Introduction
Recombinant human erythropoietin (rhEPO) has long
been used to treat anemia in many clinical settings, such as kidney
failure, bone marrow disease, chemotherapy and radiotherapy
(1). However, recently the
non-hematopoietic biological effects of erythropoietin have been
reported because of ubiquitous EPOR expression in non-erythroid
cells (2–6). Among these, deleterious effects of
therapeutically administered rhEPO on solid tumors have received a
great deal of attention.
In 2003, solid tumor patients treated with rhEPO in
two large clinical trials displayed increased mortality (2,3).
Since then, many clinical studies have reported increased tumor
progression, tumor growth and mortality in rhEPO treated solid
tumor patients (3,6–11).
Furthermore, numerous basic experimental studies also confirmed
these deleterious effects in many types of tumors, including renal,
breast, lung, prostate, ovarian, head and neck squamous cell
carcinomas (12–17). So far, whether rhEPO administration
demonstrates similar adverse effect on pituitary adenoma has not
been deciphered. Since many patients bearing pituitary adenomas
receive conservative treatment and anemia is common in these
patients, especially those with macroprolactinomas (17–19),
unveiling the potential risk of rhEPO on pituitary adenomas has
high clinical value in guiding clinicians who care for anemia
patients with pituitary adenomas.
In the present study, we first characterized EPOR
expression in different hormone secreting types of human pituitary
adenomas and found no EPOR protein expression in pituitary
adenomas. Next, we investigated for the first time the effect of
rhEPO administration on pituitary adenomas using a nude mouse
xenograft model of rat MMQ prolactin-secreting pituitary adenoma
cells. Furthermore, we also explored the underlying mechanism in
human umbilical vein endothelial cells (HUVECs) in vitro and
in chicken chorioallantoic membrane (CAM) angiogenesis model in
vivo. Our results indicate that, at least in our cases,
pituitary adenomas are EPOR-negative tumors and rhEPO
administration accelerates the growth of pituitary adenomas by
promoting tumor angiogenesis via the EPO-JAK2-STAT3-VEGF signaling
pathway.
Patients and methods
Patients and samples
A series of 31 pituitary adenoma samples were
obtained with pathological diagnosis and informed consent. All
patients (12 men, 19 women; age range 16–63, mean age 43.50±11.00)
years received surgery between April 2009 and April 2011 at the
Department of Neurosurgery, the First Affiliated Hospital of Henan
University of Science and Technology, Luoyang, China. Of these
patients, 19 had prolactinomas, four had non-functional adenomas,
two had multihormonal adenomas, four had gonadotropinomas and two
had GH-secreting adenomas. For each sample, one half was
immediately frozen at -80°C until protein extraction, the other
half was fixed with 10% formalin and embedded in paraffin for
histology and immunohistochemical analysis. The study was approved
by the Research Ethics Committee of Henan University of Science and
Technology. Samples were made anonymous according to ethical
standards.
Cell line and nude mice
The MMQ rat prolactin-secreting tumor cell line was
used in this study, because prolactinoma is the most prevalent
hormone-secreting type of pituitary adenomas and there is no mature
human pituitary adenoma cell line to date (20). The MMQ rat prolactin-secreting
tumor cell line was from ATCC and maintained in F12 culture medium
supplemented with 5% fetal bovine serum (FBS), 10% horse serum,
penicillin (100 μg/ml) and streptomycin (100 μg/ml) in a humidified
incubator (37°C, 5% carbon dioxide). Human umbilical vein
endothelial cells (HUVECs) were from cell culture center of Wuhan
University and cultured in M200 culture medium supplemented with
20% fetal bovine serum (FBS), L-gultamine (2 mM) and heparin (50
μg/ml). All animal experiments were conducted in accordance with
NIH guidelines and with the approval of the local animal use
committee. Six-week-old nude mice were inoculated subcutaneously on
the hind flank with MMQ cells (1×106). After two weeks,
xenograft tumors formed. Mice were randomized into three groups
with four mice in each group. For each group, mice were injected
with PBS, rhEPO (2000 U/kg, s.c., Kirin), or rhEPO plus bevacizumab
(a VEGF inhibitor, 10 mg/kg, i.p., Roche) twice a week for two
weeks, respectively. Tumor size was assessed by caliper
measurements twice a week. Mice were sacrificed four weeks after
tumor cell inoculation and tumors were excised for further
analysis.
Cell viability assay
MMQ cells were seeded on 96-well plates (2000
cells/well), and then cells were treated with various
concentrations (0, 1, 5 U/ml) of rhEPO for 24, 72 and 120 h in
CO2 incubator. HUVECs were seeded on 96-well plates
(5000 cells/well), after attachment, cells were left untreated or
treated with rhEPO (5 U/ml), rhEPO (5 U/ml) plus JAK2 inhibitor
AG490 (20 μM/l) for 48 h. After treatment duration, the CCK-8 assay
reagent was added to each well of the plate and incubated for
another 1 h. Absorbance was read at 450 nm using a 96-well plate
reader.
Immunohistochemisty staining
For immunohistochemisty, tissue sections were
deparaffinized and rehydrated. Antigen retrieval was accomplished
with citrate buffer (pH 6.0). Endogenous peroxidase was blocked
with 3% hydrogen peroxide. Subsequently, slides were blocked with
10% normal horse serum followed by the primary antibodies incubated
overnight at 4°C. Mouse anti-EPOR (M20, Santa Cruz, CA) antibody,
mouse anti-PCNA (Santa Cruz) and mouse anti-CD31 antibody
(Pharmingen, CA) were used. Then, slides were washed and incubated
with the biotinylated secondary antibody for 30 min, followed by
ABC reagent (Vector Labs) and diaminobenzidine. Slides were
counterstained with hematoxylin and dehydrated by sequential
ethanol and xylene. Slides were mounted and analyzed. For
microvascular density (MVD) determination, two areas of most
intense neovascularization were chosen at low magnification ×100.
Three random visual fields (x400) in each area of high
vascularisation were recorded. The final microvessel density (MVD)
was the mean value of six random visual fields of the two high
vascularisation areas.
Western blot analysis
Samples (30 μg of proteins) were electrophoresed on
a 10–12% SDS-PAGE gel under reducing conditions and electroblotted
onto nitrocellulose membrane. The membrane was blocked in 5%
non-fat milk and incubated with primary antibodies overnight at
4°C. Primary antibodies used were anti-JAK2, anti-phospho-JAK2,
anti-STAT3, anti-phospho-STAT3 (Millipore, MA), anti-ERK2,
anti-phospho-ERK2, anti-AKT1, anti-phospho-AKT1 (Santa Cruz, CA),
anti-VEGF (Pharmingen, CA). After washing, blots were incubated in
secondary antibody (1:5000) for 1 h. ECL substrate was used for
signal detection and GAPDH for internal control. The intensity of
bands was quantified by densitometric analysis. Results were
expressed as the ratio of intensity to that of internal
control.
Semiquantitative RT-PCR
Total RNA was isolated from cultured cells using
TRIzol reagent (Invitrogen) according to the manufacturer’s
protocol, complementary DNA was synthesized using reverse
transcription reagent kit (Applied Biosystems). RT-PCR analysis was
performed using the following primers: human JAK2 forward
5′-TTATGGACAACAGTCAAACAACAATTC-3′ and reverse
5′-CTTACTCTCGTCTCCACAAAA-3′. Human STAT3 forward
5′-CAAAACCCTCAAGAGCCAAGG-3′ and reverse
5′-TCACTCACAATGCTTCTCCGC-3′. Human VEGF forward
5′-CGAAGTGGTGAAGTTCATGGATG-3′ and reverse
5′-TTCTGTATCAGTCTTTCCTGGTGAG-3′. Human GAPDH forward
5′-GCTTTTAACTCTGGTAAAGTGG-3′ and reverse
5′-TCACGCCACAGTTTCCCGGAGG-3′. The PCR reactions were initiated with
denaturation at 95°C for 5 min; followed by 30 amplification cycles
at 95°C (30 sec), 58°C (30 sec) and 72°C (30 sec), then finally
72°C (10 min) and 4°C. The relative amount of gene was normalized
against GAPDH mRNA.
Chicken chorioallantoic membrane (CAM)
angiogenesis model
Chicken eggs were kept in a 37°C, 60% humidity
incubator for 9 days. The CAM was dropped and the window sealed
with stretchy tape. Fibrous membranes diluted with rhEPO (5 U/ml),
rhEPO plus AG490 (20 μM/l) or PBS were placed on the avascular area
of the Chicken chorioallantoic membrane. After 3 days, the chicken
chorioallantoic membrane was fixed in 4% paraformaldehyde in PBS,
dissected and photographed using stereomicroscope equipped with a
Digital camera for further angiogenesis analysis.
Statistical analysis
Statistical analysis was conducted with the aid of
SPSS 16.0 software. Data are expressed as mean ± SEM. Data obtained
from two groups were analyzed by Student’s t-test. P-values
<0.05 were considered significant.
Results
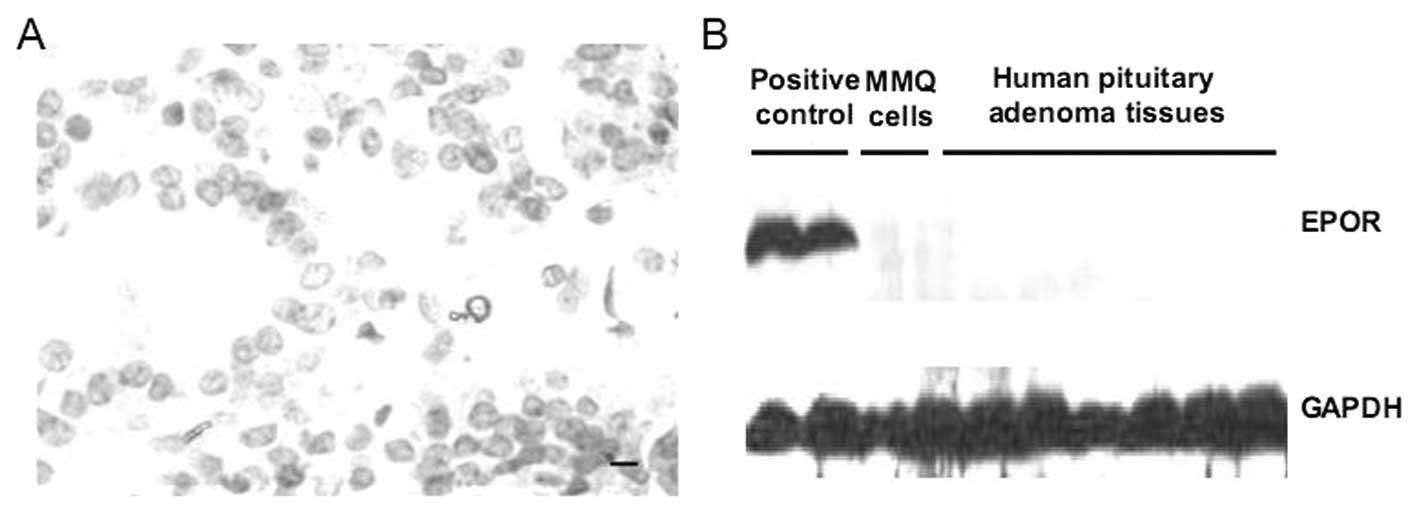
No presence of EPOR expression in human
pituitary adenoma tissue and MMQ pituitary adenoma cells
Since the EPO-EPOR signaling has never been
investigated in human pituitary adenomas, we first assessed the
EPOR expression in 31 human pituitary adenoma samples using
immunohistochemistry and Western blotting. HepG2 human hepatoma
cells and glioma tissue, known EPOR-positive cells and tissue, were
used as positive control. In this study, we found no EPOR protein
expression in pituitary adenoma samples (Fig. 1). Moreover, similar results were
also found in MMQ pituitary adenoma cells (Fig. 1). These data demonstrate that, at
least in our cases, pituitary adenomas are EPOR-negative tumors and
MMQ cells are EPOR-negative cells.
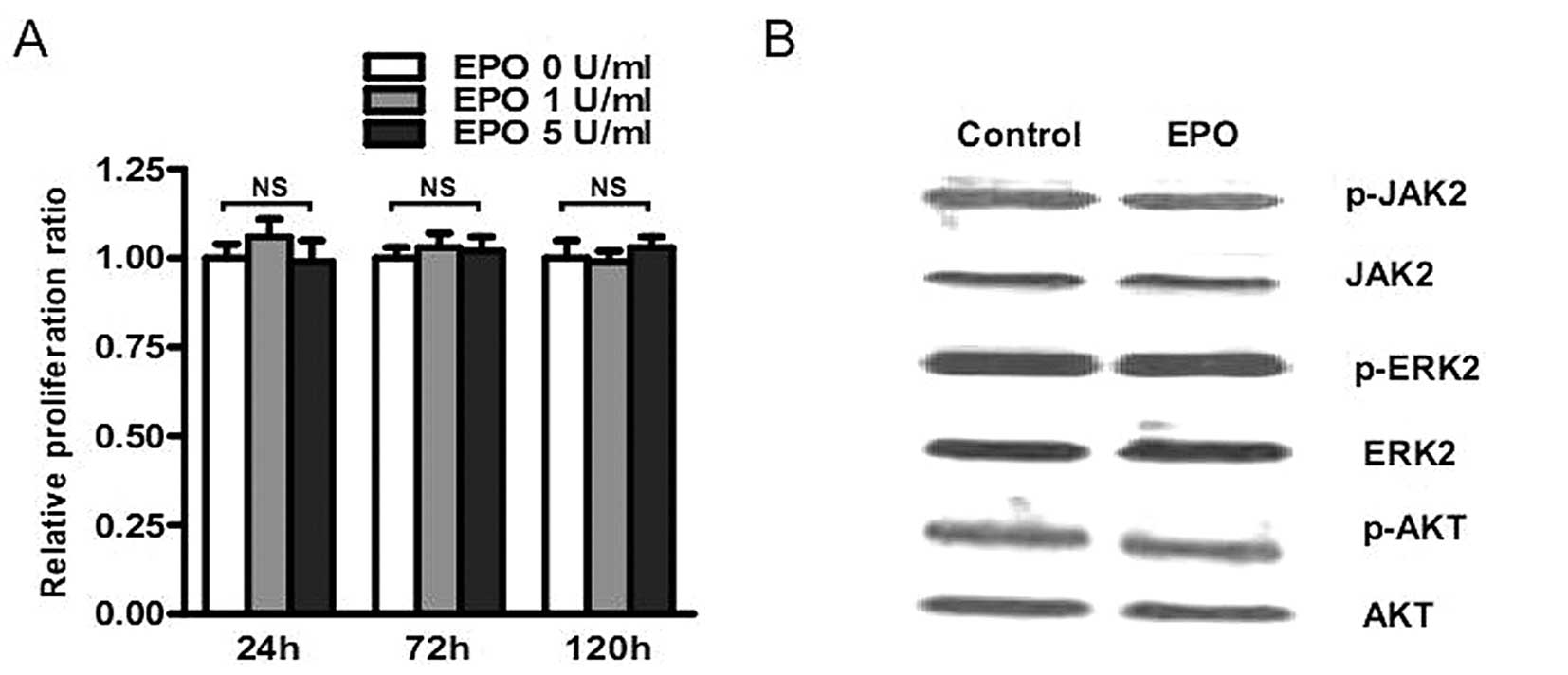
No proliferative effect of rhEPO on MMQ
cells in vitro
To further analyze the effect of rhEPO on pituitary
adenomas, the potential proliferative effect of rhEPO was evaluated
on MMQ cells in vitro. MMQ cells were starved in F12 medium
containing 1% FBS for 48 h, and then co-cultured with rhEPO (0, 1,
5 U/ml) for 24, 72 and 120 h. As expected, no proliferative
response was observed in MMQ cells when rhEPO were used as
stimulator (Fig. 2A), indicating
that rhEPO has no proliferative effect on MMQ cells in
vitro.
No rhEPO-induced signaling pathways were
activated in MMQ cells in vitro
JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways are
known to be the classic EPO-activated signaling pathways. Moreover,
some of these pathways have been reported to be activated in some
non-proliferative responding cancer cell lines. Therefore, we
determined whether 48 h rhEPO (5 U/ml) stimulation can activate
these classic pathways using Western blotting and found no
increased tyrosine phosphorylation of JAK2, ERK2 and AKT in MMQ
cells treated with rhEPO (Fig.
2B). These results indicate that rhEPO has no influence on
these classic EPO-induced signaling pathways in MMQ cells in
vitro.
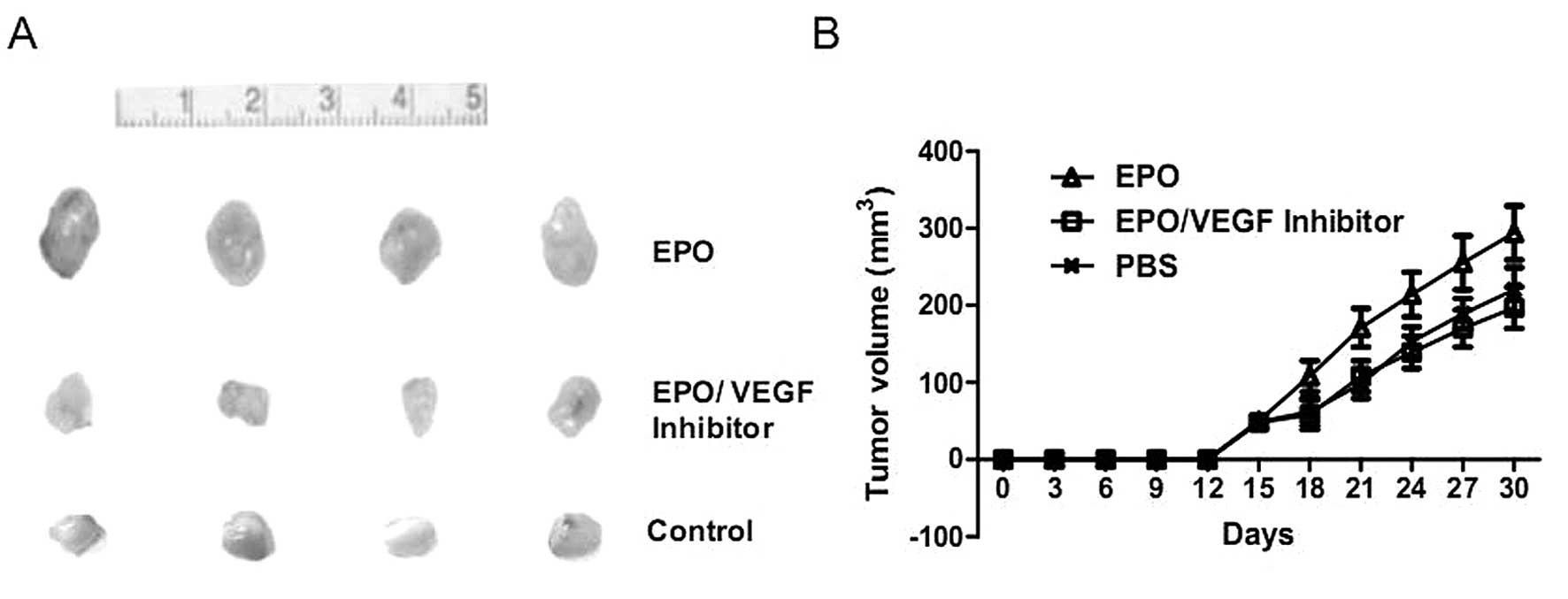
rhEPO administration is associated with
increased tumor growth and angiogenic response in vivo
As rhEPO demonstrated no direct effect on MMQ cells
in vitro, we further examined whether rhEPO regulate tumor
growth of pituitary adenomas in vivo using a nude mouse
xenograft model of MMQ prolactin-secreting pituitary adenoma cells.
Contrary to our in vitro results, we found that a two-week
rhEPO administration (2000 U/kg, twice per week) significantly
accelerated tumor growth (Fig. 3).
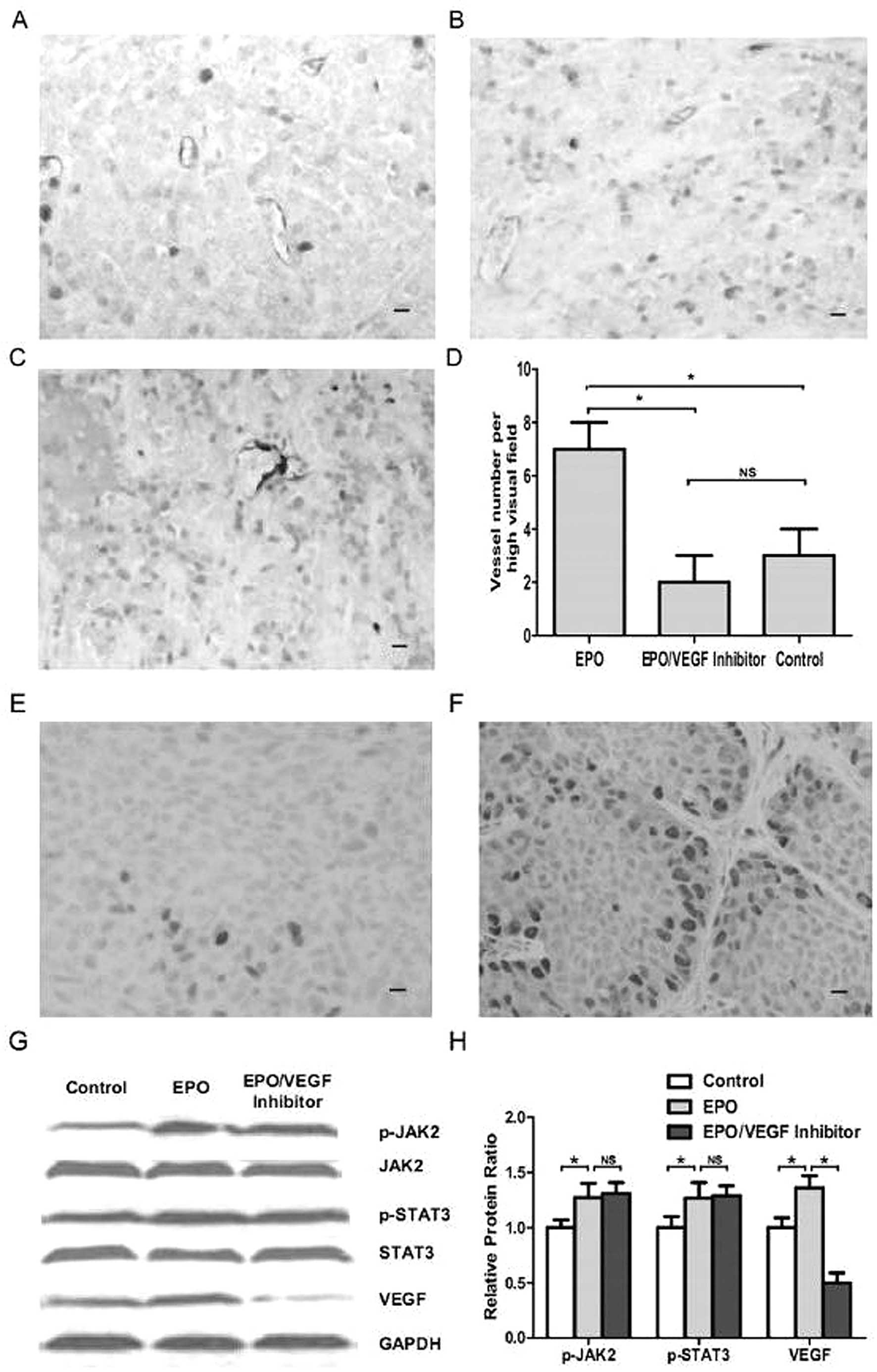
Because rhEPO has been shown to promote proliferation of human
endothelial cells and cerebral arteries express EPOR, in seeking
the underlying mechanism of the in vivo rhEPO-induced tumor
growth acceleration, we evaluated the microvessel density and tumor
cell proliferation using immunohistochemisty staining of anti-CD31
and anti-PCNA antibodies and found significantly higher microvessel
density (P<0.05, Fig. 4A–D) and
increased tumor cell proliferation (P<0.05, Fig. 4E and F) in rhEPO treated xenograft
tumors than control ones. Moreover, immunoreactivity for PCNA in
EPO treated xenografts showed that tumor cells in peri-vessel areas
displayed higher proliferation, which was indicated as increased
PCNA staining (Fig. 4F). These
results suggest a key role of angiogenesis in the rhEPO induced
tumor growth acceleration in MMQ cell xenografts.
Increased VEGF expression and
phosphorylation of JAK2 in rhEPO treated MMQ cell xenograft
tumors
Since rhEPO promotes angiogenesis in MMQ cell
xenografts in vivo without direct effect on MMQ cells in
vitro, and VEGF has been reported to play a key role in
angiogenesis, in order to characterize signaling pathways involved
in rhEPO induced in vivo tumor growth acceleration, Western
blot analysis was carried out to measure VEGF expression of rhEPO
treated xenografts. Being classic EPO proangiogenic signaling
pathway, phosphorylation of JAK2 was also evaluated (21). We found that significant enhanced
VEGF expression and phosphorylation of JAK2 in rhEPO treated
xenograft tumors (P<0.05, Fig. 4G
and H). Based on these observation, we hypothesized that rhEPO
induced VEGF signaling may play a key role in its angiogenic
effect. As STAT3 has been reported to be required for EPO induced
VEGF upregulation (22), we
further explored this issue and found significant increased
phosphorylation of STAT3 in rhEPO treated xenograft tumors
(P<0.05, Fig. 4G and H). The
data demonstrate that the EPO-JAK2-STAT3-VEGF signaling axis may be
involved in rhEPO induced angiogenesis.
VEGF blockade inhibits rhEPO induced
xenograft tumor growth and angiogenesis
To further verify the vital role of angiogenesis in
rhEPO induced tumor growth of pituitary adenoma cell xenografts, we
administered rhEPO (2000 U/kg) plus bevacizumab (10 mg/kg), a VEGF
inhibitor, to nude mice bearing xenograft tumors. After a two-week
administration, the bevacizumab administration significantly
attenuated EPO-dependent tumor growth and angiogenesis (P<0.05,
Figs. 3 and 4). These observations further confirmed
the key proangiogenic role of rhEPO in its in vivo tumor
growth acceleration.
JAK2 inhibitor AG490 attenuated
EPO-induced HUVEC survival, VEGF upregulation and phosphorylation
of JAK2 and STAT3
To further verify the involvement of
EPO-JAK2-STAT3-VEGF signaling axis in rhEPO-induced angiogenesis,
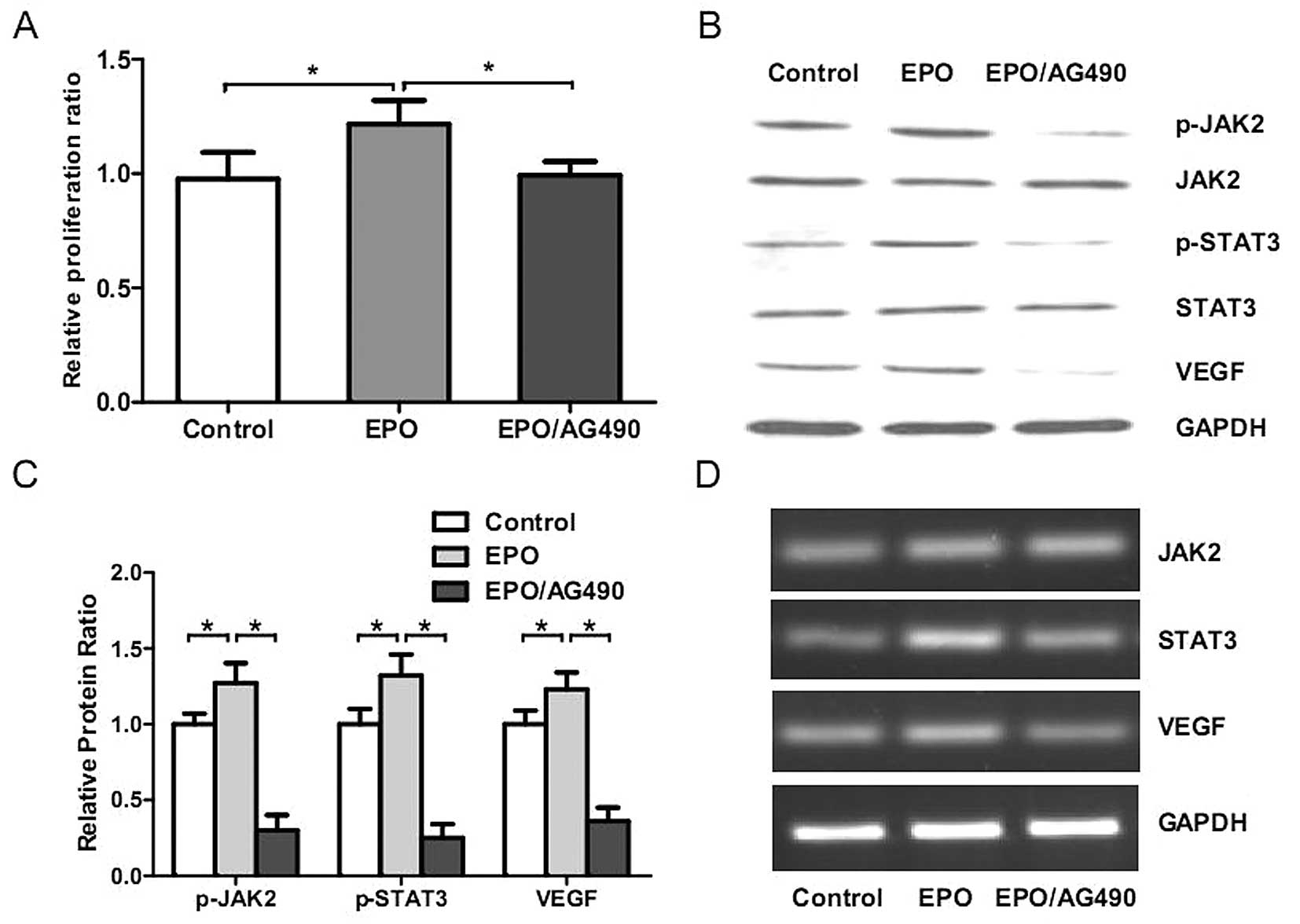
we cultured HUVECs with rhEPO (5 U/ml), rhEPO (5 U/ml) plus AG490
(20 μM/l) or low-serum medium for 48 h, and then CCK8 assay,
Western blot analysis and RT-PCR were performed. We found that
rhEPO significantly promoted the proliferation (P<0.05, Fig. 5A) and activated JAK2-STAT3-VEGF
signaling in HUVECs (P<0.05, Fig.
5B–D). Moreover, AG490 significantly inhibited these
EPO-induced effects in vitro (P<0.05, Fig. 5). These results further confirmed
the vital role of EPO-JAK2-STAT3-VEGF signaling axis in
rhEPO-induced angiogenesis.
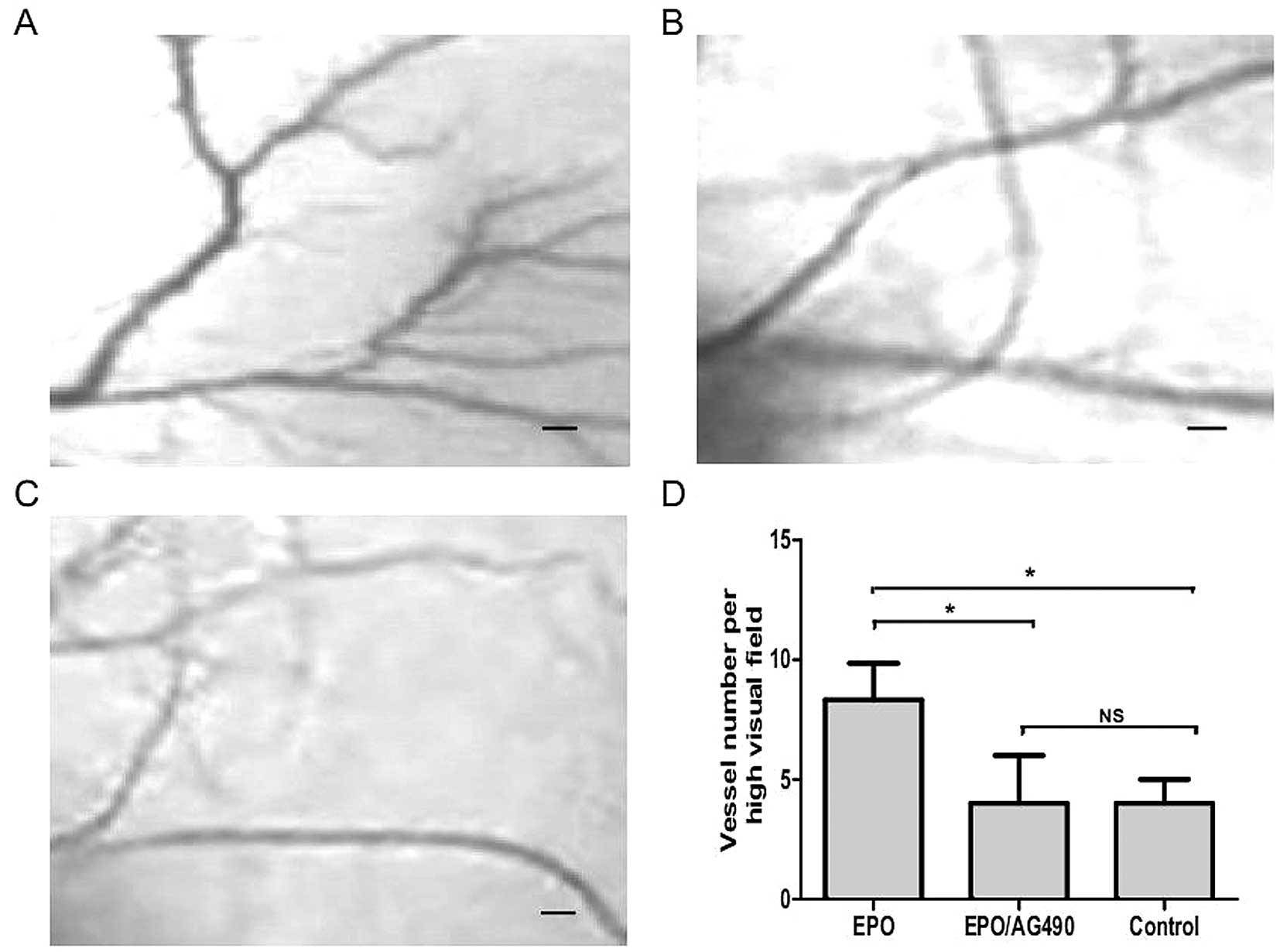
AG490 inhibits EPO-induced angiogenesis
in the chicken chorioallantoic membrane
To further demonstrate the proangiogenic role of
rhEPO more intuitively, we investigated its effect on
neovascularisation in the developing chorioallantoic membrane (CAM)
of the chicken embryo. As a trigger for angiogenesis, fibrous
membrane diluted with rhEPO (5 U/ml) or PBS were placed on the CAM
of chicken embryos. After 72 h, vessel formation was significantly
increased in EPO treated CAM compared to the control (P<0.05,
Fig. 6). As shown in Fig. 6, in contrast, when rhEPO (5 U/ml)
plus AG490 (20 μM/l) were further incubated on the CAM for 72 h,
AG490 significantly attenuated the EPO induced vessel formation
(P<0.05).
Discussion
In this study, for the first time, we present the
evidence that pituitary adenomas are EPOR-negative tumors. In
addition, rhEPO accelerates pituitary tumor growth in a nude mouse
xenograft model of MMQ pituitary adenoma cells accompanied with
increased microvessel density and upregulation of JAK2-STAT3-VEGF
signaling, whereas rhEPO displays no direct effect on MMQ cells
in vitro. Furthermore, EPO promotes HUVECs proliferation and
upregulates phosphorylation of JAK2 and STAT3 and VEGF expression,
whereas JAK2 inhibitor AG490 significantly attenuated the EPO
induced proliferation in HUVECs in vitro and vessel
formation in CAM in vivo. Our results suggest that rhEPO may
exert its in vivo proliferation effect via enhancement of
angiogenesis in pituitary adenomas through EPO-JAK2-STAT3-VEGF
signal pathway.
In the present study, a two-week rhEPO
administration resulted in accelerated tumor growth of MMQ cell
xenografts, suggesting that rhEPO administration can accelerate the
tumor growth of EPOR negative pituitary adenomas. rhEPO has to date
been described to stimulate tumor growth though both in
vitro direct and in vivo indirect effects (23). Specific in vitro growth
response to rhEPO has been reported in RCC, breast, prostate, lung
and HNSCC cell lines (14,15,23–25).
The direct proliferative effect of rhEPO seems to be EPOR-dependent
and tumor cell specific, because previous studies have shown that
growth response to rhEPO is only on those cells expressing
cell-surface EPOR (23,24). Moreover, cells from different
organs, even multiple cell lines derived from the same organ,
display different growth response to exogenous EPO (23). These discrepancies may be due to
different cell lines, different culture conditions or different
assay procedures. In the present study, the lack of growth response
and signal transduction to rhEPO in MMQ cells in vitro
excluded the possibility of the direct proliferative effect of
rhEPO on MMQ cells. This is consistent with previous experiments
suggesting no proliferative effect of rhEPO on EPOR negative cells
(26).
Besides direct proliferative effect on tumor cells,
rhEPO has also been suggested as a pro-angiogenic factor, which
indirectly promotes tumor growth via angiogenesis (21,27).
Because EPOR has been confirmed in various endothelial cells,
especially including brain capillary endothelial cells, rhEPO can
directly stimulate angiogenesis of both EPOR positive and negative
tumors, which in turn provide a growth advantage to tumor cells
in vivo (21,26,28,29).
Previous studies have documented that rhEPO can not only increase
endothelial cell proliferation but also enhance the number of
circulating endothelial cells and endothelial precursor cells
(21,26). Furthermore, it is noteworthy that
rhEPO can stimulate cerebral angiogenesis in vivo and
promote capillary tube formation of cerebral endothelial cells by
inducing vascular endothelial growth factor (VEGF), all of which
provide evidence for the proangiogenic properties of rhEPO
(28,30,31).
Based on the aforementioned, we hypothesized that rhEPO may
indirectly promote tumor growth by enhancing angiogenesis. In order
to further clarify this issue, immunohistochemisty of CD31 and PCNA
were carried out to investigate the microvessel density and tumor
proliferation of MMQ cell xenografts in both rhEPO and control
groups. In supporting of our hypothesis, we found increased
microvessel density and proliferation in rhEPO treated MMQ cell
xenografts. Moreover, tumor cells in peri-vessel areas displayed
higher proliferation. These data defined a key proangiogenic role
of rhEPO in stimulating the tumor growth of MMQ cell
xenografts.
The EPO-JAK2-STAT3-VEGF pathway has been well
documented for its essential proangiogenic role in tumor growth
acceleration (21). Previous
studies have reported that EPO can activate STAT3 and STAT5 in
primary cerebral vascular cells (21,28,29).
Furthermore, the deletion of the endogenous Epo-EPOR system in
nonhematopoietic cells in mice impairs STAT3 activation, VEGF
upregulation, and capillary growth (31). Our mechanistic study of MMQ cell
xenografts showed that rhEPO administration increased
phosphorylation of JAK2, STAT3 and VEGF expression. Moreover, VEGF
blockade attenuated rhEPO induced xenograft angiogenesis and tumor
growth. These results suggest a key role of the proangiogenic
property of rhEPO in its tumor growth promotion of pituitary
adenomas. As rhEPO has been reported to stimulate JAK2-STAT3
pathway and STAT3 is required for VEGF upregulation (22,32,33),
we further verified this issue and found that JAK2 inhibitor AG490
not only attenuated EPO induced HUVECs survival, phosphorylation of
STAT3 and VEGF upregulation in vitro, but also inhibited
EPO-induced angiogenesis in the chicken chorioallantoic membrane.
These results defined the EPO-JAK2-STAT3-VEGF pathway as an
underlying mechanism of the rhEPO proangiogenic property, which in
turn promotes tumor growth in pituitary adenomas.
In the present study, we demonstrated that, at least
in our cases, pituitary adenomas are EPOR negative tumors and
systematic rhEPO administration may promote tumor growth of
pituitary adenomans via enhancing angiogenesis though the
EPO-JAK2-STAT3-VEGF signal pathway. Based on our results, rhEPO
should be used with caution in pituitary adenoma patients due to
its potential detrimental proangiogenic and proliferative effect.
Further research is needed to fully elucidate the complex interplay
between rhEPO and pituitary adenomas, in order to identify whether
rhEPO is suitable for use in anemia patients bearing pituitary
adenoma.
Acknowledgements
This work was supported by a Grant-in-Aid for
Postdoctoral Scientific Research from Henan University of Science
and Technology.
References
|
1
|
Ng T, Marx G, Littlewood T and Macdougall
I: Recombinant erythropoietin in clinical practice. Postgrad Med J.
79:367–376. 2003. View Article : Google Scholar
|
|
2
|
Leyland-Jones B: Breast cancer trial with
erythropoietin terminated unexpectedly. Lancet Oncol. 4:459–460.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henke M, Laszig R, Rube C, et al:
Erythropoietin to treat head and neck cancer patients with anaemia
undergoing radiotherapy: randomised, double-blind,
placebo-controlled trial. Lancet. 362:1255–1260. 2003. View Article : Google Scholar
|
|
4
|
Jelkmann W, Bohlius J, Hallek M and
Sytkowski AJ: The erythropoietin receptor in normal and cancer
tissues. Crit Rev Oncol Hematol. 67:39–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glaspy JA: Erythropoiesis-stimulating
agents in oncology. J Natl Compr Cancer Netw. 6:565–575. 2008.
|
|
6
|
Glaspy J, Crawford J, Vansteenkiste J, et
al: Erythropoiesis-stimulating agents in oncology: a study-level
meta-analysis of survival and other safety outcomes. Br J Cancer.
102:301–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leyland-Jones B, Semiglazov V, Pawlicki M,
et al: Maintaining normal hemoglobin levels with epoetin alfa in
mainly nonanemic patients with metastatic breast cancer receiving
first-line chemotherapy: a survival study. J Clin Oncol.
23:5960–5972. 2005. View Article : Google Scholar
|
|
8
|
Temkin SM, Hellmann M, Serur E, Lee YC and
Abulafia O: Erythropoietin administration during primary treatment
for locally advanced cervical carcinoma is associated with poor
response to radiation. Int J Gynecol Cancer. 16:1855–1861. 2006.
View Article : Google Scholar
|
|
9
|
Wright JR, Ung YC, Julian JA, et al:
Randomized, double-blind, placebo-controlled trial of
erythropoietin in non-small-cell lung cancer with disease-related
anemia. J Clin Oncol. 25:1027–1032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smith RE Jr, Aapro MS, Ludwig H, et al:
Darbepoetin alpha for the treatment of anemia in patients with
active cancer not receiving chemotherapy or radiotherapy: results
of a phase III, multicenter, randomized, double-blind,
placebo-controlled study. J Clin Oncol. 26:1040–1050. 2008.
View Article : Google Scholar
|
|
11
|
Thomas G, Ali S, Hoebers FJ, et al: Phase
III trial to evaluate the efficacy of maintaining hemoglobin levels
above 12.0 g/dl with erythropoietin vs above 10.0 g/dl without
erythropoietin in anemic patients receiving concurrent radiation
and cisplatin for cervical cancer. Gynecol Oncol. 108:317–325.
2008. View Article : Google Scholar
|
|
12
|
Lee YS, Vortmeyer AO, Lubensky IA, et al:
Coexpression of erythropoietin and erythropoietin receptor in von
Hippel-Lindau disease-associated renal cysts and renal cell
carcinoma. Clin Cancer Res. 11:1059–1064. 2005.PubMed/NCBI
|
|
13
|
Saintigny P, Besse B, Callard P, et al:
Erythropoietin and erythropoietin receptor coexpression is
associated with poor survival in stage I non-small cell lung
cancer. Clin Cancer Res. 13:4825–4831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai SY, Childs EE, Xi S, et al:
Erythropoietin-mediated activation of JAK-STAT signaling
contributes to cellular invasion in head and neck squamous cell
carcinoma. Oncogene. 24:4442–4449. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arcasoy MO, Amin K, Vollmer RT, Jiang X,
Demark-Wahnefried W and Haroon ZA: Erythropoietin and
erythropoietin receptor expression in human prostate cancer. Mod
Pathol. 18:421–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong JY, Feldman L, Solar P, Szenajch J
and Sytkowski AJ: Characterization of erythropoietin receptor and
erythropoietin expression and function in human ovarian cancer
cells. Int J Cancer. 122:274–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Acs G, Acs P, Beckwith SM, et al:
Erythropoietin and erythropoietin receptor expression in human
cancer. Cancer Res. 61:3561–3565. 2001.PubMed/NCBI
|
|
18
|
Ellegala DB, Alden TD, Couture DE, Vance
ML, Maartens NF and Laws ER Jr: Anemia, testosterone, and pituitary
adenoma in men. J Neurosurg. 98:974–977. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimon I, Benbassat C, Tzvetov G and
Grozinsky-Glasberg S: Anemia in a cohort of men with
macroprolactinomas: increase in hemoglobin levels follows prolactin
suppression. Pituitary. 14:11–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aron DC, Tyrrell JB and Wilson CB:
Pituitary tumors. Current concepts in diagnosis and management.
West J Med. 162:340–352. 1995.PubMed/NCBI
|
|
21
|
Ribatti D: Erythropoietin and tumor
angiogenesis. Stem Cells Dev. 19:1–4. 2010. View Article : Google Scholar
|
|
22
|
Funamoto M, Fujio Y, Kunisada K, et al:
Signal transducer and activator of transcription 3 is required for
glycoprotein 130-mediated induction of vascular endothelial growth
factor in cardiac myocytes. J Biol Chem. 275:10561–10566. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szenajch J, Wcislo G, Jeong JY, Szczylik C
and Feldman L: The role of erythropoietin and its receptor in
growth, survival and therapeutic response of human tumor cells From
clinic to bench - a critical review. Biochim Biophys Acta.
1806:82–95. 2010.PubMed/NCBI
|
|
24
|
Hardee ME, Arcasoy MO, Blackwell KL,
Kirkpatrick JP and Dewhirst MW: Erythropoietin biology in cancer.
Clin Cancer Res. 12:332–339. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mohyeldin A, Lu H, Dalgard C, et al:
Erythropoietin signaling promotes invasiveness of human head and
neck squamous cell carcinoma. Neoplasia. 7:537–543. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okazaki T, Ebihara S, Asada M, Yamanda S,
Niu K and Arai H: Erythropoietin promotes the growth of tumors
lacking its receptor and decreases survival of tumor-bearing mice
by enhancing angiogenesis. Neoplasia. 10:932–939. 2008.PubMed/NCBI
|
|
27
|
Ribatti D, Vacca A, Roccaro AM, Crivellato
E and Presta M: Erythropoietin as an angiogenic factor. Eur J Clin
Invest. 33:891–896. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ribatti D, Poliani PL, Longo V, Mangieri
D, Nico B and Vacca A: Erythropoietin/erythropoietin receptor
system is involved in angiogenesis in human neuroblastoma.
Histopathology. 50:636–641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anagnostou A, Lee ES, Kessimian N,
Levinson R and Steiner M: Erythropoietin has a mitogenic and
positive chemotactic effect on endothelial cells. Proc Natl Acad
Sci USA. 87:5978–5982. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hardee ME, Cao Y, Fu P, et al:
Erythropoietin blockade inhibits the induction of tumor
angiogenesis and progression. PLoS One. 2:e5492007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Asaumi Y, Kagaya Y, Takeda M, et al:
Protective role of endogenous erythropoietin system in
nonhematopoietic cells against pressure overload-induced left
ventricular dysfunction in mice. Circulation. 115:2022–2032. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Osugi T, Oshima Y, Fujio Y, et al:
Cardiac-specific activation of signal transducer and activator of
transcription 3 promotes vascular formation in the heart. J Biol
Chem. 277:6676–6681. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hilfiker-Kleiner D, Limbourg A and Drexler
H: STAT3-mediated activation of myocardial capillary growth. Trends
Cardiovasc Med. 15:152–157. 2005. View Article : Google Scholar : PubMed/NCBI
|