Introduction
Many anticancer therapies currently in use are
inadequate not only in terms of their therapeutic efficacy but also
because they have undesirable side effects. On the other hand,
certain dietary constituents known as phytochemicals have been
shown to have significant anticancer efficacy (1) while causing minimal deleterious side
effects.
Curcumin, the active component of turmeric and a
polyphenolic compound, is one of the most widely characterized
phytochemicals. It has been a part of therapeutic preparations for
centuries due to its wide spectrum of beneficial activities and its
safety in relatively large dose (2). Evidence has been shown that curcumin
inhibits the initiation, progression and continued survival of
cancers cells (3). On basis of its
numerous anti-carcinogenic properties, curcumin has already been
the subject of several clinical trials for use as a treatment in
human cancers. However, the low bioavailability prevents its use in
chemotherapeutic application, and one potential means of
circumventing this problem has been the creation of synthetic
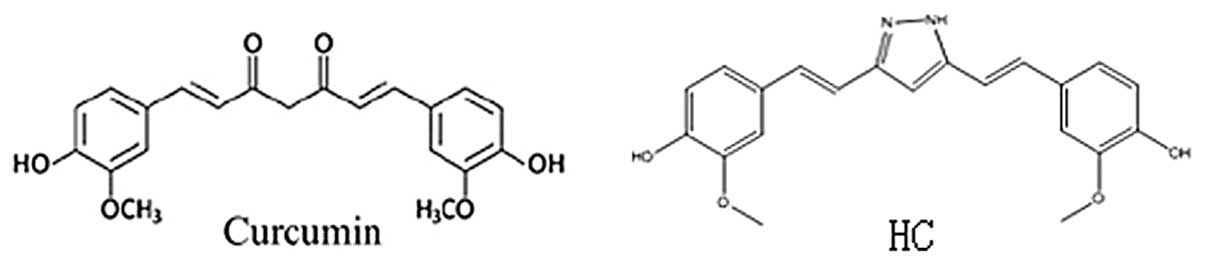
curcumin analogues. Hydrazinocurcumin (HC) (Fig. 1), a synthetic analogue of curcumin,
was obtained as pale yellow gum which was analyzed for
C21H20N2O4 by HRMS,
thus 13° of unsaturation (4).
Compared with curcumin, HC has greatly improved water solubility
and stability, and has high cell permeability, or improved
bioavailability with more favorable pharmacological activity
(5). In our study, we compared the
effects of HC and curcumin on carcinogenicity of breast cancers,
and demonstrated for the first time that HC was more effective than
curcumin in suppressing cell proliferation, colony formation, cell
migration, invasion, and induction apoptosis in human breast cancer
cells (MDA-MB-231, MCF-7), along with inhibition of STAT3
phosphorylation (Tyr705) and downregulation of STAT3 downstream
targets. Therefore, we concluded that HC is substantially more
effective than curcumin in vitro.
Materials and methods
Cell lines and reagents
The human breast cancer cell lines MDA-MB-231 and
MCF-7 were obtained from Institute of Cell Research, Chinese
Academy of Sciences. These cell lines were grown at 37˚C in
Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine
serum (FBS, Sijiqing, China) in a humidified 5% CO2
incubator. All cells were washed three times in pH 7.4 PBS before
harvesting for different experiments. Curcumin and HC were
synthesized and provided kindly by Dr Yanmei Zhang at Carlifornia
University of San Diego, the purity was >98%.
MTT cell viability assay
Breast cancer cell lines (MDA-MB-231, MCF-7) were
seeded in 96-well plates at a density of 3,000 cells per well.
Different concentrations of curcumin (2.5–40 μM) or
hydrazinocurcumin (0.5–5 μM) were added in triplicate to the plates
in the presence of 10% FBS. The cells were incubated at 37˚C for 72
h. Then 25 μl MTT (Sigma) was added to each sample. After 3.5 h,
100 μl DMSO (Sigma) was added to each well. The absorbance was read
at 490 nm. The viability of the untreated cells was arbitrarily set
at 100% and compared with the viability of curcumim,
hydrazinocurcumin-treated cells. IC50 was determined
using SPSS 16.0 software.
Colony formation assay
A base 0.6% agar gel with 10% FBS in DMEM was
prepared and added to the well of a 6-well culture dish. Breast
cancer cells were plated at a density of 3,000 cells per well on
top of the base agar for anchorage-independent growth analysis in
0.4% agar gel with 10% FBS in DMEM supplemented with curcumin,
hydrazinocurcumin or DMSO. The cells were maintained at 37˚C and
allowed to grow for 2 weeks. The colonies were stained using MTT
dye (100 μl per well). Pictures of the colonies were taken using a
Leica MZ 16FA inverted microscope (Leica Microsystems, Bannockburn,
IL) with a 7.4 Slider Camera (Diagnostic Instruments, Inc.,
Sterling Heights, MI). The colonies were scored by counting with an
inverted microscope, and numbers were normalized as a percentage of
colonies formed in DMSO treatment.
Cell cycle analysis
Cell cycle phase was determined by
fluorescence-activated cell sorting analysis. MDA-MB-231 and MCF-7
cells were inoculated into 6-well plates at a concentration of
5×105 per well, exposed to curcumin and HC at a
concentration of 10 μM, cultured for 24 and 48 h, collected, and
sorted using flow cytometry (Bekman Coulter, USA) as described
previously (6).
Apoptosis rate analysis
MDA-MB-231 and MCF-7 cells were grown for 24 h in a
6-mm plate and then treated with the 10 μM of curcumin and HC for
24 and 48 h. Cells were washed by PBS 3 times, and then digested
with 0.25% tryptan-EDTA. After centrifugation, cells were
resuspended in 0.5 ml PBS. Cells were then stained with Annexin V
and propidium iodide (PI) in the presence of 100 mg/ml RNAse and
0.1% Triton X-100 for 30 min at 37˚C. Flow cytometric (Bekman
Coulter) analysis was performed using a fluorescence-activated cell
sorter.
Wound healing/cell migration assay
Breast cancer cells (3×105 per well) were
seeded in a 6-well plate. Approximately 24 h later, when the cells
were 100% confluent, the monolayer was scratched using a 1-ml
pipette tip and washed once to remove nonadherent cells. New medium
in the presence of 10% FBS containing curcumin, hydrazinocurcumin
or DMSO was added. The treatments were removed after 4 h, the fresh
medium was added. After an additional 20 h without treatment, the
cells were observed under a microscope. When the wound in the
control was closed, the inhibition of migration was assessed by
using the ImageJ software, available from the National Institutes
of Health Web site (http://rsb.info.nih.gov/ij). The percent of wound
healed was calculated using the formula: 100−[(final area/initial
area) × 100%].
Invasion (transwell) assay
To evaluate the effects of curcumin and HC on the
invasion ability of MDA-MB-231 and MCF-7 cells, the cells were
harvested by trypsinization after treatment for 24 h with curcumin
and HC at doses of 10, 20 μmol/l. Cells were then seeded at a
density of 50,000 cells in 0.2 ml in upper compartment. In the
lower compartment 0.5 ml of medium was supplemented with 15% FBS.
After incubation for 24 h, the upper cell layer was removed with a
cotton swab, and the cells on the lower side were fixed with 4%
formaldehyde solution. Subsequently, cells were stained with
crystal viola and counted under a microscope.
Western blot analysis
Breast cancer cell lines (MDA-MB-231, MCF-7) were
treated with curcumin (10 or 20 μM, hydrazinocurcumin (10 or 20 μM)
or DMSO for 24 h. For Western blot analysis, the protein from
cancer cell lysates were subjected to SDS-PAGE and transferred to
PVDF membrane. Blots were probed with phosphospecific STAT3 (Tyr
705) antibody (Cell Signaling Technologies), with STAT3 antibody
(BD), with c-Myc, Mcl-1, Bcl-xl, survivin, MMP-9, MMP-2 antibodies
(Bioword), with cyclin D1, VEGF, Bcl-2, BAX, β-actin antibodies
(Santa Cruz Biotechnology), with PARP, caspase 9 antibodies
(Beyotime). Membranes were analyzed using enhanced
chemiluminescence (ECL) detection system (Viagene).
Results
HC and curcumin inhibit cell viability in
human breast cancer cells
A dose-dependent inhibition in tumor cell
proliferation/viability was observed after 72 h of treatment, and
IC50 values were calculated for curcumin and HC
(Table I). The results showed that
compared with curcumin, HC was substantially more potent in
inhibiting cell viability in both cell lines.
 | Table IIC50 (μM) of HC and
curcumin in breast cancer cells. |
Table I
IC50 (μM) of HC and
curcumin in breast cancer cells.
| Cell line | Curcumin | HC |
|---|
| MDA-MB-231 | 26.9 | 3.37 |
| MCF-7 | 21.22 | 2.56 |
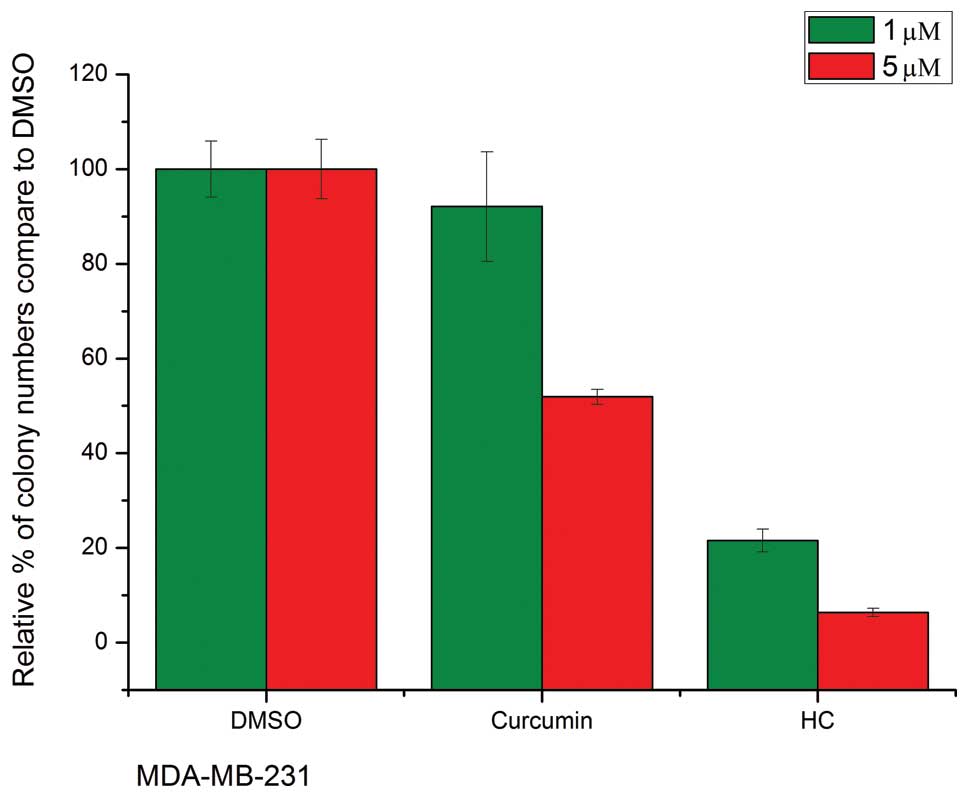
Anchorage-independent growth and cell
viability
The ability of transformed cells to grow and
proliferate in the absence of substratum attachment is one of the
hallmarks of malignancy and vitally important in the formation of
the tumor (7). It was showed that
treatment with curcumin and HC led to decreased colony formation in
soft agar in both two cell lines (Fig.
2). Compared with the DMSO control samples, a 5 μmol/l
concentration of curcumin elicited a decrease of ~50% in colony
formation, while equal concentration of HC showed ~95% reduction in
colony number.
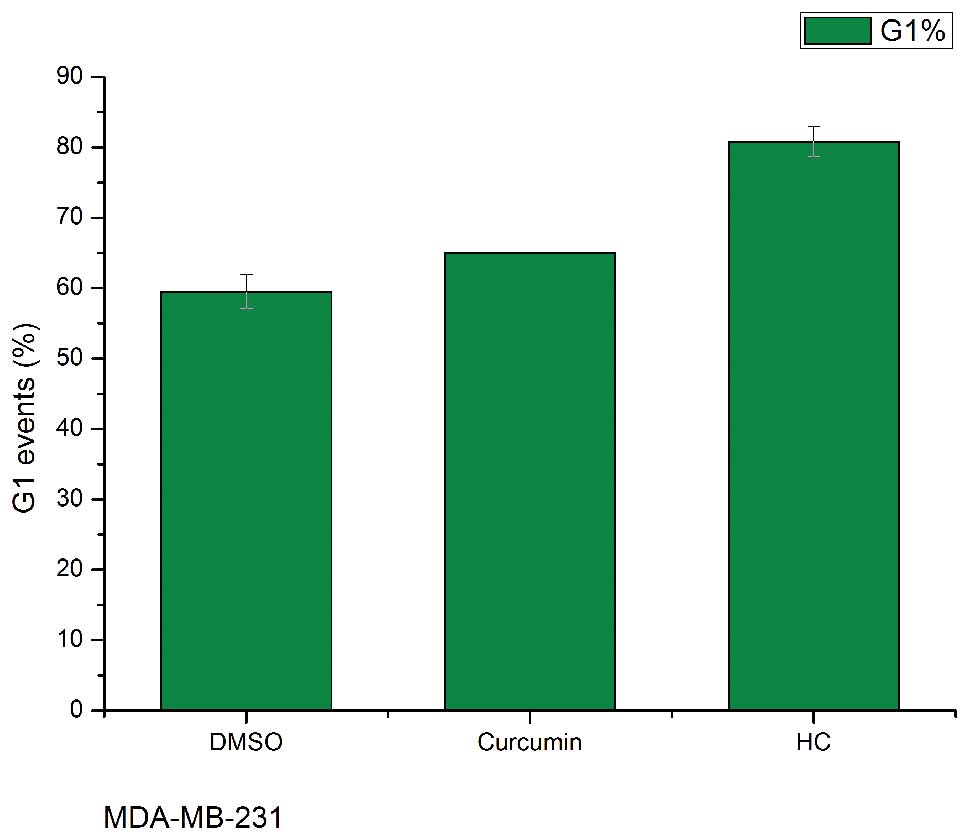
HC and curcumin arrest the cell cycle in
human breast cancer cells
As shown (Fig. 3),
HC and curcumin treatment at a concentration of 10 μM significantly
increased the cell number in the G1 phase in MDA-MB-231
and MCF-7 (p<0.05 compared with control), and HC was more potent
than curcumin in arresting the cells cycle in G1
phase.
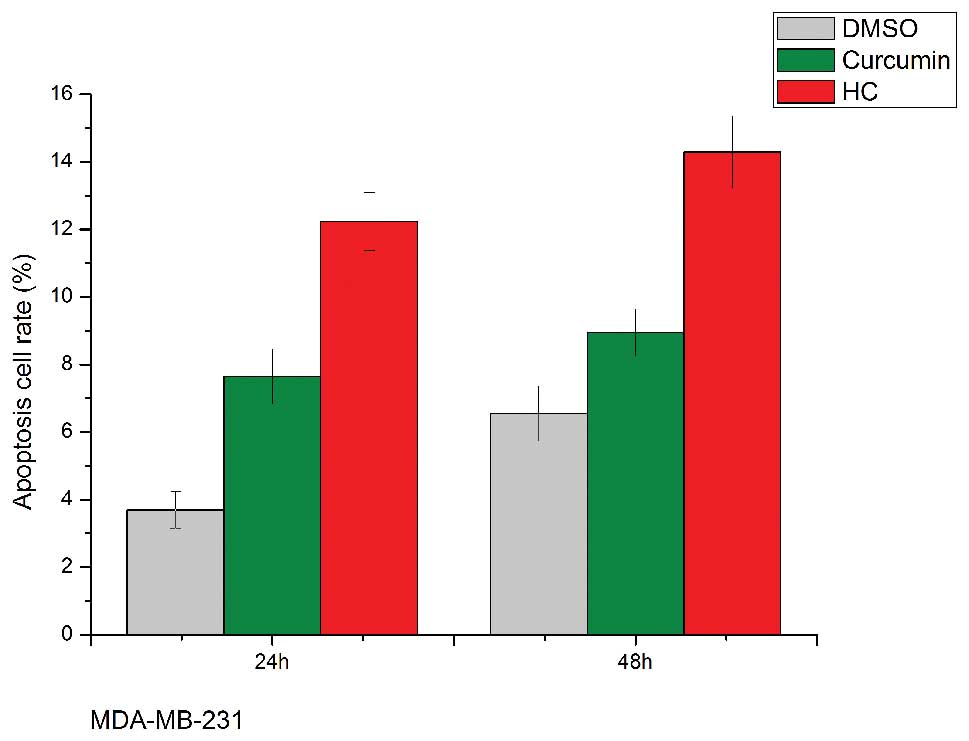
HC and curcumin induce cell apoptosis in
human breast cancer cells
At a sufficiently high dose, curcumin induces
apoptosis of many cancer cells including breast cancer cells. We
assessed the effect of HC and curcumin on the induction of
apoptosis in MDA-MB-231 and MCF-7 cells by FCM. The results
(Fig. 4) showed that HC
dose-dependently increased cells apoptotic rate after 48-h
treatment; HC at 10 μmol/l significantly induced cells apoptosis
(14% in MDA-MB-231 cells and 26% in MCF-7 cells), and at the same
concentration, curcumin only induced 9 and 20% cells apoptosis in
MDA-MB-231 and MCF-7 cells, respectively. In addition, PARP and
caspase 9 are key effector molecules in the apoptosis pathway, and
the enhancement of the level of the two molecules were also
observed within 24 h in both MDA-MB-231 and MCF-7 cells treated
with 10–20 μM curcumin and HC.
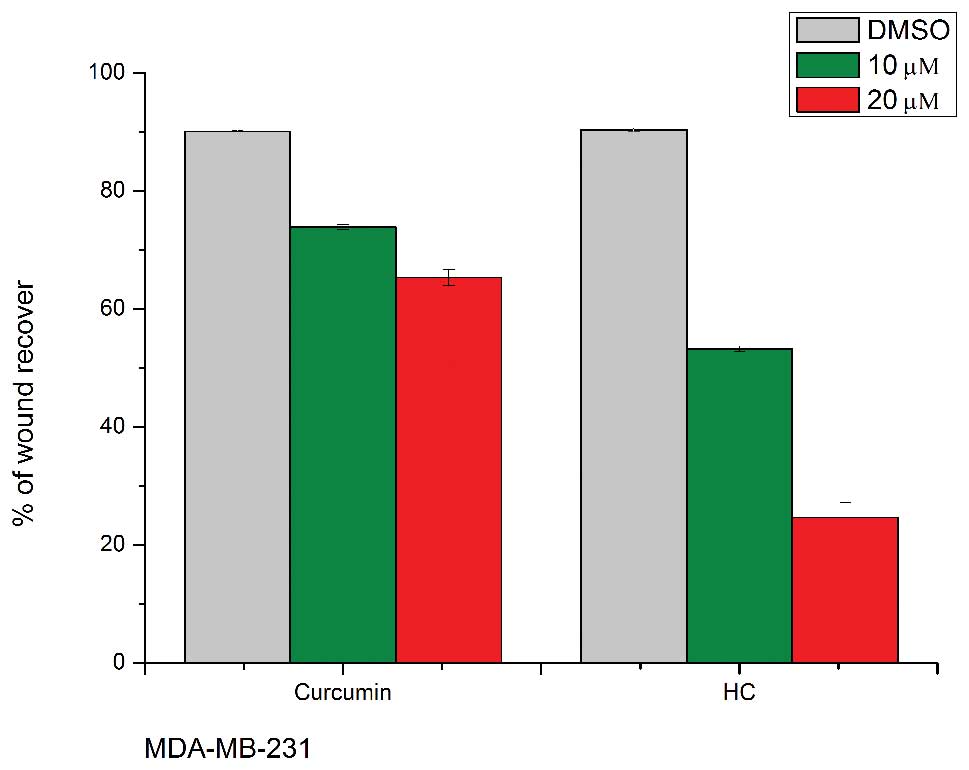
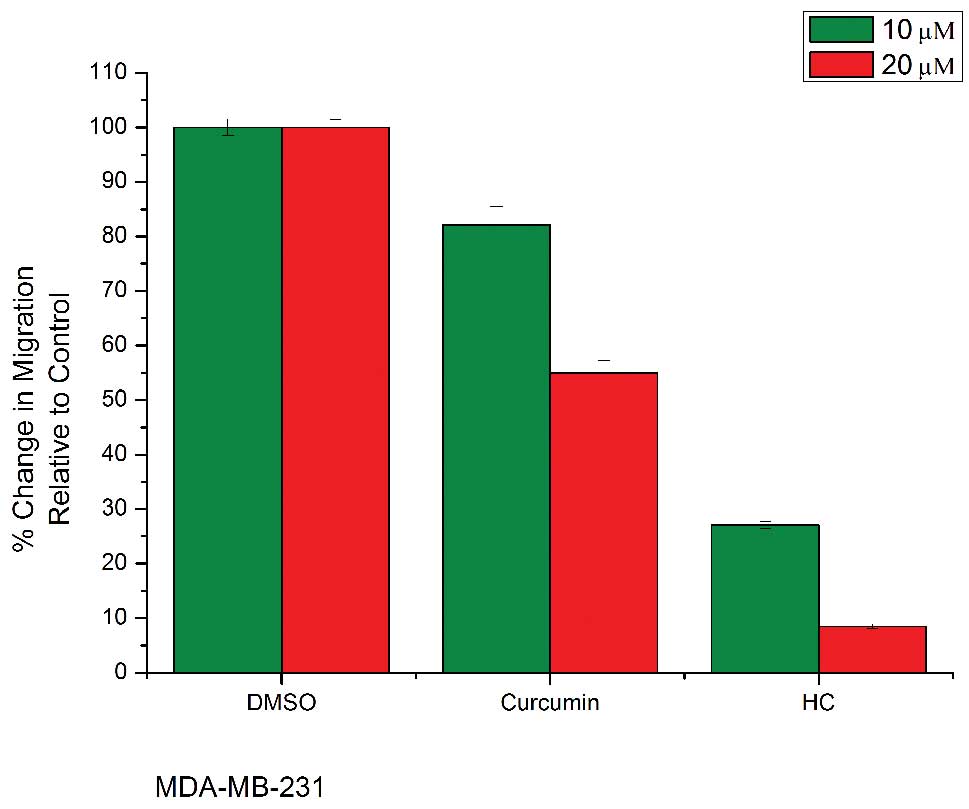
HC and curcumin inhibit cell migration in
human breast cancer cells
Cell migration is necessary in many physiological
processes, such as wound healing and tumor metastasis. To
investigate the effect of HC on cell migration, a wound healing
assay was carried out with MDA-MB-231 and MCF-7 breast cancer
cells. After the creation of a wound, cells were treated with
various concentrations of HC and curcumin. Treatment was removed
after 4 h and cells were returned to standard media in an attempt
to minimize any cytotoxic effects that could potentially confound
our observations. Following 20 h of further incubation, the areas
of the wounds were measured using ImageJ software. Treatment with
HC at a concentration of 10 μM or higher caused a significant
decrease in cell migration (p<0.05) (Fig. 5). Because MTT assay revealed that
the dosages and time points used in migration assay had minimal
impact on cell viability (Fig. 5),
the ability of HC and curcumin to inhibit cell migration might not
be due to its ability to inhibit cell proliferation.
HC and curcumin suppress cell invasion in
human breast cancer cells
To determine the role of curcumin and HC in cell
invasion, MDA-MB-231 and MCF-7 cells were treated with indicated
concentrations of curcumin and HC for 24 h. Both HC and curcumin
significantly inhibited the invasion of two cell lines (Fig. 6). Interestingly, compared with the
DMSO control, the invasion of MDA-MB-231 and MCF-7 cells was
inhibited by ~90% at the concentration of 20 μM of HC, and equal
administration of curcumin showed ~50% reduction in cell
number.
HC and curcumin inhibit the expression of
STAT3 protein and its downstream targets in human breast cancer
cells
As previously mentioned, STAT3 binding to the
promoters of the target genes induces the transcription of several
proteins involved in carcinogenicity of cancer cells. As seen in
Western blot assay (Fig. 7), HC
was more potent than curcumin in inhibiting the expression of STAT3
protein at the same concentration (10–20 μM) in MDA-MB-231 and
MCF-7 cells. To further analyze the expression of the downstream
targets of STAT3, Western blotting was run for MMP-9, MMP-2, Mcl-1,
cyclin D1, c-myc, Bcl-xl, Bcl-2, survivin and VEGF, and it found
that treatment with HC resulted in more inhibition of the
expression of these targets of STAT3 than curcumin.
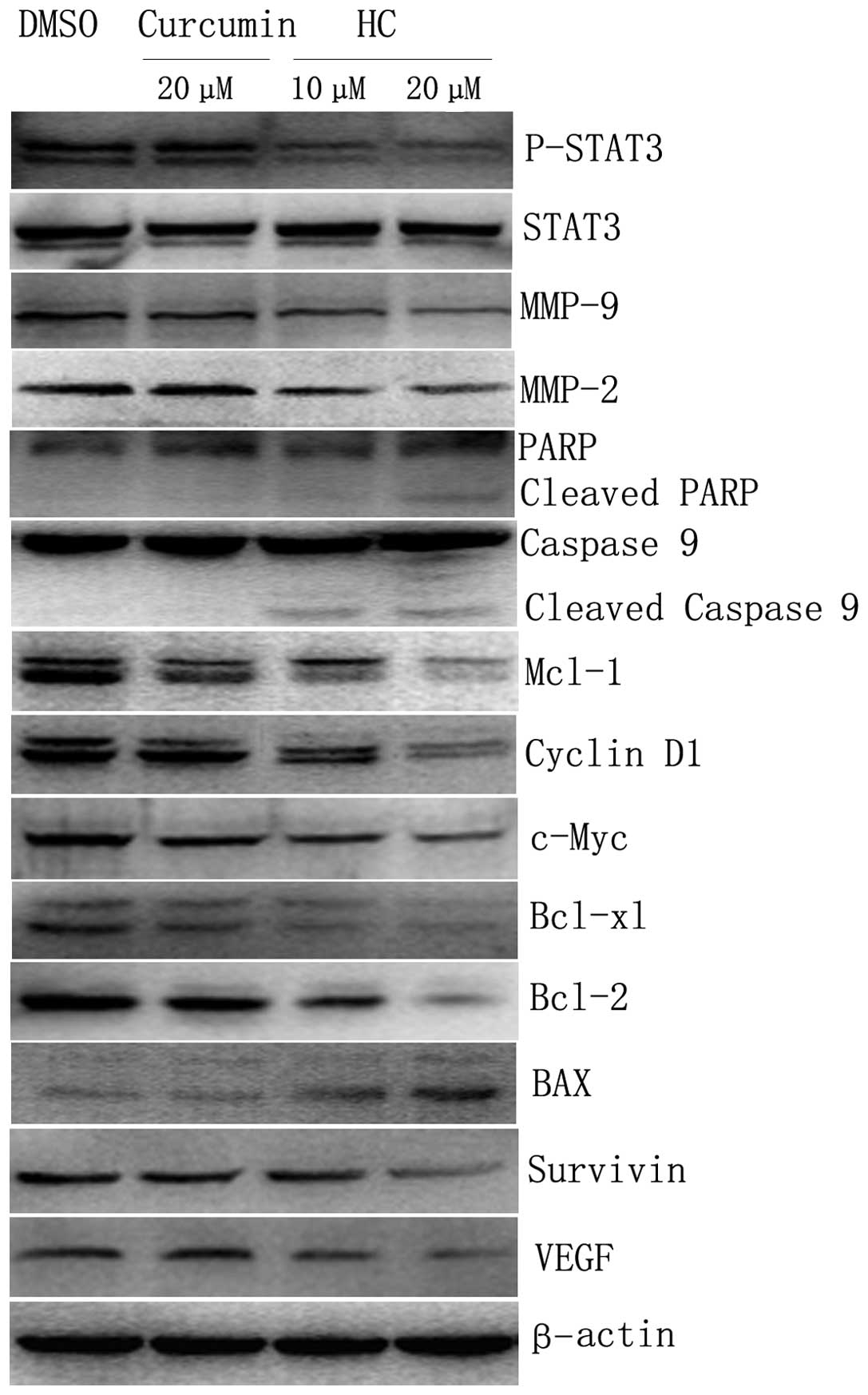 | Figure 7Expression of STAT3 and its
downstream targets by Western blot analysis. Western blot analysis
of cells treated with curcumin and HC. Both cancer cell lines
express constitutively active STAT3. (A) MDA-MB-231, (B) MCF-7,
exhibited decreased expression of STAT3 phosphorylation after
treatment with 10 and 20 μM/l curcumin and HC for 24 h. Downstream
targets of STAT3, including MMP-9, MMP-2, Mcl-1, cyclin D1, c-Myc,
Bcl-xl, Bcl-2, survivin, and VEGF were also inhibited. Cell
apoptosis is indicated by the induction of cleaved PARP, cleaved
caspase 9 and BAX. |
Discussion
The dysregulation of multiple oncogenic pathways is
common among many cancers, including breast cancer cells. Being a
phytochemical, anticancer efficacy of curcumin is mediated through
regulating various transcription factors, growth factors,
inflammatory cytokines, protein kinases, and other enzymes
(8). Persistent activation of
signal transduction and activators of transcription-3 (STAT3) is
found with high frequency in a wide range of human cancer cell
lines and tissues and implicated in stimulating cell proliferation,
promoting angiogenesis, invasion and migration, mediating immune
evasion, and conferring increased resistance to apoptosis (9–14).
As one of the most important curcumin molecular targets, STAT3 can
also be down-regulated effectively by curcumin (15,16).
STAT3 activation occurs when the tyrosine 705
(Tyr705) residue is phosphorylated, and then leads to dimerization
and translocation from the cytoplasm to the nucleus (17–19).
In the nucleus, STAT3 binding to target genes induces the
transcription (20,21). Stat3 drives malignant progression
through the deregulation of key proteins, including cell survival
proteins such as Bcl-XL, Bcl-2, Mcl-1 and survivin (10,22–26),
cell growth proteins such as cyclin D1/D2 and c-myc (9,27–31),
inducers of angiogenesis such as vascular endothelial growth factor
(VEGF) (32,33), and stimulators of invasion and
metastasis such as MMP-2, MMP-9 (34–37).
The crucial role of STAT3 in cancer progression and tumorigenesis
suggests that it is a promising molecular target for cancer
treatment.
Although curcumin has been shown to be a safe and
effective inhibitor of STAT3, its low bioavailability prevents its
chemotherapeutic application. In this study, we characterized the
biologic activity of HC, a curcumin analog with improved water
solubility, higher cell permeability and more favorable
pharmacological activity; we evaluated for the first time the
inhibitory efficacy of HC and curcumin in two breast cancer cells,
MDA-MB-231 and MCF-7, which present constitutive activation of
STAT3.
By both annexin V/PI staining and detection of
cleavage of caspase 9, and PARP, it showed that compared with
curcumin, HC increased the efficacy of induction of apoptosis of
breast cancer cells. Accordingly, Bcl-2, Bcl-xl and Mcl-1, which
are well-known apoptosis inhibitors and downstream target genes of
STAT3, decreased after treatment with HC and curcumin. Bcl-xl and
Bcl-2 are the anti-apoptotic protein within the Bcl-2 family that
inhibits apoptosis by binding proapoptotic proteins and preventing
cytochrome c release (38–40). Mcl-1 also represents a survival
factor for human cancer cells (23,41).
In addition, survivin is one of the key regulators of both cell
cycle and apoptosis (42), and was
shown in our study to be down-expressed.
The results of FCM also showed that treatment with
curcumin and HC resulted in G1 arrest of cancer cells,
and the decreased expression of cyclin D1 and c-Myc by Western
blotting might account for proliferation inhibition and cell cycle
arrest of the treated cells. Previous studies confirmed that Stat3
made an essential contribution to the regulation of cyclin D1 and
c-Myc in v-Src transformation (43). Overexpression of cyclin D1 drives
oncogenesis to regulate cell cycle progression (44). The c-Myc protooncogene is
over-expressed in Burkett's lymphoma, and in other carcinomas such
as breast cancer and colon cancer where it contributes to increased
cellular proliferation and inhibition of differentiation (45–47).
The wound healing assay and transwell assay
demonstrated that HC was more potent than curcumin in reducing the
ability of migration and invasion of MDA-MB-231 and MCF-7 cells,
and the level of MMP-2 and MMP-9 were also decreased. Constructive
expression of STAT3 can up-regulate the expression of MMP-2 and
MMP-9, which are able to efficiently degrade the extracellular
matrix and basement membrane, promoting invasion and metastasis of
cancer cells (34). Recent studies
have found that the activity of STAT3 is closely related to the
expression of MMP-9 in human breast cancer, and the activity of
MMP-9, in epithelial cells of breast cancer, was significantly
increased due to the sustained activation of the transformation of
the plasmid by STAT3 (35). Thus,
STAT3 may promote the progression of breast cancer through the
regulation on MMP-9. Other studies have confirmed that MMP-2 might
be the downstream target gene of STAT3; when it is translocated to
the nucleus, phosphorylated STAT3 can bind to the promoter of MMP-2
to regulate MMP-2 expression (36,37).
In conclusion, our results showed that compared with
curcumin, HC was more effective in inhibiting STAT3 phosphorylation
and down-regulating an array of STAT3 downstream targets which
contributed to the suppression of cell proliferation, loss of
colony formation, depressing cell migration and invasion as well as
induction of cell apoptosis. It was concluded that HC is a potent
agent in inhibiting the phosphorylation of STAT3 with a more
favorable pharmacological activity than curcumin, and HC might have
translational potential as an effective drug or preventive agent
for human breast carcinoma.
Acknowledgements
We thank Dr Yanmei Zhang at California University of
San Diego for providing HC, and Ju Cao at Chongqing Medical
University for language support. This work was supported by a grant
from National Natural Science Foundation of China (No. 30971131);
grants from Chongqing Science & Technology Commission (the
Natural Science Foundation of Chongqing, CSTC 2009BB5077); grants
from Foundation of National Key Discipline in Laboratory Medicine
(No. 2010104). This work was supported in part by Key Laboratory of
Diagnostic Medicine Designated by the Ministry of Education,
Chongqing Medical University.
References
|
1
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008.
|
|
3
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: from ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shim JS, Kim DH, Jung HJ, et al:
Hydrazinocurcumin, a novel synthetic curcumin derivative, is a
potent inhibitor of endothelial cell proliferation. Bioorg Med
Chem. 10:2987–2992. 2002. View Article : Google Scholar
|
|
5
|
Rathore R, Jain JP, Srivastava A, et al:
Simultaneous determination of hydrazinocurcumin and phenol red in
samples from rat intestinal permeability studies: HPLC method
development and validation. J Pharm Biomed Anal. 46:374–380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kakudo Y, Shibata H, Otsuka K, Kato S and
Ishioka C: Lack of correlation between p53-dependent
transcriptional activity and the ability to induce apoptosis among
179 mutant p53s. Cancer Res. 65:2108–2114. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
8
|
Heng MC: Curcumin targeted signaling
pathways: basis for anti-photoaging and anti-carcinogenic therapy.
Int J Dermatol. 49:608–622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alas S and Bonavida B: Inhibition of
constitutive STAT3 activity sensitizes resistant non-Hodgkin's
lymphoma and multiple myeloma to chemotherapeutic drug-mediated
apoptosis. Clin Cancer Res. 9:316–326. 2003.
|
|
11
|
Buettner R, Mora L and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
12
|
Shen Y, Devgan G, Darnell JJ and Bromberg
J: Constitutively activated Stat3 protects fibroblasts from serum
withdrawal and UV-induced apoptosis and antagonizes the
proapoptotic effects of activated Stat1. Proc Natl Acad Sci USA.
98:1543–1548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Real P, Sierra A, De Juan A, Segovia J,
Lopez-Vega J and Fernandez-Luna J: Resistance to chemotherapy via
Stat3- dependent overexpression of Bcl-2 in metastatic breast
cancer cells. Oncogene. 21:7611–7618. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Niu G, Kortylewski M, et al:
Regulation of the innate and adaptive immune responses by Stat-3
signaling in tumor cells. Nat Med. 10:48–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bharti A, Donato N and Aggarwal B:
Curcumin (diferuloylmethane) inhibits constitutive and
IL-6-inducible STAT3 phosphorylation in human multiple myeloma
cells. J Immunol. 171:3863–3871. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bowman T, Garcia R, Turkson J and Jove R:
STATs in oncogenesis. Oncogene. 19:2474–2488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaptein A, Paillard V and Saunders M:
Dominant negative stat3 mutant inhibits interleukin-6-induced
Jak-STAT signal transduction. J Biol Chem. 271:5961–5964. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faruqi T, Gomez D, Bustelo X, Bar-Sagi D
and Reich N: Rac1 mediates STAT3 activation by autocrine IL-6. Proc
Natl Acad Sci USA. 98:9014–9019. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar
|
|
22
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, et al: Constitutive activation of Stat3 signaling confers
resistance to apoptosis in human U266 myeloma cells. Immunity.
10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Epling-Burnette PK, Lui JH,
Catlette-Falcone R, et al: Inhibition of STAT3 signaling leads to
apoptosis of leukemic large granular lymphocytes and decreased
Mcl-1 expression. J Clin Invest. 107:351–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alas S and Bonavida B: Rituximab
inactivates signal transducer and activation of transcription 3
(STAT3) activity in B-non-Hodgkin's lymphoma through inhibition of
the interleukin 10 autocrine/paracrine loop and results in
down-regulation of Bcl-2 and sensitization to cytotoxic drugs.
Cancer Res. 61:5137–5144. 2001.PubMed/NCBI
|
|
25
|
Gritsko T, Williams A, Turkson J, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diaz N, Minton S, Cox C, et al: Activation
of stat3 in primary tumors from high-risk breast cancer patients is
associated with elevated levels of activated SRC and survivin
expression. Clin Cancer Res. 12:20–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bromberg JF: Activation of STAT proteins
and growth control. Bioessays. 23:161–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Darnell JE Jr: Transcription factors as
targets for cancer therapy. Nat Rev Cancer. 2:740–749. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnell JE: Validating Stat3 in cancer
therapy. Nat Med. 11:595–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turkson J: STAT proteins as novel targets
for cancer drug discovery. Expert Opin Ther Targets. 8:409–422.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim DJ, Chan KS, Sano S and Digiovanni J:
Signal transducer and activator of transcription 3 (Stat3) in
epithelial carcinogenesis. Mol Carcinog. 46:725–731. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xi S, Gooding WE and Grandis JR: In vivo
antitumor efficacy of STAT3 blockade using a transcription factor
decoy approach:implications for cancer therapy. Oncogene.
24:970–979. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niu G, Wright KL, Huang M, et al:
Constitutive Stat3 activity upregulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie TX, Huang FJ, Aldape KD, et al:
Activation of Stat3 in human melanoma promotes brain metastasis.
Cancer Res. 66:3188–3196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin L, Hutzen B, Ball S, et al: New
curcumin analogues exhibit enhanced growth-suppressive activity and
inhibit AKT and signal transducer and activator of transcription 3
phosphorylation in breast and prostate cancer cells. Cancer Sci.
100:1719–1727. 2009. View Article : Google Scholar
|
|
36
|
Dechow TN, Pedranzini L, Leitch A, et al:
Requirement of matrix metalloproteinase-9 for the transformation of
human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci USA.
101:10602–10607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xie TX, Wei D, Liu M, et al: Stat3
activation regulates the expression of matrix metalloproteinase-2
and tumor invasion and metastasis. Oncogene. 23:3550–3560. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Boise LH, Gonzalez-Garcia M, Postema CE,
et al: Bcl-x, a bcl-2-related gene that functions as a dominant
regulator of apoptotic cell death. Cell. 74:597–608. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gonzalez-Garcia M, Perez-Ballestero R,
Ding L, et al: Bcl-xL is the major bcl-x mRNA form expressed during
murine development and its product localizes to mitochondria.
Development. 120:3033–3042. 1994.PubMed/NCBI
|
|
40
|
Grad JM, Zeng XR and Boise LH: Regulation
of Bcl-xL: a little bit to this and a little bit to STAT. Curr Opin
Oncol. 12:543–549. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou P, Qian L, Kozopas KM and Craig RW:
Mcl-1, a bcl-2 family member, delays the death of hematopoietic
cells under a variety of apoptosis-inducing conditions. Blood.
89:630–643. 1997.PubMed/NCBI
|
|
42
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Calo V, Migliavacca M, Bazan V, et al:
STAT proteins: from normal control of cellular events to
tumorigenesis. J Cell Physiol. 197:157–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maofu FU, Wang C, Li Z, Sakamaki T and
Pestell RG: Minireview: cyclin D1: normal and abnormal functions.
Endocrinology. 145:5439–5447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Spencer CA and Groudine M: Control of
c-myc regulation in normal and neoplastic cells. Adv Cancer Res.
56:1–48. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Marcu KB, Bossone SA and Patel AJ: Myc
function and regulation. Annu Rev Biochem. 61:809–860. 1992.
View Article : Google Scholar
|
|
47
|
Facchini LM and Penn LZ: The molecular
role of Myc in growth and transformation: recent discoveries lead
to new insights. FASEB J. 12:633–651. 1998.PubMed/NCBI
|