Introduction
Ovarian cancer is the fourth most common cause of
cancer deaths in women exceeded only by breast, colon and lung
malignancies with the majority of cases being diagnosed after the
disease has become metastatic, and the 5-year survival is about 40%
(1). Although the chemotherapeutic
agents, such as cisplatin, carboplatin and paclitaxel have been
known to be effective against ovarian carcinomas, the efficacy of
which is limited by intrinsic or acquired chemoresistance in
residual cells. Therefore, it is warranted to explore new
therapeutic target for further treatment and reducing recurrence of
the disease.
Minibrain-related kinase (Mirk) is a
serine/threonine kinase which is also known as the dual specificity
tyrosine-phosphorylation-regulated kinase 1B (Dyrk1B). Mirk/Dyrk1B
is one of members of Dyrk family which have the ability to
auto-phosphorylate on tyrosine and then phosphorylate other
substrates on serine and threonine (2); therefore, they are categorized as
dual function kinases. Mirk/Dyrk1B is expressed in few normal
tissues, but in many types of human cancer (3), such as sarcomas (4,5),
pancreatic and colon carcinomas (6), and cervical cancer (7). Our recent study also found
Mirk/Dyrk1B was overexpressed in a wide spectrum of cell lines and
tumor specimens of lung cancer (8). Furthermore, the knockdown Mirk/Dyrk1B
by small interfering RNA (siRNA) induced cell apoptosis and
increased sensitivity of human cancer cells to conventional
chemotherapeutics in vitro (5,6,8). Our
previous results also showed Mirk/Dyrk1B function in an orthotopic
mouse model (8). Moreover, a study
in osteosarcoma demonstrates that the overall survival rate of
patients is negatively correlated with the levels of Mirk/Dyrk1B
protein expression (5). All of the
above suggest that Mirk/Dyrk1B could serve as a novel therapeutic
target and the overexpressed Mirk/Dyrk1B may be a diagnostic marker
and survival factor for various types of human cancer.
The mammalian forkhead subclass O (FoxO) family
members of transcriptional factors, such as FoxO1 (FHKR) and FoxO3a
(FKHR-L1) are characterized by a distinctive forkhead DNA binding
domain which function downstream of PI3K antagonist PTEN in cancer
cells, inhibit cell cycle progression and promote cell death by
modulating the expression of genes encoding apoptosis (9), growth regulatory proteins (10) and stress response (11,12).
The modulating mechanisms include: a) direct binding to the insulin
response sequence (IRS) in gene promoters (e.g., apoptotic proteins
Bim and fas ligand) and b) tethering to the other transcription
factors (cell cycle regulators cyclin G2 and cyclin D1). The
phosphorylation of FoxO factors by protein kinases, such as Akt,
serum and glucocorticoid inducible kinase (SGK) leads to their
translocation from the nucleus to the cytoplasm and loss of
proapoptotic function due to inactivation (13,14).
Whereas, the unphosphorylated active forms of FoxO reside in the
nucleus and induces cell death by up-regulation of apoptotic
proteins, such as Bim, p27, TRADD (15–17)
and repression of antiapoptotic molecule FLIP and Bcl-XL (18,19).
Furthermore, Dyrk1A, the closest family member to Mirk/Dyrk1B, has
been found to phosphorylate FoxO1 at ser329, a novel in vivo
phosphorylation site (20), and
mediates cellular localization of FoxO1 in immortalized cells
(21). More recently, the
serine/threonine kinase Mirk/Dyrk1B has been thought to be a
transcriptional co-activator which increases expression of a cohort
of antioxidants in human cancer cells (22,23).
In addition, both FoxO1 and FoxO3a have been reported to be
involved in cytotoxic stress and drug-resistance induced by
chemotherapeutics in ovarian cancers (24,25).
Taken together, we hypothesize that FoxO factors may be a novel
downstream manner by which Mirk/Dyrk1B serves as an antiapoptotic
factor and contribute to ovarian cancer cell survival.
Although a few studies show that Mirk/Dyrk1B
mediates ovarian cancer cell survival, in particular for quiescent
tumor cells, and depleting Mirk kinase increase cisplatin toxicity
associated with higher level of reactive oxygen species (ROS) in
ovarian cancer cells (23,26), insufficient data regarding the
effect of Mirk/Dyrk1B on human ovarian cancer cells are available,
and the mechanisms involved remain unclear. In this study, we have
identified that Mirk/Dyrk1B is overexpressed in a wide spectrum of
ovarian cancer cell lines and primary tumors in which it is located
in the cytoplasm. Mirk/Dyrk1B-mediated cell survival and
chemosensitivity is correlated with expression and nuclear
translocation of FoxO1 and/or FoxO3A in ovarian cancer.
Materials and methods
Antibodies
The rabbit polyclonal Dyrk1B antibody (C-term,
AP7538b) was purchased from Abgent (San Diego, CA). Anti-Bim,
anti-TRADD, and goat anti-mouse IgG horseradish peroxidase
(HRP)-conjugated secondary antibody were purchased from Santa Cruz
Biotechnology (Santa Cruz, CA). Anti-FKHR/FoxO1, anti-caspase-3,
and anti-poly(ADP-ribose) polymerase (PARP) were purchased from
Cell Signaling Technology (Danvers, MA). Anti-FKHR-L1/FoxO3a was
purchased from Upstate (Lake Placid, NY). Alexa Fluor 594 F(ab′)
fragment of goat anti-mouse IgG were purchased from Invitrogen
(Eugene, OR). Anti-β-actin and donkey anti-rabbit IgG
HRP-conjugated secondary antibody were purchased from Sigma (St.
Louis, MO) and Amersham Biosciences (Piscataway, NJ),
respectively.
Cell lines and cell culture
Human ovarian cancer cell lines used were OV2008,
OVCAR3, OVCAR5, SKOV3, MDAH2774, OVCAR10, OV1063, OVCAR8. The SKOV3
and OVCAR3 were purchased from American Type Culture Collection
(Manassas, VA); others were gifts from Dr Jin Q. Cheng (H. Lee
Moffitt Cancer Center and Research Institute, USA). All lines were
maintained in DMEM supplemented with 10% heat-inactivated (56˚C, 30
min) fetal bovine serum (FBS; Invitrogen, Grand Island, NY).
Monolayer cultures were incubated at 37˚C in a 95% humidified
atmosphere air containing 5% CO2.
Small interfering RNA treatment
Cells were reverse-transfected with small
interfering RNAs (siRNAs) using Lipofectamine 2000 transfection
reagent (Invitrogen) according to the manufacturer's instructions.
The Mirk/Dyrk1B, FoxO1 and FoxO3a siRNA duplexes as well as the
corresponding non-specific control siRNA duplexes as described
(8,27) were supplied by Dharmacon and
Ambion, respectively. After a 72-h incubation or at indicated time
points, cells were harvested or trypsinized and replated for
subsequent experiments.
Flow cytometry analysis
After 72-h treatment with siRNAs, cells were
subjected to flow cytometry analyses of apoptosis. Apoptosis was
assayed using Pharmingen (San Diego, CA) PE-conjugated monoclonal
active caspase-3 antibody apoptosis kit without modification as
described previously (8). A total
of 10,000 cells per experimental condition were used for processing
and analysis of fluorescence on Becton-Dickinson FACScan (BD,
Franklin Lakes, NJ) using CellQuest software. Apoptosis of
siRNA-transfected cells after 48-h exposure to the chemotherapeutic
agent cisplatin (CDDP) was also detected by flow cytometry
analysis.
Western blot analysis
Cells were washed twice with cold PBS and lysed with
buffer A [10 mM Tris-HCl (pH 7.4), 1% Triton X-100, 0.1% SDS, 150
mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 0.5 mM
phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 5 μg/ml
aprotinin]. After incubation for 30 min on ice, the suspensions
were centrifuged (15,000 g for 30 min). The supernatants were
removed and stored at −80˚C until analysis using gel
electrophoresis. The protein concentration was determined by
Bio-Rad protein estimation assay according to the manufacturer's
instructions. For Western blot analysis, ~60–100 μg of whole cell
proteins were separated using 10% or 12% SDS-PAGE and transferred
to nitrocellulose membranes. After blocking of the membranes with
10 mM Tris-HCl (pH 7.4), 150 mM NaCl, and 0.1% Tween-20 containing
5% non-fat dry milk at room temperature for 60 min, the membranes
were incubated with indicated antibodies at 4˚C overnight and then
with the HRP-conjugated secondary anti-rabbit or anti-mouse
antibodies at room temperature for 60 min. Each protein was
detected using the enhanced chemiluminescence (Amersham
Biosciences) system. β-actin was used as an internal control.
Patients and tumor specimens
The primary human ovarian cancer specimens were
obtained from 51 patients who underwent surgery without
chemotherapy or radiation prior to resection at the First
Affiliated Hospital of Dalian Medical University and Zhongshan
Hospital Xiamen University between 1996 and 2010. Each sample
contained at least 80% tumor cells, confirmed by microscopic
examination. As control groups, the specimens obtained from 16
patients with ovarian benign tumor and 9 cases of non-neoplastic
cyst were also examined. The clinicopathological aspects of all
samples were listed in Table I.
The study was approved by the Research Committee of the First
Affiliated Hospital of Dalian Medical University and Zhongshan
Hospital Xiamen University.
 | Table IClinicopathological aspects and Mirk
expression in patients. |
Table I
Clinicopathological aspects and Mirk
expression in patients.
| Characteristic | No. of patients |
|---|
| Total | 76 |
| Age (years)a | 47 |
| Histology (positive
for Mirk)b |
|
Cystadenocarcinomas | 51 (38) |
| Serous | 38 (31) |
| Mucinous | 13 (7) |
| Cystadenomas | 16 (6)c |
| Serous | 9 (3) |
| Mucinous | 7 (3) |
| Non-neoplastic
cysts | 9 (0)d |
Immunostaining analysis of Mirk/Dyrk1B
and FoxO
Immunohistochemistry staining using anti-Dyrk1B as
the primary antibody. After antigen retrieval with citrate, the
endogenous peroxidase activity was blocked by incubation with 0.3%
hydrogen peroxide. Slides were incubated overnight with 1:50
primary antibody at 4˚C. Antigen-antibody complexes were detected
by the avidin-biotin peroxidase method using ABC Kit (Vector
Laboratories, Inc., Burlingame, CA) and DAB (Dako, Japan) reagents.
Sections were counterstained with hematoxylin and viewed using a
microscope (Zeiss, Tokyo, Japan). For immunofluorescent staining,
cells were fixed with 4% paraformaldehyde for 20 min on ice. Cells
were incubated in 1% bovine serum albumin (BSA) in PBS for 30 min.
Primary antibody against FoxO1 or FoxO3a (1:100) was added in 1%
BSA/PBS for overnight at 4˚C. After washing, cells were incubated
with Alexa Fluor 594 F(ab′) fragment of goat anti-mouse IgG for 30
min at room temperature, and nuclei were then counterstained with
DAPI allowing visualization of nuclei with a Leica Confocal
Microscope System.
Statistical analysis
Each experiment was repeated three times. Data are
presented as mean ± SD. StatView 5.0 software was used for
statistical analyses. Statistical comparison between control and
experimental groups were performed using χ2 test (for
incidence only) and Student's t-test. The correlations between Mirk
expression and FoxO were analyzed by simple regression. Differences
were considered to be statistically significant when P<0.05.
Results
Mirk is widely overexpressed in ovarian
cancer cells and correlates with FoxO expression
In this study, we first evaluated protein expression
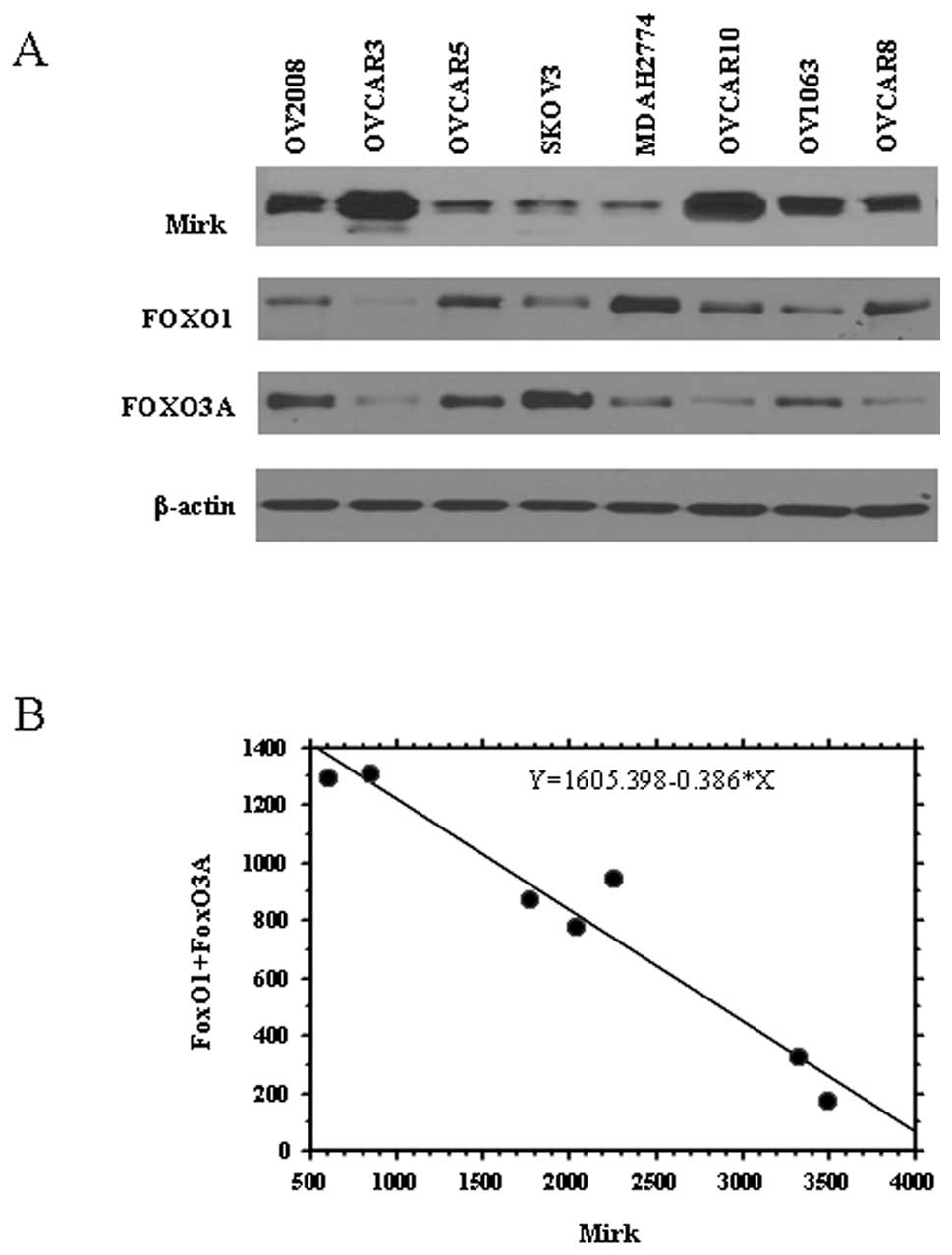
of Mirk in 8 human ovarian cancer cell lines. The 8 cell lines all
expressed Mirk protein, 5 of them with high levels (Fig. 1A), which is consistent with the
findings reported by Hu et al (26). Based on the hypothesis described
above that the FoxO transcriptional factors may be involved in Mirk
function in ovarian cancer, we further examined the expression of
both FoxO1 and FoxO3A in the 8 cell lines (Fig. 1A). As shown in Fig. 1B, correlation appears to be
negative between the expression of Mirk protein and the total level
of FoxO (FoxO1+FoxO3A) in ovarian cancer cells (R2=0.946
and P<0.001), suggesting FoxO1 and/or FoxO3A may be associated
with Mirk function or kinase activity.
Knockdown of Mirk induces apoptosis
involving the downstream signals of FoxO and results in
chemosensitivity in vitro
We have reported the concentration- and
target-dependent effects on Mirk protein and apoptosis occurred in
lung cancer cells induced by Mirk siRNA (~5–20 nM) and the
corresponding individual siRNAs #1-#4 of Mirk (8). In this study, we examined the
consequence of Mirk knockdown using 20 nM siRNA duplexes #4
targeting Mirk. We exposed 8 ovarian cancer cell lines to Mirk
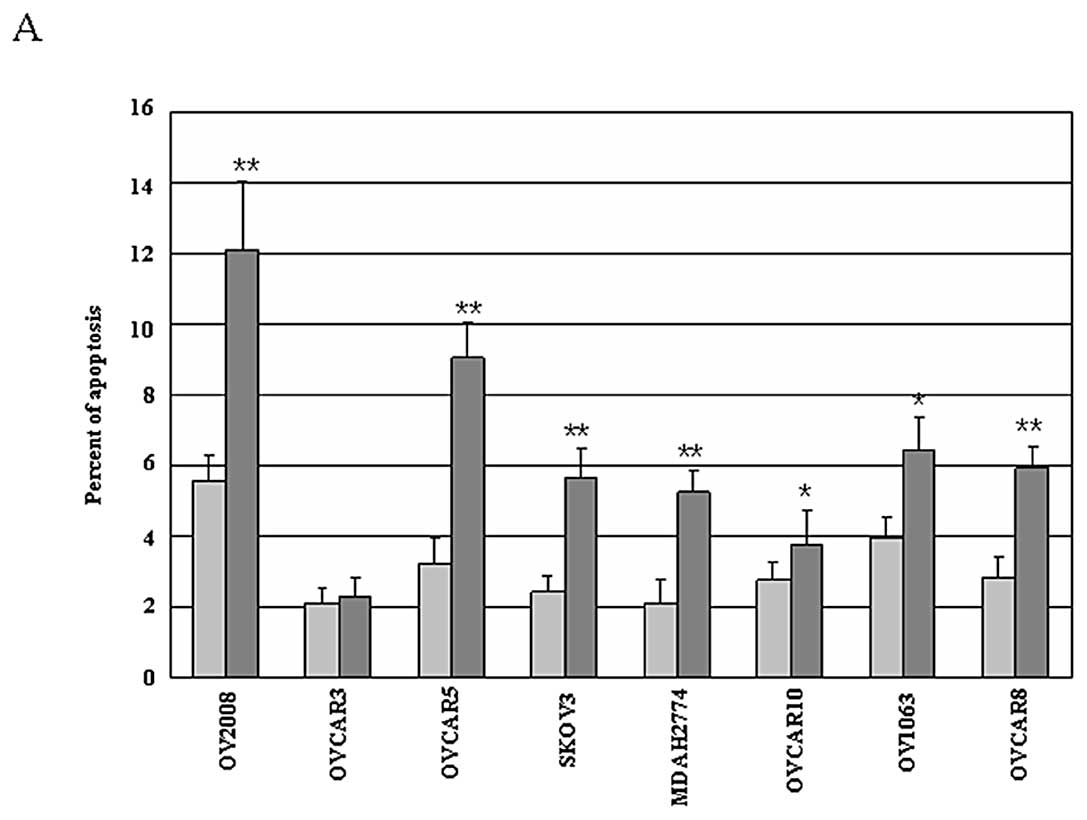
siRNA for 72 h followed by assessment of cellular apoptosis. As
shown in Fig. 2A, Mirk knockdown
by siRNA resulted in cellular apoptosis (~1.05- to 2.81-fold of
control), as evidenced by more Mirk siRNA-treated cells staining
with cleaved caspase-3. Intriguingly, the Mirk knockdown-induced
apoptosis in variant cell lines appears to be positively correlated
with FoxO (FoxO1+FoxO3A) expression. To investigate the mechanisms
involved in apoptosis induced by Mirk siRNA, the downstream signals
of FoxO factors were determined in a representative panel of three
ovarian cancer cell lines by Western blot analysis. As shown in
Fig. 2B, exposure of these cell
lines to Mirk siRNA for 72 h was associated with knockdown of Mirk,
cleavage of caspase-3 and PARP, compared with that shown with
control siRNA, and resulted in upregulation of pro-apoptotic Bim
and TRADD in all three cell lines, suggesting knockdown
Mirk-induced cellular apoptosis may be associated with FoxO factors
as well as their downstream signals. We next investigated the
effects of constitutively expressed Mirk on sensitivity of ovarian
cancer cells to conventional chemotherapeutics, Mirk siRNA-treated
OV2008, OVCAR5, and OVCAR8 cells were exposed to indicated dose of
cisplatin for apoptosis assays. Mirk siRNA treatment and exposure
to cisplatin in these cells resulted in increased apoptosis
(measured in fold) compared with cells treated with control siRNA
by caspase-3 assay (Fig. 2C),
indicating that knockdown of Mirk sensitizes ovarian cancer cells
to chemotherapy-induced apoptosis.
Mirk modulates cell survival associated
with nuclear translocation of FoxO
As described above, the phosphorylation of FoxO
factors leads to their translocation from the nucleus to the
cytoplasm and loss proapoptotic function due to inactivation.
Whereas, the unphosphorylated active forms of FoxO reside in the
nucleus and induces cellular apoptosis. Previous studies also
demonstrate Dyrk1A, the closest isoform of Mirk may phosphorylate
FoxO1 and promote nuclear output (20,21),
thus there is a possibility that it is the subcellular localization
and phosphorylation of FoxO factors but not the total protein level
that is altered in Mirk/Dyrk1B siRNA-treated ovarian cancer cells.
To explore this hypothesis, both FoxO1 and FoxO3A were detected by
immunofluorescent staining in the cell lines OV2008, OVCAR5 or
OVCAR8 treated with/without Mirk siRNA. Interestingly, both FoxO1
and FoxO3A are expressed in cytoplasm of these lines (Fig. 3A). Knockdown Mirk induced nuclear
translocation of both FoxO1 and FoxO3A in OVCAR5 (Fig. 3B), of FoxO1 alone (not FoxO3A) in
OVCAR8 (Fig. 3C), and of FoxO3A
alone (not FoxO1) in OV2008 (Fig.
3D). Taken together, these results suggest that FoxO1 and/or
FoxO3A may be a novel downstream way in which Mirk serves as an
antiapoptotic factor in ovarian cancer cells.
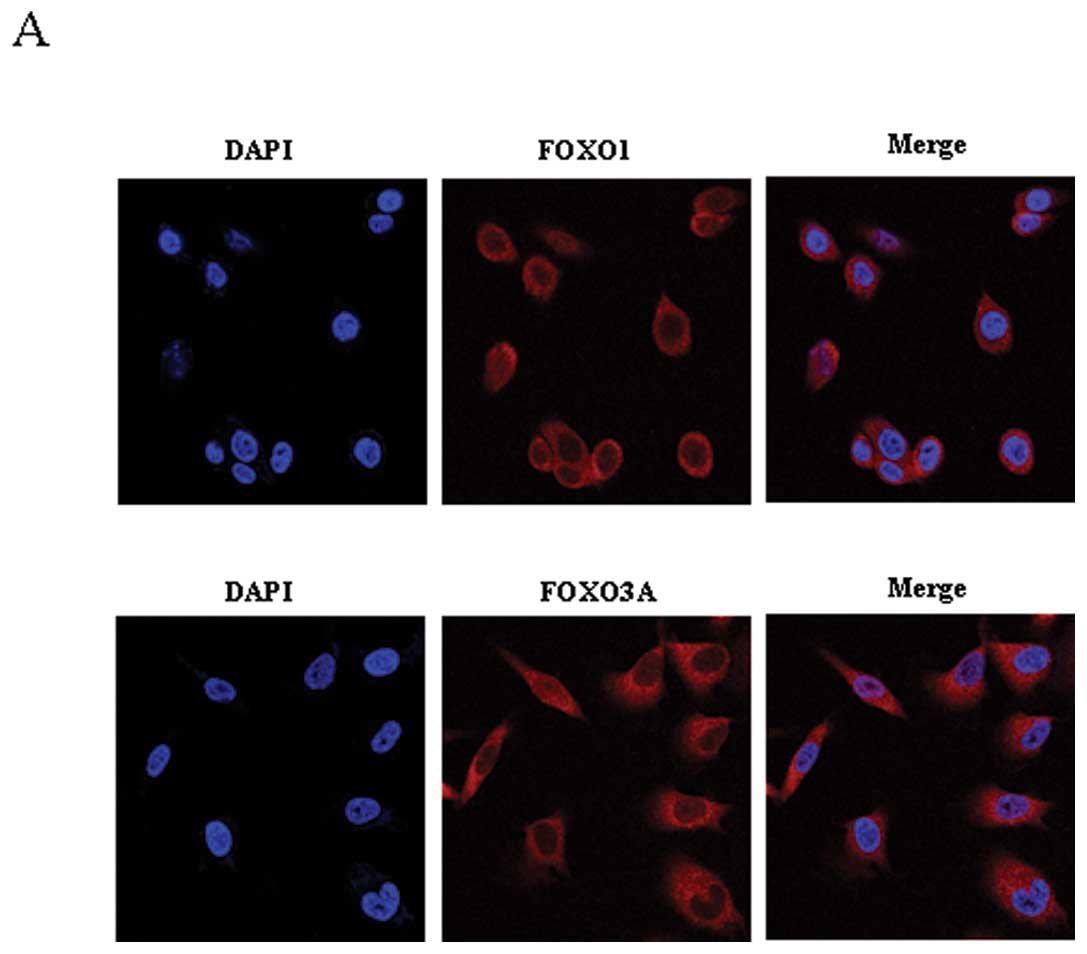 | Figure 3Confocal microscopy of FoxO1 or FoxO3A
expression in Mirk-modulated cell survival in ovarian cancer cells.
(A), Both panels show the nuclei labeled with
4′,6-diamidine-2-phenylindole (DAPI, blue), FoxO1 or Foxo3a was
cytoplasmic visualized with the use of Alexa 594 (red), and the
merge of DAPI and FoxO1 or FoxO3A, respectively. (B), Upper panels
show DAPI, FoxO1, and the merge of DAPI and FoxO1 with/without Mirk
knockdown in OVCAR5 cells. Bottom panels show DAPI, FoxO3A, and the
merge of DAPI and FoxO3A with/without Mirk knockdown in OVCAR5
cells. (C), Both panels show DAPI, FoxO1, and the merge of DAPI and
FoxO1 with/without Mirk knockdown in OVCAR8 cells. (D), Both panels
show DAPI, FoxO1, and the merge of DAPI and FoxO3A with/without
Mirk knockdown in OV2008 cells. |
Knockdown FoxO results in less Mirk
siRNA-induced apoptosis and decreased sensitivity to chemotherapy
in ovarian cancer cells
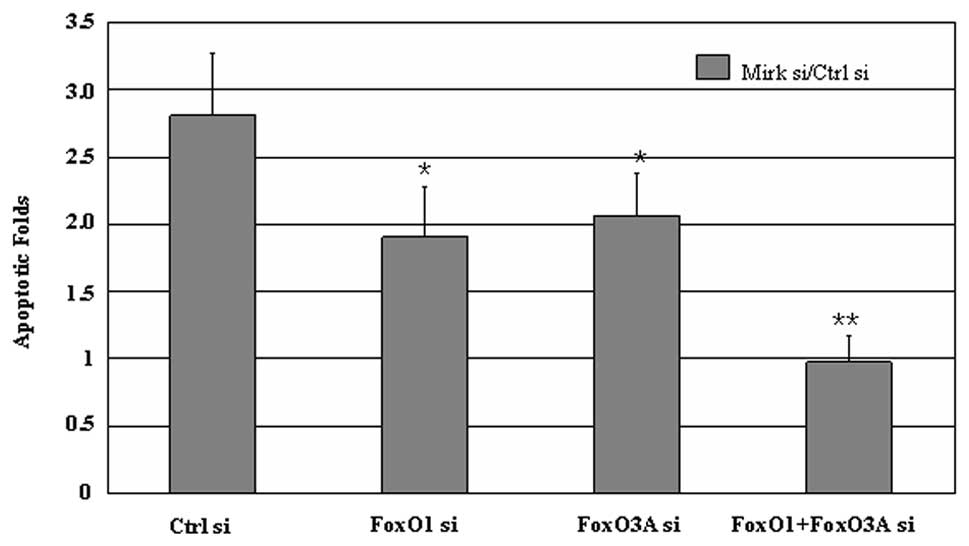
To further determine the effects of FoxO factors on
Mirk-modulated ovarian cancer cell survival, FoxO siRNAs were
exposed to the cells of OV2008, OVCAR5 or OVCAR8 treated
with/without Mirk siRNA for 72 h, then the apoptotic cells
evidenced by cleaved caspase-3 were detected by flow cytometry
analysis. Combined siRNAs of Mirk with FoxO1 and/or FoxO3A led to
less cellular apoptosis than Mirk siRNA alone in all three lines
(Fig. 4), and less sensitivity to
chemotherapeutics (data not shown). These results suggest FoxO1
and/or FoxO3A are involved in Mirk-mediated cell survival in
ovarian cancer cells.
Mirk is overexpressed in tumor specimens
from clinical ovarian cancer cases
Mirk is expressed at low level in most adult
tissues. We examined expression patterns of Mirk in ovarian cancer.
As listed in Table I, in this
study we not only examined Mirk expression in ovarian cancer
specimens included 38 serous and 13 mucinous but also 16 benign
cystadenomas and 9 non-neoplastic ovarian cysts by
immunohistochemisty. Mirk was detected in 74.5% of ovarian cancers
and overexpressed in 41% of the specimens (data not shown), and the
incidence was higher than that found in both cystadenomas and
non-neoplastic cysts (P<0.001 and P<0.05, respectively). We
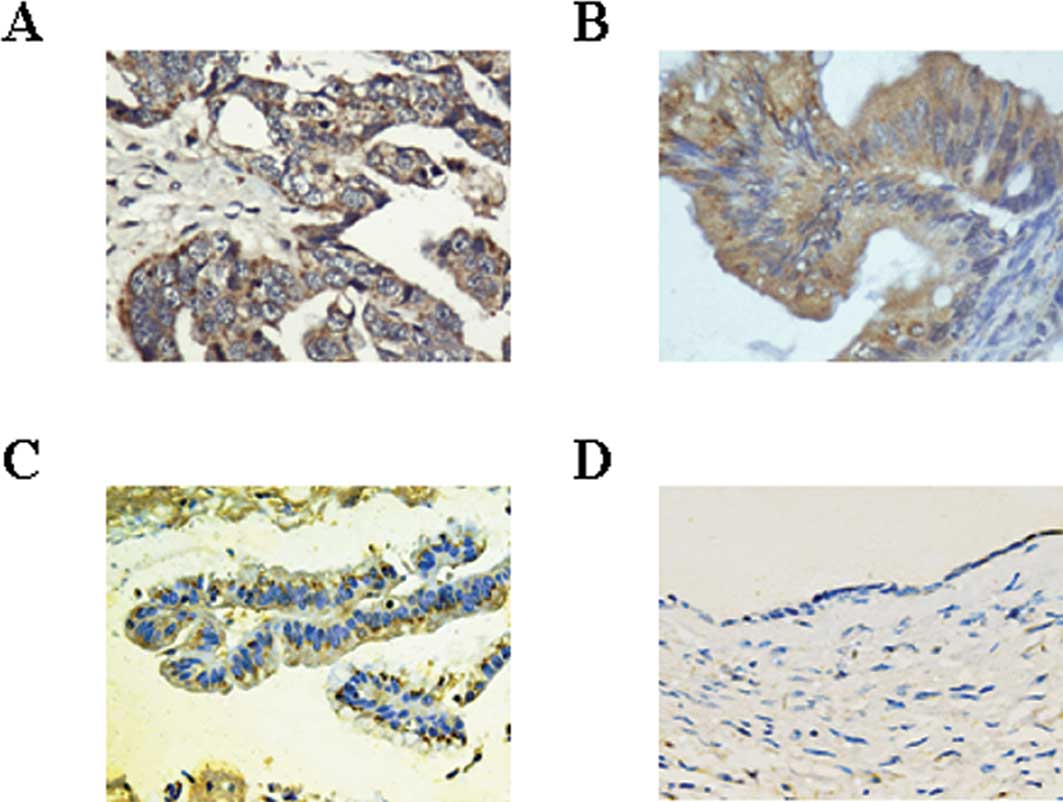
further found the overexpressed Mirk was located in the cytoplasm
of ovarian cancer specimens (Fig. 5A
and B) similarly to the findings in other organ cancers
(4,22). Compared with Mirk expression in
most of ovarian cancer specimens, it was weakly expressed only in
about 37% ovarian cystadenomas (Fig.
5C) and not expressed in the ovarian non-neoplastic cysts
(Fig. 5D), indicating Mirk may be
associated with ovarian tumorigenesis.
Discussion
Our study demonstrates that Mirk/Dyrk1B is
overexpressed in a variety of ovarian cancer cells and clinical
specimens. Knockdown of Mirk/Dyrk1B induced apoptosis of ovarian
cancer cells in vitro and sensitized ovarian cancer cells to
chemotherapeutics. These results are consistent with previous
studies (3–6,8) on
other types of human cancers, indicating that Mirk may be a novel
therapeutic target for ovarian cancer treatment. Furthermore, we
found Mirk to be expressed at higher levels in both serous and
mucinous ovarian cancers than that in cystadenomas and ovarian
non-neoplastic cysts. Therefore, our study along with others
(23,28) suggests that Mirk may also play a
role in ovarian tumorigenesis.
To date, the downstream signals of Mirk remain
unclear. Given the main function of Mirk in ovarian cancer cells in
mediating cell survival observed in this study, the mechanisms
involved may include FoxO family members, such as FoxO1 and/or
FoxO3A as well as their downstream signals, which are
constitutively expressed in ovarian cancer cells (Fig. 1A). To the best of our knowledge,
our study is the first to show an effect of FoxO involved in
Mirk-mediated cancer cell survival. It has been reported that
Dyrk1A, the closest family member to Dyrk1B/Mirk, may phosphorylate
FoxO1 at ser329, a novel in vivo phosphorylation site
(25), and promote the
phosphorylated FoxO1 nuclear output (20,21).
The knockdown of endogenous Dyrk1A by siRNA mediates cellular
localization of FoxO1 in immortalized cells (24). Thus, we hypothesize that FoxO
factors may contain a Mirk phosphorylation site, on which further
study is needed. In our study, we also found that FoxO1 and/or
FoxO3A nuclear translocation is concomitant with cell apoptosis
induced by Mirk knockdown, which together with previous studies
(24,25) suggest that it might be the
subcellular localization and phosphorylation of FoxO that is
altered in Mirk siRNA-treated ovarian cancer cells.
Taken together, Mirk/Dyrk1B is overexpressed in a
wide spectrum of ovarian cancer cell lines and human specimens. The
Mirk/Dyrk1B-mediated cell survival in ovarian cancer cells is
associated with FoxO subcellular localization. Therefore,
Mirk/Dyrk1B may be a novel target for treatment of ovarian
cancer.
Acknowledgments
We thank Dr Yuyan Zhu (University of South Florida
Medical College, Tampa, FL, USA), for helpful discussions as well
as English editing. This work was supported in part by the National
Natural Science Foundation of China (81172457 to J.G.) and Natural
Science Foundation of Fujian Province, P.R. China (2010J01236 to
X.Y.). The authors declare no conflicts of interest.
References
|
1
|
Ozols RF, Bookman MA, Connolly DC, et al:
Focus on epithelial ovarian cancer. Cancer Cell. 5:19–24. 2004.
|
|
2
|
Becker W and Joost HG: Structural and
functional characteristics of Dyrk, a novel subfamily of protein
kinases with dual specificity. Prog Nucleic Acid Res Mol Biol.
62:1–17. 1999.
|
|
3
|
Lee K, Deng X and Friedman E: Mirk protein
kinase is a mitogen-activated protein kinase substrate that
mediates survival of colon cancer cells. Cancer Res. 60:3631–3637.
2000.
|
|
4
|
Mercer SE, Ewton DZ, Shah S, Naqvi A and
Friedman E: Mirk/Dyrk1b mediates cell survival in
rhabdomyosarcomas. Cancer Res. 66:5143–5150. 2006.
|
|
5
|
Yang C, Ji D, Weinstein EJ, et al: The
kinase Mirk is a potential therapeutic target in osteosarcoma.
Carcinogenesis. 31:552–558. 2010.
|
|
6
|
Deng X, Ewton DZ, Li S, Naqvi A, Mercer
SE, Landas S and Friedman E: The kinase Mirk/Dyrk1B mediates cell
survival in pancreatic ductal adenocarcinoma. Cancer Res.
66:4149–4158. 2006.
|
|
7
|
MacKeigan JP, Murphy LO and Blenis J:
Sensitized RNAi screen of human kinases and phosphatases identifies
new regulators of apoptosis and chemoresistance. Nat Cell Biol.
7:591–600. 2005.
|
|
8
|
Gao J, Zheng Z, Rawal B, Schell MJ, Bepler
G and Haura EB: Mirk/Dyrk1B, a novel therapeutic target, mediates
cell survival in non-small cell lung cancer cells. Cancer Biol
Ther. 8:1671–1679. 2009.
|
|
9
|
Kops GJ, De Ruiter ND, De Vries-Smits AM,
Powell DR, Bos JL and Burgering BM: Direct control of the Forkhead
transcription factor AFX by protein kinase B. Nature. 398:630–634.
1999.
|
|
10
|
Nemoto S, Fergusson MM and Finkel T:
Nutrient availability regulates SIRT1 through a forkhead-dependent
pathway. Science. 306:2105–2108. 2004.
|
|
11
|
Fu W, Ma Q, Chen L, et al: MDM2 acts
downstream of p53 as an E3 ligase to promote FOXO ubiquitination
and degradation. J Biol Chem. 284:13987–14000. 2009.
|
|
12
|
Yang Y, Hou H, Haller EM, Nicosia SV and
Bai W: Suppression of FOXO1 activity by FHL2 through SIRT1-mediated
deacetylation. EMBO J. 24:1021–1032. 2005.
|
|
13
|
Brunet A, Bonni A, Zigmond MJ, et al: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999.
|
|
14
|
Nakae J, Barr V and Accili D: Differential
regulation of gene expression by insulin and IGF-1 receptors
correlates with phosphorylation of a single amino acid residue in
the forkhead transcription factor FKHR. EMBO J. 19:989–996.
2000.
|
|
15
|
Dijkers PF, Medema RH, Lammers JW,
Koenderman L and Coffer PJ: Expression of the pro-apoptotic Bcl-2
family member Bim is regulated by the forkhead transcription factor
FKHR-L1. Curr Biol. 10:1201–1204. 2000.
|
|
16
|
Dijkers PF, Medema RH, Pals C, et al:
Forkhead transcription factor FKHR-L1 modulates cytokine-dependent
transcriptional regulation of p27(KIP1). Mol Cell Biol.
20:9138–9148. 2000.
|
|
17
|
Deng R, Tang J, Xie BF, Feng GK, Huang YH,
Liu ZC and Zhu XF: SYUNZ-16, a newly synthesized alkannin
derivative, induces tumor cells apoptosis and suppresses tumor
growth through inhibition of PKB/AKT kinase activity and blockade
of AKT/FOXO signal pathway. Int J Cancer. 127:220–229. 2010.
|
|
18
|
Skurk C, Maatz H, Kim HS, Yang J, Abid MR,
Aird WC and Walsh K: The Akt-regulated forkhead transcription
factor FOXO3a controls endothelial cell viability through
modulation of the caspase-8 inhibitor FLIP. J Biol Chem.
279:1513–1525. 2004.
|
|
19
|
Tang TT, Dowbenko D, Jackson A, Toney L,
Lewin DA, Dent AL and Lasky LA: The forkhead transcription factor
AFX activates apoptosis by induction of the BCL-6 transcriptional
repressor. J Biol Chem. 277:14255–14265. 2002.
|
|
20
|
Arimoto-Ishida E, Ohmichi M, Mabuchi S, et
al: Inhibition of phosphorylation of a forkhead transcription
factor sensitizes human ovarian cancer cells to cisplatin.
Endocrinology. 145:2014–2022. 2004.
|
|
21
|
Goto T, Takano M, Hirata J and Tsuda H:
The involvement of FOXO1 in cytotoxic stress and drug-resistance
induced by paclitaxel in ovarian cancers. Br J Cancer.
98:1068–1075. 2008.
|
|
22
|
Deng X, Ewton DZ and Friedman E:
Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer
cells by reducing levels of reactive oxygen species. Cancer Res.
69:3317–3324. 2009.
|
|
23
|
Hu J and Friedman E: Depleting Mirk kinase
increases cisplatin toxicity in ovarian cancer cells. Genes Cancer.
1:803–811. 2010.
|
|
24
|
Chang HS, Lin CH, Yang CH, et al:
Increased expression of Dyrk1a in HPV16 immortalized keratinocytes
enable evasion of apoptosis. Int J Cancer. 120:2377–2385. 2007.
|
|
25
|
Von Groote-Bidlingmaier F, Schmoll D, Orth
HM, Joost HG, Becker W and Barthel A: DYRK1 is a co-activator of
FKHR (FOXO1a)-dependent glucose-6-phosphatase gene expression.
Biochem Biophys Res Commun. 300:764–769. 2003.
|
|
26
|
Hu J, Nakhla H and Friedman E: Transient
arrest in a quiescent state allows ovarian cancer cells to survive
suboptimal growth conditions and is mediated by both Mirk/dyrk1b
and p130/Rb2. Int J Cancer. 129:307–318. 2011.
|
|
27
|
Chakrabarty A, Sanchez V, Kuba MG,
Rinehart C and Arteaga CL: Breast cancer special feature: feedback
upregulation of HER3 (ErbB3) expression and activity attenuates
antitumor effect of PI3K inhibitors. Proc Natl Acad Sci USA.
22:5021–5026. 2011.
|
|
28
|
Thompson FH, Nelson MA, Trent JM, et al:
Amplification of 19q13.1–q13.2 sequences in ovarian cancer. G-band,
FISH, and molecular studies. Cancer Genet Cytogenet. 87:55–62.
1996.
|