Introduction
As it is well known, mammography (Mx) represents the
imaging procedure of reference in both the screening and the
diagnosis of primary breast cancer, in spite of some limitations in
sensitivity and specificity.
Mx performance is especially limited in patients
with extremely high dense parenchyma, with sensitivity values
dropping to approximately 50% (1),
as well as in the assessment of both multifocal/multicentric and
contralateral synchronous breast cancers, as demonstrated at
detailed serial slicing of the mastectomy specimen and at random
biopsy as well as at prophylactic mastectomy of the contralateral
breast (2,3).
Another important limitation of Mx is its intrinsic
low specificity, with positive predictive values reported in a very
wide range (2–95%) for lesions with mammographic findings belonging
to BI-RADS category 4, a category for which biopsy is indicated,
leading to numerous unnecessary biopsies. To overcome these
limitations, since the 1990s scintimammography with γ-emitting
technetium labelled radiotracers has been proposed as a
complementary tool to Mx.
However, during the course of the years, the role of
scintimammography has been progressively reduced given the low
sensitivity (generally <50%) demonstrated in the identification
of small size (<1 cm) carcinomas (4–6),
making the procedure unreliable in both the screening and the
diagnosis of tumors at a very early stage.
At present, a new interest on the employment of
breast radio-isotopic imaging is registering due to the development
of small field of view breast specific γ-cameras (BSGC) equipped
with special devices which present several physical and technical
advantages in respect of the general purpose γ-camera generally
employed in scintimammography, such as higher spatial resolution,
higher manoeuvrability, smaller lesion-to-detector distance,
exclusion of nearby organs from the field of view and reduced
radiation scattering in breast imaging. Moreover, dedicated devices
may be inserted in a mammography gantry thus permitting breast
imaging views comparable to those of Mx and a precise information
on lesion site.
In confirmation of the renovated interested on
radioisotopic breast imaging, practice guidelines for breast
scintigraphy with BSGC have been recently published (7). This new procedure, using either
99mTc-sestaMIBI or 99mTc-tetrofosmin as
radiotracer, has already demonstrated in clinical studies a high
sensitivity in the detection of small size (≤10 mm) carcinomas,
with values ranging from 86 to 91% (8–10)
which result markedly higher that those generally achieved by
conventional planar scintimammography.
In the present study we investigated the clinical
impact of breast scintigraphy acquired with a BSGC in the diagnosis
of primary breast cancer and we also evaluated its additional value
over Mx, in particular in the detection of small size
carcinomas.
Patients and methods
Patients
We studied prospectively 467 consecutive female
patients, aged 26–81 years (median age 57 years), with breast
lesions at physical examination and/or at diagnostic imaging
procedures (mammography and/or breast ultrasound or MRI). The
different indications were: suspicious lesions on physical
examination and/or on breast ultrasonography or MRI negative at Mx
(BI-RADS 1 or 3; 41 cases), characterization of lesions suspicious
at Mx (BI-RADS 4; 147 cases), preoperative local staging of lesions
highly suggestive of malignancy at Mx (BI-RADS 5; 279 cases).
Ten of the patients had already undergone
contralateral quadrantectomy (n=6) or mastectomy (n=4) for
infiltrating ductal carcinoma 2–12 years previously. All patients,
at the time of our observation had already undergone both clinical
examination and mammography.
The clinical examination had been performed in all
cases by an experienced clinician. Palpable breast lumps, skin
thickening or retraction, and nipple discharge or retraction were
noted.
Mammographic examination included routine
cranio-caudal (CC) and medio-lateral oblique (MLO) views of the
breasts and at least another projection or magnification view over
the area of suspected lesions. The lesions were described according
to the American College of Radiology Breast Imaging Reporting and
Data System (BI-RADS) Lexicon (11) for which the mammographic findings
were classified as normal (BI-RADS category 1), benign (BI-RADS
category 2), probably benign (BI-RADS category 3), suspicious
(BI-RADS category 4) or highly suggestive of malignancy (BI-RADS
category 5). In our series, 38/467 patients were classified as
BI-RADS category 1, 3/467 as BI-RADS category 3, 147/467 as BI-RADS
category 4 and 279/467 as BI-RADS category 5. Categories 1–3 were
considered negative and categories 4–5 were considered positive.
All the 41 patients negative at Mx (BI-RADS categories 1 and 3) had
unclear lesions at physical examination and/or at breast
ultrasonography or MRI.
The final diagnosis was obtained in all 467 patients
within 2 weeks of scintigraphy by surgical (52 cases) or by
percutaneous (415 cases) biopsy. Patients with proven-biopsy
primary breast cancer had surgery at the same surgical department
and the operation was planned according to the data derived from
conventional diagnostic and scintigraphic procedures. At our
institution, patients with a unifocal carcinoma <3 cm usually
undergo conservation surgery, while those with larger carcinomas or
with multicentric carcinomas are submitted to mastectomy. Patient’s
opinion on the type of surgery is considered in all cases, also
including the option of contralateral prophylactic mastectomy.
Patients with locally advanced primary breast cancer
considered eligible for neoadjuvant chemotherapy and patients who
had undergone surgical excisional biopsy were excluded from the
present study.
According to the final histopathological findings,
420/467 patients had a primary breast cancer, bilateral in 8/420
cases and multifocal/multicentric in 48/420 cases, while the
remaining 47/467 patients had a benign disease, bilateral in one
case; a benign lesion was also ascertained in 7 breast cancer
patients. Table I reports the
histological diagnosis of the 467 patients enrolled in the study,
while the patologic T stage of the 420 breast cancer patients is
reported in Table II. In total,
554 breast lesions were ascertained at histology: 499 tumor foci,
453 of which invasive (142 ≤10 mm and 311 >10 mm) and 46 in
situ, and 55 benign lesions. Two hundred and sixty-two/420
breast cancer patients were treated with conservation surgery,
while 158/420 patients had mastectomy. Moreover, contralateral
prophylactic mastectomy was performed in 3 breast cancer
patients.
 | Table IHistopathological diagnosis of the 467
patients enrolled in the study: 420 with primary breast cancer and
47 with benign disease. |
Table I
Histopathological diagnosis of the 467
patients enrolled in the study: 420 with primary breast cancer and
47 with benign disease.
| Diagnosis | No. of patients |
|---|
| Invasive ductal
carcinoma | 342a |
| Invasive lobular
carcinoma | 23 |
| Mucinous
carcinoma | 8 |
| Medullary
carcinoma | 1 |
| Ductal carcinoma
in situ (DCIS) | 42 |
| Lobular carcinoma
in situ (LCIS) | 4 |
| Sclerosing
adenosis | 25 |
| Fibrosis | 7 |
| Fibrosis/atypical
hyperplasia | 3 |
| Fibrocystic
disease | 6 |
| Fibroadenoma | 2 |
| Papillomatosis | 2 |
| Lipoma/adenosis | 1 |
| Chronic
mastitis/atypical ductal hyperplasia (bilateral) | 1 |
 | Table IIPathologic tumor (pT) stage of the 420
breast cancer patients enrolled in the study. |
Table II
Pathologic tumor (pT) stage of the 420
breast cancer patients enrolled in the study.
| pT
classification | No. of patients |
|---|
| Tis | 46 |
| T1a | 19 |
| T1b | 73 |
| T1c | 216 |
| T2 | 66 |
Breast specific γ-camera (BSGC)
scintigraphy
BSGC scintigraphy was acquired starting 10 min after
the intravenously injection of 740 MBq of
99mTc-tetrofosmin(Myoview, Amersham Health - GE
Healthcare) in the arm contralateral to the affected breast. In
patients with bilateral breast lesions, the injection was performed
in a pedal vein. Radiolabelling and quality control procedures of
the radiotracer were carried out according to the manufacturer’s
instructions. Labelling efficiency was always >95%.
Scintigraphic images were acquired at the peak of technetium (140
Kev) with a ±10% energy window using a high resolution dedicated
breast camera (LumaGEM 3200S/12k, Gamma Medica Ideas Inc.)
constituted by a small field of view (20×15 cm) high-resolution,
solid-state semiconductor (CZT) detector mounted on a modified
mammographic unit, replacing the radiographic Bucky. The camera
head is composed of a pixelated (12,288 pixels) array of CZT (pixel
size: 1.5×1.5×5 mm) coupled to an array of amplifiers, the signals
from which are conveyed to an electronics readout board. The system
is equipped with a highly sensitive (HSEN, LEAP) long-bore,
low-energy collimator (hole shape: hexagonal, hole length: 25.4 mm,
hole diameter: 2 mm, septal thickness: 0.3 mm) matched to the CZT
elements. The intrinsic spatial resolution is 1.6 mm and the energy
resolution is <5% (average 4.6% at 140 Kev).
In all cases cranio-caudal and medio-lateral oblique
projections (600 sec per view) were acquired using a 128×128 matrix
size, with the breast positioned between the detector and the
compression paddle of the mammographic unit to ensure a light
compression of the breast parenchyma, reducing its thickness,
limiting movement artefacts and improving lesion contrast.
Additional breast projections could be acquired when necessary
(i.e., breast bigger than the field of view, areas of increased
uptake at the border of the field of view or not close to the
camera, etc.), given the flexibility of mammographic gantry in
breast positioning.
The present study was performed in accordance with
the Declaration of Helsinki. All patients gave their written
informed consent prior to their inclusion in the study.
Histopathological diagnosis
Breast surgical specimens were fixed in 10% buffered
formalin and stained with hematoxylin and eosin.
Lesions were identified according to their number
and measured. The size of the carcinomas was determined according
to the largest dimension and was given in millimetres. Surgical
cancer specimens were sectioned in serial 5-mm slices and evaluated
for tumor histological type and grading.
Tumors were categorized as invasive or in
situ. According to the number of tumor foci, the carcinomas
were classified as unifocal (only one focus) or
multifocal/multicentric (two or more tumor foci within a single
quadrant of the breast or within different quadrants of the same
breast). Synchronous bilateral carcinomas were considered as
separate primary tumors in the presence of a different histologic
type or when there was no evidence of spread from contralateral
cancer. The tumor pathologic classification (TNM) system was based
on the AJCC (American Joint Commettee on Cancer) criteria according
to which the index carcinoma, considered as the largest one, is
used to designate T classification in defining multiple
simultaneous invasive primary carcinomas.
Data analysis
Scintigraphic images were independently evaluated by
two experienced nuclear medicine physicians who were blinded to the
clinical findings, to all the other diagnostic imaging procedures
data and to the final histopathological diagnoses. Scintigraphy was
considered suggestive of malignancy in the presence of moderate to
intense focal uptake with well-delineated contours (7).
A patient by patient comparative evaluation between
breast scintigraphy and mammography findings was performed,
according to Mx BI-RADS categories classification. Both
scintigraphic and mammographic data were related to the
histopathological findings obtained from surgical samples. The
incremental value of breast scintigraphy versus Mx was also
calculated.
Statistical analysis
Breast scintigraphy results were classified as
true-positive, true-negative, false-positive or false-negative
considering histology as the gold standard. Sensitivity and
specificity values were then calculated. χ2 test was
used to assess the statistical differences in sensitivity of breast
scintigraphy in the carcinomas subdivided according to size (≤10 vs
>10 mm carcinomas). The results were considered significant when
p<0.05. Positive predictive value (true-positives/true-positives
+ false-positives) of breast scintigraphy in patients with BI-RADS
category 4 mammography findings was also calculated.
Results
99mTc-tetrofosmin BSGC scintigraphy was
true-positive in 408/420 (97.1%) breast cancer patients, revealing
multifocal/multicentric disease in 43/48 (89.6%) cases and
bilateral disease in 8/8 (100%) cases. The procedure detected
480/499 (96.2%) of the overall tumor foci, including 438 of the 453
(96.7%) invasive carcinomas and 42 of the 46 (91.3%) carcinomas
in situ; according to size, it identified 130/142 (91.5%)
≤10 mm invasive carcinomas and 308 of the 311 (99%) >10 mm
(p<0.005). The smallest invasive carcinoma detected at breast
scintigraphy measured 1.8 mm. The results of scintigraphy in
relationship with the Mx findings (BI-RADS categories) are
illustrated in Table III.
 | Table IIIBSGC scintigraphy results in
relationship with mammography (Mx) findings in the 467 patients
enrolled in the study. |
Table III
BSGC scintigraphy results in
relationship with mammography (Mx) findings in the 467 patients
enrolled in the study.
Breast scintigraphy
| Mx
|
|---|
| Type of results | No. of patients | Findings | No. of patients |
|---|
| True-positive | 408 | Negative (BI-RADS
category 1) | 29 |
| | Probably benign
(BI-RADS category 3) | 2 |
| | Suspicious (BI-RADS
category 4) | 103 |
| | Highly suspicious
(BI-RADS category 5) | 274 |
| False-negative | 12 | Negative (BI-RADS
category 1) | 2 |
| | Probably benign
(BI-RADS category 3) | 1 |
| | Suspicious (BI-RADS
category 4) | 4 |
| | Highly suspicious
(BI-RADS category 5) | 5 |
| True-negative | 40 | Negative (Bi-RADS
category 1) | 7 |
| | Suspicious (BI-RADS
category 4) | 33 |
| False-positive | 7 | Suspicious (BI-RADS
category 4) | 7 |
Three hundred and seventy-seven/408 breast cancer
patients true-positive at scintigraphy were also true-positive at
Mx (BI-RADS categories 4 or 5) that was concordant with
scintigraphy in assessing the index tumor. However, breast
scintigraphy evidenced a more extensive disease in respect of Mx in
77 of these 377 patients (13 belonging to BI-RADS category 4 and 64
to BI-RADS category 5), identifying the in situ component
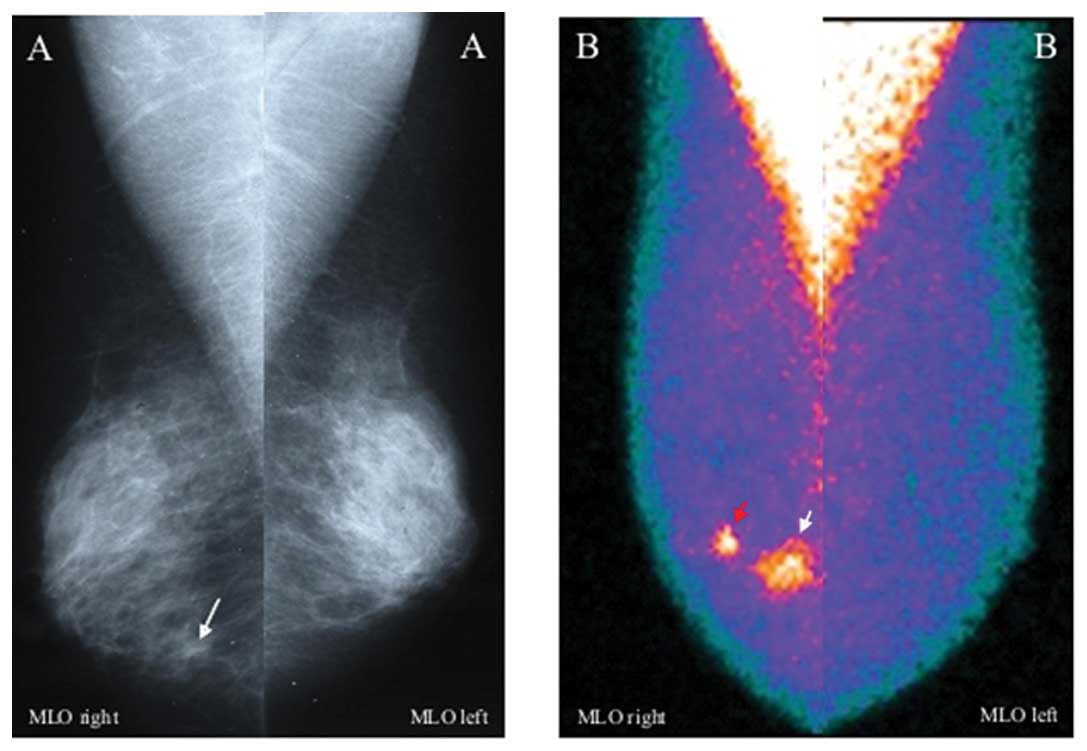
sited around invasive cancers (Fig.
1) in 56/77 cases and new tumor foci in further 21/77 cases, as
confirmed at surgery; these new 21 foci, all clinically occult,
were ipsilateral in 16 cases with surgically proven
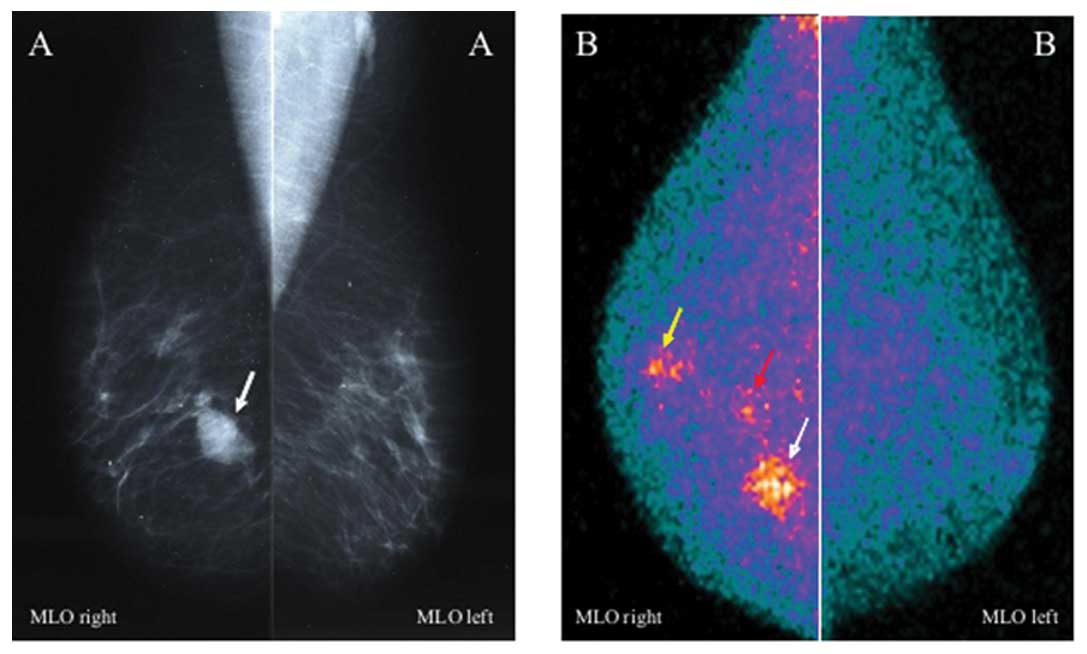
multifocal/multicentric invasive ductal carcinomas (Fig. 2) and contralateral in 5 cases, as
confirmed at surgery. Scintigraphy changed surgical management in
14 of these 77 cases (18.2%). The remaining 31/408 breast cancer
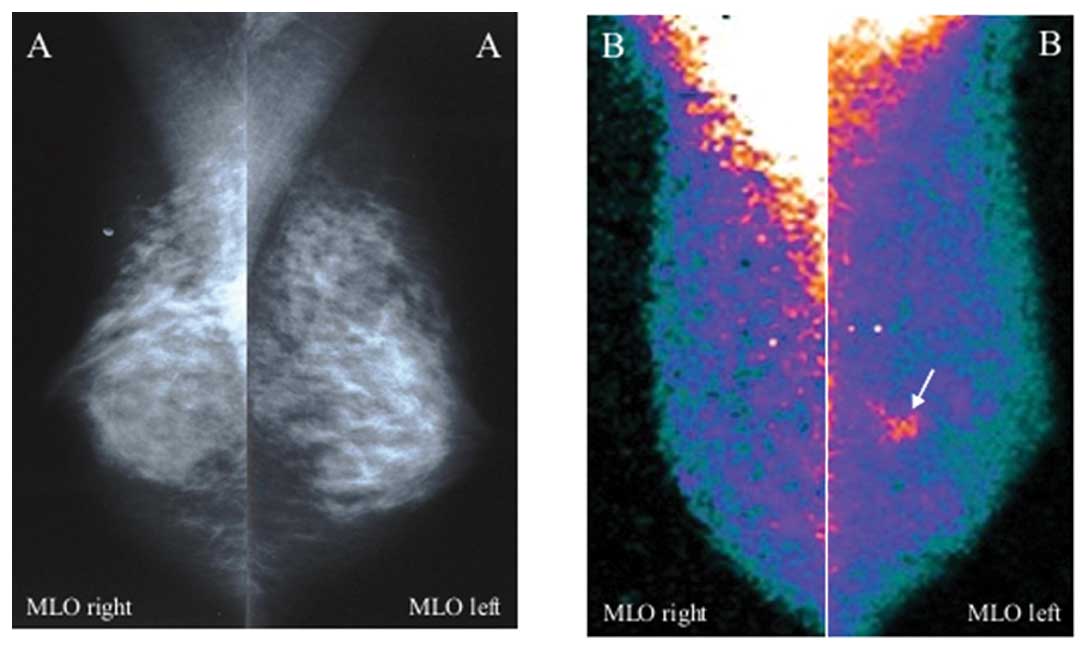
patients true-positive at scintigraphy were false-negative at Mx
(BI-RADS category 1 or 3; Fig. 3):
26 of the 31 patients had one invasive ductal carcinoma each in
heterogeneously/high dense breast (>10 mm in 7 cases and ≤10 mm
in 19), 4 had one carcinoma in situ (2 DCIS in Paget’s
disease, 1 multicentric DCIS and 1 multicentric LCIS) and 1 had one
invasive lobular carcinoma (18 mm in size) in association with a
component in situ.
Scintigraphy was false-negative in 12 breast cancer
patients, globally missing 16 tumor foci; 10 of these 12 patients
had a unifocal carcinoma each (6 subcentimetric, 5–7 mm in size,
invasive ductal carcinomas sited in internal quadrants, including 2
carcinomas that was impossible to include in the field of view of
the device since sited in internal upper quadrant close to the
chest wall, 1 LCIS and 3 DCIS), while the remaining 2/12 patients
had multicentric carcinomas (an invasive ductal with 3
subcentimetric foci, 4–5 mm in size, in one case and an invasive
mucinous with 3 foci, 11–14 mm in size, in the other case).
Scintigraphy also missed 3 additional tumor foci
(3–5 mm in size) in further 3 patients with multicentric invasive
carcinoma in whom it identified only the index tumor, failing the
diagnosis of multicentricity.
Three of the 12 breast cancer patients
false-negative at scintigraphy were also false-negative at Mx
(BI-RADS categories 1 or 3) and included the 2 patients with a
subcentimetric invasive ductal carcinoma each sited in internal
upper quadrant and excluded from the field of view of the mammogram
and the patient with LCIS. The remaining 9/12 breast cancer
patients false-negative at scintigraphy were true-positive at
Mx.
Scintigraphy was true-negative in 40/47 (85.1%)
patients with benign disease and in 47/55 (85.4%) lesions. Of the
40 patients with benign lesions true-negative at scintigraphy, 7
were also true-negative at Mx (BI-RADS category 1), while 33 were
false-positive (BI-RADS category 4). Scintigraphy was
false-positive, concordantly with Mx, in 7 patients: 4 with
sclerosing adenosis, 1 with bilateral mixed pattern of chronic
mastitis and atypical ductal hyperplasia, 1 with sclerohyaline
fibrosis and 1 with a mixed pattern of lipoma and adenosis.
Finally, of the 7 further benign lesions ascertained at surgery in
7 patients with cancer, 5 fibroadenomas and 1 fibroadenomatosis
resulted negative both at scintigraphy and at Mx, while one
papillomatosis was true negative at scintigraphy and false-positive
at Mx.
Globally, scintigraphy gave an additional value in
respect of Mx in 141/467 overall patients (30.2%) prospectively
studied, 108 of whom with breast cancer and 33 with benign lesions,
as cited above.
Finally, according to scintigraphy data and final
histopathological findings relative to the 147 patients with
BI-RADS category 4 mammographic pattern, breast biopsy would have
been avoided in 33 of these cases (22.4%). Negative predictive
value of scintigraphy in patients with BI-RADS category 4 lesions
was 82.5%.
Discussion
In the present study, 99mTc-tetrofosmin
breast scintigraphy acquired with a BSGC proved a very highly
accurate diagnostic tool in the detection of primary breast cancer.
In agreement with previous studies (8–10),
the procedure demonstrated a very high sensitivity, even in the
identification of carcinomas at a very early stage, such as
clinically occult or subcentimetric invasive carcinomas and
carcinomas in situ, while maintaining a high specificity.
Moreover, when compared with Mx, scintigraphy had an incremental
value in more than one third of cases with the improvement of both
sensitivity and specificity values, partially overcoming the
limitations of Mx.
In particular, in our series breast scintigraphy
detected 26 primary breast carcinomas of small size majority all
missed at Mx (BI-RADS category 1) for dense breast. Moreover,
scintigraphy proved more accurate than Mx in the preoperative
assessment of local disease extension, detecting a higher number of
occult additional tumor foci ipsilateral or contralateral to the
proven index cancer in respect of the findings ascertained at Mx;
scintigraphy also proved more suitable than Mx in the
identification of intraductal tumors sited around invasive
carcinomas, thus changing the local staging. The majority of our
patients downstaged at Mx belonged to BI-RADS 5, a category for
which surgery is indicated; in our series, scintigraphy gave a more
accurate local disease staging in respect of Mx in 64/279 (22.9%)
of patients with BI-RADS 5, and changed the surgical management,
from breast-conservation to more radical surgery, in 18% of overall
breast cancer patients. On the basis of these results, and even
more in the era of breast-conservation therapy, scintigraphy
acquired with a BSGC should be employed in addition to Mx in
patients scheduled to surgery, since it may contribute to achieve a
clearer margin excision and to complete surgical treatment in a
higher number of cases, thus reducing the cases to submit to second
operations and avoiding the risk of local and distant
recurrences.
Scintigraphy also demonstrated a high specificity,
excluding malignancy in 33 patients with suspicious findings at Mx
(BI-RADS 4), and demonstrated a very high negative predictive value
(82.5%) in this category of patients, thus suggesting that the
preoperative addition of breast scintigraphy to Mx should permit a
better selection of patients to submit to biopsy.
In recent years, dynamic breast magnetic resonance
imaging (MRI) is playing a growing role as a useful complementary
tool to Mx in both screening and diagnosis of primary breast cancer
as well as in the preoperative staging of the affected breast in
women with newly diagnosed primary breast cancer given its very
high sensitivity and its independence of breast density (12–14).
MRI has also been recently recommended instead of Mx by the
American Cancer Society (15) in
screening high-risk patients with mammographically dense
breasts.
BSGC scintigraphy has demonstrated its usefulness in
detecting small size (<1 cm) non palpable carcinomas occult at
Mx in women at high risk for breast cancer (16), but no comparative studies between
BSGC scintigraphy and MRI testing their sensitivity and specificity
have been carried out in patients screened for breast cancer.
However, these two procedures have been compared in the diagnosis
of primary breast cancer showing similarly high sensitivity values
(89% for breast scintigraphy and 100% for MRI), while specificity
was markedly higher for breast scintigraphy (71 vs 25%) (17). BSGC scintigraphy and MRI have also
been compared in a series of patients with newly ascertained
primary breast cancer scheduled to surgery, achieving a correct
preoperative assessment of local disease extension in a high
percentage of cases (88.5 and 80%, respectively), but MRI has lead
to an overstaging in a higher number of cases than scintigraphy
(18). The risk of surgically
overtreating patients on the basis of MRI findings thus seems to
exist, as reported by other authors (13). MRI presents other drawbacks such as
high cost, time-consuming and variability in technique and
interpretation criteria, and it requires very experienced
radiologists. Moreover, the procedure is influenced by menstrual
phase and it is also contraindicated in selected patients (i.e.,
those with allergy to contrast medium and with obesity, pacemakers,
aneurysm clips or severe claustrophobia). Thus, BSGC scintigraphy
could represent an alternative to MRI in selected cases, as also
suggested by the SNM practice guidelines (7). Moreover, BSGC scintigraphy is
relatively less expensive (3–5 times less than MRI), simple to
perform and well tolerated by the patients, and gives very high
resolution images, easy and quick to read and well comparable with
mammographic findings. However, also this scintigraphic procedure
presents some limitations, such as radiation exposure that could
limit its routine use, especially in screening programs. Tumor size
seemed to represent the most important factor affecting the
performance of scintigraphy, since the majority of false-negative
findings were related to subcentimetric carcinomas. Moreover,
lesion site may also affect sensitivity, since a high percentage of
small tumors negative at breast scintigraphy in our series were
located in internal quadrants or were excluded from the field of
view of the device as happened in two cases. The importance of
patient positioning in acquiring breast scintigraphy with dedicated
devices should be always taken into account to avoid patient
misplacement. In our series, the procedure also missed three large
carcinomas, probably due to their low cellularity and slow
proliferative growth, the histological types being mucinous or
lobular. Recent advances in technology could further increase the
performance of BSGC scintigraphy used in the present study due to
the development of dual-head systems which simultaneously acquire
opposing breast views, thus reducing lesion distance to detector
and increasing the sensitivity especially in the detection of small
size lesions. These systems also improve collimation providing a
higher number of counts per pixel in scintigraphic images thus
permitting to reduce the radiotracer dose without affecting the
quality of images.
A larger clinical application of BSGC scintigraphy
is thus suggested although many prospective trials are needed,
particularly with the aim of identifying those subgroups of
patients who would more benefit from scintigraphy employment. In
the era of breast-conservation surgery, the impact of breast
scintigraphy on changing the therapeutic approach in cases with
additional foci detected only by this procedure should also be
further analyzed in terms of improvement in surgical care and
prognosis. However, cost-benefit analysis is needed to justify the
use of BSGC scintigraphy in combination with conventional
diagnostic imaging methods as an adjunctive tool.
Another molecular breast imaging has been recently
tested in primary breast cancer patients as
18F-fluorodeoxyglucose (FDG) positron emission
mammography (PEM) which, like BSGC scintigraphy, has also showed
high sensitivity and good specificity (19,20).
However, no comparative studies between the two procedures have
been carried out in the same patient population. The detectors
employed for both procedures present very similar spatial
resolution; differences in performance thus could possibly be due
to the different characteristics of the radiotracers rather than
the technology.
In conclusion, breast scintigraphy acquired with a
BSGC proved a highly sensitive diagnostic tool, even in small size
carcinoma detection, while maintaining a high specificity. In our
series, this procedure increased both the sensitivity of Mx,
especially in dense breast and in multifocal/multicentric disease,
and the specificity as well as it better defined local tumor
extension, thus guiding the surgeon to a more appropriate surgical
treatment. A wider employment of this procedure is suggested as a
complementary tool to Mx.
Acknowledgements
The study was partly funded by the
Fondazione Banco di Sardegna.
References
|
1
|
Kolb TM, Lichy J and Newhouse JH:
Comparison of the performance of screening mammography, physical
examination, and breast US and evaluation of factors that influence
them: an analysis of 27,825 patient evaluations. Radiology.
225:165–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagios MD, Westdahal PR and Rose MR: The
concept and implications of multicentricity in breast carcinoma.
Pathology Annula. Sommers S and Rosen P: Appleton-Century-Crofts;
New York, NY: 1981, PubMed/NCBI
|
|
3
|
Holland R, Veling SHJ, Mravunac M and
Hendriks JHCL: Histologic multifocality of Tis, T1-2 breast
carcinomas: implications for clinical trials of breast-conserving
surgery. Cancer. 56:979–990. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liberman M, Sampalis F, Mulder DS and
Sampalis JS: Breast cancer diagnosis by scintimammography: a
meta-analysis and review of the literature. Breast Cancer Res
Treat. 80:115–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howarth D, Sillar R, Clark D and Lan L:
Technetium-99m sestamibi scintimammography: the influence of
histopathological characteristics, lesion size and the presence of
carcinoma in situ in the detection of breast carcinoma. Eur J Nucl
Med. 26:1475–1481. 1999. View Article : Google Scholar
|
|
6
|
Khalkhali I, Villanueva-Meyer J, Edell SL,
Connolly JL, Schnitt SJ, Baum JK, Houlihan MJ, Jenkins RM and Haber
SB: Diagnostic accuracy of 99mTc-Sestamibi breast
imaging: multicenter trial results. J Nucl Med. 41:1973–1979.
2000.
|
|
7
|
Goldsmith SJ, Parson W, Guiberteau MJ,
Stern LH, Lanzkowsky L, Weigert J, Heston TF, Jones E, Buscombe J
and Stabin MG: SNM practice guideline for breast scintigraphy with
breast specific γ-cameras 1.0. J Nucl Med Technol. 38:219–224.
2010.PubMed/NCBI
|
|
8
|
Rhodes DJ, O’Connor MK, Phillips SW, Smith
RL and Collins DA: Molecular breast imaging: a new technique using
technetiumTc scintimammography to detect small tumors of the
breast. Mayo Clin Proc. 80:24–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spanu A, Cottu P, Manca A, Chessa F, Sanna
D and Madeddu G: Scintimammography with dedicated breast camera in
unifocal and multifocal/multicentric primary breast cancer
detection: a comparative study with SPECT. Int J Oncol. 31:369–377.
2007.PubMed/NCBI
|
|
10
|
Spanu A, Chessa F, Meloni GB, Sanna D,
Cottu P, Manca A, Nuvoli S and Madeddu G: The role of planar
scintimamography with high-resolution dedicated breast camera in
the diagnosis of primary breast cancer. Clin Nucl Med. 33:739–742.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D’Orsi CJ and Kopans DB: Mammographic
features analysis. Semin Roentgenol. 18:204–230. 1993.
|
|
12
|
Pediconi F, Catalano C, Padula S, Roselli
A, Moriconi E, Pronio AM, Kirchin MA and Passariello R:
Contrast-enhanced magnetic resonance mammography: does it affect
surgical decision-making in patients with breast cancer? Breast
Cancer Res Treat. 106:65–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drew PJ, Chatterjee S, Turnbull LW, Read
J, Carleton PJ, Fox JN, Monson JR and Kerin MJ: Dynamic contrast
enhanced magnetic resonance imaging of the breasts is superior to
triple assessment for the pre-operative detection of multifocal
breast cancer. Ann Surg Oncol. 6:599–603. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Houssami N and Hayes DF: Review of
preoperative magnetic resonance imaging (MRI) in breast cancer:
should MRI be performed on all women with newly diagnosed, early
stage breast cancer? CA Cancer J Clin. 59:290–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saslow D, Boetes C, Burke W, Harms S,
Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith
RA, Warner E, Yaffe M, Andrew KS and Russel CA: American Cancer
Society guidelines for breast screening with MRI as an adjunct to
mammography. CA Cancer J Clin. 57:75–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brem RF, Schoonjans JM, Kieper DA,
Majewski S, Goodman S and Civelek C: High-resolution
scintimammography: a pilot study. J Nucl Med. 43:909–915.
2002.PubMed/NCBI
|
|
17
|
Brem RF, Petrovitch I, Rapelyea JA, Young
H, Teal C and Kelly T: Breast-specific gamma camera imaging with
99mTc-sestamibi and magnetic resonance imaging in the
diagnosis of breast cancer: a comparative study. Breast J.
13:465–469. 2007.PubMed/NCBI
|
|
18
|
Spanu A, Chessa F, Sanna D, Meloni GB,
Sanna D, Cottu P, Nuvoli S and Madeddu G:
99mTc-tetrofosmin Molecular Breast Imaging (MBI) with
high resolution dedicated breast camera (DBC) and breast dynamic
Magnetic Resonance Imaging (MRI) in the preoperative staging of
breast cancer: a comparative study. Eur J Nucl Med Mol Imaging.
36(Suppl. 2): S4712009.
|
|
19
|
Berg WA, Weinberg IN, Narayanan D, Lobrano
ME, Ross E, Amodei L, Tafra L, Adler LP, Uddo J, Stein W III and
Levine EA: High-resolution fluorodeoxyglucose positron emission
tomography with compression (positron emission mammography) is
highly accurate in depicting primary breast cancer. Breast J.
12:309–323. 2006. View Article : Google Scholar
|
|
20
|
Schilling K, Narayanan D, Kalinyak JE, The
J, Velasquez MV, Kahn S, Saady M, Mahal R and Chrystal L: Positron
emission mammography in breast cancer presurgical planning:
comparison with magnetic resonance imaging. Eur J Nucl Med Mol
Imaging. 38:23–36. 2011. View Article : Google Scholar : PubMed/NCBI
|