Introduction
Gastric cancer remains a considerable public health
problem worldwide. The incidence and mortality rates of gastric
cancer have decreased gradually. Nevertheless, gastric cancer is
second only to lung cancer as the leading cause of cancer death
around the world (1,2). Helicobacter pylori (H.
pylori) infection is now accepted as a crucial event in the
development of gastric cancer, although the etiology of this tumor
remains unclear. This infection first induces chronic superficial
gastritis, which can progress to chronic atrophic gastritis,
intestinal metaplasia, and dysplasia that leads toward gastric
carcinoma (3–6). However, only a small number of
infected patients actually develop gastric cancer. This suggests
that host genetic factors, such as genes associated with
inflammatory responses and acid secretion, may also play an
important role in gastric carcinogenesis. Therefore, the
associations between genetic polymorphisms and gastric
carcinogenesis have been investigated in several studies (7–11).
We have also revealed the significant association of polymorphisms
in TLR2 (12), MIF
(13), IL17A (14) and pre-microRNAs (15) with the susceptibility to gastric
carcinogenesis.
On the other hand, the stomach is exceedingly rich
in the peptide hormone- or active amine-producing cells such as
enterochromaffin-like (ECL) cells (histamine), D cells
(somatostatin), EC cells (serotonin), and G cells (gastrin)
(16). Histamine, one of the
active amine released in response to a variety of physiological
stimuli, is well known to be involved in the pathogenesis of
gastro-duodenal ulceration and gastric inflammation (17). Although this bio-active amine
modulates a variety of functions via interacting with specific
receptors on the target cells, H1, H2 and
H3 receptors (18),
H2 receptor has a central role only in the regulation of
acid secretion in stomach as confirmed by the widespread use of
H2 receptor blockers in the therapy of acid-related
disorders (19,20). H. pylori infection as the
main cause of gastric and duodenal ulcer heralded a new revolution
in our understanding and treatment of acid-peptic disorders
(21,22). Evidence was also provided that
increased gastric histamine contributed to the inflammatory changes
and tissue damage associated with chronic H. pylori
infection of the gastric mucosa (23,24).
Thus, intra-gastric histamine plays an important role on the
gastric inflammation acting via H2 receptor, although
H. pylori infection is one of the major contributing factors
to the development of gastro-duodenal inflammation (25).
The association between genetic polymorphisms of
histamine receptor genes and the susceptibility to psychological
and neurological disorders has been investigated (26,27).
The rs2607474 (−1018 G>A) focused in these studies is located in
an enhancer element of the HRH2 promoter, encoding histamine
H2 receptor (26). It
is likely that the HRH2 variant located in the promoter may
induce changes in the expression of receptors. Although the
investigators in the gastroenterological field have shown great
interest in histamine H2 receptors for a long time,
there has been no report whether −1018 G>A polymorphism
(rs2607474) affect on the development of and susceptibility to
gastrointestinal disorders, including gastric cancer, or not.
Furthermore, non-synonymous SNP (rs79385261, 137 A>C, Asn46Thr)
was published in dbSNP of NCBI (http://www.ncbi.nlm.nih.gov/snp). The distribution of
this genotype in Japanese is still unknown.
This study is aimed to test the hypothesis that
genetic alteration in HRH2, causing changes in the
expression, may cause an increased risk for gastric carcinogenesis.
We investigated the association between HRH2 −1018 G>A
(rs2607474) and gastric carcinogenesis. In addition, the influence
of rs79385261 (Asn46Thr) was also investigated.
Materials and methods
Clinical samples
As a gastric cancer group, 321 patients with gastric
cancer (GC group), who were enrolled at the Endoscopy Center of
Fujita Health University Hospital or Kanazawa Medical University
Hospital from January 2007 to December 2009, were selected. The
diagnoses of all gastric cancers were done histologically at the
Division of Pathology of our hospitals. As a control, 599 subjects
without malignant neoplasm on endoscopic examination were randomly
selected from our stocked DNA collected during the same period
(non-GC group). Finally, the studied population comprised 920
subjects, whose polymorphisms could be clearly analyzed. The
patients with severe systemic diseases, malignancies in other
organs, and who had received non-steroidal anti-inflammatory drugs,
antibiotics, and H. pylori eradication treatment were
excluded.
All subjects underwent upper gastrointestinal
endoscopy and, in some of them, biopsy specimens were taken from
non-cancerous mucosa. Parts of each specimen were fixed in 10%
buffered formalin and embedded in paraffin. Later, the degree of
gastritis was evaluated. The genomic DNA was isolated from
peripheral blood using FlexiGene DNA Kit (Qiagen GmbH, Hilden,
Germany).
The Ethics Committees of Fujita Health University
and Kanazawa Medical University approved the protocol, and written
informed consent was obtained from all of the participating
subjects.
Detection of H. pylori infection
H. pylori infection status was assessed by
serology, histological examination, or the urea breath test.
Patients were diagnosed as having infection when at least one of
the diagnostic tests was positive.
Genotyping of polymorphisms
Sample stocked DNA isolated from peripheral blood
was used. Polymorphisms were geno-typed by the multiplex PCR-SSCP
method as reported previously (14,28).
To detect −1018 G>A and Asn46Thr (A>C) genotypes, using the
primer pairs (−1018 forward: 5′-acctgaccc ttttctgaaaaagtttgtc-3′
and −1018 reverse: 5′-ctactcctctgaagtgctg agaaccat-3′ for −1018
G>A; and 46 forward: 5′-aatgtggtcgtctg tctggccgt-3′ and 46
reverse: 5′-agagcatcacatccaggctggtg-3′ for Asn46Thr; respectively),
one-tube multiplex PCR was carried out in a volume of 20-μl
containing 0.1 μg of genomic DNA. The DNA was denatured at
95°C for 3 min, followed by 35 cycles at 96°C for 15 sec, 60°C for
30 sec, and 72°C for 30 sec, with final extension at 72°C for 5
min. Thereafter, 2 μl of the PCR product was denatured with
10 μl of formamide (Sigma-Aldrich Co., St. Louis, MO, USA)
at 95°C for 5 min. SSCP was carried out at 6°C using a GenePhor DNA
separation system with GeneGel Excel 12.5/24 (GE Healthcare, USA),
after which the denatured single strand DNA bands were detected
using a DNA Silver Staining Kit (GE Healthcare).
Histological evaluation
In 496 of 599 control subjects, the severity of
chronic gastritis was classified according to the updated Sydney
system (29) by a pathologist who
had no access to any clinical information.
Serological evaluation
The pepsinogen (PG) I/II ratio was calculated based
on the data of the serum PG I and PG II levels measured by
radioimmunoassay in 124 of 599 control subjects. A PG I/II ratio
that showed a decrease in proportion to the severity of gastric
mucosal atrophy was used as a marker of atrophic gastritis
(30,31).
Statistical analysis
The data were expressed as mean ± SD. Mean ages
between 2 groups was compared by Student's t-test. The ratios of
H. pylori infection status and male/female were compared by
Fisher's exact test. Allele and genotype frequencies were
calculated by direct counting. The allele counts were also compared
by a Fisher's exact test. The strength of association between
allele frequencies and the disease was assessed by calculating the
odds ratio (OR) and 95% confidence intervals (CI) by logistic
regression analysis. Adjusted ORs were calculated after adjustment
for age, gender and H. pylori infection status. Each updated
Sydney system scores and PG I/II ratio between 2 groups were
compared by Mann-Whitney U-test. The relationship between age and
updated Sydney system score was assessed by ANOVA. Concerning the
power of study, when setting α=0.05, β-value was calculated. For
all analyses, the level of significance was set at p<0.05.
Results
Characteristics of subjects and the
frequencies of genotypes
Single strand DNAs of HRH2 genotypes were
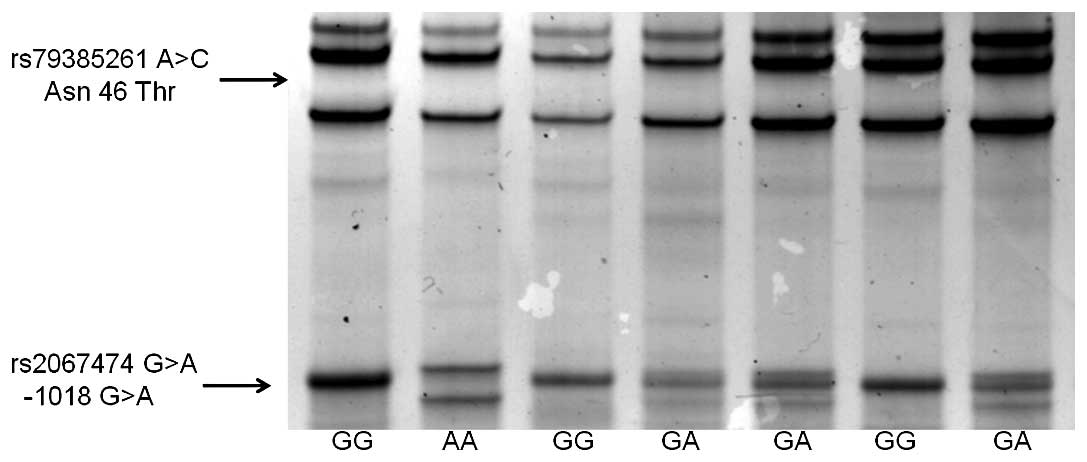
clearly separated by SSCP (Fig.
1). The minor allele of rs79385261 (Asn46Thr) could not be
detected in any subject. The distribution of −1018 G>A genotype
in control subjects was 447GG, 142GA and 10AA (Table I). It was in the Hardy-Weinberg
equilibrium (p=0.86).
 | Table ICharacteristics of the subjects and
frequencies of genotypes. |
Table I
Characteristics of the subjects and
frequencies of genotypes.
| Non-GC group | GC group | p-valuea |
|---|
| No. of
subjects | 599 | 321 | |
| Mean age ± SD | 61.7±13.2 | 65.4±11.0 | <0.0001 |
| Male:female | 345:254 | 224:97 | 0.00028 |
| H. pylori
positive ratio | 61.2% | 86.0% | <0.0001 |
| HRH2
genotype | | | |
| G/G | 447 | 269 | 0.00078b |
| G/A | 142 | 51 | |
| A/A | 10 | 1 | |
| A allele
frequency | 13.5% | 8.26% | 0.00077c |
The characteristics of subjects in this study are
summarized in Table I. The mean
age, male/female ratio and H. pylori positive ratio were
significantly higher in GC group than non-GC group. The
distribution of −1018 G>A genotype in GC group was 269GG, 51GA
and 1AA (HWE, p=0.71). The −1018 G>A minor allele frequencies in
GC and non-GC groups were 8.26% and 13.5%, respectively (p=0.00077
and β=0.93). In addition, the frequency of −1018 GG homozygote was
significantly different among GC and non-GC groups (p=0.00078 and
β=0.91).
Association between HRH2 −1018 G>A and
gastric carcinogenesis
Overall, −1018 GG homozygote had a significantly
increased risk for gastric carcinogenesis by logistic regression
analysis after adjustment for age, gender and H. pylori
infection status (OR 1.68; 95% CI 1.17–2.42; p=0.0052; Table II). When assessed by subtypes of
gastric cancer, −1018 GG homozygote had a more increased risk for
the development of intestinal type of cancer (OR 1.94; 95% CI
1.23–3.08; p=0.0047, Table II),
whereas no significant association was found between this genotype
and diffuse type of cancer.
 | Table IIThe risk of HRH2 polymorphism
(−1018 G>A) for gastric carcinogenesis. |
Table II
The risk of HRH2 polymorphism
(−1018 G>A) for gastric carcinogenesis.
| GG | GA | AA | GG vs. A carrier;
OR (95% CI) | p-value |
|---|
| Non-GC | 447 | 142 | 10 | reference | - |
| Overall GC | 269 | 51 | 1 | 1.68
(1.17–2.42) | 0.0052 |
| Intestinal | 163 | 27 | 0 | 1.94
(1.23–3.08) | 0.0047 |
| Diffuse | 104 | 23 | 1 | 1.36
(0.838–2.20) | 0.21 |
| (Unknown) | 2 | 1 | 0 | - | - |
In the subjects aged <60 years, HRH2 −1018
G>A was not associated with gastric carcinogenesis (Table III). On the other hand, in the
subjects >60 years, −1018 GG homozygote had an increased risk
for the development of gastric cancer (OR 1.87; 95% CI 1.19–2.93;
p=0.0065).
 | Table IIIThe risk of HRH2 gene
polymorphism (−1018 G>A) for gastric carcinogenesis in the
subjects aged <60 years or >60 years. |
Table III
The risk of HRH2 gene
polymorphism (−1018 G>A) for gastric carcinogenesis in the
subjects aged <60 years or >60 years.
| No. of
subjects | GG | GA | AA | GG vs. A carrier;
OR (95% CI) | p-value |
|---|
| <60 | | | | | | |
| Non-GC | 219 | 164 | 51 | 4 | reference | - |
| GC | 100 | 81 | 19 | 0 | 1.60
(0.842–3.04) | 0.15 |
| ≥60 | | | | | | |
| Non-GC | 379 | 282 | 91 | 6 | reference | - |
| GC | 221 | 188 | 32 | 1 | 1.87
(1.19–2.93) | 0.0065 |
Association between HRH2 polymorphism
(−1018 G>A) and clinicopathological features of gastric
cancer
We investigated the influences of genetic
polymorphisms on the progression of gastric cancer using various
parameters of clinicopathological features. The HRH2 −1018
GG homozygote was significantly associated with the increased risk
for the development of gastric cancer located at lower third of
stomach (OR 2.26; 95% CI 1.28–3.99; p=0.0050, Table IV). When assessed by tumor stages,
−1018 GG homozygote was associated with the increased risk for the
cases invaded beyond musclaris propria (OR 1.96; 95% CI 1.19–3.23;
p=0.0078). Regarding as lymph node metastasis, this genotype had an
increased risk for the cases both with and without lymph node
metastasis (OR 1.99; 95% CI 1.16–3.43; p=0.013 and OR 1.63; 95% CI
1.05–2.54; p=0.031, respectively).
 | Table IVAssociation between HRH2 −1018
G>A and clinicopathological features of gastric cancer. |
Table IV
Association between HRH2 −1018
G>A and clinicopathological features of gastric cancer.
| No. of
subjects | GG | GA | AA | GG vs. A carrier;
OR (95% CI) | p-value |
|---|
| Non-GC | 599 | 447 | 142 | 10 | reference | |
| Location | | | | | | |
| Upper third | 18 | 13 | 5 | 0 | 0.862
(0.297–2.50) | 0.78 |
| Middle third | 164 | 134 | 29 | 1 | 1.44
(0.917–2.26) | 0.11 |
| Lower third | 127 | 111 | 16 | 0 | 2.26
(1.28–3.99) | 0.0050 |
| Stage | | | | | | |
| ≤T1 | 159 | 131 | 27 | 1 | 1.53
(0.961–2.43) | 0.073 |
| ≥T2 | 156 | 134 | 22 | 0 | 1.96
(1.19–3.23) | 0.0078 |
| Lymph node
metastasis | | | | | | |
| n (−) | 183 | 152 | 30 | 1 | 1.63
(1.05–2.54) | 0.031 |
| n (+) | 131 | 113 | 18 | 0 | 1.99
(1.16–3.43) | 0.013 |
Histological evaluations of gastritis
among genotypes of HRH2 (−1018 G>A)
In 496 control subjects evaluated for histological
gastritis, the distribution of genotype was 397GG, 96GA and 3AA.
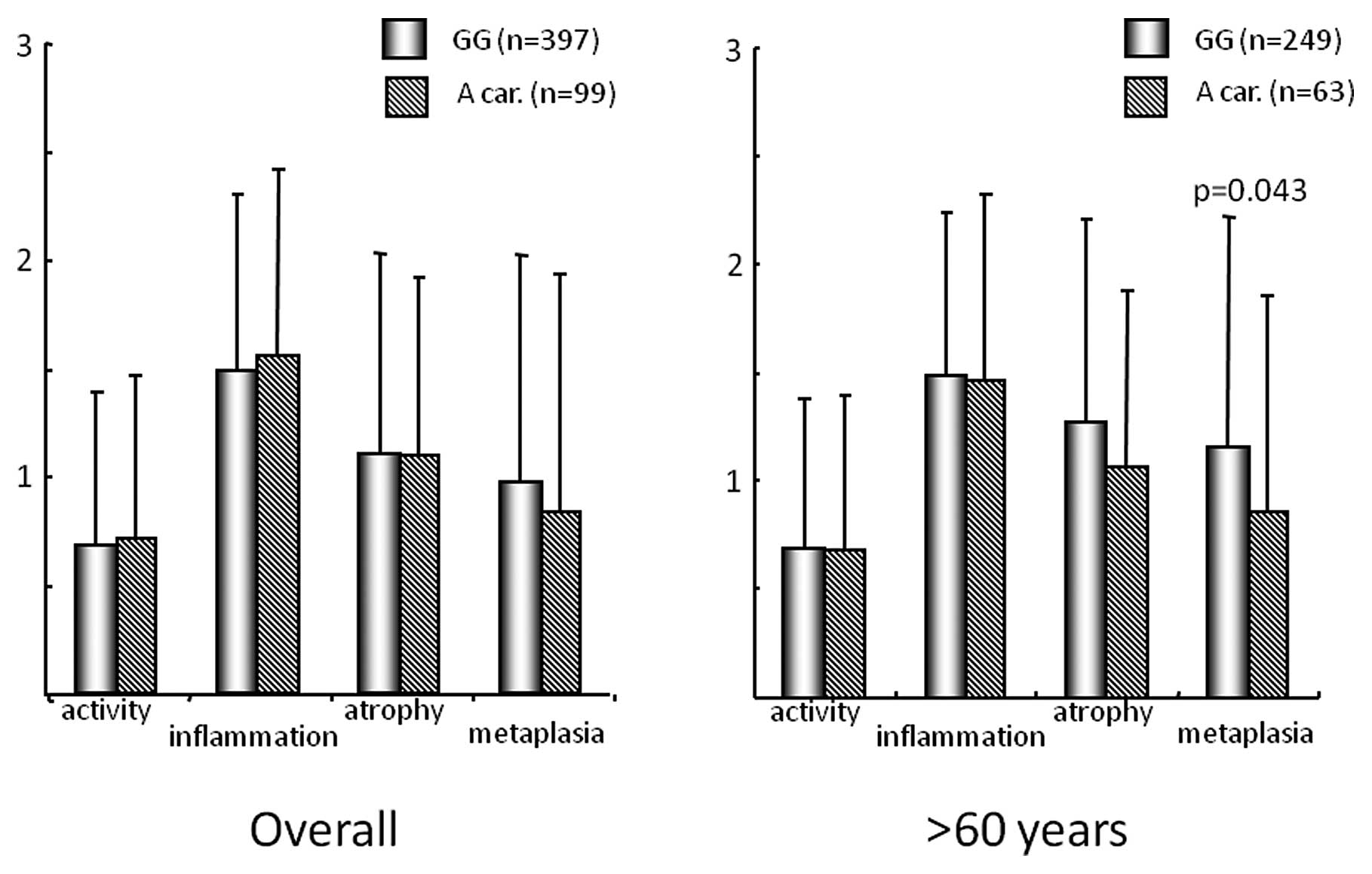
Overall, each updated Sydney system score was not different among
−1018 GG homozygote and A carrier (Fig. 2). However, in the subjects aged
>60 years, metaplasia score was significantly higher in −1018 GG
homozygote than A carrier (p=0.043).
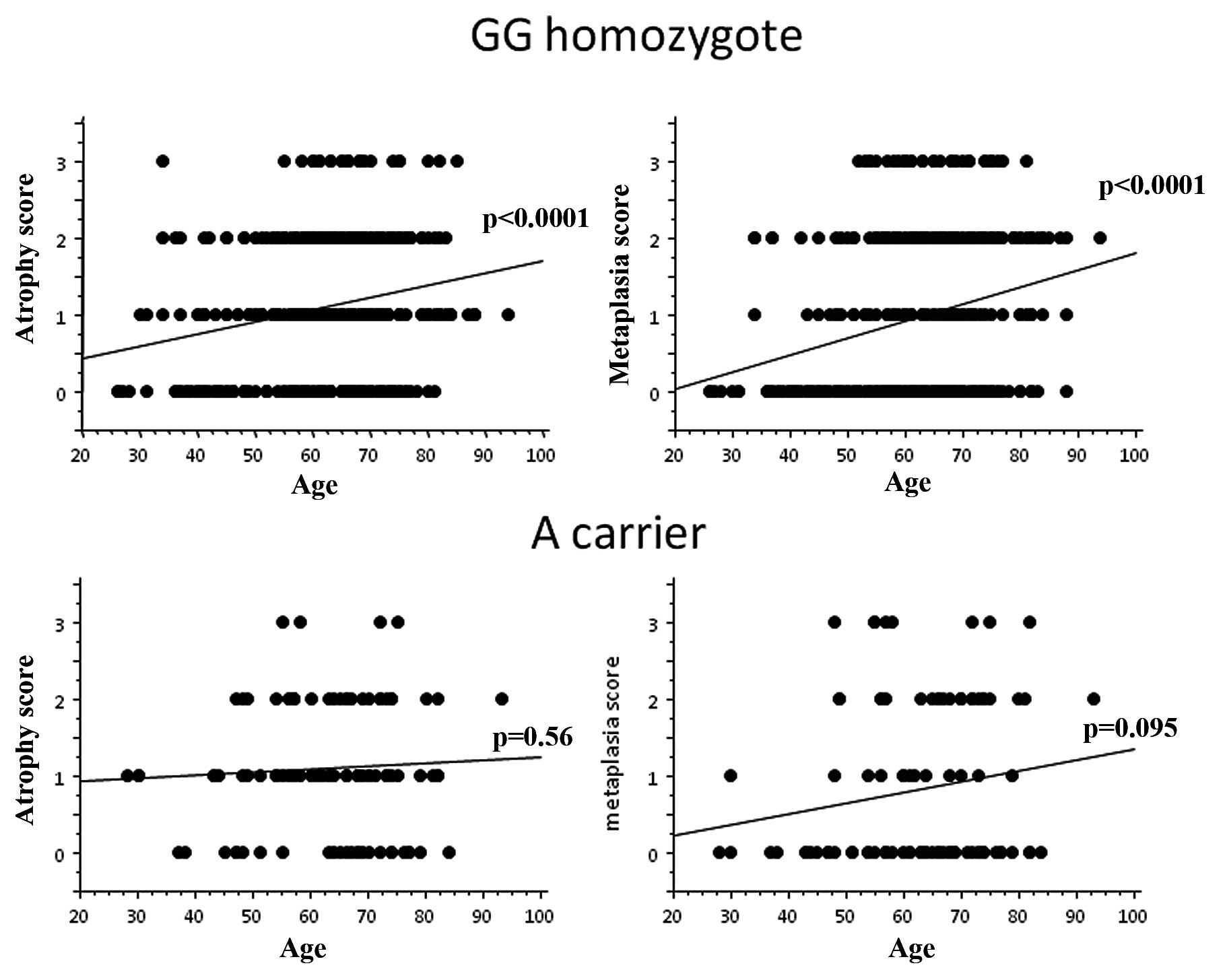
In addition, in −1018 GG homozygote, both atrophy
and metaplasia scores were significantly increased with age (both
p-values by ANOVA: p<0.0001, Fig.
3). In A carrier, however, neither atrophy nor metaplasia score
was significantly related to age.
Serum pepsinogen levels between −1018 GG
homozygote and A carrier
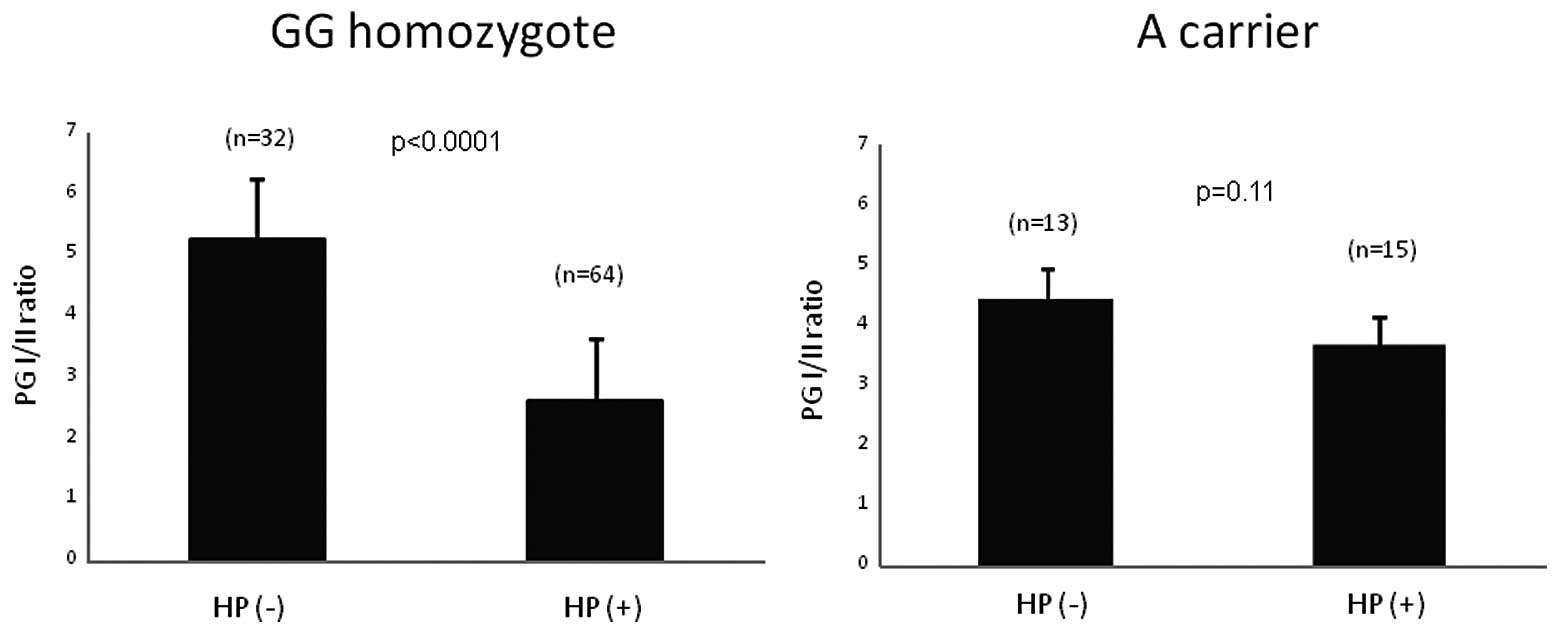
In 124 control subjects measured for serum PG
levels, 79 were H. pylori positive and 45 were negative. The
distribution of genotype in H. pylori positive was 64GG,
14GA and 1AA, whereas the distribution in H. pylori negative
was 32GG, 12GA and 1AA. In −1018 GG homozygote, PG I/II ratio was
significantly decreased under influence of H. pylori
infection (p<0.0001), whereas there was no significant
difference among H. pylori positive and negative subjects in
A carrier (Fig. 4). In H.
pylori positive subjects, PG I/II ratio was significantly lower
in GG homozygote than A carrier (p=0.036).
Discussion
In the present study, we investigated the
association between polymorphisms of HRH2, encoding
histamine H2 receptor, and gastric carcinogenesis. A
minor allele of Asn46Thr (rs79385261 A>C) could not be detected
in our subjects. So, only −1018 G>A (rs2607474), genotype of
which was in the Hardy-Weinberg equilibrium, was investigated. We
found a strongly increased association between −1018 GG homozygote
and gastric carcinogenesis, especially intestinal type of cancer.
This strong association was found in the cases with tumor located
at antrum and at the comparatively advanced stage. In addition, it
was also shown that gastric mucosal atrophy was more rapidly
progressed with age and under influence of H. pylori
infection in −1018 GG homozygote than A carrier. The HRH2
−1018 A allele frequency in non-GC group was 13.5%. This frequency
was slightly higher than that reported in Hap-Map JPT and slightly
lower than that reported in Japanese by Ito et al (32), although it was lower in Caucasians
(26,27). One limitation of this study was
that our subjects (both cases and controls) came to our hospitals
in order to have endoscopic examination for the complaint of
abdominal discomfort, or for complete check up of gastric cancer
following barium X-ray examination in the health check, not
completely healthy subjects. Therefore, minor allele frequency
might be comparatively high in our study. Another limitation was
that mean age, H. pylori infection ratio and male/female
ratio were higher in GC group than non-GC group. However, the
adjustment for age, gender and H. pylori infection status
was performed in genotype analysis using logistic regression.
There have been few reports that investigated the
influence of polymorphisms of HRH2 in the risk for human
disorders. Most of such studies revealed no association between
HRH2 −1018 G>A polymorphism and psychological or
neurological disorder (26,27,32).
On the other hand, there is no report on the association between
this polymorphism and gastric carcinogenesis. Our results provided
the first evidence that HRH2 −1018 G>A polymorphism was
significantly associated with the gastric carcinogenesis.
It has been well known that H. pylori
infection has a major role on the progression of gastric mucosal
atrophy, subsequently the development of gastric cancer. The
factors promoting H. pylori-mediated gastric atrophy have
been somewhat more controversial. H. pylori infection
results in an elevation in serum gastrin level in the early stage
of infection and precedes the development of atrophic gastritis.
Gastrin acting on ECL cell leads to increased histamine release,
which stimulate acid secretion through histamine H2
receptors on parietal cells. A hypergastrinemic mouse at the age of
5 months later shows a marked decline in acid secretion with the
spontaneous development of gastric atrophy, metaplasia, and
invasive cancer that can be markedly accelerated by concurrent
Helicobacter infection (33,34).
In addition, the majority of clinical studies have accepted that
proton pump inhibitors (PPIs), which induce achlorhydria and
hypergastrinemia, accelerate the onset of atrophic gastritis in
H. pylori-positive patients (35–37).
The above suggests that hypergastrinemia and/or insufficient acid
secretion may promote the gastric mucosal atrophy under influence
of H. pylori infection. However, Takaishi et al have
demonstrated that the gastrin-histamine axis contributes to the
development of gastric atrophy and neoplasia in a mouse model
(38). In contrast to the effects
of hypergastrinemia seen in gastrin transgenic mice, long-term
treatment of rats and mice with loxtidine, one of potent histamine
H2 receptor antagonists and inducing the ECL cells
hyperplasia after long treatment as well as omeprazole (39), did not result in loss of parietal
cells but instead appeared to result in increased parietal cells
(40,41). Histidine decarboxylase knockout
(HDC−/−) mice kept on a lowhistamine diet showed an expanded
parietal cell pool despite exhibiting marked hypergastrinemia
(42). In addition, histamine has
been shown to be important in modulating parietal cell maturation
through H2 receptors (43,44).
These observations suggest that not only the influence of
hypergastrinemia but up-regulated action of histamine with
hypergastrinemia may contribute to the gradual down-regulation of
parietal cell number, gastric atrophy.
There is no report whether HRH2 −1018 G>A
polymorphism affect on the expression and function of histamine
H2 receptors or not, although understanding the effect
of this polymorphism on the histamine signal via H2
receptor is informative. It is likely that the HRH2 genome
variant located promoter may induce changes in the expression of
receptors. In our current study, age-related gastric mucosal
atrophy gradually and rapidly progress in HRH2 −1018 GG
homozygote than −1018 A carrier. In addition, PG I/II ratio was
significantly decreased in only −1018 GG homozygotes under the
influence of H. pylori infection. These findings suggested
that the action of histamine may be up-regulated in −1018 GG
homozygote and A allele may be a loss of function allele.
According to the Lauren classification (45), there are two histologically
distinct types of gastric cancer. The intestinal type develops in
stomachs affected by chronic inflammation with passing through the
intermediate steps of atrophic gastritis or intestinal metaplasia
(46). On the other hand, the
severity of mucosal inflammation and various host features may
directly induce mutagenetic events that ultimately lead to the
onset of the diffuse type. Therefore, intestinal type of cancer
tends to arise at antrum, because more severe gastric atrophy and
metaplasia develop in the early stage of H. pylori infection
and rapidly progress at antrum. In our results, −1018 GG homozygote
was associated with intestinal type of gastric cancer, with the
cases in comparative older subjects and located at antrum. These
findings suggest that −1018 GG homozygote may have an increased
risk of which gastric mucosal atrophy progress more rapidly with
age and intestinal type of gastric cancer occur as a result.
Our data showed that −1018 GG homozygote was more
closely associated with the cases at comparatively advanced stage,
invaded beyond muscularis propria and with lymph node metastasis.
This result suggests that −1018 GG homozygote may be associated
with the gastric cancer progression, as well as development.
Previous reports suggested that cimetidine, one of H2
receptor antagonists, might be considered as an anti-cancer agent.
In 1988, it was firstly reported that post-operative treatment with
cimetidine improved survival in gastric cancer patients of all
stages (47). This effect of
cimetidine is considered to be mediated by H2 receptor
blockade of suppressor T-lymphocytes, leading to their functional
inhibition and stimulation of natural killer cell activity
(48,49) and antagonism of
histamine-stimulated growth (50).
Thus, histamine seemed to promote the tumor growth by actions other
than stimulation of gastric acid secretion, followed by gastric
mucosal atrophy. These actions of histamine may more rapidly
progress the gastric cancer in −1018 GG homozygotes.
In conclusion, the current findings indicate that
the HRH2 −1018 G>A polymorphism (rs2607474) may be
associated with the susceptibility to gastric carcinogenesis in
Japanese population. The −1018 GG homozygote may have an increased
risk for the rapid progression of severe gastric mucosal atrophy
and the subsequent development of intestinal type gastric
cancer.
References
|
1
|
Murray CJ and Lopez AD: Global mortality,
disability, and the contribution of risk factors: Global Burden of
Disease Study. Lancet. 349:1436–1442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2002.
View Article : Google Scholar
|
|
3
|
Correa P: Human gastric carcinogenesis: a
multistep and multifactorial process. First American Cancer Society
Award Lecture on Cancer Epidemiology and Prevention. Cancer Res.
52:6735–6740. 1992.
|
|
4
|
Suerbaum S and Michetti P: Helicobacter
pylori infection. N Engl J Med. 347:1175–1186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Covacci A, Telford JL, Del GG, Giudice GD,
Parsonnet J and Rappuoli R: Helicobacter pylori virlence and
genetic geography. Science. 284:1328–1333. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uemura N, Okamoto S, Yamamoto S, et al:
Helicobacter pylori infection and development of gastric cancer. N
Engl J Med. 345:784–789. 2001. View Article : Google Scholar
|
|
7
|
El-Omar EM, Carrington M, Chow WH, et al:
Interleukin-1 polymorphisms associated with increased risk of
gastric cancer. Nature. 404:398–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furuta T, El-Omar EM, Xiao F, Shirai N,
Takashima M and Sugimura H: Interleukin 1β polymorphisms increase
risk of hypochlorhydria and atrophic gastritis and reduce risk of
duodenal ulcer recurrence in Japan. Gastroenterology. 123:92–105.
2002.
|
|
9
|
Machado JC, Figueiredo C, Canedo P, et al:
A proinflammatory genetic profile increases the risk for chronic
atrophic gastritis and gastric carcinoma. Gastroenterology.
125:364–371. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohyauchi M, Imatani A, Yonechi M, et al:
The polymorphism interleukin 8–251 A/T influences the
susceptibility of Helicobacter pylori related gastric diseases in
the Japanese population. Gut. 54:330–335. 2005.
|
|
11
|
Ohmiya N, Taguchi A, Mabuchi N, et al:
MDM2 promoter polymorphism is associated with both an increased
susceptibility to gastric carcinoma and poor prognosis. J Clin
Oncol. 24:4434–4440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tahara T, Arisawa T, Wang F, et al:
Toll-like receptor 2 -196 to 174del polymorphism influences the
susceptibility of Japanese people to gastric cancer. Cancer Sci.
98:1790–1794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arisawa T, Tahara T, Shibata T, et al:
Functional promoter polymorphisms of the macrophage migration
inhibitory factor gene in gastric carcinogenesis. Oncol Rep.
19:223–228. 2008.PubMed/NCBI
|
|
14
|
Shibata T, Tahara T, Hirata I and Arisawa
T: Genetic polymorphism of interleukin-17A and -17F genes in
gastric carcinogenesis. Hum Immunol. 70:547–551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okubo M, Tahara T, Shibata T, et al:
Association between common genetic variants in pre-microRNAs and
gastric cancer risk in Japanese population. Helicobacter.
15:524–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen D, Aihara T, Zhao CM, Hakanson R and
Okabe S: Differentiation of the Gastric Mucosa I. Role of histamine
in control of function and integrity of oxyntic mucosa: 1.
understanding gastric physiology through disruption of targeted
genes. Am J Physiol Gastrointest Liver Physiol. 291:G539–G544.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rangachari PK: Histamine: mercurial
messenger in the gut. Am J Physiol. 262:G1–G13. 1992.PubMed/NCBI
|
|
18
|
Hill SJ, Ganellin CR, Timmerman H, et al:
International Union of Pharmacology. XIII Classification of
histamine receptors. Pharmacol Rev. 49:253–278. 1997.PubMed/NCBI
|
|
19
|
Black J, Duncan W and Durant D: Definition
and antagonism of histamine H2-receptors. Nature. 236:385–390.
1972. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones DB, Howden CW, Burget DW, et al:
Acid suppression in duodenal ulcer: a meta-analysis to define
optimal dosing with antisecretory drugs. Gut. 28:1120–1127. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marshall BJ and Warren JR: Unidentified
curved bacilli in the stomach of patients with gastritis and peptic
ulcer. Lancet. 1:1311–1315. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McColl KEL, El-Omar E and Gillen D:
Interactions between H. pylori infection, gastric acid secretion
and anti-secretory therapy. Br Med Bull. 54:121–138. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Courillon-Mallet A, Launay JM, Roucayrol
AM, et al: Helicobacter pylori infection: physiopathologic
implication of N-methylhistamine. Gastroenterology. 108:959–966.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McGowan CC, Cover TL and Blaser MJ:
Helicobacter pylori and gastric acid: biological and therapeutic
implications. Gastroenterology. 110:926–938. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bodger K and Crabtree JE: Helicobacter
pylori and gastric inflammation. Br Med Bull. 54:139–150. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mancama D, Arranz MJ, Munro J, et al:
Investigation of promoter variants of the histamine 1 and 2
receptors in schizophrenia and clozapine response. Neurosci Lett.
333:207–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garcia-Martin E, Ayuso P, Luengo A,
Martinez C and Agundez JAG: Genetic variability of histamine
receptors in patients with Parkinson's disease. BMC Med Genet.
9:152008.PubMed/NCBI
|
|
28
|
Arisawa T, Tahara T, Shibata T, et al: The
influence of polymorphisms of interleukin-17A and interleukin-17F
genes on the susceptibility to ulcerative colitis. J Clin Immunol.
28:44–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis: the updated Sydney
system. Am J Surg Pathol. 20:1161–1181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang GH, Shim YH, Jung HY, Kim WH, Ro JY
and Rhyu MG: CpG island methylation in premalignant stages of
gastric carcinoma. Cancer Res. 61:2847–2851. 2001.PubMed/NCBI
|
|
31
|
Pestov DG, Strezoska Z and Lau LF:
Evidence of p53-dependent cross-talk between ribosome biogenesis
and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S
transition. Mol Cell Biol. 21:4246–4255. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ito C, Morisset S, Krebs MO, et al:
Histamine H2 receptor gene variants: lack of association with
schizophrenia. Mol Psychiatry. 5:159–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fox JG, Rogers AB, Ihrig M, et al:
Helicobacter pylori-associated gastric cancer in INSGAS mice is
gender specific. Cancer Res. 63:942–950. 2003.PubMed/NCBI
|
|
34
|
Wang TC, Dangler CA, Chen D, et al:
Synergistic interaction between hypergastrinemia and Helicobacter
infection in a mouse model of gastric cancer. Gastroenterology.
118:36–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kuipers EJ, Uyterlinde AM, Pena AS, et al:
Increase of Helicobacter pylori-associated corpus gastritis during
acid suppressive therapy: implications for long-term safety. Am J
Gastroenterol. 90:1401–1406. 1995.PubMed/NCBI
|
|
36
|
Kuipers EJ, Lundell L, Klinkenberg-Knol
EC, et al: Atrophic gastritis and Helicobacter pylori infection in
patients with reflux esophagitis treated with omeprazole or
fundoplication. N Engl J Med. 334:1018–1022. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berstad AE, Hatlebakk JG, Maartmann-Moe H,
Berstad A and Brandtzaeg P: Helicobacter pylori gastritis and
epithelial cell proliferation in patients with reflux esophagitis
after treatment with lansoprazole. Gut. 41:740–747. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takaishi S, Cui G, Frederick DM, et al:
Synergistic inhibitory effects of gastrin and histamine receptor
antagonists on Helicobacter-induced gastric cancer.
Gastroenterology. 128:1965–1983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Poynter D and Selway SA: Neuroendocrine
cell hyperplasia and neuroendocrine carcinoma of the rodent fundic
stomach. Mutat Res. 248:303–319. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Brenna E, Swarts HG, Klaassen CH, De Pont
JJ and Waldum HL: Evaluation of the trophic effect of longterm
treatment with the histamine H2 receptor antagonist loxtidine on
rat oxyntic mucosa by differential counting of dispersed cells.
Gut. 35:1547–1550. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cui G, Takaishi S, Ai W, et al:
Gastrin-induced apoptosis contributes to carcinogenesis in the
stomach. Lab Invest. 86:1037–1051. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hunyady B, Zolyomi A, Czimmer J, et al:
Expanded parietal cell pool in transgenic mice unable to synthesize
histamine. Scand J Gastroenterol. 38:133–140. 2003.PubMed/NCBI
|
|
43
|
Ohtsu H and Watanabe T: New functions of
histamine found in histidine decarboxylase gene knockout mice.
Biochem Biophys Res Commun. 305:443–447. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fukushima Y, Matsui T, Saitoh T, et al:
Unique roles of G protein-coupled histamine H2 and gastrin
receptors in growth and differentiation of gastric mucosa. Eur J
Pharmacol. 502:243–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lauren P: The two histologic main types of
gastric carcinoma: diffuse and so-called intestinal type carcinoma.
An attempt at a histo-clinical classification. Acta Pathol
Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
46
|
Go MF: Review article: natural history and
epidemiology of Helicobacter pylori infection. Aliment Pharmacol
Ther. 16:3–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tonnesen H, Knigge U, Bulow S, et al:
Effect of cimetidine on survival after gastric cancer. Lancet.
2:990–992. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Griswold DE, Alessi S, Badger AM, Poste G
and Hanna N: Inhibition of T suppressor cell expression by
histamine type 2 (H2) receptor antagonists. J Immunol.
132:3054–3057. 1984.PubMed/NCBI
|
|
49
|
Kikuchi Y, Oomori K, Kizawa I and Kato K:
Augmented natural killer activity in ovarian cancer patients
treated with cimetidine. Eur J Cancer Clin Oncol. 22:1037–1043.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Watson SA, Wilkinson LJ, Robertson JR and
Hardcastle JD: Effect of histamine on the growth of human
gastro-intestinal tumours: reversal by cimetidine. Gut.
34:1091–1096. 1993. View Article : Google Scholar : PubMed/NCBI
|