Introduction
Ovarian cancer accounts for 3% of all cancers for
women in the U.S, and is the most lethal gynecologic malignancy
(1,2). Ovarian cancer histologic subtypes
include epithelioid (serous, endometrioid, mucinous, clear cell and
undifferentiated) and non-epitheliod (3), of which the epithelial ovarian cancer
(EOC) comprises about 90% of all ovarian cancers (4). Although the 5-year survival rate for
all stages has improved by combination chemotherapy with
cis-diamminedichloroplatinum (DDP) (5), it is still disappointingly low,
largely because of the finding that most patients present with
metastatic disease and due to high intrinsic resistance towards
chemotherapeutic drugs (6,7), the low overall cure rates and the
intolerable side effects of systemic chemotherapy asks for the
development of novel and more effective pharmacological
interventions.
Inhibition of apoptosis is one of the important
mechanisms for the growth of many malignant tumor cells. The
inhibitor of apoptosis proteins (IAPs) comprise a family of
structurally related cellular factors that suppress apoptosis
induced by a variety of stimuli. Up to now, eight members of IAPs
family have been identified and respectively named as: NAIP
(8), c-IAPl (MIHB, HIAP-2), c-IAP2
(HIAP-1, MIHC, API2) (9), XIAP
(hILP, MIHA, ILP-1) (10),
Survivin (11), Apollon (Bruce)
(12), ILP-2 (13) and livin (ML-IAP, KIAP) (14). Livin composed of a single
Baculovirus-Repeat (BIR) domain and a zinc-binding RING-domain,
splicing of the gene in exon 6 yields two alternatively similar
isoforms, α and β, except for 18 amino acids presented in the α
variant.
Aberrant livin expression has been demonstrated in
human malignancy and tumor tissue cells (15–19).
In most tumors, the presence or expression level of livin
correlated with in vitro drug resistance, advanced tumor
stages, and poor outcome (16,19).
Antisense oligonucleotides or small interference RNA (siRNA)
mediated livin knockdown have been shown to reduce tumor cell
proliferative potential and/or induce sensitization toward
proapoptotic simuli in renal cell carcinoma cells, lung cancer
cells or neuroblastoma cells (20–23).
Together, these studies suggest that livin may be essential for
survival of some cancer cells.
In our previous work (24), the expression of livin was detected
by immunohistochemistry (IHC) in 72% of investigated patients with
EOC. Because evaluation of staining intensity by IHC is subject to
observer variability, we precede this follow-up study to assess
livin expression in EOC tumor tissues by RT-PCR and western blot,
as the latter two methods are more quantifiable and reproducible
methods. In addition, we inhibited endogenous livin expression in
EOC cell lines by specific short hairpin RNA (shRNA). We show that
targeted silencing of the livin gene efficiently sensitized EOC
cells towards chemotherapeutic stimulus. These studies highlight
targeted inhibition of livin as a novel therapeutic strategy in
ovarian cancer.
Materials and methods
Tissue samples
Frozen tumor specimens were obtained from 50
patients with EOC. The median patient age was 56 years (range
38–82). The histological type and histological grade were as
follows: subtype (serous, 22 cases; mucinous, 12 cases;
endometrioid, 8 cases; clear cell, 8 cases), and grade (G1, 15
cases; G2, 10 cases; G3, 35 cases). Clinical stages, determined
according to the FIGO system (International Federation of
Gynecologists and Obstetricians) were stage I–II in 12 patients,
stage III–IV in 38 patients. Twenty samples of normal ovarian
tissues were obtained from patients undergoing ovarian biopsy or
ovariotomy (patients suffering from uterine fibroids, dysfunctional
uterine bleeding or uterine prolapse underwent hysterectomy
meanwhile, pathologically precluded abnormality in ovarian
tissues), which aged ranging from 42 to 68 years (median age 49).
Twenty samples of benign ovary tumor were obtained from patients
(15 cases of serous cystadenoma and 5 cases of mucinous
cystadenoma) aged ranging from 24 to 58 years (median age 43).
These samples were collected from patients admitted to the
Shengjing Hospital of China Medical University (Shenyang, China).
None of the patients had received chemotherapy, radiotherapy or
immunotherapy. This work was approved by the ethical committee of
the China Medical University. Informed consent was obtained from
each patient.
RNA extraction and semi-quantitative
RT-PCR
Total RNAs were extracted from tissue samples using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The amount of RNA was quantified in
duplicate using UV absorbance at 260 nm. Equal amounts of total RNA
was reverse-transcribed into cDNA using the Takara RNA PCR kit
(DRR037, Takara, Dalian, China) in a final volume of 20 μl
following the manufacturer’s protocol. To minimize variation in the
reverse transcription reaction, all RNA samples from a single
experimental setup were reverse transcribed simultaneously. The PCR
primers were as follows: for livin (GenBank: AF311388) specific
primers discriminating between the α- and the β-variants were:
sense, 5′-AGTTCCTGCTCCGGTCAAA-3′; antisense,
5′-GCACGGCACAAAGACGAT-3′, which yields two products of 347 bp and
293 bp, respectively; and for β-actin (GenBank: NM_001101)
(internal control), sense 5′-GATTGGCTCAGGACATTTCTG-3′ antisense
5′-GATTGCTCAGGACATTTCTG-3′ (751 bp). The amplification conditions
were as follows: denaturation at 94°C for 5 min; 32 cycles of 94°C
for 40 sec, 55°C (for livin) and 52°C (for β-actin) for 1 min, 72°C
for 1 min; and a final 5 min extension at 72°C. The PCR products
were separated on 2% agarose gels, and the bands were visualized by
ethidium bromide and photographed under UV light.
Immunoblotting assay
Frozen tissues were washed with ice-cold PBS and
homogenized in lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5
mM EDTA, 5 mM EGTA, 1 mM PMSF, 0.1% Nonidet P-40, 0.1% Triton
X-100, 50 mM NaF, 0.5 mM Na3VO4). Lysates
were centrifuged at 12,000 × g for 20 min at 4°C and supernatants
collected. Protein concentrations were determined using Bradford
protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Equal
amounts of proteins were separated on 12% SDS-polyacrylamide gel
electrophoresis and transfered onto a polyvinylidene difluoride
(PVDF) membrane (Millipore, Billerica, MA, USA). The membrane was
then blocked with 5% nonfat milk and immunoprobed with rabbit
polyclonal anti-livin antibody (IMG-347, Imgenex, San Diego, CA,
USA), followed by exposed to horseradish peroxidase-conjugated
secondary antibody and visualized using ECL plus reagent (GE
Healthcare, Piscataway, NJ, USA). Quantity One software (Bio-Rad)
was applied for analysis of the optical density of the protein
bands. The relative expression quantity of livin protein was
illustrated as the percentage of the optical density (OD) of livin
protein, adjusted with the corresponding β-actin OD.
Construction of livin shRNA vectors
Four non-overlapping segments which are located at
786–804, 647–665, 609–627 and 519–537 position in livin cDNA
(NM_022161.2) were selected as candidate targets using Target
Finder and Design Tool of Ambion (http://www.ambion.com/techlib/misc/siRNA_tools.html).
The dsDNA sequences simultaneously aimed at both of the two
variants. Another shRNA of nonspecific sequence was used as a
control (Table I). BLAST search of
the human genome database was carried out and found no homology
with other human genes. Then, these shRNAs were subcloned into
lentiviral vector pGCL-GFP (GeneChem, Shanghai, China) plasmid
between the HpaI and XhoI enzyme sites and the
recombinants generated were named Psi-1, Psi-2, Psi-3, Psi-4 and
Psi-NC. The inserts in those recombinants were confirmed by DNA
sequencing (data not presented).
 | Table ICandidate livin shRNAs and the target
sequences. |
Table I
Candidate livin shRNAs and the target
sequences.
| Target sequence
(5′→3′) | Position | GC percent (%) |
|---|
| Psi-1 |
CAGGCCATCAGGACAAGGT | 786–804 | 57.14 |
| Psi-2 |
GGAAGAGACTTTGTCCACA | 647–665 | 47.62 |
| Psi-3 |
GGAGAGAGGTCCAGTCTGA | 609–627 | 52.38 |
| Psi-4 |
AGTGGTTCCCCAGCTGTCA | 519–537 | 57.14 |
| Psi-NC |
TTCTCCGAACGTGTCACGT | - | 52.63 |
Cell culture and transfection
Ovarian cancer cell lines SKOV3 cells (purchased
from Cancer Institute, Chinese Academy of Medical Sciences) were
cultured in RPMI-1640 medium with 10% fetal bovine serum (FBS) and
seeded in 6-well plates and allowed overnight growth to reach
70–80% confluence. Cells were then transfected with each of the
siRNA recombinants using Lipofectamine™ 2000 reagent (Invitrogen).
For confirmation of downregulation of livin gene, after 72 h, cells
were harvested and analyzed for reduction of livin expression by
real-time RT-PCR and immunoblotting. The lentiviral vector giving
maximal knockdown was selected and packaged by the 293T packaging
cell and the vector particles were concentrated. During the
transfection protocol, the most suitable MOI (multiplicity of
infection) of lentivirus is MOI 20. Controls consisted of either
Lipofectamine-treated (untreated) or control siRNA-treated cells
(MOI 20) alone.
Real-time RT-PCR
Total RNA was extracted from treated cells and
reverse transcripted into cDNA as mentioned above. The real-time
PCR primers were as follows: Livin: sense,
5′-GCGTCTGGCCTCCTTCTATG-3′ antisense, 5′-AAGCACCTCACCTTGTCCTG-3′
(108 bp), which did not discriminate between the two livin
isoforms. β-actin: sense, 5′-GTGGACATCCGCAAAGAC-3′; antisense
5′-AAAGGGTGTAACGCAACTA-3′ (302 bp). Untreated group values were
used as calibrator. The relative level was calculated using the
2−ΔΔCt method after normalization with β-actin as a
housekeeping gene (25).
Cell lysis and immunoblotting
Treated cells were harvested and protein was
extracted, separated and transferred onto PVDF membrane as
described earlier. Filters were probed with either rabbit
polyclonal anti-active caspase 9 antibody (ab25759, Abcam,
Cambridge, MA, USA), anti-active caspase 7 antibody (ab2323, Abcam)
or anti-active caspase 3 antibody (ab2302, Abcam) followed by
secondary antibody and developed by ECL staining.
Cell proliferation assay
Cell proliferation was investigated by colorimetric
assay using 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium
bromide (MTT). In brief, SKOV3 cells were plated in a 96-well
flat-bottomed plate at 5×103 cells/well in triplicate
and cultured in medium for 24 h. Then the cells were treated with
lentiviral livin shRNA or control siRNA for 24, 48, 72 and 96 h. On
each experimental point, 20 μl MTT (5 mg/ml in PBS) was
added to each well, and the cells were incubated at 37°C for
additional 4 h. Then the reaction was stopped by lysing the cells
with 150 μl DMSO for 5 min. The absorbance of each well were
measured at 570 nm using a microtitre plate reader (Labsystems MK3,
Finland). The data were normalized to the untreated group. All
experimental points were set up in three replicate wells and
independently performed three times.
Apoptosis assay
Cell apoptosis assay were performed by flow
cytometry after 72 h since the cells were transfected with siRNAs.
Briefly, cells were seeded in 6-well plates in triplicate at
1×105 cells/well and transfected as described earlier.
At 72 h post-transfection, the cells were harvested, washed twice
with cold PBS, suspended in 1X binding buffer [10 mM HEPES (pH
7.4), 150 mM NaCl, 2.5 mM CaCl2, 1 mM MgCl2,
4% BSA] at a concentration of 1×106 cells/ml. Cells were
incubated in the dark for 15 min at room temperature with 5
μl Annexin V-FITC (BD Biosciences Pharmingen, Allschwill,
Switzerland). Before analysis, 2.5 μl of 7-AAD was added to
the samples in a final volume of 200 μl. A total of 10,000
cells were accumulated with a BD FACSCaliburH apparatus and data
were analyzed using the CellQuest software. Apoptotic cells were
determined by Annexin V-positive and 7-AAD negative cells.
Chemosensitivity assay
Cells were treated with lentiviral siRNA for 48 h,
and then with seven concentration gradient of DPP in PBS (100, 50,
25, 12.5, 6.25, 3.13, 1.56 mg/l) for 24 h; the effects on cell
growth were examined by MTT assay as described earlier, the growth
inhibition was calculated according to the following formula:
Inhibitory ratio (%) = [1− (OD of the lentiviral siRNA/OD of the
untreated) ×100%]. After treated with siRNA plus DPP (5 mg/l), flow
cytometry was performed to detect cells apoptosis, and active
caspase 9, 7, 3 were determined as indicated above.
Statistical analysis
All experiments were repeated in triplicate and data
were expressed as mean ± standard deviation of the mean (SD).
Statistical significance was assessed by one-way ANOVA followed by
Bonferroni multiple comparison post-tests. Statistical analyzes
were performed using SPSS 13.0 package (SPSS, Chicago, IL, USA).
p<0.05 was considered significant.
Results
Livin mRNA and protein expression in
EOC
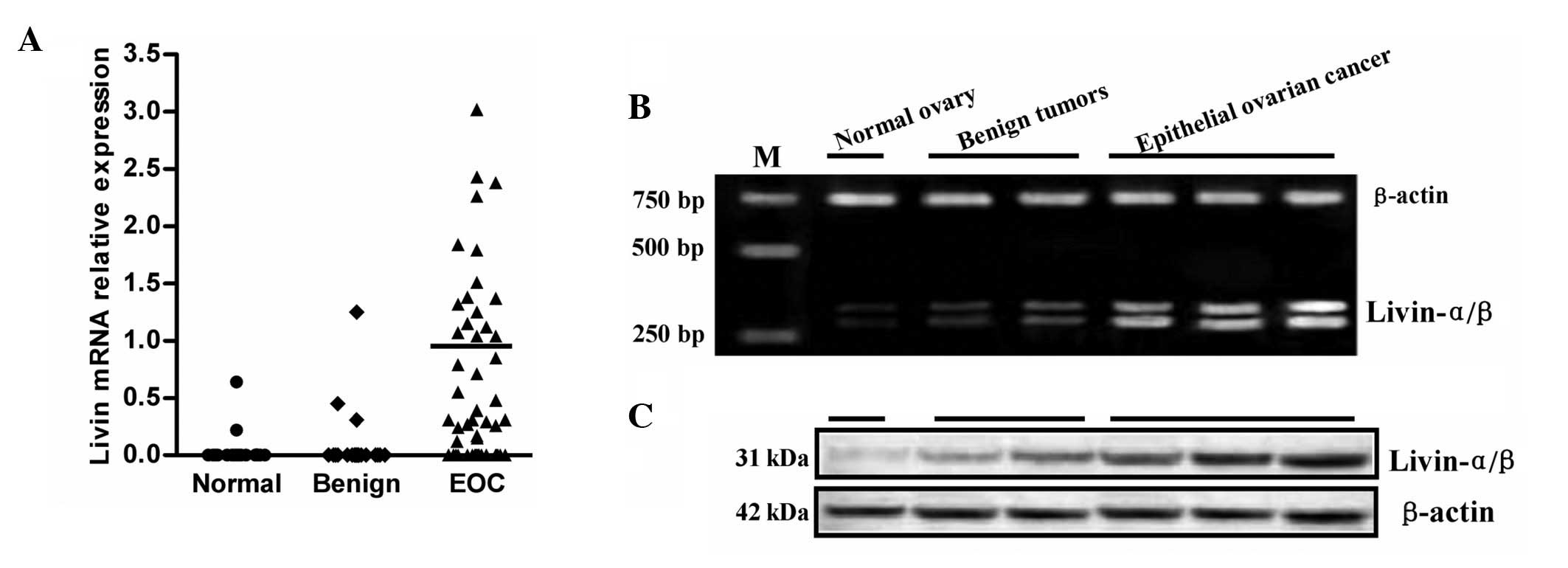
Livin mRNA was detected in 32 of 50 (64%) EOC tumor
specimens, 3 of 20 (15%) benign tumors, 2 of 20 (10%) normal ovary
tissues, respectively (χ2=24.448, P<0.01) (Fig. 1A). The expression levels of total
livin differed greatly among the 32 positive tumor samples; with no
obvious difference between two isoforms (data not shown) (Fig. 1B). The mean livin mRNA value among
the 32 positive cases was set as 1.0. High expression levels
(defined as values greater than the mean positive tumor level) were
observed in 16 tumors. These included 4/12 (33%) patients with
advanced stage (III and IV) disease, 12/38 (32%) patients with
localized stage (I and II) disease. No obvious link was observed
between the expression levels with tumor stage or tumor grade for
the limited numbers of tissue samples investigated here. Generally,
comparable changes in livin protein concentrations were also
observed. Thirty-two of the 50 (64%) tumor specimens assessed by
immunoblotting were positive for livin protein; all of these
specimens were also positive by RT-PCR (Fig. 1C). Conversely, all the specimens
negative by RT-PCR were also negative for livin protein.
Silencing of livin gene expression by
RNAi in SKOV-3 cells
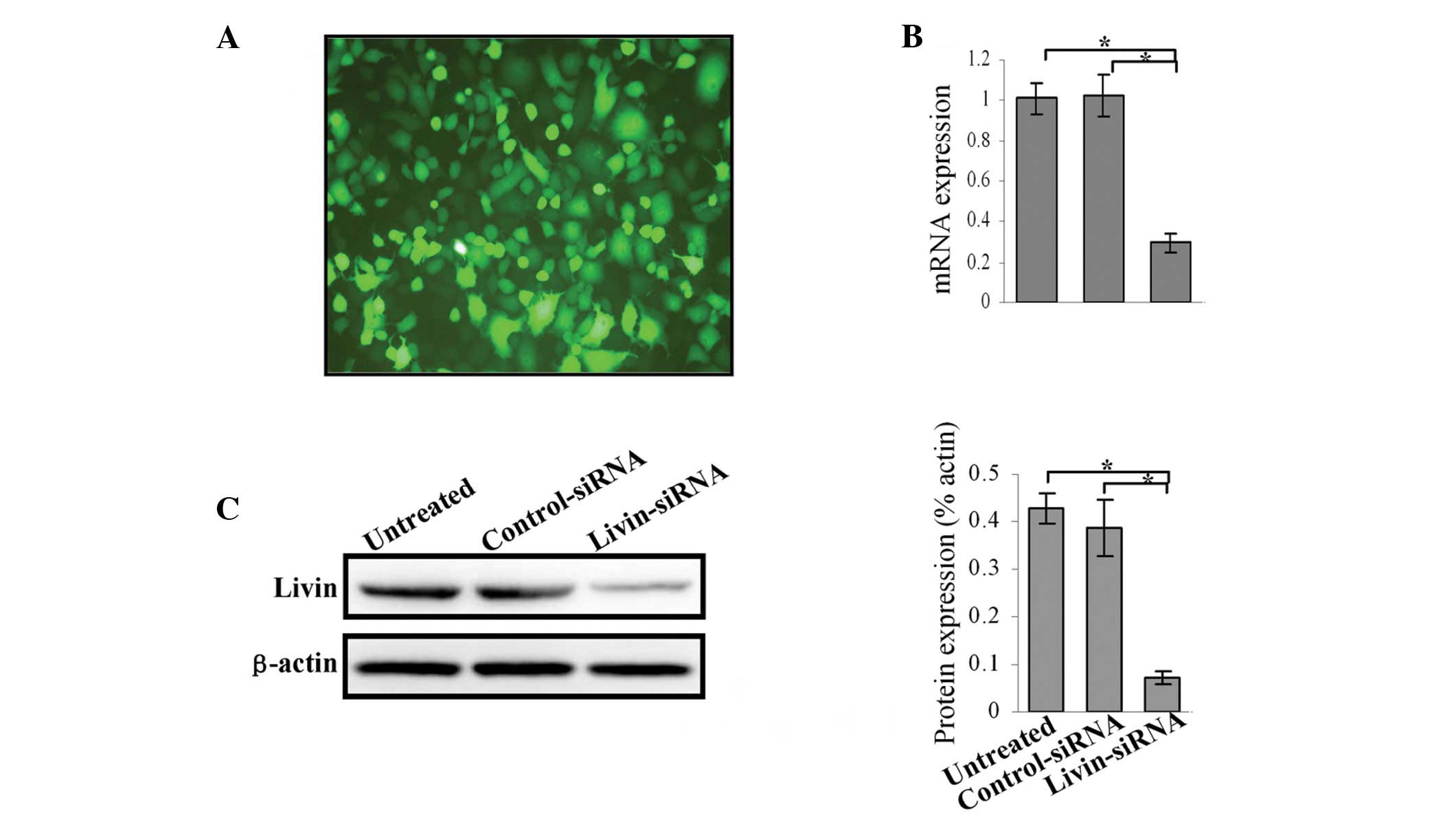
SKOV3 cells were transfected with different shRNA
constructs to screened effective targets of livin and obtained the
optimum concentration for lentivirus transfection. The knockdown
efficiency of four candidate shRNA was evaluated using western
blotting. The results disclosed that the best knockdown effect was
with Psi-4 siRNA at MOI 20 (data not shown). At 72 h
post-transfection with Psi-4 siRNA more than 90% of the survived
cells were GFP-positive (Fig. 2A).
Real-time RT-PCR analyses performed at 72 h post-transfection
showed that livin mRNA levels were significantly reduced by 70%
when compared with control transfections (Fig. 2B). To correlate the decrease in
livin mRNA expression with livin protein levels, immunoblotting
analysis was performed at 72 h after siRNA treatment. The protein
levels were reduced, thereby confirming efficient knockdown
(Fig. 2C).
Livin siRNA induces spontaneous apoptosis
in SKOV-3 cells
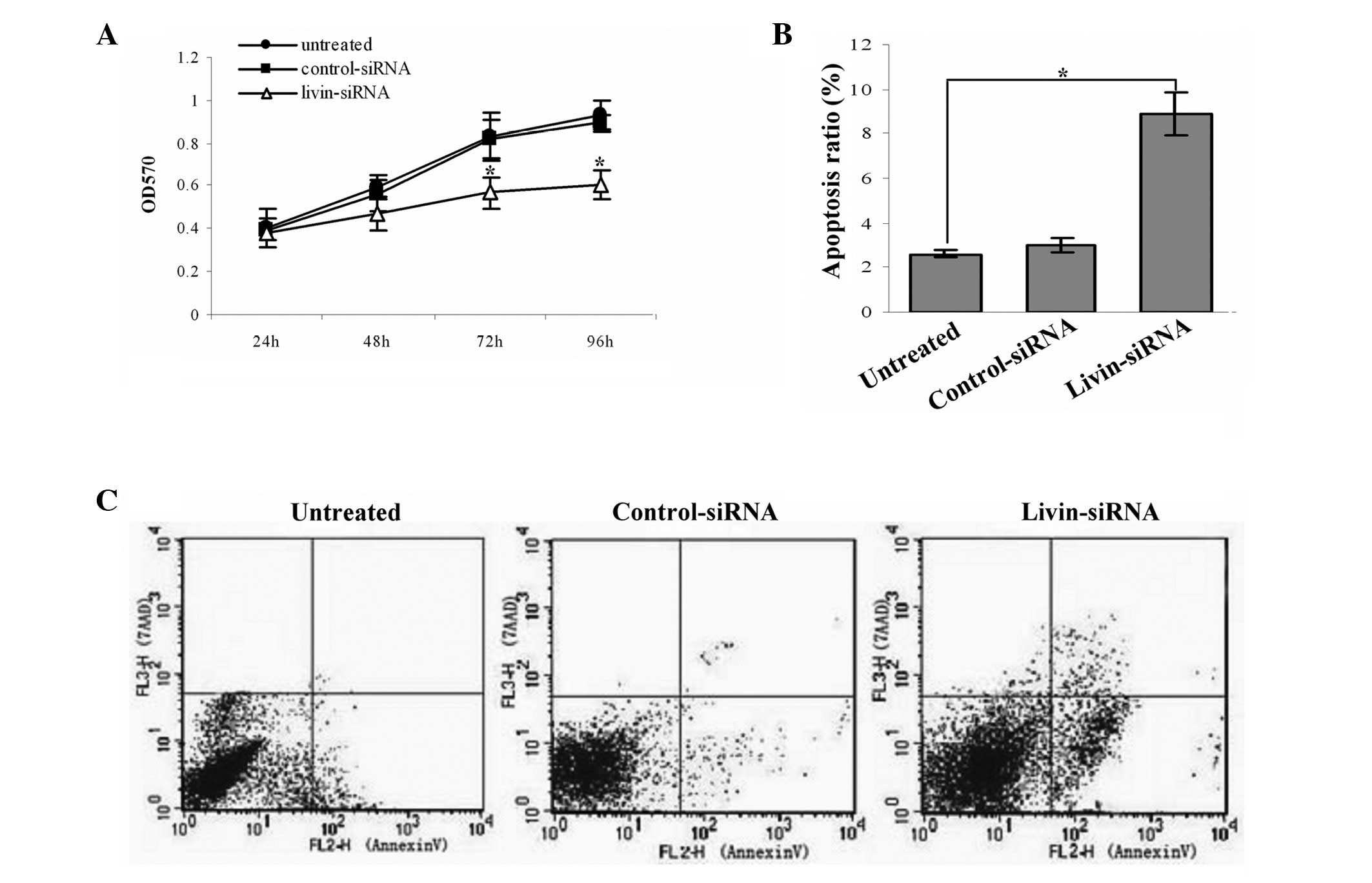
We first tested the effect of livin siRNA on SKOV-3
cell proliferation by a colorimetric assay using MTT, and the
inhibition rate was calculated with the method above. The results
showed that silencing of livin gene has substantially effect on
SKOV3 cells proliferation compared with control groups (both
P<0.05) (Fig. 3A).
The cell apoptosis was measured by flow cytometry at
72 h after transfection; apoptosis cells were determined by Annexin
V-positive and 7-AAD-negative cells (Fig. 3B and C). The results demonstrated
that the apoptosis ratio of livin siRNA cells obviously increased
compared with control groups (both P<0.05).
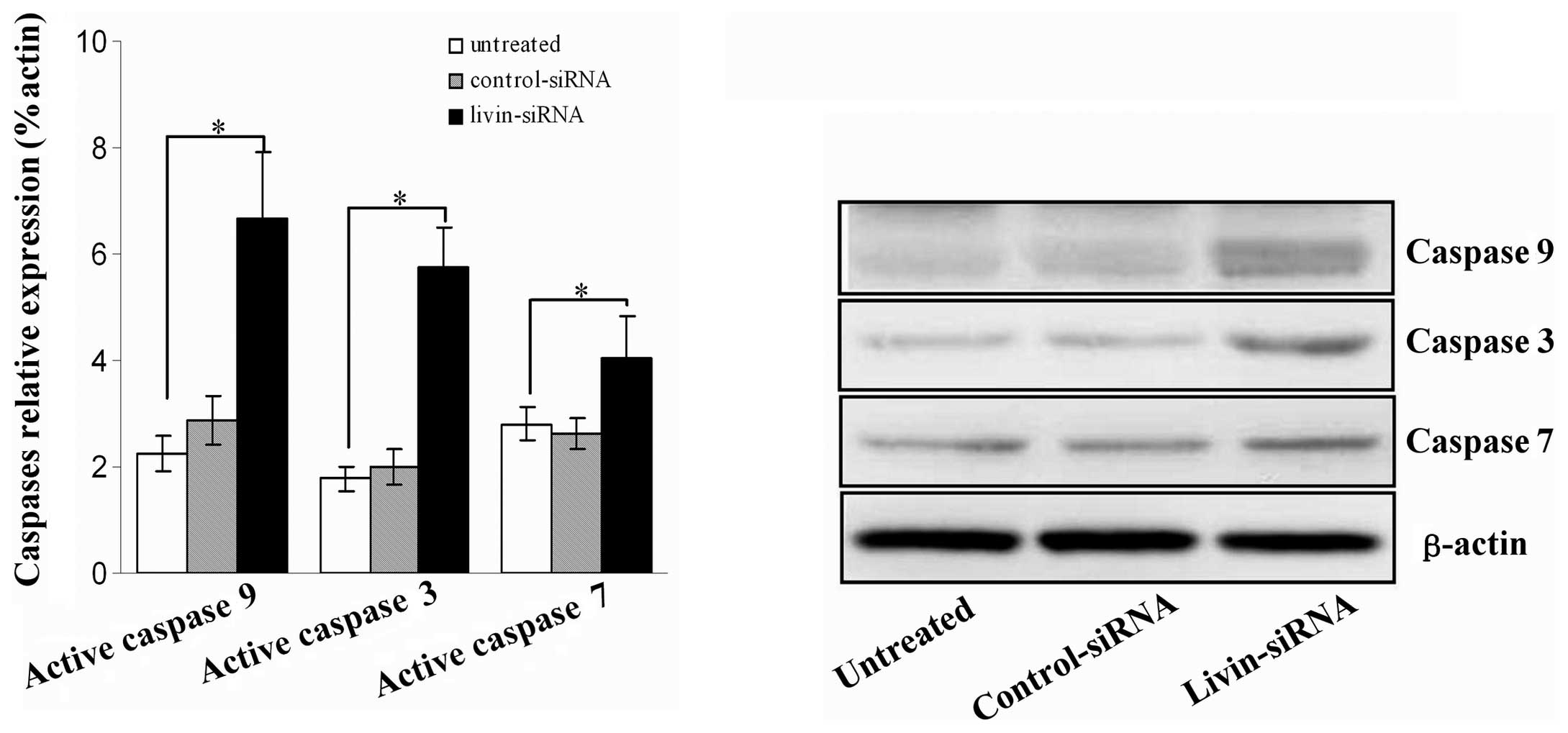
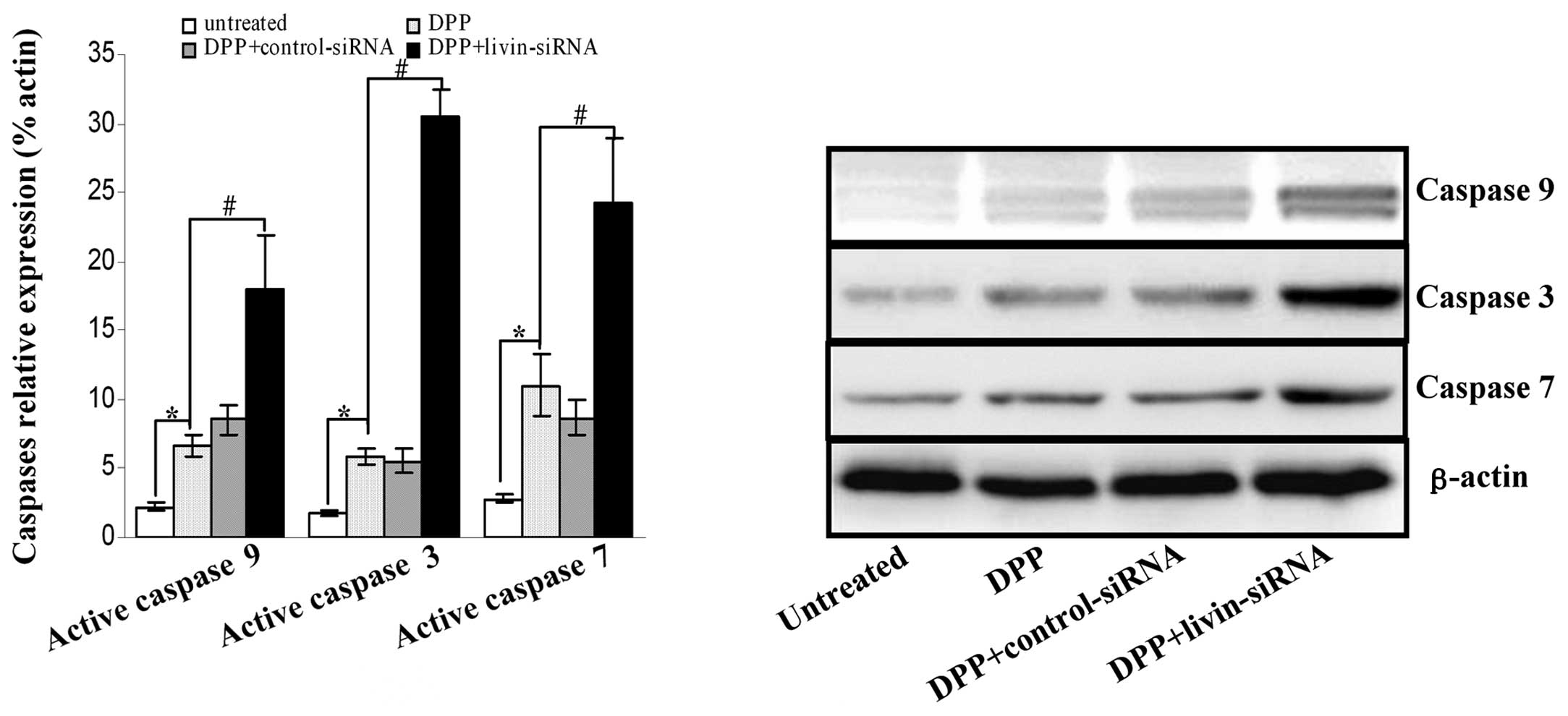
We further investigated the cleavage of several
molecular markers of mitochondrial apoptotic signaling pathway,
including caspase 9, caspase 3, and caspase 7 by immunoblotting
after SKOV-3 cells were stably transfected with livin siRNA for 72
h. As shown in Fig. 4, the results
showed that in the livin siRNA cells, cleavage of caspase 9,
caspase 3 and caspase 7 was remarkably increased, compared with
control groups (all P<0.05).
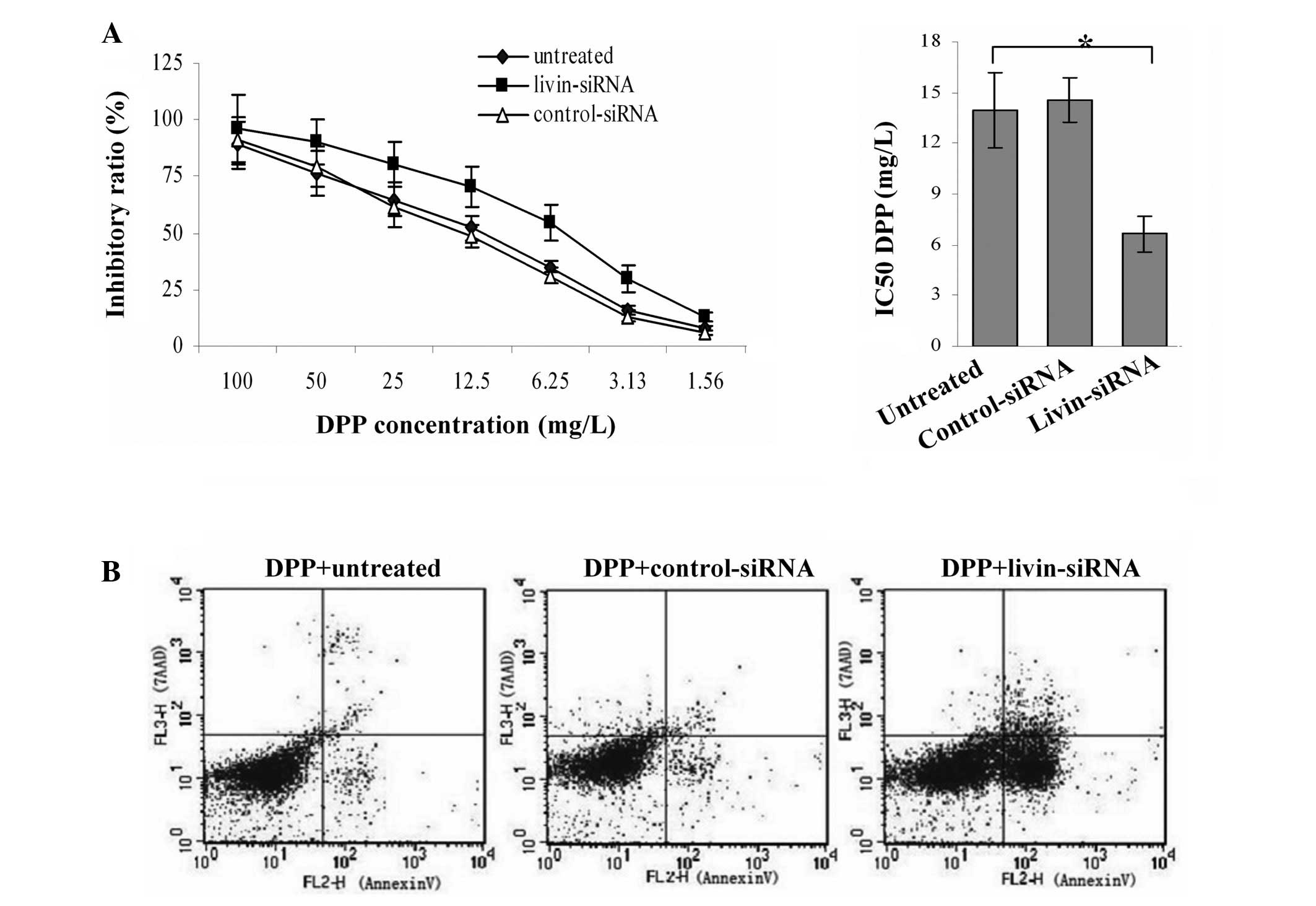
Inhibition of livin gene sensitizes SKOV3
cells towards DPP stimulus
Livin siRNA- or control siRNA-transfected cells were
treated separately with DPP for various concentrations and the
growth inhibition curves are shown in Fig. 5A. The results showed that the
silencing of livin expression resulting in strikingly higher cell
growth inhibition at different drug concentrations. The IC50 for
untreated and control-siRNA were 13.96 and 14.58, but the IC50 for
livin-siRNA dropped to 6.67 (P<0.05).
Cell apoptosis were determined by Annexin-V/7AAD
assay (Fig. 5B). In SKOV3 cells,
treatment with livin siRNA plus DPP (5 mg/l) resulted in a
significantly higher apoptosis proportion (29.89±3.29) compared to
control siRNA plus DPP (7.62±0.95) or DPP alone (6.66±0.69) (both
P<0.05). These results suggest that livin inhibition by
lentiviral shRNA resulted in remarkable enhancement in
chemosensitivity of DPP for SKOV3 cells.
Before livin inhibition, treatment with DPP alone
resulted in activation of apoptosis signaling pathway with
increased cleavage of caspase 9, caspase 3 and caspase 7 in SKOV3
cells. Targeted inhibition of livin gene existed synergistic effect
on induction of apoptosis in SKOV3 cells, as evidenced by a
dramatical enhancement of caspase 9, 7 and 3 cleavages compared
with control siRNA-treated cells after treatment with DPP (Fig. 6).
Discussion
Ovarian cancer has the highest mortality rate of all
gynecologic malignancies. Despite considerable progress in
therapies, including surgery, radiation, especially chemotherapy,
the overall survival for patients with ovarian cancer has not
improved substantially. High intrinsic resistance of ovarian cancer
cells to chemotherapeutic drugs may contribute to the failure of
the treatment. Among the reasons of chemotherapy resistance,
apoptosis deficiency is considered to be a major cause, since many
chemotherapy agents act through the induction of apoptosis
(26). So it is important to
identify the molecular determinants that mediate the apoptotic
resistance of ovarian cancer cells for the development of novel and
more effective therapy strategies.
Livin, a new member of IAPs family, has been shown
to be expressed in transformed cells and multiple malignant tumors,
such as carcinomas of the prostate, renal, gastric, bladder, lung
and breast, but not detectable in most normal differentiated
tissues with the exception of the placenta, normal testes and
spinal cord (15,27–29).
The antiapoptotic activity of livin is mediated via inhibition of
the mitochondrial apoptotic signaling pathway molecules caspase 3,
7, and 9, as well as by its E3 ubiquitin-ligase-like activity that
promotes degradation of Smac/DIABLO, a critical endogenous
regulator of all IAPs (30,31).
Pilot studies of molecular profiling and retrospective analysis
showed that livin has a strong correlation with shorter
disease-free or overall survival in most cases, and identified
livin expression as a candidate independent prognostic indicator of
poor outcome in patients with some tumor types (15,18,32).
The above studies, along with the confirmed antiapoptotic activity
of livin, have raised considerable interest in developing
strategies for the therapeutic inhibition of livin in cancers. It
has been shown that downregulation of livin gene expression by RNA
interference or antisense oligonucleotides reduces the growth of
livin-expressing cancer cells and can resensitise tumor cells
towards proapoptotic anticancer agents (33–35).
In our initial studies, we evaluated the expression
of livin in individual primary ovarian cancer frozen tissues
(Fig. 1). The presence of both
livin isoforms was demonstrated by RT-PCR and western blot in 64%
of EOC tumors studied, on the contrary only 10–15% in benign and
normal ovary tissues. This finding confirms and extends our
previous work using IHC. Contrary to our results, another study did
not detect livin expression in ovarian carcinoma, possibly due to
the limited number and complexity of specimens (only 12 solid
tumors including ovarian carcinoma, tubal carcinoma, primary
peritoneal carcinoma, and 16 peritoneal and pleural effusions)
(36). Although the number of EOC
specimens under investigation is limited, the positive percentage
for livin expression (64%) is similar to the data from previously
studies on other cancer types, such as melanoma (70.6%) (37) and lung cancer (76.3%) (27). Notably, previous studies showed
that livin expression or presence is significantly correlated with
tumor stage, increasing with tumor progression. We did not find
correlation between livin expression and tumor stage or
histological grade, possibly because of the limited specimens, but
high presence indicated that it is closely related to EOC formation
and pathological progression. Further studies involving large
number of samples of EOC cases and long-term follow-up are required
to investigate the relationship of livin with EOC tumor stage and
grade.
We further studied the livin function by
gene-silencing studies, targeted inhibition of endogenous livin
expression in SKOV3 cells resulted in downregulation of livin and
increased cell apoptosis, which was caused by activation of
mitochondrial apoptotic signaling pathway as evidenced by increased
activation of caspase 9, caspase 3, and caspase 7. Enhanced
sensitivity of siRNA-transfected tumor cells to DPP was also
observed, as suggested by increased DPP-induced apoptosis by
greater than 350%. Our results are in line with data of previous
studies for HeLa cervical carcinoma cells (38), non-small cell lung cancer (39), and renal cell carcinoma (34). All these studies suggested that the
inhibition of livin sensitize livin-expressing cancer cells to
chemotherapy stimuli.
In summary, our data suggest that the livin is
expressed in EOC and contributes to the apoptotic resistance of EOC
cells, a combination of downregulation of livin and DPP can
significantly enhance the apoptosis induced by DPP. Thus, using
combined treatment with DPP and livin inhibition may provide an
effective strategy for ovary cancer therapy. However, to realize
the therapeutic potential of RNA drugs, efficient, tissue-specific
and nonimmunogenic delivery technologies must be developed.
Recently, ultrasound-mediated and exosome-endogenous nano-vesicles
mediated siRNA delivery was reported to greatly promote the
specificity and efficiency of transfection (40,41).
Further studies are necessary to determine whether these techniques
can provide a more effective route for siRNA-mediated gene
therapy.
Abbreviations:
|
BIR
|
Baculovirus-Repeat
|
|
DDP
|
cis-diamminedichloroplatinum
|
|
EOC
|
epithelial ovarian cancer
|
|
ECL
|
enhanced chemiluminescence
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
EGTA
|
ethylene glycol tetraacetic acid
|
|
HEPES
|
hydroxyethyl piperazine ethanesulfonic
acid
|
|
IAPs
|
inhibitor of apoptosis proteins
|
|
IHC
|
immunohistochemistry
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide
|
|
MOI
|
multiplicity of infection
|
|
OD
|
optical density
|
|
PVDF
|
polyvinylidene difluoride
|
|
PMSF
|
phenylmethylsulfonyl fluoride
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
|
SDS
|
sodium dodecyl sulfate
|
|
siRNA
|
small interference RNA
|
|
shRNA
|
specific short hairpin RNA
|
Acknowledgements
We thank Yaoqiong Cao (GeneChem Co.
Ltd, Shanghai, China) for technical assistance. This work was
supported in part by grant from the National Natural Science
Foundation of China (grant no: 81100432).
References
|
1.
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
2.
|
Campos SM and Ghosh S: A current review of
targeted therapeutics for ovarian cancer. J Oncol.
1493622010.PubMed/NCBI
|
|
3.
|
Siwak DR, Carey M, Hennessy BT, et al:
Targeting the epidermal growth factor receptor in epithelial
ovarian cancer: current knowledge and future challenges. J Oncol.
5689382010.PubMed/NCBI
|
|
4.
|
Feeley KM and Wells M: Precursor lesions
of ovarian epithelial malignancy. Histopathology. 38:87–95. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kim A, Ueda Y, Naka T and Enomoto T:
Therapeutic strategies in epithelial ovarian cancer. J Exp Clin
Cancer Res. 31:142012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Trimble EL, Wright J and Christian MC:
Treatment of platinum-resistant ovarian cancer. Expert Opin
Pharmacother. 2:1299–1306. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Roy N, Mahadevan MS, McLean M, et al: The
gene for neuronal apoptosis inhibitory protein is partially deleted
in individuals with spinal muscular atrophy. Cell. 80:167–178.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Rothe M, Pan MG, Henzel WJ, Ayres TM and
Goeddel DV: The TNFR2-TRAF signaling complex contains two novel
proteins related to baculoviral inhibitor of apoptosis proteins.
Cell. 83:1243–1252. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Liston P, Roy N, Tamai K, et al:
Suppression of apoptosis in mammalian cells by NAIP and a related
family of IAP genes. Nature. 379:349–353. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen Z, Naito M, Hori S, Mashima T, Yamori
T and Tsuruo T: A human IAP-family gene, apollon, expressed in
human brain cancer cells. Biochem Biophys Res Commun. 264:847–854.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Richter BW, Mir SS, Eiben LJ, et al:
Molecular cloning of ILP-2, a novel member of the inhibitor of
apoptosis protein family. Mol Cell Biol. 21:4292–4301. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Salvesen GS and Duckett CS: IAP proteins:
blocking the road to death’s door. Nat Rev Mol Cell Biol.
3:401–410. 2002.
|
|
15.
|
Kempkensteffen C, Hinz S, Christoph F, et
al: Expression of the apoptosis inhibitor livin in renal cell
carcinomas: correlations with pathology and outcome. Tumour Biol.
28:132–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ashhab Y, Alian A, Polliack A, Panet A and
Ben Yehuda D: Two splicing variants of a new inhibitor of apoptosis
gene with different biological properties and tissue distribution
pattern. FEBS Lett. 495:56–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Vucic D, Stennicke HR, Pisabarro MT,
Salvesen GS and Dixit VM: ML-IAP, a novel inhibitor of apoptosis
that is preferentially expressed in human melanomas. Curr Biol.
10:1359–1366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Nedelcu T, Kubista B, Koller A, et al:
Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res
Clin Oncol. 134:237–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kim DK, Alvarado CS, Abramowsky CR, et al:
Expression of inhibitor-of-apoptosis protein (IAP) livin by
neuroblastoma cells: correlation with prognostic factors and
outcome. Pediatr Dev Pathol. 8:621–629. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ye L, Song X, Li S, et al: Livin-α
promotes cell proliferation by regulating G1-S cell cycle
transition in prostate cancer. Prostate. 71:42–51. 2011.
|
|
21.
|
Dasgupta A, Alvarado CS, Xu Z and Findley
HW: Expression and functional role of inhibitor-of-apoptosis
protein livin (BIRC7) in neuroblastoma. Biochem Biophys Res Commun.
400:53–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wang TS, Ding QQ, Guo RH, et al:
Expression of livin in gastric cancer and induction of apoptosis in
SGC-7901 cells by shRNA-mediated silencing of livin gene. Biomed
Pharmacother. 64:333–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Mousavi-Shafaei P, Ziaee AA, Azizi E and
Zangemeister-Wittke U: Antisense-mediated melanoma inhibitor of
apoptosis protein downregulation sensitizes G361 melanoma cells to
cisplatin. Anticancer Drugs. 17:1031–1039. 2006. View Article : Google Scholar
|
|
24.
|
Jiao YS, Wang YL, Zhang SL, Liu XM and Hao
FJ: The expression and significance of Livin and Smac in epithelial
ovarian carcinoma tissues. Modern Oncol. 17:296–299. 2009.
|
|
25.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Igney FH and Krammer PH: Death and
anti-death: tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Tanabe H, Yagihashi A, Tsuji N, Shijubo Y,
Abe S and Watanabe N: Expression of survivin mRNA and livin mRNA in
non-small-cell lung cancer. Lung Cancer. 46:299–304. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ka H and Hunt JS: Temporal and spatial
patterns of expression of inhibitors of apoptosis in human
placentas. Am J Pathol. 163:413–422. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yagihashi A, Asanuma K, Tsuji N, et al:
Detection of anti-livin antibody in gastrointestinal cancer
patients. Clin Chem. 49:1206–1208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Chang H and Schimmer AD: Livin/melanoma
inhibitor of apoptosis protein as a potential therapeutic target
for the treatment of malignancy. Mol Cancer Ther. 6:24–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ma L, Huang Y, Song Z, et al: Livin
promotes Smac/DIABLO degradation by ubiquitin-proteasome pathway.
Cell Death Differ. 13:2079–2088. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wagener N, Crnkovic-Mertens I, Vetter C,
et al: Expression of inhibitor of apoptosis protein Livin in renal
cell carcinoma and non-tumorous adult kidney. Br J Cancer.
97:1271–1276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Alvarez-Salas LM and DiPaolo JA: Molecular
approaches to cervical cancer therapy. Curr Drug Discov Technol.
4:208–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Crnkovic-Mertens I, Wagener N, Semzow J,
et al: Targeted inhibition of Livin resensitizes renal cancer cells
towards apoptosis. Cell Mol Life Sci. 64:1137–1144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Pirollo KF and Chang EH: Targeted delivery
of small interfering RNA: approaching effective cancer therapies.
Cancer Res. 68:1247–1250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Kleinberg L, Florenes VA, Silins I, et al:
Nuclear expression of survivin is associated with improved survival
in metastatic ovarian carcinoma. Cancer. 109:228–238. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Gong J, Chen N, Zhou Q, Yang B, Wang Y and
Wang X: Melanoma inhibitor of apoptosis protein is expressed
differentially in melanoma and melanocytic naevus, but similarly in
primary and metastatic melanomas. J Clin Pathol. 58:1081–1085.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Crnkovic-Mertens I, Hoppe-Seyler F and
Butz K: Induction of apoptosis in tumor cells by siRNA-mediated
silencing of the livin/ML-IAP/KIAP gene. Oncogene. 22:8330–8336.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Crnkovic-Mertens I, Muley T, Meister M, et
al: The anti-apoptotic livin gene is an important determinant for
the apoptotic resistance of non-small cell lung cancer cells. Lung
Cancer. 54:135–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Feril LB Jr: Ultrasound-mediated gene
transfection. Methods Mol Biol. 542:179–194. 2009. View Article : Google Scholar : PubMed/NCBI
|