cell types including cancer
cells due to the action of cell surface G protein-coupled
receptors. At least six LPA receptors have been described as
transducers of LPA activity (LPA1–6) (2). These six LPA receptors can be further
subdivided into two groups, the EDG family of LPA receptors
(including LPA1–3) and the purinergic family (including
LPA4–6). LPA receptors are widely expressed in tissues.
Among these receptors LPA1 has the most ubiquitous
spectrum of expression in the organism (3). LPA is implicated in several
physiological and pathological processes (immunological system,
fertility, central nervous system, renal fibrosis, lung fibrosis,
hair loss, cancer) (4). The more
important source of LPA in the organism comes from platelet
activity upon platelet aggregation (5). LPA can also be produced by cells,
including tumor cells that express the nucleotide-pyrophosphate
pyrophosphatase 2/autotaxin/ATX (6,7).
Accumulating data over the past ten years indicate that LPA is
involved in cancer progression (8). LPA might be involved in
carcinogenesis since a series of studies reported that mRNA
expression for LPA2 and LPA3 were elevated in
numerous cancers (9–12). Recently, overexpression of each EDG
family LPA receptor (LPA1, LPA2 and
LPA3) in the mammary gland of MMTV-LPAr transgenic mice
was shown to induce tumor formation and metastasis (13). We have shown that LPA-derived from
platelets controls bone metastasis formation of breast cancer cells
(14). Since its discovery as an
autocrine motility factor produced by melanoma cells, ATX was shown
to control the metastatic behavior of breast cancer cells (13,15).
These data indicate that at least in the mammary gland LPA
receptors might act as oncogenic drivers and that through the
activation of these receptors, LPA might act as a pro-metastatic
factor.
LPA1 mRNA was consistently found
expressed in human primary breast tumors (11,14).
We have shown previously that the LPA1 expressed by
breast cancer cells controls bone metastasis formation in a mouse
model (16). For that reason, the
treatment of bone metastatic animals with the competitive inhibitor
of LPA1 and LPA3 receptors, Ki16425 (17), inhibits efficiently the progression
of bone metastases (16). However,
because of the inoculation route of tumor cells directly into the
blood stream, the role of LPA1 in spontaneous
dissemination of breast cancer cells from a primary tumor site is
still misunderstood. In light of these findings, we undertook the
study of the role of LPA1 in the spontaneous
dissemination of breast cancer cells to lungs and bone taking
advantage of: i) the development of a new antagonist of
LPA1/3 receptors Debio 0719, ii) the use of a mouse
model exploiting the 4T1 mouse mammary cancer cell line which
recapitulates the distinct steps of metastasis when engrafted into
the mammary glands of syngenic BALB/C mice (18) and iii) a large collection of mRNA
from primary tumors of breast cancer patients.
Materials and methods
Drugs and reagents
Lysophosphatidic acid (LPA, Oleoyl C18:1) and
lysophosphatidylcholine (LPC) were obtained from Avanti Polar
Lipids. Debio 0719 and Debio 0719-425 (S) which correspond to the
R-stereoisomer and S-stereoisomer, respectively, of the competitive
inhibitor of LPA1 and LPA3 receptors, Ki16425
(17), were synthesized by
Debiopharm S.A.
Cell culture
The 4T1 mouse mammary cancer cell line was obtained
from the American Type Culture Collection and were cultured in
complete media, DMEM medium (Invitrogen), 10% (v/v) fetal bovine
serum (FBS, Perbio) and 1% penicillin/streptomycin (Invitrogen), at
37°C in a 5% CO2 incubator. 4T1 cells derive from a
BALB/c spontaneous mammary carcinoma and are naturally resistant to
6-thioguanine (21). For
disseminated 4T1 tumor cell (DTC) analysis, bone marrow cells were
harvested from tibias and femurs of each animal by flushing. Cells
were placed on 10-cm culture dishes in presence of complete media
supplemented with 6-thioguanine 10 μM (Aldrich). After two weeks,
resistant clones were fixed, stained and counted. The amount of
rescued DTC was expressed in terms of cell
clones/mm2.
Calcium flux assays
Assays were conducted using Chemi-Screnn™
Calcium-optimized Chem-1 stable cell line expressing human
recombinant LPA1 receptor (Milipore Corp., St. Charles,
MO). To test for LPA1 agonist activity, cells were
loaded with Fluo-4 NW and calcium flux in response to oleoyl-LPA,
Ki16425 and Debio 0719 were determined in eight point 3-fold serial
dilution dose starting at 10 μM. EC80 values for the
reference agonist were determined upon agonist addition in a
dose-dependent manner. IC50 values for Ki16425 and Debio
0719 were determined by addition of antagonist and incubation of 90
sec, followed by ligand stimulation at EC80
concentration.
Patients and tumor characteristics
Studies involving human primary breast tumors were
performed according to the principles embodied in the Declaration
of Helsinki. Tissue biopsies were obtained as part of surgical
treatments for the hormone receptor content determination.
Remaining samples were included anony-mously in this study. All
human experiments were approved by the Experimental Review Board
from the Laennec School of Medicine that waived the need for
consent. The study cohort corresponded to 104 pre-menopausal
patients treated between October 1994 and October 2001 (37). Criteria for inclusion in the study
were as follow: primary breast tumor without inflammatory features,
no previous cancer therapy, and no identified metastasis at the
time of diagnosis. Estrogen receptor (ER) and progesterone receptor
(PgR) were assayed in cytosol using the radioligand reference
method (EORTC, 1980). Results were expressed as fmol/mg cytosol
protein. ER and PgR positive tumors contained >2 and >5
fmol/mg protein, respectively.
RNA extraction
Breast cancer tissue biopsies were obtained by
surgery, selected by the pathologist and immediately snap-frozen in
liquid nitrogen until processing. The biopsies were pulverized
using a ‘Mikro-Dismembrator’ (B. Braun Biotech International,
Melsungen, Germany) and total RNAs were extracted using TRI Reagent
(Sigma, St. Louis, MO). To remove any genomic DNA contamination,
total RNAs were treated with RNAse-free DNAse I and purified using
RNeasy micro-columns (Qiagen, Hilden, Germany). RNA quality was
verified using an Agilent Bioanalyser 2100 (Agilent Technologies,
Santa Clara, CA).
Reverse transcription and quantitative
real-time polymerase chain reaction (RT-QPCR)
Expression of LPA1 mRNA was quantified by
real-time quantitative RT-PCR on an Eppendorf
Mastercycler® RealPlex (Invitrogen) using the
SYBR® Green PCR kit (Finnzymes). Quantifications were
normalized to corresponding RNA L32 and TBP values. The cDNAs were
amplified by PCR for 35 cycles with the following specific PCR
primers: human LPA1, 5′-TGGCATTAAAAATTTTACAAAAACA-3′
(forward) and 5′-AATAGTTAACAACATGGGAATGG-3′ (reverse); human L32,
5′-CAAGGAGCTGGAAGTGCTGC-3′ (forward) and 5′-CAGCTCTTTCCACGATGGC-3′
(reverse); human TBP, 5′-TGGTGTGCACAGGAGCAAG-3′ (forward) and
5′-TTCACATCACAGCTCCCCAC-3′ (reverse). Each cycle consisted of 10
sec of denaturation at 95°C, 15 sec of annealing at 60°C for
LPA1 and 67°C for L32 and TBP, followed by 10 sec of
extension at 72°C. Experimental procedures were followed as
described (15).
Cell invasion assay
Invasion assays were carried out using Bio-Coat
migration chambers (Becton-Dickinson) with 8-μm filters previously
coated with Matrigel as described previously (38). 4T1 cells (2×105) were
plated in the upper chambers in presence of absence of increasing
concentrations of Debio 0719 and LPA in the lower chambers in
presence of 1% fetal bovine serum. After incubation for 24 h at
37°C in 5% CO2 incubator, cells that had migrated
through the filters were fixed and stained. The membranes were
mounted on glass slides, and cells from 10 random microscopic
fields (magnification ×40) were counted. All experiments were run
in duplicate, and invasion was expressed in terms of
cells/mm2.
Pharmacokinetic studies
Experiments were performed at Shanghai Medicilion
Inc. (Shanghai, China) using CD-1 male mice. Debio 0719 was
administered in a solution of 10% DMA, 5% Cremophor EL and 85%
saline to mice. Debio 0719 was administered intravenously at a dose
of 5 mg/kg as a 10 ml/kg bolus into the jugular vein. For oral
exposure, Debio 0719 was administered via an oral gavage at a dose
of 50 mg/kg in a volume of 20 ml/kg. Blood samples were collected
from retro-orbital puncture in sodium heparin containing tubes (n=3
for each time-point). Plasma samples (100 μl) were transferred to
Eppendorf tube, then 20 μl methanol and 500 μl internal solution
(Lovastatin, 500 ng/ml) were added. After vortexing for 1 min and
centrifuging for 5 min at 15,000 rpm, supernatant was transferred
to new vials and 5 μl of plasma samples were analyzed for Debio
0719 concentration by liquid chromatography/mass spectrometry. The
liquid chromatography was carried out using an Agilent liquid
chromatograph (Agilent Technologies). Mass spectrometric analysis
was performed using an API3000 (triple-quadrupole) instrument from
ABI Inc (Concord, Ontorio, Canada) with an ESI interface. Data
acquisition and control system were created using Analyst 1.4
software from ABI Inc.
In vivo oncological studies
The mice used in our study were handled according to
the rules of Décret no. 87–848 du 19/10/1987, Paris. The
experimental protocol have been reviewed and approved by the
Institutional Animal Care and Use Committee of the Université
Claude Bernard Lyon-1 (Lyon, France). Studies were routinely
inspected by the attending veterinarian to ensure continued
compliance with the proposed protocols. BALB/C mice, 4 weeks of
age, were housed under barrier conditions in laminar flow isolated
hoods. Autoclaved water and mouse chow were provided ad
libitum. Animals bearing tumor xenografts were carefully
monitored for established signs of distress and discomfort and were
humanely euthanized when these were confirmed. Tumor fad pad
experiments were performed using 4T1 cells (105 in 10 μl
of PBS) injected into the fat pad of the 4th mammary gland of
female BALB/c mice of 6 weeks of age (Charles River). Animals were
treated per os with Debio 0719 (25 mg/kg twice daily or 50
mg/kg twice daily) from day 0 to 14 or from day 15 to 35 post tumor
cell injection. Primary tumors were resected 14 days after tumor
cell injection and tumor weights were measured. For spontaneous
metastasis dissemination studies, 14 days after tumor cell
injection, animals were anesthetized and primary tumors were
surgically removed. Mice were then followed for an additional
3-week observation at which time they were sacrificed, lungs were
collected for histological analysis and bone marrow cells were
harvested for DTC quantification.
Immunohistochemistry
Resected primary tumors were fixed and embedded in
paraffin. Five μm sections were subjected to immunohistochemistry.
Detection of the nuclear antigen Ki-67 was carried out as described
previously (15). The Ki-67
mitotic index was calculated as the ratio of the number of Ki-67
positive nuclei to the total nucleus number per field and results
were expressed as the percentage of Ki-67-positive nuclei. For
microvessel detection, immunostaining was performed with a rabbit
polyclonal antibody against von Willebrand factor (vWF), and a rat
monoclonal antibody against mouse CD31 (PECAM-1). Tumor
angiogenesis was evaluated using the Chalkley’s Grid. Data were
expressed as the percentage of marks on the grid that cover stained
vessels from 10 independent fields on each tumor tissue
section.
Statistical analysis
Data were analyzed with the StatView 5.0 software
using unpaired Student’s t-test for in vitro and in
vivo studies. Analysis of the distribution of LPA1
expression in relation to usual prognostic parameters was performed
with the non-parametric Mann-Whitney test or Kruskall-Wallis
test.
Results
Debio 0719 inhibits
LPA/LPA1-stimulated calcium flux with a stronger potency
than Ki16425
Since its discovery, the competitive inhibitor
Ki16425 was extensively used to address the role of LPA1
both in vitro and in vivo (16,17,19,20).
To evaluate the role of LPA1 in metastasis, we first
characterized the pharmaco-dynamic properties of two derivatives of
Ki16425, Debio 0719 and Debio 0719-425(S). Debio 0719 and Debio
0719-425(S) correspond to the R-stereoisomer and S-stereoisomer,
respectively, of the Ki16425, which is a racemic mixture of R- and
S-stereoisomers, in a ratio of ~50:50. Debio 0719 and Debio
0719-425(S) were tested for agonist activity by incubating
increasing concentrations of Debio 0719 and Debio 0719-425(S)
(0.0045–10 μM) with Chem-1 cells expressing human LPA1
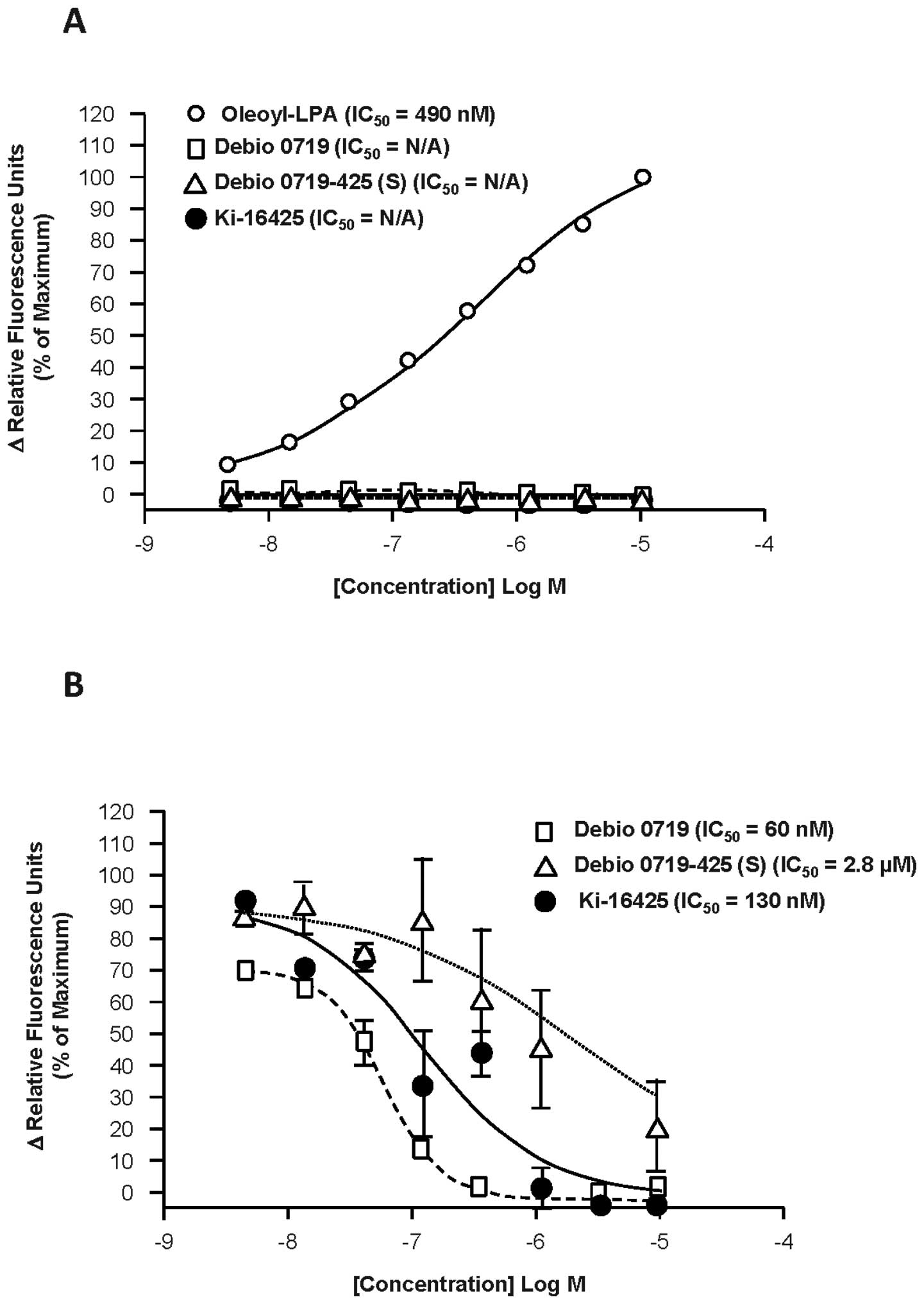
and measuring calcium flux (Fig.
1A). Like Ki16425, Debio 0719 and Debio 0719-425(S) showed no
agonist activity at the LPA1 receptor at any
concentration tested, whereas increasing concentrations of oleoyl
LPA showed dose-dependent stimulation of calcium flux with an
average EC50 of 490 nM. Debio 0719 inhibited LPA-induced
calcium flux in LPA1-expressing Chem-1 cells in a
dose-dependent manner, resulting in IC50 value of 60 nM
(Fig. 1B). Parallel experiments
showed that Ki16425 inhibited LPA-induced LPA1-dependent
calcium flux in Chem-1 cells with a higher IC50 value of
130 nM and Debio 0719-425(S) with much higher IC50 value
of 2.8 μM. Based on these results, Debio 0719 revealed 2-fold more
potent than Ki16425 at inhibiting LPA1 cell signaling
that control calcium flux. Thus, the R-stereoisomer can be
considered as the active stereoisomer of Ki16425 to inhibit
LPA/LPA1-induced calcium flux. Therefore, all subsequent
experiments presented here were carried out by using only Debio
0719.
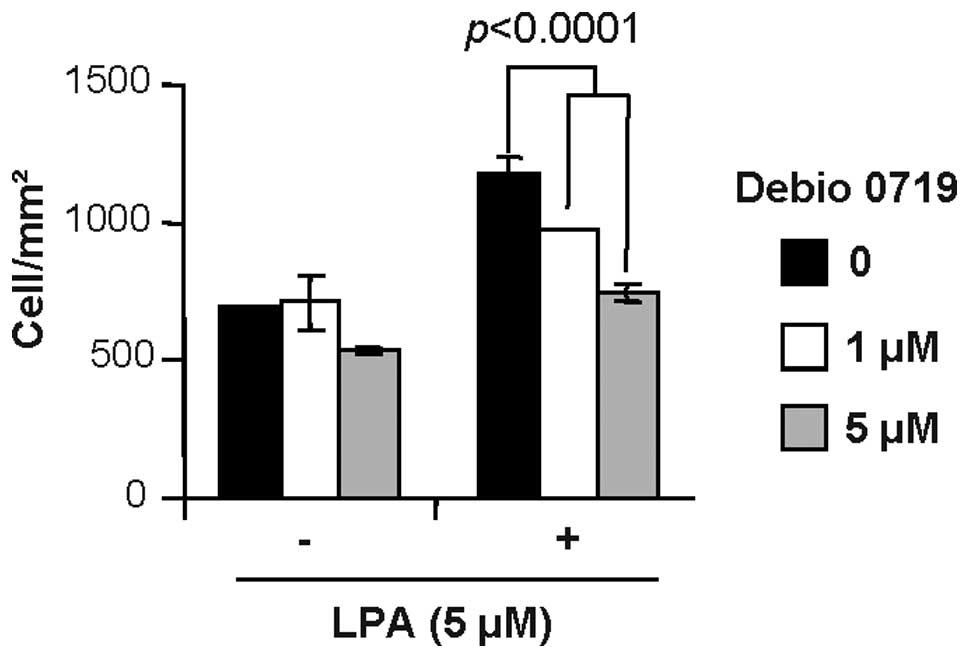
Debio 0719 inhibits 4T1 breast cancer
cell invasion in response to LPA
Recent studies have shown that LPA1 is
the main receptor that transduces the cell migratory activity of
LPA (19). The 4T1 mouse mammary
cancer cells mimic the successive steps of growth and metastasis of
breast cancers observed in clinic when injected in the mammary
fat-pad of immunocompetent BALB/c mice (18,21).
These cells express all subtypes of LPA receptors including
LPA1 (15). We found
previously that 4T1 cells respond to LPA as a chemo-attractant in a
cell invasion assay (15). We
observed here that the migratory activity of LPA was
dose-dependently blocked on cells treated with increasing
concentrations of Debio 0719 (Fig.
2).
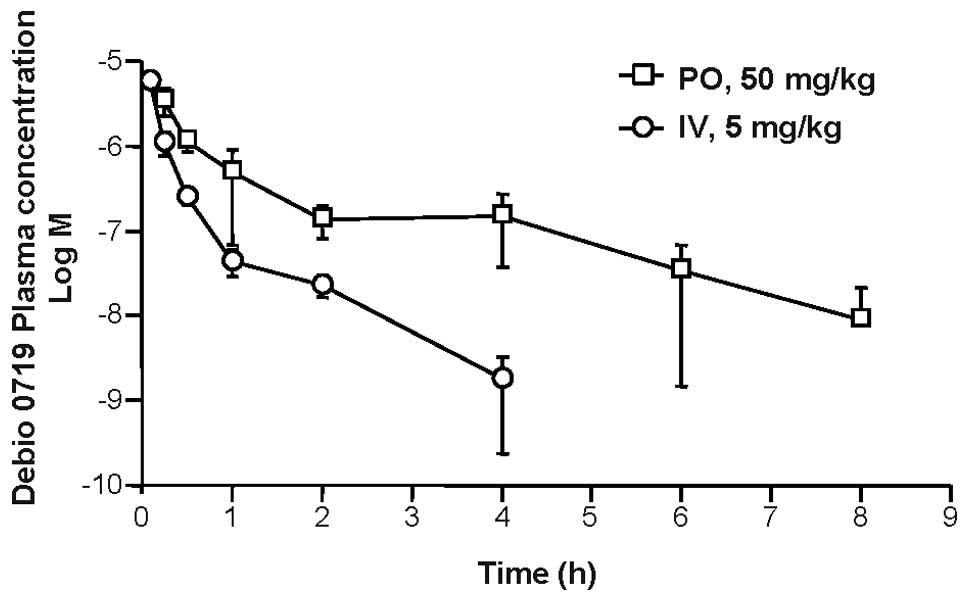
Pharmacokinetics
The therapeutic potential of a pharmacological
compound is linked to its stability and bioavailability in
vivo. Therefore, we next measured the plasma concentration time
curves for Debio 0719 in male CD-1 mice after both intravenous and
oral administration (Fig. 3).
After oral dosing (50 mg/kg), Debio 0719 concentration peaked at 15
min with a Cmax of 3.5 μM thereafter decreasing to ~10
nM by 8 h, yealding a t1/2 of 0.98 h. After intravenous
dosing (5 mg/kg) a Cmax of 5.6 μM was observed within 5
min, which decreased to ~1 nM by 4 h, yealding a t1/2 of
0.49 h. The oral exposure was high with an oral bioavailability
14.41. The detailed pharmacokinetic parameters for Debio 0719 are
shown in Table I.
 | Table IPharmacokinetic parameters for Debio
0719 in male CD-1 mice. |
Table I
Pharmacokinetic parameters for Debio
0719 in male CD-1 mice.
| Dose route | Intravenous
(n=3) | Oral (n=3) |
|---|
| Dose (mg/kg) | 5 | 50 |
| Plasma clearance
(l/h/kg) | 6.66 | |
| T1/2
(h) | 0.49 | 0.98 |
| Bioavailability (%
EF) | | 14.41 |
| Cmax
(μg/ml) | 2960 | 1658 |
| Time to maximum
concentration (h) | 0.08 | 0.25 |
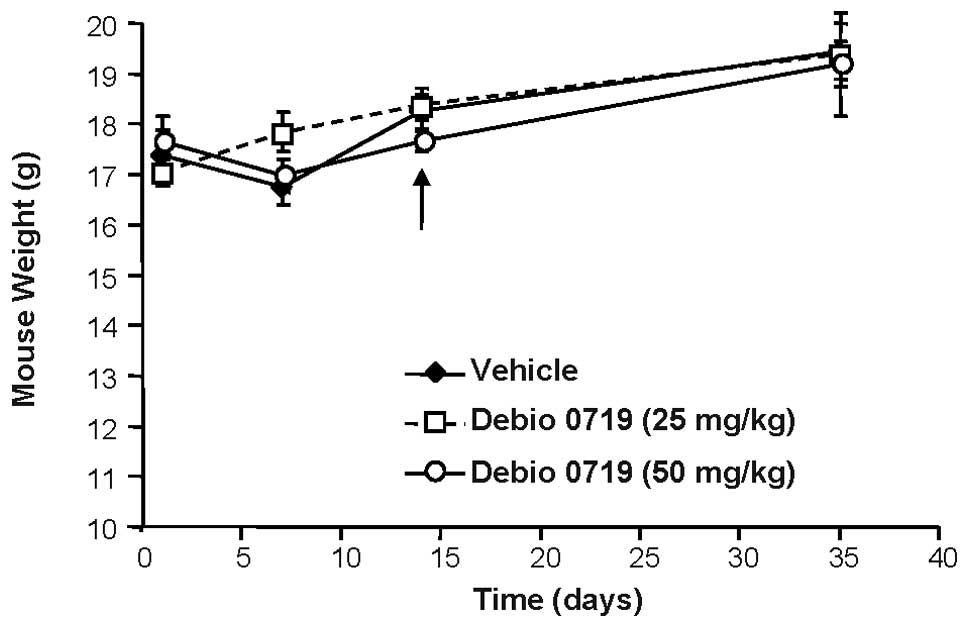
Targeting LPA1 in vivo with
Debio 0719 does not inhibit primary tumor growth of 4T1 cells
To analyze the role of LPA1 during the
early steps of the metastatic dissemination of breast cancer cells,
mice were inoculated orthotopically with 4T1 cells in the mammary
gland and treated with Debio 0719 (25 and 50 mg/kg) administered
orally per os twice daily, or with the vehicle only, from
day 0 to 14 post cell injection. Treatments were stopped at day 14
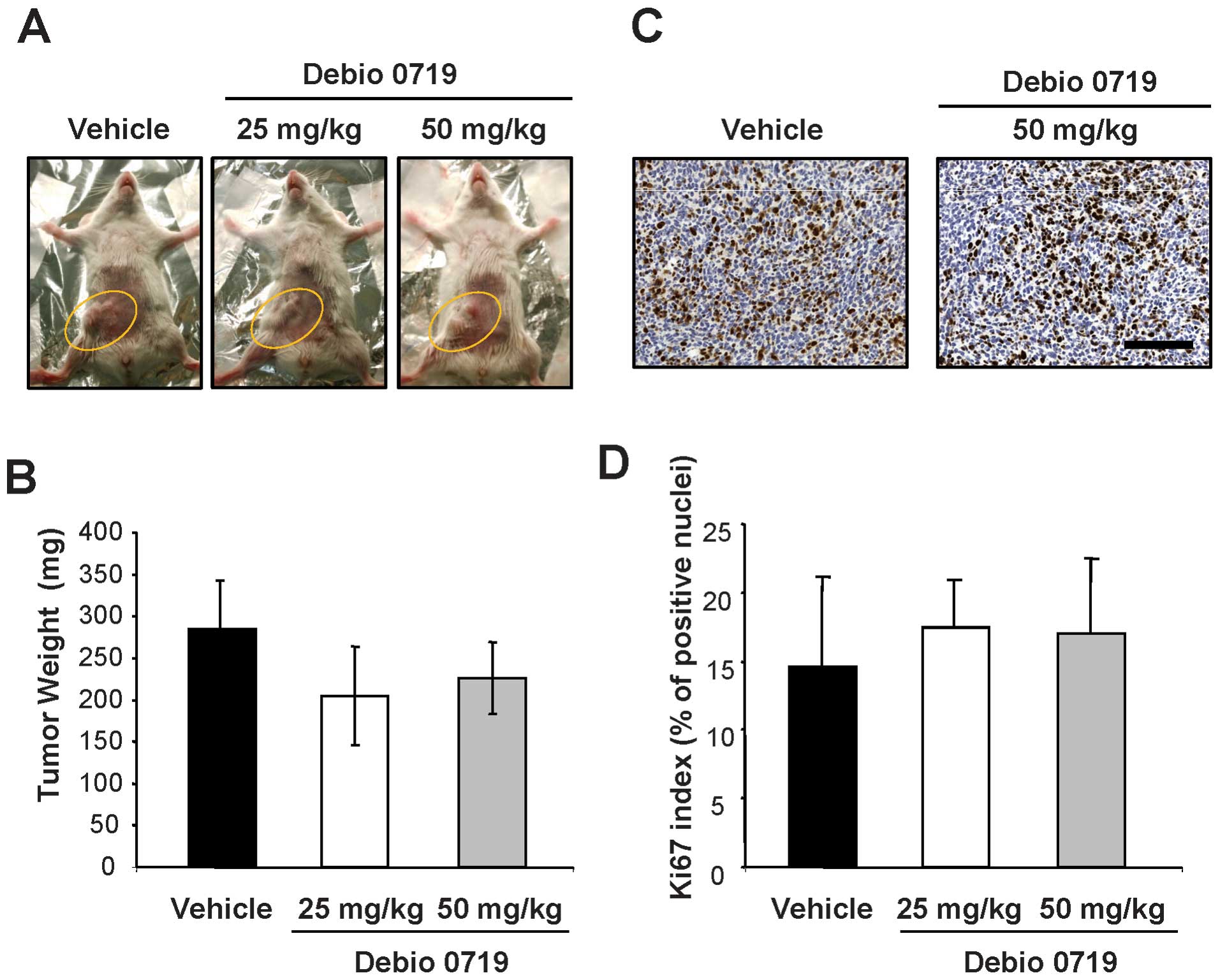
at which time primary tumors were resected. We observed that a
daily treatment of animals with Debio 0719 was well tolerated as
judged by a constant gain of weight of the mice treated with Debio
0719 (25 and 50 mg/kg) twice daily as compared with mice treated
with the vehicle (Fig. 4). Second,
by measuring the weight of resected tumors, we found no difference
in the burden of primary tumors between animals treated and not
treated with Debio 0719 (Fig. 5A and
B). This result was rather surprising based on our previous
results showing a LPA1-dependent mitogenic activity of
LPA on human MDA-B02 breast cancer cells in vitro and in
vivo (14). By performing
immunohistochemical analyses on 4T1 primary tumor sections, we
found that the expression of the mitotic marker Ki-67 in the tumors
was similar in animals treated or not treated with Debio 0719
(Fig. 5C and D). This result
indicated that the treatment of mice with Debio 0719 had no effect
on 4T1 cell proliferation in vivo at the site of
implantation.
Targeting LPA1 in vivo with
Debio 0719 inhibits spontaneous metastasis of 4T1 cells to the
lungs
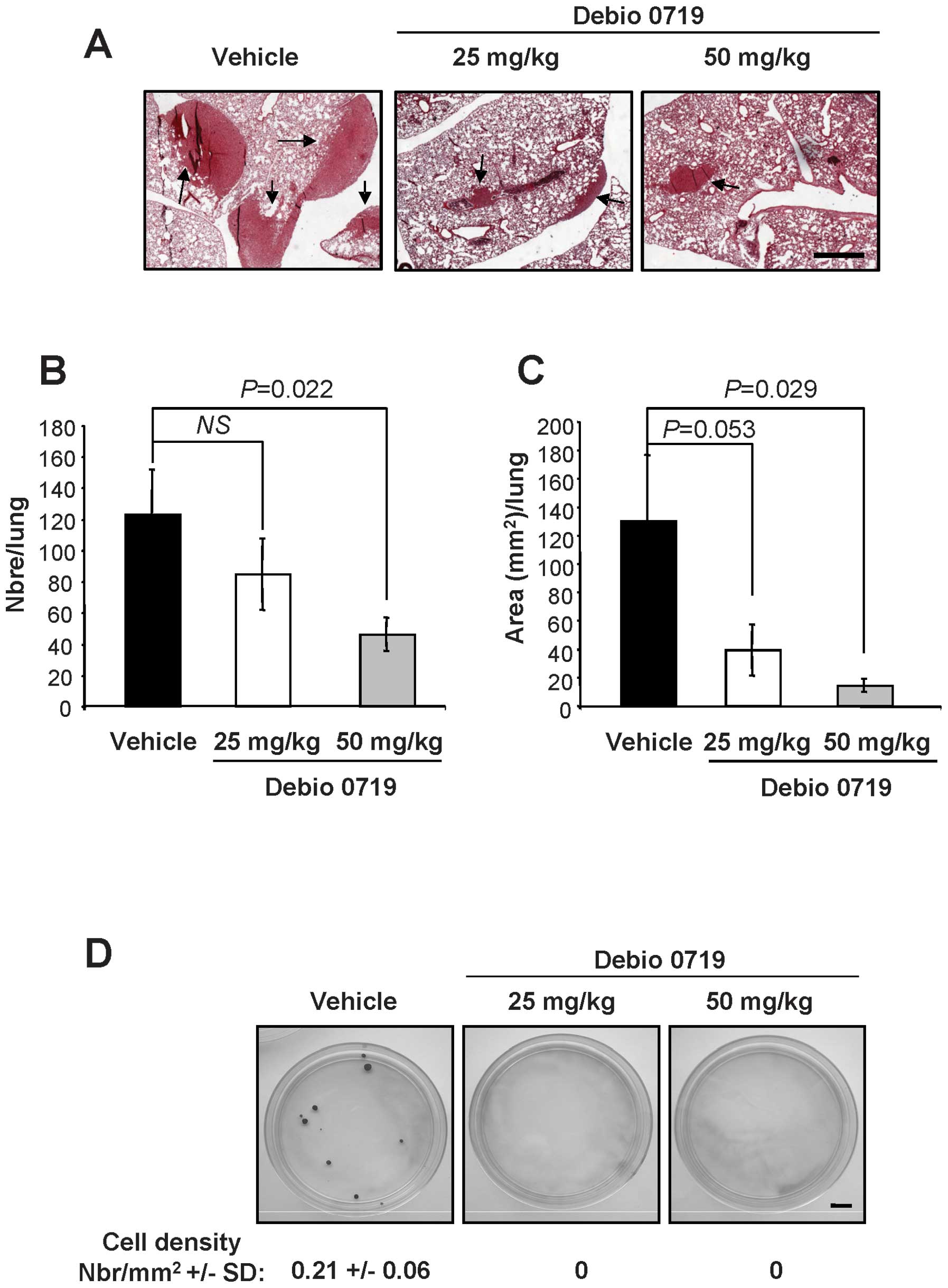
After primary tumor resections, animals treated with
Debio 0719 from day 0 to 14 post cell injection were kept for an
additional 21-day period and received no treatment until the day of
sacrifice, where lungs were collected and the number and the area
of metastatic foci were quantified by histological analysis. We
observed that the number of lung metastasis foci was significantly
reduced (inhibition = 62%, p=0.022) in animals treated with the
higher dosing regimen of Debio 0719 compared to the untreated
animals or to the group of mice receiving the lower dosing of Debio
0719 (Fig. 6A and B). Treatment of
mice with the higher dosing of Debio 0719 (50 mg/kg twice daily)
also decreased significantly the total area of lung metastases
(inhibition = 89%, p=0.029) compared to untreated animals (Fig. 6C). Animals receiving the lower
dosing of Debio 0719 presented also a reduction in the area of lung
metastasis foci but the value did not reach statistical
significance (p=0.053) (Fig.
6C).
Targeting LPA1 in vivo with
Debio 0719 inhibits dissemination of 4T1 cells (4T1-DTCs) to
bone
We then asked whether the effect of Debio 0719 was
restricted to the homing of 4T1 cells to the lungs or if its
activity impaired the overall metastatic process. To address this
question we analyzed the presence of 4T1-disseminated tumor cells
(DTCs) at the bone site, as bone is one key target tissue of breast
cancer derived metastases (22).
At the time of sacrifice, bone marrow cells were harvested and
4T1-DTCs were rescued in vitro due to their endogenous
resistance to the cytotoxic action of 6-thioguanine (21). At that day, we were not able to
rescue any 4T1-DTCs from animals treated with either high or low
dose of Debio 0719 (Fig. 6D).
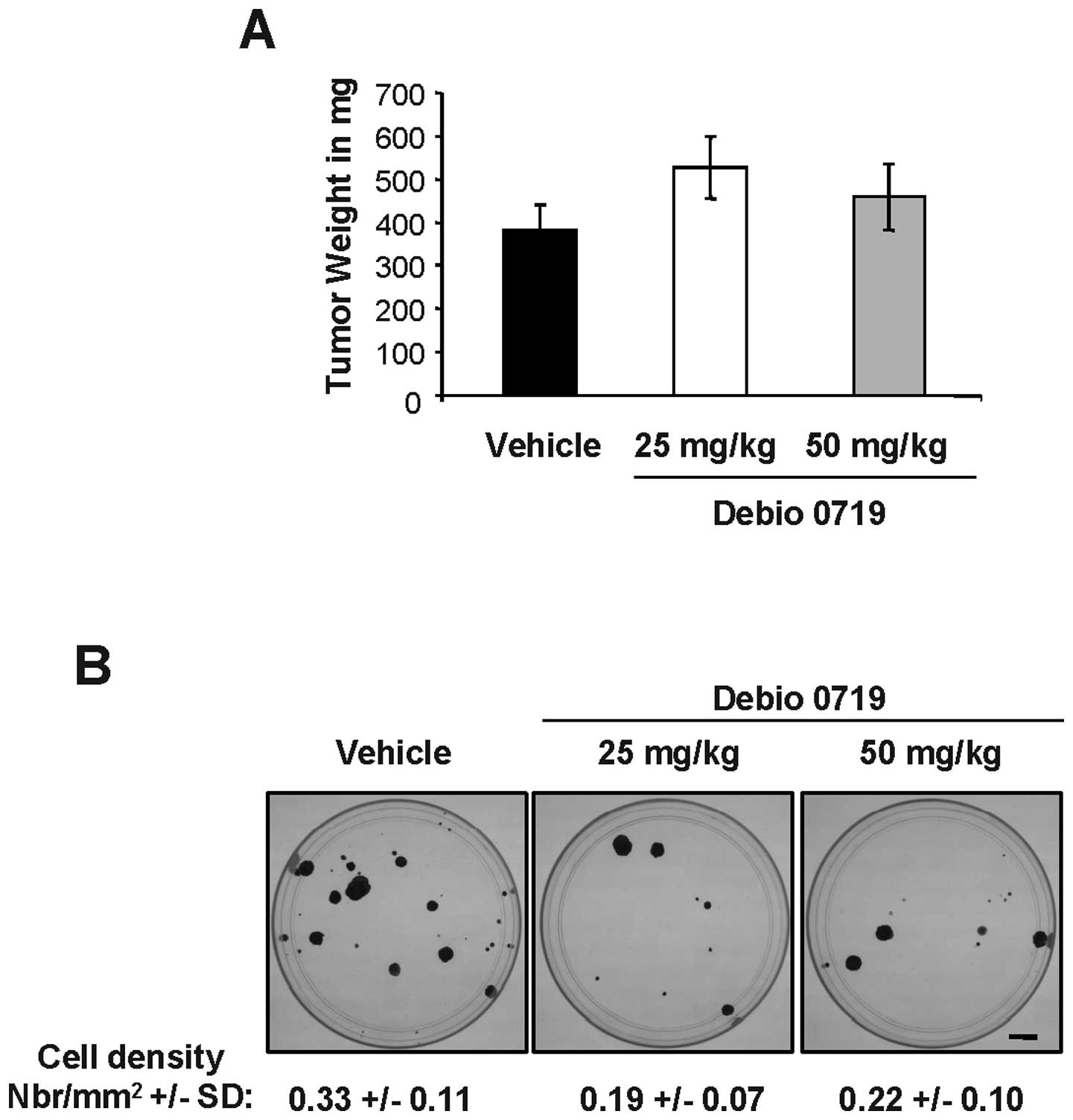
We then asked whether this observation could be due
to an unexpected side effect of the compound on tumor cell survival
in the bone marrow. We firstly generated 4T1 tumors in the mammary
fat pad of BALB/c mice and then submitted the animals to the
treatment with Debio 0719 in an adjuvant setting. Primary tumors
were resected at day 14 and animals were randomized based on the
size of resected tumors (Fig. 7A).
Then, animals were treated or not with Debio 0719 (25 or 50 mg/kg)
twice daily per os, for a 14 day period. At the time of
sacrifice, we found no statistically significant difference in the
number of 4T1-DTCs rescued from the bone marrow of animals treated
with Debio 0719 compared to animals treated with the vehicle
(Fig. 7B). These results indicated
that the activity of Debio 0719 did not impair the survival of
cancer cells already present in the bone marrow when the treatment
was started. Altogether, the results suggested that the
anti-metastatic activity of Debio 0719 was not restricted to a
specific organ but instead affected the overall metastatic capacity
of 4T1 cells.
Targeting LPA1 in vivo with
Debio 0719 inhibits spontaneous metastasis of 4T1 cells
independently of tumor angiogenesis
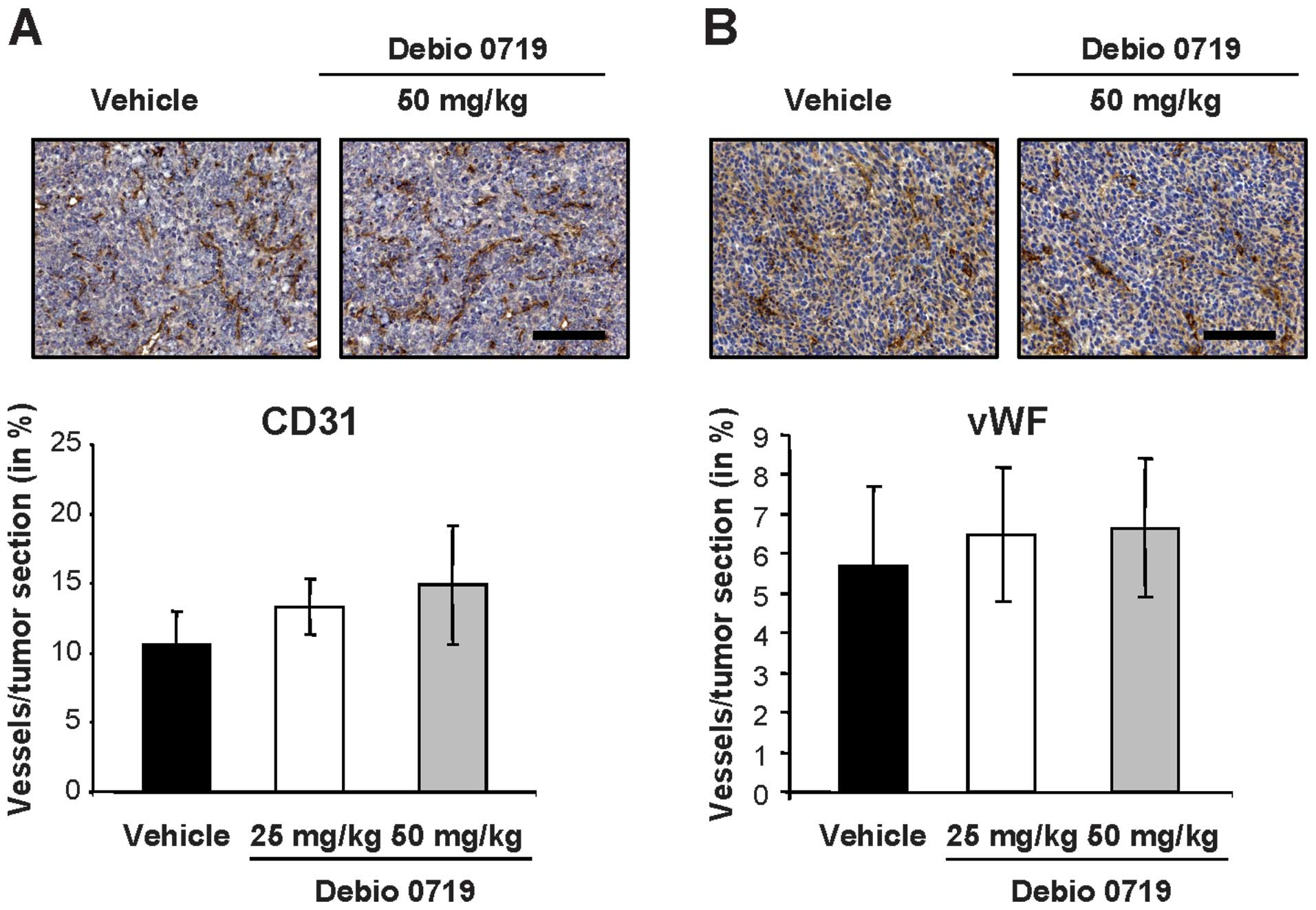
Angiogenesis is well known to control tumor growth
and metastasis, and the LPA-LPA1 track might play a
significant role in this process (23,24).
By quantifying the levels of immunohisto-chemical endothelial cell
markers (vWF and CD31) on 4T1 primary tumor sections, we found a
similar level of angiogenesis in tumors collected from animals
treated and untreated with Debio 0719 (Fig. 8). This result indicated that Debio
0719 did not significantly interfere with angiogenesis in the 4T1
mammary tumor model.
High LPA1 expression at the
primary tumor site links with positive lymph node status of
pre-menopausal breast cancer patients
In clinic, lymph nodes are the first sites invaded
by tumor cells during the metastasis process. Therefore, a high
number of positive lymph nodes contributes to the poor prognosis
for patients with breast cancers (25). We investigated whether
LPA1 might be linked to lymph node invasion in breast
cancer patients. We analyzed the expression levels of the mRNA for
LPA1 in a series of 104 primary tumors from
pre-menopausal patients without metastasis at the time of
diagnosis. Higher LPA1 mRNA expression was significantly
related to positive node tumors, p<0.001 (Table II). Any other classical prognostic
factors (surgical tumor size, histological grade, ER and PgR
status) revealed no difference (Table
II). This suggested that LPA1 expression was
associated with early steps of metastasis dissemination through
invasion of lymph nodes of patients with breast cancers.
 | Table IIDistribution of LPA1 mRNA
expression in different subsets of cases defined by the usual
prognostic factors. |
Table II
Distribution of LPA1 mRNA
expression in different subsets of cases defined by the usual
prognostic factors.
| Prognostic
factors | n | Median | P-value |
|---|
| Surgical tumor
size |
| <20 mm | 37 | 5.19 | |
| ≥20 mm | 64 | 5.51 | 0.607 |
| Histological
type |
| Ductal | 89 | 5.46 | |
| Lobular | 12 | 5.00 | 0.793 |
| Histological
gradea |
| GI | 12 | 5.36 | |
| GII | 43 | 5.46 | |
| GIII | 29 | 5.00 | 0.960 |
| Node status |
| Neg | 47 | 3.24 | |
| Pos | 57 | 6.68 | <0.001 |
| ER status |
| Neg | 27 | 5.46 | |
| Pos | 77 | 5.37 | 0.556 |
| PgR status |
| Neg | 23 | 5.55 | |
| Pos | 81 | 5.25 | 0.935 |
| ER and/or PgR |
| Neg | 34 | 5.56 | |
| ER and PgR |
| Pos | 70 | 5.04 | 0.584 |
Discussion
Metastasis is now considered as a very early event
during tumor growth (26). We
found previously that Ki16425, which is a competitive inhibitor of
LPA1 and LPA3 receptors (17), inhibits efficiently the progression
of breast cancer cell-mediated bone metastases in a mouse model
(16). Here, we present
pharmaco-kinetic and pharmacodynamic data for Debio 0719, which
corresponds to the R-stereoisomer of Ki16425. Debio 0719 was
evaluated for its ability to inhibit LPA1 activation by
LPA. Inhibition of LPA1 activation was measured as a
decrease in the LPA-stimulated calcium flux as Ki16425 was
demonstrated to block LPA-induced elevation of intracellular
calcium (17). The average
IC50 values for the Debio- and Ki16425-mediated
inhibition of LPA-stimulated human LPA1-expressing
Chem-1 stable cell line was 60 and 130 nM, respectively, indicating
that Debio 0719 was 2-fold more potent than Ki16425 at inhibiting
LPA1-induced calcium flux. This was likely due to the
presence of ~50% of the S-stereoisomer of Ki16425, which had a poor
inhibitory activity on LPA/LPA1-stimulated calcium flux.
It is noteworthy that Debio 0719 did not demonstrate any agonist
effects on LPA1 at concentrations as high as 10 μM.
Pharmacokinetic profile of Debio 0719 was assessed in male CD-1
mice. Debio 0719 was well tolerated after oral exposure with a
half-life of 0.98 h. Administration of Debio 0719 to female Balb/C
mice via an oral gavage at a dose up to 50 mg/kg twice daily for 14
days, did not induce noticeable deleterious effects. We observed
that the treatment of mice with Debio 0719 during the early phase
of tumor development inhibited efficiently the formation of
spontaneous lung and bone metastases in a preclinical breast cancer
mouse model exploiting the 4T1 mammary carcinoma cell line. In this
model, the Debio 0719-mediated reduction in metastasis was not
related to reduced angiogenesis at the primary tumor site,
suggesting that LPA1 blockade interferes with another
step of metastasis formation.
LPA is well known to have a mitogenic activity on a
wild range of normal and cancer cell types (8). Recent reports demonstrated that LPA
receptors, including LPA1 control directly the
carcinogenesis through a pro-oncogenic action. By using an
ErbB2/HER2 inducible system in the non-tumoral human MCF-10A breast
cell line, Witt and colleagues found that LPA1 exhibits
a fully oncogene activity in proliferation, migration and
3-dimentional acinar morphogenesis assays (27). However, a weak signal from
ErbB2/HER2 revealed necessary for LPA1-induced cell
action. An initial transformation step of MEF with c-myc and Tbx2
is also required for the oncogenic activity of LPA1,
LPA2 and LPA4 (28). However, in vivo, individual
over-expression of LPA1, as well as LPA2 and
LPA3, driven by the MMTV promoter in transgenic mice
leads to the formation of spontaneous mammary tumors within a year
(13). Interestingly,
over-expression of ATX, an LPA-producing enzyme, in MMTV-transgenic
animals results in a similar spontaneous breast tumor formation
(13). This work suggested that
the sensitization of the breast epithelium to LPA, by increasing
either the amount of cell surface LPA receptors or the local
formation of LPA might prevail to initiate breast carcinogenesis
rather than the control of carcinogenesis by a unique subtype of
LPA receptor. We demonstrated recently that modulating the local
production of LPA at the primary site of 4T1 breast tumors in
animals, through stable down-regulation of ATX using an shRNAi
strategy, decreases spontaneously lung metastasis formation but has
no impact on primary tumor growth (15). Here, we found no effect on the
growth of 4T1 mammary tumors in animal treated with Debio 0719.
These results suggest that targeting LPA or its receptor
LPA1 in breast cancer patients might most likely not
lead to successful inhibition of primary tumor growth. This
hypothesis is supported by the absence of link between the size or
grade, and the levels of LPA1 expression among breast
tumors analyzed throughout the present study. It could therefore be
postulated that clinical trials with compounds targeting LPA
signaling in breast cancer patients should rely on innovative
clinical end-points, instead of RECIST criteria. Recently, a
monoclonal antibody to RANKL (Denosumab) was approved to treat bone
metastasis based on clinical end-points such as time to skeletal
related events, rather than standard criteria (29).
LPA receptors share many intracellular signaling
pathways that control cell behavior (30). LPA is known to induce migration and
invasion of breast cancer cells through the mobilization of LPA
receptors and downstream activation of the β-arrestin/Ral signaling
pathway (31,32). Among LPA receptors, LPA1
was identified as the transducer of the migration activity of LPA
on neoplastic and non-neoplastic cells (19). However, the idea of having one LPA
receptor devoted to one function is probably not realistic.
Overexpression of LPA receptors (LPA1, LPA2,
LPA3) individually in the mammary gland of
MMTV-transgenic mice induces the formation of distant metastases in
up to 45% of tumor bearing animals (13). However, among LPA receptors,
LPA1 might play a key role in the metastasis process of
breast cancers. We found that high expression of LPA1
distributed with the nodal status of pre-menopausal breast cancer
patients suggesting that increased expression of this receptor
might contribute to early steps of breast cancer cell metastasis.
It was demonstrated, since its discovery as a metastasis suppressor
gene (33) that Nm23-H1
down-regulates LPA1 expression in breast cancers
(34). LPA1
re-introduction in breast cancer cells expressing Nm23-H1
was sufficient to rescue these cells from inhibited migration and
to induce metastasis formation in vivo (35,36).
In conclusion, by using an immunocompetent mouse
model, based on orthotopic implantation of breast cancer cells, we
found that blocking LPA1 activity in vivo with
Debio 0719 during the early phase of tumor growth inhibited
efficiently bone and lung metastasis formation. This
anti-metastatic effect of Debio 0719 was mainly due to inhibition
of cell invasion but not of cell proliferation and angiogenesis.
Altogether, our results suggest that the level of LPA1
expression at the site of primary tumors might control very early
events during the metastasis process of breast cancers and that
targeting LPA1 with Debio 0719 has a high therapeutic
potential against metastasis formation for breast cancer
patients.
Acknowledgments
In vitro and animal studies were sponsored by
Debiopharm S.A. (Lausanne, Switzerland). M.B. and M.M. were
employees of Debiopharm S.A. (Lausanne, Switzerland). This study
was supported by grants from the INSERM (O.P. and P.C.), the Comité
Départemental de la Loire de la Ligue Nationale Contre le Cancer
(O.P.) and the French Association pour la Recherche sur le Cancer,
ARC (OP). M.D. was a recipient of fellowship from the Ligue
Nationale contre le Cancer and the French Association pour la
Recherche sur le Cancer, ARC.
References
|
1
|
Choi JW, Herr DR, Noguchi K, et al: LPA
receptors, subtypes and biological actions. Annu Rev Pharmacol
Toxicol. 50:157–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chun J, Hla T, Lynch KR, Spiegel S and
Moolenaar WH: Inter-national Union of Basic and Clinical
Pharmacology. LXXVIII. Lysophospholipid Receptor Nomenclature.
Pharmacol Rev. 62:579–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An S, Bleu T, Hallmark OG and Goetzl EJ:
Characterization of a novel subtype of human G protein-coupled
receptor for lysophosphatidic acid. J Biol Chem. 273:7906–7910.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin ME, Herr DR and Chun J:
Lysophosphatidic acid LPA. receptors, signaling properties and
disease relevance. Prostaglandins Other Lipid Mediat. 91:130–138.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eichholtz T, Jalink K, Fahrenfort I and
Moolenaar WH: The bioactive phospholipid lysophosphatidic acid is
released from activated platelets. Biochem J. 291:677–680.
1993.PubMed/NCBI
|
|
6
|
Stracke ML, Krutzsch HC, Unsworth EJ,
Arestad A, Cioce V, Schiffmann E and Liotta LA: Identification,
purification, and partial sequence analysis of autotaxin, a novel
motility-stimulating protein. J Biol Chem. 267:2524–2529.
1992.PubMed/NCBI
|
|
7
|
Umezu-Goto M, Kishi Y, Taira A, et al:
Autotaxin has lysophospholipase D activity leading to tumor cell
growth and motility by lysophosphatidic acid production. J Cell
Biol. 158:227–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mills GB and Moolenaar WH: The emerging
role of lysophosphatidic acid in cancer. Nat Rev Cancer. 3:582–591.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schulte KM, Beyer A, Kohrer K, Oberhauser
S and Roher HD: Lysophosphatidic acid, a novel lipid growth factor
for human thyroid cells, over-expression of the high-affinity
receptor edg4 in differentiated thyroid cancer. Int J Cancer.
92:249–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang X, Schummer M, Mao M, et al:
Lysophosphatidic acid is a bioactive mediator in ovarian cancer.
Biochim Biophys Acta. 1582:257–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitayama J, Shida D, Sako A, et al:
Over-expression of lysophosphatidic acid receptor-2 in human
invasive ductal carcinoma. Breast Cancer Res. 6:R640–R646. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shida D, Watanabe T, Aoki J, et al:
Aberrant expression of lysophosphatidic acid LPA., receptors in
human colorectal cancer. Lab Invest. 84:1352–1362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Umezu-Goto M, Murph M, et al:
Expression of autotaxin and lysophosphatidic acid receptors
increases mammary tumorigenesis, invasion, and metastases. Cancer
Cell. 15:539–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boucharaba A, Serre C-M, Gres S, et al:
Platelet-derived lysophosphatidic acid supports the progression of
osteolytic bone metastases in breast cancer. J Clin Invest.
114:1714–1725. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
David M, Wannecq E, Descotes F, et al:
Cancer cell expression of autotaxin controls bone metastasis
formation in mouse through lysophosphatidic acid-dependent
activation of osteoclasts. PLoS One. 5:e97412010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boucharaba A, Serre CM, Guglielmi J,
Bordet JC, Clezardin P and Peyruchaud O: The type 1
lysophosphatidic acid receptor is a target for therapy in bone
metastases. Proc Natl Acad Sci USA. 103:9643–9648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohta H, Sato K, Murata N, et al: Ki16425,
a subtype-selective antagonist for EDG-family lysophosphatidic acid
receptors. Mol Pharmacol. 64:994–1005. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lelekakis M, Moseley JM, Martin TJ, et al:
A novel orthotopic model of breast cancer metastasis to bone. Clin
Exp Metastasis. 17:163–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hama K, Aoki J, Fukaya M, et al:
Lysophosphatidic acid and autotaxin stimulate cell motility of
neoplastic and non-neoplastic cells through LPA1. J Biol Chem.
279:17634–17639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pradere JP, Klein J, Gres S, et al: LPA1
receptor activation promotes renal interstitial fibrosis. J Am Soc
Nephrol. 18:3110–3118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aslakson CJ and Miller FR: Selective
events in the metastatic process defined by analysis of the
sequential dissemination of subpopulations of a mouse mammary
tumor. Cancer Res. 52:1399–1405. 1992.PubMed/NCBI
|
|
22
|
Akhtari M, Mansuri J, Newman KA, Guise TM
and Seth P: Biology of breast cancer bone metastasis. Cancer Biol
Ther. 7:3–9. 2007. View Article : Google Scholar
|
|
23
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeon ES, Lee IH, Heo SC, et al:
Mesenchymal stem cells stimulate angiogenesis in a murine xenograft
model of A549 human adenocarcinoma through an LPA1
receptor-dependent mechanism. Biochim Biophys Acta. 1801:1205–1213.
2010. View Article : Google Scholar
|
|
25
|
Donegan WL: Tumor-related prognostic
factors for breast cancer. CA Cancer J Clin. 47:28–51. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eyles J, Puaux AL, Wang X, et al: Tumor
cells disseminate early, but immunosurveillance limits metastatic
outgrowth, in a mouse model of melanoma. J Clin Invest.
120:2030–2039. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Witt AE, Hines LM, Collins NL, et al:
Functional proteomics approach to investigate the biological
activities of cDNAs implicated in breast cancer. J Proteome Res.
5:599–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taghavi P, Verhoeven E, Jacobs JJ, et al:
In vitro genetic screen identifies a cooperative role for LPA
signaling and c-Myc in cell transformation. Oncogene. 27:6806–6816.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stopeck AT, Lipton A, Body JJ, et al:
Denosumab compared with zoledronic acid for the treatment of bone
metastases in patients with advanced breast cancer, A R andomized,
double-blind study. J Clin Oncol. 28:5132–5139. 2010. View Article : Google Scholar
|
|
30
|
Noguchi K, Herr D, Mutoh T and Chun J:
Lysophosphatidic acid LPA. and its receptors. Curr Opin Pharmacol.
9:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boucharaba A, Guillet B, Menaa F, Hneino
M, van Wijnen AJ, Clezardin P and Peyruchaud O: Bioactive lipids
lysophosphatidic acid and sphingosine 1-phosphate mediate breast
cancer cell biological functions through distinct mechanisms. Oncol
Res. 18:173–184. 2009. View Article : Google Scholar
|
|
32
|
Li TT, Alemayehu M, Aziziyeh AI, et al:
Beta-arrestin/Ral signaling regulates lysophosphatidic
acid-mediated migration and invasion of human breast tumor cells.
Mol Cancer Res. 7:1064–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Steeg PS, Bevilacqua G, Pozzatti R, Liotta
LA and Sobel ME: Altered expression of NM23, a gene associated with
low tumor metastatic potential, during adenovirus 2 Ela inhibition
of experimental metastasis. Cancer Res. 48:6550–6554.
1998.PubMed/NCBI
|
|
34
|
Steeg PS, Horak CE and Miller KD:
Clinical-translational approaches to the Nm23-H1 metastasis
suppressor. Clin Cancer Res. 14:5006–5012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Horak CE, Lee JH, Elkahloun AG, et al:
Nm23-H1 suppresses tumor cell motility by down-regulating the
lysophosphatidic acid receptor EDG2. Cancer Res. 67:7238–7246.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Horak CE, Mendoza A, Vega-Valle E, et al:
Nm23-H1 suppresses metastasis by inhibiting expression of the
lysophosphatidic acid receptor EDG2. Cancer Res. 67:11751–11759.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berthier A, Seguin S, Sasco AJ, et al:
High expression of gabarapl1 is associated with a better outcome
for patients with lymph node-positive breast cancer. Br J Cancer.
102:1024–1031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boissier S, Ferreras M, Peyruchaud O, et
al: Bisphosphonates inhibit breast and prostate carcinoma cell
invasion, an early event in the formation of bone metastases.
Cancer Res. 60:2949–2954. 2000.PubMed/NCBI
|