Introduction
In general, pulmonary emboli originating from deep
vein thrombosis are thought to have an important role in the
development of chronic thromboembolic pulmonary hypertension
(CTEPH) (1,2). Organized and incorporated fibrous
thrombi promote the complete obliteration of the pulmonary
arteries, thus resulting in an increase in pulmonary vascular
resistance. Therefore, the current mainstay of therapy for patients
with CTEPH is pulmonary endarterectomy (PEA) (3).
Recently, the existence of not only
myofibroblast-like cells, but also endothelial-like cells, in the
endarterectomized tissues from patients with CTEPH has been
demonstrated (4–6). Moreover, our recent study suggested
that these myofibroblast-like cells might release substances that
promote the endothelial-mesenchymal transition (EnMT) and/or induce
EC dysfunction (7). Indeed, there
were transitional cells which co-expressed both endothelial (CD31)
and SM [α-smooth muscle actin (SMA)] markers in these tissues from
patients with CTEPH (7). At the
2nd passage of the myofibroblast-like cells, a pleomorphic cell
type that varied in size and shape, and was characterized by a
large nucleus, was isolated from the endarterectomized tissues of
patients with CTEPH. Because these cells seemed to be
hyperproliferative and anti-apoptotic, they were defined as
sarcoma-like cells (SCL) (preliminary data). Pulmonary intimal
sarcoma is a very uncommon neoplastic tumor of the cardiovascular
system, and only about 125 cases have been reported in the
literature (8). This tumor is
thought to originate from subendothelial-mesenchymal cells of the
pulmonary vascular wall (9).
Immunohistochemical examinations of intimal sarcoma demonstrated
that these tumors undergo endothelial, fibroblastic, or
myofibroblastic differentiation (10). Some studies have demonstrated that
intimal sarcomas have positive staining for α-SMA (11), while others have indicated that
they are only positive for vimentin, but not for desmin or α-SMA
(12). The pathobiology of
pulmonary intimal sarcoma remains largely unknown because of its
rarity.
Because the SCL were isolated from endarterectomized
tissues, i.e., the pulmonary vascular beds, of patients with CTEPH,
it was hypothesized that the analysis of these cells may contribute
to our understanding of pulmonary intimal sarcoma, which was
defined as a neoplasm arising in the tunica intima of the pulmonary
arteries. The aim of this study was to investigate the
characteristics of SCL in vivo and in vitro.
Materials and methods
Cell isolation
Endarterectomized tissues from patients with CTEPH
were obtained following PEA performed by M.M. at the Chiba Medical
Center, Japan. The details of the cell isolation techniques have
been described previously (4). The
study was approved by the Research Ethics Committee of Chiba
University School of Medicine, and written informed consent was
given by all subjects.
Cell lines and reagents
Normal human lung fibroblasts (NHLF) and human
pulmonary microvascular endothelial cells (HPMVEC) were purchased
from Lonza Inc. (Allendale, NJ, USA) and cultured using EGM and
fibroblast growth medium (FGM) supplemented with 5% fetal bovine
serum (Lonza Inc.). A549 (lung cancer cell line) cells and HT1080
(fibrosarcoma cell line) cells were obtained from Takara Biomedical
(Othsu, Shiga, Japan) and cultured in RPMI-1640 medium supplemented
with 5% fetal bovine serum (Lonza Inc.). The following Ab were used
for the analyses: mouse anti-vimentin (1:200, Dako, Carpinteria,
CA, USA), mouse anti-human desmin (1:100, Dako), mouse
anti-α-SM-actin (αSMA) (1:1,000, Sigma-Aldrich, St. Louis, MO,
USA), mouse anti-human Ki-67 (1:100, BD Biosciences Pharmingen, San
Diego, CA, USA), anti-mouse IgG conjugated with Rhodamine dye
(1:500, Molecular Probes, Eugene, OR, USA), rabbit anti-von
Willebrand factor (factor VIII) (1:1,000, Dako), and an anti-rabbit
IgG conjugated with Alexa-488 fluorescent dye (1:500, Molecular
Probes). The Bromodeoxyuridine (BrdU) Flow Kit and the BD BioCoat™
FluoroBlok™ Invasion System (24-multiwells) were purchased from BD
Biosciences.
Immunofluorescent staining
The cells were fixed in a 1:1 mixture of methanol
and acetone and incubated with the primary antibodies, followed by
incubation with the secondary antibodies. Additional details about
the method used for immunofluorescent staining have been described
previously (4).
BrdU-7-amino-actinomycin D binding
assay
The BrdU Flow Kit was used to detect the rate of DNA
synthesis. The details of this assay technique have been described
previously (4).
Colony-forming assay
Six-well flat-bottomed plates with a two-layer soft
agar system including a total of 1×104 cells/well in a
volume of 1.5 ml/well in the upper layer were adapted for the
colony-forming assay. Additional details on the method used for
this assay have been described previously (4).
Cell invasion and migration assay
The BD BioCoat FluoroBlok Invasion System
(24-multiwell) was used for the cell migration assays. The details
of the cell invasion and migration assay techniques have been
previously described (4).
Serum starvation
The cells at passage 4 were seeded at
1×105 in 25-cm2 flasks and were incubated
with serum-free medium for the indicated incubation periods. The
details of the serum starvation assay techniques have been
described previously (4).
Tumorigenicity studies
Adult male C.B-17/lcr-scid/scidJcl (SCID) mice were
purchased from a commercial vendor. Tumors were generated by
subcutaneous injection of SCL and intravenous tail vein injection
of SCL and A549 into SCID mice (n=10 per group, 20±2.5 g) under
Nembutal anesthesia (50 mg/ml). At a subcutaneous inoculation
concentration of 1×106 cells per mouse, the SCL
consistently produced subcutaneous tumors. At an intravenous
injection concentration of 2×106 cells per mouse, the
SCL and A549 cells consistently produced intravenous and lung
tumors. The mice were sacrificed, and the tumors and the organs
were quickly isolated and excised on the appropriate days after
intravenous injection. After that, they were fixed in 10% formalin
for 48 h, and embedded in paraffin. These tissues were sectioned
and prepared for the histological analysis. The animal protocol was
approved by the Animal Care and Use Committee of Chiba University
School of Medicine.
Labeling of SCL with PKH-26
The lipophilic fluorescent PKH dye, PKH-26
(Sigma-Aldrich), was used to label the SCL. The PKH26 dye was
diluted at the recommended concentration and mixed with the SLC for
5 min, according to manufacturer’s instructions. These cells were
injected intravenously via the tail vein of SCID mice and, on day
21, the mice were sacrificed, and their lungs were quickly isolated
and excised. The existence of PKH positive SCL with DAPI staining
was confirmed under fluorescent microscopy.
PCR array analysis
RT2 Profiler™ PCR Arrays (SABio-sciences, Frederick,
MD, USA) were used to analyze the expression of a focused panel of
genes involved in various biological processes. The 96-well plate
extracellular matrix and adhesion molecules PCR array (PAHS-013)
which profiles the expression of 84 key genes involved in the
formation of the extracellular matrix and adhesion molecules, were
selected to detect the differential expression of genes between SCL
and A549. Additional details on the method used for this analysis
have been described previously (7).
Statistical analysis
The statistical analyses were performed using data
from at least three independent experiments. The results are
expressed as the means ± SEM. The data were analyzed using
Student’s t-test, as appropriate. A value of p<0.05 was
considered to be significant for all tests.
Results
The cellular composition of
endarterectomized tissue from CTEPH patients
As shown previously, a few different cell types were
isolated from the endarterectomized tissues in each of the 15
patients with CTEPH (4). One of
them was determined morphologically to be the myofibroblast-like
cells (spindle-shape with cytoplasmic extensions) (4). At the 2nd passage of the
myofibroblast-like cells, another cell type that varied in size and
shape and was characterized by a large nucleus, was isolated from
one patient described in a previous study (4). These cells had the morphology of SCL
(Fig. 1A). The cells outgrown from
the thrombotic material were further characterized by
immunohistochemical staining for desmin, vimentin, factor VIII and
αSMA. The SCL were factor VIII, desmin and αSMA negative, and
vimentin positive (Fig. 1B).
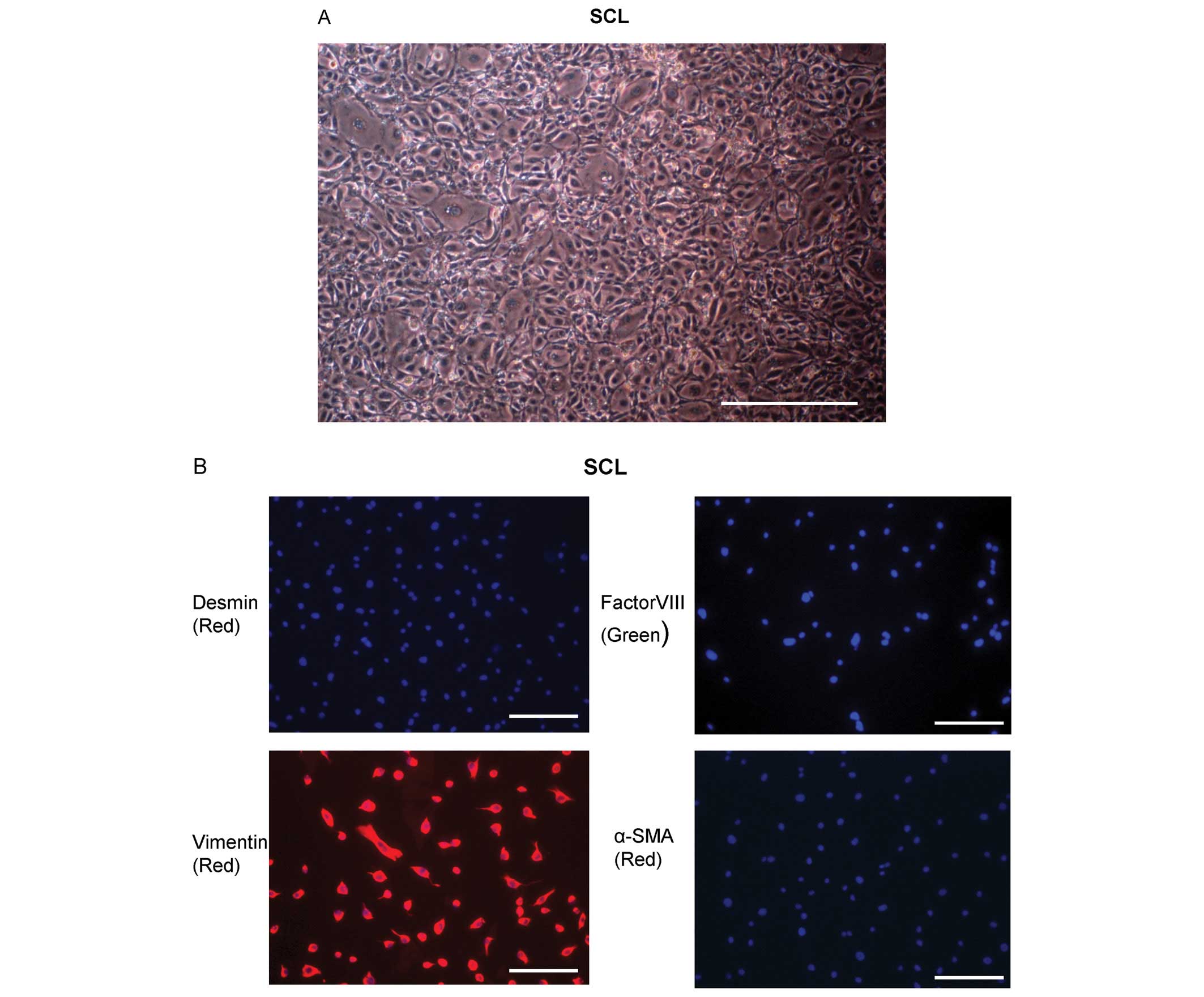 | Figure 1Characterization of the sarcoma-like
cells (SCL) from endarterectomized tissue. (A) The SCL from
endarterectomized tissue were microscopically assessed. The
magnification was ×100. Scale bar, 100 μm. (B) SCL derived from
endarterectomized tissue were assessed by immunofluorescent
staining for desmin, vimentin, factor VIII and α-SMA to confirm the
phenotypes of the cells. DAPI staining is shown in blue. The
magnification was ×200. Scale bar, 50 μm. (C) Light microscopic
examination of the SCL at the 3rd passage. The magnification was
×100. Scale bar, 100 μm. (D) Quantification of the proliferating
SCL at the 4th passage. The individual cells that had synthesized
DNA were determined by the immunofluorescent staining of
incorporated bromodeoxyuridine (BrdU). The incorporated BrdU was
stained with specific anti-BrdU fluorescent antibodies. The levels
of cell-associated BrdU were measured by flow cytometry; error bars
represent the ± SD from experiments done in triplicate.
*p<0.05. (E) The results of the colony-forming assay.
SCL at the 4th passage were trypsinized and replated on soft agar.
Microphotographs show the colonies grown in soft agar for 2 weeks.
The magnification was ×40. Scale bar, 100 μm. (F) The numbers of
colonies per microscopic field were counted; error bars represent
the ± SD from experiments done in triplicate.
*p<0.05. (G) The results from the cell invasion and
migration assay in SCL. For the BD BioCoat FluoroBlok Invasion
assay, NHLF, an invasive fibrosarcoma cell line (HT1080), an
invasive lung cancer cell line (A549) and the SCL were allowed to
invade for 16 h; error bars represent the ± SD from experiments
done in triplicate. *p<0.05. (H) Serum starvation. A
comparison of the growth of HPMVEC, NHLF and SCL in the absence of
serum. Cells at passage 4 were seeded at 1×105 in
25-cm2 flasks on day 0 and were incubated with
serum-free medium for the indicated incubation periods. On each
indicated day, a flask was trypsinized, and the cells counted. The
average values of three experiments are shown. SCL, sarcoma-like
cells; HPMVEC, human pulmonary microvascular endothelial cells;
NHLF, normal human lung fibroblasts. |
Proliferative activity of SCL
The SCL grew without cell-cell contact inhibition
and started piling up and forming foci in human fibronectin coated
culture dishes (6 cm in diameter) (Fig. 1C). In order to confirm their
proliferative activity, the number of proliferating cells which had
synthesized DNA was assessed by flow cytometry by immunofluorescent
staining of the incorporated BrdU. The number of BrdU positive
cells was increased in the SCL, but there was no increase in the
incorporation of BrdU by the HPMVEC and NHLF which were used as
controls (Fig. 1D).
Anchorage-independent growth of the
SCL
Because the growth of the SCL without cell-cell
contact inhibition suggested that they would exhibit
anchorage-independent growth, we investigated the ability of these
cells to form colonies in soft agar. The SCL showed
anchorage-independence in the soft agar colony formation assays
(Fig. 1E). The colony formation
was particularly prominent in the SCL compared with the HPMVEC and
NHLF (Fig. 1F).
Invasive and migratory activity of the
SCL
Because the SCL exhibited hyperproliferative
potential and the ability to undergo anchorage-independent growth,
which reflected cancer-related properties (13), we assessed the invasion and
migration of the SCL. Using the BD BioCoat FluoroBlok Invasion
assay, the SCL, an invasive lung cancer cell line (A549), an
invasive fibrosarcoma cell line (HT1080), and the NHLF were allowed
to invade for 16 h. The SCL showed high invasive and migratory
activity, similar to the HT1080 and A549 cells in comparison to the
NHLF (Fig. 1G). These findings
suggested that the SCL might share an in vitro invasive
potential with sarcomas and cancer cell lines.
Serum-independent growth of SCL
In the absence of serum, the HPMVEC could not
survive, and rapidly underwent apoptosis. Likewise, the NHLF
stopped proliferating and gradually died (Fig. 1H). However, the SCL survived
indefinitely in the absence of serum and kept proliferating
(Fig. 1H).
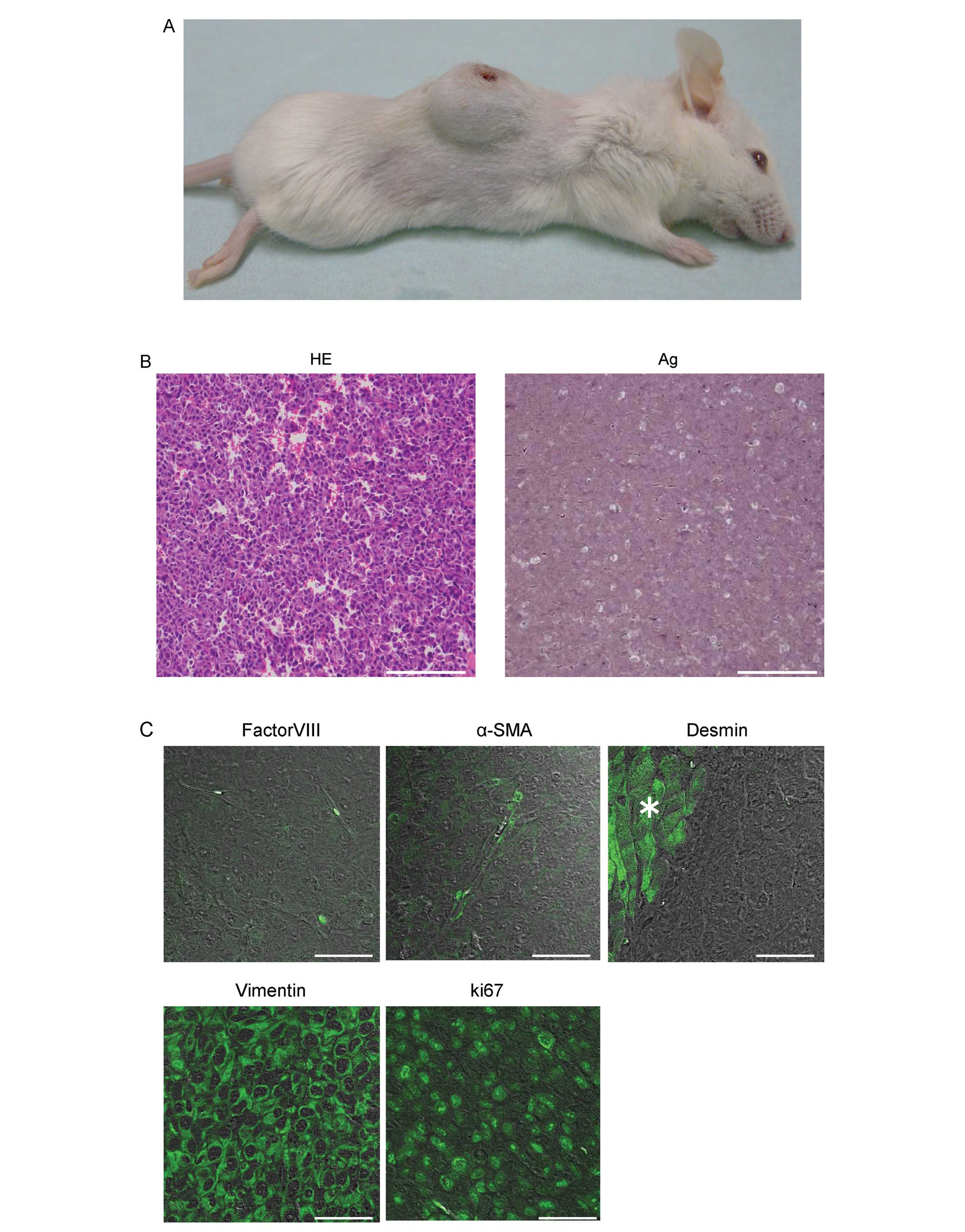
Subcutaneous tumorigenicity of SCL
In order to test whether these proliferating cells
displayed tumorigenic potential, the SCL were injected
subcutaneously into SCID mice. Within 4 weeks after s.c. injection
(i.e., when tumors had reached a diameter of about 2 cm), 8/8
animals injected with the SCL had developed solid, differentiated
tumors at the site of the injection (Fig. 2A), thus demonstrating the high
tumorigenic potential of the sarcoma-like cells.
Hematoxylin/eosin (H&E) and silver staining of
the sections of a tumor revealed the presence of pleomorphic cells
and immature blood vessels (sarcomatous vessels lacking endothelial
cells) which favored the escape of tumor metastatic cells and were
characterized by reduced collagen production (Fig. 2B). Immunofluorescent staining
demonstrated that the cells within the tumors were of mesenchymal
origin, neither EC nor SMC, since they were stained negatively for
factor VIII, αSMA, and desmin (the cells stained positively were
SMC in a SCID mouse) and positively for vimentin (Fig. 2C). The pleomorphic cells were
hyperproliferative, as indicated by their expression of the
proliferation marker, Ki67 (Fig.
2C).
Tumorigenicity of intravenously injected
SCL and A549 cells
In order to test whether the SCL can grow along the
intimal surface of the pulmonary artery in a sheet-like form and
result in artery occlusion, we investigated the time course of cell
growth within the pulmonary artery in comparison to A549 cells.
Hematoxylin/eosin staining of the sections of a lung
revealed the presence of pleomorphic cells within the peripheral
vessels on day 1, thus suggesting that they were trapped in the
pulmonary arteries after intravenous injection [Fig. 3A (small magnification) and B (large
magnification)]. The cells spread in a time-dependent manner, and
were detected in more distal pulmonary arteries on day 7, grew
along the intimal surface of the pulmonary artery in a sheet-like
form on day 14, and resulted in artery occlusion on day 21
(Fig. 3A and B). To our surprise,
there were no other microscopically visible lesions in any other
organs on day 28 after intravenous injection (data not shown).
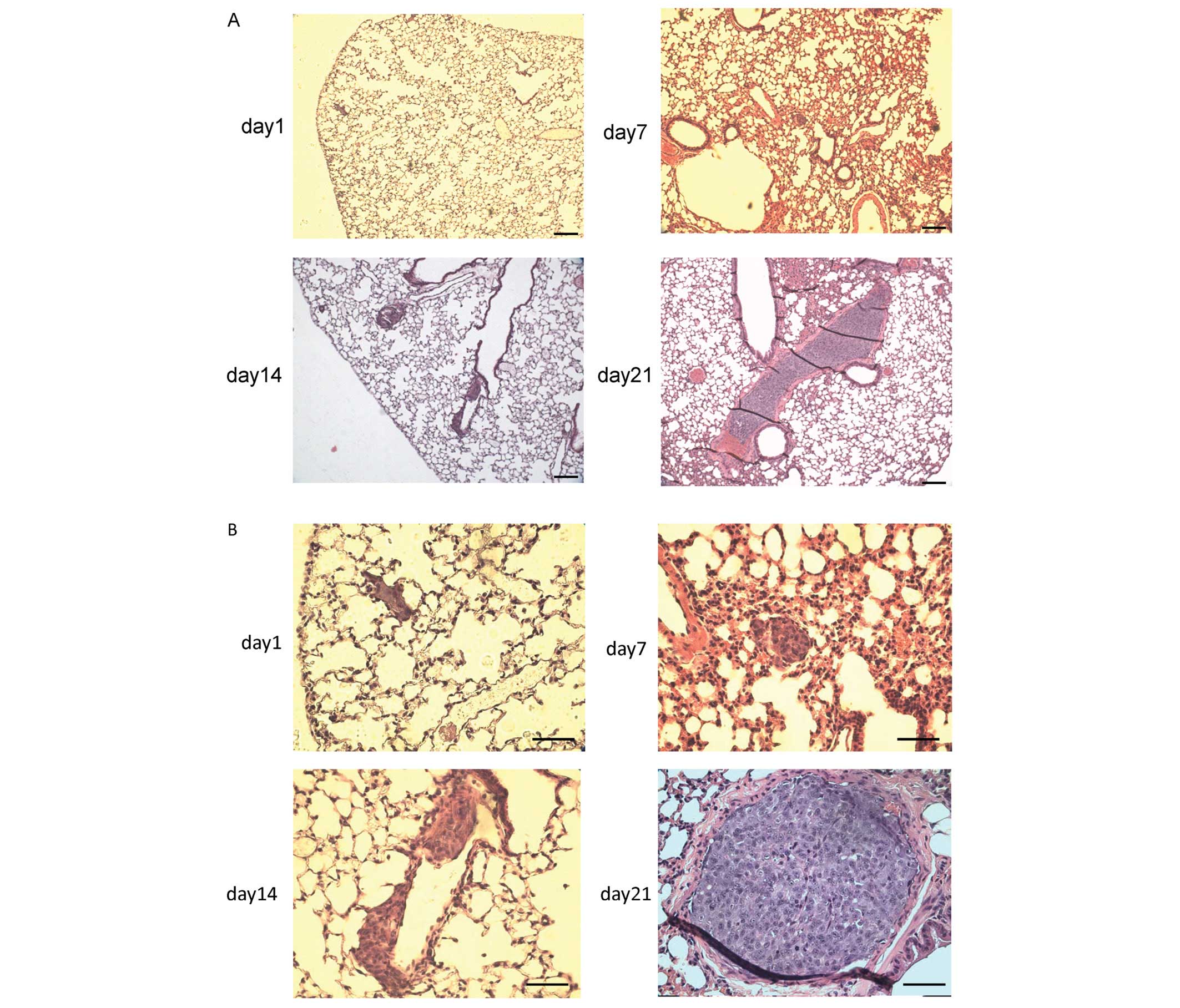 | Figure 3The tumorigenicity of intravenously
injected SCL. (A and B) Hematoxylin/eosin staining of lung tumors
from a male C.B-17/lcr-scid/scidJcl (SCID) mouse injected
intravenously with SCL (2×106 cells per mouse).
Hematoxylin/eosin staining suggested the presence of pleomorphic
cells in the pulmonary vessels that increased and migrated in a
time-dependent manner. (A) The magnification was ×40. Scale bar,
100 μm. (B) The magnification was ×100. Scale bar, 50 μm. (C)
Hematoxylin/eosin staining of lung tumors from a male
C.B-17/lcr-scid/scidJcl (SCID) mouse injected intravenously with
A549 cells (2×106 cells per mouse). Hematoxylin/eosin
staining revealed the presence of pleomorphic cells within the lung
parenchyma. Left: the magnification was x40, scale bar, 100 μm.
Right: the magnification was ×100, scale bar, 50 μm. (D)
Hematoxylin/eosin staining of the liver and pericardial tumors from
a male C.B-17/lcr-scid/scidJcl (SCID) mouse injected intravenously
with A549 cells (2×106 cells per mouse).
Hematoxylin/eosin staining revealed the presence of pleomorphic
cells in the liver and pericardium. Left: the magnification was
×40, scale bar, 100 μm. Right: the magnification was ×100, scale
bar, 50 μm. (E) Immunohistochemical staining with an anti-human
vimentin antibody, double immunofluorescent detection of PKH26 and
DAPI, and hematoxylin/eosin staining of lung tumors from a male
C.B-17/lcr-scid/scidJcl (SCID) mouse injected intravenously with
SCL (2×106 cells per mouse). These findings indicated
that pleomorphic cells originated from the SCL which were injected
intravenously via a tail vein. The magnification was ×100, scale
bar, 50 μm. |
Hematoxylin/eosin stain demonstrated that there were
no pleomorphic cells within the pulmonary vessels on day 21 after
intravenous injection of A549 cells. However, these cells developed
tumor-like lesions within the lung parenchyma (Fig. 3C). Moreover, there were visible
tumor-like lesions within not only the lungs, but also the liver
and pericardium (Fig. 3D).
The origin of pleomorphic cells in the
pulmonary vessels
To determine the origin of these pleomorphic cells,
immunohistochemical staining with an anti-human vimentin antibody
and double immunofluorescent detection of PKH26 and DAPI using
fluorescence microscopy were performed. The H&E staining of the
sections of a lung indicated the presence of pleomorphic cells in
the pulmonary vessels (Fig. 3E).
These occlusive lesions were composed of some anti-human vimentin
positive cells and some PKH26 positive cells (Fig. 3E), thus indicating that these
pleomorphic cells could originate from the SCL which were injected
intravenously via a tail vein.
The gene expression of extracellular
matrix and adhesion molecules by the SCL and A549 cells as
determined by a PCR array
The different growth patterns between the SCL and
A549 cells after intravenous injection suggested that the cells
have different pathophigiological behaviors, which may reflect a
difference between epithelial and mescenchymal tumors. Therefore,
an extracellular matrix and adhesion molecules PCR array was
performed to further characterize the SCL.
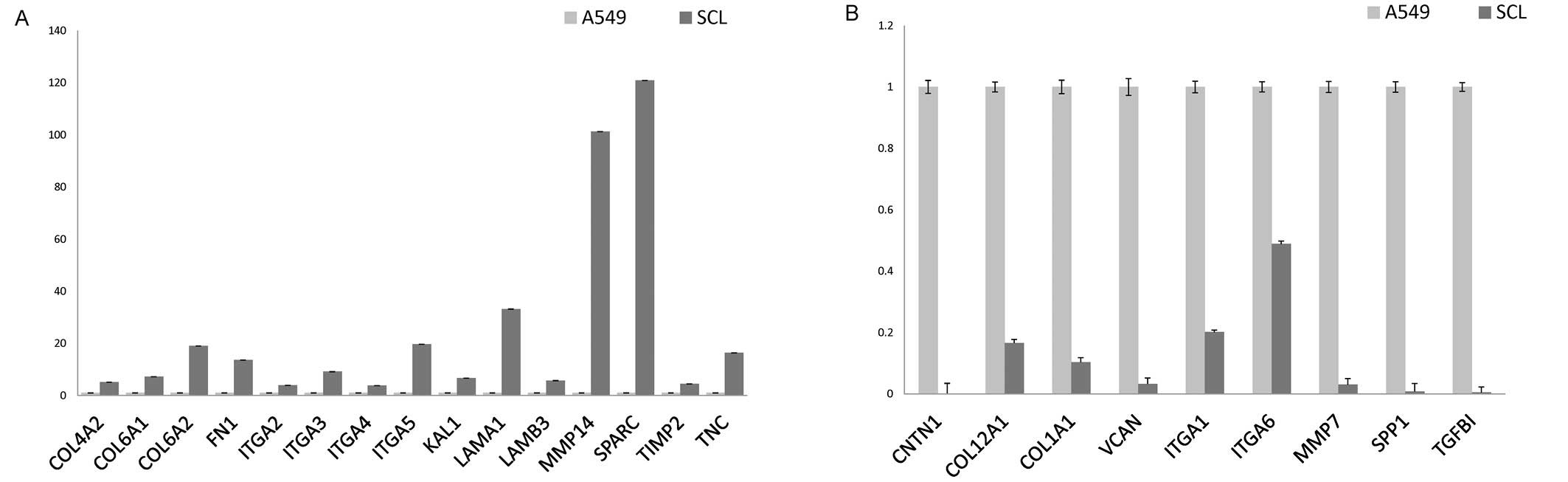
The array demonstrated that there were increases in
the expression of 15 genes and decreases in the expression of 9
genes in the SCL in comparison to the expression in A549 cells
(Fig. 4A and B). The 15 increased
genes were COL4A2, COL6A1, COL6A2, FN1, ITGA2, ITGA3, ITGA4, ITGA5,
KAL1, LAMA1, LAMB3, MMP14, SPARC, TIMP2, and TNC (Table I).
 | Table IHuman extracellular matrix and
adhesion molecules PCR array. |
Table I
Human extracellular matrix and
adhesion molecules PCR array.
| Biological process
description | Gene name | Gene symbol | Public ID | P-value |
|---|
| Extracellular
matrix proteins: collagens & ECM structural constituents | Collagen, type IV,
alpha 2 | COL4A2 | NM_001846 | 0.049403 |
| Extracellular
matrix proteins: collagens & ECM structural constituents | Collagen, type VI,
alpha 1 | COL6A1 | NM_001848 | 0.003689 |
| Cell adhesion
molecules: cell-cell adhesion | Collagen, type VI,
alpha 2 | COL6A2 | NM_001849 | 0.031248 |
| Extracellular
matrix proteins: collagens & ECM structural constituents | Fibronectin 1 | FN1 | NM_002026 | 0.005507 |
| Cell adhesion
molecules: transmembrane molecules | Integrin, alpha 2
(CD49B, alpha 2 subunit of VLA-2 receptor) | ITGA2 | NM_002203 | 0.004663 |
| Cell adhesion
molecules: transmembrane molecules | Integrin, alpha 3
(antigen CD49C, alpha 3 subunit of VLA-3 receptor) | ITGA3 | NM_002204 | 0.037106 |
| Cell adhesion
molecules: transmembrane molecules | Integrin, alpha 4
(antigen CD49D, alpha 4 subunit of VLA-4 receptor) | ITGA4 | NM_000885 | 0.005012 |
| Cell adhesion
molecules: transmembrane molecules | Integrin, alpha 5
(fibronectin receptor, alpha polypeptide) | ITGA5 | NM_002205 | 0.048419 |
| Extracellular
matrix proteins: ECM proteases inhibitors | Kallmann syndrome 1
sequence | KAL1 | NM_000216 | 0.005779 |
| Extracellular
matrix proteins: basement membrane constituents | Laminin, alpha
1 | LAMA1 | NM_005559 | 0.014549 |
| Extracellular
matrix proteins: basement membrane constituents | Laminin, beta
3 | LAMB3 | NM_000228 | 0.009781 |
| Cell adhesion
molecules: transmembrane molecules | Matrix
metallopeptidase 14 (membrane-inserted) | MMP14 | NM_004995 | 0.049414 |
| Extracellular
matrix proteins: ECM proteases | | | | |
| Extracellular
matrix proteins: basement membrane constituents | Secreted protein,
acidic, cysteine-rich (osteonectin) | SPARC | NM_003118 | 0.029498 |
| Extracellular
matrix proteins: ECM proteases inhibitors | TIMP
metallopeptidase inhibitor 2 | TIMP2 | NM_003255 | 0.026376 |
| Extracellular
matrix proteins: other ECM molecules | Tenascin C | TNC | NM_002160 | 0.014765 |
The 9 genes with an decreased expression were CNTN1,
COL12A1, COL1A1, VCAN, ITGA1, ITGA6, MMP7, SPP1, and TGFBI
(Table II).
 | Table IIHuman extracellular matrix and
adhesion molecules PCR array. |
Table II
Human extracellular matrix and
adhesion molecules PCR array.
| Biological process
description | Gene name | Gene symbol | Public ID | P-value |
|---|
| Cell adhesion
molecules: other adhesion molecules | Contactin 1 | CNTN1 | NM_001843 | 0.004029 |
| Cell adhesion
molecules: other adhesion molecules | Collagen, type XII,
alpha 1 | COL12A1 | NM_004370 | 0.014824 |
| Cell adhesion
molecules: cell-cell adhesion | Collagen, type I,
alpha 1 | COL1A1 | NM_000088 | 0.039601 |
| Cell adhesion
molecules: other adhesion molecules | Versican | VCAN | NM_004385 | 0.014957 |
| Cell adhesion
molecules: transmembrane molecules | Integrin, alpha
1 | ITGA1 | NM_181501 | 0.015272 |
| Cell-matrix
adhesion: cell adhesion molecules | Integrin, alpha
6 | ITGA6 | NM_000210 | 0.01777 |
| Transmembrane
molecules cell-matrix adhesion | | | | |
| Extracellular
matrix proteins: ECM proteases | Matrix
metallopeptidase 7 (matrilysin, uterine) | MMP7 | NM_002423 | 0.002811 |
| Cell adhesion
molecules: cell-matrix adhesion | Secreted
phosphoprotein 1 | SPP1 | NM_000582 | 0.000198 |
| Extracellular
matrix proteins: other ECM molecules | Transforming growth
factor, beta-induced, 68 kDa | TGFBI | NM_000358 | 0.003123 |
Discussion
In this study, we reported the characterization of
cells derived from endarterectomized thrombotic tissues obtained
from patients with CTEPH. These cells were defined by their
morphology and immunohistochemical staining as SCL (Fig. 1A–C). The SCL were isolated from
passaged myofibroblast-like cells which had lost their αSMA
expression (Fig. 1B) and had
morphologically changed from spindle-shaped cells to pleomorphic
cells characterized by large nuclei (Fig. 1A). In vitro, these SCL
displayed anchorage-independent growth and hyperproliferative,
invasive and tumorigenic behavior (Fig. 1D–H), raising the question whether
pulmonary vessel wall cell transdifferentiation, dedifferentiation,
and/or transformation play a role in the development of pulmonary
intimal sarcoma.
Angiogenesis, evasion of apoptosis, self-sufficiency
in growth signals, insensitivity to anti-growth signals and tissue
invasion are considered the features that characterize a malignant
cell, and were described in the landmark paper entitled ‘Hallmarks
of Cancer’ by Hanahan and Weinberg (13). We herein demonstrated that SCL from
endarterectomized tissue were hyperproliferative (Fig. 1D), anchorage-independent (Fig. 1E and F), invasive (Fig. 1G) and could grow
serum-independently (Fig. 1H). All
of these traits displayed in vitro assays reflect
cancer-defining mechanisms, thus suggesting that, as the SCL,
intimal sarcoma cells might originate from pluripotent
mesenchymal-like cells residing in the conduit vessel
endothelium.
The tumorigenic potential of the SCL that were
isolated from the myofibroblast-like cells was further demonstrated
in SCID mice (Fig. 2A–C). However,
these cells were isolated from a single patient. Therefore, it
remained unclear whether the ex vivo conditions, i.e.,
factors contained in the culture medium, allowed or induced this
transformation or transdifferentiation. Whether there is indeed
only one cell type which has the high tumorigenic potential also
remains unclear, however, there may be at least two possible
sources of the tumorgenic SCL: i) a resident stem-like fibroblast
which grows after intimal injury, and ii) a bone marrow-derived
precursor cell which migrates to injured arteries during or after
thrombus formation.
We reviewed the mechanistic basis of the vascular
lesions in PAH, comparing them with each of the cancer-defining
mechanisms (13–15). Given this quasi-neoplastic lung
vascular cell growth and the cancer-defining mechanisms
demonstrated in the cells in this study, it raises the question
whether the cells contained in the endarterectomized tissue of the
patients with CTEPH possess a latent malignant potential. However,
the SCL were isolated from specimens obtained from only one PEA
subject, and the SCID mice injected with sarcoma-like cells
developed tumors only from the cells isolated from this patient.
The conduit vessel endothelium in CTEPH does not exhibit
quasi-neoplastic potential, and CTEPH is completely different from
neoplastic disease.
Mesenchymal stem cells (MSCs) have the capacity for
limitless replicative potential as malignant cells, and could
transform themselves from a normal phenotype into a malignant
phenotype after in vitro passages (16). After intravenous injection of MSCs
into the tail veins of mice, these cells could expand rapidly
within the lung parenchyma, forming osteosarcoma-like lesions
(17). In this study, the bone
marrow-derived MSCs which migrate to injured arteries during or
after thrombus formation may have developed into the SCL under
in vitro conditions. However, the SCL could grow along the
intimal surface of the pulmonary artery in a sheet-like form and
result in the occlusion of the artery (Fig. 3A, B and D), suggesting that the SCL
were different from MSCs in terms of their behavior and tumor
progression. This indicates that the SCL may themselves have
vascular cell-like potential which leads to their affiliation with
the intimal surface of the pulmonary artery, and may be shared with
pulmonary intimal sarcoma. This is the reason why our investigation
into the characteristics of the SCL may elucidate the mechanism of
pathogenesis of pulmonary arterial intimal sarcoma.
Although the SCL grew along the intimal surface of
the pulmonary artery, the A549 cells expanded rapidly within the
lung parenchyma, forming lung cancer-like lesions (Fig. 3C), and resulted in the formation of
metastatic lesions in other organs (Fig. 3D). The differences between the
mesenchymal cells and epithelial cells may be not sufficient to
explain the observed differences between the SCL and A549 cells in
tumorigenicity, because MSCs injected intravenously have the
potential to develop into tumor-like lesions within the lung
parenchyma, similar to A549 cells. Alterations in the expression of
extracellular matrix and adhesion molecule genes (Fig. 4A and B) may explain the differences
in tumorigenicity between the SCL and A549 cells.
Cell adhesion molecules, including integrins, are
receptors located on the cell surface, through which cells can
receive important signals from their surroundings, i.e., the
basement membrane and extracellular matrix. Laminin is one of the
basement membrane proteins, signals from which are transduced
through integrin α3β1 to activate the Akt signaling pathway. This
activated pathway induces anti-apoptotic effects on these cells
(18). The increase in the
expression of laminin and integrin α1 genes in the SCL in
comparison to A549 cells (Fig. 4A)
(Table I) suggests that there was
activation of the Akt pathway in the SCL, which may be related to
the different cell behaviors between the SCL and A549 cells.
Matrix metalloproteinase (MMP)-7 exhibits
proteolytic activity against components of the extracellular matrix
(ECM). MMP-7 is frequently overexpressed in invasive cancers of
various organs, such as the colon (19), liver (20), lungs (21), and breast (22). Indeed, MMP-7 induces cancer cell
invasion under in vitro conditions (19,20,23).
The observed decrease in the expression of MMP-7 mRNA (Fig. 4B) (Table II) supports our finding that there
were no microscopically visible lesions in any other organs after
intravenous injection of SCL, although there were metastatic
lesions after intravenous injection of A549 cells (Fig. 3D).
This is, to the best of our knowledge, the first
description and investigation of cells with in vitro and
in vivo tumorigenic potential that were isolated from the
surgically removed thrombotic material of a patient with CTEPH.
Whether, and by what mechanism, these cells with a tumorigenic
potential contribute to the development of pulmonary intimal
sarcoma remains speculative. However, a further investigation of
the affinity of SCL for vascular surfaces in this mouse model may
elucidate the mechanism underlying the development of pulmonary
arterial intimal sarcoma.
Acknowledgements
This study was supported by Research
Grants for the Respiratory Failure Research Group and the
Cardiovascular Diseases (19-9, 22-33) from the Ministry of Health,
Labor and Welfare, Japan, a Grant-in-Aid for Scientific Research
(Category C 22590851) from the Japanese Ministry of Education and
Science, The Cardiovascular Research Fund, and the Takeda Science
Foundation. K.T. has received honoraria for lectures from Glaxo
Smith Kline, Actelion Pharmaceutical Ltd. N.T. has received
honoraria for lectures from Actelion, Glaxo Smith Kline, Astellas
and Pfizer and research grant support from Actelion Pharmaceutical
Ltd. We thank Toshifumi Umemiya in the Department of Molecular and
Tumor Pathology, Graduate School of Medicine, Chiba University, for
the histological analysis of the mouse tumors.
References
|
1
|
Fedullo PF, Kerr KM, Auger WR, Jamieson SW
and Kapelanski DP: Chronic thromboembolic pulmonary hypertension.
Semin Respir Crit Care Med. 21:563–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moser KM, Daily PO, Peterson K, Dembitsky
W, Vapnek JM, Shure D, Utley J and Archibald C:
Thromboendarterectomy for chronic, major vessel thromboembolic
pulmonary hypertension: immediate and long-term results in 42
patients. Ann Intern Med. 107:560–565. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jamieson SW, Kapelanski DP, Sakakibara N,
Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF and
Auger WR: Pulmonary endarterectomy: experience and lessons learned
in 1,500 cases. Ann Thorac Surg. 76:1457–1464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maruoka M, Sakao S, Kantake M, Tanabe N,
Kasahara Y, Kurosu K, Takiguchi Y, Masuda M, Yoshino I, Voelkel NF
and Tatsumi K: Characterization of myofibroblasts in chronic
thromboembolic pulmonary hypertension. Int J Cardiol. Mar 14–2011,
(Epub ahead of print).
|
|
5
|
Yao W, Firth AL, Sacks RS, Ogawa A, Auger
WR, Fedullo PF, Madani MM, Lin GY, Sakakibara N, Thistlethwaite PA,
Jamieson SW, Rubin LJ and Yuan JX: Identification of putative
endothelial progenitor cells
(CD34+CD133+Flk-1+) in
endarterectomized tissue of patients with chronic thromboembolic
pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol.
296:L870–L878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Firth AL, Yao W, Ogawa A, Madani MM, Lin
GY and Yuan JX: Multipotent mesenchymal progenitor cells are
present in endarterectomized tissues from patients with chronic
thromboembolic pulmonary hypertension. Am J Physiol Cell Physiol.
298:C1217–C1225. 2010. View Article : Google Scholar
|
|
7
|
Sakao S, Hao H, Tanabe N, Kasahara Y,
Kurosu K and Tatsumi K: Endothelial-like cells in chronic
thromboembolic pulmonary hypertension: crosstalk with
myofibroblast-like cells. Respir Res. 12:1092011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bloomberg RD, Butany JW, Cusimano RJ and
Leask RL: Primary cardiac sarcoma involving the pulmonary artery
and valve. Can J Cardiol. 19:843–847. 2003.PubMed/NCBI
|
|
9
|
Bode-Lesniewska B, Zhao J, Speel EJ,
Biraima AM, Turina M, Komminoth P and Heitz PU: Gains of 12q13–14
and overexpression of mdm2 are frequent findings in intimal
sarcomas of the pulmonary artery. Virchows Arch. 438:57–65.
2001.
|
|
10
|
Gaumann A, Petrow P, Mentzel T, Mayer E,
Dahm M, Otto M, Kirkpatrick CJ and Kriegsmann J: Osteopontin
expression in primary sarcomas of the pulmonary artery. Virchows
Arch. 439:668–674. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burke AP and Virmani R: Sarcomas of the
great vessels. Cancer. 71:1761–1773. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McGlennen RC, Manivel JC, Stanley SJ,
Slater DL, Wick MR and Dehner LP: Pulmonary artery trunk sarcoma: a
clinicopathologic, ultrastructural, and immunohistochemical study
of four cases. Mod Pathol. 2:486–494. 1989.PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg R: The hallmarks of
cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
14
|
Rai PR, Cool CD, King JA, Stevens T, Burns
N, Winn RA, Kasper M and Voelkel NF: The cancer paradigm of severe
angioproliferative pulmonary hypertension. Am J Respir Crit Care
Med. 178:558–564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakao S and Tatsumi K: Vascular remodeling
in pulmonary arterial hypertension: multiple cancer-like pathways
and possible treatment modalities. Int J Cardiol. 147:4–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loebinger MR, Sage EK and Janes SM:
Mesenchymal stem cells as vectors for lung disease. Proc Am Thorac
Soc. 5:711–716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aguilar S, Nye E, Chan J, Loebinger M,
Spencer-Dene B, Fisk N, Stamp G, Bonnet D and Janes SM: Murine but
not human mesenchymal stem cells generate osteosarcoma-like lesions
in the lung. Stem Cells. 25:1586–1594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu J, Fujibayashi A, Yamada KM and
Sekiguchi K: Laminin-10/11 and fibronectin differentially prevent
apoptosis induced by serum removal via phosphatidylinositol
3-kinase/Akt- and MEK1/ERK-dependent pathways. J Biol Chem.
277:19922–19928. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adachi Y, Yamamoto H, Itoh F, Hinoda Y,
Okada Y and Imai K: Contribution of matrilysin (MMP-7) to the
metastatic pathway of human colorectal cancers. Gut. 45:252–258.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto H, Iku S, Adachi Y, Imsumran A,
Taniguchi H, Nosho K, Min Y, Horiuchi S, Yoshida M, Itoh F and Imai
K: Association of trypsin expression with tumour progression and
matrilysin expression in human colorectal cancer. J Pathol.
199:176–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bolon I, Devouassoux M, Robert C, Moro D,
Brambilla C and Brambilla E: Expression of urokinase-type
plasminogen activator, stromelysin 1, stromelysin 3, and matrilysin
genes in lung carcinomas. Am J Pathol. 150:1619–1629.
1997.PubMed/NCBI
|
|
22
|
Heppner KJ, Matrisian LM, Jensen RA and
Rodgers WH: Expression of most matrix metalloproteinase family
members in breast cancer represents a tumor-induced host response.
Am J Pathol. 149:273–282. 1996.PubMed/NCBI
|
|
23
|
Yamamoto H, Adachi Y, Itoh F, Iku S,
Matsuno K, Kusano M, Arimura Y, Endo T, Hinoda Y, Hosokawa M and
Imai K: Association of matrilysin expression with recurrence and
poor prognosis in human esophageal squamous cell carcinoma. Cancer
Res. 59:3313–3316. 1999.PubMed/NCBI
|