Introduction
Gastric cancer is the fourth-most common cancer and
the second leading cause of cancer death worldwide (1,2).
When diagnosed in its early stages, a surgical approach with a
partial or total gastric resection are often successful (3). However, when diagnosed with primarily
unresectable disease or recurrence after surgery (4), the disease is no longer curable
despite systemic chemotherapy with cytotoxic chemotherapeutic
agents, including fluorouracil, cisplatin, doxorubicin and
paclitaxel, either alone or in combination. Response rates to these
chemotherapies are generally less than 50% and median survival time
from diagnosis is less than one year (5). New treatment strategies are therefore
urgently required.
Molecularly targeted agents, particularly those
targeting receptor tyrosine kinases (RTKs), have been successfully
used in clinical settings since the late 1990s. The most promising
of these have generally been those targeting RTKs with genetic
changes causing aberrant activation. These include trastuzumab, a
monoclonal antibody against extracellular domain of human epidermal
growth factor receptor 2 (HER2), in HER2-overexpressing breast
cancer (6); and erlotinib and
gefitinib, which inhibit epidermal growth factor receptor (EGFR)
with somatic mutations in non-small cell lung cancer (7,8). In
gastric cancer, gene amplification in HER2, MET, and
fibroblast growth factor receptor 2 (FGFR2) have been reported in
20%, 20%, and 40% of tumor samples, respectively. While HER2
amplification is predominantly found in the well-differentiated
intestinal subtype (9–14), MET and FGFR2
amplification occur more frequently in the undifferentiated diffuse
subtype (15). The potential of
these RTKs as therapeutic targets in gastric cancer was clinically
demonstrated by the success of the ToGA (Trastuzumab for Gastric
Cancer) study, at least in part (16). This open-label, international,
phase III randomized controlled trial compared cisplatin plus
fluorouracil or capecitabine (reference arm) versus the same
regimens combined with trastuzumab in HER2-overexpressing advanced
gastric cancer. Results showed that the trastuzumab-containing arm
was superior to chemotherapy alone with regard to response rate,
progression-free survival, and overall survival (16).
Although the ToGA trial shed light on the strategy
of targeting gene-amplified RTK in gastric cancer, several
questions remained to be answered, including whether trastuzumab
enhances the effect of chemotherapeutic agents even in
trastuzumab-resistant HER2-amplified gastric cancer cells,
and the control of trastuzumab-resistant HER2-amplified
cells.
Here, we compared the effects of trastuzumab in two
HER2-amplified gastric cancer cell lines in vitro,
one of which has been reported to be sensitive to anti-HER2
monoclonal antibody (NCI-N87) and the second to be insensitive
(MKN-7).
Materials and methods
Cell culture
NCI-N87 cells were purchased from American Type
Culture Collection (ATCC, Manassas, VA, USA). NUGC-4, MKN-7,
KATO-III, MKN-45, MKN-1, and MKN-74 cells were purchased from RIKEN
BioResource Center (Tsukuba, Japan). Both NCI-N87 and MKN-7 cells
have been reported to have amplification of the HER2 gene
(17,18). NCI-N87 cells were reported to be
sensitive to trastuzumab in vitro (19), while MKN-7 cells were shown to be
resistant to monoclonal antibody 4D5, which has the same
antigen-binding fragment (Fab) as trastuzumab (20).
All cell lines were maintained in RPMI-1640
(Cellgro) supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin, 100 U/ml streptomycin, and 2 mM glutamine. All cells
were grown at 37°C in a humidified atmosphere with 5%
CO2 and were in the logarithmic growth phase upon
initiation of the experiments. The cells were passaged for ≤3
months before fresh cells were obtained from frozen early-passage
stocks received from the indicated sources.
Drugs
Evelorimus (RAD001), an inhibitor of mTOR, was
kindly provided by Novartis Pharma (Basel, Switzerland).
Fluorouracil, cisplatin, doxorubicin and paclitaxel were purchased
from Wako (Osaka, Japan). Trastuzumab was obtained from the Kobe
University Hospital Pharmacy. CL-387,785, an inhibitor of
EGFR/HER2, was purchased from Calbiochem (San Diego, CA, USA).
Stock solutions were prepared in dimethyl sulfoxide (DMSO) and
stored at −20°C. The drugs were diluted in fresh media before each
experiment, with final DMSO concentrations less than 0.1%.
Antibodies and western blotting
Cells were washed with ice-cold PBS and scraped
immediately after the addition of lysis buffer [20 mM Tris (pH
7.5), 150 mM NaCl, 10% glycerol, 1% NP40, and 2 mM EDTA] containing
protease and phosphatase inhibitors [100 mM NaF, 1 mM
phenylmethylsulfonyl fluoride (PMSF), 1 mM
Na3VO4, 2 μg/ml aprotinin, and 5 μg/ml
leupeptin). Lysates were centrifuged at 14,000 relative centrifugal
force for 10 min. Supernatants were collected as protein extracts
and then separated by electrophoresis on 7.6% sodium dodecyl
sulfate (SDS)-polyacrylamide gels, followed by transfer to
nitrocellulose membranes (Millipore, Billerica, MA, USA) and
detection by immunoblotting using the enhanced chemiluminescence
system (New England Nuclear Life Science Products, Boston, MA,
USA). HER2/ErbB2 (44E7), phospho-HER2/ErbB2 (Tyr1221/1222)(6B12),
Akt, phospho-Akt (Ser473)(D9E), p70 S6 kinase, phospho-p70 S6
kinase (Thr389), and cleaved-PARP (Asp214)(D64E10) antibodies were
purchased from Cell Signaling Technology (Beverly, MA, USA).
Phospho-EGFR (Y1068), ERK1/2, phospho-ERK1/2 (pT185/pY187)
antibodies were purchased from Invitrogen (Carlsbad, CA, USA), and
β-actin antibody was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Immunoblot quantification was carried out by densitometry
using ImageJ software (21).
Cell growth assay
Growth inhibition was assessed using the MTS assay
(Promega, WI, USA), a colorimetric method for determining the
number of viable cells based on the bioreduction of
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) to a soluble forma-an product, which is detectable by
spectrophotometry at a wavelength of 490 nm. Cells were diluted in
160 μl/well of maintenance cell culture media and plated in 96-well
flat-bottom plates (Corning, Lowell, MA, USA). After a 144-h growth
period, the number of cells required to obtain an optical density
(OD) within the linear range of the assay, 1.3–2.2, was determined
for each cell line with pilot experiments. The number of cells per
well used in the subsequent experiments was as follows: NCI-N87:
5,000; MKN-7: 3,000; KATO-III: 3,000; MKN-1: 2,000; MKN-45: 1,500;
MKN-74: 2,000 and NUGC-4: 2,000. At 24 h after plating, cell
culture media were replaced with 10% FBS containing media with and
without drugs followed by incubation for an additional 120 h. A
total of 6 to 12 replicate wells were established for each
experimental point and all experiments were performed at least in
triplicate. Data are expressed as a percentage of growth relative
to that of untreated control cells.
Combination index (CI)
Interaction between trastuzumab and cytotoxic
chemotherapeutic agents was evaluated by the median-effect method
(CalcuSyn software; Biosoft) to determine the well-established CI
(22), with which CI values ≤1,
and >1 indicate synergistic, additive, and antagonistic effects,
respectively (23). The maximum
concentration of trastuzumab was set at 100 μg/ml, on the basis
that this concentration is close to the maximum plasma
concentration of trastuzumab observed clinically, and has been
widely used in previous studies (24). Maximum concentrations of
fluorouracil, doxorubicin, cisplatin, and paclitaxel was set at 100
μM, 1 μM, 10 μM, and 100 nM, respectively, based on their
approximate IC90 values as determined by pilot
experiments using the NCI-N87 cell line (data not shown). Serial
two-fold dilution with a fixed concentration ratio of
chemotherapeutic drug/trastuzumab was done, and total CI was
represented as the average of CI at each experimental point.
Results
Effect of trastuzumab on cell growth in
gastric cancer cell lines
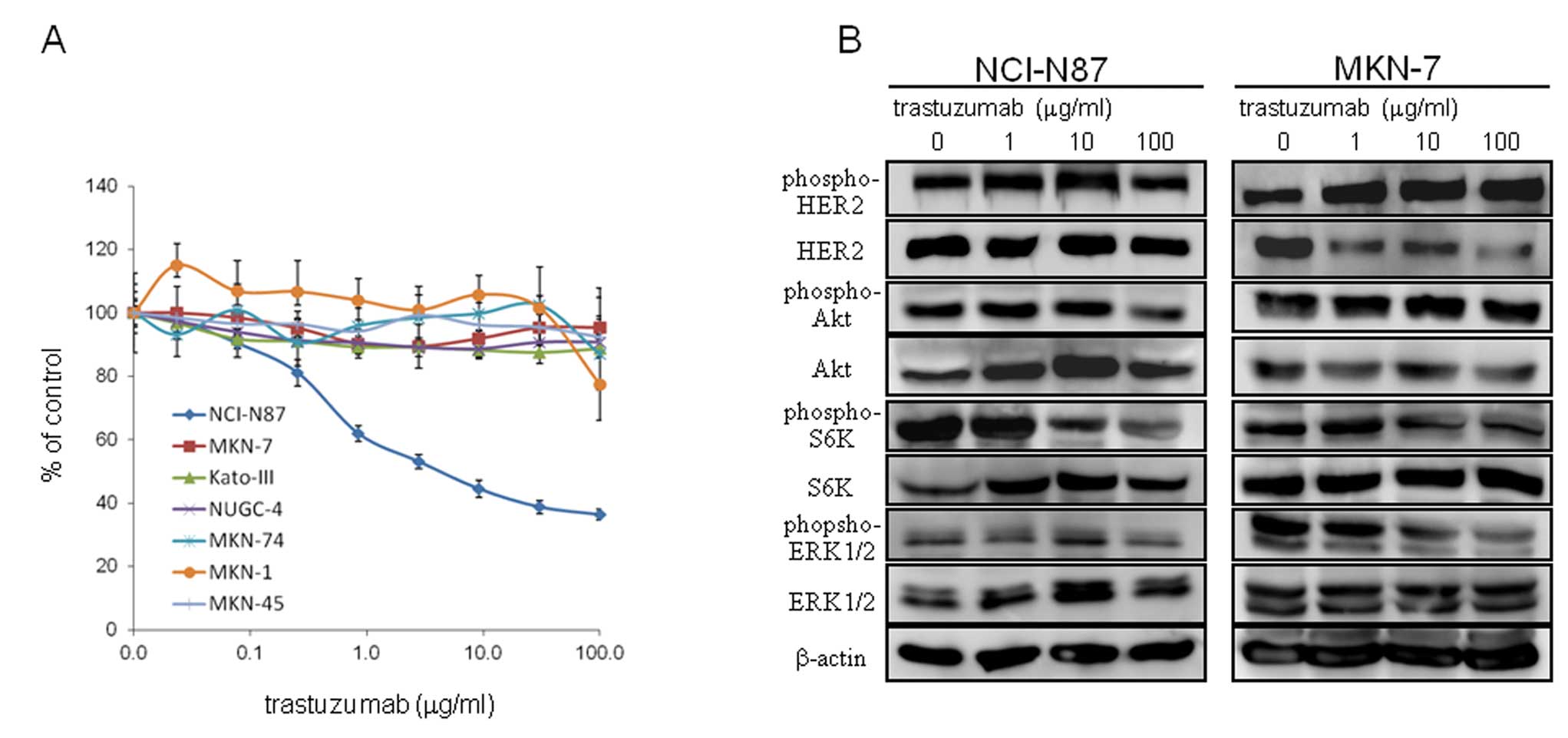
We first screened seven gastric cancer cell lines
for in vitro growth inhibition by trastuzumab (Fig. 1A). Results confirmed that
trastuzumab was not effective in five non-HER2-amplified gastric
cancer cell lines (IC50 >100 μg/ml). Of the two
HER2-amplified cell lines, only NCI-N87, and not MKN-7, was
more sensitive than non-HER2-amplified cells, consistent
with previous studies (19,20).
Effect of trastuzumab on cell signaling
in NCI-N87 and MKN-7 cell lines
To explore the underlying mechanism of the
differential effect of trastuzumab in NCI-N87 and MKN-7, we
examined phosphorylation of HER2 and representative downstream
signaling molecules in 10% FBS-containing media with and without
increasing the concentration of trastuzumab. The most remarkable
difference in signaling outcome after trastuzumab treatment was a
decrease in phosphorylation of S6K, which was observed only in
NCI-N87, and not in MKN-7 (Fig.
1B).
Synergistic effect of trastuzumab and
cytotoxic drugs in NCI-N87 cell line
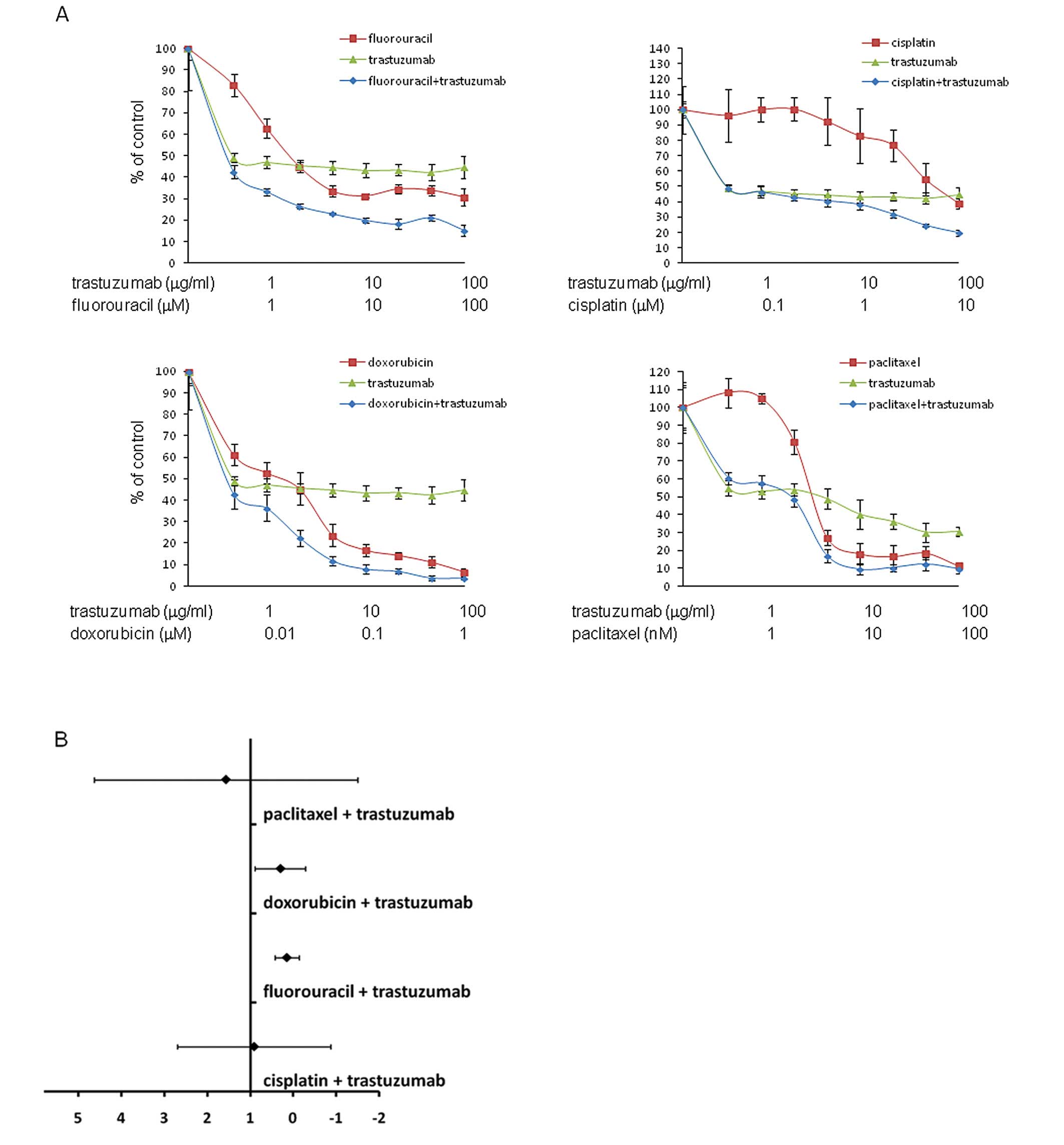
We next investigated synergy between trastuzumab and
the clinically relevant cytotoxic drugs fluorouracil, cisplatin,
doxorubicin and paclitaxcel in HER2-amplified NCI-N87 and
MKN-7. In trastuzumab-sensitive NCI-N87, the combination of
trastuzumab and fluorouracil or doxorubicin resulted in a CI value
of 0.14 [95% confidence interval (CI)-0.11–0.39] and 0.30 (95%
CI,-0.15–0.75), respectively, indicating significant synergy
between the drugs (Fig. 2A and B).
In trastuzumab-insensitive MKN-7, in contrast, trastuzumab and the
individual chemotherapeutic drugs resulted in a 95% confidence
interval for the CI value crossing 1, which indicates no
significant synergy between the drugs (Fig. 2C and D).
Fluorouracil-induced apoptosis in
HER2-amplified gastric cancer cells
Because the combination of trastuzumab and
fluorouracil showed the most promising synergy in the NCI-N87 cell
line (Fig. 2A and B), we decided
to explore the mechanism of the synergistic effect of the drugs in
subsequent experiments.
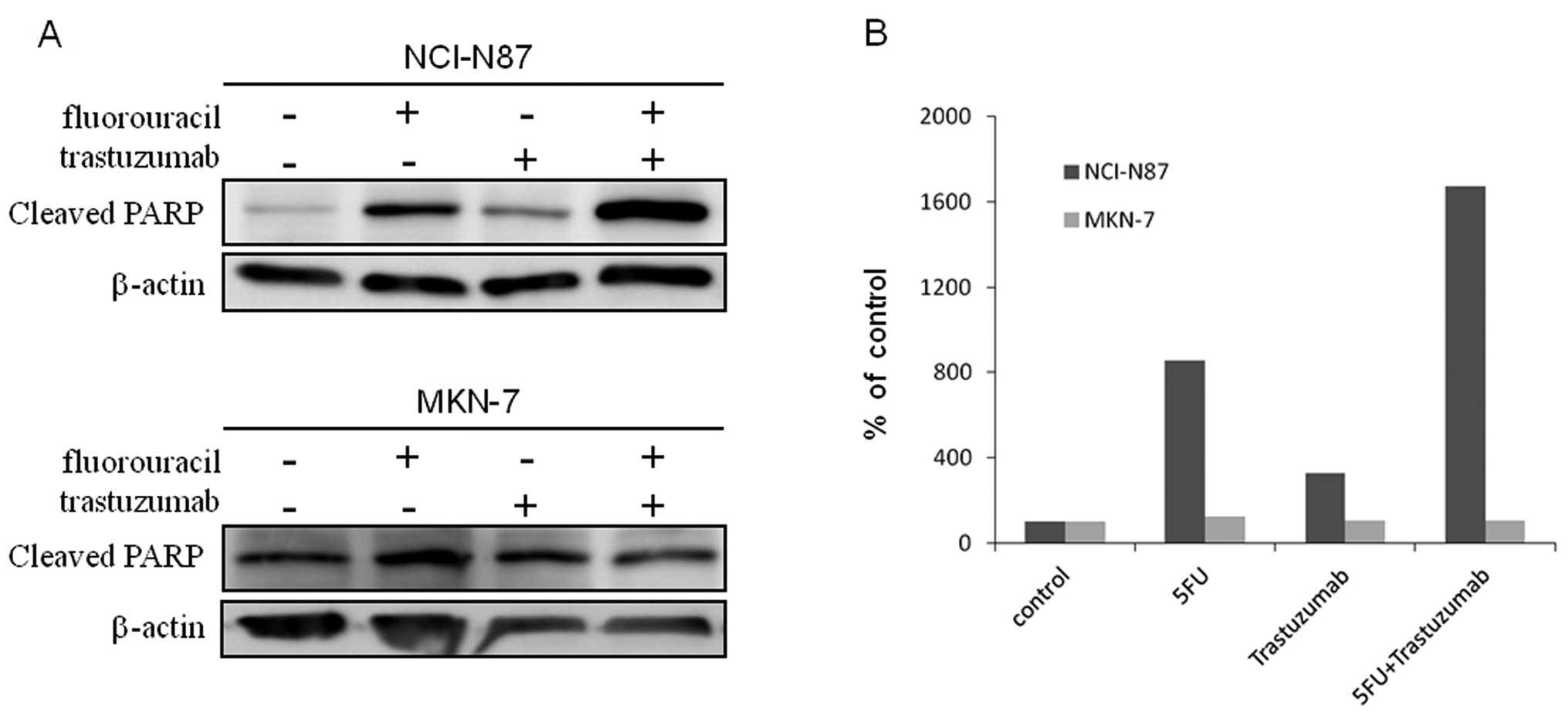
To evaluate the level of apoptosis, we used western
blot analysis for cleaved poly (ADP-ribose) polymerase (PARP),
which is indicative for apoptosis. In the NCI-N87 cell line, while
trastuzumab by itself produced a slight increase in cleaved PARP
compared to control, the combination of trastuzumab and
fluorouracil resulted in even higher level of cleaved PARP compared
to either drug alone (Fig. 3A and
B). In MKN-7, in contrast, trastuzumab alone did not produce an
increase in cleaved PARP compared to control, while the combination
of trastuzumab and fluorouracil did not increase cleaved PARP
expression compared to either drug alone (Fig. 3A and B). These results indicated
that the synergy observed in NCI-N87 cells treated with trastuzumab
and fluorouracil (Fig. 2A and B)
was likely attributable to the enhancement of fluorouracil-induced
apoptosis by trastuzumab. They also indicate that the synergistic
effect of trastuzumab and fluorouracil may be limited to cell lines
sensitive to trastuzumab itself.
Effect of everolimus in NCI-N87 and MKN-7
cell lines
Because a decrease in the phosphorylation of S6K on
treatment with trastuzumab was observed only in NCI-N87, and not in
MKN-7 (Fig. 1B), we hypothesized
that inhibition of the phosphorylation of S6K may be an important
molecular event for trastuzumab in its enhancement of
fluorouracil-induced apoptosis and synergistic effect with the
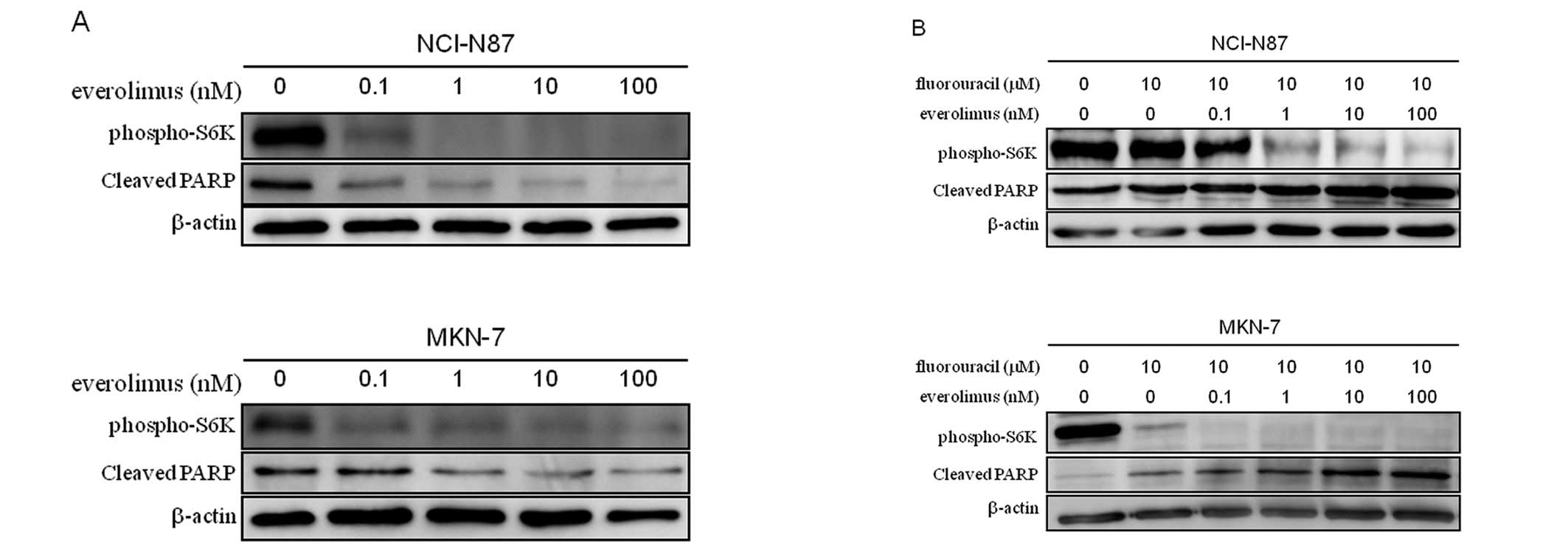
drug. Therefore, we next evaluated the effect of the mTOR inhibitor
everolimus on cell signaling and fluorouracil-induced apoptosis in
the two HER2-amplified gastric cell lines. As seen in
Fig. 4A, while everolimus alone
inhibited phosphorylation of S6K, it instead decreased the
expression of cleaved PARP as compared to control in both cell
lines. When combined with fluorouracil, however, everolimus
inhibited phosphorylation of S6K and appeared to enhance
fluorouracil-induced apoptosis in a dose-dependent manner in both
cell lines (Fig. 4B). These
results strengthen the notion that inhibition of the
phosphorylation of S6K be a key molecular event in enhancing
fluorouracil-induced apoptosis.
Effect of fluorouracil in combination
with CL-387,785 in MKN-7
MKN-7 was previously shown to be resistant to
anti-HER2 monoclonal antibody 4D5 because of alternative signaling
from overexpressing EGFR (17).
Therefore, we next evaluated the effect of CL-387,785, an EGFR/HER2
inhibitor, on cell growth, cell signaling and fluorouracil-induced
apoptosis in MKN-7.
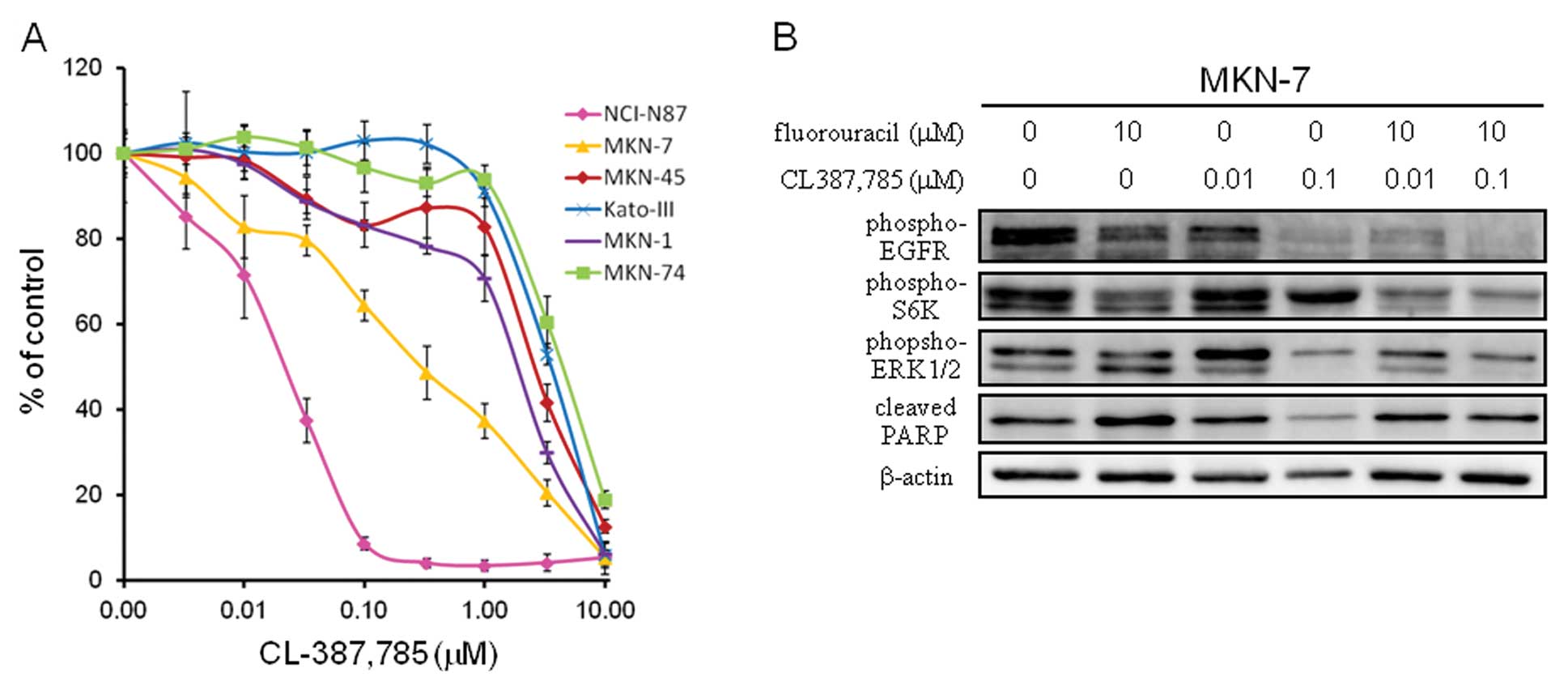
As seen in Fig. 5A,
treatment of MKN-7 with CL-387,785 resulted in growth inhibition,
albeit to a lesser extent than in NCI-N87. In the western blot
analysis, treatment of CL-387,785 resulted in decrease in
phosphorylation of ERK1/2 versus only a mild decrease in that of
S6K (Fig. 5B). Consistent with
this, the addition of CL-387,785 to fluorouracil did not increase
the expression of cleaved PARP compared to fluorouracil alone
(Fig. 5B). This finding again
supports the notion that inhibition of the mTOR-S6K signal may be
important in enhancing fluorouracil-induced apoptosis. Further, it
suggests that growth inhibitory effect of anti-RTK agent alone does
not guarantee its ability to enhance chemotherapy-induced
apoptosis.
Synergistic effect of everolimus in
combination with fluorouracil in the second gastric cancer cell
line
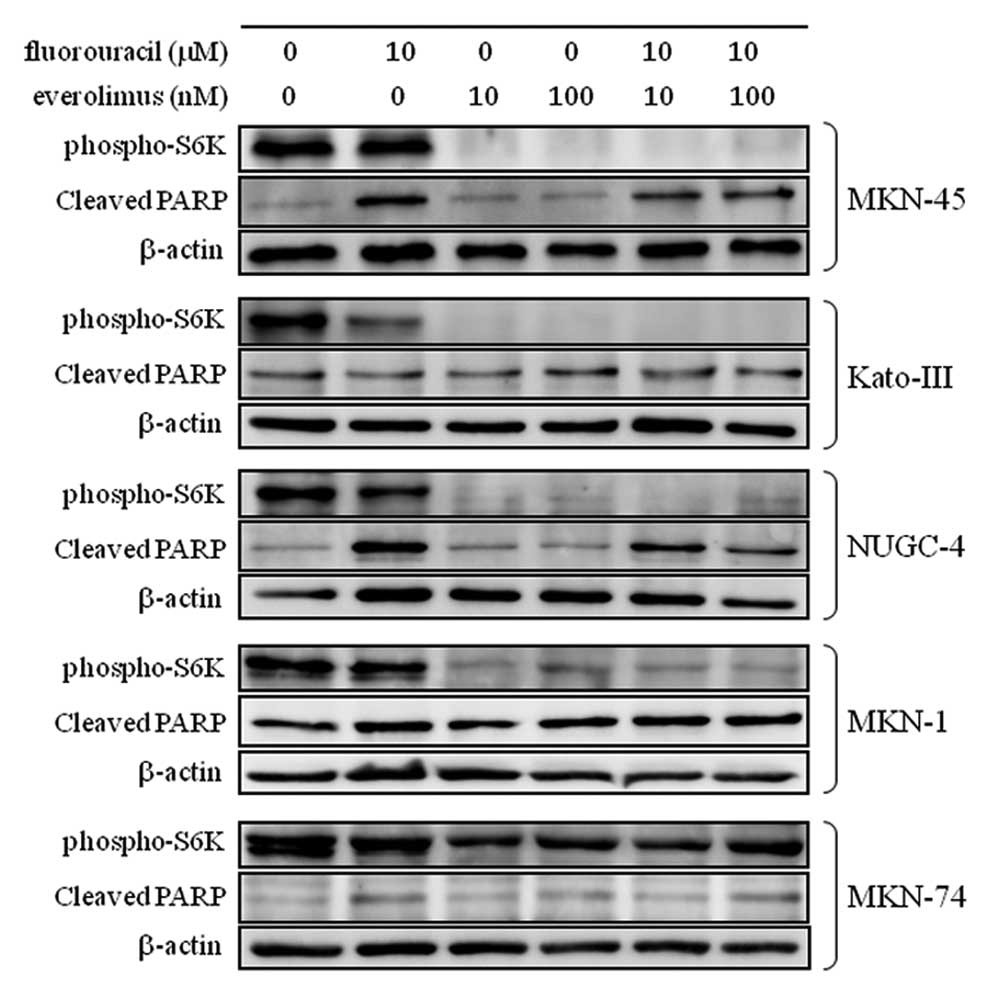
To evaluate whether the mTOR-S6K signal in
fluorouracil-induced apoptosis is universally important in all
gastric cancers, we next tested other gastric cancer cell lines for
fluorouracil-induced apoptosis. MKN-45, KATO-III, NUGC-4, MKN-1 and
MKN-74, which have been reported to harbor MET-amplification
(25), FGFR2-amplification
(25), EGFR-mutation
(E746-A750 del), PIK3CA-mutation (E545K) (26), and B-RAF mutation (G466V),
respectively (27), were treated
with everolimus, fluorouracil, or their combination.
Phosphorylation of S6K and cleavage of PARP were analyzed as shown
in Fig. 6. Everolimus dramatically
decreased the phosphorylation of S6K in all cell lines except
MKN-74, while the combination of fluorouracil and everolimus did
not increase cleaved PARP expression compared to fluorouracil alone
in any of the cell lines. This finding suggests that the mTOR-S6K
signal in fluorouracil-induced apoptosis is not universal, but
somewhat specific to HER2-amplified gastric cancer cell
lines.
Discussion
In this study, we found that while trastuzumab
administered together with fluorouracil or doxorubicin
synergistically enhanced apoptosis in the trastuzumab-sensitive
HER2-amplified gastric cancer cell line NCI-N87, no such
effect was seen in trastuzumab-insensitive HER2-amplified
MKN-7 cells. We also found that inhibition of the phosphorylation
of S6K appeared to be a key molecular event in the enhancement of
fluorouracil-induced apoptosis, indicating that the mTOR inhibitor
everolimus might be an attractive back-up drug for
HER2-amplified gastric cancers.
Several previous studies evaluated the ability of
trastuzumab to produce synergy with chemotherapeutic agents in
HER2-amplified gastric cancer cell lines. Kim et al
(28) reported a synergistic
effect between trastuzumab and cisplatin but only an additive
effect between trastuzumab and fluorouracil or oxaliplatin using
the SNU-216 cell line. Tanizaki et al (29) reported synergy between trastuzumab
and fluorouracil in three HER2-amplified gastric cancer cell
lines, NCI-N87, MKN-7, and SNU-216. These inconsistent results
among the studies, including our present study, may be due to
differences in experimental conditions. Moreover, none of the
previous studies reported the drug concentrations used to test
synergy between trastuzumab and chemotherapeutic agents.
In our study, we found that inhibition of the
phosphorylation of S6K with treatment with trastuzumab occurred
only in NCI-N87 cells, and not in MKN-7 cells. With regard to the
phosphorylation of Akt, in contrast, trastuzumab induced no or only
modest changes in both cell lines (Fig. 1B). This finding is consistent with
our previous study using HER2-amplified breast cancer cell
lines, in which the degree of growth inhibition by trastuzumab was
more closely correlated with inhibition of the phosphorylation of
S6K than of Akt (30). These
findings raise the possibility that phosphorylation of S6K might be
a pharmacodynamic marker for anti-HER2 therapy in
HER2-amplified cancer cells regardless of their origin.
We also found that a decrease in phosphorylation of
S6K was necessary to enhance fluorouracil-induced apoptosis in
HER2-amplified gastric cancer cell lines. While the mTOR-S6K signal
has been characterized as involved in G1/S cell cycle progression
by initiating protein translation, this signal is also known to
promote cell survival. This latter effect was reported to occur via
active S6K phosphorylation of the pro-apoptotic factor BAD at
Ser136 (31). The primary role of
S6K might differ depending on cell type. As we did not find that
everolimus enhanced fluorouracil-induced apoptosis in gastric
cancer cell lines which have genetic alteration in RTKs or
signaling molecules other than HER2 (Fig. 6), a predominant role of S6K in cell
survival might be characteristic in HER2-amplified gastric
cancer cells. In some cell lines, the addition of everolimus rather
decreased the level of fluorouracil-induced apoptosis compared to
fluorouracil alone (Fig. 6). This
might be due to the known effect of everolimus to induce cell cycle
arrest, under which fluorouracil would not work effectively.
Determining the predominant role of S6K in cell cycle progression
vs. cell survival in specific cells might therefore be
therapeutically important.
The clinical usefulness of everolimus is currently
under investigation. A phase II clinical trial of everolimus
monotherapy in patients with advanced gastric cancer showed a
disease control rate of 56% despite no objective tumor response
(32). Further, a randomized,
double-blind, multi-center phase III study comparing everolimus
plus best supportive care (BSC) with placebo plus BSC in patients
with advanced gastric cancer after progression on 1 or 2 prior
systemic chemotherapies is now ongoing (33). The results of this trial,
particularly the subset analysis based on HER2 status, should
provide deeper insight into the importance of the mTOR-S6K signal
in the survival of HER2-amplified gastric cancers. Although
the combination of capecitabine and everolimus showed only a modest
effect in a phase I study (34),
evaluating the trastuzumab-fluorouracil (or its derivative)
combination after enrichment of the study population with patients
whose tumors have HER2-amplification may be worthwhile.
Several limitations of this study warrant mention.
First, our use of only two HER2-amplified gastric cancer
cell lines precludes the generalization of the results. Compared to
breast cancer, substantially fewer HER2-amplified gastric
cancer cell lines are available. Confirmation of our findings in
other or newly established HER2-amplified gastric cancer
cell lines would be valuable.
Second, in addition to its inhibition of HER2
signaling, several studies have indicated the contribution of
antigen-dependent cellular cytotoxicity (ADCC) in the antitumor
effect of trastuzumab in breast cancer. Because ADCC only works in
in vivo conditions, our present data do not necessarily deny
the potential effect of trastuzumab on tumors showing resistance to
trastuzumab in vitro.
In summary, our findings suggest that inhibition of
the mTOR-S6K signal is a key molecular event in enhancing
fluorouracil-induced apoptosis in HER2-amplified gastric
cancer cells, regardless of sensitivity to trastuzumab. mTOR
inhibitors such as everolimus might be attractive back-up drugs in
a particular subset of gastric cancers. A better understanding of
these findings, however, may require further investigation in
clinical trials and associated translational studies.
Acknowledgements
This study was supported by the Global
Centers of Excellence Program (H.M.) and Grant-in-Aid for Young
Scientists (B) from the Ministry of Education, Culture, Sports,
Science and Technology of Japan (T.M), and AstraZeneca Research
Grant (T.M).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
3
|
Sue-Ling HM, Johnston D, Martin IG, et al:
Gastric cancer: a curable disease in Britain. BMJ. 307:591–596.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menges M and Hoehler T: Current strategies
in systemic treatment of gastric cancer and cancer of the
gastroesophageal junction. J Cancer Res Clin Oncol. 135:29–38.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10(Suppl 3): 49–58. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slamon DJ, Leyland-Jones B, Shak S, et al:
Use of chemotherapy plus a monoclonal antibody against HER2 for
metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paez JG, Janne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yokota J, Yamamoto T, Miyajima N, et al:
Genetic alterations of the c-erbB-2 oncogene occur frequently in
tubular adenocarcinoma of the stomach and are often accompanied by
amplification of the v-erbA homologue. Oncogene. 2:283–287.
1988.PubMed/NCBI
|
|
10
|
Hofmann M, Stoss O, Shi D, et al:
Assessment of a HER2 scoring system for gastric cancer: results
from a validation study. Histopathology. 52:797–805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanner M, Hollmen M, Junttila TT, et al:
Amplification of HER-2 in gastric carcinoma: association with
Topoisomerase IIα gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005.PubMed/NCBI
|
|
12
|
Kuniyasu H, Yasui W, Kitadai Y, Yokozaki
H, Ito H and Tahara E: Frequent amplification of the c-met gene in
scirrhous type stomach cancer. Biochem Biophys Res Commun.
189:227–232. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hattori Y, Itoh H, Uchino S, et al:
Immunohistochemical detection of K-sam protein in stomach cancer.
Clin Cancer Res. 2:1373–1381. 1996.PubMed/NCBI
|
|
14
|
Ueda T, Sasaki H, Kuwahara Y, et al:
Deletion of the carboxyl-terminal exons of K-sam/FGFR2 by short
homology-mediated recombination, generating preferential expression
of specific messenger RNAs. Cancer Res. 59:6080–6086. 1999.
|
|
15
|
Tsujimoto H, Sugihara H, Hagiwara A and
Hattori T: Amplification of growth factor receptor genes and DNA
ploidy pattern in the progression of gastric cancer. Virchows Arch.
431:383–389. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
17
|
Neve RM, Lane HA and Hynes NE: The role of
overexpressed HER2 in transformation. Ann Oncol. 12(Suppl 1): 9–13.
2001. View Article : Google Scholar
|
|
18
|
Yokoyama H, Ikehara Y, Kodera Y, et al:
Molecular basis for sensitivity and acquired resistance to
gefitinib in HER2-overexpressing human gastric cancer cell lines
derived from liver metastasis. Br J Cancer. 95:1504–1513. 2006.
View Article : Google Scholar
|
|
19
|
Matsui Y, Inomata M, Tojigamori M, Sonoda
K, Shiraishi N and Kitano S: Suppression of tumor growth in human
gastric cancer with HER2 overexpression by an anti-HER2 antibody in
a murine model. Int J Oncol. 27:681–685. 2005.PubMed/NCBI
|
|
20
|
Lane HA, Beuvink I, Motoyama AB, Daly JM,
Neve RM and Hynes NE: ErbB2 potentiates breast tumor proliferation
through modulation of p27(Kip1)-Cdk2 complex formation: receptor
overexpression does not determine growth dependency. Mol Cell Biol.
20:3210–3223. 2000. View Article : Google Scholar
|
|
21
|
Abramoff MD, Magalhaes PJ and Ram SJ:
Image processing with ImageJ. Biophotonics Int. 11:36–42. 2004.
|
|
22
|
Pegram MD, Konecny GE, O’Callaghan C,
Beryt M, Pietras R and Slamon DJ: Rational combinations of
trastuzumab with chemotherapeutic drugs used in the treatment of
breast cancer. J Natl Cancer Inst. 96:739–749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gong SJ, Jin CJ, Rha SY and Chung HC:
Growth inhibitory effects of trastuzumab and chemotherapeutic drugs
in gastric cancer cell lines. Cancer Lett. 214:215–224. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yokozaki H: Molecular characteristics of
eight gastric cancer cell lines established in Japan. Pathol Int.
50:767–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mita H, Toyota M, Aoki F, et al: A novel
method, digital genome scanning detects KRAS gene amplification in
gastric cancers: involvement of overexpressed wild-type KRAS in
downstream signaling and cancer cell growth. BMC Cancer. 9:1982009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma P and Halayko AJ: Emerging
molecular targets for the treatment of asthma. Indian J Biochem
Biophys. 46:447–460. 2009.PubMed/NCBI
|
|
28
|
Kim SY, Kim HP, Kim YJ, et al: Trastuzumab
inhibits the growth of human gastric cancer cell lines with HER2
amplification synergistically with cisplatin. Int J Oncol.
32:89–95. 2008.PubMed/NCBI
|
|
29
|
Tanizaki J, Okamoto I, Takezawa K, et al:
Synergistic antitumor effect of S-1 and HER2-targeting agents in
gastric cancer with HER2 amplification. Mol Cancer Ther.
9:1198–1207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kataoka Y, Mukohara T, Shimada H, Saijo N,
Hirai M and Minami H: Association between gain-of-function
mutations in PIK3CA and resistance to HER2-targeted agents in
HER2-amplified breast cancer cell lines. Ann Oncol. 21:255–262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harada H, Andersen JS, Mann M, Terada N
and Korsmeyer SJ: p70S6 kinase signals cell survival as well as
growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad
Sci USA. 98:9666–9670. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doi T, Muro K, Boku N, et al: Multicenter
phase II study of everolimus in patients with previously treated
metastatic gastric cancer. J Clin Oncol. 28:1904–1910. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danilkovitch-Miagkova A and Zbar B:
Dysregulation of Met receptor tyrosine kinase activity in invasive
tumors. J Clin Invest. 109:863–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lim T, Lee J, Lee DJ, et al: Phase I trial
of capecitabine plus everolimus (RAD001) in patients with
previously treated metastatic gastric cancer. Cancer Chemother
Pharmacol. 68:255–262. 2011. View Article : Google Scholar : PubMed/NCBI
|